Abstract
MicroRNAs (miRNAs) recruit the CCR4–NOT complex, which contains two deadenylases, CCR4 and CAF1, to promote shortening of the poly(A) tail. Although both CCR4 and CAF1 generally have a strong preference for poly(A) RNA substrates, it has been reported from yeast to humans that they can also remove non-A residues in vitro to various degrees. However, it remains unknown how CCR4 and CAF1 remove non-A sequences. Herein we show that Drosophila miRNAs can promote the removal of 3′-terminal non-A residues in an exonucleolytic manner, but only if an upstream poly(A) sequence exists. This non-A removing reaction is directly catalyzed by both CCR4 and CAF1 and depends on the balance between the length of the internal poly(A) sequence and that of the downstream non-A sequence. These results suggest that the CCR4–NOT complex has an intrinsic activity to remove the 3′-terminal non-A modifications downstream from the poly(A) tail.
Keywords: CCR4–NOT complex, deadenylase, deadenylation, microRNA, mRNA decay
INTRODUCTION
In eukaryotes, cytoplasmic mRNA decay pathways are generally initiated by shortening of the poly(A) tail or deadenylation. Deadenylated mRNAs are degraded by exosome from 3′-end or are decapped by the Dcp1–Dcp2 complex followed by degradation by Xrn1 from the 5′-end (Parker and Song 2004; Garneau et al. 2007; Houseley and Tollervey 2009). One of the well-known triggers of deadenylation is microRNA (miRNA) (Goldstrohm and Wickens 2008; Chen and Shyu 2011; Pérez-Ortin et al. 2013), which forms RNA-induced silencing complex (RISC) with Argonaute (Ago) proteins and guides Ago to their complementary target mRNAs (Bartel 2009; Kawamata and Tomari 2010; Hirose et al. 2014). In Drosophila melanogaster, miRNAs are loaded into Ago1 (Okamura et al. 2004; Forstemann et al. 2007; Tomari et al. 2007), which directly interacts with GW182 (also known as TNRC6 in vertebrates) (Behm-Ansmant et al. 2006; Eulalio et al. 2008). GW182 recruits two deadenylase complexes, the PAN2–PAN3 complex and the CCR4–NOT complex (Braun et al. 2011; Chekulaeva et al. 2011; Fabian et al. 2011), of which the CCR4–NOT complex provides a major contribution to miRNA-mediated deadenylation (Behm-Ansmant et al. 2006; Chen et al. 2009; Piao et al. 2010; Braun et al. 2011).
The CCR4–NOT complex is highly conserved in eukaryotes and its core components comprise eight subunits in flies (Collart and Panasenko 2012; Temme et al. 2014). Of these, CCR4 and CAF1 (also known as POP2) each possess an Mg2+-dependent deadenylase activity. While flies have only single orthologs each of CCR4 and CAF1, vertebrates such as humans have their multiple orthologs. CAF1 is a member of the DEDDh (Asp-Glu-Asp-Asp-His in the active site) family of RNases (Zuo and Deutscher 2001), and directly binds to the MIF4G fold of NOT1, the scaffold protein in the CCR4–NOT complex (Basquin et al. 2012; Petit et al. 2012). CCR4 belongs to the endonuclease-exonuclease-phosphatase (EEP) family of RNases (Dlakić 2000) and possesses the leucine-rich repeat (LRR) domain and the nuclease domain. The LRR domain of CCR4 binds to CAF1, thereby sandwiching CAF1 between CCR4 and NOT1 (Draper et al. 1995; Basquin et al. 2012). Interestingly, the nuclease domain of Saccharomyces cerevisiae CCR4 requires the neighboring LRR domain for its deadenylation activity, while the nuclease domain of human CNOT6L (CCR4 homolog) retains its activity in the absence of the LRR domain (Clark et al. 2004; Wang et al. 2010).
Although CCR4 and CAF1 generally prefer the poly(A) sequence, a number of reports suggest that they can remove non-A sequences at least in vitro (Chen et al. 2002; Thore et al. 2003; Viswanathan et al. 2004; Bianchin et al. 2005; Wang et al. 2010). For instance, recombinant yeast Pop2p (CAF1 homolog) exhibited robust RNase activity for poly(U) and poly(C) RNAs (Thore et al. 2003), although the competition assay revealed a modest preference toward poly(A) (Daugeron et al. 2001). Moreover, recombinant human CNOT7 (Caf1a) and CNOT8 (Caf1b/POP2) were able to degrade RNA substrates beyond their 3′-terminal poly(A) sequence (Bianchin et al. 2005). However, it remains unknown how CCR4 and CAF1 remove non-A sequences.
Herein we show that, in flies, miRNAs can promote the removal of 3′-terminal non-A residues, but only if an upstream poly(A) sequence is present. This activity is directly mediated by the CCR4–NOT complex and depends on the balance between the length of the internal poly(A) sequence and that of the downstream non-A sequence. Purified CCR4 or CAF1 alone shows such an internal poly(A)-dependent exonuclease activity, even though as previously reported they both strongly prefer poly(A) RNAs to other polyribonucleotides. The intrinsic property of the CCR4–NOT complex to remove 3′-terminal non-A modifications should not be restricted to miRNA triggers and may function to regulate mRNAs with widespread uridylation and guanylation downstream from the poly(A) tail.
RESULTS
miRNAs can induce the removal of a non-A sequence downstream from the poly(A) tail in S2 cells
We have previously reported that reporter RNAs bearing a 114-nucleotide (nt) poly(A) sequence internalized by a downstream 40-nt unrelated sequence (hereafter referred to as the post-poly(A) sequence), followed by the self-cleaving hammerhead ribozyme (HhR) to generate a defined 3′ end, are refractory to miRNA-mediated deadenylation and decay in Drosophila S2 cells (Fukaya and Tomari 2012). However, in the course of our study, we noticed that a shorter post-poly(A) sequence cannot completely block miRNA-mediated deadenylation. Indeed, when we expressed reporter RNAs for let-7 miRNA bearing a 50-nt poly(A) sequence with or without an unrelated 10-nt post-poly(A) sequence (Rluc-let-7-A50 and Rluc-let-7-A50C10; Fig. 1A), they were both efficiently degraded upon ectopic let-7 expression (Fig. 1B). Knockdown of Dcp2, an enzyme required for decapping, resulted in accumulation of deadenylated intermediates for both A50 and A50C10 reporters, in a manner dependent on let-7 expression (Fig. 1B). Thus, miRNAs can promote the removal of not only 3′-exposed poly(A) tails but also internalized poly(A) sequences with short post-poly(A) sequences in S2 cells.
FIGURE 1.
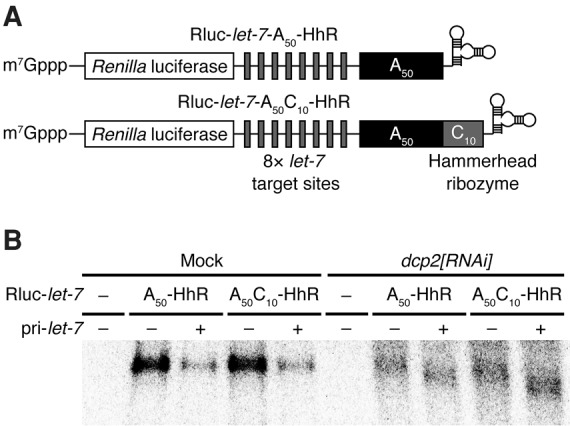
miRNAs can induce the removal of a non-A sequence downstream from the poly(A) tail in S2 cells. (A) Schematic representation of Rluc-let-7-A50-HhR and Rluc-let-7-A50C10-HhR. These reporter RNAs include Renilla luciferase (Rluc) ORF, eight let-7 target sites, and hammerhead ribozyme. The subscripts show the length of an RNA sequence including the shown base. (B) Northern blot analysis of the reporter RNAs shown in A. Expression of pri-let-7 induced degradation of the reporter RNAs in GFP knockdown (mock). In Dcp2 knockdown, deadenylated RNAs were detected dependently on expression of pri-let-7.
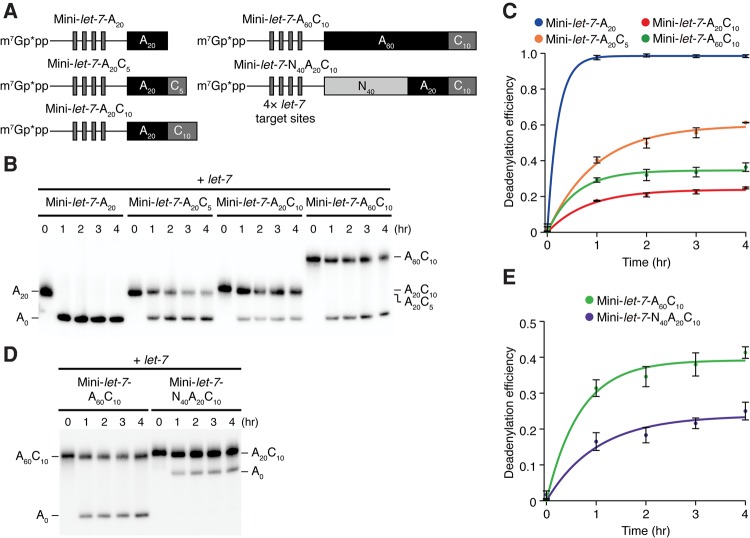
The balance between the poly(A) length and the downstream post-poly(A) length affects deadenylation efficiency
To analyze the effect of the post-poly(A) sequence on deadenylation more closely, we performed in vitro deadenylation assays. As reported previously, we prepared deadenylation-active lysate from S2 cells overexpressing Argonaute1 (Ago1) (Fukaya and Tomari 2011). We also constructed a series of reporter RNAs for let-7 with increasing lengths of the post-poly(A) sequence: Mini-let-7-A20 with a 3′ exposed 20-nt poly(A) tail, Mini-let-7-A20C5 with the poly(A) tail internalized by the 5-nt post-poly(A) sequence, and Mini-let-7-A20C10 with the 10-nt post-poly(A) sequence (Fig. 2A). We then programmed Ago1-RISC by let-7/let-7* duplex and measured the time course of reporter RNA deadenylation in vitro. As shown in Figure 2B and C, the length of the post-poly(A) sequence negatively correlated with the deadenylation efficiency. We then fixed the length of the post-poly(A) sequence at 10 nt and changed the length of the upstream poly(A) sequence from 20 nt to 60 nt. Intriguingly, the deadenylation efficiency was significantly improved by elongating the internal poly(A) sequence (Fig. 2B,C; Mini-let-7-A20C10 and Mini-let-7-A60C10). These results suggest that deadenylation is promoted by the length of the upstream poly(A) sequence and inhibited by the length of the downstream post-poly(A) sequence. To rule out the possibility that the entire length of the reporter RNAs is important, rather than the poly(A) length per se, we constructed a reporter RNA that has the same overall length as Mini-let-7-A60C10 but contains a 40-nt unrelated sequence and a 20-nt poly(A) sequence instead of the 60-nt poly(A) sequence, upstream of the 10-nt post-poly(A) sequence (Fig. 2A; Mini-let-7-N40A20C10). As shown in Figure 2D and E, deadenylation of Mini-let-7-N40A20C10 was much slower than that of Mini-let-7-A60C10. Thus, the balance between the upstream poly(A) sequence and the downstream post-poly(A) sequence determines the deadenylation efficiency.
FIGURE 2.
The balance between the poly(A) length and the downstream post-poly(A) length affects deadenylation efficiency. (A) Schematic representation of a series of Mini-let-7 reporter constructs. These reporter RNAs include four let-7 target sites and were capped with [α-32P] GTP. The subscripts show the length of an RNA sequence including the shown base. The asterisks indicate a radiolabel. (B) Deadenylation assay for Mini-let-7 reporter RNAs with S2 cell lysate overexpressed Ago1. (C) The signal intensity of the bands in B was quantified, and the ratio of the intensity from the lower band for the total intensity was calculated and plotted. Mini-let-7-A20 effectively shifted to A0. While the length of the post-poly(A) sequence negatively correlated with the deadenylation efficiency, elongating the internal poly(A) sequence improved. The graph shows means and standard deviations (n = 3). (D) Deadenylation assay for Mini-let-7-A60C10 and Mini-let-7-N40A20C10 with S2 cell lysate overexpressed Ago1. (E) The signal intensity of the bands in D was quantified, and the ratio of the intensity from the lower band for the total intensity was calculated and plotted. Mini-let-7-A60C10 was deadenylated more effectively than Mini-let-7-N40A20C10. The graph shows means and standard deviations (n = 3).
Deadenylation of the internalized poly(A) sequence involves the CCR4–NOT complex
It is well known that the CCR4–NOT deadenylase complex plays a major role in regular miRNA-directed deadenylation (Behm-Ansmant et al. 2006; Chen et al. 2009; Piao et al. 2010; Braun et al. 2011). Therefore, we asked whether deadenylation of the poly(A) sequence internalized by the post-poly(A) sequence is also mediated by the CCR4–NOT complex. To this end, we prepared lysate from S2 cells with either CCR4 or CAF1 knockdown together with Ago1 overexpression (Fig. 3A) and assayed deadenylation in vitro. Knockdown of CAF1 abolished deadenylation of Mini-let-7-A20 with the 3′-exposed poly(A) tail (Fig. 3B), while knockdown of CCR4 impaired deadenylation only modestly (Fig. 3C,D). This is consistent with the configuration of the CCR4–NOT complex, in which CAF1 bridges CCR4 and NOT1; CAF1 knockdown not only depletes CAF1 itself but also causes detachment of CCR4 from NOT1, while CAF1 is still functional on NOT1 in the absence of CCR4 (Temme et al. 2004). Importantly, both CAF1 knockdown and CCR4 knockdown inhibited deadenylation of Mini-let-7-A20N10 with the poly(A) sequence internalized by the post-poly(A) sequence, similarly to that of Mini-let-7-A20 (Fig. 3B–D). These results suggest that the CCR4–NOT complex is involved in deadenylation of internalized poly(A) sequences with post-poly(A) sequences as well as normal 3′-exposed poly(A) tails.
FIGURE 3.
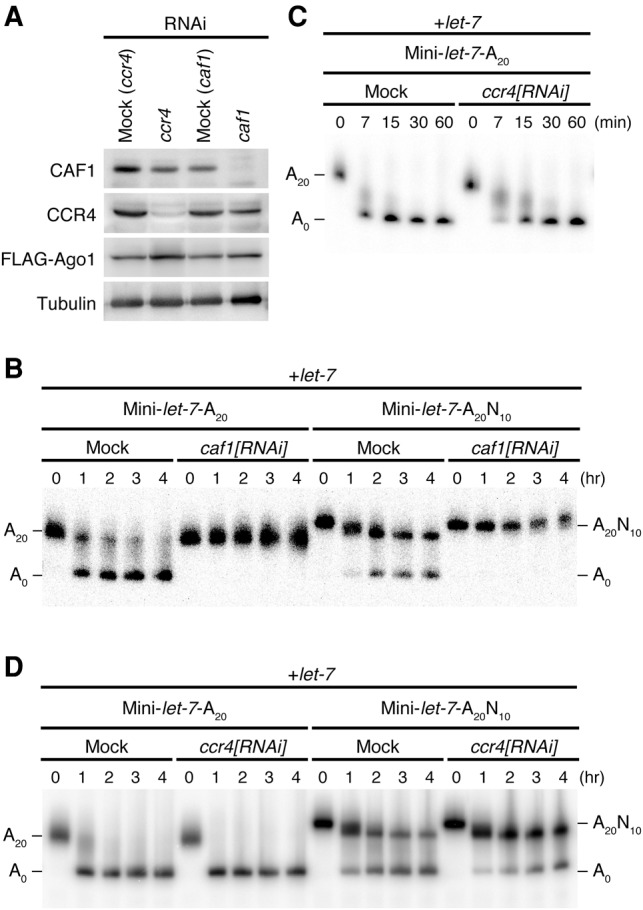
Deadenylation of the internalized poly(A) sequence involves the CCR4–NOT complex. (A) Western blot analysis. CCR4 and CAF1 were significantly reduced by treatment of the corresponding dsRNA to S2 cells. (B) Deadenylation assay for Mini-let-7-A20 and Mini-let-7-A20N10 with S2 cell lysate knocked down CAF1. While mock knockdown lysate effectively induced deadenylation for both reporter RNAs, CAF1 knockdown lysate failed to deadenylate. (C,D) Deadenylation assay for Mini-let-7-A20 and Mini-let-7-A20N10 with S2 cell lysate knocked down CCR4. Compared to mock knockdown, deadenylation was retarded in CCR4 knockdown.
CCR4 and CAF1 have an intrinsic activity to remove post-poly(A) sequences
To examine if the CCR4–NOT complex directly mediates removal of the post-poly(A) sequence, we prepared four types of recombinant Drosophila CCR4/CAF1 heterodimer using a baculovirus expression system; where both CCR4 and CAF1 are wild types (4W1W), either one of them is a catalytic mutant (4m1W and 4W1m), or both of them are catalytic mutants (4m1m) (Fig. 4A). We then constructed reporter RNAs without any miRNA target sites (Fig. 4B) and incubated them with the four types of the heterodimers. Except for 4m1m, the heterodimers showed deadenylation activity for the A20 reporter. This suggests that, as expected, the heterodimer can mediate deadenylation as long as either CCR4 or CAF1 is intact (Fig. 4C). We then tested the four heterodimers for the A20C5 reporter with the internalized poly(A) sequence. Strikingly, as we observe with the normal A20 reporter, the A20C5 reporter was also shortened by the heterodimers except when both CCR4 and CAF1 were catalytically mutated (Fig. 4D). These results indicate that Drosophila CCR4 and CAF1 have independent and intrinsic activity to remove the C5 post-poly(A) sequences downstream from the A20 poly(A) sequence. Nonetheless, when 30-nt poly(N) RNAs with each of the four different nucleotides were assayed, the CCR4/CAF1 heterodimer showed a strong preference for adenines as previously reported (Tucker et al. 2001; Viswanathan et al. 2003, 2004; Temme et al. 2004; Bianchin et al. 2005; Wang et al. 2010) and the poly(C) RNA (C30) remained completely intact (Fig. 4E). Moreover, Mini-let-7-A20N10, which contains all of four bases in the 3′-terminal N10 sequence (UUUGUCUGAC), was deadenylated by the CCR4/CAF1 heterodimer, similarly to the noC-A20C5 reporter (Supplemental Fig. S1). These results support that CCR4 and CAF1 possess an activity of removing a stretch of non-A nucleotides only when an upstream poly(A) sequence exists.
FIGURE 4.
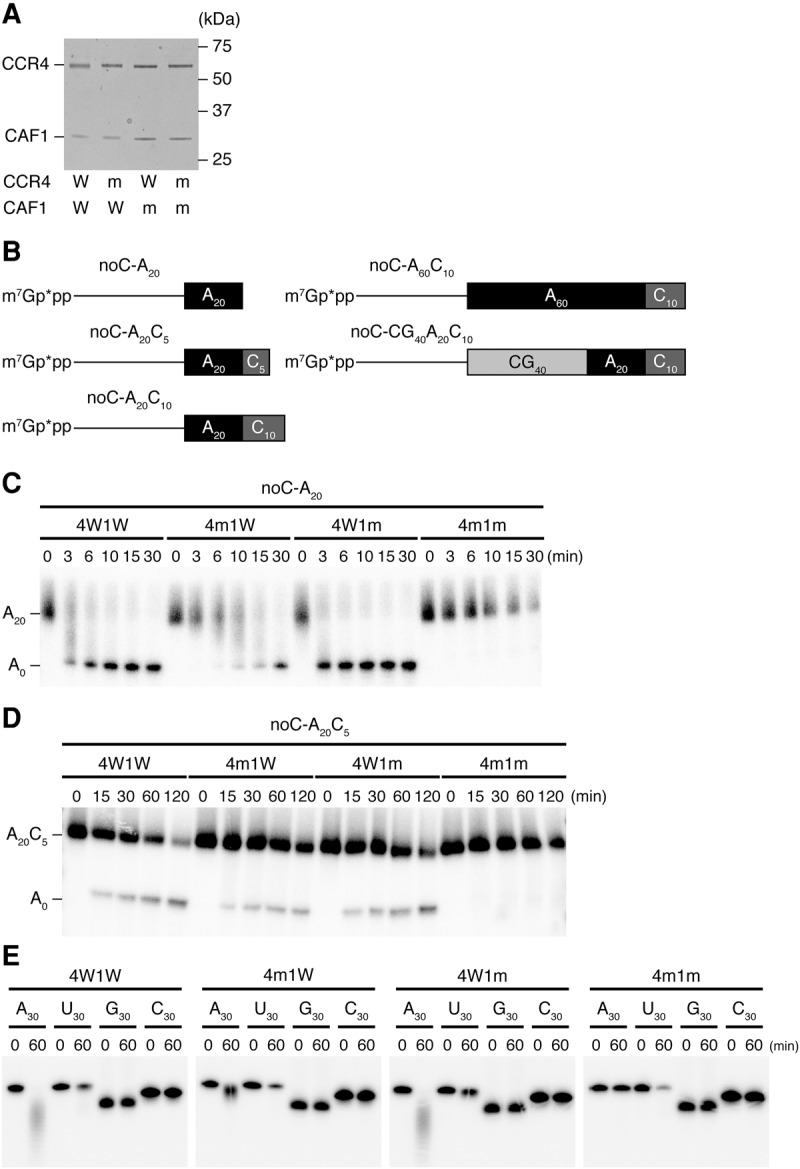
CCR4 and CAF1 have an intrinsic activity to remove post-poly(A) sequences. (A) SDS-PAGE and Coomassie brilliant blue staining for four types of recombinant Drosophila CCR4/CAF1 heterodimer, in which both CCR4 and CAF1 are wild types, either one of them is catalytic mutant, and both of them are catalytic mutants. (W) Wild type, (m) catalytic mutant. (B) Schematic representation of a series of noC reporter constructs. These reporter RNAs were capped with [α-32P] GTP. The subscripts show the length of an RNA sequence including the shown base. The asterisks indicate a radiolabel. (C) Deadenylation assay for noC-A20 with four types of recombinant CCR4/CAF1 heterodimer. The heterodimers showed deadenylation activity for the A20 reporter except for 4m1m. (4W1W) CCR4 and CAF1 are wild types, (4m1W) CCR4 is catalytic mutant and CAF1 is wild type, (4W1m) CCR4 is wild type, CAF1 is catalytic mutant, (4m1m) CCR4 and CAF1 are catalytic mutants. (D) Deadenylation assay for noC-A20C5 with four types of recombinant CCR4/CAF1 heterodimer. Despite the fact that the reporter RNA had C5 following A20, CCR4 and CAF1 could deadenylate. (E) 30 nt poly(A), (U), (G), and (C) RNAs were incubated with four types of recombinant CCR4/CAF1 heterodimer. Both CCR4 and CAF1 strongly preferred poly(A) as a substrate. Note that poly(U) was slightly degraded in all cases (even for the 4m1m double-mutant heterodimer), presumably due to contaminating nonspecific nuclease(s).
The balance between the poly(A) length and the downstream post-poly(A) length affects the activity of CCR4 and CAF1
We next set out to ask if the recombinant CCR4/CAF1 heterodimer recapitulates the dependence of deadenylation on the balance between the poly(A) length and the downstream post-poly(A) sequence, which was observed in miRNA-mediated deadenylation in cell lysate. We prepared a series of reporter RNAs with different lengths of the internal poly(A) sequence and the downstream post-poly(A) sequence (Fig. 4B) and incubated them with the four types of CCR4/CAF1 heterodimers. As we had observed in the miRNA-mediated reaction (Fig. 2), deadenylation by the wild-type 4W1W heterodimer was inhibited by the length of the post-poly(A) sequence and promoted by the length of the internal poly(A) sequence (Fig. 5A,C) but not by the entire length (Fig. 5B,D). This trend was also true for the 4m1W and 4W1m heterodimers, where either CCR4 or CAF1 was mutated. Collectively, we concluded that CCR4 and CAF1 have an intrinsic ability to remove the post-poly(A) sequence in a manner dependent on the balance between the internal poly(A) length and the downstream post-poly(A) length.
FIGURE 5.
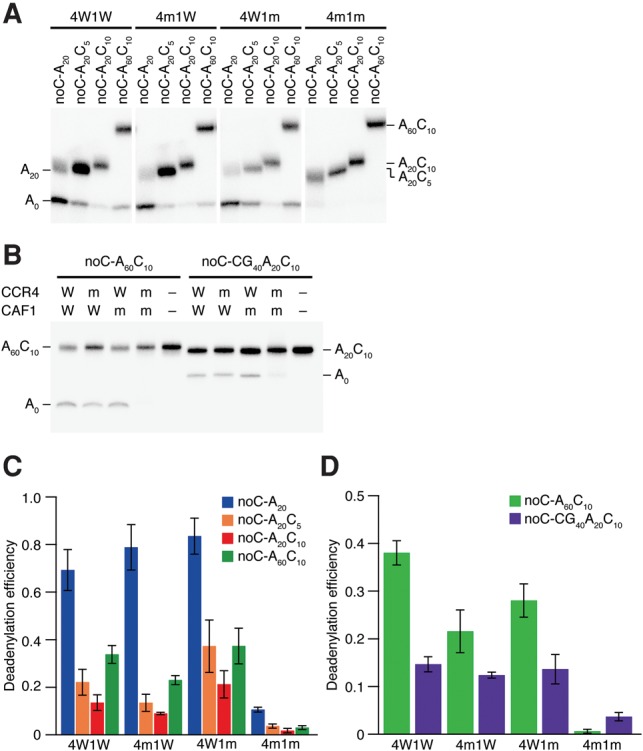
The balance between the poly(A) length and the downstream post-poly(A) length affects the activity of CCR4 and CAF1. (A) Deadenylation of noC reporter RNAs with four types of recombinant CCR4/CAF1 heterodimer after 2 h incubation. (B) Deadenylation of noC-A60C10 and noC-CG40A20C10 with four types of recombinant CCR4/CAF1 heterodimer after 2 h incubation. (C) The signal intensity of the bands in A was quantified, and the ratio of the intensity from the lower band for the total intensity was calculated and shown. As well as Figure 2C, noC-A20 effectively shifted to A0. While the length of the post-poly(A) sequence negatively correlated with the deadenylation efficiency, elongating the internal poly(A) sequence improved. The graph shows means and standard deviations (n = 3). (D) The signal intensity of the bands in B was quantified, and the ratio of the intensity from the lower band for the total intensity was calculated and shown. noC-A60C10 was deadenylated more effectively than noC-CG40A20C10. The graph shows means and standard deviations (n = 3).
CCR4 and CAF1 remove post-poly(A) sequences in an exonucleolytic manner
It has been reported that Drosophila CCR4 and CAF1 are 3′–5′ exoribonucleases with a strong A preference (Temme et al. 2004). How do CCR4 and CAF1 remove the post-poly(A) sequence? To discriminate endonucleolytic and exonucleolytic reactions, we designed reporter RNAs with an internal label in the post-poly(A) sequence. Specifically, the RNAs contained no C in the 5′ body region, while the 5-nt post-poly(A) sequence of “CGGGG” contained a single C internally (noC-A20C1G4; Fig. 6A). We then transcribed the RNAs in the presence of [α-32P] CTP so that the internal C in the post-poly(A) sequence was specifically radiolabeled. We also prepared a control reporter in which the internal 20-nt poly(A) sequence was replaced with UG repeats of 20 nt in total (noC-UG20C1G4; Fig. 6A). If CCR4 and/or CAF1 removed the post-poly(A) sequence in an endonucleolytic manner before trimming the poly(A) sequence, a 5-nt radiolabeled fragment of *CGGGG should be detected. On the other hand, if the post-poly(A) sequence was removed in an exonucleolytic manner, radiolabeled CMP should be generated. Intriguingly, the catalytically active CCR4/CAF1 heterodimers (4W1W, 4m1W, and 4W1m) generated a 1-nt reaction product from noC-A20C1G4, while the 1-nt signals from noC-UG20C1G4 or from the 4m1m heterodimer were markedly weaker (Fig. 6B). Thin-layer chromatography identified the reaction product as CMP (Fig. 6C). These results indicate that both CCR4 and CAF1 remove the post-poly(A) sequence in an exonucleolytic manner, as is the case with regular deadenylation.
FIGURE 6.
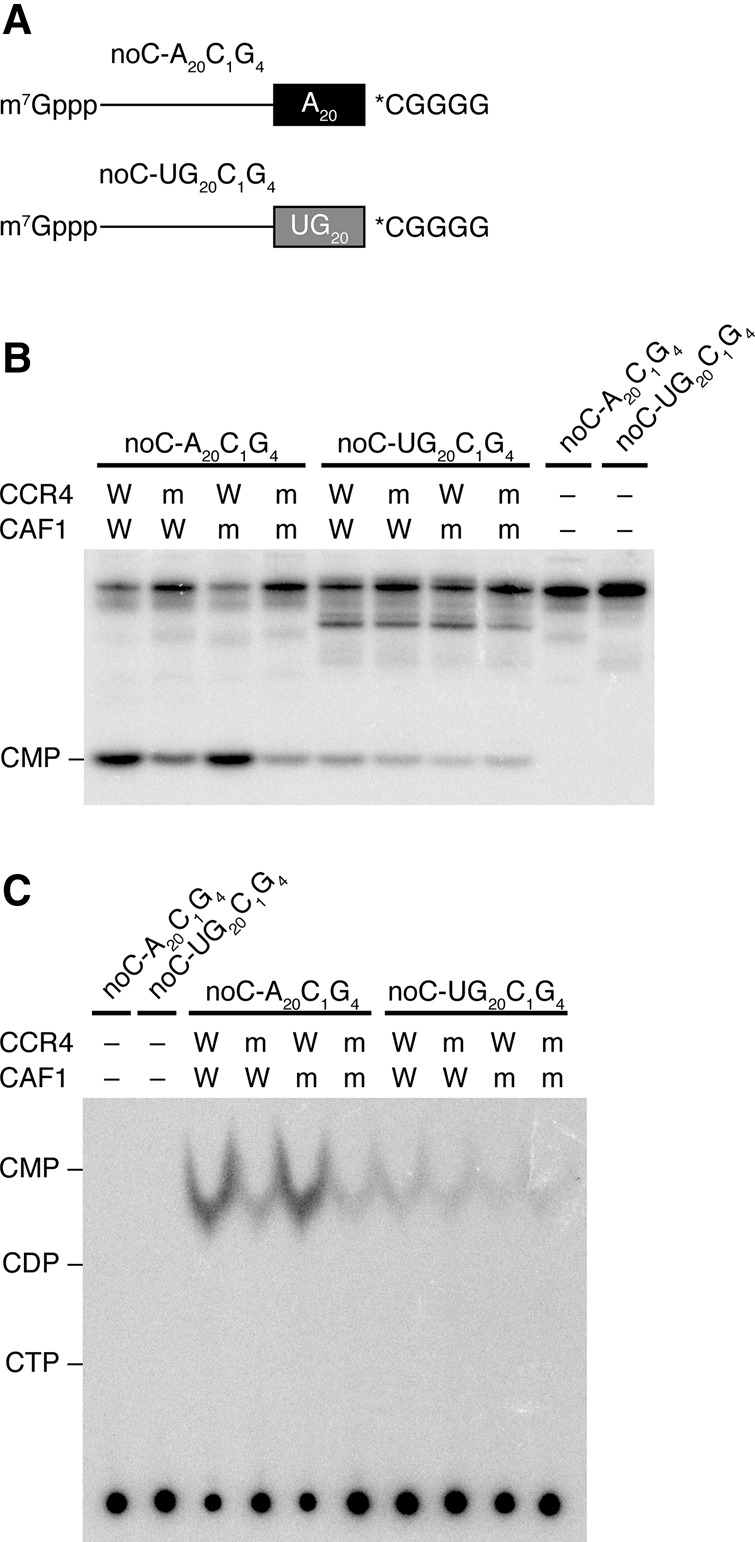
CCR4 and CAF1 remove post-poly(A) sequences in an exonucleolytic manner. (A) Schematic representation of noC-A20C1G4 and noC-UG20C1G4. These reporter RNAs include no nucleotides with C besides the moiety of post-poly(A) sequence, whereby the only post-poly(A) sequence can be labeled by transcribing with NTP including [α-32P] CTP. The asterisks indicate a radiolabel. (B) Deadenylation of noC-A20C1G4 and noC-UG20C1G4 with four types of recombinant CCR4/CAF1 heterodimer after 2 h incubation. Although CCR4 and CAF1 generated CMP from both reporter RNAs, the signal intensities from noC-A20C1G4 in active CCR4/CAF1 heterodimers were higher than from noC-A20C1G4 in inert CCR4/CAF1 heterodimer and from noC-UG20C1G4. (C) Thin layer chromatography for the samples of B.
DISCUSSION
In this study, we show that CCR4 and CAF1, the two deadenylases in the CCR4–NOT complex, can remove 3′ terminal non-A residues in an exonucleolytic manner, if an upstream poly(A) sequence is present. This noncanonical but intrinsic activity of CCR4 and CAF1 depends on the balance between the length of the upstream poly(A) sequence and that of the post-poly(A) sequence. These observations suggest that CCR4 and CAF1 can remotely contact the internal poly(A) sequence, which then promotes exonucleolytic digestion of non-A residues at the 3′ end.
Structural studies have shown that both CCR4 and CAF1 are capable of specifically recognizing an adenine adjacent to their catalytic cores (Andersen et al. 2009; Wang et al. 2010). For example, human CNOT6L (Ccr4b) recognizes an adenine by sandwiching the purine ring between the rings of Phe484 and Pro36. Additionally, the N6 group of the adenine base interacts with the carbonyl group of Asn412 (Wang et al. 2010). On the other hand, in S. pombe Pop2p (CAF1 homolog), Ser122 is thought to form a hydrogen bond to N3 of an adenine, with the side chain of Leu125 stabilizing the location of its purine ring (Andersen et al. 2009). In both cases, only a few specific interactions exist between the adenine base and the residues in the active sites, which may allow accommodation of a non-A residue for removal at the 3′ end. However, it remains unknown how the internal poly(A) sequence can be recognized. One possibility is that nonproductive yet recurring binding of the internal poly(A) sequence to the A-binding site simply increases the chance for the 3′ non-A residue to be fortuitously accommodated in the catalytic core. Another possibility is that CCR4 and CAF1 have a second, as-yet-uncharacterized poly(A) recognition site. Intriguingly, a second AMP molecule was observed ∼20 Å apart from the active site in the human CNOT6L–AMP complex structure (Wang et al. 2010); however, the adenine base is not specifically recognized there and it cannot be excluded that the second AMP site is a crystallization artifact.
It has been reported recently that human CCR4 and CAF1 work cooperatively in deadenylation; when either CNOT7 (Caf1a) or CNOT6L (Ccr4b) was mutated in the active site, the activity of recombinant BTG2–CNOT7–CNOT6L complex was abolished to the same extent as the double mutant (Maryati et al. 2015). In contrast, in our hands, the 4m1W and 4W1m fly CCR4/CAF1 heterodimers showed strong deadenylation activity compared to the 4m1m double mutant. Furthermore, 4m1W and 4W1m were able to remove post-poly(A) sequences in the same internal poly(A)-dependent manner as the 4W1W wild type. Future investigation is needed to address whether this apparent discrepancy results from the difference in species origin, the configuration of the complex, or the experimental setting including substrate RNAs.
Although we here used artificially designed reporters to detect the post-poly(A) removal activity of CCR4 and CAF1, we speculate that this activity may function in vivo. Indeed, natural post-poly(A) sequences are observed broadly in mRNAs of yeast, plants and humans and are thought to regulate the stability of mRNAs (Rissland and Norbury 2009; Sement et al. 2013; Chang et al. 2014). In human cells, oligo U is the most frequent post-poly(A) sequence and is mainly found in <25-nt poly(A) tail (Chang et al. 2014). Uridylation frequency shows a modest negative correlation with mRNA half-life, suggesting its role as a mark for mRNA decay. Recently, terminal uridylyl transferase 4 and 7 (TUT4/7) have been identified as the factors responsible for mRNA uridylation, and their activity is enhanced by a shorter poly(A) tail (Lim et al. 2014). In contrast, G is found mainly downstream from longer poly(A) tails (>40 nt) and potentially stabilizes mRNAs (Chang et al. 2014). Perhaps, the CCR4–NOT complex not only acts as a deadenylase but also may serve as an eraser of such post-poly(A) sequences, adding another layer of complexity in mRNA stability control.
MATERIALS AND METHODS
Plasmid construction
pAWS
A PCR fragment containing the SBP-tag sequence was amplified with 5′-GTGAGCTCCGCCACCATGGACGAGAAGACCACC-3′ and 5′-TCACGTGGACCGGTGGCCGGAGGGCTCCCG-3′ from pASW (Fukaya and Tomari 2012) and inserted into PCR-linearized pAWF with 5′-CACCGGTCCACGTGACGT-3′ and 5′-GGTGGCGGAGCTCACCA-3′ by using InfusionHD (Takara).
pAWS-Rluc-let-7-A50-HhR, pAWS-Rluc-let-7-A50N10-HhR
The Rluc-let-7-A50-HhR sequence was amplified by PCR from pUC57-Rluc-let-7-A114 (Fukaya and Tomari 2011) using the primers 5′-CACCATGGCTTCCAAGGTGTACGAC-3′ and 5′-CCTGTTTCGTCCTCACGGACTCATCAGACCGGAAAACACATCCGGTGACAGGTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTCTAAATGAGTCTTCGGACCT-3′ and cloned into pENTR/D-TOPO (Invitrogen), followed by recombination with pAWS destination vector using LR clonase (Invitrogen). For preparing pAWS-Rluc-let-7-A50N10-HhR, two DNA fragments, 5′-TCGACGCGGCCGCTGGCAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAATGCTTCTGGTCCTG TCACCGGATGTGTTTTCCGGTCTGATGAGTCCGTGAGGACGAAACAGGAAGGGTGGG-3′ and 5′-CGCGCCCACCCTTCCTGTTTCGTCCTCACGGACTCATCAGACCGGAAAACACATCCGGTGACAGGACCAGAAGCATTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTGCCAGCGGCCGCG-3′, were phosphorylated using T4 polynucleotide kinase (Takara) and heat annealed. The dsDNA fragment was then inserted into pENTR-Rluc-let-7-A50-HhR digested by SalI and AscI and dephosphorylated. Finally, the Rluc-let-7-A50N10-HhR sequence was recombined into pAWS destination vector using LR clonase (Invitrogen).
pFastBac Dual-CAF1-CCR4-6×His
Full-length CAF1 and CCR4 coding regions were amplified by PCR from Drosophila S2 cell cDNA using primers 5′-GAAGCGCGCGGAATTATGAAATGGACAATGCCCTC-3′ and 5′-GATTCGAAAGCGGCCTCATGAAGCGCTGTTCGT-3′, and 5′-TGCATCAGCTGCTAGCTAATGATGATGATGATGATGCATC-3′ and 5′-CACCCGGGATCTCGAATGAAAGGCAATCATTATAAAATGTCTC-3′, and cloned into the EcoRI-NotI and XhoI-NheI sites, respectively, in pFastBac Dual (Invitrogen).
Northern blot analysis
Total RNAs were extracted from S2 cells by TRI Reagent (Molecular Research Center). Two micrograms or 10 µg of total RNAs from S2 cells with the mock GFP knockdown or Dcp2 knockdown were separated on 1.3% formaldehyde agarose gel and transferred to Hybond-N+ membrane (GE Healthcare). The RNA probe 5′-UGAGGUAGAUCCUUGUAUAGU-3′ was radiolabeled with T4 polynucleotide kinase (Takara) and [γ-32P] ATP (PerkinElmer), and hybridized with the membrane using Perfecthyb Plus (Sigma) at 50°C.
Preparation of double-stranded RNAs for RNAi
Double-stranded RNAs (dsRNAs) corresponding to the open reading frame of GFP, Dcp2, CAF1, and CCR4 mRNAs were prepared as previously described (Temme et al. 2004; Forstemann et al. 2005; Rehwinkel et al. 2005). All the dsRNAs were in vitro transcribed using T7-Scribe Standard RNA IVT Kit (CELLSCRIPT) and were purified by ethanol precipitation followed by ammonium acetate precipitation.
RNA interference and transfection
Dcp2 knockdown was performed as previously described (Rehwinkel et al. 2005). After 1 d of soaking, the S2 cells were transfected with 5 µg pASW-pri-let7 (Fukaya and Tomari 2012) and 5 µg pAWS-Rluc-let-7-A50-HhR or pAWS-Rluc-let-7-A50C10-HhR using X-tremeGENE HP (Roche) and further cultured for 3 d. For CAF1 knockdown, S2 cells were seeded at a density of 1.0 × 106 cells/mL in 10-cm dishes, and 20 µg dsRNAs were added to the dishes. One day after soaking, S2 cells were transfected with 10 µg pAFW-Ago1 using X-tremeGENE HP (Roche) and further cultured for 3 d. For CCR4 knockdown, S2 cells in 10-cm dishes were soaked with 100 µg dsRNAs. After 3 d, 100 µg dsRNAs were added again, and the cells were cultured for 1 d. Then, the cells were diluted fivefold and transfected with pAFW-Ago1 similarly to CAF1 knockdown. Lysates from S2 cells were prepared as previously described (Fukaya and Tomari 2012).
Western blot analysis
Antibodies to FLAG (1:5000; Sigma), CAF1 (1:5000 [Temme et al. 2004]), CCR4 (1:5000 [Temme et al. 2004]), and Tubulin (1:1000; Sigma) were used as the primary antibodies. Chemiluminescence was induced by Luminata Forte Western HRP Substrate (Millipore) and images were acquired by LAS-3000 (Fujifilm Life Sciences).
Preparation of the target RNAs
Mini-let-7-A20 and Mini-let-7-A20N10 RNAs
DNA fragments were amplified by PCR from psiCHECK2-let-7 4× (Iwasaki et al. 2009) using primers 5′-CGTAATACGACTCACTATAGGGGCAGTAATTCTAGGCGATCG-3′ and 5′-TTTTTTTTTTTTTTTTTTTTCGCCTTAAGCGGCCCCCGCG-3′ or 5′-GTCAGACAAATTTTTTTTTTTTTTTTTTTTCGCCTTAAGCGGCCCCCGCG-3′ and used for in vitro transcription by T7-Scribe Standard RNA IVT Kit (CELLSCRIPT). RNAs were radiolabeled by using ScriptCap m7G Capping System (CELLSCRIPT) and [α-32P] GTP (PerkinElmer).
Mini-let-7-A20C5, Mini-let-7-A20C10, Mini-let-7-A60C10, and Mini-let-7-N40A20C10 RNAs
DNA fragments were amplified by PCR from psiCHECK2-let-7 4× (Iwasaki et al. 2009) using primers 5′-CGTAATACGACTCACTATAGGGGCAGTAATTCTAGGCGATCG-3′ and 5′-GGGGGTTTTTTTTTTTTTTTTTTTTGCGGCCAGCGGCCG-3′, 5′-GGGGGGGGGGTTTTTTTTTTTTTTTTTTTTGCGGCCAGCGGCCG-3′, 5′-GGGGGGGGGGTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTGCGGCCAGCGGCCG-3′, or 5′-GGGGGGGGGGTTTTTTTTTTTTTTTTTTTTAACCAACACACAGATGTAATGAAA-3′ and used for in vitro transcription by T7-Scribe Standard RNA IVT Kit (CELLSCRIPT). RNAs were radiolabeled by using ScriptCap m7G Capping System (CELLSCRIPT) and [α-32P] GTP (PerkinElmer).
noC-A20, noC-A20C5, noC-A20C10, noC-A60C10, and noC-CG40A20C10 RNAs
DNA fragments were amplified by three-way overlapping PCR using primers 5′-GGTGAGAGTGAGTAGTGTAGTATTGGTAGTTGTATTAGAGTGAGGTTGTGGTTTGTGGGT-3′ and 5′-CGTAATACGACTCACTATAGGTGAGAGTGAGTAGTGTAGTATTGG-3′ and 5′-TTTTTTTTTTTTTTTTTTTTACCCACAAACCACAACCTC-3′, 5′-GGGGGTTTTTTTTTTTTTTTTTTTTACCCACAAACCACAACCTC-3′, 5′-GGGGGGGGGGTTTTTTTTTTTTTTTTTTTTACCCACAAACCACAACCTC-3′, 5′-GGGGGGGGGGTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTACCCACAAACCACAACCTC-3′, or 5′-GGGGGGGGGGTTTTTTTTTTTTTTTTTTTTCCCGCGGGCCGGGCGGGCGGGCGGGCGGGCGGGCGGGCGGACCCACAAACCACAACCTC-3′ and used for in vitro transcription by T7-Scribe Standard RNA IVT Kit (CELLSCRIPT). RNAs were radiolabeled by using ScriptCap m7G Capping System (CELLSCRIPT) and [α-32P] GTP (PerkinElmer).
noC-A20C1G4 and noC-UG20C1G4 RNAs
DNA fragments were amplified by three-way overlapping PCR using primers 5′-GGTGAGAGTGAGTAGTGTAGTATTGGTAGTTGTATTAGAGTGAGGTTGTGGTTTGTGGGT-3′ and 5′-CGTAATACGACTCACTATAGGTGAGAGTGAGTAGTGTAGTATTGG-3′ and 5′-CCCCGCCCACCAACAAACAACAACCACCCACAAACCACAACCTC-3′, or 5′-CCCCGTTTTTTTTTTTTTTTTTTTTACCCACAAACCACAACCTC-3′ (underlines indicate 2′-O-methylated nucleotides) and used for in vitro transcription by MAXIscript T7 In Vitro Transcription Kit (Ambion) in the presence of [α-32P] CTP (PerkinElmer).
Mutagenesis
D53A and D55A mutations for CAF1 and D411A and N413A mutations for CCR4 (Temme et al. 2010) were introduced to pFastBac Dual-CAF1-CCR4-6×His by PCR with the primer pairs 5′-CACTATGTGGCCATGGCCACCGCGTTTCCAGGCGTGGTA-3′ and 5′-TACCACGCCTGGAAACGCGGTGGCCATGGCCACATAGTG-3′ for CAF1, 5′-CTGCTGCTGTGCGGTGCCTTCGCCTCGCTACCCGATTCA-3′ and 5′-TGAATCGGGTAGCGAGGCGAAGGCACCGCACAGCAGCAG-3′ for CCR4.
Protein purification
The CCR4/CAF1 heterodimers were expressed in SF9 cells by using Bac-to-Bac Baculovirus Expression system (Invitrogen). The heterodimers were purified by sequential affinity chromatography with His-trap FF crude (20–400 mM imidazole) and Mono S and Mono Q columns (20–1000 mM KCl), according to the manufacturer's instruction (GE Healthcare). The heterodimers were then buffer-exchanged into 1× lysis buffer containing 10% glycerol and 1 mM DTT using NAP-5 (GE Healthcare).
Deadenylation assay in vitro
Preparation of 40× reaction mix (containing ATP, ATP regeneration system, GTP, and RNase inhibitor), lysis buffer [30 mM HEPES–KOH (pH 7.4), 100 mM KOAc and 2 mM Mg(OAc)2], 2× proteinase K buffer, and formamide loading dye has been described in detail (Haley et al. 2003). The deadenylation assay with S2 cell lysate was previously described (Fukaya et al. 2014). Deadenylation reaction by recombinant CCR4/CAF1 heterodimers typically contained 6 µL of 1.5 µM recombinant CCR4/CAF1 heterodimer, 3 µL of 5× lysis buffer, 1.5 µL of 10 mM DTT, 0.3 µL of 40 U/µL RNasin plus (Promega), 2 µL of ∼5 nM target RNA, and 2.2 µL of water and the mixture was incubated at 25°C. For 30-nt poly(N) RNAs (Fig. 4E), Na3VO4 was added at 10 mM in the deadenylation reaction. At each time point, 2.8 µL of the reaction mixture was taken and the equal volume of formamide loading dye was added. Then, the sample was electrophoresed on 5%, 6%, or 18% denaturing polyacrylamide gel and analyzed by PhosphoImager (Typhoon FLA 7000, GE Healthcare).
Thin layer chromatography
Typically, a polyethyleneimine-cellulose plate (MACHEREY-NAGEL) was pre-run with water for 2 h. Then, the plate was dried and 2 µL of reaction mixture in formamide loading dye was spotted on the plate. The plate was run with 450 mM ammonium sulfate for 1 h and dried. Finally, the plate was analyzed by PhosphoImager (Typhoon FLA 7000, GE Healthcare).
SUPPLEMENTAL MATERIAL
Supplemental material is available for this article.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to Claudia Temme and Elmar Wahle for providing anti-CCR4 and anti-CAF1 antibodies. We thank Shintaro Iwasaki for his help in plasmid construction, and Juan Guillermo Betancur and Hiroshi Sasaki for their instructions on expression and purification of recombinant proteins. We also thank members of the Tomari laboratory for advice, discussion, and critical comments on the manuscript. This work was supported in part by a Grant-in-Aid for Scientific Research (A) to Y.T. (grant number 15H02382) from the Japan Society for the Promotion of Science. S.N. is a recipient of JSPS Research Fellowships for Young Scientists (grant number 14J08195).
Author contributions: S.N., T.F., and Y.T. conceived and designed the study. S.N. performed the experiments and analyzed the data; T.F. started the research project and obtained initial data; Y.T. supervised the study; S.N. and Y.T. wrote the manuscript; all authors discussed the results and approved the manuscript.
Footnotes
Article published online ahead of print. Article and publication date are at http://www.rnajournal.org/cgi/doi/10.1261/rna.057679.116.
REFERENCES
- Andersen KR, Jonstrup AT, Van LB, Brodersen DE. 2009. The activity and selectivity of fission yeast Pop2p are affected by a high affinity for Zn2+ and Mn2+ in the active site. RNA 15: 850–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartel DP. 2009. MicroRNAs: target recognition and regulatory functions. Cell 136: 215–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basquin J, Roudko VV, Rode M, Basquin C, Séraphin B, Conti E. 2012. Architecture of the nuclease module of the yeast Ccr4-not complex: the Not1-Caf1-Ccr4 interaction. Mol Cell 48: 207–218. [DOI] [PubMed] [Google Scholar]
- Behm-Ansmant I, Rehwinkel J, Doerks T, Stark A, Bork P, Izaurralde E. 2006. mRNA degradation by miRNAs and GW182 requires both CCR4:NOT deadenylase and DCP1:DCP2 decapping complexes. Genes Dev 20: 1885–1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchin C, Mauxion F, Sentis S, Séraphin B, Corbo L. 2005. Conservation of the deadenylase activity of proteins of the Caf1 family in human. RNA 11: 487–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JE, Huntzinger E, Fauser M, Izaurralde E. 2011. GW182 proteins directly recruit cytoplasmic deadenylase complexes to miRNA targets. Mol Cell 44: 120–133. [DOI] [PubMed] [Google Scholar]
- Chang H, Lim J, Ha M, Kim VN. 2014. TAIL-seq: genome-wide determination of poly(A) tail length and 3′ end modifications. Mol Cell 53: 1044–1052. [DOI] [PubMed] [Google Scholar]
- Chekulaeva M, Mathys H, Zipprich JT, Attig J, Colic M, Parker R, Filipowicz W. 2011. miRNA repression involves GW182-mediated recruitment of CCR4-NOT through conserved W-containing motifs. Nat Struct Mol Biol 18: 1218–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CY, Shyu AB. 2011. Mechanisms of deadenylation-dependent decay. Wiley Interdiscip Rev RNA 2: 167–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Chiang YC, Denis CL. 2002. CCR4, a 3′-5′ poly(A) RNA and ssDNA exonuclease, is the catalytic component of the cytoplasmic deadenylase. EMBO J 21: 1414–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CY, Zheng D, Xia Z, Shyu AB. 2009. Ago-TNRC6 triggers microRNA-mediated decay by promoting two deadenylation steps. Nat Struct Mol Biol 16: 1160–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark LB, Viswanathan P, Quigley G, Chiang YC, McMahon JS, Yao G, Chen J, Nelsbach A, Denis CL. 2004. Systematic mutagenesis of the leucine-rich repeat (LRR) domain of CCR4 reveals specific sites for binding to CAF1 and a separate critical role for the LRR in CCR4 deadenylase activity. J Biol Chem 279: 13616–13623. [DOI] [PubMed] [Google Scholar]
- Collart MA, Panasenko OO. 2012. The Ccr4–Not complex. Gene 492: 42–53. [DOI] [PubMed] [Google Scholar]
- Daugeron MC, Mauxion F, Séraphin B. 2001. The yeast POP2 gene encodes a nuclease involved in mRNA deadenylation. Nucleic Acids Res 29: 2448–2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dlakić M. 2000. Functionally unrelated signalling proteins contain a fold similar to Mg2+-dependent endonucleases. Trends Biochem Sci 25: 272–273. [DOI] [PubMed] [Google Scholar]
- Draper MP, Salvadore C, Denis CL. 1995. Identification of a mouse protein whose homolog in Saccharomyces cerevisiae is a component of the CCR4 transcriptional regulatory complex. Mol Cell Biol 15: 3487–3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eulalio A, Huntzinger E, Izaurralde E. 2008. GW182 interaction with Argonaute is essential for miRNA-mediated translational repression and mRNA decay. Nat Struct Mol Biol 15: 346–353. [DOI] [PubMed] [Google Scholar]
- Fabian MR, Cieplak MK, Frank F, Morita M, Green J, Srikumar T, Nagar B, Yamamoto T, Raught B, Duchaine TF, et al. 2011. miRNA-mediated deadenylation is orchestrated by GW182 through two conserved motifs that interact with CCR4-NOT. Nat Struct Mol Biol 18: 1211–1217. [DOI] [PubMed] [Google Scholar]
- Forstemann K, Tomari Y, Du T, Vagin VV, Denli AM, Bratu DP, Klattenhoff C, Theurkauf WE, Zamore PD. 2005. Normal microRNA maturation and germ-line stem cell maintenance requires Loquacious, a double-stranded RNA-binding domain protein. PLoS Biol 3: e236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forstemann K, Horwich MD, Wee L, Tomari Y, Zamore PD. 2007. Drosophila microRNAs are sorted into functionally distinct argonaute complexes after production by dicer-1. Cell 130: 287–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukaya T, Tomari Y. 2011. PABP is not essential for microRNA-mediated translational repression and deadenylation in vitro. EMBO J 30: 4998–5009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukaya T, Tomari Y. 2012. MicroRNAs mediate gene silencing via multiple different pathways in Drosophila. Mol Cell 48: 825–836. [DOI] [PubMed] [Google Scholar]
- Fukaya T, Iwakawa HO, Tomari Y. 2014. MicroRNAs block assembly of eIF4F translation initiation complex in Drosophila. Mol Cell 56: 67–78. [DOI] [PubMed] [Google Scholar]
- Garneau NL, Wilusz J, Wilusz CJ. 2007. The highways and byways of mRNA decay. Nat Rev Mol Cell Biol 8: 113–126. [DOI] [PubMed] [Google Scholar]
- Goldstrohm AC, Wickens M. 2008. Multifunctional deadenylase complexes diversify mRNA control. Nat Rev Mol Cell Biol 9: 337–344. [DOI] [PubMed] [Google Scholar]
- Haley B, Tang G, Zamore PD. 2003. In vitro analysis of RNA interference in Drosophila melanogaster. Methods 30: 330–336. [DOI] [PubMed] [Google Scholar]
- Hirose T, Mishima Y, Tomari Y. 2014. Elements and machinery of non-coding RNAs: toward their taxonomy. EMBO Rep 15: 489–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houseley J, Tollervey D. 2009. The many pathways of RNA degradation. Cell 136: 763–776. [DOI] [PubMed] [Google Scholar]
- Iwasaki S, Kawamata T, Tomari Y. 2009. Drosophila argonaute1 and argonaute2 employ distinct mechanisms for translational repression. Mol Cell 34: 58–67. [DOI] [PubMed] [Google Scholar]
- Kawamata T, Tomari Y. 2010. Making RISC. Trends Biochem Sci 35: 368–376. [DOI] [PubMed] [Google Scholar]
- Lim J, Ha M, Chang H, Kwon SC, Simanshu DK, Patel DJ, Kim VN. 2014. Uridylation by TUT4 and TUT7 marks mRNA for degradation. Cell 159: 1365–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maryati M, Airhihen B, Winkler GS. 2015. The enzyme activities of Caf1 and Ccr4 are both required for deadenylation by the human Ccr4-Not nuclease module. Biochem J 469: 169–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamura K, Ishizuka A, Siomi H, Siomi MC. 2004. Distinct roles for Argonaute proteins in small RNA-directed RNA cleavage pathways. Genes Dev 18: 1655–1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker R, Song H. 2004. The enzymes and control of eukaryotic mRNA turnover. Nat Struct Mol Biol 11: 121–127. [DOI] [PubMed] [Google Scholar]
- Pérez-Ortin JE, Alepuz P, Chávez S, Choder M. 2013. Eukaryotic mRNA decay: methodologies, pathways, and links to other stages of gene expression. J Mol Biol 425: 3750–3775. [DOI] [PubMed] [Google Scholar]
- Petit AP, Wohlbold L, Bawankar P, Huntzinger E, Schmidt S, Izaurralde E, Weichenrieder O. 2012. The structural basis for the interaction between the CAF1 nuclease and the NOT1 scaffold of the human CCR4-NOT deadenylase complex. Nucleic Acids Res 40: 11058–11072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piao X, Zhang X, Wu L, Belasco JG. 2010. CCR4-NOT deadenylates mRNA associated with RNA-induced silencing complexes in human cells. Mol Cell Biol 30: 1486–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehwinkel J, Behm-Ansmant I, Gatfield D, Izaurralde E. 2005. A crucial role for GW182 and the DCP1:DCP2 decapping complex in miRNA-mediated gene silencing. RNA 11: 1640–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rissland OS, Norbury CJ. 2009. Decapping is preceded by 3′ uridylation in a novel pathway of bulk mRNA turnover. Nat Struct Mol Biol 16: 616–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sement FM, Ferrier E, Zuber H, Merret R, Alioua M, Deragon JM, Bousquet-Antonelli C, Lange H, Gagliardi D. 2013. Uridylation prevents 3′ trimming of oligoadenylated mRNAs. Nucleic Acids Res 41: 7115–7127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temme C, Zaessinger S, Meyer S, Simonelig M, Wahle E. 2004. A complex containing the CCR4 and CAF1 proteins is involved in mRNA deadenylation in Drosophila. EMBO J 23: 2862–2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temme C, Zhang L, Kremmer E, Ihling C, Chartier A, Sinz A, Simonelig M, Wahle E. 2010. Subunits of the Drosophila CCR4-NOT complex and their roles in mRNA deadenylation. RNA 16: 1356–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temme C, Simonelig M, Wahle E. 2014. Deadenylation of mRNA by the CCR4-NOT complex in Drosophila: molecular and developmental aspects. Front Genet 5: 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thore S, Mauxion F, Séraphin B, Suck D. 2003. X-ray structure and activity of the yeast Pop2 protein: a nuclease subunit of the mRNA deadenylase complex. EMBO Rep 4: 1150–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomari Y, Du T, Zamore PD. 2007. Sorting of Drosophila small silencing RNAs. Cell 130: 299–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker M, Valencia-Sanchez MA, Staples RR, Chen J, Denis CL, Parker R. 2001. The transcription factor associated Ccr4 and Caf1 proteins are components of the major cytoplasmic mRNA deadenylase in Saccharomyces cerevisiae. Cell 104: 377–386. [DOI] [PubMed] [Google Scholar]
- Viswanathan P, Chen J, Chiang YC, Denis CL. 2003. Identification of multiple RNA features that influence CCR4 deadenylation activity. J Biol Chem 278: 14949–14955. [DOI] [PubMed] [Google Scholar]
- Viswanathan P, Ohn T, Chiang YC, Chen J, Denis CL. 2004. Mouse CAF1 can function as a processive deadenylase/3′-5′ exonuclease in vitro but in yeast the deadenylase function of CAF1 is not required for mRNA poly(A) removal. J Biol Chem 279: 23988–23995. [DOI] [PubMed] [Google Scholar]
- Wang H, Morita M, Yang X, Suzuki T, Yang W, Wang J, Ito K, Wang Q, Zhao C, Bartlam M, et al. 2010. Crystal structure of the human CNOT6L nuclease domain reveals strict poly(A) substrate specificity. EMBO J 29: 2566–2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo Y, Deutscher MP. 2001. Exoribonuclease superfamilies: structural analysis and phylogenetic distribution. Nucleic Acids Res 29: 1017–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.