Abstract
The study aimed to gain consensus on key priorities for developing breathlessness rehabilitation services for patients with chronic obstructive pulmonary disease (COPD) and chronic heart failure (CHF). Seventy-four invited stakeholders attended a 1-day conference to review the evidence base for exercise-based rehabilitation in COPD and CHF. In addition, 47 recorded their views on a series of statements regarding breathlessness rehabilitation tailored to the needs of both patient groups. A total of 75% of stakeholders supported symptom-based rather than disease-based rehabilitation for breathlessness with 89% believing that such services would be attractive for healthcare commissioners. A total of 87% thought patients with CHF could be exercised using COPD training principles and vice versa. A total of 81% felt community-based exercise training was safe for patients with severe CHF or COPD, but only 23% viewed manual-delivered rehabilitation an effective alternative to supervised exercise training. Although there was strong consensus that exercise training was a core component of rehabilitation in CHF and COPD populations, only 36% thought that this was the ‘most important’ component, highlighting the need for psychological and other non-exercise interventions for breathlessness. Patients with COPD and CHF face similar problems of breathlessness and disability on a background of multi-morbidity. Existing pulmonary and cardiac rehabilitation services should seek synergies to provide sufficient flexibility to accommodate all patients with COPD and CHF. Development of new services could consider adopting a patient-focused rather than disease-based approach. Exercise training is a core component, but rehabilitation should include other interventions to address dyspnoea, psychological and education needs of patients and needs of carers.
Keywords: Breathlessness, rehabilitation, heart failure, COPD, consensus
Introduction
Breathlessness is one of the commonest reasons for people seeking emergency department care. In older adults, common underlying medical conditions include chronic obstructive pulmonary disease (COPD) or chronic heart failure (CHF) and often both.1–3 Together, COPD and CHF account for some two million inpatient bed days per year in the United Kingdom, with COPD responsible for 1 in 8 and CHF for 1 in 20 of all emergency hospital admissions.4,5 Annual direct healthcare costs to the National Health Service (NHS) attributed to COPD and CHF are estimated to be 800 million GBP and 1.8 billion GBP, respectively.4,5
International guidelines, such as the National Institute for Health and Care Excellence (NICE), recommend CHF patients should be offered supervised, exercise-based rehabilitation6 and that exercise-based pulmonary rehabilitation (PR) should be offered to COPD patients who consider themselves functionally disabled, including those who have had a recent hospitalization for an exacerbation.6 Whereas PR is designed to cater primarily for older chronic respiratory disease patients (such as COPD), the cardiac rehabilitation (CR) population is more heterogeneous, ranging from secondary prevention in post-myocardial infarction (MI) and cardiothoracic surgery patients3 to older patients with severe CHF and multi-morbidity. Currently, only 4.4% of the 82,127 patients undergoing CR in England, Wales and Northern Ireland each year have a primary diagnosis of CHF.3 There are multiple reasons for this but existing CR services place an emphasis upon post-MI, percutaneous coronary intervention and coronary artery bypass surgery patients (77% of CR patients)3 and there may be capacity and funding issues.7 The Cardiovascular Disease Outcomes Strategy (2013) has set an ambition for CHF services to increase uptake to exercise-based CR to 33% over the next 5 years.8 Although CR for CHF patients is slowly increasing, there is limited likelihood of meeting the stated ambition of the NHS without a significant rethink of how such services are delivered.
Historically, there has been little or no collaboration between respiratory and cardiac practitioners in provision of rehabilitation services. However, there is considerable overlap between the symptom-based needs for rehabilitation of CHF and COPD patients. Both groups of patients are generally older, chronically breathless with multi-morbidity and frailty and are limited by common manifestations outside the primary site of disease such as skeletal muscle dysfunction.9
Breathlessness and frailty, common to both COPD and CHF, are two of the three research themes prioritized by the Collaboration for Leadership and Applied Health Research and Care (CLAHRC) Northwest London (http://clahrc-northwestlondon.nihr.ac.uk) with the goal of improving patient symptoms, experiences and outcomes. With these themes in mind, CLAHRC Northwest London brought together multidisciplinary stakeholders with expertise in COPD, CHF and cardiopulmonary rehabilitation to generate consensus on key elements of rehabilitation services that could accommodate the needs of chronically breathless patients.
This article reviews the evidence base for exercise-based rehabilitation in COPD and CHF. Furthermore, the article provides input from the invited stakeholders on practical considerations, including key components of a rehabilitation programme, patient uptake and adherence, and how and where rehabilitation is delivered. This should inform future consensus for wider availability of PR, CR and generic breathlessness rehabilitation services.
Methods
Seventy four invited stakeholders attended a 1-day conference, entitled ‘Common rehabilitation for breathlessness: building consensus’. In a series of presentations, speakers presented the evidence base for exercise training in CHF and COPD, described the challenges of assuring quality exercise-based rehabilitation in routine practice and reviewed ongoing hospital and community-based rehabilitation initiatives for older patients with breathlessness.
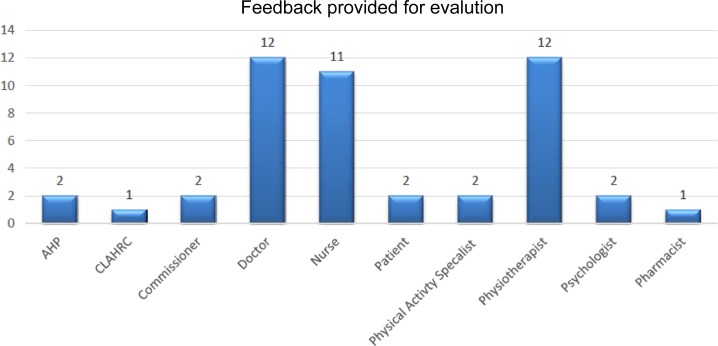
A discussion was conducted about the similarities and differences between CR and PR, the reasons why low patient uptake and adherence to rehabilitation exist and likely barriers to joint service provision. At the end of the conference, invited delegates were asked to record their views on a series of statements in relation to the development of breathlessness rehabilitation services. To maintain impartiality, the votes of invited speakers and core CLAHRC for NW London staff were excluded, leaving the views of 47 delegates to be recorded. The healthcare disciplines of respondents are summarized in Figure 1.
Figure 1.
Disciplines of those providing feedback on breathlessness services. AHP: Allied Health Professional; CLAHRC: Collaboration for Leadership and Applied Health Research and Care.
Results
Evidence base for exercise training in heart failure
The Cochrane systematic review and meta-analysis by Sagar and colleagues identified 33 randomized controlled trials (RCTs) comparing exercise training versus no exercise/usual care in a total of 4740 patients with CHF with reduced ejection fraction (HFrEF) or preserved ejection fraction (HFpEF). However, the majority had HFrEF (< 40%) and New York Heart Association (NYHA) class II and III.10 The interventions in some trials included an education component. The review only included studies with one or more of the following outcomes reported: (1) mortality, (2) hospital admission, (3) health-related quality of life (HRQoL) and (4) costs and cost-effectiveness.
This meta-analysis reported that exercise-based rehabilitation is associated with reduced risk of overall and CHF-related hospitalization at 12 months, compared with usual care (relative risk (RR): 0.75, 95% CI [0.62–0.92]; 0.61, 95% CI [0.46–0.80], respectively) and clinically important improvements in HRQoL as assessed by the Minnesota Living with Heart Failure (HF) scale.10 There was no significant impact on all-cause mortality with exercise-based rehabilitation at 12 months (RR: 0.92, 95% CI [0.67–1.26]), though there was a trend towards reduced mortality at follow-up beyond 1 year (RR: 0.80, 95% CI [0.75–1.02]).
The trial interventions were highly heterogeneous, that is, overall exercise duration from 15 minutes to 120 minutes, two to seven sessions per week, at an intensity of 40% of maximal heart rate to 85% of maximal oxygen uptake. In most trials, the need for continuous electrocardiogram (ECG) monitoring during exercise training was not specified. Meta-regression analyses showed no impact of type of rehabilitation (exercise-only interventions vs. exercise plus other interventions), type of exercise (aerobic alone vs. aerobic and strength), dose or setting (centre/hospital vs. home) on the specified outcomes.
A recent meta-analysis including six RCTs across 276 patients with HFpEF has shown similar benefits to those for patients with HFrEF, in terms of improvement in exercise capacity and HRQoL.11 However, data on the impact of exercise-based rehabilitation on mortality in HPpEF are currently lacking.
Evidence base for pulmonary rehabilitation in COPD
In stable COPD, a Cochrane review (65 RCTs, 3822 patients) compared the effects of PR versus usual care on HRQoL and functional and maximal exercise capacity.12 Meta-analysis showed statistically significant and clinically important improvements in HRQoL (four domains of the Chronic Respiratory Questionnaire (CRQ) and St. George’s Respiratory Questionnaire (SGRQ)), maximal exercise capacity (incremental shuttle walk, incremental cycle ergometry) and functional exercise capacity (6-minute walk test).12 This systematic review did not include outcomes of hospital admissions or mortality.
The role of PR for medically unstable patients has also been studied in COPD. A Cochrane review and meta-analysis (9 RCTs, 432 patients) showed that PR following a COPD exacerbation (typically severe requiring hospitalization) reduced hospital admissions (pooled odds ratio (OR) 0.22, 95% CI [0.08–0.58]), over an average of 25 weeks follow-up.13 PR also led to improvements in secondary outcomes including exercise capacity and HRQoL (CRQ and SGRQ). No adverse events in terms of increased mortality were seen with PR in this population. Indeed, PR significantly reduced mortality (OR 0.28, 95% CI [0.10–0.84]) over an average of 107 weeks follow-up, although mortality data were only recorded in a small number of patients.13
There is little RCT data examining the effects of exercise-based rehabilitation on patients with both CHF and COPD, although it is likely that previous rehabilitation trials in patients with COPD included those with undiagnosed CHF and vice versa in rehabilitation trials of patients with CHF. A recent subgroup analysis of a large multicentre RCT of exercise-based CR, HF-ACTION demonstrated that CHF patients with coexistent COPD responded as well to exercise training as those with CHF and no evidence of COPD.1
Optimal setting for rehabilitation
In COPD, there is no clear evidence showing advantages of hospital-based rehabilitation compared to community- or home-based rehabilitation.14,15 A subgroup analysis of patients in the Cochrane review of stable COPD indicated a significant difference in treatment effect for all domains of the CRQ, with higher mean changes following hospital-based PR than community-based PR, but there was no difference in SGRQ scores.12
The Self-Management Programme of Activity, Coping and Education (SPACE) for COPD is a 6-week home-based self-management intervention for COPD that has been shown to improve CRQ dyspnoea, fatigue and emotion scores, exercise performance, anxiety and disease knowledge at 6 weeks compared with usual care (excluding PR).16 At 6 months, the superiority of SPACE was sustained for measures of anxiety, exercise performance and smoking status but not for dyspnoea. An ongoing NIHR-funded trial (ISRCTN03142263) is examining the feasibility of delivering web-based rehabilitation, based on the SPACE for COPD manual, compared to conventional centre-based rehabilitation.
A recent systematic review and meta-analysis of 17 RCTs in 2172 participants undergoing CR directly compared delivery in a centre-based versus home-based setting.17 This systematic review included five studies of 345 patients with CHF with NYHA class II and III. The overall results found no significant difference in mortality, cardiac events, exercise capacity or HRQoL outcomes between the two settings.17 However the majority of studies recruited a lower risk patient and excluded those with significant arrhythmia or ischaemia.17
Rehabilitation Enablement in Heart Failure (REACH-HF) is an ongoing NIHR Programme Grant (ISRCTN25032672) investigating the effectiveness and cost-effectiveness of a self-help rehabilitation manual (with support from specially trained cardiac nurses) for HFrEF and HFpEF patients and their carers compared to a no-CR control. Outcomes of this intervention will be forthcoming.
Other rehabilitation interventions, including home-based telemonitored Nordic walking training, have proved well accepted, safe and effective, with good adherence among patients with CHF.18 There is growing evidence for the potential of web-based and other technological interventions for rehabilitation, with beneficial effects reported on HRQoL. An example includes encouraging patients with COPD to perform daily endurance walking according to the tempo of music from a programme installed on their mobile phone.19
Non-exercise interventions
The experience of breathlessness comprises both the sensation itself and the patient’s reaction to that sensation. Both can be changed by modifying central perception. Most CR and PR programmes include an educational component as well as exercise and some also include management of anxiety and depression, support for carers and other aids to reduce disability and support rehabilitation. Patients undergoing PR have rated DVD-based educational sessions, alongside a supervised exercise programme, equivalent to spoken sessions.20
Breathlessness services have also been reported in the palliative care literature. One example is the Cambridge Breathlessness Intervention Service (CBIS) that comprises a multidisciplinary team offering patients and carers a broad range of support in addition to exercise training (Table 1). This includes use of a handheld fan blowing air across the nose and mouth, which has been shown to reduce the sensation of breathlessness21 and training in recovery and pursed lip breathing.
Table 1.
Non-exercise and self-management components of a potential rehabilitation programme for breathlessness. Developed from the study by Higginson IJ et al. and Evans RA.23,24
|
|
CBIS was recently evaluated in a mixed methods RCT of patients with advanced cancer (45% lung cancer).22 In the study, the intervention comprised one to four face-to-face visits and four to six telephone contacts with the service over a period of weeks. Interventions were offered on the basis of an initial assessment and delivered mainly in patients’ homes during visits lasting 1–1.5 hours and accompanied by a medicines review. The co-morbidity burden (as measured by the Charlson index) and degree of breathlessness were high in both arms, and it is likely that the trial population included patients with coexistent COPD, CHF or both.
CBIS reduced patient distress due to breathlessness (primary outcome: −1.29; 95% CI [−2.57 to −0.005]; p = 0.049) significantly more than standard care, with 94% of respondents reporting a positive impact.22 The complex intervention reduced fear and worry, increased confidence in managing breathlessness and proved to be more cost effective than standard care, with reduced healthcare contacts and need for informal care. Patients and carers consistently identified specific and repeatable aspects of the CBIS model and interventions that were helpful. The findings have been replicated in another similar RCT of patients with advanced disease and refractory breathlessness (the majority with COPD),23 suggesting that helping patients (regardless of underlying disease) to modify the central perception of breathlessness is an important part of rehabilitation.
Joint COPD/HF rehabilitation initiatives
An outpatient PR programme designed for patients with COPD has proved equally effective for a CHF population treated in the same location by the same therapists.24
In a randomized trial of the joint intervention, 57 patients with CHF (mean left ventricular ejection fraction 30%) were assigned to 7 weeks of PR or usual care, whilst 55 patients with COPD carried out the same PR programme.24 Of these, 27 CHF and 44 COPD patients completed PR and 17 patients with CHF completed usual care.24
During a 7-week programme, patients underwent supervised physical training (endurance training and education) twice weekly for 2 hours, together with daily unsupervised home training (walking at an individually tailored speed equivalent to 85% peak oxygen consumption derived from each patient’s incremental shuttle walk test (ISWT)). Patients also performed peripheral muscle exercises three times a week (once a week supervised in hospital; twice a week at home) using free weights for the upper limbs and conditioning exercises for the lower limbs. Patients from both groups trained together and were supervised by the same therapists. No ECG monitoring was performed during exercise training, although all patients underwent a full cardiopulmonary exercise test to exclude unstable arrhythmias prior to PR.
Significant improvements in ISWT distance and endurance shuttle walk time were seen in the CHF patients undergoing PR compared to those randomized to usual care (both p < 0.001; effect sizes 0.57 and 0.95, respectively). Improvements in exercise performance and HRQoL were similar for patients with CHF and COPD who participated in the PR programme. No significant adverse events were noted, and a similar rate of dropouts was observed in the CHF groups undergoing PR and usual care. Training as a combined group did not adversely affect outcomes for patients with COPD, which were similar to those seen in patients previously treated separately from CHF patients in the programme. This study demonstrates that combined exercise rehabilitation for COPD and CHF is feasible and effective.
Quality assuring exercise-based rehabilitation
Ensuring high-quality services as part of routine practice requires continued collection and monitoring of data, and useful lessons can be learned from the National Audit of Cardiac Rehabilitation.3 Although there has been continued gradual improvement over time, the NACR 2015 audit still showed that few regions were able to meet the NICE/DH recommendation of assessing patients for CR within 10 days of their initiating event or starting rehabilitation within 25 days from referral. Whilst improvements in the proportion of patients achieving 150 minutes of exercise per week were seen across all regions and programmes, there was considerable variation both in pre- and post-CR levels across the United Kingdom. Variations also occurred in patients achieving improvements in anxiety and depression, underlining the importance of pre- and post-CR assessments to ensure that standards are met.
The NACR 2015 report also highlights shortfalls within the multidisciplinary teams supervising CR programmes3. Thus, whilst 96% of programmes include nurses and 65% include physiotherapists, only 18% include a psychologist.
The NACR and the British Association for Cardiac Prevention and Rehabilitation have embarked on an ambitious project to use data on minimum standards, collected as part of routine practice, to implement a certification process to ensure that all CR programmes achieve a basic minimum standard and achieve high-quality delivery and outcomes.
In contrast to CR, there is little national audit data for PR. The British Thoracic Society has recently developed guidelines for PR25 and quality standards for PR. The forthcoming first national audit of PR services in the United Kingdom will provide a basis for future accreditation and certification of PR services for quality assurance.
The development of such quality standards and regular audit can help to inform joint CHF/COPD services, but it is clear from the evidence presented that questions remain about where, when and how rehabilitation should be provided.
Conference stakeholder discussion
There was a high level of agreement on a number of areas (Table 2). Rehabilitation for COPD and CHF should be symptom rather than disease based and the same principles of exercise training can be used for both CHF and COPD. Whilst exercise was seen as a core component of breathlessness rehabilitation, it was not considered the only important aspect. Despite the relative lack of evidence concerning psychological aspects of rehabilitation, the contribution of mental well-being to breathlessness was considered as important as disease severity, underlining the value of psychological input for joint CR/PR services.
Table 2.
Building consensus on breathlessness rehabilitation for HF/COPD: areas of agreement. Number and percentage of participants responding to each statement.a
| S. No | Statements | Yes | No | Not sure | Blank | Total | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | ||
| 1 | Patient factors, rather than service provision, are the principal reasons for poor uptake of cardiac and pulmonary rehabilitation. | 9 | 19.1% | 18 | 38.3% | 16 | 34.0% | 4 | 8.5% | 47 |
| 2 | Mental well-being is as important a contributor to breathlessness as disease severity. | 41 | 87.2% | 3 | 6.4% | 1 | 2.1% | 2 | 4.3% | 47 |
| 3 | Common rehabilitation for breathlessness is attractive for healthcare commissioners. | 42 | 89.4% | 1 | 2.1% | 2 | 4.3% | 2 | 4.3% | 47 |
| 4 | To maximize uptake, common rehabilitation for breathlessness should be delivered in the patient’s home. | 15 | 31.9% | 13 | 27.7% | 15 | 31.9% | 4 | 8.5% | 47 |
| 5 | Rehabilitation delivered by a manual is an effective alternative to supervised exercise training. | 11 | 23.4% | 19 | 40.4% | 14 | 29.8% | 3 | 6.4% | 47 |
| 6 | Exercise training is a core component of rehabilitation for breathlessness. | 47 | 100% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 47 |
| 7 | Exercise training is the most important component of rehabilitation for breathlessness. | 17 | 36.2% | 22 | 46.8% | 4 | 8.5% | 4 | 8.5% | 47 |
| 8 | Can patients with HF be exercised using COPD training principles and vice versa. | 41 | 87.2% | 0 | 0.0% | 3 | 6.4% | 3 | 6.4% | 47 |
| 9 | Exercise training based in the community is safe for patients with severe HF or COPD. | 38 | 80.9% | 0 | 0.0% | 6 | 12.8% | 3 | 6.4% | 47 |
| 10 | Education needs of patients with HF and COPD are more similar than different. | 26 | 55.3% | 10 | 21.3% | 8 | 17.0% | 3 | 6.4% | 47 |
| 11 | Rehabilitation should be symptom based not disease based. | 35 | 74.5% | 2 | 4.3% | 7 | 14.9% | 3 | 6.4% | 47 |
| 12 | The way that interventions are delivered by healthcare professionals has an important influence on their success. | 45 | 95.7% | 0 | 0.0% | 0 | 0.0% | 2 | 4.3% | 47 |
COPD: chronic obstructive pulmonary disease; HF: heart failure.
aHighlighted areas reflect areas where consensus of >50% was achieved.
Small differences in educational requirements for patients with CHF and COPD were not seen as a barrier to joint rehabilitation. Whilst some tailoring would be needed for disease-specific information such as medications and pathophysiology, it was agreed that general requirements for health promotion are broadly similar for CHF and COPD. Advice about routine healthy exercise, outside any formalized group training, should be tailored to individual circumstances.
It was thought that community-based exercise training was safe for patients with severe CHF or COPD. In contrast to PR staff, CR practitioners might expect a recent echocardiogram or ECG for CHF patients in order to tailor exercise to individual needs as well as ECG monitoring during exercise testing or training. Previous data suggest that adverse events from exercise training in cardiac patients are rare.26 Keteyian and colleagues reported the safety of symptom-limited cardiopulmonary exercise testing in 2037 patients (NYHA class II to IV; left ventricular ejection fraction less than 35%) participating in the HF-ACTION trial; 74% of whom had an implantable cardioverter–defibrillator, biventricular pacemaker or pacemaker. In 4411 exercise tests, there were no deaths, exacerbation of HF, MI, strokes or sustained ventricular tachycardia.26 Twenty-seven tests were stopped due to non-sustained supraventricular or ventricular tachycardia. With this in mind, senior cardiology specialists from our working group felt that the potential harms of exercise to patients with CHF are overplayed. There should be focus on keeping assessment and care pathways as simple as possible in order to optimize patient uptake. Although rehabilitation programmes without continuous ECG monitoring are safe, patients are usually prescreened with symptom-limited cardiopulmonary exercise testing.
Participants were almost unanimous in agreeing that the way interventions are delivered by Healthcare Professionals (HCPs) has an important influence on their success. The personal impact of the HCP is too often dismissed as ‘placebo effect’ when it should be considered a part of the intervention. There was little support for breathlessness rehabilitation delivered exclusively in the home or by a manual as an alternative to supervised exercise training. However, it was recognized that breathlessness rehabilitation programmes need to take account of the fact that many patients with COPD or CHF are elderly with multiple morbidities and a proportion would be housebound. Some would never have participated in gym-based group exercise, so patient preference must be taken into account and a flexible, menu-based programme may best accommodate different patient needs and choices. Ongoing trials of manual-based interventions such as the REACH-HF research programme or SPACE may provide an evidence base in the future to support alternative approaches to centre-based supervised exercise training.
Discussion
Patients with COPD and CHF conditions share a similar disablement process9 and show similar clinical and physiological benefits from exercise training.27,28 Although rehabilitation services for CHF and COPD are slowly increasing, current services are unlikely to meet the needs of eligible patients without significant reconsideration of how such services are delivered. Given resource limitations7 – financial and skilled staffing – there is growing interest in exploring synergies across existing rehabilitation services and commissioning rehabilitation programmes that are symptom based rather than disease based.
There is strong evidence from meta-analyses to support exercise-based rehabilitation for both patients with COPD and CHF. However, translation of evidence to routine clinical practice remains challenging. Although NICE recommended that exercise-based training for CHF can be incorporated into existing CR programmes, the traditional CR population (post-MI and cardiothoracic surgery patients) is generally fitter and younger, and secondary prevention is the overriding concern rather than management of breathlessness. Integration of older, breathless CHF patients into existing CR groups may face potential staff and patient barriers. Although access to CR has improved for patients with CHF, 12% of CR programmes continue not to accept patients with CHF, and approximately 4% of the nearly 80,000 patients undergoing CR in England, Wales and Northern Ireland each year have a primary diagnosis of CHF.3
The PR population is more homogeneous as eligibility is dependent on the level of respiratory disability.25 Arguably the patient with CHF has more in common with the patient with COPD than one who is post-MI. However, secondary prevention is not a strong component of PR. As eligibility for PR is dependent on the level of symptoms, current PR programmes cater less well for patients with mild disease, despite good evidence to suggest that physical inactivity and skeletal muscle dysfunction are prevalent in these groups29,30 and amenable to exercise therapy.29 Furthermore, despite a growing evidence base to support PR in the peri-exacerbation setting, recent data suggest that PR may not be acceptable to patients in such populations.31 Therefore, current CR and PR programmes have strengths and weakness, but there are clear synergies where closer collaboration between CR and PR practitioners could improve prevention strategies and lead to more combined strategies in managing older patients with breathlessness.
Data on the costs and cost-effectiveness of CR in patients with CHF are limited. The one UK trial that included economic information reported a cost of centre-based CR intervention for functionally impaired, older patients with CHF of £475 per patient.32 In a broader UK-based analysis of the cost-effectiveness of secondary prevention interventions in post-MI patients, CR compared favourably (£1957 per life year gained (LYG)) with angiotensin converting enzyme inhibitors (£3398/LYG).33 PR is also associated with health economic benefits.34 The London Respiratory Team recently described the COPD Value Pyramid, which estimated the cost of PR to be between £2000 and £8000 per quality-adjusted life year (QALY), well below the £20,000 per QALY that NICE considers cost effective.
There are therefore a number of good clinical, evidence-based and economic reasons to consider wider development of cardiac, pulmonary and breathlessness rehabilitation services for patients with COPD or CHF. Looking for synergies between existing PR and CR services, and tailored exercise rehabilitation programmes for CHF may generate economies of scale that might address the current shortfall in rehabilitation services for breathless older patients. These initiatives are likely to be considered favourably by healthcare commissioners.
In areas where PR and CR services are absent, or there are gaps in service provision for patients with CHF or COPD, development of joint breathlessness rehabilitation services could be considered. Exercise training principles for COPD and CHF appear broadly comparable (Table 3),35 and there was stakeholder consensus that patients with CHF could be exercised using COPD principles and vice versa. Evans and colleagues were able to demonstrate that combined exercise rehabilitation for COPD and CHF is feasible and effective.24 However, further research is needed to corroborate this data, particularly in patients with more severe symptoms (NYHA class IV) and patients with HFpEF (who tend to be older and have more co-morbidities). Although exercise is the core component of rehabilitation programmes for both COPD and CHF, psychological and educational aspects are important. A flexible, ‘menu-based’ programme is most likely to accommodate patients of different ages, comorbidities, disease and symptom severity and previous exercise history.
Table 3.
Similarities in exercise training for patients with COPD and HF
| COPD | HF | |
|---|---|---|
| Aerobic lower limb training | High intensity (60–80% peak VO2) | High intensity (40–70% peak VO2) |
| Duration | Minimum 6–12 weeks | Minimum 12 weeks |
| Frequency | Minimum 3 times/week | Minimum 3 times/week |
| Interval | √ | √ |
| Additional strength training | √High resistance | √Low resistance |
| Moderate–high may be safe | ||
| Adjuncts | Helium/hyperoxia/one legged/NIV | ? |
COPD: chronic obstructive pulmonary disease; HF: heart failure.
Adapted from the study by Evans RA; NIV: Non-invasive ventilation.35 Note: Tick refers to Yes; Question mark refers to Unclear.
Conclusions
There is level 1A evidence (i.e. meta-analyses of RCTs) for the important health benefits of exercise-based rehabilitation in both HF with HFrEF and stable and recent exacerbation COPD populations. These benefits include important gains in HRQoL and functional capacity and reductions in hospital admissions. Due to the similarities in symptoms, needs and exercise training in COPD and CHF, there are clear advantages for seeking synergies between CR and PR programmes.
Although the current RCT evidence is limited, joint exercise rehabilitation programmes for patients with COPD or CHF, in the same location by the same therapists, appear effective, feasible and may have the potential to unblock capacity limitations for services commissioned separately. Such a service should embrace a symptom-based approach to care, that is, the management of breathlessness, rather than the more traditional disease-centred approach.
Acknowledgement
We are grateful to Jenny Bryan for writing support with the manuscript.
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Rod S Taylor is a co-author on a number of Cochrane reviews on cardiac rehabilitation and is the chief investigator on an ongoing National Institute of Health Research Programme Grants for Applied Research (RP-PG-1210-12004): Rehabilitation Enablement in Chronic Heart Failure (REACH-HF).
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was funded by the National Institute for Health Research (NIHR) Collaboration for Leadership in Applied Health Research and Care (CLAHRC) for North West London. WM and MC are supported by the NIHR Respiratory and Cardiovascular Biomedical Research Units at the Royal Brompton and Harefield NHS Foundation Trust and Imperial College London and the CLAHRC for NW London. The views expressed in this publication are those of the authors and not necessarily those of the NHS, the NIHR nor the Department of Health. RT is supported by the NIHR CLAHRC South West Peninsula at the Royal Devon and Exeter NHS Foundation Trust. SS is supported by the NIHR CLAHRC South East Midlands.
References
- 1. Mentz RJ, Schulte PJ, Fleg JL, et al. Clinical characteristics, response to exercise training, and outcomes in patients with heart failure and chronic obstructive pulmonary disease: findings from Heart Failure and a controlled trial investigating outcomes of exercise training (HF-ACTION). Am Heart J 2013; 165(2): 193–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Crisafulli E, Costi S, Luppi F, et al. Role of comorbidities in a cohort of patients with COPD undergoing pulmonary rehabilitation. Thorax 2008; 63(6): 487–492. [DOI] [PubMed] [Google Scholar]
- 3. British Heart Foundation. National audit of cardiac rehabilitation annual statistical report. London: British Heart Foundation, 2015. [Google Scholar]
- 4. National Institute for Health and Care Excellence. NICE support for commissioners and others using the quality standard for Chronic obstructive pulmonary disease (COPD), July 2011.
- 5. National Clinical Guideline Centre. Chronic heart failure: the management of chronic heart failure in adults in primary and secondary care London: National Clinical Guideline Centre, http://guidance.nice.org.uk/CG108/Guidance/pdf/English (2010). [Google Scholar]
- 6. National Institute for Health and Care Excellence. Chronic obstructive pulmonary disease. Management of chronic obstructive pulmonary disease in adults in primary and secondary care (partial update) CG 101, June 2010. [PubMed]
- 7. Dalal HM, Wingham J, Palmer J, et al. Why do so few patients with heart failure participate in cardiac rehabilitation? A cross-sectional survey from England, Wales and Northern Ireland. BMJ Open 2012; 2(2): e000787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Department of Health. Cardiovascular Disease Outcomes Strategy. Improving outcomes for people with or at risk of cardiovascular disease, www.dh.gov.uk/publications (2013).
- 9. Gosker HR, Wouters EF, van der Vusse GJ, et al. Skeletal muscle dysfunction in chronic obstructive pulmonary disease and chronic heart failure: underlying mechanisms and therapy perspectives. Am J Clin Nutr 2000; 71(5): 1033–1047. [DOI] [PubMed] [Google Scholar]
- 10. Sagar VA, Davies EJ, Briscoe S, et al. Exercise-based rehabilitation for heart failure: systematic review and meta-analysis. Open Heart 2015; 2: e000163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pandey A, Parashar A, Kumbhani DJ, et al. Exercise training in patients with heart failure and preserved ejection fraction: meta-analysis of randomized control trials. Circ Heart Fail 2015; 8(1): 33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McCarthy B, Casey D, Devane D, et al. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2015; 2: CD003793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Puhan MA, Gimeno-Santos E, Scharplatz M, et al. Pulmonary rehabilitation following exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2011; (10): CD005305. [DOI] [PubMed] [Google Scholar]
- 14. Waterhouse JC, Walters SJ, Oluboyede Y, et al. A randomised 2 × 2 trial of community versus hospital pulmonary rehabilitation for chronic obstructive pulmonary disease followed by telephone or conventional follow-up. Health Technol Assess 2010; 14(6): 1–140. [DOI] [PubMed] [Google Scholar]
- 15. Maltais F, Bourbeau J, Shapiro S, et al. Effects of home-based pulmonary rehabilitation in patients with chronic obstructive pulmonary disease: a randomized trial. Ann Intern Med 2008; 149(12): 869–878. [DOI] [PubMed] [Google Scholar]
- 16. Mitchell KE, Johnson-Warrington V, Apps LD, et al. A self-management programme for COPD: a randomised controlled trial. Eur Respir J 2014; 44(6): 1538–1547. [DOI] [PubMed] [Google Scholar]
- 17. Taylor RS, Dalal H, Jolly K, et al. Home-based versus centre-based cardiac rehabilitation. Cochrane Database Syst Rev 2015; 8: CD007130. [DOI] [PubMed] [Google Scholar]
- 18. Piotrowicz E, Zieliński T, Bodalski R, et al. Home-based telemonitored Nordic walking training is well accepted, safe, effective and has high adherence among heart failure patients, including those with cardiovascular implantable electronic devices: a randomised controlled study. Eur J Prev Cardiol 2015; 22(11): 1368–1377. [DOI] [PubMed] [Google Scholar]
- 19. Liu WT, Huang CD, Wang CH, et al. A mobile telephone-based interactive self-care system improves asthma control. Eur Respir J 2011; 37(2): 310–317. [DOI] [PubMed] [Google Scholar]
- 20. Ward S, Sewell L, Singh S. P144 Evaluation of multidisciplinary pulmonary rehabilitation education delivered by either DVD or spoken talk. Thorax 2011; 66: A125–A126. [DOI] [PubMed] [Google Scholar]
- 21. Galbraith S, Fagan P, Perkins P, et al. Does the use of a handheld fan improve chronic dyspnea? A randomized, controlled, crossover trial. J Pain Symptom Manage 2010; 39(5): 831–838. [DOI] [PubMed] [Google Scholar]
- 22. Farquhar MC, Prevost AT, McCrone P, et al. Is a specialist breathlessness service more effective and cost-effective for patients with advanced cancer and their carers than standard care? Findings of a mixed-method randomised controlled trial. BMC Med 2014; 12: 194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Higginson IJ, Bausewein C, Reilly CC, et al. An integrated palliative and respiratory care service for patients with advanced disease and refractory breathlessness: a randomised controlled trial. Lancet Respir Med 2014; 2(12): 979–987. [DOI] [PubMed] [Google Scholar]
- 24. Evans RA, Singh SJ, Collier R, et al. Generic, symptom based, exercise rehabilitation; integrating patients with COPD and heart failure. Respir Med 2010; 104(10): 1473–1481. [DOI] [PubMed] [Google Scholar]
- 25. Bolton CE, Bevan-Smith EF, Blakey JD, et al. British Thoracic Society Pulmonary Rehabilitation Guideline Development Group; British Thoracic Society Standards of Care Committee. British Thoracic Society guideline on pulmonary rehabilitation in adults. Thorax 2013; 68(Suppl 2): ii1–ii30. [DOI] [PubMed] [Google Scholar]
- 26. Keteyian SJ, Isaac D, Thadani U, et al. HF-ACTION Investigators. Safety of symptom-limited cardiopulmonary exercise testing in patients with chronic heart failure due to severe left ventricular systolic dysfunction. Am Heart J 2009; 158(4 Suppl): S72–S77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Whittom F, Jobin J, Simard PM, et al. Histochemical and morphological characteristics of the Vastus lateralis muscle in patients with chronic obstructive pulmonary disease. Med Sci Sports Exerc 1998; 30: 1467–1474. [DOI] [PubMed] [Google Scholar]
- 28. Hambrecht R, Fiehn E, Yu J, et al. Effects of endurance training on mitochondrial ultrastructure and fiber type distribution in skeletal muscle of patients with stable chronic heart failure. J Am Coll Cardiol 1997; 29: 1067–1073. [DOI] [PubMed] [Google Scholar]
- 29. Jones SE, Maddocks M, Kon SS, et al. Sarcopenia in COPD: prevalence, clinical correlates and response to pulmonary rehabilitation. Thorax 2015; 70(3): 213–218. [DOI] [PubMed] [Google Scholar]
- 30. Watz H, Waschki B, Meyer T, et al. Physical activity in patients with COPD. Eur Respir J 2009; 33(2): 262–272. Erratum in: Eur Respir J 2010; 36(2): 462. [DOI] [PubMed] [Google Scholar]
- 31. Jones SE, Green SA, Clark AL, et al. Pulmonary rehabilitation following hospitalisation for acute exacerbation of COPD: referrals, uptake and adherence. Thorax 2014; 69(2): 181–231. [DOI] [PubMed] [Google Scholar]
- 32. Witham MD, Fulton RL, Greig CA, et al. Efficacy and cost of an exercise program for functionally impaired older patients with heart failure: a randomized controlled trial. Circ Heart Fail 2012; 5(2): 209–216. [DOI] [PubMed] [Google Scholar]
- 33. Fidan D, Unal B, Critchley J, et al. Economic analysis of treatments reducing coronary heart disease mortality in England and Wales, 2000–2010. Q J Med 2007; 100: 277–289. [DOI] [PubMed] [Google Scholar]
- 34. Griffiths TL, Phillips CJ, Davies S, et al. Cost effectiveness of an outpatient multidisciplinary pulmonary rehabilitation programme. Thorax 2001; 56(10): 779–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Evans RA. Developing the model of pulmonary rehabilitation for chronic heart failure. Chron Respir Dis 2011; 8(4): 259–269. [DOI] [PubMed] [Google Scholar]