Abstract
1. FLT3 mutation status does not impact overall survival after allogeneic hematopoietic stem cell transplant.
2. Pre-emptive strategies to reduce relapse need to be investigated in FLT3 mutated patients to further improve post HCT outcomes.
Background
Patients (pts) with FMS like tyrosine kinase 3 (FLT3) mutated acute myeloid leukemia (AML) have poor prognosis and are referred for early allogeneic hematopoietic cell transplant (HCT).
Methods
Using data from the Center for International Blood and Marrow Transplant Research (CIBMTR), we evaluated 511 adult pts with de novo AML who underwent HCT during 2008-2011 to determine if FLT3 mutations (mut.) impact HCT outcomes.
Results
158 (31%) pts had FLT3 mut. Univariate analysis and multivariate analysis showed increased relapse risk at 3 years(yr.) in FLT3 mut. group when compared to wild type (WT) group (38% (95% confidence intervals [CI] 30-45) vs. 28% (95% CI 24-33), P =0.04; and relative risk [RR] 1.60 (95% CI 1.15-2.22), P =0.0048). However, FLT3 mut. status was not significantly associated with non-relapse mortality, leukemia-free survival, or overall survival (OS). Though more pts in the FLT3 mut. group died from relapsed primary disease (60% vs. 46%) as compared to WT, the 3-year OS of pts was comparable 49% (95% CI 40-57) and 55% (95% CI 50-60%) P=0.20.
Conclusions
Our data shows that FLT3 mut. status did not adversely impact the OS after HCT and about 50% of pts with this mut. who underwent HCT were long term survivors.
Keywords: Acute Myeloid Leukemia, Allogeneic stem cell transplantation, FLT3
INTRODUCTION
HCT remains the most effective post remission therapy for high-risk AML. FLT3 mut. occur in about 30% of pts with AML and the 2 most common variants are the internal tandem duplications (FLT3-ITD) and the point mut. in the tyrosine kinase domain (FLT3-TKD). FLT3-ITD mut. occur more frequently than the TKD mut. (~25% vs. ~7%), and the clinical manifestations of a FLT3-ITD mut. have a more characteristically unfavorable risk profile.1-9 In contrast with AML with FLT3-TKD, FLT3-ITD mut. is associated with short-lived remissions, resistant relapsed disease and a dismal prognosis. 2, 3, 10-14 Pts with FLT3-ITD are therefore commonly offered HCT in CR1. 15-21
Pre-HCT disease characteristics in AML such as disease status (CR1, CR2, MRD) and cytogenetics are predictors of post-HCT outcomes.22, 23 The impact of FLT3 mut. on outcomes of HCT was previously reported in predominantly normal karyotype AML in CR1 and was associated with high post-HCT relapses and poor OS (Table 1).20, 24-27 We sought to analyze within the CIBMTR database the independent impact of FLT3 mut. on HCT outcomes in AML.
Table 1.
Studies addressing Post HCT outcomes of pts with FLT3 mut.
| Reference | Total pts | FLT3 Mut. underwent HCT | Cytogenetics of FLT3 mut. pts | Relapse in FLT3 Mut.pts | OS of FLT3 Mut. pts |
|---|---|---|---|---|---|
| 20 | 103 | 40 | Intermediate | Increased | No Difference |
| 24 | 206 | 86 | Normal | Increased | Worse |
| 26 | 75 | 16 | Normal (12) Monosomal karyotype (4) |
Increased | Worse |
| 25 | 171 | 50 | Intermediate (41) Unknown (9) |
Increased | Worse ( p =.334) |
| 27 | 702 | 344 | Normal | Increased | Worse |
PATIENTS AND METHODS
The CIBMTR® is a research collaboration between the NMDP®/Be The Match® and the Medical College of Wisconsin. It comprises a group of ≥ 450 transplant centers worldwide that contribute detailed data on HCT. Studies conducted by the CIBMTR are performed in compliance with all applicable federal regulations pertaining to the protection of human research participants. Protected Health Information used in research is collected and maintained in CIBMTR's capacity as a Public Health Authority under the HIPAA Privacy Rule.
Inclusion Criteria
Adults ≥ 18 yr. with a diagnosis of de novo AML in CR1 or CR2 (M3 was excluded), with available FLT3 mut. status who underwent a HLA identical sibling or an 8/8 or 7/8 matched unrelated donor (URD) HCT after myeloablative (MAC), non-myeloablative (NMA), or reduced intensity conditioning (RIC) as previously defined28 reported to the CIBMTR from 2008-2011 were included. Graft source could be either bone marrow or peripheral blood stem cells, and any graft-versus-host-disease (GVHD) prophylaxis excluding ex- vivo T cell depletion was permitted. Cord blood and haploidentical transplants were excluded to decrease heterogeneity and due to small numbers of pts. Centers that never reported FLT3 mut. were excluded to avoid ascertainment bias. As CIBMTR data collection does not distinguish between ITD and TKD mut. or allelic ratios, all pts with FLT3 mut. were included.
Endpoints and Statistical Analysis
Patient- and HCT-related characteristics were identified, and the primary endpoints included the risk of relapse, non-relapse mortality (NRM), leukemia-free survival (LFS), and OS. Cumulative incidence (CI) of relapse was defined as the onset of recurrent AML through morphologic evidence in the bone marrow or extra medullary sites, and NRM was considered a competing risk. CI of NRM was defined as time to death from any cause while in remission, and disease relapse was considered a competing risk. LFS was calculated as the interval from HCT to time of relapse or death from any cause. OS was calculated as the interval from HCT to death from any cause. Endpoints were calculated at 3 yr.
The Kaplan-Meier estimator was used to calculate the probability of LFS and OS. Probabilities of disease relapse, NRM, incidences of acute and chronic GVHD were calculated using the CI estimates to account for competing risks. Clinical outcomes following HCT for FLT3 mut. and FLT3 WT AML pts were compared adjusting as indicated for significant patient-, disease-, and HCT-related variables. Cox proportional hazards regression was used to compare the two groups. Backward elimination was used to select significant covariates. Proportional hazards assumption was checked. If violated, it was added as time-dependent covariate in the Cox model. Interactions between the main effect and significant covariates were examined. Due to the strong correlation with FLT3 mut. status, WBC count at diagnosis was excluded in multivariate analysis (MVA).
RESULTS
Patient Characteristics
511 pts from 48 reporting centers worldwide between 2008 and 2011 were included in the analysis. Median follow-up of survivors was 37 months (12-65). 158 (31%) pts were FLT3 mut. Patient-, disease- and HCT- characteristics are outlined in Table 2.
Table 2.
Patient Characteristics
| FLT3 mut | |||
|---|---|---|---|
| Variable | Wild type | Mut. | p-value |
| Number of pts | 353 | 158 | |
| Median Age(range) | 46 (18-60) | 47 (18-60) | 0.47 |
| Gender | 0.05 | ||
| Male | 190 (54) | 70 (44) | |
| Female | 163 (46) | 88 (56) | |
| Recipient race | 0.78 | ||
| Caucasian | 320 (91) | 142 (90) | |
| Non-Caucasian | 29 (8) | 15 (9) | |
| Karnofsky score | 0.92 | ||
| <90% | 103 (29) | 49 (31) | |
| ≥90% | 234 (66) | 102 (65) | |
| Missing | 16 (5) | 7 (4) | |
| White blood count at diagnosis (×10^9/L) | <0.001 | ||
| Median (range) | 10 (<1-344) | 34 (<1-305) | <0.001 |
| HCT Co-morbidity Index | 0.95 | ||
| 0 | 131 (37) | 57 (36) | |
| 1 | 91 (26) | 40 (25) | |
| 2+ | 10 (3) | 6 (4) | |
| Missing | 121 (34) | 55 (35) | |
| Cytogenetic abnormalities | <0.001 | ||
| Favorable | 25 (7) | 5 (3) | |
| Intermediate | 216 (61) | 128 (81) | |
| Normal | 114 (32) | 102(65) | |
| Poor | 101 (29) | 20 (13) | |
| Missing | 11 (3) | 5 (3) | |
| Disease status prior to HCT | 0.07 | ||
| CR1 | 252 (71) | 125 (79) | |
| CR2 | 101 (29) | 33 (21) | |
| Status at CR1 | <0.001 | ||
| Hematologic CR only | 123 (35) | 68 (43) | |
| Cytogenetic and molecular CR | 94 (27) | 64 (41) | |
| Cytogenetic CR | 130 (37) | 22 (14) | |
| Molecular CR | 6 (2) | 4 (3) | |
| Time from diagnosis to HCT (for CR1 HCT), months | 0.82 | ||
| Median (range) | 4 (2-17) | 4 (2-19) | 0.59 |
| <6 months | 199 (79) | 100 (80) | |
| ≥6 months | 53 (21) | 25 (20) | |
| Time to achieve CR1 (for CR1 HCT), weeks | 0.05 | ||
| Median (range) | 6 ( 1-71) | 5 (1-22) | 0.07 |
| Time from CR1 to HCT (for CR1 HCT), weeks | 0.12 | ||
| Median (range) | 11 (1-53) | 13 (1-76) | 0.02 |
| Lines of induction prior to CR1 (for CR1 HCT) | 0.06 | ||
| 1 | 177 (70) | 102 (82) | |
| 2 | 63 (25) | 20 (16) | |
| ≥3 | 12 (5) | 3 (2) | |
| Type of induction therapy (for CR1 HCT) | 0.12 | ||
| 7+3 | 156 (62) | 72 (58) | |
| 7+3 + other | 78 (31) | 50 (40) | |
| Other | 16 (6) | 3 (2) | |
| Cycle of consolidation therapy prior to CR1 HCT | 0.02 | ||
| No consolidation given | 86 (34) | 24 (19) | |
| 1 | 71 (28) | 49 (39) | |
| 2 | 32 (13) | 23 (18) | |
| ≥3 cycles | 33 (13) | 17 (14) | |
| Missing | 30 (12) | 12 (10) | |
| Duration of CR1 (for CR2 HCT), months | 0.006 | ||
| Median (range) | 11 (1-98) | 6 (<1-42) | 0.006 |
| Time from relapse to HCT (for CR2 HCT), months | 0.59 | ||
| Median (range) | 3 ( 1-17) | 3 (1-19) | 0.76 |
| 0-3 months | 41 (41) | 14 (42) | |
| 3-6 months | 43 (43) | 13 (39) | |
| >6 months | 9 (9) | 5 (15) | |
| Missing | 8 (8) | 1 (3) | |
| Conditioning regimen intensity | 0.19 | ||
| MAC with TBI | 140 (40) | 74 (47) | |
| MAC without TBI | 162 (46) | 62 (39) | |
| RIC/NMA | 51 (14) | 21 (13) | |
| Type of donor | 0.69 | ||
| HLA-identical sibling | 150 (42) | 67 (42) | |
| 8/8 URD | 165 (47) | 70 (44) | |
| 7/8 URD | 38 (11) | 21 (13) | |
| Donor age of unrelated donor HCT | 0.19 | ||
| Median (range) | 30 (19-56) | 33 (19-52) | 0.04 |
| GVHD prophylaxis | 0.75 | ||
| Tacrolimus ± others | 302 (86) | 139 (88) | |
| CSA ± others | 42 (12) | 16 (10) | |
| Others | 9 (3) | 3 (2) | |
| In vivo T cell Depletion | 0.84 | ||
| ATG alone | 80 (23) | 34 (22) | |
| Alemtuzumab alone | 4 (1) | 1 (<1) | |
| No ATG or alemtuzumab | 268 (76) | 123 (78) | |
| Graft type | 0.33 | ||
| Bone marrow | 59 (17) | 21 (13) | |
| Peripheral blood | 294 (83) | 137 (87) | |
| Donor/Recipient CMV serostatus | 0.60 | ||
| R+ | 193 (55) | 96 (61) | |
| R-D+ | 46 (13) | 16 (10) | |
| R-D− | 108 (31) | 44 (28) | |
| Donor/Recipient sex match | 0.16 | ||
| M/M | 121 (34) | 43 (27) | |
| M/F | 93 (26) | 56 (35) | |
| F/M | 69 (20) | 27 (17) | |
| F/F | 70 (20) | 32 (20) |
CSA: cyclosporine; ATG: anti-thymocyte globulin; CMV: cytomegalovirus
Transplant Outcomes
The MVA of outcomes are shown in Table 3.
Table 3.
MVA of Effect of FLT3 Mut. on Outcomes
| N | RR (95%CI) | p-value | |
|---|---|---|---|
| 1. Relapse (All pts) | |||
| Main effect: | |||
| FLT3 mut. | |||
| No | 352 | 1 | |
| Yes | 157 | 1.60 (1.15-2.22) | 0.0048 |
| Other factors: | |||
| Disease status | |||
| CR1 | 375 | 1 | |
| CR2 | 134 | 1.52 (1.08-2.14) | 0.016 |
| Conditioning intensity | |||
| MAC with TBI | 214 | 1 | Poverall=0.0003 |
| MAC without TBI | 224 | 1.31 (0.92-1.89) | 0.14 |
| RIC/NMA | 71 | 2.41 (1.57-3.70) | <.0001 |
| Donor type | |||
| HLA-id sibling | 217 | 1 | Poverall=0.032 |
| 8/8 URD | 233 | 0.64 (0.45-0.90) | 0.0095 |
| 7/8 URD | 59 | 0.89 (0.53-1.49) | 0.65 |
| 2. Non Relapse mortality (All Pts) | |||
| Main effect: | |||
| FLT3 mut | |||
| No | 352 | 1 | |
| Yes | 157 | 0.77 (0.48-1.22) | 0.26 |
| Other factors: | |||
| HCT comorbidity index | |||
| 0 | 187 | 1 | Poverall=0.0018 |
| 1 | 131 | 1.77 (1.06-2.94) | 0.028 |
| 2+ | 16 | 4.50 (1.99-10.18) | 0.0003 |
| Missing | 175 | 1.24 (0.74-2.09) | 0.41 |
| Conditioning intensity | |||
| MAC with TBI | 214 | 1 | Poverall=0.047 |
| MAC without TBI | 224 | 0.58 (0.37-0.90) | 0.015 |
| RIC/NMA | 71 | 0.66 (0.35-1.26) | 0.21 |
| Donor type | |||
| HLA-id sibling | 217 | 1 | Poverall=0.0064 |
| 8/8 URD | 233 | 1.47 (0.91-2.38) | 0.12 |
| 7/8 URD | 59 | 2.82 (1.48-5.37) | 0.0016 |
| In-vivo T-cell depletion, NRM≤ 6 months | |||
| No | 390 | 1 | |
| Yes | 115 | 3.30 (1.71-6.34) | 0.0003 |
| In-vivo T-cell depletion, NRM> 6 months | |||
| No | 280 | 1 | |
| Yes | 76 | 0.69 (0.34-1.43) | 0.32 |
| 3. LeukemiaFree Survival | |||
| Main effect: | |||
| FLT3 mut | |||
| No | 352 | 1 | |
| Yes | 157 | 1.25 (0.96-1.67) | 0.099 |
| Cytogenetic abnormalities | |||
| Normal | 216 | 1 | Poverall=0.026 |
| Favorable | 30 | 0.49 (0.26-0.92) | 0.027 |
| Intermediate (excludingnormal) | 127 | 0.94 (0.68-1.29) | 0.68 |
| Poor | 121 | 1.21 (0.88-1.68) | 0.25 |
| Missing | 15 | 1.69 (0.92-3.13) | 0.094 |
| Disease status | |||
| CR1 | 375 | 1 | |
| CR2 | 134 | 1.44 (1.09-1.90) | 0.011 |
| Conditioning intensity | |||
| MAC with TBI | 214 | 1 | Poverall=0.028 |
| MAC without TBI | 224 | 0.96 (0.73-1.27) | 0.78 |
| RIC/NMA | 71 | 1.52 (1.07-2.16) | 0.021 |
| Donor type | |||
| HLA-id sibling | 217 | 1 | Poverall=0.0081 |
| 8/8 or well matched URD | 233 | 0.88 (0.67-1.16) | 0.36 |
| 7/8 URD | 59 | 1.58 (1.08-2.30) | 0.018 |
| 4. Overall mortality | |||
| Main effect: | |||
| FLT3 mut | |||
| No | 352 | 1 | |
| Yes | 157 | 1.17 (0.89-1.53) | 0.25 |
| Other factors: | |||
| Recipient age | |||
| 18-39 | 177 | 1 | |
| 40-60 | 332 | 1.77 (1.32-2.37) | 0.0001 |
| White blood count at diagnosis | |||
| <30 | 299 | 1 | Poverall=0.006 |
| 30-100 | 114 | 1.43 (1.04-1.95) | 0.027 |
| >100 | 61 | 1.82 (1.26-2.61) | 0.0013 |
| Missing | 35 | 1.05 (0.62-1.77) | 0.87 |
| Donor type | |||
| HLA-id sibling | 217 | 1 | Poverall<.0001 |
| 8/8 or well matched URD | 233 | 1.17 (0.89-1.55) | 0.27 |
| 7/8 URD | 59 | 2.33 (1.60-3.40) | <.0001 |
RR: relative risk
Relapse Risk
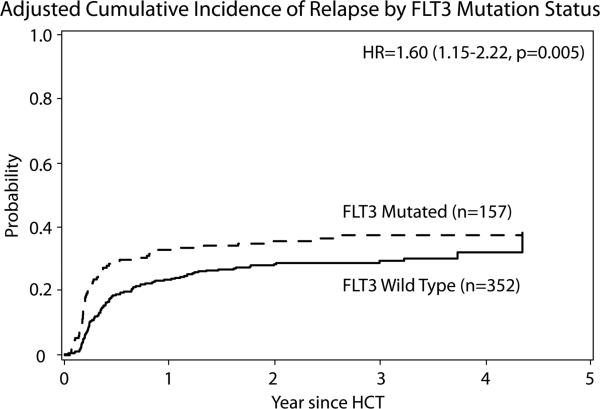
The CI of relapse at 3 yr. was 38% (95% CI 30-45) in the FLT3 mut. group compared to 28% (95% CI 24-33) in the FLT3 WT group, P=0.04. In MVA, relapse was higher in FLT3 mut. group (RR 1.6; 95% CI 1.15-2.22, P =0.005) shown in figure 1. Disease status at time of HCT (CR2 vs.CR1, RR 1.52; 95% CI 1.08-2.14, P=0.016) and conditioning intensity (RIC/NMA vs. MAC, RR 2.14; 95% CI 1.57-3.70, P<0.001) were associated with increased risk of relapse while, HCT from a URD compared to HLA-Identical sibling led to reduced relapse risk (RR 0.64; 95% CI 0.45-0.90, P=0.0095). For pts who underwent HCT in CR1, relapse was significantly impacted by the number of consolidation cycles, with pts who received ≥ 3 consolidations having lower risk of relapse compared to those with no consolidation (P= 0.046, 1 cycle [RR 0.76, 95% CI 0.47-1.23], 2 cycles [RR 0.53, 95% CI 0.28-1.00], and ≥3 cycles [RR 0.45, 95% CI 0.22-0.95]). Pts who underwent HCT in CR2 with mut. FLT3 also had higher relapse risk (RR 1.83 95% CI 1.00- 1.83, P=0.049) compared to pts in CR2 who were FLT3 WT. There was no significant statistical interaction between FLT3 status and cytogenetics (P=0.85), FLT3 status and disease status (P=0.18) nor cytogenetics and disease status (P=0.99).
Figure 1.
Adjusted CI of Relapse by FLT3 mut. status
GVHD
There was no difference in the incidence of acute GVHD between the FLT3 mut. (38% (95%CI 30-45)) and WT groups (32% (95% CI 28-37)) at 100 days (P =0.27). There was no difference in chronic GVHD between the FLT3 mut. (61% (95%CI 52-69)) and WT groups (60% (95% CI 54-65)) at 3 yr. (P =0.86).
Non-Relapse Mortality
Univariate analysis(UVA) showed no significant difference in the CI of NRM between the FLT3 mut.(11% (95% CI 7-17)) and WT groups (13% (95% CI 10-17)) at 1 year (P=0.65). In MVA, there was no significant association between FLT3 mut. status and NRM (RR 0.77; 95% CI 0.48-1.22, P=0.26) (Table 3). Compared to HCT from HLA-identical sibling donors, risk of NRM was higher in 7/8 URD (RR 2.82; 95% CI 1.48-5.37, P=0.001). Other adverse prognostic factors included the use of total body irradiation based MAC (p=0.047) and HCT comorbidity index scores of 1 or greater (P<0.001). In vivo T cell depletion was associated with higher NRM within 6 months of HCT (P <0.001).
Leukemia Free Survival
UVA showed no significant difference in the 3 year-LFS between the FLT3 mut. (47%, 95% CI 39-55) and WT groups (51%, 95 % CI 45-56) (P=0.42) which was confirmed in MVA (RR 1.25; 95% CI 0.96-1.67, P=0.1). LFS was inferior among pts who were transplanted in CR2 (RR 1.44; 95% CI 1.09-1.90, P=0.011), among those who underwent RIC/NMA preparative regimen (RR 1.52; 95% CI 1.07-2.16, P = 0.021) or had HCT from 7/8 unrelated donor (RR 1.58; 95% CI 1.08-2.30, P =0.018). In this model there was no significant interaction between FLT3 status and cytogenetics (P= 0.70).
Overall Survival
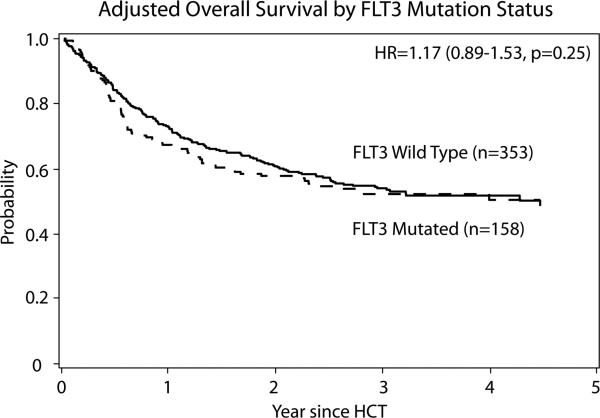
UVA showed similar OS between the FLT3 mut. (49% (95%CI 40-57)) and WT groups (55% (95% CI 50-60)) at 3 yr. (P =0.20). In MVA OS was not significantly associated with FLT3 mut. status (RR 1.17; 95% CI 0.89-1.53) as shown in figure 2. OS was inferior among pts older than 40 yr. (RR 1.77; 95% CI 1.32-2.37, P < 0.001), and after 7/8 URD (RR 2.33; 95% CI 1.60-3.40, P < 0.001).
Figure 2.
Adjusted Overall Survival by FLT3 mut. status
We re-ran the models for LFS and OS after excluding pts with poor risk cytogenetics and there was no significant difference between the two groups (data not shown)
Causes of Death
More pts in the FLT3 mut. group (60% versus 46%) died from their leukemia compared to FLT3 WT group (Table 4).
Table 4.
Causes of Death
| FLT3 mut. | ||
|---|---|---|
| WT | Mut. | |
| Total deaths | 154 | 77 |
| Primary disease | 71 (46) | 46 (60) |
| GVHD | 21 (14) | 8 (10) |
| Idiopathic pneumonia syndrome | 11 (7) | 4 (5) |
| Infection | 21 (14) | 8 (10) |
| Organ failure | 14 (9) | 8 (10) |
| Others | 14 (9) | 2 (3) |
| Missing | 2 | 1 |
DISCUSSION
Many physicians favor early HCT as the most optimal consolidation18, 19, 29 for pts with FLT3 mut. AML though this strategy remains controversial.20, 21, 30-32 In our study, we were able to examine the impact of regimen intensity, consolidation, and disease status (CR1 vs. CR2) on HCT outcomes in FLT3 mut. AML, with long median follow-up of 37 months. This study shows that though pts with FLT3 mut. AML have higher relapse risk after HCT, there is no difference in NRM, LFS, or OS, and 49% pts were alive at 3 yr. Furthermore, our results show that RIC/NMA conditioning may be a feasible strategy in pts who are unsuitable for MAC.
A possible explanation for the increase in relapse risk without concomitant detriment in OS may be the differences in post relapse intervention such as early withdrawal of immunosuppression; institution of tyrosine kinase inhibitor (TKI) based therapy in FLT3 mutated pts, or salvage treatment (chemo/donor lymphocyte infusion (DLI), second HCT), which are all difficult to capture explicitly in a registry study and may have played a role. We reviewed the CIBMTR database to determine if the pts included in this analysis received salvage DLI or a 2nd HCT post relapse, and observed that 16 /60 (27%) pts in the FLT3 mut. group who relapsed and 39/102 (38%) in the FLT3 WT group who relapsed received a DLI or 2nd HCT after relapse.
Brunet et al from the EBMT analyzed outcomes of 206 pts who underwent HLA identical sibling and matched URD HCT with only cytogenetically normal FLT3-ITD mut. AML in CR1 after MAC. They showed a higher relapse (30% vs. 16%, P=0.0006) but also noted inferior LFS (58% vs. 71%, P=0.04) in the FLT3-ITD mut. pts.24
Many pts with FLT3-ITD mut. relapse within 6 months of diagnosis and it is postulated that more consolidation in these pts may promote relapse due to upregulation of the FLT3 ligand.29 In our study, we analyzed the impact of the number of consolidations cycles in pts who underwent HCT in CR1 and found that more cycles (≥ 2) of consolidation chemo were associated with decreased the risk of relapse. However, it is noted that this data includes only pts who survived and received HCT in CR1 and cannot account for those pts who died early due to relapse.
Furthermore, our results showed that some pts with FLT3 mut. are able to achieve CR2 and undergo HCT. Our study showed that pts with mut. FLT3 in either CR1 or CR2 had similar increased relapse risk without adverse effect on NRM, LFS, and OS. While recognizing that the cohort of CR2 pts (n=134) is likely to be representative of highly selected pts (those who have chemo-sensitive disease that was kinetically stable to allow time for HCT in CR2), our data suggests that pts with FLT3 mut. AML who are able to achieve CR2 should be considered for HCT.
Though our study population included FLT3 mut. pts with abnormal cytogenetics, different conditioning regimens, and pts in CR1 or CR2, our MVA showed no significant interaction among these variables. The limitations of our study include those that are inherent in a retrospective registry study. Unfortunately, the data collected by CIBMTR during the study period did not include information about type of FLT3 mut. (ITD vs. TKD), allelic ratios, and minimal residual status at time of HCT, which precludes comment on these variables.
In summary, we show that HCT may be able to overcome the negative prognostic impact of FLT3 mut. in AML with promising LFS and OS. Because most relapses in the FLT3 mut. group were early, usually within the first year, continued investigation of pre-emptive strategies, such as post -HCT maintenance therapy with FLT3 inhibitors or hypomethylating agents, early withdrawal of immunosuppression, and DLI may have value. FLT3 inhibitors such as sorafenib33, 34, quizartinib35 and midostaurin are under investigation in the post-HCT maintenance setting, and may improve outcomes of these high risk pts.
Acknowledgments
FUNDING:
The CIBMTR is supported by Public Health Service Grant/Cooperative Agreement 5U24-CA076518 from the NCI, the NHLBI and the NIAID; a Grant/Cooperative Agreement 5U10HL069294 from NHLBI and NCI; a contract HHSH250201200016C with HRSA/DHHS; two Grants N00014-13-1-0039 and N00014-14-1-0028 from the Office of Naval Research; and grants from Alexion; *Amgen, Inc.; Anonymous donation to the Medical College of Wisconsin; Be the Match Foundation; *Bristol Myers Squibb Oncology; *Celgene Corporation; *Chimerix, Inc.; Fred Hutchinson Cancer Research Center; Gamida Cell Ltd.; Genentech, Inc.; Genzyme Corporation; *Gilead Sciences, Inc.; Health Research, Inc. Roswell Park Cancer Institute; HistoGenetics, Inc.; Incyte Corporation; *Jazz Pharmaceuticals, Inc.; Jeff Gordon Children's Foundation; The Leukemia & Lymphoma Society; The Medical College of Wisconsin; Merck & Co, Inc.; Mesoblast; *Millennium: The Takeda Oncology Co.; *Miltenyi Biotec, Inc.; National Marrow Donor Program; Neovii Biotech NA, Inc.; Novartis Pharmaceuticals Corporation; Onyx Pharmaceuticals; Optum Healthcare Solutions, Inc.; Otsuka America Pharmaceutical, Inc.; Otsuka Pharmaceutical Co, Ltd. – Japan; Oxford Immunotec; Perkin Elmer, Inc.; Pharmacyclics; *Sanofi US; Seattle Genetics; Sigma-Tau Pharmaceuticals; *Spectrum Pharmaceuticals, Inc.; St. Baldrick's Foundation; *Sunesis Pharmaceuticals, Inc.; Swedish Orphan Biovitrum, Inc.; Telomere Diagnostics, Inc.; TerumoBCT; Therakos, Inc.; University of Minnesota; and *Wellpoint, Inc. The views expressed in this article do not reflect the official policy or position of the NIH, the Department of the Navy, the Department of Defense, HRSA or any other agency of the U.S. Government.
*Corporate Members
Footnotes
Author Contribution
Designed the study, analyzed data, and wrote the manuscript: AD**
SS**
KWA
HW
WS
Designed the study and wrote the manuscript:
MA, JHA, MB, MB, JYC, BC, YBC, CSC, RPG, SG, MH, YI, MJ, RK, JK, HML, JL, MRL, DIM, TN, RFO, RR, JMR, AAS, MS, HCS, TCS, RJS, GLU, EKW, PHW, BW, AEW, DB, SD, ML, BMS, DW, HJK
CONFLICT OF INTEREST:
Authors have no relevant conflicts of interest
References
- 1.Kiyoi H, Naoe T, Nakano Y, Yokota S, Minami S, Miyawaki S, et al. Prognostic implication of FLT3 and N-RAS gene mutations in acute myeloid leukemia. Blood. 1999;93(9):3074–80. [PubMed] [Google Scholar]
- 2.Kottaridis PD, Gale RE, Frew ME, Harrison G, Langabeer SE, Belton AA, et al. The presence of a FLT3 internal tandem duplication in patients with acute myeloid leukemia (AML) adds important prognostic information to cytogenetic risk group and response to the first cycle of chemotherapy: analysis of 854 patients from the United Kingdom Medical Research Council AML 10 and 12 trials. Blood. 2001;98(6):1752–9. doi: 10.1182/blood.v98.6.1752. [DOI] [PubMed] [Google Scholar]
- 3.Frohling S, Schlenk RF, Breitruck J, Benner A, Kreitmeier S, Tobis K, et al. Prognostic significance of activating FLT3 mutations in younger adults (16 to 60 years) with acute myeloid leukemia and normal cytogenetics: a study of the AML Study Group Ulm. Blood. 2002;100(13):4372–80. doi: 10.1182/blood-2002-05-1440. [DOI] [PubMed] [Google Scholar]
- 4.Schnittger S, Schoch C, Dugas M, Kern W, Staib P, Wuchter C, et al. Analysis of FLT3 length mutations in 1003 patients with acute myeloid leukemia: correlation to cytogenetics, FAB subtype, and prognosis in the AMLCG study and usefulness as a marker for the detection of minimal residual disease. Blood. 2002;100(1):59–66. doi: 10.1182/blood.v100.1.59. [DOI] [PubMed] [Google Scholar]
- 5.Thiede C, Steudel C, Mohr B, Schaich M, Schakel U, Platzbecker U, et al. Analysis of FLT3-activating mutations in 979 patients with acute myelogenous leukemia: association with FAB subtypes and identification of subgroups with poor prognosis. Blood. 2002;99(12):4326–35. doi: 10.1182/blood.v99.12.4326. [DOI] [PubMed] [Google Scholar]
- 6.Rombouts WJ, Blokland I, Lowenberg B, Ploemacher RE. Biological characteristics and prognosis of adult acute myeloid leukemia with internal tandem duplications in the Flt3 gene. Leukemia. 2000;14(4):675–83. doi: 10.1038/sj.leu.2401731. [DOI] [PubMed] [Google Scholar]
- 7.Yamamoto Y, Kiyoi H, Nakano Y, Suzuki R, Kodera Y, Miyawaki S, et al. Activating mutation of D835 within the activation loop of FLT3 in human hematologic malignancies. Blood. 2001;97(8):2434–9. doi: 10.1182/blood.v97.8.2434. [DOI] [PubMed] [Google Scholar]
- 8.Mead AJ, Linch DC, Hills RK, Wheatley K, Burnett AK, Gale RE. FLT3 tyrosine kinase domain mutations are biologically distinct from and have a significantly more favorable prognosis than FLT3 internal tandem duplications in patients with acute myeloid leukemia. Blood. 2007;110(4):1262–70. doi: 10.1182/blood-2006-04-015826. [DOI] [PubMed] [Google Scholar]
- 9.Kok CH, Brown AL, Perugini M, Iarossi DG, Lewis ID, D'Andrea RJ. The preferential occurrence of FLT3-TKD mutations in inv(16) AML and impact on survival outcome: a combined analysis of 1053 core-binding factor AML patients. British journal of haematology. 2013;160(4):557–9. doi: 10.1111/bjh.12131. [DOI] [PubMed] [Google Scholar]
- 10.Levis M. FLT3 mutations in acute myeloid leukemia: what is the best approach in 2013? Hematology / the Education Program of the American Society of Hematology. American Society of Hematology. Education Program. 2013;2013:220–6. doi: 10.1182/asheducation-2013.1.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ravandi F, Kantarjian H, Faderl S, Garcia-Manero G, O'Brien S, Koller C, et al. Outcome of patients with FLT3-mutated acute myeloid leukemia in first relapse. Leukemia research. 2010;34(6):752–6. doi: 10.1016/j.leukres.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levis M, Small D. FLT3: ITDoes matter in leukemia. Leukemia. 2003;17(9):1738–52. doi: 10.1038/sj.leu.2403099. [DOI] [PubMed] [Google Scholar]
- 13.Nakao M, Yakota S, Iwai T. Internal tandem duplication of the flt3 gene found in acute myeloid leukemia. Leukemia. 1996;10:1911–1918. [PubMed] [Google Scholar]
- 14.Frohling S, Schlenk RF, Breitruck J, Benner A, Kreitmeier S, Tobis K, et al. Prognostic significance of activating FLT3 mutations in younger adults (16 to 60 years) with acute myeloid leukemia and normal cytogenetics: a study of the AML Study Group Ulm. Blood. 2002;100(13):4372–80. doi: 10.1182/blood-2002-05-1440. [DOI] [PubMed] [Google Scholar]
- 15.Bacher U, Haferlach C, Kern W, Haferlach T, Schnittger S. Prognostic relevance of FLT3-TKD mutations in AML: the combination matters--an analysis of 3082 patients. Blood. 2008;111(5):2527–37. doi: 10.1182/blood-2007-05-091215. [DOI] [PubMed] [Google Scholar]
- 16.Savani BN. Transplantation in AML CR1. Blood. 2010;116(11):1822–3. doi: 10.1182/blood-2010-06-291500. [DOI] [PubMed] [Google Scholar]
- 17.Schlenk RF, Kayser S, Bullinger L, Kobbe G, Casper J, Ringhoffer M, et al. Differential impact of allelic ratio and insertion site in FLT3-ITD-positive AML with respect to allogeneic transplantation. Blood. 2014;124(23):3441–9. doi: 10.1182/blood-2014-05-578070. [DOI] [PubMed] [Google Scholar]
- 18.Dezern AE, Sung A, Kim S, Smith BD, Karp JE, Gore SD, et al. Role of Allogeneic Transplantation for FLT3/ITD Acute Myeloid Leukemia: Outcomes from 133 Consecutive Newly Diagnosed Patients from a Single Institution. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2011 doi: 10.1016/j.bbmt.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schlenk RF, Dohner K, Krauter J, Frohling S, Corbacioglu A, Bullinger L, et al. Mutations and treatment outcome in cytogenetically normal acute myeloid leukemia. The New England journal of medicine. 2008;358(18):1909–18. doi: 10.1056/NEJMoa074306. [DOI] [PubMed] [Google Scholar]
- 20.Bornhauser M, Illmer T, Schaich M, Soucek S, Ehninger G, Thiede C. Improved outcome after stem-cell transplantation in FLT3/ITD-positive AML. Blood. 2007;109(5):2264–5. doi: 10.1182/blood-2006-09-047225. author reply 2265. [DOI] [PubMed] [Google Scholar]
- 21.Gale RE, Hills R, Kottaridis PD, Srirangan S, Wheatley K, Burnett AK, et al. No evidence that FLT3 status should be considered as an indicator for transplantation in acute myeloid leukemia (AML): an analysis of 1135 patients, excluding acute promyelocytic leukemia, from the UK MRC AML10 and 12 trials. Blood. 2005;106(10):3658–65. doi: 10.1182/blood-2005-03-1323. [DOI] [PubMed] [Google Scholar]
- 22.Koreth J, Schlenk R, Kopecky KJ, Honda S, Sierra J, Djulbegovic BJ, et al. Allogeneic stem cell transplantation for acute myeloid leukemia in first complete remission: systematic review and meta-analysis of prospective clinical trials. JAMA : the journal of the American Medical Association. 2009;301(22):2349–61. doi: 10.1001/jama.2009.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Araki D, Wood BL, Othus M, Radich JP, Halpern AB, Zhou Y, et al. Allogeneic Hematopoietic Cell Transplantation for Acute Myeloid Leukemia: Time to Move Toward a Minimal Residual Disease-Based Definition of Complete Remission? Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2016;34(4):329–36. doi: 10.1200/JCO.2015.63.3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brunet S, Labopin M, Esteve J, Cornelissen J, Socie G, Iori AP, et al. Impact of FLT3 internal tandem duplication on the outcome of related and unrelated hematopoietic transplantation for adult acute myeloid leukemia in first remission: a retrospective analysis. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30(7):735–41. doi: 10.1200/JCO.2011.36.9868. [DOI] [PubMed] [Google Scholar]
- 25.Song Y, Magenau J, Li Y, Braun T, Chang L, Bixby D, et al. FLT3 mutational status is an independent risk factor for adverse outcomes after allogeneic transplantation in AML. Bone marrow transplantation. 2015 doi: 10.1038/bmt.2015.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sengsayadeth SM, Jagasia M, Engelhardt BG, Kassim A, Strickland SA, Goodman S, et al. Allo-SCT for high-risk AML-CR1 in the molecular era: impact of FLT3/ITD outweighs the conventional markers. Bone marrow transplantation. 2012;47(12):1535–7. doi: 10.1038/bmt.2012.88. [DOI] [PubMed] [Google Scholar]
- 27.Schmid C, Labopin M, Socie G, Daguindau E, Volin L, Huynh A, et al. Outcome of patients with distinct molecular genotypes and cytogenetically normal AML after allogeneic transplantation. Blood. 2015;126(17):2062–9. doi: 10.1182/blood-2015-06-651562. [DOI] [PubMed] [Google Scholar]
- 28.Bacigalupo A, Ballen K, Rizzo D, Giralt S, Lazarus H, Ho V, et al. Defining the intensity of conditioning regimens: working definitions. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2009;15(12):1628–33. doi: 10.1016/j.bbmt.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Levis M. FLT3/ITD AML and the law of unintended consequences. Blood. 2011;117(26):6987–90. doi: 10.1182/blood-2011-03-340273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gupta V, Tallman MS, Weisdorf DJ. Allogeneic hematopoietic cell transplantation for adults with acute myeloid leukemia: myths, controversies, and unknowns. Blood. 2011;117(8):2307–18. doi: 10.1182/blood-2010-10-265603. [DOI] [PubMed] [Google Scholar]
- 31.Rowe JM, Tallman MS. How I treat acute myeloid leukemia. Blood. 2010;116(17):3147–56. doi: 10.1182/blood-2010-05-260117. [DOI] [PubMed] [Google Scholar]
- 32.Meshinchi S, Arceci RJ, Sanders JE, Smith FO, Woods WB, Radich JP, et al. Role of allogeneic stem cell transplantation in FLT3/ITD-positive AML. Blood. 2006;108(1):400. doi: 10.1182/blood-2005-12-4938. author reply 400-1. [DOI] [PubMed] [Google Scholar]
- 33.Chen YB, Li S, Lane AA, Connolly C, Del Rio C, Valles B, et al. Phase I trial of maintenance sorafenib after allogeneic hematopoietic stem cell transplantation for fms-like tyrosine kinase 3 internal tandem duplication acute myeloid leukemia. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2014;20(12):2042–8. doi: 10.1016/j.bbmt.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tarlock K, Chang B, Cooper T, Gross T, Gupta S, Neudorf S, et al. Sorafenib treatment following hematopoietic stem cell transplant in pediatric FLT3/ITD acute myeloid leukemia. Pediatric blood & cancer. 2015;62(6):1048–54. doi: 10.1002/pbc.25437. [DOI] [PubMed] [Google Scholar]
- 35.Sandmaier BM, Khaled SK, Oran B, Gammon G, Trone D, Frankfurt O. Results of a Phase 1 Study of Quizartinib (AC220) As Maintenance Therapy in Subjects with Acute Myeloid Leukemia in Remission Following Allogeneic Hematopoietic Cell Transplantation. Blood. 2014;124(21):428–428. doi: 10.1002/ajh.24959. [DOI] [PMC free article] [PubMed] [Google Scholar]