Abstract
Objective
Electroencephalogram (EEG) neurofeedback aimed at reducing the amplitude of the alpha-rhythm has been shown to alter neural networks associated with posttraumatic stress disorder (PTSD), leading to symptom alleviation. Critically, the amygdala is thought to be one of the central brain regions mediating PTSD symptoms. In the current study, we compare directly patterns of amygdala complex connectivity using fMRI, before and after EEG neurofeedback, in order to observe subcortical mechanisms associated with behavioural and alpha oscillatory changes among patients.
Method
We examined basolateral (BLA), centromedial (CMA), and superficial (SFA) amygdala complex resting-state functional connectivity using a seed-based approach via SPM Anatomy Toolbox. Amygdala complex connectivity was measured in twenty-one individuals with PTSD, before and after a 30-minute session of EEG neurofeedback targeting alpha desynchronization.
Results
EEG neurofeedback was associated with a shift in amygdala complex connectivity from areas implicated in defensive, emotional, and fear processing/memory retrieval (left BLA and left SFA to the periaqueductal gray, and left SFA to the left hippocampus) to prefrontal areas implicated in emotion regulation/modulation (right CMA to the medial prefrontal cortex). This shift in amygdala complex connectivity was associated with reduced arousal, greater resting alpha synchronization, and was negatively correlated to PTSD symptom severity.
Conclusion
These findings have significant implications for developing targeted non-invasive treatment interventions for PTSD patients that utilize alpha oscillatory neurofeedback, showing evidence of neuronal reconfiguration between areas highly implicated in the disorder, in addition to acute symptom alleviation.
Keywords: Neurofeedback, Electroencephalogram, Functional MRI, Functional connectivity, Posttraumatic stress disorder, Amygdala
Highlights
-
•
Alpha desynchronizing neurofeedback was associated with a shift in amygdala complex connectivity.
-
•
Connectivity shifted from areas implicated in defensive fear processing/memory retrieval, to prefrontal emotion regulation areas.
-
•
Shift in amygdala complex connectivity was associated with reduced arousal and PTSD symptom severity, and greater resting alpha synchronization after neurofeedback.
-
•
These findings have significant implications for developing targeted non-invasive treatment interventions for PTSD patients.
Individuals with posttraumatic stress disorder (PTSD) experience frequent hyperarousal, emotional numbing, and vivid re-experiencing of traumatic memories, in addition to cognitive and behavioural avoidance (APA, 2013). Here, the manifestation of PTSD symptomatology has been shown to be mediated in part through altered neurocircuitry of the amygdala (Birn et al., 2014, Etkin and Wager, 2007, Lanius et al., 2010, Patel et al., 2012, Pitman et al., 2012, Shin and Liberzon, 2010, Stevens et al., 2013, Weston, 2014, Yehuda et al., 2015). Central neurophysiological characteristics of the disorder include: aberrant amygdala and prefrontal cortex (PFC) activation during symptom provocation (Frewen et al., 2011, Hayes et al., 2012, Hopper et al., 2007), fear processing (Bruce et al., 2013, Bryant et al., 2008, Williams et al., 2006) and rest (Brown et al., 2013, Huang et al., 2014, Nicholson et al., 2015), in addition to altered amygdala connectivity at rest to the insula (Fonzo et al., 2010, Rabinak et al., 2011), hippocampus, cingulate cortex (Sripada et al., 2012) and medial PFC (Birn et al., 2014, Stevens et al., 2013). Critically, PTSD symptoms of hyperarousal have been associated with negative medial PFC-amygdala coupling (Sadeh et al., 2014), and hyper/hypo-activation of the amygdala and medial PFC, respectively, during PTSD emotional processing (Bruce et al., 2013). This may represent attenuated top-down inhibition from the PFC, on the amygdala, in PTSD patients (Lanius et al., 2010, Pitman et al., 2012, Shin and Liberzon, 2010). The amygdala also displays connectivity to lower order brain regions (Roy et al., 2009) such as the periaqueductal gray (PAG), in order to orchestrate defense, and fear related processing (Kozlowska et al., 2015), which are heavily implicated in PTSD (Lanius et al., 2014, Panksepp and Biven, 2012) and may be activated recurrently after trauma in a suboptimal way (Kozlowska et al., 2015).
Interestingly, unique amygdala connectivity patterns among patients with PTSD have been reported as a result of examining amygdalar complexes separately (Brown et al., 2013, Nicholson et al., 2015). Briefly, the basolateral amygdala complex (BLA) appears to be involved in the cortical integration of emotional processing and fear-related learning, and is regulated by inhibition from the PFC (Duvarci and Pare, 2014). In contrast, the centromedial amygdala complex (CMA) is reported to be involved in the behavioural execution of fear responses, containing projections to the PAG and brainstem (Duvarci and Pare, 2014, LeDoux, 1998, Phelps and LeDoux, 2005), and the superficial amygdala complex (SFA) is further involved in affective, social, and olfactory processing (Goossens et al., 2009, Heimer and Van Hoesen, 2006, Koelsch et al., 2013). In addition, the left amygdala has been shown to be associated with the detailed/elaborate processing of emotional stimuli, while inversely, the right amygdala engages in the rapid/automatic detection of emotional stimuli (Baas et al., 2004, Sergerie et al., 2008). In sum, as the amygdala displays a central role in the pathophysiology of PTSD (Etkin and Wager, 2007, Lanius et al., 2010, Pitman et al., 2012), characterized by unique functional connectivity patterns among amygdalar complexes (Brown et al., 2013, Nicholson et al., 2015), normalization of amygdala complex activity/connectivity in PTSD patients could significantly attenuate symptoms in this patient population.
With regard to therapeutically altering PTSD brain connectivity and downstream effects on behaviour, electroencephalogram (EEG) neurofeedback has been used as a non-invasive approach to plastically modulate large-scale neural networks – such as the salience network, default mode network (DMN), and executive functioning network (Kluetsch et al., 2014, Ros et al., 2013) – which have been shown to be implicated in PTSD (Bluhm et al., 2009, Daniels et al., 2010, Lanius et al., 2015, Rabellino et al., 2015, Shang et al., 2014, Sripada et al., 2012). Recent reports suggest covariation between alpha oscillations and spontaneous changes in the aforementioned neural networks associated with PTSD (Laufs et al., 2003, Sadaghiani et al., 2010). Alpha oscillations (8–12 Hz) reflect a state of resting wakefulness, negatively correlated to tasks requiring concentration (Nunez et al., 2001), and positively correlated to the “task-negative” DMN (Jann et al., 2009, Mantini et al., 2007) – which is of particular note given that the DMN and self-referential processing are known to be altered in patients with PTSD (Lanius et al., 2015). Among PTSD patients, alpha desynchronizing neurofeedback was found to induce a homeostatic “rebound” in alpha synchronization post-training that was associated with reductions in hyperarousal (Kluetsch et al., 2014). Elsewhere, PTSD patients have been shown to be characterized by decreased alpha oscillations (Huang et al., 2014, Ros et al., 2016).
The rationale for the current study manifests from the potential triangular relationship between the following phenomena: i) aberrant amygdala complex connectivity has been shown to be a central neural characteristic mediating PTSD psychopathology (Brown et al., 2013, Lanius et al., 2010, Nicholson et al., 2015, Pitman et al., 2012, Shin and Liberzon, 2010, Sripada et al., 2012), ii) EEG alpha desynchronization has previously been shown to plastically alter the neural networks implicated in PTSD (Ros et al., 2013), and iii) the latter has led to symptom alleviation among patients with PTSD (Kluetsch et al., 2014). Hence, using the same dataset as (Kluetsch et al., 2014) in which neural networks were successfully altered in patients with PTSD, we conducted a follow-up study investigating amygdala complex connectivity before and after one 30-minute session of alpha desynchronizing neurofeedback, in order to observe subcortical mechanisms associated with behavioural and alpha oscillatory changes among patients. Firstly, we predicted increased amygdala complex connectivity to mid-brain/brainstem areas implicated in the defense cascade (such as the PAG), before neurofeedback. Secondly, we predicted that neurofeedback would shift amygdala complex connectivity towards enhanced coupling with the medial prefrontal cortex (mPFC) and emotion regulation regions (Etkin et al., 2011), where we hypothesize this to be a modulating mechanism underlying reduced arousal after neurofeedback.
1. Method
1.1. Participants
The sample consisted of 21 participants who met DSM-IV criteria (APA, 2013) for a primary diagnosis of PTSD (see Table 1 for demographic and psychometric information), where all patients experienced childhood sexual and/or physical abuse. As previously described by Kluetsch et al. (2014), Structured Clinical Interviews for DSM-IV Axis I Disorders (First et al., 2002), and the Clinician-Administered PTSD scale (CAPS; cut-off score > 50; Blake et al., 1995), were employed by a trained psychologist to obtain Axis I diagnoses; in addition, all participants completed the Childhood Trauma Questionnaire (CTQ; Bernstein et al., 2003) and the Multiscale Dissociation Inventory (MDI; Briere, 2002). Exclusion criteria for patients with PTSD included: diagnosis of psychotic disorders over their lifetime, bipolar disorder, substance use disorders within the last 6 months, previous head trauma, significant untreated medical or neurological illness, and noncompliance with 3T fMRI safety standards. At the time of study, eleven participants were taking psychotropic medications, consisting of: citalopram (n = 2), fluoxetine (n = 1), sertraline (n = 1), clonazepam (n = 3), trazodone (n = 1), clozapine (n = 1), quetiapine (n = 1), cipralex (n = 3), and mirtazapine (n = 1).
Table 1.
Demographic and psychometric data.
| Measure | Patients with PTSD (n = 21), M ± SD |
|---|---|
| Age | 39.9 ± 13.7 |
| Sex | Females = 18 |
| CAPS | 80.62 ± 14.01 |
| MDI Average | 2.47 ± 0.91 |
| CTQ- Emotional Abuse Score | 17.48 ± 5.57 |
| CTQ- Physical Abuse Score | 11.05 ± 5.30 |
| CTQ- Sexual Abuse Score | 15.29 ± 8.21 |
| CTQ- Emotional Neglect Score | 16.33 ± 5.22 |
| CTQ Physical Neglect Score | 12.57 ± 5.49 |
| Axis I Disorder | Current/past (n) |
| Major Depressive Disorder | 8/10 |
| Dysthymic Disorder | 1/0 |
| Panic Disorder with Agoraphobia | 1/3 |
| Panic Disorder without Agoraphobia | 3/2 |
| Agoraphobia without Panic Disorder | 2/1 |
| Social Phobia | 3/0 |
| Obsessive Compulsive Disorder | 0/1 |
| Social Phobia | 3/0 |
| Somatization Disorder | 2/1 |
| Undifferentiated Somatoform Disorder | 5/0 |
| Anorexia Nervosa | 0/3 |
| Bulimia Nervosa | 1/1 |
| Unspecified Eating Disorders | 1/1 |
Abbreviations: CAPS = Clinician Administered PTSD Scale, CTQ = Childhood Trauma Questionnaire, n = number of participants corresponding to a group, M = mean, SD = standard deviation.
1.2. Procedure
Approval for the current study was obtained by Western University's ethic board, where all participants provided written informed consent. Experimental procedures were described in detail by Kluetsch et al. (2014) and consisted of the following, which occurred sequentially on the same visit: a resting state fMRI scan before neurofeedback, an EEG neurofeedback session, and a second fMRI scan after neurofeedback. The time between the first and second fMRI scan was about 1 h, where participants went back into the fMRI scanner on average about 20 min after neurofeedback. Immediately before and after the EEG neurofeedback session, participants were administered the Spielberger's State Anxiety Inventory (STAI; Spielberger, 1983) and the Thayer's Activation/Deactivation Adjective Checklist (Thayer, 1986) in order to examine state anxiety and arousal levels, respectively. Additionally, following neurofeedback training, we asked participants if they felt as though they had control over the signal feedback they were receiving, how the experience made them feel, and what strategy they found to be successful. Our proof-of-concept study with healthy controls showed that successful alpha desynchronization via EEG neurofeedback significantly alters network connectivity as compared to a sham feedback (Ros et al., 2013). Importantly, there is also evidence to suggest that effective learning of voluntary control of brain rhythms may be compromised after receiving false neurofeedback (van Boxtel et al., 2012), and therefore a sham-feedback region was not included. Moreover, neurofeedback represents a task which i) mitigates visual stimuli dependent factors in experimental designs leading to greater intrinsic effects, ii) produces the same reward contingencies across participants, and iii) has variability in terms of success between participants (Ros et al., 2014, Ros et al., 2013). Hence neurofeedback represents an elegant way to “clamp the external milieu”, in order to delineate causal, intrinsic relationships (Ros et al., 2014).
1.3. EEG neurofeedback: paradigm, recording, and preprocessing
We followed the same EEG neurofeedback experimental design, in addition to recording and preprocessing procedures, as previously reported by Kluetsch et al. (2014). The EEG session consisted of a 3-minute baseline measure both before and after a 30-minute neurofeedback training session. During baseline recordings, in which participants did not receive neurofeedback, participants were instructed to relax with their eyes open and gaze at a blank wall (limiting eye movements). The 30-minute neurofeedback session consisted of participants attempting/learning to suppress real-time alpha amplitudes (8–12 Hz) recorded from the midline parietal cortex (electrode Pz). Here signal feedback was derived from Pz, as global alpha signal averaged across multiple electrodes, may lead to a mixing of local cortical dynamics (Ros et al., 2013). This electrode was chosen based on its location over the posterior cingulate cortex (PCC)/precuneus, which are major hubs of the DMN whose blood oxygen level dependent signal changes have been correlated to EEG alpha rhythm modulations (Jann et al., 2009, Mantini et al., 2007). The neurofeedback paradigm (implemented through EEGer 4.2 neurofeedback software and the ‘SpaceRace’ game) consisted of continuous real-time visual feedback in the form of a moving spaceship, and a dynamic bar whose height was inversely proportional to instantaneous alpha amplitude. Participants were instructed that the spaceship would only move forward when they were in the zone of target brain activity (alpha lower than threshold), and that the spaceship would stop when they were outside the zone of target brain activity (alpha higher than threshold). In order to prevent demand characteristics from affecting training, we did not give participants specific instructions for regulatory strategies, nor were they informed about the type of EEG parameter/frequency that was being targeted.
1.4. EEG spectral analysis
We calculated EEG spectral amplitudes offline via short-time Fourier transformations in 4-s epochs (50% overlapping with Hanning window) in the alpha frequency band (8–12 Hz). In order to investigate whether patients were able to successfully lower their absolute alpha amplitude during neurofeedback, as compared to the first baseline, we conducted a Bonferroni corrected paired t-test for average absolute alpha amplitudes during the initial baseline and during the entire neurofeedback training session. We additionally conducted a Bonferroni corrected paired t-test comparing baseline absolute average alpha amplitudes pre and post neurofeedback training, in order to investigate if patients has plastically changed their absolute alpha amplitudes. In order to observe percent signal change, we normalized alpha values for each participant, for the following ratios of interest: a) “training alpha change” normalized by taking the ratio of average alpha amplitude during neurofeedback as compared to the average alpha amplitude during the first baseline, b) “resting alpha change” normalized using the ratio of the average alpha amplitude during the second baseline as compared to the first baseline. Hence, with changes in alpha amplitude normalized, ratios > 0 denote a relative % increase in alpha amplitude, and inversely, values < 0 demarcate a relative % decrease. In order to observe relationships between training alpha change, resting alpha change, and absolute alpha amplitude during the initial baseline, we computed a Pearson product moment correlation between the two change scores and the score for the initial baseline in SPSS (IBM Corporation, Armonk, NY, USA). This was conducted for global changes of alpha amplitude (averaged across the 19 electrodes), in addition to local changes as measured by Pz, in order to quantitatively compare local vs. global measures of alpha.
1.5. fMRI acquisition and preprocessing
Resting-state fMRI acquisition parameters are described in detail by Kluetsch et al. (2014). Image preprocessing and statistical analyses were conducted using Statistical Parametric Mapping (SPM8 and SPM12, Wellcome Department of Neurology, London, UK: http://www.fil.ion.ucl.ac.uk/spm) within Matlab 8.3 (Mathworks Inc., MA.). Images were subjected to slice-time correction (with reference to the middle slice), realignment and further motion correction using ART software (Gabrieli Lab. McGovern Institute for Brain Research, Cambridge, MA) which computes motion outlier regressors to be used in the 1st level analysis as a covariate of no interest. Images were then coregistered to the participant's corresponding T1 anatomical image, and subsequently segmented, normalized, and smoothed with an 8 mm full-width-half-maximum (FWHM) Gaussian kernel. Band-pass filtering was conducted using successive application of a high-pass and low-pass filter (frequency cut-offs 0.012 Hz and 0.1 Hz respectively) using in-house software by co-author Jean Théberge. Amygdala complex seed masks were created using SPM's Anatomy Toolbox (Eickhoff et al., 2005) featuring cytoarchitectonically-based probability maps of the amygdala. Connectivity correlations were standardized using a Fisher r-to-Z transformation in SPM.
1.6. fMRI connectivity analysis
For each participant, a mean signal intensity time course was extracted from SPM's Anatomy Toolbox for each of the six seed regions (bilateral BLA, CMA and SFA), to be used as a regressor in a correlation analysis delineating functional connectivity. This was done separately for pre and post neurofeedback resting state scans. Both positive correlations and negative correlations were examined. An initial whole-brain 2 (neurofeedback) × 2 (whole amygdala hemisphere) repeated measures full-factorial analysis of variance (ANOVA) was conducted in the second level, in order to first observe any plastic changes in amygdala functional connectivity as a result of neurofeedback, justifying subsequent examination of amygdala complexes separately. The neurofeedback factor consisted of 2 levels: pre and post neurofeedback, and the amygdala factor consisted of 2 levels: left and right whole amygdala seeds, averaging mean signal intensity time courses from all amygdala complexes in each corresponding hemisphere (BLA, CMA and SFA) extracted using SPM's Anatomy Toolbox. The levels within the neurofeedback factor and amygdala hemisphere factor were set to dependent in order to account for repeated measures. To investigate changes in amygdala complex connectivity as a function of alpha desynchronization, we examined the neurofeedback × whole amygdala hemisphere interaction, which yielded 4 significant (FWE-corrected p < 0.05, k = 10) gray matter clusters (see Table 2 for ANOVA results), including the cerebellar culmen, lentiform nucleus of the putamen/hippocampus, dorsal PAG, and the PCC. Follow-up comparisons of the peak coordinates (limited to 6 mm spheres centered around coordinate) meeting the above error rate protection were then conducted using one-sample t-tests; in addition, paired t-tests were also conducted in order to examine differences in amygdala complex connectivity to these regions as a result of neurofeedback. Follow-up analyses utilized p-uncorrected < 0.005 and k = 10, in accordance with the suggestion of Lieberman and Cunningham (2009) to balance the relative risk of Type I versus II error rates, and to protect against circularity/inflating significance values via using our own data to correct post-hoc analyses (Kriegeskorte et al., 2009, Nicholson et al., 2015, Vul and Pashler, 2012). The cerebral aqueduct was exclusively masked using PickAtlas, in order to control for CSF signal. Similarly, the mPFC was specified a-priori and used to conduct a region-of-interest analysis, utilizing the same error protection rate as the ANOVA (FWE-corrected p < 0.05 k = 10) for the aforementioned one-sample and paired-sample t-tests with a 6-mm radius sphere (MNI = 1 60–1; Bruce et al., 2013). Additionally, in order to examine if medication status had a significant effect on the current results, we included this variable as a binary covariate (0 = absent, 1 = present). This did not change the results in question, except for marginally altering the voxel number within ANOVA clusters.
Table 2.
2 (neurofeedback) × 2 (whole amygdala hemisphere) repeated measures ANOVA.
| Analysis | Gyrus/Sulcus | H | BA | Cluster Size | MNI Coordinate |
F(1, 240) | Z score | p FWE | ||
|---|---|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||||
| Neurofeedback × whole amygdala interaction | Cerebellar vermis/hemisphere, anterior lobe culmen | R | 5064 | 6 | − 40 | − 28 | 14.69 | 3.60 | < 0.05 | |
| Putamen, lentiform nucleus/hippocampus | L | 5064 | − 30 | − 18 | − 6 | 12.06 | 3.23 | < 0.05 | ||
| L | 5064 | − 30 | − 30 | − 4 | 9.75 | 2.88 | < 0.05 | |||
| Periaqueductal gray | L | 5064 | − 2 | − 28 | − 10 | 8.75 | 2.71 | < 0.05 | ||
| Posterior cingulate cortex | R | 31 | 5064 | 6 | − 32 | 28 | 8.61 | 2.68 | < 0.05 | |
Repeated measures analysis of variance for the neurofeedback by whole amygdala seed hemisphere connectivity interaction, FWE-corrected gray matter clusters (p < 0.05, k = 10). Abbreviations: BA = Brodmann area, FWE = family-wise error cluster-corrected threshold, H = hemisphere.
1.7. Multiple regression analysis
We first conducted paired t-tests on Thayer/STAI scales in order to uncover significant differences between pre and post neurofeedback measures of state arousal and anxiety, respectively. In addition to Thayer/STAI scales, we also evaluated the following measures as predictors of amygdala complex connectivity changes as a result of the neurofeedback intervention: alpha amplitude change during neurofeedback (relative to baseline), pre-post resting alpha amplitude change, and baseline measures of total CAPS total, average MDI, average MDI depersonalization/derealization subscales. For amygdala complex connectivity, we used ImgCal in SPM12, to calculate both pre > post and post > pre contrasts, on the subject level. These extracted values we then entered into a multiple regression analyses (separate analysis for each amygdala complex and each predictor), utilizing the aforementioned error protection rate for follow-up ANOVA interaction analyses, in addition to separately examining the mPFC a-priori region-of-interest (FWE-corrected p < 0.05, k = 10).
2. Results
2.1. Subjective results from neurofeedback
When examining self-reports, 80% (17 of 21) of patients felt they had a sense of control over the feedback signal, and reported that they felt more relaxed, calm, and clear-minded after the neurofeedback session (see Kluetsch et al., 2014 for details). Strategies used to make the spaceship move, or decrease alpha amplitude, most commonly included focused visual attention. A number of participants also reported that feeling positive emotions induced spaceship movement, whereas inversely, trauma-related thoughts would stop spaceship movement. Interestingly, many participants reported not being as overwhelmed by trauma-related thoughts during neurofeedback.
When examining the Thayer scores via paired t-tests, we detected a significant decrease in arousal after neurofeedback (t(20) = 2.72; p < 0.05). Therefore this measure was used as a regressor in subsequent analyses. On the other hand, when examining STAI state anxiety scores, we did not observe a significant difference in pre and post neurofeedback values.
2.2. EEG spectral analysis
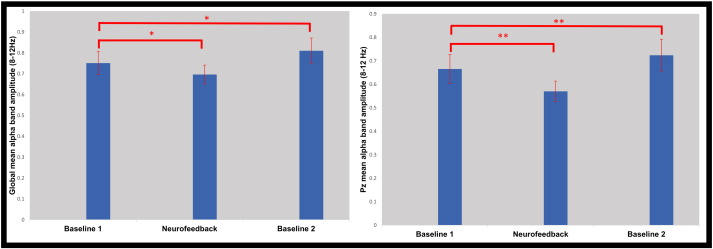
The EEG results have been previously described by Kluetsch et al. (2014). Via paired t-tests, we observed significantly reduced absolute alpha band amplitude during neurofeedback as compared to the first baseline, at both the Pz feedback site (t(20) = − 3.19, p < 0.05), as well as the global average (t(20) = − 3.21, p < 0.05), forming the basis of the “training alpha change” variable. In juxtaposition, paired t-tests showed a significant increase (rebound) in alpha amplitude during the second as compared to the first baseline, for both the Pz feedback site (t(20) = 3.54, p < 0.05) and global average (t(20) = 3.67, p < 0.05). This difference formed the basis of the “resting alpha change” variable, defined as the ratio between the average alpha amplitudes during the first and second baseline for each subject (Fig. 1). Notably, when controlling for absolute alpha amplitude at the first baseline, “training alpha change” was negatively correlated with “resting alpha change” for the global amplitude measure (global: rpartial = − 0.52, p < 0.05; Pz: rpartial = − 0.42, p = 0.06); indicating that greater alpha decreases during neurofeedback, led to stronger increases in alpha after neurofeedback (i.e., larger “rebound”).
Fig. 1.
Bar graphs showing mean alpha (8–12 Hz) amplitudes (calculated offline using average-reference montage) averaged across all participants for Baseline 1, Neurofeedback, and Baseline 2. This was done globally (left) across all 19 electrodes, and at the Pz feedback site (right). *Indicates significance threshold of p < 0.01, and **p < 0.005. Error bars represent 1 standard error of the mean.
2.3. One-sample amygdala complex functional connectivity
Briefly, both pre and post neurofeedback functional connectivity analyses displayed significant positive amygdala complex connectivity (bilateral BLA, CMA and SFA) to the PAG, lentiform nucleus of the putamen, PCC, and the anterior lobe culmen of the cerebellum (see Supplemental results; Table s1). Interestingly, when examining the mPFC a-priori region-of-interest, only the left CMA displayed significant connectivity to this region pre-neurofeedback; inversely, all amygdala complexes (bilateral BLA, CMA, SFA) displayed significant connectivity to the mPFC post-neurofeedback. Additionally, when examining pre neurofeedback functional connectivity, the left SFA displayed significant connectivity to the hippocampus, while the left CMA exhibited connectivity to red nucleus of the brainstem.
2.4. Differences in pre and post neurofeedback amygdala complex functional connectivity
2.4.1. Pre neurofeedback
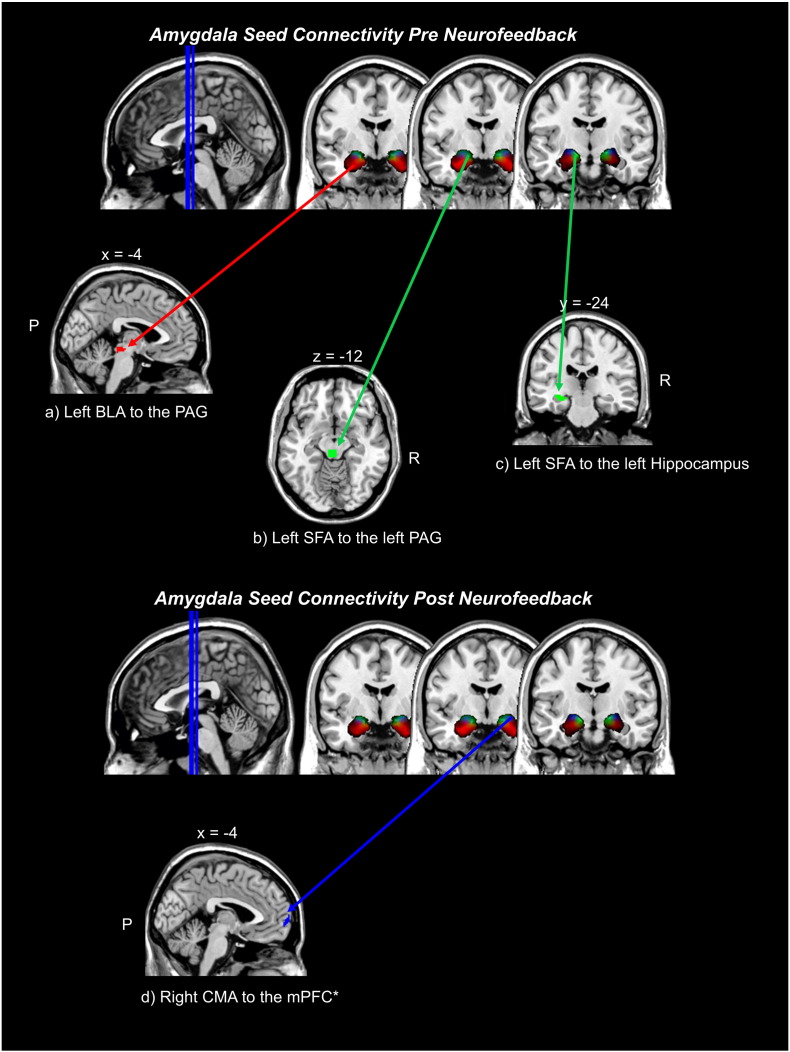
When examining increased amygdala complex connectivity for the pre-neurofeedback as compared to the post-neurofeedback condition, we observed greater connectivity between the left BLA and the PAG, as well as between the left SFA and the left PAG and left hippocampus (see Table 3; Fig. 2).
Table 3.
Pre and post neurofeedback differences in amygdala complex functional connectivity.
| Amygdala Complex Seed | Neurofeedback contrast | Gyrus/Sulcus (BA) | H | Cluster size | Beta values | MNI coordinate |
Z score | t(20) | p | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| x | y | z | |||||||||
| Left BLA | Pre > Post | Dorsal PAG | – | 63 | 0.033 | 0 | − 26 | − 10 | 2.81 | 3.16 | 0.002 |
| Post > Pre | ns | ||||||||||
| Right BLA | Pre > Post | ns | |||||||||
| Post > Pre | ns | ||||||||||
| Left CMA | Pre > Post | ns | |||||||||
| Post > Pre | ns | ||||||||||
| Right CMA | Pre > Post | ns | |||||||||
| Post > Pre | mPFC (10) | – | 31 | 0.155 | 0 | 66 | 2 | 2.40 | 2.61 | < 0 0.05 | |
| Left SFA | Pre > Post | PAG | L | 93 | 0.035 | − 2 | − 28 | − 12 | 2.78 | 3.11 | 0.003 |
| Anterior hippocampus | L | 81 | 0.060 | − 28 | − 22 | − 8 | 2.71 | 3.02 | 0.003 | ||
| Post > Pre | ns | ||||||||||
| Right SFA | Pre > Post | ns | |||||||||
| Post > Pre | ns | ||||||||||
Paired t-test for pre and post neurofeedback, examining amygdala complex functional connectivity as a follow up analysis from the ANOVA interaction, p-uncorrected < 0.005, k = 10. Asterisks indicates the a-priori region-of-interest analysis, p-FWE < 0.05, k = 10. Abbreviations: BA = Brodmann area, BLA = basolateral amygdala complex, CMA = centromedial amygdala complex, SFA = superficial amygdala complex, PAG = periaqueductal gray, mPFC = medial prefrontal cortex.
Fig. 2.
Clusters representing greater connectivity from amygdala complex seeds before neurofeedback intervention: a) left BLA to the PAG, b) left SFA to the left PAG, c) left SFA to the left hippocampus, as compared to after neurofeedback within PTSD patients. Non-significant differences were found with respect to the right BLA, right SFA, and bilateral CMA, when examining increased amygdala functional connectivity pre neurofeedback as compared to post neurofeedback. Inversely, we report increased amygdala complex connectivity after neurofeedback, as compared to before neurofeedback, d) right CMA to the mPFC. Here, non-significant differences were found when examining increased amygdala complex connectivity post neurofeedback, as compared to pre neurofeedback, for the left CMA, bilateral BLA, and bilateral SFA. The follow up comparison statistical threshold was p-uncorrected < 0.005, k = 10. *Indicates the a-priori region-of-interest analysis, in which p-FWE < 0.05, k = 10 was employed. X, Y, and Z indicate the position of brain slices displayed in MRIcron software. Abbreviations: R = right hemisphere, P = posterior, BLA = basolateral amygdala complex, CMA = centromedial amygdala complex, SFA = superficial amygdala complex, mPFC = medial prefrontal cortex, PAG = periaqueductal gray, FWE = familywise error protection rate.
2.4.2. Post neurofeedback
Inversely, when examining increased amygdala complex connectivity in the post-neurofeedback as compared to the pre-neurofeedback condition, we observed an increase in connectivity between the right CMA and the mPFC (see Table 3; Fig. 2).
2.5. Multiple regression analyses
2.5.1. CAPS total
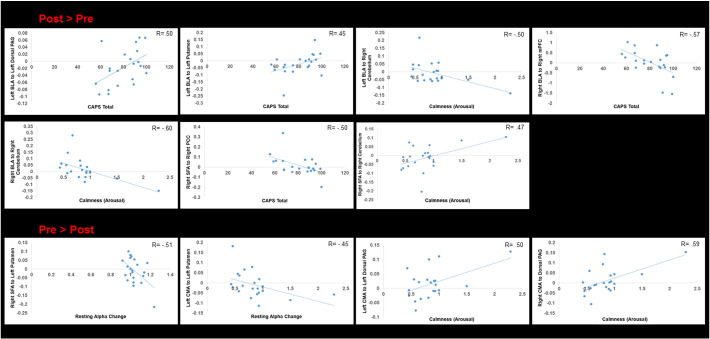
After neurofeedback, patient CAPS total scores were negatively correlated to connectivity between the right BLA and right mPFC, and the right SFA and right PCC. Furthermore, greater CAPS scores after neurofeedback were positively correlated to connectivity between the left BLA and the left dorsal PAG, and between the left BLA and the left putamen (see Table 4; Fig. 3).
Table 4.
Predictors of amygdala complex functional connectivity within PTSD patients.
| Amygdala seed & predictor | Contrast, correlation | Gyrus/sulcus (BA) | H | Cluster size | Beta value | MNI coordinate |
Z score | t(19) | p | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| x | y | z | |||||||||
| Left BLA | |||||||||||
| CAPS total | post > pre, positive | Dorsal PAG | L | 121 | − 0.002 | − 2 | − 22 | − 10 | 3.20 | 3.74 | < 0.001 |
| post > pre, positive | Putamen | L | 111 | − 0.002 | − 28 | − 18 | − 2 | 2.71 | 3.04 | < 0.005 | |
| Arousal | post > pre, positive | Cerebellum, anterior lobe culmen | R | 46 | − 0.105 | 2 | − 38 | − 24 | 2.66 | 2.98 | < 0.005 |
| Resting alpha change (rebound) | ns | ||||||||||
| Right BLA | |||||||||||
| CAPS total | post > pre, negative | *mPFC (10) | R | 47 | − 0.030 | 2 | 64 | 2 | 3.11 | 3.61 | < 0.005 |
| Arousal | post > pre, positive | Cerebellum, anterior lobe culmen | R | 96 | 0.149 | 6 | − 38 | − 30 | 3.16 | 3.69 | < 0.0001 |
| Resting alpha change (rebound) | ns | ||||||||||
| Left CMA | |||||||||||
| CAPS total | ns | ||||||||||
| Arousal | pre > post, negative | Dorsal PAG | L | 25 | − 0.067 | − 2 | − 24 | − 10 | 2.74 | 3.08 | < 0.005 |
| Resting alpha change (rebound) | pre > post, negative | Putamen | L | 117 | − 0.544 | − 34 | − 14 | − 8 | 2.54 | 2.82 | < 0.005 |
| Right CMA | |||||||||||
| CAPS total | ns | ||||||||||
| Arousal | pre > post, negative | Dorsal PAG | 27 | − 0.091 | 0 | − 24 | − 6 | 2.79 | 3.14 | < 0.005 | |
| Resting alpha change (rebound) | ns | ||||||||||
| Left SFA | |||||||||||
| CAPS total | ns | ||||||||||
| Arousal | ns | ||||||||||
| Resting alpha change (rebound) | ns | ||||||||||
| Right SFA | |||||||||||
| CAPS total | post > pre, negative | PCC (31) | R | 95 | − 0.004 | 10 | − 30 | 24 | 2.56 | 2.84 | < 0.005 |
| Arousal | post > pre, positive | Cerebellum, anterior lobe culmen | R | 106 | 0.176 | 2 | − 38 | − 28 | 2.80 | 3.16 | < 0.005 |
| Resting alpha change (rebound) | pre > post, negative | Putamen | L | 48 | − 0.545 | − 34 | − 18 | − 4 | 2.76 | 3.10 | < 0.005 |
CAPS total scores, arousal, and resting alpha change, evaluated as a predictors of amygdala complex connectivity changes pre and post neurofeedback, p-uncorrected < 0.005, k = 10. Asterisks indicates the a-priori region-of-interest analysis, p-FWE < 0.05, k = 10. Abbreviations: CAPS = Clinician Administered PTSD Scale, FWE = family wise error protection rate, PAG = periaqueductal gray, mPFC = medial prefrontal cortex, PCC = posterior cingulate cortex.
Fig. 3.
Representative scatter plots depicting amygdala complex connectivity regression results with calmness (arousal), the Clinician Administered PTSD Scale (CAPS total), and resting alpha change. The x-axis pertains to either calmness, CAPS total, or resting alpha change, while the y-axis indicates the beta value representing amygdala complex connectivity. The top portion illustrates greater connectivity post neurofeedback as compared to pre neurofeedback, while the lower portion illustrates greater connectivity pre neurofeedback as compared to post neurofeedback. Abbreviations: BLA = basolateral amygdala complex, CMA = centromedial amygdala complex, SFA = superficial amygdala complex, mPFC = medial prefrontal cortex, PAG = periaqueductal gray, PCC = posterior cingulate cortex.
2.5.2. Arousal
Before neurofeedback, arousal was positively correlated with connectivity between the bilateral CMA and the dorsal PAG. After neurofeedback, greater calmness (decreased arousal) was significantly associated with connectivity between the bilateral BLA and right SFA to the right anterior lobe cerebellar culmen (see Table 4; Fig. 3).
2.5.3. Resting alpha change
Relative changes in baseline alpha amplitude (resting alpha change), was negatively correlated to pre-neurofeedback connectivity between the left CMA and right SFA to the putamen (see Table 4; Fig. 3).
MDI averaged scores, and MDI depersonalization/derealization averages, in addition to training alpha change, did not significantly predict differences in pre and post neurofeedback amygdala complex connectivity. Additionally, we identified outliers in both arousal and resting alpha change scores for the regression analyses via Cook's Distance criteria of > 1, where distribution normality was also violated via Shapiro Wilks test. However, outliers and normality violations were resolved via a log 10 transformation of arousal and resting alpha change scores, where significant results remained throughout the analyses. Thus, our results proved to be outlier- independent, and statistical distribution assumptions were met.
3. Discussion
We previously demonstrated that large-scale functional brain networks implicated in PTSD could be plastically modified using EEG alpha rhythm desynchronization – which was associated with symptom alleviation and a “rebound” in alpha synchronization post neurofeedback (Kluetsch et al., 2014). Here we show that a 30-minute session of neurofeedback is also capable of shifting amygdala complex connectivity from “bottom-up” areas implicated in defensive, emotional, and fear processing/memory retrieval, to “top-down” prefrontal emotion regulation regions. Consistent with neurocognitive models of PTSD (Lanius et al., 2010), this shift in amygdala complex connectivity was positively associated with reduced hyperarousal among patients, together with a stronger “rebound” of alpha synchronization, and negatively correlated to PTSD symptom severity.
3.1. Therapeutic shift away from lower order regions
We report increased connectivity before neurofeedback from the left BLA and left SFA to the PAG, as compared to the post neurofeedback condition, which may reflect increased fear and defense circuit processing in PTSD patients before the intervention. The PAG is a midbrain structure that is implicated in pain perception, analgesia, anxiety, defense circuits, and fear processing/expectancy (Bandler et al., 2000, Johansen et al., 2010, Linnman et al., 2012, Merker, 2007). Moreover, the dorsolateral (dl)PAG, constituting current clusters, has been shown to be involved in “active” sympathetic nervous system threat defenses (Bandler et al., 2000, Lanius et al., 2014), fear learning during life threatening situations (Kincheski et al., 2012), and fight-or-flight responses, in which a critical amygdala-hypothalamus-PAG circuit has been delineated (Kozlowska et al., 2015). This suggests that the BLA and SFA amygdala complexes may be integrating emotional processes (Goossens et al., 2009, Heimer and Van Hoesen, 2006, Koelsch et al., 2013) from the PAG related to active defense mechanisms and fight-or-flight responses, reflecting hyperarousal of emotions characteristic of PTSD patients prior to neurofeedback. In support of this, Porges, 2009, Porges, 2007 has proposed a model of “neuroception”, which allows individuals to subcortically assess environmental risks and safety, involving both the amygdala and PAG. Thus, hypermonitoring of neuroception may also be attenuated after neurofeedback.
The PAG is a central convergence zone involved in all basic emotional circuits in humans (Panksepp, 2011). Interestingly, Damasio et al. (2000) has demonstrated that the PAG is involved in a broad spectrum of emotions, including fear and sadness, related to re-experiencing personal events. However, the PAG has largely been neglected in the PTSD and neuroimaging literature (Kincheski et al., 2012). Here, when examining post vs. pre neurofeedback connectivity, CAPS scores were positively correlated to connectivity between the left BLA- left PAG, and reductions in PTSD arousal were negatively correlated to bilateral CMA- PAG connectivity, suggesting that higher levels of PTSD symptoms and arousal are associated with stronger amygdala-PAG connectivity profiles remaining after neurofeedback.
In line with these findings, hyperactivation has been reported in the amygdala and PAG during PTSD symptom provocation (Pissiota et al., 2002), and Mobbs et al. (2009) show that as a perceived threat moves closer, neural activity shifts from the ventromedial PFC to the PAG, where PAG activation was associated with pain expectancy, dread and perceived inability to escape. This has strong implications for PTSD hyperarousal due to chronic activation of defensive cascades and stress responses, involving the PAG, which may be activated recurrently after trauma in a suboptimal way (Kozlowska et al., 2015) (i.e., trauma threats may always be perceived as proximal in PTSD patients). Hence it has been suggested that decreasing amygdala-PAG activity may be a potential treatment to decrease anxiety (Kozlowska et al., 2015).
In the current study we also report increased connectivity from the left SFA to the hippocampus, before neurofeedback as compared to after, which may represent an exacerbated connection between emotional processing and autobiographical memory within PTSD patients before neurofeedback intervention. The hippocampus is reciprocally linked to the amygdala, where both are involved in fear and anxiety circuits (Pitman et al., 2012, Ravindran and Stein, 2009), as well as autobiographical memory retrieval (Greenberg et al., 2005). This suggests increased fear/anxiety circuit processing in relation to autobiographical memory in PTSD patients before neurofeedback, where the SFA is associated with affective/social processing (Goossens et al., 2009, Heimer and Van Hoesen, 2006, Koelsch et al., 2013). Additionally, the hippocampus integrates contextual information into memories, gating amygdala emotional activity according to fear context (Sotres-Bayon et al., 2012), where it has been suggested that hippocampal contextual functioning may be altered in patients with PTSD (Shin and Liberzon, 2010). Hence, hippocampal dysfunction may reflect a failure to recognize safe contexts and aberrant memory function for neutral material (Pitman et al., 2012), paralleling hyperaroused emotional states within PTSD patients prior to intervention. On balance, aberrant hippocampal connectivity, and hyperactivation with the amygdala, have been associated with PTSD symptoms of re-experiencing, avoidance, and disorder severity (Sadeh et al., 2014, Shin et al., 2004, Sripada et al., 2013). This parallels our current findings as decreased PTSD symptoms were found to concatenate with decreased amygdala-hippocampal connectivity. Similarly, Cisler et al. (2014) report increased connectivity between the amygdala and hippocampus among PTSD patients during traumatic reminders as compared to neutral processing, where the authors suggest this reflects over-generalized fear conditioning after trauma. In sum, decreased connectivity with the amygdala post neurofeedback treatment suggests normalization of this neural network, and perhaps less emotional arousal triggered by autobiographical trauma related memories.
Lastly, when examining post vs. pre neurofeedback connectivity, CAPS scores were positively correlated to connectivity between the left BLA and the left putamen. The putamen has recently been identified as a key region in the “hate circuit” (Zeki and Romaya, 2008), and has been associated with sensorimotor and stimulus response coordination (Grahn et al., 2008), showing implications for hypervigilance and readiness for action in PTSD. Furthermore, the putamen has been shown to be active during threat processing related to pain (Butler et al., 2007) and during flashbacks of trauma in PTSD patients (Osuch et al., 2001). Similarly, increased alpha synchronization after desynchronizing neurofeedback (rebound), was negatively correlated with pre vs. post neurofeedback connectivity from the left CMA and right SFA to the putamen. Speculatively, putamen functions associated with threat processing, flashbacks, and hate circuitry, may be related to exacerbated PTSD symptoms pre neurofeedback, thereby negatively correlating to successful resting alpha change.
3.2. Therapeutic shift towards emotion regulation/modulation regions
In the current study, we report a shift in amygdala connectivity from regions involved in fear processing and fear memory, to ventromedial emotion regulation cortical areas (Etkin et al., 2011). Here, we observed increased right CMA amygdala connectivity to the medial PFC after neurofeedback, as compared to pre neurofeedback. This suggests top-down emotional regulation over the amygdala (Etkin et al., 2011), where specifically the CMA is involved in the execution of fear responses (Duvarci and Pare, 2014). Speculatively, this regulatory connection may underlie the alleviation of symptoms observed in PTSD patients after neurofeedback. In support of this mechanism, when examining post vs. pre neurofeedback connectivity, greater CAPS scores were negatively correlated to connectivity between the right BLA and right mPFC, where the BLA is involved in integrating cortical emotional processing (Duvarci and Pare, 2014). Notably, when examining 1-sample t-tests, all amygdala complexes displayed functional connectivity to the medial PFC after neurofeedback, in juxtaposition to only the left CMA complex showing connectivity to the medial PFC before neurofeedback.
Of importance, the medial PFC has direct anatomical projections to the amygdala and plays a central role in the “top-down regulation” of amygdala processing (Ghashghaei et al., 2007), the inhibition of negative affect (Banks et al., 2007, Phan et al., 2005), and amygdala activity during real-time fMRI neurofeedback emotion regulation (Paret et al., 2015, Zotev et al., 2013, Zotev et al., 2011). On balance, Banks et al. (2007) show that amygdala-dmPFC coupling is negatively correlated to negative affect, where inversely, hyperarousal has been associated with negative mPFC-amygdala coupling in PTSD patients (Sadeh et al., 2014). In line with the current findings, it is widely accepted that PTSD patients are characterized by failed top-down inhibition of emotion generation regions, such as the amygdala (Etkin and Wager, 2007, Lanius et al., 2010, Lanius et al., 2007, Pitman et al., 2012, Shin and Liberzon, 2010, Weston, 2014), where mPFC activation has been negatively correlated to PTSD symptom severity (Dickie et al., 2008, Hopper et al., 2007, Shin and Liberzon, 2010, Williams et al., 2006, Yin et al., 2011). Notably, increased mPFC activation has been reported when examining neural activity post treatment among PTSD patients (Peres et al., 2007, Ravindran and Stein, 2009, Seedat et al., 2004, Shin and Liberzon, 2010). Hence, increased mPFC-amygdala coupling in the current study may be related to increased cortical modulation of the amygdala and negative affect, showing implications for arousal attenuation mechanisms through neurofeedback.
Additionally, for post vs. pre neurofeedback connectivity, reduced arousal was positively correlated with connectivity from the bilateral BLA and right SFA to the right anterior lobe cerebellar culmen, and greater CAPS scores were negatively correlated to connectivity between the right SFA and right PCC. Here, the cerebellar culmen has been shown to be involved in executive functioning and emotional processing (Stoodley and Schmahmann, 2009), suggesting emotional modulation over amygdala complexes after neurofeedback concomitant with reduced arousal. Furthermore, the posterior cingulate cortex is involved in directed attention (Leech and Sharp, 2014), suggesting that focused attention related to the neurofeedback task may help to facilitate calmness (reduced arousal).
In the present study, we have demonstrated that the cortex of PTSD patients is sufficiently plastic such that 30-minutes of targeted volitional activity via neurofeedback is capable of reconfiguring amygdala complex connectivity. Other recent studies have reported functional connectivity changes as a result of neurofeedback (Hamilton et al., 2011, Kluetsch et al., 2014, Ros et al., 2013). Specifically, the average elapsed time of 20 min following neurofeedback in which we obtained our second fMRI scan, is superior to the 15 min cut-off used to substantiate LTP (long-term potentiation) from STP (short-term potentiation) -like brain plasticity (Schulz and Fitzgibbons, 1997). Hence, our experiment provides a temporally direct association between neurofeedback and plastic modulation of amygdala complex connectivity. Based on recent work, we expect that repeated sessions would be required to induce longer lasting effects that are stable for weeks/months (Engelbregt et al., 2016), which we hope to address with a randomized control trial currently underway in our laboratory.
4. Limitations
First, as our EEG protocol has been validated in a randomized, placebo-controlled study with healthy individuals (Ros et al., 2013), a sham-feedback region was not included in the current analysis. This was also done for ethical reasons as to mitigate feelings of frustration and failure, as well as to not attenuate benefits of neurofeedback treatment in the future (Van Boxtel et al., 2012). Although, the objective of the current study was to investigate subcortical mechanisms related to behavioural and alpha oscillatory changes in patients. Here, the reported results can be viewed as more dependent, “cause and effect” relationships, as neurofeedback mitigates visual stimuli dependent factors in experimental designs, leading to greater intrinsic effects, produces the same reward contingencies across participants, and has variability in terms of success between participants (Ros et al., 2014, Ros et al., 2013). Hence neurofeedback represents an elegant way to “clamp the external milieu”, in order to delineate causal relationships (Ros et al., 2014), as participants' entrained neuronal differences may be considered as resulting minimally from external factors and can instead be regarded as being driven by the modulation of intrinsic, stimulus-independent brain states. As a future direction, studies should include a psychiatric control group with disorders other than PTSD, and separately examine the dissociative subtype of PTSD. Moreover, subsequent experiments should employ simultaneous EEG/fMRI recordings during resting state and neurofeedback, and examine the effects of general relaxation or focused attention. Our sample consisted of a large portion of patients on medication (although this did not appear to affect our results when conducting regression analyses). Effects of medication on neurofeedback should be more directly examined. Finally, effects of repeated administration of EEG neurofeedback should be investigated and studied longitudinally.
5. Conclusions
In summary, we show that after a 30-minute session of alpha amplitude reduction via EEG neurofeedback, amygdala complex connectivity concomitantly shifts from areas implicated in defensive, emotional, and fear processing/memory retrieval, to prefrontal emotion regulation regions. This shift in amygdala complex connectivity was positively associated with reduced arousal among PTSD patients and more alpha “rebound”, and negatively correlated to PTSD symptom severity. These results have significant implications for developing non-invasive interventions that target alpha oscillations, and provides evidence of neuronal reconfiguration after neurofeedback between areas highly implicated in PTSD. This therapeutic shift from lower order regions to emotion regulation regions further suggests that future PTSD studies should examine the entire neural axis, devoting more attention to lower order brain regions, such as the PAG, given their central role in emotional processing.
The following is the supplementary data related to this article.
1 sample amygdala complex resting-state functional connectivity for pre and post neurofeedback.
Funding and disclosure
This research was supported by funding from Lawson Health Research Institute and from the Canadian Institute for Military and Veteran Health Research. The authors declare no conflicts of interest.
References
- APA . American Journal of Psychiatry. fifth ed. American Psychiatric Publishing; Washington DC: Arlington, VA: 2013. Diagnostic and statistical manual of mental disorders. [Google Scholar]
- Baas D., Aleman A., Kahn R.S. Lateralization of amygdala activation: a systematic review of functional neuroimaging studies. Brain Res. Rev. 2004;45:96–103. doi: 10.1016/j.brainresrev.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Bandler R., Keay K.a., Floyd N., Price J. Central circuits mediating patterned autonomic activity during active vs. passive emotional coping. Brain Res. Bull. 2000;53:95–104. doi: 10.1016/s0361-9230(00)00313-0. [DOI] [PubMed] [Google Scholar]
- Banks S.J., Eddy K.T., Angstadt M., Nathan P.J., Luan Phan K. Amygdala-frontal connectivity during emotion regulation. Soc. Cogn. Affect. Neurosci. 2007;2:303–312. doi: 10.1093/scan/nsm029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein D.P., Stein J.A., Newcomb M.D., Walker E., Pogge D., Ahluvalia T., Stokes J., Handelsman L., Medrano M., Desmond D., Zule W. Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abus. Negl. 2003;27:169–190. doi: 10.1016/s0145-2134(02)00541-0. [DOI] [PubMed] [Google Scholar]
- Birn R.M., Patriat R., Phillips M.L., Germain A., Herringa R.J. Childhood maltreatment and combat posttraumatic stress differentially predict fear-related fronto-subcortical connectivity. Depress. Anxiety. 2014;31:880–892. doi: 10.1002/da.22291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake D.D., Weathers F.W., Nagy L.M., Kaloupek D.G., Gusman F.D., Charney D.S., Keane T.M. The development of a Clinician-Administered PTSD Scale. J. Trauma. Stress. 1995;8:75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- Bluhm R.L., Williamson P.C., Osuch E.a., Frewen P.a., Stevens T.K., Boksman K., Neufeld R.W.J., Théberge J., Ra Lanius. Alterations in default network connectivity in posttraumatic stress disorder related to early-life trauma. J. Psychiatry Neurosci. 2009;34:187–194. [PMC free article] [PubMed] [Google Scholar]
- Briere J. Psychol. Assess. Resour; Odessa, Florida: 2002. Multiscale Dissociation Inventory Professional Manual. [Google Scholar]
- Brown V.M., LaBar K.S., Haswell C.C., Gold A.L., Beall S.K., Van Voorhees E., Marx C.E., Calhoun P.S., Fairbank J.a., Green K.T., Tupler L.a., Weiner R.D., Beckham J.C., Brancu M., Hoerle J.M., Pender M., Kudler H., Swinkels C.M., Nieuwsma J.a., Runnals J.J., Youssef N.a., McDonald S.D., Davison R., Yoash-Gantz R., Taber K.H., Hurley R., McCarthy G., Morey R.a. Altered resting-state functional connectivity of basolateral and centromedial amygdala complexes in posttraumatic stress disorder. Neuropsychopharmacology. 2013;39:361–369. doi: 10.1038/npp.2013.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce S.E., Buchholz K.R., Brown W.J., Yan L., Durbin A., Sheline Y.I. Altered emotional interference processing in the amygdala and insula in women with post-traumatic stress disorder. NeuroImage Clin. 2013;2:43–49. doi: 10.1016/j.nicl.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant R.A., Kemp A.H., Felmingham K.L., Liddell B., Olivieri G., Peduto A., Gordon E., Williams L.M. Enhanced amygdala and medial prefrontal activation during nonconscious processing of fear in posttraumatic stress disorder: an fMRI study. Hum. Brain Mapp. 2008;29:517–523. doi: 10.1002/hbm.20415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler T., Pan H., Tuescher O., Engelien A., Goldstein M., Epstein J., Weisholtz D., Root J.C., Protopopescu X., Cunningham-Bussel a.C., Chang L., Xie X.-H., Chen Q., Phelps E.a., Ledoux J.E., Stern E., Silbersweig D.a. Human fear-related motor neurocircuitry. Neuroscience. 2007;150:1–7. doi: 10.1016/j.neuroscience.2007.09.048. [DOI] [PubMed] [Google Scholar]
- Cisler J.M., Steele J.S., Lenow J.K., Smitherman S., Everett B., Messias E., Kilts C.D. Functional reorganization of neural networks during repeated exposure to the traumatic memory in posttraumatic stress disorder: an exploratory fMRI study. J. Psychiatr. Res. 2014;48:47–55. doi: 10.1016/j.jpsychires.2013.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damasio A.R., Grabowski T.J., Bechara A., Damasio H., Ponto L., Parvizi J., Hichwa R. Subcortical and cortical brain activity during the feeling of self-generated emotions. Nat. Neurosci. 2000;3:1049–1056. doi: 10.1038/79871. [DOI] [PubMed] [Google Scholar]
- Daniels J.K., Mcfarlane A.C., Bluhm R.L., Moores K.A., Clark C.R., Shaw M.E., Williamson P.C., Densmore M., Lanius R.A. Switching between executive and default mode networks in posttraumatic stress disorder: alterations in functional connectivity. 2010;35:258–267. doi: 10.1503/jpn.090175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickie E.W., Brunet A., Akerib V., Armony J.L. An fMRI investigation of memory encoding in PTSD: influence of symptom severity. Neuropsychologia. 2008;46:1522–1531. doi: 10.1016/j.neuropsychologia.2008.01.007. [DOI] [PubMed] [Google Scholar]
- Duvarci S., Pare D. Amygdala microcircuits controlling learned fear. Neuron. 2014;82:966–980. doi: 10.1016/j.neuron.2014.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff S.B., Stephan K.E., Mohlberg H., Grefkes C., Fink G.R., Amunts K. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. NeuroImage. 2005;25:1325–1335. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Engelbregt H.J., Keeser D., van Eijk L., Suiker E.M., Eichhorn D., Karch S., Deijen J.B., Pogarell O. Short and long-term effects of sham-controlled prefrontal EEG-neurofeedback training in healthy subjects. Clin. Neurophysiol. 2016 doi: 10.1016/j.clinph.2016.01.004. [DOI] [PubMed] [Google Scholar]
- Etkin A., Wager T.D. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am. J. Psychiatry. 2007;164:1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A., Egner T., Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn. Sci. 2011;15:85–93. doi: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M.B., Spitzer R.L., Gibbon M., Williams J.B.W. Biometrics Research, New York State; New York: 2002. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Patient Edition (SCID-I/P, 11/2002 revision). for DSMIV. [Google Scholar]
- Fonzo G.a., Simmons A.N., Thorp S.R., Norman S.B., Paulus M.P., Stein M.B. Exaggerated and disconnected insular-amygdalar blood oxygenation level-dependent response to threat-related emotional faces in women with intimate-partner violence posttraumatic stress disorder. Biol. Psychiatry. 2010;68:433–441. doi: 10.1016/j.biopsych.2010.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frewen P.a., Dozois D.J.a., Neufeld R.W.J., Densmore M., Stevens T.K., Lanius R.a. Neuroimaging social emotional processing in women: fMRI study of script-driven imagery. Soc. Cogn. Affect. Neurosci. 2011;6:375–392. doi: 10.1093/scan/nsq047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghashghaei H.T., Hilgetag C.C., Barbas H. Sequence of information processing for emotions based on the anatomic dialogue between prefrontal cortex and amygdala. NeuroImage. 2007;34:905–923. doi: 10.1016/j.neuroimage.2006.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goossens L., Kukolja J., Onur O.a., Fink G.R., Maier W., Griez E., Schruers K., Hurlemann R. Selective processing of social stimuli in the superficial amygdala. Hum. Brain Mapp. 2009;30:3332–3338. doi: 10.1002/hbm.20755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grahn J.a., Parkinson J.a., Owen A.M. The cognitive functions of the caudate nucleus. Prog. Neurobiol. 2008;86:141–155. doi: 10.1016/j.pneurobio.2008.09.004. [DOI] [PubMed] [Google Scholar]
- Greenberg D.L., Rice H.J., Cooper J.J., Cabeza R., Rubin D.C., LaBar K.S. Co-activation of the amygdala, hippocampus and inferior frontal gyrus during autobiographical memory retrieval. Neuropsychologia. 2005;43:659–674. doi: 10.1016/j.neuropsychologia.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Hamilton J.P., Glover G.H., Hsu J.-J., Johnson R.F., Gotlib I.H. Modulation of subgenual anterior cingulate cortex activity with real-time neurofeedback. Hum. Brain Mapp. 2011;32(1):22–31. doi: 10.1002/hbm.20997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes J.P., Hayes S.M., Mikedis A.M. Quantitative meta-analysis of neural activity in posttraumatic stress disorder. Biol. Mood Anxiety Disord. 2012;2:9. doi: 10.1186/2045-5380-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimer L., Van Hoesen G.W. The limbic lobe and its output channels: implications for emotional functions and adaptive behavior. Neurosci. Biobehav. Rev. 2006;30:126–147. doi: 10.1016/j.neubiorev.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Hopper J.W., Frewen P.a., Sack M., Lanius R.a., Van Der Kolk B.a. The responses to script-driven imagery scale (RSDI): assessment of state posttraumatic symptoms for psychobiological and treatment research. J. Psychopathol. Behav. Assess. 2007;29:249–268. [Google Scholar]
- Huang M.-X., Yurgil K.a., Robb A., Angeles A., Diwakar M., Risbrough V.B., Nichols S.L., McLay R., Theilmann R.J., Song T., Huang C.W., Lee R.R., Baker D.G. Voxel-wise resting-state MEG source magnitude imaging study reveals neurocircuitry abnormality in active-duty service members and veterans with PTSD. NeuroImage Clin. 2014;5:408–419. doi: 10.1016/j.nicl.2014.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jann K., Dierks T., Boesch C., Kottlow M., Strik W., Koenig T. BOLD correlates of EEG alpha phase-locking and the fMRI default mode network. NeuroImage. 2009;45:903–916. doi: 10.1016/j.neuroimage.2009.01.001. [DOI] [PubMed] [Google Scholar]
- Johansen J.P., Tarpley J.W., LeDoux J.E., Blair H.T. Neural substrates for expectation-modulated fear learning in the amygdala and periaqueductal gray. Nat. Neurosci. 2010;13:979–986. doi: 10.1038/nn.2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kincheski G.C., Mota-Ortiz S.R., Pavesi E., Canteras N.S., Carobrez A.P. The dorsolateral periaqueductal gray and its role in mediating fear learning to life threatening events. PLoS One. 2012;7 doi: 10.1371/journal.pone.0050361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluetsch R.C., Ros T., Théberge J., Frewen P.a., Calhoun V.D., Schmahl C., Jetly R., Lanius R.a. Plastic modulation of PTSD resting-state networks and subjective wellbeing by EEG neurofeedback. Acta Psychiatr. Scand. 2014;130:123–136. doi: 10.1111/acps.12229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koelsch S., Skouras S., Fritz T., Herrera P., Bonhage C., Küssner M.B., Jacobs A.M. The roles of superficial amygdala and auditory cortex in music-evoked fear and joy. NeuroImage. 2013;81:49–60. doi: 10.1016/j.neuroimage.2013.05.008. [DOI] [PubMed] [Google Scholar]
- Kozlowska K., Walker P., McLean L., Carrive P. Fear and the defense Cascade. Harv. Rev. Psychiatry. 2015;5:1. doi: 10.1097/HRP.0000000000000065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegeskorte N., Simmons K.W., Bellgowan P.S.F., Baker C. Circular analysis in systems neuroscience: the dangers of double dipping. Nat. Neurosci. 2009;535 doi: 10.1038/nn.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanius R.a., Frewen P.a., Girotti M., Neufeld R.W.J., Stevens T.K., Densmore M. Neural correlates of trauma script-imagery in posttraumatic stress disorder with and without comorbid major depression: a functional MRI investigation. Psychiatry Res. Neuroimaging. 2007;155:45–56. doi: 10.1016/j.pscychresns.2006.11.006. [DOI] [PubMed] [Google Scholar]
- Lanius R.a., Vermetten E., Loewenstein R.J., Brand B., Christian S., Bremner J.D., Spiegel D. Emotion modulation in PTSD: clinical and neurobiological evidence for a dissociative subtype. Am. J. Psychiatry. 2010;167:640–647. doi: 10.1176/appi.ajp.2009.09081168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanius U.F., Paulsen S., Corrigan F.M. Springer Publishing Company; New York: 2014. Neurobiology and Treatment of Traumatic Dissociation: Towards an Embodied Self. [Google Scholar]
- Lanius R.A., Frewen P.A., Tursich M., Jetly R., Mckinnon M.C. 2015. Restoring Large-Scale Brain Networks in PTSD and Related Disorders: A Proposal for Neuroscientifically-Informed Treatment Interventions 1, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laufs H., Kleinschmidt a., Beyerle a., Eger E., Salek-Haddadi a., Preibisch C., Krakow K. EEG-correlated fMRI of human alpha activity. NeuroImage. 2003;19:1463–1476. doi: 10.1016/s1053-8119(03)00286-6. [DOI] [PubMed] [Google Scholar]
- LeDoux J. Fear and the brain: where have We been, and where are we going? Biol. Psychiatry. 1998;44:1229–1238. doi: 10.1016/s0006-3223(98)00282-0. [DOI] [PubMed] [Google Scholar]
- Leech R., Sharp D.J. The role of the posterior cingulate cortex in cognition and disease. Brain. 2014;137:12–32. doi: 10.1093/brain/awt162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman M.D., Cunningham W.A. Type I and Type II error concerns in fMRI research: re-balancing the scale. Soc. Cogn. Affect. Neurosci. 2009;4:423–428. doi: 10.1093/scan/nsp052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnman C., Moulton E.a., Barmettler G., Becerra L., Borsook D. Neuroimaging of the periaqueductal gray: state of the field. NeuroImage. 2012;60:505–522. doi: 10.1016/j.neuroimage.2011.11.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantini D., Perrucci M.G., Del Gratta C., Romani G.L., Corbetta M. Electrophysiological signatures of resting state networks in the human brain. Proc. Natl. Acad. Sci. U. S. A. 2007;104:13170–13175. doi: 10.1073/pnas.0700668104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merker B. Consciousness without a cerebral cortex: a challenge for neuroscience and medicine. Behav. Brain Sci. 2007;30:63–81. doi: 10.1017/S0140525X07000891. [DOI] [PubMed] [Google Scholar]
- Mobbs D., Petrovic P., Marchant J.L., Hassabis D., Weiskopf N., Seymour B., Dolan R.J., Frith C.D. Vol. 317. 2009. Europe PMC Funders Group When Fear Is Near; pp. 1079–1083. [Google Scholar]
- Nicholson A., Densmore M., Frewen P.A., Théberge J., Neufeld R.W.J., McKinnon M.C., Lanius The dissociative subtype of posttraumatic stress disorder: unique resting-state functional connectivity of basolateral and centromedial amygdala complexes. Neuropsychopharmacology. 2015 doi: 10.1038/npp.2015.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez P.L., Wingeier B.M., Silberstein R.B. Spatial-temporal structures of human alpha rhythms: theory, microcurrent sources, multiscale measurements, and global binding of local networks. Hum. Brain Mapp. 2001;13:125–164. doi: 10.1002/hbm.1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osuch E.a., Benson B., Geraci M., Podell D., Herscovitch P., McCann U.D., Post R.M. Regional cerebral blood flow correlated with flashback intensity in patients with posttraumatic stress disorder. Biol. Psychiatry. 2001;50:246–253. doi: 10.1016/s0006-3223(01)01107-6. [DOI] [PubMed] [Google Scholar]
- Panksepp J. The basic emotional circuits of mammalian brains: do animals have affective lives? Neurosci. Biobehav. Rev. 2011;35:1791–1804. doi: 10.1016/j.neubiorev.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Panksepp J., Biven L. first ed. W.W. Norton & Co.; New York: 2012. The Archaeology of Mind: Neuroevolutionary Origins of Human Emotions. [Google Scholar]
- Paret C., Ruf M., Fungisai Gerchen M., Kluetsch R., Demirakca T., Jungkunz M., Bertsch K., Schmahl C., Ende G. fMRI neurofeedback of amygdala response to aversive stimuli enhances prefrontal-limbic brain connectivity. NeuroImage. 2015;125:182–188. doi: 10.1016/j.neuroimage.2015.10.027. [DOI] [PubMed] [Google Scholar]
- Patel R., Spreng R.N., Shin L.M., Girard T.a. Neurocircuitry models of posttraumatic stress disorder and beyond: a meta-analysis of functional neuroimaging studies. Neurosci. Biobehav. Rev. 2012;36:2130–2142. doi: 10.1016/j.neubiorev.2012.06.003. [DOI] [PubMed] [Google Scholar]
- Peres J.F.P., Newberg A.B., Mercante J.P., Simão M., Albuquerque V.E., Peres M.J.P., Nasello A.G. Cerebral blood flow changes during retrieval of traumatic memories before and after psychotherapy: a SPECT study. Psychol. Med. 2007;37:1481–1491. doi: 10.1017/S003329170700997X. [DOI] [PubMed] [Google Scholar]
- Phan K.L., Fitzgerald D.a., Nathan P.J., Moore G.J., Uhde T.W., Tancer M.E. Neural substrates for voluntary suppression of negative affect: a functional magnetic resonance imaging study. Biol. Psychiatry. 2005;57:210–219. doi: 10.1016/j.biopsych.2004.10.030. [DOI] [PubMed] [Google Scholar]
- Phelps E.a., LeDoux J.E. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron. 2005;48:175–187. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Pissiota A., Frans Ö., Fernandez M., Von Knorring L., Fischer H., Fredrikson M. Neurofunctional correlates of posttraumatic stress disorder: a PET symptom provocation study. Eur. Arch. Psychiatry Clin. Neurosci. 2002;252:68–75. doi: 10.1007/s004060200014. [DOI] [PubMed] [Google Scholar]
- Pitman R.K., Rasmusson A.M., Koenen K.C., Shin L.M., Orr S.P., Gilbertson M.W., Milad M.R., Liberzon I. Biological studies of post-traumatic stress disorder. Nat. Rev. Neurosci. 2012;13:769–787. doi: 10.1038/nrn3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porges S.W. The polyvagal perspective. Biol. Psychol. 2007;74:116–143. doi: 10.1016/j.biopsycho.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porges S.W. The polyvagal theory: new insights into adaptive reactions of the autonomic nervous system. Cleve. Clin. J. Med. 2009;76:S86–S90. doi: 10.3949/ccjm.76.s2.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabellino D., Tursich M., Pa F., Jk D., Densmore M. 2015. Intrinsic Connectivity Networks in post-traumatic stress disorder during sub- and supraliminal processing of threat-related stimuli; pp. 1–14. [DOI] [PubMed] [Google Scholar]
- Rabinak C.A., Angstadt M., Welsh R.C., Kenndy A.E., Lyubkin M., Martis B., Luan Phan K. Altered amygdala resting-state functional connectivity in post-traumatic stress disorder. Front. Psychiatry. 2011:2. doi: 10.3389/fpsyt.2011.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravindran L.N., Stein M.B. Pharmacotherapy of PTSD: premises, principles, and priorities. Brain Res. 2009;1293:24–39. doi: 10.1016/j.brainres.2009.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ros T., Théberge J., Frewen P.a., Kluetsch R., Densmore M., Calhoun V.D., Lanius R.a. Mind over chatter: plastic up-regulation of the fMRI salience network directly after EEG neurofeedback. NeuroImage. 2013;65:324–335. doi: 10.1016/j.neuroimage.2012.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ros T., Baars J., Lanius R.a., Vuilleumier P. Tuning pathological brain oscillations with neurofeedback: a systems neuroscience framework. Front. Hum. Neurosci. 2014;8:1–22. doi: 10.3389/fnhum.2014.01008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ros T., Frewen P., Theberge J., Kluetsch R., Mueller A., Candrian G., Jetly R., Vuilleumier P., Lanius R. Neurofeedback Tunes Long-Range Temporal Correlations in Spontaneous Brain Activity. 2016. http://arxiv.org/abs/1512.09133 [DOI] [PubMed]
- Roy A.K., Shehzad Z., Margulies D.S., Kelly a.M.C., Uddin L.Q., Gotimer K., Biswal B.B., Castellanos F.X., Milham M.P. Functional connectivity of the human amygdala using resting state fMRI. NeuroImage. 2009;45:614–626. doi: 10.1016/j.neuroimage.2008.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadaghiani S., Scheeringa R., Lehongre K., Morillon B., Giraud A.-L., Kleinschmidt A. Intrinsic connectivity networks, alpha oscillations, and tonic alertness: a simultaneous electroencephalography/functional magnetic resonance imaging study. J. Neurosci. 2010;30:10243–10250. doi: 10.1523/JNEUROSCI.1004-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeh N., Spielberg J.M., Warren S.L., Miller G.a., Heller W. Aberrant neural connectivity during emotional processing associated with posttraumatic stress. Clin. Psychol. Sci. 2014 doi: 10.1177/2167702614530113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz P.E., Fitzgibbons J.C. Differing mechanisms of expression for short- and long-term potentiation. J. Neurophysiol. 1997;78(1):321–334. doi: 10.1152/jn.1997.78.1.321. [DOI] [PubMed] [Google Scholar]
- Seedat S., Warwick J., Van Heerden B., Hugo C., Zungu-Dirwayi N., Van Kradenburg J., Stein D.J. Single photon emission computed tomography in posttraumatic stress disorder before and after treatment with a selective serotonin reuptake inhibitor. J. Affect. Disord. 2004;80:45–53. doi: 10.1016/S0165-0327(03)00047-8. [DOI] [PubMed] [Google Scholar]
- Sergerie K., Chochol C., Armony J.L. The role of the amygdala in emotional processing: a quantitative meta-analysis of functional neuroimaging studies. Neurosci. Biobehav. Rev. 2008;32:811–830. doi: 10.1016/j.neubiorev.2007.12.002. [DOI] [PubMed] [Google Scholar]
- Shang J., Lui S., Meng Y., Zhu H., Qiu C., Gong Q., Liao W., Zhang W. Alterations in Low-Level Perceptual Networks Related to Clinical Severity in PTSD after an Earthquake: A Resting-State fMRI Study. PLoS One. 2014;9:e96834. doi: 10.1371/journal.pone.0096834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin L.M., Liberzon I. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology. 2010;35:169–191. doi: 10.1038/npp.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin L.M., Shin P.S., Heckers S., Krangel T.S., Macklin M.L., Orr S.P., Lasko N., Segal E., Makris N., Richert K., Levering J., Schacter D.L., Alpert N.M., Fischman A.J., Pitman R.K., Rauch S.L. Vol. 300. 2004. Hippocampal Function in Posttraumatic Stress Disorder; pp. 292–300. [DOI] [PubMed] [Google Scholar]
- Sotres-Bayon F., Sierra-Mercado D., Pardilla-Delgado E., Quirk G.J. Gating of fear in prelimbic cortex by hippocampal and amygdala inputs. Neuron. 2012;76:804–812. doi: 10.1016/j.neuron.2012.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger C.D. Consulting Psychologists; Palo Alto, CA: 1983. Manual for the State-Trait Anxiety Inventory; p. 1983. [Google Scholar]
- Sripada R.K., King A.P., Garfinkel S.N., Wang X., Sripada C.S., Welsh R.C., Liberzon I. Altered resting-state amygdala functional connectivity in men with posttraumatic stress disorder. J. Psychiatry Neurosci. 2012;37:241–249. doi: 10.1503/jpn.110069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripada R.K., Garfinkel S.N., Liberzon I. Avoidant symptoms in PTSD predict fear circuit activation during multimodal fear extinction. Front. Hum. Neurosci. 2013;7:672. doi: 10.3389/fnhum.2013.00672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens J.S., Jovanovic T., Fani N., Ely T.D., Glover E.M., Bradley B., Ressler K.J. Disrupted amygdala-prefrontal functional connectivity in civilian women with posttraumatic stress disorder. J. Psychiatr. Res. 2013;47:1469–1478. doi: 10.1016/j.jpsychires.2013.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoodley C.J., Schmahmann J.D. Functional topography in the human cerebellum: a meta-analysis of neuroimaging studies. NeuroImage. 2009;44:489–501. doi: 10.1016/j.neuroimage.2008.08.039. [DOI] [PubMed] [Google Scholar]
- Thayer R.E. Activation-deactivation adjective check list: current overview and structural analysis. Psychol. Rep. 1986;58:607–614. [Google Scholar]
- Van Boxtel G.J.M., Denissen A.J.M., Jäger M., Vernon D., Dekker M.K.J., Mihajlović V., Sitskoorn M.M. A novel self-guided approach to alpha activity training. Int. J. Psychophysiol. 2012;83:282–294. doi: 10.1016/j.ijpsycho.2011.11.004. [DOI] [PubMed] [Google Scholar]
- Vul E., Pashler H. Voodoo and circularity errors. NeuroImage. 2012;62(2):945–948. doi: 10.1016/j.neuroimage.2012.01.027. [DOI] [PubMed] [Google Scholar]
- Weston C.S.E. Posttraumatic stress disorder: a theoretical model of the hyperarousal subtype. Front. Psychiatry. 2014;5:1–20. doi: 10.3389/fpsyt.2014.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams L.M., Kemp A.H., Felmingham K., Barton M., Olivieri G., Peduto A., Gordon E., Bryant R.a. Trauma modulates amygdala and medial prefrontal responses to consciously attended fear. NeuroImage. 2006;29:347–357. doi: 10.1016/j.neuroimage.2005.03.047. [DOI] [PubMed] [Google Scholar]
- Yehuda R., Hoge C.W., McFarlane A.C., Vermetten E., Lanius R.A., Nievergelt C.M.…Hyman S.E. Post-traumatic stress disorder. Nat. Rev. Dis. Primers. 2015;1:1–22. doi: 10.1038/nrdp.2015.57. ( http://doi.org/10.1038/nrdp.2015.57) [DOI] [PubMed] [Google Scholar]
- Yin Y., Jin C., Hu X., Duan L., Li Z., Song M., Chen H., Feng B., Jiang T., Jin H., Wong C., Gong Q., Li L. Altered resting-state functional connectivity of thalamus in earthquake-induced posttraumatic stress disorder: a functional magnetic resonance imaging study. Brain Res. 2011;1411:98–107. doi: 10.1016/j.brainres.2011.07.016. [DOI] [PubMed] [Google Scholar]
- Zeki S., Romaya J.P. Neural correlates of hate. PLoS One. 2008;3 doi: 10.1371/journal.pone.0003556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zotev V., Krueger F., Phillips R., Alvarez R.P., Simmons W.K., Bellgowan P., Drevets W.C., Bodurka J. Self-regulation of amygdala activation using real-time FMRI neurofeedback. PLoS One. 2011;6 doi: 10.1371/journal.pone.0024522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zotev V., Phillips R., Young K.D., Drevets W.C., Bodurka J. Prefrontal control of the amygdala during real-time fMRI neurofeedback training of emotion regulation. PLoS One. 2013;8 doi: 10.1371/journal.pone.0079184. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
1 sample amygdala complex resting-state functional connectivity for pre and post neurofeedback.