ABSTRACT
Bacterial antibiotic efflux pumps are key players in antibiotic resistance. Although their role in conferring multidrug resistance is well documented, the emergence of “super” efflux pump variants that enhance bacterial resistance to multiple drugs has not been reported. Here, we describe the emergence of a resistance-enhancing variant (named RE-CmeABC) of the predominant efflux pump CmeABC in Campylobacter, a major zoonotic pathogen whose resistance to antibiotics is considered a serious antibiotic resistance threat in the United States. Compared to the previously characterized CmeABC transporters, RE-CmeABC is much more potent in conferring Campylobacter resistance to antibiotics, which was shown by increased MICs and reduced intracellular accumulation of antibiotics. Structural modeling suggests that sequence variations in the drug-binding pocket of CmeB possibly contribute to the enhanced efflux function. Additionally, RE-CmeABC expands the mutant selection window of ciprofloxacin, enhances the emergence of antibiotic-resistant mutants, and confers exceedingly high-level resistance to fluoroquinolones, an important class of antibiotics for clinical therapy of campylobacteriosis. Furthermore, RE-CmeABC is horizontally transferable, shifts antibiotic MIC distribution among clinical isolates, and is increasingly prevalent in Campylobacter jejuni isolates, suggesting that it confers a fitness advantage under antimicrobial selection. These findings reveal a new mechanism for enhanced multidrug resistance and an effective strategy utilized by bacteria for adaptation to selection from multiple antibiotics.
IMPORTANCE
Bacterial antibiotic efflux pumps are ubiquitously present in bacterial organisms and protect bacteria from the antibacterial effects of antimicrobials and other toxic compounds by extruding them out of cells. Thus, these efflux transporters represent an important mechanism for antibiotic resistance. In this study, we discovered the emergence and increasing prevalence of a unique efflux pump variant that is much more powerful in the efflux of antibiotics and confers multidrug resistance in Campylobacter, which is a major foodborne pathogen transmitted to humans via the food chain. Unlike other specific resistance determinants that only allow bacteria to resist a particular antimicrobial, the acquisition of a functionally enhanced efflux pump will empower bacteria with simultaneous resistance to multiple classes of antibiotics. These findings reveal a previously undescribed mechanism for enhanced multidrug resistance and open a new direction for us to understand how bacteria adapt to antibiotic treatment.
INTRODUCTION
As evidenced by the recent emergence and spread of bacteria resistant to the last-resort antibiotics (e.g., carbapenems and colistin), the increasing prevalence of antibiotic-resistant pathogens has become a public health crisis (1–4). Different from other antibiotic resistance mechanisms, bacterial multidrug efflux pumps confer resistance to structurally diverse antimicrobials. Among various types of efflux transporters, members of the resistance-nodulation-cell division (RND) family are the most important in mediating antibiotic resistance in Gram-negative bacteria (5, 6). Typically, an RND efflux transporter is an inner membrane protein. It interacts with a periplasmic fusion protein and an outer membrane channel protein to assemble as a tripartite efflux complex that spans the entire cell envelope and extrudes antimicrobials directly out of bacterial cells (6). These RND efflux transporters are commonly controlled by regulatory factors, and overexpression of these efflux transporters is typically required for mediating clinically relevant levels of antibiotic resistance (5–10). Recently, Blair et al. reported that a single amino acid substitution in the drug-binding pocket of the AcrB transporter enhanced the function of the efflux pump and conferred clinically relevant resistance in Salmonella (11), suggesting an overexpression-independent mechanism for enhancing the function of a multidrug-resistant transporter.
Thermophilic Campylobacter species, including Campylobacter jejuni and Campylobacter coli, are major foodborne pathogens and are among the most frequent causes of bacterial gastroenteritis in humans, accounting for 400 million to 500 million cases of diarrhea each year worldwide (12). Transmission of Campylobacter bacteria to humans occurs mainly via the foodborne route, and contaminated poultry meat, water, and milk are the main sources of infection (13, 14). Because of the use of antibiotics in animal agriculture and medical settings, antibiotic-resistant Campylobacter is increasingly prevalent worldwide (15, 16). Because of its rising prevalence and impact on public health, antibiotic-resistant Campylobacter has been listed by the Centers for Disease Control and Prevention as a serious antibiotic resistance threat in the United States (17). In Campylobacter bacteria, the tripartite multidrug efflux pump CmeABC is the predominant efflux system and plays a key role in mediating resistance to structurally diverse antimicrobials (18). CmeABC constitutes the CmeB inner membrane transporter, belonging to the RND protein family; the CmeA periplasmic protein, a member of the membrane fusion protein family; and the CmeC outer membrane channel protein. This powerful efflux system is regulated primarily by CmeR and secondarily by CosR, both of which bind to the promoter region of the cmeABC operon and function as transcriptional repressors for this efflux system (19, 20). Notably, CmeABC is important for both intrinsic and acquired resistance to antibiotics, but overexpression of cmeABC confers only a modest increase in the MICs of antibiotics compared with the base-level expression of the efflux pump (19, 21).
In this study, we report the identification of a potent variant of CmeABC that shows an enhanced efflux function. We show that this variant CmeABC is highly potent against multiple antibiotics, promotes the emergence of antibiotic-resistant mutants, and confers exceedingly high-level resistance to fluoroquinolones. Additionally, we discovered that the cmeABC variant is increasingly prevalent in C. jejuni isolates, indicating that it confers on Campylobacter a fitness advantage under antibiotic selection pressure. To the best of our knowledge, this is the first report on the emergence of a “super” efflux pump variant that empowers bacteria with simultaneous resistance to multiple classes of drugs.
RESULTS
Identification of a unique CmeABC variant associated with resistance to florfenicol and other antimicrobials.
Florfenicol, the fluorinated derivative of chloramphenicol, has been used for treatment of respiratory diseases in food-producing animals, and previously we reported a high prevalence of florfenicol resistance in Campylobacter isolates in China (22). A C. coli isolate, DH161 (Table 1), was florfenicol resistant but did not harbor any known mechanisms (e.g., mutations in rRNA or acquisition of floR, fexA, fexB, cfr, or optrA) that confer florfenicol resistance, as determined by PCR (see Table S3 in the supplemental material). A natural-transformation assay revealed that florfenicol resistance in DH161 was transferrable between C. coli and C. jejuni. Specifically, the MIC of florfenicol for transformant NT161 was 32-fold higher than that for the recipient C. jejuni NCTC 11168 (Table 1). Notably, NT161 also showed increased resistance to other antibiotics, including chloramphenicol (16-fold), ciprofloxacin (8-fold), erythromycin (4-fold), and tetracycline (4-fold). These findings indicated that the transformation resulted in the transfer of multidrug resistance, suggesting that either multiple antibiotic resistance genes/mutations or a multidrug resistance mechanism was transferred from DH161 to NT161. Subsequently, random transposon mutagenesis of NT161 was conducted with EZ-Tn5 to identify the specific mechanism responsible for florfenicol resistance in the transformant, which led to the identification of the transposon mutant NT161::aphA-3, which was susceptible to florfenicol. Determination of the transposon insertion site in NT161::aphA-3 revealed the insertion of the EZ-Tn5 transposon into a gene whose nucleotide sequence was 78.7% identical to that of the native cmeB gene in NCTC 11168. Notably, compared with NT161, NT161-aphA-3 showed a marked decrease in the MICs of florfenicol (64-fold), chloramphenicol (64-fold), ciprofloxacin (>32-fold), erythromycin (>32-fold), and tetracycline (>64-fold) (Table 1). These results suggest that a cmeABC-related mechanism was likely to be responsible for the elevated resistance to florfenicol and other antimicrobials.
TABLE 1 .
Antimicrobial susceptibilities of the key Campylobacter strains used in this study
| Strain | Description | MIC (mg/liter)a of: |
||||
|---|---|---|---|---|---|---|
| FFC | CHL | CIP | ERY | TET | ||
| DH161 | C. coli from duck, isolated in 2009 | 16 | 256 | 256 | 4 | 512 |
| NCTC 11168 | C. jejuni reference strain | 0.5 | 1 | 0.125 | 1 | 1 |
| NT161 | Transformant of NCTC 11168 carrying RE-cmeABC from DH161 | 16 | 16 | 1 | 4 | 4 |
| NT161-aphA-3 | NT161cmeABC::aphA-3 | 0.25 | 0.25 | <0.03 | <0.125 | <0.06 |
| 11168-V1 | NCTC 11168 with RE-cmeABC of NT161 and its promoter sequence | 16 | 16 | 1 | 4 | 4 |
| 11168-V2 | NCTC 11168 with RE-cmeABC of NT161 but original promoter of cmeABC in NCTC 11168 | 4 | 4 | 0.5 | 2 | 2 |
| 81–176 | C. jejuni reference strain | 0.5 | 2 | 0.125 | 0.25 | - |
| 81–176-V | 81-176 with RE-cmeABC of DH161 | 32 | >64 | 1 | 512 | - |
| 11168-C257T | NCTC 11168 with C257T mutation in gyrA | 0.5 | 1 | 16 | 1 | 1 |
| NT161-C257T | NT161 with C257T mutation in gyrA | 16 | 16 | 256 | 4 | 4 |
CHL, chloramphenicol; CIP, ciprofloxacin; ERY, erythromycin; FFC, florfenicol; TET, tetracycline.
To further examine this possibility, we performed whole-genome sequence analysis of NT161. Comparative analysis of the draft genome of NT161 with the complete genome of NCTC 11168 (GenBank accession number NC_002163) revealed that a 5,691-bp segment containing the entire cmeABC operon of DH161 replaced the same locus of NCTC 11168. Beyond this replacement, no other insertions/deletions or plausible single-nucleotide polymorphisms were observed in NT161. These results indicated that the natural transformation led to allelic exchange of cmeABC between the donor and recipient strains and acquisition of cmeABC from DH161 elevated multidrug resistance in NT161. Here we designate this CmeABC variant of DH161 RE-CmeABC (resistance-enhancing CmeABC).
Functional confirmation of RE-CmeABC.
To verify the function of RE-CmeABC, we replaced the cmeABC operon in NCTC 11168 with RE-cmeABC by gene-specific replacement by natural transformation. PCR and Sanger sequencing confirmed the successful allelic replacement in the transformant. As shown in Table 1, transformant 11168-V1 showed 32-, 16-, 8-, 4-, and 4-fold increases in the MICs of florfenicol, chloramphenicol, ciprofloxacin, erythromycin, and tetracycline, respectively, compared to the recipient strain NCTC 11168 (Table 1). In addition, we introduced RE-cmeABC into a different C. jejuni strain, 81-176. We first deleted the whole cmeABC operon natively present in 81-176 and then introduced RE-cmeABC into a different locus via natural transformation. The 81-176 transformant with RE-cmeABC (named 81-176-V) showed a marked increase in the MICs of florfenicol (64-fold), chloramphenicol (>32-fold), and ciprofloxacin (8-fold) compared with 81-176 with its native cmeABC operon (Table 1). These results clearly show that RE-cmeABC confers significantly higher MICs of different antibiotics.
Sequence analysis of the promoter region of RE-cmeABC indicated the presence of an A → G mutation compared to the cmeABC promoter in NCTC 11168, resulting in an imperfect inverted repeat in the CmeR-binding site. Previous studies have indicated that the mutations in the CmeR-binding site result in overexpression of CmeABC in Campylobacter (20, 23). We compared the cmeABC expression levels in 11168-V1 and NCTC 11168 by real-time PCR. The results show a 5-fold increase in the expression of cmeABC in 11168-V1 over that in NCTC 11168. To differentiate the functional differences attributable to overexpression and sequence variation itself, we further constructed transformant 11168-V2 in which the original cmeABC coding region of NCTC 11168 was replaced with RE-cmeABC but it retained the native promoter sequence of NCTC 11168. Real-time quantitative reverse transcription (RT)-PCR showed that RE-cmeABC in 11168-V2 was expressed at a level similar (P > 0.05) to that of cmeABC in NCTC 11168. However, the MICs of florfenicol, chloramphenicol, ciprofloxacin, erythromycin, and tetracycline were 8-, 4-, 4-, 2-, and 2-fold higher, respectively, in 11168-V2 than in NCTC 11168 (Table 1). These MIC differences were attributable to sequence variations between the two cmeABC copies. Comparison of 11168-V1 with 11168-V2 revealed 4-fold (for phenicols) and 2-fold differences (for ciprofloxacin, erythromycin, and tetracycline), which were attributable to the increased expression of RE-cmeABC. Thus, both sequence variation and enhanced expression contributed to the resistance-enhancing function of RE-CmeABC.
We further performed accumulation assays to assess whether RE-CmeABC extrudes more ciprofloxacin. Compared to NCTC 11168, 11168-V1 and 11168-V2 accumulated significantly less ciprofloxacin (Fig. 1), while there was no significant difference in ciprofloxacin accumulation between 11168-V1 and 11168-V2. This result indicated that RE-CmeABC indeed has an enhanced efflux function.
FIG 1 .
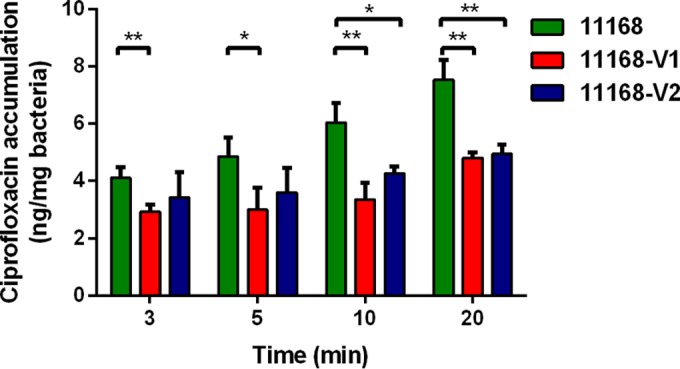
Accumulation of ciprofloxacin in NCTC 11168, 11168-V1, and 11168-V2. Each bar represents the mean and standard deviation of triplicate samples. A t test was used to perform the statistical analysis. *, P < 0.05; **, P < 0.01.
Contribution of RE-CmeABC to extremely high-level ciprofloxacin resistance in Campylobacter isolates.
Fluoroquinolone-resistant Campylobacter is increasingly prevalent, and its resistance is mediated by GyrA mutations in conjunction with the function of CmeABC (15). Typically, fluoroquinolone-resistant Campylobacter isolates have ciprofloxacin MICs ranging from 4 to 32 mg/liter (24). However, we noticed that DH161 had an extremely high ciprofloxacin MIC (256 mg/liter; Table 1). In addition, multiple C. jejuni isolates in our previous surveillance work showed ciprofloxacin MICs of ≥256 mg/liter (see Table S1 in the supplemental material). These isolates harbored the known C257T (Thr-86-Ile) mutation in gyrA, but there were no plasmid-mediated quinolone resistance (PMQR) genes, as determined by PCR (see Table S3). Thus, the C257T mutation alone could not explain the extremely high ciprofloxacin MIC. We then sequenced and analyzed the CmeABC operon in these C. jejuni isolates. CmeA and CmeC in these isolates were homologous (>98% amino acid identities) to CmeA and CmeC in NCTC 11168, while CmeB in these C. jejuni strains showed only ~81% amino acid sequence identity to CmeB in NCTC 11168 but was highly homologous (>99.5% amino acid sequence identity) to CmeB in C. coli DH161, indicating that these C. jejuni isolates harbored RE-CmeABC. Given that RE-CmeABC showed an enhanced function in conferring antibiotic resistance, we hypothesized that the extremely high ciprofloxacin MIC was due to the synergistic action of the CmeABC variant and the gyrA mutation. To prove this possibility, the C257T mutation in gyrA was introduced into both NCTC 11168 and NT161, which differed only in the cmeABC operon. As shown in Table 1, the ciprofloxacin MIC for NT161 containing the C257T mutation was 256 mg/liter, while the ciprofloxacin MIC for NCTC 11168 containing the C257T mutation was 16 mg/liter, a 16-fold difference. These results strongly indicate that RE-CmeABC together with the C257T mutation conferred extremely high-level ciprofloxacin resistance.
RE-CmeABC increases the frequencies of emergence of fluoroquinolone-resistant Campylobacter mutants.
Previous studies demonstrated that CmeABC contributes to the emergence of fluoroquinolone-resistant Campylobacter mutants (21, 25). Since RE-CmeABC, identified in this study, shows an enhanced efflux function compared with the typical CmeABC efflux pump, we speculated that RE-CmeABC could even further promote the emergence of fluoroquinolone-resistant Campylobacter mutants under selection pressure. As shown in Table S2 in the supplemental material, when ciprofloxacin was used at 1.25 or 4 mg/liter to select the mutants, the frequency of emergence of fluoroquinolone-resistant mutants was about 9-fold (P < 0.05) and 25-fold higher (P < 0.05), respectively, in NT161 than in NCTC 11168. Importantly, when a ciprofloxacin concentration of 8 or 16 mg/liter was used on the selective agar plates, no mutant colonies of NCTC 11168 were detected, while the frequency of mutant emergence in NT161 remained at ~10−8. These results clearly indicate that the cmeABC variant expanded the mutant selection window for fluoroquinolones and increased the frequency of emergence of fluoroquinolone-resistant mutants under antibiotic selection.
Structural modeling of CmeB suggests enhanced drug binding by the transporter.
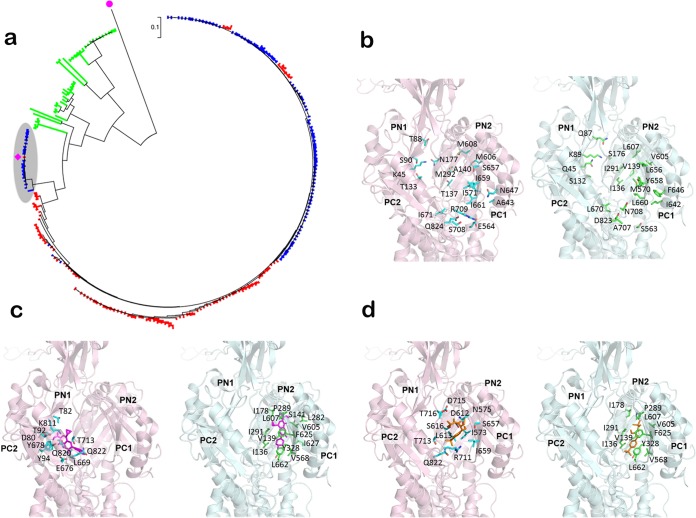
Generally, the CmeB amino acid sequences of C. jejuni and C. coli isolates are at least 93% identical (Fig. 2a), but CmeB in RE-CmeABC is divergent, showing ~81% homology with that of NCTC 11168. Interestingly, there are 18 CmeB homologs (from 18 different C. jejuni isolates) that are similar to CmeB in RE-CmeABC in the GenBank database, most of which were deposited in 2015. On the basis of phylogenetic analysis, CmeB in RE-CmeABC and its 18 homologs formed a unique subtree (shaded gray in Fig. 2a), which is apart from the majority of the CmeB sequences of C. jejuni and C. coli in the database.
FIG 2 .
Sequence analysis and structure prediction of CmeB. (a) Phylogenetic analysis of Campylobacter CmeB sequences identified in this study and deposited in the GenBank database. Different Campylobacter species are shown in different colors as follows: red, C. coli; blue, C. jejuni; green, other Campylobacter species. The purple square indicates CmeB of isolate DH161. The unique subtree of RE-CmeB and its homologs from C. jejuni and C. coli is shaded gray. The purple dot indicates multidrug efflux RND transporter AcrB (accession number WP_044694729.1) from E. coli, which was used as the outgroup. (b) The CmeB structures predicted by the Modeller program. For clarity, only the predicted periplasmic domain structures of NCTC 11168 CmeB (pink) and RE-CmeB (DH161, light green) are shown. The 22 mutated residues located in the periplasmic drug-binding cavity of the mutant transporter are represented by green sticks. The corresponding residues in wild-type CmeB are represented by cyan sticks. (c) The predicted bound ciprofloxacin molecules are magenta. The residues involved in binding are represented by sticks. (d) The predicted bound florfenicol molecules are orange. The residues involved in binding are represented by sticks.
To help understand how RE-CmeABC has an enhanced efflux function, we compared the predicted structures of CmeB in RE-CmeABC and CmeB in NCTC 11168 by homology modeling, based on the crystal structure of the MexB membrane protein (Protein Data Bank ID 2V50) (26) and with the Modeller program (27). Protein sequence alignment suggests that MexB is >40% identical to these two CmeB proteins. As expected, each predicted CmeB structure constitutes 12 transmembrane helices (TMs 1 to 12) and two large extracellular loops protruding out of the inner membrane and into the periplasmic space (Fig. 2b). These two periplasmic loops are located between TMs 1 and 2 and between TMs 7 and 8, respectively, generating a periplasmic domain of the pump. As in AcrB (28, 29) and MexB (26), this periplasmic domain can be divided into six subdomains (PN1, PN2, PC1, PC2, DN, and DC) (Fig. 2b). A cleft is formed between subdomains PC1 and PC2. This cleft is likely to create an entrance for different substrates, allowing them to enter the pump through the periplasm and outer leaflet of the inner membrane. Deep inside the cleft, the pump forms a large internal cavity. In AcrB, this cavity has been shown to form an important binding site that plays a predominant role in recognizing substrates for export. Interestingly, of the 198 amino acid differences between the NCTC 11168 and variant CmeB transporters, 22 are localized in this drug-binding cavity (Fig. 2b).
To understand if these mutated residues in the variant transporter are important for recognizing drug molecules, we used AutoDock Vina (30) to determine how CmeB binds drugs. Specifically, Vina was employed to predict the binding modes for ciprofloxacin and florfenicol. The data suggest that these two drugs are bound within the cavity between subdomains PC1 and PC2 in both CmeB and the variant transporter. However, these two transporters utilize different sets of residues to contact these drugs. In CmeB of NCTC 11168, residues D80, T82, T92, Y94, K811, L669, E676, Y678, T713, Q820, and Q822 are predicted to be involved in the binding of ciprofloxacin (Fig. 2c), while the variant transporter may use I136, V139, S141, I178, P289, I291, L282, V605, L607, F625, I627, Y328, V568, and L662 to bind ciprofloxacin (Fig. 2c). For florfenicol binding, CmeB of NCTC 11168 is likely to employ residues I573, N575, D612, L613, S616, S657, I659, T713, D715, R711, T716, and Q822 to interact with the drug (Fig. 2d); however, the variant transporter may utilize residues I178, I136, V139, P289, I291, Y328, V568, V605, L607, F625, and L662 to bind florfenicol (Fig. 2d). Interestingly, on the basis of the prediction, it was found that the variant transporter tends to use the mutated residues, including I136, V139, I291, V605, and L607, to bind these drugs. The ciprofloxacin- and florfenicol-binding energies of CmeB of NCTC 11168 are predicted to be −7.4 and −6.6 kcal/mol, respectively, while the variant transporter seems to secure these drugs more tightly, with predicted binding energies of −8.1 kcal/mol for ciprofloxacin and −7.1 kcal/mol for florfenicol. These findings suggest that the amino acid changes enhance drug binding in the cmeB variant.
Rising prevalence of RE-CmeABC in C. jejuni isolates.
To understand the epidemiological significance of RE-CmeABC, we examined its distribution among 2,002 Campylobacter isolates (1,458 of C. coli and 544 of C. jejuni) derived from chickens and swine in China from 2012 to 2014 (Table 2). Among the isolates examined, 236 (11.8%) carried RE-CmeABC, including 189 C. jejuni and 47 C. coli isolates (Table 2). The percentage of Campylobacter isolates harboring RE-cmeABC increased from 7.0% (55/784) in 2012 to 9.8% (60/611) in 2013 and to 19.9% (121/607) in 2014. A chi-square test revealed a significant linear trend in the ordered years 2012 to 2014 (P < 0.0001), indicating an emergent trend of the RE-CmeABC variant in Campylobacter isolates in China. Remarkably, RE-cmeABC was highly prevalent in the C. jejuni isolates: 30.8% (37/120) in 2012, 16.7% (44/264) in 2013, 67.5% (108/160) in 2014, and 34.7% (189/544) overall (Table 2). The chi-square test also revealed a significant linear trend in the ordered years 2012 to 2014 (P < 0.0001). The decreased incidence in 2013 might be due to the effect of sampling bias, as more than half (146) of the C. jejuni isolates were from Ningxia Province, where the prevalence of RE-cmeABC was much lower than that in the isolates from other regions (Table 2). These results indicated that RE-cmeABC is increasingly prevalent in C. jejuni isolates in China. On the contrary, the percentage of C. coli isolates containing RE-cmeABC was low (ranging from 2.7 to 4.6%) and did not show a significant linear trend during 2012 to 2014 (P = 0.73).
TABLE 2 .
Distribution of RE-cmeABC in various Campylobacter isolates
| Yr and location of isolation | Host | (%) no. of positive isolates/total no. of isolates | (%) no. of positive isolates/total no. of isolates |
|
|---|---|---|---|---|
| C. jejuni | C. coli | |||
| 2012 | ||||
| Guangdong | Chicken | (8.9) 11/124 | (20.6) 7/34 | (4.4) 4/90 |
| Swine | (1.0) 2/191 | (0.0) 0/1 | (1.1) 2/190 | |
| Ningxia | Chicken | (15.2) 5/33 | (31.3) 5/16 | (0.0) 0/17 |
| Swine | (1.4) 3/208 | (1.4) 3/208 | ||
| Shandong | Swine | (2.8) 2/71 | (2.8) 2/71 | |
| Shanghai | Chicken | (20.4) 32/157 | (36.2) 25/69 | (8.0) 7/88 |
| Total | (7.0) 55/784 | (30.8) 37/120 | (2.7) 18/664 | |
| 2013 | ||||
| Guangdong | Chicken | (17.0) 18/106 | (66.7) 10/15 | (8.8) 8/91 |
| Swine | (3.7) 2/54 | (100.0) 1/1 | (1.9) 1/53 | |
| Ningxia | Chicken | (0.5) 1/196 | (0.7) 1/144 | (0.0) 0/52 |
| Swine | (0.0) 0/24 | (0.0) 0/2 | (0.0) 0/22 | |
| Shandong | Chicken | (25.0) 18/72 | (19.4) 13/67 | (100.0) 5/5 |
| Shanghai | Chicken | (9.8) 13/132 | (40.7) 11/27 | (1.9) 2/105 |
| Henan | Chicken | (80.0) 8/10 | (100.0) 8/8 | (0.0) 0/2 |
| Swine | (0.0) 0/17 | (0.0) 0/17 | ||
| Total | (9.8) 60/611 | (16.7) 44/264 | (4.6) 16/347 | |
| 2014 | ||||
| Guangdong | Chicken | (15.6) 21/135 | (81.3) 13/16 | (6.7) 8/119 |
| Ningxia | Chicken | (6.7) 4/60 | (8.1) 3/37 | (4.3) 1/23 |
| Swine | (30.8) 8/26 | (66.7) 4/6 | (20.0) 4/20 | |
| Shandong | Chicken | (0.0)0/164 | (0.0) 0/1 | (0.0) 0/163 |
| Shanghai | Chicken | (18.2) 14/77 | (56.0) 14/25 | (0.0) 0/52 |
| Swine | (0.0) 0/52 | (0.0) 0/52 | ||
| Henan | Chicken | (93.7) 74/79 | (98.7) 74/75 | (0.0) 0/4 |
| Swine | (0.0) 0/14 | (0.0) 0/14 | ||
| Total | (19.9) 121/607 | (67.5) 108/160 | (2.9) 13/447 | |
| All | ||||
| Chicken | (16.3) 219/1,345 | (34.4) 184/534 | (4.3) 35/811 | |
| Swine | (2.6) 17/657 | (50.0) 5/10 | (1.9) 12/647 | |
| Total | (11.8) 236/2,002 | (34.7) 189/544 | (3.2) 47/1,458 | |
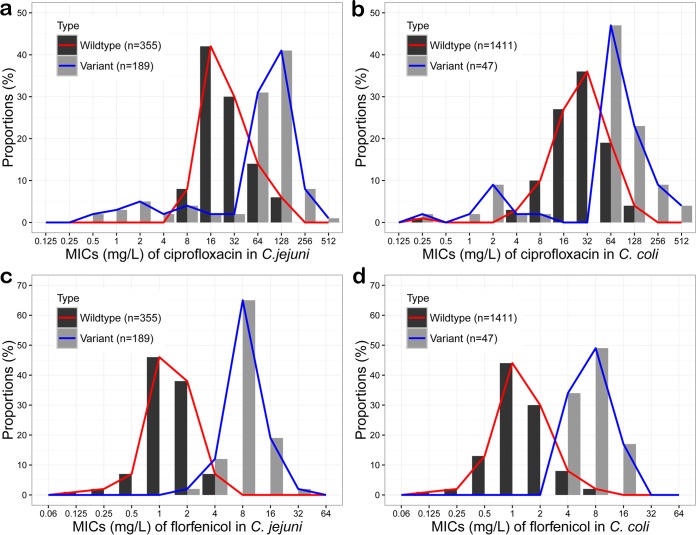
We further analyzed the influence of RE-CmeABC on the distribution of MICs of ciprofloxacin and florfenicol among the 2,002 Campylobacter isolates (16). Notably, the isolates containing RE-cmeABC or other types of cmeABC showed a bimodal pattern of distribution (Fig. 3). This is especially obvious with C. jejuni and the antibiotic florfenicol. The isolates harboring RE-CmeABC shifted the MIC distribution to the higher range. A Fisher exact test confirmed that the MICs of ciprofloxacin and florfenicol were significantly higher for the isolates containing RE-CmeABC than for other isolates (P < 0.0001). These results indicate that RE-CmeABC increases antibiotic MICs at the population level.
FIG 3 .
Distribution of ciprofloxacin and florfenicol MICs for Campylobacter isolates with RE-cmeABC or other CmeABC types. (a) Distribution of ciprofloxacin MICs for C. jejuni. (b) Distribution of ciprofloxacin MICs for C. coli. (c) Distribution of florfenicol MICs for C. jejuni. (d) Distribution of florfenicol MICs for C. coli. In all panels, “Variant” indicates strains that contain RE-cmeABC while “Wildtype” depicts isolates containing a cmeABC operon that is not RE-cmeABC.
Molecular typing and phylogenetic analysis of RE-cmeABC-carrying C. jejuni and C. coli isolates.
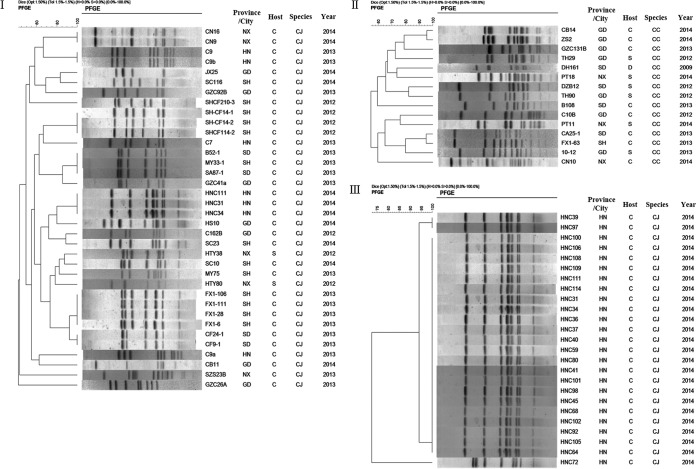
To determine if RE-cmeABC-carrying Campylobacter isolates were genetically related, 51 isolates (36 of C. jejuni and 15 of C. coli) representing different regions, years, host species, and antimicrobial susceptibility patterns were selected for pulsed-field gel electrophoresis (PFGE) analysis by SmaI digestion (Fig. 4I and II). With 80% genetic similarity as the cutoff, the 36 C. jejuni isolates were grouped into 17 clusters (PFGE patterns represented by multiple strains) and 24 unique PFGE patterns (PFGE patterns represented by a single strain) (Fig. 4I), while the 15 C. coli isolates were grouped into 11 clusters and 15 unique PFGE patterns (Fig. 4II). In general, the C. jejuni isolates from different regions or different years were genetically diverse; however, identical PFGE patterns were obtained with some isolates derived from the same region and the same year. Some examples included the four isolates from Shanghai (Fx1-106, Fx1-111, Fx1-6, and Fx-1-28) and the three isolates from Henan (HNC111, HNC31, and HNC34) (Fig. 4I). Additionally, we did a more detailed analysis of the RE-cmeABC-positive C. jejuni isolates collected in 2014 from Henan and found that most of them had the same PFGE pattern (Fig. 4III). These results indicated that RE-cmeABC-carrying isolates showed both overall genetic diversity and regional clonality, suggesting that both clonal expansion and horizontal transmission were involved in the dissemination of this cmeABC variant.
FIG 4 .
PFGE analysis of representative RE-cmeABC-positive Campylobacter isolates. SmaI was used for digestion. The regions of isolation (provinces and cities) include Guangdong (GD), Shandong (SD), Ningxia (NX), Henan (HN), and Shanghai (SH). The host species include chickens (C), swine (S), and ducks (D). (I) Representative RE-cmeABC-positive C. jejuni (CJ) isolates (n = 36). With 80% genetic similarity as a cutoff, the C. jejuni isolates were grouped into 17 clusters (PFGE patterns represented by multiple strains) and 24 unique PFGE patterns. (II) Representative RE-cmeABC-positive C. coli (CC) isolates (n = 15). C. coli isolates were grouped into 11 clusters and 15 unique PFGE patterns. (III) Representative RE-cmeABC-positive C. jejuni isolates from Henan Province in 2014 (n = 25). The isolates were grouped into two clusters and three unique PFGE patterns.
DISCUSSION
In this study, we identified an emergent CmeABC variant (RE-CmeABC) that is more potent in the efflux of antibiotics, conferring enhanced resistance to multiple antimicrobials, especially to florfenicol and fluoroquinolones (Fig. 1; Table 1). Additionally, RE-CmeABC in conjunction with the C257T mutation in gyrA confers exceedingly high-level resistance to ciprofloxacin (MIC, ≥256 mg/liter). This RE-cmeABC variant is especially prevalent in C. jejuni and is emergent in chickens and swine in China, suggesting that it facilitates the adaptation of Campylobacter in the food-producing environment, where antibiotics are frequently used. Genotyping analysis suggested that both clonal expansion and horizontal transmission are involved in the spread of this unique CmeABC variant.
The elevated resistance to multiple antimicrobials seen is due to both overexpression and sequence variation of RE-cmeABC. Previous studies indicated that overexpression of cmeABC conferred only a moderate, usually 2-fold, increase in the MICs of several antimicrobials (19, 31). In this study, however, overexpressed RE-cmeABC resulted in 32- and 8-fold increases in the MICs of florfenicol and ciprofloxacin, respectively (Table 1), indicating a much enhanced function of RE-CmeABC. Similar to well-characterized AcrB (32), CmeB is responsible for the substrate recognition and interaction in the CmeABC efflux system. Thus, the sequence variations in CmeB likely had the most impact on its function. Indeed, structural modeling predicted that ciprofloxacin and florfenicol are bound within the cavity between subdomains PC1 and PC2 in both CmeB and its variant transporter RE-CmeABC (Fig. 2). However, these two transporters utilize different sets of residues to contact these drugs. On the basis of the prediction, it was found that the variant transporter tends to use the mutated residues, including I136, V139, I291, V605, and L607, to bind these drugs, which secure these drugs more tightly than the typical CmeB protein. Thus, the enhanced function of CmeB in RE-CmeABC may be explained, at least in part, by its altered antibiotic-binding kinetics. This possibility remains to be verified by structural analysis in future studies.
Results of this study also reveal a clear rising trend in the prevalence of RE-CmeABC in C. jejuni (Table 2). The difference in the prevalence of RE-cmeABC between C. jejuni and C. coli is probably due to the fact that C. coli is intrinsically more resistant to antimicrobials than C. jejuni is (16). Thus, under antimicrobial selection, C. jejuni may have evolved to acquire RE-cmeABC as a means of enhanced resistance and adaptation. Previously, Cagliero et al. reported that a multidrug-resistant C. jejuni strain (154KU) isolated in France harbored a cmeB gene that was quite divergent from the cmeB genes of other Campylobacter strains. Interestingly, the CmeB amino acid sequence of C. jejuni 154KU was 99.3% identical to that of RE-CmeABC identified in this study, indicating that it is a homolog of RE-CmeABC (33). This finding and the RE-CmeABC homologs recently deposited in GenBank from different countries suggest that this potent variant has emerged in other countries beyond China. The emergence and increasing prevalence of RE-CmeABC in China were likely driven by the extensive use of antimicrobials in animal production. Florfenicol, fluoroquinolones, macrolides, and tetracyclines are commonly used to prevent and control bacterial diseases in food animal production in China (16). Because of its enhanced function in multidrug resistance, harboring RE-CmeABC provides Campylobacter with an enhanced ability to adapt in the animal production environment.
Interestingly, PFGE analysis of the RE-CmeABC-harboring isolates indicated that they were genetically diverse, despite certain clonality among isolates from the same region and the same year. This finding suggests that RE-cmeABC can spread by horizontal gene transfer. Indeed, we showed under laboratory conditions that RE-cmeABC was easily transferred between Campylobacter isolates by natural transformation. Given that Campylobacter is naturally transformable, it is expected that RE-cmeABC will continue to increase in prevalence under selection pressure from antimicrobial use.
In conclusion, our findings reveal a “super” CmeABC variant utilized by Campylobacter for enhanced multidrug resistance. It desensitizes Campylobacter to multiple classes of antibiotics and promotes the emergence of fluoroquinolone-resistant mutants under selection pressure. Additionally, RE-CmeABC, together with GyrA mutations, confers exceedingly high-level resistance to fluoroquinolone, a key antibiotic used for the treatment of Campylobacter infections. Thus, dissemination of RE-CmeABC will likely have a significant impact on the clinical treatment of campylobacteriosis. Given that RE-cmeABC can be transferred by natural transformation, it is possible that this variant will continue to spread in clinical isolates. Thus, enhanced efforts are needed to prevent its further dissemination. Furthermore, our study reveals an effective strategy utilized by bacteria for adaptation to antibiotic selection. Gaining a specific resistance determinant only allows bacteria to resist a particular antimicrobial, while acquisition of a functionally enhanced efflux pump empowers bacteria with simultaneous resistance to multiple classes of antibiotics. Such a mechanism provides an efficient means for bacterial adaptation to selection pressure from the use of diverse antimicrobials.
MATERIALS AND METHODS
Campylobacter strains and antimicrobial susceptibility testing.
C. coli DH161 was isolated from a duck slaughterhouse in Shandong Province, China, in 2009. Additionally, a total of 2,002 Campylobacter isolates (1,458 of C. coli and 544 of C. jejuni) were tested for the presence of RE-cmeABC (Table 2). These Campylobacter strains were isolated from cecal contents, carcasses, and feces of swine and chickens from Shandong, Henan, Ningxia, Guangdong, and Shanghai under the surveillance program for antimicrobial resistance in Campylobacter of animal origin during 2012 to 2014 (16, 18). All of the Campylobacter strains were grown on Mueller-Hinton (MH) agar (Sigma-Aldrich, St. Louis, MO) at 42°C under microaerobic conditions (5% O2, 10% CO2, 85% N2).
Antimicrobial susceptibility testing was conducted by the standard agar dilution method according to the guidelines of the Clinical and Laboratory Standards Institute (34). C. jejuni ATCC 33560 was used for quality control. All of the experiments described above were repeated three times.
Detection of florfenicol and fluoroquinolone resistance determinants.
Mutations involved in florfenicol resistance in the 23S rRNA gene and L4 and L22 ribosomal protein genes in the strains used in this study were detected by PCR and DNA sequencing. In addition, the floR, fexA, fexB, cfr, and optrA genes, known to confer resistance to florfenicol, were examined by PCR. The primers used are listed in Table S3 in the supplemental material. A mismatch amplification mutation assay (MAMA)-PCR was performed to detect the C257T (Thr-86-Ile) mutation in the quinolone resistance-determining region of the gyrA gene (35). Moreover, the strains were screened for the presence of parC and PMQR genes by PCR amplification with the primers in Table S3.
Natural transformation.
Natural transformation was conducted as described previously (36). Briefly, genomic DNA of C. coli DH161 served as the donor DNA and C. jejuni NCTC 11168, susceptible to florfenicol, was used as the recipient strain. The transformants (including NT161) were selected on MH agar plates containing florfenicol (4 mg/liter). Transformation without donor DNA was used as a negative control. Transformants were examined for known mutations involved in florfenicol resistance.
The cmeABC operon in NCTC 11168 was replaced with RE-cmeABC of DH161 by natural transformation. The donor DNA was the PCR product of the entire RE-cmeABC operon. Briefly, RE-cmeABC was amplified from DH161 with primers CmeABC-F and CmeABC-R (see Table S3 in the supplemental material) and cloned into the pMD19-T vector (TaKaRa). This suicide vector was then transferred into C. jejuni NCTC 11168 by natural transformation (36), and the transformants (11168-V1 and 11168-V2) were selected on MH agar plates containing florfenicol (2 to 4 mg/liter). 11168-V1 and 11168-V2 have the same RE-cmeABC coding sequence, while 11168-V1 has the same promoter as DH161 and 11168-V2 has the same promoter as NCTC 11168. PCR and DNA sequencing were performed to confirm the presence of RE-cmeABC in these transformants. The three copies of 23S rRNA, as well as the L4 and L22 ribosomal protein genes, were also examined for potential mutations by PCR and DNA sequencing.
Random transposon mutagenesis.
To identify the determinant involved in florfenicol resistance, we conducted random mutagenesis of transformant NT161 with the EZ-Tn5 <KAN-2> Insertion kit (Epicentre Biotechnologies) (18). The transposon mutants were screened on MH agar plates containing kanamycin (50 mg/liter). Clones selected on the kanamycin plates were inoculated simultaneously onto two types of MH agar plates containing kanamycin (50 mg/liter) and florfenicol (4 mg/liter), respectively. Clones that failed to grow on plates containing 4 mg/liter florfenicol but grew on plates containing kanamycin at 50 mg/liter were selected to confirm the kanamycin resistance gene aphA-3 insertion by PCR and its flanking regions by a modified random primer walking sequencing method (37).
Whole-genome sequencing.
The whole genome of transformant NT161 was sequenced on an Illumina HiSeq 2000 platform at the Hudson Alpha Institute (Huntsville, AL). The backbone genome sequence of NT161 is NCTC 11168.
Introduction of RE-cmeABC into C. jejuni 81-176.
The native cmeABC operon in C. jejuni 81-176 was deleted and replaced with a chloramphenicol resistance gene (cat) by the homologous-recombination method (19). The intact RE-cmeABC operon from DH161 was amplified with primers cmeAKpnI-F and cmeCKpnI-R. The amplicon was digested with KpnI and ligated into the corresponding sites of plasmid pUC-cadF-ermB-cj1476c (38). pUC-cadF-ermB-cj1476c contains a macrolide resistance gene (ermB) between the sequences homologous to the cadF and cj1476c genes. The constructed suicide plasmid pUC-cadF-RE-cmeABC-ermB-cj1476c was used to introduce RE-cmeABC into the 81-176 cmeABC deletion mutant strain. The transformants (81-176-V) were selected on MH agar plates containing erythromycin (10 mg/liter) and verified by PCR and DNA sequence analysis.
Selection of the C257T mutation in the gyrA gene.
In order to assess the level of resistance to fluoroquinolones in strains coharboring RE-cmeABC and the DNA gyrase C257T mutation, the C257T mutation in gyrA was selected in C. jejuni NT161 and NCTC 11168 by exposure to ciprofloxacin. Briefly, strains NCTC 11168 and NT161 were grown overnight on antibiotic-free MH agar and then resuspended in MH broth to achieve a density of 109 CFU/ml. Each suspension of 100 µl was separately spread onto MH agar plates supplemented with ciprofloxacin (4 mg/liter) and cultivated at 42°C for 2 to 3 days under microaerophilic conditions. Colonies on the plates were selected, and the C257T mutation in gyrA was confirmed by MAMA-PCR (35).
Determining frequencies of emergence of fluoroquinolone-resistant mutants.
The frequency of emergence of fluoroquinolone-resistant Campylobacter mutants was detected as described previously (25). Briefly, strains NCTC 11168 and NT161 were grown on MH agar plates for 24 h at 42°C under microaerobic conditions (5% O2, 10% CO2, 85% N2). The cells were collected and suspended in 500 µl of MH broth. The total CFU count was determined by serial dilution and plating. The same amount (100 µl) of a bacterial suspension of each strain was plated onto MH agar plates containing four different concentrations of ciprofloxacin, i.e., 1.25, 4, 8, and 16 mg/liter, respectively. The frequency of emergence of fluoroquinolone-resistant mutants was calculated as the ratio of the CFU counts on ciprofloxacin-containing MH agar plates to the CFU counts on ciprofloxacin-free MH agar plates after 48 h of incubation at 42°C under microaerobic conditions. This experiment was repeated three times.
Sequence analysis and structure prediction of CmeB.
The Protein Basic Local Alignment Search Tool (BLASTP) was used to search Campylobacter CmeB protein sequences (taxid:194 and taxid:72294) in the database of nonredundant protein sequences. The maximum number of target sequences was set at 250. Three sequences were eliminated from the analysis because of the low query coverage. A total of 247 sequences from C. jejuni (n = 105), C. coli (n = 98), and other species (n = 44) were used for further analysis. ClustalW was used to carry out multiple-sequence alignments of the 247 searched sequences together with CmeB of DH161 and a multidrug efflux RND transporter (accession no. WP_044694729.1) from E. coli (used as the outgroup). The maximum-likelihood phylogenetic tree was constructed with MEGA6.
The structures of CmeB in NCTC 11168 and DH161 were predicted by homology modeling with the Modeller program (27). The predictions were based on profile-profile sequence alignments (FFAS03) (39) by utilizing the structure of MexB (2V50) (26) as the template. The predicted structures were idealized with PHENIX (40) and then input into AutoDock Vina (version 1.1.2) (30). The structures of both ligands, ciprofloxacin and florfenicol, were obtained from the PubChem database (http://pubchem.ncbi.nlm.nih.gov). AutoDockTools (41) was then used to prepare the ligand files. The protein was set as a rigid structure, whereas the conformation of each ligand was optimized during all modeling and docking procedures. The iterated local search global optimizer algorithm was employed to predict the binding free energies for these compounds.
Real-time RT-PCR analysis of cmeABC transcription.
Real-time RT-PCR was conducted to determine the expression levels of cmeABC in various strains as described previously (31). Briefly, NCTC 11168, 11168-V1, and 11168-V2 were grown in MH broth for 16 h at 42°C under microaerobic conditions. Two volumes of Sample Protector for RNA/DNA (TaKaRa) were added to the cultures to stabilize the total bacterial RNA. The mixtures were incubated at room temperature for 5 min, and then bacterial cells were collected by centrifugation at 5,000 × g for 10 min. Total RNA was purified by using an RNeasy minikit (Qiagen) according to the instructions included. Purified RNA was treated with TURBO DNA-free (Ambion) to remove DNA contamination. The primers used for real-time PCR are shown in Table S4 in the supplemental material. The two pairs of primers were designed to target the conserved regions of cmeA and cmeC, respectively, in NCTC 11168, 11168-V1, and 11168-V2. Ten-fold dilution series between 2.5 pg and 25 ng were made for RNA templates of NCTC 11168, 11168-V1, and 11168-V2 to generate the standard curve. RT-PCR assays were performed with the One Step SYBR PrimeScript PLUS RT-PCR kit (Perfect Real Time) (TaKaRa). The thermal cycling conditions were as follows: 10 min at 50°C, 5 min at 60°C, 5 min at 95°C, and then 40 cycles of 10 s at 95°C and 30 s at 58°C. Samples were normalized by using 16S RNA as an internal standard. Three biological replicates were prepared for each strain, and three technical replicates were performed for each RNA template. The relative changes (n-fold) in cmeABC transcription between cmeABC variant strains (11168-V1 and 11168-V2) and NCTC 11168 were calculated by the 2−ΔΔCT method as described previously (42).
Ciprofloxacin accumulation assay.
The ciprofloxacin accumulation assay used was based on the method described previously (18). Overnight broth culture of C. jejuni was harvested and washed once in phosphate-buffered saline (PBS; pH 7.2), and the cell density was adjusted to an optical density at 600 nm of 20. Three different tubes were prepared for each sample. A cell suspension of each strain was incubated for 10 min at 37°C in a normal atmosphere, and then ciprofloxacin was added to a final concentration of 10 mg/liter. Aliquots (0.5 ml) were taken after 3, 5, 10, and 20 min and immediately diluted with 2.5 ml of ice-cold PBS. Bacterial cells were harvested by centrifugation at 4°C at 6,000 × g for 5 min and washed once with 2 ml of ice-cold PBS. Bacterial cells were resuspended in 0.2 ml of 0.1 M glycine hydrochloride (pH 3.0) and shaken at room temperature for 16 h. The supernatant was taken after centrifugation at 15,000 × g for 5 min, and fluorescence was measured with FLUOstar Omega with excitation and emission wavelengths of 279 and 447 nm, respectively. A standard curve was prepared by measuring the fluorescence emitted from 0.1 M glycine hydrochloride containing serially diluted ciprofloxacin and used to determine the concentration of ciprofloxacin in the samples.
Detection of RE-cmeABC in Campylobacter isolates.
To measure the distribution of RE-cmeABC in Campylobacter isolates, primers BV-F (5′-CGTATTGCACGATTATTTGGAC) and BV-R (5′-ATCGTTATCAAACCCTCTATGTGCC) were designed according to the sequences specific for the cmeB variant in DH161 and used to specifically amplify the cmeABC variant. The PCR cycling conditions were as follows: initial denaturation at 95°C for 5 min; 30 cycles of 95°C for 30 s, 59°C for 30 s, and 72°C for 40 s; and a final extension at 72°C for 7 min. NCTC 11168 was used as a negative control, while DH161 was used as a positive control in the PCRs. Representative PCR products were selected for DNA sequencing analysis for verification.
PFGE.
Representative Campylobacter isolates containing RE-cmeABC were genotyped by PFGE, which was conducted with a CHEF-DR III apparatus (Bio-Rad Laboratories, Hercules, CA) in accordance with the protocol for Campylobacter (43). The DNA of Campylobacter was digested with SmaI, and the DNA of Salmonella H9812 digested with XbaI was used as the reference marker. The results were analyzed with the InfoQuest FP software version 4.5 (Bio-Rad Laboratories).
Accession number.
The cmeABC variant sequences of the strains involved in this study have been submitted to GenBank under accession number KT778507.
SUPPLEMENTAL MATERIAL
Antimicrobial susceptibilities of Campylobacter isolates that show exceedingly high-level resistance to fluoroquinolones.
Frequencies of emergence of fluoroquinolone-resistant Campylobacter mutants under different ciprofloxacin selection pressures.
Key primers used for PCRs in this study.
Primers used for real-time RT-PCRs in this study.
ACKNOWLEDGMENTS
This study was supported by grants from the National Basic Research Program of China and the National Natural Science Foundation of China (2013CB127200 and 31370046; to J.S. and Y.W.) and grant R01AI118283 from the National Institute of Allergy and Infectious Diseases (to Q.Z. and E.W.Y.).
Funding Statement
The founders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Footnotes
Citation Yao H, Shen Z, Wang Y, Deng F, Liu D, Naren G, Dai L, Su C, Wang B, Wang S, Wu C, Yu EW, Zhang Q, Shen J. 2016. Emergence of a potent multidrug efflux pump variant that enhances Campylobacter resistance to multiple antibiotics. mBio 7(5):e01543-16. doi:10.1128/mBio.01543-16.
REFERENCES
- 1.Yong D, Toleman MA, Giske CG, Cho HS, Sundman K, Lee K, Walsh TR. 2009. Characterization of a new metallo-beta-lactamase gene, blaNDM-1, and a novel erythromycin esterase gene carried on a unique genetic structure in Klebsiella pneumoniae sequence type 14 from India. Antimicrob Agents Chemother 53:5046–5054. doi: 10.1128/AAC.00774-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kumarasamy KK, Toleman MA, Walsh TR, Bagaria J, Butt F, Balakrishnan R, Chaudhary U, Doumith M, Giske CG, Irfan S, Krishnan P, Kumar AV, Maharjan S, Mushtaq S, Noorie T, Paterson DL, Pearson A, Perry C, Pike R, Rao B, Ray U, Sarma JB, Sharma M, Sheridan E, Thirunarayan MA, Turton J, Upadhyay S, Warner M, Welfare W, Livermore DM, Woodford N. 2010. Emergence of a new antibiotic resistance mechanism in India, Pakistan, and the UK: a molecular, biological, and epidemiological study. Lancet Infect Dis 10:597–602. doi: 10.1016/S1473-3099(10)70143-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu YY, Wang Y, Walsh TR, Yi LX, Zhang R, Spencer J, Doi Y, Tian G, Dong B, Huang X, Yu LF, Gu D, Ren H, Chen X, Lv L, He D, Zhou H, Liang Z, Liu JH, Shen J. 2016. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: a microbiological and molecular biological study. Lancet Infect Dis 16:161–168. doi: 10.1016/S1473-3099(15)00424-7. [DOI] [PubMed] [Google Scholar]
- 4.Wang Y, Wu C, Zhang Q, Qi J, Liu H, Wang Y, He T, Ma L, Lai J, Shen Z, Liu Y, Shen J. 2012. Identification of New Delhi metallo-beta-lactamase 1 in Acinetobacter lwoffii of food animal origin. PLoS One 7:e37152. doi: 10.1371/journal.pone.0037152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blair JM, Richmond GE, Piddock LJ. 2014. Multidrug efflux pumps in Gram-negative bacteria and their role in antibiotic resistance. Future Microbiol 9:1165–1177. doi: 10.2217/fmb.14.66. [DOI] [PubMed] [Google Scholar]
- 6.Li XZ, Plésiat P, Nikaido H. 2015. The challenge of efflux-mediated antibiotic resistance in Gram-negative bacteria. Clin Microbiol Rev 28:337–418. doi: 10.1128/CMR.00117-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Srikumar R, Kon T, Gotoh N, Poole K. 1998. Expression of Pseudomonas aeruginosa multidrug efflux pumps MexA-MexB-OprM and MexC-MexD-OprJ in a multidrug-sensitive Escherichia coli strain. Antimicrob Agents Chemother 42:65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yoon EJ, Courvalin P, Grillot-Courvalin C. 2013. RND-type efflux pumps in multidrug-resistant clinical isolates of Acinetobacter baumannii: major role for AdeABC overexpression and AdeRS mutations. Antimicrob Agents Chemother 57:2989–2995. doi: 10.1128/AAC.02556-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baucheron S, Tyler S, Boyd D, Mulvey MR, Chaslus-Dancla E, Cloeckaert A. 2004. AcrAB-TolC directs efflux-mediated multidrug resistance in Salmonella enterica serovar Typhimurium DT104. Antimicrob Agents Chemother 48:3729–3735. doi: 10.1128/AAC.48.10.3729-3735.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Llanes C, Hocquet D, Vogne C, Benali-Baitich D, Neuwirth C, Plésiat P. 2004. Clinical strains of Pseudomonas aeruginosa overproducing MexAB-OprM and MexXY efflux pumps simultaneously. Antimicrob Agents Chemother 48:1797–1802. doi: 10.1128/AAC.48.5.1797-1802.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blair JM, Bavro VN, Ricci V, Modi N, Cacciotto P, Kleinekathӧfer U, Ruggerone P, Vargiu AV, Baylay AJ, Smith HE, Brandon Y, Galloway D, Piddock LJ. 2015. AcrB drug-binding pocket substitution confers clinically relevant resistance and altered substrate specificity. Proc Natl Acad Sci U S A 112:3511–3516. doi: 10.1073/pnas.1419939112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ruiz-Palacios GM. 2007. The health burden of Campylobacter infection and the impact of antimicrobial resistance: playing chicken. Clin Infect Dis 44:701–703. doi: 10.1086/509936. [DOI] [PubMed] [Google Scholar]
- 13.Humphrey T, O’Brien S, Madsen M. 2007. Campylobacters as zoonotic pathogens: a food production perspective. Int J Food Microbiol 117:237–257. doi: 10.1016/j.ijfoodmicro.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 14.Taylor EV, Herman KM, Ailes EC, Fitzgerald C, Yoder JS, Mahon BE, Tauxe RV. 2013. Common source outbreaks of Campylobacter infection in the USA, 1997–2008. Epidemiol Infect 141:987–996. doi: 10.1017/S0950268812001744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luangtongkum T, Jeon B, Han J, Plummer P, Logue CM, Zhang Q. 2009. Antibiotic resistance in Campylobacter: emergence, transmission and persistence. Future Microbiol 4:189–200. doi: 10.2217/17460913.4.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Y, Dong Y, Deng F, Liu D, Yao H, Zhang Q, Shen J, Liu Z, Gao Y, Wu C, Shen Z. 2016. Species shift and multidrug resistance of Campylobacter from chicken and swine, China, 2008–14. J Antimicrob Chemother 71:666–669. doi: 10.1093/jac/dkv382. [DOI] [PubMed] [Google Scholar]
- 17.Anonymous. 2014. Antibiotic resistance threats in the United States, 2013. Centers for Disease Control and Prevention; http://www.cdc.gov/drugresistance/threat-report-2013/. [Google Scholar]
- 18.Lin J, Michel LO, Zhang Q. 2002. CmeABC functions as a multidrug efflux system in Campylobacter jejuni. Antimicrob Agents Chemother 46:2124–2131. doi: 10.1128/AAC.46.7.2124-2131.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin J, Akiba M, Sahin O, Zhang Q. 2005. CmeR functions as a transcriptional repressor for the multidrug efflux pump CmeABC in Campylobacter jejuni. Antimicrob Agents Chemother 49:1067–1075. doi: 10.1128/AAC.49.3.1067-1075.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grinnage-Pulley T, Zhang Q. 2015. Genetic basis and functional consequences of differential expression of the CmeABC efflux pump in Campylobacter jejuni isolates. PLoS One 10:e0131534. doi: 10.1371/journal.pone.0131534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shen Z, Pu XY, Zhang Q. 2011. Salicylate functions as an efflux pump inducer and promotes the emergence of fluoroquinolone-resistant Campylobacter jejuni mutants. Appl Environ Microbiol 77:7128–7133. doi: 10.1128/AEM.00763-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen X, Naren GW, Wu CM, Wang Y, Dai L, Xia LN, Luo PJ, Zhang Q, Shen JZ. 2010. Prevalence and antimicrobial resistance of Campylobacter isolates in broilers from China. Vet Microbiol 144:133–139. doi: 10.1016/j.vetmic.2009.12.035. [DOI] [PubMed] [Google Scholar]
- 23.Cagliero C, Maurel MC, Cloeckaert A, Payot S. 2007. Regulation of the expression of the CmeABC efflux pump in Campylobacter jejuni: identification of a point mutation abolishing the binding of the CmeR repressor in an in vitro-selected multidrug-resistant mutant. FEMS Microbiol Lett 267:89–94. doi: 10.1111/j.1574-6968.2006.00558.x. [DOI] [PubMed] [Google Scholar]
- 24.Luangtongkum T, Morishita TY, El-Tayeb AB, Ison AJ, Zhang Q. 2007. Comparison of antimicrobial susceptibility testing of Campylobacter spp. by the agar dilution and the agar disk diffusion methods. J Clin Microbiol 45:590–594. doi: 10.1128/JCM.00986-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yan M, Sahin O, Lin J, Zhang Q. 2006. Role of the CmeABC efflux pump in the emergence of fluoroquinolone-resistant Campylobacter under selection pressure. J Antimicrob Chemother 58:1154–1159. doi: 10.1093/jac/dkl412. [DOI] [PubMed] [Google Scholar]
- 26.Sennhauser G, Bukowska MA, Briand C, Grütter MG. 2009. Crystal structure of the multidrug exporter MexB from Pseudomonas aeruginosa. J Mol Biol 389:134–145. doi: 10.1016/j.jmb.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 27.Sali A, Blundell TL. 1993. Comparative protein modelling by satisfaction of spatial restraints. J Mol Biol 234:779–815. doi: 10.1006/jmbi.1993.1626. [DOI] [PubMed] [Google Scholar]
- 28.Murakami S, Nakashima R, Yamashita E, Matsumoto T, Yamaguchi A. 2006. Crystal structures of a multidrug transporter reveal a functionally rotating mechanism. Nature 443:173–179. doi: 10.1038/nature05076. [DOI] [PubMed] [Google Scholar]
- 29.Nakashima R, Sakurai K, Yamasaki S, Nishino K, Yamaguchi A. 2011. Structures of the multidrug exporter AcrB reveal a proximal multisite drug-binding pocket. Nature 480:565–569. doi: 10.1038/nature10641. [DOI] [PubMed] [Google Scholar]
- 30.Trott O, Olson AJ. 2010. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem 31:455–461. doi: 10.1002/jcc.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin J, Cagliero C, Guo B, Barton YW, Maurel MC, Payot S, Zhang Q. 2005. Bile salts modulate expression of the CmeABC multidrug efflux pump in Campylobacter jejuni. J Bacteriol 187:7417–7424. doi: 10.1128/JB.187.21.7417-7424.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tikhonova EB, Wang Q, Zgurskaya HI. 2002. Chimeric analysis of the multicomponent multidrug efflux transporters from Gram-negative bacteria. J Bacteriol 184:6499–6507. doi: 10.1128/JB.184.23.6499-6507.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cagliero C, Cloix L, Cloeckaert A, Payot S. 2006. High genetic variation in the multidrug transporter cmeB gene in Campylobacter jejuni and Campylobacter coli. J Antimicrob Chemother 58:168–172. doi: 10.1093/jac/dkl212. [DOI] [PubMed] [Google Scholar]
- 34.Clinical and Laboratory Standards Institute 2008. Performance standards for antimicrobial disk and dilution susceptibility tests for Bacteria isolated from animals: approved standard M31-A3. CLSI, Wayne, PA. [Google Scholar]
- 35.Zirnstein G, Helsel L, Li Y, Swaminathan B, Besser J. 2000. Characterization of gyrA mutations associated with fluoroquinolone resistance in Campylobacter coli by DNA sequence analysis and MAMA PCR. FEMS Microbiol Lett 190:1–7. doi: 10.1111/j.1574-6968.2000.tb09253.x. [DOI] [PubMed] [Google Scholar]
- 36.Wang Y, Taylor DE. 1990. Natural transformation in Campylobacter species. J Bacteriol 172:949–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang K, McClure JA, Elsayed S, Conly JM. 2009. Novel staphylococcal cassette chromosome mec type, tentatively designated type VIII, harboring class A mec and type 4 ccr gene complexes in a Canadian epidemic strain of methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 53:531–540. doi: 10.1128/AAC.01118-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cloak OM, Solow BT, Briggs CE, Chen CY, Fratamico PM. 2002. Quorum sensing and production of autoinducer-2 in Campylobacter spp., Escherichia coli O157:H7, and Salmonella enterica serovar Typhimurium in foods. Appl Environ Microbiol 68:4666–4671. doi: 10.1128/AEM.68.9.4666-4671.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jaroszewski L, Rychlewski L, Li Z, Li W, Godzik A. 2005. FFAS03: a server for profile—profile sequence alignments. Nucleic Acids Res 33:W284–W288. doi: 10.1093/nar/gki418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Adams PD, Afonine PV, Bunkóczi G, Chen VB, Davis IW, Echols N, Headd JJ, Hung LW, Kapral GJ, Grosse-Kunstleve RW, McCoy AJ, Moriarty NW, Oeffner R, Read RJ, Richardson DC, Richardson JS, Terwilliger TC, Zwart PH. 2010. PHENIX: a comprehensive python-based system for macromolecular structure solution. Acta Crystallogr D Biol Crystallogr 66:213–221. doi: 10.1107/S0907444909052925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morris GM, Huey R, Lindstrom W, Sanner MF, Belew RK, Goodsell DS, Olson AJ. 2009. AutoDock4 and AutoDockTools4: automated docking with selective receptor flexibility. J Comput Chem 30:2785–2791. doi: 10.1002/jcc.21256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 43.Ribot EM, Fitzgerald C, Kubota K, Swaminathan B, Barrett TJ. 2001. Rapid pulsed-field gel electrophoresis protocol for subtyping of Campylobacter jejuni. J Clin Microbiol 39:1889–1894. doi: 10.1128/JCM.39.5.1889-1894.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Antimicrobial susceptibilities of Campylobacter isolates that show exceedingly high-level resistance to fluoroquinolones.
Frequencies of emergence of fluoroquinolone-resistant Campylobacter mutants under different ciprofloxacin selection pressures.
Key primers used for PCRs in this study.
Primers used for real-time RT-PCRs in this study.