Abstract
Background
The main focus of studies of individuals at ultra-high risk for psychosis (UHR) has been on identifying brain changes in those individuals who will develop psychosis. However, longitudinal studies have shown that up to half of UHR individuals are resilient, with symptomatic remission and good functioning at follow-up. Yet little is known about brain development in resilient individuals. Therefore, the aim of this study was to investigate differences in brain development between resilient and non-resilient individuals.
Methods
A six-year longitudinal structural MRI study was performed with up to three scans per individual. The final sample consisted of 48 UHR individuals and 48 typically developing controls with a total of 225 MRI-scans, aged 12–20 years at the time of the first MRI-scan and matched for age, gender and number of follow-up scans. At six-year follow-up, 35 UHR individuals were divided in resilient (good functional outcome) and non-resilient (poor functional outcome) subgroups, defined by the modified Global Assessment of Functioning. The main outcome measures were developmental changes in MR-based measures of cortical and subcortical anatomy.
Results
We found widespread differences in volume of frontal, temporal and parietal cortex between resilient and non-resilient individuals. These were already present at baseline and remained stable over development (12–24 years). Furthermore, there were differences in the development of cortical surface area in frontal regions including cingulate gyrus.
Conclusions
Developmental differences may reflect compensatory neural mechanisms, where better functioning in resilient individuals leads to less tissue loss over development.
Keywords: Ultra-high risk, Psychosis, Resilience, MRI, Functional outcome, Brain development
Highlights
-
•
Is brain development different between resilient and non-resilient UHR individuals?
-
•
We performed a longitudinal MRI study with up to three scans per individual.
-
•
Resilience was defined by functional outcome at 6-year follow-up.
-
•
Widespread differences were found, primarily in volume and cortical surface area.
-
•
Developmental differences may reflect compensatory neural mechanisms.
1. Introduction
Traditionally, studies of individuals at ultra-high risk (UHR) for psychosis have attempted to identify neurobiological markers to predict which UHR individuals will go on to develop psychosis (i.e. undergo a ‘transition to psychosis’). Thus, the field has focused on identifying differences in the brains of those subjects who will worsen over time compared to those who will not. However, transition rates have plummeted since the earliest reports of rates of over 50% (Miller et al., 2002) to an average of 29% in more recent reports (Fusar-Poli et al., 2012a). At the same time, there has been a steady increase in the remission rates reported, of up to 54% (Simon et al., 2011). A recent meta-analysis of eight longitudinal studies (Simon et al., 2013) reported that 73% of 773 UHR subjects did not develop psychosis over a 2-year follow-up and 46% fully remitted from their baseline symptoms. We conducted a longer follow-up, with a mean of six years, and found that 41% of adolescents at UHR fully remitted from their at-risk state (de Wit et al., 2014). Therefore, it is at least as relevant to investigate neurobiological changes in UHR subjects who show resilience and go on to function well, as it is to investigate those who undergo a transition to psychosis.
In addition, the criterion of ‘transition to psychosis’ has been criticized as a measure to identify which individuals will have a truly poor clinical outcome: the threshold for transition is essentially arbitrary and is based entirely on positive symptoms (Fusar-Poli et al., 2013, Ziermans et al., 2014). There is increasing evidence that negative symptoms and the level of cognitive and social functioning are equally important for the long-term outcome of UHR individuals (Fusar-Poli and Borgwardt, 2007, Carrión et al., 2013, Fusar-Poli et al., 2013). Moreover, some individuals may develop psychosis before going on to recover completely, while some individuals who do not develop psychosis may have worse outcomes (Yung et al., 2010, Fusar-Poli and Van Os, 2013, Cotter et al., 2014, de Wit et al., 2014). Taken together, this underscores the importance of studying resilience, as much as transition to psychosis. We follow the American Psychological Associate in defining resilience as “the process of adapting well in the face of adversity, trauma, tragedy, threats or significant sources of stress — such as family and relationship problems, serious health problems or workplace and financial stressors. It means “bouncing back” from difficult experiences.” We therefore focus on how well individuals function at follow-up, rather than whether they have experienced a transition to psychosis and operationalise resilience as having good functional outcome. To permit comparison to the extant literature, we also perform complementary analyses using the more traditional operationalisation based on remission of positive symptoms (included as Supplemental material).
Compared to a volunteer sample of typically developing controls, UHR individuals have been reported to show reduced gray matter volume in the frontal and temporal lobes, anterior cingulate gyrus and hippocampal regions. (for reviews, see Fusar-Poli et al., 2011, Wood et al., 2013, Bois et al., 2014). However, many imaging studies of UHR individuals have been cross-sectional in design and have therefore been limited in their ability to show developmental differences between UHR individuals with different outcomes. The longitudinal studies that have been conducted were only partially successful in predicting transition to psychosis and have reported many inconsistent findings (for review, see Wood et al., 2013). This may in part be related to limited follow-up times and differences in the methods used (Wood et al., 2013), but is likely also related to the diverse clinical outcomes of UHR individuals and the relatively arbitrary criterion of transition to psychosis (de Wit et al., 2014). One recent study of particular interest is that of Cropley et al. (2016): in subjects with attenuated positive symptoms, reduced gray matter volume was associated with more severe positive, negative and depressive symptoms and lower global functioning in the UHR subgroup without transition to psychosis. Unfortunately however, there is a lack of studies investigating brain development with MRI scans at different time points.
Therefore, we investigated brain development in resilient versus non-resilient UHR individuals over, on average, six years. We conducted a comprehensive assessment of symptoms and functioning and examined brain development, with MRI scans at three different time points. This study includes a long follow-up of six years and more than two MRI scans per individual. This permits a better assessment of outcome and non-linear modeling of developmental trajectories.
2. Methods and materials
2.1. Participants
All data were collected at the Department of Psychiatry at the University Medical Center Utrecht, Brain Center Rudolf Magnus in the Netherlands. Participants were between 12 and 18 years of age at the time of recruitment and were included after written informed consent. The study was approved by the Dutch Central Committee on Research Involving Human Subjects.
Recruitment details have been described previously (Sprong et al., 2008, Ziermans et al., 2011). Briefly, adolescents at UHR were referred by general practitioners or other psychiatry clinics. For inclusion at baseline, subjects in the UHR group had to fulfill at least one of the following criteria: 1) attenuated positive symptoms, 2) brief, limited, or intermittent psychotic symptoms, 3) genetic risk for psychosis combined with a deterioration in overall level of social, occupational/school, and psychological functioning in the past year or 4) two or more of nine basic symptoms of mild cognitive disturbances. The first three inclusion criteria were assessed using the Structured Interview for Prodromal Syndromes (McGlashan et al., 2001) and the Family Interview for Genetic Studies (Maxwell, 1982). The fourth inclusion criterion was assessed using the Bonn Scale for the Assessment of Basic Symptoms-Prediction List that was assessed by a clinical expert (TZ) working with child populations (Schultze-Lutter and Klosterkötter, 2002). Exclusion criteria consisted of a past or present psychotic episode lasting more than one week, traumatic brain injury or any known neurological disorder, and verbal intellectual IQ < 75, as assessed using the Wechsler Intelligence Scales by one of the co-authors (TZ) as well as fully trained research assistants. (Wechsler, 1997, Wechsler, 2002). The typically developing control group consisted of typically developing adolescents recruited through secondary schools in the region of Utrecht. They were excluded if they met one of the UHR-criteria, if they or any first degree relative had a history of a psychiatric illness, or if they had a second-degree relative with a psychotic disorder.
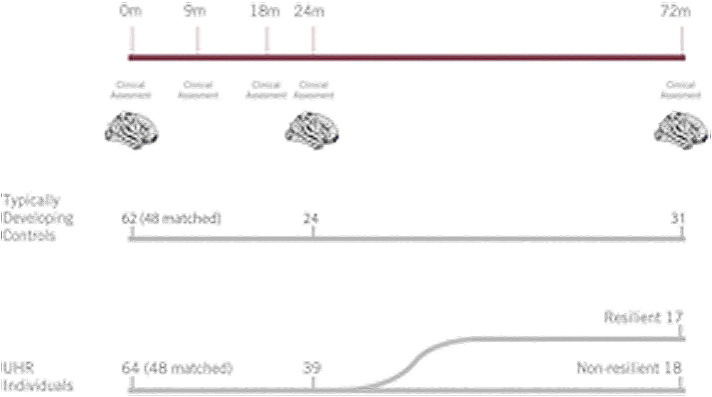
At baseline, 64 UHR individuals and 62 typically developing controls completed the clinical assessment and an MRI scan. These groups were then matched for gender, age, and number of follow-up scans, resulting in a longitudinal dataset of 48 UHR individuals and 48 typically developing controls with one, two or three scans and a total of 225 MRI scans. Participants were between 12,2 and 19,6 years of age at the time of the first MRI scan (Table 1). Follow-up assessments were conducted 9 months, 18 months, 2 years, and 6 years post baseline (range 3,5–8,0 years). The follow-ups at 9 and 18 months only included clinical assessments. For an overview of the timeline, see Fig. 1. We split the UHR group into two groups based on the 6-year clinical follow-up data, one ‘resilient’ and one ‘non-resilient’ subgroup. Clinical outcome was available for 35 UHR individuals at 6 year follow up. Resilience was defined by functional outcome using the modified Global Assessment of Functioning (mGAF) scale (Hall, 1995) as either a) Good functional outcome (resilient): mGAF score of ≥ 65 or b) Poor functional outcome (non-resilient): mGAF score of < 65. The cut off of 65 has been used before (Allen et al., 2014) and was chosen as the 60–70 range corresponds to ‘generally good function with meaningful interpersonal relationships, and some persistent mild symptoms and/or some persistent difficulty in social, occupational, or school functioning’ (Hall, 1995). A score below 60 indicates ‘moderate to severe symptoms and/or moderate to severe difficulty in social, work, or school functioning,’ while scores above 70 correspond to ‘some transient mild symptoms to absent or minimal symptoms and/or slight to no impairment in social, work, or school functioning’.
Table 1.
Demographic data for typically developing controls (TDC) and UHR individuals.
| TDC | UHR | UHR vs. TDC | |
|---|---|---|---|
| Number of individuals, n (males) | 48 (29) | 48 (29) | n.s. |
| Hand preference, n, right/non-right | 40/8 | 44/4 | n.s. |
| Parental education, years, mean (SD) | |||
| Mother | 13.45 (2.39) | 12.96 (2.16) | n.s. |
| Father | 14.22 (2.17) | 13.74 (2.18) | n.s. |
| Premorbid IQ, mean (SD) | 107.04 (13.12) | 100.40 (11.97) | t = 2.85, p = 0.01 |
| Age at baseline scan, years | |||
| Mean (SD) | 15.72 (1.54) | 15.43 (2.11) | n.s. |
| Range | 12.19–18.76 | 12.28–19.64 | |
| Age at 6-year FU scan, years | |||
| Mean (SD) | 21.40 (1.57) | 21.16 (2.42) | n.s. |
| Range | 17.57–24.54 | 16.84–25.79 | |
| Intra Cranial Volume (mm3) | 1,621,000 (148220) | 1,586,000 (167740) | n.s. |
| Number of scans, n | n.a. | ||
| Total number of scans, n | 103 | 122 | |
| 1 | 48 | 48 | |
| 2 | 24 | 39 | |
| 3 | 31 | 35 |
Notes: TDC = typically developing controls; UHR = Individuals at ultra-high risk for psychosis; IQ = intelligence quotient; SD = standard deviation; FU = follow-up.
Fig. 1.
Timeline of the study. The top bar shows the time points at which individuals had a clinical assessment and/or MRI scanning session, at 0, 9, 18, 24 and 72 months follow-up. The number of individuals included at baseline and follow up are displayed for the typical developing control group (middle) and UHR individuals (bottom). At six-year follow up, 17 individuals were defined as resilient and 18 individuals as non-resilient.
To make our results comparable to the existing literature we included an extra analysis in our supplemental material, where we used the “classic” operationalisation by McGlashan and colleagues based on UHR criteria (McGlashan et al., 2001), comparing individuals who had remitted from UHR criteria and individuals who had not. Further details on both subgroupings are provided in Supplemental information S1.
2.2. Image acquisition
MRI scans were acquired on a single 1.5-T scanner at all time points (Philips, Best, The Netherlands). Whole brain T1-weighted three-dimensional fast field echo scans were made with 1.5-mm contiguous coronal slices of the whole head (256 × 256 matrix, FoV = 256 mm, echo time (TE) = 4.6 ms, repetition time (TR) = 30 ms, flip angle = 30°).
2.3. Image processing
Scans were processed and analyzed using FreeSurfer v 5.1.0 software. Technical details of the automated reconstruction scheme of this well-validated software program are described elsewhere (Dale et al., 1999, Fischl et al., 1999, Carmona et al., 2009). We calculated average volume (mm3), cortical thickness (mm), surface area (mm2), and gyrification for the 34 cortical structures in each hemisphere from the Desikan-Killiany atlas (Desikan et al., 2006), as well as cortical thickness and surface area for each lobe and the whole brain, and total gray and white matter volume per hemisphere and the whole brain. Gyrification could not be estimated for 7 scans, because of FreeSurfer processing errors. We also measured the volume of subcortical structures, as well as subcortical gray matter volume and gray and white matter separately for the cerebellum. In order to reduce within-subject variability between scan sessions, the longitudinal analysis processing pipeline of FreeSurfer was used for subjects with more than one scan (Gogtay et al., 2006, Reuter and Fischl, 2011). Manual edits were performed as necessary by a trained rater (SdW) blind to subject identity and group membership.
2.4. Statistical analyses
The statistical software package R version 2.14.0 was used (1) to test for between-group differences in the demographic and clinical data and (2) to investigate the effects of age, group and their interaction on the brain measures using a linear mixed model. The mixed model procedure investigated the relationship between age, group and our measures of interest (brain measures) using a top down selection procedure to test for the best-fit growth model. We were particularly interested in two effects: (1) main effects of group, where differences between groups were present at first assessment and stable over developmental time (range 12–24 years), and (2) group ∗ age interaction effects where the developmental trajectories differed between the groups (e.g. structure × increased over time in group A while it decreased over time in group B). Details of this model and selection procedure are provided in Supplemental information S2.
3. Results
3.1. Between-group differences in demographic and clinical characteristics
Demographic and clinical characteristics of typically developing controls and UHR individuals are provided in Table 1. Both groups were matched for age, gender and number of scans. Hand preference and intracranial volume did not differ between groups, but IQ was lower for UHR individuals than for typically developing controls.
Demographic details of resilient and non-resilient individuals are given in Table 2. The subgroups did not differ in terms of the use of psychotropic medication (Table 2). Our definition of resilience largely overlaps with the definition as used in the classical definition based on remission of UHR symptoms (see Supplementary information S3, Supplementary information S4): of 17 individuals classified as resilient based on functional outcome, 12 were classified as being in remission; of 16 individuals classified being in remission, 12 were classified as resilient based on functional outcome. Of the five individuals classified as resilient based on functional outcome but not as being in remission, two had a psychotic episode and later remitted with high mGAF scores at 6-year follow-up. The other three still scored in the UHR range for positive symptoms, but reported good functioning (mGAF scores between 70 and 80) at 6-year follow-up. In clinical terms, resilience led to statistical improvements on symptom measures at follow-up, while there were few differences in symptoms at baseline.
Table 2.
Demographic and clinical data of resilient (R) and non-resilient (NON-R) UHR individuals based on functional outcomea.
| R | NON-R | R vs. NON-R | |
|---|---|---|---|
| Number of individuals, n (males) | 17 (13) | 18 (10) | n.s. |
| Premorbid IQ, mean (SD) | 101.18 (12.89) | 103.22 (10.28) | n.s. |
| Age at baseline, years | |||
| Mean (SD) | 15.42 (2.20) | 15.54 (2.48) | n.s. |
| Range | 12.31–19.64 | 12.28–19.43 | |
| Age at 6-year FU, years | |||
| Mean (SD) | 21.34 (2.58) | 20.99 (2.32) | n.s. |
| Range | 17.88–25.79 | 16.84–24.80 | |
| SIPS/SOPS baseline, mean (SD) | |||
| Total score | 21.35 (10.74) | 25.06 (11.47) | n.s. |
| Positive symptoms | 7.41 (4.53) | 8.50 (3.13) | n.s. |
| Negative symptoms | 4.24 (4.66) | 4.17 (3.67) | n.s. |
| Disorganized symptoms | 3.41 (3.30) | 5.89 (2.83) | U = 68.0, p = 0.004 |
| General symptoms | 6.29 (4.33) | 6.50 (4.51) | n.s. |
| mGAF baseline, mean (SD) | 57.06 (13.57) | 56.83 (17.44) | n.s. |
| SIPS/SOPS 6-year FU, mean (SD) | |||
| Total score | 11.75 (8.36) | 35.11 (14.11) | U = 20.5, p ≤ 0.001 |
| Positive symptoms | 3.88 (3.59) | 10.00 (5.54) | U = 45.5, p ≤ 0.001 |
| Negative symptoms | 3.88 (3.72) | 11.17 (5.79) | U = 44.0, p ≤ 0.001 |
| Disorganized symptoms | 3.19 (2.74) | 7.22 (3.37) | t = 3.80, p = 0.001 |
| General symptoms | 1.50 (1.59) | 6.72 (4.86) | U = 42.0, p ≤ 0.001 |
| mGAF 6-year FU, mean (SD) | 77.94 (7.30) | 47.50 (9.94) | t = − 10.27, p ≤ 0.001 |
| Psychotropic medication baseline, any | n.s. | ||
| No | 9 | 9 | |
| Yes | 8 | 9 | |
| Psychotropic medication 6-year FU, any | n.s. | ||
| No | 13 | 11 | |
| Yes | 4 | 7 |
TDC = typically developing controls; UHR = Individuals at ultra-high risk for psychosis; R = resilience; NON-R = non-resilience; IQ = intelligence quotient; SD = standard deviation; FU = follow-up; SIPS/SOPS = Structured Interview for Prodromal Symptoms/Scale of Prodromal Symptoms; mGAF = Modified Global Assessment of Functioning.
Subgroups are based on outcome at 6-year FU; outcome was unknown for 13 UHR individuals.
3.2. Brain development in UHR compared to typical development
To allow comparison to earlier studies, we first tested for differences in brain development between the whole group of UHR individuals and typically developing controls. In the cortex, we found the largest differences in cortical surface area. Here, we primarily found group ∗ age interactions, with less steep decreases over developmental time in UHR individuals in frontal and parietal areas compared to typically developing controls (p = 0.004–0.043). We found fewer differences in cortical volume, cortical thickness and gyrification. Furthermore, we found that UHR individuals showed stable as well as steeper decreases over developmental time in hippocampus (p = 0.012/0.006) and thalamus volume (p = 0.015/0.022), as well as smaller volume at baseline and steeper increases over developmental time in the volume of third (p = 0.019) and inferior lateral ventricle (p = 0.049) than typically developing controls. All results are listed in Supplemental information S5.
3.3. Brain development in resilient versus non-resilient UHR individuals
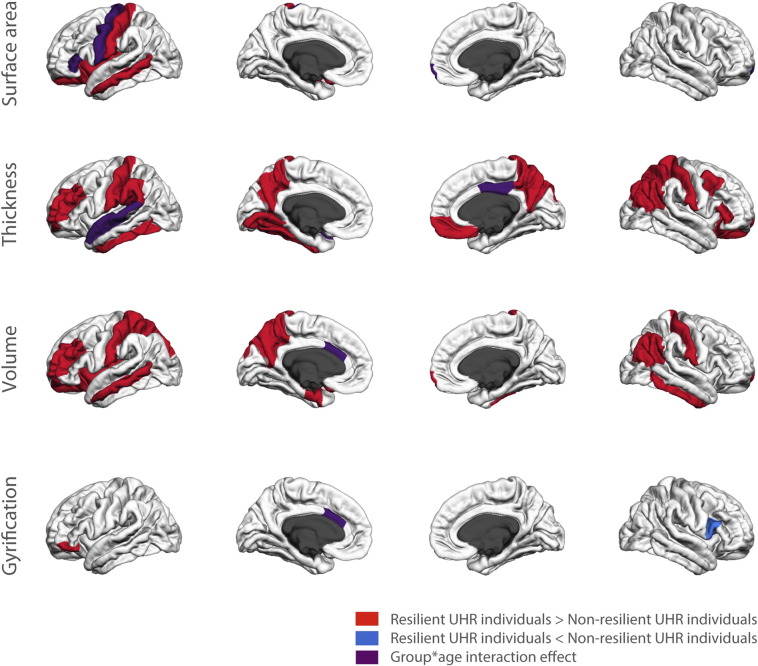
Main effects of group and group ∗ age interaction effects of resilient versus non-resilient individuals are shown in Fig. 2 and listed in Supplemental information S6. We additionally provided results from the analyses using the classic operationalisation (remission from UHR criteria) in Supplemental information S7. These were highly similar to those reported in the main paper.
Fig. 2.
Differences in cortical morphology between resilient and non-resilient UHR individuals.
3.3.1. Stable differences between groups
There were widespread differences between resilient and non-resilient UHR individuals that were stable over developmental time (i.e., main effects of group). Resilient individuals had larger volumes of frontal, temporal and parietal cortex, corpus callosum and nucleus accumbens than non-resilient individuals (p = 0.003–0.025, see Supplemental information S4). Furthermore, overall cortical thickness was larger for resilient compared to non-resilient individuals, mainly due to increases in thickness in frontal, parietal and temporal lobes (p = 0.000–0.046). Cortical surface area was larger throughout the left hemisphere and there were some differences in gyrification (p = 0.003–0.042, see Supplemental information S4). When we added the trajectories of typically developing controls in these areas, we found that they fell between those of resilient and non-resilient individuals. This is illustrated in the supplemental information where some of the graphs of these trajectories are provided (S8).
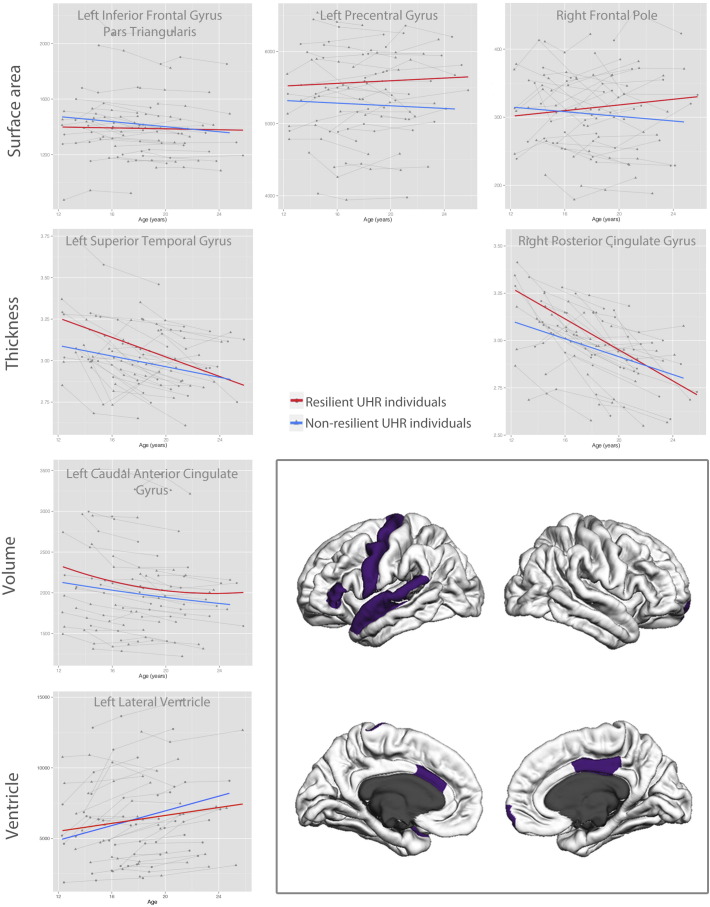
3.3.2. Developmental differences
There were some developmental differences between resilient and non-resilient UHR individuals where developmental trajectories diverged between groups over time (group ∗ age interactions; see Fig. 3). Resilient individuals showed smaller decrease in volume over developmental time in anterior cingulate gyrus (p = 0.049) and smaller increase over developmental time in lateral ventricle volume (p = 0.036). Furthermore, resilient individuals showed greater decreases in cortical thickness in superior temporal cortex (p = 0.038) and posterior cingulate gyrus (p = 0.011) than non-resilient individuals. Resilient individuals also showed increases over developmental time in cortical surface area in precentral gyrus (p = 0.037) and frontal pole (p = 0.033) compared to decreases in non-resilient individuals. Finally, resilient individuals showed decreases in gyrification in anterior cingulate gyrus over developmental time compared to increases in non-resilient individuals (p = 0.049).
Fig. 3.
Distinct developmental trajectories between resilient and non-resilient UHR individuals.
4. Discussion
We investigated brain development over six years in a matched sample of 48 adolescents at ultra-high risk for developing psychosis and 48 typically developing controls, with up to three MRI scans per individual. Our main goal was to compare brain development between resilient and non-resilient UHR individuals. We operationalized resilience as good functioning at 6-year follow-up, information that was available for 35 of the UHR individuals. We found widespread differences in volume of frontal, temporal and parietal cortex that were already present at baseline and remained stable over development. Furthermore, there were differences between resilient and non-resilient individuals in the development of cortical surface area in multiple frontal regions including cingulate gyrus. These diverging developmental trajectories may reflect compensatory neural mechanisms, where the better functioning resilient individuals results in less tissue loss with development.
When we compared brain development between all UHR individuals and typically developing volunteers, we found diverging developmental trajectories for cortical surface area in frontal and parietal regions. Here, typically developing controls showed greater decreases over development than UHR individuals. Furthermore, we found decreases in hippocampus and thalamus volume and increases in the volume of the third and inferior lateral ventricles in UHR individuals that were already present at baseline. These findings are in keeping with other studies on psychosis and UHR (for reviews, see Fusar-Poli et al., 2011, Fusar-Poli et al., 2012b, Wood et al., 2013, Bois et al., 2014).
When we compared brain development between resilient and non-resilient UHR individuals, we found three types of changes: First, there were differences that were already present at baseline and that were stable over development, with the developmental trajectories parallel for both groups. These included greater cortical thickness and cortical volume and larger volume of the nucleus accumbens and corpus callosum in resilient individuals. Interestingly, when we added the trajectories of typically developing controls in these areas, we found that they fell between those of resilient and non-resilient individuals (see Supplemental information S6). As such, the volume of resilient individuals exceeded that of typically developing volunteers, one possible mechanism that may be at play is that the greater volume may be protective for worse outcome, including less tissue loss. In addition, these volumetric differences might be a predictive marker of good outcome. Earlier longitudinal studies of development in UHR individuals primarily focused on transition to psychosis. Most of these have shown differences between UHR individuals with different clinical outcomes in insular, temporal, parietal and superior frontal regions (Fornito et al., 2008, Sun et al., 2009, Ziermans et al., 2012, Wood et al., 2013). One study investigated differences in baseline gray matter volume between non-transitioned individuals with and without persistent attenuated positive symptoms and found differences in frontal, temporal, posterior and cingulate regions (Cropley et al., 2015). These regions highly overlap with our results. Our data suggest that these regions may hold promise for predicting, at a young age, which UHR individuals will get better and which will worsen over time. This was also suggested by Cropley et al. (2015).
A second pattern in our data is developmental trajectories that overlap at young age but diverge over developmental time. Areas showing this pattern include precentral gyrus, frontal pole, anterior cingulate gyrus and lateral ventricle (see Fig. 3). The enlargement of lateral ventricles was one of the first brain findings to be reported in schizophrenia and is still one of the most consistently reported (Shenton et al., 2001). In our study, the volume of lateral ventricles increased more over development for non-resilient individuals than for resilient ones. The anterior cingulate gyrus showed decreases in volume over development for both resilient and non-resilient individuals. However, its volume stabilized in early adulthood for resilient individuals, whereas it continued to decrease in the non-resilient group. This structure is important for goal-directed behavior and involved in error and conflict monitoring and has often been reported to show changes in structure and function in UHR and schizophrenia (Fornito et al., 2008, Fornito et al., 2009, Reid et al., 2010). The stabilization in the resilient group may reflect neural changes as a result of recovery in this group, whereas the continuing loss of volume in non-resilient individuals may reflect their continuing difficulties with cognitive and emotional integration (Fornito et al., 2008, Fornito et al., 2009). The left precentral gyrus and right frontal pole showed developmental increases in surface area in resilient individuals compared to slight decreases in non-resilient individuals. Changes in frontal areas have often been associated with UHR and schizophrenia (for review, see Wood et al., 2013). As such, these increases may also represent compensatory mechanisms, related to better functioning in resilience. Other structures, such as the posterior cingulate and superior temporal gyrus, showed converging trajectories, where baseline differences disappeared with development. Interestingly, both resilient and non-resilient individuals showed decreases over development in cortical thickness in these areas, similar to what is seen in typical development. However, the non-resilient group showed a slower rate of change than the resilient group. These findings concur with a study by Zalesky et al. (2015): these authors reported maturational delays in cortical thickness in childhood onset schizophrenia, and normalization of these changes over development for those subjects who showed symptomatic improvement.
Finally, there were brain areas that differed between UHR individuals and typically developing controls, but not between resilient and non-resilient UHR individuals. These included hippocampus, thalamus and frontal and parietal cortical surface area. These changes are unlikely to be useful for predicting long-term functioning in UHR individuals, as they do not differ between resilient and non-resilient individuals. Rather, they may be related to non-specific UHR risk factors, such as obstetric complications (Stefanis et al., 1999, Wood et al., 2008).
In our supplemental materials, we have included the results of a second analysis where we compared those UHR individuals who had remitted from their UHR status to those who had not. The results from this analysis were similar but noisier (see Supplemental information S7). The biggest difference was found for gyrification: while there were few differences in gyrification found with the operationalisation based on functional outcome, more were found with the analysis using remission from UHR criteria. This might suggest that differences in gyrification are more specific to positive symptoms than global functioning. We chose to present the results from the operationalisation based on functional outcome as we felt that was the definition with the most clinical relevance. Hence, when interpreting the results, we should keep in mind that the main findings of this paper relate to global functioning.
There are some limitations to this study. First is the relatively modest sample size, especially for the follow-up analyses on resilience (17 versus 18 subjects). This is in part because it was necessary to exclude some subjects from these analyses to match subgroups for gender and age. We felt it was important to maintain this matching given the skewed distribution of gender in UHR and schizophrenia (McGrath et al., 2008, Walder et al., 2013, Cocchi et al., 2014) and findings of gender differences in brain size and development (Lenroot et al., 2007, Wierenga et al., 2014). The limited sample size means we may have been underpowered to detect subtle differences, and may be causal to the relatively small number of developmental changes found. On the other hand, longitudinal samples require far fewer participants than cross-sectional studies in order to detect small differences in brain structure. Steen and colleagues showed that the required sample to detect a 5% difference in whole brain volume is 73 patients and 73 typically developing controls in a 2-sample cross-sectional study, against just 5 patients and 5 typically developing controls in a longitudinal study design (Steen et al., 2007). Moreover, we were able to report longitudinal data with more than two MRI scans per individual, potentially permitting us to fit more complex developmental models to the data.
A second limitation is that a large number of UHR individuals were on medication for the duration of this study. This is often the case in UHR studies and one can argue that medication may have been prescribed for individuals who were clinically more severely affected, or that it may have helped prevent the onset of psychosis. However, medication use did not differ between resilient and non-resilient individuals, not at baseline or at follow-up. This suggests that medication use did not overly influence our results. The lack of a difference between resilient and non-resilient individuals could even be taken to suggest that medication does not play a role in individual outcome, although the sample size is too modest to permit any such definitive conclusion.
In conclusion, brain development in resilient individuals initially at UHR for psychosis differs from that of non-resilient individuals. Widespread differences in cortical thickness and volume were evident at baseline and remained stable over development. However, several frontal areas showed diverging developmental trajectories. The stable differences that were already present at baseline may hold promise for predicting, at a young age, who will go on to recover and who will not, whereas the divergence in frontal areas may reflect neural changes related to better functioning.
The following are the supplementary data related to this article.
Operationalisations of resilience.
Linear mixed models.
Demographic and clinical data of remitted and non-remitted UHR individuals based on UHR remission criteria.
Overlap between the two different analyses, (1) resilience based on functional outcome, (2) subgrouping based on remission from UHR criteria.
Stable and developmental differences between UHR individuals and typically developing controls (TDC).
Stable and developmental differences in brain development between resilient (R) and non-resilient (NR) UHR individuals based on functional outcome.
Stable and developmental differences between remitted (R) and non-remitted (NR) UHR individuals based on UHR remission criteria.
Acknowledgements
We would like to thank all subjects for their participation in this study. We thank Dr. Mirjam Simons-Sprong, Anneke Schouten, Nieke Kobussen, Petra Klaassen and Juliette Weusten for their help with subject recruitment and testing.
References
- Allen P., Chaddock C.A., Egerton A., Howes O.D., Barker G., Bonoldi I., Fusar-Poli P., Murray R., McGuire P. Functional outcome in people at high risk for psychosis predicted by thalamic glutamate levels and Prefronto-striatal activation. Schizophr. Bull. 2014;41:429–439. doi: 10.1093/schbul/sbu115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bois C., Whalley H., McIntosh A., Lawrie S. Structural magnetic resonance imaging markers of susceptibility and transition to schizophrenia: A review of familial and clinical high risk population studies. Journal of Psychopharmacology (Oxford, England) 2014;29:144–154. doi: 10.1177/0269881114541015. [DOI] [PubMed] [Google Scholar]
- Carmona S., Proal E., Hoekzema E.A., Gispert J.-D., Picado M., Moreno I., Soliva J.C., Bielsa A., Rovira M., Hilferty J., Bulbena A., Casas M., Tobeña A., Vilarroya O. Ventro-striatal reductions underpin symptoms of hyperactivity and impulsivity in attention-deficit/hyperactivity disorder. Biol. Psychiatry. 2009;66:972–977. doi: 10.1016/j.biopsych.2009.05.013. [DOI] [PubMed] [Google Scholar]
- Carrión R.E., McLaughlin D., Goldberg T.E., Auther A.M., Olsen R.H., Olvet D.M., Correll C.U., Cornblatt B.A. Prediction of functional outcome in individuals at clinical high risk for psychosis. JAMA Psychiatry (Chicago, Ill.) 2013;70:1133–1142. doi: 10.1001/jamapsychiatry.2013.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocchi A., Lora A., Meneghelli A., La Greca E., Pisano A., Cascio M.T., Preti A. Sex differences in first-episode psychosis and in people at ultra-high risk. Psychiatry Res. 2014;215:314–322. doi: 10.1016/j.psychres.2013.11.023. [DOI] [PubMed] [Google Scholar]
- Cotter J., Drake R.J., Bucci S., Firth J., Edge D., Yung A.R. What drives poor functioning in the at-risk mental state? A systematic review. Schizophr. Res. 2014;159:267–277. doi: 10.1016/j.schres.2014.09.012. [DOI] [PubMed] [Google Scholar]
- Cropley V.L., Lin A., Nelson B., Reniers R.L.E.P., Yung A.R., Bartholomeusz C.F., Klauser P., Velakoulis D., McGorry P., Wood S.J., Pantelis C. Baseline grey matter volume of non-transitioned ‘ultra high risk’ for psychosis individuals with and without attenuated psychotic symptoms at long-term follow-up. Schizophr. Res. 2016;173:1527–1528. doi: 10.1016/j.schres.2015.05.014. [DOI] [PubMed] [Google Scholar]
- Dale A.M., Fischl B., Sereno M.I. Cortical surface-based analysis. I. Segmentation and surface reconstruction. NeuroImage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- de Wit S., Schothorst P.F., Oranje B., Ziermans T.B., Durston S., Kahn R.S. Adolescents at ultra-high risk for psychosis: long-term outcome of individuals who recover from their at-risk state. European Neuropsychopharmacology: The Journal of the European College of Neuropsychopharmacology. 2014;24:865–873. doi: 10.1016/j.euroneuro.2014.02.008. [DOI] [PubMed] [Google Scholar]
- Desikan R.S., Ségonne F., Fischl B., Quinn B.T., Dickerson B.C., Blacker D., Buckner R.L., Dale A.M., Maguire R.P., Hyman B.T., Albert M.S., Killiany R.J. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Fischl B., Sereno M.I., Dale A.M. Cortical surface-based analysis. II: inflation, flattening, and a surface-based coordinate system. NeuroImage. 1999;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Fornito A., Yung A.R., Wood S.J., Phillips L.J., Nelson B., Cotton S., Velakoulis D., McGorry P.D., Pantelis C., Yücel M. Anatomic abnormalities of the anterior cingulate cortex before psychosis onset: an MRI study of ultra-high-risk individuals. Biol. Psychiatry. 2008;64:758–765. doi: 10.1016/j.biopsych.2008.05.032. [DOI] [PubMed] [Google Scholar]
- Fornito A., Yücel M., Dean B., Wood S.J., Pantelis C. Anatomical abnormalities of the anterior cingulate cortex in schizophrenia: bridging the gap between neuroimaging and neuropathology. Schizophr. Bull. 2009;35:973–993. doi: 10.1093/schbul/sbn025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusar-Poli P., Borgwardt S. Integrating the negative psychotic symptoms in the high risk criteria for the prediction of psychosis. Med. Hypotheses. 2007;69:959–960. doi: 10.1016/j.mehy.2006.12.044. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P., Van Os J. Lost in transition: setting the psychosis threshold in prodromal research. Acta Psychiatr. Scand. 2013;127:248–252. doi: 10.1111/acps.12028. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P., Borgwardt S., Crescini A., Deste G., Kempton M.J., Lawrie S., Mc Guire P., Sacchetti E. Neuroanatomy of vulnerability to psychosis: a voxel-based meta-analysis. Neurosci. Biobehav. Rev. 2011;35:1175–1185. doi: 10.1016/j.neubiorev.2010.12.005. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P., Bonoldi I., Yung A.R., Borgwardt S., Kempton M.J., Valmaggia L., Barale F., Caverzasi E., McGuire P. Predicting psychosis: meta-analysis of transition outcomes in individuals at high clinical risk. Arch. Gen. Psychiatry. 2012;69:220–229. doi: 10.1001/archgenpsychiatry.2011.1472. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P., McGuire P., Borgwardt S. Mapping prodromal psychosis: a critical review of neuroimaging studies. European Psychiatry: The Journal of the Association of European Psychiatrists. 2012;27:181–191. doi: 10.1016/j.eurpsy.2011.06.006. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P., Borgwardt S., Bechdolf A., Addington J., Riecher-Rössler A., Schultze-Lutter F., Keshavan M., Wood S., Ruhrmann S., Seidman L.J., Valmaggia L., Cannon T., Velthorst E., De Haan L., Cornblatt B., Bonoldi I., Birchwood M., McGlashan T., Carpenter W., McGorry P., Klosterkötter J., McGuire P., Yung A. The psychosis high-risk state: a comprehensive state-of-the-art review. JAMA psychiatry. 2013;70:107–120. doi: 10.1001/jamapsychiatry.2013.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N., Nugent T.F., Herman D.H., Ordonez A., Greenstein D., Hayashi K.M., Clasen L., Toga A.W., Giedd J.N., Rapoport J.L., Thompson P.M. Dynamic mapping of normal human hippocampal development. Hippocampus. 2006;16:664–672. doi: 10.1002/hipo.20193. [DOI] [PubMed] [Google Scholar]
- Hall R.C. Global assessment of functioning. A modified scale. Psychosomatics. 1995;36:267–275. doi: 10.1016/S0033-3182(95)71666-8. [DOI] [PubMed] [Google Scholar]
- Lenroot R., Gogtay N., Greenstein D. Sexual dimorphism of brain developmental trajectories during childhood and adolescence. NeuroImage. 2007;36:1065–1073. doi: 10.1016/j.neuroimage.2007.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell M.E. Clinical Neurogenetics Branch, Intramural Research Program, National Institute of Mental Health; Bethesda, Maryland: 1982. Family Interview for Genetic Studies (FIGS): A Manual for FIGS. [Google Scholar]
- McGlashan T.H., Miller T.J., Woods S.W. PRIME Research Clinic, Yale School of Medicine; New Haven: 2001. Structured Interview for Prodromal Syndromes (SIPS) — Version 3.0. [Google Scholar]
- McGrath J., Saha S., Chant D., Welham J. Schizophrenia: a concise overview of incidence, prevalence, and mortality. Epidemiol. Rev. 2008;30:67–76. doi: 10.1093/epirev/mxn001. [DOI] [PubMed] [Google Scholar]
- Miller T.J., McGlashan T.H., Rosen J.L., Somjee L., Markovich P.J., Stein K., Woods S.W. Prospective diagnosis of the initial prodrome for schizophrenia based on the structured interview for prodromal syndromes: preliminary evidence of interrater reliability and predictive validity. Am. J. Psychiatry. 2002;159:863–865. doi: 10.1176/appi.ajp.159.5.863. [DOI] [PubMed] [Google Scholar]
- Reid M.A., Stoeckel L.E., White D.M., Avsar K.B., Bolding M.S., Akella N.S., Knowlton R.C., den Hollander J.A., Lahti A.C. Assessments of function and biochemistry of the anterior cingulate cortex in schizophrenia. Biol. Psychiatry. 2010;68:625–633. doi: 10.1016/j.biopsych.2010.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter M., Fischl B. Avoiding asymmetry-induced bias in longitudinal image processing. NeuroImage. 2011;57:19–21. doi: 10.1016/j.neuroimage.2011.02.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultze-Lutter F., Klosterkötter J. University of Cologne; Cologne: 2002. Bonn Scale for the Assessment of Basic Symptoms - Prediction List (BSABS-P) [Google Scholar]
- Shenton M.E., Dickey C.C., Frumin M., McCarley R.W. A review of MRI findings in schizophrenia. Schizophr. Res. 2001;49:1–52. doi: 10.1016/s0920-9964(01)00163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon A.E., Velthorst E., Nieman D.H., Linszen D., Umbricht D., de Haan L. Ultra high-risk state for psychosis and non-transition: a systematic review. Schizophr. Res. 2011;132:8–17. doi: 10.1016/j.schres.2011.07.002. [DOI] [PubMed] [Google Scholar]
- Simon A.E., Borgwardt S., Riecher-Rössler A., Velthorst E., de Haan L., Fusar-Poli P. Moving beyond transition outcomes: meta-analysis of remission rates in individuals at high clinical risk for psychosis. Elsevier Psychiatry Research. 2013;209:266–272. doi: 10.1016/j.psychres.2013.03.004. [DOI] [PubMed] [Google Scholar]
- Sprong M., Becker H.E., Schothorst P.F., Swaab H., Ziermans T.B., Dingemans P.M., Linszen D., van Engeland H. Pathways to psychosis: a comparison of the pervasive developmental disorder subtype multiple complex developmental disorder and the ‘at risk mental state’. Schizophr. Res. 2008;99:38–47. doi: 10.1016/j.schres.2007.10.031. [DOI] [PubMed] [Google Scholar]
- Steen R.G., Hamer R.M., Lieberman J.A. Measuring brain volume by MR imaging: impact of measurement precision and natural variation on sample size requirements. AJNR Am. J. Neuroradiol. 2007;28:1119–1125. doi: 10.3174/ajnr.A0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanis N., Frangou S., Yakeley J., Sharma T., O'Connell P., Morgan K., Sigmudsson T., Taylor M., Murray R. Hippocampal volume reduction in schizophrenia: effects of genetic risk and pregnancy and birth complications. Biol. Psychiatry. 1999;46:697–702. doi: 10.1016/s0006-3223(99)00089-x. [DOI] [PubMed] [Google Scholar]
- Sun D., Phillips L., Velakoulis D., Yung A., McGorry P.D., Wood S.J., van Erp T.G.M., Thompson P.M., Toga A.W., Cannon T.D., Pantelis C. Progressive brain structural changes mapped as psychosis develops in “at risk” individuals. Schizophr. Res. 2009;108:85–92. doi: 10.1016/j.schres.2008.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walder D.J., Holtzman C.W., Addington J., Cadenhead K., Tsuang M., Cornblatt B., Cannon T.D., McGlashan T.H., Woods S.W., Perkins D.O., Seidman L.J., Heinssen R., Walker E.F. Sexual dimorphisms and prediction of conversion in the NAPLS psychosis prodrome. Schizophr. Res. 2013;144:43–50. doi: 10.1016/j.schres.2012.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Psychological Corporation Ltd., Harcourt Publishers; 1997. Wechsler Adult Intelligence Scale-III NL: Afname en scorings handleiding [Manual] [Google Scholar]
- Wechsler D. Psychological Corporation Ltd., Harcourt Assessment; 2002. Wechsler Intelligence Scale for Children-III NL: Handleiding en verantwoording [Manual] [Google Scholar]
- Wierenga L.M., Langen M., Oranje B., Durston S. Unique developmental trajectories of cortical thickness and surface area. NeuroImage. 2014;87:120–126. doi: 10.1016/j.neuroimage.2013.11.010. [DOI] [PubMed] [Google Scholar]
- Wood S.J., Pantelis C., Velakoulis D., Yücel M., Fornito A., McGorry P.D. Progressive changes in the development toward schizophrenia: studies in subjects at increased symptomatic risk. Schizophr. Bull. 2008;34:322–329. doi: 10.1093/schbul/sbm149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood S.J., Reniers R.L.E.P., Heinze K. Neuroimaging findings in the at-risk mental state: a review of recent literature. Can. J. Psychiatr. 2013;58:13–18. doi: 10.1177/070674371305800104. [DOI] [PubMed] [Google Scholar]
- Yung A.R., Nelson B., Thompson A., Wood S.J. The psychosis threshold in ultra high risk (prodromal) research: is it valid? Schizophr. Res. 2010;120:1–6. doi: 10.1016/j.schres.2010.03.014. [DOI] [PubMed] [Google Scholar]
- Zalesky A., Pantelis C., Cropley V., Fornito A., Cocchi L., McAdams H., Clasen L., Greenstein D., Rapoport J.L., Gogtay N. Delayed development of brain connectivity in adolescents with schizophrenia and their unaffected siblings. JAMA Psychiatry. 2015;72:900–908. doi: 10.1001/jamapsychiatry.2015.0226. [DOI] [PubMed] [Google Scholar]
- Ziermans T.B., Schothorst P.F., Sprong M., van Engeland H. Transition and remission in adolescents at ultra-high risk for psychosis. Schizophr. Res. 2011;126:58–64. doi: 10.1016/j.schres.2010.10.022. [DOI] [PubMed] [Google Scholar]
- Ziermans T.B., Schothorst P.F., Schnack H.G., Koolschijn P.C.M.P., Kahn R.S., van Engeland H., Durston S. Progressive structural brain changes during development of psychosis. Schizophr. Bull. 2012;38:519–530. doi: 10.1093/schbul/sbq113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziermans T., de Wit S., Schothorst P., Sprong M., van Engeland H., Kahn R., Durston S. Neurocognitive and clinical predictors of long-term outcome in adolescents at ultra-high risk for psychosis: a 6-year follow-up. PLoS One. 2014;9 doi: 10.1371/journal.pone.0093994. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Operationalisations of resilience.
Linear mixed models.
Demographic and clinical data of remitted and non-remitted UHR individuals based on UHR remission criteria.
Overlap between the two different analyses, (1) resilience based on functional outcome, (2) subgrouping based on remission from UHR criteria.
Stable and developmental differences between UHR individuals and typically developing controls (TDC).
Stable and developmental differences in brain development between resilient (R) and non-resilient (NR) UHR individuals based on functional outcome.
Stable and developmental differences between remitted (R) and non-remitted (NR) UHR individuals based on UHR remission criteria.