Abstract
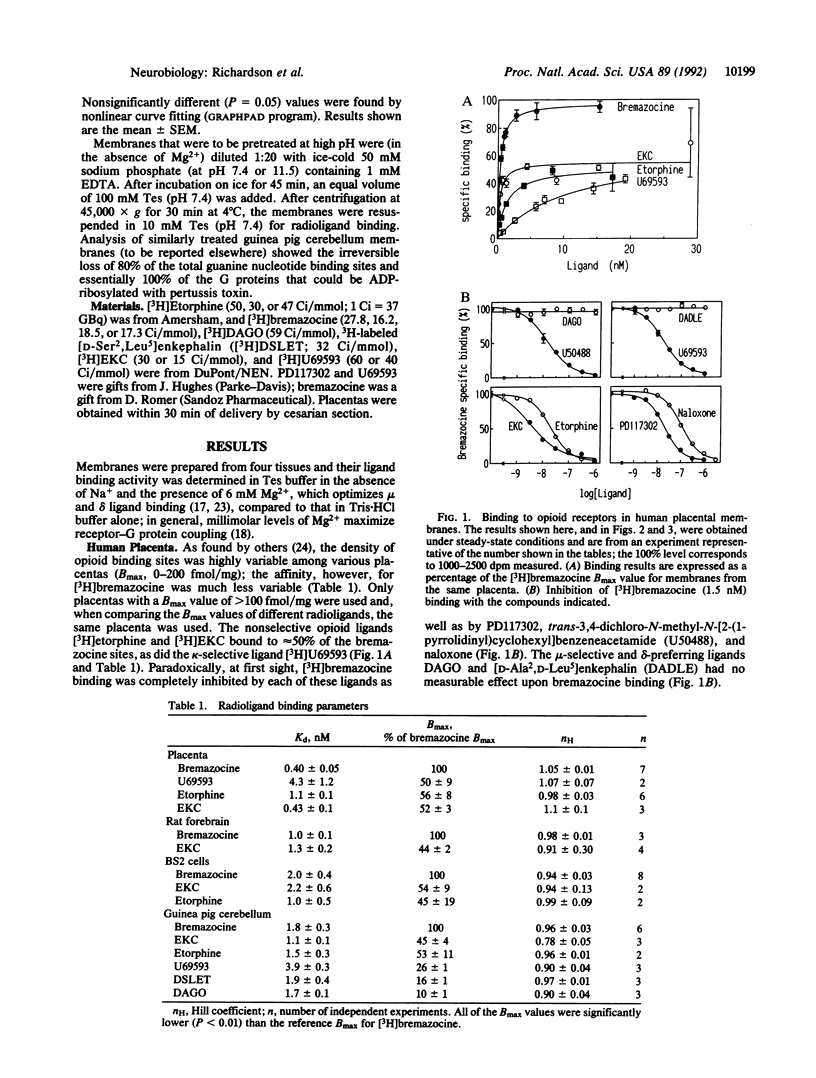
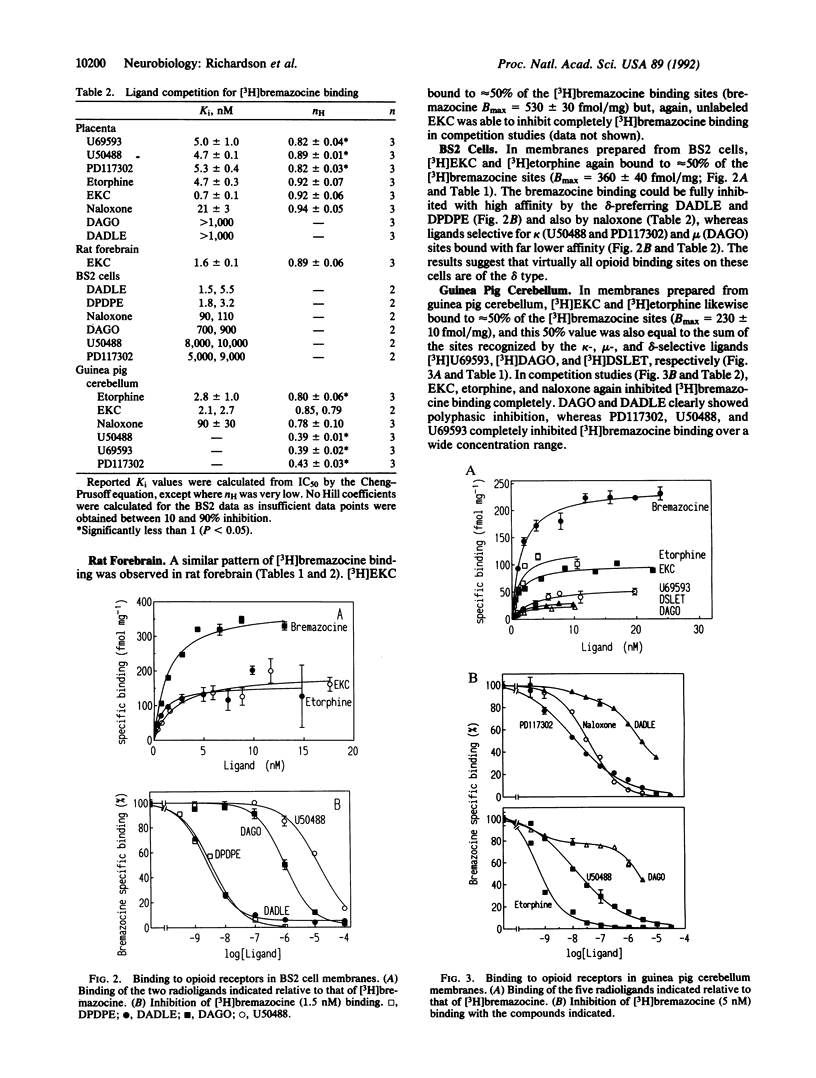
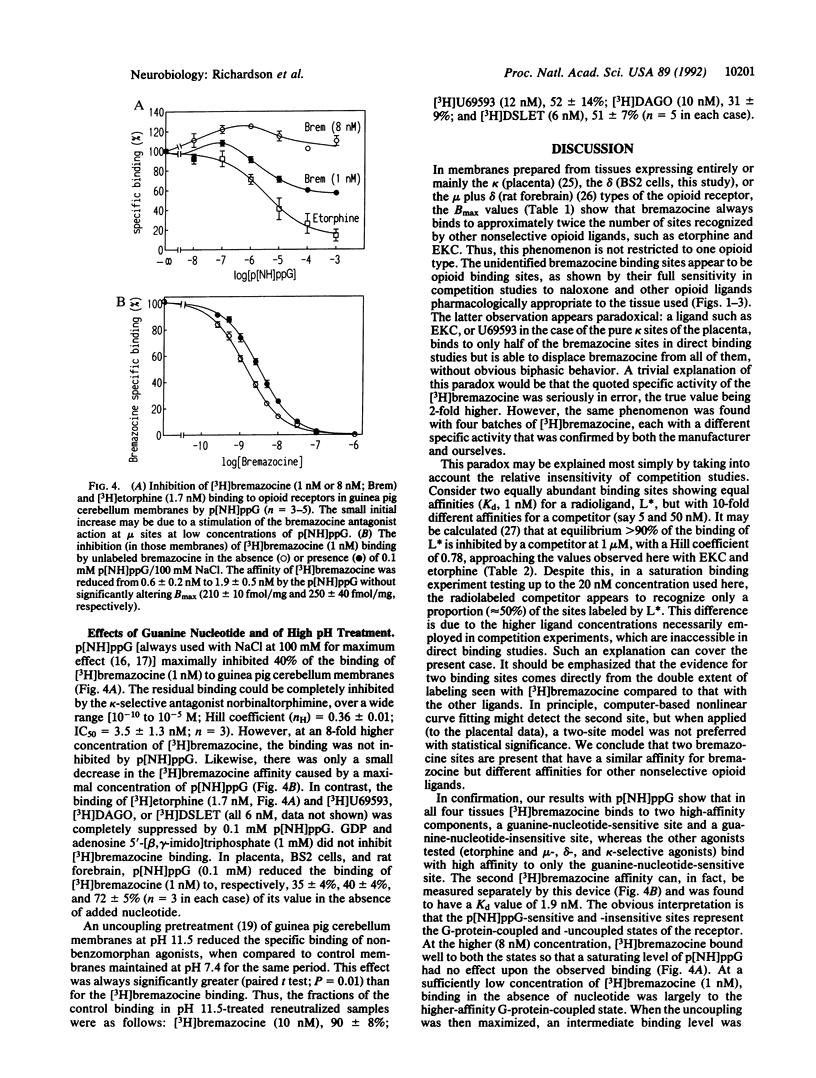
Opioid receptors are currently classified as mu, delta, and kappa types, but various subtypes have also been proposed. We have investigated whether subtypes exist by using [3H]bremazocine. [3H]Bremazocine binds to twice as many naloxone-sensitive sites as other nonselective opioid agonists, as shown in four membrane types that have very different ratios of mu, delta, and kappa receptor types. [3H]Bremazocine binding is completely inhibited by an excess (in unlabeled form) of other opioid ligands, with Hill coefficients of 0.8-0.95. These paradoxes can be explained if there are high- and low-affinity states of the mu, delta, and kappa receptors and bremazocine binds with similar affinities to both states. We propose that these states are the guanine nucleotide-binding protein (G-protein)-coupled form and the uncoupled form of each receptor. As evidence for this proposal, the [3H]bremazocine binding suffered little or no loss with G-protein-uncoupling treatments, whereas binding of other opioid agonists was fully sensitive. We conclude that [3H]bremazocine offers a tool for the measurement of the total pools of coupled and uncoupled opioid receptors and that much of the previous characterization of opioid receptor subtypes reflects, instead, a significant pool of G-protein-uncoupled opioid receptors.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed M. S., Schinfeld J. S., Jones R., Cavinato A. G., Baker C. Correlation between the number of placental opioid receptors, mode of delivery, and maternal narcotic use. Membr Biochem. 1986;6(3):255–267. doi: 10.3109/09687688609065452. [DOI] [PubMed] [Google Scholar]
- Birnbaumer L., Abramowitz J., Brown A. M. Receptor-effector coupling by G proteins. Biochim Biophys Acta. 1990 May 7;1031(2):163–224. doi: 10.1016/0304-4157(90)90007-y. [DOI] [PubMed] [Google Scholar]
- Citri Y., Schramm M. Resolution, reconstitution and kinetics of the primary action of a hormone receptor. Nature. 1980 Sep 25;287(5780):297–300. doi: 10.1038/287297a0. [DOI] [PubMed] [Google Scholar]
- Clark C. R., Birchmore B., Sharif N. A., Hunter J. C., Hill R. G., Hughes J. PD117302: a selective agonist for the kappa-opioid receptor. Br J Pharmacol. 1988 Mar;93(3):618–626. doi: 10.1111/j.1476-5381.1988.tb10319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark J. A., Liu L., Price M., Hersh B., Edelson M., Pasternak G. W. Kappa opiate receptor multiplicity: evidence for two U50,488-sensitive kappa 1 subtypes and a novel kappa 3 subtype. J Pharmacol Exp Ther. 1989 Nov;251(2):461–468. [PubMed] [Google Scholar]
- Corbett A. D., Kosterlitz H. W. Bremazocine is an agonist at kappa-opioid receptors and an antagonist at mu-opioid receptors in the guinea-pig myenteric plexus. Br J Pharmacol. 1986 Sep;89(1):245–249. doi: 10.1111/j.1476-5381.1986.tb11141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demoliou-Mason C. D., Barnard E. A. Distinct subtypes of the opioid receptor with allosteric interactions in brain membranes. J Neurochem. 1986 Apr;46(4):1118–1128. doi: 10.1111/j.1471-4159.1986.tb00626.x. [DOI] [PubMed] [Google Scholar]
- Evans T., Hepler J. R., Masters S. B., Brown J. H., Harden T. K. Guanine nucleotide regulation of agonist binding to muscarinic cholinergic receptors. Relation to efficacy of agonists for stimulation of phosphoinositide breakdown and Ca2+ mobilization. Biochem J. 1985 Dec 15;232(3):751–757. doi: 10.1042/bj2320751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frances B., Moisand C., Meunier J. C. Na+ ions and Gpp(NH)p selectively inhibit agonist interactions at mu- and kappa-opioid receptor sites in rabbit and guinea-pig cerebellum membranes. Eur J Pharmacol. 1985 Nov 5;117(2):223–232. doi: 10.1016/0014-2999(85)90607-7. [DOI] [PubMed] [Google Scholar]
- Gillan M. G., Kosterlitz H. W. Spectrum of the mu, delta- and kappa-binding sites in homogenates of rat brain. Br J Pharmacol. 1982 Nov;77(3):461–469. doi: 10.1111/j.1476-5381.1982.tb09319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes A., Kelly A. Profile of activity of kappa receptor agonists in the rabbit vas deferens. Eur J Pharmacol. 1985 Apr 16;110(3):317–322. doi: 10.1016/0014-2999(85)90558-8. [DOI] [PubMed] [Google Scholar]
- Hunter J. C., Leighton G. E., Meecham K. G., Boyle S. J., Horwell D. C., Rees D. C., Hughes J. CI-977, a novel and selective agonist for the kappa-opioid receptor. Br J Pharmacol. 1990 Sep;101(1):183–189. doi: 10.1111/j.1476-5381.1990.tb12110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent R. S., De Lean A., Lefkowitz R. J. A quantitative analysis of beta-adrenergic receptor interactions: resolution of high and low affinity states of the receptor by computer modeling of ligand binding data. Mol Pharmacol. 1980 Jan;17(1):14–23. [PubMed] [Google Scholar]
- Lahti R. A., Mickelson M. M., McCall J. M., Von Voigtlander P. F. [3H]U-69593 a highly selective ligand for the opioid kappa receptor. Eur J Pharmacol. 1985 Feb 26;109(2):281–284. doi: 10.1016/0014-2999(85)90431-5. [DOI] [PubMed] [Google Scholar]
- Leff S. E., Creese I. Interactions of dopaminergic agonists and antagonists with dopaminergic D3 binding sites in rat striatum. Evidence that [3H]dopamine can label a high affinity agonist-binding state of the D1 dopamine receptor. Mol Pharmacol. 1985 Feb;27(2):184–192. [PubMed] [Google Scholar]
- Lynch C. J., Taylor S. J., Smith J. A., Exton J. H. Formation of the high-affinity agonist state of the alpha 1-adrenergic receptor at cold temperatures does not require a G-protein. FEBS Lett. 1988 Feb 29;229(1):54–58. doi: 10.1016/0014-5793(88)80796-8. [DOI] [PubMed] [Google Scholar]
- MacDermot J., Higashida H., Wilson S. P., Matsuzawa H., Minna J., Nirenberg M. Adenylate cyclase and acetylcholine release regulated by separate serotonin receptors of somatic cell hybrids. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1135–1139. doi: 10.1073/pnas.76.3.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKnight A. T., Corbett A. D., Marcoli M., Kosterlitz H. W. The opioid receptors in the hamster vas deferens are of the delta-type. Neuropharmacology. 1985 Nov;24(11):1011–1017. doi: 10.1016/0028-3908(85)90184-4. [DOI] [PubMed] [Google Scholar]
- Miller L., Shaw J. S., Whiting E. M. The contribution of intrinsic activity to the action of opioids in vitro. Br J Pharmacol. 1986 Mar;87(3):595–601. doi: 10.1111/j.1476-5381.1986.tb10202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris B. J., Herz A. Control of opiate receptor number in vivo: simultaneous kappa-receptor down-regulation and mu-receptor up-regulation following chronic agonist/antagonist treatment. Neuroscience. 1989;29(2):433–442. doi: 10.1016/0306-4522(89)90070-5. [DOI] [PubMed] [Google Scholar]
- Mosberg H. I., Hurst R., Hruby V. J., Gee K., Yamamura H. I., Galligan J. J., Burks T. F. Bis-penicillamine enkephalins possess highly improved specificity toward delta opioid receptors. Proc Natl Acad Sci U S A. 1983 Oct;80(19):5871–5874. doi: 10.1073/pnas.80.19.5871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nock B., Rajpara A., O'Connor L. H., Cicero T. J. Autoradiography of [3H]U-69593 binding sites in rat brain: evidence for kappa opioid receptor subtypes. Eur J Pharmacol. 1988 Sep 1;154(1):27–34. doi: 10.1016/0014-2999(88)90359-7. [DOI] [PubMed] [Google Scholar]
- Porthé G., Valette A., Cros J. Kappa opiate binding sites in human placenta. Biochem Biophys Res Commun. 1981 Jul;101(1):1–6. doi: 10.1016/s0006-291x(81)80002-2. [DOI] [PubMed] [Google Scholar]
- Robson L. E., Gillan M. G., Kosterlitz H. W. Species differences in the concentrations and distributions of opioid binding sites. Eur J Pharmacol. 1985 May 28;112(1):65–71. doi: 10.1016/0014-2999(85)90239-0. [DOI] [PubMed] [Google Scholar]
- Rothman R. B., Bykov V., de Costa B. R., Jacobson A. E., Rice K. C., Brady L. S. Interaction of endogenous opioid peptides and other drugs with four kappa opioid binding sites in guinea pig brain. Peptides. 1990 Mar-Apr;11(2):311–331. doi: 10.1016/0196-9781(90)90088-m. [DOI] [PubMed] [Google Scholar]
- Tiberi M., Magnan J. Demonstration of the heterogeneity of the kappa-opioid receptors in guinea-pig cerebellum using selective and nonselective drugs. Eur J Pharmacol. 1990 Jun 12;188(6):379–389. doi: 10.1016/0922-4106(90)90198-7. [DOI] [PubMed] [Google Scholar]
- Werling L. L., Puttfarcken P. S., Cox B. M. Multiple agonist-affinity states of opioid receptors: regulation of binding by guanyl nucleotides in guinea pig cortical, NG108-15, and 7315c cell membranes. Mol Pharmacol. 1988 Apr;33(4):423–431. [PubMed] [Google Scholar]
- Wood M. S., Traynor J. R. [3H]diprenorphine binding to kappa-sites in guinea-pig and rat brain: evidence for apparent heterogeneity. J Neurochem. 1989 Jul;53(1):173–178. doi: 10.1111/j.1471-4159.1989.tb07310.x. [DOI] [PubMed] [Google Scholar]
- Zukin R. S., Eghbali M., Olive D., Unterwald E. M., Tempel A. Characterization and visualization of rat and guinea pig brain kappa opioid receptors: evidence for kappa 1 and kappa 2 opioid receptors. Proc Natl Acad Sci U S A. 1988 Jun;85(11):4061–4065. doi: 10.1073/pnas.85.11.4061. [DOI] [PMC free article] [PubMed] [Google Scholar]


