Although adipose-derived stem cells (ASCs) hold the promise of effective therapy for myocardial infarction (MI), low cardiac retention of implanted ASCs has hindered their therapeutic efficiency. We investigated whether an externally applied static magnetic field (SMF) enhanced cardiac localization of “magnetic” ASCs preloaded with superparamagnetic iron oxide (SPIO) nanoparticles and further improved heart function recovery. In conclusion, the SMF increased the cardiac retention of implanted “magnetic” SPIO-labeled ASCs and enhanced heart function recovery after MI.
Keywords: Adipose-derived stem cells, Static magnetic field, Myocardial infarction, Superparamagnetic iron oxide, Cell retention
Abstract
Although adipose-derived stem cells (ASCs) hold the promise of effective therapy for myocardial infarction, low cardiac retention of implanted ASCs has hindered their therapeutic efficiency. We investigated whether an externally applied static magnetic field (SMF) enhances cardiac localization of "magnetic" cells and promotes heart function recovery when ASCs are preloaded with superparamagnetic iron oxide (SPIO) nanoparticles. The influence of SMF (0.1 Tesla) on the biological activities of SPIO-labeled ASCs (SPIOASCs) was investigated first. Fifty-six female rats with myocardial infarction underwent intramyocardial injection of cell culture medium (CCM) or male SPIOASCs with or without the subcutaneous implantable magnet (CCM-magnet or SPIOASC-magnet). Four weeks later, endothelial differentiation, angiogenic cytokine secretion, angiogenesis, cardiomyocyte apoptosis, cell retention, and cardiac performance were examined. The 0.1-Tsela SMF did not adversely affect the viability, proliferation, angiogenic cytokine secretion, and DNA integrity of SPIOASCs. The implanted SPIOASCs could differentiate into endothelial cell, incorporate into newly formed vessels, and secrete multiple angiogenic cytokines. Four weeks after cell transplantation, the number of cardiac SPIOASCs was significantly increased, vascular density was markedly enlarged, fewer apoptotic cardiomyocytes were present, and heart contractile function was substantially improved in the SPIOASC-magnet treated rats in comparison with the SPIOASC-treated rats. The SPIOASCs could differentiate into endothelial cells, incorporate into vessels, promote angiogenesis, and inhibit ischemic cardiomyocyte apoptosis. An externally applied SMF offered a secure environment for biological properties of SPIOASCs, increased the cardiac retention of implanted magnetic SPIOASCs, and further enhanced heart function recovery after myocardial infarction.
Significance
This pilot proof-of-concept study suggests that a 0.1-Tesla static magnetic field does not adversely affect the viability, proliferation, angiogenic cytokine secretion, or DNA integrity of the superparamagnetic iron oxide-labeled adipose-derived stem cells (SPIOASCs). Implantation of adipose-derived stem cells promotes myocardial neovascularization and inhibits ischemic cardiomyocyte apoptosis through endothelial differentiation, incorporation into vessels, and paracrine factor secretion. An externally applied static magnetic field enhanced myocardial retention of intramyocardially injected "magnetic" SPIOASCs and promoted cardiac function recovery after myocardial infarction. With further preclinical optimization, this approach may improve the outcome of current stem cell therapy for ischemic myocardial infarction.
Introduction
Adipose tissue comprises a heterogeneous cell population including endothelial cells and mesenchymal stem cells. The latter are called adipose-derived stem cells (ASCs) [1, 2]. Adipose tissue has several advantages over other tissues as a resource for mesenchymal stem cells: the abundance in most of population, easily and repetitive harvest with a minimally invasive procedure, and rapid proliferation [3]. ASCs have been demonstrated to be able to differentiate into endothelial cells and smooth muscle cells [4, 5] and to secrete proangiogenic and antiapoptotic growth factors [6, 7]. The transplantation of ASCs improves cardiac function following myocardial infarction (MI), primarily by induced neovascularization and prevention of cardiomyocyte apoptosis [8–11].
Although stem cell transplantation is a promising therapy for MI, low cardiac cell retention and engraftment are major obstacles to achieving a significant functional benefit. At 6 days after cell transplantation, the percentages of the delivered cells trapped in the lung are approximately 26%, 47%, and 43% for intramyocardial, intracoronary, and intravenous delivery, respectively [12]. The corresponding cardiac retention rates are 11%, 2.6%, and 3.2%, respectively [12]. Intra-aortic delivery accompanies the cell distribution in heart, kidney, lung, liver, and spleen [13]. After intravenous delivery, many cells are observed in the lungs, with only a few implanted cells presented in the hearts [13]. Although intramyocardial delivery produces the greatest cell retention in the hearts in comparison with other approaches, approximately 50% of cells escape from the heart to other organs, such as the lungs, within 1 hour of cell delivery [14]. Evidently, most implanted cells are trapped and colonized, none specifically in untargeted extracardiac organs. Most studies only demonstrated that ejection fraction (EF) was mildly or moderately increased by the transplantation of stem cells [8–11].
Externally applied static magnetic field (SMF) has been used to direct drugs, cytokines, and other molecules chemically bound to magnetic particles into targeted organisms for maximization of therapeutic effects and minimization of systemic side effects [15]. The SMF around the tumor significantly enhanced the delivery of magnetic particle-conjugated anticancer drugs into the targeted solid tumor after intravascular administration [16, 17]. Externally applied SMF increased the uptake of biocompatible magnetic nanoparticle-labeled monocytes into tumor by threefold after intravenous injection [18]. The main mechanism was predominantly related to the fact that the magnetic targeting approach induced extravasation of magnetic particle-combined agents through the vascular wall and led to localization and retention in the targeted site [17]. Superparamagnetic iron oxide (SPIO) nanoparticles coated with dextrin (Feridex, Bayer HealthCare Pharmaceuticals, Whippany, NJ, http://www.bayer.com/) are nontoxic and biodegradable and have been approved by the U.S. Food and Drug Administration for clinical practice as magnetic resonance contrast agents in humans [19, 20]. SPIOs are efficiently internalized by a wide variety of cells, including ASCs. The "magnetic" iron-labeled ASCs were thus obtained. The labeling of ASCs with SPIOs is safe without affecting the viability, proliferation, differentiation potential, antigenic phenotype, and cell factor secretion of ASCs [21, 22].
In this study, ASCs were preloaded with SPIOs and an SMF was generated above the heart by subcutaneous insertion of a magnet over the chest cavity to attract these magnetic cells to the targeted area. We first examined the magnetic safety and magnetophoretic attraction of the SPIO-labeled ASCs (SPIOASCs) in vitro, with the same magnet as that subcutaneously implanted in vivo. We further investigate whether externally applied SMF enhances the cardiac retention and functional benefit of magnetic SPIOASCs in infarcted hearts.
Materials and Methods
All animals received care by humans, and the study was approved by the Institutional Review Board and Animal Care Committee of Huazhong University of Science and Technology and National Research Council of Canada.
Preparation of ASCs
ASCs were isolated from 12-month-old male transgenic rats expressing green fluorescent protein (GFP). Subcutaneous adipose tissue was obtained from the abdominal and inguinal regions of the rats, minced and digested with collagenase I (2 mg/ml, Worthington Biochemical Corp., Lakewood, NJ, http://www.worthington-biochem.com) at 37°C for 20–30 minutes. Collagenase activity was neutralized by adding DMEM-F12 (HyClone, GE Healthcare, Logan, UT, http://www.gelifesciences.com) containing 15% fetal bovine serum (FBS, HyClone, GE Healthcare). The digested adipose tissue was filtered twice with a 100-μm and then with a 25-μm nylon membrane to eliminate the undigested fragments. Cellular suspension was centrifuged at 1,000 g for 10 minutes. Cell pellets were resuspended in cell-culture medium (CCM) and cultivated for 24 hours at 37°C in 5% CO2.
The GFP-positive ASCs were incubated for 2 days in a CCM containing 50 μg/ml SPIO nanoparticles (Feridex) and 6 μg/ml protamine sulfate. On the day of cell transplantation, the SPIOASCs were trypsinized and the detached cells were then centrifuged. The supernatant was removed, and FBS-free medium was added to the cell pellet.
Flow Cytometry Analysis
Adherent ASCs were resuspended with 0.25% trypsin. Then the suspended ASCs were fixed for 10 minutes in 1% paraformaldehyde. The ASCs were then washed twice with phosphate-buffered saline (PBS) and incubated with primary antibodies at room temperature for 30 minutes. The antibodies used were fluorescein isothiocyanate-conjugated anti-rat CD11b, CD34, CD45, CD59, CD29, and CD90.1 (Thermo Fisher Scientific Life Sciences, Waltham, MA, http://www.thermofisher.com). Flow cytometric analysis was performed on a fluorescence-activated cell sorter (BD Biosciences, San Jose, CA, https://www.bdbiosciences.com).
In Vitro SPIOASCs Under the Exposure of SMF
The SPIOASCs were resuspended in one column of a 24-well plate, which was superimposed on the top of the same magnet as that subsequently used in vivo. The magnetic field intensity was approximately 0.1 Tesla on the inner wall of plate by measurement with a handheld Gauss meter. The ASCs and SPIOASCs cultured without SMF were used as control. One week later, the cells from three cell groups (ASCs, SPIOASCs, SPIOASCs-magnet) were collected for assessment of growth, proliferation, cytokine secretion, and DNA integrity.
To investigate the ability of a magnet to capture SPIOASCs in vitro, the magnetic SPIOASCs were suspended in a cell-cultured dish. A circular magnet was directly applied to the outside dish bottom wall. After 2 days of culture, cell condensation was assessed visually under a phase-contrast microscopy.
MTT Assay
The viability of ASCs was measured by the (3-[4,5-methylthiazol-2-yl]-2,5-diphenyl-tetrazolium bromide) (MTT) assay (Sigma-Aldrich, St. Louis, MO, http://www.sigmaaldrich.com). Briefly, a 5-mg/ml MTT salt solution was added to cells to give a final concentration of 2.5 mg/ml MTT. Cells were then incubated at 37°C for 1 hour. The final formazan product was dissolved in dimethyl sulfoxide and absorbance was measured at 570 nm. The amount of formazan was directly proportional to the number of live cells.
Cell Proliferation Assay
Proliferation of ASCs was assessed by cell counting kit assays. A cell counting kit reagent (Sigma-Aldrich) was added to the well and incubated for 2 hours. Absorbance value was measured at 450 nm using a microplate reader.
Reverse Transcriptase-Polymerase Chain Reaction
Total RNA from the ASCs was extracted using the TRIzol Reagent (Thermo Fisher Scientific Life Sciences) protocol. One microgram of RNA was reversely transcribed using SuperScript III reverse transcriptase (RT; Thermo Fisher Scientific Life Sciences). cDNA was used as a template for polymerase chain reaction (PCR) amplification. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an internal control for RNA extraction and RT-PCR assay. The following primers were used: for vascular endothelial growth factor (VEGF), were upstream primer 5′-TCTTCCCTGGAGACCTGAGA-3′ and downstream primer 5′- ACGGAATATCTCGGAAAACTGCTC-3′; for hepatocyte growth factor (HGF), upstream primer 5′- ACACAAACAACAGTAGGGTGGATGG-3′ and downstream primer 5′- TTGGGATGGC ACATCCACGA CCAGG-3′; for insulin-like growth factor-1 (IGF-1), upstream primer 5′- CATGTCGTCT TCACATCTCTTCTAC-3′ and downstream primer 5′- CTTGTGTGTCGATAGGGGCTGGGAC-3′; and for GAPDH, upstream primer 5′- ATCTGACATGCCGCCTGGAGAAACC-3′ and downstream primer 5′- CAGGGTTTCTTACTCCTTGGAGGCC-3′.
Single Cell Gel Electrophoresis (Comet Assay)
ASCs at the cell density 2 × 104 cells/ml were spread into the agarose-covered surface of a precoated slide. After the agarose has gelled, the sides were submerged in a covered dish containing alkaline lysis solution overnight at 4°C. After three rinses, the electrophoresis was conducted in the slides submerged in a chamber for 25 minutes at a voltage of 0.6 V/cm. Then, the slides were rinsed with distilled water and stained in 2.5 μg/ml propidium iodide for 20 minutes. The stained slides were analyzed under a fluorescence microscope. Normal ASCs treated with 2.5 mM H2O2 at room temperature for 10 minutes were used as positive control.
Animal Model and Experimental Protocol
Female rats (n = 56) with an average body weight of 200 g were anesthetized through inhalation of 1.5%–2% isoflurane in oxygen, followed by intubation and mechanical ventilation with a rodent ventilator at a rate of 60–70 breaths/min and tidal volume of 2–3 ml. A left anterior thoracotomy was made through the fourth intercostal space. The left anterior descending artery (LAD) was permanently occluded at about 2–3 mm from its origin using a 7-0 silk suture. Coronary ligation generated an infarct demonstrated by macroscopic blanching (supplemental online Fig. 1A). Cardiac cine magnetic resonance imaging (MRI) was performed before and after surgery with double inversion recovery fast spin echo black-blood sequence. The postinfarct hearts displayed the depressed shortening of left ventricular (LV) anterio-lateral wall (supplemental online Fig. 2A). Absolute reduction of approximately 15% in the shortening fraction and 26% in the LV ejection fraction (LVEF) indicated the efficiency of the surgical procedure to induce MI (supplemental online Fig. 2B and 2C). Moreover, in the magnet groups, postsurgery MRI was impossible because of the subcutaneous implantation of magnet.
Immediately after LAD occlusion, the rats were randomly divided into four groups (CCM, n = 8; CCM-magnet, n = 8; SPIOASCs, n = 20; and SPIOASCs-magnet, n = 20). In the rats receiving ASC implantation, four injections of approximately 1.5 × 106 GFP-positive SPIOASCs with total volume of 150 µl were made into the infarct border using a 30-gauge needle (supplemental online Fig. 1B). The CCM-magnet treated rats and SPIOASCs-magnet-treated rats underwent the placement of a circular magnet above the heart for 60 seconds (supplemental online Fig. 1C) and the subsequent subcutaneous insertion of the disc magnet over the chest cavity immediately after suture closure of the costal margin (supplemental online Fig. 1D). There was a 1.6 ± 0.2-mm distance from the disc magnet to the surface of infarcted myocardium. The disc magnet produced the approximately 0.1-Tesla SMF on the surface of LV anterio-lateral wall. CCM control rats and CCM-magnet-treated rats underwent four injections of 150 µl CCM in the same regions. Rats (n = 6 for each cell-injected group) were euthanized and hearts were cryo-sectioned 1 week after surgery to assess short-term cell localization and retention. The numbers of GFP-positive cells were counted in at least 3 high-power fields (HPFs) in infarcted regions of each section under a fluorescence microscope. The histological analysis indicated that the preinfarct region contained the cellular cluster of GFP-positive SPIOASCs and cells containing blue-stained particles (supplemental online Fig. 1E and 1F).
Hematoxylin-Eosin and Prussian Blue Staining
Heart tissue sections from rats (n = 6 for each cell-injected group at 1 week after surgery) were immersed in Prussian blue reagent (4% potassium ferrocyanide/12% HCl, 50:50 vol/vol) for 40 minutes under agitation. The tissue sections were then washed once in PBS and twice in deionized water. The tissue sections were counterstained through standard hematoxylin-eosin procedure. Then, the slides were mounted by antifade mounting media. The sections were examined under a light microscope.
Cardiac Cine MRI
Rats (n = 8 for each CCM-injected group, n = 14 for each cell-injected group at 4 weeks after surgery) were endotracheally incubated, mechanically ventilated with oxygen comprising 1.5%–2% isoflurane using a ventilator, and positioned prone in a cradle. Standard limb leads were constructed from electrodes placed on both forepaws and the left hind paw. The cradle was inserted into an in-house manufactured quadrature MR coil. The coil was positioned in the center of the horizontal bore of a 7-Tesla Bruker magnet interfaced to Bruker Biospec console (Bruker, Karlsruhe, Germany, https://www.bruker.com/).
Cardiac cine imaging was performed using a gradient-echo sequence with a repetition time of 9.2 milliseconds, echo time of 3.5 milliseconds, field of view of 8 × 8 cm2, and matrix size of 256 × 256. The cine images were acquired from 5 consecutive slices along the short cardiac axis with a slice thickness of 2.0 mm and no gap between the slices.
Real-Time PCR
Male SPIOASCs were intramyocardially injected into the myocardium of female rats, enabling detection of Sry gene located on the Y chromosome as an index of engraftment. Quantitative real-time PCR was performed 4 weeks after cell injection (n = 6 for each cell-injected group at 4 weeks after surgery). The DNA of the hearts (n = 6 for each cell-injected group at 4 weeks after surgery) was extracted with the Qiagen kit (Qiagen, Mississauga, Ontario, Canada, https://www.qiagen.com). The total amount of DNA was measured by spectrophotometry. Real-time PCR was performed with SYBR-green (Thermo Fisher Scientific Life Sciences). The SYBR-green I dye binds to the double-stranded product, resulting in an increase in fluorescence detected by the ABI 7900HT Sequence Detection System (Thermo Fisher Scientific Life Sciences). A specific sequence of rat Sry3 gene in the Y chromosome was targeted. The genomic DNA taken from male SMCs was used to obtain a standard curve as described previously. The primer pairs are 5′-GTT CAG CCC TAC AGC CTG AGG ACA T-3′ and 5′-GGA TTC TGT TGA GCC AAC TTG CGC C-3′. The cycling conditions were 10 minutes at 95°C for activation of polymerase and then 10 seconds at 95°C for denaturation, 10 seconds at 60°C inducing annealing, and 15 seconds at 72°C for extension. Forty-five cycles were used.
Immunofluorescence Analysis
The frozen rat hearts (n = 8 for each rat group at 4 weeks after surgery) were transversely cryosectioned into 8-µm-thick slices from apex to the base. Tissue sections were fixed with 4% paraformaldehyde for 10 minutes at room temperature, washed three times with PBS containing 0.3% Triton X-100, and blocked with 2% goat serum and 1% bovine serum albumin for 30 minutes at room temperature. Slides were then incubated with primary antibodies against von Willebrand factor (vWF), VEGF, HGF, and IGF-1 for 1 hour at 37°C. After 3 washes with PBS, slides were incubated with Alexa Fluor 594-conjugated rabbit anti-goat IgG (Thermo Fisher Scientific Life Sciences) for 45 minutes at 37°C. After 3 more washes with PBS, the slides were stained for nuclei with 4′,6-diamidino-2-phenylindole (DAPI; Sigma-Aldrich). Capillaries were counted in at least 3 random fields in infracted regions of each section under a fluorescence microscope. The capillary density was expressed as counts/mm2.
Staining Analysis of Apoptosis
The cytopreserved tissue sections (n = 8 for each rat group at 4 weeks after surgery) were fixed in 4% paraformaldehyde in phosphate-buffed saline (pH, 7.4) for 20 minutes at room temperature. Then tissue sections were stained by terminal deoxynucleotidyl transferase-mediated digoxigenin-deoxyuridine nick-end labeling (TUNEL) with In Situ Cell Death Detection Kit (Roche Diagnostics, Indianapolis, IN, https://usdiagnostics.roche.com) according to the manufacturer’s instructions. After TUNEL, the sections were counterstained for nuclei with DAPI. The TUNEL- or DAPI-positive nuclei were counted in 3 random fields in the border zone of each section under a fluorescence microscope. The percentage of apoptotic cells was calculated as the ratio of TUNEL-positive nuclei to total DAPI-positive nuclei.
Image Processing and Data Analysis
Cine MRIs were analyzed by using the image processing software Marevisi (Institute for Biodiagnostics, Winnipeg, Canada). LV endothelial and epicardial contours were drawn semiautomatically on serial short-axis slices of both end-diastolic and end-systolic images. The volume of the LV chamber was calculated using a simple numerical integration algorithm. LV end-diastolic volume (LVEDV) and LV end-systolic volume (LVESV) in each short-axis slice were determined and added to obtain the maximum volume of blood within the LV over a cardiac cycle. Stroke volume (SV) was calculated as the value of LVEDV minus LVESV. EF was calculated as the ratio of stroke volume to LVEDV.
Statistical Analysis
All data are expressed as means ± SD. Statistical significance was evaluated with unpaired Student’s t test for comparisons between two means, with analysis of variance for more than two means. Data were considered significant when the p-value was <.05. All statistical analyses were performed with SPSS software, version 12.0 (IBM, Inc., Chicago, IL, http://www.ibm.com).
Results
Characterization of Cultured ASCs
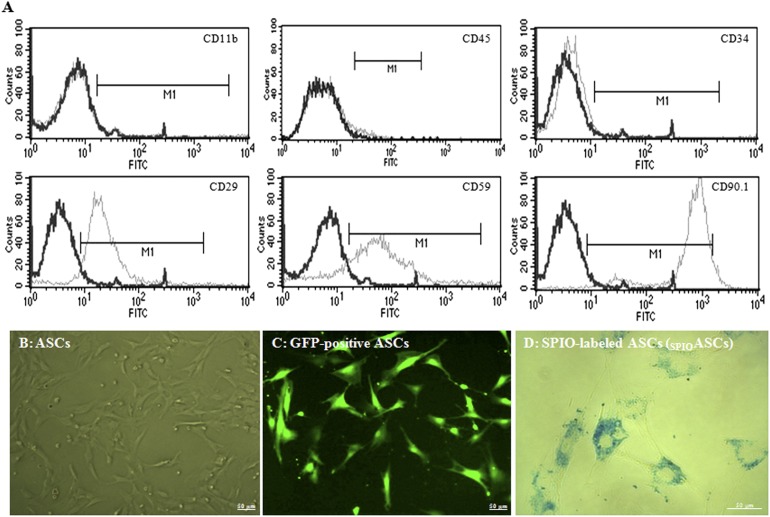
The cultured ASCs contained a significant number of mesenchymal stem cells, identified by the expression of CD29 (82.07% ± 8.62%), CD59 (83.43% ± 8.45%), and CD90.1 (94.29% ± 5.65%), a very small percentage of endothelial cells, identified by the expression of CD34 (4.56% ± 1.07%), but no hematopoietic lineages, identified by a negative expression of CD11b (0.07% ± 0.04%) and CD45 (0.06% ± 0.02%) (Fig. 1A). These results indicated that the cultured ASCs contained a large population of mesenchymal stem cells and a small percentage of endothelial cells.
Figure 1.
Characterization of ASCs. (A): The cultured ASCs (n = 3 experiments) were positive for CD29, CD59, and CD90.1 and negative for CD11b and CD45. (B): The ASCs appeared spindle-shaped under phase-contrast microscopy. (C): Fluorescence microscopy images of ASCs expressed strong blue GFP fluorescence. (D): Prussian blue staining showed blue magnetic SPIO particles distributed around the nuclei of SPIOASCs. Scale bar = 50 μm. Abbreviations: ASC, adipose-derived stem cell; FITC, fluorescein isothiocyanate; GFP, green fluorescent protein; SPIO, superparamagnetic iron oxide; SPIOASC, superparamagnetic iron oxide-labeled adipose-derived stem cell.
Cultured ASCs showed a fibroblast-like morphology (Fig. 1B). ASCs derived from GFP transgenic rats expressed green fluorescence (Fig. 1C). Prussian blue staining confirmed particle uptake by ASCs (Fig. 1D).
Effect of SMF on Cell Growth, Paracrine Cytokines, and DNA Integrity
At 1 week after SMF exposure, cell viability evaluated by MTT assay did not differ among ASCs (100% ± 1.6%), SPIOASCs (97.3% ± 2.5%), and SPIOASCs-magnet (96.8% ± 2.5%) (supplemental online Fig. 3A). Cell proliferation percentage reflected by direct cell counting did not differ among ASCs (100% ± 2.2%), SPIOASCs (100.3% ± 3.7%), and SPIOASCs-magnet (99.2% ± 3.6%) (supplemental online Fig. 3B).
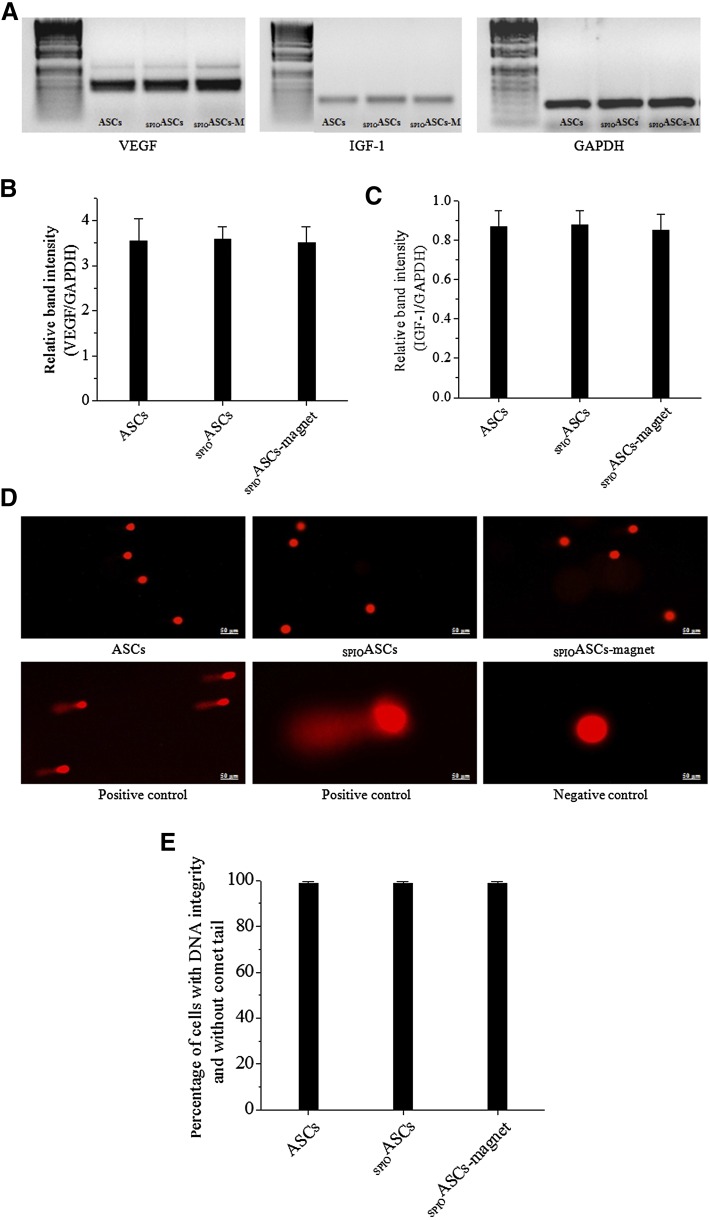
Figure 2A showed the mRNA bands of angiogenic cytokines expressed in three cell groups. The relative band intensity (VEGF/GAPDH) was not different among the ASCs (3.55 ± 0.48 arbitrary units [A.U.]), SPIOASCs (3.58 ± 0.27), and SPIOASCs-magnet (3.50 ± 0.36) (Fig. 2B). Moreover, the relative band intensity (IGF-1/GAPDH) did not differ among the ASCs (0.86 ± 0.08 A.U.), SPIOASCs (0.87 ± 0.07), and SPIOASCs-magnet (0.85 ± 0.08) (Fig. 2C).
Figure 2.
Angiogenic cytokines secretion and DNA integrity of ASCs under the exposure of 0.1-Tesla static magnetic field. (A): Typical example of reverse-transcriptase polymerase chain reaction analysis of VEGF and IGF-1 expression in ASCs, SPIOASCs, and SPIOASCs-magnet. (B and C): Expression of two cytokines from three groups of ASCs were indistinguishable (n = 3 experiments for each cell group). (D): DNA damages with comet tail were not observed at three cell groups. (E): The percentage of cells with integral DNA was identical among three cell groups (n = 3 experiments for each cell group). Scale bar = 50 μm. Abbreviations: ASC, adipose-derived stem cell; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; IGF-1, insulin-like growth factor-1; SPIOASC, superparamagnetic iron oxide-labeled adipose-derived stem cell; VEGF, vascular endothelial growth factor.
Figure 2D showed the comet assay results in three cell groups. DNA damage with comet tail was not observed in ASCs, SPIOASCs, and SPIOASCs-magnet by visual estimation. The percentage of cells with integral DNA without comet tail did not differ among the ASCs (98.8% ± 0.41%), SPIOASCs (98.7% ± 0.33%), and SPIOASCs-magnet (98.6% ± 0.29%) (Fig. 2E). These indicated that the SMF (0.1 Tesla) did not adversely affect viability, proliferation, cytokine secretion, and DNA integrity of SPIOASCs.
Proangiogenic Effect of Implanted ASCs
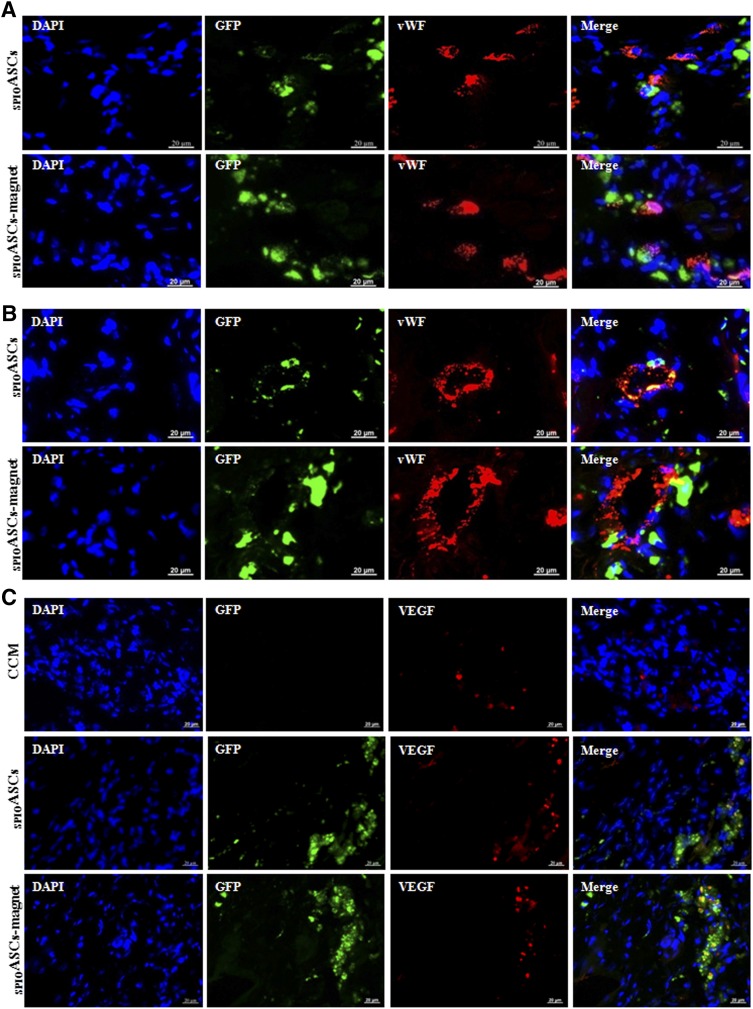
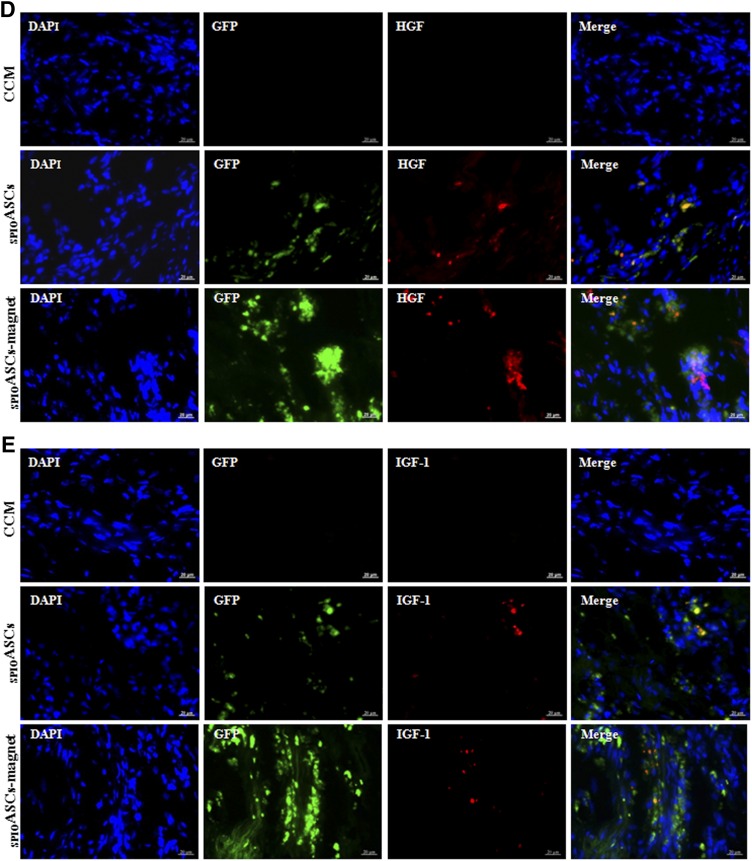
The implanted ASCs expressed vWF, indicating that some injected cells differentiated into endothelial cells (Fig. 3A). The GFP-positive ASCs directly incorporated into newly formed blood vessels (Fig. 3B). The implanted ASCs expressed the angiogenic cytokines, such as VEGF, HGF, and IGF-1 (Fig. 3C–3E). These indicated that ASC transplantation promoted neovascularization by differentiation into endothelial cells, incorporation into vasculature, and secretion of multiple angiogenic cytokines.
Figure 3.
Immunofluorescence analysis of the injected ASCs 4 weeks after cell transplantation. The sections of heart intramyocardially injected with GFP-positive ASCs (green) were costained with DAPI (blue) and vWF or three cytokines (red) in the SPIOASCs treated rats (n = 8) and the SPIOASC-magnet-treated rats (n = 8). (A): Merge images in right column showed that some implanted ASCs differentiated into endothelial cells. (B): Merge images in right column showed that some implanted ASCs directly incorporated into newly formed vessels. (C–E): Merge images in right column showed that the injected ASCs secreted VEGF (C), HGF (D), and IGF-1 (E) under myocardial microenvironment. Scale bar = 20 μm. Abbreviations: DAPI, 4′,6-diamidino-2-phenylindole; GFP, green fluorescent protein; HGF, hepatocyte growth factor; IGF-1, insulin-like growth factor-1; SPIOASC, superparamagnetic iron oxide-labeled adipose-derived stem cell; vWF, von Willebrand factor.
Externally Applied SMF Captures Magnetic SPIOASCs In Vitro
The SPIOASCs were resuspended in a cell-cultured dish and a circular magnet was positioned behind the dish (supplemental online Fig. 4A). The magnet was the same as that subsequently used in vivo. After two days of cultivation, the ‘magnetic’ SPIOASCs were obviously attracted toward magnet and accumulated focally on the adjacent inner wall (supplemental online Fig. 4B). In contrast, less SPIOASC accumulation was observed on the remote area (supplemental online Fig. 4C).
Externally Applied SMF Enhances Cardiac Retention of Implanted SPIOASCs
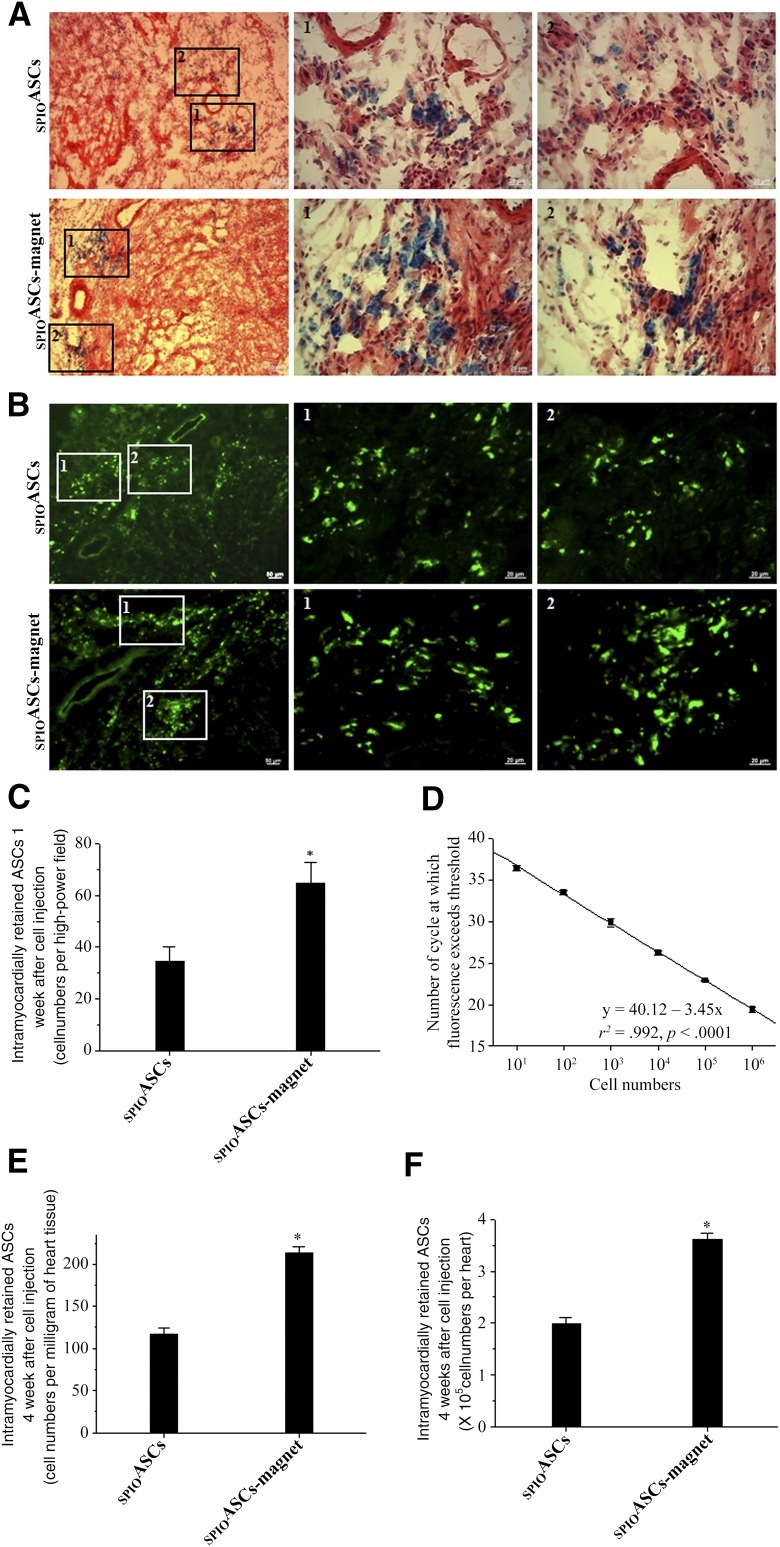
One week after cell transplantation, more cells containing blue-stained particles were distributed in the SPIOASC-magnet-treated rats than in the SPIOASC-treated rats (Fig. 4A). SPIOs released upon the death of transplanted cells have been engulfed in macrophages [23]. Prussian blue staining might reflect the location of SPIOs in the myocardium, but not necessarily the number of engrafted SPIOASCs. Likewise, more GFP-positive cells were evident in the SPIOASC-magnet-treated rats than in the SPIOASC-treated rats (Fig. 4B). The number of SPIOASCs, quantified as GFP-positive cells per HPF, was significantly greater in the SPIOASC-magnet-treated rats (64.7 ± 8.01 cells per HPF) than in the SPIOASC-treated rats (34.7 ± 5.31) (Fig. 4C).
Figure 4.
Effect of the externally applied static magnetic field on cell retention and engraftment. (A, B): One week after cell implantation, more GFP-positive SPIOASCs and cells containing blue-stained particles were detected in the SPIOASC-magnet-treated rats (n = 6) than in the SPIOASC-treated rats (n = 6). Regions 1 and 2 selected in left column are amplified in two right columns, respectively. (C): At 1 week after cell transplantation, the SPIOASC-magnet-treated rats (n = 6) exhibited an approximately 1.86-fold greater cell numbers per high-power field than the SPIOASC-treated rats (n = 6). (D): The reproducibility of the standard curve obtained by real-time polymerase chain reaction. A serial 10-fold dilution of DNA was tested 6 times in separate experiments. Each circle corresponds to the result of one dilution in one assay. The solid line corresponds to the regression analysis. (E, F): At 4 weeks after cell transplantation, the SPIOASC-magnet treated rats (n = 6) showed an approximately 1.82-fold greater cell numbers per milligram of heart tissue (E) and per heart (F) than the SPIOASC-treated rats (n = 6). Scale bar = 20 μm. ∗, p < .05 versus SPIOASCs. Abbreviation: SPIOASC, superparamagnetic iron oxide-labeled adipose-derived stem cell.
Figure 4D shows the reproducibility of the Y-chromosome quantification by real-time PCR. The correlation between the number of copies of male cell DNA and number of cycles for detecting threshold was excellent (r2 = .992; p < .0001). The number of alive male ASCs transplanted into female heart was significantly greater in the SPIOASC-magnet-treated rats (213.96 ± 6.28 cells per milligram of heart tissue or 3.62 ± 0.11 ×105 cells per heart) than in the SPIOASC-treated rats (116.88 ± 7.22 or 1.98 ± 0.12) at 4 weeks after cell transplantation (Fig. 4E and 4F). These indicated that externally applied SMF enhanced myocardial retention of the magnetic SPIOASCs implanted using intramyocardial injection.
Externally Applied SMF Enhances ASC-Induced Angiogenesis and Inhibition of Apoptosis
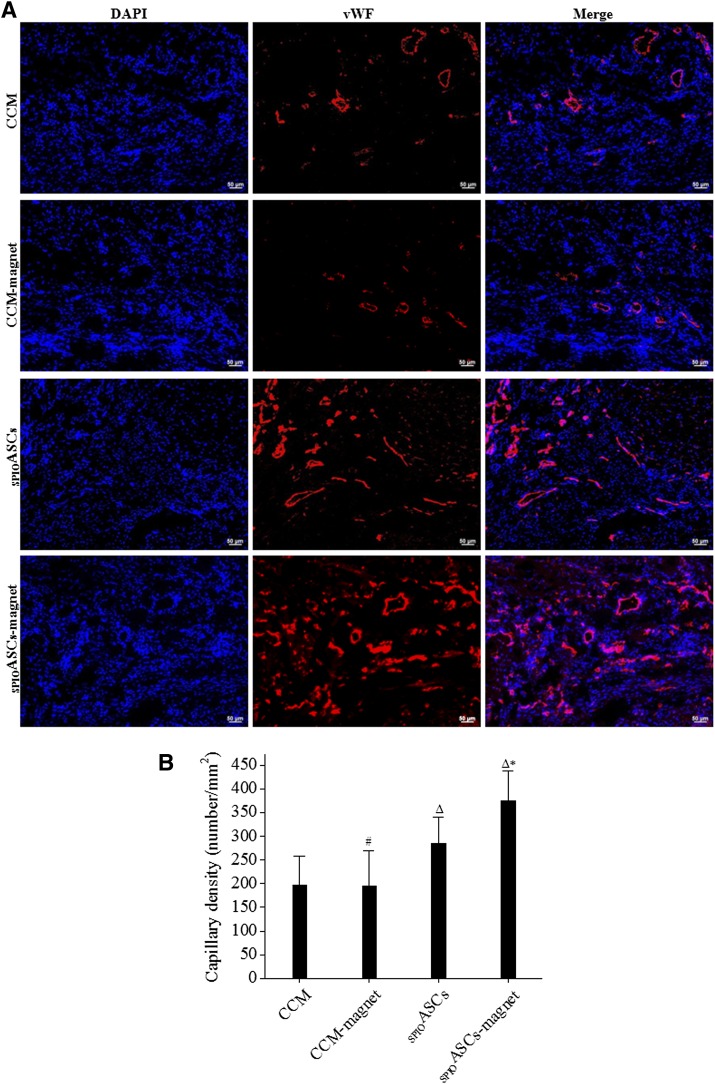
Capillaries were identified according to positive vWF staining in the peri-infarct zone 4 weeks after cell transplantation (Fig. 5A). Capillary density was substantially higher in the SPIOASC-magnet-treated rats (374.49 ± 64.54 vessels/mm2) than in the SPIOASC-treated rats (285.82 ± 55.33), in the CCM-magnet-treated rats (195.71 ± 74.87), and in the CCM control rats (197.67 ± 60.70) (Fig. 5B). This indicated that externally applied SMF significantly strengthened SPIOASC-induced angiogenesis.
Figure 5.
Immunofluorescence analysis of capillary density in the peri-infarct zone 4 weeks after cell transplantation. (A): Cardiac sections from four rat groups were stained with DAPI (blue) and antibody to endothelial cell marker vWF (red). (B): Capillary density was greatest in the SPIOADSC-magnet-treated rats (n = 8) compared with the SPIOADSC-treated rats (n = 8), the CCM-magnet rats (n = 8), and the CCM control rats (n = 8). Scale bar = 50 μm. #, p > .05 versus the CCM; Δ, p < .05 versus the CCM or CCM-magnet; ∗, p < .05 versus the SPIOASCs, CCM-magnet, or CCM. Abbreviations: CCM, cell culture medium; DAPI, 4′,6-diamidino-2-phenylindole; SPIOASC, superparamagnetic iron oxide-labeled adipose-derived stem cell; vWF, von Willebrand factor.
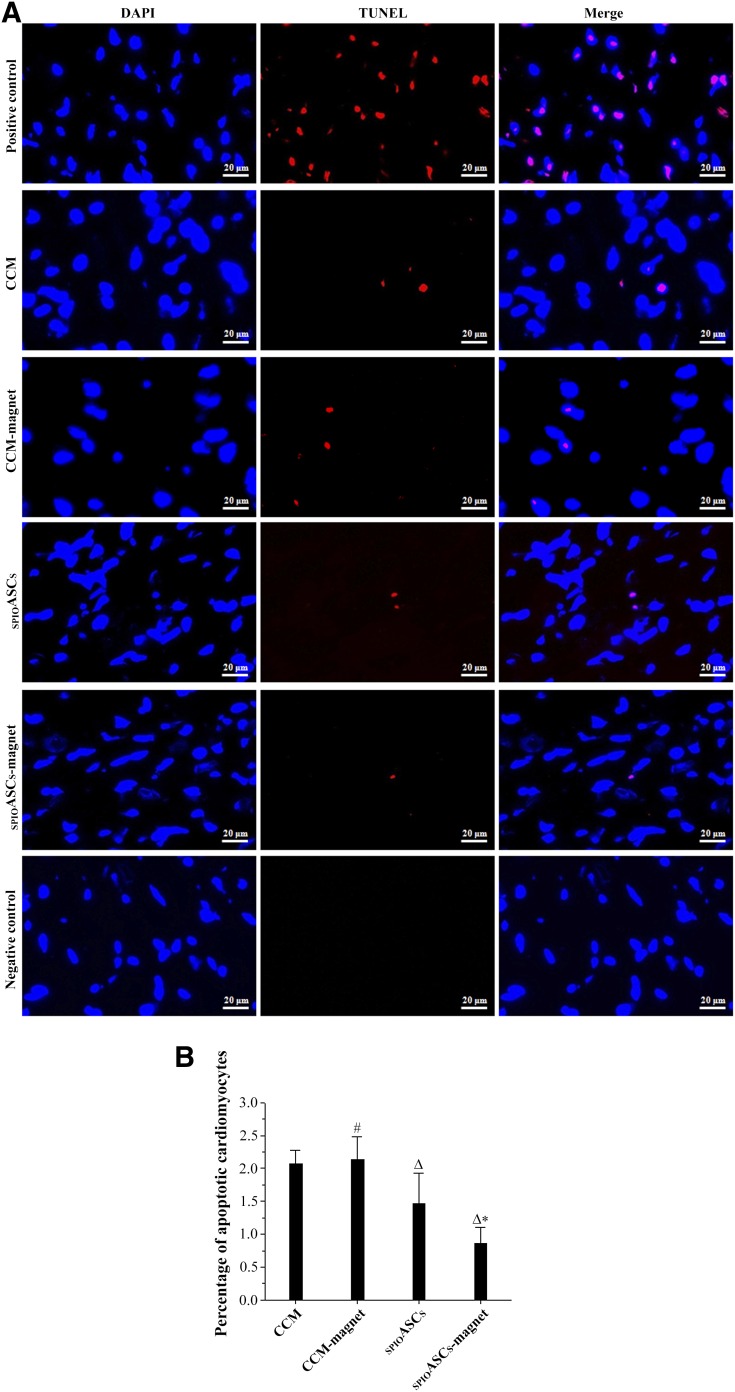
Apoptotic cardiomyocytes were stained red with TUNEL reagent in the peri-infarct zone 4 weeks after cell transplantation (Fig. 6A). TUNEL-positive cardiomyocytes in the CCM-magnet-treated rats (2.13% ± 0.35%) and the CCM control rats (2.07% ± 0.20%) were indistinguishable. Apoptotic cardiomyocytes was significantly lower in the SPIOASC-magnet-treated rats (0.86% ± 0.23%) than in the SPIOASC-treated rats (1.46% ± 0.46%) (Fig. 6B). These findings suggested that externally applied SMF did not induce cardiomyocyte apoptosis but enhanced SPIOASC-induced inhibition of ischemic cardiomyocyte apoptosis.
Figure 6.
TUNEL staining of apoptotic cardiomyocytes in the peri-infarct zone 4 weeks after cell transplantation. (A): Cardiac sections from four rat groups were stained with DAPI (blue) and with TUNEL reagent for apoptosis (red). (B): TUNEL-positive cardiomyocytes were fewest in the SPIOASC-magnet-treated rats (n = 8) compared with the SPIOASC-treated rats (n = 8), the CCM-magnet rats (n = 8), and the CCM control rats (n = 8). Scale bar = 20 μm. #, p > .05 versus the CCM; Δ, p < .05 versus the CCM or CCM-magnet; ∗, p < .05 versus the SPIOASCs, CCM-magnet, or CCM. Abbreviations: CCM, cell culture medium; DAPI, 4′,6-diamidino-2-phenylindole; SPIOASC, superparamagnetic iron oxide-labeled adipose-derived stem cell; TUNEL, terminal deoxynucleotidyl transferase-mediated digoxigenin-deoxyuridine nick-end labeling.
Externally Applied SMF Enhances Functional Benefit of SPIOASC Transplantation
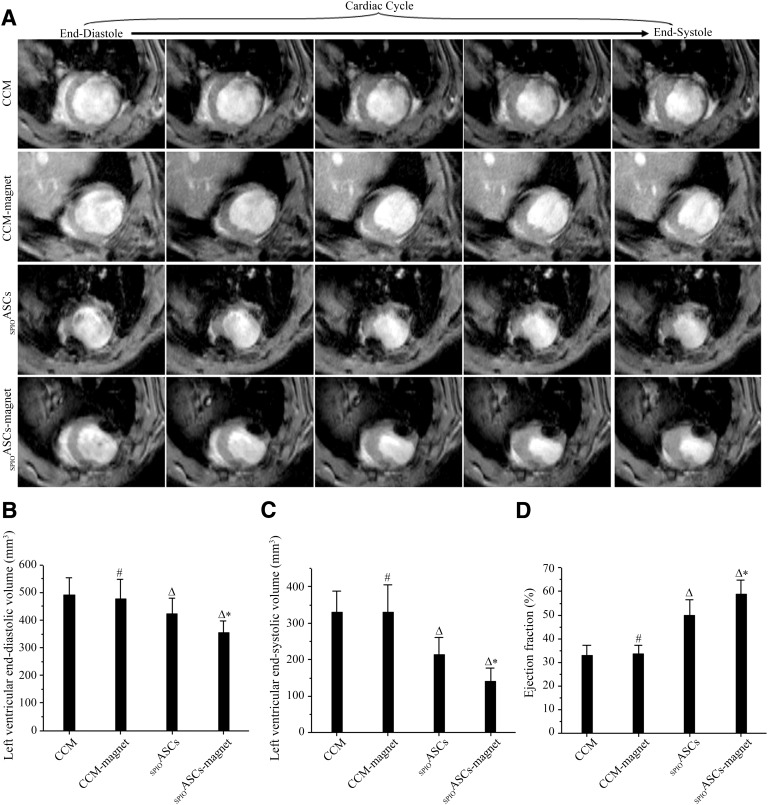
Figure 7A showed three stacks of representative cine MR images acquired at 4 weeks after cell transplantation. The SPIOASC-magnet-treated rats displayed a smaller LV slice volume in comparison with the SPIOASC-treated rats, the CCM-magnet-treated rats, and the CCM control rats.
Figure 7.
Magnetic resonance imaging (MRI) analysis of cardiac function. (A): Typical short-axis cine MRIs from end-diastole to end-systole during the whole cardiac cycle. The SPIOASC-magnet-treated rats (n = 14) displayed a smaller left ventricular (LV) slice volume in comparison with the SPIOASC-treated rats (n = 14), the CCM-magnet-treated rats (n = 8), and the CCM control rats (n = 8). (B, C): LV end-diastolic (B) and end-systolic (C) volumes were lowest in the SPIOASC-magnet-treated rats (n = 14) compared with the SPIOASC-treated rats (n = 14), the CCM-magnet rats (n = 8), and the CCM control rats (n = 8). (D): LV ejection fraction was highest in the SPIOASC-magnet-treated rats (n = 14) compared with the SPIOASC-treated rats (n = 14), the CCM-magnet rats (n = 8), and the CCM control rats (n = 8). #, p > .05 versus the CCM; Δ, p < .05 versus the CCM or CCM-magnet; ∗, p < .05 versus the SPIOASCs, CCM-magnet, or CCM. Abbreviations: CCM, cell culture medium; SPIOASC, superparamagnetic iron oxide-labeled adipose-derived stem cell.
There were no significant differences in LVEDV, LVESV, and LVEF between the CCM-magnet-treated rats and the CCM control rats (476.77 ± 72.49 versus 489.56 ± 62.61 mm3 for LVEDV, 329.43 ± 76.31 versus 330.06 ± 57.03 mm3 for LVESV, 33.42% ± 3.92% versus 32.87% ± 4.34% for LVEF). This suggested that externally applied SMF did not adversely affect cardiac contractile function.
The LVEDV and LVESV were significantly lower in the SPIOASC-magnet-treated rats than in the SPIOASCs treated rats (353.26 ± 43.88 versus 421.62 ± 57.01 mm3 for LVEDV, 140.34 ± 36.80 versus 213.02 ± 47.52 mm3 for LVESV) (Fig. 7B and 7C). LVEF was substantially greater in the SPIOASC-magnet-treated rats (58.87% ± 5.87%) than in the SPIOASC-treated rats (49.73% ± 6.85%) (Fig. 7D). This suggested that improved cell retention by externally applied SMF indeed translated into superior heart functional benefit. Supplemental online videos 1–4 showed MRI short-axis cine views of representative hearts from the four rats group 4 weeks after MI.
Discussion
The safety of SMF on ASCs is the prerequisite for the feasibility of magnetic targeting technique. The subcutaneously implanted magnet produced the approximately 0.1-Tesla SMF on the LV anterior wall. The SPIOASCs under the 0.1 Tesla of SMF had viability and proliferative ability similar to those of the normal SPIOASCs. Exposure to 0.1-Tesla SMF for 1 week did not alter the expression of VEGF and IGF-1 of the ASCs. Comet assay demonstrated that no DNA single-strand break was observed in the SPIOASCs regardless of whether the SMF was present. Those findings agree with those of previous investigations. Victora et al. found that the number of hematopoietic stem cells was not reduced by the 3- to 5-month exposure of magnetic field (180–200 gauss) and that proliferative capacity was likewise unimpaired [24]. Schäfer et al. found that the exposure to 0.6-Tesla magnetic field for 24 hours had no influence on the migration capacity, viability, proliferation rate, and chondrogenic differentiation capacity of SPIO-labeled or unlabeled mesenchymal stem cells [25]. Reddig et al. reported that exposure of human peripheral blood mononuclear cells to 7-Tesla SMF did not induce DNA double-strand breaks [26]
The magnetic field has enhanced the delivery of magnetic particle- conjugated anticancer drugs to the targeted tumor organ in both preclinical and clinical studies [15–17]. A magnet positioned near the subcutaneous tumor increased the retention percentage of intravenously delivered SPIO-labeled monocytes from 4.9% ± 3.5% to 16.9% ± 4.2% [18]. This study found that locally applied SMF (0.1 Tesla, 7 days) enhanced cardiac retention of magnetic SPIOASCs by approximately 1.82-fold at 4 weeks after intramyocardial injection. A subcutaneously implanted magnet (0.1 Tesla, 24 hours) over the chest cavity increased cardiac retention of intraventricularly delivered iron-labeled endothelial progenitor cells by approximately 10-fold in ischemic reperfused MI [27]. Placement of a 1.3-Tesla magnet 1 cm above the heart enhanced cell retention by 5.2- to 6.2-fold at 24 hours after intracoronary delivery of cardiac-derived stem cells [28]. A 0.6-Tesla magnet placed above the heart (1–10 mm) significantly increased the cardiac retention of intravenously injected bone marrow stem cells at 24 hours by 2.73- to 2.87-fold [29]. Consequently, magnetic targeting is a simple, effective, and noninvasive technique to improve cell retention and engraftment in targeted organ. Intravenous drainage and mechanical contraction account for the significant loss of transplanted cells by means of washing out and squeezing out [30]. Therefore, magnetic attraction might attenuate cell loss via venous drainage, increase intravascular cell stay, and enhance the formation of multicellular clusters, which was considered the theoretic basis for magnetic targeting [31].
In this study, some ASCs differentiated into endothelial cells, as evidence of coexpression of the markers specific for the endothelial cells. Other investigators also demonstrated that a certain percentage of human ASCs were able to differentiate into endothelial cells in infarcted myocardium [9, 10, 32]. This study found the engrafted ASCs incorporated into newly formed vessels. This phenomenon has also been demonstrated in infarcted pig hearts receiving intracoronary injection of human ASCs [10]. Paracrine secretion of ASCs was a critical mechanism underlying their therapeutic benefits. This study found that implanted ASCs secreted VEGF, HGF, and IGF-1. Xiaowen Bai et al. demonstrated that injected human ASCs secreted basic fibroblast growth factor and IGF-1 at 10 weeks after cell transplantation [32]. Sadat et al. demonstrated that human ASCs secreted significant amounts of VEGF and IGF-1, which were responsible for antiapoptosis and proangiogenesis [6]. Rehmans et al. found that VEGF secretion in ASCs increased 5-fold when cultured under hypoxic conditions [7]. Therefore, proangiogenic effect of ASCs might be attributed to the differentiation into endothelial cells, incorporation into newly formed vessels, and multiple cytokines secretion.
The critical step for success in cell therapy is to deliver a sufficiently high number of cells to infarcted hearts [33]. The positive dose dependence of therapeutic benefits has been demonstrated with intramyocardial, intracoronary arterial, and intravenous delivery of the umbilical cord blood cells [34]. At a same cell dose, direct intramyocardial injection resulted in greater improvement in LVEF relative to intracoronary arterial infusion [35]. More importantly, the superior regenerative effect of direct intramyocardial injection was the result of its greater cardiac retention of the implanted cells relative to intracoronary arterial infusion [35]. The benefit of cell implantation on heart function is proportional to the numbers of injected cells homing to ischemic heart [27, 30]. Externally applied SMF could attract more implanted magnetic iron-labeled stem cells to the heart. The enhanced cell retention indeed translated into increased capillary density, attenuated LV remodeling, and improved cardiac function [27–29]. We found that neovascularization, reduction of cardiomyocyte apoptosis, and improvement of heart function were significantly greater in the SPIOASC-magnet-treated hearts relative to the SPIOASC-treated hearts.
The present study has several limitations. We did not explore the relationship of different magnetic duration and varying magnet strength with targeting efficacy; further studies will be needed to identify the optimal magnetic intensity and duration for effective targeting. The present study failed to clarify the kinetics of cell retention in different time points after cell implantation. The data concerning cell retention behavior should be explored in future studies. We have used only a proof-of-concept small animal model, and large-animal data are necessary to advance the process of translation.
Conclusion
A 0.1-Tesla SMF did not adversely affect the viability, proliferation, angiogenic cytokine secretion, or DNA integrity of the SPIOASCs. ASC implantation promotes myocardial neovascularization and inhibits ischemic cardiomyocyte apoptosis through endothelial differentiation, incorporation into vessels, and paracrine secretion. An externally applied SMF enhanced myocardial retention of intramyocardially injected magnetic SPIOASCs and promoted cardiac function recovery after MI.
Supplementary Material
Acknowledgments
This work was supported by the National Natural Sciences Foundation of China (81200105), the Natural Science Foundation of Hubei Province of China (2015CFB457), the China Postdoctoral Science Foundation funded Project (20100470050), the Canadian Institute of Health Research, and the National Research Council of Canada.
Author Contributions
J.W.: financial support, manuscript writing, final approval of manuscript; B.X. and D.Z.: collection and/or assembly of data, data analysis and interpretation; J.D.: administrative support, data analysis and interpretation; H.-Y.L.: administrative support, collection and/or assembly of data; D.H.F. and R.C.A.: provision of study material or patients, conception and design; G.T.: conception and design, financial support, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
R.C.A. has an unrestricted education grant from Pfizer. The other authors indicated no potential conflicts of interest.
References
- 1.Planat-Benard V, Silvestre JS, Cousin B, et al. Plasticity of human adipose lineage cells toward endothelial cells: Physiological and therapeutic perspectives. Circulation. 2004;109:656–663. doi: 10.1161/01.CIR.0000114522.38265.61. [DOI] [PubMed] [Google Scholar]
- 2.Zuk PA, Zhu M, Mizuno H, et al. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng. 2001;7:211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 3.De Ugarte DA, Morizono K, Elbarbary A, et al. Comparison of multi-lineage cells from human adipose tissue and bone marrow. Cells Tissues Organs. 2003;174:101–109. doi: 10.1159/000071150. [DOI] [PubMed] [Google Scholar]
- 4.Ning H, Liu G, Lin G, et al. Fibroblast growth factor 2 promotes endothelial differentiation of adipose tissue-derived stem cells. J Sex Med. 2009;6:967–979. doi: 10.1111/j.1743-6109.2008.01172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rodríguez LV, Alfonso Z, Zhang R, et al. Clonogenic multipotent stem cells in human adipose tissue differentiate into functional smooth muscle cells. Proc Natl Acad Sci USA. 2006;103:12167–12172. doi: 10.1073/pnas.0604850103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sadat S, Gehmert S, Song YH, et al. The cardioprotective effect of mesenchymal stem cells is mediated by IGF-I and VEGF. Biochem Biophys Res Commun. 2007;363:674–679. doi: 10.1016/j.bbrc.2007.09.058. [DOI] [PubMed] [Google Scholar]
- 7.Rehman J, Traktuev D, Li J, et al. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation. 2004;109:1292–1298. doi: 10.1161/01.CIR.0000121425.42966.F1. [DOI] [PubMed] [Google Scholar]
- 8.Wang L, Deng J, Tian W, et al. Adipose-derived stem cells are an effective cell candidate for treatment of heart failure: An MR imaging study of rat hearts. Am J Physiol Heart Circ Physiol. 2009;297:H1020–H1031. doi: 10.1152/ajpheart.01082.2008. [DOI] [PubMed] [Google Scholar]
- 9.Bai X, Yan Y, Song YH, et al. Both cultured and freshly isolated adipose tissue-derived stem cells enhance cardiac function after acute myocardial infarction. Eur Heart J. 2010;31:489–501. doi: 10.1093/eurheartj/ehp568. [DOI] [PubMed] [Google Scholar]
- 10.Valina C, Pinkernell K, Song YH, et al. Intracoronary administration of autologous adipose tissue-derived stem cells improves left ventricular function, perfusion, and remodelling after acute myocardial infarction. Eur Heart J. 2007;28:2667–2677. doi: 10.1093/eurheartj/ehm426. [DOI] [PubMed] [Google Scholar]
- 11.Perin EC, Sanz-Ruiz R, Sánchez PL, et al. Adipose-derived regenerative cells in patients with ischemic cardiomyopathy: The PRECISE Trial. Am Heart J. 2014;168:88–95.e2. doi: 10.1016/j.ahj.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 12.Hou D, Youssef EA, Brinton TJ, et al. Radiolabeled cell distribution after intramyocardial, intracoronary, and interstitial retrograde coronary venous delivery: implications for current clinical trials. Circulation. 2005;112(suppl):I150–I156. doi: 10.1161/CIRCULATIONAHA.104.526749. [DOI] [PubMed] [Google Scholar]
- 13.Li SH, Lai TY, Sun Z, et al. Tracking cardiac engraftment and distribution of implanted bone marrow cells: Comparing intra-aortic, intravenous, and intramyocardial delivery. J Thorac Cardiovasc Surg. 2009;137:1225–33.e1. doi: 10.1016/j.jtcvs.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 14.Yasuda T, Weisel RD, Kiani C, et al. Quantitative analysis of survival of transplanted smooth muscle cells with real-time polymerase chain reaction. J Thorac Cardiovasc Surg. 2005;129:904–911. doi: 10.1016/j.jtcvs.2004.06.027. [DOI] [PubMed] [Google Scholar]
- 15.Lübbe AS, Bergemann C, Huhnt W, et al. Preclinical experiences with magnetic drug targeting: tolerance and efficacy. Cancer Res. 1996;56:4694–4701. [PubMed] [Google Scholar]
- 16.Lübbe AS, Bergemann C, Riess H, et al. Clinical experiences with magnetic drug targeting: a phase I study with 4′-epidoxorubicin in 14 patients with advanced solid tumors. Cancer Res. 1996;56:4686–4693. [PubMed] [Google Scholar]
- 17.Goodwin SC, Bittner CA, Peterson CL, et al. Single-dose toxicity study of hepatic intra-arterial infusion of doxorubicin coupled to a novel magnetically targeted drug carrier. Toxicol Sci. 2001;60:177–183. doi: 10.1093/toxsci/60.1.177. [DOI] [PubMed] [Google Scholar]
- 18.Muthana M, Scott SD, Farrow N, et al. A novel magnetic approach to enhance the efficacy of cell-based gene therapies. Gene Ther. 2008;15:902–910. doi: 10.1038/gt.2008.57. [DOI] [PubMed] [Google Scholar]
- 19.Castaneda RT, Khurana A, Khan R, et al. Labeling stem cells with ferumoxytol, an FDA-approved iron oxide nanoparticle. J Vis Exp 2011. (57):e3482. [DOI] [PMC free article] [PubMed]
- 20.Renshaw PF, Owen CS, McLaughlin AC, et al. Ferromagnetic contrast agents: A new approach. Magn Reson Med. 1986;3:217–225. doi: 10.1002/mrm.1910030205. [DOI] [PubMed] [Google Scholar]
- 21.Wang L, Deng J, Wang J, et al. Superparamagnetic iron oxide does not affect the viability and function of adipose-derived stem cells, and superparamagnetic iron oxide-enhanced magnetic resonance imaging identifies viable cells. Magn Reson Imaging. 2009;27:108–119. doi: 10.1016/j.mri.2008.05.013. [DOI] [PubMed] [Google Scholar]
- 22.Blaber SP, Hill CJ, Webster RA, et al. Effect of labeling with iron oxide particles or nanodiamonds on the functionality of adipose-derived mesenchymal stem cells. PLoS One. 2013;8:e52997. doi: 10.1371/journal.pone.0052997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Terrovitis J, Stuber M, Youssef A, et al. Magnetic resonance imaging overestimates ferumoxide-labeled stem cell survival after transplantation in the heart. Circulation. 2008;117:1555–1562. doi: 10.1161/CIRCULATIONAHA.107.732073. [DOI] [PubMed] [Google Scholar]
- 24.Viktora L, Fiala J, Petz R. Effect of prolonged exposure to a magnetic field on the haematopoietic stem cell. Physiol Bohemoslov. 1976;25:359–364. [PubMed] [Google Scholar]
- 25.Schäfer R, Bantleon R, Kehlbach R, et al. Functional investigations on human mesenchymal stem cells exposed to magnetic fields and labeled with clinically approved iron nanoparticles. BMC Cell Biol. 2010;11:22. doi: 10.1186/1471-2121-11-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reddig A, Fatahi M, Friebe B, et al. Analysis of DNA double-strand breaks and cytotoxicity after 7 Tesla magnetic resonance imaging of isolated human lymphocytes. PLoS One. 2015;10:e0132702. doi: 10.1371/journal.pone.0132702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chaudeurge A, Wilhelm C, Chen-Tournoux A, et al. Can magnetic targeting of magnetically labeled circulating cells optimize intramyocardial cell retention? Cell Transplant. 2012;21:679–691. doi: 10.3727/096368911X612440. [DOI] [PubMed] [Google Scholar]
- 28.Cheng K, Malliaras K, Li TS, et al. Magnetic enhancement of cell retention, engraftment, and functional benefit after intracoronary delivery of cardiac-derived stem cells in a rat model of ischemia/reperfusion. Cell Transplant. 2012;21:1121–1135. doi: 10.3727/096368911X627381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang Z, Shen Y, Sun A, et al. Magnetic targeting enhances retrograde cell retention in a rat model of myocardial infarction. Stem Cell Res Ther. 2013;4:149. doi: 10.1186/scrt360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Teng CJ, Luo J, Chiu RC, et al. Massive mechanical loss of microspheres with direct intramyocardial injection in the beating heart: implications for cellular cardiomyoplasty. J Thorac Cardiovasc Surg. 2006;132:628–632. doi: 10.1016/j.jtcvs.2006.05.034. [DOI] [PubMed] [Google Scholar]
- 31.Cheng K, Li TS, Malliaras K, et al. Magnetic targeting enhances engraftment and functional benefit of iron-labeled cardiosphere-derived cells in myocardial infarction. Circ Res. 2010;106:1570–1581. doi: 10.1161/CIRCRESAHA.109.212589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bai X, Yan Y, Coleman M, et al. Tracking long-term survival of intramyocardially delivered human adipose tissue-derived stem cells using bioluminescence imaging. Mol Imaging Biol. 2011;13:633–645. doi: 10.1007/s11307-010-0392-z. [DOI] [PubMed] [Google Scholar]
- 33.Pouzet B, Vilquin JT, Hagège AA, et al. Factors affecting functional outcome after autologous skeletal myoblast transplantation. Ann Thorac Surg. 2001;71:844–850; discussion 850–851. doi: 10.1016/s0003-4975(00)01785-9. [DOI] [PubMed] [Google Scholar]
- 34.Henning RJ, Burgos JD, Vasko M, et al. Human cord blood cells and myocardial infarction: Effect of dose and route of administration on infarct size. Cell Transplant. 2007;16:907–917. doi: 10.3727/096368907783338299. [DOI] [PubMed] [Google Scholar]
- 35.Perin EC, Silva GV, Assad JA, et al. Comparison of intracoronary and transendocardial delivery of allogeneic mesenchymal cells in a canine model of acute myocardial infarction. J Mol Cell Cardiol. 2008;44:486–495. doi: 10.1016/j.yjmcc.2007.09.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.