Mature blood cells such as red blood cells, platelets, and engineered T cells have been increasingly used to treat several diseases. The current state of knowledge on the directed differentiation of embryonic stem cells and the reprogramming of somatic cells toward the generation of blood stem cells and derivatives is reviewed.
Keywords: Embryonic stem cells, Hematopoietic cells, Stem cell transplantation, Reprogramming, Differentiation, Hemangioblast, Hematopoietic stem cells
Abstract
Hematopoietic cell-based therapies are currently available treatment options for many hematological and nonhematological disorders. However, the scarcity of allogeneic donor-derived cells is a major hurdle in treating these disorders. Embryonic stem cell-based directed differentiation and direct reprogramming of somatic cells provide excellent tools for the potential generation of hematopoietic stem cells usable in the clinic for cellular therapies. In addition to blood stem cell transplantation, mature blood cells such as red blood cells, platelets, and engineered T cells have also been increasingly used to treat several diseases. Besides cellular therapies, induced blood progenitor cells generated from autologous sources (either induced pluripotent stem cells or somatic cells) can be useful for disease modeling of bone marrow failures and acquired blood disorders. However, although great progress has been made toward these goals, we are still far from the use of in vitro-derived blood products in the clinic. We review the current state of knowledge on the directed differentiation of embryonic stem cells and the reprogramming of somatic cells toward the generation of blood stem cells and derivatives.
Significance
Hematopoietic cell-based therapies are currently available treatment options for many hematological and nonhematological disorders. However, the scarcity of allogeneic donor-derived cells is a major hurdle in treating these disorders. The current state of knowledge on the directed differentiation of embryonic stem cells and the reprogramming of somatic cells toward the generation of blood stem cells and derivatives is reviewed.
A Need for In Vitro-Derived Blood Cell Populations
For several decades now, bone marrow transplantation (BMT) has been the only known cure for a number of hematological disorders. Cancer patients also benefit from this procedure, which helps overcome chemotherapy-mediated immune system depletion. However, several limitations are still associated with BMT, the major one being the need for donors matching the human leukocyte antigen system of the recipients. In 1968, the Bone Marrow Transplant Program was started at the John Hopkins Institute, with the aim of improving the outcome of BMT and increasing the rate of success of haploidentical or half-matching transplantations [1]. Considerable efforts were made toward this goal, and more than 26 million adult donors are currently registered in the Bone Marrow Donor Worldwide system. However, approximately 37,000 patients worldwide are still on waiting lists for BMT [2].
The therapeutic potential of BMT relies on the presence of long-term repopulating hematopoietic stem cells (HSCs) within the bone marrow cell compartment, which are able to engraft the recipient and reconstitute all blood cell lineages. Their self-renewal capacity and multilineage potential make HSCs essential for the precise development and continuous supply of all blood cells throughout life. In adults, HSCs home exclusively to the bone marrow, but bone marrow withdrawal from donors is painful and invasive. Alternative sources of HSCs have been explored to overcome this issue, such as mobilized peripheral blood and umbilical cord blood. After cytokine infusion, a large number of HSCs are released into the peripheral blood and are easily accessible for withdrawal [3]. Nevertheless, cytokine-mediated mobilization releases a mixture of hematopoietic progenitors in which the amount of bona fide HSCs is still often limited. Despite increasing advances in cord blood biobanking, the same issues are encountered during umbilical cord blood transplantation. The rate of success in HSC transplantation is strictly dependent on the number of injected HSCs; therefore, a need exists for protocols aimed at expanding HSC populations. The ex vivo expansion of HSCs has been pursued by many research groups for decades. Disparate approaches have been used for this purpose, varying from alterations in the expression level of self-renewal-related genes to the sophisticated mimicking of the bone marrow niche [4, 5]. Certainly, the presence of hematopoietic cytokines in ex vivo cultures is determinant for the proliferation and survival of HSCs in vitro. However, the self-renewing potential of these cells seems to be progressively lost in culture, in favor of lineage commitment and leading to HSC exhaustion. Thus, the combination of intrinsic and extrinsic factors that enhance HSC expansion still needs to be explored further.
An alternative approach to solving this issue is to produce HSCs and blood derivatives in vitro, either from limitless sources such as embryonic stem cells (ESCs) or through the reprogramming of somatic cells.
In Vitro Differentiation of Embryonic Stem Cells
ESCs were first derived from mouse blastocysts in the early 1980s by two groups using different protocols [6, 7]. Both studies generated fully pluripotent cells able to self-renew if maintained on mouse embryonic fibroblasts or in feeder-free conditions supplemented with leukemia inhibitory factor and interleukin-6. The addition of growth factors and cytokines and exposure to stromal coculture was sufficient to drive ESC differentiation toward many adult cell types. The most common differentiation protocol is based on suspension cultures in which ESCs form aggregates termed embryoid bodies (EBs), containing derivatives of the three embryonic germ layers.
ESC-Derived Hematopoiesis
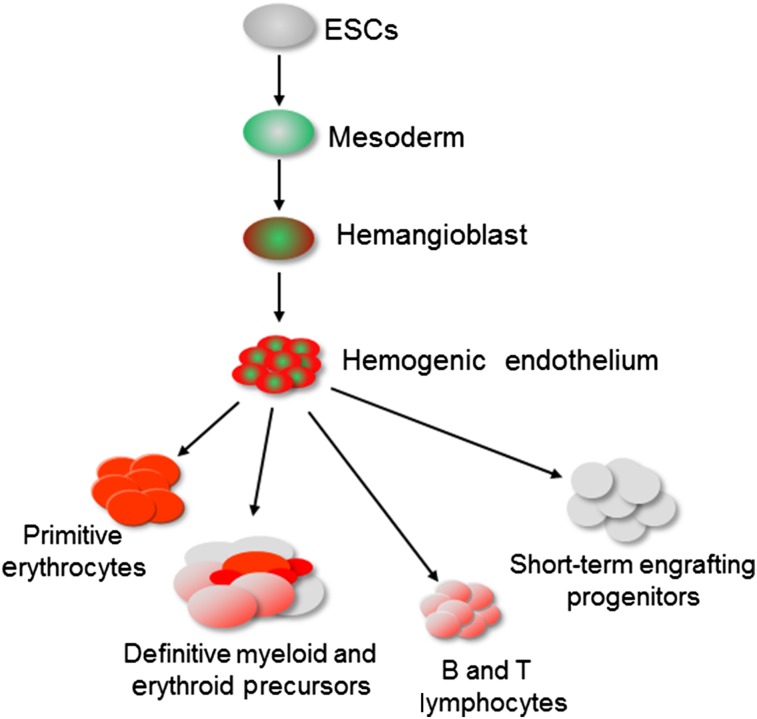
The in vitro differentiation of mouse ESCs toward the hematopoietic program has been carefully dissected over the last decades, with the help of gain- and loss-of-function, gene expression, cell surface marker analysis, and single cell approaches. This has allowed mapping of the different stages of hematopoietic specification in a temporal sequence of events that are highly reproducible (Fig. 1). Strong evidence supports the hypothesis that mouse ESC differentiation accurately recapitulates the early developmental events occurring during embryogenesis. Pioneering studies initially demonstrated the generation of hematopoietic cells from differentiating mouse ESCs [8, 9]. Further studies, still using mouse ESCs, revealed the presence of a Brachyury+FLK1+ mesodermal progenitor, giving rise to all blood cells at the early stage of EB culture [10–12]. This progenitor, the blast colony-forming cell (BL-CFC), was shown to represent the in vitro equivalent of the hemangioblast and to give rise to smooth muscle, endothelium, and both primitive and definitive hematopoiesis with further culture [13–16]. More recently, we, and others, have demonstrated that mouse BL-CFCs generate blood progenitors through a transient and specialized endothelial population, termed hemogenic endothelium [17–19]. These endothelial cells form tight clusters from which round hematopoietic cells emerge after further culture [18]. Eilken et al. showed that mouse ESC-derived FLK1+ cells gave rise to vascular endothelial cadherin-positive endothelium that differentiated into CD41+CD45+ hematopoietic cells [17]. The latter emerged from the adherent fraction as round and bright cells, progressively detaching to float in the culture supernatant. When exposed to lineage-specific cytokines, these cells were able to generate definitive hematopoietic lineages, such as megakaryocytes and myeloid cells. These findings are consistent with in vivo observations of the emergence of blood progenitors from the endothelial layer of the yolk sac [20] and dorsal aorta in chicken and murine embryos [21, 22], thus confirming the reliability of the mouse ESC system in recapitulating embryonic hematopoiesis [23]. Several studies have now also demonstrated that human ESCs undergo a similar differentiation process with the generation of intermediate BL-CFCs [24, 25] and hemogenic endothelium [26, 27], leading to the generation of blood progenitors.
Figure 1.
The in vitro differentiation of ESCs proceeds in well-defined sequential steps leading to the generation of all hematopoietic lineages. Abbreviation: ESCs, embryonic stem cells.
Directed Differentiation of ESCs
To date, erythroid, myeloid, and lymphoid lineages have all been reproducibly generated from in vitro differentiating mouse [28, 29] and human [26, 27] ESCs. Nonetheless, the most challenging goal remains the derivation of long-term engrafting HSCs, which has eluded the field for several decades now. It is still not clear whether it is possible to generate a cell population in vitro equivalent to the hemogenic endothelium found in the dorsal aorta and giving rise to HSCs. Despite the number of approaches tested, few studies have shown the engrafting capacity of in vitro-derived blood progenitors, and long-term engraftment remains an unachieved goal. Many studies using mouse ESCs have shown promising results with clearly detectable engraftment on transplantation in mouse recipients [30–33]. However, all these experimental protocols relied on serum-supplemented cultures to derive engrafting progenitors and to date, none of these results have been reproduced or pursued. Most likely, specific, but unknown, serum-related factors have determined the successful derivation of repopulating blood cells but have been as yet impossible to identify and thus to reproduce.
To overcome this issue, we, and others, have developed serum-free culture conditions in which the addition of specific growth factors and cytokines efficiently drives the specification of mouse [28, 34, 35] or human [36] ESCs toward hematopoiesis. Recently, we showed, using mouse ESCs, that hemogenic endothelium cells derived from these serum-free cultures were able to give rise to erythroid, myeloid, and lymphoid lineages upon engraftment in immunocompromised recipients [29]. Mature blood cells derived from the injected progenitors were detected in both spleen and bone marrow up to 22 weeks after engraftment. However, secondary transplantation assays were not successful, suggesting that these culture conditions produced multipotent progenitors with poor self-renewal potential. More strikingly, the repopulating ability was only observed on engraftment of hemogenic endothelium, emerging during the onset of mesoderm specification toward hematopoiesis. In addition, engrafting progenitors were lost after 1 or 2 days of culture, indicating that this population is extremely transient.
Important progress has also been described in the directed differentiation of human ESCs toward engrafting blood progenitors. Two studies reported the generation of blood cells with engrafting capacity using serum-supplemented cultures [37] and/or stroma cell lines [38]. However, despite these encouraging results, the use of serum and stroma compromises the reproducibility of the differentiation protocols, hindering their validation. A recent study by Gori et al. reported high levels and long-term engraftment of blood progenitors derived from Macaca nemestrina-induced pluripotent stem cells (iPSCs) and human ESCs when exposed to an endothelial-like niche activating the Notch pathway [4]. However, secondary engraftment was only observed when the in vitro-derived blood progenitors were genetically modified to express the chemotherapy-resistant methylguanine methyltransferase transgene, suggesting limited self-renewal capacity of the unmanipulated cells.
Important advances have been made toward the derivation of long-term multilineage blood progenitors from in vitro differentiating ESCs; however, we are still far from generating cells usable in the clinic. One main area of investigation will be to focus on defining culture conditions that better mimic the microenvironment supporting the maintenance and expansion of these in vitro-derived blood progenitors. To this extent, future research will benefit from investigating the microenvironment supporting HSC maintenance and expansion in vivo, which could be thus reproduced in vitro more accurately.
Reprogramming Directed Differentiated Cells
By combining directed differentiation and reprogramming approaches, Kyba et al. achieved a first breakthrough success in the generation of engrafting hematopoietic progenitors from mouse ESCs through the enforced expression of Hoxb4, a gene involved in HSC self-renewal [5]. Multilineage engraftment of primary and secondary recipient mice was obtained, with persistence of donor chimerism for up to 5 months. However, a large amount of cells was required for engraftment and lymphoid reconstitution was transient and incomplete. The generation of engrafting blood progenitors from mouse ESCs upon Hoxb4 overexpression has now been reproduced and validated by many groups [39, 40]. Further studies demonstrated that the induced overexpression of Cdx4 in mouse EBs and blast colony cultures, along with Hoxb4 overexpression, resulted in increased lymphoid commitment [41]. Moreover, OP9 stromal coculture was used in these studies to promote hematopoietic commitment and proliferation, with enhanced success in rescuing irradiated recipients. The Hoxb4 approach, however, did not prove successful in the generation of engrafting cells from differentiating human ESCs [37, 42]. Recently, Doulatov et al. have shown that the enforced expression of Hoxa9, Erg, Rora, Sox4, and Myb in CD34+CD45+ cells derived from differentiating human ESCs led to the generation of myeloid and erythroid progenitors [43]. They also demonstrated short-term in vivo engraftment of these blood progenitors; however, lymphoid potential was not observed. The overexpression of SOX17 in human ESC-derived CD34+CD43− cells generated hemogenic endothelial cells with hematopoietic potential [44]. Following the developmental hematopoietic program, the ectopic expression of selected transcription factors (e.g., SCL/GATA2 or ETV2/GATA2) in human ESCs led to the generation of hemogenic endothelial cells with erythroid-megakaryocytic or myeloid lineage potential, respectively [45]. Along similar lines, the overexpression of RUNX1a, an isoform of RUNX1, in human ESCs promoted definitive hematopoiesis and conferred short-term multilineage hematopoietic reconstitution [46].
Taken together, although ESC reprogramming approaches to generate functional engrafting hematopoietic cells have taken the field of blood cell regenerative medicine to the next level, several considerations need to be addressed to make them more functional and safe. In particular, the use of gene overexpression and stromal cocultures represent major hurdles for further translation to clinical applications.
Direct Reprogramming of Somatic Cells to Hematopoietic Progenitors
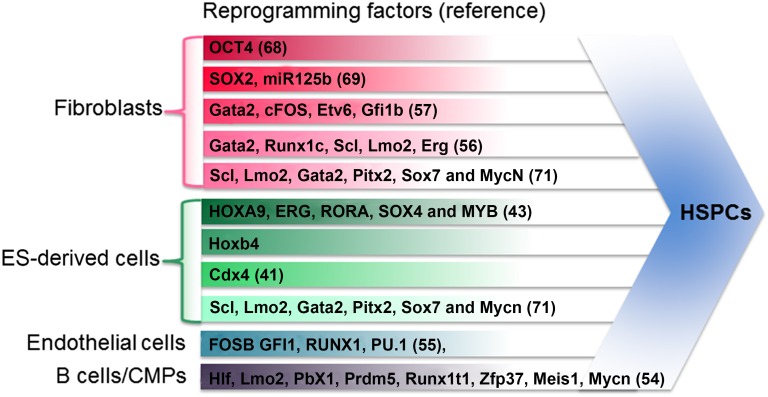
Direct reprogramming, an alternative approach to the directed differentiation of ESCs, is based on the enforced expression of transcription factors (TFs), whose sequential action dictates lineage specification during development [47]. TF-induced lineage conversion was first reported in a seminal study by Davis et al., who showed that fibroblasts could be reprogrammed to myoblasts by the enforced expression of MyoD [48]. This ground-breaking work inspired many direct reprogramming studies for the generation of clinically relevant cell populations such as pancreatic β cells, hepatocytes, neuronal cells, and cardiomyocytes [49–53]. From these studies and because of a lack of robust protocols to generate HSCs from ESCs, we, and others, have attempted to generate HSCs through the enforced expression of hematopoietic-specifying TFs in somatic cells (Fig. 2) [54–57]. This approach is akin to the generation of iPSCs from somatic cells [58]. The results from these studies have clearly demonstrated the success of this approach as evidenced by the generation of hematopoietic stem and progenitor cells (HSPCs), with varying levels of multilineage engraftment capacity.
Figure 2.
List of transcription factors and starting cell types used in different studies to generate HSPCs via direct reprogramming. Abbreviations: CMPs, common myeloid progenitors; ES, embryonic stem cell; HSPCs, hematopoietic stem and progenitor cells.
Transdifferentiation of One Blood Lineage to Another
Vast knowledge of the gene regulatory networks involved in blood cell commitment was instrumental in establishing transdifferentiation protocols of one blood cell type to another by the enforced expression or silencing of one or two key TFs. Interplay between the lineage-specific TFs GATA1 and PU.1, which regulate erythroid versus myeloid specification is a primary example of cross-antagonism [59]. The enforced expression of PU.1, along with CEBPα, is able to reprogram B cells, T cells, and fibroblasts to macrophages without retro-differentiation to HSCs [60–63]. GATA1, along with SCL and CEBPα, can efficiently reprogram terminally differentiated mature B cells into the erythroid lineage [64]. The ectopic expression of GATA1 can also reprogram avian myelomonocytic cell lines into eosinophils, thromboblasts, or erythroblasts [65]. The overexpression of FLI1 and ERG was shown to reprogram human bone marrow-derived erythroblasts to megakaryocytes with the ability to produce functional platelets [66]. Ectopic expression of PU.1 in leukemic B cells not only reprogramed these malignant cells to macrophages but also impaired tumorigenicity [67]. Together, these studies indicate that transdifferentiation approaches can be used to generate many functional mature blood cell types.
Reprogramming to Hematopoietic Progenitor Cells
The first reprogramming study reported by Szabo et al. showed that the overexpression of the pluripotency TF OCT4 could confer hematopoietic progenitor capacity to human neonatal and adult dermal fibroblasts when cultured in hematopoietic-permissive culture conditions [68]. OCT4-induced progenitors had erythroid, myeloid, and megakaryocytic potential. Gene expression analyses showed that blood cell specification did not occur via an intermediate pluripotent state. However, OCT4-induced hematopoietic progenitors lacked lymphoid potential and had limited engraftment capacity. In a more recent report, Pulecio et al. showed that the overexpression of another pluripotency TF SOX2, along with mir125B, reprogrammed human fibroblasts to monocyte-like progenitor cells with in vivo specification toward monocytic and macrophage lineages [69]. However, a major limitation with these two studies was that SOX2 or OCT4 could induce a partial pluripotency state, which is potentially tumorigenic. Our team and Pereira et al. [57] have relied on the overexpression of hematopoietic-specifying factors to reprogram murine fibroblasts to blood progenitors. Pereira et al. showed that the overexpression of four TFs, Gata2, Gfi1b, cFos, and Etv6, induced a hemogenic endothelial cell fate in murine fibroblasts [57]. Despite transcriptional similarity with aorta-gonads-mesonephros and fetal liver HSCs, these reprogrammed cells displayed poor in vitro multilineage clonogenic capacity and no in vivo repopulation capacity. Using a similar approach, we showed that the ectopic expression of five TFs (Erg, Gata2, Lmo2, Runx1c, and Scl) reprogrammed fibroblasts to hematopoietic progenitors with erythrocyte, granulocyte, macrophage, and megakaryocyte potential [56]. We also observed that reprogramming occurred via a hemogenic endothelial stage. However, in contrast to the study by Pereira et al. [57], the hemogenic endothelium induced by the five TFs showed multilineage clonogenic capacity in vitro [56]. Furthermore, combinatorial experiments revealed that Scl and Lmo2 together were sufficient to reprogram fibroblasts to HSPCs [70]. However, only limited short-term engraftment was observed with the cells induced by the five TFs, suggesting that the culture conditions need to be further optimized to generate functional HSPCs. Finally, in a third study, the overexpression of Scl, Lmo2, Gata2, Pitx2, Sox17, and MycN in murine ESCs, fetal liver cells, or fibroblasts generated expandable hemangioblasts with smooth muscle, endothelial, and hematopoietic differentiation potential [71]. However, the multilineage engraftment of these hemangioblast-derived cells was not demonstrated.
Role of the Microenvironment in Direct Reprogramming
A large body of work has demonstrated the primordial role of the surrounding niche in the maintenance of HSCs in the bone marrow [72]. Work from Riddell et al. has now revealed the role of the microenvironment in the generation of HSCs through reprogramming of committed blood cells [54]. In their study, murine pre-/pro-B cells and common myeloid progenitor cells were transduced with 33 HSC-specific TFs and three translational regulators and injected into lethally irradiated recipients. Analysis of the donor-derived cells revealed that Hlf, Lmo2, Pbx1, Prdm5, Runx1t1, and Zfp37 TFs were sufficient to generate functional self-renewing HSCs with multilineage engraftment. The addition of Mesi1 and Mycn further increased the reprogramming efficiency and enabled serial transplantation ability. This study provided the first evidence of the generation of functional murine HSCs by reprogramming mature somatic cells and also established the importance of functional screening strategies for lineage conversions. However, translating this approach to human settings is not feasible because it depends on the endogenous niche. Another major limitation is the unsuitability of using committed blood cells that could carry acquired genetic mutations in diseased conditions. Although this approach is not ideal for the generation of patient-specific inducible HSCs, it should guide our future efforts to generate functional HSCs in vitro by mimicking the HSC niche. In another exciting study, Sandler et al. reported that human umbilical vein and adult dermal microvascular endothelial cells, which are developmentally close to HSCs, can also be reprogrammed to long-term engrafting multipotent progenitors by the overexpression of Fosb, Gfi1, Runx1, and Pu.1 [55]. Reprograming endothelial cells to multipotent blood progenitors requires coculturing with engineered endothelial cells, further supporting the importance of the niche in generating functional HSPCs in vitro. An adequate source of the starting population could be a limitation in using this approach for the generation of inducible multipotent blood progenitors. Moreover, the T lymphoid potential of endothelial cell-derived blood progenitors was not clearly demonstrated. The importance of the in vivo niche was also highlighted by two studies that described the derivation of functional HSCs within teratoma from transplanted human PSCs into NOD scid γ mice [73, 74]. HSCs generated in this approach were able to reconstitute the entire hematopoietic system of immunocompromised recipients and possessed secondary repopulation capacity. More importantly, HSCs derived from gene-corrected iPSCs cured X-linked severe combined immunodeficiency in mouse models. However, the clinical applicability of this approach is extremely limited owing to the possibility of tumor formation by the transplanted PSCs. Collectively, these findings clearly demonstrate the importance of cell, matrix, and molecular cues from the endogenous niche in providing functionality to HSCs. Recent advances in tissue engineering to mimic the bone marrow niche showed promising, yet not conclusive, methods to maintain HSCs derived from bone marrow or cord blood [75]. Future efforts to mimic the authentic milieu of cell, matrix, and biomolecule cues by integrating novel tissue engineering approaches will be useful to maintain and expand HSCs.
Direct Reprogramming Versus Directed Differentiation
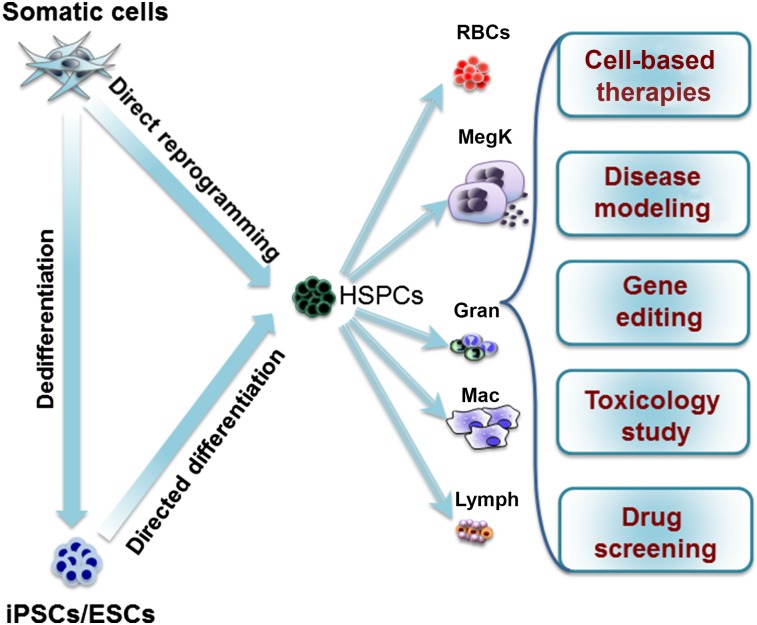
Direct reprogramming to blood progenitor cells has the potential to change the field of hematopoietic cell-based therapies. Several respective advantages and disadvantages exist to using direct reprogramming strategies compared with ESC- or iPSC-based differentiation to blood progenitor cells. The primary advantage of ESC/iPSC technology is the unlimited self-renewing capacity of these cells to provide large numbers of blood cells for therapeutic purposes. However, a potential drawback is the risk of teratoma formation from undifferentiated ESCs/iPSCs. Another important limitation is that the ESC/iPSC-derived hematopoietic system mainly recapitulates embryonic hematopoiesis, which might not be clinically relevant. In contrast, direct reprogramming offers more direct and faster conversion to HSPCs. Adult somatic cell-derived blood cells might be more mature than ESC/iPSC-derived blood cells and therefore might be better suited for modeling blood cell disorders. However, the number of somatic cells available for reprogramming limits the number of reprogrammed blood cells that can be generated. Additionally, a major drawback to the direct reprogramming approach is the potential for mutagenesis mediated by the insertion of the reprogramming constructs and the enforced expression of TFs that are potentially oncogenic. However, both direct reprogramming and directed differentiation approaches are useful methods that can be deployed to generate or engineer blood cells (Fig. 3).
Figure 3.
Generation of HSPCs via reprogramming somatic cells to iPSCs followed by directed differentiation, directed differentiation of ESCs, or direct reprogramming. Clinical utility of HSPCs and differentiated blood cells, including RBCs, MegK, platelets, Gran, Mac, and Lymph. Abbreviations: ESCs, embryonic stem cells; Gran, granulocytes; HSPCs, hematopoietic stem and progenitor cells; iPSCs, induced pluripotent stem cells; Lymph, lymphocytes; Mac, macrophages; MegK, megakaryocytes; RBCs, red blood cells.
Future Challenges
In several studies, the generation of phenotypic HSPCs from both murine and human pluripotent stem and somatic cells has been achieved [43, 54–56]. However, complete in vivo functionality of these in vitro-generated HSPCs, the safety and reproducibility of reprogramming, and directed differentiation procedures have not been clearly demonstrated. Moreover, translation of mouse studies to humans remains a major challenge. This is primarily a result of the lack of an adequate model to test human HSCs and the significant differences between mouse and human hematopoietic systems [76]. Humanized mice or reconstituted human bone marrow could act as alternate models for testing human HSCs [77, 78]. A major limitation in the use of lentiviral or retroviral expression-based reprogramming strategies is the risk of insertional mutagenesis that could activate oncogenes and that would therefore not be clinically compatible. Transfection of synthetic mRNA, self-replicating episomes, nonintegrating viruses, or protein transduction represent attractive alternatives to generate transgene free cells, which are all safer for therapeutic applications [79–82]. Multiple combinations of TFs have been shown to induce a blood cell progenitor phenotype in different somatic cell types with varying multilineage in vitro and in vivo potential [5, 43, 54–57, 68, 69]. However, the reproducibility of these results across different laboratories remains a major concern. The lack of consensus among the TFs reported by different laboratories could have resulted from (a) different cell types of origin, (b) different dosages and stoichiometry of TFs, (c) different epigenetic status of starting cell types, and/or (d) complex regulation among TFs [83, 84]. All these factors should be carefully considered, because they will not only affect reprogramming efficiency but will also affect the functionality of the cells generated. Moreover, the compositions of the media need to be specifically defined to provide better control of reprogramming and differentiation experiments to ensure reproducibility under current Good Manufacturing Practice standards. The epigenetic landscape of the starting population is also clearly important, as it has been shown that endothelial and blood cells can be reprogrammed successfully to functional HSPCs owing to their epigenetic similarities. Furthermore, the epigenetic memory retained from the starting cell type needs to be carefully evaluated in the reprogrammed cells. Testing inhibitors of DNA methyltransferases or histone deacetylases, which are known to increase chromatin accessibility, or modulators of other epigenetic modifiers might help in solving these issues. In addition to epigenetic memory, the age of the starting population also plays a crucial role in reprogramming to pluripotency and in lineage conversion [56, 85]. Ageing is often associated with the accumulation of mutations and alternations in telomere length and therefore could indirectly affect the reprogramming process; however, this could also pose a threat to the safe use of the reprogrammed cells [86]. For example, it has been shown that mutations in tumor suppressor genes or genes involved in cellular maintenance adversely affect chromosome stability and induce oncogenic transformation in reprogrammed cells [87]. In addition, transplantation procedures to treat hematological nonmalignant disorders need to be optimized to improve the engraftment capacity of the in vitro-derived cells while minimizing the side effects of transfusion. Even after the generation of successfully engraftable HSCs, the safety of these cells, their homing to the proper niche, and their molecular profiles must be thoroughly tested before moving toward clinical applications.
Conclusion
Despite many challenges to overcome in generating and using in vitro-derived HSPCs for clinical proposes, it is clear that blood progenitor cells generated from autologous sources will have immense potential to benefit patients in the future. An integration of niche cues, epigenetic signals, and finer refinement of TF combinations will take us toward generating functional HSCs through the differentiation of ESCs or through a direct reprogramming approach. In addition, mimicking the developmental processes involved in the generation and maintenance of HSCs and screening small molecules that promote HSC generation/maintenance will aid in generating the required numbers of clinically relevant cell populations.
Author Contributions
K.B., S.M., E.G.-A., M.F., G.L., and V.K.: manuscript writing, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
References
- 1.Luznik L, O'Donnell PV, Symons HJ, et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transpl. 2008;14:641–650. doi: 10.1016/j.bbmt.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gratwohl A, Pasquini MC, Aljurf M, et al. One million haemopoietic stem-cell transplants: A retrospective observational study. Lancet Haematol. 2015;2:e91–e100. doi: 10.1016/S2352-3026(15)00028-9. [DOI] [PubMed] [Google Scholar]
- 3.Jansen J, Hanks S, Thompson JM, et al. Transplantation of hematopoietic stem cells from the peripheral blood. J Cell Mol Med. 2005;9:37–50. doi: 10.1111/j.1582-4934.2005.tb00335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gori JL, Butler JM, Chan YY, et al. Vascular niche promotes hematopoietic multipotent progenitor formation from pluripotent stem cells. J Clin Invest. 2015;125:1243–1254. doi: 10.1172/JCI79328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kyba M, Perlingeiro RC, Daley GQ. HoxB4 confers definitive lymphoid-myeloid engraftment potential on embryonic stem cell and yolk sac hematopoietic progenitors. Cell. 2002;109:29–37. doi: 10.1016/s0092-8674(02)00680-3. [DOI] [PubMed] [Google Scholar]
- 6.Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- 7.Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci USA. 1981;78:7634–7638. doi: 10.1073/pnas.78.12.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Keller G, Kennedy M, Papayannopoulou T, et al. Hematopoietic commitment during embryonic stem cell differentiation in culture. Mol Cell Biol. 1993;13:473–486. doi: 10.1128/mcb.13.1.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doetschman TC, Eistetter H, Katz M, et al. The in vitro development of blastocyst-derived embryonic stem cell lines: Formation of visceral yolk sac, blood islands and myocardium. J Embryol Exp Morphol. 1985;87:27–45. [PubMed] [Google Scholar]
- 10.Choi K, Kennedy M, Kazarov A, et al. A common precursor for hematopoietic and endothelial cells. Development. 1998;125:725–732. doi: 10.1242/dev.125.4.725. [DOI] [PubMed] [Google Scholar]
- 11.Nishikawa SI, Nishikawa S, Hirashima M, et al. Progressive lineage analysis by cell sorting and culture identifies FLK1+VE-cadherin+ cells at a diverging point of endothelial and hemopoietic lineages. Development. 1998;125:1747–1757. doi: 10.1242/dev.125.9.1747. [DOI] [PubMed] [Google Scholar]
- 12.Fehling HJ, Lacaud G, Kubo A, et al. Tracking mesoderm induction and its specification to the hemangioblast during embryonic stem cell differentiation. Development. 2003;130:4217–4227. doi: 10.1242/dev.00589. [DOI] [PubMed] [Google Scholar]
- 13.Huber TL, Kouskoff V, Fehling HJ, et al. Haemangioblast commitment is initiated in the primitive streak of the mouse embryo. Nature. 2004;432:625–630. doi: 10.1038/nature03122. [DOI] [PubMed] [Google Scholar]
- 14.Kennedy M, Firpo M, Choi K, et al. A common precursor for primitive erythropoiesis and definitive haematopoiesis. Nature. 1997;386:488–493. doi: 10.1038/386488a0. [DOI] [PubMed] [Google Scholar]
- 15.Stefanska M, Costa G, Lie-A-Ling M, et al. Smooth muscle cells largely develop independently of functional hemogenic endothelium. Stem Cell Res (Amst) 2014;12:222–232. doi: 10.1016/j.scr.2013.10.009. [DOI] [PubMed] [Google Scholar]
- 16.Lancrin C, Sroczynska P, Serrano AG, et al. Blood cell generation from the hemangioblast. J Mol Med (Berl) 2010;88:167–172. doi: 10.1007/s00109-009-0554-0. [DOI] [PubMed] [Google Scholar]
- 17.Eilken HM, Nishikawa S, Schroeder T. Continuous single-cell imaging of blood generation from haemogenic endothelium. Nature. 2009;457:896–900. doi: 10.1038/nature07760. [DOI] [PubMed] [Google Scholar]
- 18.Lancrin C, Sroczynska P, Stephenson C, et al. The haemangioblast generates haematopoietic cells through a haemogenic endothelium stage. Nature. 2009;457:892–895. doi: 10.1038/nature07679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pearson S, Lancrin C, Lacaud G, et al. The sequential expression of CD40 and Icam2 defines progressive steps in the formation of blood precursors from the mesoderm germ layer. Stem Cells. 2010;28:1089–1098. doi: 10.1002/stem.434. [DOI] [PubMed] [Google Scholar]
- 20.Frame JM, Fegan KH, Conway SJ, et al. Definitive hematopoiesis in the yolk sac emerges from Wnt-responsive hemogenic endothelium independently of circulation and arterial identity. Stem Cells. 2016;34:431–444. doi: 10.1002/stem.2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jaffredo T, Gautier R, Eichmann A, et al. Intraaortic hemopoietic cells are derived from endothelial cells during ontogeny. Development. 1998;125:4575–4583. doi: 10.1242/dev.125.22.4575. [DOI] [PubMed] [Google Scholar]
- 22.Boisset JC, van Cappellen W, Andrieu-Soler C, et al. In vivo imaging of haematopoietic cells emerging from the mouse aortic endothelium. Nature. 2010;464:116–120. doi: 10.1038/nature08764. [DOI] [PubMed] [Google Scholar]
- 23.Costa G, Kouskoff V, Lacaud G. Origin of blood cells and HSC production in the embryo. Trends Immunol. 2012;33:215–223. doi: 10.1016/j.it.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 24.Kennedy M, D’Souza SL, Lynch-Kattman M, et al. Development of the hemangioblast defines the onset of hematopoiesis in human ES cell differentiation cultures. Blood. 2007;109:2679–2687. doi: 10.1182/blood-2006-09-047704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang L, Li L, Shojaei F, et al. Endothelial and hematopoietic cell fate of human embryonic stem cells originates from primitive endothelium with hemangioblastic properties. Immunity. 2004;21:31–41. doi: 10.1016/j.immuni.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 26.Kennedy M, Awong G, Sturgeon CM, et al. T lymphocyte potential marks the emergence of definitive hematopoietic progenitors in human pluripotent stem cell differentiation cultures. Cell Reports. 2012;2:1722–1735. doi: 10.1016/j.celrep.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 27.Choi KD, Vodyanik MA, Togarrati PP, et al. Identification of the hemogenic endothelial progenitor and its direct precursor in human pluripotent stem cell differentiation cultures. Cell Reports. 2012;2:553–567. doi: 10.1016/j.celrep.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pearson S, Sroczynska P, Lacaud G, et al. The stepwise specification of embryonic stem cells to hematopoietic fate is driven by sequential exposure to Bmp4, activin A, bFGF and VEGF. Development. 2008;135:1525–1535. doi: 10.1242/dev.011767. [DOI] [PubMed] [Google Scholar]
- 29.Pearson S, Cuvertino S, Fleury M, et al. In vivo repopulating activity emerges at the onset of hematopoietic specification during embryonic stem cell differentiation. Stem Cell Rep. 2015;4:431–444. doi: 10.1016/j.stemcr.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Müller AM, Dzierzak EA. ES cells have only a limited lymphopoietic potential after adoptive transfer into mouse recipients. Development. 1993;118:1343–1351. doi: 10.1242/dev.118.4.1343. [DOI] [PubMed] [Google Scholar]
- 31.Hole N, Graham GJ, Menzel U, et al. A limited temporal window for the derivation of multilineage repopulating hematopoietic progenitors during embryonal stem cell differentiation in vitro. Blood. 1996;88:1266–1276. [PubMed] [Google Scholar]
- 32.Potocnik AJ, Kohler H, Eichmann K. Hemato-lymphoid in vivo reconstitution potential of subpopulations derived from in vitro differentiated embryonic stem cells. Proc Natl Acad Sci USA. 1997;94:10295–10300. doi: 10.1073/pnas.94.19.10295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burt RK, Verda L, Kim DA, et al. Embryonic stem cells as an alternate marrow donor source: Engraftment without graft-versus-host disease. J Exp Med. 2004;199:895–904. doi: 10.1084/jem.20031916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nostro MC, Cheng X, Keller GM, et al. Wnt, activin, and BMP signaling regulate distinct stages in the developmental pathway from embryonic stem cells to blood. Cell Stem Cell. 2008;2:60–71. doi: 10.1016/j.stem.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lengerke C, Schmitt S, Bowman TV, et al. BMP and Wnt specify hematopoietic fate by activation of the Cdx-Hox pathway. Cell Stem Cell. 2008;2:72–82. doi: 10.1016/j.stem.2007.10.022. [DOI] [PubMed] [Google Scholar]
- 36.Pick M, Azzola L, Mossman A, et al. Differentiation of human embryonic stem cells in serum-free medium reveals distinct roles for bone morphogenetic protein 4, vascular endothelial growth factor, stem cell factor, and fibroblast growth factor 2 in hematopoiesis. Stem Cells. 2007;25:2206–2214. doi: 10.1634/stemcells.2006-0713. [DOI] [PubMed] [Google Scholar]
- 37.Wang L, Menendez P, Shojaei F, et al. Generation of hematopoietic repopulating cells from human embryonic stem cells independent of ectopic HOXB4 expression. J Exp Med. 2005;201:1603–1614. doi: 10.1084/jem.20041888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ledran MH, Krassowska A, Armstrong L, et al. Efficient hematopoietic differentiation of human embryonic stem cells on stromal cells derived from hematopoietic niches. Cell Stem Cell. 2008;3:85–98. doi: 10.1016/j.stem.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 39.Bonde S, Dowden AM, Chan KM, et al. HOXB4 but not BMP4 confers self-renewal properties to ES-derived hematopoietic progenitor cells. Transplantation. 2008;86:1803–1809. doi: 10.1097/TP.0b013e31818fe741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miyake N, Brun AC, Magnusson M, et al. HOXB4-induced self-renewal of hematopoietic stem cells is significantly enhanced by p21 deficiency. Stem Cells. 2006;24:653–661. doi: 10.1634/stemcells.2005-0328. [DOI] [PubMed] [Google Scholar]
- 41.Wang Y, Yates F, Naveiras O, et al. Embryonic stem cell-derived hematopoietic stem cells. Proc Natl Acad Sci USA. 2005;102:19081–19086. doi: 10.1073/pnas.0506127102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bowles KM, Vallier L, Smith JR, et al. HOXB4 overexpression promotes hematopoietic development by human embryonic stem cells. Stem Cells. 2006;24:1359–1369. doi: 10.1634/stemcells.2005-0210. [DOI] [PubMed] [Google Scholar]
- 43.Doulatov S, Vo LT, Chou SS, et al. Induction of multipotential hematopoietic progenitors from human pluripotent stem cells via respecification of lineage-restricted precursors. Cell Stem Cell. 2013;13:459–470. doi: 10.1016/j.stem.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nakajima-Takagi Y, Osawa M, Oshima M, et al. Role of SOX17 in hematopoietic development from human embryonic stem cells. Blood. 2013;121:447–458. doi: 10.1182/blood-2012-05-431403. [DOI] [PubMed] [Google Scholar]
- 45.Elcheva I, Brok-Volchanskaya V, Kumar A, et al. Direct induction of haematoendothelial programs in human pluripotent stem cells by transcriptional regulators. Nat Commun. 2014;5:4372. doi: 10.1038/ncomms5372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ran D, Shia WJ, Lo MC, et al. RUNX1a enhances hematopoietic lineage commitment from human embryonic stem cells and inducible pluripotent stem cells. Blood. 2013;121:2882–2890. doi: 10.1182/blood-2012-08-451641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Graf T, Enver T. Forcing cells to change lineages. Nature. 2009;462:587–594. doi: 10.1038/nature08533. [DOI] [PubMed] [Google Scholar]
- 48.Davis RL, Weintraub H, Lassar AB. Expression of a single transfected cDNA converts fibroblasts to myoblasts. Cell. 1987;51:987–1000. doi: 10.1016/0092-8674(87)90585-x. [DOI] [PubMed] [Google Scholar]
- 49.Huang P, He Z, Ji S, et al. Induction of functional hepatocyte-like cells from mouse fibroblasts by defined factors. Nature. 2011;475:386–389. doi: 10.1038/nature10116. [DOI] [PubMed] [Google Scholar]
- 50.Ieda M, Fu JD, Delgado-Olguin P, et al. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell. 2010;142:375–386. doi: 10.1016/j.cell.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sekiya S, Suzuki A. Direct conversion of mouse fibroblasts to hepatocyte-like cells by defined factors. Nature. 2011;475:390–393. doi: 10.1038/nature10263. [DOI] [PubMed] [Google Scholar]
- 52.Vierbuchen T, Ostermeier A, Pang ZP, et al. Direct conversion of fibroblasts to functional neurons by defined factors. Nature. 2010;463:1035–1041. doi: 10.1038/nature08797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhou Q, Brown J, Kanarek A, et al. In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature. 2008;455:627–632. doi: 10.1038/nature07314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Riddell J, Gazit R, Garrison BS, et al. Reprogramming committed murine blood cells to induced hematopoietic stem cells with defined factors. Cell. 2014;157:549–564. doi: 10.1016/j.cell.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sandler VM, Lis R, Liu Y, et al. Reprogramming human endothelial cells to haematopoietic cells requires vascular induction. Nature. 2014;511:312–318. doi: 10.1038/nature13547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Batta K, Florkowska M, Kouskoff V, et al. Direct reprogramming of murine fibroblasts to hematopoietic progenitor cells. Cell Reports. 2014;9:1871–1884. doi: 10.1016/j.celrep.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pereira CF, Chang B, Qiu J, et al. Induction of a hemogenic program in mouse fibroblasts. Cell Stem Cell. 2013;13:205–218. doi: 10.1016/j.stem.2013.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 59.Arinobu Y, Mizuno S, Chong Y, et al. Reciprocal activation of GATA-1 and PU.1 marks initial specification of hematopoietic stem cells into myeloerythroid and myelolymphoid lineages. Cell Stem Cell. 2007;1:416–427. doi: 10.1016/j.stem.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 60.Feng R, Desbordes SC, Xie H, et al. PU.1 and C/EBPalpha/beta convert fibroblasts into macrophage-like cells. Proc Natl Acad Sci USA. 2008;105:6057–6062. doi: 10.1073/pnas.0711961105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xie H, Ye M, Feng R, et al. Stepwise reprogramming of B cells into macrophages. Cell. 2004;117:663–676. doi: 10.1016/s0092-8674(04)00419-2. [DOI] [PubMed] [Google Scholar]
- 62.Laiosa CV, Stadtfeld M, Xie H, et al. Reprogramming of committed T cell progenitors to macrophages and dendritic cells by C/EBP alpha and PU.1 transcription factors. Immunity. 2006;25:731–744. doi: 10.1016/j.immuni.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 63.Di Tullio A, Vu Manh TP, Schubert A, et al. CCAAT/enhancer binding protein alpha (C/EBP(alpha))-induced transdifferentiation of pre-B cells into macrophages involves no overt retrodifferentiation. Proc Natl Acad Sci USA. 2011;108:17016–17021. doi: 10.1073/pnas.1112169108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sadahira K, Fukuchi Y, Kunimono H, et al. Direct reprogramming of terminally differentiated B cells into erythroid lineage. FEBS Lett. 2012;586:3645–3652. doi: 10.1016/j.febslet.2012.08.019. [DOI] [PubMed] [Google Scholar]
- 65.Kulessa H, Frampton J, Graf T. GATA-1 reprograms avian myelomonocytic cell lines into eosinophils, thromboblasts, and erythroblasts. Genes Dev. 1995;9:1250–1262. doi: 10.1101/gad.9.10.1250. [DOI] [PubMed] [Google Scholar]
- 66.Siripin D, Kheolamai P, U-Pratya Y, et al. Transdifferentiation of erythroblasts to megakaryocytes using FLI1 and ERG transcription factors. Thromb Haemost. 2015;114:593–602. doi: 10.1160/TH14-12-1090. [DOI] [PubMed] [Google Scholar]
- 67.McClellan JS, Dove C, Gentles AJ, et al. Reprogramming of primary human Philadelphia chromosome-positive B cell acute lymphoblastic leukemia cells into nonleukemic macrophages. Proc Natl Acad Sci USA. 2015;112:4074–4079. doi: 10.1073/pnas.1413383112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Szabo E, Rampalli S, Risueño RM, et al. Direct conversion of human fibroblasts to multilineage blood progenitors. Nature. 2010;468:521–526. doi: 10.1038/nature09591. [DOI] [PubMed] [Google Scholar]
- 69.Pulecio J, Nivet E, Sancho-Martinez I, et al. Conversion of human fibroblasts into monocyte-like progenitor cells. Stem Cells. 2014;32:2923–2938. doi: 10.1002/stem.1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Goode DK, Obier N, Vijayabaskar MS, et al. Dynamic gene regulatory networks drive hematopoietic specification and differentiation. Dev Cell. 2016;36:572–587. doi: 10.1016/j.devcel.2016.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vereide DT, Vickerman V, Swanson SA, et al. An expandable, inducible hemangioblast state regulated by fibroblast growth factor. Stem Cell Rep. 2014;3:1043–1057. doi: 10.1016/j.stemcr.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Boulais PE, Frenette PS. Making sense of hematopoietic stem cell niches. Blood. 2015;125:2621–2629. doi: 10.1182/blood-2014-09-570192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Suzuki N, Yamazaki S, Yamaguchi T, et al. Generation of engraftable hematopoietic stem cells from induced pluripotent stem cells by way of teratoma formation. Mol Ther. 2013;21:1424–1431. doi: 10.1038/mt.2013.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Amabile G, Welner RS, Nombela-Arrieta C, et al. In vivo generation of transplantable human hematopoietic cells from induced pluripotent stem cells. Blood. 2013;121:1255–1264. doi: 10.1182/blood-2012-06-434407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Choi JS, Mahadik BP, Harley BA. Engineering the hematopoietic stem cell niche: Frontiers in biomaterial science. Biotechnol J. 2015;10:1529–1545. doi: 10.1002/biot.201400758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mestas J, Hughes CC. Of mice and not men: Differences between mouse and human immunology. J Immunol. 2004;172:2731–2738. doi: 10.4049/jimmunol.172.5.2731. [DOI] [PubMed] [Google Scholar]
- 77.Drake AC, Chen Q, Chen J. Engineering humanized mice for improved hematopoietic reconstitution. Cell Mol Immunol. 2012;9:215–224. doi: 10.1038/cmi.2012.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Torisawa YS, Spina CS, Mammoto T, et al. Bone marrow-on-a-chip replicates hematopoietic niche physiology in vitro. Nat Methods. 2014;11:663–669. doi: 10.1038/nmeth.2938. [DOI] [PubMed] [Google Scholar]
- 79.Fusaki N, Ban H, Nishiyama A, et al. Efficient induction of transgene-free human pluripotent stem cells using a vector based on Sendai virus, an RNA virus that does not integrate into the host genome. Proc Jpn Acad, Ser B, Phys Biol Sci. 2009;85:348–362. doi: 10.2183/pjab.85.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mandal PK, Rossi DJ. Reprogramming human fibroblasts to pluripotency using modified mRNA. Nat Protoc. 2013;8:568–582. doi: 10.1038/nprot.2013.019. [DOI] [PubMed] [Google Scholar]
- 81.Warren L, Manos PD, Ahfeldt T, et al. Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell. 2010;7:618–630. doi: 10.1016/j.stem.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang H, Ma Y, Gu J, et al. Reprogramming of somatic cells via TAT-mediated protein transduction of recombinant factors. Biomaterials. 2012;33:5047–5055. doi: 10.1016/j.biomaterials.2012.03.061. [DOI] [PubMed] [Google Scholar]
- 83.Tiemann U, Sgodda M, Warlich E, et al. Optimal reprogramming factor stoichiometry increases colony numbers and affects molecular characteristics of murine induced pluripotent stem cells. Cytometry A. 2011;79:426–435. doi: 10.1002/cyto.a.21072. [DOI] [PubMed] [Google Scholar]
- 84.Wilson NK, Foster SD, Wang X, et al. Combinatorial transcriptional control in blood stem/progenitor cells: Genome-wide analysis of ten major transcriptional regulators. Cell Stem Cell. 2010;7:532–544. doi: 10.1016/j.stem.2010.07.016. [DOI] [PubMed] [Google Scholar]
- 85.Wang B, Miyagoe-Suzuki Y, Yada E, et al. Reprogramming efficiency and quality of induced pluripotent stem cells (iPSCs) generated from muscle-derived fibroblasts of mdx mice at different ages. PLoS Curr. 2011;3:RRN1274. doi: 10.1371/currents.RRN1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang F, Yin Y, Ye X, et al. Molecular insights into the heterogeneity of telomere reprogramming in induced pluripotent stem cells. Cell Res. 2012;22:757–768. doi: 10.1038/cr.2011.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sarig R, Rivlin N, Brosh R, et al. Mutant p53 facilitates somatic cell reprogramming and augments the malignant potential of reprogrammed cells. J Exp Med. 2010;207:2127–2140. doi: 10.1084/jem.20100797. [DOI] [PMC free article] [PubMed] [Google Scholar]