Abstract
Background
Aerobic exercise training (AET) has been shown to provide general health benefits, and to improve motor behaviours in particular, in individuals with Parkinson's disease (PD). However, the influence of AET on their motor learning capacities, as well as the change in neural substrates mediating this effect remains to be explored.
Objective
In the current study, we employed functional Magnetic Resonance Imaging (fMRI) to assess the effect of a 3-month AET program on the neural correlates of implicit motor sequence learning (MSL).
Methods
20 healthy controls (HC) and 19 early PD individuals participated in a supervised, high-intensity, stationary recumbent bike training program (3 times/week for 12 weeks). Exercise prescription started at 20 min (+ 5 min/week up to 40 min) based on participant's maximal aerobic power. Before and after the AET program, participants' brain was scanned while performing an implicit version of the serial reaction time task.
Results
Brain data revealed pre-post MSL-related increases in functional activity in the hippocampus, striatum and cerebellum in PD patients, as well as in the striatum in HC individuals. Importantly, the functional brain changes in PD individuals correlated with changes in aerobic fitness: a positive relationship was found with increased activity in the hippocampus and striatum, while a negative relationship was observed with the cerebellar activity.
Conclusion
Our results reveal, for the first time, that exercise training produces functional changes in known motor learning related brain structures that are consistent with improved behavioural performance observed in PD patients. As such, AET can be a valuable non-pharmacological intervention to promote, not only physical fitness in early PD, but also better motor learning capacity useful in day-to-day activities through increased plasticity in motor related structures.
Keywords: Parkinson's disease, Exercise, Motor learning, fMRI
Highlights
-
•
Three months of aerobic exercise training (AET) improves fitness in PD patients.
-
•
Effects of AET on cognitive and motor skills in PD were evaluated concurrently.
-
•
Some executive functions and procedural learning capacity improved after AET.
-
•
Striatum, hippocampus and cerebellar brain plasticity was observed after AET.
-
•
AET can be used as a non-pharmacological intervention to improve functioning in PD.
1. Introduction
Parkinson's disease (PD) is a progressive disorder of multifactorial etiology characterized by motor symptoms such as tremor, rigidity, bradykinesia and gait difficulties. Motor deficits in PD are due to an abnormal neuronal activity of the motor circuit in the basal ganglia, predominantly involving the striatum and the putamen (Amano et al., 2013). Such brain dysfunctions lead to motor functional difficulties, including impairments in learning new motor skills (Clark, 2014), hence adding burden to day-to-day activities and leading sometimes to inactivity and exacerbation of disease manifestation (van Nimwegen et al., 2011). As a result, both the known striatal pathology in PD and the lack of physical activity imposed by the disease can jeopardize the patients' capacity to acquire new motor skilled behaviours throughout the progression of the neurodegenerative process.
Apart from the treatments commonly used to managed PD symptoms (e.g., dopaminergic-derived drug treatments and DBS), alternative treatments, such as physical activity, have recently been investigated in PD. Such a new approach is based on an increasing number of studies in animals and humans, which indicate that physical exercise may attenuate symptoms of the disease, and even exert a neuroprotective effect (Hirsch and Farley, 2009). In rodents for example, mice model of PD forced to exercise on a treadmill did not develop behavioural deficits and significantly preserved nigrostriatal neuronal connections as well as striatal dopamine levels compared to sedentary mice (Pothakos et al., 2009, Yoon et al., 2007). Petzinger et al. (2007) have also found that a similar exercise intervention improves efficiency of brain dopamine cells more in mice who exercised as compared to those that did not. Altogether, these results suggest that exercise improves dopaminergic neurotransmission efficiency by modifying the areas of the brain (e.g., the substantia nigra and basal ganglia) where dopamine signals originate (Petzinger et al., 2007). Given that movement is modulated through dopamine, findings in rodents suggest a possible interaction between behaviour and neuronal cerebral viability in the striatum after cardiovascular exercise (Petzinger et al., 2010).
In humans, studies in PD individuals have demonstrated significant improvement in gait and mobility following supervised physical exercise (Beall et al., 2013, Herman et al., 2007, Nadeau et al., 2014, Ridgel et al., 2011, Ridgel et al., 2009, van Eijkeren et al., 2008). Among the many different types of physical training programs (e.g., resistance training, flexibility, coordination, etc.), aerobic exercise training (AET) has been the most studied so far, and the one that has shown the most unequivocal benefits on health across the life span (Voss et al., 2011). Although the underlying mechanisms in PD still remain conjectural, it has been hypothesized that exercise-dependent plasticity following AET acts on the brain in a similar manner as the dopaminergic-derived treatments, using the same pathways to produce symptomatic relief (Beall et al., 2013). It is thus possible that exercise dependent change can have beneficial effects on motor skill learning processes. More specifically, AET may restore motor sequence learning (MSL), a capacity that is frequently impaired in PD (Clark, 2014), by improving brain functioning at the level of the striatum or motor-related associated circuits.
Despite behavioural impairment observed when performing MSL tasks, studies have demonstrated that similar motor-related brain regions seem to be recruited in individuals with PD (Carbon et al., 2010, Schendan et al., 2013, Werheid et al., 2003) as in healthy aging controls (Albouy et al., 2015, Doyon et al., 2003). Specifically, functional brain imaging studies with healthy participants have shown that brain plasticity associated with MSL relies on recruitment of striatum and hippocampus, as well as the cerebellum, motor cortical regions, and prefrontal and parietal cortex (Albouy et al., 2015, Doyon et al., 2009). Studies with PD individuals yielded similar regions, but different patterns of interaction between them, in the cortico-striatal and medio-temporal lobe networks (Carbon et al., 2010, Schendan et al., 2013, Werheid et al., 2003). Furthermore, these findings could be influenced by the disease stage and the impact of dopaminergic medication (see Ruitenberg et al., 2015 for a review). However, studies directly comparing the neuronal substrate involved in MSL in healthy and PD are few and those directly comparing the effects of aerobic exercise on the MSL neuronal substrate in healthy and PD are non-existent. In fact, one study demonstrated that a single bout of high-intensity aerobic exercise facilitates motor sequence learning in healthy individuals (Mang et al., 2014), but the neural correlates of these effects have never been investigated at the whole brain level; and furthermore, they have never been specific to the chronic effects of exercise.
Thus, to date, there is a lack of evidence regarding the effects of aerobic training exercise on the MSL functional neuronal substrate in both healthy and PD. In response to this knowledge gap, the principal aim of the present study was to investigate, with whole brain functional magnetic resonance imaging (fMRI), the neural substrate mediating the effects of cardiorespiratory exercise on MSL in both PD and healthy control (HC) individuals. We have already presented behavioural evidence that 3-month AET improved MSL capacity in both healthy and PD individuals, as well as executive functions in PD (Duchesne et al., 2015). In the current article, we present the neuroimaging data related to MSL from the same study and we hypothesize that 1) AET will improve MSL capacity through augmented activity in the cortico-striatal (CS) system in PD, and 2) MSL-related cerebral activity changes observed after AET will be linked to improvement in aerobic fitness in both PD and HC groups.
2. Methods
2.1. Participants
Forty-two participants (21 PD patients and 23 HC) were eligible for participation after the completion of the first evaluation. However, between the evaluation and prior to the onset of the exercise regimen, two HC participants decided to withdraw from the project for personal reasons. One PD participant and one HC were excluded after the beginning for health security. One PD patient was excluded from analysis because of extreme results on several outcomes, even if this person respected all inclusion criteria. PD patients had to be classified as stage 1 or 2 according to Hoehn and Yahr's scale (Hoehn and Yahr, 1967) based upon evaluation of a certified neurologist. Participants who were under medication continued their treatment all throughout the study (testing and training). A total of 39 participants (18 women) aged between 40 and 80 years old participated in the present study; 19 were diagnosed with PD and 20 were healthy control (HC) subjects. They were screened for appropriateness for testing in an MR environment (e.g., no metallic implants that could interfere with testing, no claustrophobia etc.). Then, the Physical Activity Readiness Questionnaire (PAR-Q) (Warburton et al., 2011) was used to verify the participant's safety in participating in a physical program. To control for pre-existing experience in tasks requiring highly coordinated finger dexterities, PD and HC subjects had to have no previous training in playing a musical instrument, nor any training as a professional typist. Other exclusion criteria included the presence of other neurological disorders, comorbidities likely to affect gait, or any history of smoking or heart diseases, and high baseline physical activity.
These two groups were well matched with respect to their sex distribution, age, number of years of education and fitness level (as there were no significant differences on any of these variables prior to the AET intervention program, see Table 1). Yet, and as expected, significant group differences were found on Beck's depression and anxiety scales, with PD patients showing higher scores on both scales as compared with HC participants.
Table 1.
Demographics of the two groups of participants.
| Variablesa | HC (n = 20) | PD (n = 19) | p-Value |
|---|---|---|---|
| Sex (male/female) | 8/12 | 13/6 | 0,07b |
| Age (years) | 64 (8.19) | 59 (7.11) | 0.06c |
| Education (years) | 15.7 (2.36) | 15.05 (2.78) | 0.43c |
| Fitnessd | 2.1 (1.17) | 1.84 (1.26) | 0.51c |
| Cognition (MMSE/MOCA)e | 29.18/28.56 (1.25/1.51) | 28.4/27.21 (1.34/1.85) | 0.275/0.08c |
| Depressionf | 4.8 (4.5) | 10.5 (8.3) | *0.01c |
| Anxietyf | 2.1 (2.7) | 8.6 (8.4) | *0.002c |
| Hoehn and Yahr score | N/A | 2 (0) | N/A |
| UPDRS total score | N/A | 21.84 (6.16) | N/A |
| Years diagnosed | N/A | 8.1 (9.12) | N/A |
Values represent mean (standard deviation), except for ‘sex’, where values represent counts.
p-Value from chi-square test.
p-Value from ANOVA.
Jackson's questionnaire assessing activity level at baseline.
5 PD and 11 HC were assessed with MMSE and 14 PD and 9 HC with MOCA.
Beck depression inventory and Beck anxiety were used.
indicate statistically significant differences.
2.2. Clinical assessment of health and cardiovascular fitness
First, PD subjects were recruited based on a neurological assessment performed by an expert neurologist. Clinical assessments of the participants' health and physical activity level were then performed by a geriatrist, neuropsychologist and physiologist using the following tests: the United Parkinson's Disease Rating Scale (UPDRS) (Goetz et al., 2008), the Montreal Cognitive Assessment (MOCA) or Mini Mental State Evaluation (MMSE) (Nazem et al., 2009), the Beck Depression Inventory (BDI) (Beck and Beamesderfer, 1974) and Beck Anxiety Inventory (BAI) (Osman et al., 1997), as well as the Jackson's Questionnaire (Jackson et al., 1990).
Participants' cardiovascular fitness level was evaluated using either a submaximal aerobic test (11 HC, 5 PD) or a medically supervised maximal oxygen uptake test (9 HC, 14 PD). The protocol required a five minutes resting period before measuring heart rate, blood pressure, and aerobic capacity. For the evaluated with a sub-maximal test (using a recumbent bike), the test started with a workload stage of 35 or 50 W (for women and men, respectively), lasting one minute; the intensity increasing progressively by 15 W every minute until they reached 85% of their predicted maximal heart rate (HRmax = 208 − 0.7 × age). When the target heart rate was reached, participants were asked to complete the current stage (if possible). The power level (in W) of the last completed stage was used to extrapolate the maximal aerobic power (MAP). Then, VO2max estimation was calculated with body weight (kg) and MAP (W) measures, using the American College Sport Medicine's formula: VO2 = (10,8 × W/weight) + 7; MAP estimation was used for exercise prescription. For the participants that were evaluated with a graded cycling (one minute workload stage) maximal test, the protocol started again at 35 W or 50 W (for women and men, respectively), and the intensity progressively increased by 15 W every minute until maximal efforts criteria were fulfilled (e.g., age predicted maximal heart rate ± 10 bpm and VO₂ plateau), or until subjects could not maintain the required workload anymore (fatigue). Aerobic capacity (VO2max) was measured directly using a breath-by-breath system. The last completed stage power (W) was recorded and used for exercise prescription (American College of Sport Medicine, 2006). Importantly, PD individuals performed the stress test while following their usual medication regimen, which did not change during the study period. In addition, for each patient, the stress tests were administered at the same time of day and did not change pre-to-post AET.
2.3. Motor sequence learning task
Subjects' implicit MSL capacity was evaluated using a version of the serial reaction time (SRT) task (Nissen and Bullemer, 1987), in which participants were asked to press buttons on a keyboard in response to corresponding visual stimuli presented on a screen. Unbeknown to the participants, stimuli were presented in a random or in a repeating sequential order, in blocks of 40 stimuli each. These blocks were presented in triplets (sequence, random, sequence), with a total of 12 triplets during the entire training session. For the sequential blocks, two 8-item, second order conditional sequences (2-4-3-1-4-2-1-3 and 3-1-2-4-1-3-4-2) were used and counterbalanced between sessions (pre-, post-AET) and subjects, in order to avoid repetition of the learned sequence for the same individual. For each trial, reaction time (i.e., the time between the onsets of the stimulus to the completion of the response. To assess sequence learning, reaction times (RTs) and accuracy were compared between the sequence and random blocks, with faster RTs and better accuracy for sequence as compared to the random blocks reflecting greater motor sequence learning capacity. More information on motor task procedure can be found in previous paper (Duchesne et al., 2015).
2.4. Behavioural statistical data analysis
The main independent variables included in our analyses were the presence/absence of the disease (i.e., PD vs. HC groups, between group variable) and the effect of aerobic training, expressed as the time of testing (pre- vs. post- intervention, within-group variable). Dependent variables included the following: physical fitness (expressed as VO2max estimate) and the subject's performance (learning score) on the MSL task. We first assessed the relationship between independent and dependent variables using a mixed repeated measures analysis of variance (ANOVA), with group as between-subjects factor and time of testing as within-subjects factor. In order to account for the effect of multiple comparisons, the statistical significance was adjusted using the Bonferroni method. The two psychological variables (depression and anxiety) were used as covariates in all subsequent analyses involving behavioural data, to control for group differences at baseline on these variables. All analyses were performed with SPSS 20 (IBM Inc., Armonk, NY).
2.5. Experimental procedure
Participants were tested on two time points: pre and post training. Each occasion was similar and included two different days of testing that occurred at least 48 h apart. On day 1, participants were first informed in details about the study protocol before signing a consent form, which was approved by the “Regroupement Neuroimagerie Québec” Ethics Review Committee. Participants were then evaluated with respect to their functional capacities and cardiorespiratory fitness level, as well as to their medical and psychological status. This was followed by giving instructions to participants regarding the scanning procedure and the motor sequence learning task while lying in a mock scanner. During this familiarization session, participants had time to practice a randomly assigned trial to correct the positioning of the fingers on the response box. On Day 2, participants' brain was then scanned while completing the motor sequence task while positioned in the magnetic resonance imaging system available at the “Unité de Neuroimagerie Fonctionnelle” from the “Université de Montréal”, the latter session occurring before and after the AET program.
2.5.1. The exercise protocol procedure
The cardiorespiratory fitness program involved three months of recumbent bike-training, three times a week, 1 h/session, which occurred at the same time of day. The exercise intensity prescription was based on the subject's maximal aerobic power output from the maximum volume of oxygen (VO₂peak) uptake conducted on pre-test day (American College of Sport Medicine, 2006). Duration of the exercise program started at 20 min and 60% of intensity per session, and was then increased by steps of 5 min and 5% of intensity every week until participants reached 40 min of training at 80% intensity. To reach a high-intensity level, bike speed was maintained at 60 rpm. As such, to achieve the desired bike resistance power and adjust intensity level (if needed), the work intensity was based on power output (Watt), controlling for subject's heart rate. In addition, rate of perceived exertion (Borg scale) (Borg, 1982) was assessed on each training session. The target of 75% or more in participation rate in the fitness training program was achieved. In addition, PD individuals did not change their medication regimen for the entire duration of the study.
2.6. fMRI data acquisition and analysis
Functional MRI-series were acquired using a 3.0T TIM TRIO scanner system (Siemens, Erlangen, Germany), equipped with a 12-channel head coil. Multislice T2*-weighted fMRI images were obtained with a gradient echo-planar sequence using axial slice orientation in an ascending order (TR = 2650 ms, TE = 30 ms, FA = 90°, 43 transverse slices, 3 mm slice thickness, 10% inter-slice gap, FoV = 220 × 220 mm2, matrix size = 64 × 64 × 43, voxel size = 3.4 × 3.4 × 3 mm3). A structural T1-weigthed 3D MP-RAGE sequence (TR = 2300 ms, TE = 2.98 ms, TI = 900 ms, FA = 9°, 176 slices, FoV = 256 × 256 mm2, matrix size = 256 × 256 × 176, voxel size = 1 × 1 × 1 mm3) was also acquired in all subjects. Head movements were minimized using cushions and instructions prior to scanning.
Functional volumes were pre-processed and analysed using SPM8 (http://www.fil.ion.ucl.ac.uk/spm/software/spm8/; Wellcome Department of Imaging Neuroscience, London, UK) implemented in MATLAB. Functional scans of each session were realigned using rigid body transformations, iteratively optimized to minimize the residual sum of squares between the first and each subsequent image separately for each session, hence creating a mean realigned image. The mean functional image was then coregistered to the structural T1-image using a rigid body transformation optimized to maximize the normalized mutual information between the two images. Coregistration parameters were then applied to the realigned BOLD time series. An average subject-based template was created using DARTEL in SPM8, and registered to MNI space (Montreal Neurological Institute, http://www.bic.mni.mcgill.ca). All functional and anatomical images were then normalized using the resulting template. Finally, all functional images were spatially smoothed using an isotropic 8-mm full-width at half-maximum (FWHM) Gaussian kernel.
The analysis of fMRI data, based on a summary statistics approach, was conducted in 2 serial steps, accounting respectively for fixed and random effects. For each subject, changes in brain regional responses were estimated through a model including responses to the sequence and random conditions separately in each practice session (pre and post). These four regressors consisted of boxcars convolved with the canonical hemodynamic response function. Instructions as well as movement parameters derived from realignment of the functional volumes were also included as covariates of no interest. High-pass filtering was implemented in the design matrix using a cut-off period of 128 s to remove slow drifts from the time series. Serial correlations in fMRI signal were estimated using an autoregressive (order 1) plus white noise model and a restricted maximum likelihood (ReML) algorithm.
The contrast of interest explored the main motor sequence learning effect (Sequence - Random) between sessions (POST - PRE). The resulting contrast images of each subject were then further spatially smoothed (Gaussian kernel 6 mm FWHM) and entered in a second-level analysis, accounting for inter-subject variance. In the second level analyses, one sample t-tests were performed to explore main motor sequence learning effect between-sessions within each group (HC and PD) separately. Subsequently, two-sample t-tests were performed in order to compare this effect between groups (HC vs PD).
Furthermore, we performed an additional analysis in order to assess the relationship between these changes in brain activation and aerobic fitness across sessions. Specifically, the main sequence learning effect between sessions was regressed against changes in aerobic fitness to ascertain whether these effects were related to the AET. As before, one sample t-tests were performed to explore the regression effects within each group (HC and PD) separately, and two-sample t-tests were performed in order to compare the regression effects between groups (HC vs PD).
The set of voxel activation values resulting from each analysis described above (activation and regression analyses) were displayed in statistical maps at a threshold of p < 0.005 (uncorrected for multiple comparisons). However, all statistical inferences were performed at a threshold of p < 0.05 after family-wise error (FWE) correction for multiple comparisons over small spherical volumes (10 mm radius) located in a priori defined structures of interest reported in published work on motor learning.
3. Results
3.1. Behavioural results
3.1.1. Aerobic exercise training
The 3-months aerobic training regimen yielded a significant improvement in aerobic fitness as indicated by the estimated VO2max in the two groups combined (F1,35 = 17.333, p < 0.001), as well as in both HC (F1,35 = 15.188, p < 0.001) and PD (F1,35 = 9.984, p < 0.003) groups separately (see Supplemental materials for more details). Also the estimated power level increased significantly following AET in the two groups combined (F1,35 = 16.461, p < 0.001) as well as in the HC (F1,35 = 14.930, p < 0.001) and PD (F1,35 = 10.500, p < 0.003) groups separately. However, there was no significant group × session interaction, nor any group differences for all of these fitness measures. Altogether, these results indicate that the effect of exercise had similar physiological benefits in both groups of participants who significantly increased their individual level of aerobic fitness (Duchesne et al., 2015).
3.1.2. Motor sequence learning
The detailed results of the MSL task for the two conditions (sequence and random) and groups (PD and HC), both pre and post training session are reported elsewhere (Duchesne et al., 2015) and here in Supplemental materials. Even though the interaction between groups and conditions regardless of time of testing (i.e. pre- vs. post-AET) was not significant (F1,35 = 0,266, p = 0.6), across all participants, we observed a significant difference in reaction time between conditions (F1,35 = 17.712, p < 0.001), with performance during the sequence being faster than during the random condition; an indicator of motor sequence learning capacity. In addition, there was a marginally significant interaction between condition and session (F1,35 = 3.650, p = 0.064) across the two groups, hence demonstrating a trend that the participant's motor sequence learning capacity tended to improve more than their performance in the random condition following AET.
Within-group analyses revealed between-session improvement in reaction time for both sequence (F1,35 = 12.525, p < 0.001) and random conditions (F1,35 = 8.422, p < 0.006); whereas PD individuals showed AET-related improvement in RT only for the sequential condition (F1,35 = 5.173, p < 0.029). Most importantly, when assessing motor sequence learning capacity in each group, we identified a significant sequence learning effect (i.e., sequential - random conditions) pre-AET in HC (F1,35 = 4.059, p = 0.052), but not in PD (F1,35 = 1.427, p = 0.24). After AET, however, both groups showed significant sequence learning (F1,35 = 7.134, p < 0.011, for HC and F1,35 = 8.878, p < 0.005).
3.2. Brain imaging results
3.2.1. Functional brain changes related to motor learning capacity following AET in PD
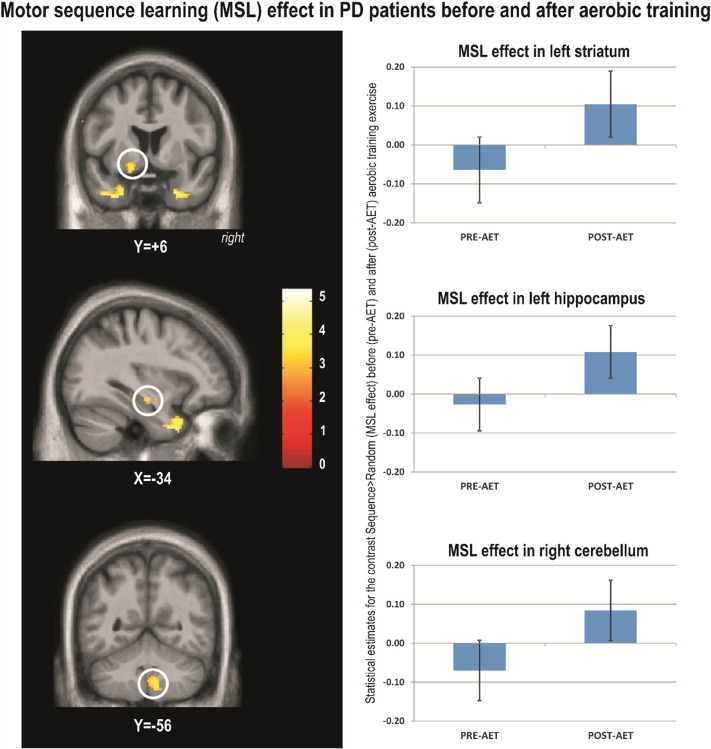
To investigate the effects of AET on the neural correlates specific to motor sequence learning, we contrasted the main sequence learning effect [Sequence – Random] by session [POST – PRE] within PD group. The results of this analysis revealed significant training-related changes for PD's individuals. Specifically, brain responses reflecting motor sequence learning capacity increased significantly post-AET in the temporal lobes, left ventral striatum, left hippocampus, cerebellar lobules 8 and 9 bilaterally and right crus (Table 2, Fig. 1).
Table 2.
Functional imaging results of the changes in the main learning effect following aerobic exercise training in PD.
| Area | X mm | Y mm | Z mm | K | Z | psvc |
|---|---|---|---|---|---|---|
| 2.1. Main effect of session on sequence learning [POST – PRE] × [Sequence – Random] | ||||||
| PD | ||||||
| Right temporal lobe | 30 | 12 | − 36 | 92 | 3,85 | 0,002 |
| Left temporal lobe | − 30 | 12 | − 38 | 36 | 3,45 | 0,008 |
| Left striatum ventral | − 18 | 6 | − 12 | 48 | 3,76 | 0,003 |
| Left hippocampus | − 34 | − 16 | − 12 | 99 | 3,66 | 0,004 |
| Right cerebellum lobules 8 and 9 | 16 | − 46 | − 54 | 38 | 3,1 | 0,022 |
| Left cerebellum lobules 8 and 9 | − 20 | − 42 | − 52 | 38 | 3,14 | 0,02 |
| Right cerebellum crus 1 | 28 | − 72 | − 38 | 51 | 3,1 | 0,022 |
| 2.2. Main effect of session on sequence learning [POST – PRE] × [Sequence – Random] regressed against changes in VO2max | ||||||
| Positive | ||||||
| Left hippocampus | − 18 | − 34 | − 2 | 182 | 3,19 | 0,017 |
| Left hippocampus | − 18 | − 24 | − 10 | 23 | 3,12 | 0,021 |
| Right hippocampus | 36 | − 32 | − 8 | 170 | 3,4 | 0,01 |
| Left putamen dorsal | − 28 | 0 | 18 | 45 | 3,04 | 0,025 |
| Negative | ||||||
| Cerebellum lobule 7 | − 2 | − 76 | − 24 | 167 | 3,98 | 0,001 |
| Left cerebellum lobules 8 and 9 | − 4 | − 62 | − 36 | 65 | 3,17 | 0,018 |
| − 16 | − 60 | − 46 | 110 | 3,11 | 0,021 | |
| Right cerebellum lobules 8 and 9 | 26 | − 64 | − 46 | 58 | 3,23 | 0,015 |
Statistical inferences were performed at a threshold of p < 0.05 after correction for multiple comparisons over small spherical volumes (SVC). K represents the number of voxels in each cluster reported (determined at a threshold of p = 0.005).
Fig. 1.
Motor sequence learning (MSL) effect in PD patients before and after aerobic training.
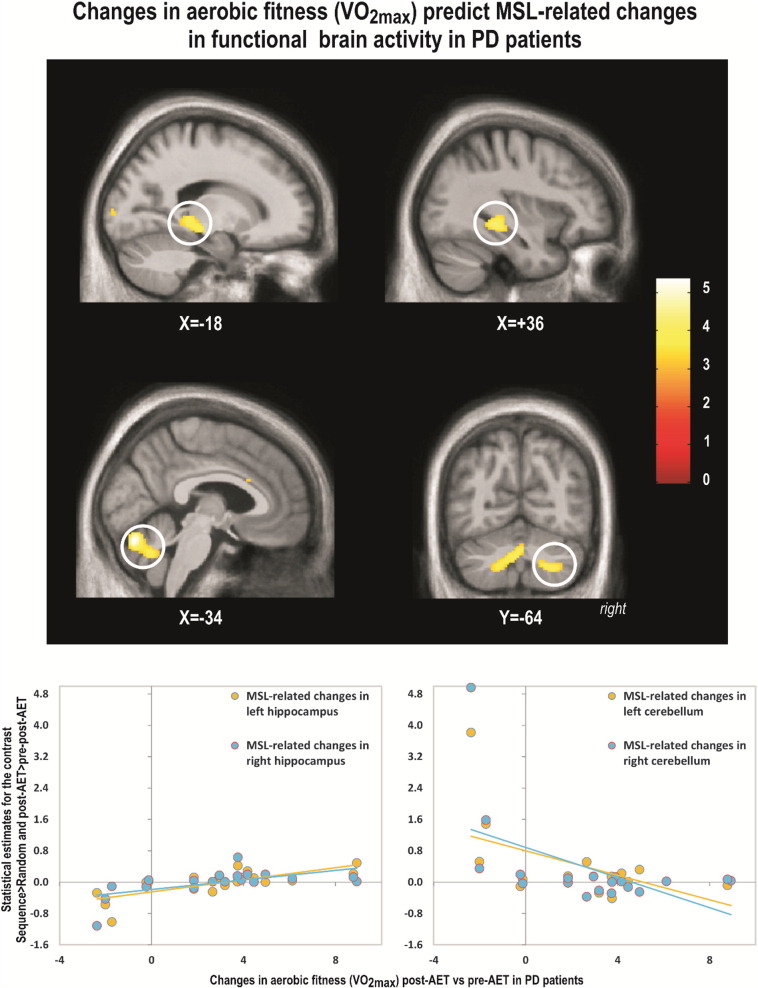
To test whether pre-post functional brain changes expressing motor learning capacity were indeed related to improvement in aerobic fitness, we conducted a regression analysis between changes in estimated VO2max and changes in MSL brain responses ([Sequence – Random] × session [POST – PRE]). In PD patients, increases in VO2max (indicating improved aerobic fitness as a result of training) correlated positively and significantly with increases activity in the hippocampus bilaterally (Table 2. Fig. 2) and the left dorsal striatum. This indicated that as the aerobic capacity improved in PD patients, the motor learning specific activation also increased in these brain regions. In contrast, a significant negative correlation was observed in bilateral cerebellum lobe 7, 8 and 9 (Table 2, Fig. 2).
Fig. 2.
Changes in aerobic fitness (VO2max) predict MSL-related changes in functional brain activity in PD patients.
While the regions corresponding to the main sequence learning effect and those resulting from the regression analysis were found in similar macro-structures (i.e. hippocampus, striatum), they did not spatially overlap.
3.2.2. Functional brain changes related to motor learning capacity following AET in HC
The main learning effect [Sequence – Random] by session [POST – PRE] assessed within the HC group revealed no significant change in activity between sessions. The regression analysis [Sequence – Random] × session [POST – PRE] also revealed no significant correlation between AET-related changes in aerobic capacity and brain changes related to motor learning capacity for HC group alone.
3.2.3. Between group differences in functional brain activity related to motor learning capacity following AET
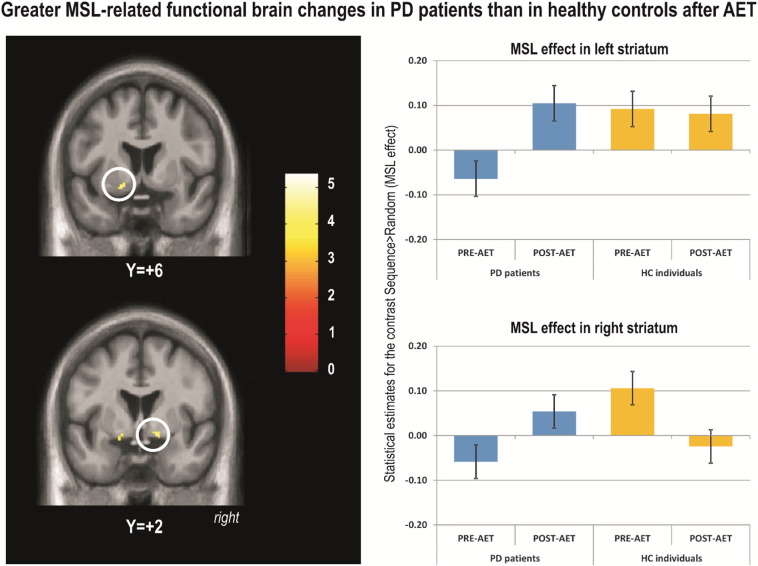
When comparing the two groups (PD vs. HC) on the main learning effect [Sequence – Random] by session [POST – PRE], PD patients showed significantly higher levels of motor learning related activation than HC, post-AET as compared to pre-AET, in the left cerebellum (lobules 8 and 9), right globus pallidus, and left ventral striatum (Table 3, Fig. 3). The left hippocampus was marginally significant (0.06) in this contrast. However, no significant differences were found in the opposite direction (i.e., HC greater than PD) for the same contrast.
Table 3.
Functional imaging results of the main sequence learning effect following aerobic exercise training between PD and HC.
| Area | X mm | Y mm | Z mm | K | Z | psvc |
|---|---|---|---|---|---|---|
| 3.1. Main effect of session on sequence learning [POST – PRE] × [Sequence – Random] | ||||||
| PD-HC | ||||||
| Left cerebellum lobules 8 and 9 | − 2 | − 62 | − 40 | 95 | 3,5 | 0,007 |
| − 20 | − 48 | − 54 | 181 | 3,41 | 0,009 | |
| − 24 | − 48 | − 44 | 169 | 3,3 | 0,013 | |
| Right globus pallidus | 14 | 2 | − 10 | 11 | 3,15 | 0,019 |
| Left striatum ventral | − 18 | 6 | − 12 | 15 | 3,1 | 0,022 |
| Left hippocampus | − 36 | − 16 | − 14 | 5 | 2,68 | 0,06a |
| HC-PD | ||||||
| No significant responses | ||||||
| 3.2. Main effect of session on sequence learning [POST – PRE] × [Sequence – Random] regressed against changes in VO2max | ||||||
| PD-HC | ||||||
| Positive | ||||||
| Right hippocampus | 20 | − 28 | − 6 | 38 | 3,15 | 0,019 |
| Negative | ||||||
| Cerebellum lobule 7 | − 2 | − 76 | − 24 | 95 | 3,48 | 0,007 |
| Left cerebellum lobules 8 and 9 | 0 | − 64 | − 32 | 2,85 | 0,041 | |
| HC-PD | ||||||
| Positive | ||||||
| No significant responses | ||||||
| Negative | ||||||
| No significant responses | ||||||
Statistical inferences were performed at a threshold of p < 0.05 after correction for multiple comparisons over small spherical volumes (SVC). K represents the number of voxels in each cluster reported (determined at a threshold of p = 0.005).
Marginally significant.
Fig. 3.
Greater MSL-related functional brain changes in PD patients than in healthy controls after AET.
3.2.4. Functional brain changes following AET in PD and HC regressed against changes in aerobic fitness
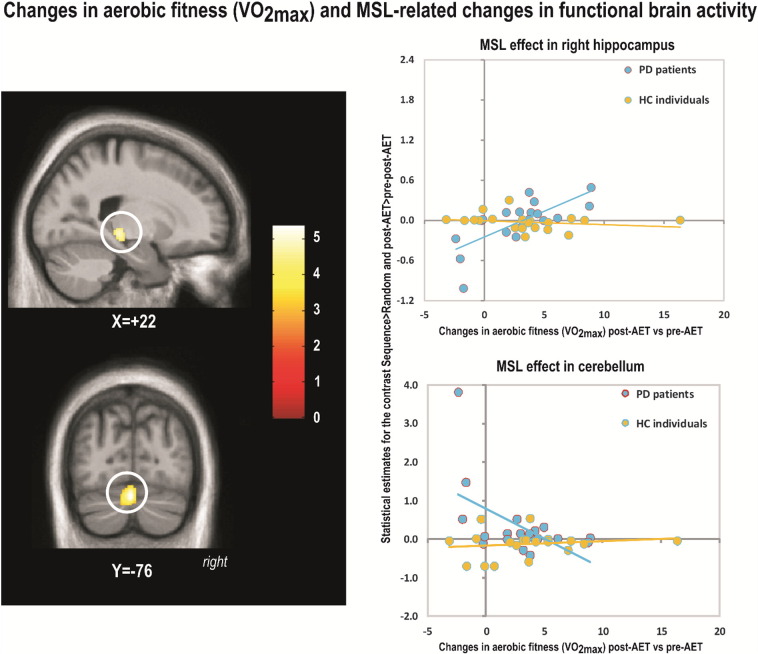
Between-group differences in regression between VO2max changes and MSL-related brain activity changes were observed in the right hippocampus (Table 3, Fig. 4) and in left cerebellum lobules 8, 9 and 7 (negative correlation). These between-group effects were both driven by the findings in the PD group. Again, there was no spatial overlap between the regions obtained from the regression analysis (Section 3.2.4) and those obtained from the group contrast (Section 3.2.3), albeit they were found in the same macro-structures (i.e. hippocampus, cerebellum lobule 8 and 9). Also, no significant differences were found in the opposite direction (i.e., HC greater than PD).
Fig. 4.
Changes in aerobic fitness (VO2max) and MSL-related changes in functional brain activity.
4. Discussion
We recently reported that 12 weeks of progressive intense AET resulted in significant improvement in aerobic fitness as well as motor skill learning in both PD and HC groups (Duchesne et al., 2015). AET-related MSL improvements were global in HC individuals as they were observed in both conditions (sequence and random), but were specific to the sequence condition in PD patients. In addition, PD patients improved their sequence-specific motor learning capacity after aerobic training as indicated by the significant difference between sequence and random conditions after the training period only. Interestingly, in the present study, the neuroimaging results corroborate those behavioural findings and suggest that AET-related changes in motor sequence learning capacity (i.e., difference between sequence and random conditions) are mainly seen in PD individuals. Specifically, increases in MSL-specific brain activity due to training were found in the temporal lobe, hippocampus, striatum and cerebellum in the PD group. Importantly, there was also a positive relation between the patients' change in aerobic fitness and MSL-related changes in the hippocampal and striatal activity, while we found a negative relation between the change in fitness level and activity in the cerebellum in PD patients. Most importantly, the effects at the cerebral level are larger in the PD than in the HC group.
4.1.1.1. MSL exercise-dependent plasticity related to the striatum and hippocampus in PD individuals
The current study is the first to examine the changes in neural substrate supporting MSL following aerobic exercise training in both healthy controls and PD patients. Our findings thus extend the neuroimaging literature on implicit MSL in PD individuals and HC participants (Gamble et al., 2014, Ruitenberg et al., 2015, Siegert et al., 2006), by specifically highlighting the positive effects of AET on the MSL-related activity in the striatum, cerebellum and; all structures that are typically involved in motor learning (Albouy et al., 2013, Doyon et al., 2009, Doyon et al., 2011). In fact, our hypothesis regarding changes in MSL specific to striatal changes following AET was confirmed in PD individuals. This finding is consistent with previous studies, which have revealed AET-dependent neuroplasticity in the central nervous system due to neurochemical changes in the striatum, the latter having been proposed as one possible mechanism of action to explain the gains in performance on motor tasks in PD (Petzinger et al., 2010, Petzinger et al., 2011, Petzinger et al., 2013, Petzinger et al., 2015). Indeed, research in animal models of PD using dopaminergic neurotoxins has shown that behavioural motor ameliorations following physical exercise are associated with increased efficiency in dopaminergic and glutamatergic neurotransmission (Fisher et al., 2013, Paillard et al., 2015, Petzinger et al., 2013, van Nimwegen et al., 2011, Petzinger et al., 2015), which together are thought to reduce the cortically-driven hyper-excitability observed in PD patients. These authors have reported an increase in dopamine availability coupled with a greater expression of dopamine D2 receptors, hence producing a better dopaminergic signaling in the striatum overall. Furthermore, physical exercise has been found to diminish the amount of synaptic glutamate release, which is known to decrease the level of cortical excitability (Fisher et al., 2004). Although conjectural, it is thus probable that the AET-specific changes in striatal activity observed in the current study may reflect this type of neurochemical mechanism, which would in turn explain the improvement in motor learning.
Interestingly, our results revealed that AET-related and MSL-specific functional brain changes are not only observed in the striatum, but within the hippocampus and cerebellum as well. These findings could suggest that such brain structures are part of the functional network compensating for the typical dysfunction within the cortico-striatal circuits seen in PD. In fact, the reported positive relationship between changes in aerobic fitness and AET-related MSL brain changes in the same macro-structures (hippocampus and striatum) is consistent with emerging data suggesting that links between the hippocampus and the dopaminergic systems in PD are important in memory and learning (Calabresi et al., 2013). In their review, Calabresi et al. (2013) suggested that, while dopamine-dependent impairment (involving a complex molecular dysfunction at glutamatergic synapses) of hippocampal long-term potentiation (LTP) might contribute to cognitive impairments in PD, such interactions could also be a potential source of symptoms reduction in PD patients. Clinical interventions such as AET, for example, could potentially stimulate neurogenesis in the dysfunctional hippocampus of PD's individuals, as it has been shown in rodent's studies (Voss, Vivar, Kramer, and van Praag, 2013). This type of interventions could also be responsible for the associated involvement of neurotrophic factors, as a greater expression in brain-derived neurotrophic factor (BDNF) and LTP mechanisms following exercise has also been proposed as a putative mechanism responsible for the neuroprotective effects and functional improvements in this clinical population (Audiffren et al., 2011, Dishman et al., 2006, Fabel and Kempermann, 2008, Gomez-Pinilla et al., 2011, Mustroph et al., 2012, Voss et al., 2013). Based on evidence that exercise-dependent brain changes within the hippocampus have been associated with functional changes in memory (Erickson et al., 2011, Szabo et al., 2011), we can thus conjecture that the improvement observed during MSL in PD following AET could be explained by LTP-like processes, due to greater expression of BDNF in the hippocampus.
In light of these recent findings (Calabresi et al., 2013), it can be hypothesized that the beneficial effect that AET has on MSL-related activity in both of these regions, may be based on the interaction or functional connectivity between them. It is possible that plastic AET- and MSL-related changes may first occur within the hippocampus, and then propagate in basal ganglia via interactions between the MTL and dopaminergic system. This assumption is based on the fact that while MSL-related changes were observed in both hippocampus and striatum in PD individuals, only in the hippocampus we found regions in which MSL-related changes in activity remained positively correlated with improvements in aerobic fitness when PD and HC participants were compared. Yet further functional connectivity investigations are still necessary to elucidate such AET-related brain reorganisation.
4.1.1.2. MSL exercise-dependent plasticity related to the cerebellum in PD individuals
In the current study, we found that AET-related and MSL specific changes in the cerebellum correlated negatively with changes in aerobic capacity. Although apparently contradictory, this result can be interpreted in light of several lines of evidence from previous studies: First, changes in the cerebellum have been interpreted as a compensatory functional system in animal models of PD model following exercise (Holschneider et al., 2007, Wang et al., 2015a, Wang et al., 2015b), and a similar assumption has been proposed following striatal dysfunction in PD (Doyon, 2008). Second, human studies have indicated that both the cortico-striatal (CS) and cortico-cerebellar (CC) systems play distinctive roles in MSL (Doyon, 2008, Doyon and Benali, 2005, Doyon et al., 2003, Leggio and Molinari, 2015), despite the fact that cerebellum and basal ganglia are known to be interconnected (Bostan and Strick, 2010, Hoshi et al., 2005). Given the interaction between the CC and CS systems during MSL (Doyon, 2008, Doyon et al., 2009, Doyon and Benali, 2005) and the fact that previous functional imaging data revealed that cerebellar hemispheres are hyperactivated, while the striatum is hypoactivated in PD patients as compared to healthy controls (Yu, Sternad, Corcos, and Vaillancourt, 2007), it is thus possible that the cerebellum is capable of compensating for the deficient basal ganglia activity observed in PD. Hence, in the current study, the PD participants who improved less their aerobic capacity used predominantly the cerebellum during MSL, whereas those who had higher changes in aerobic fitness used more the hippocampus and striatum, indicating a ‘restoration’ of functionality in the network typically seen in MSL.
According to the motor sequence learning literature, both the cerebellum and striatum are known to be involved in the early MSL phase, while only the striatum has been shown to maintain its activity in later learning stages when the memory trace has been consolidated (Doyon et al., 2009, Doyon et al., 2011, Doyon et al., 2002). Therefore, while modest improvements in aerobic capacity linked with increases in cerebellar activity may reflect learning in the early stage tested here, the fact that large improvements in aerobic fitness were associated with an enhanced hippocampal and striatal functioning may reflect a transition towards a more durable stage of MSL. These MSL findings are thus in line with previous structural and physiological investigations, which parallel the functional changes that aerobic exercise can exert in general (e.g., (Hillman, Erickson, and Kramer, 2008) for a review), and in PD, in particular (Ahlskog, 2011, Alberts et al., 2011, Goodwin et al., 2008, Hirsch and Farley, 2009, Speelman et al., 2011) for reviews).
4.1.1.3. MSL and exercise in HC individuals
Compared to PD individuals, AET did not have a statistically significant effect on changes in the neural correlates of MSL in the HC group. Such divergence between the HC and PD groups could be explained by a potential ceiling effect observed at baseline for the HC group in regards to their MSL capacity at both the behavioural and cerebral level. Given that HC individuals already showed a good MSL capacity prior to AET, they obviously had less room for sequence-specific behavioural improvement compared to PD patients. This is supported by the fact that analysis of the behavioural results revealed that, while HC individuals improved their motor performance in both random and sequence condition after AET, they also maintained a similar magnitude in RT difference between these two conditions (Duchesne et al., 2015). This pattern of results can be interpreted as HC individuals having a more efficient medial temporal lobe, CS, CC functioning networks prior to AET, as compared to PD patients, which leaves also less room for improvements to be seen in functional activity in these brain areas.
5. Limitations and conclusions
One of the limitations in our study was the heterogeneity of the patient population. Similar to many other studies with PD patients, disease characteristics were diverse (e.g., motor, cognitive, neuropsychiatric, etc.). In fact, it was often a challenge to control for all symptoms and obtain a homogeneous sample. However, we used several strategies to address this limitation. First, we matched the PD group with the HC group with respect to sex distribution, age, years of education, cognitive and fitness level. In addition, given that the two groups differed in their depression and anxiety symptoms, we accounted for these variables in our statistical analyses by considering them as covariates in the analyses of the behavioural data. Another limitation of our study was the lack of a PD control group for the type of exercise. Despite this constraint, however, it is worth noting the fact that we conducted a regression analysis highlighting that there were functional brain changes that were related to changes in aerobic fitness level, which indicate that there were brain changes specific to the AET program in PD patients.
To conclude, this study is the first to assess the neural correlates of MSL following AET in PD. Functional reorganisation of brain activity in early PD following aerobic training was observed within the hippocampus, the striatum and the cerebellum, resulting in improvement of their learning capacity. Most importantly, in the same brain structures, albeit in different sub-regions, we have also showed that MSL specific brain plasticity correlated with changes in aerobic fitness, showing a positive relationship within the hippocampus and the striatum and a negative relationship within the cerebellum. Altogether our study makes two important contributions to the field. First, we show that a 12-week progressive aerobic training regimen has beneficial effects on motor skill learning in sedentary PD and healthy individuals. Second, we highlight the neurophysiological changes underlying exercise-dependent plasticity in PD, with particular emphasis on the neuronal substrate of MSL. Our results thus pave the way for other studies to further explore the structural and functional organization within brain regions and how exercise-dependent plasticity manifests itself within the medial temporal lobe, CS and CC systems following chronic or acute bouts of aerobic activities in PD. As a result, these findings have important clinical implications for the rehabilitation of PD individuals, as the design and the use of non-pharmacological interventions based on physical exercise can be implemented to improve motor learning capacity and restore motor functions in patients afflicted with this debilitating disease.
Acknowledgments
-
1.
This work was supported by the Fonds de Recherche du Québec - Santé under Grant 26409.
-
2.
The authors wish to thank Dr. Juan Manuel Villalpando and Dr. Thien Tuong Minh Vu who kindly accepted to supervise physical assessment during testing.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.nicl.2016.09.011.
Appendix A. Supplementary data
Supplementary material
References
- Ahlskog J.E. Does vigorous exercise have a neuroprotective effect in Parkinson disease? Neurology. 2011;77(3):288–294. doi: 10.1212/WNL.0b013e318225ab66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberts J.L., Linder S.M., Penko A.L., Lowe M.J., Phillips M. It is not about the bike, it is about the pedaling: forced exercise and Parkinson's disease. Exerc. Sport Sci. Rev. 2011;39(4):177–186. doi: 10.1097/JES.0b013e31822cc71a. [DOI] [PubMed] [Google Scholar]
- Albouy G., King B.R., Maquet P., Doyon J. Hippocampus and striatum: dynamics and interaction during acquisition and sleep-related motor sequence memory consolidation. Hippocampus. 2013;23(11):985–1004. doi: 10.1002/hipo.22183. [DOI] [PubMed] [Google Scholar]
- Albouy G., Fogel S., King B.R., Laventure S., Benali H., Karni A.…Doyon J. Maintaining vs. enhancing motor sequence memories: respective roles of striatal and hippocampal systems. NeuroImage. 2015;108:423–434. doi: 10.1016/j.neuroimage.2014.12.049. [DOI] [PubMed] [Google Scholar]
- Amano S., Roemmich R.T., Skinner J.W., Hass C.J. Ambulation and Parkinson disease. Phys. Med. Rehabil. Clin. N. Am. 2013;24(2):371–392. doi: 10.1016/j.pmr.2012.11.003. [DOI] [PubMed] [Google Scholar]
- American College of Sport Medicine . 6th ed. Lippincott Williams & Wilkins, Lea & Febiger; USA, Philadelphia: 2006. ACSM's Guidelines for Exercise Testing and Prescription. [Google Scholar]
- Audiffren M., André N., Albinet C. Effets positifs de l'exercice physique chronique sur les fonctions cognitives des seniors: bilan et perspectives. Revue de neuropsychologie neurosciences cognitives et cliniques. 2011;3(4):207–225. [Google Scholar]
- Beall E.B., Lowe M.J., Alberts J.L., Frankemolle A.M.M., Thota A.K., Shah C., Phillips M.D. The effect of forced-exercise therapy for Parkinson's disease on motor cortex functional connectivity. Brain Connectivity. 2013;3(2):190–198. doi: 10.1089/brain.2012.0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck A.T., Beamesderfer A. Assessment of depression: the depression inventory. Mod. Probl. Pharmacopsychiatry. 1974;7(0):151–169. doi: 10.1159/000395074. http://www.ncbi.nlm.nih.gov/pubmed/4412100 [DOI] [PubMed] [Google Scholar]
- Borg G. Psychophysical bases of perceived exertion. Med. Sci. Sports Exerc. 1982;14:377–381. http://www.ncbi.nlm.nih.gov/pubmed/7154893. [PubMed] [Google Scholar]
- Bostan A.C., Strick P.L. The cerebellum and basal ganglia are interconnected. Neuropsychol. Rev. 2010;20(3):261–270. doi: 10.1007/s11065-010-9143-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabresi P., Castrioto A., Di Filippo M., Picconi B. New experimental and clinical links between the hippocampus and the dopaminergic system in Parkinson's disease. Lancet Neurol. 2013;12(8):811–821. doi: 10.1016/S1474-4422(13)70118-2. [DOI] [PubMed] [Google Scholar]
- Carbon M., Reetz K., Ghilardi M.F., Dhawan V., Eidelberg D. Early Parkinson's disease: longitudinal changes in brain activity during sequence learning. Neurobiol. Dis. 2010;37(2):455–460. doi: 10.1016/j.nbd.2009.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark G.M., Lum J.A.G., Ullman M.T. A meta-analysis and meta-regression of serial reaction time tast performance in Parkinson's disease. Neuropsychology. 2014 doi: 10.1037/neu0000121. [DOI] [PubMed] [Google Scholar]
- Dishman R.K., Berthoud H.R., Booth F.W., Cotman C.W., Edgerton V.R., Fleshner M.R.…Zigmond M.J. Neurobiology of exercise. Obesity (Silver Spring) 2006;14(3):345–356. doi: 10.1038/oby.2006.46. [DOI] [PubMed] [Google Scholar]
- Doyon J. Motor sequence learning and movement disorders. Curr. Opin. Neurol. 2008;21(4):478–483. doi: 10.1097/WCO.0b013e328304b6a3. [DOI] [PubMed] [Google Scholar]
- Doyon J., Benali H. Reorganization and plasticity in the adult brain during learning of motor skills. Curr. Opin. Neurobiol. 2005;15(2):161–167. doi: 10.1016/j.conb.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Doyon J., Song A.W., Karni A., Lalonde F., Adams M.M., Ungerleider L.G. Experience-dependent changes in cerebellar contributions to motor sequence learning. Proc. Natl. Acad. Sci. U. S. A. 2002;99(2):1017–1022. doi: 10.1073/pnas.022615199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyon J., Penhune V., Ungerleider L.G. Distinct contribution of the cortico-striatal and cortico-cerebellar systems to motor skill learning. Neuropsychologia. 2003;41(3):252–262. doi: 10.1016/s0028-3932(02)00158-6. [DOI] [PubMed] [Google Scholar]
- Doyon J., Bellec P., Amsel R., Penhune V., Monchi O., Carrier J.…Benali H. Contributions of the basal ganglia and functionally related brain structures to motor learning. Behav. Brain Res. 2009;199(1):61–75. doi: 10.1016/j.bbr.2008.11.012. [DOI] [PubMed] [Google Scholar]
- Doyon J., Orban P., Barakat M., Debas K., Lungu O., Albouy G.…Benali H. Functional brain plasticity associated with motor learning. Med. Sci. (Paris) 2011;27(4):413–420. doi: 10.1051/medsci/2011274018. [DOI] [PubMed] [Google Scholar]
- Duchesne C., Lungu O., Nadeau A., Robillard M.E., Bore A., Bobeuf F.…Doyon J. Enhancing both motor and cognitive functioning in Parkinson's disease: aerobic exercise as a rehabilitative intervention. Brain Cogn. 2015;99:68–77. doi: 10.1016/j.bandc.2015.07.005. [DOI] [PubMed] [Google Scholar]
- Erickson K.I., Voss M.W., Prakash R.S., Basak C., Szabo A., Chaddock L.…Kramer A.F. Exercise training increases size of hippocampus and improves memory. Proc. Natl. Acad. Sci. U. S. A. 2011;108(7):3017–3022. doi: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabel K., Kempermann G. Physical activity and the regulation of neurogenesis in the adult and aging brain. Neruomol. Med. 2008;10(2):59–66. doi: 10.1007/s12017-008-8031-4. [DOI] [PubMed] [Google Scholar]
- Fisher B.E., Petzinger G.M., Nixon K., Hogg E., Bremmer S., Meshul C.K., Jakowec M.W. Exercise-induced behavioral recovery and neuroplasticity in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned mouse basal ganglia. J. Neurosci. Res. 2004;77(3):378–390. doi: 10.1002/jnr.20162. [DOI] [PubMed] [Google Scholar]
- Fisher B.E., Li Q., Nacca A., Salem G.J., Song J., Yip J.…Petzinger G.M. Treadmill exercise elevates striatal dopamine D2 receptor binding potential in patients with early Parkinson's disease. NeuroReport. 2013;24(10):509–514. doi: 10.1097/WNR.0b013e328361dc13. [DOI] [PubMed] [Google Scholar]
- Gamble K.R., Cummings T.J., Jr., Lo S.E., Ghosh P.T., Howard J.H., Jr., Howard D.V. Implicit sequence learning in people with Parkinson's disease. Front. Hum. Neurosci. 2014;8:563. doi: 10.3389/fnhum.2014.00563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetz C.G., Tilley B.C., Shaftman S.R., Stebbins G.T., Fahn S., Martinez-Martin P.…LaPelle N. Movement Disorder Society-sponsored revision of the Unified Parkinson's Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov. Disord. 2008;23(15):2129–2170. doi: 10.1002/mds.22340. [DOI] [PubMed] [Google Scholar]
- Gomez-Pinilla F., Zhuang Y., Feng J., Ying Z., Fan G. Exercise impacts brain-derived neurotrophic factor plasticity by engaging mechanisms of epigenetic regulation. Eur. J. Neurosci. 2011;33(3):383–390. doi: 10.1111/j.1460-9568.2010.07508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin V.A., Richards S.H., Taylor R.S., Taylor A.H., Campbell J.L. The effectiveness of exercise interventions for people with Parkinson's disease: a systematic review and meta-analysis. Mov. Disord. 2008;23(5):631–640. doi: 10.1002/mds.21922. [DOI] [PubMed] [Google Scholar]
- Herman T., Giladi N., Gruendlinger L., Hausdorff J.M. Six weeks of intensive treadmill training improves gait and quality of life in patients with Parkinson's disease: a pilot study. Arch. Phys. Med. Rehabil. 2007;88(9):1154–1158. doi: 10.1016/j.apmr.2007.05.015. [DOI] [PubMed] [Google Scholar]
- Hillman C.H., Erickson K.I., Kramer A.F. Be smart, exercise your heart: exercise effects on brain and cognition. Nat. Rev. Neurosci. 2008;9(1):58–65. doi: 10.1038/nrn2298. [DOI] [PubMed] [Google Scholar]
- Hirsch M.A., Farley B.G. Exercise and neuroplasticity in persons living with Parkinson's disease. Eur. J. Phys. Rehabil. Med. 2009;45(2):215–229. [PubMed] [Google Scholar]
- Hoehn M.M., Yahr M.D. Parkinsonism: onset, progression and mortality. Neurology. 1967;17(5):427–442. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- Holschneider D.P., Yang J., Guo Y., Maarek J.M. Reorganization of functional brain maps after exercise training: importance of cerebellar-thalamic-cortical pathway. Brain Res. 2007;1184:96–107. doi: 10.1016/j.brainres.2007.09.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshi E., Tremblay L., Feger J., Carras P.L., Strick P.L. The cerebellum communicates with the basal ganglia. Nat. Neurosci. 2005;8(11):1491–1493. doi: 10.1038/nn1544. http://www.nature.com/neuro/journal/v8/n11/suppinfo/nn1544_S1.html [DOI] [PubMed] [Google Scholar]
- Jackson A.S., Blair S.N., Mahar M.T., Wier L.T., Ross R.M., Stuteville J.E. Prediction of functional aerobic capacity without exercise testing. Med. Sci. Sports Exerc. 1990;22(6):863–870. doi: 10.1249/00005768-199012000-00021. http://www.ncbi.nlm.nih.gov/pubmed/2287267 [DOI] [PubMed] [Google Scholar]
- Leggio M., Molinari M. Cerebellar sequencing: a trick for predicting the future. Cerebellum. 2015;14(1):35–38. doi: 10.1007/s12311-014-0616-x. [DOI] [PubMed] [Google Scholar]
- Mang C.S., Snow N.J., Campbell K.L., Ross C.J., Boyd L.A. A single bout of high-intensity aerobic exercise facilitates response to paired associative stimulation and promotes sequence-specific implicit motor learning. J. Appl. Physiol. (1985) 2014;117(11):1325–1336. doi: 10.1152/japplphysiol.00498.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustroph M.L., Chen S., Desai S.C., Cay E.B., DeYoung E.K., Rhodes J.S. Aerobic exercise is the critical variable in an enriched environment that increases hippocampal neurogenesis and water maze learning in male C57BL/6J mice. Neuroscience. 2012;219:62–71. doi: 10.1016/j.neuroscience.2012.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadeau A., Pourcher E., Corbeil P. Effects of 24 wk of treadmill training on gait performance in Parkinson's disease. Med. Sci. Sports Exerc. 2014;46(4):645–655. doi: 10.1249/MSS.0000000000000144. [DOI] [PubMed] [Google Scholar]
- Nazem S., Siderowf A.D., Duda J.E., Ten Have T., Colcher A., Horn S.S.…Weintraub D. Montreal cognitive assessment performance in patients with Parkinson's disease with “normal” global cognition according to mini-mental state examination score. J. Am. Geriatr. Soc. 2009;57(2):304–308. doi: 10.1111/j.1532-5415.2008.02096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nissen, Bullemer Attentional requirements of learning: evidence from performance measures. Cogn. Psychol. 1987;19(1):32. [Google Scholar]
- Osman A., Kopper B.A., Barrios F.X., Osman J.R., Wade T. The Beck Anxiety Inventory: reexamination of factor structure and psychometric properties. J. Clin. Psychol. 1997;53(1):7–14. doi: 10.1002/(sici)1097-4679(199701)53:1<7::aid-jclp2>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Paillard T., Rolland Y., de Souto Barreto P. Protective effects of physical exercise in Alzheimer's disease and Parkinson's disease: a narrative review. J. Clin. Neurophysiol. 2015;11(3):212–219. doi: 10.3988/jcn.2015.11.3.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petzinger G.M., Walsh J.P., Akopian G., Hogg E., Abernathy A., Arevalo P.…Jakowec M.W. Effects of treadmill exercise on dopaminergic transmission in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned mouse model of basal ganglia injury. J. Neurosci. 2007;27(20):5291–5300. doi: 10.1523/JNEUROSCI.1069-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petzinger G.M., Fisher B.E., Van Leeuwen J.E., Vukovic M., Akopian G., Meshul C.K.…Jakowec M.W. Enhancing neuroplasticity in the basal ganglia: the role of exercise in Parkinson's disease. Mov. Disord. 2010;25(Suppl. 1):S141–S145. doi: 10.1002/mds.22782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petzinger G.M., Fisher B.E., Akopian G., Holschneider D.P., Wood R., Walsh J.P.…Jakowec M.W. The role of exercise in facilitating basal ganglia function in Parkinson's disease. Neurodegener. Dis. Manag. 2011;1(2):157–170. doi: 10.2217/nmt.11.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petzinger G.M., Fisher B.E., McEwen S., Beeler J.A., Walsh J.P., Jakowec M.W. Exercise-enhanced neuroplasticity targeting motor and cognitive circuitry in Parkinson's disease. Lancet Neurol. 2013;12(7):716–726. doi: 10.1016/S1474-4422(13)70123-6. (S1474-4422(13)70123-6 [pii] ) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petzinger G.M., Holschneider D.P., Fisher B.E., McEwen S., Kintz N., Halliday M.…Jakowec M.W. The effects of exercise on dopamine neurotransmission in Parkinson's disease: targeting neuroplasticity to modulate basal ganglia circuitry. Brain Plast. 2015;1(1):29–39. doi: 10.3233/BPL-150021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pothakos K., Kurz M.J., Lau Y.S. Restorative effect of endurance exercise on behavioral deficits in the chronic mouse model of Parkinson's disease with severe neurodegeneration. BMC Neurosci. 2009;10:6. doi: 10.1186/1471-2202-10-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridgel A.L., Vitek J.L., Alberts J.L. Forced, not voluntary, exercise improves motor function in Parkinson's disease patients. Neurorehabil. Neural Repair. 2009;23(6):600–608. doi: 10.1177/1545968308328726. [DOI] [PubMed] [Google Scholar]
- Ridgel A.L., Kim C.H., Fickes E.J., Muller M.D., Alberts J.L. Changes in executive function after acute bouts of passive cycling in Parkinson's disease. J. Aging Phys. Act. 2011;19(2):87–98. doi: 10.1123/japa.19.2.87. http://www.ncbi.nlm.nih.gov/pubmed/21558565 [DOI] [PubMed] [Google Scholar]
- Ruitenberg M.F., Duthoo W., Santens P., Notebaert W., Abrahamse E.L. Sequential movement skill in Parkinson's disease: a state-of-the-art. Cortex. 2015;65:102–112. doi: 10.1016/j.cortex.2015.01.005. [DOI] [PubMed] [Google Scholar]
- Schendan H.E., Tinaz S., Maher S.M., Stern C.E. Frontostriatal and mediotemporal lobe contributions to implicit higher-order spatial sequence learning declines in aging and Parkinson's disease. Behav. Neurosci. 2013;127(2):204–221. doi: 10.1037/a0032012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegert R.J., Taylor K.D., Weatherall M., Abernethy D.A. Is implicit sequence learning impaired in Parkinson's disease? A meta-analysis. Neuropsychology. 2006;20(4):490–495. doi: 10.1037/0894-4105.20.4.490. [DOI] [PubMed] [Google Scholar]
- Speelman A.D., van de Warrenburg B.P., van Nimwegen M., Petzinger G.M., Munneke M., Bloem B.R. How might physical activity benefit patients with Parkinson disease? Nat. Rev. Neurol. 2011;7(9):528–534. doi: 10.1038/nrneurol.2011.107. [DOI] [PubMed] [Google Scholar]
- Szabo A.N., McAuley E., Erickson K.I., Voss M., Prakash R.S., Mailey E.L.…Kramer A.F. Cardiorespiratory fitness, hippocampal volume, and frequency of forgetting in older adults. Neuropsychology. 2011;25(5):545–553. doi: 10.1037/a0022733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Eijkeren F.J.M., Reijmers R.S.J., Kleinveld M.J., Minten A., Bruggen J.P., Bloem B.R. Nordic walking improves mobility in Parkinson's disease. Mov. Disord. 2008;23(15):2239–2243. doi: 10.1002/mds.22293. [DOI] [PubMed] [Google Scholar]
- van Nimwegen M., Speelman A., Hofman-van Rossum E., Overeem S., Deeg D., Borm G.…Munneke M. Physical inactivity in Parkinson's disease. J. Neurol. 2011:1–8. doi: 10.1007/s00415-011-6097-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss M.W., Nagamatsu L.S., Liu-Ambrose T., Kramer A.F. Exercise, brain, and cognition across the life span. J. Appl. Physiol. 2011;111(5):1505–1513. doi: 10.1152/japplphysiol.00210.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss M.W., Vivar C., Kramer A.F., van Praag H. Bridging animal and human models of exercise-induced brain plasticity. Trends Cogn. Sci. 2013;17(10):525–544. doi: 10.1016/j.tics.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Guo Y., Myers K.G., Heintz R., Holschneider D.P. Recruitment of the prefrontal cortex and cerebellum in Parkinsonian rats following skilled aerobic exercise. Neurobiol. Dis. 2015;77(0):71–87. doi: 10.1016/j.nbd.2015.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Guo Y., Myers K.G., Heintz R., Peng Y.-H., Maarek J.-M.I., Holschneider D.P. Exercise alters resting-state functional connectivity of motor circuits in parkinsonian rats. Neurobiol. Aging. 2015;36(1):536–544. doi: 10.1016/j.neurobiolaging.2014.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warburton D.E., Jamnik V.K., Bredin S.D., Gledhill N. The Physical Activity Readiness Questionnaire for Everyone (PAR-Q +): French North America Version (Questionnaire sur l'aptitude à l'activité physique pour tous (Q-AAP +)) Health Fit. J. Canada. 2011;4(2):1–4. [Google Scholar]
- Werheid K., Zysset S., Müller A., Reuter M., von Cramon D.Y. Rule learning in a serial reaction time task: an fMRI study on patients with early Parkinson's disease. Cogn. Brain Res. 2003;16(2):273–284. doi: 10.1016/s0926-6410(02)00283-5. [DOI] [PubMed] [Google Scholar]
- Yoon M.C., Shin M.S., Kim T.S., Kim B.K., Ko I.G., Sung Y.H.…Kim C.J. Treadmill exercise suppresses nigrostriatal dopaminergic neuronal loss in 6-hydroxydopamine-induced Parkinson's rats. Neurosci. Lett. 2007;423(1):12–17. doi: 10.1016/j.neulet.2007.06.031. [DOI] [PubMed] [Google Scholar]
- Yu H., Sternad D., Corcos D.M., Vaillancourt D.E. Role of hyperactive cerebellum and motor cortex in Parkinson's disease. NeuroImage. 2007;35(1):222–233. doi: 10.1016/j.neuroimage.2006.11.047. (S1053-8119(06)01175-X [pii]) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material