Abstract
Retinitis Pigmentosa (RP) in the human is a progressive, currently irreversible neural degenerative disease usually caused by gene defects that disrupt the function or architecture of the photoreceptors. While RP can initially be a disease of photoreceptors, there is increasing evidence that the inner retina becomes progressively disorganized as the outer retina degenerates. These alterations have been extensively described in animal models, but remodeling in humans has not been as well characterized. This study, using computational molecular phenotyping (CMP) seeks to advance our understanding of the retinal remodeling process in humans. We describe cone mediated preservation of overall topology, retinal reprogramming in the earliest stages of the disease in retinal bipolar cells, and alterations in both small molecule and protein signatures of neurons and glia. Furthermore, while Müller glia appear to be some of the last cells left in the degenerate retina, they are also one of the first cell classes in the neural retina to respond to stress which may reveal mechanisms related to remodeling and cell death in other retinal cell classes. Also fundamentally important is the finding that retinal network topologies are altered. Our results suggest interventions that presume substantial preservation of the neural retina will likely fail in late stages of the disease. Even early intervention offers no guarantee that the interventions will be immune to progressive remodeling. Fundamental work in the biology and mechanisms of disease progression are needed to support vision rescue strategies.
Keywords: retinitis pigmentosa (RP), retinal pigment epithelium (RPE), computational molecular phenotyping (CMP), retina, cone photoreceptor, rod photoreceptor, amacrine cell, bipolar cell, ganglion cell, Müller cell, retinal remodeling, neural remodeling
1. Introduction
Animal models of retinitis pigmentosa (RP) have been extensively studied. However, with a couple of exceptions (Li et al., 1995, Fariss et al., 2000, Marc et al., 2007), detailed documentation of retinal remodeling in humans has not been coherently summarized. Our goal was to assess human RP retinas in advanced stages of remodeling and evaluate how they compare with animal models in terms of cellular metabolism, rewiring and cell survival. We examined the dependence of phase III (late stage) retinal remodeling on cone survival and document alterations in glutamine synthetase (GS) expression in human RP.
RP is a collection of progressive degenerative diseases with tissue-wide defects at the molecular and cellular level in natural (Baehr and Frederick, 2009) and engineered models of retinal degeneration (Jones et al., 2003). As of October 1st, 2015, there are 238 genes and 278 separate loci involved in retinal degenerations (RetNet, http://www.sph.uth.tmc.edu/RetNet/). RP presents in different forms with varying etiologies including genetic abnormalities in the retinal pigment epithelium (RPE) (Gu et al., 1997, Morimura et al., 1998), ABCR gene and ATP binding cassette defects (Allikmets et al., 1997a, Allikmets et al., 1997b, Cremers et al., 1998, Allikmets, 2000, Molday et al., 2000), defects in tyrosine kinase receptors (D'Cruz et al., 2000, Duncan et al., 2003), a number of cilliopathies and transport defects (Li et al., 2004, Yen et al., 2006), transducin and arrestin abnormalities (Dryja et al., 1993, Sommer et al., 2005, Sommer and Farrens, 2006, Sommer et al., 2006, Sommer et al., 2007, Zeitz et al., 2008), mutations in the machinery of rhodopsin processing and trafficking including peripherin defects (Clarke et al., 2000), defects in rod cGMP phosphodiesterase (McLaughlin et al., 1993, Huang et al., 1995, McLaughlin et al., 1995), defects in metabotropic glutamate receptors (mGluRs) (Dryja et al., 2005, Zeitz et al., 2005), synthetic enzymatic defects (Vasireddy et al., 2007), and defects in genes associated with signaling (Chen et al., 2000, Hu and Wensel, 2002, Hu et al., 2003, Hu and Wensel, 2004, Wensel, 2008).
It is still commonly and incorrectly held that retinal degenerative diseases affect only the sensory retina, leaving the neural retina relatively unscathed. This position overlooks remodeling, a series of changes to retinal organization that extend beyond the photoreceptor layer. Loss of sensory rod and cone input to the neural retina constitutes deafferentation and the neural retina responds in the same manner as deafferented brain (Marc and Jones, 2003; Jones et al., 2003).
Neural retinal deafferentation results in remodeling at the cellular level and reprogramming at the molecular level and progressive neural degeneration becomes unavoidable (Li et al., 1995, de Raad et al., 1996, Fletcher and Kalloniatis, 1996, Fariss et al., 2000, Machida et al., 2000, Strettoi and Pignatelli, 2000, Strettoi et al., 2002, Jones et al., 2003, Marc and Jones, 2003, Marc et al., 2003b, Marc et al., 2003a, Strettoi et al., 2003, Cuenca et al., 2004, Jones and Marc, 2005, Jones et al., 2005, Marc et al., 2005a, Jones et al., 2006, Pu et al., 2006, Aleman et al., 2007, Marc et al., 2007, Marc et al., 2008, Jones et al., 2011, Jones et al., 2012). Remodeling consists of three major phases. Phase I is the pre-degeneration period characterized primarily by early markers of photoreceptor stress. Phase II is the period of photoreceptor loss accompanied by glial remodeling of the outer nuclear layer, leaving a glial seal (not a scar) between the remnant neural retina and the remnant RPE/choroid. Phase III is a protracted, life-long period of neural, glial and vascular remodeling of the survivor retina involving over thirty different modifications (Marc et al., 2003), including neuronal cell death, neuronal morphologic change and migration, de novo neuritogenesis, microneuroma formation, network rewiring, altered glial metabolism and form, and RPE invasion of the neural retina.
RP remodeling progresses differently depending on the degree of cone survival. In cone decimating RP the phase II → III transition is marked by aggressive remodeling of the neural retina, including neuronal cell death. In cone sparing RP, islands of survivor cones somehow delay remodeling and cell death (Marc et al., 2003; Jones et al., 2003), even the though the survivor cones may lack opsin expression and appear unresponsive to light (Marc et al., 2007). However, it appears that cone sparing RP itself may be a sub-phase that can devolve to cone decimated forms.
It should also be noted that retinal degeneration and remodeling can result from retinal detachment (Chang et al., 1995, Lewis et al., 1998), AMD (Sullivan et al., 2003, Marc et al., 2008, Jones et al., 2012) or any other situation where photoreceptors are lost, especially cones. Photoreceptor loss triggers a series of phased negatively “plastic” revisions to the neural retina called retinal remodeling. In detail, remodeling events are similar to neurodegenerative events in CNS including trauma and epilepsy (Prince et al., 2009). Fundamentally, regardless of the initiating event (rod based, mixed rod/cone degenerative events or debris-associated forms), the subsequent retinal changes and apparent plasticity result in revisions to neuronal morphology and organization through neuritogenesis and cell migration, reprogramming of gene expression, protein expression, glutamate channel function, synaptic and possibly gap-junctional connectivities. The negative implications of these changes for rescue of vision are substantial and must be addressed if vision rescue strategies are to succeed.
2. Materials and Methods
2.1 Human tissue
Exploring the metabolism of small molecules requires rapid access to eyes for fixation post-mortem. Retinal samples RP1 and RP2 from 2 human donors with a diagnosis of RP were collected within 3–5 hours post-mortem. Some samples were incubated with the excitation marker 1-amino-4-guanidobutane (AGB) with and without ionotropic glutamate receptor (iGluR) agonists (Marc, 1999b, a, Marc and Jones, 2002). Samples of 4 normal human subjects were included for controls. Portions of every eye collected for histological purposes were prepared and processed for light and transmission electron microscope (TEM) imaging. For CMP, eyes are immersion-fixed overnight in buffered 2.5% glutaraldehyde, 1% formaldehyde, resin embedded and serially sectioned at 70-250 nm (Marc et al., 1995). Normal and RP samples ranged from subjects ranging in age from 30–80 years. RP tissues were obtained from two sources: The Foundation Fighting Blindness Retina Donor Program, Stanford University and the University of Utah Lions Eye Bank. Genotyping of donors was not available and the mutations involved are unknown. Institutional approval for use of human eyes was obtained from the University of Utah and Stanford University and followed the tenets of the Declaration of Helsinki. All retinal tissues and data were de-identified in accordance with HIPPA Privacy Rules.
2.2 Primate tissue
Eyes from 1 adult male and 1 adult female olive baboons (Papio anubis) were obtained during necropsy from the Southwest Foundation for Biomedical Research (San Antonio, TX). Anesthesia and euthanasia conform to institutional animal care and use authorizations and the ARVO Statement for the Use of Animals in Ophthalmic and Visual Research
2.3 OCT
Enucleated globes with the anterior segment removed were submerged post-fixation in a large scintillation chamber filled with normal saline for high resolution mapping and correlation with histological/CMP analysis. High resolution scans were performed with a Heidelberg Spectralis, OCT Plus (3 mode) w/ BluePeak Imaging was performed on regions of interest and data saved with landmarks. Globes were then removed, portions corresponding to regions imaged by OCT were trephined, and processed for CMP. Since the eyes were post-mortem, long collection times could be used with 391 frames per scan. Frames were exported as png channels and imported into Osirix DICOM viewing software http://www.osirix-viewer.com/AboutOsiriX.html for re-slicing to explore and find the optimal region for correlation with histological images. Once precise regions were identified, IR-Tweak, part of the NCR toolset https://www.sci.utah.edu/download/ncrtoolset.html was used to register the OCT images to the histological images.
2.4 Computational Molecular Phenotyping (CMP)
| Reagent | SKU | RRID | Source | Dilution |
|---|---|---|---|---|
| anti-L-aspartate IgG | D100R | AB_2341093 | Signature Immunologics | 1:100 |
| anti-L-glutamate IgG | E100R | AB_2532055 | Signature Immunologics | 1:100 |
| anti-glycine IgG | G100R | AB_2532057 | Signature Immunologics | 1:100 |
| anti-glutathione IgG | J100R | AB_2532058 | Signature Immunologics | 1:100 |
| anti-L-glutamine IgG | Q100R | AB_2532059 | Signature Immunologics | 1:100 |
| anti-taurine IgG | TT100R | AB_2532060 | Signature Immunologics | 1:100 |
| AGB | B100R | AB_2532053 | Signature Immunologics | 1:100 |
| anti-arginine IgG | Marc Laboratory | 1:100 | ||
| anti-GABA IgG | YY100R | AB_2532061 | Signature Immunologics | 1:100 |
| anti-GS IgG | 610517 | AB_397879 | BD Biosciences | 1:50 |
| anti-CRALBP IgG | NA | AB_2314227 | Gift of Dr. Jack Saari | 1:400 |
| anti-rod opsin 1D4 | AB_2315015 | Gift of Dr. Robert Molday | 1:4000 | |
| anti-cone opsin | AB5405 | AB_177456 | EMD Millipore | 1:1000 |
Tissues were probed with IgGs selective for individual small or macro molecules (aspartate (D), arginine (R), glutamate (E), glycine (G), glutathione (J), glutamine (Q), taurine (τ), GABA (γ), CRALBP, rod opsin (1D4), cone opsin (rg-opsin) or GS (Table 1), visualized with secondary antibodies conjugated to 1.4 nm gold, followed by silver intensification (Marc et al., 1995).
Images were captured as 8-bit high-resolution (243 nm/pixel) images (Marc and Jones, 2002), mosaicked, and registered with ir-tweak https://www.sci.utah.edu/download/ncrtoolset.html into large image databases. Cell classification was performed on N-dimensional (N-space) monochrome images via k-means or ISODATA clustering (Marc et al., 1995, Marc and Cameron, 2002, Marc and Jones, 2002) using PCI Geomatica (PCI Geomatics, Inc.) for pixel based clustering and mask generation into theme maps. Detailed theme map generation first involves production of raw classification theme maps (Marc et al., 1995, Kalloniatis et al., 1996), which is the mathematical division of regions into statistically separable classes based on multiple channel inputs. Adobe Photoshop (Adobe, San Jose, CA) was used for final image generation. For display only, raw data channels are linearly contrast-stretched over a 30–220 pixel value range and sharpened with unsharp masking. Molecular signals were visualized as selected rgb maps encoding three molecular signals as red, green, and blue, respectively, e.g., γG.E → rgb which assigns γ-aminobutyric acid, glycine and L-glutamate to red, green, and blue color channels, respectively. Monochrome images are density mapped and rgb images intensity mapped (Marc et al., 1995). Both high and low magnification EM images montages are captured digitally as 12-bit monochrome channels and assembled into large mosaics (Anderson et al., 2009).
2.5 Electron Microscopy (EM)
Tissues were postfixed in 1% buffered osmium tetroxide, followed by resin embedding and thin sectioning. Large scale transmission electron microscopy was then performed, creating EM mosaics as previously described on 90-nm lead-stained sections on single-slot grids (Marc et al., 2012).
2.6 Imaging Terminology
The convention for display of image data (Marc et al., 1995, Marc et al., 2005b) in this text and figures is defined by the use of single-letter biochemical codes for amino acids (e.g. G glycine, E glutamate, Q glutamine, etc...) and augmented by notations for non-protein amino acids and other small molecules (B AGB, τ taurine, γ γ GABA). Color images are rendered as true-color rgb-mapped images where each molecule is mapped to a channel. For example γ G.E → rgb implies that GABA signals are mapped to the red channel, glycine signals to the green channel and glutamate signals to the blue channel.
3.0 Results
Progressive rod-specific degeneration leads to complete elimination of rods and rod signaling and often cone decimation. In cone-sparing forms of RP, there is extensive survival of substantially altered cones until late in the disease, whereupon complete topological or structural remodeling occurs as well as extensive neuronal death. The presence of survivor cones grossly stabilizes the remodeling process, and preserves iGluR signaling in cone pathways (Marc et al., 2007, Marc et al., 2008, Jones et al., 2012). Surviving cells in the neural retina demonstrate neurite sprouting, cell migration, and reprogramming of GluR expression (Marc et al., 2007, Lin et al., 2012a, Lin et al., 2012b). Additionally, marked reduction in glutamine synthetase (GS) expression occurs in retinal degeneration as remodeling progresses. By late stages of retinal degenerative diseases, GS expression is largely absent. Remarkably, metabolic reprogramming revealed by CMP shows chaotic patterns not predicted by changes in GS levels (Pfeiffer et al., 2016), in press. Finally, we see no evidence that remodeling ever stops.
Normal human tissues were harvested within 3–5hrs post mortem for analysis and comparison with RP samples. Normal human retina was identical to primate retina with the exception of minor ischemic changes observable in human tissues from post-mortem changes. This occurs in both normal and RP retinas. Alterations in small molecule patterns in retinal post-mortem ischemia include with some loss of GABA from amacrine cells which causes clear banding in the IPL, and increases in GABA concentrations in Müller cells. Figure 1 shows the normal overall topology, structure and lamination of the human retina, and demonstrates minor changes in small molecule levels 1.5hrs post mortem. For unknown reasons, post-mortem, ischemic retina results in small molecule signals being better preserved in the peripheral retina than in central retina. Rapid fixation in the macaque retina (Fig. 1C, D) captures normal metabolic signatures with no signs of ischemia (Kalloniatis et al., 1996). We speculate that differences in vascularization of the central vs. peripheral retina or differences in metabolic demands of central vs. peripheral retina may influence the signals we observe post-mortem.
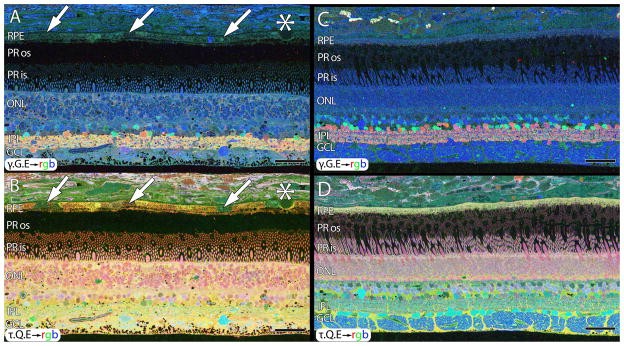
Figure 1.
CMP of 77 year old normal human male (A , B) and primate (C,D). A. γ.G.E → rgb mapping of human peripheral retina from a 77 year old male. Inhomogeneity in the small molecule signals of the RPE indicated by arrows in B. Small druse, asterisk. Mild elevation of GABA in the Müller cells. B. τ.Q.E rgb mapping reveals Müller cells in yellow. Mild postmortem elevations of glutamine and taurine in Müller cells. RPE heterogeneity (arrows). C. Normal macaque retina, γ.G.E → rgb mapping. No RPE heterogeneity. No elevation of GABA in Müller cells. D. Normal macaque retina, τ.G.E → rgb mapping. No RPE heterogeneity. No abnormal elevation of glutamine or taurine in Müller cells. Scale = 40 μm.
Aside from alterations in GABA content in ischemic retina, normal aged human retinas (Fig. 1A,B, sample HS1) show the expected retinal stratification with no signs of remodeling or cell loss. However, aged human RPE often shows small molecule heterogeneity in the RPE that we call “bricking”, especially in the glutamine and taurine channels suggestive of changes due to aging, oxidative stress or even potential indications of early age-related macular degeneration (AMD). However, heterogenous RPE signals are common in our samples and suggest that RPE stress is widespread in humans with age, independent of any disease diagnosis. Importantly, Figure 1C, D demonstrates CMP results from a macaque retina harvested immediately post-mortem, demonstrating normal signatures, lamination, and cell layer thickness with γ .G.E→rgb mapping in C and τ .Q.E → rgb mapping in D revealing normal small molecule levels without ischemic changes. Additionally, primate retinas never show bricking in the RPE, but rather a homogenous positive τ.Q.E signature across the entire retina.
3.1 RP human retina, fundoscopy
Human RP retinas are dramatically different from normal retinas. Figure 2 shows fundoscopic exams from an 67yo normal male (Sample HS1) and an 77yo male (Sample RP1) diagnosed with RP at age 25. Fortunately, we have some medical records from donor RP1 giving insight into the progression of the disease. At age 31 patient RP1 had bilateral 20/30 acuity, but a wide ring scotoma sparing only a central tunnel spanning 10-15° with some peripheral islands of vision. The RP1 fundus also showed marked vascular attenuation and peripheral bone spicule pigmentation, indicative of advanced remodeling. He retained moderately good visual acuity until age 55 (bliateral 20/80 acuity) and visual fields constricted to 10°. His last ophthalmological exam at age 66 showed end-stage RP with dense bone spicule pigmentation, and visual acuity at light-perception in OD and hand-motion at 3 feet in OS. By age 77, the fundus showed the high pallor characteristic of very late stage RP.
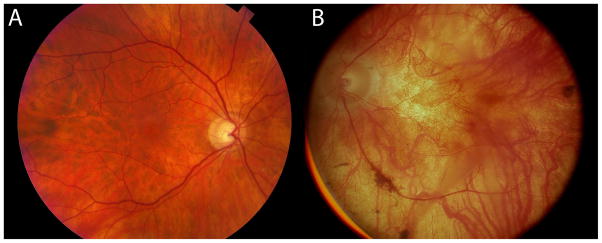
Figure 2.
Fundoscopic images of a 67 year old normal human retina (HS1) in A and a fundoscopic image from an RP donor (RP1), then aged 58 when imaged in 1991 in B, 20 years prior to death. The fundoscopic image of RP1 demonstrates a pale fundus, optic nerve atrophy, and vessel attenuation. This retina provided the tissue for sample RP1.
3.2 Pigmented bone spicules, imaging
Pigmented bone spicules are a common finding in RP. They are accumulations of pigment granules from the RPE processes that have invaded the neural retina and often surround blood vessels (Milam et al., 1998). Many processes extend from the internal limiting membrane, through the external limiting membrane and into the remnant sub-retinal space (Figure 3). Figure 4A demonstrates a hypertrophic blood vessel surrounded by RPE processes containing pigment granules. Figure 4B demonstrates similar processes traversing the neural retina. These pigment granules resemble those found at the top of the retina by the remnant RPE layer seen in Figure 4C and the inset D derived from remnant RPE cells being pulled down into the neural retina (Figure 4C, D) and not the larger caliber, round pigment granules associated with vascular pigment.
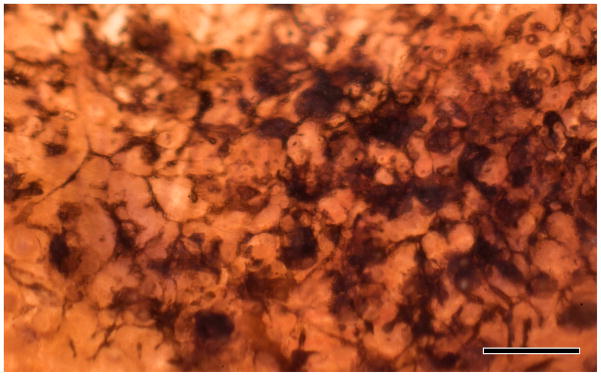
Figure 3.
Backlit extended depth-of-focus view of sample RP2 retina, visualizing pigmented bone spicules invading the neural retina. Spicules appear to fill the spaces between cells and function as highways or facilitated pathways for migration of other cell classes that ultimately alter the topology throughout the retina. Scale = 200 μm.
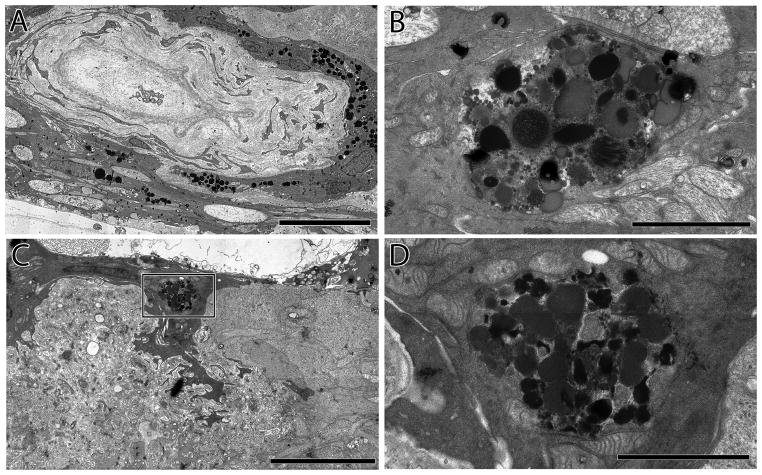
Figure 4.
A. Hypertrophic blood vessel adjacent to the inner limiting membrane invested by pigmented RPE processes.Scale = 15 μm B. RPE pigment granules deep within the neural retina in the former ganglion cell layer, Scale = 3 μm. C. A remnant RPE cell invading the neural retina. Scale = 9 μm. D. An inset from C showing detail of the pigment granules and intracellular debris, pigment granules surrounding vascular elements. Scale = 2 μm.
3.3 Human retina, CMP (Mid-stage RP)
As in animal models of RP (Jones et al., 2003), such as the P347L transgenic rabbit model of autosomal dominant, cone sparing RP (Jones et al., 2011, Jones et al., 2012), human RP progresses through phases I-II-III (Jones and Marc, 2005, Jones et al., 2005, Marc et al., 2005a, Marc et al., 2007, Jones et al., 2012). Early neural plasticity begins in phase II prior to evidence of large scale histological alterations in the neural retina (Marc et al., 2007, Jones et al., 2011). Obtaining samples of phase I and early phase II human RP is difficult, especially since different genotypes progress through phases I and II at different speeds. However, cone-sparing RP patterns the retina into islands of decimated phase III retina in a sea of spared late phase II retina, with graduated transition zones. Patient RP2, with aggressive, presumed autosomal recessive RP and limited visual fields (Figure 5) showed late phase II remodeling and reprogramming characteristic of cone-sparing RP, as first reported in Marc et al., 2007. More detailed analysis shows neuronal remodeling, apparent from the glycine signal in Figure 5A (γ .G.E → rgb) where aberrant neuronal sprouts from the glycinergic amacrine cells can be seen in the outer plexiform layer.
Figure 5.
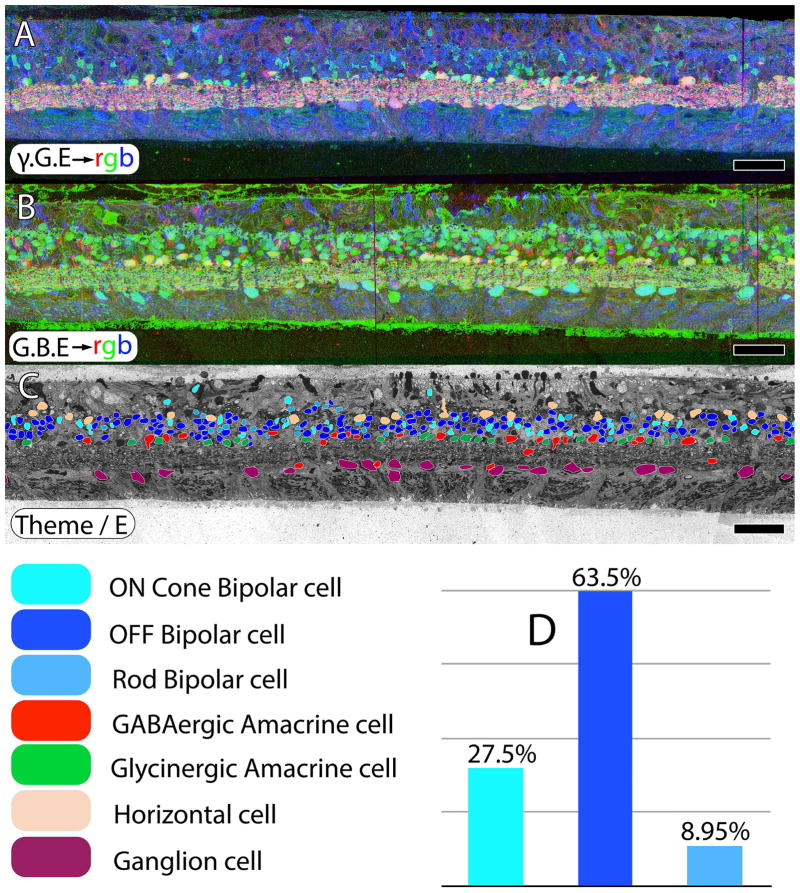
CMP and excitation mapping in human sample RP2. A. γ.G.E → rgb mapping. B. G.AGB.E→ mapping. The AGB signal is induced by activating iGluRs with 25 μM kainic acid. C. Classified retinal neurons, revealing ON cone bipolar, OFF bipolar, rod bipolar, GABAergic amacrine, glycinergic amacrine, horizontal and ganglion cells. Retinal bipolar cell classes in normal retina should approximate 33% each for ON, OFF and rod bipolar cell classes. However, in retinitis pigmentosa, excitation mapping reveals extensive class switching of rod bipolar cells from ON to OFF subclasses as manifested by iGluR functional display D. Scale = 60 μm.
Reprogramming also begins in late phase II retinas, even in the presence of survivor cones. In normal primate tissues, expected retinal bipolar cell proportions should be ≈ 30% ON cone, 40% OFF cone, and 30% rod bipolar cells (Marc et al., 2007). Marc et al., (2007) also showed that both mouse and human rod bipolar cells switch their glutamate receptors after losing rod input and become OFF bipolar cells. Jones et al., (2011) demonstrated that the rabbit RP model does the same. In a reanalysis of a different regions of human RP from retina RP2, excitation mapping with AGB in response to 25 μM kainic acid (Fig. 5B) shows the extensive class switching of rod bipolar cells to an OFF pharmacology, again despite the presence of cones. Classifying retinal cells based on small molecule signatures (Fig. 5C) allows them to be segmented into ON cone bipolar, OFF cone bipolar, rod bipolar, GABAergic amacrine, glycinergic amacrine, horizontal and ganglion cells and highlights the increase of OFF signals that correspond to a loss of normal rod BCs (Fig. 5D).
The spatial heterogeneity in cone-sparing and cone-decimated regions can be visualized in a single human retina. Cone sparing in late phase II human RP, (Fig. 5,6) duplicates phase II remodeling observed in the 10 month old Tg P347L rabbit retina, a large eye model of human cone-sparing RP where most rod photoreceptors were lost in a regional distribution (Jones et al., 2011). Even though the retina has not moved into full scale phase III remodeling, it is clear that aberrant neuritogenesis is beginning (Figure 6E).
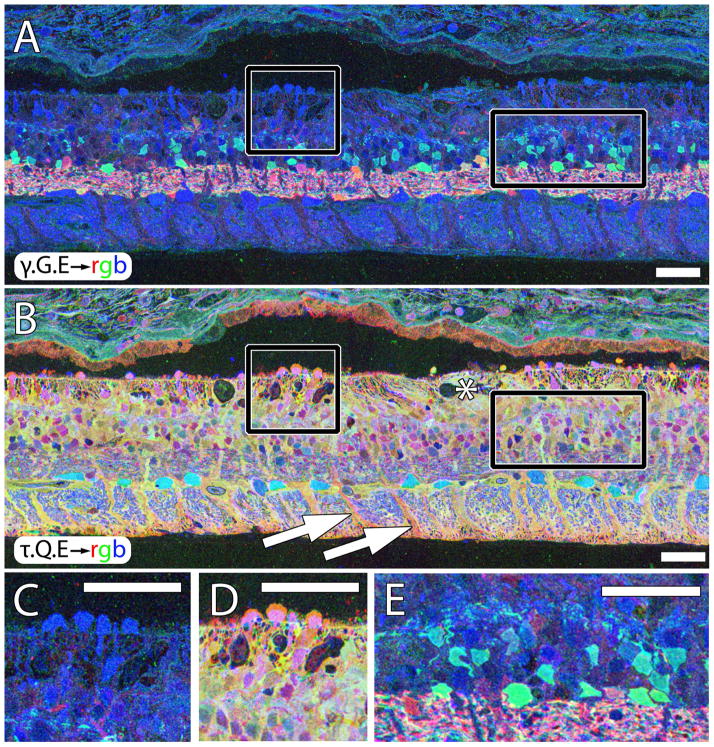
Figure 6.
Sparse cone sparing in sample RP2. A. γ.G.E → rgb mapping showing high blue glutamate signals in a cluster of cones (square) and isolated cones projecting into the subretinal space (arrows). B. τ.Q.E rgb mapping showing characteristic magenta cone and yellow-gold Müller cell signatures. C. Square Inset from A. D. Square inset from B. E. Rectangle inset from in A showing excessive glycine+ neuritogenesis in the outer retina. Asterisk in B demonstrates onset of Müller glial seal. All scales = 42 μm.
Müller cell metabolism also begins to shift in late phase II in a remarkable way. Rather than a wholesale change in metabolic signatures of all Müller cells, individual Müller cell signatures begin to alter, even when immediately adjacent to normal Müller cells. This Müller cell reactivity and indications of morphological alteration can precede cone photoreceptor loss. But after complete photoreceptor loss, large-scale revision and topological restructuring of the retina occurs. Extensive Müller cell hypertrophy, coalescence of neighboring Müller cells into large vertical glial columns, and formation of the distal seal begin to segment the retina into irregular domains that appear poorly connected in animal models (Jones et al., 2003). Among the severe remodeling defects found in animals models is the emigration of survivor neurons into the remnant sub-retinal space. This occurs in the human RP retina as well (Fig. 7).
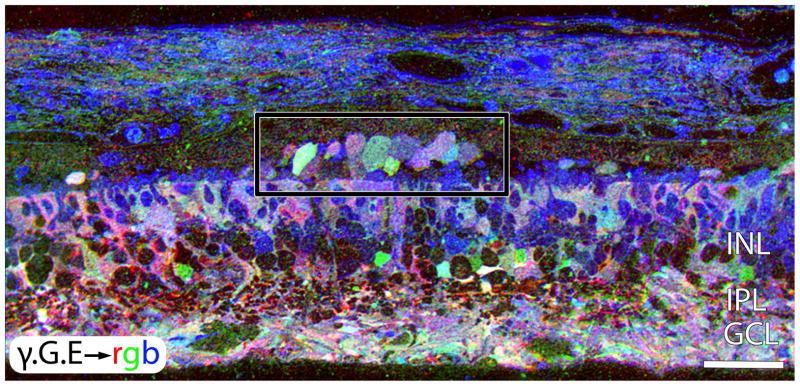
Figure 7.
γ .G.E → mapping in a peripheral region of retina from Sample RP2. This region is devoid of remnant rods or cones. The area in the rectangle shows GABAergic and glycinergic amacrine cells emigrating into the remnant sub-retinal space. Scale = 42 μm.
3.5 Human RP Retina Phase III Remodeling (CMP)
Sample RP1 demonstrates severe neural retina remodeling, including a complete loss of the normal topology and stratification. Figure 7 demonstrates γ.G.E → mapping in a peripheral region of retina from Sample RP2 devoid of any remnant rods or cones, but also showing dramatic GABAergic and glycinergic amacrine cells emigrating out of the neural retina. Figure 8 shows CMP images directly correlated with post-mortem OCT imaging of a 3mm biopsy punch of human RP retina. Scaling of the OCT data with histology at this scale is not very informative other than as a demonstration of what current clinical imaging can resolve in end stage RP. While it is not possible to identify any normal cells or layering once the normal lamination of the retina is lost in end-stage RP, OCT results in earlier stages of retinal degeneration are more feasible as shown by (Pinilla et al., 2015) with the P23H rat. Müller cells and large zones of unknown cells now dominate the remnant retina. There is no evidence of functional neural retina, particularly if the retina is classified and segmented into normal (Figure 9A) and advanced RP, well into phase III (Figure 9B) demonstrating clear disruption of the normal structure of the IPL and GCL.
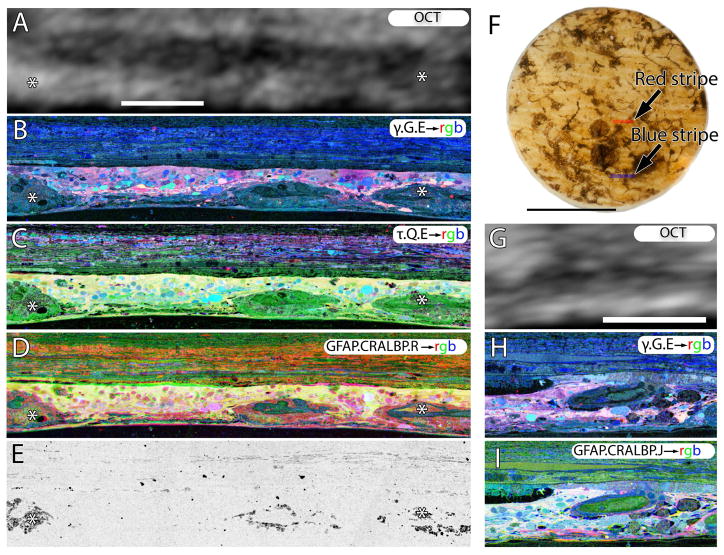
Figure 8.
Retina from late phase III remodeling in sample RP1. A, OCT of the area of retina subserved by the red stripe, registered to the histology shown in B-E. B. γ.G.E → rgb mapping, showing nearly complete loss of bipolar and ganglion cells. C. tau.Q.E→ rgb mapping revealing that Müller cells in yellow now dominate the retina. D. GFAP.CRALBP.R→rgb mapping. E. Phase contrast image showing pigment granules around vascular elements and in the neural retina. F. Backlit image of the 3mm biopsy punch. G. OCT of the area of retina subserved by the blue stripe, registered to the histology shown in H and I. H.γ G.E → rgb mapping. I. GFAP. CRALBP. J.→ rgb mapping. Scales A-E, G-I = 100 μm. Scale, F = 2 mm.
Figure 9.
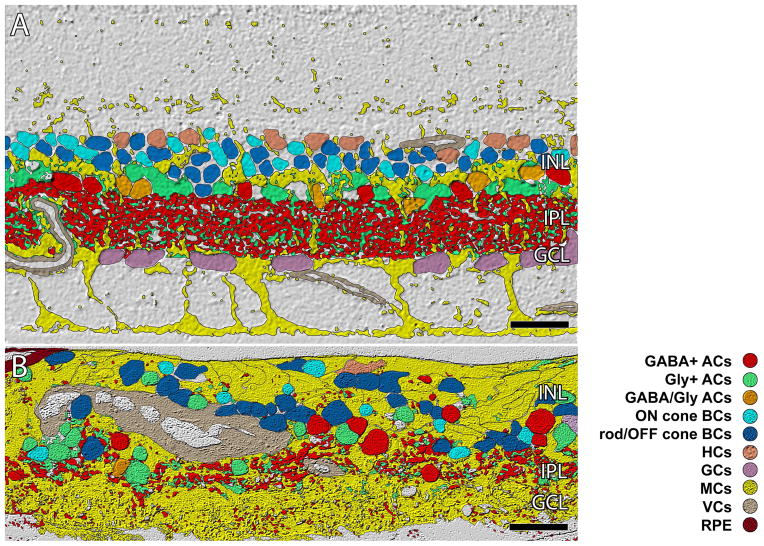
ISODATA clustering of retinal cell classes. A. In normal primate retina lamination is precise (outer plexiform layer, OPL; inner nuclear layer, INL; inner plexiform layer IPL, ganglion cell layer GCL, optif fiber layer, OFL, inner limiting membrane ILM). The IPL is ≈ 40 μm thick B. Sample RP3.(republished from Jones et. al. 2003) showing remodeling clearly disrupting normal lamination of the retina. The IPL and GCL are essentially gone. Scale = 40 μm.
3.7 Metabolic changes
Can Müller cells in the remodeling retina support normal retinal metabolism, including neurotransmission, reactive oxygen species regulation and carbon skeleton recycling? Previous CMP of Müller cells in rodent models of RP and AMD showed significant increase changes in glutamine content upon transitioning into phase III with formation of the distal glial seal (Marc et al., 2008, Jones et al., 2011). Further, it was possible to capture evidence of early metabolic dysregulation in phase II while photoreceptors were still present (Jones et al., 2011). The same early drift in metabolic signatures occurs in human RP (Fig. 6B, 11B).
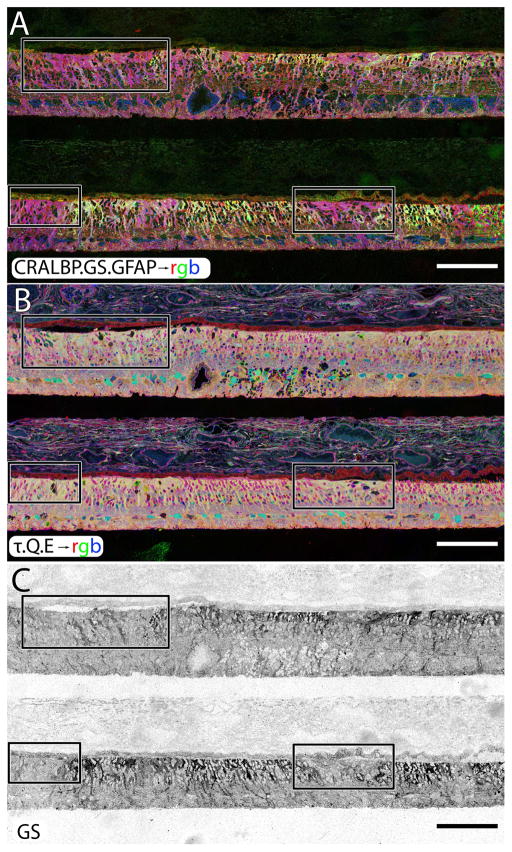
Figure 11.
A two regions of human RP retina, stacked, probed and registered in serial sections for CRALBP, GS, GFAP in A, taurine, glutamine and glutamate in B. The isolated GS channel is shown in C. All three panels demonstrate inhomogeneous levels of GS expression occurring in human RP in response to retinal degeneration and subsequent retinal remodeling. Before all photoreceptors die off, GS increases in concentration only to disappear in regions where Müller cells are engaging in seal formation. Scale = 200 μm.
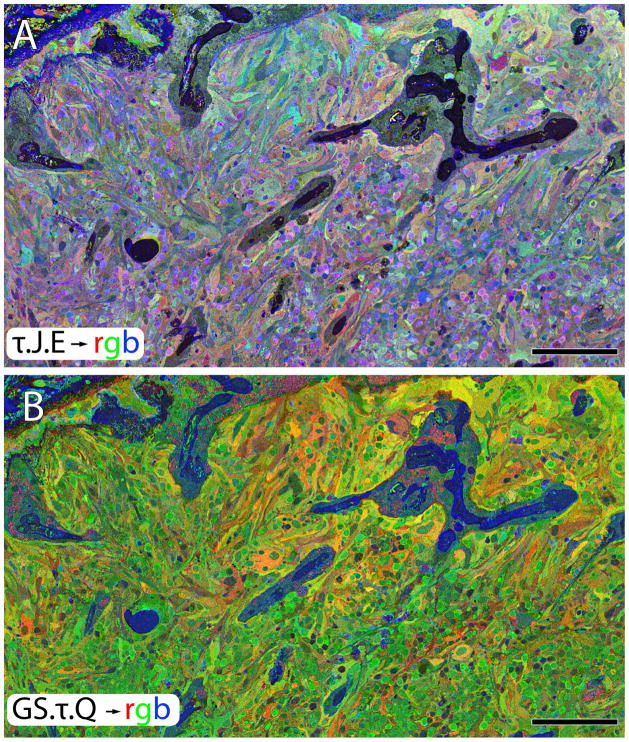
In analyses of human retina where zones of complete phase III retina are flanked by late phase II retina containing patches of survivor cones, the presence of the glial seal in phase III areas is marked by both a paradoxical decrease in GS levels (Fig. 10B, 11C) and an increase in glutamine (Fig. 8C, 10B, 11B) in the same cells. Thus, the change in glial metabolism observed in animal models is mimicked precisely in human RP. In detail however, the time course of metabolic variation reveals transient upregulation of GS expression in stressed phase II Müller cells, followed by collapse in expression as Müller cells begin to form the distal seal in phase III (Figure 11). But animals models of RP are short-lived and we did not know whether the metabolic change associated with the transition to phase III was an end point. Analysis of late stage human RP suggests not. Rather, Müller cell metabolic profiles diverge into a chaotic assembly of unrelated signatures (Figs. 10, 11) with uncorrelated variations in taurine, glutamine, glutathione, glutamate and GS. This suggests that there is no single path to metabolic variation in Müller cells and the late phase III Müller cell cohort is unlikely to support normal metabolic homeostasis in the RP retina. Interestingly, GS expression then begins to decline and by the latest, terminal stage of retinal degeneration, GS expression is almost completely undetectable (Fig. 11C).
Figure 10.
A near horizontal section through the Müller cell end feet revealing metabolic instability. A. T.J.E.→ rgb. B. GS.T.Q.→ rgb. Scale, = 200 μm.
4.0 Discussion
4.1 Retinal Degeneration and Remodeling in the Human Retina
Retinal degeneration is a primary degenerative process that devolves to retinal remodeling, a progressive neurodegeneration encompassing a spectrum of molecular, cellular and tissue-level pathologies. One of our key questions has been the degree to which human RP mimics the events described in many animal models. We use CMP as a tool that can be used on curated legacy samples of tissues to reveal metabolic states defined by a combination of small molecules and CMP-compliant protein markers. In instances where live human RP retina becomes available, excitation mapping with AGB allows rapid profiling of neural activity. These techniques have allowed us to show that human retina remodels in the same fashion as animal models of disease. In particular, phase III remodeling in cone decimating disease which leads to extensive loss of neurons, reorganization of the retina into chaotic tissue patterns and glial metabolic reprogramming that appears incompatible with homeostatic functions of retina.
4.2 Retinal Remodeling is Negative Neural Plasticity in the Adult Retina
In natural models of retinal degenerations (Baehr and Frederick, 2009), trauma such as retinal detachment (Chang et al., 1995, Lewis et al., 1998), toxin exposure (Peichl and Bolz, 1984), or any of the forms of RP (Humphries et al., 1997, Machida et al., 2000, Frederick et al., 2001, Baehr and Frederick, 2009) can trigger remodeling. Regardless of cause, if photoreceptor loss deafferents the neural retina, it induces negative plastic remodeling of the neural retina (Jones et al., 2003, Marc and Jones, 2003, Marc et al., 2003b, Jones and Marc, 2005, Jones et al., 2005, Marc et al., 2005a, Jones et al., 2006, Marc et al., 2007, Marc et al., 2008, Jones et al., 2011, Jones et al., 2012),. The progression of retinal remodeling is similar to the negative plasticity that occurs in central nervous system (CNS) pathologies like physical trauma (Harel and Strittmatter, 2006), epilepsy (Morimoto et al., 2004) and other forms of CNS deafferentation such as Alzheimer’s disease (Anderton et al., 1998) and spinal injury (Sugimoto et al., 1990). The neural responses that ultimately result in negative plasticity and remodeling constitute substantial impediments to rescue strategies of all types.
Retinal remodeling is not unique to RP disorders as other genetic and environmental diseases like AMD (Sullivan et al., 2003, Jones et al., 2012) as well as insults including trauma and retinal detachment (Lewis et al., 1998) also induce this negative plasticity which results in neuronal rewiring and reprogramming that induces alterations in gene expression (Hackam et al., 2004), de novo neuritogenesis (Li et al., 1995) as well as formation of new circuitry and novel synapses in microneuromas (Jones et al., 2003, Marc and Jones, 2003, Marc et al., 2003a). These changes are likely creating corruptive circuitry in nominally, bipolar cell populations through alterations in the connectivities of dendritic trees and supernumerary axons (Marc et al., 2007). It is likely that corruption of neural conectivities in other retinal cell classes also occur.
4.3 Retinal Degeneration and Remodeling Occurs In Phases
Retinal remodeling occurs in phases: In phase I, photoreceptor stress initiates early remodeling & reprogramming events as well as metabolic alterations in Müller glia. In phase II, microglia and RPE cells become involved and Müller glia begin to hypertrophy. Outer nuclear layer (ONL) ablation also occurs in phase III and cell stress pathways are engaged while Müller cells begin sealing the retina off from the choroid. In phase III, de novo neurite formation, rewiring and neuronal death start a process that continues to progress with neuronal translocation and massive restructuring of the retina (Jones et al., 2003, Marc and Jones, 2003, Marc et al., 2003b, Marc et al., 2004, Jones and Marc, 2005, Jones et al., 2005, Marc et al., 2007, Marc et al., 2008, Jones et al., 2011, Jones et al., 2012).
Fundamentally, this process leaves open questions of precisely when retinal plasticity and remodeling begins and, perhaps more importantly, when and if they ever end. These questions have implications for when and how interventional therapies should be implemented. However, it will be difficult to track the earliest stages of retinal plasticity given current clinical imaging methodologies. We simply do not have enough resolution with current OCT to distinguish this early plasticity. Even recent advances in fundus autofluorescence will only visualize later stages of disease processes, far later than phase I retinal remodeling, particularly in the periphery where RP will be most involved. Indeed, there we demonstrate that retinal remodeling in the human adult retina begins early, before clinically observable signs become apparent via fundoscopic imaging or behavior or traditional histological analysis reveal it (Jones et al., 2011).
Clinical sequelae depend upon the form of RP involved, but commonly, the initial rod/cone dystrophies manifest with complaints about nyctalopia beginning in the teens or early 20's and changes in the electroretinogram (ERG) can often be observed prior to fundoscopic findings (Young et al., 1996). This reveals something fundamental: Photoreceptor function is altered or impaired prior to photoreceptor cell death and clinical fundoscopic findings or even before light histological analysis become evident. By the time the classic fundoscopic image of pigmented bone spicules appears in human patients, retinal degeneration is advanced with hypertrophic Müller cells, acting as highways for neuronal translocation as well as pigment translocations into the inner retina from the RPE (Jones et al., 2003). At this point, neuronal populations have been lost and the image processing substrate of the neural retina is forever altered.
4.4 Retinal remodeling alters the molecular identity of retinal neurons
Alternations to neurons in the retina manifest themselves morphologically, but also in alterations that are detectable at the molecular level. Examining just the dendritic loss from bipolar cells would reveal a number of proteins that are impacted from gap junctions to iGluR and cytoskeletal elements. Indeed, many hypothesis have been constructed attempting to explain dendritic arbor remodeling after loss of afferent excitatory activity (Turrigiano and Nelson, 2000), which could result from changes to a variety of molecular alterations including early immediate genes (Byers et al., 2000), extracellular matrix proteins (Johnson and Anderson, 2004) and glutamate receptors (Gazzaley et al., 1997, Marc et al., 2007, Jones et al., 2011, Lin et al., 2012a, Lin et al., 2012b) as well as exciting work in the roles of microglia (Almasieh et al., 2012, Roh et al., 2012) in the degenerative process. Additionally, a number of groups have explored reduced neurotransmission and ectopic synaptic formation (Ball et al., 2003, Haeseleer et al., 2004, Mansergh et al., 2005, Chang et al., 2006, Bayley and Morgans, 2007) in various mutants. Yet despite some localized recording of degenerate retina indicating aberrant processing (Homma et al., 2009) and retinal output from GCs (Sekirnjak et al., 2011), as well as efforts to examine gene expression in early ectopic synaptogenesis (Michalakis et al., 2013), very few studies have examined how these changes impact the precise retinal circuitry and function of the neural retina in degenerative disease in any of the various cell classes, despite over a decade of work from labs studying retinal degeneration and remodeling. That said, substantial efforts are being made to examine gap junctional coupling is altered (Ivanova et al., 2015a, Ivanova et al., 2015b) as well as exploring how retinal processing pathways are undergoing changes as a result of degeneration (Yee et al., 2014, Ivanova et al., 2016).
4.5 Retinal Reprogramming
Because we were able to get some RP samples (RP2) within 4 hrs post-mortem, we were able to explore the glutamate mediated signaling in RP retina through excitation mapping with AGB (Marc, 1999b, a, Marc and Jones, 2002, Marc et al., 2005b, Marc et al., 2007). AGB permeates ionotropic glutamate gated AMPA, KA and NMDA receptors/channels and mGluR6-gated channels, revealing activity in those neuronal populations.
Human and non-human primate retina exhibits three major classes of bipolar cells: ON cone bipolar cells, OFF cone bipolar cells and rod bipolar cells (Wassle and Boycott, 1991). The use of CMP demonstrates two major classes of bipolar cells, ON cone bipolar cells and a complex of rod and OFF cone bipolar cells (Kalloniatis et al., 1996, Marc et al., 1998). Activity probing of glutamate channel permeation with AGB contributes physiological response profiles to neurons identified through CMP as well as acting as an additional discriminant (Marc, 1999b, a, Marc and Jones, 2002), that recapitulates data from physiological experiments showing that ON bipolar cells utilize metabotropic GluRs and are thus KA insensitive (Slaughter and Miller, 1981, 1983, Karschin and Wassle, 1990, Yamashita and Wassle, 1991, Sasaki and Kaneko, 1996). OFF bipolar cell responses possess substantial response profiles to KA administration and label strongly with AGB whereas rod bipolar cells show no labeling after KA administration (Marc, 1999a). Therefore, application of KA in the presence of AGB, allows visualization of neuronal classes including bipolar cell classes presenting with AMPA/KA receptor pharmacological phenotypes (Marc, 1999a).
Human RP retina shows identical findings observed in the mouse (Marc et al., 2007), and the rabbit (Jones et al., 2011), exhibiting physiological response profiles that demonstrate phenotypic revisions of bipolar cell classes in response to photoreceptor cell death. These profiles suggest that mGluR6 and iGluR channel expression has been radically altered in the degenerate retina, revealing a common biological motif in response to neural deafferentation that effectively reprograms the remaining retina by converting rod and ON bipolar cells to an OFF bipolar cell phenotype (Figure 5). These findings hold in all instances of cone sparing animal models of retinitis pigmentosa and are consistent with immunohistochemical work by Cuenca et al (Cuenca et al., 2014) and work examining the rapid alterations of mGluR post photoreceptor ablation (Lin et al., 2012b, Dunn, 2015). Additional work in the rd1 mouse shows that oscillatory activity may transferred from the ON to OFF pathways via a glycergic synapse (Poria and Dhingra, 2015).
The finding of altered bipolar cell populations implying a Rod BC → OFF BC functional transition from rod OFF-like bipolar cell phenotypes in mouse models of RP, human RP and in a large eye model of autosomal dominant RP, the P347L rabbit (Jones et al., 2011) indicates a common mechanism responsible for maintaining bipolar cell identity and a common path of reprogramming from loss of rod photoreceptors. It is important to note that these changes occur before bipolar cell connections are lost and certainly before significant histopathologic changes in the ONL.
It has been shown that some retinal neurons including bipolar cells exhibit spontaneous membrane oscillations suggesting that cone bipolar cells become intrinsically active (Borowska et al., 2011). These oscillations appear to be light-independent spiking in ON and OFF ganglion cells in the rd1 mouse model (Stasheff, 2008, Menzler and Zeck, 2011, Margolis et al., 2014). It should be noted that self-signaling in the absence of photoreceptors in the rd1 mouse includes a large spectrum of amacrine and ganglion cells (Marc et al., 2007) and the presence of well preserved regions of the retina in RP prior to phase III remodeling does not mean the retina functions normally.
4.6 Altered metabolism of Müller cells and retinal topological restructuring
Müller cells are responsible for recycling of carbon skeleton elements of the retina and, via their uniquely high expression of GS, appear essential for the recycling of transported glutamate into glutamine (Pow and Crook, 1996), a major precursor for both excitatory and inhibitory neurons (Marc, 2004). Müller cell metabolic status appears to be exquisitely sensitive to photoreceptor cell stress. In animal models, Müller cells demonstrate metabolic alterations prior to photoreceptor cell loss (Jones et al., 2003, Marc and Jones, 2003, Marc et al., 2003a, Jones and Marc, 2005, Jones et al., 2005, Jones et al., 2011, Jones et al., 2012) and may reveal potential targets of intervention important in preserving retinal topology and function.
While Müller cells exhibit metabolic alterations prior to obvious photoreceptor cell loss, they also undergo a series of morphological transformations after cone photoreceptors are lost, ultimately forming a seal between the remnant neural retina and the remnant choroid and also form conduits for the translocation of migrating neurons. The mechanism for Müller cell hypertrophy is unknown as are mechanisms for migration of cell classes along Müller cell columns in advanced remodeling.
4.7 Remodeling Alters Circuitry to Form Unknown Circuit Topologies
Considering the changes to retinal topology in late stage RP, there is a significant question as to the fates of the ≈80 individual cell classes in the retina as well as their connectivities. It is clear that given the alterations in retinal neuronal cell classes shown here and previously documented (Marc et al., 2007, Jones et al., 2011), along with alterations in glutamate receptors (Lin et al., 2012b, Dunn, 2015) and alterations in electrical activity of bipolar cells (Borowska et al., 2011) and ganglion cells (Stasheff, 2008, Menzler and Zeck, 2011, Margolis et al., 2014), retinal function as well as circuitry and glutamate channel expression are clearly compromised early in the retinal degenerative process. By late phase III, networks involving major cell classes such as the AII amacrine cell (Marc et al., 2014) will be dramatically altered as neurons die, though the precise timing and sequence of cell class loss and circuitry alterations are not known. Furthermore, given the changes in retinal circuitry, there are implications for downstream changes in CNS in response to lesions (Boucard et al., 2009, Aguilar et al., 2010).
4.8 Summary of the progression to non-functional retina
RP involves extensive remodeling that ultimately destroys the organization of the retina to such an extent that many cell classes are no longer remaining by advanced stages of the disease. Standard histology does not segment or quantify the extent of remodeling, but CMP demonstrates the extent of alterations observed in human retina compared to normal human retina or young primate retina (Fig. 9). For simplicity sake, the photoreceptor cell classes were omitted from the normal primate retina in figure 9, but the remaining amacrine, bipolar, horizontal, ganglion, Müller, vascular and RPE cells are shown after CMP and image based clustering, revealing the dramatic alterations in topology compared with a normal primate retina. At this stage, the late phase III RP retina has no recognizable features of a normal retina and former INL cells in this region have migrated down into the remnant ganglion cell layer or up to the distal retina.
4.9 Impact of remodeling on therapeutics
Retinal remodeling has substantial impact on potential therapeutic interventions. Phase I and II retinal remodeling alters gene expression and the neuronal class switching/reprogramming resembles normal plastic behavior observed in CNS which might be amenable to the right therapeutic interventions. Müller cell metabolic changes, glial seal formation, RPE bricking, vascular remodeling are all potential complications that may or may not be amenable to treatment. However, by the time phase III remodeling happens with rewiring, neuritogenesis, Müller seal formation, microneuroma formation, neuronal migration, and cell death, therapeutic interventions to the surviving retina are likely impossible. As we have shown in this paper, human RP patients exhibit all of the remodeling defects observed in models of RP which have substantial import to most therapies.
4.10 Optogenetics
Optogenetics has been enthusiastically adopted as an experimental approach and has substantial promise as a therapy for vision rescue. While the implications for remodeling in optogenetics has recently been reviewed by (Henriksen et al., 2014), optogenetically targeting remaining cell classes in the retina (Sahel and Roska, 2013) presumes minimal abnormalities of signal processing associated with remodeling that may alter processing of signals from photosensitized cells.
Early targeting of surviving retinal bipolar cells may allow preservation of retinal processing and many view bipolar cells as a compromise between preserving as much of the retina’s signal networks as possible and the risk of cell loss late in degeneration. However, there are several important limitations. Optogenetically transducing large numbers of bipolar cells with current approaches requires a subretinal injection which may induce more damage to the neural retina due to the Müller cell seal, while phase II or III remodeling may cause enough rewiring to distort processing. In bipolar cells, one of the most commonly targeted cell types for optogenetics, we know this is already a compromised pathway as they become polyaxonal and engage in microneuromas before progressing to cell death. Targeting of ganglion cells in late-stage degeneration is appealing in those patients where bipolar or amacrine cell death and remodeling may render upstream targets unavailable (Jones et al., 2003, Marc and Jones, 2003, Marc et al., 2003a, Marc et al., 2003b). However, we don’t know whether or how long the photic signals from optogenetically modified ganglion cells will be able to overcome self-signaling from corrupt networks or even when ganglion cell signaling no longer coherently signals visual information to cortex due to upstream retinal remodeling. Furthermore, it appears that all retinal neurons continue to die (e.g. Fig. 9), and given the progressive nature of remodeling, it is likely that optogenetically transduced neurons will be co-opted into plasticity as the remodeling “program” continues. At the very least, windows of opportunity that define when optogenetic interventions will be possible or most successful need to be explored. We argue that phase I or II retina is the most viable period. Further complicating definition of temporal interventions, retinal remodeling is not coherent across retina and many forms of RP possess regions of retina that are mixtures of phase II and III retina (Fig. 10). Therefore, remodeling and cell death must be addressed if optogenetic rescues are to succeed.
4.11 Gene Therapy
Like optogenetics and survival factor therapies, gene therapy approaches will also have to take into account progressive negative plasticity in the retina as photoreceptors are lost and alterations in Müller cells and neuronal networks follow. Windows of opportunity will be important and different between individuals and will require some means of monitoring patients, such as documenting the presence of phase I retina by OCT. Administration of gene therapy via subretinal injections is complicated by the emergence of the glial seal in later phases of remodeling where again, surgical detachment of the glial seal may be traumatic and likely do more harm than good. Therefore, in late stage retinal degenerations, intravitreal injections might be preferred, though this may not be the ideal approach for many viral delivery schemes as targeting therapies to penetrate the ILM.
Gene therapies that depend on photoreceptor survival will likely be the most successful. However, it is clear that photoreceptor involvement begins long before any obvious photoreceptor death. The initial successes with gene therapy for Leber Congenital Amurosis (LCA) have been stunning, but it a distinctly small percentage of retinal degenerative diseases. Furthermore, in the case of LCA, it is clear that the retinal degeneration continues to progress despite rescue of RPE65 (Jacobson et al., 2015) and it may be that LCA and stationary night blindness that do not induce photoreceptor degeneration may still have restricted windows of opportunity for intervention, notably phase I of retinal remodeling.
4.12 Survival factor therapy
Another strategy to retard retinal degeneration involves use of survival factors such as neurotrophins (e.g. ciliary neurotrophic factor, CNTF) to slow or delay photoreceptor apoptosis. These strategies have been validated for slower models of adRP such as the rat P23H and S344ter rhodopsin transgenic models (Sieving et al., 2006), though some argue that structural preservation afforded by CNTF does not necessarily include a functional rescue (Liang et al., 2001). There is also evidence that CNTF infusion in other models of RP negatively alters photoreceptor morphology through loss of outer and inner segments and alters inner retinal organization (Beltran et al., 2007). Therefore survival factor therapy without known cellular or molecular targets and undefined windows of opportunity are not likely to be effective in the diverse forms of human RP. Survival factors in of themselves currently offer little prospect of reversing or altering the course of remodeling and if effective, would be restricted to phase I and early phase II remodeling. That said, finding generalizable targets that slow down or delay cell death and/or the subsequent retinal remodeling are attractive and may hold the best short term promise for preserving vision.
4.13 Stem / neuroprogenitor cell therapy
Several groups have shown that isolated stem / progenitor cells, and possibly adult cells have the potential to integrate in the outer nuclear layer and possibly repopulate the retina (Gust and Reh, 2011, Mansergh et al., 2015). This approach can take the form of replacing lost cells or regenerating them and may hold incredible promise for particularly RPE based therapies. But to date, the efficiency of cell integration in degenerate tissues is low unless the remodeling process itself is restrained, as stem cells or transplanted neuroprogenitors will not survive phase III remodeling. Additionally, as discussed above, getting transplants past the Müller cell seal is problematic and engineering exogenous photoreceptors to extend synapses through the seal will be problematic. Direct injection into the retina is also likely to activate microglial activation which would inhibit migration (Singhal et al., 2008) or even result in microglial attack (Bull et al., 2008). If stem and neuroprogenitor cell therapies are to work and lead to new photoreceptor growth and integration, they must establish connections with existing bipolar, horizontal and ganglion cells prior to onset of morphologic or molecular remodeling. As such, most current RP patients will be excluded and any stem or neuroprogenitor cell therapies will be restricted to phase I and early phase II remodeling.
4.14 Retinal Transplantation
There have been efforts to deliver fetal retina sheets into the remnant subretinal space of degenerating retina to rescue vision loss. These efforts have demonstrated long-term photoreceptor survival and some visual driving in rats (Seiler et al., 2012), though retinal remodeling remains a major barrier. The glial seal, once formed will prevent easy retinal detachment and the extent (if any) of transplant-to-host neurite intermingling is small (Seiler et al., 2012). Furthermore, the transplanted retina begins to remodel even more aggressively than the host retina (Seiler et al., 2012). If phase 3 remodeling in the host retina ensues, an intact, or semi-intact retinal transplant cannot recapitulate normal circuitry and a functional outcome is impossible. Without understanding how to constrain retinal remodeling, any retinal transplantation approaches will be largely restricted to phases I-II.
4.15 Bionic Interventions
There have been a number of efforts to restore vision to the profoundly blind with both subretinal (Stingl et al., 2013) and epiretinal bionic implants (Rizzo et al., 2014). The epiretinal implants appear to be the most successful to date that provide direct current stimulation of retinal ganglion cells or the broader retinal circuitry. In late stage retinal degeneration shown in this paper, the viability of such implants will be limited both in function and how long they may be expected to stimulate a retina that is progressively remodeling, especially if extensive neuronal death is present. It has not been demonstrated that bionic implants of any kind arrest retinal remodeling. In fact, there is some evidence that implants induce further or more aggressive remodeling (Butterwick et al., 2009).
It should also be noted that we don’t have a fundamental, detailed understanding of how signals flow in the normal retina, much less the degenerate retina. Even early in the retinal degenerative process, there is iGluR reprogramming of bipolar cell populations that presumably alter the ON/OFF weighting of visual pathways (Marc et al., 2007, Jones et al., 2011). Later in the retinal degenerative process, retinal rewiring is more substantial with novel synaptic formation between unknown classes of cells (Jones et al., 2003). The likelihood that this synaptology is “normal” is low. That said, a number of implanted patients can successfully navigate with implants, at least for the near term. Regardless, if bionic implants of the epiretinal or subretinal variety are to succeed in providing a long term rescue, remodeling will have to be addressed in these solutions as well.
5.0 Conclusions
RP is a primary sensory retina disease that converts to progressive neurodegenerative remodeling. Disease progression in human RP shows that animal models of retinal degeneration accurately recapitulate the human condition from early to late stages of degeneration and remodeling. Cone mediated preservation of bipolar cell signaling, retinal reprogramming and retinal remodeling seen in the human RP retina are all duplicated in animal models. Müller cell metabolic changes are also present throughout degeneration both in animal models and human retina. The mechanisms for negative plasticity in the neural retina are unknown. If we are to understand the fundamental nature of retinal plasticity and its impact on designing and implementing strategies for rescue or arrest of vision loss, we need to explore mechanisms of retinal remodeling. Understanding precisely how retinas rewire at the earliest stages of retinal plasticity will define how we intervene as these alterations serve as functional markers for progression through various aspects of retinal disease that unfortunately, for diseases like RP and age-related macular degeneration AMD are both progressive and currently irreversible. A fundamental problem with retinal degenerative diseases is the diversity of potential targets and complex specialization of various cell classes as well as their cell-cell connectivities and retinal networks. Therefore, knowing which cell classes are involved, and which anatomical structures are altered (gap junctions, adherens junctions, synapses), as well as their physiological responses and metabolic dependencies will provide additional molecular targets for therapeutic interventions. Any therapy, bionic or biological, that attempts to interface with a plastic and continuously revising structure may have, at best, limited success.
Demonstrate advanced negative plasticity and remodeling in human retinitis pigmentosa (RP).
Advances our understanding of the extent of human retinal disease.
Retinal reprogramming prior to photoreceptor loss.
Alterations in both small molecule and protein signatures of neurons and glia.
Müller cells are the first cells to show metabolic stress in retina and the last cells left in degenerate retina.
Retinal network topologies are altered in RP.
Unless we understand the mechanisms of retinal degeneration, interventions designed to rescue vision loss will likely fail.
Acknowledgments
James Gilman of the Moran Eye Center for the normal fundoscopic image.
Supported by: EY015128, NIH EY02576, EY014800 Vision Core, an Unrestricted Grant from Research to Prevent Blindness, Inc., New York, NY, to the Department of Ophthalmology & Visual Sciences, University of Utah; Edward N. and Della L. Thome Memorial Foundation grant for Age-Related Macular Degeneration Research, a Research to Prevent Blindness Career Development Award.
Abbreviations footnote
The following abbreviations are used in this paper:
- RP
retinitis pigmentosa
- RPE
retinal pigment epithelium
- GS
glutamine synthetase
- GABA
γ-aminobutyric acid
- CRALBP
cellular retinaldehyde binding protein 1
- EM
electron microscopy
- RGB
red green blue
- ONL
outer nuclear layer
- ISODATA
Iterative Self-Organizing Data Analysis Technique
- CMP
Computational Molecular Phenotyping
Footnotes
Author responsibilities:
Jones: 1° Author, CMP, TEM, imaging, manuscript pre paration.
Pfeiffer: 2° Author, CMP, TEM, imaging, manuscript preparation
Ferrell: 2° Author, CMP, TEM, imaging, manuscript preparation
Watt: 2° Author, CMP, TEM, imaging, manuscript pre paration.
Marmor: 3° Author, manuscript preparation.
Marc: 1° Author, CMP, TEM, imaging, manuscript prep aration.
Definitions:
1° Author: Generated primary manuscript
2° Author: Generated manuscript sections and contri buted substantial revisions
3° Author: Generated area-specific manuscript revisi ons
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- Aguilar J, Humanes-Valera D, Alonso-Calvino E, Yague JG, Moxon KA, Oliviero A, Foffani G. Spinal cord injury immediately changes the state of the brain. J Neurosci. 2010;30:7528–7537. doi: 10.1523/JNEUROSCI.0379-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aleman TS, Cideciyan AV, Sumaroka A, Schwartz SB, Roman AJ, Windsor EA, Steinberg JD, Branham K, Othman M, Swaroop A, Jacobson SG. Inner retinal abnormalities in X-linked retinitis pigmentosa with RPGR mutations. Invest Ophthalmol Vis Sci. 2007;48:4759–4765. doi: 10.1167/iovs.07-0453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allikmets R. Simple and complex ABCR: genetic predisposition to retinal disease. American journal of human genetics. 2000;67:793–799. doi: 10.1086/303100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allikmets R, Shroyer NF, Singh N, Seddon JM, Lewis RA, Bernstein PS, Peiffer A, Zabriskie NA, Li Y, Hutchinson A, Dean M, Lupski JR, Leppert M. Mutation of the Stargardt disease gene (ABCR) in age-related macular degeneration. Science. 1997a;277:1805–1807. doi: 10.1126/science.277.5333.1805. [DOI] [PubMed] [Google Scholar]
- Allikmets R, Singh N, Sun H, Shroyer NF, Hutchinson A, Chidambaram A, Gerrard B, Baird L, Stauffer D, Peiffer A, Rattner A, Smallwood P, Li Y, Anderson KL, Lewis RA, Nathans J, Leppert M, Dean M, Lupski JR. A photoreceptor cell-specific ATP-binding transporter gene (ABCR) is mutated in recessive Stargardt macular dystrophy. Nature genetics. 1997b;15:236–246. doi: 10.1038/ng0397-236. [DOI] [PubMed] [Google Scholar]
- Almasieh M, Wilson AM, Morquette B, Cueva Vargas JL, Di Polo A. The molecular basis of retinal ganglion cell death in glaucoma. Prog Retin Eye Res. 2012;31:152–181. doi: 10.1016/j.preteyeres.2011.11.002. [DOI] [PubMed] [Google Scholar]
- Anderson JR, Jones BW, Yang JH, Shaw MV, Watt CB, Koshevoy P, Spaltenstein J, Jurrus E, UVK, Whitaker RT, Mastronarde D, Tasdizen T, Marc RE. A computational framework for ultrastructural mapping of neural circuitry. PLoS Biol. 2009;7:e1000074. doi: 10.1371/journal.pbio.1000074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderton BH, Callahan L, Coleman P, Davies P, Flood D, Jicha GA, Ohm T, Weaver C. Dendritic changes in Alzheimer's disease and factors that may underlie these changes. Prog Neurobiol. 1998;55:595–609. doi: 10.1016/s0301-0082(98)00022-7. [DOI] [PubMed] [Google Scholar]
- Baehr W, Frederick JM. Naturally occurring animal models with outer retina phenotypes. Vision research. 2009;49:2636–2652. doi: 10.1016/j.visres.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball SL, Pardue MT, McCall MA, Gregg RG, Peachey NS. Immunohistochemical analysis of the outer plexiform layer in the nob mouse shows no abnormalities. Vis Neurosci. 2003;20:267–272. doi: 10.1017/s0952523803203059. [DOI] [PubMed] [Google Scholar]
- Bayley PR, Morgans CW. Rod bipolar cells and horizontal cells form displaced synaptic contacts with rods in the outer nuclear layer of the nob2 retina. J Comp Neurol. 2007;500:286–298. doi: 10.1002/cne.21188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltran WA, Wen R, Acland GM, Aguirre GD. Intravitreal injection of ciliary neurotrophic factor (CNTF) causes peripheral remodeling and does not prevent photoreceptor loss in canine RPGR mutant retina. Exp Eye Res. 2007;84:753–771. doi: 10.1016/j.exer.2006.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borowska J, Trenholm S, Awatramani GB. An intrinsic neural oscillator in the degenerating mouse retina. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:5000–5012. doi: 10.1523/JNEUROSCI.5800-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucard CC, Hernowo AT, Maguire RP, Jansonius NM, Roerdink JB, Hooymans JM, Cornelissen FW. Changes in cortical grey matter density associated with long-standing retinal visual field defects. Brain : a journal of neurology. 2009;132:1898–1906. doi: 10.1093/brain/awp119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bull ND, Limb GA, Martin KR. Human Muller stem cell (MIO-M1) transplantation in a rat model of glaucoma: survival, differentiation, and integration. Invest Ophthalmol Vis Sci. 2008;49:3449–3456. doi: 10.1167/iovs.08-1770. [DOI] [PubMed] [Google Scholar]
- Butterwick A, Huie P, Jones BW, Marc RE, Marmor M, Palanker D. Effect of shape and coating of a subretinal prosthesis on its integration with the retina. Exp Eye Res. 2009;88:22–29. doi: 10.1016/j.exer.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers MR, Chudler EH, Iadarola MJ. Chronic tooth pulp inflammation causes transient and persistent expression of Fos in dynorphin-rich regions of rat brainstem. Brain Res. 2000;861:191–207. doi: 10.1016/s0006-8993(00)01936-3. [DOI] [PubMed] [Google Scholar]
- Chang B, Heckenlively JR, Bayley PR, Brecha NC, Davisson MT, Hawes NL, Hirano AA, Hurd RE, Ikeda A, Johnson BA, McCall MA, Morgans CW, Nusinowitz S, Peachey NS, Rice DS, Vessey KA, Gregg RG. The nob2 mouse, a null mutation in Cacna1f: anatomical and functional abnormalities in the outer retina and their consequences on ganglion cell visual responses. Vis Neurosci. 2006;23:11–24. doi: 10.1017/S095252380623102X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CJ, Lai WW, Edward DP, Tso MO. Apoptotic photoreceptor cell death after traumatic retinal detachment in humans. Arch Ophthalmol. 1995;113:880–886. doi: 10.1001/archopht.1995.01100070054025. [DOI] [PubMed] [Google Scholar]
- Chen CK, Burns ME, He W, Wensel TG, Baylor DA, Simon MI. Slowed recovery of rod photoresponse in mice lacking the GTPase accelerating protein RGS9-1. Nature. 2000;403:557–560. doi: 10.1038/35000601. [DOI] [PubMed] [Google Scholar]
- Clarke G, Goldberg AF, Vidgen D, Collins L, Ploder L, Schwarz L, Molday LL, Rossant J, Szel A, Molday RS, Birch DG, McInnes RR. Rom-1 is required for rod photoreceptor viability and the regulation of disk morphogenesis. Nature genetics. 2000;25:67–73. doi: 10.1038/75621. [DOI] [PubMed] [Google Scholar]
- Cremers FP, van de Pol DJ, van Driel M, den Hollander AI, van Haren FJ, Knoers NV, Tijmes N, Bergen AA, Rohrschneider K, Blankenagel A, Pinckers AJ, Deutman AF, Hoyng CB. Autosomal recessive retinitis pigmentosa and cone-rod dystrophy caused by splice site mutations in the Stargardt's disease gene ABCR. Human molecular genetics. 1998;7:355–362. doi: 10.1093/hmg/7.3.355. [DOI] [PubMed] [Google Scholar]
- Cuenca N, Fernandez-Sanchez L, Campello L, Maneu V, De la Villa P, Lax P, Pinilla I. Cellular responses following retinal injuries and therapeutic approaches for neurodegenerative diseases. Prog Retin Eye Res. 2014;43:17–75. doi: 10.1016/j.preteyeres.2014.07.001. [DOI] [PubMed] [Google Scholar]
- Cuenca N, Pinilla I, Sauve Y, Lu B, Wang S, Lund RD. Regressive and reactive changes in the connectivity patterns of rod and cone pathways of P23H transgenic rat retina. Neuroscience. 2004;127:301–317. doi: 10.1016/j.neuroscience.2004.04.042. [DOI] [PubMed] [Google Scholar]
- D'Cruz PM, Yasumura D, Weir J, Matthes MT, Abderrahim H, LaVail MM, Vollrath D. Mutation of the receptor tyrosine kinase gene Mertk in the retinal dystrophic RCS rat. Human molecular genetics. 2000;9:645–651. doi: 10.1093/hmg/9.4.645. [DOI] [PubMed] [Google Scholar]
- de Raad S, Szczesny PJ, Munz K, Reme CE. Light damage in the rat retina: glial fibrillary acidic protein accumulates in Muller cells in correlation with photoreceptor damage. Ophthalmic research. 1996;28:99–107. doi: 10.1159/000267881. [DOI] [PubMed] [Google Scholar]
- Dryja TP, Berson EL, Rao VR, Oprian DD. Heterozygous missense mutation in the rhodopsin gene as a cause of congenital stationary night blindness. Nature genetics. 1993;4:280–283. doi: 10.1038/ng0793-280. [DOI] [PubMed] [Google Scholar]
- Dryja TP, McGee TL, Berson EL, Fishman GA, Sandberg MA, Alexander KR, Derlacki DJ, Rajagopalan AS. Night blindness and abnormal cone electroretinogram ON responses in patients with mutations in the GRM6 gene encoding mGluR6. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:4884–4889. doi: 10.1073/pnas.0501233102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan JL, Yang H, Vollrath D, Yasumura D, Matthes MT, Trautmann N, Chappelow AV, Feng W, Earp HS, Matsushima GK, LaVail MM. Inherited retinal dystrophy in Mer knockout mice. Advances in experimental medicine and biology. 2003;533:165–172. doi: 10.1007/978-1-4615-0067-4_21. [DOI] [PubMed] [Google Scholar]
- Dunn FA. Photoreceptor ablation initiates the immediate loss of glutamate receptors in postsynaptic bipolar cells in retina. J Neurosci. 2015;35:2423–2431. doi: 10.1523/JNEUROSCI.4284-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fariss RN, Li ZY, Milam AH. Abnormalities in rod photoreceptors, amacrine cells, and horizontal cells in human retinas with retinitis pigmentosa. Am J Ophthalmol. 2000;129:215–223. doi: 10.1016/s0002-9394(99)00401-8. [DOI] [PubMed] [Google Scholar]
- Fletcher EL, Kalloniatis M. Neurochemical architecture of the normal and degenerating rat retina. Journal of Comparative Neurology. 1996;376:343–360. doi: 10.1002/(SICI)1096-9861(19961216)376:3<343::AID-CNE1>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Frederick JM, Krasnoperova NV, Hoffmann K, Church-Kopish J, Ruther K, Howes K, Lem J, Baehr W. Mutant rhodopsin transgene expression on a null background. Invest Ophthalmol Vis Sci. 2001;42:826–833. [PubMed] [Google Scholar]
- Gazzaley AH, Benson DL, Huntley GW, Morrison JH. Differential subcellular regulation of NMDAR1 protein and mRNA in dendrites of dentate gyrus granule cells after perforant path transection. J Neurosci. 1997;17:2006–2017. doi: 10.1523/JNEUROSCI.17-06-02006.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu SM, Thompson DA, Srikumari CR, Lorenz B, Finckh U, Nicoletti A, Murthy KR, Rathmann M, Kumaramanickavel G, Denton MJ, Gal A. Mutations in RPE65 cause autosomal recessive childhood-onset severe retinal dystrophy. Nature genetics. 1997;17:194–197. doi: 10.1038/ng1097-194. [DOI] [PubMed] [Google Scholar]
- Gust J, Reh TA. Adult donor rod photoreceptors integrate into the mature mouse retina. Invest Ophthalmol Vis Sci. 2011;52:5266–5272. doi: 10.1167/iovs.10-6329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackam AS, Strom R, Liu D, Qian J, Wang C, Otteson D, Gunatilaka T, Farkas RH, Chowers I, Kageyama M, Leveillard T, Sahel JA, Campochiaro PA, Parmigiani G, Zack DJ. Identification of gene expression changes associated with the progression of retinal degeneration in the rd1 mouse. Invest Ophthalmol Vis Sci. 2004;45:2929–2942. doi: 10.1167/iovs.03-1184. [DOI] [PubMed] [Google Scholar]
- Haeseleer F, Imanishi Y, Maeda T, Possin DE, Maeda A, Lee A, Rieke F, Palczewski K. Essential role of Ca2+-binding protein 4, a Cav1.4 channel regulator, in photoreceptor synaptic function. Nat Neurosci. 2004;7:1079–1087. doi: 10.1038/nn1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harel NY, Strittmatter SM. Can regenerating axons recapitulate developmental guidance during recovery from spinal cord injury? Nat Rev Neurosci. 2006;7:603–616. doi: 10.1038/nrn1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriksen BS, Marc RE, Bernstein PS. Optogenetics for retinal disorders. Journal of ophthalmic & vision research. 2014;9:374–382. doi: 10.4103/2008-322X.143379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homma K, Osakada F, Hirami Y, Jin ZB, Mandai M, Takahashi M. Detection of localized retinal malfunction in retinal degeneration model using a multielectrode array system. J Neurosci Res. 2009;87:2175–2182. doi: 10.1002/jnr.22024. [DOI] [PubMed] [Google Scholar]
- Hu G, Wensel TG. R9AP, a membrane anchor for the photoreceptor GTPase accelerating protein, RGS9-1. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:9755–9760. doi: 10.1073/pnas.152094799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu G, Wensel TG. Characterization of R9AP, a membrane anchor for the photoreceptor GTPase-accelerating protein, RGS9-1. Methods in enzymology. 2004;390:178–196. doi: 10.1016/S0076-6879(04)90012-2. [DOI] [PubMed] [Google Scholar]
- Hu G, Zhang Z, Wensel TG. Activation of RGS9-1GTPase acceleration by its membrane anchor, R9AP. The Journal of biological chemistry. 2003;278:14550–14554. doi: 10.1074/jbc.M212046200. [DOI] [PubMed] [Google Scholar]
- Huang SH, Pittler SJ, Huang X, Oliveira L, Berson EL, Dryja TP. Autosomal recessive retinitis pigmentosa caused by mutations in the alpha subunit of rod cGMP phosphodiesterase. Nature genetics. 1995;11:468–471. doi: 10.1038/ng1295-468. [DOI] [PubMed] [Google Scholar]
- Humphries MM, Rancourt D, Farrar GJ, Kenna P, Hazel M, Bush RA, Sieving PA, Sheils DM, McNally N, Creighton P, Erven A, Boros A, Gulya K, Capecchi MR, Humphries P. Retinopathy induced in mice by targeted disruption of the rhodopsin gene. Nat Genet. 1997;15:216–219. doi: 10.1038/ng0297-216. [DOI] [PubMed] [Google Scholar]
- Ivanova E, Yee CW, Baldoni R, Jr, Sagdullaev BT. Aberrant activity in retinal degeneration impairs central visual processing and relies on Cx36-containing gap junctions. Exp Eye Res. 2015a doi: 10.1016/j.exer.2015.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanova E, Yee CW, Sagdullaev BT. Increased phosphorylation of Cx36 gap junctions in the AII amacrine cells of RD retina. Frontiers in cellular neuroscience. 2015b;9:390. doi: 10.3389/fncel.2015.00390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanova E, Yee CW, Sagdullaev BT. Disruption in dopaminergic innervation during photoreceptor degeneration. J Comp Neurol. 2016;524:1208–1221. doi: 10.1002/cne.23899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson SG, Cideciyan AV, Roman AJ, Sumaroka A, Schwartz SB, Heon E, Hauswirth WW. Improvement and decline in vision with gene therapy in childhood blindness. N Engl J Med. 2015;372:1920–1926. doi: 10.1056/NEJMoa1412965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson LV, Anderson DH. Age-related macular degeneration and the extracellular matrix. N Engl J Med. 2004;351:320–322. doi: 10.1056/NEJMp048131. [DOI] [PubMed] [Google Scholar]
- Jones BW, Kondo M, Terasaki H, Lin Y, McCall M, Marc RE. Retinal remodeling. Japanese journal of ophthalmology. 2012;56:289–306. doi: 10.1007/s10384-012-0147-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones BW, Kondo M, Terasaki H, Watt CB, Rapp K, Anderson J, Lin Y, Shaw MV, Yang JH, Marc RE. Retinal remodeling in the Tg P347L rabbit, a large-eye model of retinal degeneration. J Comp Neurol. 2011;519:2713–2733. doi: 10.1002/cne.22703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones BW, Marc RE. Retinal remodeling during retinal degeneration. Exp Eye Res. 2005;81:123–137. doi: 10.1016/j.exer.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Jones BW, Marc RE, Watt CB, Vaughan DK, Organisciak DT. Neural plasticity revealed by light-induced photoreceptor lesions. Adv Exp Med Biol. 2006;572:405–410. doi: 10.1007/0-387-32442-9_57. [DOI] [PubMed] [Google Scholar]
- Jones BW, Watt CB, Frederick JM, Baehr W, Chen CK, Levine EM, Milam AH, LaVail MM, Marc RE. Retinal remodeling triggered by photoreceptor degenerations. Journal of Comparative Neurology. 2003;464:1–16. doi: 10.1002/cne.10703. [DOI] [PubMed] [Google Scholar]
- Jones BW, Watt CB, Marc RE. Retinal remodelling. Clin Exp Optom. 2005;88:282–291. doi: 10.1111/j.1444-0938.2005.tb06712.x. [DOI] [PubMed] [Google Scholar]
- Kalloniatis M, Marc RE, Murry RF. Amino acid signatures in the primate retina. Journal of Neuroscience. 1996;16:6807–6829. doi: 10.1523/JNEUROSCI.16-21-06807.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karschin A, Wassle H. Voltage- and transmitter-gated currents in isolated rod bipolar cells of rat retina. J Neurophysiol. 1990;63:860–876. doi: 10.1152/jn.1990.63.4.860. [DOI] [PubMed] [Google Scholar]
- Lewis GP, Linberg KA, Fisher SK. Neurite outgrowth from bipolar and horizontal cells after experimental retinal detachment. Invest Ophthalmol Vis Sci. 1998;39:424–434. [PubMed] [Google Scholar]
- Li JB, Gerdes JM, Haycraft CJ, Fan Y, Teslovich TM, May-Simera H, Li H, Blacque OE, Li L, Leitch CC, Lewis RA, Green JS, Parfrey PS, Leroux MR, Davidson WS, Beales PL, Guay-Woodford LM, Yoder BK, Stormo GD, Katsanis N, Dutcher SK. Comparative genomics identifies a flagellar and basal body proteome that includes the BBS5 human disease gene. Cell. 2004;117:541–552. doi: 10.1016/s0092-8674(04)00450-7. [DOI] [PubMed] [Google Scholar]
- Li ZY, Kljavin IJ, Milam AH. Rod photoreceptor neurite sprouting in retinitis pigmentosa. J Neurosci. 1995;15:5429–5438. doi: 10.1523/JNEUROSCI.15-08-05429.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang FQ, Aleman TS, Dejneka NS, Dudus L, Fisher KJ, Maguire AM, Jacobson SG, Bennett J. Long-term protection of retinal structure but not function using RAAV.CNTF in animal models of retinitis pigmentosa. Molecular therapy : the journal of the American Society of Gene Therapy. 2001;4:461–472. doi: 10.1006/mthe.2001.0473. [DOI] [PubMed] [Google Scholar]
- Lin Y, Jones BW, Liu A, Tucker JF, Rapp K, Luo L, Baehr W, Bernstein PS, Watt CB, Yang JH, Shaw MV, Marc RE. Retinoid receptors trigger neuritogenesis in retinal degenerations. FASEB J. 2012a;26:81–92. doi: 10.1096/fj.11-192914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Jones BW, Liu A, Vazquez-Chona FR, Lauritzen JS, Ferrell WD, Marc RE. Rapid glutamate receptor 2 trafficking during retinal degeneration. Mol Neurodegener. 2012b;7:7. doi: 10.1186/1750-1326-7-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machida S, Kondo M, Jamison JA, Khan NW, Kononen LT, Sugawara T, Bush RA, Sieving PA. P23H rhodopsin transgenic rat: correlation of retinal function with histopathology. Invest Ophthalmol Vis Sci. 2000;41:3200–3209. [PubMed] [Google Scholar]
- Mansergh F, Orton NC, Vessey JP, Lalonde MR, Stell WK, Tremblay F, Barnes S, Rancourt DE, Bech-Hansen NT. Mutation of the calcium channel gene Cacna1f disrupts calcium signaling, synaptic transmission and cellular organization in mouse retina. Hum Mol Genet. 2005;14:3035–3046. doi: 10.1093/hmg/ddi336. [DOI] [PubMed] [Google Scholar]
- Mansergh FC, Carrigan M, Hokamp K, Farrar GJ. Gene expression changes during retinal development and rod specification. Mol Vis. 2015;21:61–87. [PMC free article] [PubMed] [Google Scholar]
- Marc RE. Kainate activation of horizontal, bipolar, amacrine, and ganglion cells in the rabbit retina. Journal of Comparative Neurology. 1999a;407:65–76. [PubMed] [Google Scholar]
- Marc RE. Mapping glutamatergic drive in the vertebrate retina with a channel-permeant organic cation. Journal of Comparative Neurology. 1999b;407:47–64. doi: 10.1002/(sici)1096-9861(19990428)407:1<47::aid-cne4>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Marc RE. Retinal Neurotransmitters. In: Chalupa LM, Werner J, editors. The Visual Neurosciences. Vol. 1. Cambridge, MA: MIT Press; 2004. pp. 315–330. [Google Scholar]
- Marc RE, Anderson JR, Jones BW, Sigulinsky CL, Lauritzen JS. The AII amacrine cell connectome: a dense network hub. Frontiers in neural circuits. 2014;8:104. doi: 10.3389/fncir.2014.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marc RE, Cameron DA. A molecular phenotype atlas of the zebrafish retina. Journal of Neurocytology. 2002 doi: 10.1023/a:1016516818393. in press. [DOI] [PubMed] [Google Scholar]
- Marc RE, Jones BW. Molecular phenotyping of retinal ganglion cells. J Neurosci. 2002;22:413–427. doi: 10.1523/JNEUROSCI.22-02-00413.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marc RE, Jones BW. Retinal remodeling in inherited photoreceptor degenerations. Mol Neurobiol. 2003;28:139–147. doi: 10.1385/MN:28:2:139. [DOI] [PubMed] [Google Scholar]
- Marc RE, Jones BW, Anderson JR, Kinard K, Marshak DW, Wilson JH, Wensel T, Lucas RJ. Neural reprogramming in retinal degeneration. Investigative Ophthalmology & Visual Science. 2007;48:3364–3371. doi: 10.1167/iovs.07-0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marc RE, Jones BW, Lauritzen JS, Watt CB, Anderson JR. Building retinal connectomes. Curr Opin Neurobiol. 2012;22:568–574. doi: 10.1016/j.conb.2012.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marc RE, Jones BW, Lucas RJ. Excitatory Self–signaling In Retinal Remodeling. Invest Ophthalmol Vis Sci. 2004;45 E-abstract 833. [Google Scholar]
- Marc RE, Jones BW, Watt CB. Retinal remodeling: Circuitry revisions triggered by photoreceptor degeneration. Springer/Kluwer Academic Press Corporation; 2005a. [Google Scholar]
- Marc RE, Jones BW, Watt CB, Strettoi E. Neural remodeling in retinal degeneration. Progress in retinal and eye research. 2003a;22:607–655. doi: 10.1016/s1350-9462(03)00039-9. [DOI] [PubMed] [Google Scholar]
- Marc RE, Jones BW, Watt CB, Strettoi E. Neural Remodeling in Retinal Degeneration. Prog Retina Eye Res. 2003b;22:607–655. doi: 10.1016/s1350-9462(03)00039-9. [DOI] [PubMed] [Google Scholar]
- Marc RE, Jones BW, Watt CB, Vazquez-Chona F, Vaughan DK, Organisciak DT. Extreme retinal remodeling triggered by light damage: implications for age related macular degeneration. Molecular Vision. 2008;14:782–806. [PMC free article] [PubMed] [Google Scholar]
- Marc RE, Kalloniatis M, Jones BW. Excitation mapping with the organic cation AGB2+ Vision Res. 2005b;45:3454–3468. doi: 10.1016/j.visres.2005.07.025. [DOI] [PubMed] [Google Scholar]
- Marc RE, Murry RF, Basinger SF. Pattern recognition of amino acid signatures in retinal neurons. Journal of Neuroscience. 1995;15:5106–5129. doi: 10.1523/JNEUROSCI.15-07-05106.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marc RE, Murry RF, Fisher SK, Linberg KA, Lewis GP, Kalloniatis M. Amino acid signatures in the normal cat retina. Investigative Ophthalmology & Visual Science. 1998;39:1685–1693. [PubMed] [Google Scholar]
- Margolis DJ, Gartland AJ, Singer JH, Detwiler PB. Network oscillations drive correlated spiking of ON and OFF ganglion cells in the rd1 mouse model of retinal degeneration. PloS one. 2014;9:e86253. doi: 10.1371/journal.pone.0086253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin ME, Ehrhart TL, Berson EL, Dryja TP. Mutation spectrum of the gene encoding the beta subunit of rod phosphodiesterase among patients with autosomal recessive retinitis pigmentosa. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:3249–3253. doi: 10.1073/pnas.92.8.3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin ME, Sandberg MA, Berson EL, Dryja TP. Recessive mutations in the gene encoding the beta-subunit of rod phosphodiesterase in patients with retinitis pigmentosa. Nature genetics. 1993;4:130–134. doi: 10.1038/ng0693-130. [DOI] [PubMed] [Google Scholar]
- Menzler J, Zeck G. Network oscillations in rod-degenerated mouse retinas. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:2280–2291. doi: 10.1523/JNEUROSCI.4238-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalakis S, Schaferhoff K, Spiwoks-Becker I, Zabouri N, Koch S, Koch F, Bonin M, Biel M, Haverkamp S. Characterization of neurite outgrowth and ectopic synaptogenesis in response to photoreceptor dysfunction. Cell Mol Life Sci. 2013;70:1831–1847. doi: 10.1007/s00018-012-1230-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milam AH, Li ZY, Fariss RN. Histopathology of the human retina in retinitis pigmentosa. Prog Retin Eye Res. 1998;17:175–205. doi: 10.1016/s1350-9462(97)00012-8. [DOI] [PubMed] [Google Scholar]
- Molday LL, Rabin AR, Molday RS. ABCR expression in foveal cone photoreceptors and its role in Stargardt macular dystrophy. Nature genetics. 2000;25:257–258. doi: 10.1038/77004. [DOI] [PubMed] [Google Scholar]
- Morimoto K, Fahnestock M, Racine RJ. Kindling and status epilepticus models of epilepsy: rewiring the brain. Prog Neurobiol. 2004;73:1–60. doi: 10.1016/j.pneurobio.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Morimura H, Fishman GA, Grover SA, Fulton AB, Berson EL, Dryja TP. Mutations in the RPE65 gene in patients with autosomal recessive retinitis pigmentosa or leber congenital amaurosis. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:3088–3093. doi: 10.1073/pnas.95.6.3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peichl L, Bolz J. Kainic acid induces sprouting of retinal neurons. Science. 1984;223:503–504. doi: 10.1126/science.6691162. [DOI] [PubMed] [Google Scholar]
- Pfeiffer RL, Marc RE, Jones BW. Experimental Eye Research. 2016. Müller cell metabolic chaos during retinal degeneration. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinilla I, Fernandez-Sanchez L, Segura FJ, Sanchez-Cano AI, Tamarit JM, Fuentes-Broto L, Eells JT, Lax P, Cuenca N. Long time remodeling during retinal degeneration evaluated by optical coherence tomography, immunocytochemistry and fundus autofluorescence. Exp Eye Res. 2015 doi: 10.1016/j.exer.2015.10.012. [DOI] [PubMed] [Google Scholar]
- Poria D, Dhingra NK. Spontaneous oscillatory activity in rd1 mouse retina is transferred from ON pathway to OFF pathway via glycinergic synapse. J Neurophysiol. 2015;113:420–425. doi: 10.1152/jn.00702.2014. [DOI] [PubMed] [Google Scholar]
- Pow DV, Crook DK. Direct immunocytochemical evidence for the transfer of glutamine from glial cells to neurons: use of specific antibodies directed against the d-stereoisomers of glutamate and glutamine. Neuroscience. 1996;70:295–302. doi: 10.1016/0306-4522(95)00363-n. [DOI] [PubMed] [Google Scholar]
- Prince DA, Parada I, Scalise K, Graber K, Jin X, Shen F. Epilepsy following cortical injury: cellular and molecular mechanisms as targets for potential prophylaxis. Epilepsia. 2009;50(Suppl 2):30–40. doi: 10.1111/j.1528-1167.2008.02008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pu M, Xu L, Zhang H. Visual response properties of retinal ganglion cells in the royal college of surgeons dystrophic rat. Invest Ophthalmol Vis Sci. 2006;47:3579–3585. doi: 10.1167/iovs.05-1450. [DOI] [PubMed] [Google Scholar]
- Rizzo S, Belting C, Cinelli L, Allegrini L, Genovesi-Ebert F, Barca F, di Bartolo E. The Argus II Retinal Prosthesis: 12-month outcomes from a single-study center. Am J Ophthalmol. 2014;157:1282–1290. doi: 10.1016/j.ajo.2014.02.039. [DOI] [PubMed] [Google Scholar]
- Roh M, Zhang Y, Murakami Y, Thanos A, Lee SC, Vavvas DG, Benowitz LI, Miller JW. Etanercept, a widely used inhibitor of tumor necrosis factor-alpha (TNF-alpha), prevents retinal ganglion cell loss in a rat model of glaucoma. PLoS One. 2012;7:e40065. doi: 10.1371/journal.pone.0040065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahel JA, Roska B. Gene therapy for blindness. Annu Rev Neurosci. 2013;36:467–488. doi: 10.1146/annurev-neuro-062012-170304. [DOI] [PubMed] [Google Scholar]
- Sasaki T, Kaneko A. L-Glutamate-induced responses in OFF-type bipolar cells of the cat retina. Vision research. 1996;36:787–795. doi: 10.1016/0042-6989(95)00176-x. [DOI] [PubMed] [Google Scholar]
- Seiler MJ, Jones BW, Aramant RB, Yang PB, Keirstead HS, Marc RE. Computational molecular phenotyping of retinal sheet transplants to rats with retinal degeneration. Eur J Neurosci. 2012;35:1692–1704. doi: 10.1111/j.1460-9568.2012.08078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekirnjak C, Jepson LH, Hottowy P, Sher A, Dabrowski W, Litke AM, Chichilnisky EJ. Changes in physiological properties of rat ganglion cells during retinal degeneration. J Neurophysiol. 2011;105:2560–2571. doi: 10.1152/jn.01061.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieving PA, Caruso RC, Tao W, Coleman HR, Thompson DJ, Fullmer KR, Bush RA. Ciliary neurotrophic factor (CNTF) for human retinal degeneration: phase I trial of CNTF delivered by encapsulated cell intraocular implants. Proc Natl Acad Sci U S A. 2006;103:3896–3901. doi: 10.1073/pnas.0600236103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singhal S, Lawrence JM, Bhatia B, Ellis JS, Kwan AS, Macneil A, Luthert PJ, Fawcett JW, Perez MT, Khaw PT, Limb GA. Chondroitin sulfate proteoglycans and microglia prevent migration and integration of grafted Muller stem cells into degenerating retina. Stem cells. 2008;26:1074–1082. doi: 10.1634/stemcells.2007-0898. [DOI] [PubMed] [Google Scholar]
- Slaughter MM, Miller RF. 2-amino-4-phosphonobutyric acid: a new pharmacological tool for retina research. Science. 1981;211:182–185. doi: 10.1126/science.6255566. [DOI] [PubMed] [Google Scholar]
- Slaughter MM, Miller RF. Bipolar cells in the mudpuppy retina use an excitatory amino acid neurotransmitter. Nature. 1983;303:537–538. doi: 10.1038/303537a0. [DOI] [PubMed] [Google Scholar]
- Sommer ME, Farrens DL. Arrestin can act as a regulator of rhodopsin photochemistry. Vision research. 2006;46:4532–4546. doi: 10.1016/j.visres.2006.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer ME, Farrens DL, McDowell JH, Weber LA, Smith WC. Dynamics of arrestin-rhodopsin interactions: loop movement is involved in arrestin activation and receptor binding. The Journal of biological chemistry. 2007;282:25560–25568. doi: 10.1074/jbc.M702155200. [DOI] [PubMed] [Google Scholar]
- Sommer ME, Smith WC, Farrens DL. Dynamics of arrestin-rhodopsin interactions: arrestin and retinal release are directly linked events. The Journal of biological chemistry. 2005;280:6861–6871. doi: 10.1074/jbc.M411341200. [DOI] [PubMed] [Google Scholar]
- Sommer ME, Smith WC, Farrens DL. Dynamics of arrestin-rhodopsin interactions: acidic phospholipids enable binding of arrestin to purified rhodopsin in detergent. The Journal of biological chemistry. 2006;281:9407–9417. doi: 10.1074/jbc.M510037200. [DOI] [PubMed] [Google Scholar]
- Stasheff SF. Emergence of sustained spontaneous hyperactivity and temporary preservation of OFF responses in ganglion cells of the retinal degeneration (rd1) mouse. Journal of neurophysiology. 2008;99:1408–1421. doi: 10.1152/jn.00144.2007. [DOI] [PubMed] [Google Scholar]
- Stingl K, Bartz-Schmidt KU, Besch D, Braun A, Bruckmann A, Gekeler F, Greppmaier U, Hipp S, Hortdorfer G, Kernstock C, Koitschev A, Kusnyerik A, Sachs H, Schatz A, Stingl KT, Peters T, Wilhelm B, Zrenner E. Artificial vision with wirelessly powered subretinal electronic implant alpha-IMS. Proceedings Biological sciences / The Royal Society. 2013;280:20130077. doi: 10.1098/rspb.2013.0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strettoi E, Pignatelli V. Modifications of retinal neurons in a mouse model of retinitis pigmentosa. Proc Natl Acad Sci U S A. 2000;97:11020–11025. doi: 10.1073/pnas.190291097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strettoi E, Pignatelli V, Rossi C, Porciatti V, Falsini B. Remodeling of second-order neurons in the retina of rd/rd mutant mice. Vision Res. 2003;43:867–877. doi: 10.1016/s0042-6989(02)00594-1. [DOI] [PubMed] [Google Scholar]
- Strettoi E, Porciatti V, Falsini B, Pignatelli V, Rossi C. Morphological and functional abnormalities in the inner retina of the rd/rd mouse. J Neurosci. 2002;22:5492–5504. doi: 10.1523/JNEUROSCI.22-13-05492.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto T, Bennett GJ, Kajander KC. Transsynaptic degeneration in the superficial dorsal horn after sciatic nerve injury: effects of a chronic constriction injury, transection, and strychnine. Pain. 1990;42:205–213. doi: 10.1016/0304-3959(90)91164-E. [DOI] [PubMed] [Google Scholar]
- Sullivan R, Penfold P, Pow DV. Neuronal migration and glial remodeling in degenerating retinas of aged rats and in nonneovascular AMD. Invest Ophthalmol Vis Sci. 2003;44:856–865. doi: 10.1167/iovs.02-0416. [DOI] [PubMed] [Google Scholar]
- Turrigiano GG, Nelson SB. Hebb and homeostasis in neuronal plasticity. Curr Opin Neurobiol. 2000;10:358–364. doi: 10.1016/s0959-4388(00)00091-x. [DOI] [PubMed] [Google Scholar]
- Vasireddy V, Uchida Y, Salem N, Jr, Kim SY, Mandal MN, Reddy GB, Bodepudi R, Alderson NL, Brown JC, Hama H, Dlugosz A, Elias PM, Holleran WM, Ayyagari R. Loss of functional ELOVL4 depletes very long-chain fatty acids (> or =C28) and the unique omega-O-acylceramides in skin leading to neonatal death. Human molecular genetics. 2007;16:471–482. doi: 10.1093/hmg/ddl480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassle H, Boycott BB. Functional architecture of the mammalian retina. Physiol Rev. 1991;71:447–480. doi: 10.1152/physrev.1991.71.2.447. [DOI] [PubMed] [Google Scholar]
- Wensel TG. Signal transducing membrane complexes of photoreceptor outer segments. Vision research. 2008;48:2052–2061. doi: 10.1016/j.visres.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita M, Wassle H. Responses of rod bipolar cells isolated from the rat retina to the glutamate agonist 2-amino-4-phosphonobutyric acid (APB) J Neurosci. 1991;11:2372–2382. doi: 10.1523/JNEUROSCI.11-08-02372.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee CW, Toychiev AH, Ivanova E, Sagdullaev BT. Aberrant synaptic input to retinal ganglion cells varies with morphology in a mouse model of retinal degeneration. J Comp Neurol. 2014;522:4085–4099. doi: 10.1002/cne.23660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen HJ, Tayeh MK, Mullins RF, Stone EM, Sheffield VC, Slusarski DC. Bardet-Biedl syndrome genes are important in retrograde intracellular trafficking and Kupffer's vesicle cilia function. Human molecular genetics. 2006;15:667–677. doi: 10.1093/hmg/ddi468. [DOI] [PubMed] [Google Scholar]
- Young NM, Mets MB, Hain TC. Early diagnosis of Usher syndrome in infants and children. Am J Otol. 1996;17:30–34. [PubMed] [Google Scholar]
- Zeitz C, Gross AK, Leifert D, Kloeckener-Gruissem B, McAlear SD, Lemke J, Neidhardt J, Berger W. Identification and functional characterization of a novel rhodopsin mutation associated with autosomal dominant CSNB. Investigative ophthalmology & visual science. 2008;49:4105–4114. doi: 10.1167/iovs.08-1717. [DOI] [PubMed] [Google Scholar]
- Zeitz C, van Genderen M, Neidhardt J, Luhmann UF, Hoeben F, Forster U, Wycisk K, Matyas G, Hoyng CB, Riemslag F, Meire F, Cremers FP, Berger W. Mutations in GRM6 cause autosomal recessive congenital stationary night blindness with a distinctive scotopic 15-Hz flicker electroretinogram. Investigative ophthalmology & visual science. 2005;46:4328–4335. doi: 10.1167/iovs.05-0526. [DOI] [PubMed] [Google Scholar]