Abstract
The ability to reconstitute sodium channel function and pharmacology in vitro using cloned subunits of known structure has greatly enhanced our understanding of the action of pyrethroid insecticides at this target and the structural determinants of resistance and interspecies selectivity. However, the use of reconstituted channels raises three critical questions: (1) Which subunits and subunit combinations should be used? (2) Which heterologous expression system is preferred? (3) Which combination of subunits and expression system best represents the function of native neuronal channels in the organism of interest? This review considers these questions from the perspective of recent research in this laboratory on the action of pyrethroid insecticides on rat Nav1.6 sodium channels by comparing the effects of heteroligomeric complex composition on channel function and insecticide response when channels are expressed in either Xenopus oocytes or stably-transformed HEK293 cells. These comparisons provide new insight into the influence of cellular context on the functional and pharmacological properties of expressed channels, the modulatory effects of sodium channel auxiliary subunits on the action of pyrethroids, and the relative fidelity of the Xenopus oocyte and HEK293 cell expression systems as model systems for studying of channel function and pyrethroid action.
Introduction
Pyrethroids owe their insecticidal activity to their ability to modify the gating of voltage-gated sodium channels (VGSCs), which mediate the transient increase in the sodium permeability of the nerve membrane that underlies the rising phase of the nerve action potential (Bloomquist, 1993; Soderlund, 1995; Narahashi, 1996). The identification of single amino acid substitutions in the VGSC sequences of resistant insects that reduce the susceptibility of expressed channels to pyrethroid modification provides further evidence that action on VGSCs underlies the primary insecticidal actions of pyrethroids (Soderlund and Knipple, 2003; Soderlund, 2005; Rinkevich et al., 2013).
The compelling evidence for effects on VGSCs as the mechanism of insecticidal activity of pyrethroids and the strong conservation of VGSC structure, function and pharmacology across animal taxa (Goldin, 2002) implicates VGSCs in the central nervous system (CNS) as important target sites for the acute neurotoxic effects of pyrethroids in mammals. However, individual CNS neurons express multiple VGSC isoforms and contain multiple functionally and pharmacologically distinct VGSC heteromultimeric complexes (Felts et al., 1997; Whitaker et al., 2000; Whitaker et al., 2001). Thus, the relative sensitivity of different isoforms and subunit complexes to pyrethroids, and therefore the relative importance of these isoforms and complexes as targets in intoxication, cannot be determined in studies using native neurons. This difficulty can be overcome by using in vitro systems for the heterologous expression and functional characterization of VGSC complexes of defined subunit structure.
This brief review summarizes and synthesizes work from this laboratory during the past decade using two heterologous expression systems – the unfertilized oocytes of the frog Xenopus laevis and the human embryonic kidney-derived HEK293 cell line - to express the rat Nav1.6 sodium channel, either alone or in combination with the rat β1 and β2 auxiliary subunits, and characterize both their functional properties and their pharmacological modification by pyrethroid insecticides. We also provide a provisional assessment of the relative merits of these two systems for predicting the action of pyrethroids on VGSCs in their native neuronal environment.
Structural and Pharmacological Heterogeneity of Mammalian Sodium Channels
Structural heterogeneity
Potential VGSC targets for pyrethroid intoxication in mammals comprise nine different pore-forming α subunit isoforms (Nav1.1 – Nav1.9) that exhibit unique patterns of developmental and anatomical expression and varied functional and pharmacological properties (Goldin, 2001). Four α subunits (Nav1.1, Nav1.2, Nav1.3, and Nav1.6) are abundantly expressed in either the embryonic or adult brain and represent possible targets for pyrethroid neurotoxicity in the CNS. The Nav1.3 and Nav1.6 isoforms are of particular interest because they are the most abundantly-expressed isoforms in the embryonic (Nav1.3) and adult (Nav1.6) brain (Felts et al., 1997; Whitaker et al., 2000; Shah et al., 2001; Whitaker et al., 2001).
Additional diversity among sodium channels results from the coassembly in the nerve membrane of an α subunit with one or two auxiliary β subunits that modulate channel gating and regulate expression (Isom, 2001). VGSCs in the adult brain are heterotrimeric complexes of one α subunit and two β subunits that differ in structure and their mode of association (covalent or noncovalent) with the α subunit (Hartshorne and Catterall, 1984). Although there are four β subunits in mammals, the ubiquitous expression of the β1 and β2 subunits in the adult brain implies that the majority of brain VGSCs are α+β1+β2 complexes (Whitaker et al., 2000; Shah et al., 2001; Schaller and Caldwell, 2003). However, the actual subunit composition of native sodium channel complexes remains to be determined.
Pharmacological heterogeneity
The correlation of pyrethroid sensitivity with mammalian VGSC structure using neuronal tissue preparations is complicated by the fact that neurons are now known to express multiple VGSC isoforms. However, a limited number of physiological studies suggest that sodium channel isoforms expressed in various mammalian tissues exhibit differential sensitivity to pyrethroids. The clearest evidence of the pharmacological heterogeneity among VGSC isoforms is found in the responses of the tetrodotoxin (TTX)-sensitive and TTX-resistant VGSC populations in dorsal root ganglion neurons to pyrethroids. The TTX-resistant sodium current in these cells is much more sensitive than the TTX-sensitive current to allethrin (Ginsburg and Narahashi, 1993), tetramethrin (Tatebayashi and Narahashi, 1994; Song et al., 1996) and deltamethrin (Tabarean and Narahashi, 1998).
Several studies have employed transient expression in Xenopus laevis oocytes to assess the action of pyrethroids on individual rat sodium channel isoforms and defined subunit complexes (Smith and Soderlund, 1998; Vais et al., 2000; Smith and Soderlund, 2001; Soderlund and Lee, 2001; Choi and Soderlund, 2006; Meacham et al., 2008; Tan and Soderlund, 2009; Tan and Soderlund, 2010; Tan and Soderlund, 2011a). Among the five rat isoforms examined to date, the Nav1.3, Nav1.6 and Nav1.8 isoforms are relatively sensitive to pyrethroid modification; in particular, the Nav1.8 isoform is likely responsible for the TTX-resistant, pyrethroid-sensitive current in dorsal root ganglion sensory neurons. By contrast the Nav1.2 and Nav1.7 isoforms are relatively resistant to pyrethroid modification.
The identification of Nav1.6 as a pyrethroid-sensitive isoform is of particular interest because Nav1.6 is the most abundant sodium channel α subunit in the adult rat brain (Auld et al., 1988), where it is preferentially expressed in regions of brain axons associated with action potential initiation (Hu et al., 2009). Nav1.6 is also the predominant isoform at nodes of Ranvier and is expressed in presynaptic and postsynaptic membranes of the neocortex and cerebellum (Caldwell et al., 2000). Thus, Nav1.6 sodium channels play important roles in both electrical and chemical signaling in the brain. The remainder of this review focuses on our studies of the function and pyrethroid pharmacology of the rat Nav1.6 isoform, either alone or in complexes with the β1 and β2 auxiliary subunits, expressed either in Xenopus oocyte or HEK293 cells.
Rat Nav1.6 Sodium Channels Expressed in Xenopus Oocytes
The Xenopus oocyte expression system
The Xenopus oocyte expression system is arguably the most widely-employed heterologous expression system for the reconstitution and study of both ligand-gated and voltage-gated ion channels (Goldin, 2006). When injected with synthetic mRNA the oocyte efficiently translates the message, performs post-translational modifications on the nascent protein, and inserts the mature protein into the cell membrane. For some channels, the oocyte system is the only heterologous expression system that will permit functional and pharmacological characterization in vitro. For example, all of our knowledge of heterologously-expressed insect VGSCs, including numerous studies identifying the functional role of putative insecticide resistance mutations (Rinkevich et al., 2013), is derived from the oocyte system, and all efforts to achieve the functional expression of insect VGSCs in other systems have so far failed.
The oocyte expression system is particularly well-suited to studies in which channel structure is the principal experimental variable. Expression in oocytes, when coupled with site-directed mutagenesis, also facilitates the testing of specific hypotheses regarding the effects of channel structure on both functional and pharmacological properties and the role of specific domains and amino acid residues in drug and insecticide binding to the channel.
Despite its considerable strengths the oocyte system also possesses two limitations that are intrinsic to the biology of the oocyte itself (Goldin, 2006). First, the biochemistry of post-translational modification is specific to the amphibian oocyte and therefore may differ markedly from both the processing of proteins that normally reside in the membranes of mammalian or insect neurons. Second, the large cell surface area and yolk of the oocyte provide a significant sink for lipophilic compounds such as pyrethroids. As a result, oocytes continue to accumulate pyrethroid during perfusion for up to 3 hours (Harrill et al., 2005); thus, nominal pyrethroid concentrations in the perfusion medium may not reach equilibrium during an experiment and also may not reflect the concentrations available to bind to expressed sodium channels. However, we found that the extent sodium channel modification by pyrethroids reached an apparent equilibrium after perfusion for 20 min (Choi and Soderlund, 2006), suggesting that a portion of the oocyte burden of pyrethroid is not accessible to channels that are expressed in the cell membrane. Nevertheless, the experimental benefits afforded by the oocyte system must be balanced against the uncertain extent to which the results obtained accurately reflect the properties of the same channels in their native neuronal environment.
Action of pyrethroids on rat Nav1.6 sodium channels
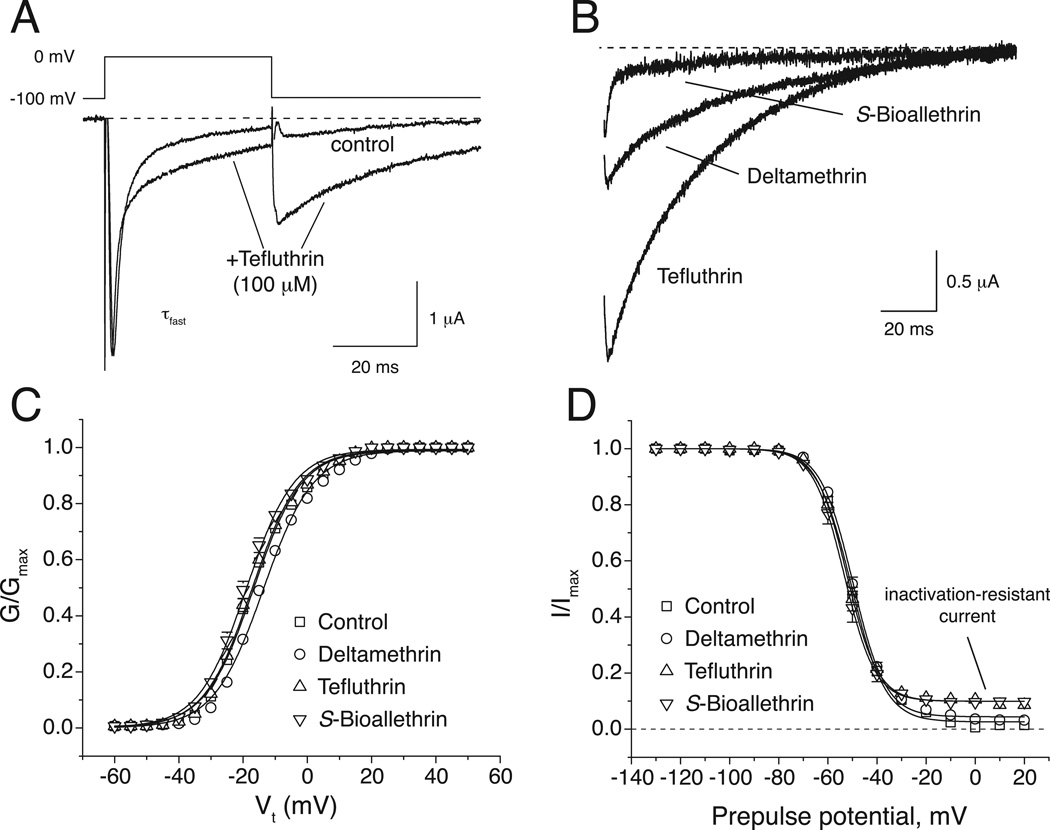
We expressed the rat Nav1.6 α subunit with the rat β1 and β2 subunits to give a channel complex in oocytes that reflected the inferred composition of the most abundant CNS complex and assessed the action of S-bioallethrin, tefluthrin and deltamethrin on sodium currents under voltage-clamp conditions (Tan and Soderlund, 2010). We assessed channel modification in the resting state by equilibrating oocytes with a high concentration of pyrethroid in perfusion medium and measuring the pyrethroid-modified current during the first depolarizing pulse. The effects of tefluthrin (100 µM, Fig. 1A) illustrate typical pyrethroid effects: a slowing of inactivation during depolarization, evident as the broadening and incomplete decay of the peak transient sodium current; and, slowing of deactivation, evident as a sodium tail current visible following repolarization.
Fig. 1.
Action of pyrethroids on Nav1.6+β1+β2 sodium channels expressed in Xenopus oocytes (re-drawn from data in Tan and Soderlund, 2010). (A) Representative control and tefluthrin (100 µM)-modified sodium currents recorded from an oocyte expressing rat Nav1.6 sodium channels using the indicated depolarization protocol. The dashed line indicates zero current. (B) Representative sodium tail currents recorded from oocyte expressing rat Nav1.6 sodium channels following exposure to S-bioallethrin, tefluthrin or deltamethrin (100 µM). Traces show currents recorded beginning 0.5 ms after repolarization to −100 mV from a step depolarization to 0 mV. The dashed line indicates zero current. (C) Effects of S-bioallethrin, tefluthrin or deltamethrin (100 µM) on the voltage dependence of activation of rat Nav1.6 sodium channels expressed in oocytes. Conductances of peak transient sodium currents measured upon depolarization from −100 mV to a range of test potentials are plotted as a function of test potential. Values are means; of 19 (control), 4 (S-bioallethrin), 6 (tefluthrin) or 9 (deltamethrin) separate experiments with different oocytes; bars show SE values larger than the data point symbols. Conductance – voltage curves were fitted to mean conductance values using the Boltzmann equation. (D) Effects of S-bioallethrin, tefluthrin or deltamethrin (100 µM) on the voltage dependence of steady-state inactivation of rat Nav1.6 sodium channels expressed in oocytes. Amplitudes of peak transient currents obtained during a 40-ms test depolarization to 0 mV following 100-msec conditioning prepulses from −140 mV to a range of conditioning potenticals are plotted as a function of prepulse potential. Values are means of 19 (control), 4 (S-bioallethrin), 6 (tefluthrin) or 9 (deltamethrin) separate experiments with different oocytes; bars show SE values larger than the data point symbols. The dashed line indicates zero current (complete inactivation). Current -voltage curves were fitted to mean current amplitude values using the Boltzmann equation.
The gating kinetics of pyrethroid-modified channels varied with the compound employed. Figure 1B illustrates the sodium tail currents produced by S-bioallethrin (rapid decay), tefluthrin (intermediate decay), and deltamethrin (slow decay). In contrast to their strong effects on channel kinetics, the three pyrethroids had little detectable effect on the voltage dependence of channel activation (Fig. 1C) or steady-state inactivation (Fig. 1D). However, all three pyrethroids caused a small but statistically-significant increase in the fraction of channels that were refractory to inactivation following strong depolarizations (see Fig. 1D).
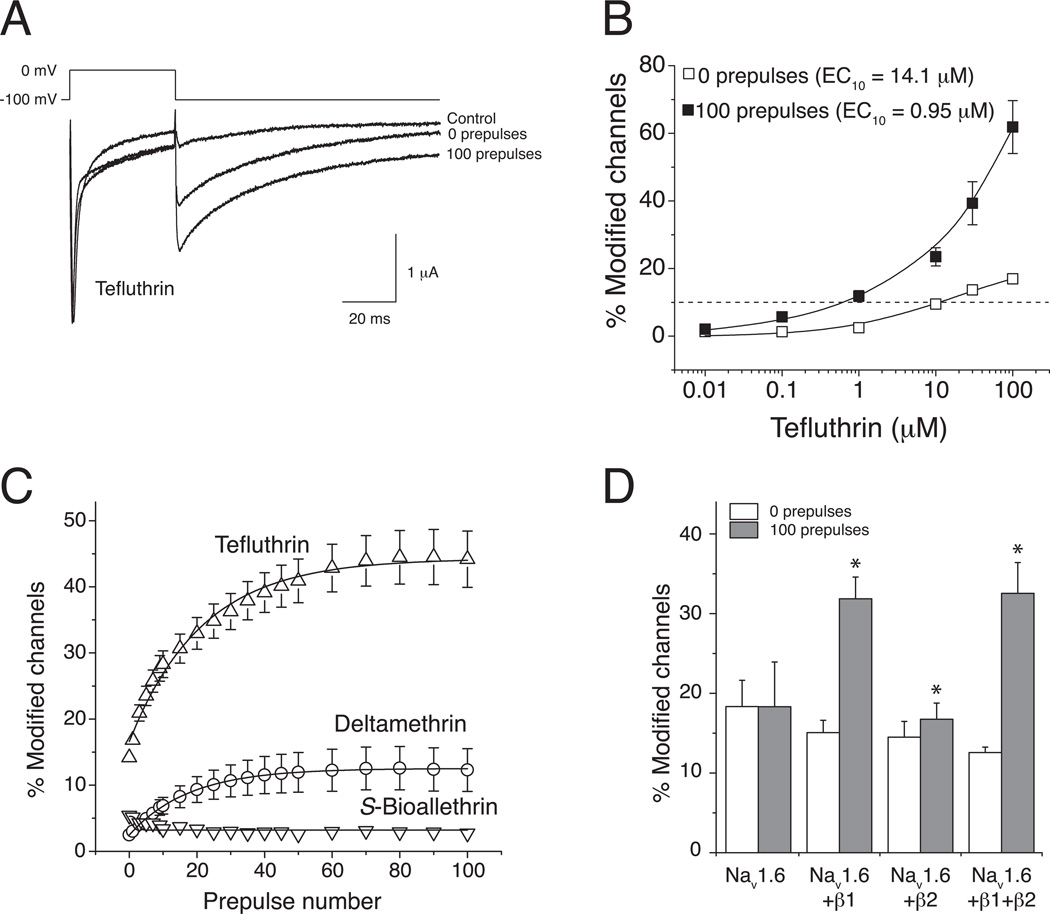
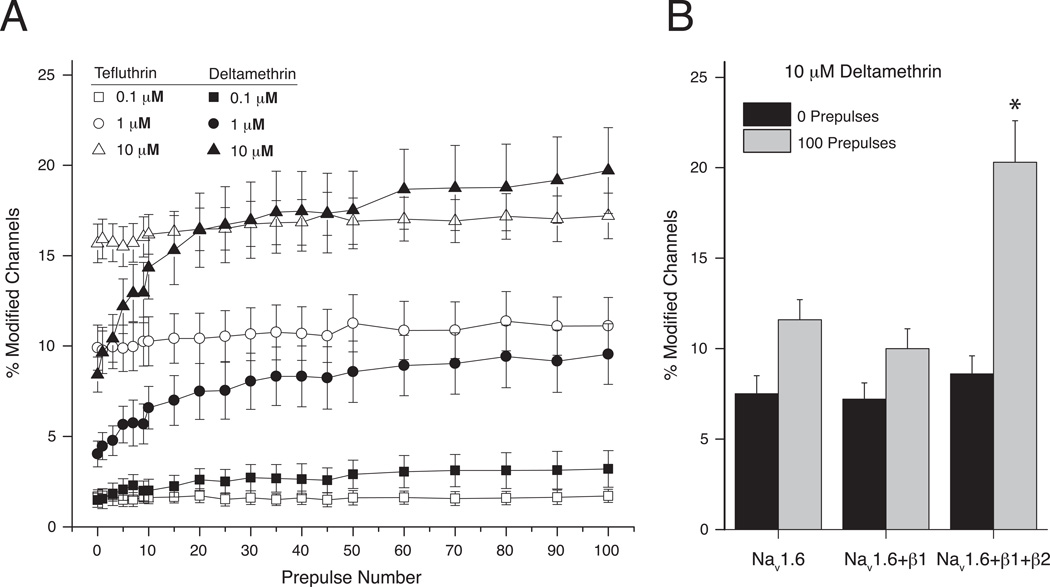
Modification of both insect and mammalian VGSCs expressed in Xenopus oocytes is enhanced by repeated depolarization (Soderlund, 2010). We therefore assessed the use-dependent modification rat Nav1.6 channel complexes by examining the impact of trains of up to 100 brief, high-frequency depolarizations on the extent of channel modification by S-bioallethrin, tefluthrin and deltamethrin in a subsequent test depolarization of normal duration (Tan and Soderlund, 2010). Repeated depolarization strongly enhanced channel modification by tefluthrin, increasing the amplitude of the tail current (Fig. 2A). Experiments with a range of tefluthrin concentrations showed that repeated depolarization increased its apparent potency by more than 10-fold (Fig. 2B). Repeated depolarization also increased the extent of channel modification by deltamethrin but had no effect on channel modification by S-bioallethrin (Fig. 2C). Experiments comparing Nav1.6 α subunits expressed alone or in combination with the β1 and β2 subunits, either singly or in combination, showed that the β1 subunit was required to observe the use-dependent component of Nav1.6 channel modification by tefluthrin (Fig. 2D).
Fig. 2.
Resting and use-dependent modification of rat Nav1.6 sodium channels expressed in Xenopus oocytes by pyrethroids (redrawn from data in Tan and Soderlund, 2010; Tan and Soderlund, 2011b). (A) Representative traces recorded from an oocyte expressing rat Nav1.6+β1+β2 sodium channels exposed to 100 µM tefluthrin. The control trace was recorded prior to insecticide exposure. Following equilibration with tefluthrin traces were recorded before or after the application of a high-frequency train of 100 depolarizing prepulses (5 ms, 66.7 Hz). (B) Concentration dependence of resting (0 prepulses) and use-dependent (100 prepulses) modification of rat Nav1.6+β1+β2 sodium channels by tefluthrin. Values are means ± SE of 5 separate experiments. The dashed line indicates 10% channel modification. (C) Effect of repeated depolarizing prepulses on the extent of modification of rat Nav1.6+β1+β2 sodium channels by S-bioallethrin, tefluthrin and deltamethrin. Values are means ± SE of 4 (S-bioallethrin), 15 (tefluthrin) or 12 (deltamethrin) separate experiments with different oocytes. (D) Comparison of the extent of resting (after 0 prepulses) and maximal use-dependent (after 100 prepulses) modification of rat Nav1.6, Nav1.6+β1, Nav1.6+β2, and Nav1.6+β1+β2 by tefluthrin. Values for use-dependent modification marked with asterisks were significantly different from values for the resting modification of the same channel (paired t-tests, P < 0.05).
Rat Nav1.6 Sodium Channels Expressed in HEK293 Cells
The HEK293 cell expression system
HEK293 cells were derived from human embryonic kidney cells by transformation with sheared adenovirus type 5 DNA (Graham et al., 1977). HEK293 cells and various subclones derived from them have been employed extensively as a platform for the transient or stable heterologous expression of neuroreceptor and ion channel proteins (Thomas and Smart, 2005). Despite their putative origin in embryonic kidney tissue, HEK293 cells exhibit some characteristics of neurons. They express more than 60 neuronal genes including neurofilament proteins and neuroreceptor and ion channel subunits (Shaw et al., 2002; Thomas and Smart, 2005), and electrophysiological studies confirm the functional expression of endogenous voltage-gated calcium and potassium channels (Berjulow et al., 1996; Jiang et al., 2002). Moreover, studies in this laboratory identified a small TTX-sensitive sodium current in HEK293 cells that was associated primarily with the expression of the human Nav1.7 sodium channel isoform (He and Soderlund, 2010).
As a system for the heterologous expression of mammalian VGSCs, HEK293 cells avoid the problematic aspects of oocyte biology that limit the utility of the Xenopus oocyte system. The stable transformation and clonal selection of HEK293 cells provide immortalized cell lines expressing high levels of specific ion channel subunit combinations. These cell lines facilitate detailed pharmacological studies and are suitable biological substrates for high-throughput screening against ion channel targets. The principal limitation of HEK293 cells for the study of mammalian VGSCs lies in their endogenous expression, albeit at low levels, of sodium channel subunits and voltage-gated sodium currents. This problem can be overcome by a high level of heterologous expression, so that large whole-cell sodium currents recorded from transformed cells can be reliably attributed to the channels formed by heterologous expression of exogenous subunits.
Action of pyrethroids on rat Nav1.6 sodium channels
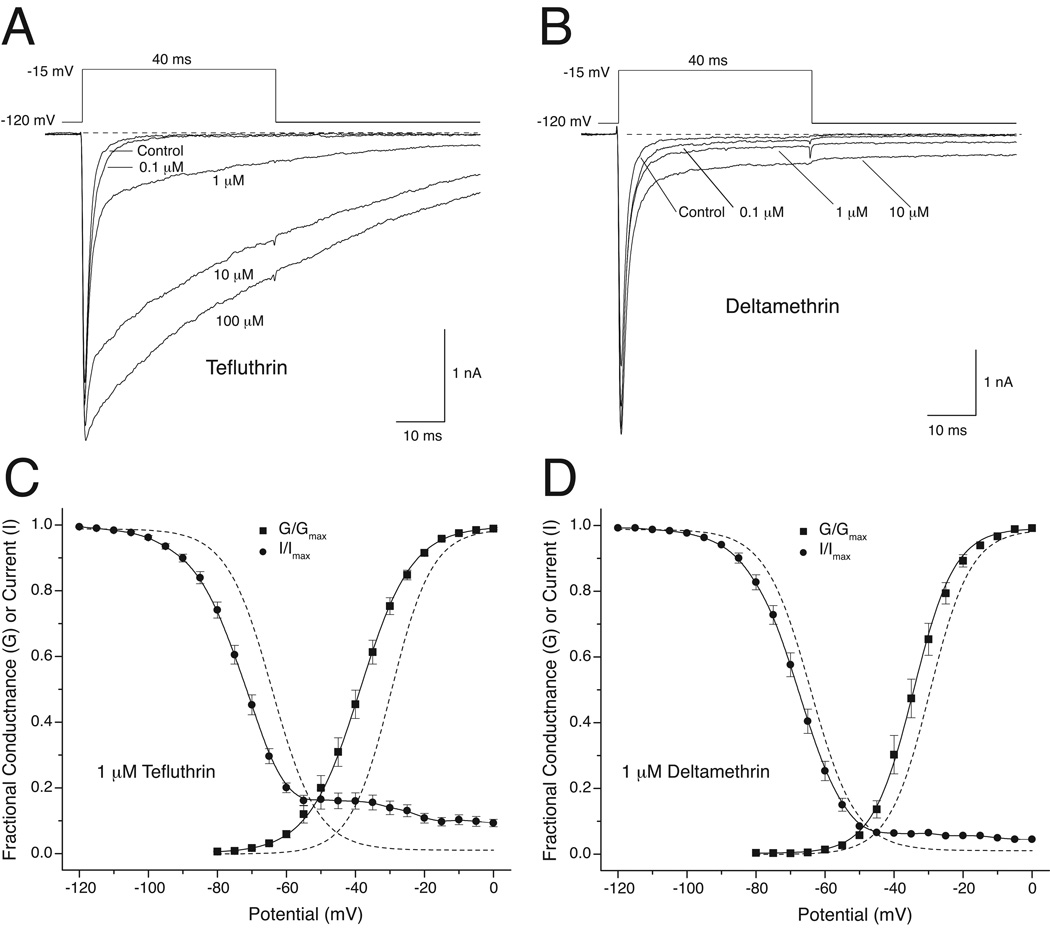
Figure 3 summarizes the action of tefluthrin and deltamethrin on Nav1.6+β1+β2 sodium channels expressed in HEK293 cells (He and Soderlund, 2011). Both insecticides caused persistent sodium currents during and after a depolarizing pulse (Fig. 3A and 3B). Whereas tefluthrin-induced currents decayed slowly (Fig. 3A), deltamethrin-induced currents did not exhibit any detectable decay (Fig. 3B). In contrast to their modification of Nav1.6+β1+β2 sodium channels expressed in oocytes, both tefluthrin (Fig. 3C) and deltamethrin (Fig. 3D) shifted the voltage dependence of both activation and steady-state inactivation to more hyperpolarized potentials. Both insecticides also created subpopulations of channels that were resistant to inactivation following very strong depolarizations. The impact of both insecticides on voltage-dependent activation and steady-state inactivation significantly enhanced sodium window currents. Thus, both pyrethroids, but especially tefluthrin, increased the probability of channel opening across a wide range of membrane potentials at which unmodified channels were refractory to activation.
Fig. 3.
Resting-state modification by tefluthrin and deltamethrin of rat Nav1.6+β1+β2 sodium channels expressed in HEK293 cells (redrawn from data in He and Soderlund, 2011). (A) Concentration-dependent modification of sodium currents cells by tefluthrin. Traces were recorded from a single cell prior to pyrethroid exposure (control) and following equilibration with increasing concentrations of pyrethroid. Dashed lines indicate zero current. (B) Concentration-dependent modification of sodium currents by deltamethrin. Traces were recorded from a single cell prior to pyrethroid exposure (control) and following equilibration with increasing concentrations of pyrethroid. Dashed lines indicate zero current. (C) Effects of 1 µM tefluthrin on sodium window currents in HEK293 cells expressing rat Nav1.6+β1+β2 sodium channels. (D) Effects of 1 µM deltamethrin on sodium window currents in HEK293 cells expressing rat Nav1.6+β1+β2 sodium channels. Panels C and D show voltage dependence plots for activation and steady-state inactivation in the presence of 1 µM pyrethroid (He and Soderlund, 2011; He and Soderlund, 2014). Dashed lines show the activation and inactivation curves recorded from HEK293 cells expressing rat Nav1.6+β1+β2 sodium channels in the absence of pyrethroid.
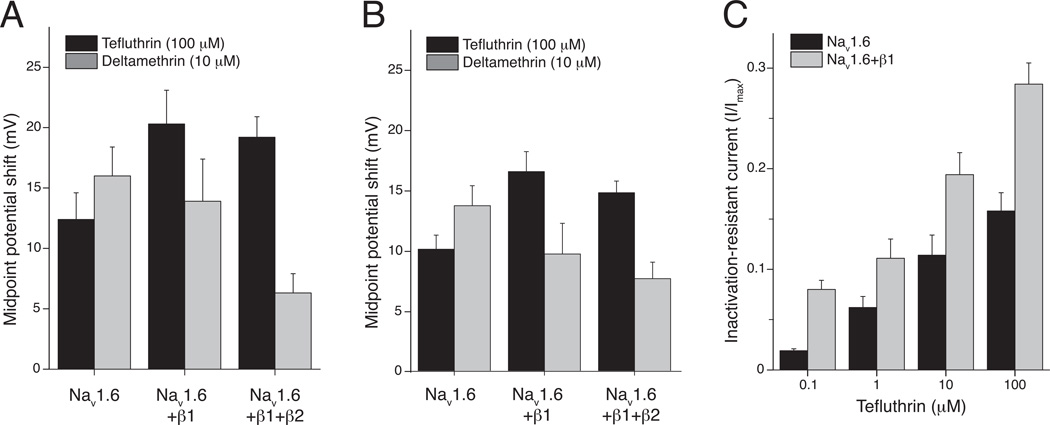
We explored the impact of the auxiliary β subunits on Nav1.6 channel modification by tefluthrin and deltamethrin by comparing the action of these insecticides on Nav1.6, Nav1.6+β1, or Nav1.6+β1+β2 channels (He and Soderlund, 2011; He and Soderlund, 2016) Coexpression with β subunits exerted opposite effects on the shifts in voltage-dependent gating of Nav1.6 channels caused by tefluthrin and deltamethrin. The β1 subunit increased the magnitude of the tefluthrin-dependent hyperpolarizing shifts in both activation (Fig. 4A) and steady-state inactivation (Fig. 4B) by approximately 1.5-fold, but the addition of the β2 subunit to form a ternary complex had no additional effect. In contrast to these results, coexpression with the β subunits decreased the magnitude of deltamethrin-dependent hyperpolarizing shifts in activation and steady-state inactivation (Fig. 4A and 4B). Coexpression with the β1 subunit also significantly increased the magnitude of the fraction of the tefluthrin-modified current that was resistant to inactivation at all tefluthrin concentrations examined (Fig. 4C) but had no effect on the magnitude of the inactivation-resistant component of the deltamethrin-modified current (not shown). Thus the opposite effects of coexpression with the β subunits on the voltage-dependent gating of tefluthrin- or deltamethrin modified channels contributed to the differences observed in the relative magnitudes of tefluthrin- and deltamethrin-induced window currents in assays with Nav1.6+β1+β2 channels (see Fig. 3C and 3D).
Fig. 4.
Effect of coexpression with β subunits on the modification of the voltage-dependent gating of rat Nav1.6 channels expressed in HEK293 cells by tefluthrin and deltamethrin (redrawn from data in He and Soderlund, 2011; He and Soderlund, 2016). (A) Effect of coexpression with β subunits on the magnitude of the shift in V0.5 values for Nav1.6 sodium channel activation caused by tefluthrin (100 µM) or deltamethrin (10 µM). (B) Effect of coexpression with β subunits on the magnitude of the shift in V0.5 values for Nav1.6 sodium channel inactivation caused by tefluthrin (100 µM) or deltamethrin (10 µM). Values in panels A and B were calculated by subtracting the mean control V0.5 values from the mean V0.5 values measured in the presence of insecticide; bars show SE values. (C) Effect of coexpression with the β 1 subunit on tefluthrin-induced, inactivation-resistant currents carried by Nav1.6 sodium channels expressed in HEK293 cells. Values are means ±SE of normalized fractional current (I/Imax) measured following conditioning depolarizations to 0 mV in either HEK293 cells expressing either Nav1.6 or Nav1.6+β1 sodium channels following exposure to four concentrations of tefluthrin.
The importance of use-dependent channel modification in the action of pyrethroids on both insect and mammalian channels expressed in Xenopus oocytes led us to examine use-dependent modification of rat Nav1.6 channels expressed in HEK293 cells and the impact of coexpression with the β1 and β2 subunits on use-dependent modification (He and Soderlund, 2011; He and Soderlund, 2016). In the HEK293 cell system the action of deltamethrin was enhanced by more than two-fold by repetitive depolarization, whereas the action of tefluthrin was unaffected (Fig. 5A). Comparison of the action of deltamethrin on Nav1.6, Nav1.6+β1, or Nav1.6+β1+β2 channels showed that use-dependent enhancement of modification required the presence of the β2 subunit in a heterotrimeric complex (Fig. 5B). It is possible that the β2 subunit alone is sufficient to confer use-dependent enhancement of modification by deltamethrin. However, we did not investigate this effect because there is no evidence for that sodium channels in neurons exist as α+β2 heterodimers.
Fig. 5.
Resting and use-dependent modification of rat Nav1.6 sodium channels expressed in HEK293 cells by pyrethroids (redrawn from data in He and Soderlund, 2011; He and Soderlund, 2016). (A) Effects of repeated 5-ms depolarizing prepulses delivered at 20 Hz on the extent of modification of sodium channels in HEK293 cells expressing Nav1.6+β1+β2 sodium channels by tefluthrin or deltamethrin. Values are the means of 6–19 determinations with different cells; bars show SE values larger than the data point symbols. (B) Effect of coexpression with the β1 and β2 subunits on the resting (0 prepulses) and use-dependent (100 prepulses) modification of Nav1.6 sodium channels by 10 µM deltamethrin. Values are means ± SE of 6–7 determinations with different cells; asterisk indicates a value for use-dependent modification significantly different from that for resting modification of the same channel (paired t-test, P < 0.05).
Conclusions
Effects of cellular expression context on Nav1.6 channel modification by pyrethroids
Our studies of the action of tefluthrin and deltamethrin on rat Nav1.6+β1+β2 channels expressed either in Xenopus oocytes (Tan and Soderlund, 2010) or HEK293 cells (He and Soderlund, 2011) provide an unique opportunity to assess the impact of the cellular context for heterologous expression on the properties of pyrethroid-modified channels. These studies employed identical sodium channel subunit sequences and sodium channel complex compositions as well as comparable experimental approaches to assess channel function and pyrethroid modification. Differences in the pharmacological properties of tefluthrin and deltamethrin in assays with Xenopus oocytes and HEK293 cells can therefore be reliably attributed to the cellular context in which these channels were expressed.
Our studies focused on three aspects of channel modification by pyrethroids: alteration of channel kinetics; effects on voltage-dependent gating; and, the relative significance of resting and use-dependent (i.e., open channel) modification. In both expression systems, tefluthrin and deltamethrin produced sodium currents with prolonged inactivation and deactivation kinetics. These modified currents are the hallmarks of pyrethroid action in native neurons and heterologous expression systems. In both systems, deltamethrin-induced currents were more persistent than tefluthrin-produced currents, but currents induced by both compounds were more persistent in assays using HEK293 cells than in assays using Xenopus oocytes.
In contrast to the similarity between expression systems in the kinetics of pyrethroid-modified Nav1.6 sodium channels, we identified marked differences between these systems in the impact of pyrethroids on voltage-dependent gating. Neither tefluthrin nor deltamethrin had a significant effect on the voltage dependence of channel activation or steady-state fast inactivation in the oocyte system; the only detectable effect was a small increase in the fraction of current that was refractory to inactivation following strong depolarizations. In HEK293 cells, unlike in oocytes, both pyrethroids caused substantial hyperpolarizing shifts in the voltage dependence of both activation and steady-state inactivation and also caused a large fraction of channels (up to 30% in the case of 100 µM tefluthrin; see Fig. 4C) to be resistant to voltage-dependent inactivation. The combined effects of pyrethroids on channel activation and inactivation in HEK293 cells produced large window currents, which describe an enhanced probability of persistent channel opening at membrane potentials at which unmodified channels are either closed or inactivated. Thus, in HEK293 cells tefluthrin and deltamethrin function as persistent activators of sodium channels in addition to their effects on the kinetics of channel inactivation and deactivation, but such effects are not observed for the same pyrethroid - channel combinations assayed in the Xenopus oocyte system.
The relative importance of resting and use-dependent modification of rat Nav1.6 sodium channels by pyrethroids also differed between expression systems. In oocytes, modification of Nav1.6 channels by deltamethrin required repetitive depolarization, indicating that this compound binds almost exclusively to open Nav1.6 channels in this system. Tefluthrin produced readily detectable modification of resting Nav1.6 channels, but repetitive depolarization significantly enhanced the extent of modification, increasing the apparent affinity of channels for this compound by ~15-fold. This result indicates that tefluthrin binds to both resting and open Nav1.6 channels but exhibits preference for binding to the open state. In contrast to the results obtained in oocytes, both tefluthrin and deltamethrin produced detectable resting modification of Nav1.6 channels expressed in HEK293 cells, but only in the case of deltamethrin was modification further enhanced by repeated depolarization. Based on results with these two pyrethroids, it appears that Nav1.6 channels expressed in oocytes exhibit a preference for open-state modification, whereas the same channels expressed in HEK293 cells may undergo more extensive modification in the resting state.
Modulation of pyrethroid action by β subunits
Indirect evidence suggests that Nav1.6 sodium channels in the rat CNS exist predominantly as heterotrimeric complexes with the auxiliary β1 and β2 subunits. Our studies of rat Nav1.6 sodium channels expressed either alone or in heteromultimeric complexes with the β subunits provide insight into the ways in which the auxiliary subunits shape pyrethroid action and the influence of cellular expression context on those effects.
In oocytes coexpression with β subunits, and in particular the β1 subunit, was necessary for use-dependent modification by tefluthrin. Neither Nav1.6 channels nor Nav1.6+β2 channels exhibited enhanced modification upon repeated depolarization, and inclusion of the β2 subunit in Nav1.6+β1+β2 channel complexes did not further modify the effects of the β1 subunit. This effect is not limited to Nav1.6 sodium channels; the rat Nav1.3 sodium channel isoform also did not exhibit use-dependent enhancement of modification by tefluthrin unless expressed with the β1 and β2 subunits (Tan and Soderlund, 2011b). In HEK293 cells coexpression with β subunits was also required to observe use-dependent enhancement of the modification of Nav1.6 sodium channels by deltamethrin. In this case, however, use-dependent effects required the presence of the β2 subunit in Nav1.6+β1+β2 channel complexes whereas coexpression with only the β1 subunit did not confer use-dependent effects. The mechanism by which auxiliary β subunits indirectly facilitate binding of some pyrethroids to pyrethroid receptor domains on the open state of sodium channel α subunits remains to be determined.
In addition to effects on use-dependent modification, assays in the HEK293 cell system revealed substantial and compound-specific effects of β subunits on voltage-dependent gating that were not observed in the Xenopus oocyte system. Coexpression of Nav1.6 channels with the β subunits increased the magnitude of tefluthrin-dependent hyperpolarizing shifts in the voltage dependence of activation and steady-state inactivation but decreased the magnitude of the hyperpolarizing shifts in activation and steady-state inactivation caused by deltamethrin. Coexpression with β1 subunit also increased the fraction of tefluthrin-modified Nav1.6 channels that were resistant to inactivation but had no corresponding effect on deltamethrin-modified channels.
Taken together, our results identify important but unpredictable effects of auxiliary β subunits on the pharmacology of pyrethroid action on VGSCs. The effects of β subunits vary depending on cellular expression context, and also vary depending on the pyrethroid examined. These results underscore the need to reconstitute complete sodium channel complexes to accurately assess channel modification by pyrethroids.
Heterologous expression systems as neuron surrogates
The ultimate value of heterologous expression systems depends on their ability to mimic the functional and pharmacological properties of VGSCs in their native neuronal environment. It is therefore important to ask how pyrethroid action on mammalian VGSCs expressed either in Xenopus oocytes or HEK293 cells compares to the action of pyrethroids on neuronal sodium channels. There are surprisingly few studies available in the literature of the action of pyrethroids on VGSCs in mammalian neurons. However, the principal features of pyrethroid action on Nav1.6 channels expressed in HEK293 cells, including the large shifts in voltage-dependent gating that are not observed for the same channels expressed in Xenopus oocytes, are largely consistent with the action of pyrethroids on sodium channels in mammalian neurons (Tatebayashi and Narahashi, 1994; Tabarean and Narahashi, 1998; Wu et al., 2009). Thus, our data suggest that the HEK293 cell system is a more appropriate model than the Xenopus oocyte system for studies of pyrethroid action on mammalian VGSCs.
Research Highlights.
Mammalian neurons express multiple sodium channel subunit complexes.
Reconstitution in vitro allows the study of individual subunits and complexes.
Xenopus oocytes and HEK293 yield channels with different properties.
Channels in HEK293 cells exhibit properties similar to channels in neurons.
Acknowledgments
This work was supported in part by a grant (R01-ES013686) from the National Institute of Environmental Health Sciences, National Institutes of Health. The contents of this paper are solely the responsibility of the authors and do not necessarily represent the official views of the National Institute of Environmental Health Sciences.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This paper is based on a contribution to the symposium “Insecticide Action on Ion Channels: A Tribute to Toshio Narahashi” in the Division of Agrochemicals at the 250th American Chemical Society National Meeting on August 16, 2015.
Conflict of Interest Statement
The authors declare that they have no conflicts of interest with regard to sources of funding for this research or the design and interpretation of the experiments described herein.
References
- Auld VJ, Goldin AL, Krafte DS, Marshall J, Dunn JM, Catterall WA, Lester HA, Davidson N, Dunn RJ. A rat brain Na+ channel a subunit with novel gating properties. Neuron. 1988;1:449–461. doi: 10.1016/0896-6273(88)90176-6. [DOI] [PubMed] [Google Scholar]
- Berjulow S, Doring F, Froschmayr M, Grabner M, Glossmann H, Hering S. Endogenous calcium channels in human embryonic kidney (HEK293) cells. British Journal of Pharmacology. 1996;118:748–754. doi: 10.1111/j.1476-5381.1996.tb15463.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloomquist JR. Neuroreceptor mechanisms in pyrethroid mode of action and resistance. In: Roe M, Kuhr RJ, editors. Reviews in Pesticide Toxicology. Raleigh, NC: Toxicology Communications; 1993. pp. 181–226. [Google Scholar]
- Caldwell JH, Schaller KL, Lasher RS, Peles E, Levinson SR. Sodium channel Nav1.6 is localized nodes of Ranvier, dendrites, and synapses. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:5616–5620. doi: 10.1073/pnas.090034797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J-S, Soderlund DM. Structure-activity relationships for the action of 11 pyrethroid insecticides on rat Nav1.8 sodium channels expressed in Xenopus oocytes. Toxicology and Applied Pharmacology. 2006;211:233–244. doi: 10.1016/j.taap.2005.06.022. [DOI] [PubMed] [Google Scholar]
- Felts PA, Yokoyama S, Dib-Hajj S, Black JA, Waxman SG. Sodium channel a-subunit mRNAs I, II, III, NaG, Na6 and hNE (PN1): different expression patters in developing rat nervous system. Molecular Brain Research. 1997;45:71–82. doi: 10.1016/s0169-328x(96)00241-0. [DOI] [PubMed] [Google Scholar]
- Ginsburg KS, Narahashi T. Differential sensitivity of tetrodotoxin-sensitive and tetrodotoxin-resistant sodium channels to the insecticide allethrin in rat dorsal root ganglion neurons. Brain Research. 1993;627:239–248. doi: 10.1016/0006-8993(93)90326-i. [DOI] [PubMed] [Google Scholar]
- Goldin AL. Resurgence of sodium channel research. Annual Review of Physiology. 2001;63:871–894. doi: 10.1146/annurev.physiol.63.1.871. [DOI] [PubMed] [Google Scholar]
- Goldin AL. Evolution of voltage-gated Na+ channels. Journal of Experimental Biology. 2002;205:575–584. doi: 10.1242/jeb.205.5.575. [DOI] [PubMed] [Google Scholar]
- Goldin AL. Expression of ion channels in Xenopus oocytes. In: Clare JJ, Trezise DJ, editors. Expression and analysis of recombinant ion channels. KGaA, Weinheim: Wiley VCH Verlag GmbH & Co; 2006. pp. 1–25. [Google Scholar]
- Graham FL, Smiley J, Russell WC, Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. Journal of General Virology. 1977;36:59–77. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- Harrill JA, Meacham CA, Shafer TJ, Hughes MF, Crofton KM. Time and concentration dependent accumulation of [3H]-deltamethrin in Xenopus laevis oocytes. Toxicology Letters. 2005;157:79–88. doi: 10.1016/j.toxlet.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Hartshorne RP, Catterall WA. The sodium channel from rat brain: purification and subunit composition. Journal of Biological Chemistry. 1984;259:1667–1685. [PubMed] [Google Scholar]
- He B, Soderlund DM. Human embryonic kidney (HEK293) cells express endogenous voltage-gated sodium currents and Nav1.7 sodium channels. Neuroscience Letters. 2010;469:268–272. doi: 10.1016/j.neulet.2009.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B, Soderlund DM. Differential state-dependent modification of rat Nav1.6 sodium channels expressed in human embryonic kidney (HEK293) cells by the pyrethroid insecticides tefluthrin and deltamethrin. Toxicology and Applied Pharmacology. 2011;257:377–387. doi: 10.1016/j.taap.2011.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B, Soderlund DM. Functional expression of rat Nav1.6 voltage-gated sodium channels in HEK293 cells: modulation by the auxiliary β1 subunit. PLoS One. 2014;9:e85188. doi: 10.1371/journal.pone.0085188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B, Soderlund DM. Effects of the β1 auxiliary subunit on modification of rat Nav1.6 sodium channels expressed in HEK293 cells by the pyrethroid insecticides tefluthrin and deltamethrin. Toxicology and Applied Pharmacology. 2016;291:58–69. doi: 10.1016/j.taap.2015.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W, Tian C, Yang M, Hou H, Shu Y. Distinct contributions of Nav1.6 and Nav1.2 in action potential initiation and backpropagation. Nature Neuroscience. 2009;12:996–1002. doi: 10.1038/nn.2359. [DOI] [PubMed] [Google Scholar]
- Isom LL. Sodium channel β subunits: anything but auxiliary. Neuroscientist. 2001;7:42–54. doi: 10.1177/107385840100700108. [DOI] [PubMed] [Google Scholar]
- Jiang B, Sun X, Cao K, Wang R. Endogenous Kv channels in human embryonic kidney (HEK-293) cells. Molecular and Cellular Biochemistry. 2002;238:69–79. doi: 10.1023/a:1019907104763. [DOI] [PubMed] [Google Scholar]
- Meacham CA, Brodfuehrer PD, Watkins JA, Shafer TJ. Developmentally-regulated sodium channel subunits are differentially sensitive to α-cyano containing pyrethroids. Toxicology and Applied Pharmacology. 2008;231:273–281. doi: 10.1016/j.taap.2008.04.017. [DOI] [PubMed] [Google Scholar]
- Narahashi T. Neuronal ion channels as the target sites of insecticides. Pharmacology & Toxicology. 1996;78:1–14. doi: 10.1111/j.1600-0773.1996.tb00234.x. [DOI] [PubMed] [Google Scholar]
- Rinkevich FD, Du Y, Dong K. Diversity and convergence of sodium channel mutations involved in resistance to pyrethroids. Pestic. Biochem. Physiol. 2013;106:93–100. doi: 10.1016/j.pestbp.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller KL, Caldwell JH. Expression and distribution of voltage-gated sodium channels in the cerebellum. Cerebellum. 2003;2:2–9. doi: 10.1080/14734220309424. [DOI] [PubMed] [Google Scholar]
- Shah BS, Stevens EB, Pinnock RD, Dixon AK, Lee K. Developmental expression of the novel voltage-gated sodium channel auxiliary subunit b3, in rat CNS. Journal of Physiology. 2001;534:763–776. doi: 10.1111/j.1469-7793.2001.t01-1-00763.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw G, Morse S, Ararat M, Graham FL. Preferential transformation of human neuronal cells by human adeonviruses and the origin of HEK 293 cells. FASEB Journal. 2002;16:869–871. doi: 10.1096/fj.01-0995fje. [DOI] [PubMed] [Google Scholar]
- Smith TJ, Soderlund DM. Action of the pyrethroid insecticide cypermethrin on rat brain IIa sodium channels expressed in Xenopus oocytes. NeuroToxicology. 1998;19:823–832. [PubMed] [Google Scholar]
- Smith TJ, Soderlund DM. Potent actions of the pyrethroid insecticides cismethrin and cypermethrin on rat tetrodotoxin-resistant peripheral nerve (SNS/PN3) sodium channels expressed in Xenopus . oocytes. Pestic. Biochem. Physiol. 2001;70:52–61. doi: 10.1002/(SICI)1520-6327(1998)38:3<126::AID-ARCH3>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Soderlund DM. Mode of action of pyrethrins and pyrethroids. In: Casida JE, Quistad GB, editors. Pyrethrum Flowers: Production, Chemistry, Toxicology, and Uses. New York: Oxford University Press; 1995. pp. 217–233. [Google Scholar]
- Soderlund DM. Sodium channels. In: Gilbert L, Iatrou K, Gill S, editors. Comprehensive Molecular Insect Science. New York: Elsevier; 2005. pp. 1–24. [Google Scholar]
- Soderlund DM. State-dependent modification of voltage-gated sodium channels by pyrethroids. Pestic. Biochem. Physiol. 2010;97:78–86. doi: 10.1016/j.pestbp.2009.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderlund DM, Knipple DC. The molecular biology of knockdown resistance to pyrethroid insecticides. Insect Biochemistry and Molecular Biology. 2003;33:563–577. doi: 10.1016/s0965-1748(03)00023-7. [DOI] [PubMed] [Google Scholar]
- Soderlund DM, Lee SH. Point mutations in homology domain II modify the sensitivity of rat Nav1.8 sodium channels to the pyrethroid cismethrin. Neurotoxicology. 2001;22:755–765. doi: 10.1016/s0161-813x(01)00065-1. [DOI] [PubMed] [Google Scholar]
- Song J-H, Nagata K, Tatebayashi H, Narahashi T. Interactions of tetramethrin, fenvalerate and DDT at the sodium channel in rat dorsal root ganglion neurons. Brain Research. 1996;708:29–37. doi: 10.1016/0006-8993(95)01239-7. [DOI] [PubMed] [Google Scholar]
- Tabarean IV, Narahashi T. Potent modulation of tetrodotoxin-sensitive and tetrodotoxin-resistant sodium channels by the Type II pyrethroid deltamethrin. Journal of Pharmacology and Experimental Therapeutics. 1998;284:958–965. [PubMed] [Google Scholar]
- Tan J, Soderlund DM. Human and rat Nav1.3 voltage-gated sodium channels differ in inactivation properties and sensitivity to the pyrethroid insecticide tefluthrin. Neurotoxicology. 2009;30:81–89. doi: 10.1016/j.neuro.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan J, Soderlund DM. Divergent actions of the pyrethroid insecticides S-bioallethrin, tefluthrin, and deltamethrin on rat Nav1.6 sodium channels. Toxicology and Applied Pharmacology. 2010;247:229–237. doi: 10.1016/j.taap.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan J, Soderlund DM. Action of tefluthrin on rat Nav1.7 voltage-gated sodium channels expressed in Xenopus . oocytes. Pestic. Biochem. Physiol. 2011a;101:21–26. doi: 10.1016/j.pestbp.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan J, Soderlund DM. Coexpression with auxiliary β subunits modulates the action of tefluthrin on rat Nav1.6 and Nav1.3 sodium channels. Pestic. Biochem. Physiol. 2011b;101:256–264. doi: 10.1016/j.pestbp.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatebayashi H, Narahashi T. Differential mechanism of action of the pyrethroid tetramethrin on tetrodotoxin-sensitive and tetrodotoxin-resistant sodium channels. Journal of Pharmacology and Experimental Therapeutics. 1994;270:595–603. [PubMed] [Google Scholar]
- Thomas P, Smart TG. HEK293 cell line: a vehicle for the expression of recombinant proteins. Journal of Pharmacological and Toxicological Methods. 2005;51:187–200. doi: 10.1016/j.vascn.2004.08.014. [DOI] [PubMed] [Google Scholar]
- Vais H, Atkinson S, Eldursi N, Devonshire AL, Williamson MS, Usherwood PNR. A single amino acid change makes a rat neuronal sodium channel highly sensitive to pyrethroid insecticides. FEBS Letters. 2000;470:135–138. doi: 10.1016/s0014-5793(00)01305-3. [DOI] [PubMed] [Google Scholar]
- Whitaker WRJ, Clare JJ, Powell AJ, Chen YH, Faull RLM, Emson PC. Distribution of voltage-gated sodium channel α-subunit and β-subunit mRNAs in human hippocampal formation, cortex, and cerebellum. Journal of Comparative Neurology. 2000;422:123–139. doi: 10.1002/(sici)1096-9861(20000619)422:1<123::aid-cne8>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Whitaker WRJ, Faull RLM, Waldvogel HJ, Plumpton CJ, Emson PC, Clare JJ. Comparative distribution of voltage-gated sodium channel proteins in human brain. Molecular Brain Research. 2001;88:37–53. doi: 10.1016/s0169-328x(00)00289-8. [DOI] [PubMed] [Google Scholar]
- Wu S-N, Wu Y-H, Chen B-S, Liu Y-C. Underlying mechanism of action of tefluthrin, a pyrethroid insecticide, on voltage-gated ion currents and on action currents in pituitary tumor (GH3) cells and GnRH-secreting (GT1-7) neurons. Toxicology. 2009;2009:70–77. doi: 10.1016/j.tox.2009.01.009. [DOI] [PubMed] [Google Scholar]