Abstract
Meiosis is a key event of sexual life cycles in eukaryotes. Its mechanistic details have been uncovered in several model organisms, and most of its essential features have received various and often contradictory evolutionary interpretations. In this perspective, we present an overview of these often ‘weird’ features. We discuss the origin of meiosis (origin of ploidy reduction and recombination, two-step meiosis), its secondary modifications (in polyploids or asexuals, inverted meiosis), its importance in punctuating life cycles (meiotic arrests, epigenetic resetting, meiotic asymmetry, meiotic fairness) and features associated with recombination (disjunction constraints, heterochiasmy, crossover interference and hotspots). We present the various evolutionary scenarios and selective pressures that have been proposed to account for these features, and we highlight that their evolutionary significance often remains largely mysterious. Resolving these mysteries will likely provide decisive steps towards understanding why sex and recombination are found in the majority of eukaryotes.
This article is part of the themed issue ‘Weird sex: the underappreciated diversity of sexual reproduction’.
Keywords: genetic conflict, modified meiosis, origin of meiosis, epigenetics, recombination hotspots, automixis
1. Introduction
In eukaryotic sexual life cycles, haploid cells fuse to give rise to diploids, before diploid cells are converted back to haploids in a process known as meiosis. Meiosis reduces a cell's chromosome number by half, while also creating new allele combinations distributed across daughter cells through segregation and recombination. This genetic reshuffling reduces genetic associations within and between loci and is thought to be the basis of the success of sexual reproduction. Mechanistic studies of meiosis have been carried out in different fields, such as cell biology, genetics and epigenetics, encompassing a wide range of eukaryotes. However, these studies rarely focus on the evolutionary significance of meiotic mechanisms, rather mentioning them in passing and often in a simplified manner. In evolutionary biology studies, meiosis is often simplified and represented by random assortment of chromosomes and recombination maps expressing the probability of recombination events between ordered loci, with little attention to the molecular and cellular details. While these simplifications are legitimate and useful in many cases, the wealth of mechanistic findings being uncovered points to a considerable number of evolutionary puzzles surrounding meiosis that have yet to be resolved. Indeed, in the following perspective, we will show that close scrutiny of almost every aspect of meiosis will reveal ‘weird’ features that constitute evolutionary mysteries.
2. The origins of meiosis
The origin of meiosis through gradual steps is among the most intriguing evolutionary enigmas [1,2]. Meiosis is one of the ‘major innovations’ of eukaryotes that evolved before their subsequent radiation over 1 billion years ago [3–5]. Extant eukaryotes share a set of genes specifically associated with meiosis, implying that it evolved only once before their last common ancestor [6,7]. Identifying the selective scenario that led to its early evolution is difficult, but clues can be obtained by determining (i) which mitotic cellular processes were reused in meiosis (e.g. DNA repair through homologous recombination and possibly reduction), (ii) which selective steps were involved in the assembly of the full cellular process, and (iii) why different forms of meiosis were perhaps less successful.
(a). The origin of ploidy reduction
A form of reductional cell division (aka ‘proto-meiosis’) probably evolved in early asexual unicellular eukaryotes. Two scenarios for this have been proposed. The first is that diploidy accidentally occurred by replication of the nuclear genome without subsequent cell division (endoreplication) [8–12], and that returning to haploidy was selected for to correct this. Because either haploidy or higher ploidy levels may be favoured in different ecological situations [13,14], a variant of this scenario is that a proto-meiosis–endoreplication cycle evolved to switch between ploidy levels [5]. The resulting life cycle may have resembled modern ‘parasexual’ fungi in which diploid cells lose chromosomes in subsequent mitotic divisions, leading to haploidy via aneuploid intermediates [15]. Many other modern eukaryotes also increase and decrease their ploidy somatically, depending on growth stage or specific environmental stimuli [16]. The second scenario is that proto-meiosis evolved in response to the fusion of two haploid cells (syngamy), as in standard modern eukaryotic sexual life cycles. Syngamy may have been favoured because it allows recessive deleterious mutations to be masked in diploids [1,12]. A difficulty with this idea is that such masking may not be sufficient to favour diploidy in asexuals [17]. In a variant of this scenario, early syngamy evolved as a result of ‘manipulation’ by selfish elements (plasmids, transposons) to promote their horizontal transmission [18]. In support of this view, mating-type switching (which can allow syngamy in haploid colonies) has evolved multiple times in yeasts and involves domesticated mobile genetic elements [19].
(b). The origin of homologue pairing and meiotic recombination
Meiosis requires the correct segregation of homologues, which is achieved by homologue pairing at the beginning of prophase I (figure 1). This homology search is mediated by the active formation of numerous DNA double-strand breaks (DSBs) followed by chiasmata formation, but less well-known mechanisms of recombination-independent pairing also exist [20]. Non-homologous centromere coupling is also often observed at this stage, but the functional and evolutionary significance of this coupling is elusive [21]. In many species, chromosome pairing is further strengthened by ‘synapsis’, which is the formation of a protein structure known as the synaptonemal complex [22] and the pairing of homologous centromeres [21]. Chiasmata are then resolved as either crossovers (hereafter ‘COs’) resulting in the exchange of large chromatid segments, or non-crossovers (NCOs), where both situations cause gene-conversion events [23]. The synaptonemal complex then disappears, and homologues remain tethered at CO positions and centromeres. The precise function of the synaptonemal complex is not entirely understood [20]; one possibility is that it may serve to stabilize homologues during CO maturation. Some pairing mechanism must be advantageous to ensure proper segregation of homologues, but the origins and selective advantage of extensive pairing, synapsis, gene conversion and recombination remain poorly understood [24].
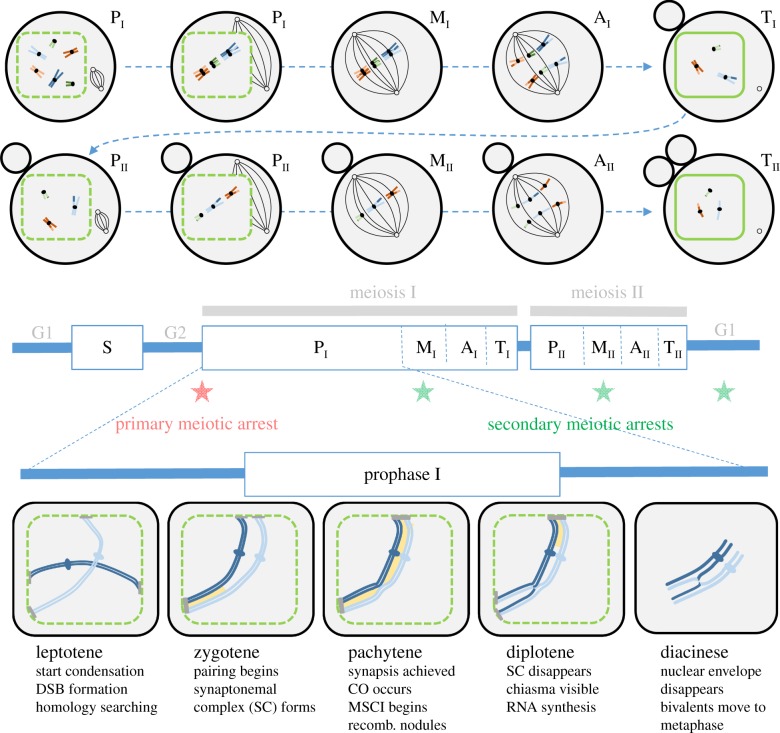
Figure 1.
Schematic of the different steps in standard meiosis. The top panel illustrates the different phases of a typical female meiosis for each of the two meiotic divisions: prophase (P, with early and late prophase distinguished), metaphase (M), anaphase (A) and telophase (T). The nuclear membrane is indicated by the green contour (dashed when it starts fragmenting). The small black circles represent microtubule organizing centres and the black lines represent microtubules of the meiotic spindle. First and second polar bodies are shown as grey circles next to the oocyte (chromosomes inside the polar bodies are not shown). Homologous chromosomes are represented with the same colour with slightly different shades (e.g. orange and light orange). Homologues pair and segregate in meiosis I, then sister chromatids segregate in meiosis II. The middle panel shows the meiotic cell cycle. The timing of the primary meiotic arrest is indicated by a red star, while the timing of the most common secondary arrests in different organisms is indicated by green stars (see §4a). The lower panel indicates the important steps (DSB formation, crossing overs) occurring during prophase I. The synaptonemal complex is shown in yellow. Chromatin condenses in chromosomes throughout prophase I (only one pair of homologues is illustrated). In most species, telomeres attach to the nuclear envelope. The attachment plate is indicated by a grey bar. MSCI, meiotic sex chromosome inactivation (see §4d).
Most evidence suggests that homologous recombination evolved long before meiosis, as it occurs in all domains of life and involves proteins that share strong homology [25,26]. One hypothesis is that meiotic pairing and extensive homologous recombination in meiosis evolved to avoid the burden and consequences of non-allelic ectopic recombination in the large genomes of early eukaryotes, which presumably had many repetitive sequences [9,27,28]. Such sequences may have been related to the spread of retrotransposons in early eukaryotes, of which many types are very ancient in eukaryotes, but absent in bacteria and archaea [29]. A second possibility is that recombination arose by the spread of self-promoting genetic elements exploiting the machinery of DNA repair and associated gene conversion [30]. Another hypothesis is that pairing and recombination initially arose as a way to repair mutational damage caused by increased oxidative stress due to rising atmospheric oxygen or endosymbiosis [7,31–33]. This scenario presupposes that DNA maintenance is inefficient in the absence of meiosis; however, prokaryotes (including archaea) have efficient repair mechanisms that involve recombination, but not meiosis [9]. In addition, this scenario does not fit well with the observation that a large number of DSBs are actively generated at the onset of meiosis [1,34].
(c). The origin of two-step meiosis
A particular feature of meiosis is that it starts with chromosome doubling (S phase; figure 1) before meiosis occurs (figure 2a). For ploidy reduction, the initial steps appear superfluous [35]. A simpler single-step cell division, without the initial DNA replication phase, could in principle achieve ploidy reduction (figure 2b). Recombination may not be a crucial difference between one- and two-step meiosis, as both can involve COs, even if with one CO, the two meiotic products carry recombinant chromosomes in one-step meiosis, whereas only two out of four are recombinant in two-step meiosis [36]. Three hypotheses have been proposed to account for two-step meiosis. The first postulates that two-step meiosis better protects against particular selfish genetic elements (SGEs) that increase their transmission frequencies by sabotaging the meiotic products in which they do not end up (known as ‘sister killers’, distinct from the ‘sperm killers’ discussed below) [37]. In a two-step meiosis, there is uncertainty as to whether the reductional division is meiosis I or II, meaning that the sabotage mechanism has a much reduced efficacy. Microsporidia and red algae show specific modifications to meiosis that increase such uncertainty even more [38]. However, such sister killers are hypothetical, and theoretical studies based on assumptions about how different killers might act suggest that this mechanism does not inevitably promote the development of a two-step meiosis [39]. The second hypothesis is that sexual species with one-step meiosis would be vulnerable to invasion by asexual mutants, and have thus gone disproportionally extinct in the past. Contrary to one-step meiosis, most automictic modifications of two-step meiosis involve a loss of heterozygosity with each generation (see §3c), which would cause expression of recessive and partially recessive deleterious mutational effects, and reduce the fitness of newly emerging asexual mutants [36]. Finally, a third hypothesis posits that a one-step meiosis is more complex and thus less likely to evolve than a two-step meiosis [9]. Mitotic and meiotic cell cycles start similarly with DNA replication in response to increasing cyclin-dependent kinase (CDK) activity. Two-step meiosis can be achieved simply by modulating CDK activity at the end of a cell cycle to add a second division event [40]. By contrast, a one-step meiosis would require extensive modification of the mitotic cycle. Despite earlier suggestions of its presence in some basal eukaryotes (protists) [8,41], there are presently no firm indications that one-step meioses exist in nature [38,42], although inverted meiosis (see below) is genetically similar to mitosis followed by single-step meiosis.
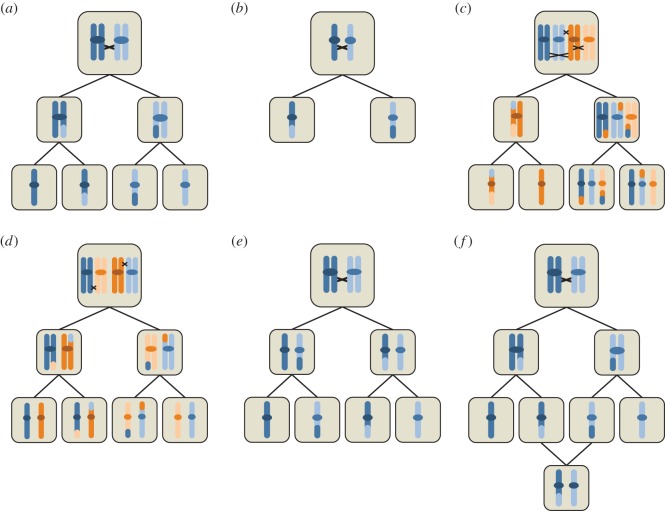
Figure 2.
Schematic of meiosis and some of its modifications. (a) Regular meiosis. Following DNA replication, homologous chromosomes are separated in the first meiotic division, whereas sister chromatids are separated in the second division. COs result in chromosomes in the final meiotic products that carry genetic material from both homologous chromosomes. (b) Hypothetical ‘one-step’ meiosis, in which DNA replication before entering meiosis is suppressed and, therefore, only a single meiotic division is required. (c) Multivalent formation in a neo-tetraploid. Blue and orange chromosome pairs are assumed to be identical or very similar so that pairing can occur. Chiasmata of one chromosome with three other chromosomes leads to mis-segregation. (d) Bivalent formation in a tetraploid with exactly one CO per chromosome. Chromosomes may pair randomly (leading to polysomic inheritance), but segregation proceeds normally. (e) Inverted meiosis, in which sister chromatids are separated in the first division and homologous chromosomes in the second division. Note that although centromeres are shown here for clarity, all described species consistently using inverted meiosis are holokinetic (no centromeres). (f) Central fusion automixis, a mechanism of producing diploid eggs that can then develop parthenogenetically without fertilization. As a consequence of COs, heterozygosity may be lost with this mechanism in regions distal to the centromere.
3. Secondary modifications of meiosis
Meiosis is remarkably conserved across eukaryotes. Nevertheless, in many species, variants exist that may offer insights into the evolutionary origins and mechanistic constraints of meiosis. Here, we discuss three of these modifications: meiosis in polyploids, inverted meiosis and meiosis in asexual organisms.
(a). Meiosis and polyploidy
Polyploidy is surprisingly common in eukaryotes given the considerable problems it poses to meiosis [43–45]. In diploids, homologous chromosomes recognize each other and align to form bivalents during Prophase I, but when there are three or more chromosomes with sufficient homology, these chromosomes may all align to varying degrees, forming multivalents. This can occur when all chromosome sets originate from the same species (autopolyploidy), and also when polyploidy is a result of hybridization (allopolyploidy). Multivalent formation is often associated with mis-segregation of chromosomes (figure 2c) as well as chromosomal rearrangements arising from recombination within multivalents, leading to reduced fertility and low-fitness offspring (e.g. [46–48]). These problems may be compounded in allopolyploids because recombination homogenises partially differentiated chromosomes, thereby further increasing the likelihood that they will pair (the ‘polyploid ratchet’ [49]).
Given these detrimental effects, the existence of successful polyploid species and lineages indicates that natural selection can often promote transitions from multi- to bivalents that will then segregate as in diploids (cf. figure 2c,d) (e.g. [50,51]). However, how such transitions are achieved at the molecular level remains a mystery. Part of the answer seems to be a reduction in the number of COs, since multivalents can only form with at least two COs per chromosome [51–53]. This mechanism seems particularly important in autopolyploids and may be achieved through increasing CO interference (see §5b for definition) [54]. Several candidate genes that may effect such modifications have been identified in the autotetraploid Arabidopsis arenosa [51,55]. In allopolyploids, there is evidence for genes that have been selected to strengthen the preferential pairing of homologous (i.e. of the same origin, rather than ‘homeologous’) chromosomes, including ph1 in hexaploid wheat [56]. This preferential pairing can also be achieved through reducing CO numbers, but specifically those between homeologues; this could indirectly produce an increase in CO numbers and hence recombination rates between homologues [43]. Intriguingly, because most extant organisms have a history of polyploidy, many features of ‘standard’ meiosis such as CO interference may have been shaped by the problems involved in multivalent segregation.
Polyploidy with odd numbers of chromosome sets poses an even greater problem because aneuploid gametes are generally produced (e.g. [57]). However, there are some plant species where solutions to even this problem have evolved, and where odd-number polyploidy appears to persist in a stable manner. In these species, the problem of unequal segregation during meiosis is solved through exclusion of univalents in one sex but inclusion in the other, leading, for example, to haploid sperm and tetraploid eggs in pentaploid dog roses [58].
(b). Inverted meiosis
In normal meiosis, homologous chromosomes are separated during meiotic division I, whereas sister chromatids are separated during meiosis II. Why meiosis generally follows this order is unknown, but interestingly, in some species meiosis takes place in the reverse order (figure 2e), including some flowering plants [59–61], mites [62], true bugs [63] and mealybugs [64]. All species with this ‘inverted’ meiosis described to date seem to have holocentric chromosomes (i.e. the kinetochores are assembled along the entire chromosome, rather than at localized centromeres). Inverted meiosis is viewed as a possible solution to specific problems of kinetochore geometry in such meiosis [65]. Yet, intriguing as they are, these systems provide little insight into why inverted meiosis is absent or very rare in monocentric species.
It is conceivable that a reverse order of divisions would make meiosis more vulnerable to exploitation by meiotic drive or sister killer SGEs, but to the best of our knowledge, there is currently neither theoretical nor empirical support for this idea. Another possibility is that meiosis I tends to be reductional because it allows for DSB repair by sister chromatid exchange in arrested female meiosis [66]. Alternatively, the order of meiotic divisions could merely be a ‘frozen accident’, i.e. a solution that has been arrived at a long time ago by chance, and that reversal is difficult (at least with monocentric chromosomes). However, a recent paper investigating human female meioses in unprecedented detail casts doubt on this view [67]. The careful genotyping of eggs (or embryos) and polar bodies at many markers indicated that surprisingly often, chromosomes followed an ‘inverted meiosis’ pattern of segregation, even though this led to aneuploidies in approx. 23% of cases. The question of why one order of meiotic divisions is almost universal, therefore, remains unresolved.
(c). Meiosis modifications and loss of sex
Many organisms have abandoned canonical sexual reproduction, reproducing asexually by suppressing or modifying meiosis and producing diploid eggs that can develop without fertilization. This raises two connected mysteries: why are some types of modifications much more frequent than others, and how can mitotic (or mitosis-like) asexual reproduction (‘apomixis’ or ‘clonal parthenogenesis’ in animals, ‘mitotic apomixis’ in plants) evolve from meiosis? Examples of meiosis-derived modes of asexual reproduction include chromosome doubling prior to meiosis (‘endomitosis’ or ‘pre-meiotic doubling’), fusion of two of the four products of a single meiosis (‘automixis’ in animals, ‘within-tetrad mating’ in fungi), and suppression of one of the two meiotic divisions (included under ‘automixis’ or ‘meiotic apomixis’, depending on the author; see [68–74] for detailed descriptions of these processes).
Two particularly common modes of asexuality are the suppression of meiosis I, and automixis involving fusing meiotic products that were separated during meiosis I (‘central fusion’, figure 2f). Both are genetically equivalent and lead to reduced heterozygosity when there is recombination between a locus and the centromere of the chromosome on which it is located. Most other forms of meiosis-derived asexual reproduction lead to a much stronger reduction in offspring heterozygosity [75–79], and it has been hypothesized that the reduced fitness of homozygous progeny explains the rarity of these other forms [71,78,80]. Indirect support comes from the observation that species with regular asexual reproduction usually do so by central fusion or suppression of meiosis I, often accompanied by very low levels of recombination, thus maintaining heterozygosity. By contrast, species that only rarely reproduce asexually show a wider variety of asexual modes and higher levels of recombination [1,71,73,81,82]. Nonetheless, this hypothesis cannot explain some observations, for instance, the rarity of pre-meiotic doubling with sister-chromosome pairing, which would also efficiently maintain heterozygosity [71]. Perhaps, evolving a mechanism that ensures exclusive sister pairing (i.e. the complete absence of non-sister pairing) is difficult, though it seems to occur in some lizard species [83]. In addition, such a system would make it difficult to repair DSBs occurring before doubling (as both sister chromatids would have the same DSBs) [71].
The question of how a mitotic asexual mutant can invade a sexual species is at the heart of the debate on the evolutionary maintenance of sex, as this is what is investigated in most theoretical models and is the situation where the cost of sex is most evident [1]. However, unless meiosis can be entirely bypassed (e.g. as with vegetative reproduction), secondary asexuality is likely to evolve via modification of meiosis, keeping much of the cell signalling and machinery intact ([65,76,80,81], see also §4). Indeed, detailed cytological and genetic investigations in several asexual species thought to reproduce clonally by mitotic apomixis have uncovered remnants of meiosis [73,84–86]. In Daphnia, meiosis I is aborted mid-way and a normal meiosis II follows. Hence, clonality in Daphnia is meiotically derived [84]. This should lead to loss of heterozygosity in centromere-distal regions, but if recombination is fully suppressed the genetic outcome resembles mitosis. Importantly, this suggests a possible stepwise route to evolution of mitosis-like asexuality. Rare automixis (spontaneous development of unfertilized eggs) occurs in many species [1,81]. If this becomes more common, forms of automixis maintaining heterozygosity in centromere regions might be selectively favoured and recombination suppressed, eventually leading to meiosis-derived asexuality with the same genetic consequences as mitosis [87–91]. Indeed, in Arabidopsis, meiosis can be transformed to genetically resemble mitosis, but modification of several genes is needed to achieve this [92–94]. In angiosperms, there is also the difficulty to overcome the absence of endosperm fertilization to achieve proper seed development, which further stresses that meiosis-derived asexuality is unlikely to evolve in a single step. To fully understand the evolutionary maintenance of sex, we may therefore need to understand the selection pressures acting in the intermediate stages, which probably involve loss of heterozygosity, and thus inbreeding depression [77,80]. In many cases, the initial evolution of asexuality may thus resemble the evolution of self-fertilization, and several traits may pre-exist (such as low recombination rates) that make the successful transition to asexuality more likely in some taxa.
4. Meiosis punctuates life cycles
Meiosis is a key step in sexual life cycles, as well as some asexual life cycles derived from sexual ancestors. In multicellular eukaryotes, where meiosis is tightly associated with reproduction (unlike in many protists), meiosis is also a cellular and genetic bottleneck at the critical transition between the diploid and the haploid phases.
(a). Meiosis timing and arrest
In early haploid eukaryotes, meiosis probably quickly followed endomitosis or syngamy. Today, multicellular eukaryotes exhibit a variety of life cycles in which the haploid or diploid phase may predominate. The duration of the different phases was perhaps initially controlled, in part, by the timing of meiosis—for instance, a multicellular, extended diploid phase likely evolved by postponing meiosis. However, in metazoans, life cycles are mostly determined by the extent of somatic development within each phase rather than by the timing of meiosis, which can be halted or postponed. In animals, where haploid mitosis is suppressed, syngamy immediately follows meiosis. Furthermore, specific cells are ‘destined’ at an early stage to eventually undergo meiosis (aka germline), whereas this cell fate is determined much later in fungi, plants and some algae.
The timing of meiosis in the germline of animals has been intensively investigated. Whereas male meiosis occurs continuously, female meiosis usually stops twice (figure 1). These ‘meiotic arrests’ are under the control of various factors that are not completely identified across animals [95–97]. Arrest 1 occurs in prophase I during early development and can last years until sexual maturity. The timing of arrest 2 is more variable (ranging from metaphase I in many invertebrates, to metaphase II in vertebrates and G1 phase after meiosis II in some echinoderms), and may have evolved to prevent the risk of premature parthenogenetic cleavage of oocytes or inappropriate DNA replication before fertilization [97,98]; this is supported by the fact that this arrest is usually released by fertilization. However, the evolutionary significance of its precise timing in diverse groups is not well understood. Three ideas have been put forward to explain arrest 1 [66]. First, its occurrence at prophase I may allow the repair of accidental DSBs by sister chromatid exchange during long periods between arrests 1 and 2. Second, if arrest 1 was to occur during an earlier mitotic division within the germline, this might decrease the variance in the number of deleterious mutations among gametes within individuals, which may be detrimental if some defective gametes or early embryos can be eliminated and replaced during reproduction. Third, it may be easier to prevent uncontrolled proliferation in a non-dividing meiotic oocyte, as once the cell starts the meiotic cell division, it cannot engage in further mitotic divisions. Arrest 1 may thus have evolved to control (and minimize) the number of possibly wasteful and mutagenic mitotic divisions in the female germline. Similar meiotic arrests in plants are unknown. Plants seem to completely lack strict mechanisms to arrest the meiotic cell division. Contrary to animals and fungi that may arrest the cell cycle and abort meiosis once DSBs are not repaired, plants will progress through meiosis irrespective of such major defects [40].
(b). Meiosis and epigenetic reset
Meiosis and syngamy represent critical transitions between haploid and diploid phases in each generation. It has been suggested that a primary function of meiosis is to allow for epigenetic resetting in eukaryotes [99]. For instance, metazoan development is under the control of many epigenetic changes (cytosine methylation and chromatin marks) that are irreversibly maintained throughout life and must be reset twice each generation (at the n → 2n and 2n → n transitions). This ensures proper development, the acquisition of parent-specific imprints, and may allow for mechanisms limiting the maximal number of possible successive mitoses (‘Hayflick limit’, reducing tumour development [99]). Some loci escape these resets, which can lead to transgenerational epigenetic inheritance [100]. This occurs much less frequently in animals than in plants (e.g. in Arabidopsis, demethylation is largely restricted to asymmetric CHH methylation sites, and contrary to mouse, does not occur on most symmetric CG and CHG methylation sites) [100]. Although the 2n → n resetting occurs at or very close to meiosis in some cases (in female meiosis in animals), its timing may not be strictly tied to meiosis. For instance, it occurs pre-meiotically in the male germ line of animals (as shown in mice) or post-meiotically in male plant gametophytes (as shown in Arabidopsis) [100].
The evolutionary significance of these timing differences are poorly understood. Meiosis may simply not be the optimal time for epigenetic resetting. Many epigenetic pathways repress the activity of transposable elements (TEs), and so resetting epigenetic marks exposes the genome to mobilization of these elements, which may be particularly detrimental when producing gametes. In addition, meiosis may be specifically vulnerable to TE activity for several reasons [101,102]. These include (i) deficient synapsis and repair due to the reshuffling of the meiotic machinery towards TE-induced DSBs; (ii) ectopic recombination among TEs, and (iii) interference with synapsis due to TE transcriptional activity. Alternative TE silencing mechanisms, such as those involving small RNAs, may have evolved to ensure proper TE control during epigenetic resetting. For example, these mechanisms involve piRNA and/or endo-siRNA in mammal male and female germlines, respectively [103], and transfer of siRNA from the central cell to the egg cell in plant female gametophytes [104]. It is also possible that stringent synapsis checkpoints evolved, in part, to prevent the formation of defective gametes due to TE activity, along with other possible causes of meiotic errors.
(c). Meiosis asymmetry
Symmetrical meiosis results in four viable gametes, whereas asymmetrical meiosis results in a single gamete. Symmetrical meiosis is ancestral and is found in male meiosis in animals, seed plants, ‘homosporous’ species (e.g. mosses, many ferns) and isogamous eukaryotes. Asymmetrical meiosis, on the other hand, has evolved multiple times, and occurs in female meiosis in animals, seed plants and some ciliates. The selective scenarios underlying the evolution of meiotic asymmetry are unresolved. In some cases, such as in ciliates, there is no requirement for four meiotic products, as sex occurs by the cytoplasmic exchange of haploid micronuclei (conjugation). In other cases, asymmetrical meiosis in females results in a large oocyte full of resources, which may favour the production of a single cell rather than four [66,105,106]. However, females could also achieve this symmetrically by undergoing fewer meioses. Therefore, is it possible that asymmetrical meiosis allows better control of resource allocation to oocytes, as symmetrical meiosis may not ensure an even distribution of resources across four meiocytes; one difficulty here is that it is not clear why female control of resource allocation would be more efficient among meiocytes derived from the same or different meiosis. A solution may be that meiocytes must compete for resources during meiosis, so that a symmetrical female meiosis is vulnerable to SGEs that bias resource allocation in their favour, possibly by killing other products of meiosis [106]. Asymmetrical meiosis may therefore have evolved to suppress such costly competition within tetrads [107], but as discussed in the next section, it also opens the possibility of new conflicts [106]. Hence, the evolution of asymmetrical female meiosis is a question that remains not entirely resolved.
(d). Fairness of meiosis
A striking feature of meiosis is its apparent fairness: under Mendel's first Law of inheritance, each allele has a 50% chance of ending up in any given gamete. However, there are many SGEs that increase their chances above 50% by subverting the mechanism of meiosis. These SGEs fall into two classes. The first class is killer SGEs, which kill cells that have not inherited the element. In principle, such killers could operate during meiosis (the hypothetical ‘sister killers’ as discussed above), but the numerous killer SGEs that have been identified so far operate post-meiotically, e.g. by killing sibling sperm [108–111]. The second class consists of meiotic drivers that exploit the asymmetry of female meiosis discussed in the previous section. These elements achieve transmission in excess of 50% by preferentially moving into the meiotic products that will eventually become the eggs or megaspores [109,112]. There is a similarity between this kind of meiotic drive, where alleles preferentially go where resources are (i.e. the egg), and SGEs expressed later and biasing resource allocation in their favour [113]. Parents make decisions of allocations to offspring before the ‘meiotic veil of ignorance’, whereas offspring compete for resources ‘from behind the veil’ [114,115]. These genetic conflicts (between parent and offspring and between paternally and maternally derived alleles) are likely at the origin of parental imprints that differentially occur at male and female meiosis on some genes controlling embryo growth [114].
SGEs that undermine the fairness of meiosis provide explanations for otherwise puzzling observations. Perhaps most strikingly, centromere DNA regions often evolve rapidly, in contrast with what one would expect given their important and conserved function in meiosis. Henikoff et al. [116], therefore, proposed that expansion of repeat sequences in centromeric DNA produces a ‘stronger’ centromere, with increased kinetochore binding, which exhibits drive towards the future egg during meiosis I and, consequently, spreads in the population. Some of the best support for this hypothesis comes from a female meiotic driver in the monkeyflower Mimulus guttatus [117]. Although conclusive evidence for a direct centromere function of this element is lacking, it is physically associated with large centromere-specific satellite DNA arrays [118]. Female meiotic drive may also explain rapid karyotype evolution and the distribution of meta- versus acrocentric chromosomes [112] because Robertsonian fusion chromosomes (fusions of two acrocentric chromosomes into one metacentric) can behave like meiotic drivers and segregate preferentially into the future egg during meiosis I [119].
Other features of meiosis may be adaptations to suppress killer or meiotic drive SGEs. Such adaptations are expected, because these elements are generally costly for the rest of the genome (e.g. [108,120]). Defence against killer elements can be achieved by limiting gene expression. Accordingly, meiotic sex chromosome inactivation (MSCI, starting at pachytene of prophase I, figure 1) has been proposed to have evolved to control sex chromosome meiotic drive elements [121], and more generally this same principle may explain limited gene expression during meiosis and in its haploid products, as well as sharing of RNA and proteins among these cells. There is also evidence for rapid evolution and positive selection in the DNA-binding regions of centromere-associated proteins, which accords with the expectation of selection for countermeasures to limit preferential segregation of centromere drive elements towards the egg [106,116]. The evolution of holokinetic chromosomes may be an extreme form of defence against centromere drive [106].
5. Meiosis and recombination
A ubiquitous feature of meiosis is the exchange of genetic material between homologous chromosomes. While we have discussed arguments on its origin (see §2b), the maintenance of recombination is even more debated [122–124]. Here, we do not review this question, but discuss the evolutionary significance of patterns of recombination variation within and across species, as these present many mysteries connected to the functioning of meiosis.
(a). The number of crossovers per chromosome: constrained or not?
In many species, the number of COs per bivalent appears to follow highly constrained patterns, showing little variation compared to the variation of chromosome sizes, themselves spanning several orders of magnitude [125]. Within species, the correlation between genetic map length (in cM, with 50 cM being equivalent to 1 CO per bivalent) and physical length (in megabases, Mb) per chromosome is very strong (R2 > 0.95) [126–131], and often has an intercept of approximately 50 cM, consistent with occurrence of one obligate CO per bivalent. There is direct evidence indicating that bivalents lacking a CO have an increased probability of non-disjunction, resulting in unviable or unfit aneuploid offspring [132,133]. Indeed, COs establish physical connections between homologues, promoting accurate disjunction by providing the tension needed for the bipolar spindle to establish [134–136]. Therefore, this constraint has likely led to the evolution of regulation of CO numbers per bivalent across the eukaryotes [137,138]. However, the reasons underlying the evolutionary persistence of this constraint are not well understood. In several species (e.g. Arabidopsis, [139]), the intercept is less than 50 cM, but the smallest chromosome is at least 50 cM, thus still consistent with one obligate CO. More decisively, many species are achiasmate (i.e. have an absence of recombination) in one sex [140], with alternative mechanisms to ensure proper disjunction of achiasmate bivalents [141,142]. This indicates that COs are not always obligatory and are maintained for reasons other than ensuring proper disjunction.
In addition to the obligate CO, additional CO events can occur within bivalents. The strong cM–Mb relationship within species indicates that the number of surplus COs correlates strongly with physical chromosome size (see above). However, the rate at which surplus COs are added per Mb (i.e. the slope of the correlation) varies strongly between species [125,131,143]. This may be partly explained by selection for different CO rates in different species [144–146]. The strong correlations observed within most species may be explained by variation in trans-acting factors, such as the locus RNF212 and its protein, which affects the propensity for DSBs to form surplus COs [147,148]; indeed, the identification of loci affecting variation in CO rates indicates the potential for rapid evolution of CO rates within and between species [149].
A further constraint on bivalent disjunction may exist: the separation of different bivalents on the meiotic spindle may need to be collectively synchronized to avoid aneuploidy. If the number of COs correlates with the amount of tension exerted on the homologues, then a tight control of excess COs may minimize disjunction asynchrony. This hypothesis may explain the observation that some disjunction problems in humans occur in a global manner without involving effects driven by specific chromosomes [150–152]. Generally, high CO numbers are, on the other hand, not necessarily problematic with respect to proper disjunction [136,153].
(b). Crossover interference
A CO in one position may strongly reduce the likelihood of another CO occurring in the vicinity and/or on the same bivalent. This ‘crossover interference’ is widespread [125,136,154,155], but its function and mechanistic basis remains largely unknown. In many species, two classes of COs have been identified: Class I COs, which are sensitive to interference; and Class II COs, which are not [156]. Class I COs are thought to play a major role in ensuring obligate COs, and so interference may limit the frequency or variance of COs, which may be important in ensuring proper disjunction [157]. For instance, as with autopolyploids (see above), increased interference may limit the number of COs to just one per chromosome, preventing aberrant multivalent segregation [54]. A variant of this idea is that interference is a mechanism to avoid COs occurring in close proximity, which might reduce cohesion between homologues [158] or slip and cancel each other out when they involve two or four non-interlocking chromatids, resulting in no CO occurring [159]; however, these mechanisms do not explain long-distance interference. A further suggestion is that CO interference may be adaptive by breaking up genetic associations. First, adjacent COs may be avoided because they cancel their effects on genetic associations [160]. Second, it has been speculated that CO interference may reduce the chances of breaking up co-adapted gene complexes (supergenes) [157]. Some support for the idea that CO interference is not a purely mechanistic constraint comes from the fact that some species lack interference [154] and, more importantly, that there is some evidence suggesting that interference levels evolve in long-term evolution experiments in Drosophila [161].
(c). Differences in recombination rates between the sexes
In many species, CO rates and localization differ between male and female meioses, and these differences can vary in degree and direction even between closely related species [162–164]. The most extreme case is achiasmy, an absence of recombination in one sex, nearly always the heterogametic sex [162]. This may have evolved either as a side effect of selection to suppress recombination between the sex chromosomes [165,166], or as a way to promote tight linkage without suppressing recombination on the X or Z chromosomes [163]. More intriguing are the quantitative differences between males and females, known as heterochiasmy, which are found in many taxa, but whose mechanistic and evolutionary drivers are not yet fully understood. A number of explanations have been proposed, relating to mechanistic factors such as differences in chromatin structure [167–169], sexual dimorphism in the action of loci associated with CO rate (e.g. RNF212, [127,128,148]), and evolutionarily widespread processes such as sperm competition, sexual dimorphism and dispersal [162,170,171]. Some models point to a role of sex differences in selection during the haploid phase [172]. While a viable explanation in plants [163], there is little empirical support for this in animals [171], where meiosis in females is only completed after fertilization (i.e. there is no true haploid phase), and where only few genes are expressed in sperm. However, meiotic drive systems are often entirely distinct between males and females [173] and may be a primary cause of haploid selection [174]. These systems often require genetic associations between two loci (a distorter and responder, or a distorter and a centromere in males and females, respectively). These driving elements might thus be very important in shaping heterochiasmy patterns [107]. Indeed, COs in female meiosis are located closer to centromeres, which would be consistent with the view that this localization evolved to limit centromeric drive [175] (see also §4d). Similarly, meiotic drive in favour of recombinant chromatids has been detected in human female meiosis [67], which may limit centromere drive.
(d). The localization of crossovers and recombination hotspots
The localization of recombination events differs between species. In many species, recombination occurs in localized regions known as ‘recombination hotspots’ of approximately 1–2 kb in length [176–179], although some species (e.g. Caenorhabditis elegans and Drosophila) lack well-defined hotspots [180,181]. There are at least two types of hotspots (figure 3). The first type, probably ancestral, is found in fungi, plants, birds and some mammals; these hotspots are temporally stable (up to millions of years) and concentrated near promoter regions and transcription start sites [178,182–185]. The second type is likely derived and is found in other mammals, including mice and humans, where the positioning of hotspots is determined by the zinc-finger protein PRDM9. This system differs in two respects from the former: first, it appears to direct DSBs away from regulatory regions [186], and second, mutations in the DNA-binding zinc-finger array change the sequence motif targeted by the protein, leading to rapid evolution of hotspot positions over short time scales [187,188]. This system is not present in all mammals: in dogs, hotspots target promoter regions [189], and the knock-out of Prdm9 in mouse makes recombination target promoter regions instead, underlining its derived nature [186].
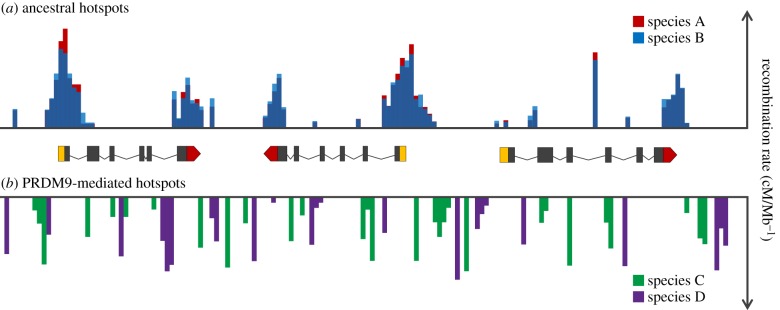
Figure 3.
Hypothetical genome sequence containing three genes showing the distribution of ancient recombination hotspots in most model species (a) compared with derived PRDM9-mediated recombination hotspots (b). Studies in fungi, plants, birds and dogs indicate that ancestral hotspots are stable over long evolutionary timescales (up to millions of years) and concentrate at promoter regions and transcription start sites (and at stop sites in some species). These start and stop sites for each gene are indicated in yellow and red blocks, with their introns and exons represented by lines and black blocks, respectively. PRDM9-mediated hotspots are found in some mammals, including humans and mice, and are directed away from promotor regions. The DNA-binding zinc-finger in the PRDM9 protein targets specific sequence motifs; mutations in the zinc-finger array change the targeted motif, leading to rapid evolution of hotspot positions and an absence of hotspot conservation over short evolutionary timescales (at the population and species level).
The evolutionary significance of both kinds of hotspots remains unclear. For the first type, the positions of hotspots may be caused by chromatin accessibility in transcribed regions or have evolved to favour recombination in gene rich regions (where it might be worth reducing genetic association). However, this does not clearly account for their precise location in regulatory regions. Another possibility might be that the co-occurrence of both COs and gene-conversion events (i.e. where resolution of DSBs without CO is achieved by exchanging small segments of DNA) specifically in regulatory regions could repress enhancer runaway, a mechanism that can lead to suboptimal expression levels [190]. The evolutionary significance of the second kind of hotspot is similarly elusive. These hotspots are self-destructing because the target sequence motifs are eroded by biased gene conversion (BGC) during DSB repair [191]. This leads to a ‘hotspot paradox’: how can hotspots and recombination be maintained in the long term in the face of BGC [192]? A possible solution is that trans-acting factors like PRDM9 may mutate sufficiently fast to constantly ‘chase’ new and frequent targets (hotspots), switching to new ones when these targets become rare due to BGC [193]. This ‘Red Queen’ model does not require strong stabilizing selection on the number of COs, and closely mimics the pattern of hotspot turnover observed in some cases [194]. However, this model does not explain how the second kind of hotspots evolved in the first place, as when it arose proper segregation was presumably already ensured by the first kind of hotspots (which, as seen in mice, are still active). Also, it does not explain why PRDM9 action is self-destructing: there is no necessity to induce DSBs exactly at the position of the target sequence for a trans-acting factor. In fact, there is no logical necessity to rely on a target sequence to maintain one CO per chromosome, as fixed chromosomal features could serve this purpose. It is worth noting here that recruiting promoter sequences for this purpose (as found for hotspots of the first kind) would be very efficient, as these sequences are highly stable and dispersed in the genome on all chromosomes. There is also no evidence so far that targeted binding motifs of PRDM9 correspond to some SGEs whose elimination would be beneficial. Overall, while spectacular progress has been made recently in elucidating hotspot mechanisms in detail (and patterns in recombination landscapes), there are still major gaps in our understanding of their evolutionary significance.
6. Conclusion
The evolutionary significance of meiosis has often been interpreted in an oversimplified manner, restricted mainly to the direct (DSB repair, proper disjunction) or indirect (genetic associations) effects of meiotic recombination. Yet, many features of meiosis are unlikely to be explained by effects of recombination alone, and the fields of cellular and molecular biology are uncovering new meiotic features at a high rate. One of the main take-home messages of this review is that many, if not most features of meiosis are still awaiting an evolutionary explanation. Nonetheless, the recent advances in all detailed aspects of meiosis now offer the chance to investigate these questions in a far more comprehensive manner. This will require continued dialogue between cell, molecular and evolutionary biologists (as advocated e.g. in [195]), and perhaps also the realization that similarities between features may in fact have different evolutionary explanations (e.g. different kinds of hotspots).
One of the most salient themes in most meiosis mysteries is the impact of genetic conflicts and SGEs. As for the evolution of genome size and structure, their impact is probably central [196], but in many cases they remain hypothetical and difficult to demonstrate and study directly: many SGEs reach fixation quickly and leave almost no visible footprint. Showing that some meiotic features evolved to control SGEs represents an even greater challenge. Indeed, if successful, such features would prevent these SGEs from spreading, further limiting their detection. In addition, demonstrating a role in SGEs control requires ruling out that these features evolved for more mechanistic and simpler alternatives. This is usually extremely difficult, as many ad hoc mechanistic constraints can be imagined.
Although meiosis is highly conserved in eukaryotes, deviations from the norm are ubiquitous and may provide important insights into its evolution. This is already apparent when considering model organisms (e.g. point centromeres in yeast, achiasmy in male Drosophila, holokinetic chromosomes in C. elegans, fast evolving recombination hotspots in mice and humans). However, the true diversity of meiotic features is likely to be revealed only when considering non-model organisms, and unicellular eukaryotes appear especially promising in this respect. Obtaining a clearer understanding of the evolutionary significance of the myriad of meiotic features will certainly be crucial to inspire and guide mechanistic investigations. Conversely, as often, ‘all theory is grey, but green is the tree of life’ [Goethe, Faust, Part I], the mysteries of meiosis call for new developments of evolutionary theory, to make it less grey and more closely connected to the biological details. Overall, all these mysteries tend to have been overshadowed by the famous question of the maintenance of sex. However, resolving them might provide decisive steps towards solving this major question of evolutionary biology.
Acknowledgement
We thank M. Archetti, K. Bomblies, D. Charlesworth, B. de Massy, L. Duret, E. Jenczewski, R. Mercier, P. van Dijk and J. Wilkins for comments on this manuscript. Our intention was to cover all meiosis-related evolutionary mysteries in a single perspective. We apologize to all authors of the many interesting and relevant papers we were unable to cite in the final condensed version.
Authors' contributions
T.L. conceived and coordinated the review. All authors contributed to and approved this manuscript.
Competing interests
We have no competing interests.
Funding
We received no funding for this study.
References
- 1.Maynard Smith J. 1978. The evolution of sex. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 2.Hamilton WD. 2001. Narrow roads of gene land: vol. 2: evolution of sex. Oxford, UK: Oxford University Press. [Google Scholar]
- 3.Knoll AH, Javaux EJ, Hewitt D, Cohen P. 2006. Eukaryotic organisms in Proterozoic oceans. Phil. Trans. R. Soc. B 361, 1023–1038. ( 10.1098/rstb.2006.1843) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Douzery EJP, Snell EA, Bapteste E, Delsuc F, Philippe H. 2004. The timing of eukaryotic evolution: does a relaxed molecular clock reconcile proteins and fossils? Proc. Natl Acad. Sci. USA 101, 15 386–15 391. ( 10.1073/pnas.0403984101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cavalier-Smith T. 2002. Origins of the machinery of recombination and sex. Heredity 88, 125–141. ( 10.1038/sj.hdy.6800034) [DOI] [PubMed] [Google Scholar]
- 6.Ramesh M, Malik S-B, Logsdon JJ. 2005. A phylogenomic inventory of meiotic genes: evidence for sex in Giardia and an early eukaryotic origin of meiosis. Curr. Biol. 15, 185–191. ( 10.1016/j.cub.2005.01.003) [DOI] [PubMed] [Google Scholar]
- 7.Speijer D, Lukeš J, Eliáš M. 2015. Sex is a ubiquitous, ancient, and inherent attribute of eukaryotic life. Proc. Natl Acad. Sci. USA 112, 8827–8834. ( 10.1073/pnas.1501725112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cleveland L. 1947. The origin and evolution of meiosis. Science 105, 287–289. ( 10.1126/science.105.2724.287) [DOI] [PubMed] [Google Scholar]
- 9.Wilkins AS, Holliday R. 2009. The evolution of meiosis from mitosis. Genetics 181, 3–12. ( 10.1534/genetics.108.099762) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hurst L, Nurse P. 1991. A note on the evolution of meiosis. J. Theor. Biol. 150, 561–563. ( 10.1016/S0022-5193(05)80447-3) [DOI] [PubMed] [Google Scholar]
- 11.Margulis L, Sagan D. 1990. Origins of sex: three billion years of genetic recombination. New Haven, CT: Yale University Press. [Google Scholar]
- 12.Maynard Smith J, Szathmary E. 1995. The major transitions in evolution. Oxford, UK: Oxford University Press. [Google Scholar]
- 13.Mable BK, Otto SP. 1998. The evolution of life cycles with haploid and diploid phases. BioEssays 20, 453–462. ( 10.1002/(SICI)1521-1878(199806)20:6%3C453::AID-BIES3%3E3.0.CO;2-N) [DOI] [Google Scholar]
- 14.Rescan M, Lenormand T, Roze D. 2016. Interactions between genetic and ecological effects on the evolution of life cycles. Am. Nat. 187, 19–34. ( 10.1086/684167) [DOI] [PubMed] [Google Scholar]
- 15.Souza-Júnior SA, Becker TCA, Castro-Prado MAA. 2007. Asexual recombination in a uvsH mutant of Aspergillus nidulans. Biol. Res. 40, 65–71. ( 10.4067/S0716-97602007000100007) [DOI] [PubMed] [Google Scholar]
- 16.Parfrey LW, Lahr DJG, Katz LA. 2008. The dynamic nature of eukaryotic genomes. Mol. Biol. Evol. 25, 787–794. ( 10.1093/molbev/msn032) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Otto SP, Goldstein DB. 1992. Recombination and the evolution of diploidy. Genetics 131, 745–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hickey DA, Rose MR. 1988. The role of gene transfer in the evolution of eukaryotic sex. In The evolution of sex: an examination of current ideas (eds Michod RE, Levin BR), pp. 161–175. Sunderland, MA: Sinauer Associates. [Google Scholar]
- 19.Rusche LN, Rine J. 2010. Switching the mechanism of mating type switching: a domesticated transposase supplants a domesticated homing endonuclease. Genes Dev. 24, 10–14. ( 10.1101/gad.1886310) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zickler D, Kleckner N. 2015. Recombination, pairing, and synapsis of homologs during meiosis. Cold Spring Harb. Perspect. Biol. 7, a016626 ( 10.1101/cshperspect.a016626) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Obeso D, Pezza R, Dawson D. 2014. Couples, pairs, and clusters: mechanisms and implications of centromere associations in meiosis. Chromosoma 123, 43–55. ( 10.1007/s00412-013-0439-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kleckner N. 1996. Meiosis: how could it work? Proc. Natl Acad. Sci. USA 93, 8167–8174. ( 10.1073/pnas.93.16.8167) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baudat F, Imai Y, de Massy B. 2013. Meiotic recombination in mammals: localization and regulation. Nat. Rev. Genet. 14, 794–806. ( 10.1038/nrg3573) [DOI] [PubMed] [Google Scholar]
- 24.Birdsell J, Wills C. 2003. The evolutionary origin and maintenance of sexual recombination: a review of contemporary models. In Evolutionary biology (eds MacIntyre R, Clegg M), pp. 27–138. Dordrecht, The Netherlands: Kluwer Academic Publishers. [Google Scholar]
- 25.Marcon E, Moens PB. 2005. The evolution of meiosis: recruitment and modification of somatic DNA-repair proteins. BioEssays 27, 795–808. ( 10.1002/bies.20264) [DOI] [PubMed] [Google Scholar]
- 26.White MF. 2011. Homologous recombination in the archaea: the means justify the ends. Biochem. Soc. Trans. 39, 15–19. ( 10.1042/BST0390015) [DOI] [PubMed] [Google Scholar]
- 27.Heng HHQ. 2007. Elimination of altered karyotypes by sexual reproduction preserves species identity. Genome 50, 517–524. ( 10.1139/G07-039) [DOI] [PubMed] [Google Scholar]
- 28.Sasaki M, Lange J, Keeney S. 2010. Genome destabilization by homologous recombination in the germ line. Nat. Rev. Mol. Cell Biol. 11, 182–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arkhipova I, Meselson M. 2005. Deleterious transposable elements and the extinction of asexuals. BioEssays 27, 76–85. ( 10.1002/bies.20159) [DOI] [PubMed] [Google Scholar]
- 30.Archetti M. 2003. A selfish origin for recombination. J. Theor. Biol. 223, 335–346. ( 10.1016/S0022-5193(03)00102-4) [DOI] [PubMed] [Google Scholar]
- 31.Bernstein H, Hopf FA, Michod RE. 1988. Is meiotic recombination and adaptation for repairing DNA, producing genetic variation, or both? In The evolution of sex (eds Michod RE, Levin BR), pp. 139–160. Sunderland, MA: Sinauer Associates. [Google Scholar]
- 32.Bernstein H, Bernstein C, Michod RE. 2011. Meiosis as an evolutionary adaptation for DNA repair. In DNA repair (ed. I Kruman), pp. 357–382. Slavka Krautzeka, Croatia: InTech.
- 33.Hörandl E, Hadacek F. 2013. The oxidative damage initiation hypothesis for meiosis. Plant Reprod. 26, 351–367. ( 10.1007/s00497-013-0234-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maynard-Smith J. 1988. The evolution of recombination. In The evolution of sex: an examination of current ideas (eds Michod RE, Levin BR), pp. 106–125. Sunderland, MA: Sinauer Associates. [Google Scholar]
- 35.Hurst LD. 1993. Drunken walk of the diploid. Nature 365, 206–207. ( 10.1038/365206a0) [DOI] [PubMed] [Google Scholar]
- 36.Archetti M. 2004. Loss of complementation and the logic of two-step meiosis. J. Evol. Biol. 17, 1098–1105. ( 10.1111/j.1420-9101.2004.00726.x) [DOI] [PubMed] [Google Scholar]
- 37.Haig D, Grafen A. 1991. Genetic scrambling as a defence against meiotic drive. J. Theor. Biol. 153, 531–558. ( 10.1016/S0022-5193(05)80155-9) [DOI] [PubMed] [Google Scholar]
- 38.Haig D. 1993. Alternatives to meiosis: the unusual genetics of red algae, microsporidia, and others. J. Theor. Biol. 163, 15–31. ( 10.1006/jtbi.1993.1104) [DOI] [PubMed] [Google Scholar]
- 39.Hurst LD, Randerson JP. 2000. Transitions in the evolution of meiosis. J. Evol. Biol. 13, 466–479. ( 10.1046/j.1420-9101.2000.00182.x) [DOI] [Google Scholar]
- 40.Wijnker E, Schnittger A. 2013. Control of the meiotic cell division program in plants. Plant Reprod. 26, 143–158. ( 10.1007/s00497-013-0223-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Raikov IB. 1982. The protozoan nucleus—morphology and evolution. Vienna, Austria: Springer. [Google Scholar]
- 42.Raikov IB. 1995. Meiosis in protists: recent advances and persisting problems. Eur. J. Protistol. 31, 1–7. ( 10.1016/S0932-4739(11)80349-4) [DOI] [Google Scholar]
- 43.Grandont L, Jenczewski E, Lloyd A. 2013. Meiosis and its deviations in polyploids. Cytogenet. Genome Res. 140, 171–184. ( 10.1159/000351730) [DOI] [PubMed] [Google Scholar]
- 44.Stenberg P, Saura A. 2013. Meiosis and its deviations in polyploid animals. Cytogenet. Genome Res. 140, 185–203. ( 10.1159/000351731) [DOI] [PubMed] [Google Scholar]
- 45.Lloyd A, Bomblies K. 2016. Meiosis in autopolyploid and allopolyploid Arabidopsis. Curr. Opin. Plant Biol. 30, 116–122. ( 10.1016/j.pbi.2016.02.004) [DOI] [PubMed] [Google Scholar]
- 46.Madlung A, Tyagi AP, Watson B, Jiang H, Kagochi T, Doerge RW, Martienssen R, Comai L. 2005. Genomic changes in synthetic Arabidopsis polyploids. Plant J. 41, 221–230. ( 10.1111/j.1365-313X.2004.02297.x) [DOI] [PubMed] [Google Scholar]
- 47.Gaeta RT, Pires JC, Iniguez-Luy F, Leon E, Osborn TC. 2007. Genomic changes in resynthesized Brassica napus and their effect on gene expression and phenotype. Plant Cell 19, 3403–3417. ( 10.1105/tpc.107.054346) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang Y, Jha AK, Chen R, Doonan JH, Yang M. 2010. Polyploidy-associated genomic instability in Arabidopsis thaliana. Genesis 48, 254–263. ( 10.1002/dvg.20629) [DOI] [PubMed] [Google Scholar]
- 49.Gaeta RT, Chris Pires J. 2010. Homoeologous recombination in allopolyploids: the polyploid ratchet. New Phytol. 186, 18–28. ( 10.1111/j.1469-8137.2009.03089.x) [DOI] [PubMed] [Google Scholar]
- 50.Santos JL, Alfaro D, Sanchez-Moran E, Armstrong SJ, Franklin FCH, Jones GH. 2003. Partial diploidization of meiosis in autotetraploid Arabidopsis thaliana. Genetics 165, 1533–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yant L, Hollister JD, Wright KM, Arnold BJ, Higgins JD, Franklin FCH, Bomblies K. 2013. Meiotic adaptation to genome duplication in Arabidopsis arenosa. Curr. Biol. 23, 2151–2156. ( 10.1016/j.cub.2013.08.059) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cifuentes M, Grandont L, Moore G, Chèvre AM, Jenczewski E. 2010. Genetic regulation of meiosis in polyploid species: new insights into an old question. New Phytol. 186, 29–36. ( 10.1111/j.1469-8137.2009.03084.x) [DOI] [PubMed] [Google Scholar]
- 53.Le Comber SC, Ainouche ML, Kovarik A, Leitch AR. 2010. Making a functional diploid: from polysomic to disomic inheritance. New Phytol. 186, 113–122. ( 10.1111/j.1469-8137.2009.03117.x) [DOI] [PubMed] [Google Scholar]
- 54.Bomblies K, Jones G, Franklin C, Zickler D, Kleckner N. 2016. The challenge of evolving stable polyploidy: could an increase in ‘crossover interference distance’ play a central role? Chromosoma 125, 287–300. ( 10.1007/s00412-015-0571-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hollister JD, Arnold BJ, Svedin E, Xue KS, Dilkes BP, Bomblies K. 2012. Genetic adaptation associated with genome-doubling in autotetraploid Arabidopsis arenosa. PLoS Genet. 8, e1003093 ( 10.1371/journal.pgen.1003093) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Griffiths S, Sharp R, Foote TN, Bertin I, Wanous M, Reader S, Colas I, Moore G. 2006. Molecular characterization of Ph1 as a major chromosome pairing locus in polyploid wheat. Nature 439, 749–752. ( 10.1038/nature04434) [DOI] [PubMed] [Google Scholar]
- 57.St Charles J, Hamilton ML, Petes TD. 2010. Meiotic chromosome segregation in triploid strains of Saccharomyces cerevisiae. Genetics 186, 537–550. ( 10.1534/genetics.110.121533) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lim KY, et al. 2005. Evolutionary implications of permanent odd polyploidy in the stable sexual, pentaploid of Rosa canina L. Heredity 94, 501–506. ( 10.1038/sj.hdy.6800648) [DOI] [PubMed] [Google Scholar]
- 59.Pazy B, Plitmann U. 1991. Unusual chromosome separation in meiosis of Cuscuta L. Genome 10, 533–536. ( 10.1139/g91-082) [DOI] [Google Scholar]
- 60.Heckmann S, Jankowska M, Schubert V, Kumke K, Ma W, Houben A. 2014. Alternative meiotic chromatid segregation in the holocentric plant Luzula elegans. Nat. Commun. 5, 4979 ( 10.1038/ncomms5979) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cabral G, Marques A, Schubert V, Pedrosa-Harand A, Schlögelhofer P. 2014. Chiasmatic and achiasmatic inverted meiosis of plants with holocentric chromosomes. Nat. Commun. 5, 5070 ( 10.1038/ncomms6070) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wrensch DL, Kethley J, Norton RA. 1994. Cytogenetics of holokinetic chromosomes and inverted meiosis: keys to the evolutionary success of mites, with generalizations on eukaryotes. In Mites: ecological and evolutionary analyses of life-history patterns (ed. MA Houck), pp. 282–343. Dordrecht, The Netherlands: Springer. [Google Scholar]
- 63.John B. 1990. Meiosis. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 64.Bongiorni S, Fiorenzo P, Pippoletti D, Prantera G. 2004. Inverted meiosis and meiotic drive in mealybugs. Chromosoma 112, 331–341. ( 10.1007/s00412-004-0278-4) [DOI] [PubMed] [Google Scholar]
- 65.Melters DP, Paliulis LV, Korf IF, Chan SWL. 2012. Holocentric chromosomes: convergent evolution, meiotic adaptations, and genomic analysis. Chromosom. Res. 20, 579–593. ( 10.1007/s10577-012-9292-1) [DOI] [PubMed] [Google Scholar]
- 66.Mira A. 1998. Why is meiosis arrested? J. Theor. Biol. 194, 275–287. ( 10.1006/jtbi.1998.0761) [DOI] [PubMed] [Google Scholar]
- 67.Ottolini CS, et al. 2015. Genome-wide maps of recombination and chromosome segregation in human oocytes and embryos show selection for maternal recombination rates. Nat. Genet. 47, 727–735. ( 10.1038/ng.3306) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mogie M. 1986. Automixis: its distribution and status. Biol. J. Linn. Soc. 28, 321–329. ( 10.1111/j.1095-8312.1986.tb01761.x) [DOI] [Google Scholar]
- 69.Suomalainen E, Saura A, Lokki J. 1987. Cytology and evolution in parthenogenesis. Boca Raton, FL: CRC Press. [Google Scholar]
- 70.Stenberg P, Saura A. 2009. Cytology of asexual animals. In Lost sex. The evolutionary biology of parthenogenesis (eds Schön I, Martens K, van Dijk P), pp. 63–74. Dordrecht, The Netherlands: Springer Science. [Google Scholar]
- 71.Archetti M. 2010. Complementation, genetic conflict, and the evolution of sex and recombination. J. Hered. 101, 1–13. ( 10.1093/jhered/esq009) [DOI] [PubMed] [Google Scholar]
- 72.Neiman M, Schwander T. 2011. Using parthenogenetic lineages to identify advantages of sex. Evol. Biol. 38, 115–123. ( 10.1007/s11692-011-9113-z) [DOI] [Google Scholar]
- 73.Nougué O, Rode N, Zahab R, Ségard A, Chevin L-M, Haag CR, Lenormand T. 2015. Automixis in Artemia: solving a century-old problem. J. Evol. Biol. 28, 2337–2348. ( 10.1111/jeb.12757) [DOI] [PubMed] [Google Scholar]
- 74.van Dijk PJ. 2009. Apomixis: basics for non-botanists. In Lost sex. The evolutionary biology of parthenogenesis (eds Schön I, Martens K, van Dijk PJ), pp. 47–62. Dordrecht, The Netherlands: Springer Science. [Google Scholar]
- 75.Asher JH. 1970. Parthenogenesis and genetic variability. II. One-locus models for various diploid populations. Genetics 66, 369–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pearcy M, Hardy O, Aron S. 2006. Thelytokous parthenogenesis and its consequences on inbreeding in an ant. Heredity 96, 377–382. ( 10.1038/sj.hdy.6800813) [DOI] [PubMed] [Google Scholar]
- 77.Engelstädter J, Sandrock C, Vorburger C. 2011. Contagious parthenogenesis, automixis, and a sex determination meltdown. Evolution 65, 501–511. ( 10.1111/j.1558-5646.2010.01145.x) [DOI] [PubMed] [Google Scholar]
- 78.Engelstädter J. 2008. Constraints on the evolution of asexual reproduction. BioEssays 30, 1138–1150. ( 10.1002/bies.20833) [DOI] [PubMed] [Google Scholar]
- 79.Svendsen N, et al. 2015. Uncovering cryptic asexuality in Daphnia magna by RAD-sequencing. Genetics 201, 1143–1155. ( 10.1534/genetics.115.179879) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Archetti M. 2004. Recombination and loss of complementation: a more than two-fold cost for parthenogenesis. J. Evol. Biol. 17, 1084–1097. ( 10.1111/j.1420-9101.2004.00745.x) [DOI] [PubMed] [Google Scholar]
- 81.Schwander T, Crespi BJ. 2009. Multiple direct transitions from sexual reproduction to apomictic parthenogenesis in Timema stick insects. Evolution 63, 84–103. ( 10.1111/j.1558-5646.2008.00524.x) [DOI] [PubMed] [Google Scholar]
- 82.Baudry E, Kryger P, Allsopp M, Koeniger N, Vautrin D, Mougel F, Cornuet J-M, Solignac M. 2004. Whole-genome scan in thelytokous-laying workers of the Cape honeybee (Apis mellifera capensis): central fusion, reduced recombination rates and centromere mapping using half-tetrad analysis. Genetics 167, 243–252. ( 10.1534/genetics.167.1.243) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lutes AA, Neaves WB, Baumann DP, Wiegraebe W, Baumann P. 2010. Sister chromosome pairing maintains heterozygosity in parthenogenetic lizards. Nature 464, 283–286. ( 10.1038/nature08818) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hiruta C, Nishida C, Tochinai S. 2010. Abortive meiosis in the oogenesis of parthenogenetic Daphnia pulex. Chromosom. Res. 18, 833–840. ( 10.1007/s10577-010-9159-2) [DOI] [PubMed] [Google Scholar]
- 85.Miura T, Braendle C, Shingleton A, Sisk G, Kambhampati S, Stern DL. 2003. A comparison of parthenogenetic and sexual embryogenesis of the pea aphid Acyrthosiphon pisum (Hemiptera: Aphidoidea). J. Exp. Zool. B. Mol. Dev. Evol. 295, 59–81. ( 10.1002/jez.b.3) [DOI] [PubMed] [Google Scholar]
- 86.van Baarlen P, van Dijk PJ, Hoekstra RF, de Jong JH. 2000. Meiotic recombination in sexual diploid and apomictic triploid dandelions (Taraxacum officinale L.). Genome 43, 827–835. ( 10.1139/g00-047) [DOI] [PubMed] [Google Scholar]
- 87.Bell G. 1982. The masterpiece of nature: the evolution and genetics of sexuality. Berkeley, CA: University of California Press.
- 88.Neiman M, Sharbel TF, Schwander T. 2014. Genetic causes of transitions from sexual reproduction to asexuality in plants and animals. J. Evol. Biol. 27, 1346–1359. ( 10.1111/jeb.12357) [DOI] [PubMed] [Google Scholar]
- 89.Nunney L. 1989. The maintenance of sex by group selection. Evolution 43, 245–257. ( 10.2307/2409205) [DOI] [PubMed] [Google Scholar]
- 90.Simon J-C, Rispe C, Sunnucks P. 2002. Ecology and evolution of sex in aphids. Trends Ecol. Evol. 17, 34–39. ( 10.1016/S0169-5347(01)02331-X) [DOI] [Google Scholar]
- 91.Schwander T, Vuilleumier S, Dubman J, Crespi BJ. 2010. Positive feedback in the transition from sexual reproduction to parthenogenesis. Proc. R. Soc. B 277, 1435–1442. ( 10.1098/rspb.2009.2113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Marimuthu MPA, et al. 2011. Synthetic clonal reproduction through seeds. Science 331, 876 ( 10.1126/science.1199682) [DOI] [PubMed] [Google Scholar]
- 93.D'Erfurth I, Jolivet S, Froger N, Catrice O, Novatchkova M, Mercier R. 2009. Turning meiosis into mitosis. PLoS Biol. 7, e1000124 ( 10.1371/journal.pbio.1000124) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ravi M, Marimuthu MPA, Siddiqi I. 2008. Gamete formation without meiosis in Arabidopsis. Nature 451, 1121–1124. ( 10.1038/nature06557) [DOI] [PubMed] [Google Scholar]
- 95.Masui Y. 2000. The elusive cytostatic factor in the animal egg. Nat. Rev. Mol. Cell Biol. 1, 228–232. ( 10.1038/35043096) [DOI] [PubMed] [Google Scholar]
- 96.Kishimoto T. 2003. Cell-cycle control during meiotic maturation. Curr. Opin. Cell Biol. 15, 654–663. ( 10.1016/j.ceb.2003.10.010) [DOI] [PubMed] [Google Scholar]
- 97.Extavour C. 2009. Oogenesis: making the Mos of meiosis. Curr. Biol. 19, R489–R491. ( 10.1016/j.cub.2009.05.015) [DOI] [PubMed] [Google Scholar]
- 98.Sagata N. 1996. Meiotic metaphase arrest in animal oocytes: its mechanisms and biological significance. Trends Cell Biol. 6, 22–28. ( 10.1016/0962-8924(96)81034-8) [DOI] [PubMed] [Google Scholar]
- 99.Gorelick R, Carpinone J. 2009. Origin and maintenance of sex: the evolutionary joys of self sex. Biol. J. Linn. Soc. 98, 707–728. ( 10.1111/j.1095-8312.2009.01334.x) [DOI] [Google Scholar]
- 100.Heard E, Martienssen RA. 2014. Transgenerational epigenetic inheritance: myths and mechanisms. Cell 157, 95–109. ( 10.1016/j.cell.2014.02.045) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zamudio N, Barau J, Teissandier A, Walter M, Borsos M, Servant N, Bourc'his D. 2015. DNA methylation restrains transposons from adopting a chromatin signature permissive for meiotic recombination. Genes Dev. 29, 1256–1270. ( 10.1101/gad.257840.114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Soper SFC, Van Der Heijden GW, Hardiman TC, Goodheart M, Martin SL, De Boer P, Bortvin A. 2008. Mouse maelstrom, a component of nuage, is essential for spermatogenesis and transposon repression in meiosis. Dev. Cell. 15, 285–297. ( 10.1016/j.devcel.2008.05.015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Claycomb JM. 2014. Ancient endo-siRNA pathways reveal new tricks. Curr. Biol. 24, R703–R715. ( 10.1016/j.cub.2014.06.009) [DOI] [PubMed] [Google Scholar]
- 104.Castel E, Martienssen R. 2013. RNA interference (RNAi) in the nucleus: roles for small RNA in transcription, epigenetics and beyond. Nat. Rev. Genet. 14, 100–112. ( 10.1038/nrg3355) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Schuh M, Ellenberg J. 2008. A new model for asymmetric spindle positioning in mouse oocytes. Curr. Biol. 18, 1986–1992. ( 10.1016/j.cub.2008.11.022) [DOI] [PubMed] [Google Scholar]
- 106.Malik HS, Henikoff S. 2009. Major evolutionary transitions in centromere complexity. Cell 138, 1067–1082. ( 10.1016/j.cell.2009.08.036) [DOI] [PubMed] [Google Scholar]
- 107.Haig D. 2010. Games in tetrads: segregation, recombination, and meiotic drive. Am. Nat. 176, 404–413. ( 10.1086/656265) [DOI] [PubMed] [Google Scholar]
- 108.Jaenike J. 2001. Sex chromosome meiotic drive. Annu. Rev. Ecol. Syst. 32, 25–49. ( 10.1146/annurev.ecolsys.32.081501.113958) [DOI] [Google Scholar]
- 109.Burt A, Trivers R. 2006. Genes in conflict: the biology of selfish genetic elements. Boston, MA: Harvard University Press. [Google Scholar]
- 110.Larracuente AM, Presgraves DC. 2012. The selfish segregation distorter gene complex of Drosophila melanogaster. Genetics 192, 33–53. ( 10.1534/genetics.112.141390) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lindholm AK, et al. 2016. The ecology and evolutionary dynamics of meiotic drive. Trends Ecol. Evol. 31, 315–326. ( 10.1016/j.tree.2016.02.001) [DOI] [PubMed] [Google Scholar]
- 112.De Villena FPM, Sapienza C. 2001. Nonrandom segregation during meiosis: the unfairness of females. Mamm. Genome 12, 331–339. ( 10.1007/s003350040003) [DOI] [PubMed] [Google Scholar]
- 113.Haig D. 1996. Gestational drive and the green-bearded placenta. Proc. Natl Acad. Sci. USA 93, 6547–6551. ( 10.1073/pnas.93.13.6547) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Haig D. 2014. Coadaptation and conflict, misconception and muddle, in the evolution of genomic imprinting. Heredity 113, 96–103. ( 10.1038/hdy.2013.97) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Okasha S. 2012. Social justice, genomic justice and the veil of ignorance: Harsanyi meets Mendel. Econ. Philos. 28, 43–71. ( 10.1017/S0266267112000119) [DOI] [Google Scholar]
- 116.Henikoff S, Ahmad K, Malik HS. 2001. The centromere paradox: stable inheritance with rapidly evolving DNA. Science 293, 1098–1102. ( 10.1126/science.1062939) [DOI] [PubMed] [Google Scholar]
- 117.Fishman L. 2013. Female meiotic drive in monkeyflowers: insight into the population genetics of selfish centromeres. In Plant centromere biology (eds Jiang J, Birchler JA), pp. 129–145. Oxford, UK: Wiley-Blackwell. [Google Scholar]
- 118.Fishman L, Saunders A. 2008. Centromere-associated female meiotic drive entails male fitness costs in monkeyflowers. Science 322, 1559–1562. ( 10.1126/science.1161406) [DOI] [PubMed] [Google Scholar]
- 119.Chmátal L, Gabriel SI, Mitsainas GP, Martínez-Vargas J, Ventura J, Searle JB, Schultz RM, Lampson MA. 2014. Centromere strength provides the cell biological basis for meiotic drive and karyotype evolution in mice. Curr. Biol. 24, 2295–2300. ( 10.1016/j.cub.2014.08.017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Fishman L, Kelly JK. 2015. Centromere-associated meiotic drive and female fitness variation in Mimulus. Evolution 69, 1208–1218. ( 10.1111/evo.12661) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tao Y, Araripe L, Kingan SB, Ke Y, Xiao H, Hartl DL. 2007. A sex-ratio meiotic drive system in Drosophila simulans. II. An X-linked distorter. PLoS Biol. 5, 2576–2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Barton NH, Charlesworth B. 1998. Why sex and recombination? Science 281, 1986–1990. ( 10.1126/science.281.5385.1986) [DOI] [PubMed] [Google Scholar]
- 123.Otto SP, Lenormand T. 2002. Resolving the paradox of sex and recombination. Nat. Rev. Genet. 3, 252–261. ( 10.1038/nrg761) [DOI] [PubMed] [Google Scholar]
- 124.Otto SP. 2009. The evolutionary enigma of sex. Am. Nat. 174, S1–S14. ( 10.1086/599084) [DOI] [PubMed] [Google Scholar]
- 125.Mercier R, Mézard C, Jenczewski E, Macaisne N, Grelon M. 2015. The molecular biology of meiosis in plants. Annu. Rev. Plant Biol. 66, 297–327. ( 10.1146/annurev-arplant-050213-035923) [DOI] [PubMed] [Google Scholar]
- 126.Kong X, Murphy K, Raj T, He C, White PS, Matise TC. 2004. A combined linkage-physical map of the human genome. Am. J. Hum. Genet. 75, 1143–1148. ( 10.1086/426405) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Johnston SE, Berenos C, Slate J, Pemberton JM. 2016. Conserved genetic architecture underlying individual recombination rate variation in a wild population of Soay sheep (Ovis aries). Genetics 203, 583–598. ( 10.1534/genetics.115.185553) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ma L, et al. 2015. Cattle sex-specific recombination and genetic control from a large pedigree analysis. PLoS Genet. 11, 1–24. ( 10.1371/journal.pgen.1005387) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kawakami T, Smeds L, Backström N, Husby A, Qvarnström A, Mugal CF, Olason P, Ellegren H. 2014. A high-density linkage map enables a second-generation collared flycatcher genome assembly and reveals the patterns of avian recombination rate variation and chromosomal evolution. Mol. Ecol. 23, 4035–4058. ( 10.1111/mec.12810) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Groenen MAM, et al. 2009. A high-density SNP-based linkage map of the chicken genome reveals sequence features correlated with recombination rate. Genome Res. 19, 510–519. ( 10.1101/gr.086538.108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Li W, Freudenberg J. 2009. Two-parameter characterization of chromosome-scale recombination rate. Genome Res. 19, 2300–2307. ( 10.1101/gr.092676.109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Nagaoka SI, Hassold TJ, Hunt PA. 2012. Human aneuploidy: mechanisms and new insights into an age-old problem. Nat. Rev. Genet. 13, 493–504. ( 10.1038/nrg3245) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hassold T, Hall H, Hunt P. 2007. The origin of human aneuploidy: where we have been, where we are going. Hum. Mol. Genet. 16, 203–208. ( 10.1093/hmg/ddm243) [DOI] [PubMed] [Google Scholar]
- 134.Petronczki M, Siomos MF, Nasmyth K. 2003. Un ménage à quatre: the molecular biology of chromosome segregation in meiosis. Cell 112, 423–440. ( 10.1016/S0092-8674(03)00083-7) [DOI] [PubMed] [Google Scholar]
- 135.Hirose Y, Suzuki R, Ohba T, Hinohara Y, Matsuhara H, Yoshida M, Itabashi Y, Murakami H, Yamamoto A. 2011. Chiasmata promote monopolar attachment of sister chromatids and their co-segregation toward the proper pole during meiosis I. PLoS Genet. 7, e1001329 ( 10.1371/journal.pgen.1001329) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hunter N. 2015. Meiotic recombination: the essence of heredity. Cold Spring Harb. Perspect. Biol. 7, a016618 ( 10.1101/cshperspect.a016618) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hunt PA. 2006. Meiosis in mammals: recombination, non-disjunction and the environment. Biochem. Soc. Trans. 34, 574–577. ( 10.1042/BST0340574) [DOI] [PubMed] [Google Scholar]
- 138.Hassold T, Hunt P. 2001. To err (meiotically) is human: the genesis of human aneuploidy. Nat. Rev. Genet. 2, 280–291. ( 10.1038/35066065) [DOI] [PubMed] [Google Scholar]
- 139.Giraut L, Falque M, Drouaud J, Pereira L, Martin OC, Mézard C. 2011. Genome-wide crossover distribution in Arabidopsis thaliana meiosis reveals sex-specific patterns along chromosomes. PLoS Genet. 7, e1002354 ( 10.1371/journal.pgen.1002354) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Bell G. 1982. The masterpiece of nature: the evolution and genetics of sexuality. Berkeley, CA: University of California Press. [Google Scholar]
- 141.Hawley S, Theurkauf W. 1993. Requiem for distributive segregation: achiasmate segregation in Drosophila females. Trends Genet. 9, 310–316. ( 10.1016/0168-9525(93)90249-H) [DOI] [PubMed] [Google Scholar]
- 142.Krishnaprasad GN, Anand MT, Lin G, Tekkedil MM, Steinmetz LM, Nishant KT. 2015. Variation in crossover frequencies perturb crossover assurance without affecting meiotic chromosome segregation in Saccharomyces cerevisiae. Genetics 199, 399–412. ( 10.1534/genetics.114.172320) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Fledel-Alon A, Wilson DJ, Broman K, Wen X, Ober C, Coop G, Przeworski M. 2009. Broad-scale recombination patterns underlying proper disjunction in humans. PLoS Genet. 5, e1000658 ( 10.1371/journal.pgen.1000658) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wilfert L, Gadau J, Schmid-Hempel P. 2007. Variation in genomic recombination rates among animal taxa and the case of social insects. Heredity 98, 189–197. ( 10.1038/sj.hdy.6800950) [DOI] [PubMed] [Google Scholar]
- 145.Ross L, Blackmon H, Lorite P, Gokhman VE, Hardy NB. 2015. Recombination, chromosome number and eusociality in the Hymenoptera. J. Evol. Biol. 28, 105–116. ( 10.1111/jeb.12543) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Dumont BL, White MA, Steffy B, Wiltshire T, Payseur BA. 2011. Extensive recombination rate variation in the house mouse species complex inferred from genetic linkage maps. Genome Res. 21, 114–125. ( 10.1101/gr.111252.110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Reynolds A, et al. 2013. RNF212 is a dosage-sensitive regulator of crossing-over during mammalian meiosis. Nat. Genet. 45, 269–278. ( 10.1038/ng.2541) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kong A, et al. 2014. Common and low-frequency variants associated with genome-wide recombination rate. Nat. Genet. 46, 11–16. ( 10.1038/ng.2833) [DOI] [PubMed] [Google Scholar]
- 149.Hunter CM, Huang W, Mackay TFC, Singh ND. 2016. The genetic architecture of natural variation in recombination rate in Drosophila melanogaster. PLoS Genet. 12, e1005951 ( 10.1371/journal.pgen.1005951) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Brown A, Feingold E, Broman K, Sherman S. 2000. Genome-wide variation in recombination in female meiosis: a risk factor for non-disjunction of chromosome 21. Hum. Mol. Genet. 9, 515–523. ( 10.1093/hmg/9.4.515) [DOI] [PubMed] [Google Scholar]
- 151.Louis EJ, Borts RH. 2003. Meiotic recombination: too much of a good thing? Curr. Biol. 13, R953–R955. ( 10.1016/j.cub.2003.11.040) [DOI] [PubMed] [Google Scholar]
- 152.Hassold T, Sherman S, Hunt P. 2000. Counting cross-overs: characterizing meiotic recombination in mammals. Hum. Mol. Genet. 9, 2409–2419. ( 10.1093/hmg/9.16.2409) [DOI] [PubMed] [Google Scholar]
- 153.Séguéla-Arnaud M, et al. 2015. Multiple mechanisms limit meiotic crossovers: TOP3α and two BLM homologs antagonize crossovers in parallel to FANCM. Proc. Natl Acad. Sci. USA 112, 4713–4718. ( 10.1073/pnas.1423107112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Berchowitz LE, Copenhaver GP. 2010. Genetic interference: don't stand so close to me. Curr. Genomics 11, 91–102. ( 10.2174/138920210790886835) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Hillers KJ. 2004. Crossover interference. Curr. Biol. 14, R1036–R1037. ( 10.1016/j.cub.2004.11.038) [DOI] [PubMed] [Google Scholar]
- 156.Housworth EA, Stahl FW. 2003. Crossover interference in humans. Am. J. Hum. Genet. 73, 188–197. ( 10.1086/376610) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wang S, Zickler D, Kleckner N, Zhang L. 2015. Meiotic crossover patterns: obligatory crossover, interference and homeostasis in a single process. Cell Cycle 14, 305–314. ( 10.4161/15384101.2014.991185) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Van Veen JE, Hawley RS. 2003. Meiosis: when even two is a crowd. Curr. Biol. 13, 831–833. ( 10.1016/j.cub.2003.10.014) [DOI] [PubMed] [Google Scholar]
- 159.Maguire M. 1980. Adaptive advantage for chiasma interference: a novel suggestion. Heredity 45, 127–131. ( 10.1038/hdy.1980.56) [DOI] [PubMed] [Google Scholar]
- 160.Goldstein DB, Bergman A, Feldman MW. 1993. The evolution of interference: reduction of recombination among three loci. Theor. Popul. Biol. 44, 246–259. ( 10.1006/tpbi.1993.1028) [DOI] [PubMed] [Google Scholar]
- 161.Aggarwal DD, Rashkovetsky E, Michalak P, Cohen I, Ronin Y, Zhou D, Haddad GG, Korol AB. 2015. Experimental evolution of recombination and crossover interference in Drosophila caused by directional selection for stress-related traits. BMC Biol. 13, 101 ( 10.1186/s12915-015-0206-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Burt A, Bell G, Harvey PH. 1991. Sex differences in recombination. J. Evol. Biol. 4, 259–277. ( 10.1046/j.1420-9101.1991.4020259.x) [DOI] [Google Scholar]
- 163.Lenormand T, Dutheil J. 2005. Recombination difference between sexes: a role for haploid selection. PLoS Biol. 3, 396–403. ( 10.1371/journal.pbio.0030063) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Hedrick PW. 2007. Sex: differences in mutation, recombination, selection, gene flow, and genetic drift. Evolution 61, 2750–2771. ( 10.1111/j.1558-5646.2007.00250.x) [DOI] [PubMed] [Google Scholar]
- 165.Haldane JBS. 1922. Sex ratio and unisexual sterility in hybrid animals. J. Genet. 12, 101–109. ( 10.1007/BF02983075) [DOI] [Google Scholar]
- 166.Huxley JS. 1928. Sexual difference of linkage in Gammarus chevreuxi. J. Genet. 20, 145–156. ( 10.1007/BF02983136) [DOI] [Google Scholar]
- 167.Gruhn JR, Al-Asmar N, Fasnacht R, Maylor-Hagen H, Peinado V, Rubio C, Broman KW, Hunt PA, Hassold T. 2015. Correlations between synaptic initiation and meiotic recombination: a study of humans and mice. Am. J. Hum. Genet. 98, 102–115. ( 10.1016/j.ajhg.2015.11.019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Petkov PM, Broman KW, Szatkiewicz JP, Paigen K. 2007. Crossover interference underlies sex differences in recombination rates. Trends Genet. 23, 539–542. ( 10.1016/j.tig.2007.08.015) [DOI] [PubMed] [Google Scholar]
- 169.Morelli MA, Cohen PE. 2005. Not all germ cells are created equal: aspects of sexual dimorphism in mammalian meiosis. Reproduction 130, 761–781. ( 10.1530/rep.1.00865) [DOI] [PubMed] [Google Scholar]
- 170.Burt A. 2000. Perspective: sex, recombination and the efficacy of selection: was Weismann right? Evolution 54, 337–351. ( 10.1111/j.0014-3820.2000.tb00038.x) [DOI] [PubMed] [Google Scholar]
- 171.Mank JE. 2009. The evolution of heterochiasmy: the role of sexual selection and sperm competition in determining sex-specific recombination rates in eutherian mammals. Genet. Res. 91, 355–363. ( 10.1017/S0016672309990255) [DOI] [PubMed] [Google Scholar]
- 172.Lenormand T. 2003. The evolution of sex dimorphism in recombination. Genetics 163, 811–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Úbeda F, Haig D. 2005. On the evolutionary stability of Mendelian segregation. Genetics 170, 1345–1357. ( 10.1534/genetics.104.036889) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Joseph SB, Kirkpatrick M. 2004. Haploid selection in animals. Trends Ecol. Evol. 19, 592–597. ( 10.1016/j.tree.2004.08.004) [DOI] [Google Scholar]
- 175.Brandvain Y, Coop G. 2012. Scrambling eggs: meiotic drive and the evolution of female recombination rates. Genetics 190, 709–723. ( 10.1534/genetics.111.136721) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Paigen K, Petkov P. 2010. Mammalian recombination hot spots: properties, control and evolution. Nat. Rev. Genet. 11, 221–233. ( 10.1038/nrg2712) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.de Massy B. 2013. Initiation of meiotic recombination: how and where? Conservation and specificities among eukaryotes. Annu. Rev. Genet. 47, 563–599. ( 10.1146/annurev-genet-110711-155423) [DOI] [PubMed] [Google Scholar]
- 178.Choi K, Henderson IR. 2015. Meiotic recombination hotspots—a comparative view. Plant J. 83, 52–61. ( 10.1111/tpj.12870) [DOI] [PubMed] [Google Scholar]
- 179.Keeney S. 2008. Spo11 and the formation of DNA double-strand breaks in meiosis. Genome Dyn. Stab. 2, 81–123. ( 10.1007/7050_2007_026) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Kaur T, Rockman MV. 2014. Crossover heterogeneity in the absence of hotspots in Caenorhabditis elegans. Genetics 196, 137–148. ( 10.1534/genetics.113.158857) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Comeron JM, Ratnappan R, Bailin S. 2012. The many landscapes of recombination in Drosophila melanogaster. PLoS Genet. 8, 33–35. ( 10.1371/journal.pgen.1002905) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Pan J, et al. 2011. A hierarchical combination of factors shapes the genome-wide topography of yeast meiotic recombination initiation. Cell 144, 719–731. ( 10.1016/j.cell.2011.02.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Choi K, et al. 2013. Arabidopsis meiotic crossover hot spots overlap with H2A.Z nucleosomes at gene promoters. Nat. Genet. 45, 1327–1336. ( 10.1038/ng.2766) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Singhal S, et al. 2015. Stable recombination hotspots in birds. Science 350, 928–932. ( 10.1126/science.aad0843) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Wijnker E, et al. 2013. The genomic landscape of meiotic crossovers and gene conversions in Arabidopsis thaliana. Elife 2013, 1–22. ( 10.7554/eLife.01426) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Brick K, Smagulova F, Khil P, Camerini-Otero RD, Petukhova GV. 2012. Genetic recombination is directed away from functional genomic elements in mice. Nature 485, 642–645. ( 10.1038/nature11089) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Baudat F, Buard J, Grey C, Fledel-Alon A, Ober C, Przeworski M, Coop G, de Massy B. 2010. PRDM9 is a major determinant of meiotic recombination hotspots in humans and mice. Science 327, 836–840. ( 10.1126/science.1183439) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Stevison LS, Woerner AE, Kidd JM, Kelley JL, Veeramah KR, McManus KF, Bustamante CD, Hammer MF, Wall JD. 2016. The time scale of recombination rate evolution in great apes. Mol. Biol. Evol. 33, 928–945. ( 10.1093/molbev/msv331) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Auton A, et al. 2013. Genetic recombination is targeted towards gene promoter regions in dogs. PLoS Genet. 9, e1003984 ( 10.1371/journal.pgen.1003984) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Fyon F, Cailleau A, Lenormand T. 2015. Enhancer runaway and the evolution of diploid gene expression. PLoS Genet. 11, e1005665 ( 10.1371/journal.pgen.1005665) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Jeffreys AJ, Neumann R. 2002. Reciprocal crossover asymmetry and meiotic drive in a human recombination hot spot. Nat. Genet. 31, 267–271. ( 10.1038/ng910) [DOI] [PubMed] [Google Scholar]
- 192.Boulton A, Myers RS, Redfield RJ. 1997. The hotspot conversion paradox and the evolution of meiotic recombination. Proc. Natl Acad. Sci. USA 94, 8058–8063. ( 10.1073/pnas.94.15.8058) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Úbeda F, Wilkins JF. 2011. The Red Queen theory of recombination hotspots. J. Evol. Biol. 24, 541–553. ( 10.1111/j.1420-9101.2010.02187.x) [DOI] [PubMed] [Google Scholar]
- 194.Lesecque Y, Glémin S, Lartillot N, Mouchiroud D, Duret L. 2014. The Red Queen model of recombination hotspots evolution in the light of archaic and modern human genomes. PLoS Genet. 10, 1–14. ( 10.1371/journal.pgen.1004790) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Lynch M, Field MC, Goodson HV, Malik HS, Pereira-Leal JB, Roos DS, Turkewitz AP, Sazer S. 2014. Evolutionary cell biology: two origins, one objective. Proc. Natl Acad. Sci. USA 111, 16 990–4. ( 10.1073/pnas.1415861111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Rice WR. 2013. Nothing in genetics makes sense except in light of genomic conflict. Annu. Rev. Ecol. Evol. Syst. 44, 217–237. ( 10.1146/annurev-ecolsys-110411-160242) [DOI] [Google Scholar]