Abstract
Quantitative analysis with mass spectrometry (MS) is important but challenging. Matrix-assisted laser desorption/ionization (MALDI) coupled with time-of-flight (TOF) MS offers superior sensitivity, resolution and speed, but such techniques have numerous disadvantages that hinder quantitative analyses. This review summarizes essential obstacles to analyte quantification with MALDI-TOF MS, including the complex ionization mechanism of MALDI, sensitive characteristics of the applied electric fields and the mass-dependent detection efficiency of ion detectors. General quantitative ionization and desorption interpretations of ion production are described. Important instrument parameters and available methods of MALDI-TOF MS used for quantitative analysis are also reviewed.
This article is part of the themed issue ‘Quantitative mass spectrometry’.
Keywords: ionization mechanism, timing characteristic, ion optics, detection efficiency, photoreaction, instrument parameters
1. Introduction
Quantifying molecular analytes is fundamental to scientific research and applications. Along with molecular identification, quantification reveals molecular information on physical and chemical properties as well as how molecules interact with their surroundings. Molecular abundance variations are also important indicators of chemical reactions, diseases and pollutants [1, see ch. 5, pp. 157–167 and chs. 10–13, pp. 249–331]. Among the important analytical techniques, mass spectrometry (MS) offers great accuracy, sensitivity and speed. In modern MS, a matrix-assisted laser desorption/ionization (MALDI) ion source coupled with a time-of-flight (TOF) mass spectrometer is one of the most widely used instruments for protein, carbohydrate and polymer studies. Quantification of analytes with MALDI-TOF MS, however, is highly challenging and should be done with care.
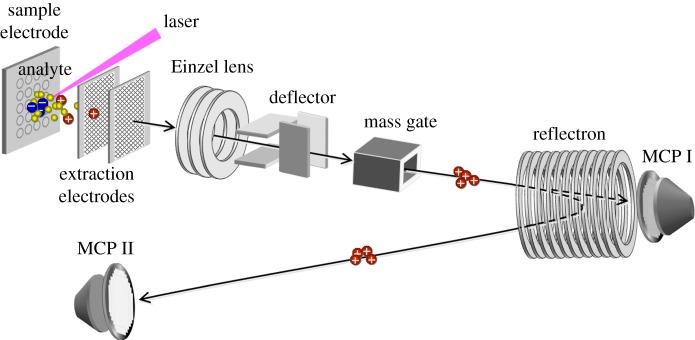
MALDI-TOF MS's ability to quantify the presence of different analytes depends on many factors. These factors are based on three MS components: the ion source, mass analyser and detector. Figure 1 depicts a typical configuration of a MALDI-TOF mass spectrometer. The MALDI ion source comprises a sample electrode and two extraction electrodes. The mass analyser contains an Einzel lens to focus the ion beam, deflector plates to adjust the ion trajectory, a mass gate (or timed ion selector) to suppress unwanted ions, and a reflectron to improve the mass resolving power. The most critical factor associated with the ion source is the ionization efficiency of the analytes. Such efficiency depends on the intrinsic properties of the analytes and ionization mechanisms. Sample morphologies also affect the reproducibility of spectra. In the mass analyser, quantitative capability is affected by ion transmission efficiency through the TOF mass analyser. Transmission efficiency reflects the configurations and timing characteristics of the electric fields used to guide the ions to the ion detectors. Ion lifetimes are also important, as ions spend most of their time in the TOF mass analyser [1, see ch. 5, pp. 157–167 and chs. 10–13, pp. 249–331]. The third factor is the detection efficiency of the ion detectors. On the whole, however, with properly and carefully designed experiments, including standard calibrations, quantitative analyses in MALDI-TOF MS can be achieved [2–4].
Figure 1.
Configuration of MALDI-TOF mass spectrometer. The ion source comprises a sample electrode and two extraction electrodes. The mass analyser contains an Einzel lens, deflectors, a mass gate and a reflector. Ions are detected with MCP I and II in linear and reflectron modes, respectively. (Online version in colour.)
This review discusses the current status of analyte quantification with MALDI-TOF MS. The first part of this review discusses MALDI ionization and desorption mechanisms, including a general quantitative interpretation of ion yields based on chemical equilibria. The impact of mass spectrometric components and the methods used to obtain quantitative information are discussed in §3, including ways of improving the ion transmission efficiency through the TOF mass analyser and the detection properties of the ion detectors. Finally, in §4, current strategies for MALDI-TOF MS usage in quantitative measurement- and quantification-related issues are reviewed.
2. Ion production in matrix-assisted laser desorption/ionization
MALDI generates protonated (and deprotonated) analytes via complex reactions. To facilitate ionization, the analytes are typically dissolved in solution and co-crystallized with matrix compounds before measurements. After crystallization, ionization of analytes is achieved with photochemical reactions induced by a pulsed UV laser beam [5]. The initial photochemical reactions are followed by fast desorption of irradiated species from the solid to the gas phase. Decomposition of ions may occur if ions gain high internal energy through these processes.
The ionization efficiency of analytes depends on the matrix [6,7], analyte-to-matrix ratio [8,9], crystal morphology [10–13], laser energy [14,15] and others. Because no single experimental condition offers equal ionization efficiency for every analyte, a general reaction model is necessary for the optimization of experimental conditions in quantitative analysis. However, the MALDI reaction models proposed in the literature remain controversial owing to inconsistent experimental results. For example, the observed ion-to-neutral ratios of desorbed materials range from 10−3 to 10−8 [16–20]. This inconsistency is mainly attributed to the complexity of the ionization mechanisms.
(a). Ionization
The ionization reactions in MALDI have been qualitatively described by numerous models. These models can be summarized into two categories: photoreaction and pre-charging. The pre-charging model proposes that analytes preserve their charges in solution after crystallization, and lasers serve as energy sources to liberate ions during the transition from the solid to the gas phase [21]. The pre-charging model is not included here because a quantitative interpretation of this model is not yet available. In the photoreaction model, on the other hand, the principal ionization reaction comprises two consecutive steps. The first step is the generation of matrix ions by photochemical reactions, and the second step is charge transfer from the matrix ions to neutral analytes [22–25]. For the initial ionization of matrices, two important reaction pathways are electron depletion [22,26,27], as shown in scheme 1, and proton disproportionation, as shown in scheme 2 [28–30].
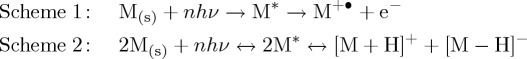 |
Here M and M* stand for matrices in the ground and excited electronic states, respectively. The matrix radical cation in scheme 1 undergoes complex ion--molecule reactions to produce protonated matrices. The early quantitative ionization model based on scheme 1 proposed that electron depletion is facilitated via multiphoton absorption or annihilation processes [31,32]. Knochenmuss proposed that three excited matrix molecules pool their energy to promote one of them to the ionization continuum [26,33]. By considering the decrease in the ionization energy of the matrix in the solid phase, Liu et al. demonstrated that electron depletion can be achieved by an energy corresponding to fewer than two UV photons [27].
In contrast with electron depletion, thermal or quasi-thermal ionization models were proposed to produce matrix proton pairs based on scheme 2 [28,30,34]. For example, Lai and co-workers estimated ion abundance based on chemical equilibrium between two 2,4,6-trihydroxyacetophenone (THAP) molecules,
The equilibrium constant (k1) can be estimated from the predicted Gibbs free energy [28].
The second step of the photoreaction model is proton transfer from protonated matrices to neutral analytes via ion--molecule collision. Such reactive collisions occur numerously in the initial phase-transition process. The efficiency of such proton-transfer reactions relies on the proton affinity (PA) of the analytes and matrices [22,35–37]. The higher the PA of the analyte, the higher the analyte ion yield is. For analytes with low PA, such as carbohydrates, ionization commonly forms alkali ion adducts [38,39]. Such ionization products are presumably produced via pre-charging reactions [21].
(b). Desorption
Desorption is mainly induced by sudden heating of matrix crystals during laser excitation. Desorption processes in MALDI have been described quantitatively in more detail than ionization [40–43]. The desorption efficiency of materials depends on the intrinsic properties of the samples and the excitation conditions, such as the molecular weight [43], crystal morphology [44–46] and laser conditions [5,47–49].
A comprehensive interpretation of ion yield should simultaneously consider ionization and desorption processes. Dreisewerd et al. [5] quantitatively described the desorption process by Arrhenius-type equations. In this interpretation, ion abundance (Iion) is a function of desorption activation energy (Ea, J), initial crystal temperature (T0, K) and laser fluence (F, J m−2). By using transition state theory in the estimation of the desorption rate, Lai et al. [50] developed a comprehensive interpretation of ion yield:
| 2.1 |
Here, A is the pre-exponential factor, k is the Boltzmann constant, h is the Planck constant, γ is the conversion factor between laser fluence and surface temperature change, and ν is the vibrational frequency of the intermolecular bond. The concentration of surface ions [ion]s can be estimated using the equilibrium constant of the reactions in scheme 1 or 2. Observation shows crystal temperature rising instantaneously by approximately 1000 K and rapidly decreasing due to expansion cooling [51]. When the laser fluence is above the ion production threshold, the signal intensity increases exponentially with the increase in laser fluence, as shown in figure 2 [5]. As a high ion signal produced with high laser energy causes signal saturation, the best laser fluence for quantitative analysis is slightly above the threshold value [52].
Figure 2.
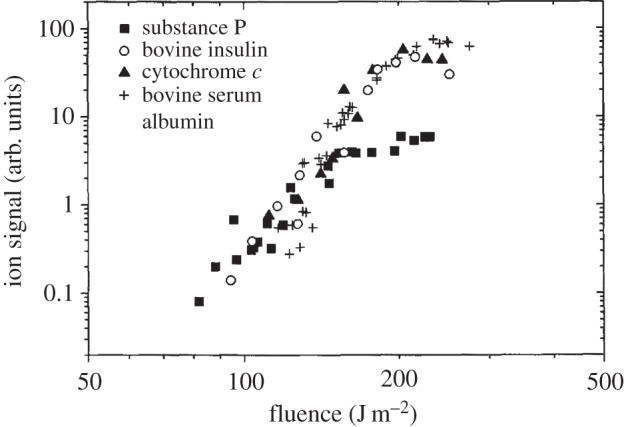
Change of ion abundance of various biomolecules with laser fluence. The result shows that ion signal intensity increases exponentially with the increase of laser fluence. (Reproduced with permission from Dreisewerd et al. [5] Copyright © 1995 Elsevier.)
(c). Decomposition
Ions produced in MALDI may decompose extensively because they typically have high internal energy. Decomposition results in unwanted ion loss and chemical noise that seriously affect the accuracy of quantitative analysis. Ion decomposition can be reduced by selecting ‘soft’ matrices [53] or decreasing the laser fluence [54].
3. Instrument performance
Instrument performance impacts quantification ability via the electric field and ion optics, ion extraction times and ion detection. Because individual ions exhibit distinct desorption properties and stability, their optimal extraction conditions in the ion source region vary. In TOF mass analysers, ion transmission is optimized with electrical components. The detection efficiency of ion detectors also critically affects the spectral pattern of MALDI-TOF MS data.
(a). Electric field and ion optics
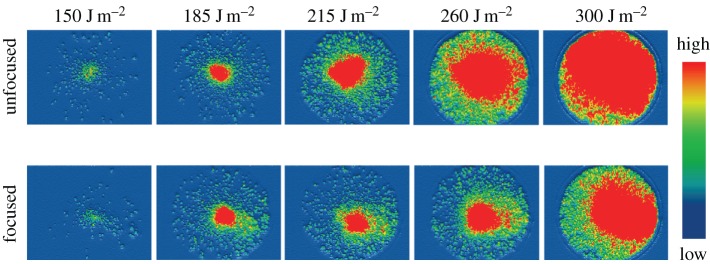
Reliable quantitative information is available when proper ion transmission efficiency is achieved. Ion transmission efficiency reduces due to ion decomposition and poor ion trajectory. To suppress ion decomposition, as mentioned, the ionization conditions need to be changed. In addition, ions move both axially and radially after desorption [55], and the resultant expansion of the ion packets arriving at the detector increase with laser energy. Figure 3 gives ion populations for a 40 mm diameter detector surface using different laser fluences [50]. With an unfocused ion beam (upper row), a considerable portion of the ion beam misses the detection area when the highest laser fluence is used. Ion loss due to oversized distributions can be minimized using an Einzel lens and electrostatic deflectors to focus and adjust the ion beams (lower row), respectively [56,57]. To avoid signal saturation, as also mentioned above, it is important to use medium or low laser fluences.
Figure 3.
Spatial distribution of ions at detector surface under various experimental conditions. The upper row shows that ion distribution increases with laser fluence. The ion distribution exceeds that of the detection area with the highest laser fluence used in the measurement. The lower row shows that ion distribution can be reduced to obtain correct quantity information by using Einzel lens systems. (Reproduced with permission from Lai et al. [50]. Copyright © 2010 American Chemical Society.)
(b). Timing characteristics of electric fields
MALDI-TOF MS sensitivity changes with the timing of ion extraction, or extraction delay. Delayed extraction is a well-established means of enhancing mass resolving power by reducing the spatial spread among ion packets during MALDI [58]. Another notable advantage is the significant suppression of metastable decay [59,60] due to the reduced number of ion--molecule collisions after extraction delay. Although higher sensitivity provides more accurate quantitative information, optimal extraction delay is mass-sensitive. Observations show that heavier ions require longer extraction delays and vice versa [61]. In this case, estimating analyte amounts based on relative signal intensity in MALDI-TOF MS is unreliable for ions with significant mass differences.
The timing characteristics of mass gates (figure 1) also affect the accuracy of quantification. High-mass range sensitivity is dependent on ion detector saturation with lighter ions. In order to obtain a correct signal intensity for quantity estimations, all unwanted low-mass ions are deflected by appropriate timing of mass gating [62]. When examining proteins and peptides with MALDI-TOF MS, a low-mass cut-off is typically used to enhance the sensitivity for high-mass ions [63].
(c). Detector performance
MALDI-TOF mass analysers detect ions using microchannel plate (MCP) detectors [64]. An MCP detects incoming ions by amplifying secondary electrons ejected during high-velocity ion--surface collisions through a cascade reaction within microchannels. The resultant electric current collected by the anode is digitized. Thus, the detection efficiency of MCPs depends not only on the magnitude of amplification of secondary electrons, but more importantly on the conversion efficiency of primary ions to secondary electrons. A conventional single-disc MCP works when it is supplied with 600 V across the MCP. It reaches maximum gain (approx. 104) at 1000 V. As MCP signal intensity depends critically on the supplied voltage, it is important to ensure that the voltage supply does not vary.
Notably, operating MCPs in high-gain mode can easily cause saturation of microchannels when the secondary electron density reaches the space-charge limit [65], or when MCP surfaces are unable to deliver enough electric current to sustain an amplification reaction. Further, longer microchannels result in longer response times [64], which may distort the shape of the spectral signal, resulting in inaccurate peaks and signal intensity. If signal saturation or peak distortion occurs, ion abundance may be underestimated.
The conversion efficiency of secondary electrons from primary ions is mass-dependent [66–68]. Mass dependence varies with the efficiency of the MCP to generate secondary electrons, which in turn depends on ion velocity during MCP ion--surface collisions [68]. Figure 4 shows observed secondary electron yields with changing ion mass and ion kinetic energy [68]. As all ions possess the same kinetic energy, high-mass ions travel with lower speed than low-mass ions. This distinction in ion speed is the key principle behind TOF MS. Therefore, ions with higher mass generate fewer secondary electrons [69–71]. Although increasing acceleration voltage at the sample electrode restores high-mass ion detection efficiency, it is unable to compensate for the sensitivity difference between high- and low-mass ions. The only feasible way to account for this problem is by using internal standard compounds, which will be described in §4b.
Figure 4.
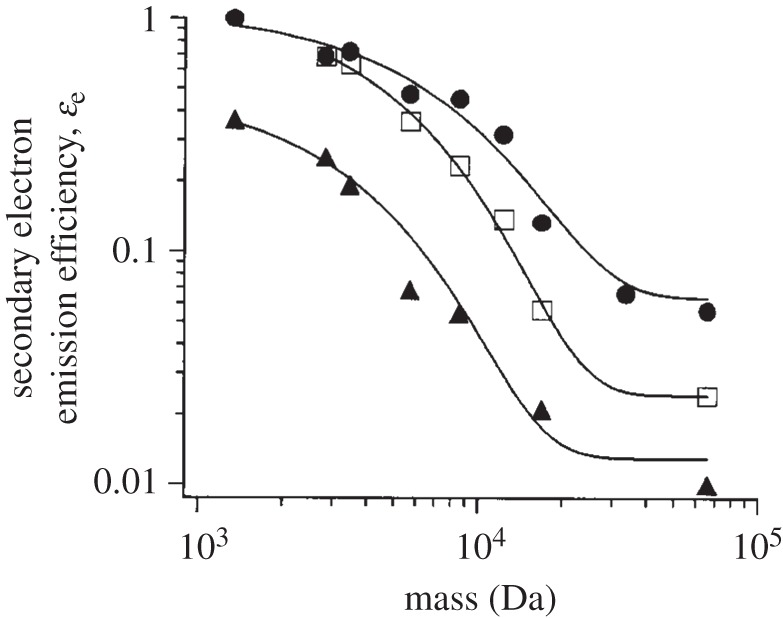
Change of secondary electron emission efficiency with ion mass and ion kinetic energy. The solid circles, open squares and solid triangles represent ion kinetic energy of 30, 20 and 10 keV, respectively. (Adapted from Westmacott et al. [68].)
When an ion enters a microchannel, amplification reactions proceed until all the secondary electrons exit the microchannel. Before amplification is complete, in a given microchannel, it is unable to deliver amplification to another incoming ion. The period for a microchannel to fully recover is termed dead time, and it is typically in the millisecond range [64,72]. As most data acquisition times under MALDI-TOF MS are in the tens of microseconds, every microchannel can only receive one effective ion per acquisition event. Although MCP discs usually comprise a million microchannels, the dead time is still a property that needs to be considered in quantitative mass analysis [73]. If large numbers of ions are produced or the ion beam is focused on to a small area of the MCP, ion abundance may be considerably underestimated. Therefore, it is recommended to project ion beams across the entire area of the MCP.
Aside from the intrinsic properties of MCP detectors, accurate data analysis also depends on the properties of digitizers [74]. Electric signals delivered from MCPs are typically converted to electronic signals with analogue-to-digital (ADC) or time-to-digital (TDC) converters. An ADC directly converts electric currents to voltages, so ion abundance can be estimated based on the corresponding signal intensity. ADCs are suitable for detecting mid- to high-flux ion beams, but their accuracy in determining low-flux ion beams is limited by electronic noise. By contrast, TDCs count individual ions present at a time segment with typical resolution in the sub- to low-nanosecond range. TDCs are suitable for identifying low-flux ion beams, but ion abundance may be considerably underestimated if high ion flux is present. To achieve the highest analytical performance, it is possible to combine multiple digitizers in series [75,76]. Another novel strategy proposed is the use of an anode array (known as sectioned anodes) to collect electrons generated by MCPs [77,78].
4. Available methods
Quantitative analysis with MALDI-TOF MS is challenging because signal intensity in mass spectra varies with sample composition, sample morphology, laser conditions and depletion of samples during continuous laser exposure. Inhomogeneous sample morphology is also a crucial obstacle that leads to poor signal reproducibility (including the so-called sweet spot and coffee-ring effects) [79,80]. In order to perform quantitative analysis with the highest accuracy, it is important to find the optimal sample preparation method and to perform standard calibrations.
(a). Sample preparation methods
A major reason for poor reproducibility is inhomogeneous sample morphologies resulting from the dried-droplet (DD) method [43,81]. Such a problem is matrix-dependent. For example, samples prepared with THAP and α-cyano-4-hydroxycinnamic acid (CHCA) form homogeneous crystal morphologies, whereas those with 2,5-dihydroxybenzoic acid and sinapinic acid (SA) form irregular sample morphologies. To reduce data variation, hundreds of laser shots across randomly selected sample positions are used to obtain averaged spectra [82]. As CHCA provides higher ion yields than other matrices, it is the most commonly used matrix for quantitative studies [2,3,83]. Signal reproducibility is also improved by ionic liquid matrices [84,85], solvent-free sample preparation [86,87], small droplet deposition [88] and prestructured sample supports [89–91]. However, changing sample composition and preparation methods change ion yields, and the durability of prestructured sample supports is limited. Therefore, one has to compromise with all factors during analysis.
Alternatively, changing sample drying methods or surface properties change the resultant sample morphologies. Improved sample morphologies are available by preparing samples with fast evaporation [10,92], seeded layer (SL) [79,93], sandwich [94], electrospray deposition [95] and sublimation [44,96] methods. These methods provide fine and uniform crystals that provide superior signal reproducibility compared to conventional methods. Figure 5 shows the crystal morphologies of various matrices prepared with DD and SL methods [79]. With the SL method, significantly finer crystals than those with the DD method are produced. Drying samples under vacuum [97] or on a substrate with well-controlled surface and environmental temperatures [98] also provides finer sample homogeneity and signal reproducibility.
Figure 5.
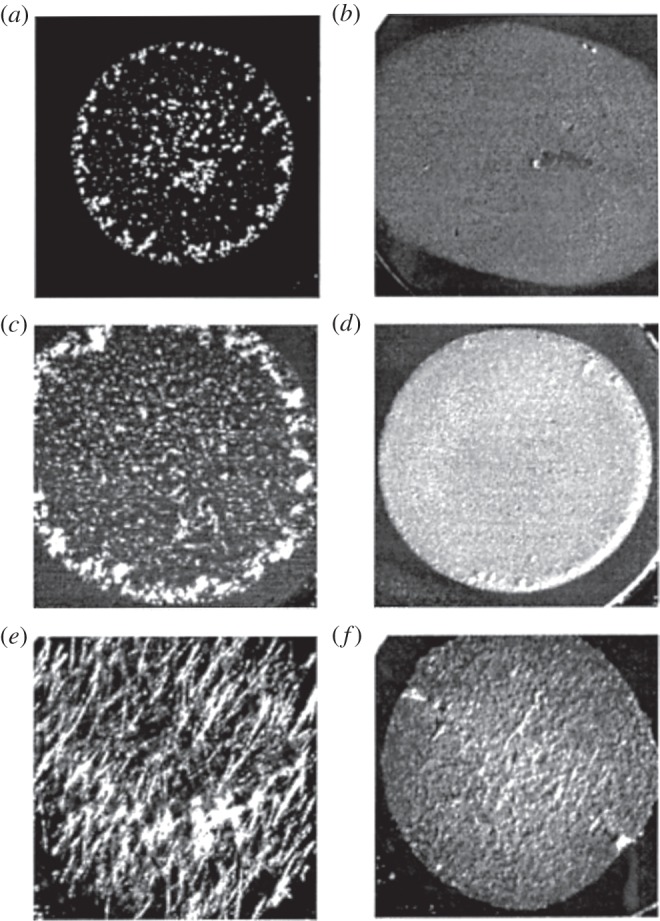
Crystal morphologies of three matrices prepared with DD and SL methods. (a) DD CHCA, (b) SL CHCA, (c) DD SA, (d) SL SA, (e) DD ferulic acid (FA) and (f) SL FA. (Adapted from Onnerfjord et al. [79].)
(b). Standard calibration
Standard calibration can be conducted externally or internally. In external calibration, the signal intensity of analytes is correlated to calibration curves determined with analytes of known concentration. However, external calibration may result in error because the ionization efficiencies of analytes change with composition. One such phenomenon is ion suppression effects observed in complex samples, especially when analysing trace analytes in biological fluids. On the other hand, internal calibration by mixing standard compounds in analyte mixtures minimizes uncertainties in quantitative analysis [99,100]. Ideal internal standards are compounds with similar chemical structures as the analyte. Isotopically labelled analyte analogues offer the best results for quantification analyses [4,101], including isotope-coded affinity tags [102], and isobaric tags for relative and absolute quantitation [103].
5. Conclusion
Quantitative analysis with MALDI-TOF MS is feasible but requires consideration of the sample and instrument conditions. The most critical problems are sample properties, ionization chemistry, electric fields and detector properties. Because the parameters are interrelated, adjusting one parameter changes the optimal conditions of others. Even though high-quality mass spectra can be obtained with optimal experimental conditions, quantitative analysis of MALDI-TOF mass spectra should be interpreted with special care. The most critical challenges lie in ionization chemistry and detector efficiency. For example, the development of a universal matrix providing equal ionization efficiency to all analytes is highly necessary. On the other hand, new ion detectors providing equal efficiency to light and heavy ions and free of dead time problems are critical for improving data reliability.
Authors' contribution
C.-C.W., Y.-H.L. and Y.-M.O. summarized the literature articles, drafted and revised the manuscript; H.-T.C. supervised Y.-M.O. and reviewed the manuscript; and Y.-S.W. summarized the literature articles, drafted and prepared the final manuscript.
Competing interests
We declare we have no competing interests.
Funding
This work was supported by the Genomics Research Center, Academia Sinica and the Ministry of Science and Technology of Taiwan under contract no. 104-2119-M-001-014.
References
- 1.Urban P, Chen Y-C, Wang Y-S. 2016. Time-resolved mass spectrometry. New York, NY: Wiley. [Google Scholar]
- 2.Sleno L, Volmer DA. 2005. Some fundamental and technical aspects of the quantitative analysis of pharmaceutical drugs by matrix-assisted laser desorption/ionization mass spectrometry. Rapid Commun. Mass Spectrom. 19, 1928–1936. ( 10.1002/rcm.2006) [DOI] [PubMed] [Google Scholar]
- 3.Szajli E, Feher T, Medzihradszky KF. 2008. Investigating the quantitative nature of MALDI-TOF MS. Mol. Cell Proteomics 7, 2410–2418. ( 10.1074/mcp.M800108-MCP200) [DOI] [PubMed] [Google Scholar]
- 4.Bucknall M, Fung KYC, Duncan MW. 2002. Practical quantitative biomedical applications of MALDI-TOF mass spectrometry. J. Am. Soc. Mass Spectrom. 13, 1015–1027. ( 10.1016/s1044-0305(02)00426-9) [DOI] [PubMed] [Google Scholar]
- 5.Dreisewerd K, Schurenberg M, Karas M, Hillenkamp F. 1995. Influence of the laser intensity and spot size on the desorption of molecules and ions in matrix-assisted laser desorption/ionization with a uniform beam profile. Int. J. Mass Spectrom. 141, 127–148. ( 10.1016/0168-1176(94)04108-j) [DOI] [Google Scholar]
- 6.Krause J, Stoeckli M, Schlunegger UP. 1996. Studies on the selection of new matrices for ultraviolet matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom. 10, 1927–1933. ( 10.1002/(SICI)1097-0231(199612)10:15%3C1927::AID-RCM709%3E3.0.CO;2-V) [DOI] [PubMed] [Google Scholar]
- 7.Zenobi R, Knochenmuss R. 1998. Ion formation in MALDI mass spectrometry. Mass Spectrom. Rev. 17, 337–366. ( 10.1002/(Sici)1098-2787(1998)17:5%3C337::AID-MAS2%3E3.0.CO;2-S) [DOI] [Google Scholar]
- 8.Yao J, Scott JR, Young MK, Wilkins CL. 1998. Importance of matrix : analyte ratio for buffer tolerance using 2,5-dihydroxybenzoic acid as a matrix in matrix-assisted laser desorption/ionization Fourier transform mass spectrometry and matrix-assisted laser desorption/ionization time of flight. J. Am. Soc. Mass Spectrom. 9, 805–813. ( 10.1016/s1044-0305(98)00046-4) [DOI] [PubMed] [Google Scholar]
- 9.Tsai ST, Chen CH, Lee YT, Wang YS. 2008. Desorption dynamics of neutral molecules in matrix-assisted laser desorption/ionization. Mol. Phys. 106, 239–247. ( 10.1080/00268970701779671) [DOI] [Google Scholar]
- 10.Vorm O, Roepstorff P, Mann M. 1994. Improved resolution and very high sensitivity in MALDI TOF of matrix surfaces made by fast evaporation. Anal. Chem. 66, 3281–3287. ( 10.1021/ac00091a044) [DOI] [Google Scholar]
- 11.Chen Y, Vertes A. 2003. Pumping rate and surface morphology dependence of ionization processes in matrix-assisted laser desorption ionization. J. Phys. Chem. A 107, 9754–9761. ( 10.1021/jp035844u) [DOI] [Google Scholar]
- 12.Shimma S, Takashima Y, Hashimoto J, Yonemori K, Tamura K, Hamada A. 2013. Alternative two-step matrix application method for imaging mass spectrometry to avoid tissue shrinkage and improve ionization efficiency. J. Mass Spectrom. 48, 1285–1290. ( 10.1002/jms.3288) [DOI] [PubMed] [Google Scholar]
- 13.Westman A, Huthfehre T, Demirev P, Sundqvist BUR. 1995. Sample morphology effects in matrix-assisted laser desorption/ionization mass spectrometry of proteins. J. Mass Spectrom. 30, 206–211. ( 10.1002/jms.1190300131) [DOI] [Google Scholar]
- 14.Westmacott G, Ens W, Hillenkamp F, Dreisewerd K, Schurenberg M. 2002. The influence of laser fluence on ion yield in matrix-assisted laser desorption ionization mass spectrometry. Int. J. Mass Spectrom. 221, 67–81. ( 10.1016/S1387-3806(02)00898-9) [DOI] [Google Scholar]
- 15.Guenther S, Koestler M, Schulz O, Spengler B. 2010. Laser spot size and laser power dependence of ion formation in high resolution MALDI imaging. Int. J. Mass Spectrom. 294, 7–15. ( 10.1016/j.ijms.2010.03.014) [DOI] [Google Scholar]
- 16.Tsai M-T, Lee S, Lu IC, Chu KY, Liang C-W, Lee CH, Lee YT, Ni C-K. 2013. Ion-to-neutral ratio of 2,5-dihydroxybenzoic acid in matrix-assisted laser desorption/ionization. Rapid Commun. Mass Spectrom. 27, 955–963. ( 10.1002/rcm.6534) [DOI] [PubMed] [Google Scholar]
- 17.Bae YJ, Shin YS, Moon JH, Kim MS. 2012. Degree of ionization in MALDI of peptides: thermal explanation for the gas-phase ion formation. J. Am. Soc. Mass Spectrom. 23, 1326–1335. ( 10.1007/s13361-012-0406-y) [DOI] [PubMed] [Google Scholar]
- 18.Ens W, Mao Y, Mayer F, Standing KG. 1991. Properties of matrix-assisted laser desorption measurements with a time-to-digital converter. Rapid Commun. Mass Spectrom. 5, 117–123. ( 10.1002/rcm.1290050306) [DOI] [PubMed] [Google Scholar]
- 19.Mowry CD, Johnston MV. 1993. Simultaneous detection of ions and neutrals produced by matrix-assisted laser desorption. Rapid Commun. Mass Spectrom. 7, 569–575. ( 10.1002/rcm.1290070702) [DOI] [Google Scholar]
- 20.Quist AP, Huthfehre T, Sundqvist BUR. 1994. Total yield measurements in matrix-assisted laser desorption using a quartz crystal microbalance. Rapid Commun. Mass Spectrom. 8, 149–154. ( 10.1002/rcm.1290080204) [DOI] [Google Scholar]
- 21.Karas M, Kruger R. 2003. Ion formation in MALDI: the cluster ionization mechanism. Chem. Rev. 103, 427–439. ( 10.1021/cr010376a) [DOI] [PubMed] [Google Scholar]
- 22.Ehring H, Karas M, Hillenkamp F. 1992. Role of photoionization and photochemistry in ionization processes of organic molecules and relevance for matrix-assisted laser desorption ionization mass spectrometry. Org. Mass Spectrom. 27, 472–480. ( 10.1002/oms.1210270419) [DOI] [Google Scholar]
- 23.Liao PC, Allison J. 1995. Ionization processes in matrix-assisted laser desorption/ionization mass spectrometry: matrix-dependent formation of [M + H]+ vs [M + Na]+ ions of small peptides and some mechanistic comments. J. Mass Spectrom. 30, 408–423. ( 10.1002/jms.1190300304) [DOI] [Google Scholar]
- 24.Breuker K, Knochenmuss R, Zhang J, Stortelder A, Zenobi R. 2003. Thermodynamic control of final ion distributions in MALDI: in-plume proton transfer reactions. Int. J. Mass Spectrom. 226, 211–222. ( 10.1016/S1387-3806(02)00965-X) [DOI] [Google Scholar]
- 25.Knochenmuss R, Stortelder A, Breuker K, Zenobi R. 2000. Secondary ion--molecule reactions in matrix-assisted laser desorption/ionization. J. Mass Spectrom. 35, 1237–1245. ( 10.1002/1096-9888(200011)35:11%3C1237::AID-JMS74%3E3.0.CO;2-O) [DOI] [PubMed] [Google Scholar]
- 26.Knochenmuss R. 2002. A quantitative model of ultraviolet matrix-assisted laser desorption/ionization. J. Mass Spectrom. 37, 867–877. ( 10.1002/jms.349) [DOI] [PubMed] [Google Scholar]
- 27.Liu B-H, Charkin OP, Klemenko NM, Chen C-W, Wang Y-S. 2010. Initial ionization reaction in matrix-assisted laser desorption/ionization. J. Phys. Chem. B 114, 10 853–10 859. ( 10.1021/jp104178m) [DOI] [PubMed] [Google Scholar]
- 28.Lai Y-H, Chen B-G, Lee YT, Wang Y-S, Lin SH. 2014. Contribution of thermal energy to initial ion production in matrix-assisted laser desorption/ionization observed with 2,4,6-trihydroxyacetophenone. Rapid Commun. Mass Spectrom. 28, 1716–1722. ( 10.1002/rcm.6952) [DOI] [PubMed] [Google Scholar]
- 29.Chu KY, Lee S, Tsai MT, Lu IC, Dyakov YA, Lai YH, Lee YT, Ni CK. 2014. Thermal proton transfer reactions in ultraviolet matrix-assisted laser desorption/ionization. J. Am. Soc. Mass Spectrom. 25, 310–318. ( 10.1007/s13361-013-0792-9) [DOI] [PubMed] [Google Scholar]
- 30.Bae YJ, Kim MS. 2015. A thermal mechanism of ion formation in MALDI. Annu. Rev. Anal. Chem. 8, 41–60. ( 10.1146/annurev-anchem-081413-024102) [DOI] [PubMed] [Google Scholar]
- 31.Ludemann HC, Redmond RW, Hillenkamp F. 2002. Singlet--singlet annihilation in ultraviolet matrix-assisted laser desorption/ionization studied by fluorescence spectroscopy. Rapid Commun. Mass Spectrom. 16, 1287–1294. ( 10.1002/rcm.712) [DOI] [PubMed] [Google Scholar]
- 32.Allwood DA, Dyer PE, Dreyfus RW. 1997. Ionization modelling of matrix molecules in ultraviolet matrix-assisted laser desorption/ionization. Rapid Commun. Mass Spectrom. 11, 499–503. ( 10.1002/(Sici)1097-0231(199703)11:5%3C499::AID-RCM880%3E3.0.CO;2-2) [DOI] [Google Scholar]
- 33.Knochenmuss R. 2003. A quantitative model of ultraviolet matrix-assisted laser desorption/ionization including analyte ion generation. Anal. Chem. 75, 2199–2207. ( 10.1021/ac034032r) [DOI] [PubMed] [Google Scholar]
- 34.Lu IC, Lee C, Lee YT, Ni CK. 2015. Ionization mechanism of matrix-assisted laser desorption/ionization. Annu. Rev. Anal. Chem. 8, 21–39. ( 10.1146/annurev-anchem-071114-040315) [DOI] [PubMed] [Google Scholar]
- 35.Harrison AG. 1997. The gas-phase basicities and proton affinities of amino acids and peptides. Mass Spectrom. Rev. 16, 201–217. ( 10.1002/(SICI)1098-2787(1997)16:4%3C201::AID-MAS3%3E3.0.CO;2-L) [DOI] [Google Scholar]
- 36.Jebber KA, Zhang K, Cassady CJ, Chung-Phillips A. 1996. Ab initio and experimental studies on the protonation of glucose in the gas phase. J. Am. Chem. Soc. 118, 10 515–10 524. ( 10.1021/Ja960427z) [DOI] [Google Scholar]
- 37.Knochenmuss R. 2006. Ion formation mechanisms in UV-MALDI. Analyst 131, 966–986. ( 10.1039/b605646f) [DOI] [PubMed] [Google Scholar]
- 38.Mohr MD, Bornsen KO, Widmer HM. 1995. Matrix-assisted laser-desorption ionization mass-spectrometry—improved matrix for oligosaccharides. Rapid Commun. Mass Spectrom. 9, 809–814. ( 10.1002/rcm.1290090919) [DOI] [PubMed] [Google Scholar]
- 39.Zhang J, Ha TK, Knochenmuss R, Zenobi R. 2002. Theoretical calculation of gas-phase sodium binding energies of common MALDI matrices. J. Phys. Chem. A 106, 6610–6617. ( 10.1021/Jp0203548) [DOI] [Google Scholar]
- 40.Vertes A, Irinyi G, Gijbels R. 1993. Hydrodynamic model of matrix-assisted laser-desorption mass-spectrometry. Anal. Chem. 65, 2389–2393. ( 10.1021/Ac00065a036) [DOI] [Google Scholar]
- 41.Johnson RE. 1994. Models for matrix-assisted desorption by a laser-pulse. Int. J. Mass Spectrom. 139, 25–38. ( 10.1016/0168-1176(94)04070-2) [DOI] [Google Scholar]
- 42.Zhigilei LV, Leveugle E, Garrison BJ, Yingling YG, Zeifman MI. 2003. Computer simulations of laser ablation of molecular substrates. Chem. Rev. 103, 321–347. ( 10.1021/cr010459r) [DOI] [PubMed] [Google Scholar]
- 43.Dreisewerd K. 2003. The desorption process in MALDI. Chem. Rev. 103, 395–425. ( 10.1021/cr010375i) [DOI] [PubMed] [Google Scholar]
- 44.Jaskolla TW, Karas M, Roth U, Steinert K, Menzel C, Reihs K. 2009. Comparison between vacuum sublimed matrices and conventional dried droplet preparation in MALDI-TOF mass spectrometry. J. Am. Soc. Mass Spectrom. 20, 1104–1114. ( 10.1016/j.jasms.2009.02.010) [DOI] [PubMed] [Google Scholar]
- 45.Gluckmann M, Pfenninger A, Kruger R, Thierolf M, Karas M, Horneffer V, Hillenkamp F, Strupat K. 2001. Mechanisms in MALDI analysis: surface interaction or incorporation of analytes? Int. J. Mass Spectrom. 210, 121–132. ( 10.1016/s1387-3806(01)00450-x) [DOI] [Google Scholar]
- 46.Horneffer V, Dreisewerd K, Ludemann HC, Hillenkamp F, Lage M, Strupat K. 1999. Is the incorporation of analytes into matrix crystals a prerequisite for matrix-assisted laser desorption/ionization mass spectrometry? A study of five positional isomers of dihydroxybenzoic acid. Int. J. Mass Spectrom. 185, 859–870. ( 10.1016/s1387-3806(98)14218-5) [DOI] [Google Scholar]
- 47.Little MW, Laboy J, Murray KK. 2007. Wavelength dependence of soft infrared laser desorption and ionization. J. Phys. Chem. C 111, 1412–1416. ( 10.1021/jp063154v) [DOI] [Google Scholar]
- 48.Soltwisch J, Jaskolla TW, Dreisewerd K. 2013. Color matters---material ejection and ion yields in UV-MALDI mass spectrometry as a function of laser wavelength and laser fluence. J. Am. Soc. Mass Spectrom. 24, 1477–1488. ( 10.1007/s13361-013-0699-5) [DOI] [PubMed] [Google Scholar]
- 49.Nordhoff E, Ingendoh A, Cramer R, Overberg A, Stahl B, Karas M, Hillenkamp F, Crain PF. 1992. Matrix-assisted laser desorption ionization mass-spectrometry of nucleic-acids with wavelengths in the ultraviolet and infrared. Rapid Commun. Mass Spectrom. 6, 771–776. ( 10.1002/rcm.1290061212) [DOI] [PubMed] [Google Scholar]
- 50.Lai Y-H, Wang C-C, Lin S-H, Lee Y-T, Wang Y-S. 2010. Solid-phase thermodynamic interpretation of ion desorption in matrix-assisted laser desorption/ionization. J. Phys. Chem. B 114, 13 847–13 852. ( 10.1021/jp104250g) [DOI] [PubMed] [Google Scholar]
- 51.Koubenakis A, Frankevich V, Zhang J, Zenobi R. 2004. Time-resolved surface temperature measurement of MALDI matrices under pulsed UV laser irradiation. J. Phys. Chem. A 108, 2405–2410. ( 10.1021/Jp037811k) [DOI] [Google Scholar]
- 52.Williams TL, Andrzejewski D, Lay JO, Musser SM. 2003. Experimental factors affecting the quality and reproducibility of MALDI TOF mass spectra obtained from whole bacteria cells. J. Am. Soc. Mass Spectrom. 14, 342–351. ( 10.1016/S1044-0305(03)00065-5) [DOI] [PubMed] [Google Scholar]
- 53.Schulz E, Karas M, Rosu F, Gabelica V. 2006. Influence of the matrix on analyte fragmentation in atmospheric pressure MALDI. J. Am. Soc. Mass Spectrom. 17, 1005–1013. ( 10.1016/j.jasms.2006.03.009) [DOI] [PubMed] [Google Scholar]
- 54.Luo GH, Marginean I, Vertes A. 2002. Internal energy of ions generated by matrix-assisted laser desorption/ionization. Anal. Chem. 74, 6185–6190. ( 10.1021/Ac020339z) [DOI] [PubMed] [Google Scholar]
- 55.Zhang W, Chait BT. 1997. Radial velocity distributions of molecular ions produced by matrix-assisted laser desorption/ionization. Int. J. Mass Spectrom. Ion Process. 160, 259–267. ( 10.1016/S0168-1176(96)04526-0) [DOI] [Google Scholar]
- 56.Mamyrin BA, Karataev VI, Shmikk DV, Zagulin VA. 1973. Mass-reflectron a new nonmagnetic time-of-flight high-resolution mass-spectrometer. Zh. Eksp. Teor. Fiz. 64, 82–89. [Google Scholar]
- 57.Jonsson G, Hedin A, Håkansson P, Sundqvist BUR, Bennich H, Roepstorff P. 1989. Compensation for non-normal ejection of large molecular ions in plasma-desorption mass spectrometry. Rapid Commun. Mass Spectrom. 3, 190–191. ( 10.1002/rcm.1290030607) [DOI] [Google Scholar]
- 58.Cotter RJ. 1997. Pulsed extraction, continuous ionization, and ion storage instruments. In Time of flight mass spectrometry: instrumentation and applications in biological research, pp. 137–168. Washington, DC: American Chemical Society. [Google Scholar]
- 59.Kaufmann R, Chaurand P, Kirsch D, Spengler B. 1996. Post-source decay and delayed extraction in matrix-assisted laser desorption/ionization-reflectron time-of-flight mass spectrometry. Are there trade-offs? Rapid Commun. Mass Spectrom. 10, 1199–1208. ( 10.1002/(SICI)1097-0231(19960731)10:10%3C1199::AID-RCM643%3E3.0.CO;2-F) [DOI] [PubMed] [Google Scholar]
- 60.Tsarbopoulos A, Bahr U, Pramanik BN, Karas M. 1997. Glycoprotein analysis by delayed extraction and post-source decay MALDI-TOF-MS. Int. J. Mass Spectrom. 169, 251–261. ( 10.1016/S0168-1176(97)00222-X) [DOI] [Google Scholar]
- 61.Barbacci DC, Edmondson RD, Russell DH. 1997. Evaluation of the variables that affect resolution in delayed extraction MALDI-TOF. Int. J. Mass Spectrom. 165, 221–235. ( 10.1016/S0168-1176(97)00169-9) [DOI] [Google Scholar]
- 62.Hanson CD, Just CL. 1994. Selective background suppression in MALDI-TOF mass-spectrometry. Anal. Chem. 66, 3676–3680. ( 10.1021/Ac00093a022) [DOI] [Google Scholar]
- 63.Beavis RC, Chait BT. 1989. Factors affecting the ultraviolet laser desorption of proteins. Rapid Commun. Mass Spectrom. 3, 233–237. ( 10.1002/rcm.1290030708) [DOI] [PubMed] [Google Scholar]
- 64.Wiza JL. 1979. Microchannel plate detectors. Nucl. Instrum. Methods 162, 587–601. ( 10.1016/0029-554X(79)90734-1) [DOI] [Google Scholar]
- 65.Westman A, Brinkmalm G, Barofsky DF. 1997. MALDI induced saturation effects in chevron microchannel plate detectors. Int. J. Mass Spectrom. Ion Process. 169–170, 79–87. ( 10.1016/S0168-1176(97)00205-X) [DOI] [Google Scholar]
- 66.Geno PW, Macfarlane RD. 1989. Secondary-electron emission induced by impact of low-velocity molecular-ions on a microchannel plate. Int. J. Mass Spectrom. Ion Process. 92, 195–210. ( 10.1016/0168-1176(89)83028-9) [DOI] [Google Scholar]
- 67.Meier R, Eberhardt P. 1993. Velocity and ion species dependence of the gain of microchannel plates. Int. J. Mass Spectrom. Ion Process. 123, 19–27. ( 10.1016/0168-1176(93)87050-3) [DOI] [Google Scholar]
- 68.Westmacott G, Frank M, Labov SE, Benner WH. 2000. Using a superconducting tunnel junction detector to measure the secondary electron emission efficiency for a microchannel plate detector bombarded by large molecular ions. Rapid Commun. Mass Spectrom. 14, 1854–1861. ( 10.1002/1097-0231(20001015)14:19%3C1854::AID-RCM102%3E3.0.CO;2-M) [DOI] [PubMed] [Google Scholar]
- 69.Martens J, Ens W, Standing KG, Verentchikov A. 1992. Secondary-ion and electron production from surfaces bombarded by large polyatomic ions. Rapid Commun. Mass Spectrom. 6, 147–157. ( 10.1002/rcm.1290060215) [DOI] [PubMed] [Google Scholar]
- 70.Brunelle A, Chaurand P, Della-Negra S, Le Beyec Y, Baptista GB. 1993. Surface secondary electron and secondary ion emission induced by large molecular ion impacts. Int. J. Mass Spectrom. Ion Process. 126, 65–73. ( 10.1016/0168-1176(93)80071-L) [DOI] [Google Scholar]
- 71.Verentchikov A, Ens W, Martens J, Standing KG. 1993. Detection of large molecular ions by secondary ion and secondary electrom emission. Int. J. Mass Spectrom. Ion Process. 126, 75–83. ( 10.1016/0168-1176(93)80072-M) [DOI] [Google Scholar]
- 72.Seko A, Kobayashi H. 1973. Application of channel multiplier plates as image information preprocessors. Rev. Sci. Instrum. 44, 400–405. ( 10.1063/1.1686143) [DOI] [Google Scholar]
- 73.Wei S, Tzeng WB, Castleman AW. 1990. Dissociation dynamics: measurements of decay fractions of metastable ammonia cluster ions. J. Chem. Phys. 93, 2506–2512. ( 10.1063/1.459033) [DOI] [Google Scholar]
- 74.Da Costa G, Vurpillot F, Bostel A, Bouet M, Deconihout B. 2005. Design of a delay-line position-sensitive detector with improved performance. Rev. Sci. Instrum. 76, 013304 ( 10.1063/1.1829975) [DOI] [Google Scholar]
- 75.Baptista GB, Brunelle A, Chaurand P, Della-Negra S, Depauw J, Le Bayec Y, Chait BT. 1991. Ion counting and ion intensity measurements in time-of-flight mass spectrometry. Application to matrix-assisted laser desorption. Rapid Commun. Mass Spectrom. 5, 632–637. ( 10.1002/rcm.1290051214) [DOI] [Google Scholar]
- 76.Beavis RC. 1996. Increasing the dynamic range of a transient recorder by using two analog-to-digital converters. J. Am. Soc. Mass Spectrom. 7, 107–113. ( 10.1016/1044-0305(95)00592-7) [DOI] [PubMed] [Google Scholar]
- 77.Bouneau S, Cohen P, Della Negra S, Jacquet D, Le Beyec Y, Le Bris J, Pautrat M, Sellem R. 2003. 256-Anode channel plate device for simultaneous ion detection in time of flight measurements. Rev. Sci. Instrum. 74, 57–67. ( 10.1063/1.1527721) [DOI] [Google Scholar]
- 78.Barbacci DC, Russell DH, Schultz JA, Holocek J, Ulrich S, Burton W, Van Stipdonk M. 1998. Multi-anode detection in electrospray ionization time-of-flight mass spectrometry. J. Am. Soc. Mass Spectrom. 9, 1328–1333. ( 10.1016/S1044-0305(98)00113-5) [DOI] [PubMed] [Google Scholar]
- 79.Onnerfjord P, Ekstrom S, Bergquist J, Nilsson J, Laurell T, Marko-Varga G. 1999. Homogeneous sample preparation for automated high throughput analysis with matrix-assisted laser desorption/ionisation time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom. 13, 315–322. ( 10.1002/(SICI)1097-0231(19990315)13:5%3C315::AID-RCM483%3E3.0.CO;2-C) [DOI] [PubMed] [Google Scholar]
- 80.Albrethsen J. 2007. Reproducibility in protein profiling by MALDI-TOF mass spectrometry. Clin. Chem. 53, 852–858. ( 10.1373/clinchem.2006.082644) [DOI] [PubMed] [Google Scholar]
- 81.Garden RW, Sweedler JV. 2000. Heterogeneity within MALDI samples as revealed by mass spectrometric imaging. Anal. Chem. 72, 30–36. ( 10.1021/ac9908997) [DOI] [PubMed] [Google Scholar]
- 82.Chaurand P, Schwartz SA, Reyzer ML, Caprioli RA. 2005. Imaging mass spectrometry: principles and potentials. Toxicol. Pathol. 33, 92–101. ( 10.1080/01926230590881862) [DOI] [PubMed] [Google Scholar]
- 83.Sleno L, Volmer DA. 2006. Assessing the properties of internal standards for quantitative matrix-assisted laser desorption/ionization mass spectrometry of small molecules. Rapid Commun. Mass Spectrom. 20, 1517–1524. ( 10.1002/rcm.2498) [DOI] [PubMed] [Google Scholar]
- 84.Zabet-Moghaddam M, Heinzle E, Tholey A. 2004. Qualitative and quantitative analysis of low molecular weight compounds by ultraviolet matrix-assisted laser desorption/ionization mass spectrometry using ionic liquid matrices. Rapid Commun. Mass Spectrom. 18, 141–148. ( 10.1002/Rcm.1293) [DOI] [PubMed] [Google Scholar]
- 85.Li YL, Gross ML. 2004. Ionic-liquid matrices for quantitative analysis by MALDI-TOF mass spectrometry. J. Am. Soc. Mass Spectrom. 15, 1833–1837. ( 10.1016/j.jasms.2004.08.011) [DOI] [PubMed] [Google Scholar]
- 86.Trimpin S, Keune S, Rader HJ, Mullen K. 2006. Solvent-free MALDI-MS: developmental improvements in the reliability and the potential of MALDI in the analysis of synthetic polymers and giant organic molecules. J. Am. Soc. Mass Spectrom. 17, 661–671. ( 10.1016/j.jasms.2006.01.007) [DOI] [PubMed] [Google Scholar]
- 87.Trimpin S, Weidner SM, Falkenhagen J, McEwen CN. 2007. Fractionation and solvent-free MALDI-MS analysis of polymers using liquid adsorption chromatography at critical conditions in combination with a multisample on-target homogenization/transfer sample preparation method. Anal. Chem. 79, 7565–7570. ( 10.1021/ac070986w) [DOI] [PubMed] [Google Scholar]
- 88.Tu T, Sauter ADJ, Sauter ADR, Gross ML. 2008. Improving the signal intensity and sensitivity of MALDI mass spectrometry by using nanoliter spots deposited by induction-based fluidics. J. Am. Soc. Mass Spectrom. 19, 1086–1090. ( 10.1016/j.jasms.2008.03.017) [DOI] [PubMed] [Google Scholar]
- 89.Schuerenbeg M, Luebbert C, Eickhoff H, Kalkum M, Lehrach H, Nordhoff E. 2000. Prestructured MALDI-MS sample supports. Anal. Chem. 72, 3436–3442. ( 10.1021/Ac000092a) [DOI] [PubMed] [Google Scholar]
- 90.Urban PL, Jefimovs K, Amantonico A, Fagerer SR, Schmid T, Madler S, Puigmarti-Luis J, Goedecke N, Zenobi R. 2010. High-density micro-arrays for mass spectrometry. Lab on a Chip 10, 3206–3209. ( 10.1039/c0lc00211a) [DOI] [PubMed] [Google Scholar]
- 91.Hu J-B, Chen Y-C, Urban PL. 2013. Coffee-ring effects in laser desorption/ionization mass spectrometry. Anal. Chim. Acta 766, 77–82. ( 10.1016/j.aca.2012.12.044) [DOI] [PubMed] [Google Scholar]
- 92.Nicola AJ, Gusev AI, Proctor A, Jackson EK, Hercules DM. 1995. Application of the fast-evaporation sample preparation method for improving quantification of angiotensin-II by matrix-assisted laser-desorption ionization. Rapid Commun. Mass Spectrom. 9, 1164–1171. ( 10.1002/rcm.1290091216) [DOI] [PubMed] [Google Scholar]
- 93.Dai YQ, Whittal RM, Li L. 1999. Two-layer sample preparation: a method for MALDI-MS analysis of complex peptide and protein mixtures. Anal. Chem. 71, 1087–1091. ( 10.1021/ac980684h) [DOI] [PubMed] [Google Scholar]
- 94.Li L, Golding RE, Whittal RM. 1996. Analysis of single mammalian cell lysates by mass spectrometry. J. Am. Chem. Soc. 118, 11 662–11 663. ( 10.1021/ja9627499) [DOI] [Google Scholar]
- 95.Hensel RR, King RC, Owens KG. 1997. Electrospray sample preparation for improved quantitation in matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom. 11, 1785–1793. ( 10.1002/(SICI)1097-0231(19971030)11:16%3C1785::AID-RCM78%3E3.0.CO;2-Q) [DOI] [PubMed] [Google Scholar]
- 96.Hankin JA, Barkley RM, Murphy RC. 2007. Sublimation as a method of matrix application for mass spectrometric imaging. J. Am. Soc. Mass Spectrom. 18, 1646–1652. ( 10.1016/j.jasms.2007.06.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cohen SL, Chait BT. 1996. Influence of matrix solution conditions on the MALDI-MS analysis of peptides and proteins. Anal. Chem. 68, 31–37. ( 10.1021/Ac9507956) [DOI] [PubMed] [Google Scholar]
- 98.Lai Y-H, Cai Y-H, Lee H, Ou Y-M, Hsiao C-H, Tsao C-W, Chang H-T, Wang Y-S. 2016. Reducing spatial heterogeneity of MALDI samples with Marangoni flows during sample preparation. J. Am. Soc. Mass Spectrom. 27, 1314–1321. ( 10.1007/s13361-016-1406-0) [DOI] [PubMed] [Google Scholar]
- 99.Kang MJ, Tholey A, Heinzle E. 2001. Application of automated matrix-assisted laser desorption/ionization time-of-flight mass spectrometry for the measurement of enzyme activities. Rapid Commun. Mass Spectrom. 15, 1327–1333. ( 10.1002/rcm.376) [DOI] [PubMed] [Google Scholar]
- 100.Gusev AI, Wilkinson WR, Proctor A, Hercules DM. 1996. Direct quantitative analysis of peptides using matrix assisted laser desorption ionization. Fresenius J. Anal. Chem. 354, 455–463. ( 10.1007/s0021663540455) [DOI] [PubMed] [Google Scholar]
- 101.Mirgorodskaya OA, Kozmin YP, Titov MI, Korner R, Sonksen CP, Roepstorff P. 2000. Quantitation of peptides and proteins by matrix-assisted laser desorption/ionization mass spectrometry using 18O-labeled internal standards. Rapid Commun. Mass Spectrom. 14, 1226–1232. ( 10.1002/1097-0231(20000730)14:14%3C1226::AID-RCM14%3E3.3.CO;2-M) [DOI] [PubMed] [Google Scholar]
- 102.Griffin TJ, Gygi SP, Rist B, Aebersold R, Loboda A, Jilkine A, Ens W, Standing KG. 2001. Quantitative proteomic analysis using a MALDI quadrupole time-of-flight mass spectrometer. Anal. Chem. 73, 978–986. ( 10.1021/ac001169y) [DOI] [PubMed] [Google Scholar]
- 103.Wiese S, Reidegeld KA, Meyer HE, Warscheid B. 2007. Protein labeling by iTRAQ: a new tool for quantitative mass spectrometry in proteome research. Proteomics 7, 340–350. ( 10.1002/pmic.200600422) [DOI] [PubMed] [Google Scholar]