Abstract
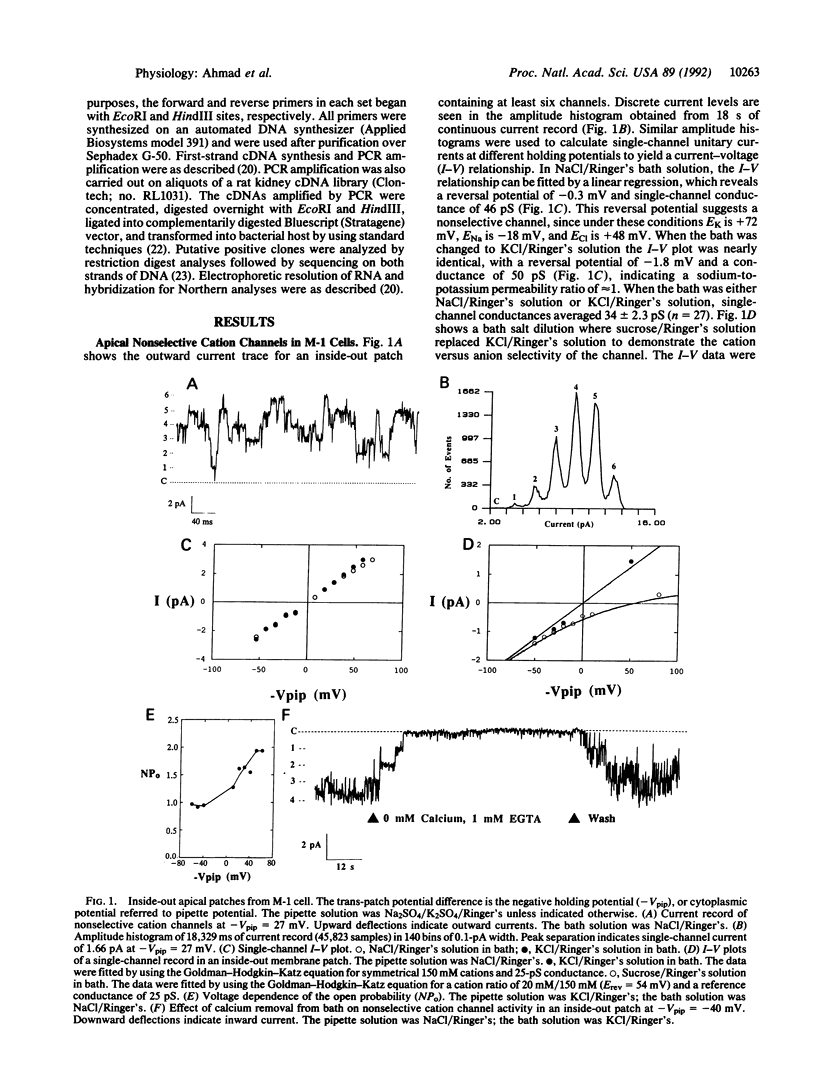
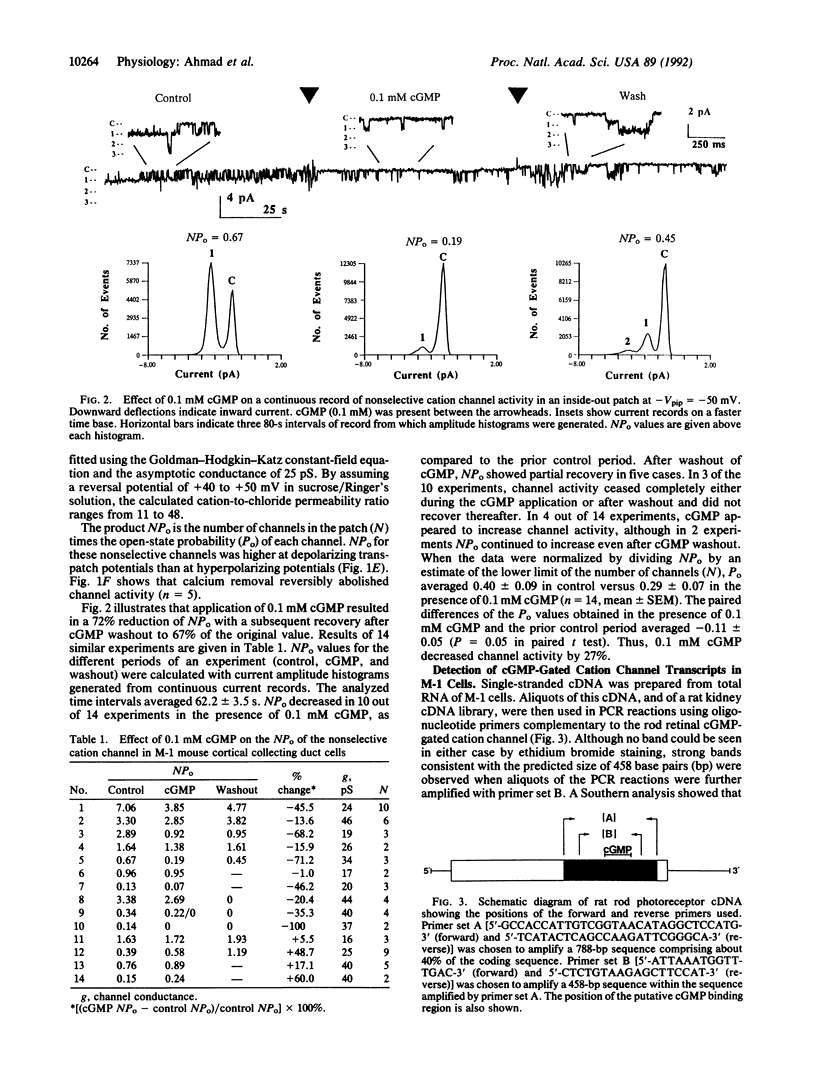
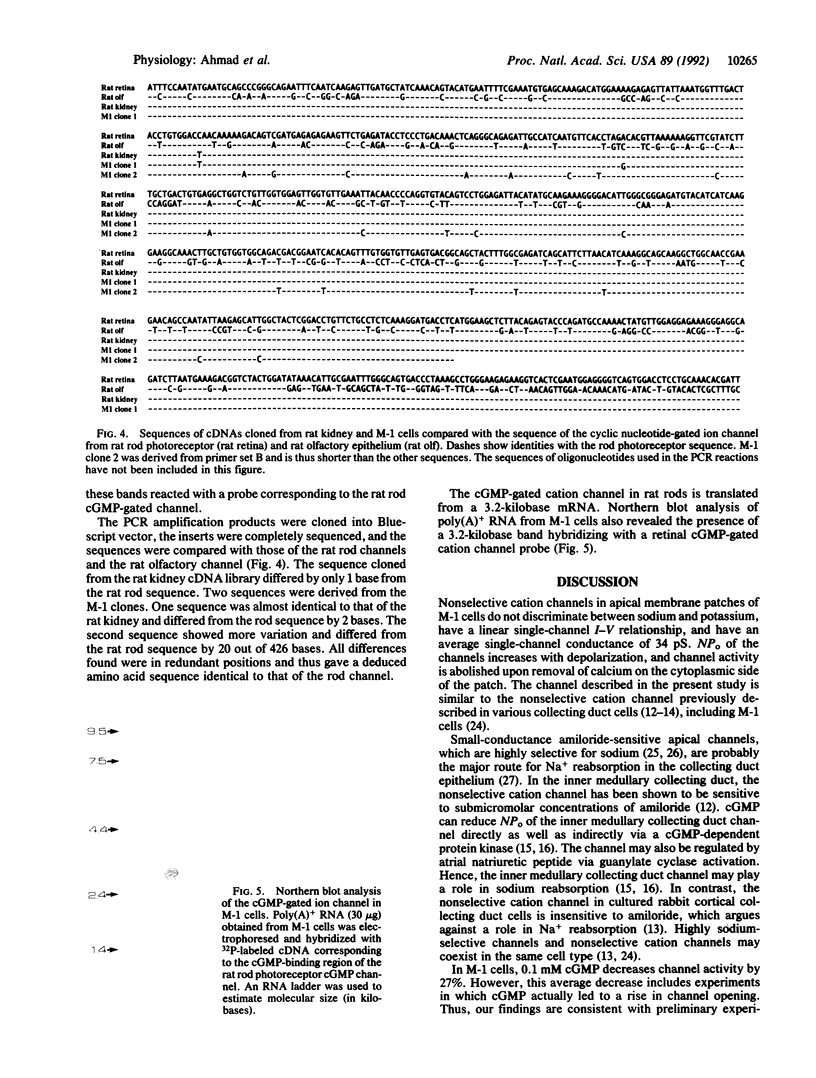
Apical nonselective cation channels with an average single-channel conductance of 34 +/- 2.3 pS were found in M-1 mouse cortical collecting duct cells. Channel activity is increased by depolarization and abolished by cytoplasmic calcium removal. Cytoplasmic application of 0.1 mM cGMP decreases channel open probability by 27%. cDNAs corresponding to approximately 40% of the coding region of the photoreceptor channel were isolated by the polymerase chain reaction from M-1 cells and a rat kidney cDNA library. The rat kidney-derived sequence differs by a single base, and the M-1-cell-derived sequence differs by only two bases, from the photoreceptor sequence. A second clone from M-1 cells differs by 20 out of 426 bases from the photoreceptor sequence. In all three clones, the deduced amino acid sequence is identical to that of the rat photoreceptor channel. Northern blot analysis of poly(A)+ RNA from M-1 cells reveals the presence of a 3.2-kilobase band hybridizing with a retinal cGMP-gated cation channel probe. The results suggest the expression in M-1 cells of more than one gene coding for nonselective cation channels or channel subunits, one of which is identical to the cGMP-gated cation channel gene of rod photoreceptors.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmad I., Redmond L. J., Barnstable C. J. Developmental and tissue-specific expression of the rod photoreceptor cGMP-gated ion channel gene. Biochem Biophys Res Commun. 1990 Nov 30;173(1):463–470. doi: 10.1016/s0006-291x(05)81081-2. [DOI] [PubMed] [Google Scholar]
- Cook D. I., Poronnik P., Young J. A. Characterization of a 25-pS nonselective cation channel in a cultured secretory epithelial cell line. J Membr Biol. 1990 Mar;114(1):37–52. doi: 10.1007/BF01869383. [DOI] [PubMed] [Google Scholar]
- Das S., Garepapaghi M., Palmer L. G. Stimulation by cGMP of apical Na channels and cation channels in toad urinary bladder. Am J Physiol. 1991 Feb;260(2 Pt 1):C234–C241. doi: 10.1152/ajpcell.1991.260.2.C234. [DOI] [PubMed] [Google Scholar]
- Dhallan R. S., Yau K. W., Schrader K. A., Reed R. R. Primary structure and functional expression of a cyclic nucleotide-activated channel from olfactory neurons. Nature. 1990 Sep 13;347(6289):184–187. doi: 10.1038/347184a0. [DOI] [PubMed] [Google Scholar]
- Filipovic D., Sackin H. A calcium-permeable stretch-activated cation channel in renal proximal tubule. Am J Physiol. 1991 Jan;260(1 Pt 2):F119–F129. doi: 10.1152/ajprenal.1991.260.1.F119. [DOI] [PubMed] [Google Scholar]
- Garty H., Benos D. J. Characteristics and regulatory mechanisms of the amiloride-blockable Na+ channel. Physiol Rev. 1988 Apr;68(2):309–373. doi: 10.1152/physrev.1988.68.2.309. [DOI] [PubMed] [Google Scholar]
- Guy H. R., Durell S. R., Warmke J., Drysdale R., Ganetzky B. Similarities in amino acid sequences of Drosophila eag and cyclic nucleotide-gated channels. Science. 1991 Nov 1;254(5032):730–730. doi: 10.1126/science.1658932. [DOI] [PubMed] [Google Scholar]
- Gögelein H., Greger R. A voltage-dependent ionic channel in the basolateral membrane of late proximal tubules of the rabbit kidney. Pflugers Arch. 1986;407 (Suppl 2):S142–S148. doi: 10.1007/BF00584943. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hunter M., Lopes A. G., Boulpaep E., Giebisch G. Regulation of single potassium ion channels from apical membrane of rabbit collecting tubule. Am J Physiol. 1986 Oct;251(4 Pt 2):F725–F733. doi: 10.1152/ajprenal.1986.251.4.F725. [DOI] [PubMed] [Google Scholar]
- Kaupp U. B., Niidome T., Tanabe T., Terada S., Bönigk W., Stühmer W., Cook N. J., Kangawa K., Matsuo H., Hirose T. Primary structure and functional expression from complementary DNA of the rod photoreceptor cyclic GMP-gated channel. Nature. 1989 Dec 14;342(6251):762–766. doi: 10.1038/342762a0. [DOI] [PubMed] [Google Scholar]
- Kaupp U. B. The cyclic nucleotide-gated channels of vertebrate photoreceptors and olfactory epithelium. Trends Neurosci. 1991 Apr;14(4):150–157. doi: 10.1016/0166-2236(91)90087-b. [DOI] [PubMed] [Google Scholar]
- Laskowski F. H., Christine C. W., Gitter A. H., Beyenbach K. W., Gross P., Frömter E. Cation channels in the apical membrane of collecting duct principal cell epithelium in culture. Ren Physiol Biochem. 1990 Jan-Apr;13(1-2):70–81. doi: 10.1159/000173349. [DOI] [PubMed] [Google Scholar]
- Light D. B., Corbin J. D., Stanton B. A. Dual ion-channel regulation by cyclic GMP and cyclic GMP-dependent protein kinase. Nature. 1990 Mar 22;344(6264):336–339. doi: 10.1038/344336a0. [DOI] [PubMed] [Google Scholar]
- Light D. B., McCann F. V., Keller T. M., Stanton B. A. Amiloride-sensitive cation channel in apical membrane of inner medullary collecting duct. Am J Physiol. 1988 Aug;255(2 Pt 2):F278–F286. doi: 10.1152/ajprenal.1988.255.2.F278. [DOI] [PubMed] [Google Scholar]
- Light D. B., Schwiebert E. M., Karlson K. H., Stanton B. A. Atrial natriuretic peptide inhibits a cation channel in renal inner medullary collecting duct cells. Science. 1989 Jan 20;243(4889):383–385. doi: 10.1126/science.2463673. [DOI] [PubMed] [Google Scholar]
- Ling B. N., Hinton C. F., Eaton D. C. Potassium permeable channels in primary cultures of rabbit cortical collecting tubule. Kidney Int. 1991 Sep;40(3):441–452. doi: 10.1038/ki.1991.231. [DOI] [PubMed] [Google Scholar]
- Merot J., Poncet V., Bidet M., Tauc M., Poujeol P. Apical membrane ionic channels in the rabbit cortical thick ascending limb in primary culture. Biochim Biophys Acta. 1991 Dec 9;1070(2):387–400. doi: 10.1016/0005-2736(91)90079-n. [DOI] [PubMed] [Google Scholar]
- Nawy S., Jahr C. E. cGMP-gated conductance in retinal bipolar cells is suppressed by the photoreceptor transmitter. Neuron. 1991 Oct;7(4):677–683. doi: 10.1016/0896-6273(91)90380-i. [DOI] [PubMed] [Google Scholar]
- O'Neil R. G., Boulpaep E. L. Effect of amiloride on the apical cell membrane cation channels of a sodium-absorbing, potassium-secreting renal epithelium. J Membr Biol. 1979 Nov 30;50(3-4):365–387. doi: 10.1007/BF01868898. [DOI] [PubMed] [Google Scholar]
- Palmer L. G., Frindt G. Amiloride-sensitive Na channels from the apical membrane of the rat cortical collecting tubule. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2767–2770. doi: 10.1073/pnas.83.8.2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge L. D., Swandulla D. Calcium-activated non-specific cation channels. Trends Neurosci. 1988 Feb;11(2):69–72. doi: 10.1016/0166-2236(88)90167-1. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoos B. A., Náray-Fejes-Tóth A., Carretero O. A., Ito S., Fejes-Tóth G. Characterization of a mouse cortical collecting duct cell line. Kidney Int. 1991 Jun;39(6):1168–1175. doi: 10.1038/ki.1991.148. [DOI] [PubMed] [Google Scholar]
- Yau K. W., Baylor D. A. Cyclic GMP-activated conductance of retinal photoreceptor cells. Annu Rev Neurosci. 1989;12:289–327. doi: 10.1146/annurev.ne.12.030189.001445. [DOI] [PubMed] [Google Scholar]
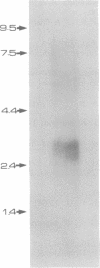
- Yellen G. Single Ca2+-activated nonselective cation channels in neuroblastoma. Nature. 1982 Mar 25;296(5855):357–359. doi: 10.1038/296357a0. [DOI] [PubMed] [Google Scholar]