Abstract
Dasatinib (DAS) has been licensed for the frontline treatment in chronic myeloid leukemia (CML). However, very few data are available regarding its efficacy and toxicity in elderly patients with CML outside clinical trials. To address this issue, we set out a “real-life” cohort of 65 chronic phase CML patients older than 65 years (median age 75.1 years) treated frontline with DAS in 26 Italian centers from June 2012 to June 2015, focusing our attention on toxicity and efficacy data. One third of patients (20/65: 30.7%) had 3 or more comorbidities and required concomitant therapies; according to Sokal classification, 3 patients (4.6%) were low risk, 39 (60.0%) intermediate risk, and 20 (30.8%) high risk, whereas 3 (4.6%) were not classifiable. DAS starting dose was 100 mg once a day in 54 patients (83.0%), whereas 11 patients (17.0%) received less than 100 mg/day. Grade 3/4 hematologic and extrahematologic toxicities were reported in 8 (12.3%) and 12 (18.5%) patients, respectively. Overall, 10 patients (15.4%) permanently discontinued DAS because of toxicities. Pleural effusions (all WHO grades) occurred in 12 patients (18.5%) and in 5 of them occurred during the first 3 months. DAS treatment induced in 60/65 patients (92.3%) a complete cytogenetic response and in 50/65 (76.9%) also a major molecular response. These findings show that DAS might play an important role in the frontline treatment of CML patients >65 years old, proving efficacy and having a favorable safety profile also in elderly subjects with comorbidities.
Introduction
Imatinib (IM) frontline treatment in chronic myeloid leukemia (CML) led to an excellent disease control in the vast majority of the patients [1], [2], [3]. However, about one third of the patients discontinue the drug due to treatment failure or toxicity [4], [5] and require a salvage therapy with second-generation tyrosine kinase inhibitors (2G-TKIs) such as dasatinib (DAS) or nilotinib (NIL) [6], [7], [8].
Both these 2G-TKIs were very effective in patients resistant/intolerant to IM, and their use was extended to the frontline treatment of newly diagnosed CML patients. The DASISION and ENESTnd clinical trials recently demonstrated a faster and deeper clinical efficacy of the 2G-TKIs as compared with IM, leading to the approval of DAS and NIL as alternative first-line option in CML patients [9], [10].
However, both these company-sponsored studies recruited CML patients according to well-defined protocol criteria, excluding many patients for several reasons with a resulting median age of enrolled subjects <50 years, compared with a median age of about 60 years in all newly diagnosed CML patients in Italy [11]. Thus, these trials introduced a selection bias, and the enrolled cohorts did not really reflect the whole CML population observed in daily clinical practice [12].
This topic is particularly important in CML patients older than 65 years [13]. Older age and comorbidities were often considered as exclusion criteria from clinical trials [14]. Recently, both the EUTOS population-based registry and other studies provided more information on the clinical characteristics, comorbidities, and epidemiology of patients with CML in Europe and in the United States [15], [16]. However, only few and sparse data are reported in the current literature on 2G-TKIs in patients older than 65 years with newly diagnosed CML, and this is still a matter of debate.
To address this issue, we collected a “real-life” cohort of CML patients in chronic phase older than 65 years and treated frontline with DAS in 26 Italian centers from June 2012 to June 2015, focusing our attention on toxicity and efficacy data.
Patients and Methods
Patient Population
From June 2012 to June 2015, we identified a series of 65 patients older than 65 years (M/F 32/33, median age 75.1 years, absolute range 65.1-89.3, interquartile range [IR] 70.5-78.7) who were diagnosed at 26 institutions as having early chronic phase CML with the following characteristics:
-
-
no prior TKIs treatment; but only hydroxyurea was permitted if given for less than 3 months to reduce white blood cell count
-
-
DAS as first-line treatment
Twelve of these patients (18.5%) were older than 80 years. The main clinical features at diagnosis of the whole cohort are shown in Table 1. As to comorbidities, only 6 patients (9.2%) did not have any concomitant disease; on the contrary, 1 or 2 concomitant diseases requiring specific treatments were present in 39 patients (60.1%), and 3 or more concomitant diseases were present in 20 patients (30.7%). The five most common comorbidities are reported (Table 1).
Table 1.
Clinical Features at Diagnosis
| No. of patients | 65 |
| Male, n (%) | 32 (49.2) |
| Median age, years (absolute range/IR) |
75.1 (65.0-89.3/70.5-78.7) |
| Sokal risk: n (%) | |
| Low | 3 (4.6) |
| Intermediate | 39 (60.0) |
| High | 20 (30.8) |
| Not evaluable | 3 (4.6) |
| Performance status (ECOG), n (%) | |
| Grade 0-1 | 55 (84.6%) |
| Grade 2 | 10 (15.4%) |
| Median Hb, g/dl (IR) |
12.5 (11.4-13.8) |
| Median white blood cell count, ×109/l (IR) |
48.3 (27.8-93.7) |
| Median PLTs, ×109/l (IR) |
430 (243-803) |
| Spleen enlargement, cm below costal margin (%) | |
| 0 | 37 (57.0) |
| 1-5 | 22 (33.8) |
| > 5 | 5 (7.7) |
| Not evaluable | 1 (1.5) |
| Most common comorbidities, n (%): | |
| Arterial hypertension | 37 (56.9) |
| Diabetes | 14 (21.5) |
| Dyslipidemia | 13 (20.0) |
| Cardiovascular diseases | 10 (15.3) |
| Previous/concomitant neoplasia | 10 (15.3) |
ECOG, Eastern Cooperative Oncology Group; Hb, hemoglobin; PLTs, platelets.
Cytogenetic and Molecular Evaluation
Cytogenetic analyses were performed on bone marrow samples by chromosome standard G or Q banding techniques in at least 20 cell metaphases from direct or short-term (24-48 hours) cultures. If less than 20 metaphases were evaluable, fluorescence in situ hybridization on interphase cells was performed with BCR-ABL extrasignal, dual-color, dual-fusion probes.
Real-time quantitative polymerase chain reaction to assess BCR-ABL1 transcript levels was performed according to suggested procedures and recommendations; results were expressed as BCR-ABL1/ABL1 ratio according to the International Scale [17].
Cytogenetic and molecular responses were categorized according to standard criteria: major cytogenetic response (MCyR) was defined by the presence of more than 66% Ph-negative metaphases; complete cytogenetic response (CCyR) by the presence of 100% Ph-negative metaphases or as a number of positive marrow cell interphase nuclei less than 2 of 200 (<1%) at fluorescence in situ hybridization analysis; major molecular response (MMR) was defined as a BCR-ABL1/ABL1 ratio <0.1% (MR 3.0), and deep molecular response as a BCR-ABL1/ABL1 ratio <0.01% (MR 4.0) or <0.0032% (MR 4.5) International Scale (after internal standardization) [18]. Those patients who were evaluated only with molecular analysis and had a BCR-ABL1/ABL1 ratio <1.0 were considered as in CCyR also [19].
Primary hematologic resistance to DAS was defined as failure to achieve a complete hematologic remission after 3 months of treatment; primary cytogenetic resistance to DAS was defined as failure to achieve CCyR after 6 months of treatment. Secondary resistance to DAS was defined as the loss of CCyR or the loss of MMR at any time after the achievement of a previous CCyR or MMR.
Statistical Analysis
Calculations were expressed as mean ± SD for normally distributed data, as median and IR for not-normally distributed data, or as percentage frequencies; comparisons between groups of patients were made by paired t test, χ2 test, and Fisher exact test, as appropriate, at significance levels of P < .05. The Kaplan-Meier product-limit method was used to estimate univariate survival curves, and the log-rank test was adopted to compare the survival curves. Event-free survival (EFS) was calculated from the date of DAS therapy start to any of the following events: primary resistance to DAS, permanent DAS discontinuation due to toxicity or any other unrelated cause, secondary resistance to DAS, death due to any cause. Overall survival (OS) was calculated from the date of DAS start to death due to any cause. All calculations were made using a standard statistical package (SPSS for Windows Version 15.0, Chicago, IL).
Results
Patient Therapy
Median interval from CML diagnosis to DAS therapy was 23 days (IR 14-34). DAS starting dose was 100 mg once a day (OAD) in 54 patients (83.0%), 80 mg OAD in 3 patients (4.7%), and 50 mg OAD in 8 patients (12.3%), respectively. The main reasons for a reduction in the DAS starting dose were age over 80 years in 5 patients, severe comorbidities in 4 patients, and physician judgment in 2 patients.
Treatment Toxicity
Hematologic toxicities of grade 3/4 were reported in 8 patients (12.3%) after a median period of treatment of 1.1 months (IR 0.7-2.5), whereas grade 3/4 extrahematologic toxicities were reported in 12 patients (18.5%) after a median treatment period of 4.6 months (IR 0.8-13.7). There was no significant difference in hematologic and extrahematologic toxicities, either of all WHO grades than of grade 3/4, according to the initial dose of DAS.
Pleural effusions of all WHO grades occurred in 12 patients (18.5%): in 5 of them, pleural effusions occurred during the first 3-month period of treatment. Permanent DAS discontinuation due to pleural effusions was necessary in 6 patients (50.0% of those who developed pleural effusions and 9.5% of the entire evaluated cohort). The main clinical features of pleural effusions are shown in Table 2.
Table 2.
Main Clinical Features of Pleural Effusions
| Patients with pleural effusion, n (%) | 12 (18.5%) |
| Grade 1-2 | 9 |
| Grade 3 | 3 |
| Concomitant pericardial effusion, n | 0 |
| Median time from DAS start (months) (IR) |
3.4 (1.2-6.2) |
| Recurrence of pleural effusion, n (%) | 7/12 (58.3%) |
Overall, 10 patients (15.4%) permanently discontinued DAS due to toxicity (2 patients in the first 3 months of treatment, 4 between the 4th and 12th month, and 4 beyond that period). A different profile of dose adaptation and permanent drug discontinuation was observed according to the initial DAS dose. Twenty-six of 54 patients (48.1%) required a dose reduction in the group treated with 100 mg as initial dose as compared with 2 of 11 patients (18.1%) receiving less than 100 mg (P = .056). Moreover, all 10 patients who needed a permanent treatment discontinuation because of toxicity have been treated with 100 mg as initial dose. All dose modifications at different time points are shown in Figure 1.
Figure 1.
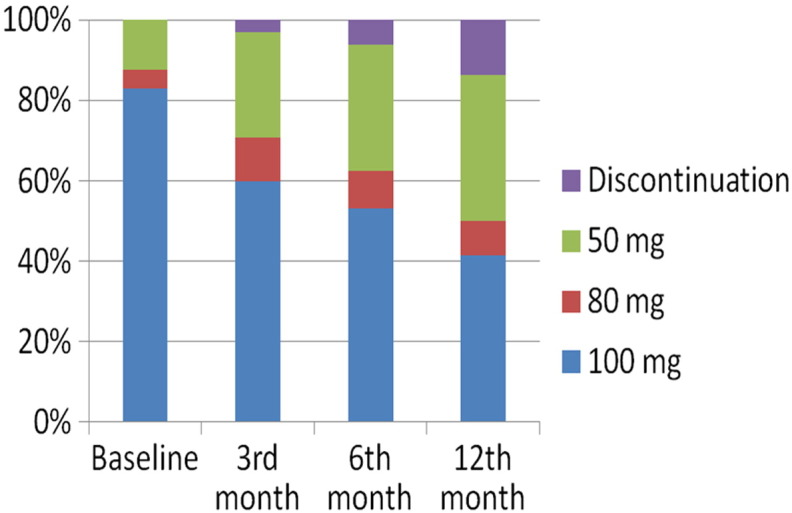
Dose modifications at different time points.
Treatment Results
All patients attained a complete hematologic response. After a median period of treatment of 17.0 months (IR 11.9-24.2), all of them were evaluable for cumulative response, with at least one cytogenetic and/or molecular evaluation performed. Two patients (3.1%) needed an early treatment discontinuation due to toxicity and were considered as failure; another 2 (3.1%) had a primary cytogenetic resistance, and 1 (1.5%) achieved a PCyR only. The remaining 60 patients (92.3%) achieved a CCyR and 50 of them (76.9%) also an MMR. Response to treatment at different time points is shown in Table 3. At the 3-month time point, early molecular response (BCR-ABL1/ABL1 ratio <10%) was achieved by 49 of 52 evaluable patients (94.2%); at the 6-month time point, early molecular response (BCR-ABL1/ABL1 ratio <1.0%) was achieved by 46 of 49 evaluable patients (93.8%).
Table 3.
Cytogenetic and Molecular Response to DAS at Different Time Points
| 3rd Month | 6th Month | 12th Month | |
|---|---|---|---|
| Too early | / | 1 | 7 |
| Evaluable | 65 | 64 | 58 |
| Not done | 6 (9.3%) | 6 (9.4%) | / |
| Discontinuation | 2 (3.1%) | 4 (6.2%) | 7 (12.1%) |
| Less than CCyR | 10 (15.3%) | 5 (7.8%) | 3 (5.1%) |
| CCyR* | 47 (72.3%) | 49 (76.6%) | 48 (82.8%) |
| MMR | 19 (29.2%) | 36 (56.2%) | 37 (63.8%) |
| MR 3.0 | 13 | 16 | 20 |
| MR 4.0 | 4 | 13 | 9 |
| MR 4.5 | 2 | 7 | 8 |
Patients at any time point with molecular analysis only and BCR-ABL1/ABL1 ratio <1.0 were also considered in CCyR.
Follow-Up
At the last follow-up, after a median period of observation of 19.3 months (IR 14.3-25.6), 59 patients are still alive and 48 of them are still receiving DAS treatment. Only 1 patient (1.5%) developed a blast phase after 13.5 months of therapy and is still alive and in treatment with ponatinib. Four patients died while in MMR (three cases) or in CHR (one case) from CML-unrelated causes (heart failure in two cases; acute myocardial infarction and senectus in one case, respectively), and two patients were lost to follow-up.
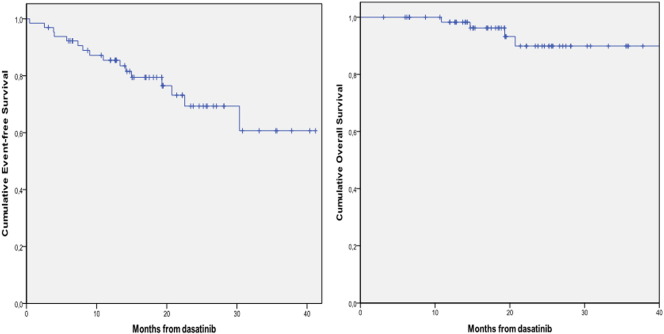
The 12-month and 24-month cumulative EFSs were 85.4% (95% confidence interval [CI] 76.6-94.2) and 69.3% (95% CI 55.2-83.4), respectively (Figure 2A): the 12-month and 24-month cumulative OSs were 98.3% (95% CI 95.0-100) and 89.9% (95% CI 80.1-99.7), respectively (Figure 2B).
Figure 2.
Cumulative EFS (A) and OS (B).
Discussion
Efficacy and safety of IM in patients older than 65 years with CML were already demonstrated in many clinical studies and real-life analyses; however, as in younger subjects, the response to IM remains unsatisfactory in about one third of patients.
The introduction of more potent but also more toxic 2G-TKIs in the frontline therapy of CML leads to the question of whether or not patients older than 65 years could be safely treated with these drugs.
In the setting of controlled clinical trials, we can only have data on tolerability and efficacy in very fit and compliant patients older than 65 years but not on the whole real-life elderly population. The selection bias present in these trials becomes evident when we look at the exclusion criteria and at the median age of the enrolled cohorts, which is lower than the median age of CML unselected patients in the most important epidemiological registries [20]. In both the DASISION and ENESTnd trials, median age was lower than 50 years and the rate of patients aged over 65 years was lower than 15% (36/246 [12.7%] and 27/281 [9.6%] in the NIL 300-mg and in the NIL 400-mg arms of ENESTnd, respectively, and 20/259 [7.7%] in the DAS arm of DASISION).
Therefore, to test feasibility of 2G-TKIs in patients older than 65 years, postapproval data from the current clinical practice are needed. At present, however, there is no report available in the literature on this issue.
We are aware that a selection was also made in our cohort, as many physicians still consider IM therapy the best frontline drug for patients older than 65 years. It is reasonable that, in our centers, low-risk or very frail patients older than 65 years probably did not receive DAS as frontline therapy and were not included in our real-life cohort.
However, the median age of our cohort was remarkably high, with about 50% of patients older than 75 years. Furthermore, a major point was the incidence in our cohort of concomitant diseases: it is worth noting that one third of patients had 3 or more comorbidities requiring active treatments and that an Eastern Cooperative Oncology Group performance status = 2 was reported in 16% of patients.
In this context, DAS treatment was safe, grade 3 to 4 hematological and extrahematological toxicities were acceptable, and the incidence of pleural effusions was comparable to that observed in clinical controlled trials in the same age group. The rate of permanent treatment discontinuation at 12 months was less than 15%, and it was similar to the rate observed in the DASISION trial [21].
Treatment results were also encouraging, with a high rate of cumulative CCyR (>90%) and MMR (>70%). These results were not affected by the initial DAS dosage: moreover, none of the patients who received lower DAS initial dose needed to stop the treatment for toxicities. Recent data from the OPTIM-Dasatinib study suggested that also a dose of DAS <100 mg could be effective with a lower toxicity [22]; the small sample size of our cohort did not allow us to spread any conclusion on the clinical value of a reduced DAS dose in this group of patients. In the future, however, an individualized approach, consisting of a reduced starting dose with progressive modifications based on response and toxicity, could be useful at least in some patient older than 65 years with comorbidities.
In conclusion, present data show that DAS could play a major role in the frontline treatment also in real-life patients aged over 65 years and with comorbidities, being effective and having a favorable safety profile.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.Druker BJ, Guilhot F, O’Brien SG, Gathmann I, Kantarjian H, Gattermann N, Deininger MW, Silver RT, Goldman JM, Stone RM. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006;355:2408–2417. doi: 10.1056/NEJMoa062867. [DOI] [PubMed] [Google Scholar]
- 2.Branford S, Seymour J, Grigg A, Arthur C, Rudzki Z, Lynch K, Hughes T. BCR-ABL messenger RNA levels continue to decline in patients with chronic phase chronic myeloid leukemia treated with imatinib for more than 5 years and approximately half of all first-line treated patients have stable undetectable BCR-ABL using strict sensitivity criteria. Clin Cancer Res. 2007;13:7080–7086. doi: 10.1158/1078-0432.CCR-07-0844. [DOI] [PubMed] [Google Scholar]
- 3.Stagno F, Stella S, Spitaleri A, Pennisi MS, Di Raimondo F, Vigneri P. Imatinib mesylate in chronic myeloid leukemia: frontline treatment and long-term outcomes. Expert Rev Anticancer Ther. 2016;16(3):273–278. doi: 10.1586/14737140.2016.1151356. [DOI] [PubMed] [Google Scholar]
- 4.Marin D, Milojkovic D, Olavarria E, Khorashad JS, de Lavallade H, Reid AG, Foroni L, Rezvani K, Bua M, Dazzi F. European LeukemiaNet criteria for failure or suboptimal response reliably identify patients with CML in early chronic phase treated with imatinib whose eventual outcome is poor. Blood. 2008;112:4437–4444. doi: 10.1182/blood-2008-06-162388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Castagnetti F, Gugliotta G, Breccia M, Stagno F, Iurlo A, Albano F, Abruzzese E, Martino B, Levato L, Intermesoli T. Long-term outcome of chronic myeloid leukemia patients treated frontline with imatinib. Leukemia. 2015;29(9):1823–1831. doi: 10.1038/leu.2015.152. [DOI] [PubMed] [Google Scholar]
- 6.Shah NP, Kantarjian HM, Kim DW, Réa D, Dorlhiac-Llacer PE, Milone JH, Vela-Ojeda J, Silver RT, Khoury HJ, Charbonnier A. Intermittent target inhibition with Dasatinib 100 mg once daily preserves efficacy and improves tolerability in imatinib-resistant and–intolerant chronic-phase chronic myeloid leukemia. J Clin Oncol. 2008;26:3204–3212. doi: 10.1200/JCO.2007.14.9260. [DOI] [PubMed] [Google Scholar]
- 7.Latagliata R, Breccia M, Castagnetti F, Stagno F, Luciano L, Gozzini A, Ulisciani S, Cavazzini F, Annunziata M, Sorà F. Dasatinib is safe and effective in unselected chronic myeloid leukaemia elderly patients resistant/intolerant to imatinib. Leuk Res. 2011;35(9):1164–1169. doi: 10.1016/j.leukres.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 8.Kantarjian HM, Giles FJ, Bhalla KN, Pinilla-Ibarz J, Larson RA, Gattermann N, Ottmann OG, Hochhaus A, Radich JP, Saglio G. Nilotinib is effective in patients with chronic myeloid leukemia in chronic phase after imatinib resistance or intolerance: 24-month follow-up results. Blood. 2011;117:1141–1145. doi: 10.1182/blood-2010-03-277152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kantarjian H, Shah NP, Hochhaus A, Cortes J, Shah S, Ayala M, Moiraghi B, Shen Z, Mayer J, Pasquini R. Dasatinib versus Imatinib in Newly Diagnosed Chronic-Phase Chronic Myeloid Leukemia. N Engl J Med. 2010;362:2260–2270. doi: 10.1056/NEJMoa1002315. [DOI] [PubMed] [Google Scholar]
- 10.Saglio G, Kim DW, Issaragrisil S, le Coutre P, Etienne G, Lobo C, Pasquini R, Clark RE, Hochhaus A, Hughes TP. Nilotinib versus imatinib for newly diagnosed chronic myeloid leukemia. N Engl J Med. 2010;362:2251–2259. doi: 10.1056/NEJMoa0912614. [DOI] [PubMed] [Google Scholar]
- 11.Gugliotta G, Castagnetti F, Fogli M, Cavo M, Baccarani M, Rosti G. Impact of comorbidities on the treatment of chronic myeloid leukemia with tyrosine-kinase inhibitors. Expert Rev Hematol. 2013;6:563–574. doi: 10.1586/17474086.2013.837279. [DOI] [PubMed] [Google Scholar]
- 12.Latagliata R, Carmosino I, Vozella F, Volpicelli P, De Angelis F, Loglisci MG, Salaroli A, De Luca ML, Montagna C, Serrao A. Impact of exclusion criteria for the DASISION and ENESTnd trials in the front-line treatment of a “real-life” patient population with Chronic Myeloid Leukemia. Hematol Oncol. 2015 doi: 10.1002/hon.2274. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 13.Rohrbacher M, Berger U, Hochhaus A, Metzgeroth G, Adam K, Lahaye T, Saussele S, Müller MC, Hasford J, Heimpel H. Clinical trials underestimate the age of chronic myeloid leukemia (CML) patients. Incidence and median age of Ph/BCR-ABL-positive CML and other chronic myeloproliferative disorders in a representative area in Germany. Leukemia. 2009;23:602–604. doi: 10.1038/leu.2008.245. [DOI] [PubMed] [Google Scholar]
- 14.Hamaker ME, Stauder R, van Munster BC. Exclusion of Older Patients From Ongoing Clinical Trials for Hematological Malignancies: An Evaluation of the National Institutes of Health Clinical Trial Registry. Oncologist. 2014;19:1069–1075. doi: 10.1634/theoncologist.2014-0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoffmann VS, Baccarani M, Hasford J, Lindoerfer D, Burgstaller S, Sertic D, Costeas P, Mayer J, Indrak K, Everaus H. The EUTOS population-based registry: incidence and clinical characteristics of 2904 CML patients in 20 European Countries. Leukemia. 2015;29(6):1336–1343. doi: 10.1038/leu.2015.73. [DOI] [PubMed] [Google Scholar]
- 16.Jabbour E, Makenbaeva D, Lingohr-Smith M, Lin J. Use of Real-World Claim Databases to Assess Prevalence of Comorbid Conditions Relevant to the Treatment of Chronic Myelogenous Leukemia Based on National Comprehensive Network Treatment Guidelines. Clin Lymphoma Myeloma Leuk. 2015;12:797–802. doi: 10.1016/j.clml.2015.09.008. [DOI] [PubMed] [Google Scholar]
- 17.Hughes T, Deininger M, Hochhaus A, Branford S, Radich J, Kaeda J, Baccarani M, Cortes J, Cross NC, Druker BJ. Monitoring CML patients responding to treatment with tyrosine kinase inhibitors: review and recommendations for harmonizing current methodology for detecting BCR-ABL transcripts and kinase domain mutations and for expressing results. Blood. 2006;108:28–37. doi: 10.1182/blood-2006-01-0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cross NC, White H, Müller MC, Saglio G, Hochhaus A. Standardized definitions of molecular response in chronic myeloid leukemia. Leukemia. 2012;26:2172–2175. doi: 10.1038/leu.2012.104. [DOI] [PubMed] [Google Scholar]
- 19.Falchi L, Kantarjian HM, Wang X, Verma D, Quintás-Cardama A, O'Brien S, Jabbour EJ, Ravandi-Kashani F, Borthakur G, Garcia-Manero G. Significance of deeper molecular responses in patients with chronic myeloid leukemia in early chronic phase treated with tyrosine kinase inhibitors. Am J Hematol. 2013;88(12):1024–1029. doi: 10.1002/ajh.23560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Höglund M, Sandin F, Simonsson B. Epidemiology of chronic myeloid leukaemia: an update. Ann Hematol. 2015;94(Suppl. 2):241–247. doi: 10.1007/s00277-015-2314-2. [DOI] [PubMed] [Google Scholar]
- 21.Jabbour E, Kantarjian HM, Saglio G, Steegmann JL, Shah NP, Boqué C, Chuah C, Pavlovsky C, Mayer J, Cortes J. Early response with dasatinib or imatinib in chronic myeloid leukemia: 3-year follow-up from a randomized phase 3 trial (DASISION) Blood. 2014;123(4):494–500. doi: 10.1182/blood-2013-06-511592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rousselot P, Mollica L, Guerci-Bresler A, Nicolini FE, Etienne G, Legros L, Charbonnier A, Coiteux V, Dartigeas C, Escoffre-Barbe M. Abstract S678 EHA. 2014. Dasatinib daily dose optimization based on residual drug levels resulted in reduced risk of pleural effusions and high molecular response rates: final results of the randomized OPTIM dasatinib trial. [Google Scholar]