Abstract
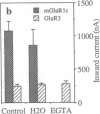
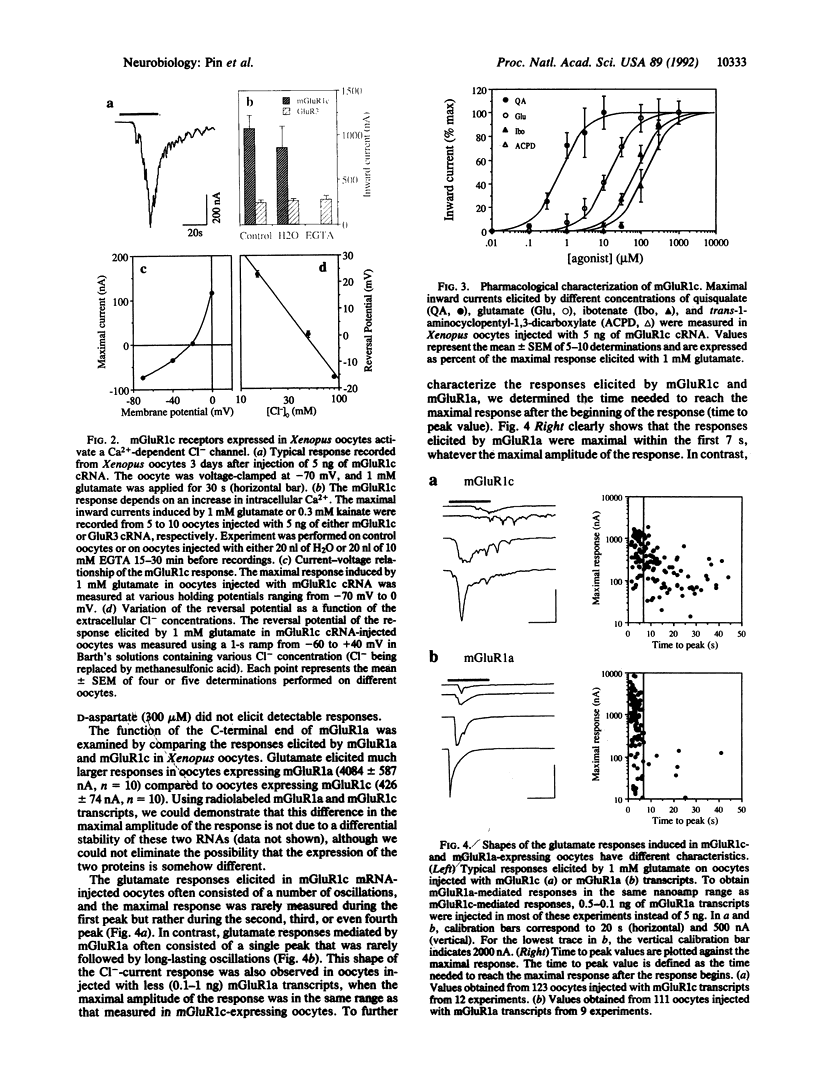
A splice variant of the metabotropic glutamate receptor (mGluR) 1a, named mGluR1c, was isolated. Compared to mGluR1a, the predicted mGluR1c protein is 302 amino acids shorter at its C-terminal end. Despite this difference, mGluR1c activates phospholipase C in Xenopus oocytes with a pharmacological profile identical to that of mGluR1a. However, in contrast to the large fast transient responses induced by mGluR1a, mGluR1c receptors elicit a small more slowly generated long-lasting oscillatory current, suggesting that these two receptors do not generate the same pattern of Ca2+ release in Xenopus oocytes. In situ hybridization data show that mGluR1c mRNA is expressed at a lower level than the other splice variants of mGluR1. Some differences in the regional distribution of these transcripts were observed in the cerebellum, the olfactory bulb, and the striatum.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bladon T. S., McBurney M. W. The rodent B2 sequence can affect expression when present in the transcribed region of a reporter gene. Gene. 1991 Feb 15;98(2):259–263. doi: 10.1016/0378-1119(91)90183-c. [DOI] [PubMed] [Google Scholar]
- Boulter J., Connolly J., Deneris E., Goldman D., Heinemann S., Patrick J. Functional expression of two neuronal nicotinic acetylcholine receptors from cDNA clones identifies a gene family. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7763–7767. doi: 10.1073/pnas.84.21.7763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnashev N., Monyer H., Seeburg P. H., Sakmann B. Divalent ion permeability of AMPA receptor channels is dominated by the edited form of a single subunit. Neuron. 1992 Jan;8(1):189–198. doi: 10.1016/0896-6273(92)90120-3. [DOI] [PubMed] [Google Scholar]
- Charpak S., Gähwiler B. H., Do K. Q., Knöpfel T. Potassium conductances in hippocampal neurons blocked by excitatory amino-acid transmitters. Nature. 1990 Oct 25;347(6295):765–767. doi: 10.1038/347765a0. [DOI] [PubMed] [Google Scholar]
- Clemens M. J. A potential role for RNA transcribed from B2 repeats in the regulation of mRNA stability. Cell. 1987 Apr 24;49(2):157–158. doi: 10.1016/0092-8674(87)90553-8. [DOI] [PubMed] [Google Scholar]
- Cornell-Bell A. H., Finkbeiner S. M., Cooper M. S., Smith S. J. Glutamate induces calcium waves in cultured astrocytes: long-range glial signaling. Science. 1990 Jan 26;247(4941):470–473. doi: 10.1126/science.1967852. [DOI] [PubMed] [Google Scholar]
- Courtney M. J., Lambert J. J., Nicholls D. G. The interactions between plasma membrane depolarization and glutamate receptor activation in the regulation of cytoplasmic free calcium in cultured cerebellar granule cells. J Neurosci. 1990 Dec;10(12):3873–3879. doi: 10.1523/JNEUROSCI.10-12-03873.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dal Toso R., Sommer B., Ewert M., Herb A., Pritchett D. B., Bach A., Shivers B. D., Seeburg P. H. The dopamine D2 receptor: two molecular forms generated by alternative splicing. EMBO J. 1989 Dec 20;8(13):4025–4034. doi: 10.1002/j.1460-2075.1989.tb08585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohlman H. G., Thorner J., Caron M. G., Lefkowitz R. J. Model systems for the study of seven-transmembrane-segment receptors. Annu Rev Biochem. 1991;60:653–688. doi: 10.1146/annurev.bi.60.070191.003253. [DOI] [PubMed] [Google Scholar]
- Fagni L., Bossu J. L., Bockaert J. Activation of a Large-conductance Ca2+-Dependent K+ Channel by Stimulation of Glutamate Phosphoinositide-coupled Receptors in Cultured Cerebellar Granule Cells. Eur J Neurosci. 1991;3(8):778–789. doi: 10.1111/j.1460-9568.1991.tb01674.x. [DOI] [PubMed] [Google Scholar]
- Glaum S. R., Holzwarth J. A., Miller R. J. Glutamate receptors activate Ca2+ mobilization and Ca2+ influx into astrocytes. Proc Natl Acad Sci U S A. 1990 May;87(9):3454–3458. doi: 10.1073/pnas.87.9.3454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harootunian A. T., Kao J. P., Paranjape S., Tsien R. Y. Generation of calcium oscillations in fibroblasts by positive feedback between calcium and IP3. Science. 1991 Jan 4;251(4989):75–78. doi: 10.1126/science.1986413. [DOI] [PubMed] [Google Scholar]
- Hirono C., Ito I., Sugiyama H. Neurotensin and acetylcholine evoke common responses in frog oocytes injected with rat brain messenger ribonucleic acid. J Physiol. 1987 Jan;382:523–535. doi: 10.1113/jphysiol.1987.sp016382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollmann M., Hartley M., Heinemann S. Ca2+ permeability of KA-AMPA--gated glutamate receptor channels depends on subunit composition. Science. 1991 May 10;252(5007):851–853. doi: 10.1126/science.1709304. [DOI] [PubMed] [Google Scholar]
- Houamed K. M., Kuijper J. L., Gilbert T. L., Haldeman B. A., O'Hara P. J., Mulvihill E. R., Almers W., Hagen F. S. Cloning, expression, and gene structure of a G protein-coupled glutamate receptor from rat brain. Science. 1991 May 31;252(5010):1318–1321. doi: 10.1126/science.1656524. [DOI] [PubMed] [Google Scholar]
- Jensen A. M., Chiu S. Y. Fluorescence measurement of changes in intracellular calcium induced by excitatory amino acids in cultured cortical astrocytes. J Neurosci. 1990 Apr;10(4):1165–1175. doi: 10.1523/JNEUROSCI.10-04-01165.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller B. U., Hollmann M., Heinemann S., Konnerth A. Calcium influx through subunits GluR1/GluR3 of kainate/AMPA receptor channels is regulated by cAMP dependent protein kinase. EMBO J. 1992 Mar;11(3):891–896. doi: 10.1002/j.1460-2075.1992.tb05127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechleiter J., Girard S., Clapham D., Peralta E. Subcellular patterns of calcium release determined by G protein-specific residues of muscarinic receptors. Nature. 1991 Apr 11;350(6318):505–508. doi: 10.1038/350505a0. [DOI] [PubMed] [Google Scholar]
- Lechleiter J., Girard S., Peralta E., Clapham D. Spiral calcium wave propagation and annihilation in Xenopus laevis oocytes. Science. 1991 Apr 5;252(5002):123–126. doi: 10.1126/science.2011747. [DOI] [PubMed] [Google Scholar]
- Lechleiter J., Hellmiss R., Duerson K., Ennulat D., David N., Clapham D., Peralta E. Distinct sequence elements control the specificity of G protein activation by muscarinic acetylcholine receptor subtypes. EMBO J. 1990 Dec;9(13):4381–4390. doi: 10.1002/j.1460-2075.1990.tb07888.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester R. A., Jahr C. E. Quisqualate receptor-mediated depression of calcium currents in hippocampal neurons. Neuron. 1990 May;4(5):741–749. doi: 10.1016/0896-6273(90)90200-y. [DOI] [PubMed] [Google Scholar]
- Masu M., Tanabe Y., Tsuchida K., Shigemoto R., Nakanishi S. Sequence and expression of a metabotropic glutamate receptor. Nature. 1991 Feb 28;349(6312):760–765. doi: 10.1038/349760a0. [DOI] [PubMed] [Google Scholar]
- Mengod G., Vilaró M. T., Niznik H. B., Sunahara R. K., Seeman P., O'Dowd B. F., Palacios J. M. Visualization of a dopamine D1 receptor mRNA in human and rat brain. Brain Res Mol Brain Res. 1991 May;10(2):185–191. doi: 10.1016/0169-328x(91)90110-j. [DOI] [PubMed] [Google Scholar]
- Meyer T., Stryer L. Molecular model for receptor-stimulated calcium spiking. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5051–5055. doi: 10.1073/pnas.85.14.5051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaghan D. T., Bridges R. J., Cotman C. W. The excitatory amino acid receptors: their classes, pharmacology, and distinct properties in the function of the central nervous system. Annu Rev Pharmacol Toxicol. 1989;29:365–402. doi: 10.1146/annurev.pa.29.040189.002053. [DOI] [PubMed] [Google Scholar]
- Murphy S. N., Miller R. J. A glutamate receptor regulates Ca2+ mobilization in hippocampal neurons. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8737–8741. doi: 10.1073/pnas.85.22.8737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoletti F., Wroblewski J. T., Novelli A., Alho H., Guidotti A., Costa E. The activation of inositol phospholipid metabolism as a signal-transducing system for excitatory amino acids in primary cultures of cerebellar granule cells. J Neurosci. 1986 Jul;6(7):1905–1911. doi: 10.1523/JNEUROSCI.06-07-01905.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Dowd B. F., Lefkowitz R. J., Caron M. G. Structure of the adrenergic and related receptors. Annu Rev Neurosci. 1989;12:67–83. doi: 10.1146/annurev.ne.12.030189.000435. [DOI] [PubMed] [Google Scholar]
- Schoepp D., Bockaert J., Sladeczek F. Pharmacological and functional characteristics of metabotropic excitatory amino acid receptors. Trends Pharmacol Sci. 1990 Dec;11(12):508–515. doi: 10.1016/0165-6147(90)90052-a. [DOI] [PubMed] [Google Scholar]
- Sladeczek F., Pin J. P., Récasens M., Bockaert J., Weiss S. Glutamate stimulates inositol phosphate formation in striatal neurones. Nature. 1985 Oct 24;317(6039):717–719. doi: 10.1038/317717a0. [DOI] [PubMed] [Google Scholar]
- Sugiyama H., Ito I., Hirono C. A new type of glutamate receptor linked to inositol phospholipid metabolism. Nature. 1987 Feb 5;325(6104):531–533. doi: 10.1038/325531a0. [DOI] [PubMed] [Google Scholar]
- Tanabe Y., Masu M., Ishii T., Shigemoto R., Nakanishi S. A family of metabotropic glutamate receptors. Neuron. 1992 Jan;8(1):169–179. doi: 10.1016/0896-6273(92)90118-w. [DOI] [PubMed] [Google Scholar]
- den Dunnen J. T., Schoenmakers J. G. Consensus sequences of the Rattus norvegicus B1- and B2 repeats. Nucleic Acids Res. 1987 Mar 25;15(6):2772–2772. doi: 10.1093/nar/15.6.2772. [DOI] [PMC free article] [PubMed] [Google Scholar]