Abstract
Genome-wide association studies (GWAS) have found few common variants that influence fasting measures of insulin sensitivity. We hypothesized that a GWAS of an integrated assessment of fasting and dynamic measures of insulin sensitivity would detect novel common variants. We performed a GWAS of the modified Stumvoll Insulin Sensitivity Index (ISI) within the Meta-Analyses of Glucose and Insulin-Related Traits Consortium. Discovery for genetic association was performed in 16,753 individuals, and replication was attempted for the 23 most significant novel loci in 13,354 independent individuals. Association with ISI was tested in models adjusted for age, sex, and BMI and in a model analyzing the combined influence of the genotype effect adjusted for BMI and the interaction effect between the genotype and BMI on ISI (model 3). In model 3, three variants reached genome-wide significance: rs13422522 (NYAP2; P = 8.87 × 10−11), rs12454712 (BCL2; P = 2.7 × 10−8), and rs10506418 (FAM19A2; P = 1.9 × 10−8). The association at NYAP2 was eliminated by conditioning on the known IRS1 insulin sensitivity locus; the BCL2 and FAM19A2 associations were independent of known cardiometabolic loci. In conclusion, we identified two novel loci and replicated known variants associated with insulin sensitivity. Further studies are needed to clarify the causal variant and function at the BCL2 and FAM19A2 loci.
Introduction
Genome-wide association studies (GWAS) have identified common genetic variants associated with type 2 diabetes (1), a disease marked by a reduction in β-cell function and insulin sensitivity (2). While both β-cell function and insulin sensitivity traits are partly heritable, GWAS have demonstrated relatively few single nucleotide polymorphisms (SNPs) associated with insulin sensitivity (3).
Traits used to estimate insulin sensitivity from fasting measurements in prior large GWAS, including fasting insulin and the HOMA–insulin resistance (HOMA-IR), demonstrate approximately half the heritability of traits that incorporate both fasting and dynamic assessments of insulin sensitivity following a glucose load (4). Moreover, there is only modest genetic correlation between HOMA-IR and measures of insulin sensitivity by euglycemic clamp, which is considered the gold standard measure of peripheral insulin sensitivity (5,6). Thus, an alternative approach to discover new common genetic variants associated with insulin sensitivity is to perform GWAS using a dynamic measure of whole-body insulin sensitivity. As an example, a recent GWAS identified a novel insulin sensitivity locus at NAT2 using euglycemic clamp and insulin suppression test techniques in 2,764 subjects, with replication in another 2,860 individuals (7). However, these direct, whole-body measures of insulin sensitivity are time- and resource-intensive interventions, which limits the feasible sample size of such experiments. Indices derived from an oral glucose tolerance test that integrate fasting and dynamic measures of insulin sensitivity reasonably approximate euglycemic clamp measures and can be applied in existing large cohorts with glycemic traits, potentially increasing the statistical power to detect novel variant associations.
We tested the hypothesis that a well-powered GWAS would detect common genetic variants for the modified Stumvoll Insulin Sensitivity Index (ISI). Insulin sensitivity assessed by the euglycemic-hyperinsulinemic clamp (average glucose infusion rate/average plasma insulin concentration [M/I]) has a stronger correlation with the ISI than with HOMA-IR (r = 0.79 vs. 0.59, respectively) (8). In addition, the ISI is well correlated (r = 0.69) with M/I, even when calculated using only fasting insulin values and glucose and insulin values 120 min after a 75-g oral glucose load (9); this modified version is widely available in existing cohorts, providing a larger sample size for association analyses than the sample size that would be available if indices requiring additional time points were used. We further hypothesized that a subset of these common genetic variants would influence the ISI independently or through their effect on BMI. Thus we tested the association of the modified ISI in statistical models without adjusting for BMI, in statistical models adjusting for BMI, and in a validated model (10,11) analyzing the combined influence of the genotype effect adjusted for BMI and the interaction effect between the genotype and BMI on ISI.
Research Design and Methods
Cohort Descriptions
The cohorts participating in the Meta-Analyses of Glucose and Insulin-related Traits Consortium (MAGIC) contributed a total of 30,107 individuals to the analyses. Detailed information on the study cohorts and methods is provided in Supplementary Table 1. All participants were of white European ancestry from the United States or Europe and did not have diabetes. All studies were approved by local research ethic committees, and all participants gave informed consent.
Modified Stumvoll ISI
Missing trait data were not imputed, and outliers were not excluded from analyses. The ISI was calculated as previously described (9), according to the following formula:
 |
Discovery Effort: GWAS
Cohorts that were able to contribute genome-wide genotyping results during the course of the project were included in the discovery effort. These were the Framingham Heart Study (FHS), Sorbs, the Finland–United States Investigation of NIDDM (FUSION), the Cardiovascular Health Study (CHS), Ludwigshafen Risk and Cardiovascular Health (LURIC) study, the Uppsala Longitudinal Study of Adult Men (ULSAM), and Metabolic Syndrome in Men (METSIM) study. For the discovery GWAS, all samples with call rates <95% were excluded, and SNPs departing from Hardy-Weinberg equilibrium (at P < 10−6), genotype rate <95%, or minor allele frequency <1% were excluded. Poorly imputed SNPs were excluded if R2 <0.3 or proper-info was <0.4.
Each SNP was tested for association with ISI in three different additive genetic models: model 1 was adjusted for age and sex; model 2 was adjusted for age, sex, and BMI; and model 3 analyzed the combined influence of the genotype effect adjusted for BMI and the interaction effect between the genotype and BMI on ISI (10,11). The associations in model 3 result from a test with two degrees of freedom. When no interaction is present, the additional degree of freedom results in a modest loss of statistical power. When interaction is present, however, the statistical power of the model is greater (11). To adjust for differences in insulin measurement between cohorts, effect estimates were normalized to the SD of the ISI in each cohort (Supplementary Table 1). A robust estimate of the standard error was calculated in the interaction analysis using ProbAbel, QUICKtest, or generalized estimating equations using the R geepack package. An inverse-variance meta-analysis using METAL was performed on the β coefficient/SD from each cohort.
Following meta-analysis, SNPs with total sample size less than 8,500 (approximately half of the maximum sample size) or with heterogeneity P values ≤10−6 (a value chosen to take into account multiple hypothesis testing but below the level of strict Bonferroni correction) in the meta-analysis of the discovery cohorts were removed. Genomic correction of cohort-specific association statistics (i.e., correction for each individual study) was performed. In total, up to 2.4 million SNPs were meta-analyzed for association with ISI in the discovery effort.
Selection of SNPs for Replication
Candidate SNPs for replication were identified by their association P value ≤10−7 in one or more of the analysis models. For gene loci with multiple replication candidates, the SNP with the lowest P value and any other SNP in low linkage disequilibrium (LD; r2 < 0.5) with the index SNP in Europeans were retained. Using these filters, 23 unique candidate SNPs from 23 loci were identified for replication. The SNP Annotation and Proxy Search site was used to find up to three proxies in high LD (r2 > 0.8) in Europeans for each candidate SNP.
Replication Effort
Cohorts that did not contribute to the discovery effort but were able to contribute association results during the course of the project were included in the replication effort. These were the European Network on Functional Genomics of Type 2 Diabetes (EUGENE2) study, Amish Studies, the Relationship between Insulin Sensitivity and Cardiovascular Risk Study (RISC), the Tübingen Family Study for Type 2 Diabetes (Tübingen), Inter99 Study, the Segovia Study, the Pizarra Study, the Botnia Study, the 1936 Birth Cohort, and the Ely Study. Genotype data were obtained using in silico data from preexisting GWAS or de novo genotyping. In replication cohorts, SNPs with a minor allele count (MAC) <20 were excluded. Additional details of the replication cohort effort are provided in Supplementary Table 1.
Combined Meta-analysis
We required the absence of heterogeneity in the combined analysis of discovery and replication cohorts (P > 10−6) as well as nominal significance (P < 0.05) in the replication effort and genome-wide significance (P < 5 × 10−8) in the combined meta-analysis for statistical evidence of association between a novel SNP and the ISI. To assess the effect of removing lower-frequency SNPs in model 3, a sensitivity analysis was performed using the MAC <20 filter on a cohort-wise basis in both the discovery and replication cohorts.
Assessment for Association of Known Insulin Sensitivity Loci With ISI
The associations of published insulin sensitivity loci were tested for association with the ISI in the discovery cohorts. Loci associated with fasting insulin without (12) and with adjustment for BMI (3,12), with fasting insulin using the approach in model 3 (10), and with direct measures of insulin sensitivity were included in these analyses (7). The published results for associations with fasting insulin with or without BMI adjustment (N = ∼50,000–100,000) (3,12) or exploiting potential BMI-by-gene interaction (model 3; N = ∼80,000) (10) used the same statistical approach as in the current study but were derived in a sample size approximately three to six times larger than that of the current study discovery cohort (N = ∼16,000). The sample sizes of the published fasting insulin analyses were much greater because only fasting insulin and BMI phenotypes were required for cohort participation. To analyze the association with fasting insulin and ISI in a comparable sample, we also examined the subset of discovery cohorts that contributed to the current assessment of ISI and prior assessments of fasting insulin: FHS, Sorbs, FUSION, and CHS. In models 2 and 3, only data from FHS, Sorbs, and FUSION were analyzed because participant-level BMI data were not available in CHS. A binomial sign test was used to determine whether the expected direction of the effect for these published loci with ISI occurred more often than by chance.
Conditional Analyses and Assessment of the Association of Top Findings With Direct Measures of Insulin Sensitivity
Findings that reached genome-wide significance were assessed for association with direct measures of insulin sensitivity in the Genetics of Insulin Sensitivity (GENESIS) consortium (7). Direct measures of insulin sensitivity were inverse normal transformed M value in cohorts with euglycemic insulin clamp assessments and inverse normal transformation of the steady state plasma glucose from cohorts with an insulin suppression test. These two traits are highly correlated (r = −0.85; P < 0.001) (13), and tests of association with the direct measure of insulin sensitivity showed no evidence of heterogeneity (P value for heterogeneity = 0.34 for the BCL2 variant and 0.66 for the FAM19A2 variant). Therefore, we did not perform separate tests of association in the smaller subsets of data with either the M value or the insulin suppression test phenotype. Statistical models were adjusted for age, sex, and BMI.
The top findings of the ISI analyses were also assessed in a MAGIC association analysis from Manning et al. (10) with fasting insulin using the approach in model 3. These ISI variants were only available in the discovery cohort from Manning et al. (n = 38,649 for rs12454712; n = 45,290 for rs10506418). We also performed association analyses with fasting insulin and ISI in a subset of the discovery cohort—FHS, Sorbs, and FUSION—to ascertain values in a comparable sample.
Approximate conditional analyses were performed to understand whether known loci contributed to the associations of novel findings with the ISI (14). These analyses were based on the summary-level statistics from the meta-analysis and the estimated LD using individual-level genotype data from the FHS discovery cohort. The software implementation for this approach does not incorporate the interaction term from model 3, and therefore conditional analyses were not performed in model 3.
Results
The demographic characteristics of the participants included in the discovery and replication efforts are presented in Table 1. In total, the discovery, replication, and combined meta-analyses included up to 16,753, 13,354, and 30,107 participants, respectively.
Table 1.
Cohort and participant demographics
| Cohort | Participants (n) | Female (%) | Age (years) | BMI (kg/m2) | Fasting glucose (mmol/L) | Fasting insulin (pmol/L) | Stumvoll ISI ([μmol * pmol]/[kg * min * L]) |
|---|---|---|---|---|---|---|---|
| Discovery | |||||||
| FHS | 2,602 | 54 | 54.0 ± 9.9 | 26.8 ± 4.5 | 5.2 ± 0.5 | 28.6 ± 9.9 | 0.111 ± 0.012 |
| Sorbs | 802 | 60 | 46.3 ± 15.9 | 26.5 ± 6.4 | 5.5 ± 1.2 | 40.7 ± 26.5 | 0.105 ± 0.023 |
| FUSION | 462 | 63 | 66.5 ± 6.7 | 27.6 ± 4.2 | 5.1 ± 0.5 | 68.5 ± 36.0 | 0.087 ± 0.023 |
| CHS | 2,761 | 62 | 72.3 ± 5.3 | 26.0 ± 4.3 | 5.5 ± 0.5 | 93.3 ± 47.8 | 0.059 ± 0.038 |
| LURIC | 962 | 24 | 61.9 ± 27.1 | 27.1 ± 3.8 | 5.5 ± 0.6 | 61.6 ± 49.9 | 0.065 ± 0.038 |
| ULSAM | 962 | 0 | 71.0 ± 0.6 | 26.0 ± 3.2 | 5.4 ± 0.6 | 73.0 ± 40.1 | 0.074 ± 0.031 |
| METSIM | 7,388 | 0 | 57.0 ± 6.96 | 26.8 ± 3.8 | 5.7 ± 0.5 | 49.8 ± 35.3 | 0.093 ± 0.030 |
| Replication | |||||||
| EUGENE2 | 885 | 56 | 39.4 ± 9.2 | 26.5 ± 4.8 | 5.1 ± 0.5 | 49.0 ± 34.9 | 0.091 ± 0.028 |
| Amish Studies | 334 | 61 | 45 ± 12.7 | 27.4 ± 4.7 | 4.9 ± 0.5 | 63.4 ± 26.0 | 0.09 ± 0.02 |
| RISC | 921 | 56 | 44 ± 8.37 | 25.5 ± 4.0 | 5.1 ± 0.6 | 34.4 ± 18.7 | 0.106 ± 0.018 |
| Tübingen | 2,470 | 65 | 40.2 ± 13.2 | 30.9 ± 9.6 | 5.2 ± 0.6 | 83.4 ± 72.2 | 0.070 ± 0.049 |
| Inter99 | 5,318 | 51 | 45.9 ± 7.9 | 26.1 ± 4.4 | 5.5 ± 0.5 | 41.1 ± 26.3 | 0.101 ± 0.021 |
| Segovia | 420 | 53 | 52.1 ± 11.4 | 26.7 ± 3.8 | 4.5 ± 0.6 | 71.2 ± 39.7 | 0.087 ± 0.025 |
| Pizarra | 640 | 66 | 43.6 ± 13.0 | 27.8 ± 4.9 | 5.4 ± 0.7 | 46.6 ± 34.2 | 0.101 ± 0.023 |
| Botnia Study | 1,235 | 52 | 58.3 ± 10.2 | 27.1 ± 3.9 | 5.4 ± 0.5 | 44.7 ± 28.7 | 0.099 ± 0.020 |
| 1936 Birth Cohort | 576 | 54 | 60.5 ± 0.5 | 26.5 ± 4.0 | 5.2 ± 0.5 | 42.5 ± 23.7 | 0.098 ± 0.022 |
| Ely Study | 1,442 | 54 | 61.1 ± 9.2 | 27.3 ± 4.8 | 5.0 ± 0.6 | 57.1 ± 35.7 | 0.088 ± 0.031 |
Continuous results are shown as mean ± SD. Additional information for each cohort can be found in Supplementary Table 1.
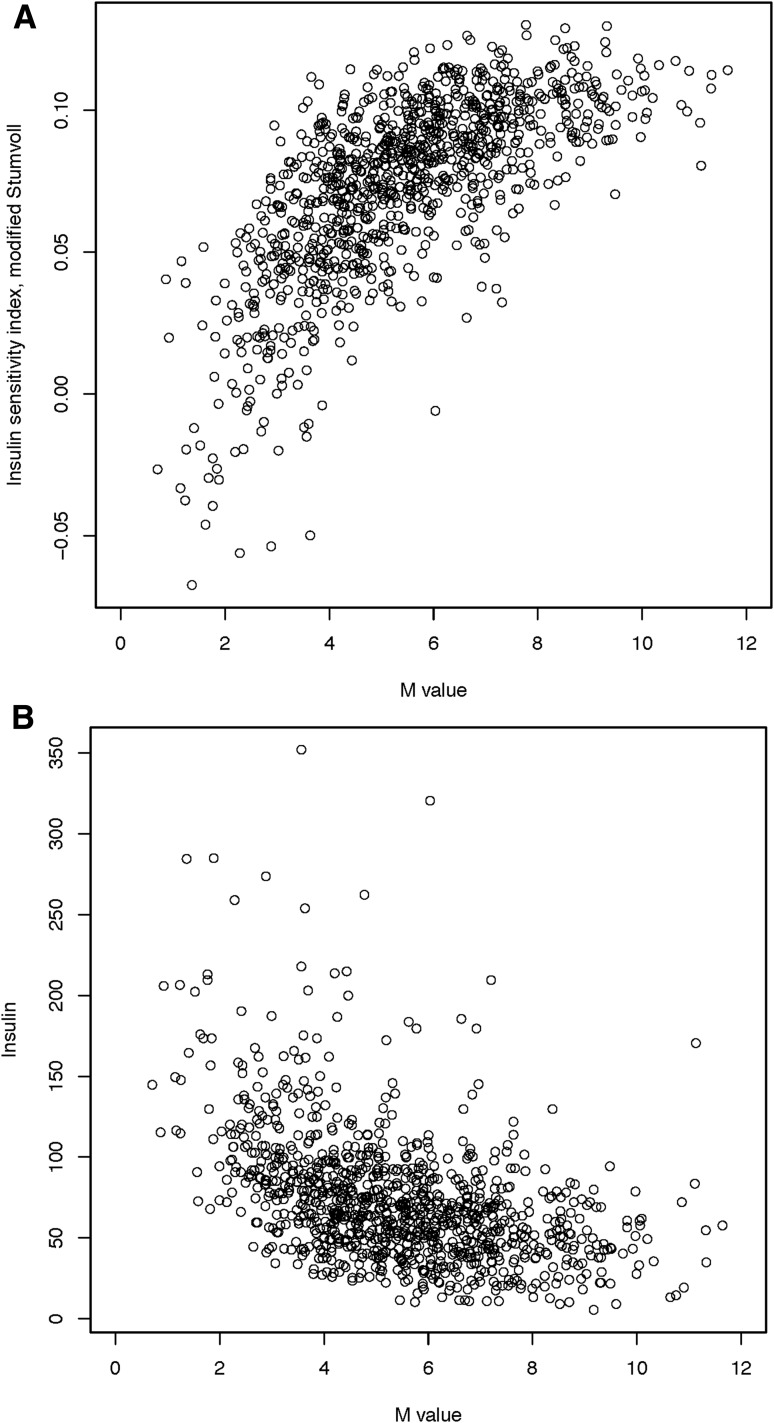
Using a variance component approach implemented in SOLAR software (15), the heritability of the ISI (H2r ± SE) in related FHS participants (n = 2,833) was very similar without or with adjustment for BMI (34.6 ± 6.8%; P = 2.8 ×10−8 and 33.4 ± 6.8%; P = 1.0 ×10−6, respectively). Within the ULSAM discovery cohort, the Spearman correlation between the ISI and M value from the euglycemic-hyperinsulinemic clamp was 0.71 (Fig. 1), consistent with reports from the literature (9); the Spearman correlation between ISI and fasting insulin was −0.49 (Fig. 1).
Figure 1.
Correlation of ISI with the M value from the insulin clamp (A) and fasting insulin (B) in ULSAM. Insulin sensitivity was measured within the ULSAM discovery cohort (n = 1,025) using a hyperinsulinemic-euglycemic clamp (M value), the modified Stumvoll ISI, and fasting insulin. The ULSAM cohort contains only men, and individuals with known diabetes were excluded from these analyses. For the comparison of the M value with ISI, the Pearson correlation was 0.69 and the Spearman correlation was 0.71, which are consistent with prior published reports. For the comparison of the ISI with fasting insulin, the Pearson correlation was −0.45 and the Spearman correlation was −0.49.
When tested in the full discovery cohort, 12 of 13 loci previously associated with fasting insulin in the literature (12) (P = 0.002 for binomial sign test) and 13 of 15 loci previously associated with fasting insulin after adjusting for BMI in the literature (3,12) (P = 0.004 for binomial sign test) showed the expected direction of effect with the ISI in the discovery cohorts (Supplementary Table 2). When these associations were examined in a subset of the current study discovery cohort (Supplementary Table 2), statistical significance was reduced, but effects at each loci remained in the expected direction (10 of 13 loci for ISI vs. fasting insulin without BMI adjustment, P = 0.03 for binomial sign test; 11 of 15 loci for ISI vs. fasting insulin with BMI adjustment, P = 0.04 for binomial sign test). Using a variant in LD with rs1208 (rs7815686; r2 = 0.67), we also found the expected direction of effect with ISI in the discovery cohorts (n = 16,753) at the NAT2 locus (model 1; β= −0.029; P = 9 × 10−3) (7).
The QQ plots for models 1, 2, and 3 are shown in Supplementary Figs. 1–3, respectively. Measures of genomic control were consistent with low inflation (model 1 λGC = 1.015; model 2 λGC = 1.006; model 3 λGC = 1.079). While genomic control was used to correct for each individual study, no additional corrections were applied to the meta-analysis results. The separate results of the discovery and replication results for model 1 (adjusting for age and sex), model 2 (adjusting for age, sex, and BMI), and model 3 (adjusting for age, sex, and BMI and analyzing the combined influence of the genotype effect adjusted for BMI and1the interaction effect between the genotype and BMI on ISI) are shown in Supplementary Table 3. Four SNPs selected from the discovery effort reached nominal significance (P < 0.05) in the replication analyses: rs13422522 (NYAP2) in models 1, 2, and 3; rs12454712 (BCL2) in models 2 and 3; rs10506418 (FAM19A2) in model 3; and rs6013915 (PFDN4) in model 3. Although the association with rs4548846 (CDH13) reached nominal significance in the replication effort for model 3, the association was in the opposite direction of effect, as in the discovery analyses; consequently, the association of this variant also had high heterogeneity in the combined meta-analysis.
We compared the β coefficients for the 22 SNPs identified in the discovery effort (rs4548846 [CDH13] was excluded given its high heterogeneity) with fasting insulin and ISI in a subset of the discovery cohort. Pearson correlations between the β for fasting insulin and the β for ISI were −0.494 in model 1, −0.797 in model 2, and −0.461 (for SNP effect) and −0482 (for interaction) in model 3.
The results of the combined discovery and replication cohort meta-analyses in each of the three models are shown in Table 2 and Supplementary Table 3. No association reached genome-wide significance in model 1. In model 2, rs13422522 (NYAP2; P = 1.8 × 10−11) and rs12454712 (BCL2; P = 1.9 × 10−8) achieved genome-wide significance. In model 3, rs13422522 (NYAP2; P = 8.9 × 10−11), rs12454712 (BCL2; P = 2.7 × 10−8), and rs10506418 (FAM19A2; P = 1.9 × 10−8) reached genome-wide significance. In model 3, rs6027072 (ARHGAP40; P = 4.4 × 10−9) also reached genome-wide significance but did not achieve nominal significance in the replication cohort, and rs6013915 (PFND4) had high heterogeneity in the combined meta-analysis of discovery and replication cohorts (P for heterogeneity = 6.03 × 10−7); therefore associations with these SNPs were not included as trustworthy findings.
Table 2.
Meta-analysis results for variant association with the ISI
| SNP | Chromosome | Locus | Allele (effect/other) | Frequency of the effect allele | Model 1, β ± SE (P value) | Model 2, β ± SE (P value) | Model 3, β ± SE (main/interaction) (joint P value) | N (minimum/maximum) |
|---|---|---|---|---|---|---|---|---|
| rs13422522 | 2 | NYAP2 | C/G | 0.77 | −0.04 ± 0.01 (1.6 × 10−5) | −0.06 ± 0.01 (1.2 × 10−11) | 0.10 ± 0.06/−0.01 ± 0.002 (8.9 × 10−11) | 30,057/30,078.3 |
| rs4078023 | 16 | GP2 | T/G | 0.98 | −0.028 ± 0.04 (0.49) | −0.05 ± 0.04 (0.20) | 0.80 ± 0.17/−0.03 ± 0.01 (3.2 × 10−7) | 24,727/24,742 |
| rs12372926 | 15 | ARRDC4 | T/C | 0.41 | −0.03 ± 0.01 (0.001) | −0.03 ± 0.01 (1.6 × 10−5) | 0.11 ± 0.05/−0.005 ± 0.002 (4.2 × 10−4) | 30,073/30,095 |
| rs16924527 | 8 | TOX | A/C | 0.02 | 0.12 ± 0.04 (0.001‡) | 0.07 ± 0.03 (0.02) | −0.08 ± 0.14/0.01 ± 0.01 (3.7 × 10−6‡) | 24,994/25,005 |
| rs2828537 | 21 | MRPL39 | A/T | 0.97 | −0.04 ± 0.03 (0.12) | −0.03 ± 0.02 (0.16) | 0.42 ± 0.10/−0.02 ± 0.004 (2.6 × 10−5) | 29,733/29,753.9 |
| rs3900087 | 4 | ADAMTS3 | T/C | 0.98 | −0.04 ± 0.05 (0.33) | −0.04 ± 0.04 (0.31) | 0.74 ± 0.21/−0.03 ± 0.01 (4.7 × 10−4) | 22,350/22,351 |
| rs6027072 | 20 | ARHGAP40 | A/G | 0.03 | 0.10 ± 0.02 (0.0001) | 0.08 ± 0.02 (4.1 × 10−4) | −0.39 ± 0.12/0.02 ± 0.005 (4.4 × 10−9) | 28,877/28,896 |
| rs12454712 | 18 | BCL2 | T/C | 0.58 | −0.04 ± 0.01 (0.0003) | −0.05 ± 0.01 (1.9 × 10−8) | 0.04 ± 0.05/−0.003 ± 0.002 (2.7 × 10−8) | 25,973/26,761 |
| rs10506418 | 12 | FAM19A2 | A/G | 0.03 | 0.06 ± 0.03 (0.05) | 0.06 ± 0.03 (0.01) | −0.62 ± 0.13/0.03 ± 0.005 (1.9 × 10−8) | 26,011/26,024 |
| rs1857095 | 1 | ELTD1 | T/C | 0.98 | −0.01 ± 0.03 (0.84) | −0.02 ± 0.03 (0.37) | 0.08 ± 0.12/−0.0003 ± 0.005 (7.9 × 10−9‡) | 26,596/26,608.9 |
| rs11594101 | 10 | NRG3 | A/G | 0.98 | 0.02 ± 0.04 (0.57) | −0.002 ± 0.03 (0.94) | 0.62 ± 0.14/−0.02 ± 0.005 (9.5 × 10−5) | 27,885/27,904 |
| rs12583553 | 13 | FGF9 | A/T | 0.97 | −0.04 ± 0.03 (0.19) | −0.05 ± 0.03 (0.04) | 0.55 ± 0.12/−0.02 ± 0.005 (3.6 × 10−9‡) | 29,195/29,215 |
| rs4548846 | 16 | CDH13 | T/C | 0.02 | 0.02 ± 0.04 (0.72) | −0.002 ± 0.04 (0.96) | 0.59 ± 0.18/−0.03 ± 0.01 (1.1 × 10−5‡) | 18,401/18,405.99 |
| rs12522198 | 5 | FAM134B | A/G | 0.02 | −0.03 ± 0.04 (0.48) | 0.01 ± 0.04 (0.84) | 0.79 ± 0.19/−0.03 ± 0.01 (1.6 × 10−4‡) | 19,798/20,589 |
| rs10483182 | 22 | ISX | A/G | 0.01 | 0.06 ± 0.04 (0.17) | 0.03 ± 0.04 (0.39) | −1.16 ± 0.18/0.05 ± 0.01 (7.8 × 10−12‡) | 20,399/20,409 |
| rs10520638 | 15 | AGBL1 | T/C | 0.01 | 0.004 ± 0.05 (0.93) | −0.01 ± 0.04 (0.77) | 0.89 ± 0.19/−0.04 ± 0.01 (1.2 × 10−7‡) | 12,369/12,383 |
| rs6013915 | 20 | PFDN4 | A/G | 0.03 | 0.05 ± 0.03 (0.14) | 0.06 ± 0.03 (0.05) | −0.84 ± 0.19/0.04 ± 0.01 (1.5 × 10−9‡) | 23,111/23,121.9 |
| rs9658121 | 6 | PPARD | A/G | 0.02 | −0.01 ± 0.04 (0.80) | 0.02 ± 0.04 (0.63) | −0.40 ± 0.15/0.02 ± 0.01 (7.3 × 10−4‡) | 16,973/16,985 |
| rs10508754 | 10 | KIAA1462 | A/G | 0.08 | −0.03 ± 0.02 (0.08) | −0.05 ± 0.02 (0.01) | 0.14 ± 0.09/−0.01 ± 0.004 (0.07) | 25,146/25,150 |
| rs11627967 | 14 | NPAS3 | T/G | 0.016 | −0.02 ± 0.05 (0.69) | −0.03 ± 0.04 (0.44) | −0.94 ± 0.21/0.04 ± 0.01 (1.6 × 10−7‡) | 17,593/17,595.98 |
| rs10495667 | 2 | VSNL1 | A/G | 0.04 | 0.02 ± 0.02 (0.32) | 0.01 ± 0.02 (0.69) | −0.51 ± 0.12/0.02 ± 0.005 (3.8 × 10−5) | 27,332/27,345.9 |
| rs13059110 | 3 | TXNDC6 | T/G | 0.13 | −0.05 ± 0.02 (0.0001) | −0.04 ± 0.01 (2.3 × 10−4) | 0.03 ± 0.07/−0.002 ± 0.003 (0.01) | 26,420/26,425 |
| rs11790816 | 9 | SH3GL2 | T/C | 0.02 | 0.01 ± 0.03 (0.63) | 0.02 ± 0.03 (0.45) | −0.37 ± 0.14/0.02 ± 0.01 (0.001‡) | 21,814/21,833.92 |
Model 1 is adjusted for age and sex; model 2 is adjusted for age, sex, and BMI; model 3 assesses the combined influence of the SNP effect adjusted for BMI and the interaction effect between the genotype and BMI on ISI. For models 1 and 2, the effect (β), SE, and P values for the SNP are shown. For model 3, the β and SE are provided for the SNP and the interaction; a P value is provided for the joint influence of the SNP and interaction effect. N = sample size for the combined analysis of discovery and replication cohorts. Effect sizes are presented as the SD per effect allele. ‡P value for heterogeneity in the combined analysis of discovery and replication cohorts (P ≤ 10−6).
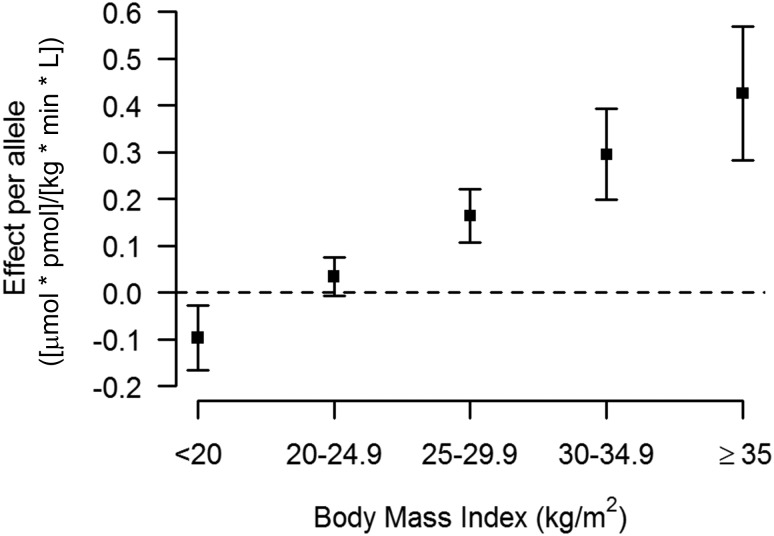
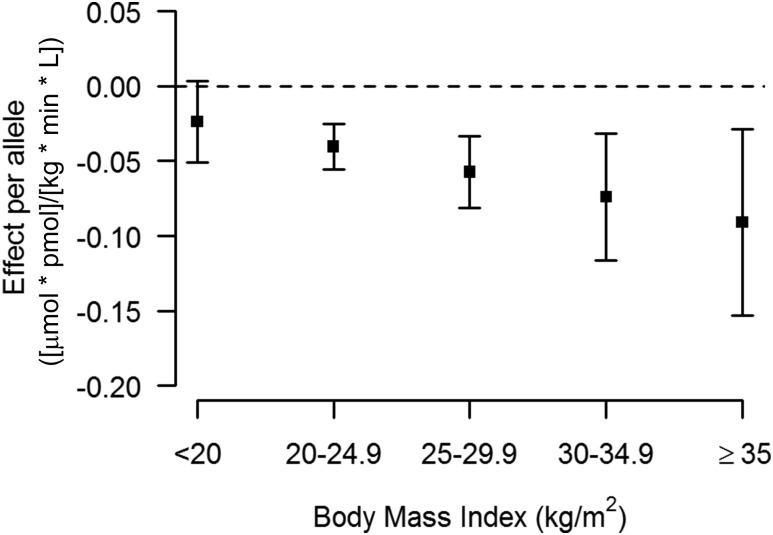
Hence, rs13422522 (NYAP2), rs12454712 (BCL2), and rs10506418 (FAM19A2) were the three SNPs that reached our a priori requirements for claiming statistical evidence. The association at rs13422522 (NYAP2) was in LD (r2 = 0.7) with previously reported results at the known insulin sensitivity signal rs2943641 (IRS1) (10), and the association with the ISI in model 2 was greatly reduced by conditioning rs13422522 on rs2943641 in the discovery cohort (β = −0.066 ± 0.01; P = 4.29 × 10−8 to β = −0.025 ± 0.01; P = 0.01). Thus, this SNP was considered a reflection of the known IRS1 signal and not an independent signal. The associations for rs12454712 (BCL2) and rs10506418 (FAM19A2) with the ISI were consistent across the discovery and replication cohorts (Supplementary Figs. 4 and 5, respectively). When stratifying by BMI, the effect of the minor allele (A) at rs10506418 (FAM19A2) on insulin sensitivity was negative at lower BMI and became positive and stronger with increasing BMI (Fig. 2), and the effect of the major allele (T) at rs12454712 (BCL2) on ISI was more negative with increasing BMI (Fig. 3).
Figure 2.
The effect of rs10506418 (FAM19A2) on insulin sensitivity by BMI category. The effect of the minor allele (A) at rs10506418 (FAM19A2) on the ISI is shown by BMI category. At a low BMI (<20 kg/m2), the effect is negative. At each category of increasing BMI above 20 kg/m2, the effect is positive and stronger.
Figure 3.
The effect of rs10506418 (BCL2) on insulin sensitivity by BMI category. The effect of the major allele (T) at rs10506418 (BCL2) on the ISI is shown by BMI category. At each category of increasing BMI, the effect is negative and stronger.
The genomic inflation of models 1 and 2 was low and slightly higher in model 3. Because the same individuals were used in each model, inflation in model 3 was unlikely to arise from population stratification. We performed an additional sensitivity analysis that applied the MAC < 20 filter to both the discovery and replication cohorts (Supplementary Table 4), which tended to reduce the statistical significance of associations with high heterogeneity and slightly reduced the statistical significance of the association at the FAM19A2 locus in model 3 without markedly reducing the magnitude of effect or affecting heterogeneity (β = −0.62 ± 0.13; P = 1.9 ×10−8; P for heterogeneity = 0.11 to β = −0.58 ± 0.13; P = 8.0 ×10−7; P for heterogeneity = 0.07). The sample size for the FAM19A2 locus association in model 3 was 462 individuals fewer when the MAC filter was applied in the discovery cohorts versus when the minor allele frequency filter was applied, and the resulting loss in power was likely responsible for the slight reduction in statistical significance.
Conditioning the results at either variant with known signals at least 1 Mb away did not attenuate the association with the ISI in the discovery cohorts of model 2 (a full description is provided in Supplementary Table 5). The rs10506418 (FAM19A2) variant was not associated with fasting insulin using model 3 in a separate GWAS result (10) or with direct measures of insulin sensitivity in GENESIS. The major allele (T) of rs12454712 (BCL2), which was associated with lower insulin sensitivity in this study, was also associated with a trend toward higher fasting insulin in a separate GWAS result using model 3 (SNP effect −0.006 ± 0.003; interaction effect 0.001 ± 0.001; P = 5.9 × 10−5; N = 38,649) (10). Similar trends were observed when the variant was tested for association with ISI and fasting insulin in the same discovery cohort subset (Supplementary Table 5).
Discussion
In a study of over 30,000 participants, we found novel, independent, genome-wide significant associations for the ISI at rs12454712 (BCL2) and rs10506418 (FAM19A2). Strengths of this study’s design include a large sample size, individuals with glycemic and metabolic phenotyping, high-quality genomic data, and use of traditional and contemporary statistical models to account for the influence of BMI on insulin sensitivity. In addition, our approach targeted a phenotype not previously examined in GWAS: the modified Stumvoll ISI. By incorporating glucose and insulin measures before and after a glucose load, this phenotype captures information that fasting assessments such as HOMA-IR or insulin alone would not. Indeed, the correlation between ISI and the M value is higher than that between the M value and fasting insulin (16), which has been used in prior genetic studies of insulin sensitivity (10,12). At the same time, the use of measures obtained at only two time points (fasting and 120 min into an oral glucose tolerance test) permitted the assembly of the large sample size required to achieve adequate statistical power.
Several findings serve as positive controls for our results and demonstrate that the ISI is a robust measure of fasting and whole-body insulin sensitivity. First, we observe a strong correlation of ISI with direct measures of insulin sensitivity. Second, we show that the ISI can detect genetic influences on measures of fasting insulin sensitivity (3,10,12), generally ascribed to hepatic physiology, as well as on measures of whole-body insulin sensitivity, which also incorporates contributions from muscle and adipose tissue. Integrated measures of insulin sensitivity may have clinical relevance, since a reduction in peripheral insulin sensitivity may be an early contributor to the development of type 2 diabetes (17–19).
Consistent with prior genetic explorations of insulin sensitivity (10), the association of variants at the BCL2 and FAM19A2 loci became stronger and genome-wide significant after accounting for the effect of BMI on ISI. Notably, the ISI can be calculated with or without BMI in the formula, and the correlation of the ISI with M/I is greater when BMI is included (r = 0.69 vs. 0.79) (8,9). We note that the effect of these loci on insulin sensitivity is modest, consistent with published findings on other common genetic variants for glycemic traits, such as glucose (12) and fasting insulin (3,10,12). Yet our findings are meaningful because they provide a more complete understanding of the contribution of common genetic variations to insulin sensitivity.
The existing literature bolsters our finding of BCL2 as a novel candidate insulin sensitivity locus. The major allele (T) at rs12454712, which was associated with lower insulin sensitivity in our analysis, has been previously associated with type 2 diabetes in a multiethnic GWAS (odds ratio 1.09 [95% CI 1.05–1.11]; P = 2.1 × 10−8) (20) in analyses adjusted for BMI. Further, this same variant was recently associated with higher BMI-adjusted waist-to-hip ratio in women (β = 0.035; P = 1.1 × 10−9; N = 96,182) but not in men (β = 0.007; P = 0.25; N = 73,576) (21). All these findings suggest that the metabolically deleterious effects of the BCL2 locus become more evident after adjusting for BMI. Last, we find that the statistical association of rs12454712 (BCL2) is stronger with the ISI than with fasting insulin (10). Notably, the published fasting insulin results were from a study much larger than ours. The ability of the ISI to detect a genome-wide significant finding in a smaller sample suggests that the BCL2 locus may have a greater influence on insulin sensitivity when fasting and postprandial phenotypes are assessed together.
The mechanism by which BCL2 influences insulin sensitivity remains unclear. The BCL2 family of proteins regulate apoptosis through control of mitochondrial permeability (22). Mouse models suggest that inhibiting Bcl2 improves glucose tolerance through effects on pancreatic β-cells (23). Conversely, pharmacological inhibition of the BCL2 protein causes hyperglycemia among a subset of patients with chronic lymphocytic leukemia (24), but the mechanism of this observation is unknown. By contrast, there is little direct published literature to support the role of FAM19A2 in insulin sensitivity. We found that the association of the minor allele (A) at the FAM19A2 locus with reduced insulin sensitivity was detected at BMI <30 kg/m2. This may suggest the variant is more deleterious among individuals with lower levels of adiposity. While BCL2 and FAM19A2 are the closest genes to rs12454712 and rs10506418, respectively, we have not excluded other genes in the region (Supplementary Figs. 6 and 7). Additional in silico findings at the BCL2 and FAM19A2 variants are provided in Supplementary Table 5.
We recognize limitations to our study. First, analyses were performed exclusively in white individuals of European ancestry. Exploring these loci in other racial and ethnic groups is necessary. Second, we used an estimate of whole-body insulin sensitivity derived from measures of glucose and insulin after a glucose load, rather than direct measures of insulin sensitivity. The wide availability of the ISI provided increased statistical power of the association analyses relative to that of other indices that are better correlated with euglycemic measures of insulin sensitivity, such as the Matsuda Index (25). Assessment of our novel findings in the GENESIS consortium suggests that the ISI may be capturing different information on insulin sensitivity than that provided by the insulin clamp or the insulin suppression test, or that the power in the GENESIS analyses was limited to detect this association. Third, conditional analyses could not be performed in model 3, which would have been the best method of assessing the dependence of the signals at BCL2 and FAM19A2. However, the LD for each variant with other known glucose and insulin loci in the region was low, and the nominally significant associations of the BCL2 and FAM19A2 variants with ISI were stable after conditioning in model 2, suggesting that analyses in model 3 would have probably confirmed secondary loci. Fourth, given our desire for the early dissemination of these results, no experimental attempts at determining the causal gene and mechanisms of action in our novel candidate insulin sensitivity loci were performed.
In conclusion, we identified two novel candidate insulin sensitivity loci through a GWAS of the modified Stumvoll ISI. Our results demonstrate that the ISI is a robust measure of fasting and whole-body measures of insulin sensitivity and suggest that genetic variation in the FAM19A2 and BCL2 loci influence insulin sensitivity. While further functional work is needed to clarify the causal genes and mechanisms of action of these loci, our work and the published literature provide support for genes in these loci having an effect on human glycemic metabolism.
Supplementary Material
Article Information
Acknowledgments. The authors thank all the participants of each cohort for their cooperation and contribution to this study. For FHS, this research was conducted in part using data and resources from the FHS of the National Heart Lung, and Blood Institute of the National Institutes of Health and Boston University School of Medicine; the analyses reflect intellectual input and resource development from the FHS investigators participating in the SNP Health Association Resource (SHARe) project. A full list of principal CHS investigators and institutions can be found at chs-nhlbi.org. For ULSAM, the authors thank the SNP&SEQ Technology Platform in Uppsala (www.genotyping.se) for excellent genotyping. Computations were performed on resources provided by SNIC through Uppsala Multidisciplinary Center for Advanced Computational Science (UPPMAX) under Project b2011036. For Sorbs, the authors thank Knut Krohn (Microarray Core Facility, Institute of Pharmacology, University of Leipzig) for genotyping support and Joachim Thiery (Institute of Laboratory Medicine, Clinical Chemistry and Molecular Diagnostics, University of Leipzig) for clinical chemistry services. For LURIC, the authors thank the LURIC study team members who are either temporarily or permanently involved in patient recruitment and sample and data handling. Furthermore, the authors thank the laboratory staff at the Ludwigshafen General Hospital and the Universities of Freiburg, Ulm, and Graz. For Amish Studies, the authors gratefully thank their Amish community and research volunteers for their long-standing partnership in research, and they acknowledge the dedication of their Amish liaisons, field workers, and the Amish Research Clinic staff, without whom these studies would not have been possible. For the Ely Study, the authors are grateful to the staff of St. Mary’s Surgery, Ely, U.K., and the study team.
Funding. This work was supported by National Institutes of Health grant DK099249 (to G.A.W.). Grant support was provided to cohorts. For METSIM, the study was funded by the Academy of Finland (grants 77299 and 124243). For Sorbs, the work was supported by grants from the German Research Council (DFG-SFB 1052, “Obesity mechanisms,” A01, C01, B03, and SPP 1629 TO 718/2-1), the German Diabetes Association, and the Diabetes Hilfs- und Forschungsfonds Deutschland (DHFD). This work was further supported by the Federal Ministry of Education and Research (BMBF), Germany, FKZ (01EO1501, AD2-060E to P.K.), and by the Boehringer Ingelheim Foundation. For FHS, the study was supported by the National Heart, Lung, and Blood Institute (contract nos. N02-HL-6-4278 [supporting its contract with Affymetrix, Inc., for genotyping services], N01-HC-25195, HL084756, and HHSN268201500001I) and the National Institute of Diabetes and Digestive and Kidney Diseases (R01 DK078616 to J.D., U01-DK085526 to H.C. and J.D., 2R01 DK078616 and K24 DK080140 to J.M.). A portion of this research utilized the Linux Cluster for Genetic Analysis (LinGA‐II) funded by the Robert Dawson Evans Endowment of the Department of Medicine at Boston University School of Medicine and Boston Medical Center. A.K.M. was supported by American Diabetes Association Research Foundation (grant 7-12-MN-02). For CHS, research was supported by National Heart, Lung, and Blood Institute contract nos. HHSN268201200036C, HHSN268200800007C, N01HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, and N01HC85086 and grant nos. U01HL080295, HL105756, and HL120393, with additional contribution from the National Institute of Neurological Disorders and Stroke. Additional support was provided by R01AG023629 from the National Institute on Aging (NIA). For ULSAM, the project was supported by the Knut and Alice Wallenberg Foundation (Wallenberg Academy Fellow), the European Research Council (ERC Starting Grant), the Swedish Diabetes Foundation (grant 2013-024), the Swedish Research Council (grant 2012-1397), and the Swedish Heart-Lung Foundation (20120197). A.P.M. is a Wellcome Trust Senior Fellow in Basic Biomedical Science (grant WT098017 from the Nordic Center of Excellence in Disease Genetics). For LURIC, the study was supported by the 7th Framework Program (AtheroRemo, grant agreement no. 201668, and RiskyCAD, grant agreement no. 305739) of the European Union and by the INTERREG-IV-Oberrhein-Program (Project A28, Genetic mechanisms of cardiovascular diseases) with support from the European Regional Development Fund (ERDF) and the Wissenschaftsoffensive. M.E.K. and W.M. are supported by the German Federal Ministry of Education and Research as part of the Competence Cluster of Nutrition and Cardiovascular Health (nutriCARD). For Inter99, the study was financially supported by research grants from the Danish Research Council, the Danish Centre for Health Technology Assessment, Novo Nordisk, the Research Foundation of Copenhagen County, Ministry of Internal Affairs and Health of Denmark, the Danish Heart Foundation, the Danish Pharmaceutical Association, the Augustinus Foundation, the Ib Henriksen Foundation, the Becket Foundation, and the Danish Diabetes Association. For FUSION, the study was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (DK093757, DK072193, DK062370) and the National Human Genome Research Institute (ZIA-HG000024). H.A.K. has received funding from the Academy of Finland (support for clinical research careers, grant no. 258753). Work on the Amish Studies was supported by National Institutes of Health awards K23GM102678 (from National Institute of General Medical Sciences) to J.P.L. and HL084756 (from National Heart, Lung, and Blood Institute) to J.R.O. The Botnia study has been financially supported by grants from the Sigrid Juselius Foundation, the Folkhälsan Research Foundation, the Nordic Center of Excellence in Disease Genetics, a European Union grant (EXGENESIS, GA FP6 2004-005272), the Signe and Ane Gyllenberg Foundation, the Swedish Cultural Foundation in Finland, the Finnish Diabetes Research Foundation, the Foundation for Life and Health in Finland, the Finnish Medical Society, the Paavo Nurmi Foundation, the Helsinki University Central Hospital Research Foundation, the Perklén Foundation, the Ollqvist Foundation, the Närpes Health Care Foundation, and the Ahokas Foundation. The DGI study was further supported by a Swedish Research Council Linné grant (2006-237) and by the Ministry of Education in Finland, the Municipal Health Care Center and Hospital in Jakobstad, and the Health Care Centers in Vasa, Närpes, and Korsholm. The Tübingen study was supported in part by a grant from the German Federal Ministry of Education and Research to the German Center for Diabetes Research (DZD e.V.). For the Ely Study, J.L., C.Lang, R.A.S., and N.J.W. acknowledge support from the Medical Research Council (MC_UU_12015/1). The Ely Study was funded by the Medical Research Council (MC_U106179471) and Diabetes UK. Genotyping in the Ely and Fenland studies was supported in part by a Medical Research Council–GlaxoSmithKline pilot program grant (G0701863). For the Birth Cohort 1936 and Inter99 studies, work was support by the Novo Nordisk Foundation Center for Basic Metabolic Research, an independent research center at the University of Copenhagen partially funded by an unrestricted donation from the Novo Nordisk Foundation (www.metabol.ku.dk). Work on the Pizarra study was support by Instituto de Salud Carlos III PI11/00880 and Instituto de Salud Carlos III PS09/02117. For Segovia, this work was supported by the Fondo Europeo para el Desarrollo Regional, Red de Centros RCMN (C03/08) grants FEDER 2FD 1997/2309, the Instituto de Salud Carlos III-RETIC RD06/0015/0012, Madrid, Spain (FIS 03/1618); CIBER in Diabetes and Associated Metabolic Disorders (Instituto de Salud Carlos III, Ministerio de Ciencia e Innovación); and the Madrid Autonomous Community (MOIR S2010/BMD-2423), and by educational grants from Eli Lilly Lab, Spain; Bayer Pharmaceutical Co., Spain; and the Fundación Mutua Madrileña 2008, Spain.
Duality of Interest. M.E.K. (LURIC) has received lecture fees from AstraZeneca. G.S. (LURIC) is member of an Amgen advisory board (Thousand Oaks, CA). W.M. (LURIC) is employed by Synlab Services GmbH and holds shares of Synlab Holding GmbH. He has received grants from Siemens Diagnostics, Aegerion Pharmaceuticals, Amgen, AstraZeneca, Danone, Sanofi/Genzyme, Pfizer, BASF, and Abbott Diagnostics. B.M.P. (CHS) serves on the data safety and monitoring board of a clinical trial funded by the manufacturer (Zoll LifeCor Corp.) and the Yale Open Data Access Project Steering Committee funded by Johnson & Johnson. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. G.A.W. (Massachusetts General Hospital [MGH]) and J.C.F. (MGH and FHS) conceived the study. G.A.W. (MGH), S.G. (ULSAM), and D.R. (FHS) wrote the manuscript under the guidance of J.D. (FHS), E.I. (ULSAM), and J.C.F. (MGH, FHS); the manuscript was finalized based on detailed comments from other authors. S.G. (ULSAM) and D.R. (FHS) constructed the figures and performed the meta-analysis of data from all cohorts. A.K.M. (FHS) performed analysis. S.G. (ULSAM), D.R. (FHS), A.Le. (FHS), A.P.M. (FHS), W.X. (Genetics of Insulin Sensitivity, GENESIS), and Z.Z. (GENESIS) performed in silico analyses for follow-up studies. D.R., (FHS), H.C. (FHS), C.-T.L. (FHS), J.H. (FHS), A.P.M. (ULSAM), N.G. (Inter99 and Birth Cohort 1936), J.P.L. (Amish), H.S. (Tübingen), J.L. (Ely Study), M.T.M.-L. (Segovia), M.L.B. (CHS), Y.-D.I.C. (CHS), A.-C.A. (Segovia), J.M.G.-Z. (Pizarra), M.O.G. (CHS), L.L. (ULSAM), F.M. (Tübingen), G.M.M.-N. (Pizzara), J.I.R. (CHS), M.S.-R. (Segovia), R.A.S. (Ely Study), N.J.W. (Ely Study), M.L. (METSIM), J.D. (FHS), C.M.L. (ULSAM), and C.Lang. (Ely Study) performed genotyping within cohorts. A.S. (METSIM, EUGENE2), M.E.K. (LURIC), G.D. (LURIC), G.S. (LURIC), A.C.-A. (Segovia), K.F. (Inter99), J.R.K. (CHS), H.A.K. (FUSION), A.Li. (Inter99 and Birth Cohort 1936), C.Lang. (Ely Study), G.R.-M. (Pizzara), D.S.S. (CHS), T.H. (Inter99 and Birth Cohort 1936), T.J.J. (Inter99 and Birth Cohort 1936), O.P. (Inter99 and Birth Cohort 1936), R.A.S. (Ely Study), N.J.W. (Ely Study), A.F. (Tübingen), N.S. (Tübingen), R.N.B. (FUSION), J.T. (FUSION), P.K. (Sorbs), M.S. (Sorbs), J.K. (METSIM), J.B.M. (FHS), J.D. (FHS), and E.I. (ULSAM) performed phenotyping within cohorts. P.K. (Sorbs), C.Land. (Botnia Study), S.G. (ULSAM), D.R. (FHS), H.C. (FHS), C.-T.L. (FHS), J.H. (FHS), R.A.J. (CHS), K.R. (CHS), A.P.M. (ULSAM), R.M. (Sorbs), A.T. (Sorbs), I.P. (Sorbs), A.U.J. (FUSION, METSIM), E.V.A. (Inter99 and Birth Cohort 1936), N.G. (Inter99 and Birth Cohort 1936), J.P.L. (Amish), M.E.M. (Amish), H.S. (Tübingen), J.L. (Ely Study), T.M.F. (RISC), M.N.W. (RISC), W.X. (RISC), S.M. (Pizarra), M.T.M.-L. (Segovia), M.W. (RISC), and J.R.O. (Amish) analyzed data within cohorts. A.Li. (Inter99 and Birth Cohort 1936), C.Lang. (Ely Study), J.H. (METSIM), M.S.-R. (Segovia), U.S. (EUGENE2), F.S. (Pizarra), T.H. (Inter99 and Birth Cohort 1936), T.J.J. (Inter99 and Birth Cohort 1936), O.P. (Inter99 and Birth Cohort 1936), R.A.S. (Ely Study), N.J.W. (Ely Study), A.F. (Tübingen), H.U.H. (Tübingen), N.S. (Tübingen), L.G. (Botnia Study), J.R.O. (Amish Studies), M.B. (FUSION), R.N.B. (FUSION), F.S.C. (FUSION), K.L.M. (FUSION), J.T. (FUSION), W.M. (LURIC), P.K. (Sorbs), M.S. (Sorbs), B.M.P. (CHS), J.K. (METSIM), M.L. (METSIM), and E.I. (ULSAM) were the cohort principal investigators. G.A.W. is the guarantor of this work and, as such, had full access to the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db16-0199/-/DC1.
G.A.W., S.G., and D.R. contributed equally as first authors.
E.I. and J.C.F. contributed equally as senior authors.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Morris AP, Voight BF, Teslovich TM, et al.; Wellcome Trust Case Control Consortium; Meta-Analyses of Glucose and Insulin-related traits Consortium (MAGIC) Investigators; Genetic Investigation of ANthropometric Traits (GIANT) Consortium; Asian Genetic Epidemiology Network–Type 2 Diabetes (AGEN-T2D) Consortium; South Asian Type 2 Diabetes (SAT2D) Consortium; DIAbetes Genetics Replication And Meta-analysis (DIAGRAM) Consortium . Large-scale association analysis provides insights into the genetic architecture and pathophysiology of type 2 diabetes. Nat Genet 2012;44:981–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kahn SE. The relative contributions of insulin resistance and beta-cell dysfunction to the pathophysiology of type 2 diabetes. Diabetologia 2003;46:3–19 [DOI] [PubMed] [Google Scholar]
- 3.Dimas AS, Lagou V, Barker A, et al.; MAGIC Investigators . Impact of type 2 diabetes susceptibility variants on quantitative glycemic traits reveals mechanistic heterogeneity. Diabetes 2014;63:2158–2171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bergman RN, Zaccaro DJ, Watanabe RM, et al. Minimal model-based insulin sensitivity has greater heritability and a different genetic basis than homeostasis model assessment or fasting insulin. Diabetes 2003;52:2168–2174 [DOI] [PubMed] [Google Scholar]
- 5.Rasmussen-Torvik LJ, Pankow JS, Jacobs DR, et al. Heritability and genetic correlations of insulin sensitivity measured by the euglycaemic clamp. Diabet Med 2007;24:1286–1289 [DOI] [PubMed] [Google Scholar]
- 6.Ingelsson E, Langenberg C, Hivert MF, et al.; MAGIC investigators . Detailed physiologic characterization reveals diverse mechanisms for novel genetic Loci regulating glucose and insulin metabolism in humans. Diabetes 2010;59:1266–1275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Knowles JW, Xie W, Zhang Z, et al.; RISC (Relationship between Insulin Sensitivity and Cardiovascular Disease) Consortium; EUGENE2 (European Network on Functional Genomics of Type 2 Diabetes) Study; GUARDIAN (Genetics UndeRlying DIAbetes in HispaNics) Consortium; SAPPHIRe (Stanford Asian and Pacific Program for Hypertension and Insulin Resistance) Study . Identification and validation of N-acetyltransferase 2 as an insulin sensitivity gene [published correction appears in J Clin Invest 2016;126:403]. J Clin Invest 2015;125:1739–175125798622 [Google Scholar]
- 8.Stumvoll M, Mitrakou A, Pimenta W, et al. Use of the oral glucose tolerance test to assess insulin release and insulin sensitivity. Diabetes Care 2000;23:295–301 [DOI] [PubMed] [Google Scholar]
- 9.Stumvoll M, Van Haeften T, Fritsche A, Gerich J. Oral glucose tolerance test indexes for insulin sensitivity and secretion based on various availabilities of sampling times. Diabetes Care 2001;24:796–797 [DOI] [PubMed] [Google Scholar]
- 10.Manning AK, Hivert M-F, Scott RA, et al.; DIAbetes Genetics Replication And Meta-analysis (DIAGRAM) Consortium; Multiple Tissue Human Expression Resource (MUTHER) Consortium . A genome-wide approach accounting for body mass index identifies genetic variants influencing fasting glycemic traits and insulin resistance. Nat Genet 2012;44:659–669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Manning AK, LaValley M, Liu CT, et al. Meta-analysis of gene-environment interaction: joint estimation of SNP and SNP × environment regression coefficients. Genet Epidemiol 2011;35:11–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scott RA, Lagou V, Welch RP, et al.; DIAbetes Genetics Replication and Meta-analysis (DIAGRAM) Consortium . Large-scale association analyses identify new loci influencing glycemic traits and provide insight into the underlying biological pathways. Nat Genet 2012;44:991–1005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Knowles JW, Assimes TL, Tsao PS, et al. Measurement of insulin-mediated glucose uptake: direct comparison of the modified insulin suppression test and the euglycemic, hyperinsulinemic clamp. Metabolism 2013;62:548–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang J, Ferreira T, Morris AP, et al.; Genetic Investigation of Anthropometric Traits (GIANT) Consortium; DIAbetes Genetics Replication And Meta-analysis (DIAGRAM) Consortium . Conditional and joint multiple-SNP analysis of GWAS summary statistics identifies additional variants influencing complex traits. Nat Genet 2012;44:369–375, S1–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Almasy L, Blangero J. Multipoint quantitative-trait linkage analysis in general pedigrees. Am J Hum Genet 1998;62:1198–1211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Otten J, Ahrén B, Olsson T. Surrogate measures of insulin sensitivity vs the hyperinsulinaemic-euglycaemic clamp: a meta-analysis. Diabetologia 2014;57:1781–1788 [DOI] [PubMed] [Google Scholar]
- 17.Kashyap SR, Belfort R, Berria R, et al. Discordant effects of a chronic physiological increase in plasma FFA on insulin signaling in healthy subjects with or without a family history of type 2 diabetes. Am J Physiol Endocrinol Metab 2004;287:E537–E546 [DOI] [PubMed] [Google Scholar]
- 18.Perseghin G, Ghosh S, Gerow K, Shulman GI. Metabolic defects in lean nondiabetic offspring of NIDDM parents: a cross-sectional study. Diabetes 1997;46:1001–1009 [DOI] [PubMed] [Google Scholar]
- 19.Vaag A, Henriksen JE, Beck-Nielsen H. Decreased insulin activation of glycogen synthase in skeletal muscles in young nonobese Caucasian first-degree relatives of patients with non-insulin-dependent diabetes mellitus. J Clin Invest 1992;89:782–788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saxena R, Elbers CC, Guo Y, et al.; Look AHEAD Research Group; DIAGRAM consortium . Large-scale gene-centric meta-analysis across 39 studies identifies type 2 diabetes loci [published correction appears in Am J Hum Genet 2012;90:753]. Am J Hum Genet 2012;90:410–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shungin D, Winkler TW, Croteau-Chonka DC, et al.; ADIPOGen Consortium; CARDIOGRAMplusC4D Consortium; CKDGen Consortium; GEFOS Consortium; GENIE Consortium; GLGC; ICBP; International Endogene Consortium; LifeLines Cohort Study; MAGIC Investigators; MuTHER Consortium; PAGE Consortium; ReproGen Consortium . New genetic loci link adipose and insulin biology to body fat distribution. Nature 2015;518:187–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brenner D, Mak TW. Mitochondrial cell death effectors. Curr Opin Cell Biol 2009;21:871–877 [DOI] [PubMed] [Google Scholar]
- 23.Luciani DS, White SA, Widenmaier SB, et al. Bcl-2 and Bcl-xL suppress glucose signaling in pancreatic β-cells. Diabetes 2013;62:170–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roberts AW, Davids MS, Pagel JM, et al. Targeting BCL2 with Venetoclax in relapsed chronic lymphocytic leukemia. N Engl J Med 2016;374:311–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stancáková A, Javorský M, Kuulasmaa T, Haffner SM, Kuusisto J, Laakso M. Changes in insulin sensitivity and insulin release in relation to glycemia and glucose tolerance in 6,414 Finnish men. Diabetes 2009;58:1212–1221 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.