Abstract
We tested the hypothesis that acute administration of oral dehydroepiandrosterone (DHEA) during episodes of repeated hypoglycemia can prevent the development of hypoglycemia-associated neuroendocrine and autonomic failure in healthy humans. Twenty-seven individuals (16 men, 11 women) participated in two separate randomized, single-blind, 2-day protocols. Day 1 consisted of morning and afternoon 2-h hypoglycemic clamps (2.9 mmol/L) with 800 mg of DHEA or placebo administered before each clamp. Day 2 consisted of a single 2-h hypoglycemic clamp (2.9 mmol/L) following either DHEA (1,600 mg) or placebo. A 3-tritiated glucose was used to determine glucose kinetics during hypoglycemia on day 2. Antecedent hypoglycemia with placebo resulted in significant reductions of epinephrine, norepinephrine, glucagon, growth hormone, cortisol, endogenous glucose production, and lipolytic and symptom responses. During hypoglycemia on day 2, DHEA prevented blunting of all neuroendocrine, autonomic nervous system (ANS), metabolic, and symptom counterregulatory responses following hypoglycemia on day 1. In summary, DHEA can acutely preserve a wide range of key neuroendocrine, ANS, and metabolic counterregulatory homeostatic responses during repeated hypoglycemia. We conclude that DHEA may have acute effects to protect against hypoglycemia-associated neuroendocrine and autonomic failure in healthy humans.
Introduction
Hypoglycemia remains a major obstacle for obtaining good metabolic control in clinical practice and is associated with severe adverse events in individuals with either type 1 or type 2 diabetes (1,2). Hypoglycemia occurs clinically as a result of a relative excess of insulin, often coupled with acquired deficient neuroendocrine and autonomic nervous system (ANS) counterregulatory responses (3). These acquired deficient counterregulatory responses (called hypoglycemia-associated autonomic failure) can occur rapidly after only one episode of antecedent hypoglycemia (4). Significant progress has been achieved in understanding the mechanisms responsible for the acquired deficient counterregulatory responses present in individuals with diabetes (5). Previous work has demonstrated that, because of the importance of maintaining an adequate glucose supply to the brain, there is a wide network of central nervous system (CNS) receptors, nuclei, and neurotransmitters that can regulate counterregulatory responses during hypoglycemia (6–10). Thus, although several CNS mechanisms have been implicated, a single cause for the syndrome of hypoglycemia-associated counterregulatory failure has not been defined. Despite this, several experimental approaches have been used to improve counterregulatory responses during hypoglycemia, including a period of scrupulously avoiding hypoglycemia (5) and a series of innovative pharmacologic interventions to block or activate CNS pathways (11–14). All have provided some success, but none have been able to completely reverse the syndromes of hypoglycemia-associated autonomic failure.
Dehydroepiandrosterone (DHEA) and its sulfated metabolite (DHEA-S) are naturally occurring steroid hormones that have been demonstrated to have anti–γ-aminobutyric acid (GABA), anticorticosteroid, stimulatory nitric oxide (15), and N-methyl-d-aspartate agonist effects—all of which would be predicted to augment counterregulatory responses during repeated hypoglycemia. Supporting this approach, we previously demonstrated that high-dose intravenous infusions of DHEA during repeated hypoglycemia in a conscious rat model preserves neuroendocrine and ANS counterregulatory responses (16). Rats, unlike humans, do not have circulating concentrations of DHEA, and thus it is unknown whether the hormone would also have similar protective counterregulatory effects in humans. Therefore in this study we tested the hypothesis that oral DHEA could acutely protect and prevent hypoglycemia-associated counterregulatory failure during repeated hypoglycemia in healthy humans. The glucose clamp technique was used during 2-day repeated hypoglycemia studies to control insulin and glycemic levels. DHEA (800 mg twice on day 1 and 1,600 mg on day 2) or placebo were administered before hypoglycemic clamps to determine the acute effects of the hormone on subsequent neuroendocrine, ANS, and metabolic counterregulatory responses.
Research Design and Methods
Participants
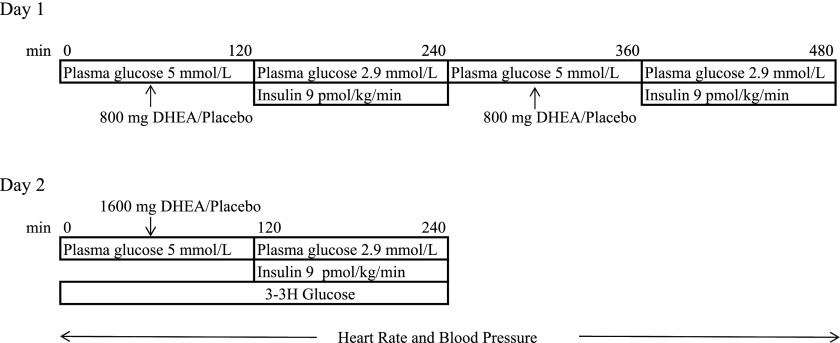
Twenty-seven healthy individuals (16 men and 11 women; mean ± standard deviation age, 28 ± 1 years; BMI 26 ± 1 kg/m2) were randomly allocated to participate in two separate, single-blind, 2-day protocols (Fig. 1). Four individuals participated in both studies (with a minimum of 3 months between studies), resulting in 15 participants (7 men, 8 women) in the placebo group and 16 participants (11 men, 5 women) in the DHEA group.
Figure 1.
Diagram of study procedures.
Participants were nonsmokers with normal liver, renal, and hematologic parameters. Subjects who were 40 years old underwent a standard Bruce cardiac stress test to exclude silent ischemia (17). Studies were approved by the University of Maryland human subjects institutional review board, and all subjects gave informed written and verbal consent.
Experimental Design
All participants were instructed to avoid intense exercise and alcohol and to consume their usual weight-maintaining diet for 3 days before each study. Each participant was admitted to the University of Maryland Clinical Research Center the evening before an experiment. The next morning, after an 10-h overnight fast, participants had intravenous cannula placed in both arms under local 1% lidocaine anesthesia. One cannula was placed in a retrograde fashion into a vein in the back of a hand. This hand was placed in a heated box (55–60°C) so that arterialized blood could be obtained (18). The other cannula was placed in the contralateral arm for infusions of dextrose solution, insulin, potassium chloride, and tritiated labeled glucose.
Day 1 Hypoglycemia
Day 1 began with a baseline period (0–120 min) followed by a 2-h hyperinsulinemic-hypoglycemic experimental clamp period (120–240 min). One hour before each experimental clamp period (morning and afternoon), participants were given orally either 800 mg DHEA or placebo (100% parity; KEBD Enterprises, LLC, Lakewood, CO). At the start of the experimental period, a primed continuous infusion of insulin (Eli Lilly, Indianapolis, IN) was administered at a rate of 9 pmol/kg/min for 120 min (Fig. 1). Potassium chloride (5 mmol/h) was also infused during the morning and afternoon clamp periods to reduce insulin-induced hypokalemia. Plasma glucose concentrations were measured every 5 min, the rate of glucose reduction was controlled (∼0.08 mmol/min), and the hypoglycemic nadir (2.9 ± 0.1 mmol/L) was achieved and held constant using a modification of the glucose clamp technique (19). After completing the initial 2-h clamp period, the insulin infusion was stopped and a 2-h period of euglycemia was maintained using 20% dextrose infusion. At that point, insulin was restarted, and a second hyperinsulinemic-hypoglycemic clamp (similar to that used in the morning study) was performed (Fig. 1). Electrocardiography was recorded continuously and blood pressure every 10 min throughout all 2-h hyperinsulinemic-hypoglycemic clamps. Upon completion of the second glucose clamp, subjects consumed a standardized meal, a bedtime snack, and remained in the Clinical Research Center.
Day 2 Hypoglycemia
Day 2 followed a similar pattern to the day 1 procedures. All studies started after a 10-h overnight fast and consisted of a tracer equilibration period (0–120 min) and a 120-min experimental period (120–240 min). A primed (18 μCi) continuous infusion (0.18 μCi/min) of high-performance liquid chromatography–purified [3–3H] glucose (11.5 mCi/mmol/L; PerkinElmer Life Sciences, Boston, MA) was administered starting at 0 min and continued throughout the study to measure glucose kinetics. One hour before the morning hypoglycemic clamp, participants received 1,600 mg DHEA or placebo. As in day 1, an electrocardiogram was recorded continuously and blood pressure every 10 min throughout the 2-h hyperinsulinemic-hypoglycemic clamps.
Tracer Calculations
Endogenous glucose production (EGP) was calculated according to the method described by Wall et al. (20), in which the total rate of appearance (comprising both EGP and any exogenous glucose infused to maintain the desired euglycemia) is determined and then the amount of exogenous glucose infused is subtracted from it. It is now recognized that this approach is not fully quantitative, since total rate of appearance and rate of disappearance can be underestimated. The use of a highly purified tracer and taking measurements under steady-state conditions (i.e., constant specific activity) eliminates most, if not all, of the problems. To minimize changes in specific activity, the tracer infusion was proportionally increased, as necessary, commensurate with the changes of the exogenous glucose infusion rate. Glucose kinetics are only reported in this article when glucose specific activity (disintegrations per minute [dpm]/μmol) is proven to be in a steady state (coefficient of variation [CV] <5.0%) and include baseline values and the final 30 min of glucose clamps.
Analytical Methods
The collection and processing of blood samples have been described elsewhere (11). Plasma glucose concentrations were measured in triplicate with a glucose analyzer (Beckman, Fullerton, CA) using the glucose oxidase method. Glucagon was measured according to the method described by Aguilar-Parada et al. (21) with an interassay CV of 12%. Insulin was measured as previously described (22), with an interassay CV of 11%. Catecholamines were determined by high-pressure liquid chromatography as previously described (23), with an interassay CV of 12% for epinephrine and 8% for norepinephrine. Growth hormone (24) (interassay CV 8%), cortisol (Clinical Assays γ Coat Radioimmunoassay Kit; interassay CV 6%), and pancreatic polypeptide (25) (interassay CV 8%) were measured using radioimmunoassay techniques. Lactate and glycerol were measured from deproteinized whole blood using the method described by Lloyd et al. (26). Nonesterified fatty acids (NEFAs) were measured using a kit from Wako Diagnostics (27). DHEA (interassay CV 8.3%), and DHEA-S (interassay CV 9.17%) were measured using ELISA techniques using kits from Rocky Mountain Diagnostics Inc. (Colorado Springs, CO) and Alpco (Salem, NH), respectively.
Cardiovascular Parameters
Heart rate and systolic, diastolic, and mean arterial blood pressures were measured noninvasively using a Dinamap vitals monitor (Critikon, Tampa, FL) every 10 min.
Autonomic and Neuroglycopenic Symptom Responses
Autonomic and neuroglycopenic symptom responses were assessed by a questionnaire administered before and every 15 min throughout the hypoglycemic clamps (28).
Statistical Analysis
Data are expressed as mean ± SE. Data from the DHEA and placebo groups were analyzed using standard, unpaired, parametric one- and two-way ANOVA with repeated measures as appropriate (Graph Pad Software, Inc., San Diego, CA). Tukey post hoc analysis was used to delineate significance within each group. Baseline to final 30 min day 1 and day 2 values and responses within hypoglycemic clamps for each group (DHEA and placebo) were also compared using paired, two-tailed t tests. A P value <0.05 was accepted as statistically significant.
Results
Day 1 Glucose and Insulin
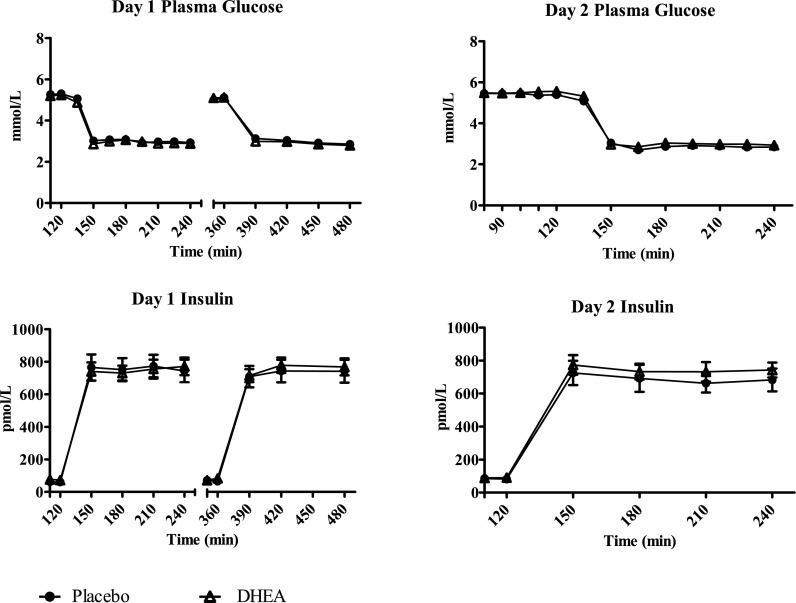
Plasma glucose concentrations were equivalent (2.9 ± 0.1 mmol/L) during day 1 morning and afternoon hypoglycemia studies with or without DHEA (Fig. 2). Plasma insulin concentrations during day 1 studies were similar among all groups (764 ± 91 pmol/L) (Fig. 2).
Figure 2.
Plasma glucose and plasma insulin concentrations during day 1 and day 2 studies.
Day 2 Glucose and Insulin
During day 2, plasma glucose (2.9 ± 0.04 mmol/L) and insulin concentrations (736 ± 86 pmol/L) were similar in both groups (Fig. 2).
ANS Responses
Baseline values of norepinephrine and pancreatic polypeptide were similar at the start of the day 1 and day 2 studies. Day 2 baseline epinephrine levels in the placebo group versus day 1 baseline epinepherine level values in the DHEA group (Table 1).
Table 1.
Baseline neuroendocrine, intermediary metabolite, EGP, and cardiovascular values on days 1 and 2 in overnight-fasted healthy individuals in the placebo and DHEA groups
| Day 1 placebo | Day 2 placebo | Day 1 DHEA | Day 2 DHEA | |
|---|---|---|---|---|
| Epinephrine (pmol/L) | 93 ± 11 | 82 ± 11*† | 158 ± 33 | 120 ± 16 |
| Norepinephrine (nmol/L) | 0.8 ± 0.1 | 0.9 ± 0.1 | 1.0 ± 0.1 | 0.9 ± 0.1 |
| Glucagon (ng/L) | 60 ± 4 | 62 ± 5 | 58 ± 4 | 55 ± 4 |
| Growth hormone (µg/L) | 3 ± 1 | 2 ± 1 | 2 ± 1 | 3 ± 1 |
| Cortisol (nmol/L) | 414 ± 83 | 359 ± 55 | 331 ± 55 | 303 ± 28 |
| Pancreatic polypeptide (pmol/L) | 33 ± 12 | 30 ± 11 | 29 ± 10 | 31 ± 14 |
| EGP (µmol/kg/min) | 10.5 ± 0.6 | 9.7 ± 0.6 | ||
| NEFA (µmol/L) | 376 ± 37 | 398 ± 45 | 456 ± 57 | 431 ± 54 |
| Glycerol (µmol/L) | 62 ± 8 | 65 ± 8 | 74 ± 13 | 81 ± 11 |
| Lactate (mmol/L) | 0.6 ± 0.1 | 0.8 ± 0.1 | 0.6 ± 0.1 | 0.8 ± 0.1 |
| Symptoms | ||||
| Autonomic | 7 ± 1 | 7 ± 0 | 7 ± 0 | 6 ± 0 |
| Neuroglycopenic | 6 ± 0 | 7 ± 1 | 7 ± 1 | 6 ± 0 |
| Total | 13 ± 1 | 14 ± 2 | 14 ± 1 | 12 ± 1 |
*P < 0.03 compared with day 1 DHEA;
†P = 0.051 compared with day 2 DHEA.
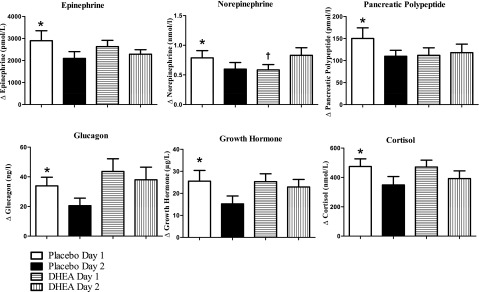
Day 2 plasma epinephrine responses in the placebo group (Δ 2,094 ± 311 pmol/L) (Fig. 3) were significantly reduced (P < 0.001) compared with day 1 responses (Δ 2,901 ± 450 pmol/L). Day 1 responses following DHEA (2,631 ± 286 pmol/L) were not significantly different from day 2 DHEA (2,284 ± 208 pmol/L).
Figure 3.
Epinephrine, norepinephrine, pancreatic polypeptide, glucagon, growth hormone, and cortisol concentrations (change from baseline to final 30 min of morning hypoglycemic clamps) on days 1 and 2 in healthy individuals fasted overnight following either placebo or DHEA administration. *P < 0.04–0.0001 compared with day 2 placebo; †P < 0.04 compared with day 2 DHEA.
Placebo day 2 norepinephrine responses (Δ 0.60 ± 0.11 nmol/L) (Fig. 3) were also significantly lower (P < 0.05) compared with day 1 placebo (Δ 0.78 ± 0.12 nmol/L). After administration of DHEA, day 2 norepinephrine responses to hypoglycemia (Δ 0.83 ± 0.12 nmol/L) were increased (P < 0.05) compared with day 1 responses to hypoglycemia (Δ 0.54 ± 0.08 nmol/L).
Day 2 pancreatic polypeptide responses (Fig. 3) in the placebo group (110 ± 14 pmol/L) were significantly lower (P < 0.05) compared with day 1 hypoglycemia (151 ± 14 pmol/L). Day 1 and day 2 pancreatic polypeptide responses were similar during DHEA administration (112 ± 17 and 118 ± 20 pmol/L, respectively).
Neuroendocrine Counterregulatory Hormones
Baseline values of glucagon, growth hormone, and cortisol were similar at the start of all study days (Table 1). Day 2 plasma glucagon responses in the placebo group (Δ 21 ± 5 ng/L) (Fig. 3) were significantly blunted (P < 0.03) following day 1 hypoglycemia (Δ 34 ± 6 ng/L). DHEA prevented any blunting of glucagon responses during hypoglycemia on day 2 (day 1 Δ 44 ± 9 ng/L vs. day 2 Δ 39 ± 8 ng/L).
Day 2 growth hormone responses in the placebo group (Δ 15 ± 4 µg/L) (Fig. 3) were also blunted (P < 0.04) compared with day 1 responses (Δ 26 ± 4 µg/L). Growth hormone responses during DHEA administration were similar during hypoglycemia on day 1 (Δ 25 ± 4 µg/L) and day 2 (Δ 23 ± 3 µg/L).
Day 2 plasma cortisol responses (Fig. 3) were also lower (P < 0.04) following placebo (Δ 349 ± 57 nmol/L) compared with day 1 hypoglycemia (Δ 474 ± 52 nmol/L). Following DHEA, cortisol responses during day 1 (Δ 471 ± 47 nmol/L) and day 2 (Δ 392 ± 52 nmol/L) were similar.
Glucose Kinetics
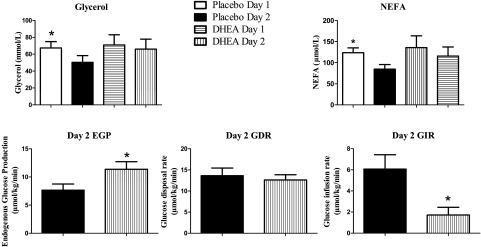
Baseline rates of glucose kinetics were similar at the start of day 2 studies (Table 1). Steady-state values of glucose specific activity (CV <3.0%) were obtained in both groups at the start and during the final 30 min of the glucose clamps (Table 2). Rates of EGP during the final 30 min of hypoglycemia on day 2 were increased (P < 0.04) following DHEA (11.4 ± 1.3 µmol/kg/min) compared with placebo (7.7 ± 1.1 µmol/kg/min) (Fig. 4). Glucose infusion rates during the final 30 min of hypoglycemia on day 2 were significantly reduced (P < 0.009) following DHEA administration (1.7 ± 0.7 µmol/kg/min) compared with placebo (6.1 ± 1.4 µmol/kg/min). The glucose disposal rates during the final 30 min on day 2 were similar in both groups: 13.7 ± 1.8 µmol/kg/min in the placebo group, 13.2 ± 0.9 µmol/kg/min in the DHEA group (Fig. 4).
Table 2.
Glucose specific activity and CVs at baseline and during the final 30 min of hypoglycemic clamps (2.9 ± 0.1 mmol/L) in overnight-fasted healthy individuals
| Baseline |
Final 30 min |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 100* | 110* | 120* | CV (%) | 210* | 225* | 232* | 240* | CV (%) | |
| Placebo (dpm/µmol) | 359 ± 30 | 351 ± 32 | 364 ± 29 | 2.94 | 331 ± 23 | 321 ± 19 | 328 ± 20 | 327 ± 21 | 1.90 |
| DHEA (dpm/µmol) | 368 ± 33 | 370 ± 33 | 373 ± 32 | 0.99 | 337 ± 33 | 327 ± 30 | 323 ± 29 | 322 ± 30 | 3.01 |
*Time points during the experimental period.
Figure 4.
Glycerol and NEFA responses, rates of EGP, glucose disposal rate (GDR), and glucose infusion rate (GIR) (final 30 min of day 2 clamps) on days 1 and 2 in healthy individuals fasted overnight following either placebo or DHEA administration. *P < 0.04–0.009 compared with day 2 placebo.
Intermediary Metabolism
Baseline levels of glycerol and NEFAs were similar at the start of all study days (Table 1). Day 2 plasma glycerol concentrations were lower (P = 0.01) (50 ± 8 µmol/L) compared with day 1 hypoglycemia (67 ± 7 µmol/L). After DHEA administration, day 1 plasma glycerol concentrations (71 ± 12 µmol/L) were maintained at values similar to day 2 hypoglycemia (66 ± 12 µmol/L) (Fig. 4).
Plasma NEFA concentrations during the final 30 min on day 2 in the placebo group (85 ± 11 µmol/L) were also reduced (P < 0.0004) compared with day 1 hypoglycemia (124 ± 11 µmol/L) but were not significantly changed in the DHEA group (day 1: 136 ± 28, day 2: 116 ± 22 µmol/L).
Plasma lactate responses were reduced during day 2 hypoglycemia (Δ 0.5 ± 0.1 mmol/L; P = 0.005) compared with day 1 hypoglycemia (Δ 0.9 ± 0.1 mmol/L) in the placebo group. DHEA administration resulted in similar plasma lactate responses during repeated hypoglycemia (day 1 Δ 0.7 ± 0.1 mmol/L vs. day 2 Δ 0.5 ± 0.1mmol/L).
Cardiovascular Responses
Baseline values of cardiovascular parameters (systolic blood pressure and heart rate) were similar at the start of both study days in the placebo and DHEA groups (Table 1). Heart rate and systolic and mean arterial blood pressures were increased during day 2 hypoglycemia following DHEA administration (Table 3).
Table 3.
Cardiovascular parameters in overnight-fasted healthy individuals following either placebo or DHEA administration during hypoglycemia (2.9 ± 0.1 mmol/L) on days 1 and 2
| Day 1 placebo |
Day 2 placebo |
Day 1 DHEA |
Day 2 DHEA |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Final | Δ | Baseline | Final | Δ | Baseline | Final | Δ | Baseline | Final | Δ | |
| Blood pressure (mmHg) | ||||||||||||
| Systolic | 115 ± 3 | 127 ± 4* | 12 ± 2† | 120 ± 2 | 124 ± 3 | 4 ± 2 | 118 ± 3 | 124 ± 4* | 7 ± 2‡ | 118 ± 3 | 132 ± 4* | 14 ± 3† |
| Diastolic | 68 ± 2 | 63 ± 2* | −5 ± 2 | 69 ± 2 | 63 ± 2* | −6 ± 2 | 66 ± 3 | 61 ± 2* | −6 ± 2 | 69 ± 3 | 65 ± 3* | −4 ± 2 |
| Mean arterial pressure (mmHg) | 84 ± 2 | 84 ± 2 | 1 ± 1 | 85 ± 2 | 83 ± 2 | −2 ± 1 | 84 ± 2 | 82 ± 2 | −2 ± 1‡ | 85 ± 2 | 87 ± 3 | 2 ± 1 |
| Heart rate (bpm) | 64 ± 3 | 78 ± 2* | 14 ± 3† | 67 ± 3 | 75 ± 3* | 8 ± 2 | 65 ± 3 | 74 ± 3* | 9 ± 2‡ | 67 ± 3 | 82 ± 2* | 15 ± 2 |
*P < 0.03–0.0001 compared with baseline;
†P < 0.04–0.009 compared with Δ in day 2 placebo;
‡P < 0.04–0.02 compared with Δ in day 1 DHEA.
Hypoglycemic Symptoms
Baseline autonomic, neuroendocrine, and total hypoglycemic symptom scores were similar at the start of each series of hypoglycemic studies on days 1 and 2 (Table 1). Autonomic symptom scores were reduced during day 2 compared with day 1 hypoglycemia in the placebo group (15 ± 3 vs. 20 ± 3; P < 0.004). Following DHEA administration, autonomic symptom scores were similar during hypoglycemia on day 1 and day 2 (16 ± 2 vs. 16 ± 3). Neuroglycopenic symptom scores were similar during all day 1 and day 2 studies. Total symptom scores were reduced during day 2 compared with day 1 hypoglycemia in the placebo group (28 ± 4 vs. 34 ± 5; P < 0.02). Total symptom scores were similar during day 1 and day 2 DHEA studies (29 ± 4 vs. 26 ± 4).
DHEA and DHEA-S Levels
Baseline DHEA and DHEA-S levels remained unchanged during hypoglycemia following placebo on days 1 and 2 (Table 4). DHEA and DHEA-S levels increased several fold compared with placebo following administration of the hormone during hypoglycemia on days 1 and 2 (Table 4).
Table 4.
DHEA and DHEA-S levels at baseline and during the final 30 min of hypoglycemic clamps (2.9 ± 0.1 mmol/L) on days 1 and 2 in overnight-fasted healthy individuals
| Day 1 placebo |
Day 2 placebo |
Day 1 DHEA |
Day 2 DHEA |
|||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Final | Baseline | Final | Baseline | Final | Baseline | Final | |
| DHEA (ng/mL) | 20 ± 3 | 20 ± 4 | 20 ± 4 | 21 ± 3 | 14 ± 2 | 33 ± 5* | 20 ± 2† | 44 ± 5* |
| DHEA-S (μg/mL) | 1 ± 0.2 | 2 ± 0.3 | 1 ± 0.2 | 1 ± 0.3 | 2 ± 0.2 | 10 ± 2* | 6 ± 0.6† | 14 ± 2* |
*P < 0.001–0.0001 compared with baseline and placebo;
†P < 0.005–0.0001 compared with day 1 DHEA.
Discussion
This study investigated whether high-dose DHEA can acutely preserve neuroendocrine, ANS, and metabolic homeostatic counterregulatory responses during repeated hypoglycemia in healthy humans. Antecedent hypoglycemia resulted in significant blunting of ANS (epinephrine, norepinephrine, pancreatic polypeptide), neuroendocrine (glucagon, growth hormone, cortisol), metabolic (EGP, lipolysis, glycogenolysis), and hypoglycemic symptom scores during next-day hypoglycemia. Oral DHEA given before each hypoglycemic clamp on days 1 and 2 preserved the wide spectrum of integrated ANS, neuroendocrine, and metabolic physiologic counterregulatory responses and acutely prevented hypoglycemia-associated neuroendocrine and autonomic failure.
Humans (and other mammals) have sophisticated and multiple mechanisms to preserve adequate fuel for the brain. As blood glucose decreases, synchronized neuroendocrine, ANS, and metabolic mechanisms are activated to defend against hypoglycemia. Unfortunately, hypoglycemia itself causes a reduction in homeostatic counterregulatory defenses against subsequent hypoglycemia (2,3). Because of the complexity of preserving blood glucose delivery acutely to the brain, several mechanisms have been identified that can contribute to deficient counterregulatory responses to subsequent hypoglycemia (5). More recent animal and human studies have demonstrated that differing pharmacologic approaches can protect or even improve counterregulatory responses during hypoglycemia (11–13,16,29). However, only one approach has reported a successful intervention to preserve counterregulatory responses during repeated hypoglycemia in humans (13). In this study we used DHEA, which is interconverted into DHEA-S and has anti-GABA, antiglucocorticoid, and stimulatory N-methyl-d-aspartate and nitric oxide synthase activities (15,30–36), all of which would be predicted to enhance and protect neuroendocrine and ANS counterregulatory responses.
Study participants were randomized to receive either placebo or DHEA before hypoglycemic clamps on days 1 and 2 and were blinded to that randomization. Repeated hypoglycemia (2.9 mmol/L) in the placebo group blunted all key neuroendocrine, ANS, metabolic, and symptom responses on day 2 compared with day 1, whereas 800 mg of DHEA before the two hypoglycemic clamps on day 1 and 1,600 mg before the single hypoglycemic clamp on day 2 preserved the wide spectrum of all counterregulatory responses during repeated hypoglycemia. Baseline values of the neuroendocrine, ANS, metabolic, and cardiovascular parameters measured were similar at the start of the day 1 and day 2 studies in both groups. It therefore seems that DHEA was exerting effects during hypoglycemia to affect responses to the physiologic stress rather than a constitutive action to change basal neuroendocrine, ANS, and metabolic tones. In addition, the day 1 counterregulatory responses were similar between the two groups, demonstrating that DHEA did not induce an inherent acute increase or suppression of counterregulatory responses during hypoglycemia. This, in turn, provided a stable basis on which to compare the differing repeated hypoglycemia results from day 2.
Although all the major metabolic counterregulatory hormones were preserved during repeated hypoglycemia with DHEA, it is notable that epinephrine and glucagon responses, which are the two principal acute-acting counterregulatory hormones, were maintained following DHEA. Furthermore, norepinephrine, which serves as a biomarker for sympathetic nervous system neural activity, was in fact increased during hypoglycemia following DHEA on day 2.
The maintained epinephrine, glucagon, and direct sympathetic nervous system drive resulted in an important preservation of metabolic counterregulatory mechanisms on day 2. EGP was increased during hypoglycemia following DHEA on day 2. There was also a commensurate reduction in the glucose infusion rate needed to maintain hypoglycemia on day 2. However, peripheral glucose disposal rates were similar in the DHEA and placebo groups. This indicates that the improved counterregulatory response following DHEA was acting primarily and selectively to enhance EGP rather than limiting peripheral glucose disposal. Lipolysis was also maintained following DHEA. This allowed glycerol to be used as a gluconeogenic precursor and NEFAs to provide energy for gluconeogenesis and reduce the inhibitory action of insulin on hepatic glycogenolysis.
Although growth hormone and cortisol have limited action in the acute defense against a falling plasma glucose, it is worth noting that DHEA preserved the responses of these two hormones during repeated hypoglycemia. Pancreatic polypeptide responses were also maintained following DHEA. Pancreatic polypeptide is used as a biomarker of parasympathetic nervous system activity during hypoglycemia (37). This indicates that DHEA is exerting simultaneous widespread acute effects on the hypothalamic–pituitary axis, the ANS, and differing systemic neuroendocrine organs (pancreas and adrenal glands) to modulate counterregulatory responses to hypoglycemia in healthy humans.
Cardiovascular responses of systolic blood pressure and heart rate were also increased during hypoglycemia followed DHEA on day 2. These effects are plausibly explained by the increased sympathetic nervous system drive following DHEA. DHEA also preserved autonomic hypoglycemic symptoms during the day 2 studies. Autonomic hypoglycemic symptoms typically originate at a glucose concentration of ∼3.3 mmol/L and are an important cue to take action in the defense against a decreasing glucose value. Neuroglycopenic symptoms typically occur at threshold of ∼2.9 mmol/L in healthy individuals and so provided a relatively weak experimental signal at our glucose clamps of 2.9 mmol/L (38).
DHEA is rapidly interconverted to DHEA-S in the adrenals and other peripheral organs. DHEA-S has powerful neuro-steroidal activity, can be produced de novo in the brain, and is the major circulating DHEA metabolite (39). DHEA and DHEA-S have been classically thought to exert their physiologic roles by acting as precursors to testosterone and estradiol and then interacting with sex hormone receptors. To date, no classical individual hormone receptor for DHEA or DHEA-S has been identified. Nevertheless, recent work has identified several receptors in vitro that can interact with DHEA-S and may translate into clinical relevance (36).
Both DHEA and DHEA-S have been reported to interact with GABA receptors to antagonize GABA effects (30). This would likely protect counterregulatory responses during hypoglycemia, since GABAA activation has been demonstrated to reduce neuroendocrine, ANS, and metabolic homeostatic responses during hypoglycemia and exercise in humans and rodents (14,40). Second, DHEA has been reported to have an antiglucocorticoid action in rat models (33). This action may also result in improved ANS counterregulatory responses during hypoglycemia, as glucocorticoids have been reported to reduce neuroendocrine and catecholamine responses to differing subsequent physiologic stress, including hypoglycemia (41). Furthermore, Steckelbroeck et al. (42) have demonstrated that DHEA can be metabolized in the human brain by membrane bound 7α-hydroxylase enzymes to protect against the effects of neurotoxic glucocorticoids. DHEA-S has also been reported to interact with N-methyl-aspartate excitatory amino acid receptors that can enhance the effects of glutamate (32). This seems to be relevant; recent work by Szepietowska et al. (43) demonstrated that increases in hypothalamic glutamate concentrations have the potential to increase counterregulatory responses to hypoglycemia. Finally, DHEA has been reported to stimulate nitric oxide synthase, and thus nitric oxide, via a specific cell surface receptor in vascular endothelial cells (15). This also seems notable; recent work from Fioramonti et al. (44) reported that ventromedial hypothalamic nitric oxide production is required to detect hypoglycemia and activate counterregulatory responses.
DHEA has been used in numerous metabolic studies at doses ranging from 20 to 1,600 mg/day (45–50). In this study we used the highest reported dose in the literature to maximize any possible experimental signal. Although DHEA-S has been associated with a number of adverse events, none occurred in this study. DHEA-S levels increased quickly and reached plasma concentrations similar to those in previous reports using equivalent doses (47–50).
A limitation of this study is that we are unable to comment on the dose-response effects of DHEA-S on the protection of counterregulatory responses during repeated hypoglycemia. Thus there is an opportunity for future work to determine whether lower doses of DHEA-S can preserve counterregulatory responses during repeated hypoglycemia.
In summary, this study determined that high-dose oral DHEA taken 1 h before repeated 2-day hypoglycemic clamps in healthy individuals can acutely preserve ANS (epinephrine, norepinephrine, pancreatic polypeptide), neuroendocrine (glucagon, growth hormone, cortisol), metabolic (EGP, lipolysis), and hypoglycemic symptom responses. These results seem to be clinically relevant. An oral adjunct therapeutic approach to maintain ANS and neuroendocrine counterregulatory responses during intensified glycemic control would be welcomed. Future studies examining the effects of DHEA on preserving counterregulatory responses and preventing hypoglycemia-associated autonomic failure in type 1 and type 2 diabetes would also be helpful.
We conclude that DHEA-S can have rapid effects to protect a wide range of ANS and neuroendocrine counterregulatory responses during antecedent hypoglycemia in healthy individuals, and to prevent the development of hypoglycemia-associated autonomic and neuroendocrine failure.
Article Information
Acknowledgments. The authors thank Wanda Snead and Eric Allen (Vanderbilt Hormone Assay Core Laboratory), Lindsay Pulliam (University of Maryland), and the Johns Hopkins Core Laboratory for their excellent technical assistance. The authors also thank the nursing staff of the University of Maryland, Baltimore General Clinical Research Center, for their excellent care. The authors thank Cheryl Young (University of Maryland) for her assistance in recruiting and screening participants.
Funding. This work was supported by the National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases grants RO1 DK069803 and P60 DK020593 (a Vanderbilt Diabetes Research and Training grant) and National Heart, Lung, and Blood Institute grant PO1 HL056693.
Duality of Interest. No conflicts of interest relevant to this article were reported.
Author Contributions. M.M. performed studies, researched and analyzed data, and wrote, reviewed, and edited the manuscript. M.S.H., N.J., and I.D. performed studies. D.B.T. and L.M.Y. performed studies, researched data, and reviewed and edited the manuscript. S.N.D. devised the study, reviewed data, and wrote, reviewed, and edited the manuscript. S.N.D. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Clinical trial reg. no. NCT00607646, clinicaltrials.gov.
References
- 1.Cryer PE. The barrier of hypoglycemia in diabetes. Diabetes 2008;57:3169–3176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cryer PE. Hypoglycaemia: the limiting factor in the glycaemic management of type I and type II diabetes. Diabetologia 2002;45:937–948 [DOI] [PubMed] [Google Scholar]
- 3.Cryer PE, Davis SN, Shamoon H. Hypoglycemia in diabetes. Diabetes Care 2003;26:1902–1912 [DOI] [PubMed] [Google Scholar]
- 4.Heller SR, Cryer PE. Reduced neuroendocrine and symptomatic responses to subsequent hypoglycemia after 1 episode of hypoglycemia in nondiabetic humans. Diabetes 1991;40:223–226 [DOI] [PubMed] [Google Scholar]
- 5.Cryer PE. Mechanisms of hypoglycemia-associated autonomic failure in diabetes. N Engl J Med 2013;369:362–372 [DOI] [PubMed] [Google Scholar]
- 6.Marty N, Dallaporta M, Thorens B. Brain glucose sensing, counterregulation, and energy homeostasis. Physiology (Bethesda) 2007;22:241–251 [DOI] [PubMed] [Google Scholar]
- 7.Frizzell RT, Jones EM, Davis SN, et al. . Counterregulation during hypoglycemia is directed by widespread brain regions. Diabetes 1993;42:1253–1261 [DOI] [PubMed] [Google Scholar]
- 8.Borg WP, Sherwin RS, During MJ, Borg MA, Shulman GI. Local ventromedial hypothalamus glucopenia triggers counterregulatory hormone release. Diabetes 1995;44:180–184 [DOI] [PubMed] [Google Scholar]
- 9.Teves D, Videen TO, Cryer PE, Powers WJ. Activation of human medial prefrontal cortex during autonomic responses to hypoglycemia. Proc Natl Acad Sci U S A 2004;101:6217–6221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arbelaez AM, Powers WJ, Videen TO, Price JL, Cryer PE. Attenuation of counterregulatory responses to recurrent hypoglycemia by active thalamic inhibition: a mechanism for hypoglycemia-associated autonomic failure. Diabetes 2008;57:470–475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Briscoe VJ, Ertl AC, Tate DB, Dawling S, Davis SN. Effects of a selective serotonin reuptake inhibitor, fluoxetine, on counterregulatory responses to hypoglycemia in healthy individuals. Diabetes 2008;57:2453–2460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McCrimmon RJ, Evans ML, Fan X, et al. . Activation of ATP-sensitive K+ channels in the ventromedial hypothalamus amplifies counterregulatory hormone responses to hypoglycemia in normal and recurrently hypoglycemic rats. Diabetes 2005;54:3169–3174 [DOI] [PubMed] [Google Scholar]
- 13.Vele S, Milman S, Shamoon H, Gabriely I. Opioid receptor blockade improves hypoglycemia-associated autonomic failure in type 1 diabetes mellitus. J Clin Endocrinol Metab 2011;96:3424–3431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hedrington MS, Farmerie S, Ertl AC, Wang Z, Tate DB, Davis SN. Effects of antecedent GABAA activation with alprazolam on counterregulatory responses to hypoglycemia in healthy humans. Diabetes 2010;59:1074–1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu D, Dillon JS. Dehydroepiandrosterone stimulates nitric oxide release in vascular endothelial cells: evidence for a cell surface receptor. Steroids 2004;69:279–289 [DOI] [PubMed] [Google Scholar]
- 16.Sandoval DA, Ping L, Neill RA, Morrey S, Davis SN. The effects of dehydroepiandrosterone sulfate on counterregulatory responses during repeated hypoglycemia in conscious normal rats. Diabetes 2004;53:679–686 [DOI] [PubMed] [Google Scholar]
- 17.Bruce RA. Exercise testing of patients with coronary heart disease. Principles and normal standards for evaluation. Ann Clin Res 1971;3:323–332 [PubMed] [Google Scholar]
- 18.Abumrad NN, Rabin D, Diamond MP, Lacy WW. Use of a heated superficial hand vein as an alternative site for the measurement of amino acid concentrations and for the study of glucose and alanine kinetics in man. Metabolism 1981;30:936–940 [DOI] [PubMed] [Google Scholar]
- 19.Amiel SA, Tamborlane WV, Simonson DC, Sherwin RS. Defective glucose counterregulation after strict glycemic control of insulin-dependent diabetes mellitus. N Engl J Med 1987;316:1376–1383 [DOI] [PubMed] [Google Scholar]
- 20.Wall JS, Steele R, De Bodo RC, Altszuler N. Effect of insulin on utilization and production of circulating glucose. Am J Physiol 1957;189:43–50 [DOI] [PubMed] [Google Scholar]
- 21.Aguilar-Parada E, Eisentraut AM, Unger RH. Pancreatic glucagon secretion in normal and diabetic subjects. Am J Med Sci 1969;257:415–419 [DOI] [PubMed] [Google Scholar]
- 22.Wide L, Porath J. Radioimmunoassay of proteins with the use of Sephadex-coupled antibodies. Biochim Biophys Acta Gen Subj 1966;130:257–260 [Google Scholar]
- 23.Causon RC, Carruthers ME, Rodnight R. Assay of plasma catecholamines by liquid chromatography with electrochemical detection. Anal Biochem 1981;116:223–226 [DOI] [PubMed] [Google Scholar]
- 24.Hunter WM, Greenwood FC. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature 1962;194:495–496 [DOI] [PubMed] [Google Scholar]
- 25.Hagopian W, Lever EG, Cohen D, et al. . Predominance of renal and absence of hepatic metabolism of pancreatic polypeptide in the dog. Am J Physiol 1983;245:E171–E177 [DOI] [PubMed] [Google Scholar]
- 26.Lloyd B, Burrin J, Smythe P, Alberti KG. Enzymic fluorometric continuous-flow assays for blood glucose, lactate, pyruvate, alanine, glycerol, and 3-hydroxybutyrate. Clin Chem 1978;24:1724–1729 [PubMed] [Google Scholar]
- 27.Ho RJ. Radiochemical assay of long-chain fatty acids using 63Ni as tracer. Anal Biochem 1970;36:105–113 [DOI] [PubMed] [Google Scholar]
- 28.Deary IJ, Hepburn DA, MacLeod KM, Frier BM. Partitioning the symptoms of hypoglycaemia using multi-sample confirmatory factor analysis. Diabetologia 1993;36:771–777 [DOI] [PubMed] [Google Scholar]
- 29.Sanders NM, Wilkinson CW, Taborsky GJ Jr, et al. . The selective serotonin reuptake inhibitor sertraline enhances counterregulatory responses to hypoglycemia. Am J Physiol Endocrinol Metab 2008;294:E853–E860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Imamura M, Prasad C. Modulation of GABA-gated chloride ion influx in the brain by dehydroepiandrosterone and its metabolites. Biochem Biophys Res Commun 1998;243:771–775 [DOI] [PubMed] [Google Scholar]
- 31.Demirgören S, Majewska MD, Spivak CE, London ED. Receptor binding and electrophysiological effects of dehydroepiandrosterone sulfate, an antagonist of the GABAA receptor. Neuroscience 1991;45:127–135 [DOI] [PubMed] [Google Scholar]
- 32.Compagnone NA, Mellon SH. Dehydroepiandrosterone: a potential signalling molecule for neocortical organization during development. Proc Natl Acad Sci U S A 1998;95:4678–4683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wright BE, Porter JR, Browne ES, Svec F. Antiglucocorticoid action of dehydroepiandrosterone in young obese Zucker rats. Int J Obes Relat Metab Disord 1992;16:579–583 [PubMed] [Google Scholar]
- 34.Debonnel G, Bergeron R, de Montigny C. Potentiation by dehydroepiandrosterone of the neuronal response to N-methyl-D-aspartate in the CA3 region of the rat dorsal hippocampus: an effect mediated via sigma receptors. J Endocrinol 1996;150(Suppl.):S33–S42 [PubMed] [Google Scholar]
- 35.McNelis JC, Manolopoulos KN, Gathercole LL, et al. . Dehydroepiandrosterone exerts antiglucocorticoid action on human preadipocyte proliferation, differentiation, and glucose uptake. Am J Physiol Endocrinol Metab 2013;305:E1134–E1144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wen S, Dong K, Onolfo JP, Vincens M. Treatment with dehydroepiandrosterone sulfate increases NMDA receptors in hippocampus and cortex. Eur J Pharmacol 2001;430:373–374 [DOI] [PubMed] [Google Scholar]
- 37.Veedfald S, Plamboeck A, Hartmann B, et al. . Pancreatic polypeptide responses to isoglycemic oral and intravenous glucose in humans with and without intact vagal innervation. Peptides 2015;71:229–231 [DOI] [PubMed] [Google Scholar]
- 38.Cryer PE. Symptoms of hypoglycemia, thresholds for their occurrence, and hypoglycemia unawareness. Endocrinol Metab Clin North Am 1999;28:495–500, v–vi [DOI] [PubMed] [Google Scholar]
- 39.Baulieu EE, Robel P. Dehydroepiandrosterone and dehydroepiandrosterone sulfate as neuroactive neurosteroids. J Endocrinol 1996;150(Suppl.):S221–S239 [PubMed] [Google Scholar]
- 40.Chan O, Paranjape S, Czyzyk D, et al. . Increased GABAergic output in the ventromedial hypothalamus contributes to impaired hypoglycemic counterregulation in diabetic rats. Diabetes 2011;60:1582–1589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Davis SN, Shavers C, Davis B, Costa F. Prevention of an increase in plasma cortisol during hypoglycemia preserves subsequent counterregulatory responses. J Clin Invest 1997;100:429–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Steckelbroeck S, Watzka M, Lütjohann D, et al. . Characterization of the dehydroepiandrosterone (DHEA) metabolism via oxysterol 7alpha-hydroxylase and 17-ketosteroid reductase activity in the human brain. J Neurochem 2002;83:713–726 [DOI] [PubMed] [Google Scholar]
- 43.Szepietowska B, Zhu W, Czyzyk J, Eid T, Sherwin RS. EphA5-EphrinA5 interactions within the ventromedial hypothalamus influence counterregulatory hormone release and local glutamine/glutamate balance during hypoglycemia. Diabetes 2013;62:1282–1288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fioramonti X, Marsollier N, Song Z, et al. . Ventromedial hypothalamic nitric oxide production is necessary for hypoglycemia detection and counterregulation. Diabetes 2010;59:519–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yen SS, Morales AJ, Khorram O. Replacement of DHEA in aging men and women. Potential remedial effects. Ann N Y Acad Sci 1995;774:128–142 [DOI] [PubMed] [Google Scholar]
- 46.Villareal DT, Holloszy JO. Effect of DHEA on abdominal fat and insulin action in elderly women and men: a randomized controlled trial. JAMA 2004;292:2243–2248 [DOI] [PubMed] [Google Scholar]
- 47.Mortola JF, Yen SS. The effects of oral dehydroepiandrosterone on endocrine-metabolic parameters in postmenopausal women. J Clin Endocrinol Metab 1990;71:696–704 [DOI] [PubMed] [Google Scholar]
- 48.Nestler JE, Barlascini CO, Clore JN, Blackard WG. Dehydroepiandrosterone reduces serum low density lipoprotein levels and body fat but does not alter insulin sensitivity in normal men. J Clin Endocrinol Metab 1988;66:57–61 [DOI] [PubMed] [Google Scholar]
- 49.Welle S, Jozefowicz R, Statt M. Failure of dehydroepiandrosterone to influence energy and protein metabolism in humans. J Clin Endocrinol Metab 1990;71:1259–1264 [DOI] [PubMed] [Google Scholar]
- 50.Usiskin KS, Butterworth S, Clore JN, et al. . Lack of effect of dehydroepiandrosterone in obese men. Int J Obes 1990;14:457–463 [PubMed] [Google Scholar]