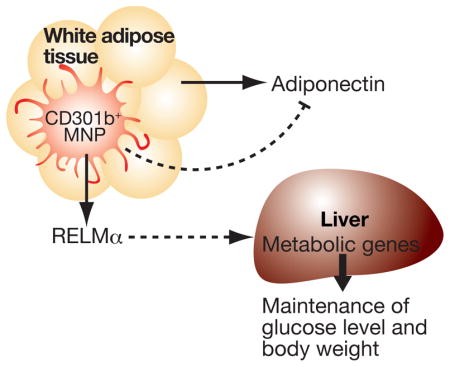
Summary
Mononuclear phagocytes (MNPs) are a highly heterogeneous group of cells that play important roles in maintaining the body’s homeostasis. Here, we found CD301b (also known as MGL2), a lectin commonly used as a marker for alternatively-activated macrophages (M2), was selectively expressed by a subset of CD11b+CD11c+MHCII+ MNPs in multiple organs including adipose tissues. Depleting CD301b+ MNPs in vivo led to a significant weight loss with increased insulin sensitivity and a marked reduction in serum RELM α, a multifunctional cytokine produced by MNPs. Reconstituting RELM α in CD301b+ MNP-depleted animals restored body weight and normoglycemia. Thus, CD301b+ MNPs play crucial roles in maintaining glucose metabolism and net energy balance.
Keywords: Alternatively activated macrophage, glucose metabolism, adipose tissue macrophage
Graphical Abstract
Introduction
Maintaining the whole body energy balance is vital to our lives. Mononuclear phagocytes (MNPs), including monocytes, macrophages and dendritic cells (DCs) are present in most organs throughout the body and play an important role in maintaining whole body homeostasis. MNPs respond to and counteract metabolic stresses such as those imposed by high fat diet (HFD) feeding in mouse models (Biswas and Mantovani, 2012; Chawla et al., 2011; Olefsky and Glass, 2010). However, little is known regarding their role in maintaining metabolic homeostasis in metabolically normal conditions under regular chow feeding.
Macrophages are thought to adopt distinct phenotypes depending on the external stimuli. M1, or classical activation, refers to the activation status whereby MNPs are stimulated with pro-inflammatory type 1 cytokines or inflammatory stimuli such as interferon-γ or lipopolysaccharide (LPS). M2, or alternative activation, in contrast, refers to the activation status of MNPs that are stimulated with type 2 cytokines such as interleukin (IL)-4 or IL-13 (Martinez et al., 2009). In general, M1 MNPs are considered to play pro-inflammatory roles, while M2 MNPs are generally specialized on homeostasis and tissue repair. Although the concept of M1 and M2 activation has come originally from in vitro observations (Stein et al., 1992), it is now widely applied to in vivo MNP populations that have similar gene expression profile to those activated in vitro (Martinez et al., 2009).
Chronic low-grade inflammation has long been linked to metabolic complications associated with obesity such as insulin resistance. Accordingly, several types of immune cells within the adipose tissue have been linked to obesity-associated metabolic disease (Mathis, 2013). Contributions of MNPs in metabolic disease have been well documented (Hotamisligil, 2006; Weisberg et al., 2003; Xu et al., 2003). Early during the development of obesity, MNPs in the adipose tissue ‘switch’ their phenotype from an M2-like status in the lean conditions to an M1-like phenotype (Lumeng et al., 2007). Importantly, genetic and pharmacological reduction in the number of M1 MNPs has been shown to result in a marked improvement in insulin sensitivity (Feng et al., 2011; Kanda et al., 2006; Patsouris et al., 2008; Weisberg et al., 2006). In contrast, blocking M2 activation cascade by deleting macrophage expression of IL-4 receptor downstream components, such as PPARγ, PPARδ and KLF4, results in exacerbated insulin resistance and, in some models, increase in weight gain upon HFD feeding (Kang et al., 2008; Liao et al., 2011; Odegaard et al., 2007; Odegaard et al., 2008). However, MNP populations in vivo are highly heterogeneous, and do not necessarily conform to either the M1 or M2 phenotypes (Shaul et al., 2010; Wentworth et al., 2010). Therefore, dissection of the role of MNP subsets beyond the simple M1-versus-M2 classification is needed to understand the role of various MNPs in the context of metabolic diseases.
The Mgl2 gene (encoding the CD301b protein) is often considered to be a prototypical M2 marker both in vivo and in vitro (Auffray et al., 2007; Byles et al., 2013; Fujisaka et al., 2009; Han et al., 2013; Kang et al., 2008; Li et al., 2011; Lumeng et al., 2007; Raes et al., 2005). Importantly, a marked reduction of Mgl2 expression in the adipose tissue MNPs has been reported in MNP-specific PPARδ-deficient mice, in which exacerbated insulin resistance with more weight gain upon HFD feeding has also been observed (Kang et al., 2008). These studies suggest that CD301b+ MNPs play a protective role against metabolic stress and that depletion of CD301b+ MNPs would result in a similar phenotype to the above mentioned M2-deficient models.
Here, to characterize the role of CD301b+ MNPs in whole body metabolism, we examined the expression pattern of the CD301b protein among MNP subsets in the adipose tissue and other organs. In a mouse model expressing diphtheria toxin receptor (DTR) under the control of the Mgl2 gene (Mgl2-DTR mouse) (Kumamoto et al., 2013), we examined the metabolic consequences of acute depletion of CD301b+ MNPs by diphtheria toxin (DT) treatment in both regular chow fed mice as well as those on high fat diet. Contrary to previous studies that have suggested an anti-diabetogenic role of CD301b+MNPs, our results reveal their requirement for the maintenance of positive energy balance under both steady state and high fat diet metabolic conditions, and identify RELM α as a key downstream effector molecule.
Results
CD301b is expressed in a subset of MNPs in vitro and in vivo
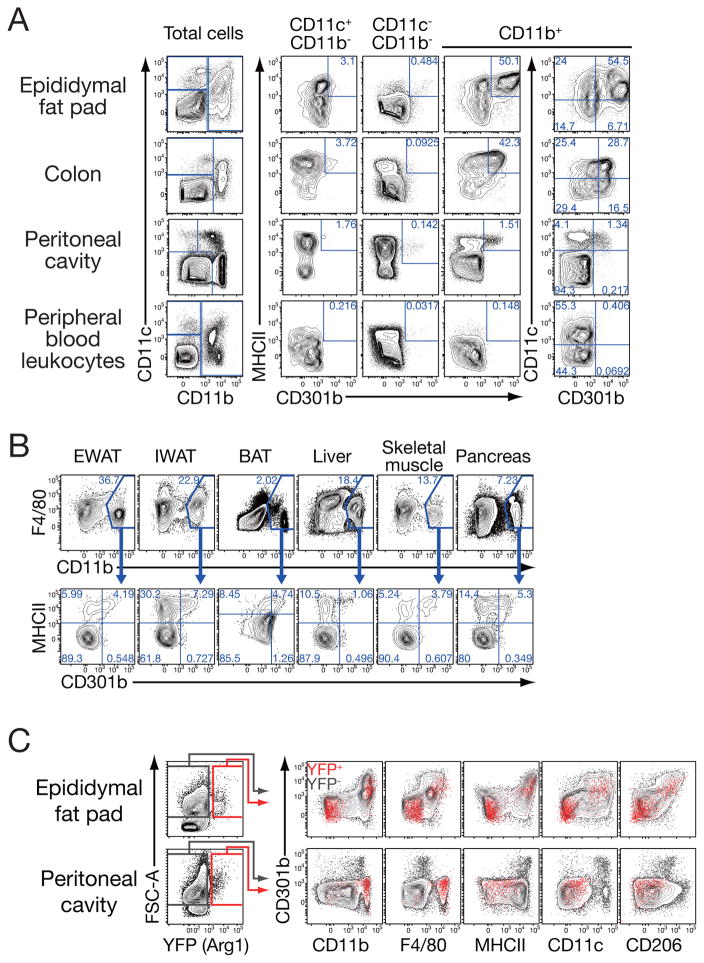
Mgl2 mRNA, encoding the CD301b protein, has been shown to be highly expressed in interleukin-4 (IL-4)-stimulated M2 macrophages in vitro and M2-like MNPs in vivo (Raes et al., 2005). However, the proportion of CD301b+ cells out of all M2 macrophages in vivo, or the phenotype of CD301b+ cells in metabolic organs is unknown. To this end, we investigated the expression of CD301b in MNPs isolated from various non-lymphoid tissues of WT mice fed regular chow. CD301b was found on a subset of MNPs in most of the peripheral organs tested, including the epididymal fat, colon, and the peritoneum (Figure 1A). Little if any CD301b+ cells were found in the blood, consistent with a previous observation that Mgl2 expression is upregulated in a fraction of circulating MNPs after their entry into the peripheral tissue (Figure 1A) (Auffray et al., 2007). A previous study has shown that adipose tissue macrophages express a high amount of Mgl2 mRNA particularly in lean mice (Lumeng et al., 2007). Indeed, a fraction of CD11b+F4/80int to high macrophage-like cells in the visceral adipose tissue, as well as other metabolic organs, expressed CD301b protein (Figure 1B). Importantly, as was observed previously with the CD301b+ cells in other sites of the body (Denda-Nagai et al., 2010; Kumamoto et al., 2009; Kumamoto et al., 2013), the majority of CD301b+ cells in the peripheral organs uniformly expressed a high amount of MHC class II and CD11b, an intermediate to high amount of CD11c, and only a moderate amount of F4/80 (Figure 1A, B).
Figure 1. CD301b is expressed in a subset of MNPs.
Expression of CD301b in MNPs isolated from indicated organs of regular chow-fed male WT (A, B) or YARG (C) mice. Cells were gated and overlayed as indicated. Representative data from more than five (A), three (B), and two (C) independent experiments are shown. EWAT: epididymal WAT, IWAT: inguinal WAT, BAT: interscapular brown adipose tissue.
Since Mgl2 mRNA expression has often been used as a marker for adipose tissue-resident M2-like MNPs in vivo (Fujisaka et al., 2009; Han et al., 2013; Li et al., 2011; Lumeng et al., 2007), we next compared the expression of CD301b and two other M2 markers, Arginase1 and CD206. Cells expressing Arg1 mRNA were analyzed using the YARG reporter mice in which expression of Arginase 1 is monitored by YFP expression from the Arg1 locus (Reese et al., 2007). Expression of CD206 (also known as macrophage mannose receptor), another commonly-used M2 marker, was assessed by a specific antibody. In the epididymal fat, the expression of CD301b was observed selectively in the cells expressing CD206. In contrast, Arg1 expression was observed in two clearly distinct populations in the adipose tissue: those expressing common MNP markers including CD11b, F4/80, MHCII, CD11c, CD206 and CD301b, and those that were negative for all MNP markers tested (Figure 1C). In the peritoneal cavity, the expression of CD301b showed distinct patterns from that of Arg1, though it still partially overlapped with that of CD206. In the peritoneum, Arg1 expression was confined to CD11b+ F4/80+ MHCII− CD11c− CD206− cells. In both organs, CD301b was specifically expressed in CD11c+ CD11b+ MHCIIhi F4/80int CD206+ MNPs (Figure 1). These results indicate that not all M2 markers examined (CD301b, CD206, Arg1) are expressed in the same MNP subset in vivo and that CD301b+ MNPs represent a relatively consistent subset throughout different organs. Therefore, the “M2” macrophage markers delineate multiple distinct cell subsets in different organs, and CD301b+ MNPs do not clearly fit either M1 or M2 macrophage definition.
Depletion of CD301b+ MNPs results in weight loss in regular chow and HFD fed mice
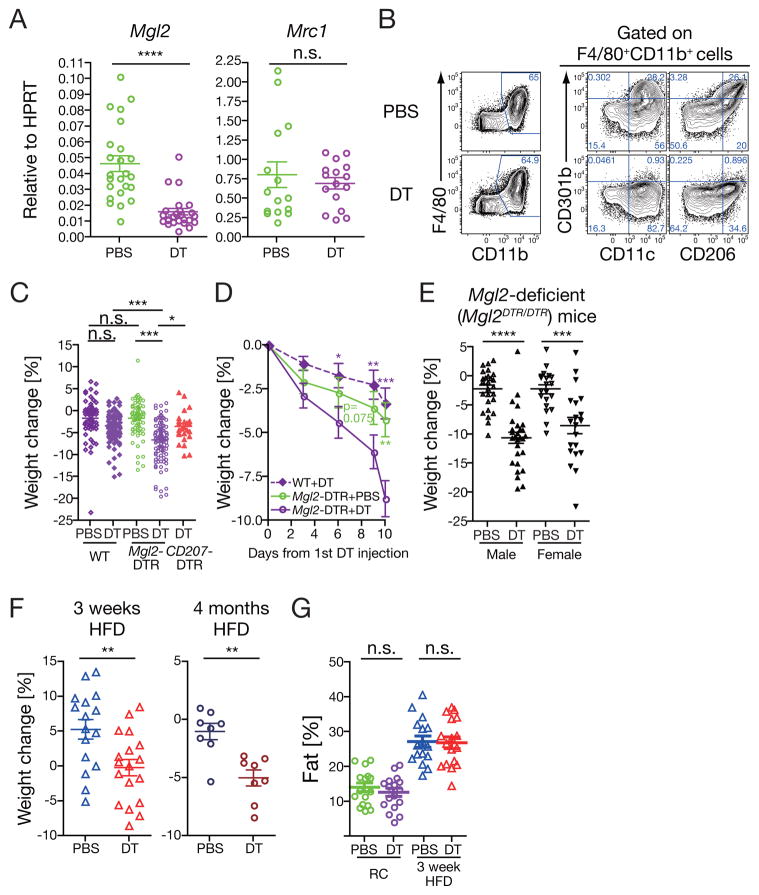
Next, we examined the role of CD301b+ MNPs in possible regulation of in vivo metabolism by using Mgl2+/DTReGFP (Mgl2-DTR) mice, in which CD301b+ MNPs can be specifically and inducibly depleted by injecting diphtheria toxin (DT) (Kumamoto et al., 2013). Quantitative real-time PCR experiments in the epididymal white adipose tissue revealed that the DT treatment successfully abolished expression of Mgl2 but not Mrc1 (encoding CD206) (Figure 2A). Consistent with the mRNA expression data, flow-cytometric experiments revealed that DT treatment of Mgl2-DTR mice did not affect the rest of the MNPs that did not express this marker (Figure 2B). After DT injections given every-third-day for 10 days, the regular chow-fed Mgl2-DTR mice had a significant reduction in body weight compared to the wild-type (WT) animals treated with DT or Mgl2-DTR mice treated with phosphate-buffered saline (PBS) (Figure 2C,D and Supplementary Figure 1B). The weight loss was not due to the loss of CD301b protein induced by the DT treatment but rather to the loss of CD301b-expressing cells and was generally observed in both males and females, as indicated by the significant weight loss after the DT-induced cell depletion in Mgl2DTReGFP/DTReGFP homozygotic mice of both sexes that are deficient for the CD301b protein (DTR-eGFP is genetically targeted into the Mgl2 locus) (Figure 2E).
Figure 2. Depletion of CD301b+ MNPs results in weight loss.
(A) Relative expression of Mgl2 (CD301b) and Mrc1 (CD206) in the epididymal WAT in DT-treated regular chow-fed Mgl2-DTR mice. Mgl2-DTR mice were injected intraperitoneally with 0.5 μg DT or PBS on days 0, 3, 6 and 9. On day 10, mice were sacrificed and mRNA expression was determined by real-time PCR. (B) Depletion of CD301b+ MNPs by DT treatment in Mgl2-DTR mice. Stromal vascular fraction was isolated from the epididymal WAT in Mgl2-DTR mice fed with HFD for 3 weeks and treated with DT or PBS for 10 days. Cells were stained and gated as indicated. Reperesentative data from five independent experiments are shown. (C,D) Regular chow-fed mice of indicated strains were treated with DT as in (A) and weighed at day 10 (C) or at indicated time-points (D). Changes in body weight are indicated as percentage of the weight on day 0. Statistics in C was calculated by One-way ANOVA with Tukey’s post test. Statistics in D is calculated from 16 WT+DT, 19 Mgl2-DTR + PBS and 20 Mgl2-DTR +DT mice and indicates comparison to DT-treated Mgl2-DTR mice with two-sided Student’s t-test. (E) Male or female regular chow-fed homozygotic Mgl2DTR/DTR mice were treated with DT for 10 days. Changes in body weight between day 0 and day 10 of DT treatment are shown. (F) Mgl2-DTR mice were fed with HFD for indicated period of time and treated with DT during the last 10 days of the HFD treatment as in (A), and the changes in body weight were calculated as in (C). (G) Adiposity was measured by nuclear magnetic resonance in Mgl2-DTR mice that were fed with regular chow or HFD for 3 weeks and treated with DT for 10 days. Data were pooled from two (A), six (B), five (D) and three (E–G) independent experiments. Each dot in graphs indicates an individual mouse and bars indicate mean ± s.e.m. *p<0.05, **p<0.01, ***<0.001, ****p<0.0001. n.s., not significant by two-sided Student’s t-test unless otherwise indicated. Please see Figure S1.
It was formally possible that DT injection led to either an off target effect or that depletion of MNPs in general accounts for our observation. DT treatments did not deplete leukocyte subsets other than MNPs including eosinophils, natural killer and natural killer T cells, or type 2 innate lymphoid cells that are important in regulating various metabolic pathways (Supplementary Figure 1A) (Brestoff et al., 2015; Ji et al., 2012; Lee et al., 2016; Lynch et al., 2012; Molofsky et al., 2013; Ohmura et al., 2010; Satoh et al., 2012; Schipper et al., 2012; Strodthoff et al., 2013; Wensveen et al., 2015; Wu et al., 2011; Wu et al., 2012). However, we noted previously that DT treatment of Mgl2-DTR mice results in deletion of skin Langerhans cells and dermal DCs in addition to internal tissue MNPs (Kumamoto et al., 2013). Thus, to address the contribution of Langerhans cell loss in the observed weight loss, we compared weight loss in another DTR based system, CD207-DTR transgenic mice, in which DT treatment results in the selective depletion of skin Langerhans cells (Bobr et al., 2010). The weight loss was not induced in CD207-DTR transgenic mice treated with DT (Figure 2C and Supplementary Figure 1B). In addition, as opposed to the Itgax-DTR mice (Jung et al., 2002; Patsouris et al., 2008), Mgl2-DTR mice showed no apparent sickness or lethality even with multiple DT injections (Kumamoto et al., 2013). These data indicate that depletion of CD301b+ MNPs, but not Langerhans cells, results in weight loss.
To determine whether this phenotype is recapitulated in obese animals, we depleted CD301b+ MNPs after short term or long term HFD feeding. Similarly to the regular chow-fed animals, Mgl2-DTR mice fed with HFD either for 3 weeks or for 4 months gained significantly less weight, or lost more weight, respectively, upon DT treatment (Figure 2F and Supplementary Figure 1C). The DT treatment did not affect the fat ratio in the total body mass as determined by nuclear magnetic resonance, suggesting that the depletion leads to a reduction in both lean and fat mass (Figure 2G). These data indicate that depletion of CD301b+ MNPs results in weight loss even in animals that are already obese.
Depletion of CD301b+ MNPs alters whole body energy balance
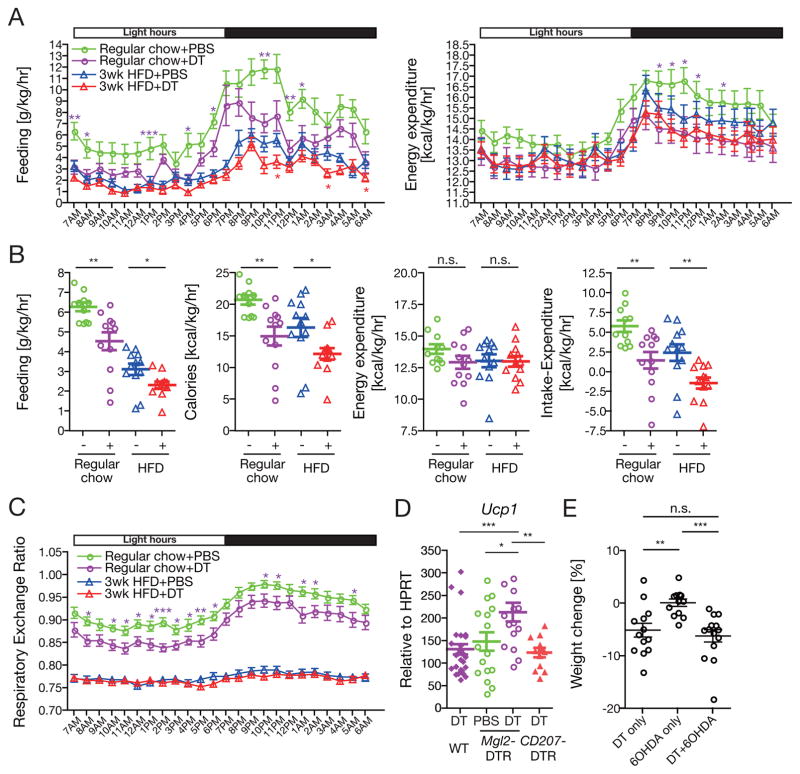
Next, we probed the mechanism of weight loss seen following CD301b+ MNP depletion. Behavioral and calorimetric analyses in metabolic cages revealed a significant reduction in food intake per body weight as well as per mouse in both regular chow- and HFD-fed Mgl2-DTR mice treated with DT (Figure 3A and Supplementary Figure 2A,B). There was also a trend for reduction in energy expenditure in regular chow-fed DT-treated animals during the night, but it did not reach statistical significance as a whole-day average when normalized by body weight (Figure 3A,B). At the whole animal level, the reduction in energy expenditure was evident throughout the day when mice were fed with regular chow (Supplementary Figure 2A,B). Consistent with the weight loss, the net energy balance (energy expenditure subtracted from the food intake) was significantly lower in the DT-treated animals compared to the PBS-treated animals (Figure 3B and Supplementary Figure 2B). In regular chow-fed groups, the depletion significantly lowered the respiratory exchange ratio (RER), indicating that they relied more on lipid oxidation rather than carbohydrate oxidation compared to the PBS-treated controls (Figure 3C). Reduction in RER is often observed in calorically restricted animals (Bruss et al., 2010; Wueest et al., 2014), and may reflect the reduction in food intake in CD301b+ MNP-depleted mice (Figure 3A, B).
Figure 3. Depletion of CD301b+ MNPs results in a negative shift in the energy balance.
(A–C) Mgl2-DTR mice fed on regular chow or HFD for 3 weeks were individually housed and treated with DT for 10 days. Indirect calorimetry was performed in metabolic cages during the last 5 days of treatment. Hourly (A, C) and a whole-day daily (B) average data are shown. Data were pooled from two independent calorimetry experiments with total n=11–12 per group. (D) Regular chow-fed mice of indicated genotype were treated with DT for 10 days. Relative expression of Ucp1 in the interscapular brown adipose tissue was determined by real-time PCR. (E) Regular chow-fed Mgl2-DTR mice were intraperitoneally injected with 6-hydroxydopamine (6OHDA) on days −3 and −1, then treated with DT on days 0, 3, 6 and 9. Changes in body weight between day 0 and day 10 were calculated. Bars indicate mean ± s.e.m. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001. n.s., not significant by two-sided Student’s t-test. Please see Figure S2.
Feeding behavior is regulated by the hunger-satiety circuit in the central nervous system. Since c-Fos is transiently expressed in recently activated neurons, immunohistochemical staining for c-Fos provides a reliable method to label activated neurons. Thus, we predicted that, if the feeding behavior is controlled centrally, we should see increased c-Fos expression in the proopiomelanocortin (POMC) neurons in the arcuate nucleus (ARC), which inhibit food intake. Furthermore, we predicted that neurons in the parabrachial nucleus (PBN) which when activated trigger profound anorexia, would also express higher amounts of c-Fos. However, we did not see significant changes in c-Fos expression in either the POMC neurons in the ARN or neurons within the PBN following depletion of CD301b+ MNPs (Supplementary Figure 2C). In addition, we did not find CD301b+ cells in the hypothalamus. Nevertheless, the alteration in the net energy balance was accompanied by increased sympathetic output, as indicated by the upregulation of Ucp1 gene expression in the brown adipose tissue induced by the depletion of CD301b+ MNPs but not by DT treatment alone (Figure 3D). Again, increased expression of Ucp1 gene was only seen after depletion of CD301b+ MNP but not Langerhans cells. Although the enhanced sympathetic output could directly explain the reduced food intake, chemical sympathectomy by intraperitoneal 6-hydroxydopamine injection did not rescue the weight loss in DT-treated animals, suggesting that the sympathetic output in the periphery alone cannot account for the weight loss phenotype (Figure 3E). Collectively, these results suggest a role for CD301b+ MNPs in active maintenance of positive energy balance, and suggest that these cells do so without directly interacting with cells of the feeding control center of the central nervous system.
Depletion of CD301b+ MNPs causes hypoglycemia in both regular chow- and HFD-fed animals
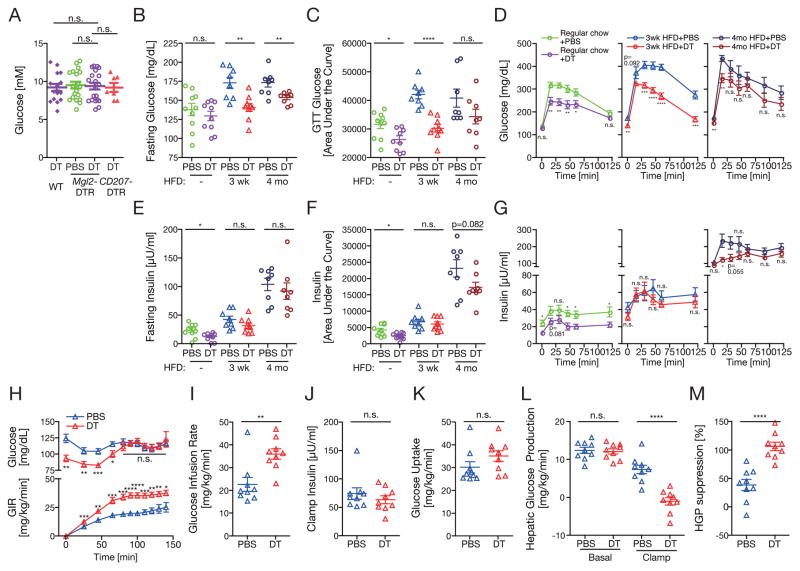
We next explored the role of CD301b+ MNPs in the regulation of glucose metabolism. The CD301b+ MNP depletion did not affect plasma glucose concentrations in lean animals that were fed ad libitum (Figure 4A). In a standard glucose-tolerance test (GTT) in overnight fasted animals, however, the plasma glucose concentrations were significantly reduced in DT-treated animals in both regular chow and HFD feeding conditions (Figure 4B–D). In DT-treated regular chow-fed animals, the plasma insulin concentrations were also lower than the PBS-treated control animals in the fasting state and during the GTT, suggesting that the depletion of CD301b+ MNPs enhanced insulin sensitivity (Figure 4E–G). To test this possibility, we performed hyperinsulinemic-euglycemic clamp studies on DT- or PBS-treated Mgl2-DTR mice that had been on HFD for 3 weeks, in which glucose-reducing effect of DT was most prominent in our GTTs. Consistent with the GTT results, the DT-treated animals displayed improved whole-body insulin sensitivity as demonstrated by the higher glucose infusion rate required to maintain euglycemia under experimental conditions in which plasma insulin concentrations were matched (Figure 4H–J). The higher glucose infusion rate in the depleted mice was largely explained by the suppression of hepatic glucose production compared with controls, whereas insulin-stimulated glucose uptake was not different between these groups (Figure 4K–M).
Figure 4. Depletion of CD301b+ MNPs results in hypoglycemia in both lean and obese mice.
(A) Serum glucose concentrations in DT-treated mice fed ad libitum. (B–G) GTTs were performed after an overnight fasting in Mgl2-DTR mice fed with regular chow, HFD for 3 weeks or HFD for 4 months and treated with DT for 10 days. Plasma glucose (B–D) and insulin (E–G) concentrations are shown. (H–M) Hyperinsulinaemic-euglycemic clamp was performed on Mgl2-DTR mice fed with HFD for 3 weeks and treated with DT for 10 days.3H-glucose tracer was included in the infusate so that rates of whole-body glucose uptake (K) and hepatic glucose production (L, M) could be determined during steady-state (final 40 min of study). Data were pooled from two independent experiments. Bars indicate mean ± s.e.m. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001. n.s., not significant by two-sided Student’s t-test.
Depletion of CD301b+ MNPs alters metabolic gene expression patterns and increases serum adiponectin
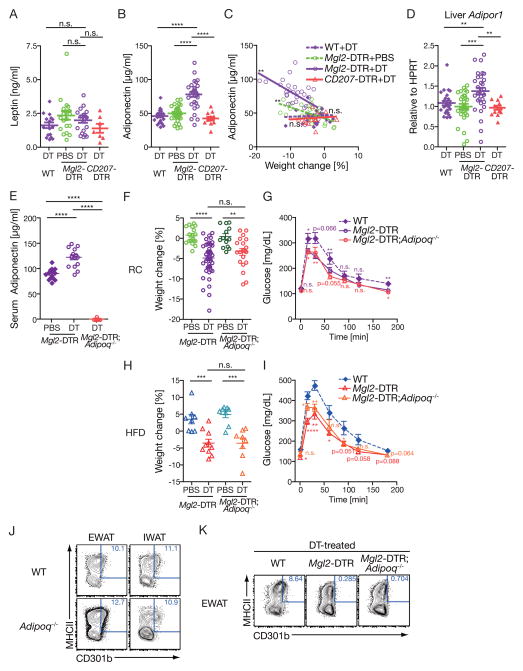
Our data thus far indicated the requirement for CD301b+ MNPs in maintaining normal weight and glucose metabolism. We next examined how the CD301b+ MNPs maintain such metabolic balance. To this end, we examined circulating concentrations of two key adipokines, leptin and adiponectin, which are known to affect body weight and insulin sensitivity through distinct mechanisms (Ouchi et al., 2011; Rosen and Spiegelman, 2006). Measurement of concentrations of these factors in the sera showed no difference in serum leptin concentrations between CD301b+ MNP-intact and depleted animals (Figure 5A). In contrast, serum adiponectin was significantly increased in CD301b+ MNP-depleted lean mice (Figure 5B). The elevation of circulating adiponectin concentration was not observed in CD207-DTR mice, further confirming the subset specificity of this phenomenon (Figure 5B). Comparison of serum adiponectin concentration and the body weight revealed inverse correlation in mice depleted of CD301b+ MNPs (Figure 5C). In parallel, in the liver, the expression of adiponectin receptor Adipor1 was significantly elevated in CD301b+ MNP-depleted mice, suggesting that adiponectin signaling may be selectively enhanced by the depletion of CD301b+ MNPs (Figure 5D). To test whether the increased adiponectin reflects the cause or the consequence of metabolic imbalance, we crossed the Mgl2-DTR mice to Adipoq−/− mice. Our results demonstrated clearly that weight loss and better glucose clearance upon DT treatment in both regular chow- and HFD-feeding conditions were preserved in the absence of Adipoq gene following CD301b+ MNP depletion (Figure 5E–I). The expression of CD301b protein in the Adipoq−/− mice was comparable to that in WT mice (Figure 5J), and CD301b+ MNP depletion was complete (Figure 5K). Collectively, these results indicate that the elevated serum adiponectin concentrations in CD301b+ MNP-depleted mice are the consequence, and not the cause, of weight loss in these animals.
Figure 5. Depletion of CD301b+ MNPs results in elevated circulating adiponectin concentrations.
Regular chow-fed mice of indicated genotype were treated with DT for 10 days. (A,B,E) Circulating Leptin (A) and adiponectin (B,E) concentrations were determined by ELISA. (C) Correlation between weight loss and circulating adiponectin concentrations is shown. (D) Relative expression of Adipor1 (Adiponectin Receptor 1) in the liver. (F–J) Weight change (F,H) and GTT (G,I) in Mgl2-DTR mice on Adipoq−/− background fed with regular chow (F,G) or HFD for 3 weeks (H,I). (J, K) CD301b expression (J) and depletion (K) in mice on Adipoq−/− background. Data are gated on F4/80+CD11b+ cells in epididymal (EWAT) or inguinal (IWAT) fat pads. Blood and tissue samples were collected in two (A–D), five (E–G) and two (H,I) independent experiments. Bars indicate mean ± s.e.m. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001. n.s., not significant by two-sided Student’s t-test. Statistics in GTT curves indicates comparison to Mgl2-DTR mice.
Depletion of CD301b+MNPs alters distinct gene sets in the white adipose tissue and the liver
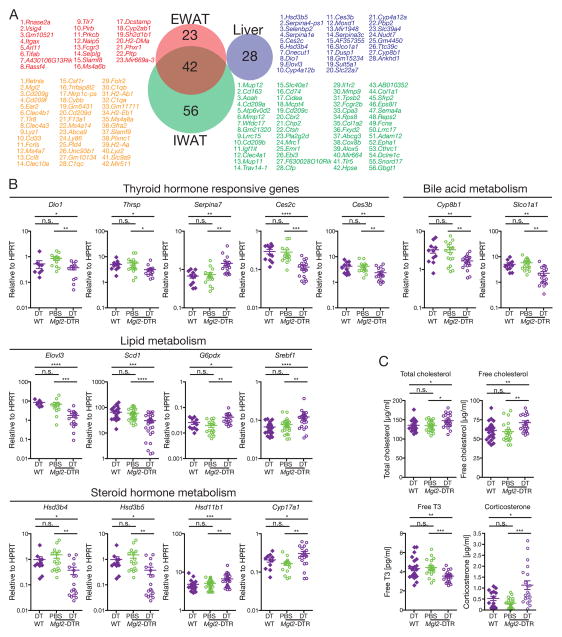
To understand the molecular basis for the altered metabolism in the CD301b+MNP-depleted mice, we next examined the transcriptome profiles in the visceral and subcutaneous white adipose tissues (WAT) and the liver. Among genes that were highly significantly downregulated by the depletion (p<0.01, more than 50% reduction), 42 genes were shared between the inguinal and epididymal WAT, many of which were myeloid cell markers that were likely expressed in CD301b+MNPs themselves (Figure 6A). In contrast, we found a very different set of genes downregulated in the liver, compared to WAT (Figure 6A). Consistent with the improved hepatic insulin sensitivity, many of the 28 genes that were specifically downregulated in the liver had metabolic function. Gene set enrichment analysis (Subramanian et al., 2005) also revealed significant reduction in ‘immune-related’ pathways in the WAT and in metabolic pathways in the liver, but little overlap between these two organs (Supplementary Figure 3). The top differentially expressed genes in the WAT belonged to the genes expressed by the CD301b+ MNPs themselves (Mgl2, Cd209g, Cd209f, Clec4b1, Clec4a3, Clec10a, Csf1r). Further quantitative analyses by real-time PCR in the liver identified several genes related to metabolism of fatty acids, thyroid hormones, steroids and bile acids that were either significantly up- or down-regulated by the depletion of CD301b+MNPs (Figure 6B). Consistently, serum cholesterol, free thyroid hormone T3 and corticosterone concentrations were significantly changed in mice depleted of CD301b+MNPs (Figure 6C). Collectively, these results indicate that depletion of CD301b+MNPs affects multiple metabolic pathways in the liver, while it mainly results in a reduction in the genes expressed by the CD301b+MNPs in the WAT.
Figure 6. Depletion of CD301b+MNPs alters hepatic metabolism.
Regular chow-fed mice were treated with DT or PBS for 10 days. (A) Gene expression in indicated organs was compared between DT-treated WT and DT-treated Mgl2-DTR mice by microarray. Genes significantly downregulated more than 50% (two mice per group, p<0.01) are shown in the order of magnitude of reduction. (B) Expression of indicated genes in the whole liver was examined by real-time PCR. (C) Cholesterol, T3 and conrticosterone were measeured in the sera. Each dot in graphs indicates independent mouse and bars indicate mean ± s.e.m. Tissue samples were collected in four independent experiments (B,C). *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001. n.s., not significant by two-sided Student’s t-test. Please see Figure S3.
Resistin-like molecule α (RELM α) is responsible for the weight loss and enhanced glucose clearance induced by CD301b+MNP depletion
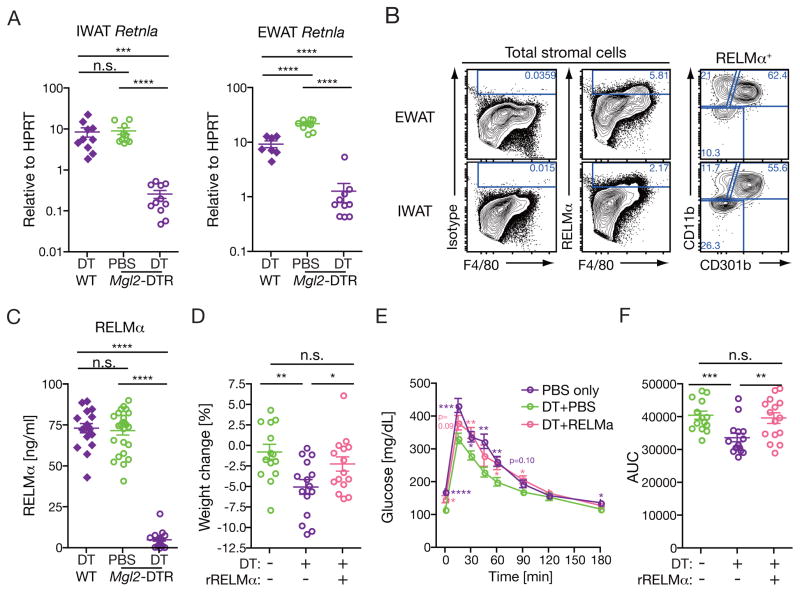
Among the 42 genes commonly downregulated in both inguinal and epididymal WAT, the expression of Retnla gene was most dramatically reduced upon depletion of CD301b+MNPs (Figure 6A), which was further confirmed by real-time PCR analysis of the WAT (Figure 7A). The Retnla gene encodes RELM α protein (also known as FIZZ1), which was originally identified as a tissue-specific cytokine derived from WAT and lung epithelia but later found to be highly expressed in M2 macrophages (Holcomb et al., 2000; Raes et al., 2002; Steppan et al., 2001b). In WT mice, CD301b+ MNPs were the dominant cell type that expressed intracellular RELMα protein in both epididymal and inguinal WAT (Figure 7B). In addition, the depletion of CD301b+MNPs in Mgl2-DTR mice resulted in a severe reduction in the amount of circulating RELMα protein (Figure 7C). To directly address if the reduced RELM α concentration is responsible for the metabolic changes in CD301b+MNP-depleted mice, we supplemented the mice with recombinant RELM α intraperitoneally as previously described (Osborne et al., 2013) in parallel with the DT treatment. The reconstitution of RELM α fully restored the body weight as well the glucose concentrations in GTT in CD301b+MNP-depleted mice (Figure 7C–E). Collectively, these results revealed that CD301b+ MNPs are the major source of circulating RELM α, and place RELM α as the major effector mechanism by which CD301b+MNPs maintain the whole body metabolism.
Figure 7. Reconstitution of RELMα restores body weight and normoglycemia in CD301b+MNP-depleted mice.
(A,B) Regular chow-fed mice were treated with DT or PBS for 10 days. (A) RELMα mRNA expression was measured by real-time PCR in the whole IWAT and EWAT. (B) Intracellular RELMα expression in WAT MNPs. Representative data from two independent experiments are shown. (C) RELMα was quantitated in the sera by ELISA. (D–F) Regular chow-fed Mgl2-DTR mice were treated with DT with or without recombinant RELMα for 10 days. Weight change (D) and GTT (E,F) were examined as in Figure 4. Statistics in GTT curves indicates comparison to Mgl2-DTR mice with DT treatment alone. Bars indicate mean ± s.e.m. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001. n.s., not significant by two-sided Student’s t-test. Pooled data from three independent experiments are shown.
Discussion
In the present study, we have demonstrated that CD301b molecule marks a distinct subset of MNPs present in various organs involved in systemic metabolism including the adipose tissue, skeletal muscle, colon and pancreas. A transient depletion of CD301b+ MNPs induced weight loss and enhances insulin sensitivity in both lean and obese animals. Depletion of CD301b+ MNPs led to reduced food intake, increased fatty acid oxidation, and reduced gluconeogenesis. These phenotypes were accompanied by increased concentrations of adiponectin and cholesterols, and decreased free thyroid hormone T3 and RELM α in circulation. Importantly, reconstituting RELM α normalizes the weight loss and hypoglycemia in CD301b+ MNP-depleted mice. Our results indicate a role for CD301b+ MNPs in maintenance of net energy balance even under lean conditions.
RELMα is a member of the resistin family of hormones that has been identified in the adipose tissue with roles in promoting insulin resistance and linked to obesity with insulin resistance (Steppan et al., 2001a). Although RELMα has not been identified in humans, human resistin expression pattern is more similar to mouse RELMα than mouse resistin, and is expressed in myeloid cells (Nair et al., 2006). In addition, RELMα has been shown to inhibit adipocyte differentiation (Blagoev et al., 2002), suppress type 2 inflammation (Nair et al., 2009; Pesce et al., 2009), and promote T helper 17 cell-dependent colitis and skin wound healing (Knipper et al., 2015; Munitz et al., 2008; Osborne et al., 2013). Retnla−/− mice show faster glucose clearance in GTT in mice with dextran sodium sulfate-induced colitis (Munitz et al., 2009). Furthermore, RELMα deficiency in Ldlr−/− atherosclerotic mice results in hypercholesterolemia, while its overexpression in WT animals reduces serum cholesterol and directly induces bile acid synthases Cyp7a1 and Cyp8b1 in the liver (Lee et al., 2014). Our results of the elevated serum cholesterol and reduced hepatic Cyp8b1 expression in CD301b+MNP-depleted mice are thus consistent with these studies. While the reason for the lack of metabolic phenotype reported in Retnla−/− mice under steady-state conditions is unclear, these animals seem to have developed a compensatory mechanism to maintain normal metabolic homeostasis, as they maintain lower leptin concentrations in the sera despite their normal body weight and normal weight gain upon HFD feeding (Munitz et al., 2009). Thus, leptin sensitivity may be enhanced in Retnla−/− mice. Following acute depletion of CD301b+MNPs, or in colitic or atherosclerotic animals, the drop in RELMα concentrations has a strong impact on glucose and cholesterol metabolism, perhaps because of the host’s inability to engage compensatory mechanisms that are present in animals genetically deficient for RELMα. How RELMα secreted by CD301b+ MNP controls feeding behavior and energy balance remains to be determined.
Along with Mgl2 (CD301b), Mrc1 (CD206) and Arg1, Retnla (RELMα) mRNA expression is one of the most commonly used indicators of the M2 activation status. Indeed, all of these genes are highly inducible by IL-4 stimulation in peritoneal macrophages in an IL-4R-dependent manner (Raes et al., 2005; Raes et al., 2002). The expression of Retnla in MNPs in vivo seems to be dependent on IL-4R signaling cascade (Knipper et al., 2015; Pesce et al., 2009), and our data show that CD301b+MNPs are required to maintain systemic RELMα protein concentrations in vivo. However, the depletion of CD301b+MNPs results in a selective loss of certain M2 marker genes such as Retnla but not all (those that were not lost include Mrc1 and Arg1), reflecting the heterogeneity within the so-called M2-like population.
Although the M1 and M2 paradigm has been helpful in our current understanding of the role of MNP subsets in metabolic homeostasis in vivo (Biswas and Mantovani, 2012; Odegaard and Chawla, 2011), CD301b+ MNP in the adipose tissue described here does not fit into either M1 or M2 classification. In vivo analyses of CD301b expression revealed that while CD301b expressing cells were uniformly CD11b+ and CD11c+ (typically considered M1 marker) in different organs, some but not all M2 markers were expressed by the same MNP subset. Consistently, phenotypic heterogeneity among M1- and M2-like populations have been previously described in both mice and humans (Li et al., 2010; Shaul et al., 2010; Wentworth et al., 2010), which collectively necessitated a major revision in the simplified M1–M2 view in describing in vivo MNP subsets (Mantovani, 2016; Martinez and Gordon, 2014; Murray et al., 2014). Nevertheless, the IL-4 receptor (IL-4R)-driven signaling cascade, a prototypical M2 differentiation driver (Stein et al., 1992), in MNPs seems to play crucial roles in regulating homeostasis under metabolic stress conditions such as over-nutrition (Kang et al., 2008; Odegaard et al., 2007; Odegaard et al., 2008) or cold exposure (Nguyen et al., 2011; Qiu et al., 2014). For instance, MNP-specific deletion of PPARγ or PPARδ, two major transcription factors downstream of the IL-4R-STAT6 axis, results in increased weight gain and insulin resistance upon HFD feeding, along with a marked reduction in M2 marker expression including the Mgl2 gene (Kang et al., 2008). In contrast, inactivating inflammatory signaling component such as IKK-β or JNK in MNPs suppresses expression of inflammatory M1 signature genes and ameliorates insulin resistance in HDF-fed mice without affecting body weight (Arkan et al., 2005; Han et al., 2013; Solinas et al., 2007). In addition, depletion of CD11c+ MNPs in bone marrow (BM) chimeric mice transplanted with Itgax-DTR BM cells also dampens the inflammatory cytokine expression and improves the insulin sensitivity in diet-induced obese mice but not in lean mice, without affecting the body weight (Patsouris et al., 2008).
Collectively, in light of our findings, these observations suggest the importance of the IL-4R signaling cascade within CD301b− M2-like MNPs in maintaining insulin sensitivity and in controlling body weight under HFD conditions. Alternatively, since Lyz2 promoter has been widely used in previous studies as an expression driver for Cre recombinase to generate MNP-specific gene-deficient mice, it is also possible that common MNP precursors that express Lyz2 promoter are involved in promoting insulin sensitivity under HFD-induced stress. In contrast, our results reveal the role of CD301b+CD11c+ MNPs in maintaining the blood glucose and the body weight in both lean and obese animals. Taken together, this study contributes to the mounting evidence for MNPs in regulating whole body metabolic homeostasis, and reveals the role of CD301b+ MNP in maintaining positive energy balance through the secretion of RELMα.
Experimental Procedures
Mice and treatments
WT C57BL/6 (WT), Mgl2-DTR (Mgl2+/DTReGFP) and homozygotic Mgl2DTReGFP/DTReGFP mice were maintained in our specific pathogen-free facility at room temperature (20–22°C). CD207-DTR mice were a gift from Daniel Kaplan and maintained in our colony. YARG mice were a gift from Ruslan Medzhitov. Adipoq−/− mice were purchased from the Jackson Laboratory and crossed with Mgl2-DTR mice. Unless otherwise stated, 3–6 month-old males were used for experiments. Where indicated, mice were fed with HFD (60% kcal% fat, D12492, Research Diets) for indicated period of time. For in vivo cell depletion, DT (0.5 μg/mouse/dose, List Biological Laboratories) was given intraperitoneally every three days for four times, then mice were sacrificed on day 10. In HFD-fed animals, DT was given in the last 10 days of the indicated HFD feeding period. For chemical sympathectomy, WT mice were injected intraperitoneally twice with 3.5 mg/mouse/dose 6-hydroxydopamine (~100 μg/g body weight, MP Biomedicals) dissolved in 0.07% (w/v) ascorbic acid (Sigma) in PBS on 1 and 3 days prior to the first DT treatment, as previously described (Riol-Blanco et al., 2014). For reconstitution of RELM α, 10 μg of bacteria-expressed RELM α (Peprotech) was injected intraperitoneally every three days for four times as previously described (Osborne et al., 2013), at the same time with the DT treatments. In general, animals with different treatments were co-housed in the same cages to avoid cage-to-cage variation. For indirect calorimetry, mice were individually housed during the 10-day DT treatment period and their metabolic activity was monitored in metabolic cages in the last five days. All animal protocols were approved by the Institutional Animal Care and Use Committee at Yale University.
Cell preparation and flow cytometry
Cells were isolated from tissues by digestion with collagenase D (Roche) and stained with monoclonal antibodies against CD11b (clone M1/70), CD11c (N418), F4/80 (BM8), MHCII (M5/114.15.2), CD206 (C068C2), CD301b (11A10-B7), Siglec F (E50–2440), CD3e (17A2), NK1.1 (PK136), CD25 (PC61), CD127 (A7R34), CD4 (GK1.5), CD8a (53–6.7), CD45 (30-F11), CD49b (DX5), and FceRI (MAR-1). All antibodies were purchased from Biolegend except anti-SglecF and anti-CD301b, which were purcahsed from BD Biosciences and prepared in-house, respectively (Kumamoto et al., 2013). For intracellular RELMα staining, cells pre-stained for MNP markers were fixed and permeabilized, then stained with biotinylated rabbit anti-mouse RELMα (Peprotech).
Gene expression analyses
Total RNA was isolated from tissues with Trizol (invitrogen) and RNeasy Mini Kit (Qiagen) and analyzed with Affymetrix Mouse Gene 2.0ST Microarray (GEO accession number for the microarray data is GSE84809). For real-time PCR, cDNA was synthesized with iScript cDNA synthesis kit (Bio-Rad). Genes were amplified with iTaq Universal SYBR Green Supermix (Bio-Rad) with the primers listed in Supplementary Information and their relative amount to Hprt was calculated.
ELISA and metabolite measurements
Serum leptin (Sigma), adiponectin (Adipogen), free and total cholesterol (BioVision), free and total T3 (Alpha Diagnostic) and corticosterone (Abnova) were measured by commercial ELISA kits. For quantifying RELM α, ELISA plates were coated with rabbit anti-murine RELM α and serum RELM α was detected by biotinylated rabbit anti-murine RELM α (all reagents are from Peprotech).
Glucose tolerance tests and hyperinsulinemic euglycemic clamps
Experiments evaluating glucose homeostasis were performed according to recommendations by the NIH-funded Mouse Metabolic Phenotyping Center (MMPC) consortium (Ayala et al., 2010). GTTs were performed by intraperitoneally injecting 1 mg/g body weight dextrose. Glucose was measured in the tail blood using a YSI Glucose Analyzer or a Breeze2 Glucometer (Bayer) and insulin was measured by radioimmunoassay (Linco). Hyperinsulinemic euglycemic clamps were performed as previously described (Jurczak et al., 2012).
c-Fos staining in the brain
Under deep anesthesia, mice were perfused with a fixative containing 4% paraformaldehyde, 15% picric acid, 0.1% glutaraldehyde in 0.1 M phosphate buffer. Fifty micron sections were cut through the ARN and PBN of the brain, and every third section was used for immunostaining. Sections were stained with goat anti-c-Fos (Santa Cruz) and rabbit anti-POMC (Phoenix Pharmaceuticals).
Supplementary Material
Acknowledgments
We thank Wang Guillin and Yong Kong for microarray analysis, and Daniel Okin and Ruslan Medzhitov for critical reading of the manuscript. This study was funded by support from HHMI, and grants from NIH AI054359, MMPC NIH DK059635 and Pilot and Feasibility Project Grants from Diabetes Research Center at Yale.
Footnotes
Author Contributions
Y.K. and A.I. designed research. Y.K., M.J., J.G.C. and M.S. performed experiments. Y.K., T.H., G.S. and A.I. analyzed data. Y.K. and A.I. wrote the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arkan MC, Hevener AL, Greten FR, Maeda S, Li ZW, Long JM, Wynshaw-Boris A, Poli G, Olefsky J, Karin M. IKK-beta links inflammation to obesity-induced insulin resistance. Nat Med. 2005;11:191–198. doi: 10.1038/nm1185. [DOI] [PubMed] [Google Scholar]
- Auffray C, Fogg D, Garfa M, Elain G, Join-Lambert O, Kayal S, Sarnacki S, Cumano A, Lauvau G, Geissmann F. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science. 2007;317:666–670. doi: 10.1126/science.1142883. [DOI] [PubMed] [Google Scholar]
- Ayala JE, Samuel VT, Morton GJ, Obici S, Croniger CM, Shulman GI, Wasserman DH, McGuinness OP. Standard operating procedures for describing and performing metabolic tests of glucose homeostasis in mice. Dis Model Mech. 2010;3:525–534. doi: 10.1242/dmm.006239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas SK, Mantovani A. Orchestration of metabolism by macrophages. Cell Metab. 2012;15:432–437. doi: 10.1016/j.cmet.2011.11.013. [DOI] [PubMed] [Google Scholar]
- Blagoev B, Kratchmarova I, Nielsen MM, Fernandez MM, Voldby J, Andersen JS, Kristiansen K, Pandey A, Mann M. Inhibition of adipocyte differentiation by resistin-like molecule alpha. Biochemical characterization of its oligomeric nature. J Biol Chem. 2002;277:42011–42016. doi: 10.1074/jbc.M206975200. [DOI] [PubMed] [Google Scholar]
- Bobr A, Olvera-Gomez I, Igyarto BZ, Haley KM, Hogquist KA, Kaplan DH. Acute ablation of Langerhans cells enhances skin immune responses. J Immunol. 2010;185:4724–4728. doi: 10.4049/jimmunol.1001802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brestoff JR, Kim BS, Saenz SA, Stine RR, Monticelli LA, Sonnenberg GF, Thome JJ, Farber DL, Lutfy K, Seale P, et al. Group 2 innate lymphoid cells promote beiging of white adipose tissue and limit obesity. Nature. 2015;519:242–246. doi: 10.1038/nature14115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruss MD, Khambatta CF, Ruby MA, Aggarwal I, Hellerstein MK. Calorie restriction increases fatty acid synthesis and whole body fat oxidation rates. Am J Physiol Endocrinol Metab. 2010;298:E108–116. doi: 10.1152/ajpendo.00524.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byles V, Covarrubias AJ, Ben-Sahra I, Lamming DW, Sabatini DM, Manning BD, Horng T. The TSC-mTOR pathway regulates macrophage polarization. Nat Commun. 2013;4:2834. doi: 10.1038/ncomms3834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chawla A, Nguyen KD, Goh YP. Macrophage-mediated inflammation in metabolic disease. Nat Rev Immunol. 2011;11:738–749. doi: 10.1038/nri3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denda-Nagai K, Aida S, Saba K, Suzuki K, Moriyama S, Oo-Puthinan S, Tsuiji M, Morikawa A, Kumamoto Y, Sugiura D, et al. Distribution and function of macrophage galactose-type C-type lectin 2 (MGL2/CD301b): efficient uptake and presentation of glycosylated antigens by dendritic cells. J Biol Chem. 2010;285:19193–19204. doi: 10.1074/jbc.M110.113613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng B, Jiao P, Nie Y, Kim T, Jun D, van Rooijen N, Yang Z, Xu H. Clodronate liposomes improve metabolic profile and reduce visceral adipose macrophage content in diet-induced obese mice. PLoS One. 2011;6:e24358. doi: 10.1371/journal.pone.0024358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujisaka S, Usui I, Bukhari A, Ikutani M, Oya T, Kanatani Y, Tsuneyama K, Nagai Y, Takatsu K, Urakaze M, et al. Regulatory mechanisms for adipose tissue M1 and M2 macrophages in diet-induced obese mice. Diabetes. 2009;58:2574–2582. doi: 10.2337/db08-1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han MS, Jung DY, Morel C, Lakhani SA, Kim JK, Flavell RA, Davis RJ. JNK expression by macrophages promotes obesity-induced insulin resistance and inflammation. Science. 2013;339:218–222. doi: 10.1126/science.1227568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holcomb IN, Kabakoff RC, Chan B, Baker TW, Gurney A, Henzel W, Nelson C, Lowman HB, Wright BD, Skelton NJ, et al. FIZZ1, a novel cysteine-rich secreted protein associated with pulmonary inflammation, defines a new gene family. EMBO J. 2000;19:4046–4055. doi: 10.1093/emboj/19.15.4046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- Ji Y, Sun S, Xu A, Bhargava P, Yang L, Lam KS, Gao B, Lee CH, Kersten S, Qi L. Activation of natural killer T cells promotes M2 Macrophage polarization in adipose tissue and improves systemic glucose tolerance via interleukin-4 (IL-4)/STAT6 protein signaling axis in obesity. J Biol Chem. 2012;287:13561–13571. doi: 10.1074/jbc.M112.350066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung S, Unutmaz D, Wong P, Sano G, De los Santos K, Sparwasser T, Wu S, Vuthoori S, Ko K, Zavala F, et al. In vivo depletion of CD11c+ dendritic cells abrogates priming of CD8+ T cells by exogenous cell-associated antigens. Immunity. 2002;17:211–220. doi: 10.1016/s1074-7613(02)00365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurczak MJ, Lee AH, Jornayvaz FR, Lee HY, Birkenfeld AL, Guigni BA, Kahn M, Samuel VT, Glimcher LH, Shulman GI. Dissociation of inositol-requiring enzyme (IRE1alpha)-mediated c-Jun N-terminal kinase activation from hepatic insulin resistance in conditional X-box-binding protein-1 (XBP1) knock-out mice. J Biol Chem. 2012;287:2558–2567. doi: 10.1074/jbc.M111.316760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanda H, Tateya S, Tamori Y, Kotani K, Hiasa K, Kitazawa R, Kitazawa S, Miyachi H, Maeda S, Egashira K, et al. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest. 2006;116:1494–1505. doi: 10.1172/JCI26498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang K, Reilly SM, Karabacak V, Gangl MR, Fitzgerald K, Hatano B, Lee CH. Adipocyte-derived Th2 cytokines and myeloid PPARdelta regulate macrophage polarization and insulin sensitivity. Cell Metab. 2008;7:485–495. doi: 10.1016/j.cmet.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knipper JA, Willenborg S, Brinckmann J, Bloch W, Maass T, Wagener R, Krieg T, Sutherland T, Munitz A, Rothenberg ME, et al. Interleukin-4 Receptor alpha Signaling in Myeloid Cells Controls Collagen Fibril Assembly in Skin Repair. Immunity. 2015;43:803–816. doi: 10.1016/j.immuni.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumamoto Y, Denda-Nagai K, Aida S, Higashi N, Irimura T. MGL2 Dermal dendritic cells are sufficient to initiate contact hypersensitivity in vivo. PLoS One. 2009;4:e5619. doi: 10.1371/journal.pone.0005619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumamoto Y, Linehan M, Weinstein JS, Laidlaw BJ, Craft JE, Iwasaki A. CD301b(+) dermal dendritic cells drive T helper 2 cell-mediated immunity. Immunity. 2013;39:733–743. doi: 10.1016/j.immuni.2013.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BC, Kim MS, Pae M, Yamamoto Y, Eberle D, Shimada T, Kamei N, Park HS, Sasorith S, Woo JR, et al. Adipose Natural Killer Cells Regulate Adipose Tissue Macrophages to Promote Insulin Resistance in Obesity. Cell Metab. 2016;23:685–698. doi: 10.1016/j.cmet.2016.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MR, Lim CJ, Lee YH, Park JG, Sonn SK, Lee MN, Jung IH, Jeong SJ, Jeon S, Lee M, et al. The adipokine Retnla modulates cholesterol homeostasis in hyperlipidemic mice. Nat Commun. 2014;5:4410. doi: 10.1038/ncomms5410. [DOI] [PubMed] [Google Scholar]
- Li P, Fan W, Xu J, Lu M, Yamamoto H, Auwerx J, Sears DD, Talukdar S, Oh D, Chen A, et al. Adipocyte NCoR knockout decreases PPARgamma phosphorylation and enhances PPARgamma activity and insulin sensitivity. Cell. 2011;147:815–826. doi: 10.1016/j.cell.2011.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Lu M, Nguyen MT, Bae EJ, Chapman J, Feng D, Hawkins M, Pessin JE, Sears DD, Nguyen AK, et al. Functional heterogeneity of CD11c-positive adipose tissue macrophages in diet-induced obese mice. J Biol Chem. 2010;285:15333–15345. doi: 10.1074/jbc.M110.100263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao X, Sharma N, Kapadia F, Zhou G, Lu Y, Hong H, Paruchuri K, Mahabeleshwar GH, Dalmas E, Venteclef N, et al. Kruppel-like factor 4 regulates macrophage polarization. J Clin Invest. 2011;121:2736–2749. doi: 10.1172/JCI45444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch L, Nowak M, Varghese B, Clark J, Hogan AE, Toxavidis V, Balk SP, O’Shea D, O’Farrelly C, Exley MA. Adipose tissue invariant NKT cells protect against diet-induced obesity and metabolic disorder through regulatory cytokine production. Immunity. 2012;37:574–587. doi: 10.1016/j.immuni.2012.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani A. Reflections on immunological nomenclature: in praise of imperfection. Nat Immunol. 2016;17:215–216. doi: 10.1038/ni.3354. [DOI] [PubMed] [Google Scholar]
- Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep. 2014;6:13. doi: 10.12703/P6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez FO, Helming L, Gordon S. Alternative activation of macrophages: an immunologic functional perspective. Annu Rev Immunol. 2009;27:451–483. doi: 10.1146/annurev.immunol.021908.132532. [DOI] [PubMed] [Google Scholar]
- Mathis D. Immunological goings-on in visceral adipose tissue. Cell Metab. 2013;17:851–859. doi: 10.1016/j.cmet.2013.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molofsky AB, Nussbaum JC, Liang HE, Van Dyken SJ, Cheng LE, Mohapatra A, Chawla A, Locksley RM. Innate lymphoid type 2 cells sustain visceral adipose tissue eosinophils and alternatively activated macrophages. J Exp Med. 2013;210:535–549. doi: 10.1084/jem.20121964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munitz A, Seidu L, Cole ET, Ahrens R, Hogan SP, Rothenberg ME. Resistin-like molecule alpha decreases glucose tolerance during intestinal inflammation. J Immunol. 2009;182:2357–2363. doi: 10.4049/jimmunol.0803130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munitz A, Waddell A, Seidu L, Cole ET, Ahrens R, Hogan SP, Rothenberg ME. Resistin-like molecule alpha enhances myeloid cell activation and promotes colitis. J Allergy Clin Immunol. 2008;122:1200–1207. e1201. doi: 10.1016/j.jaci.2008.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, Gordon S, Hamilton JA, Ivashkiv LB, Lawrence T, et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41:14–20. doi: 10.1016/j.immuni.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair MG, Du Y, Perrigoue JG, Zaph C, Taylor JJ, Goldschmidt M, Swain GP, Yancopoulos GD, Valenzuela DM, Murphy A, et al. Alternatively activated macrophage-derived RELM-{alpha} is a negative regulator of type 2 inflammation in the lung. J Exp Med. 2009;206:937–952. doi: 10.1084/jem.20082048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair MG, Guild KJ, Artis D. Novel effector molecules in type 2 inflammation: lessons drawn from helminth infection and allergy. J Immunol. 2006;177:1393–1399. doi: 10.4049/jimmunol.177.3.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen KD, Qiu Y, Cui X, Goh YP, Mwangi J, David T, Mukundan L, Brombacher F, Locksley RM, Chawla A. Alternatively activated macrophages produce catecholamines to sustain adaptive thermogenesis. Nature. 2011;480:104–108. doi: 10.1038/nature10653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odegaard JI, Chawla A. Alternative macrophage activation and metabolism. Annu Rev Pathol. 2011;6:275–297. doi: 10.1146/annurev-pathol-011110-130138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odegaard JI, Ricardo-Gonzalez RR, Goforth MH, Morel CR, Subramanian V, Mukundan L, Red Eagle A, Vats D, Brombacher F, Ferrante AW, et al. Macrophage-specific PPARgamma controls alternative activation and improves insulin resistance. Nature. 2007;447:1116–1120. doi: 10.1038/nature05894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odegaard JI, Ricardo-Gonzalez RR, Red Eagle A, Vats D, Morel CR, Goforth MH, Subramanian V, Mukundan L, Ferrante AW, Chawla A. Alternative M2 activation of Kupffer cells by PPARdelta ameliorates obesity-induced insulin resistance. Cell Metab. 2008;7:496–507. doi: 10.1016/j.cmet.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohmura K, Ishimori N, Ohmura Y, Tokuhara S, Nozawa A, Horii S, Andoh Y, Fujii S, Iwabuchi K, Onoe K, et al. Natural killer T cells are involved in adipose tissues inflammation and glucose intolerance in diet-induced obese mice. Arterioscler Thromb Vasc Biol. 2010;30:193–199. doi: 10.1161/ATVBAHA.109.198614. [DOI] [PubMed] [Google Scholar]
- Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol. 2010;72:219–246. doi: 10.1146/annurev-physiol-021909-135846. [DOI] [PubMed] [Google Scholar]
- Osborne LC, Joyce KL, Alenghat T, Sonnenberg GF, Giacomin PR, Du Y, Bergstrom KS, Vallance BA, Nair MG. Resistin-like molecule alpha promotes pathogenic Th17 cell responses and bacterial-induced intestinal inflammation. J Immunol. 2013;190:2292–2300. doi: 10.4049/jimmunol.1200706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011;11:85–97. doi: 10.1038/nri2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patsouris D, Li PP, Thapar D, Chapman J, Olefsky JM, Neels JG. Ablation of CD11c-positive cells normalizes insulin sensitivity in obese insulin resistant animals. Cell Metab. 2008;8:301–309. doi: 10.1016/j.cmet.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesce JT, Ramalingam TR, Wilson MS, Mentink-Kane MM, Thompson RW, Cheever AW, Urban JF, Jr, Wynn TA. Retnla (relmalpha/fizz1) suppresses helminth-induced Th2-type immunity. PLoS Pathog. 2009;5:e1000393. doi: 10.1371/journal.ppat.1000393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu Y, Nguyen KD, Odegaard JI, Cui X, Tian X, Locksley RM, Palmiter RD, Chawla A. Eosinophils and type 2 cytokine signaling in macrophages orchestrate development of functional beige fat. Cell. 2014;157:1292–1308. doi: 10.1016/j.cell.2014.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raes G, Brys L, Dahal BK, Brandt J, Grooten J, Brombacher F, Vanham G, Noel W, Bogaert P, Boonefaes T, et al. Macrophage galactose-type C-type lectins as novel markers for alternatively activated macrophages elicited by parasitic infections and allergic airway inflammation. J Leukoc Biol. 2005;77:321–327. doi: 10.1189/jlb.0304212. [DOI] [PubMed] [Google Scholar]
- Raes G, De Baetselier P, Noel W, Beschin A, Brombacher F, Hassanzadeh Gh G. Differential expression of FIZZ1 and Ym1 in alternatively versus classically activated macrophages. J Leukoc Biol. 2002;71:597–602. [PubMed] [Google Scholar]
- Reese TA, Liang HE, Tager AM, Luster AD, Van Rooijen N, Voehringer D, Locksley RM. Chitin induces accumulation in tissue of innate immune cells associated with allergy. Nature. 2007;447:92–96. doi: 10.1038/nature05746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riol-Blanco L, Ordovas-Montanes J, Perro M, Naval E, Thiriot A, Alvarez D, Paust S, Wood JN, von Andrian UH. Nociceptive sensory neurons drive interleukin-23-mediated psoriasiform skin inflammation. Nature. 2014;510:157–161. doi: 10.1038/nature13199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen ED, Spiegelman BM. Adipocytes as regulators of energy balance and glucose homeostasis. Nature. 2006;444:847–853. doi: 10.1038/nature05483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh M, Andoh Y, Clingan CS, Ogura H, Fujii S, Eshima K, Nakayama T, Taniguchi M, Hirata N, Ishimori N, et al. Type II NKT cells stimulate diet-induced obesity by mediating adipose tissue inflammation, steatohepatitis and insulin resistance. PLoS One. 2012;7:e30568. doi: 10.1371/journal.pone.0030568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schipper HS, Rakhshandehroo M, van de Graaf SF, Venken K, Koppen A, Stienstra R, Prop S, Meerding J, Hamers N, Besra G, et al. Natural killer T cells in adipose tissue prevent insulin resistance. J Clin Invest. 2012;122:3343–3354. doi: 10.1172/JCI62739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaul ME, Bennett G, Strissel KJ, Greenberg AS, Obin MS. Dynamic, M2-like remodeling phenotypes of CD11c+ adipose tissue macrophages during high-fat diet--induced obesity in mice. Diabetes. 2010;59:1171–1181. doi: 10.2337/db09-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solinas G, Vilcu C, Neels JG, Bandyopadhyay GK, Luo JL, Naugler W, Grivennikov S, Wynshaw-Boris A, Scadeng M, Olefsky JM, et al. JNK1 in hematopoietically derived cells contributes to diet-induced inflammation and insulin resistance without affecting obesity. Cell Metab. 2007;6:386–397. doi: 10.1016/j.cmet.2007.09.011. [DOI] [PubMed] [Google Scholar]
- Stein M, Keshav S, Harris N, Gordon S. Interleukin 4 potently enhances murine macrophage mannose receptor activity: a marker of alternative immunologic macrophage activation. J Exp Med. 1992;176:287–292. doi: 10.1084/jem.176.1.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steppan CM, Bailey ST, Bhat S, Brown EJ, Banerjee RR, Wright CM, Patel HR, Ahima RS, Lazar MA. The hormone resistin links obesity to diabetes. Nature. 2001a;409:307–312. doi: 10.1038/35053000. [DOI] [PubMed] [Google Scholar]
- Steppan CM, Brown EJ, Wright CM, Bhat S, Banerjee RR, Dai CY, Enders GH, Silberg DG, Wen X, Wu GD, et al. A family of tissue-specific resistin-like molecules. Proc Natl Acad Sci USA. 2001b;98:502–506. doi: 10.1073/pnas.98.2.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strodthoff D, Lundberg AM, Agardh HE, Ketelhuth DF, Paulsson-Berne G, Arner P, Hansson GK, Gerdes N. Lack of invariant natural killer T cells affects lipid metabolism in adipose tissue of diet-induced obese mice. Arterioscler Thromb Vasc Biol. 2013;33:1189–1196. doi: 10.1161/ATVBAHA.112.301105. [DOI] [PubMed] [Google Scholar]
- Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisberg SP, Hunter D, Huber R, Lemieux J, Slaymaker S, Vaddi K, Charo I, Leibel RL, Ferrante AW., Jr CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J Clin Invest. 2006;116:115–124. doi: 10.1172/JCI24335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wensveen FM, Jelencic V, Valentic S, Sestan M, Wensveen TT, Theurich S, Glasner A, Mendrila D, Stimac D, Wunderlich FT, et al. NK cells link obesity-induced adipose stress to inflammation and insulin resistance. Nat Immunol. 2015;16:376–385. doi: 10.1038/ni.3120. [DOI] [PubMed] [Google Scholar]
- Wentworth JM, Naselli G, Brown WA, Doyle L, Phipson B, Smyth GK, Wabitsch M, O’Brien PE, Harrison LC. Pro-inflammatory CD11c+CD206+ adipose tissue macrophages are associated with insulin resistance in human obesity. Diabetes. 2010;59:1648–1656. doi: 10.2337/db09-0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D, Molofsky AB, Liang HE, Ricardo-Gonzalez RR, Jouihan HA, Bando JK, Chawla A, Locksley RM. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science. 2011;332:243–247. doi: 10.1126/science.1201475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, Parekh VV, Gabriel CL, Bracy DP, Marks-Shulman PA, Tamboli RA, Kim S, Mendez-Fernandez YV, Besra GS, Lomenick JP, et al. Activation of invariant natural killer T cells by lipid excess promotes tissue inflammation, insulin resistance, and hepatic steatosis in obese mice. Proc Natl Acad Sci USA. 2012;109:E1143–1152. doi: 10.1073/pnas.1200498109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wueest S, Item F, Boyle CN, Jirkof P, Cesarovic N, Ellingsgaard H, Boni-Schnetzler M, Timper K, Arras M, Donath MY, et al. Interleukin-6 contributes to early fasting-induced free fatty acid mobilization in mice. Am J Physiol Regul Integr Comp Physiol. 2014;306:R861–867. doi: 10.1152/ajpregu.00533.2013. [DOI] [PubMed] [Google Scholar]
- Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.