Abstract
In recent decades, there has been a growing interest in the assessment of patients in altered states of consciousness. There is a need for accurate and early prediction of awakening and recovery from coma. Neurophysiological assessment of coma was once restricted to brainstem auditory and primary cortex somatosensory evoked potentials elicited in the thirty millisecond range, which have both shown good predictive value for poor coma outcome only. In this paper, we review how passive auditory oddball paradigms including deviant and novel sounds have proved their efficiency in assessing brain function at a higher level, without requiring the patient’s active involvement, thus providing an enhanced tool for the prediction of coma outcome. The presence of an MMN in response to deviant stimuli highlights preserved automatic sensory memory processes. Recorded during coma, MMN has shown high specificity as a predictor of recovery of consciousness. The presence of a novelty P3 in response to the subject’s own first name presented as a novel (rare) stimulus has shown a good correlation with coma awakening. There is now a growing interest in the search for markers of consciousness, if there are any, in unresponsive patients (chronic vegetative or minimally conscious states). We discuss the different ERP patterns observed in these patients. The presence of novelty P3, including parietal components and possibly followed by a late parietal positivity, raises the possibility that some awareness processes are at work in these unresponsive patients.
Introduction
Coma is a severe medical condition defined by loss of consciousness and absence of reactivity to external stimuli. It is a disorder of consciousness following severe brain damage, mainly traumatic head injury or anoxic encephalopathy due to cardiac arrest for example. Other causes may be vascular, metabolic, or infectious diseases. Coma can last for a maximum of one month (Bernat 2006), during which time it is difficult to predict outcome. Patients who do not die during this acute phase evolve toward a vegetative state, a clinical condition in which they open their eyes, appear to be awake, but do not communicate and show no objective signs of awareness. This vegetative phase may be transient or permanent. In most cases, patients return to full conscious awareness, some sooner than others, and some with more severe disabilities than others, depending on the case. In some cases the vegetative state may last for months or years and the patient never recovers. For a long time, the outcome of coma has been scored using the Glasgow Outcome Scale (GOS), which includes death, vegetative state, severe disability, moderate disability and good recovery (Jennett and Bond 1975). The more recently identified category of minimally conscious states (MCS) may be added to these 5 categories, and is characterized by the presence of non-reflex or voluntary movements (Giacino et al. 2002).
For therapeutic, ethical, and economic reasons, predicting the functional outcome of patients as soon and as reliably as possible is an important aspect of patient care. The salient questions are: will the patient awaken from coma or not? If the patient awakens, will he or she be disabled or not? Coma outcome mainly depends on the actual functional state of the patient following brain injury, and also on the cause of the coma. In clinical practice during coma, outcome prognosis is mainly based on a set of clinical observations and on neuroimaging and electrophysiological techniques. In terms of clinical variables, the Glasgow Coma Scale (GCS), which is based on eye, verbal, and motor responses (Teasdale and Jennett 1974) and pupillary response are the strongest predictive measures (Marmarou et al. 2007; Mushkudiani et al. 2008; Lee et al. 2010). In routine practice, electrophysiological scores are based on the classical resting state EEG and on evoked responses to sensory stimulations. Early-evoked potentials like primary somatosensory responses in the 30-millisecond range and brainstem auditory potentials have been used for more than two decades due to their high predictive value for poor coma outcome. Indeed, in anoxic coma the abolition of somatosensory evoked potentials (SEP) is related to a poor outcome, defined as death or survival in a vegetative state, with 100 % specificity (Madl et al. 1996; Zandbergen et al. 1998; Robinson et al. 2003; Fischer et al. 2006). Following traumatic brain injury, the predictive value of SEP abolition for unfavorable outcome is 98.5 % when there are no focal injuries likely to abolish their cortical components (Fischer and Luaute 2005). Conversely, the presence of early-evoked potentials provides evidence of the integrity of sensory pathways and primary sensory cortices, but it does not serve as a guarantee of good coma outcome. Long-latency event-related potentials (ERPs) have been more recently introduced for coma prognosis, on the basis that these cortical markers of cognitive brain function could be useful in detecting patients prone to favorable outcome (Lew et al. 2006).
Even in the absence of explicit instructions to the subject (or when the subject’s attention is diverted by a primary task), appropriate auditory paradigms elicit long-latency ERPs that provide information on several cortical processing stages. On this basis, we developed a passive novelty oddball paradigm (Holeckova et al. 2006), which highlights successive stages of auditory processing. In this paradigm, repetitive standard tones are interspersed with slightly deviant stimuli (shorter duration deviants, occurring 15 % of the time) and rare salient novel sounds (the subject’s own name, 4 % of the time). This paradigm assesses 1) the encoding of acoustic inputs in the auditory cortex as indexed by the obligatory sensory response (N1) evoked by each tone in the sequence, 2) their representation in auditory sensory memory as indexed by the automatic response to deviant tones (Mismatch Negativity or MMN), and 3) the orienting of attention indexed by the automatic response to rare and salient stimuli (novelty P3).
The MMN and novelty P3 ERP components provide objective information on the functional state of the patient’s cortex without requiring his or her active participation. We have extensively investigated oddball paradigms in healthy subjects during wakefulness (Holeckova et al. 2006; Eichenlaub et al. 2012) and during sleep (Ruby et al. 2008; Eichenlaub et al. 2013). In this review we will show how MMN and novelty P3 have proved their usefulness in predicting coma outcome when they are detected in comatose patients. The prognostic power of a component is assessed through sensitivity, specificity, and positive and negative predictive values. Sensitivity for good outcome is assessed through the proportion of patients who display the component among the patients with good outcome; specificity is the proportion of patients who do not show the component among the patients with poor outcome. Predictive values are also often used, although they depend on the prevalence of good outcomes in the study population. The positive predictive value is the proportion of patients who actually show good outcome among the patients who display the component; the negative predictive value is the proportion of patients who do not show good outcome among the patients who do not display the component.
Firstly for N1 and MMN, and then for novelty P3, we will describe the patterns of responses in healthy subjects and give some recommendations for their recording in coma patients. We will then review the studies that have investigated their predictive power for awakening from comas of different etiologies. Finally, we will discuss the value of these passively obtained ERP components as markers of brain function in chronic disorders of consciousness.
N1 and MMN
Recording N1 and MMN
A very simple auditory paradigm presenting successive tones binaurally to passive subjects enables N1 and MMN elicitation. Classically, tone bursts (including one or more sine curves) with a duration of around 30 to 100 ms and short rise and fall times (5 to 10 ms) are used. The interval between successive onsets, or stimulus onset asynchrony (SOA), is set to around 500 to 800 ms. A small proportion (about 15 %) of tone bursts differing from the others according to one or several features (duration, intensity, or pitch) are randomly introduced into the sequence; these oddball stimuli are called Deviants.
Investigated for a long time as “vertex potential”, the N1 is evoked by a relatively abrupt change in the level of energy impinging on the sensory receptors (Näätänen and Picton 1987). Due to short rise times, the tones (Standards and Deviants) elicit an N1, which appears as a large negative deflection at central sites around 100 ms after stimulus onset. Several generators have been shown to contribute to the scalp-recorded N1. The obligatory sensory N1 response is generated in the auditory cortex, as attested by an inversion of the potential (i.e. a positive deflection) at the mastoids when the reference electrode is placed on the tip of the subject’s nose.
Described 35 years ago by Näätänen et al. (1978), the MMN is elicited by Deviants, generated by automatic change detection processes, in which the current auditory input is found to differ from the representation of the preceding auditory stimuli. It can be detected even if subjects are not aware of the auditory changes, but only if the deviance exceeds the subject’s discrimination threshold (Sams et al. 1985). It appears around 120 ms after a detectable change in the stimulation. It is identified in the deviance-specific response, i.e. in the difference curve between the responses to Deviants and Standards, as a fronto-central negative wave associated with positive deflections at both mastoids (with nose reference). The scalp topography of MMN varies slightly according to the type of stimulus deviance, suggesting that MMNs for different attributes originate, at least in part, from distinct neuronal populations in the auditory cortex (Giard et al. 1995). Among other deviances, duration decrement MMN shows the most replicable measures in healthy subjects (Tervaniemi et al. 1999). The magnitude of deviance to be used in clinical practice results from a compromise. It should be large enough to elicit mismatch processes in all patients (Sams et al. 1985). However, an excessive magnitude of deviance leads to dissimilar sensory N1s to Standards and Deviants, and may distort MMN results. We used shorter Deviants than Standards, namely 75 ms Standards and 30 ms Deviants, the SOA lasting 610 ms (Fischer et al. 1999). In a series of 45 patients and 15 healthy subjects, we presented these long and short stimuli alternately as Standards or Deviants in successive blocks of stimuli (“classical” blocks with long Standards and short Deviants, and other blocks with short Standards and long Deviants). An MMN unspoiled by physical differences between standard and deviant stimuli was obtained by subtracting the ERP for 30 ms Standards from the ERP for 30 ms Deviants (“true” MMN). We compared this “true” MMN with the MMN “classically” obtained in the “classical” blocks. All healthy subjects and 15 of the patients showed a “classical” MMN and a “true” MMN at the same latency, suggesting that the MMN obtained from 75 ms standards and 30 ms deviants actually discloses mismatch processes (Morlet and Fischer 2001). In coma populations, MMNs to frequency deviance are also used. In this case, complex musical tones are preferred to pure sinusoidal tones, as demonstrated in a study of 79 patients with extremely diffuse brain injuries (Kotchoubey et al. 2003).
In the Intensive Care Unit (ICU), a reduced number of electrodes are used for practical reasons. To record N1 and MMN, electrodes placed at medial frontal and central sites (Fz and Cz) are compulsory; electrodes placed laterally (e.g. F3, F4, C3, C4) may be useful, firstly due to a slight right preponderance of MMN scalp topography, but also due to distorted topographies observed in some patients, for example after brain trauma or after decompression surgery. It is strongly recommended to record responses at the mastoids (either M1 and M2 or both mastoids linked), the reference electrode being placed on the tip of the nose, because an inversion at the mastoids provides an additional clue for N1 and MMN detection. If recording at the mastoids is not feasible, linked ears may be chosen as the reference, which enhances negative potentials at fronto-central sites as compared with the nose reference condition.
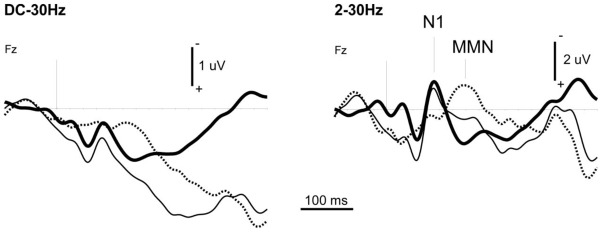
A major concern in routine neurophysiological investigation is the interpretation of individual pathological data recorded in the ICU. Electrical surroundings (other equipment close to the patient) or patient condition (muscle artifacts) can blur wave detection. This is especially true regarding the MMN, which shows a poor signal-to-noise ratio. At least 250 Deviants should be averaged after careful artifact rejection. Low-pass filtering (20 Hz or 30 Hz) is recommended to smooth averaged data and we advise an additional high-pass filter (2 Hz or 3 Hz) to better highlight N1 and MMN components in pathological recordings (Figure 1). Ideally, wave detection is performed in two steps. The first step involves visual inspection by a skilled neurophysiologist, mainly based on response coherency gauging (e.g. detect typical response patterns at expected latencies, rule out residual EEG slow waves in the response). In the second step, a statistical approach based on the responses to single events validates the consistency of visually detected waves. The two steps may also be inverted, waves complying with statistical criteria being submitted to the neurophysiologist. However, with regard to event related potentials in coma patients, visual analysis cannot be omitted, because some key items of the patient’s history (e.g. surgery) need to be taken into account for data reading. Most statistical methods for objective wave detection are based on the comparison of single-trial responses with baseline (e.g. a negative deflection for N1), or the comparison between single-trial responses to different stimuli (e.g. between Standards and Deviants for MMN). In both comatose patients and healthy sleeping subjects (Ruby et al. 2008), N1 may appear as a small inflection toward negativity in more positive potentials. In most cases, high-pass filtering turns this N1 pattern into a genuine negative wave, as illustrated in Figure 1. We also proposed an original detection method based on cross-correlating odd and even responses in a window centered on the visually detected wave (Fischer et al. 2008; Fischer et al. 1999).
Figure 1.
Example of the usefulness of high-pass filtering to properly detect N1 and MMN in coma recordings. The data were recorded in a patient during the first week of a coma of vascular origin using a classical oddball paradigm (standard tone-bursts 75 ms; deviant tone-bursts 30 ms, probability 0.15; stimulus onset asynchrony 610 ms).
Averaged response to Standards (thick line, 1145 events averaged), to Deviants (thin line, 242 events averaged) and deviance-specific response (Deviants minus Standards, dotted line) at the frontal electrode Fz.
Left panel: 30 Hz low-pass filter (Butterworth, order 6). Right panel: with additional 2 Hz high-pass filter (Butterworth, order 4).
Alternatively, a comprehensive assessment of sensory and mismatch processes may be provided without classical ERP component recognition. Two recent studies have introduced multivariate algorithms that quantify the performance of individual patients in differentiating between standard and deviant stimuli in mismatch paradigms (King et al. 2013; Tzovara et al. 2013). Such methods provide each patient’s decoding accuracy without any a priori hypothesis about N1 and MMN responses. Based on multivariate classifiers, they take advantage of a large number of electrodes.
N1 and MMN during coma: predictive value for awakening
In 1993 Kane et al. published a short report of the first study investigating the prognostic value of MMN in coma patients (Kane et al. 1993). It dealt with a series of 18 patients in coma after blunt head trauma (GCS ≤ 7 at the time of recording). Frequency MMN (90 % of the tones at 800 Hz and 10 % at 1600 Hz) was recorded every 3–7 days up to the return to consciousness. Normative values had been established in 10 healthy volunteers, who all showed MMN responses. In each patient who recovered, MMN was present in the recording that preceded the comprehension of simple commands (GCS ≥ 10). Kane concluded that the MMN response could be the earliest available indicator of awakening from coma and might reflect the recovery of neurochemical mechanisms for information processing in the cerebral cortex essential to cognition. Three years later, the same team (Kane et al. 1996) published a larger series including 54 comatose traumatic brain injury patients. The main outcome measure was GOS assessed 3 months after coma onset. Electrophysiological recordings were repeatedly carried out. The best response obtained while the patient was still in coma was retained. The presence of MMN predicted the return to consciousness (89.7 % sensitivity and 100 % specificity) and preceded changes in GCS.
Our own contribution to the assessment of N1 and MMN ability to predict awakening from coma included 3 studies (Fischer et al. 1999; Fischer et al. 2004; Fischer et al. 2006) using duration MMN (86 % of the tones lasting 75 ms and 14 % of the tones lasting 30 ms). We had previously tested this paradigm in a series of 52 healthy subjects using both visual analysis and objective confirmation based on cross-correlation of odd and even responses. N1 and MMN had been successfully detected in all healthy subjects (Fischer et al. 1999).
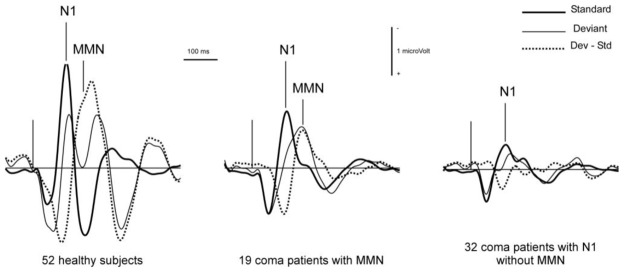
In 1999, our first exploratory series included 128 comatose patients (Fischer et al. 1999). The causes of coma were: head injury (34 cases), cardiac and respiratory failure (19 cases), stroke (41 cases), complications of neurosurgery (31 cases) and encephalitis (3 cases). The recordings took place on average 8 days after coma onset. An N1 was observed in 84 patients, and an MMN in only 33 patients. MMN was never found when N1 was absent. Both components detected in comatose patients showed pathological patterns, with significantly lower amplitudes than in healthy subjects (Figure 2). The patients were evaluated three months after coma onset using standard GOS criteria. The patients with GOS levels 1 and 2 (death and vegetative state) were classified as “non-awake”. By 3 months after coma onset, 95 patients had awakened and 33 had not, including 22 who had died. MMN showed a poor sensitivity to awakening, but a good specificity. Thirty of the 95 patients who had regained consciousness showed an MMN (31.6 % sensitivity) and 30 of the 33 patients who were considered as non-awake showed no MMN (90.9 % specificity). Out of the 33 patients in whom MMN was detected, 30 returned to consciousness, which gives a positive predictive value of 90.9 % in this coma population. The presence of N1 irrespective of MMN showed a better sensitivity (73.7 %) and a lower specificity (57.6 %). As a whole, the percentage of patients with MMNs in our population was lower than in Kane’s studies. This discrepancy could be explained by different methods of identification, but particularly by the fact that in Kane’s studies recordings were repeated until the return to consciousness, thus increasing the chances of observing MMN. In our study, ERPs were recorded during the acute phase of coma, but for the patients who did awaken, the delay between recording and awakening was significantly shorter in patients showing an N1 (with MMN or not) than in patients with no N1. The shortest delays were observed when N1 and MMN were both present (Figure 3). Out of 30 patients in whom MMN was detected, 22 awoke within 7 days. Our results do not rule out the possibility of a later appearance of MMN in some patients who recovered late.
Figure 2.
Grand averages obtained from 52 healthy subjects, 33 coma patients with N1 and MMN, and 51 coma patients showing an N1 but no MMN. Responses to Standards (thick line), to Deviants (thin line) and deviance-specific response (Deviants minus Standards, dotted line). The data were recorded from a single derivation, with Fz as active electrode and the right mastoid as reference. Digital filter 3–30 Hz (Butterworth). Adapted from Fischer et al. 1999.
Figure 3.
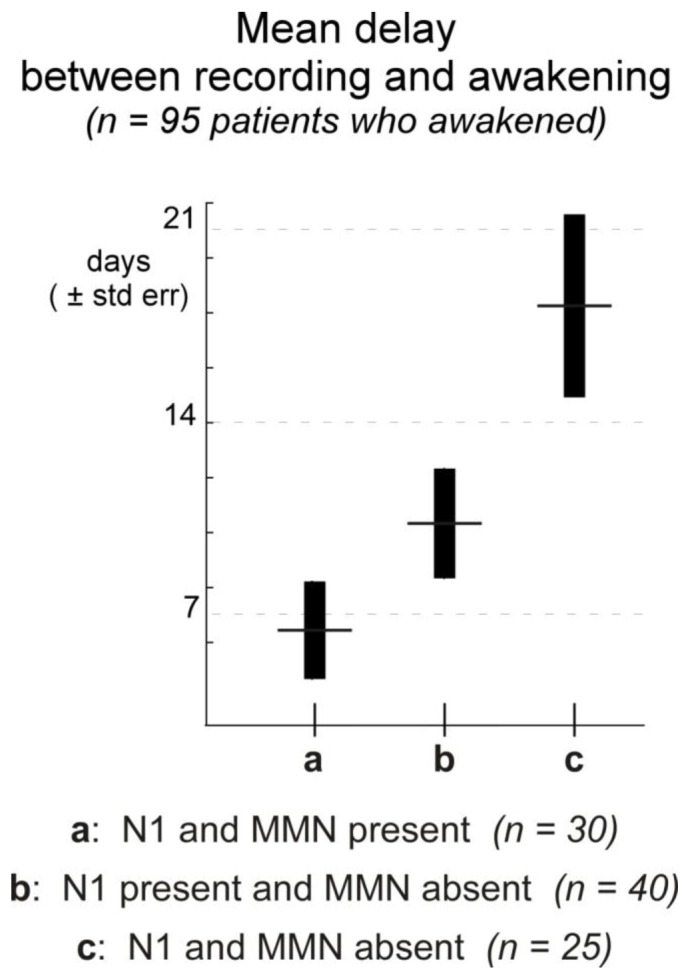
Time elapsed between recording and awakening in the 95 patients who awakened from a series of 128 patients recorded during coma, as a function of the presence of N1 and MMN at the time of recording. The delay was found to be significantly longer in patients with no N1 than in the other two populations with N1, whether MMN was present or not (Kruskal-Wallis p < 0.01). Adapted from Fischer et al. 1999.
Then, in 2004, we tried to determine the predictive role of N1 and MMN, together with brainstem auditory evoked potentials (BAEPs), middle-latency auditory evoked potentials (MLAEPs) and clinical variables recorded on average 10 days after coma onset, in a large prospective cohort including 346 consecutive patients (Fischer et al. 2004). The causes of coma were: stroke (125 cases), trauma (96 cases), anoxia (64 cases), complications of neurosurgery (54 cases) and encephalitis (7 cases). Patients were monitored for 12 months and classified as awake or non-awake. Multivariate analyses were performed using regression logistic and Cox models. The strongest predictive variable for awakening was the widely used pupillary reflex (estimated probability 80 %), immediately followed by N1 (estimated probability rose to 87 % when N1 was present) and by MLAEPs (estimated probability rose to 90 % with normal MLAEPs). This large population confirmed the good positive predictive value of MMN for awakening (when MMN was present, 88.6 % of the patients awakened). Moreover, as outcome was assessed one year after coma onset, we were able to observe that MMN precludes comatose patients from moving to a permanent vegetative state: no patient in whom MMN was present became permanently vegetative. Finally, our data confirmed that the overall outcome and prognosis for coma awakening depend on etiology: traumatic etiologies had the best chances of recovery (with possibly long-lasting recovery) while post-anoxic coma showed a globally poor outcome.
The next study was dedicated to severe anoxic coma (Fischer et al. 2006). Current trends in ethical decision-making in anoxic coma have increased the need for accurate and early prediction of reversibility or irreversibility of disorders of consciousness in patients who suffered from cardiac arrest. We gathered clinical variables and recorded the SEPs, BAEPs, MLAEPs, and the auditory event-related potentials (N1 and MMN) within an average period of 8 days after cardiac arrest. Patients were free of sedative drugs at the time of recording. The patients were monitored for 12 months and classified as awake or non-awake (permanent vegetative state or death). All patients in whom SEPs were abolished, or MLAEPs abolished, or BAEPs abnormal, did not awaken (100 % negative predictive value for SEPs, MLAEPs and BAEPs). In contrast, all patients in whom MMN was present awakened (100 % positive predictive value). A tree-based classification was performed. On the decision tree, the awakening/non-awakening explicative variables were, by order of importance, MMN, pupillary reactivity and SEPs. So, a prognostic tree for the prediction of awakening in severe anoxic coma could be designed, predicting awakening with certainty when MMN is present and non-awakening when MMN and pupillary light reflex are absent or SEPs abolished.
In 2005 Naccache et al. (2005) closely replicated our results. They recorded frequency MMN (85 % of trials at 1 kHz and 15 % at 2 kHz) in 30 comatose patients suffering from various causes of coma. All patients were free of sedative drugs. The delay between coma onset and recording was 25 days. Clinical outcome was evaluated at 1 month after MMN recordings. From the 30 patients, 10 had an MMN, 9 of them awakened, and the last one died from an unrelated event. Within the group without MMN (20 patients), 13 died or progressed to a vegetative state while 7 of them awakened. In this study, MMN predicted awakening with a specificity of 93 %, a sensitivity of 56 % and a positive predictive value of 90 %, therefore further highlighting the usefulness of bedside recording of auditory ERPs to predict awakening in comatose patients.
A recent study (Tzovara et al. 2013) investigated mismatch discrimination (with pitch, duration and location deviants) in 30 comatose patients early after cardiac arrest using a multivariate decoding algorithm. The data were recorded twice in a row, within 24 h after coma onset and under mild therapeutic hypothermia, and after 1 day and under normothermic conditions. Decoding accuracy in discriminating sounds (with no distinction of sensory and mismatch processes) was found independent of patients’ chances of survival in both recordings. However, the progression of auditory discrimination between the first and second recordings was informative; all patients with an improvement in the decoding performance from therapeutic hypothermia to normothermic conditions awoke from coma and survived at 3 months.
N1 and MMN during coma: Predictive value for functional outcome
The prediction of functional outcome months after coma is a critical issue for medical care and public health. Clinical variables have been used for decades with a rather poor specificity. Until now, the potential of prognostic tools available for making decisions about the management of comatose patients has not been clear. In the case of anoxic coma, validated indicators using somatosensory evoked potentials only predict poor outcome. Beyond mere awakening, the challenge here is to recognize patients who might recover with a good functional outcome as soon as possible.
In 2005, we investigated the usefulness of N1 and MMN recorded during coma for the prediction of good functional outcome 12 months after coma onset in our large prospective cohort (Luauté et al. 2005). The analysis also included BAEPs, MLAEPs and clinical variables. We considered death (GOS 1), permanent vegetative state (GOS 2) and minimally conscious state as poor outcomes (141 patients), and we considered moderate disability (GOS 4) and good recovery (GOS 5) as good outcomes (122 patients). As a single predictor, MMN had the highest estimated predictive value (69.8 %) and the highest specificity for good outcome (87.9 %), but a very poor sensitivity (32.0 %). Logistic multivariate regression analysis showed that age, pupillary light reflex and MMN were the most discriminating predictive factors for good outcome.
N1 and MMN in Vegetative and Minimally Conscious States
After the acute phase of coma, patients enter a phase during which no further ventilation is required and some sleep-wake cycles appear. In this post-coma phase, most body functions are normal, but patients are unable to communicate. A distinction has been made between the vegetative state (also termed “unresponsive wakefulness syndrome”, see Laureys et al. (2010)) and the minimally conscious state (Giacino et al. 2002). The latter is characterized by inconsistent but clearly discernible behavioral evidence of consciousness. An MCS patient shows orientation to pain, eye tracking or reproducible albeit inconsistent response to commands, but his or her state may vary even within short periods of time. The differential diagnosis between VS and MCS is performed through the repeated assessment of the patient’s neurobehavioral function by trained examiners. The most commonly used scale is the Coma Recovery Scale (CRS-R - Giacino et al. (2004)). Diagnosing a patient as being in MCS is a key issue. Either the patient is in a transient state and the minimally conscious state may be precursory to a return to consciousness, or the patient is in a permanent state and the diagnosis should be taken into account for his or her management by caregivers. Event-related potentials, and particularly the MMN, have been proposed to detect remaining cortical functions in post-comatose patients, to differentiate VS from MCS and to predict their outcome.
Kotchoubey et al. (2005) investigated the presence of electrophysiological indicators of remaining cortical functions in 98 post-comatose patients showing extremely severe diffuse brain injuries. They used, among others, two different passive oddball paradigms (with simple tones and harmonic chords) to elicit N1 and frequency MMN. VS and MCS patients differed significantly in the frequency of MMNs, but surprisingly, VS patients, whose diagnosis was more severe, exhibited better MMN results than MCS patients. In contrast, the N1 was recorded more frequently in MCS patients, but with marginal significance. The authors defined clinical improvement as follows: (a) for VS patients, the diagnosis of MCS or better, (b) for MCS patients, any diagnosis better than MCS, including distinct communication ability, (c) for patients already communicating during the examination, an improvement in cognitive functions observed by at least two independent neuropsychologists. Follow-up data at 6 months after ERP recording revealed that the presence of an MMN was related to a better outcome.
Wijnen et al. (2007) investigated longitudinal changes in MMN responses during recovery from VS to consciousness in 10 patients suffering from traumatic brain injury. The patients were repeatedly recorded every 2 weeks for an average period of 3.5 months. MMN amplitude increased with recovery of consciousness and showed a sudden increase when patients started to exhibit inconsistent behavioral responses to simple commands. The authors concluded that this sudden enhancement in MMN amplitude preceding overt communication with the environment might be indicative of consolidation of the neural networks underlying overt communication.
We investigated (Fischer et al. 2010) a series of 27 patients in a permanent vegetative state, i.e. more than one year after coma onset for traumatic coma and more than 6 months after coma onset for other causes of coma (The Multi-Society Task Force on PVS: Medical aspects of the persistent vegetative state (1). 1994; The Multi-Society Task Force on PVS: Medical aspects of the persistent vegetative state (2). 1994). They were diagnosed as PVS (16 cases) or MCS (11 cases), due to anoxia (18 cases) or other etiologies (9 cases). In this study, N1 was detected in only 12 patients, and MMN in only 5 patients. N1 abolition was preferentially found in anoxia over other etiologies and in PVS over MCS. MMN was not correlated with either behavioral assessment or coma etiology.
Furthermore, in a more recent study (Höller et al. 2011) MMN failed to distinguish patients in a vegetative state (16 cases) from those in a minimally conscious state (6 cases): it was detected in only 2 VS patients and in none of the MCS patients. However, it should be noted that the paradigm used allowed MMN detection in only 11 out of 15 healthy subjects, probably due to the small frequency deviance (85 % of the tones at 500 Hz and 15 % of the tones at 513 Hz).
To further detect possible conscious processing in vegetative patients, Naccache and collaborators introduced a new active auditory paradigm (Bekinschtein et al. 2009). With this so-called “local-global” paradigm, an MMN is automatically elicited by changes in pitch that are local in time, whereas a change in sound sequence that appears across several seconds and is designed as a target elicits a parietal P300. The P300 is thought to only be present when subjects consciously perceive this global rule violation. In a recent study, the same team investigated the local response (MMN) and the global response (P300) in a series of 104 patients (vegetative, minimally conscious and conscious state), using single trial decoding (King et al. 2013). The proportion of patients showing a significant global decoding score was smaller in VS patients than in the other two groups, suggesting that the global response (P300) could provide an index of the level of consciousness. However, there was no difference in the proportion of VS patients showing a local response (MMN) compared to MCS patients.
Taken together, these results globally suggest the limited capacity of MMN to distinguish vegetative from minimally conscious patients. These studies highlight the difficulty in establishing a clear boundary between the two states. The subjective dimension of awareness is difficult to test through mere behavioral assessment (Bernat 2002).
However, as also shown by a previous meta-analysis (Daltrozzo et al. 2007), all studies that have investigated a relationship between MMN recording and patient outcome have provided evidence of the good positive predictive value of the presence of MMN for favorable outcome irrespective of the time of recording.
Novelty P3
Recording Novelty P3
The presence of N1 in response to repetitive stimuli demonstrates basic sensory processing of auditory stimulation and the presence of MMN in response to randomly presented deviant stimuli demonstrates automatic recognition of oddball events, without attention processes necessarily being engaged. These two components provide evidence of basic brain function. They have proved their efficiency in assessing the functional state of comatose brains, thus yielding prognostic markers for good coma recovery. To test higher-level processes without requesting the active participation of the patient, unexpected salient stimuli (called novel stimuli, or Novels) can be added to the passive auditory oddball paradigm, with a very small frequency of presentation (p < 0.05). By virtue of their salience and scarcity, these novel stimuli elicit attention-orienting processes beyond mismatch processes. These processes are revealed by a positive wave about 300 ms after stimulus onset, the novelty P3 (Friedman et al. 2001). Novel stimuli might be extremely deviant tones, environmental sounds (like dog barking or bell ringing) or vocal stimuli. The subject’s own name (SON) is a good candidate for mobilizing a large population of neurons. Indeed, this stimulus holds a specific relevance to the listener because of its subjective importance and its frequent occurrence in daily life. We further proposed that SON uttered by a familiar voice, which increases the emotional aspect of the stimulus, would increase the chances of getting a response from comatose patients. In a previous coma study, presentations of SON interspersed with tones and used as “emotional conditioners” increased the probability of obtaining P300 components in response to the tones (Signorino et al. 1995).
Our team has extensively investigated the response to SON presented as Novel among classical standard and deviant tones in healthy subjects watching a silenced movie. A Positron Emission Tomography (PET) study (Holeckova et al. 2008) showed robust cerebral blood flow activation over several brain regions (temporal, frontal and parietal cortices, hippocampus and precuneus) that could be associated with speech, novelty and self-recognition processing, thus corroborating the activation of a large neuronal network when the subject unexpectedly hears his or her own name. We also compared the pattern of activation when SON was uttered by a familiar person and by an unfamiliar person. Only small differential contributions of the left and right prefrontal cortices were observed, which can be explained by different memory processing when hearing a familiar or an unknown voice (Holeckova et al. 2008).
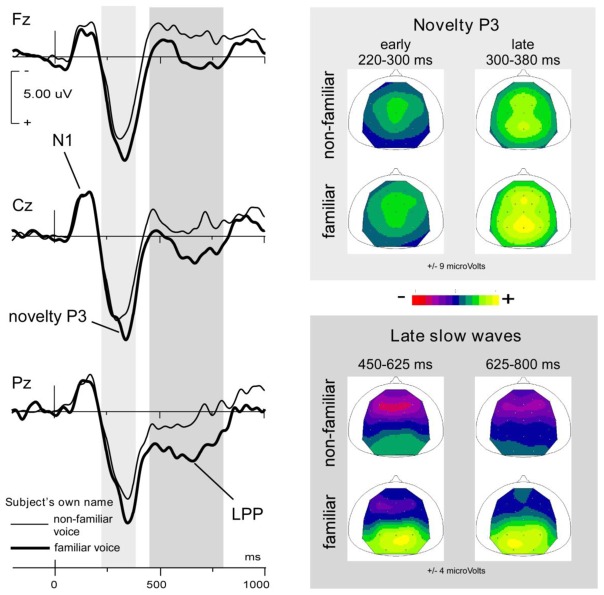
The novelty P3 in response to SON in healthy subjects was a complex positive response peaking around 350 ms and showing two successive stages with stable topography, as displayed in Figure 4 (Holeckova et al. 2006). From 220 ms to 300 ms, a first subcomponent peaked at central sites. This early P3a has been associated with alerting processes governing the direction of attentional move (Ceponiene et al. 2004). The second phase of novelty P3, from 300 ms to 380 ms, showed both a frontal and a parietal maximum (Holeckova et al. 2006), in agreement with most novelty P3 data from the literature (Friedman et al. 2001). In our data, like in the data from Yago et al. (2003), frontal and parietal aspects of the novelty P3 appeared synchronously (see Figure 4). The frontal subcomponent has been associated with orientation processes (P3a). The parietal component has been associated with stimulus categorization, significance as a target and degree of information delivery, therefore representing an instance of the P3b component (Sutton et al. 1965; Friedman et al. 2001; Gaeta et al. 2003).
Figure 4.
Responses to the subject’s own first name uttered by a familiar and a non-familiar voice presented as novel stimuli in a novelty oddball paradigm (probability p = 0.02 for each Novel). Grand averages obtained from 15 healthy subjects.
Left panel: averaged waveforms recorded at 3 midline electrodes (Fz, Cz, Pz) with nose reference (non-familiar voice, thin line; familiar voice, thick line).
Right panels: Mean scalp potential distributions for the 2 types of stimuli; above in the 2 stages of the novelty P3 averaged over the 220–300 ms and 300–380 ms, respectively; below over the 450–625 ms and 625–800 time intervals including late slow waves.
Adapted from Holeckova et al. 2006.
In healthy subjects, with first names uttered by a familiar voice and by an unknown voice presented in the same stimulation block, the familiar voice not only brought about increased amplitude of the parietal aspect of the novelty P3, but also elicited a large additional parietal slow wave at later latencies, as shown in Figure 4 (Holeckova et al. 2006). In a more recent study in healthy subjects, we presented SON and another first name as Novels in an oddball paradigm, in order to investigate the specificity of the response to SON (Eichenlaub et al. 2012). In this study, an experimenter who was not familiar with the subject uttered both names and a late parietal positivity (LPP) appeared in response to SON and not to the other name. Taken together, our results question the brain processes inherent in such LPPs and call for further investigation. In the literature, late slow waves with parietal topography have been described after target P300s in conditions where the active participation of the subject was requested. They emerged after target identification and were associated with response selection (Falkenstein et al. 1994), retrieval of information from working memory (Garcia-Larrea and Cezanne-Bert 1998; Lefebvre et al. 2005) or recollection of old items (Curran 2004). In each of our experiments (Holeckova et al. 2006; Eichenlaub et al. 2012), LPPs were found in response to the relatively more self-related stimulus (familiar versus unknown voice, own versus other name) although no active response was required. In response to these particular stimuli, specific processes might well have been covertly activated while the subject’s attention was engaged in film watching. However, our paradigms did not make it possible to reveal the nature of these high-level cognitive processes.
In unresponsive patients as well as in healthy subjects, the Novels were inserted in the classical oddball paradigm with duration Deviants. First name stimuli last on average 600 ms with a large range of durations. We therefore chose to keep a large time interval between the Novel onset and the onset of the following Standard (1220 ms i.e. twice the interval between tones).
In clinical practice, at least 50 responses to Novels should be averaged for adequate signal-to-noise ratio. The response, when present, is a large positive wave. To assess the topography of the response, midline electrodes from frontal to parietal sites (Fz, Cz, Pz) are necessary. Wave detection in individual patients may be objectivized by statistical analysis based on the whole set of single trials. For example, at each sampling point, a bootstrap estimate of the confidence interval may be assessed (Efron 1979). In our studies of unresponsive coma and post-coma patients (Fischer et al. 2008; Fischer et al. 2010), we considered that a novelty P3 wave was detected when at least 62 consecutive points (i.e. a duration of 60 ms) were found to be significantly different from the baseline and positive (unilateral p < 0.05) around a positive maximum at Cz (Guthrie and Buchwald 1991). Like for N1 or MMN, visual analysis is of utmost importance for definitive wave recognition. Response topography differs from one patient to the next due to the pathology, mainly in traumatic coma. Waves showing a maximum around prefrontal electrodes should be treated with caution as they most likely reflect some ocular artifact spreading over the scalp. Indeed, unexpected Novels often elicit an eye blink, especially in post-coma patients, whose eyes are open. When a sufficient number of scalp electrodes are used, an independent component analysis (ICA) efficiently filters out such artifacts. However, in clinical routine, a small number of electrodes are used and a trial by trial correction of the raw EEG signal using regression with EOG signals (Gratton et al. 1983) gives good results (Fischer et al. 2010).
Prognostic value of novelty P3 during coma
The novelty oddball paradigm was presented with the patient’s own first name as Novel over 20 minutes in a series of 50 severe comatose patients (GCS ≤ 8) of various etiologies (anoxia, 20 cases; brain injury, 15 cases; stroke, 15 cases) on average 20 days after coma onset (during the first week for 22 patients and during the first month for 41 patients) (Fischer et al. 2008).
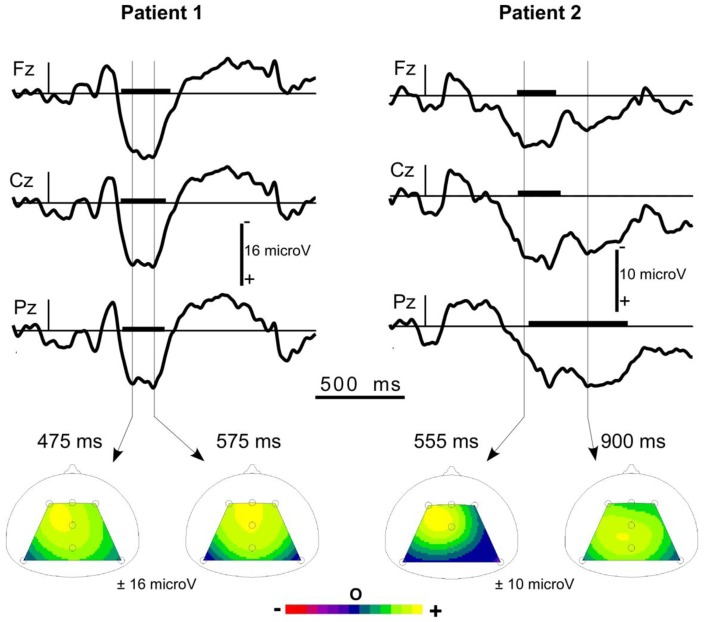
A sensory N1 in response to standard tones was observed in 27 patients, 14 of whom also showed an MMN in response to deviant tones. A novelty P3 was observed in 21 patients. It was a large wave (about 13 μV) peaking around 650 ms (see Figure 5).
Figure 5.
Typical responses to the subject’s own name presented as a novel in 2 individual coma patients.
Upper panel: responses at the three midline electrodes Fz, Cz, Pz (with a nose reference). At each electrode, the window with significantly positive potentials (unilateral bootstrap p<0.05) is highlighted by a thick line on the time axis. The 2 patients show a novelty P3.
Lower panel: Scalp potential maps at 2 latencies during the detected wave. In Patient 1, the topography proved to be stable during the detected wave. In Patient 2, a more parietal topography can be observed in the late part of the wave (LPP).
Three months after the onset of coma, Patient 1 (female, 30 years old, stroke) was in a minimally conscious state and Patient 2 (male, 61 years old, anoxia) had recovered with moderate disability.
Adapted from Fischer et al. 2008.
Three months after coma onset, the patients were considered as non-awake if they were in MCS, in VS or dead. Awakenings included good recovery, and moderate and severe disabilities. There was a high correlation between the presence of novelty P3 and awakening at three months (χ2 = 13.6, p = 0.0002). Compared to MMN, novelty P3 showed as large a specificity for awakening (84.6 % for both waves), but a much higher sensitivity (70.8 % for novelty P3 versus 41.6 % for MMN). As in previous studies, only a small proportion of the patients showed an MMN but a surprising result in these data was the presence of novelty P3s in 11 patients showing no MMN. Attention orienting processes were not expected to occur in patients showing no basic automatic mismatch processes. In fact, different anatomical structures are activated in MMN and novelty P3 elicitation and the different brain injuries encountered in the coma patients might have affected each structure differently. Moreover, much larger neuronal networks are activated by the Novels than by the Deviants (Holeckova et al. 2008) and the signal-to-noise ratio is much higher for large novelty P3s than for MMNs. Small MMNs buried in noise might not have been detected and could have caused some false negative results. Thus, an important result of this study was that the use of novelty P3 increases the prognostic value of MMN alone.
Interestingly, although for nine patients a single stable topography was observed along the interval of novelty P3 detection (central, frontal-central or central-parietal, depending on the patient), for the other 12 patients we clearly observed two successive stages with, in late latencies, a more pronounced parietal topography. In these patients, wave offset appeared on average 330 ms later at Pz than at Fz, thus isolating a late parietal component after the novelty P3 (see Figure 5, right panel). The unusual pattern of the responses to novelty observed in coma patients prevents these late parietal components from being labeled with confidence. However, should the late parietal components be instances of the parietal aspect of the novelty P3 or instances of LPPs appearing after the novelty P3, their presence provides evidence of some implicit cognitive processes. Indeed, 11 of the 12 patients with a late parietal component did awaken within 3 months of coma onset.
Novelty P3 in permanent vegetative patients
We presented the novelty oddball paradigm in a series of 27 patients in a permanent unresponsive state (16 diagnosed as PVS, 11 as MCS) after a coma due to anoxia (18 cases), traumatic brain injury (4 cases), stroke (4 cases), and viral encephalitis (1 case). The proportion of PVS was larger in anoxic patients (13/18) than in other etiologies (3/9), but the difference did not reach significance (Fischer et al. 2010). Out of the 18 anoxic patients, only two (one PVS and one MCS) showed a small atypical response to their own name. In contrast, 5 patients (2 PVS and 3 MCS) out of the 9 non-anoxic patients showed a novelty P3 with a very similar amplitude and topography to those in healthy subjects. In two of them, a late parietal positive component was observed at the end of the novelty P3.
This study revealed the involvement of automatic brain processes in the orientation of attention to a specific stimulus (SON) in patients who were considered as permanently unresponsive. In 2 patients, higher order processes were also highlighted. These responses favoring some “islands of cognition” were preferentially obtained in patients who had not suffered from anoxia, irrespective of their clinical assessment, which was based on mere behavior. These results question the reliability of routine clinical assessment and call for further investigation into the functional state of these patients.
Conclusion
Our clinical studies, based on large cohorts of coma patients, demonstrate the value of MMN and novelty P3 as early and reliable markers of coma outcome. The ERP technique is widely applicable in clinical routine at the patient’s bedside and these components have brought substantial additional information to the available clinical tools, like behavioral assessment, EEG analysis, and early sensory-evoked potentials.
MMN alterations have been shown to index functional and cognitive decline shared by different disorders, irrespective of their etiology or symptomatology (Näätänen et al. 2012). In disorders of consciousness, the presence of an MMN demonstrates proper functioning of basic cognitive processes and is therefore an efficient marker for the possibility of awakening. In brains that have suffered from anoxia, the numerous poor outcomes are due to immediate irreversible and diffuse damage. In anoxic comas, the presence of an MMN during the early stages of coma excludes poor outcomes (Fischer et al. 2006). In a different way, most traumatic injuries induce more localized brain damage, allowing a gradual recovery of brain function and leading to progressive MMN recovery (Kane et al. 1993; Kane et al. 1996; Fischer et al. 1999; Wijnen et al. 2007).
Because of its salience, the own first name presented rarely within an oddball paradigm elicits an orienting response, the novelty P3, which provides evidence of at least basic attention orienting processes. We showed that the presence of novelty P3 in coma patients is highly correlated with further awakening (Fischer et al. 2008). The novelty P3 shows a better sensitivity for awakening than MMN, probably because SON activates larger neuronal networks than mere deviant tones do. Because of its very special meaning for the subject, his or her own first name may trigger more complex processes, like those revealed by late parietal components (Holeckova et al. 2006; Eichenlaub et al. 2012). A late parietal positive wave (LPP) following the novelty P3 suggests some covert cognitive processing. In coma patients it signals impending awakening (Fischer et al. 2008). In permanently unresponsive patients, it suggests some “islands of cognition” without providing clear-cut responses about possible awareness (Fischer et al. 2010).
The MMN reveals preconscious change detection processes that may reach conscious perception by activating frontal processes, which, in turn, initiate further higher-level cerebral processes (Rinne et al. 2000). In this way, MMN processes, elicited through passive auditory paradigms, find themselves at the junction between exogenous sensory processes (like those revealed by the N1 auditory component) and high-level cognitive endogenous processes (like, among others, those revealed by the P300 family). Boly et al. (2011) measured effective cortical connectivity during a mismatch paradigm in patients with disorders of consciousness in order to detect impairments in the underlying cortical networks. At the group level, through Dynamic Causal Modeling (DCM), they observed impaired backward connection from frontal to temporal cortices in VS patients only (as compared with MCS patients and controls), thus suggesting that a specific top-down failure of effective connectivity is related to consciousness level. If applicable at the level of single patients, this DCM approach would greatly refine the interpretation of scalp markers.
More generally, over the last decade, the possibility that unresponsive patients could be covertly conscious has been the subject of much debate. Numerous attempts have been made to assess cognition and to detect awareness in these patients, using functional neuroimaging and electrophysiological investigations (for a review, see Harrison and Connolly (2013)). This rapidly expanding field of research promises exciting developments toward a better understanding of consciousness impairments and the possibility of establishing communication with some of these patients showing no voluntary behavior.
Acknowledgments
This work was conducted in the framework of the LabEx Cortex (“Construction, Function and Cognitive Function and Rehabilitation of the Cortex”, ANR-10-LABX-0042) of Université de Lyon.
References
- Bekinschtein TA, Dehaene S, Rohaut B, Tadel F, Cohen L, Naccache L. Neural signature of the conscious processing of auditory regularities. Proc Natl Acad Sci U S A. 2009;106(5):1672–1677. doi: 10.1073/pnas.0809667106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernat JL. Questions remaining about the minimally conscious state. Neurology. 2002;58(3):337–338. doi: 10.1212/wnl.58.3.337. [DOI] [PubMed] [Google Scholar]
- Bernat JL. Chronic disorders of consciousness. The Lancet. 2006;367(9517):1181–1192. doi: 10.1016/S0140-6736(06)68508-5. [DOI] [PubMed] [Google Scholar]
- Boly M, Garrido MI, Gosseries O, Bruno MA, Boveroux P, Schnakers C, Massimini M, Litvak V, Laureys S, Friston K. Preserved feedforward but impaired top-down processes in the vegetative state. Science. 2011;332(6031):858–862. doi: 10.1126/science.1202043. [DOI] [PubMed] [Google Scholar]
- Ceponiene R, Lepisto T, Soininen M, Aronen E, Alku P, Näätänen R. Event-related potentials associated with sound discrimination versus novelty detection in children. Psychophysiology. 2004;41(1):130–141. doi: 10.1111/j.1469-8986.2003.00138.x. [DOI] [PubMed] [Google Scholar]
- Curran T. Effects of attention and confidence on the hypothesized ERP correlates of recollection and familiarity. Neuropsychologia. 2004;42(8):1088–1106. doi: 10.1016/j.neuropsychologia.2003.12.011. [DOI] [PubMed] [Google Scholar]
- Daltrozzo J, Wioland N, Mutschler V, Kotchoubey B. Predicting coma and other low responsive patients outcome using event-related brain potentials: A meta-analysis. Clinical Neurophysiology. 2007;118(3):606–614. doi: 10.1016/j.clinph.2006.11.019. [DOI] [PubMed] [Google Scholar]
- Efron B. Bootstrap methods: another look at the Jackknife. The Annals of Statistics. 1979;7(1):1–26. [Google Scholar]
- Eichenlaub JB, Bertrand O, Morlet D, Ruby P. Brain Reactivity Differentiates Subjects with High and Low Dream Recall Frequencies during Both Sleep and Wakefulness. Cerebral cortex. 2013 doi: 10.1093/cercor/bhs388. [DOI] [PubMed] [Google Scholar]
- Eichenlaub JB, Ruby P, Morlet D. What is the specificity of the response to the own first-name when presented as a novel in a passive oddball paradigm? An ERP study. Brain Res. 2012;1447(0):65–78. doi: 10.1016/j.brainres.2012.01.072. [DOI] [PubMed] [Google Scholar]
- Falkenstein M, Hohnsbein J, Hoormann J. Effects of choice complexity on different subcomponents of the late positive complex of the event-related potential. Electroencephalogr Clin Neurophysiol. 1994;92(2):148–160. doi: 10.1016/0168-5597(94)90055-8. [DOI] [PubMed] [Google Scholar]
- Fischer C, Dailler F, Morlet D. Novelty P3 elicited by the subject’s own name in comatose patients. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology. 2008;119(10):2224–2230. doi: 10.1016/j.clinph.2008.03.035. [DOI] [PubMed] [Google Scholar]
- Fischer C, Luaute J. Evoked potentials for the prediction of vegetative state in the acute stage of coma. Neuropsychological rehabilitation. 2005;15(3–4):372–380. doi: 10.1080/09602010443000434. [DOI] [PubMed] [Google Scholar]
- Fischer C, Luaute J, Adeleine P, Morlet D. Predictive value of sensory and cognitive evoked potentials for awakening from coma. Neurology. 2004;63(4):669–673. doi: 10.1212/01.wnl.0000134670.10384.e2. [DOI] [PubMed] [Google Scholar]
- Fischer C, Luaute J, Morlet D. Event-related potentials (MMN and novelty P3) in permanent vegetative or minimally conscious states. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology. 2010;121(7):1032–1042. doi: 10.1016/j.clinph.2010.02.005. [DOI] [PubMed] [Google Scholar]
- Fischer C, Luaute J, Nemoz C, Morlet D, Kirkorian G, Mauguiere F. Improved prediction of awakening or nonawakening from severe anoxic coma using tree-based classification analysis. Critical care medicine. 2006;34(5):1520–1524. doi: 10.1097/01.CCM.0000215823.36344.99. [DOI] [PubMed] [Google Scholar]
- Fischer C, Morlet D, Bouchet P, Luaute J, Jourdan C, Salord F. Mismatch negativity and late auditory evoked potentials in comatose patients. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology. 1999;110(9):1601–1610. doi: 10.1016/s1388-2457(99)00131-5. [DOI] [PubMed] [Google Scholar]
- Friedman D, Cycowicz YM, Gaeta H. The novelty P3: an event-related brain potential (ERP) sign of the brain’s evaluation of novelty. Neurosci Biobehav Rev. 2001;25(4):355–373. doi: 10.1016/s0149-7634(01)00019-7. [DOI] [PubMed] [Google Scholar]
- Gaeta H, Friedman D, Hunt G. Stimulus characteristics and task category dissociate the anterior and posterior aspects of the novelty P3. Psychophysiology. 2003;40(2):198–208. doi: 10.1111/1469-8986.00022. [DOI] [PubMed] [Google Scholar]
- Garcia-Larrea L, Cezanne-Bert G. P3, positive slow wave and working memory load: a study on the functional correlates of slow wave activity. Electroencephalogr Clin Neurophysiol. 1998;108(3):260–273. doi: 10.1016/s0168-5597(97)00085-3. [DOI] [PubMed] [Google Scholar]
- Giacino JT, Ashwal S, Childs N, Cranford R, Jennett B, Katz DI, Kelly JP, Rosenberg JH, Whyte J, Zafonte RD, Zasler ND. The minimally conscious state: definition and diagnostic criteria. Neurology. 2002;58(3):349–353. doi: 10.1212/wnl.58.3.349. [DOI] [PubMed] [Google Scholar]
- Giacino JT, Kalmar K, Whyte J. The JFK Coma Recovery Scale-Revised: measurement characteristics and diagnostic utility. Arch Phys Med Rehabil. 2004;85(12):2020–2029. doi: 10.1016/j.apmr.2004.02.033. S0003999304004770 [pii] [DOI] [PubMed] [Google Scholar]
- Giard MH, Lavikainen J, Reinikainen K, Perrin F, Bertrand O, Pernier J, Näätänen R. Separate representation of stimulus frequency, intensity, and duration in auditory sensory memory: An event-related potential and dipole-model analysis. J Cogn Neurosci. 1995;7(2):133–143. doi: 10.1162/jocn.1995.7.2.133. [DOI] [PubMed] [Google Scholar]
- Gratton G, Coles MG, Donchin E. A new method for off-line removal of ocular artifact. Electroencephalogr Clin Neurophysiol. 1983;55(4):468–484. doi: 10.1016/0013-4694(83)90135-9. [DOI] [PubMed] [Google Scholar]
- Guthrie D, Buchwald JS. Significance testing of difference potentials. Psychophysiology. 1991;28(2):240–244. doi: 10.1111/j.1469-8986.1991.tb00417.x. [DOI] [PubMed] [Google Scholar]
- Harrison AH, Connolly JF. Finding a way in: A review and practical evaluation of fMRI and EEG for detection and assessment in disorders of consciousness. Neurosci Biobehav Rev. 2013;37(8):1403–1419. doi: 10.1016/j.neubiorev.2013.05.004. [DOI] [PubMed] [Google Scholar]
- Holeckova I, Fischer C, Giard MH, Delpuech C, Morlet D. Brain responses to a subject’s own name uttered by a familiar voice. Brain Res. 2006;1082(1):142–152. doi: 10.1016/j.brainres.2006.01.089. [DOI] [PubMed] [Google Scholar]
- Holeckova I, Fischer C, Morlet D, Delpuech C, Costes N, Mauguiere F. Subject’s own name as a novel in a MMN design: a combined ERP and PET study. Brain Res. 2008;1189:152–165. doi: 10.1016/j.brainres.2007.10.091. [DOI] [PubMed] [Google Scholar]
- Höller Y, Bergmann J, Kronbichler M, Crone JS, Schmid EV, Golaszewski S, Ladurner G. Preserved oscillatory response but lack of mismatch negativity in patients with disorders of consciousness. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology. 2011;122(9):1744–1754. doi: 10.1016/j.clinph.2011.02.009. [DOI] [PubMed] [Google Scholar]
- Jennett B, Bond M. Assessment of outcome after severe brain damage. Lancet. 1975;1(7905):480–484. doi: 10.1016/s0140-6736(75)92830-5. [DOI] [PubMed] [Google Scholar]
- Kane NM, Curry SH, Butler SR, Cummins BH. Electrophysiological indicators of awakening from coma. The Lancet. 1993;341:688. doi: 10.1016/0140-6736(93)90453-n. [DOI] [PubMed] [Google Scholar]
- Kane NM, Curry SH, Rowlands CA, Manara AR, Lewis T, Moss T, Cummins BH, Butler SR. Event-related potentials - neurophysiological tools for predicting emergence and early outcome from traumatic coma. Intensive Care Med. 1996;22(1):39–46. doi: 10.1007/BF01728329. [DOI] [PubMed] [Google Scholar]
- King JR, Faugeras F, Gramfort A, Schurger A, El Karoui I, Sitt JD, Rohaut B, Wacongne C, Labyt E, Bekinschtein T, Cohen L, Naccache L, Dehaene S. Single-trial decoding of auditory novelty responses facilitates the detection of residual consciousness. Neuroimage. 2013;83C:726–738. doi: 10.1016/j.neuroimage.2013.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotchoubey B, Lang S, Herb E, Maurer P, Schmalohr D, Bostanov V, Birbaumer N. Stimulus complexity enhances auditory discrimination in patients with extremely severe brain injuries. Neurosci Lett. 2003;352(2):129–132. doi: 10.1016/j.neulet.2003.08.045. [DOI] [PubMed] [Google Scholar]
- Kotchoubey B, Lang S, Mezger G, Schmalohr D, Schneck M, Semmler A, Bostanov V, Birbaumer N. Information processing in severe disorders of consciousness: vegetative state and minimally conscious state. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology. 2005;116(10):2441–2453. doi: 10.1016/j.clinph.2005.03.028. [DOI] [PubMed] [Google Scholar]
- Laureys S, Celesia GG, Cohadon F, Lavrijsen J, Leon-Carrion J, Sannita WG, Sazbon L, Schmutzhard E, von Wild KR, Zeman A, Dolce G European Task Force on Disorders of C. Unresponsive wakefulness syndrome: a new name for the vegetative state or apallic syndrome. BMC Med. 2010;8:68. doi: 10.1186/1741-7015-8-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YC, Phan TG, Jolley DJ, Castley HC, Ingram DA, Reutens DC. Accuracy of clinical signs, SEP, and EEG in predicting outcome of hypoxic coma: a meta-analysis. Neurology. 2010;74(7):572–580. doi: 10.1212/WNL.0b013e3181cff761. [DOI] [PubMed] [Google Scholar]
- Lefebvre CD, Marchand Y, Eskes GA, Connolly JF. Assessment of working memory abilities using an event-related brain potential (ERP)-compatible digit span backward task. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology. 2005;116(7):1665–1680. doi: 10.1016/j.clinph.2005.03.015. [DOI] [PubMed] [Google Scholar]
- Lew HL, Poole JH, Castaneda A, Salerno RM, Gray M. Prognostic value of evoked and event-related potentials in moderate to severe brain injury. The Journal of head trauma rehabilitation. 2006;21(4):350–360. doi: 10.1097/00001199-200607000-00006. [DOI] [PubMed] [Google Scholar]
- Luauté J, Fischer C, Adeleine P, Morlet D, Tell L, Boisson D. Late auditory and cognitive evoked potentials can be useful to predict good functional outcome after coma. Arch Phys Med Rehabil. 2005;86:917–923. doi: 10.1016/j.apmr.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Madl C, Kramer L, Yeganehfar W, Eisenhuber E, Kranz A, Ratheiser K, Zauner C, Schneider B, Grimm G. Detection of nontraumatic comatose patients with no benefit of intensive care treatment by recording of sensory evoked potentials. Arch Neurol. 1996;53(6):512–516. doi: 10.1001/archneur.1996.00550060054017. [DOI] [PubMed] [Google Scholar]
- Marmarou A, Lu J, Butcher I, McHugh GS, Murray GD, Steyerberg EW, Mushkudiani NA, Choi S, Maas AI. Prognostic value of the Glasgow Coma Scale and pupil reactivity in traumatic brain injury assessed pre-hospital and on enrollment: an IMPACT analysis. Journal of neurotrauma. 2007;24(2):270–280. doi: 10.1089/neu.2006.0029. [DOI] [PubMed] [Google Scholar]
- Morlet D, Fischer C. The mismatch negativity (MMN) recorded in comatose patients actually discloses mismatch processes. EPIC XIII. 2001;41(3):199. [Google Scholar]
- The Multi-Society Task Force on PVS. Medical aspects of the persistent vegetative state (1) N Engl J Med. 1994;330(21):1499–1508. doi: 10.1056/NEJM199405263302107. [DOI] [PubMed] [Google Scholar]
- The Multi-Society Task Force on PVS. Medical aspects of the persistent vegetative state (2) N Engl J Med. 1994;330(22):1572–1579. doi: 10.1056/NEJM199406023302206. [DOI] [PubMed] [Google Scholar]
- Mushkudiani NA, Hukkelhoven CW, Hernandez AV, Murray GD, Choi SC, Maas AI, Steyerberg EW. A systematic review finds methodological improvements necessary for prognostic models in determining traumatic brain injury outcomes. Journal of clinical epidemiology. 2008;61(4):331–343. doi: 10.1016/j.jclinepi.2007.06.011. [DOI] [PubMed] [Google Scholar]
- Näätänen R, Gaillard AW, Mantysalo S. Early selective-attention effect on evoked potential reinterpreted. Acta psychologica. 1978;42(4):313–329. doi: 10.1016/0001-6918(78)90006-9. [DOI] [PubMed] [Google Scholar]
- Näätänen R, Kujala T, Escera C, Baldeweg T, Kreegipuu K, Carlson S, Ponton C. The mismatch negativity (MMN)--a unique window to disturbed central auditory processing in ageing and different clinical conditions. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology. 2012;123(3):424–458. doi: 10.1016/j.clinph.2011.09.020. [DOI] [PubMed] [Google Scholar]
- Näätänen R, Picton T. The N1 wave of the human electric and magnetic response to sound: a review and an analysis of the component structure. Psychophysiology. 1987;24(4):375–425. doi: 10.1111/j.1469-8986.1987.tb00311.x. [DOI] [PubMed] [Google Scholar]
- Naccache L, Puybasset L, Gaillard R, Serve E, Willer JC. Auditory mismatch negativity is a good predictor of awakening in comatose patients: a fast and reliable procedure. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology. 2005;116(4):988–989. doi: 10.1016/j.clinph.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Rinne T, Alho K, Ilmoniemi RJ, Virtanen J, Näätänen R. Separate time behaviors of the temporal and frontal mismatch negativity sources. Neuroimage. 2000;12(1):14–19. doi: 10.1006/nimg.2000.0591. [DOI] [PubMed] [Google Scholar]
- Robinson LR, Micklesen PJ, Tirschwell DL, Lew HL. Predictive value of somatosensory evoked potentials for awakening from coma. Critical care medicine. 2003;31(3):960–967. doi: 10.1097/01.CCM.0000053643.21751.3B. [DOI] [PubMed] [Google Scholar]
- Ruby P, Caclin A, Boulet S, Delpuech C, Morlet D. Odd sound processing in the sleeping brain. J Cogn Neurosci. 2008;20(2):296–311. doi: 10.1162/jocn.2008.20023. [DOI] [PubMed] [Google Scholar]
- Sams M, Paavilainen P, Alho K, Näätänen R. Auditory frequency discrimination and event-related potentials. Electroencephalogr Clin Neurophysiol. 1985;62(6):437–448. doi: 10.1016/0168-5597(85)90054-1. [DOI] [PubMed] [Google Scholar]
- Signorino M, D’Acunto S, Angeleri F, Pietropaoli P. Eliciting P300 in comatose patients. Lancet. 1995;345(8944):255–256. doi: 10.1016/s0140-6736(95)90252-x. [DOI] [PubMed] [Google Scholar]
- Sutton S, Braren M, Zubin J, John ER. Evoked-Potential Correlates of Stimulus Uncertainty. Science. 1965;150(3700):1187–1188. doi: 10.1126/science.150.3700.1187. [DOI] [PubMed] [Google Scholar]
- Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974;2(7872):81–84. doi: 10.1016/s0140-6736(74)91639-0. [DOI] [PubMed] [Google Scholar]
- Tervaniemi M, Lehtokoski A, Sinkkonen J, Virtanen J, Ilmoniemi RJ, Näätänen R. Test-retest reliability of mismatch negativity for duration, frequency and intensiy changes. Clinical Neurophysiology. 1999;110:1388–1393. doi: 10.1016/s1388-2457(99)00108-x. [DOI] [PubMed] [Google Scholar]
- Tzovara A, Rossetti AO, Spierer L, Grivel J, Murray MM, Oddo M, De Lucia M. Progression of auditory discrimination based on neural decoding predicts awakening from coma. Brain. 2013;136(1):81–89. doi: 10.1093/brain/aws264. [DOI] [PubMed] [Google Scholar]
- Wijnen VJ, van Boxtel GJ, Eilander HJ, de Gelder B. Mismatch negativity predicts recovery from the vegetative state. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology. 2007;118(3):597–605. doi: 10.1016/j.clinph.2006.11.020. [DOI] [PubMed] [Google Scholar]
- Yago E, Escera C, Alho K, Giard MH, Serra-Grabulosa JM. Spatiotemporal dynamics of the auditory novelty-P3 event-related brain potential. Brain Res Cogn Brain Res. 2003;16(3):383–390. doi: 10.1016/s0926-6410(03)00052-1. [DOI] [PubMed] [Google Scholar]
- Zandbergen EG, de Haan RJ, Stoutenbeek CP, Koelman JH, Hijdra A. Systematic review of early prediction of poor outcome in anoxic- ischaemic coma [see comments] Lancet. 1998;352(9143):1808–1812. doi: 10.1016/S0140-6736(98)04076-8. [DOI] [PubMed] [Google Scholar]