Abstract
Objective
Glucose-stimulated insulin secretion in pancreatic beta cells requires metabolic signals including the generation of glucose-derived short chain acyl-CoAs in the cytosol from mitochondrially-derived metabolites. One concept of insulin secretion is that ATP citrate lyase generates short chain acyl-CoAs in the cytosol from mitochondrially-derived citrate. Of these, malonyl-CoA, is believed to be an important signal in insulin secretion. Malonyl-CoA is also a precursor for lipids. Our recent evidence suggested that, in the mitochondria of beta cells, glucose-derived pyruvate can be metabolized to acetoacetate that is exported to the cytosol and metabolized to the same short chain acyl-CoAs and fatty acids that can be derived from citrate. We tested for redundancy of the citrate pathway.
Methods
We inhibited ATP citrate lyase activity using hydroxycitrate as well as studying a stable cell line generated with shRNA knockdown of ATP citrate lyase in the pancreatic beta cell line INS-1 832/13.
Results
In both instances glucose-stimulated insulin release was not inhibited. Mass spectrometry analysis showed that the flux of carbon from [U-13C]glucose and/or [U-13C]α-ketoisocaproic acid (KIC) into short chain acyl-CoAs in cells with hydroxycitrate-inhibited ATP citrate lyase or in the cell line with stable severe (>90%) shRNA knockdown of ATP citrate lyase was similar to the controls. Both 13C-glucose and 13C-KIC introduced substantial 13C labeling into acetyl-CoA, malonyl-CoA, and HMG-CoA under both conditions. Glucose flux into fatty acids was not affected by ATP citrate lyase knockdown.
Conclusion
The results establish the involvement of the acetoacetate pathway in insulin secretion in pancreatic beta cells.
Keywords: Acetoacetate pathway, Malonyl-CoA, Acetyl-CoA, Palmitate, Mass spectrometry, Mitochondrial biosynthesis, Citrate
Abbreviations: ACC, acetyl-CoA carboxylase; ACLY, ATP citrate lyase; KIC, α-ketoisocaproic acid; SCOT, succinyl-CoA:3-ketoacid-CoA transferase; ZMP, 5-aminoimidazole-4-carboxamide ribonucleotide
Highlights
-
•
In pancreatic beta cells mitochondria synthesize metabolites from glucose.
-
•
Mitochondria-derived citrate and acetoacetate can transfer carbon to the cytosol.
-
•
The citrate pathway requires ATP citrate lyase (ACLY).
-
•
Inhibition of ACLY did not stop metabolite export to the cytosol or insulin release.
-
•
The results establish the role of the acetoacetate pathway in insulin secretion.
1. Introduction
In addition to ATP production, an important role of mitochondria in the pancreatic beta cell is anaplerosis, which is the net synthesis of citric acid cycle intermediates that are exported to the cytosol where they are converted to numerous other metabolites that stimulate and/or support insulin secretion. Among these cytosolic products derived from mitochondrial metabolites are short chain acyl-CoAs, such as acetyl-CoA, malonyl-CoA, acetoacetyl-CoA and HMG-CoA [1], [2], [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15]. Short chain acyl-CoAs are produced inside mitochondria, but cells are unable to transport them across the mitochondrial inner membrane. In order for a cell to provide short chain acyl-CoAs to the cytosol, they are converted to metabolites in the mitochondria that can be exported to the cytosol where they are converted back to short chain acyl-CoAs. During glucose-stimulated insulin secretion in pancreatic beta cells, including human pancreatic islets, short chain acyl-CoAs can be derived from pyruvate in mitochondria by two different pathways. Pyruvate can be metabolized through pyruvate carboxylase to oxaloacetate and through pyruvate dehydrogenase to form acetyl-CoA. Oxaloacetate and acetyl-CoA can combine to form citrate in the citrate synthase reaction in the mitochondria. Citrate is then exported out of the mitochondria to the cytosol where ATP citrate lyase (ACLY) catalyzes citrate's conversion into both oxaloacetate and acetyl-CoA [5], [6]. Cytosolic acetyl-CoA can be carboxylated by acetyl-CoA carboxylase (ACC) to form malonyl-CoA, which can itself act as a signal or be used for fatty acid synthesis. Cytosolic acetyl-CoA can also be converted to acetoacetyl-CoA and HMG-CoA.
In a second biosynthesis pathway, the acetoacetate pathway, that also originates in mitochondria, pyruvate can be converted to acetyl-CoA catalyzed by pyruvate dehydrogenase in the mitochondria. Two acetyl-CoA molecules can combine to form acetoacetyl-CoA. Acetoacetyl-CoA can be converted into acetoacetate by succinyl-CoA:3-ketoacid-CoA transferase (SCOT) and transported out of the mitochondria to the cytosol where it is converted to acetoacetyl-CoA by acetoacetyl-CoA synthetase [9], [10], [11], [12], [13], [14], [15], [16]. Cytosolic acetoacetyl-CoA can then be converted to cytosolic acetyl-CoA and malonyl-CoA and HMG-CoA as well as fatty acids.
There are several reports that knockdown of ATP citrate lyase with siRNA technology in beta cells does not impair glucose-stimulated insulin release [15], [17] while the inhibition of SCOT [15] or acetoacetyl-CoA synthetase [15] does lower insulin release suggesting that the formation of short chain acyl-CoAs through acetoacetate is important during insulin secretion. α-Ketoisocaproic acid (KIC), which stimulates insulin release by itself, is used experimentally to study insulin secretion. KIC is the first intermediate in leucine metabolism. Leucine is the only other normal physiologic stimulator of insulin secretion besides glucose. Both KIC and leucine form substantial amounts of acetoacetate that can exit the mitochondria and increase the levels of short chain fatty acyl-CoAs in the cytosol [14]. To understand the relative contribution of the pathway involving ATP citrate lyase versus the acetoacetate pathway in generating cytosolic short chain acyl-CoAs in insulin secretion, we used 13C-labeled glucose and KIC and monitored 13C flux into short chain acyl-CoAs in the INS-1 832/13 cell line, a cell line of homogeneous pancreatic beta cells in which we decreased ATP citrate lyase enzyme activity. We lowered ATP citrate lyase enzyme activity with hydroxycitrate, an inhibitor of with ATP citrate lyase or used an ATP citrate lyase knockdown cell line generated from INS-1 832/13 cells using shRNA technology [15]. Flux of 13C glucose into fatty acids was also monitored as a final end product of cytosolic acetyl-CoA and malonyl-CoA.
2. Materials and methods
2.1. Materials
[U-13C6]α-Ketoisocaproic was purchased from Cambridge Isotopes (Cambridge, MA, USA). All other chemicals were purchased from Sigma–Aldrich in the highest purity available unless otherwise mentioned. INS-1 832/13 cells were from Chris Newgard [18]
2.2. Cell culture
The ATP citrate lyase knockdown cell line ACLY-940-12 and the control cell line with a scrambled shRNA (U6) were generated from the INS-1 832/13 cell line as previously described. The ACLY 940-12 cell line was one of four stable shRNA ATP citrate lyase knockdown cell lines that previously showed strong knockdown of ATP citrate lyase enzyme activity and no inhibition of glucose-stimulated insulin release [15]. Cells were maintained in RPMI 1640 tissue culture medium (contains 11.1 mM glucose) supplemented with 2 mM glutamine, 1 mM sodium pyruvate, 10% fetal bovine serum, 10 mM HEPES buffer, 100 units/ml penicillin, 100 μg/ml streptomycin, 250 ng/ml amphotericin B, and 50 μM β-mercaptoethanol (INS-1 medium). Hygromycin-B 150 μg/ml was used for continual selection of the ACLY 940-12 and U6 cell lines. For metabolomics experiments, cells were cultured in 6 cm tissue culture plates until the cells were 80% confluent. Cells were then maintained for 6 h in RPMI 1640 medium modified to contain 3 mM glucose (with fetal bovine serum, sodium pyruvate, HEPES buffer and β-mercaptoethanol) before replacing the medium with Krebs–Henseleit solution (KRHB) containing no glucose [19] for another 30 min. This 30 min pre-incubation was followed by stimulation with 10 mM uniformly 13C-labeled glucose or 2 mM uniformly 13C-labeled α-ketoisocaproic acid (KIC) for another 30 min. One set of cells was harvested after 30 min in the presence of KRHB alone (zero time) for the measurement of metabolites and a second set of cells after further incubation for the second 30 min in the presence of the 13C-labeled glucose or α-ketoisocaproic acid.
When INS-1 832/13 cells were incubated with hydroxycitrate, it was present for the 30 min pre-incubation time period in Krebs–Henseleit solution as well as for the subsequent 30 min in the presence of glucose or α-ketoisocaproaic acid.
2.3. Insulin release and enzyme activity
Glucose-stimulated insulin release [11], [13], [14] and ATP citrate lyase enzyme activity [15] were measured as previously described.
2.4. Metabolite extraction and measurements
Metabolites were extracted in 8:1:1 methanol:chloroform:water. Polar metabolites were separated on a Luna NH2 column (2 mm × 150 mm) from Phenomenex, (Torrance, CA). Metabolites were detected by time of flight mass spectrometry in the negative mode as described previously [19], [20], [21]. Metabolites were identified using accurate mass or authentic standards (if available). Relative peak areas were used for relative quantification of identified metabolites [19], [20], [21]. For absolute quantification, metabolites were quantified using a standard addition approach in which a set of standard concentrations was spiked into a control sample and the endogenous concentration was measured. Metabolite levels were expressed on the basis of protein content. Whole cell protein content was measured on identical companion tissue culture plates using the Pierce BCA assay (Thermo-Scientific) according to the manufacturer's instructions.
3. Results
3.1. Inhibition of ATP citrate lyase with hydroxycitrate does not inhibit glucose-stimulated insulin release and does not affect flux of glucose into short chain acyl-CoAs
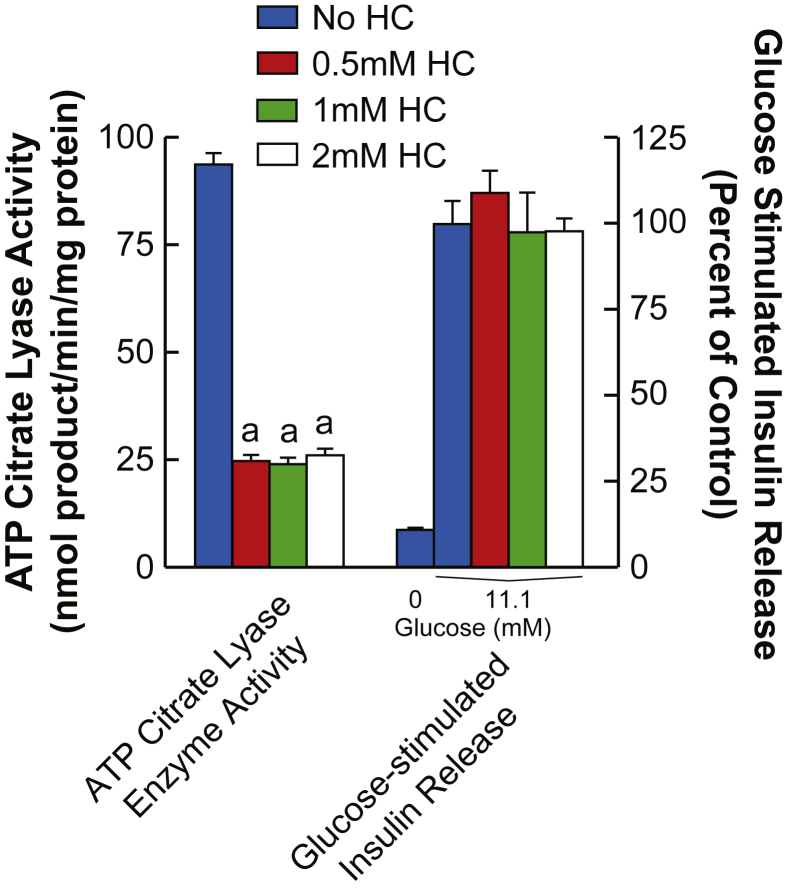
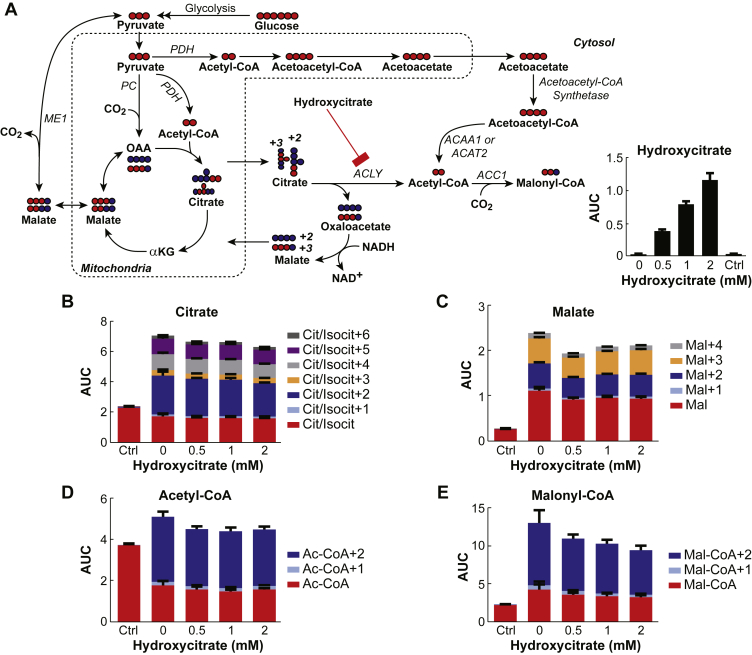
Concentrations of hydroxycitrate, a specific inhibitor of ATP citrate lyase [22], [23], of 20 μm, 50 μm, 100 μm, and 200 μm inhibited ATP citrate lyase 28–45% and showed no effect on glucose-stimulated insulin release or on the levels and incorporation of 13C into malate, citrate, acetyl-CoA, and malonyl-CoA (data not shown). Therefore, further studies were carried out with higher concentrations of hydroxycitrate. Figure 1 shows that although 0.5 mM, 1 mM, and 2 mM concentrations of hydroxycitrate inhibit ATP citrate lyase enzyme activity in the cytosol of INS-1 832/13 cells at the most by 73%, the same concentrations of hydroxycitrate do not inhibit glucose-stimulated insulin release from these cells. In a separate experiment not shown here, we found that 10 mM hydroxycitrate inhibited ATP citrate lyase enzyme activity by no more than 60%. To study the flux of glucose into metabolites INS-1 832/13 cells were incubated with concentrations of hydroxycitrate of 0.5 mM, 1 mM, and 2 mM starting 30 min before and continuing for another 30 min after 10 mM [U-13C]glucose was added to the cells (Figure 2A). Measurements of the intracellular concentration of hydroxycitrate showed that it was increased (Figure 2A). If the only pathway for the formation of short chain acyl-CoA in pancreatic beta cells requires ATP citrate lyase, its inhibition by hydroxycitrate should decrease the conversion of citrate into acetyl-CoA and oxaloacetate that is converted into malate in the cytosol (Figure 2A). All concentrations of hydroxycitrate showed a minimal effect on flux of glucose into citrate and malate (Figure 2B and C). Importantly, hydroxycitrate showed no effect on the increase in the +2 isotopomer of both acetyl-CoA and malonyl-CoA (Figure 2D and E). This +2 labeling begins with the formation of acetyl-CoA from glucose-derived pyruvate in the pyruvate dehydydrogenase reaction. Although acetyl-CoA is formed in both the mitochondria and cytosol, malonyl-CoA is formed only in the cytosol indicating the presence of a pathway that does not involve ATP citrate lyase. The lack of inhibition of the increase in the +2 isotopomer of malonyl-CoA suggests that in beta cells [U-13C]glucose is not metabolized to short chain acyl-CoAs solely in a pathway that involves citrate and the ATP citrate lyase reaction.
Figure 1.
Inhibition of ATP citrate lyase with hydroxycitrate does not inhibit glucose-stimulated insulin release in pancreatic beta cells. ATP citrate lyase enzyme activity in a cytosol fraction of INS-1 832/13 cells was assayed in the absence and in the presence of 0.5 mM, 1 mM, and 2 mM hydroxycitrate. ap < 0.01 vs control without hydroxycitrate. Insulin release from INS-1 832/13 cells incubated for 1 h in the absence of glucose or in the presence of 11.1 mM glucose (a maximal stimulus for INS-1 832/13 cells) without hydroxycitrate (control) or in the presence of 0.5, 1 mM, or 2 mM hydroxycitrate. ATP citrate lyase enzyme activity and glucose-stimulated insulin release without hydroxycitrate were 98 ± 3 (mean ± SE, N = 16) nmol product formed/min/mg cytosol protein and 18.5 ± 0.8 (mean ± SE, N = 12) mU insulin/mg cell protein/h, respectively. Insulin release results are presented as percent of the control with 11.1 mM glucose and without hydroxycitrate.
Figure 2.
Inhibition of ATP citrate lyase with hydroxycitrate does not inhibit flux of [U-13C]glucose through citrate into various metabolites in pancreatic beta cells. A. Metabolic pathways showing normal flux of glucose to cytosolic acyl-CoAs through the acetoacetate pathway and diminished flux of glucose through the citrate pathway by inhibition of ATP citrate lyase with hydroxycitrate. Abbreviations: ACAA1, acetyl-CoA acyltransferase 1; ACAT2, acetyl-CoA acetyltransferase 2; ACC1, acetyl-CoA carboxylase 1; ACLY, ATP citrate lyase; αKG, α-ketoglutarate; ME1, malic enzyme 1; OAA, oxaloacetic acid; PC, pyruvate carboxylase; PDH, pyruvate dehydrogenase. B–E. Relative total concentrations and mass isotopomer distributions in citrate, malate, acetyl-CoA and malonyl-CoA. INS-1 832/13 cells were incubated in KRHB containing no glucose and no hydroxycitrate as a control (Ctrl) and in the presence of zero to 2 mM hydroxycitrate for 30 min. Cells were kept in the presence of hydroxycitrate and stimulated with 10 mM [U-13C]glucose for an additional 30 min before metabolism was stopped and cells were harvested. Results are shown as the mean ± SE, n = 4 for each condition. None of the total metabolite or isotopomer concentrations in the cells stimulated with glucose in the presence of hydroxycitrate were statistically different from the concentrations of the same metabolites in the cells stimulated with glucose alone.
3.2. ATP citrate lyase knockdown does not inhibit glucose-stimulated insulin release and does not affect the flux of glucose into short chain acyl-CoAs
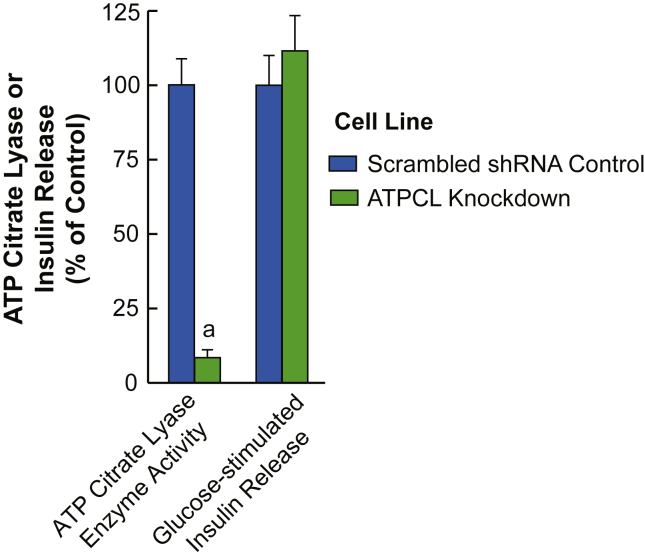
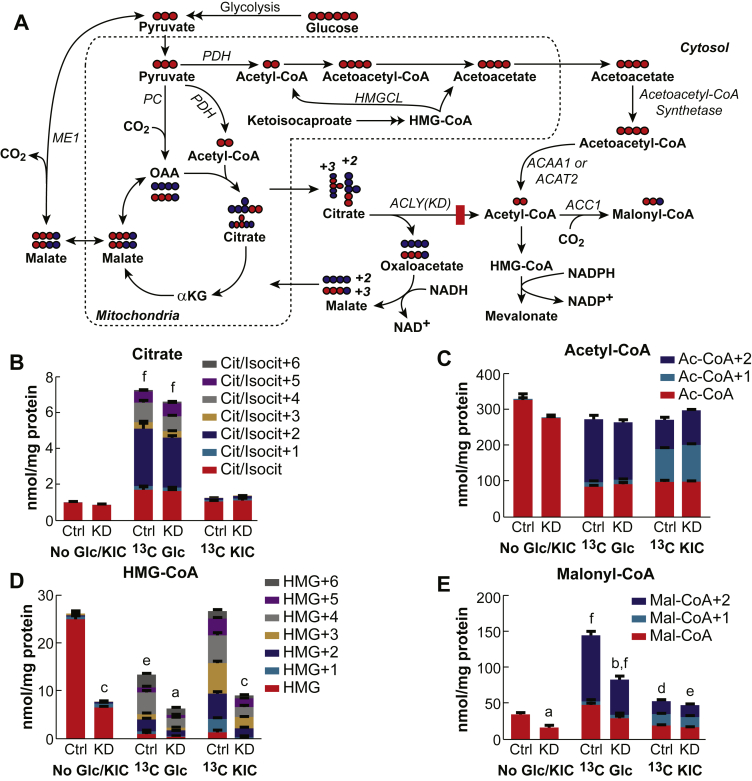
Using more specific and almost complete inhibition of ATP citrate lyase, we studied the ATP citrate lyase stable knockdown cell line ACLY 940-12 [15]. Figure 3 shows that >90% knockdown of ATP citrate lyase enzyme activity in this INS-1 832/13-derived cell line does not inhibit glucose-stimulated insulin release. Figure 4A shows the acetoacetate pathway and the citrate pathways for the incorporation of glucose carbon into acetyl-CoA and malonyl-CoA. With the block at ATP citrate lyase in the citrate pathway, the only pathway for the formation of malonyl-CoA is from the extramitochondrial acetyl-CoA derived from acetoacetate exported from the mitochondria to the cytosol (Figure 4A).
Figure 3.
Strong knockdown of ATP citrate lyase enzyme activity does not inhibit glucose-stimulated insulin release. ATP citrate lyase enzyme activity and 11.1 mM glucose-stimulated insulin release were compared in the INS-1 832/13-derived cell lines containing either a scrambled shRNA or the ATP citrate lyase knockdown cell line ACLY 940-12. Results are the mean ± SE of 8 comparisons of cell preparations of enzyme activity and 11 experiments of 4 replicate incubations of each cell line comparing insulin release. ap < 0.001 vs control.
Figure 4.
Knockdown of ATP citrate lyase does not decrease the flux of [U-13C]glucose and [U-13C]KIC into various metabolites. A. Metabolic pathways showing normal flux of glucose flux to cytosolic acyl-CoAs through the acetoacetate pathway and blocked flux through the citrate pathway by >90% knockdown of ATP citrate lyase in the cell line ACLY 940-12. Abbreviations are the same as in Figure 2A except ACLY(KD) indicates ATP citrate lyase knockdown and HMGCL indicates HMG-CoA lyase. B–E. Relative total concentrations and mass isotopomer distributions in citrate, acetyl-CoA, HMG-CoA, and malonyl-CoA. The ATP citrate lyase knockdown cell line ACLY-940-12 (KD) or the control cell line containing a scrambled shRNA (Ctrl) were incubated in the presence of KRHB without glucose for 30 min before stimulation with 10 mM [U-13C]glucose or 2 mM [U-13C]KIC for an additional 30 min. As an additional control, cells were also incubated with no glucose and no KIC for the entire 60 min (No Glc/KIC). Results are shown as the mean ± SE, n = 4 for each condition. ap < 0.05, bp < 0.01 and cp < 0.001 total metabolite concentration in KD cells vs control (Ctrl) with same stimulus. For the same stimulus comparisons, none of the differences in isotopomer concentrations between the control and ATP citrate lyase knockdown cell line achieved statistical significance with the single exception of KIC stimulation where almost all isotopomers in HMG-CoA were statistically lower in the KD cell line. dp < 0.05, ep < 0.01 and fp < 0.001 total metabolite concentration in a stimulated cell line (13C Glc or 13C KIC) vs same cell line with no stimulus (No Glc/KIC).
Prior to stimulation with glucose or KIC, both cell lines showed similar levels of citrate and acetyl-CoA while the ATP citrate lyase knockdown cell line showed decreased levels of malonyl-CoA and HMG-CoA (Figure 4D and E). Stimulation with 10 mM [U-13C]glucose increased the total levels of citrate and acetyl-CoA equally in both cell lines (Figure 4B and C) with similar isotopomer distribution. The main isotopomers of citrate were the +2 isotopomer coming from pyruvate dehydrogenase-derived acetyl-CoA and +3 isotopomer coming from pyruvate carboxylase-derived oxaloacetate. Acetyl-CoA showed mainly the +2 isotopomer that is coming from pyruvate dehydrogenase activity in the mitochondria and from the breakdown of acetoacetyl-CoA derived from acetoacetate exported into the cytosol. HMG-CoA levels are known to decrease in pancreatic beta cells after glucose stimulation [19], [20], [21] due to the increased flux and consumption into the mevalonate pathway [5]. The level of HMG-CoA decreased in both the control cell line and the ATP citrate lyase knockdown cell line after glucose stimulation. The total amount of HMG-CoA was lower in the knockdown cells most likely because its level in these cells was lower before glucose stimulation (Figure 4D). The increase in the malonyl-CoA concentration and 13C-glucose incorporation into malonyl-CoA were similar in both cell lines, but the total concentration was less in the knockdown cell line due to the difference in the initial levels prior to stimulation with glucose (Figure 4E). Thus, during the course of glucose-stimulated insulin secretion, similar to with hydroxycitrate inhibition, the knockdown of ATP citrate lyase did not prevent the flux of glucose into malonyl-CoA that is formed only in the cytosol and, with knockdown of ATP citrate lyase, can only be formed via the acetoacetate pathway (Figure 4A).
KIC can stimulate insulin secretion by itself and is also the first intermediate in the leucine metabolism pathway. In the mitochondria, KIC is metabolized into HMG-CoA, which is converted into acetyl-CoA and acetoacetate (Figure 4A). [U-13C6]KIC increased the +1 and +2 isotopomers of acetyl-CoA and malonyl-CoA equally in both the control and the ATP citrate lyase knockdown cell lines (Figure 4C and E). The +1 isotopomer of acetyl-CoA and malonyl-CoA is likely coming from the degradation of +3 labeled cytosolic acetoacetate-derived acetoaceetyl-CoA into two molecules of acetyl-CoA. Although the increase in malonyl-CoA using KIC was much lower than the increase evoked by glucose, it showed increases and isotopomer distribution in the ATP citrate lyase knockdown cell line similar to those of the control cell line. This indicates that cytosolic acetyl-CoA required for malonyl-CoA formation was coming from the acetoacetate pathway and not from the citrate pathway, as KIC cannot be metabolized to citrate (Figure 4A). The HMG-CoA levels in the control and the ATP citrate knockdown cell lines with stimulation with KIC were similar to the HMG-CoA levels in the same cell line without stimulation with KIC or glucose (Figure 4D). This extensive labeling of HMG-CoA in both the control cell line and the ATP citrate lyase knockdown cell lines indicates a high rate of flux into the acetoacetate and mevalonate pathways. The total level of HMG was not deceased compared to glucose, because the consumption of HMG was replenished by KIC.
3.3. Knockdown of ATP citrate lyase does not affect flux of glucose into lipid
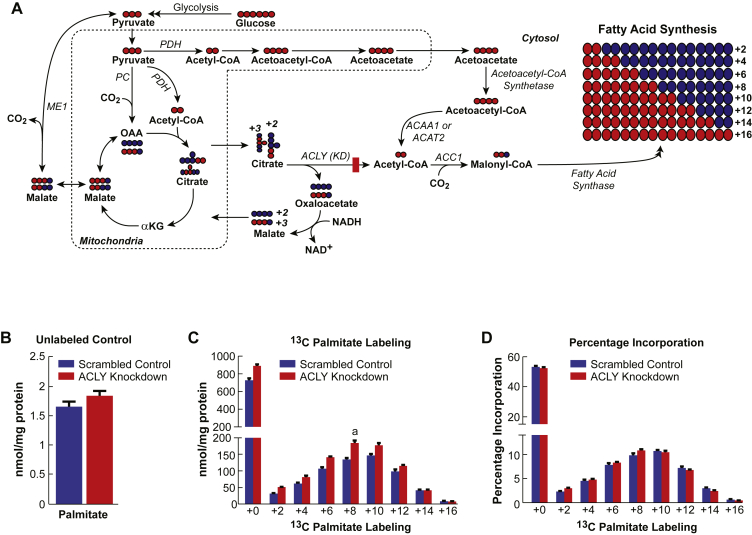
To further discern whether glucose flux into acetyl-CoA and malonyl-CoA is not affected by ATP citrate lyase knockdown, we monitored glucose flux into the de novo synthesis of fatty acid in the ATP citrate lyase knockdown cell line ACLY 940-12. This is possible because fatty acid synthesis depends on continued rounds of incorporation of 2-carbon units derived from malonyl-CoA formed in the cytosol from acetyl-CoA (Figure 5A). Cells with knocked down ATP citrate lyase or the control cells were incubated with [U-13C]glucose in RPMI cell culture medium for ∼16 h. Metabolism was then stopped, and all lipids were extracted and converted into free fatty acid methyl esters and measured using GC/MS. [U-13C]glucose incorporation into fatty acids can be determined by measuring the sequential introduction of 2 daltons (coming from acetyl-CoA via malonyl-CoA) into palmitic acid (Figure 5A). Before labeling, both cell lines showed similar levels of total palmitic acid (Figure 5B). The addition of [U-13C6]glucose to the cells revealed a substantial labeling in palmitate in both the ATP citrate lyase knockdown cell line and the control U6 cell line (Figure 5C). Although the total amount of palmitate labeling was slightly higher in knockdown cells (Figure 5C), the percentage of total incorporation of glucose into palmitate was similar between the ATP citrate lyase knockdown cell line and the control cell line confirming that acetyl-CoA and malonyl-CoA can be formed via a pathway redundant to the citrate pathway that requires ATP citrate lyase. The only known redundant pathway is the acetoacetate pathway.
Figure 5.
Knockdown of ATP citrate lyase does not decrease the flux of [U-13C]glucose into fatty acids. A. Schematic showing incorporation of 2-carbon units into palmitate via an intact acetoacetate pathway in the presence of a block at the ATP citrate lyase reaction in the citrate pathway. Abbreviations are the same as in Figure 2A except ACLY(KD) indicates ATP citrate lyase knockdown. B–D The ATP citrate lyase knockdown cell line ACLY 940-12 (ACLY Knockdown) or the scrambled shRNA control cell line (Scrambled Control) were incubated in RPMI 1640 cell culture medium containing 10 mM [U-13C]glucose for 16 h before cell metabolism was stopped. All lipids were converted into free fatty acids and measured using GC/MS. B. Levels of palmitate without [U-13C]glucose. C. Absolute amount of various isotopomers of palmitate after exposure to the [U-13C]glucose. D. Percentage labeling of each isotopomer, normalized to the unlabeled palmitate. Results are shown as the mean ± SE with n = 4 for each condition. ap < 0.05 vs Scrambled Control.
4. Discussion
4.1. Importance of short chain acyl-CoAs in insulin secretion
There are numerous metabolic signals that mediate the glucose-stimulated insulin secretion in pancreatic beta cells. Among the most important metabolic signals are short chain acyl-CoAs, such as acetyl-CoA, acetoacetyl-CoA, malonyl-CoA, and HMG-CoA [4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15]. All of these short chain acyl-CoAs can be generated in the cytosol from glucose after glucose-derived carbon is exported from the mitochondria as citrate or acetoacetate. In the cytosol ATP citrate lyase converts citrate into acetyl-CoA from which other short chain acyl-CoAs can be formed (Figure 2, Figure 4, Figure 5A). Human pancreatic islets have a much lower activity of pyruvate carboxylase required for the citrate pathway than rodent islets or rodent-derived cell lines while human islets still maintain robust glucose-stimulated insulin secretion [9]. ATP citrate lyase knockdown with siRNA technology in INS-1 832/13 cells does not affect glucose-stimulated insulin secretion (15, 17 and Figure 3), suggesting the presence of a pathway independent of citrate and ATP citrate lyase that beta cells utilize to generate short chain acyl-CoAs. This alternative pathway can only be the acetoacetate pathway shown in Figure 2, Figure 4, Figure 5A.
4.2. Glucose flux with inhibition of ATP citrate lyase
The current work shows the redundancy of the ATP citrate lyase reaction using stable isotope labeling and mass spectrometry. ATP citrate lyase activity was lowered using hydroxycitrate to inhibit its enzyme activity by about 70% or by generating an INS-1 832/13-derived cell line with ATP citrate lyase enzyme activity knocked down >90%. This was followed by monitoring the incorporation of carbon from 13C-labeled glucose into various metabolites. The levels of citrate and acetyl-CoA, as well as the isotopomer distribution in these metabolites, did not change after ATP citrate lyase inhibition with hydroxycitrate (Figure 2B and D). However, as these two metabolites are present in both the cytosol and mitochondria, measuring their levels might not clearly show the alternative cytosolic pathway of glucose that can lead to the acetyl-CoA formation. Malonyl-CoA, on the other hand, is formed only in the cytosol, and malonyl-CoA showed no difference in flux from glucose after hydroxycitrate treatment that inhibited ATP citrate lyase (Figure 2E). In the cytosol, malonyl-CoA is the precursor for fatty acid synthesis. In the ATP citrate lyase knockdown cells, flux from glucose into malonyl-CoA was substantial, but the unstimulated levels of malonyl-CoA were lower before glucose treatment (Figure 4E). Although the absolute level of malonyl-CoA in the ATP citrate lyase knockdown cells was lower before and after the addition of glucose in the ATP citrate lyase knockdown cells, the increase in incorporation of 13C-glucose carbon into malonyl-CoA (Figure 4E) and palmitate (Figure 5C) in the ATP citrate lyase knockdown cells after the addition of glucose was similar to that in the control cells. This suggests that the citrate pathway alone, which uses ATP citrate lyase, with or without the assistance from the acetoacetate pathway, maintains malonyl-CoA levels in resting beta cells, but, with glucose stimulation, the acetoacetate pathway is the pathway that supplies short chain acyl-CoAs to the cytosol. This observation is consistent with data from the current study (Figure 3) and previous studies [15], [17] that show that knockdown of ATP citrate lyase does not inhibit glucose-stimulated insulin secretion, but knockdown of acetoacetyl-CoA synthetase [15] and SCOT [10] that catalyze reactions in the acetoacetate pathway does inhibit glucose-stimulated insulin release. HMG-CoA levels showed a pattern similar to malonyl-CoA (Figure 4D), where 13C glucose was significantly incorporated into the HMG-CoA while it showed lower starting levels in the ATP citrate lyase knockdown cells.
4.3. α-Ketoisocaproic acid flux with inhibition of ATP citrate lyase
To monitor the flow of carbons not derived from glucose through acetyl-CoA and malonyl-CoA, we used 13C-labeled KIC. KIC forms HMG-CoA that is then converted to acetyl-CoA and acetoacetate catalyzed by HMG-CoA lyase in the mitochondria. The acetoacetate can then be exported to the cytosol and converted to acetoacetyl-CoA followed by the other short chain acyl-CoAs (Figure 4A). As expected, KIC did not induce any labeling in citrate. However, it caused a significant labeling in acetyl-CoA and malonyl-CoA (Figure 4C and 4E). As expected, the levels and labeling of both metabolites were similar between the ATP citrate lyase knockdown and the control cell lines because ATP citrate lyase is not used in the pathway from KIC to cytosolic acetyl-CoA and malonyl-CoA formation. This further implicates the acetoacetate pathway in insulin secretion. HMG-CoA levels in the KIC-stimulated ATP citrate lyase knockdown cells and control cells were similar to their levels in the same cell lines prior to stimulation (Figure 4D).
5. Conclusion
The flux of glucose into cytosolic short chain acyl-CoAs was maintained in pure beta cells in the presence of inhibition of ATP citrate lyase with hydroxycitrate and also in a cell line with >90% ATP citrate lyase knockdown suggesting that a pathway other than the citrate pathway is responsible for the flow of carbons into short chain acyl-CoAs during insulin secretion. The only known pathway other than through the ATP citrate lyase reaction leading to the formation of short chain acyl-CoAs in the cytosol is the acetoacetate pathway (Figure 4, Figure 5A). In the ATP citrate lyase knockdown beta cell line prior to stimulation with glucose the level of malonyl-CoA was lower compared to the control cell line, but with glucose stimulation the increase in the level of malonyl-CoA and glucose incorporated into malonyl-CoA in the ATP citrate lyase knockdown cell line was similar to that in the control cell line (Figure 4E). The results suggest that in resting beta cells the citrate pathway, with or without help from the acetoacetate pathway, maintains short chain acyl-CoA levels in the cytosol, but during insulin stimulatory conditions, such as in the presence of increased concentrations of glucose, the acetoacetate pathway is primarily responsible for supplying cytosolic short chain acyl-CoAs to the cytosol. This idea is consistent with the fact that during glucose stimulation the mitochondrial concentration of succinate increases to high levels and SCOT, which uses succinate as a substrate to form acetoacetate, has a relatively high Km for succinate [10], [15] and, therefore, the acetoacetate pathway can act as a sensor during glucose-stimulated insulin secretion. Glucose incorporation into palmitate in the ATP citrate lyase knockdown cells was even slightly higher in the ATP citrate lyase knockdown cell line than in the control cell line (Figure 5C). This also supports the idea of an active acetoacetate pathway supplying cytosolic acetyl-CoA and malonyl-CoA during glucose-stimulated insulin secretion.
Author contribution
MEA designed and executed experiments, researched the data and wrote the manuscript. MJL and IHA designed and executed experiments. RTK and CFB reviewed the data and contributed to the writing of the manuscript. MJM designed experiments, researched the data and wrote the manuscript.
Funding
This work was supported by the National Institutes of Health [grant numbers NIH DK28348 to MJM and NIH DK046960 to RTK]; the Nowlin Family Trust of the InFaith Community Foundation (to MJM); The Michigan Regional Comprehensive Metabolomics Resource Core Grant U24 [grant number DK097153]; and the A. Alfred Taubman Institute (to C.F.B).
Acknowledgements
The authors thank Scott Stoker for excellent technical assistance.
Disclosure Statement
The authors declare no conflict of interest.
References
- 1.Henquin J.C. Triggering and amplifying pathways of regulation of insulin secretion by glucose. Diabetes. 2000;49:1751–1760. doi: 10.2337/diabetes.49.11.1751. [DOI] [PubMed] [Google Scholar]
- 2.Straub S.G., Sharp G.W. Glucose-stimulated signaling pathways in biphasic insulin secretion. Diabetes/Metabolism Research and Reviews. 2002;18:451–463. doi: 10.1002/dmrr.329. [DOI] [PubMed] [Google Scholar]
- 3.Corkey B.E., Deeney J.T., Yaney G.C., Tornheim K., Prentki M. The role of long-chain fatty acyl-CoA esters in beta-cell signal transduction. Journal of Nutrition. 2000;130:299S–304S. doi: 10.1093/jn/130.2.299S. [DOI] [PubMed] [Google Scholar]
- 4.Farfari S., Schulz V., Corkey B., Prentki M. Glucose-regulated anaplerosis and cataplerosis in pancreatic beta-cells: possible implication of a pyruvate/citrate shuttle in insulin secretion. Diabetes. 2000;49:718–726. doi: 10.2337/diabetes.49.5.718. [DOI] [PubMed] [Google Scholar]
- 5.MacDonald M.J., Fahien L.A., Brown L.J., Hasan N.M., Buss J.D., Kendrick M.A. Perspective: emerging evidence for signaling roles of mitochondrial anaplerotic products in insulin secretion. American Journal of Physiology. Endocrinology and Metabolism. 2005;288:E1–E15. doi: 10.1152/ajpendo.00218.2004. [DOI] [PubMed] [Google Scholar]
- 6.Jitrapakdee S., Wutthisathapornchai A., Wallace J.C., MacDonald M.J. Regulation of insulin secretion: role of mitochondrial signalling. Diabetologia. 2010;53:1019–1032. doi: 10.1007/s00125-010-1685-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prentki M., Matschinsky F., Madiraju S.R. Metabolic signaling in fuel-induced insulin secretion. Cell Metabolism. 2013;18:162–185. doi: 10.1016/j.cmet.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 8.Flamez D., Berger V., Kruhoffer M., Orntoft T., Pipeleers D., Schuit F.C. Critical role for cataplerosis via citrate in glucose-regulated insulin release. Diabetes. 2002;51:2018–2024. doi: 10.2337/diabetes.51.7.2018. [DOI] [PubMed] [Google Scholar]
- 9.MacDonald M.J., Longacre M.J., Stoker S.W., Kendrick M., Thonpho A., Brown L.J. Differences between human and rodent pancreatic islets: low pyruvate carboxylase, ATP citrate lyase, and pyruvate carboxylation and high glucose-stimulated acetoacetate in human pancreatic islets. Journal of Biological Chemistry. 2011;286:18383–18396. doi: 10.1074/jbc.M111.241182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hasan N.M., Longacre M.J., Seed Ahmed M., Kendrick M.A., Gu H., Ostenson C.G. Lower succinyl-CoA:3-ketoacid-CoA transferase (SCOT) and ATP citrate lyase in pancreatic islets of a rat model of type 2 diabetes: knockdown of SCOT inhibits insulin release in rat insulinoma cells. Archives of Biochemistry and Biophysics. 2010;499:62–68. doi: 10.1016/j.abb.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Macdonald M.J., Hasan N.M., Longacre M.J. Studies with leucine, beta-hydroxybutyrate and ATP citrate lyase-deficient beta cells support the acetoacetate pathway of insulin secretion. Biochimica et Biophysica Acta. 2008;1780:966–972. doi: 10.1016/j.bbagen.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.MacDonald M.J., Stoker S.W., Hasan N.M. Anaplerosis from glucose, alpha-ketoisocaproate, and pyruvate in pancreatic islets, INS-1 cells and liver mitochondria. Molecular and Cellular Biochemistry. 2008;313:195–202. doi: 10.1007/s11010-008-9757-x. [DOI] [PubMed] [Google Scholar]
- 13.MacDonald M.J., Longacre M.J., Stoker S.W., Brown L.J., Hasan N.M., Kendrick M.A. Acetoacetate and beta-hydroxybutyrate in combination with other metabolites release insulin from INS-1 cells and provide clues about pathways in insulin secretion. American Journal of Physiology. Cell physiology. 2008;294:C442–C450. doi: 10.1152/ajpcell.00368.2007. [DOI] [PubMed] [Google Scholar]
- 14.MacDonald M.J. Synergistic potent insulin release by combinations of weak secretagogues in pancreatic islets and INS-1 cells. Journal of Biological Chemistry. 2007;282:6043–6052. doi: 10.1074/jbc.M606652200. [DOI] [PubMed] [Google Scholar]
- 15.MacDonald M.J., Smith A.D., 3rd, Hasan N.M., Sabat G., Fahien L.A. Feasibility of pathways for transfer of acyl groups from mitochondria to the cytosol to form short chain acyl-CoAs in the pancreatic beta cell. Journal of Biological Chemistry. 2007;282:30596–30606. doi: 10.1074/jbc.M702732200. [DOI] [PubMed] [Google Scholar]
- 16.Panten U., Willenborg M., Schumacher K., Hamada A., Ghaly H., Rustenbeck I. Acute metabolic amplification of insulin secretion in mouse islets is mediated by mitochondrial export of metabolites, but not by mitochondrial energy generation. Metabolism. 2013;62:1375–1386. doi: 10.1016/j.metabol.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 17.Joseph J.W., Odegaard M.L., Ronnebaum S.M., Burgess S.C., Muehlbauer J., Sherry A.D. Normal flux through ATP-citrate lyase or fatty acid synthase is not required for glucose-stimulated insulin secretion. Journal of Biological Chemistry. 2007;282:31592–31600. doi: 10.1074/jbc.M706080200. [DOI] [PubMed] [Google Scholar]
- 18.Hohmeier H.E., Mulder H., Chen G., Henkel-Rieger R., Prentki M., Newgard C.B. Isolation of INS-1-derived cell lines with robust ATP-sensitive K+ channel-dependent and -independent glucose-stimulated insulin secretion. Diabetes. 2000;49:424–430. doi: 10.2337/diabetes.49.3.424. [DOI] [PubMed] [Google Scholar]
- 19.Lorenz M.A., El Azzouny M.A., Kennedy R.T., Burant C.F. Metabolome response to glucose in the beta-cell line INS-1 832/13. Journal of Biological Chemistry. 2013;288:10923–10935. doi: 10.1074/jbc.M112.414961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.ElAzzouny M.A., Evans C.R., Burant C.F., Kennedy R.T. Metabolomics Analysis reveals that AICAR affects glycerolipid, ceramide and nucleotide synthesis pathways in INS-1 cells. PLoS One. 2015;10:e0129029. doi: 10.1371/journal.pone.0129029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.El-Azzouny M., Evans C.R., Treutelaar M.K., Kennedy R.T., Burant C.F. Increased glucose metabolism and glycerolipid formation by fatty acids and GPR40 receptor signaling underlies the fatty acid potentiation of insulin secretion. Journal of Biological Chemistry. 2014;289:13575–13588. doi: 10.1074/jbc.M113.531970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheema-Dhadli S., Halperin M.L., Leznoff C.C. Inhibition of enzymes which interact with citrate by (–)hydroxycitrate and 1,2,3,-tricarboxybenzene. European Journal of Biochemistry. 1973;38:98–102. doi: 10.1111/j.1432-1033.1973.tb03038.x. [DOI] [PubMed] [Google Scholar]
- 23.Board M., Newsholme E. Hydroxycitrate causes altered pyruvate metabolism by tumorigenic cells. Biochemistry and Molecular Biology International. 1996;40:1047–1056. doi: 10.1080/15216549600201683. [DOI] [PubMed] [Google Scholar]