SUMMARY
Humanin (HN) has cytoprotective action on male germ cells after testicular stress induced by heat and hormonal deprivation. To examine whether HN has protective effects on chemotherapy-induced male germ cell apoptosis, we treated four groups of adult rats with (i) vehicle (control), (ii) HN, (iii) cyclophosphamide (CP); or (iv) HN+CP. To investigate whether the protective effects of HN on germ cells require the presence of Leydig cells, another four groups of rats were pre-treated with ethane dimethanesulfonate (EDS), a Leydig cell toxicant, to eliminate Leydig cells. After 3 days, when Leydig cells were depleted by EDS, we administered: (i) vehicle, (ii) HN, (iii) CP; or (iv) HN+CP to rats. All rats were killed 12 h after the injection of HN and/or CP. Germ cell apoptosis was detected by TUNEL assay and quantified by numerical count. Compared with control and HN (alone), CP significantly increased germ cell apoptosis; HN +CP significantly reduced CP-induced apoptosis at early (I–VI) and late stages (IX–XIV) but not at middle stages (VII–VIII) of the seminiferous epithelial cycle. Pre-treatment with EDS markedly suppressed serum and intratesticular testosterone (T) levels, and significantly increased germ cell apoptosis at the middle (VII–VIII) stages. CP did not further increase germ cell apoptosis in the EDS-pre-treated rats. HN significantly attenuated germ cell apoptosis at the middle stages in EDS pre-treated rats. To investigate whether HN has any direct effects on Leydig cell function, adult Leydig cells were isolated and treated with ketoconazole (KTZ) to block testosterone synthesis. HN was not effective in preventing the reduction of T production by KTZ in vitro. We conclude that HN decreases CP and/or EDS-induced germ cell apoptosis in a stage-specific fashion. HN acts directly on germ cells to protect against EDS-induced apoptosis in the absence of Leydig cells and intratesticular testosterone levels are very low.
Keywords: cyclophosphamide, ethane dimethanesulfonate, germ cell apoptosis, humanin, ketoconazole, Leydig cells
INTRODUCTION
Humanin (HN) is a 24-amino acid, evolutionarily conserved, pro-survival peptide encoded from an open reading frame within the 16S rRNA gene in the mitochondria. HN was first discovered in the surviving neurons of human Alzheimer's disease brain (Hashimoto et al., 2001). Subsequently, HN was found to be cytoprotective against injury-induced apoptosis in many tissues including neuronal tissue (Hashimoto et al., 2001; Kariya et al., 2002, 2005; Nishimoto et al., 2004; Matsuoka et al., 2006; Xu et al., 2006, 2010), blood-derived cells (Wang et al., 2005), heart and blood vessels (Jung & Van Nostrand, 2003; Bachar et al., 2010; Muzumdar et al., 2010), pancreatic beta cells (Muzumdar et al., 2009; Hoang et al., 2010), and testis (Lue et al., 2010; Moretti et al., 2010; Jia et al., 2013). HN has no reported action on normal cells not under stress. HN protects cells from apoptosis due its ability to directly interact with pro-apoptotic proteins, including Bax-related proteins (Guo et al., 2003) and insulin-like growth factor binding protein-3 (IGFBP3) (Ikonen et al., 2003). These two HN binding pro-apoptotic proteins interact to promote apoptosis in the testis (Jia et al., 2010). The intracellular binding of HN with pro-apoptotic Bax inhibits Bax translocation to mitochondria, and attenuates activation of Bax-mediated apoptosis in cultured neuronal cells and in the testis (Guo et al., 2003; Jia et al., 2010). The anti-apoptotic action of HN on neurons is also mediated through an interleukin-like heterotrimeric cell membrane receptor (Hashimoto et al., 2009; Matsuoka & Hashimoto, 2010). While there are advances in understanding of the action of HN in various tissues and cells, the mechanisms of HN action on specific testicular cells remain to be defined.
HN was reported to be present in Leydig, endothelial, peritubular, and germ cells in rat testes (Colon et al., 2006). HN was identified as one of the most frequently expressed genes in the primary spermatocytes from the human testis (Liang et al., 2004), and in human spermatozoa (Moretti et al., 2010). HN protected immature Leydig cell from apoptosis induced by dexamethasone in young rats (Colon et al., 2006). We have previously reported that germ cell apoptosis induced by intratesticular hormone deprivation by GnRH antagonist and by direct pro-apoptotic action of IGFBP3 treatment (Lue et al., 2010) was significantly reduced by the co-administration of HN in rats. Although HN is expressed in the Leydig cells, whether Leydig cells play any role in the anti-apoptotic action of HN remains unknown.
Cancer chemotherapy has made considerable advances over the last several decades and has produced an increased population of post-chemotherapy cancer-free survivors. Onco-infertility is recognized as an important issue in cancer survivors in the reproductive age. How to prevent and treat chemotherapy-induced onco-infertility has become a clinically relevant and increasingly active research area in reproductive biology and oncology. This issue is important in boys where sperm banking is not possible and where testicular tissue is cryopre-served as an experimental procedure (Dohle, 2010; Trost & Brannigan, 2012; Loren et al., 2013). We have studied the potential of HN to protect against chemotherapy-induced germ cell apoptosis in murine models. In this study, we investigated whether HN can prevent cyclophosphamide (CP) and ethane dimethanesulfonate (EDS) induced germ cell apoptosis. The chemotherapeutic agent CP has been shown to increase germ cell apoptosis in rodents by a direct toxic effect on germ cells (Meistrich et al., 1982; Cai et al., 1997). EDS is a Leydig cell toxicant (Morris et al., 1986, 1997; Rommerts et al., 1988) depleting Leydig cells, decreasing intratesticular testosterone, and causing germ cell apoptosis (Sharpe et al., 1988a,b). EDS may also have a direct toxic effect on seminiferous epithelium (Sprando et al., 1990). In this study, we used EDS to deplete Leydig cells and asked the question of whether the anti-apoptotic peptide HN is effective in protecting male germ cells. We also determined whether HN has any direct effect on testosterone production using isolated adult Leydig cells exposed to ketoconazole (KTZ) in vitro. KTZ interferes with steroidogenesis and suppresses testosterone production in cultured Leydig cells (Rajfer et al., 1985; Sikka et al., 1985). Our present studies show that (i) humanin protects against chemotherapy-induced stage-specific male germ cell apoptosis, (ii) the cytoprotective actions of exogenous HN treatment on EDS-induced male germ cell apoptosis occur despite depletion of Leydig cells and low intratesticular testosterone level in adult rats.
MATERIALS AND METHODS
Animals
Young adult (3-month-old) male Sprague–Dawley rats used for the in vivo and in vitro studies were purchased from Charles River Laboratories (Wilmington, MA, USA) and housed in a standard animal facility under controlled temperature (22 °C) and photoperiod of 12 h of light and 12 h of darkness with free access to food and water. The animal use protocol was reviewed and approved by the Institutional Animal Care and Use Committee of Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center.
Experiment 1. The effects of HN on chemotherapy-induced male germ cell apoptosis with or without pre-treatment with EDS to deplete Leydig cells in rats
Thirty-two young adult (90-day-old) male rats were randomly divided into eight groups with four rats per group. To examine the effects of HN on CP-induced male germ cell apoptosis, four groups of rats were treated with (i) vehicle (control), (ii) a single intraperitoneal (IP) injection of HN (40 mg/kg BW) (synthesized by CP scientific, Sunnyvale, CA, USA), (iii) a single IP injection of CP (Cyclophosphamide Monohydrate, 70 mg/kg BW, Sigma, St. Louis, MO, USA); or 4) CP+HN to rats. To determine whether Leydig cells were involved in the cytoprotective action of HN on germ cell apoptosis, we pre-treated four groups of rats with IP injection of ethane dimethanesulfonate (EDS, 80 mg/kg) to eliminate Leydig cells. EDS was a gift from M. Meistrich, PhD, MD Anderson Cancer Center. EDS was synthesized by the M.D. Anderson Translational Chemistry Core Facility under the direction of Dr. William Bornmann. After 3 days, when depletion of Leydig cells occurred (Morris et al., 1997), we administered a single IP injection of vehicle (control); HN; CP; or HN+CP to four groups of EDS pre-treated rats. Twelve hours after treatment, all rats were injected with heparin (130 IU/100 g BW, i.p.) 15 min before being killed by a lethal injection of sodium pentobarbital (200 mg/kg BW i.p.) to facilitate testicular perfusion using a whole-body perfusion technique (Lue et al., 2010). Body weight was recorded at autopsy and blood samples were collected from the right ventricle of each animal immediately after death, and plasma was separated and stored at −20 C for subsequent testosterone (T) measurement. One testis from each animal was snap-frozen in liquid nitrogen. The contralateral testis was then fixed by vascular perfusion with Bouin's solution for 40 min, preceded by a brief saline wash. Testes were removed and placed into the same fixative overnight. One slice from the middle region of Bouin's fixed testis was processed for routine paraffin embedding for histological evaluation and terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay (Lue et al., 2010).
Experiment 2. The effects of HN on cultured Leydig cells treated with Ketoconazole
Leydig cells were isolated based on a previously described procedure (Klinefelter et al., 1987; Gao et al., 1996; Salva et al., 2001). Briefly, the testes were decapsulated, and then dissociated with collagenase, dispase, DNase, and shaking for 15 min at 34 °C. The seminiferous tubules were removed by filtration through 40 μm nylon mesh (BD Falcon, Franklin Lakes, NJ, USA). Leydig cells were then harvested after centrifugation and purified using a Percoll gradient (GE Healthcare, Uppsala, Sweden) and centrifugation (60 min at 20 000 g at 4 °C). Enriched Leydig cells were harvested at densities between 1.065 (red) and 1.075 (blue) g/cm3 from the percoll gradient. These Leydig cells were washed by diluting the percoll and excluding residual germ cells and other cells using a BSA density gradient with centrifugation. The purity of the Leydig cells was >90%, as determined by histochemical staining for 3β-hydroxysteroid dehydrogenase. The cell viability, as assessed by trypan blue exclusion, was >90%. In all the in vitro experiments, 2 × 105 purified Leydig cells were added to each well of the 6-well plates in 2 mL Leydig cell culture media (Dulbecco's Modified Eagles Medium-Ham's nutrient mixture F-12, Life Technologies, Grand Island) containing penicillin and streptomycin (Invitrogen Life Technologies, Inc., Paisley, UK). Eight replicate experiments were performed where Leydig cells were incubated, respectively, with vehicle (control), HN (10 mcg/mL), KTZ (10 mcg/mL, Sigma Aldrich, St. Louis, MO, USA), or KTZ + HN at 34 °C for 4 h. After treatment, the culture medium from each well was collected and stored at −20 °C for testosterone measurement.
Immunohistochemistry for localization of HN in testes
Endogenous HN localization in rat testes was detected by immunohistochemistry using rat humanin (rattin)-specific antibody. In brief, after deparaffinization and rehydration, testicular sections were first incubated with a rabbit polyclonal anti-rat humanin (rattin) antibody (Abcam, Cambridge, MA, USA) at a concentration of 10 mcg/mL at 4 °C overnight and then followed by Alexa Fluor-594 conjugated anti-rabbit secondary antibody (Invitrogen, Life Technologies, Grand Island, NY, USA) at a concentration of 20 mcg/mL for 1 h at room temperature. For negative controls, sections were treated only with secondary antibody. Slides were counterstained with 4′, 6-diamidino-2-phenylindole (DAPI) and reviewed with a Zeiss Axioskop 40 fluorescent microscope.
TUNEL assay for assessment of apoptotic cells in testicular sections
The in situ detection of cells with DNA strand breaks by terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) was performed in paraffin-embedded testicular sections using ApopTag Peroxidase in Situ Apoptosis Detection Kit (Milli-pore, Billerica, MA, USA) as described earlier (Lue et al., 2000). Negative and positive controls were carried out in every assay. For negative controls, tissue sections were processed in an identical manner, except that the terminal deoxynucleotidyl transferase enzyme was substituted with the same volume of phosphate buffered saline (PBS). The apoptotic germ cell population was enumerated out by counting TUNEL positive germ cells using the Axioskop 40 microscope (Zeiss, Thornwood, NY, USA). The rate of germ cell apoptosis (Apoptotic Index, AI) was expressed as the percentage (%) of the number of the cross sections of seminiferous tubules containing TUNEL positive apoptotic germ cells/number of cross sections of all seminiferous tubules at early (I–VI) and late (IX–XIV), and middle (VII–VIII) stages on the slide. In addition, the different types of apoptotic germ cells were assessed using the same testicular sections.
Testosterone measurement
Testosterone concentrations in plasma, testes, and culture medium were measured by a previously described radioimmunoassay (Lue et al., 2010) with a minimal detection limit of 0.1 ng/mL. The intra- and inter-assay coefficients of variations were 8 and 11%, respectively.
Statistical analysis
Statistical analyses were performed using SAS 9.3 (SAS Institute, Carey, NC, USA). The in vivo and in vitro data were analyzed by unpaired Student's t-test after confirmation by Shapiro–Wilk test to reject statistically significant non-normality. For the in vivo experiments, we first assessed whether or not CP, EDS or both increased the AI using the unpaired Student's t-test by comparing CP vs control, EDS vs control, and EDS+CP vs control, respectively. We then examined contrasts of a priori interest which were focused on the effects of HN: CP vs CP+HN; EDS vs EDS+HN; and EDS+CP vs EDS+CP+HN. Statistical comparison of AI in these groups of interest were shown in the figures, whereas those between other groups were not shown in the figures but included in the description in the text. For the in vitro analyses, the experiment was designed to determine if HN could salvage testosterone production (treated with KTZ) from Leydig cells in vitro. Accordingly, we first assessed whether or not KTZ reduced testosterone by unpaired Student's t-test by comparing KTZ vs control. We next assessed by unpaired Student's t-test whether HN preventing this occurring by comparing KTZ vs KTZ+HN. Statistical significance was construed when <0.05. All tests were two-sided.
RESULTS
Rat HN protein (Rattin) is expressed in Leydig cells and germ cells in rat testes
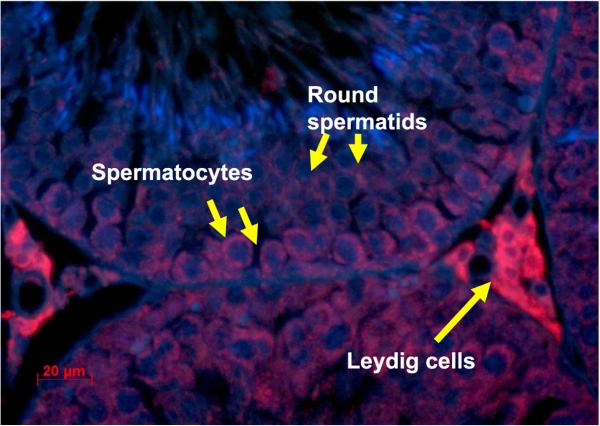
Using immunohistochemistry and a specific antibody against rat HN (also named rattin in rat), we showed that rat HN was expressed in Leydig cells, spermatocytes, and round spermatids. HN appeared predominantly localized in the cytoplasm of Leydig cells, and moderately in cytoplasm of spermatocytes and round spermatids in adult rat testes (Fig. 1).
Figure 1.
Expression of rat HN (ratting) in a testis section of an adult rat. HN detected with antibody against rat HN was present in Leydig cells (arrows), primary spermatocytes, round spermatids, and peritubular myoid cells.
EDS eliminated Leydig cells and suppressed testosterone production
Histology of testes obtained from rats treated with EDS for 3 days showed the expected depletion of Leydig cells in the interstitial spaces compared with control animals. HN administered 3 days after EDS pre-treatment did not reverse the EDS-induced Leydig cell loss (Figure S1). In all groups treated with EDS, intratesticular (Fig. 2A) and serum testosterone levels (Fig. 2B) were decreased to very low levels (both p < 0.0005) (dark bars) compared with those without EDS pre-treatment. CP or HN had no significant effects (p > 0.05 in all comparisons) on serum and intratesticular testosterone levels with or without EDS pre-treatment (Fig. 2A and B).
Figure 2.
Intratesticular (A) and serum (B) testosterone (T) levels in animals not pre-treated with ethane dimethanesulfonate (EDS) (HN, CP, CP+HN) were not significantly different from vehicle-treated rats (Light bars). In the EDS-treated animals, intratesticular and serum testosterone levels were markedly reduced in all groups (EDS, EDS + HN, EDS + CP, EDS + CP + HN). HN did not restore intratesticular and serum testosterone levels in the EDS-treated rats (Dark bars). There were four rats in each group.
HN attenuates CP-induced germ cell apoptosis in early (I-VI) and late (IX-XIV) stages of the seminiferous epithelial cycle
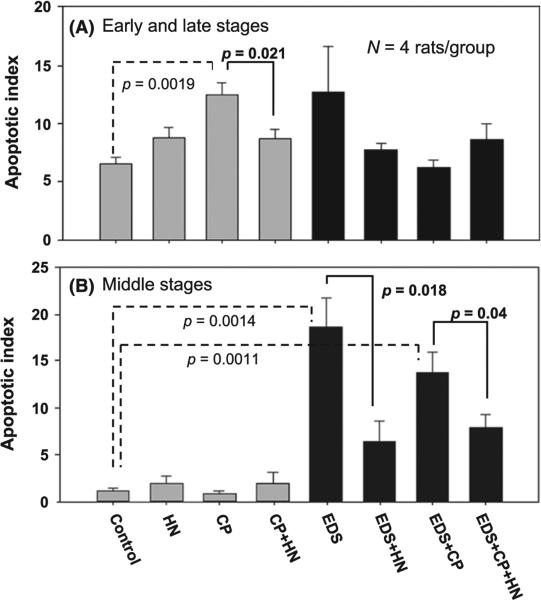
We used CP to induce germ cell apoptosis and examined the cytoprotective efficacy of HN on germ cells against apoptosis in adult rats with or without EDS pre-treatment. In both Fig. 3 A and B, the light bars represent animals not pre-treated with EDS where Leydig cells were present in the interstitial space, and the dark bars represent EDS pre-treated animals with testes depleted of Leydig cells. Figure 3A presents germ cell apoptosis index (AI) obtained in early (I–VI) and late (IX–XIV) stages, and Fig. 3B middle (VII–VIII) stages of the seminiferous epithelium cycle.
Figure 3.
(A) Apoptotic Index [% of cross sections of seminiferous tubules (ST) containing TUNEL positive germ cells/total cross sections of seminiferous tubules] at early (I–VI) and late (IX–XIV) stages of seminiferous epithelium cycle. In the groups of rats with the presence of Leydig cells (light bars), CP increased apoptosis compared with vehicle (p = 0.0019, dashed bracket) control, and HN reduced the CP-induced apoptosis (p = 0.021). Ethane dimethanesulfonate (EDS) pre-treatment (dark bars) had a minimal effect on apoptosis of germ cells (p = 0.169), and HN had no significant effect on apoptosis in the EDS (p = 0.251) (Fig. 3A, dark bars) at early and late stages. B) Apoptotic Index at middle (VII–VIII) stages of seminiferous epithelium cycle. CP did not increase apoptosis in the middle stages in animals not pre-treated with EDS (Fig. 3B. light bars). In the EDS pre-treated rats (dark bars), EDS increased apoptosis of germ cells compared with control (p = 0.0014, dashed bracket) and HN decreased apoptosis levels in EDS-treated rats (p = 0.018). Addition of CP to EDS did not further increased germ cell apoptosis. CP+EDS increased apoptosis compared with control (p = 0.0011, dashed bracket) and HN prevented apoptosis in EDS+CP-treated rats (p = 0.04) (Fig. 3B. Dark bars). There were four rats in each group.
In these short-term experiments, CP significantly increased germ cell apoptosis at early (I–VI) and late (IX–XIV) stages of the seminiferous epithelial cycle compared with control (p = 0.0019) (Fig. 3A. light bars). At these early and late stages, CP treatment mainly induced apoptosis of spermatocytes, round spermatid, and differentiated spermatogonia. HN cotreatment significantly reduced CP-induced apoptosis at early and late stages (p = 0.021, Fig. 3A, light bars) of seminiferous epithelial cycle in adult rats. Because CP treatment at this dose level for such a short duration did not increase apoptosis in the middle (VII–VIII) stages of the seminiferous epithelial cycle (p = 0.328), HN had no effect on CP-treated testis (p = 0.427) (Fig. 3B, light bars).
HN attenuates EDS-induced germ cell apoptosis in middle stages (VII-VIII) of the seminiferous epithelial cycle
Pre-treatment with EDS had significant acute cytotoxic effects on germ cells (p = 0.0014) when compared with control in the androgen sensitive middle (VII–VIII) stages (Fig. 3B, dark bars). EDS pre-treatment mainly induced apoptosis of preleptotene and pachytene spermatocytes, round spermatids, and elongated spermatids at the middle stages. The EDS effect may be a direct cytotoxic effect or mediated by the extremely low intratesticular testosterone levels and the depletion of Leydig cells. HN administered 3 days after treatment by EDS significantly reduced the EDS-induced apoptosis of germ cells (p = 0.018). Addition of CP treatment in EDS pre-treated rats increased germ cell apoptosis (p = 0.0011) as compared with control, but did not additively increased germ cell apoptosis caused by EDS treatment at middle stages (p = 0.248). The increase in apoptosis in CP+EDS group was reduced by concomitant HN treatment (p = 0.04) at the middle stages (Fig. 3B dark bars).
EDS pre-treatment did not significantly increase germ cell apoptosis at the early (I–VI) and late (IX–XIV) stages compared with controls (p = 0.169) and addition of HN did not significantly reduced germ cell apoptosis (p = 0.251) (Fig. 3 A, dark bars). Similarly in these early and late stages, CP also did not significantly increased germ cell apoptosis compared with control (p = 0.617) and HN did not change the CP effects in EDS pre-treated animals where Leydig cells were absent (p = 0.173) (Fig. 3A dark bars). However, the comparison of the CP effect on germ cell apoptosis in rats with or without EDS pre-treatment (CP in Fig 3A, light vs. dark bars) showed that when Leydig cells were depleted and intratesticuar testosterone was very low, CP-induced germ cell apoptosis was significantly lower in EDS pre-treated rats (p = 0.0023).
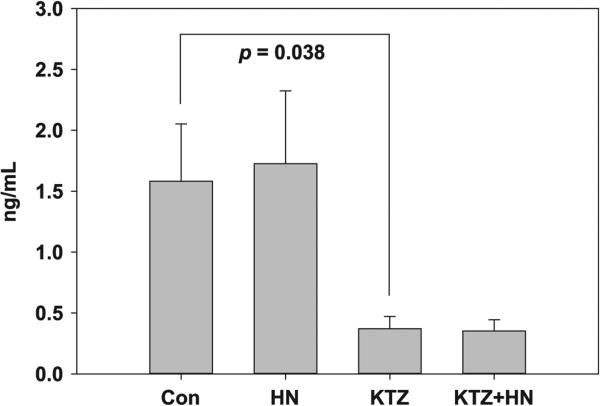
HN has no effect on KTZ-induced suppression of T of Leydig cells in vitro
KTZ, compared with control, lowered the testosterone levels significantly (p = 0.038, Fig. 4) in 4 h. HN treatment, compared with control, did not salvage the KTZ-induced decrease in testosterone production by the Leydig cells in vitro.
Figure 4.
Effect of HN on Leydig cells exposed to ketoconazole (KTZ) for 4 h in vitro. KTZ markedly reduced testosterone levels in cultured Leydig cells. HN did not restore testosterone levels suppressed by KTZ to baseline. We performed eight replicate experiments where culture Leydig cells were treated with vehicle (control), HN, KTZ, and HN+KTZ, respectively.
DISCUSSION
Testicular stressors that induce germ cell apoptosis include agents that lower intratesticular testosterone (e.g., GnRH-A, dexamethasone, EDS) (Sharpe et al., 1988a,b; Sprando et al., 1990; Sinha-Hikim & Swerdloff, 1993; Sinha Hikim et al., 1997; Woolveridge et al., 1999; Yazawa et al., 2000; Orazizadeh et al., 2009), testicular hyperthermia (Lue et al., 1999, 2000), and agents that can cause direct germ cell damage (e.g., alkylating agents such as CP) (Meistrich et al., 1982; Trasler et al., 1986; Delbes et al., 2010; Marcon et al., 2011). We have previously demonstrated that exogenous HN can mitigate male germ cell apoptosis induced by GnRH-A, and IGFBP-3 (Lue et al., 2010; Jia et al., 2013).
In this study using a rat humanin (rattin)-specific antibody, we observed that rat HN was expressed in stress-sensitive primary spermatocytes and round spermatids raising the possibility of a possible protective role of HN in these germ cells. The finding by Colon et al. (2006), as well as from this study of expression of HN in rat Leydig cells suggests Leydig cells may play a role in the cytoprotective effects of HN as Leydig cells may be one of the sources of endogenous HN with paracrine action on germ cells.
The commonly used chemotherapeutic agent CP was used to induce germ cell apoptosis. CP has been studied in both rats and mice and CP treatment induced germ cell apoptosis mainly at early and late stages of seminiferous epithelium cycle in rodents (Meistrich et al., 1982; Cai et al., 1997). Consistent with earlier report (Cai et al., 1997), we showed in this study that CP increased germ cell apoptosis at early and late stages. In these short-term experiments, we demonstrated HN was able to differentially protect against CP-induced stage-specific (early and late) germ cell apoptosis in rats. Presumably the androgen responsive middle stages were protected in short-term experiments by the presence of high intra-testicular concentrations of testosterone.
In these acute experiments, when Leydig cells were eliminated by EDS, CP failed to increase germ cell apoptosis in early and late stages which were not sensitive to androgens. Our data suggest that Leydig cells may be involved in CP-induced germ cell apoptosis and absence of Leydig cell was protective for germ cells. In prior studies, GnRH antagonist plus the anti-androgen flutamide treatment suppressed testosterone levels and action in rats and protected spermatogonial stem cell damage induced by CP or irradiation and restored spermatogenesis and fertility (Meistrich et al., 1995; Shetty & Meistrich, 2005). However, when the same principle was translated to non-human primates, suppression of testosterone by GnRH antagonist did not restore spermatogenesis induced by irradiation indicating species differences in testicular responses to GnRH antagonist and irradiation (Boekelheide et al., 2005; Shetty & Meistrich, 2005). The observation that CP-induced apoptosis of germ cells requires Leydig cells and testosterone does not distract from the principal point of this manuscript that HN protects against germ cell apoptosis in the absence of Leydig cells and despite the fact that intratesticular testosterone levels are very low.
To show that the cytoprotective effect of HN against chemotherapy-induced apoptosis of male germ cells was a direct effect on germ cells required evidence that the cytoprotective effects of HN did not require Leydig cell and its products. EDS is a cytotoxic agent that effectively eliminates Leydig cell by activating caspase-3 inducing Leydig cell apoptosis (Rommerts et al., 1988, 2004; Sprando et al., 1990; Morris et al., 1997). By selectively eliminating Leydig cells with EDS in this study, we demonstrated that EDS-induced germ cell apoptosis in short-term treatment mainly at the androgen sensitive middle stages of the seminiferous epithelium but not at non-androgen sensitive early and late stages. This suggests that EDS induces apoptosis by reducing intratesticular testosterone to very low levels. Administration of exogenous HN protected germ cells at the middle stages from apoptosis in response to EDS despite the absence of Leydig cells and testosterone. This finding was similar to our previous finding that HN protected germ cells from apoptosis induced by GnRH antagonist despite abolition of Leydig cell steroidogenesis when both intratesticular and serum testosterone levels were very low (Lue et al., 2010; Jia et al., 2013). Although we did not find any effects of HN on serum and intratesticular testosterone level in vivo (Fig 2), we could not rule out the possibility that this is because of a large variation in serum and intratesticular T levels and small number of animals examined. We also observed that CP failed to enhance EDS-induced germ cell apoptosis in the middle stages. This might be because of the following: (i) absence of testosterone protects germ cell from CP action (Meistrich et al., 1995; Shetty & Meistrich, 2005), (ii) CP does not affect germ cell apoptosis in the middle stages after 12 h treatment, and (iii) the very potent cytotoxic effect of pre-treatment of EDS may have obliterated the small effect if any of CP in the middle stages. When there was no increase in CP-induced apoptosis in middle stages or EDS-induced apoptosis in early or late stages, HN has no effect on promoting germ cell survival. This is consistent with our prior findings that HN has no effect on germ cells not under stress (Lue et al., 2010; Jia et al., 2013).
Thus, in this study, we showed that HN protects against CP-induced germ cell apoptosis at early and late stages. HN reduced EDS-induced germ cell apoptosis at middle stages despite depletion of Leydig cells by EDS. Thus, we provide strong supportive evidence that HN has direct cytoprotective action on male germ cells after chemotherapy in a stage-specific fashion. It should be emphasized that the stage specificity of the cytoprotective effects of humanin are evident in short term experiments. We anticipate that chronic treatment with cytotoxic agent will spread from the early and late stages where they begin, to all stages of the seminiferous epithelium. Once apoptosis occurs in one stage(s), with time and with continued treatment, the spermatogenic cycle will be arrested. If spermatogonia are damaged by the cytotoxic agent, the spermatogenic cycle may not recover leading to onco-infertility.
Endogenous HN is expressed in both immature and adult Leydig cells (Colon et al., 2006); the latter is confirmed in this study. To investigate the direct effects of HN on testosterone production from Leydig cells, we performed in vitro experiments to investigate synthetic HN's action on cultured adult Leydig cells treated with ketoconazole (KTZ). KTZ is known to reduce testosterone levels by acting at multiple steps in testosterone synthesis (Rajfer et al., 1985; Sikka et al., 1985). When HN was added to Leydig cells in vitro, synthetic HN at a dose examined was not able to prevent the decreased testosterone production of Leydig cells induced by treatment with KTZ. Although we failed to demonstrate the HN's protective action on testosterone production from adult Leydig cells in vitro, we cannot exclude the possibility that endogenous HN present in Leydig cells could play a role in preserving germ cells and Leydig cell function from different stressors in vivo.
In summary, we have demonstrated that with short-term treatment: (i) HN significantly reduced CP- and EDS-induced germ cell apoptosis at different stages of the seminiferous epithelium, (ii) HN protected EDS-induced germ cell apoptosis in middle stages of the seminiferous cycle despite depletion of Leydig cells and lower intratesticular testosterone levels in adult rats. Although we have previously demonstrated that HN inhibited Bax translocation from cytosol to mitochondria might be one of the intracellular mechanisms of HN preventing germ cell apoptosis (Jia et al., 2013), the underlying molecular mechanisms of the paracrine action of HN on other testicular cells remain to be determined.
Supplementary Material
ACKNOWLEDGMENTS
This study supported by the Endocrinology, Metabolism and Nutrition training Grant (T32 DK007571) and UCLA Clinical and Translational Science Institute (UL1TR000124). We thank Andrew Leung and Sima Baravarian from the Endocrine and Metabolic Research Laboratory for all assays and Vince Atienza for preparing tissues and handling of animals.
Footnotes
Data from this manuscript were presented in abstract form at the 10th International Congress of Andrology, Melbourne, 2013 and American Society of Andrology, San Antonio, Texas, 2013.
AUTHOR's CONTRIBUTIONS
Prasanth Surampudi contributed to the study design of in vitro experiment, performed in vitro experiments, and wrote the manuscript; Ivy Chang contributed to the study design of in vivo experiment, and performed quantitative assessment of germ cell apoptosis; Yanhe Lue contributed to study design, performed in vivo experiments, and did major revision of the manuscript; Tracy Doumit performed TUNEL assay; Yue Jia assisted in the in vitro experiments; Vince Atienza assisted with Leydig cell isolation, culture, and animal perfusion; Peter Y. Liu performed statistical data analysis; Ronald S. Swerdloff was involved in study design and writing and revising the manuscript; Christina Wang contributed to study design and the writing and reviewing of the revised manuscript.
DISCLOSURES
Authors have nothing to declare for this study and manuscript.
SUPPORTING INFORMATION
Additional Supporting Information may be found in the online version of this article:
REFERENCES
- Bachar AR, Scheffer L, Schroeder AS, Nakamura HK, Cobb LJ, Oh YK, Lerman LO, Pagano RE, Cohen P, Lerman A. Humanin is expressed in human vascular walls and has a cytoprotective effect against oxidized LDL-induced oxidative stress. Cardiovasc Res. 2010;88:360–366. doi: 10.1093/cvr/cvq191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boekelheide K, Schoenfeld HA, Hall SJ, Weng CC, Shetty G, Leith J, Harper J, Sigman M, Hess DL, Meistrich ML. Gonadotropin-releasing hormone antagonist (Cetrorelix) therapy fails to protect nonhuman primates (Macaca arctoides) from radiation-induced spermatogenic failure. J Androl. 2005;26:222–234. doi: 10.1002/j.1939-4640.2005.tb01089.x. [DOI] [PubMed] [Google Scholar]
- Cai L, Hales BF, Robaire B. Induction of apoptosis in the germ cells of adult male rats after exposure to cyclophosphamide. Biol Reprod. 1997;56:1490–1497. doi: 10.1095/biolreprod56.6.1490. [DOI] [PubMed] [Google Scholar]
- Colon E, Strand ML, Carlsson-Skwirut C, Wahlgren A, Svechnikov KV, Cohen P, Soder O. Anti-apoptotic factor humanin is expressed in the testis and prevents cell-death in leydig cells during the first wave of spermatogenesis. J Cell Physiol. 2006;208:373–385. doi: 10.1002/jcp.20672. [DOI] [PubMed] [Google Scholar]
- Delbes G, Vaisheva F, Luu T, Marcon L, Hales BF, Robaire B. Reversibility of the effects of the chemotherapeutic regimen for non-Hodgkin lymphoma, cyclophosphamide, doxorubicin, vincristine, and prednisone, on the male rat reproductive system and progeny outcome. Reprod Toxicol. 2010;29:332–338. doi: 10.1016/j.reprotox.2010.01.007. [DOI] [PubMed] [Google Scholar]
- Dohle GR. Male infertility in cancer patients: review of the literature. Int J Urol. 2010;17:327–331. doi: 10.1111/j.1442-2042.2010.02484.x. [DOI] [PubMed] [Google Scholar]
- Gao HB, Shan LX, Monder C, Hardy MP. Suppression of endogenous corticosterone levels in vivo increases the steroidogenic capacity of purified rat Leydig cells in vitro. Endocrinology. 1996;137:1714–1718. doi: 10.1210/endo.137.5.8612506. [DOI] [PubMed] [Google Scholar]
- Guo B, Zhai D, Cabezas E, Welsh K, Nouraini S, Satterthwait AC, Reed JC. Humanin peptide suppresses apoptosis by interfering with Bax activation. Nature. 2003;423:456–461. doi: 10.1038/nature01627. [DOI] [PubMed] [Google Scholar]
- Hashimoto Y, Niikura T, Tajima H, Yasukawa T, Sudo H, Ito Y, Kita Y, Kawasumi M, Kouyama K, Doyu M, Sobue G, Koide T, Tsuji S, Lang J, Kurokawa K, Nishimoto I. A rescue factor abolishing neuronal cell death by a wide spectrum of familial Alzheimer's disease genes and Abeta. Proc Natl Acad Sci USA. 2001;98:6336–6341. doi: 10.1073/pnas.101133498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto Y, Kurita M, Aiso S, Nishimoto I, Matsuoka M. Humanin inhibits neuronal cell death by interacting with a cytokine receptor complex or complexes involving CNTF receptor alpha/WSX-1/gp130. Mol Biol Cell. 2009;20:2864–2873. doi: 10.1091/mbc.E09-02-0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoang PT, Park P, Cobb LJ, Paharkova-Vatchkova V, Hakimi M, Cohen P, Lee KW. The neurosurvival factor Humanin inhibits beta-cell apoptosis via signal transducer and activator of transcription 3 activation and delays and ameliorates diabetes in nonobese diabetic mice. Metabolism. 2010;59:343–349. doi: 10.1016/j.metabol.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikonen M, Liu B, Hashimoto Y, Ma L, Lee KW, Niikura T, Nishimoto I, Cohen P. Interaction between the Alzheimer's survival peptide humanin and insulin-like growth factor-binding protein 3 regulates cell survival and apoptosis. Proc Natl Acad Sci USA. 2003;100:13042–13047. doi: 10.1073/pnas.2135111100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Y, Lee KW, Swerdloff R, Hwang D, Cobb LJ, Sinha Hikim A, Lue YH, Cohen P, Wang C. Interaction of insulin-like growth factor-binding protein-3 and BAX in mitochondria promotes male germ cell apoptosis. J Biol Chem. 2010;285:1726–1732. doi: 10.1074/jbc.M109.046847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Y, Lue YH, Swerdloff R, Lee KW, Cobb LJ, Cohen P, Wang C. The cytoprotective peptide humanin is induced and neutralizes Bax after pro-apoptotic stress in the rat testis. Andrology. 2013;1:651–659. doi: 10.1111/j.2047-2927.2013.00091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung SS, Van Nostrand WE. Humanin rescues human cerebrovascular smooth muscle cells from Abeta-induced toxicity. J Neurochem. 2003;84:266–272. doi: 10.1046/j.1471-4159.2003.01524.x. [DOI] [PubMed] [Google Scholar]
- Kariya S, Takahashi N, Ooba N, Kawahara M, Nakayama H, Ueno S. Humanin inhibits cell death of serum-deprived PC12 h cells. NeuroReport. 2002;13:903–907. doi: 10.1097/00001756-200205070-00034. [DOI] [PubMed] [Google Scholar]
- Kariya S, Hirano M, Nagai Y, Furiya Y, Fujikake N, Toda T, Ueno S. Humanin attenuates apoptosis induced by DRPLA proteins with expanded polyglutamine stretches. J Mol Neurosci. 2005;25:165–169. doi: 10.1385/JMN:25:2:165. [DOI] [PubMed] [Google Scholar]
- Klinefelter GR, Hall PF, Ewing LL. Effect of luteinizing hormone deprivation in situ on steroidogenesis of rat Leydig cells purified by a multistep procedure. Biol Reprod. 1987;36:769–783. doi: 10.1095/biolreprod36.3.769. [DOI] [PubMed] [Google Scholar]
- Liang G, Zhang XD, Wang LJ, Sha YS, Zhang JC, Miao SY, Zong SD, Wang LF, Koide SS. Identification of differentially expressed genes of primary spermatocyte against round spermatid isolated from human testis using the laser capture microdissection technique. Cell Res. 2004;14:507–512. doi: 10.1038/sj.cr.7290254. [DOI] [PubMed] [Google Scholar]
- Loren AW, Mangu PB, Beck LN, Brennan L, Magdalinski AJ, Partridge AH, Quinn G, Wallace WH, Oktay K. Fertility preservation for patients with cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2013;31:2500–2510. doi: 10.1200/JCO.2013.49.2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lue YH, Hikim AP, Swerdloff RS, Im P, Taing KS, Bui T, Leung A, Wang C. Single exposure to heat induces stage-specific germ cell apoptosis in rats: role of intratesticular testosterone on stage specificity. Endocrinology. 1999;140:1709–1717. doi: 10.1210/endo.140.4.6629. [DOI] [PubMed] [Google Scholar]
- Lue Y, Hikim AP, Wang C, Im M, Leung A, Swerdloff RS. Testicular heat exposure enhances the suppression of spermatogenesis by testosterone in rats: the “two-hit” approach to male contraceptive development. Endocrinology. 2000;141:1414–1424. doi: 10.1210/endo.141.4.7416. [DOI] [PubMed] [Google Scholar]
- Lue Y, Swerdloff R, Liu Q, Mehta H, Hikim AS, Lee KW, Jia Y, Hwang D, Cobb LJ, Cohen P, Wang C. Opposing roles of insulin-like growth factor binding protein 3 and humanin in the regulation of testicular germ cell apoptosis. Endocrinology. 2010;151:350–357. doi: 10.1210/en.2009-0577. [DOI] [PubMed] [Google Scholar]
- Marcon L, Zhang X, Hales BF, Robaire B, Nagano MC. Effects of chemotherapeutic agents for testicular cancer on rat spermatogonial stem/progenitor cells. J Androl. 2011;32:432–443. doi: 10.2164/jandrol.110.011601. [DOI] [PubMed] [Google Scholar]
- Matsuoka M, Hashimoto Y. Humanin and the receptors for humanin. Mol Neurobiol. 2010;41:22–28. doi: 10.1007/s12035-009-8090-z. [DOI] [PubMed] [Google Scholar]
- Matsuoka M, Hashimoto Y, Aiso S, Nishimoto I. Humanin and colivelin: neuronal-death-suppressing peptides for Alzheimer's disease and amyotrophic lateral sclerosis. CNS Drug Rev. 2006;12:113–122. doi: 10.1111/j.1527-3458.2006.00113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meistrich ML, Finch M, da Cunha MF, Hacker U, Au WW. Damaging effects of fourteen chemotherapeutic drugs on mouse testis cells. Cancer Res. 1982;42:122–131. [PubMed] [Google Scholar]
- Meistrich ML, Parchuri N, Wilson G, Kurdoglu B, Kangasniemi M. Hormonal protection from cyclophosphamide-induced inactivation of rat stem spermatogonia. J Androl. 1995;16:334–341. [PubMed] [Google Scholar]
- Moretti E, Giannerini V, Rossini L, Matsuoka M, Trabalzini L, Collodel G. Immunolocalization of humanin in human sperm and testis. Fertil Steril. 2010;94:2888–2890. doi: 10.1016/j.fertnstert.2010.04.075. [DOI] [PubMed] [Google Scholar]
- Morris ID, Phillips DM, Bardin CW. Ethylene dimethanesulfonate destroys Leydig cells in the rat testis. Endocrinology. 1986;118:709–719. doi: 10.1210/endo-118-2-709. [DOI] [PubMed] [Google Scholar]
- Morris AJ, Taylor MF, Morris ID. Leydig cell apoptosis in response to ethane dimethanesulphonate after both in vivo and in vitro treatment. J Androl. 1997;18:274–280. [PubMed] [Google Scholar]
- Muzumdar RH, Huffman DM, Atzmon G, Buettner C, Cobb LJ, Fishman S, Budagov T, Cui L, Einstein FH, Poduval A, Hwang D, Barzilai N, Cohen P. Humanin: a novel central regulator of peripheral insulin action. PLoS ONE. 2009;4:e6334. doi: 10.1371/journal.pone.0006334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzumdar RH, Huffman DM, Calvert JW, Jha S, Weinberg Y, Cui L, Nemkal A, Atzmon G, Klein L, Gundewar S, Ji SY, Lavu M, Predmore BL, Lefer DJ. Acute humanin therapy attenuates myocardial ischemia and reperfusion injury in mice. Arterioscler Thromb Vac Biol. 2010;30:1940–1948. doi: 10.1161/ATVBAHA.110.205997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimoto I, Matsuoka M, Niikura T. Unravelling the role of Humanin. Trends Mol Med. 2004;10:102–105. doi: 10.1016/j.molmed.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Orazizadeh M, Hashemitabar M, Khorsandi L. Protective effect of minocycline on dexamethasone induced testicular germ cell apoptosis in mice. Eur Rev Med Pharmacol Sci. 2009;13:1–5. [PubMed] [Google Scholar]
- Rajfer J, Sikka SC, Xie HW, Swerdloff RS. Effect of in vitro ketoconazole on steroid production in rat testis. Steroids. 1985;46:867–881. doi: 10.1016/0039-128x(85)90035-2. [DOI] [PubMed] [Google Scholar]
- Rommerts FF, Teerds KJ, Hoogerbrugge JW. In vitro effects of ethylene-dimethane sulfonate (EDS) on Leydig cells: inhibition of steroid production and cytotoxic effects are dependent on species and age of rat. Mol Cell Endocrinol. 1988;55:87–94. doi: 10.1016/0303-7207(88)90094-9. [DOI] [PubMed] [Google Scholar]
- Rommerts FF, Kuhne L, van Cappellen GW, Stocco DM, King SR, Jankowska A. Specific dose-dependent effects of ethane 1,2-dimethanesulfonate in rat and mouse Leydig cells and non-steroidogenic cells on programmed cell death. J Endocrinol. 2004;181:169–178. doi: 10.1677/joe.0.1810169. [DOI] [PubMed] [Google Scholar]
- Salva A, Klinefelter GR, Hardy MP. Purification of rat leydig cells: increased yields after unit-gravity sedimentation of collagenase-dispersed interstitial cells. J Androl. 2001;22:665–671. [PubMed] [Google Scholar]
- Sharpe RM, Donachie K, Cooper I. Re-evaluation of the intratesticular level of testosterone required for quantitative maintenance of spermatogenesis in the rat. J Endocrinol. 1988a;117:19–26. doi: 10.1677/joe.0.1170019. [DOI] [PubMed] [Google Scholar]
- Sharpe RM, Fraser HM, Ratnasooriya WD. Assessment of the role of Leydig cell products other than testosterone in spermatogenesis and fertility in adult rats. Int J Androl. 1988b;11:507–523. doi: 10.1111/j.1365-2605.1988.tb01024.x. [DOI] [PubMed] [Google Scholar]
- Shetty G, Meistrich ML. Hormonal approaches to preservation and restoration of male fertility after cancer treatment. J Natl Cancer Inst Monogr. 2005;34:36–39. doi: 10.1093/jncimonographs/lgi002. [DOI] [PubMed] [Google Scholar]
- Sikka SC, Swerdloff RS, Rajfer J. In vitro inhibition of testosterone biosynthesis by ketoconazole. Endocrinology. 1985;116:1920–1925. doi: 10.1210/endo-116-5-1920. [DOI] [PubMed] [Google Scholar]
- Sinha Hikim AP, Rajavashisth TB, Sinha HI, Lue Y, Bonavera JJ, Leung A, Wang C, Swerdloff RS. Significance of apoptosis in the temporal and stage-specific loss of germ cells in the adult rat after gonadotropin deprivation. Biol Reprod. 1997;57:1193–1201. doi: 10.1095/biolreprod57.5.1193. [DOI] [PubMed] [Google Scholar]
- Sinha-Hikim AP, Swerdloff RS. Temporal and stage-specific changes in spermatogenesis of rat after gonadotropin deprivation by a potent gonadotropin-releasing hormone antagonist treatment. Endocrinology. 1993;133:2161–2170. doi: 10.1210/endo.133.5.8404667. [DOI] [PubMed] [Google Scholar]
- Sprando RL, Santulli R, Awoniyi CA, Ewing LL, Zirkin BR. Does ethane 1,2-dimethanesulphonate (EDS) have a direct cytotoxic effect on the seminiferous epithelium of the rat testis? J Androl. 1990;11:344–352. [PubMed] [Google Scholar]
- Trasler JM, Hales BF, Robaire B. Chronic low dose cyclophosphamide treatment of adult male rats: effect on fertility, pregnancy outcome and progeny. Biol Reprod. 1986;34:275–283. doi: 10.1095/biolreprod34.2.275. [DOI] [PubMed] [Google Scholar]
- Trost LW, Brannigan RE. Oncofertility and the male cancer patient. Curr Treat Options Oncol. 2012;13:146–160. doi: 10.1007/s11864-012-0191-7. [DOI] [PubMed] [Google Scholar]
- Wang D, Li H, Yuan H, Zheng M, Bai C, Chen L, Pei X. Humanin delays apoptosis in K562 cells by downregulation of P38 MAP kinase. Apoptosis. 2005;10:963–971. doi: 10.1007/s10495-005-1191-x. [DOI] [PubMed] [Google Scholar]
- Woolveridge I, de Boer-Brouwer M, Taylor MF, Teerds KJ, Wu FC, Morris ID. Apoptosis in the rat spermatogenic epithelium following androgen withdrawal: changes in apoptosis-related genes. Biol Reprod. 1999;60:461–470. doi: 10.1095/biolreprod60.2.461. [DOI] [PubMed] [Google Scholar]
- Xu X, Chua CC, Gao J, Hamdy RC, Chua BH. Humanin is a novel neuroprotective agent against stroke. Stroke. 2006;37:2613–2619. doi: 10.1161/01.STR.0000242772.94277.1f. [DOI] [PubMed] [Google Scholar]
- Xu X, Chua KW, Chua CC, Liu CF, Hamdy RC, Chua BH. Synergistic protective effects of humanin and necrostatin-1 on hypoxia and ischemia/reperfusion injury. Brain Res. 2010;1355:189–194. doi: 10.1016/j.brainres.2010.07.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazawa H, Sasagawa I, Nakada T. Apoptosis of testicular germ cells induced by exogenous glucocorticoid in rats. Hum Reprod. 2000;15:1917–1920. doi: 10.1093/humrep/15.9.1917. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.