Abstract
BACKGROUND
Bacillus anthracis, the causative agent of anthrax, is a potential bioterrorism agent. Anthrax meningitis may be a manifestation of B. anthracis infection, has high mortality, and requires more aggressive treatment than anthrax without meningitis. Rapid identification and treatment of anthrax meningitis are essential for successful management of an anthrax mass casualty incident.
METHODS
Three hundred six published reports from 1880 through 2013 met pre-defined inclusion criteria. We calculated descriptive statistics for abstracted cases and conducted multivariable regression on separate derivation and validation cohorts to identify clinical diagnostic and prognostic factors for anthrax meningitis.
RESULTS
One hundred thirty-two of 363 (36%) cases with systemic anthrax met anthrax meningitis criteria. Severe headache, altered mental status, meningeal signs, and other neurological signs at presentation independently predicted meningitis in the derivation cohort and are proposed as a four-item screening tool for use during mass casualty incidents. Presence of any one factor on admission had a sensitivity for finding anthrax meningitis of 89% (83%) in the adult (pediatric) validation cohorts. Anthrax meningitis was unlikely in the absence of any of these signs or symptoms ([LR−]=0.12 [0.19] for adult [pediatric] cohorts), while presence of two or more factors made meningitis very likely ([LR+]=26.5 [29.2]). Survival of anthrax meningitis was predicted by treatment with a bactericidal agent (P=0.005) and use of multiple antimicrobials (P=0.012).
CONCLUSIONS
We developed an evidence-based triage tool for screening patients for meningitis during an anthrax mass casualty incident; its use could improve both patient outcomes and resource allocation in such an event.
Keywords: anthrax, Bacillus anthracis, mass casualty incident, meningitis, resource allocation
Introduction
Bacillus anthracis, the bacterium that causes anthrax, is a significant public health concern due to its high mortality rate, near global ubiquity, and prior use as a biological weapon. Although modern critical care has improved outcomes, the mortality rate of inhalation anthrax remains high – ~90% pre-2001 [1]and 43% since 2001 [2–6]. Anthrax meningitis, a common complication of anthrax, has a 94% reported fatality rate [7]. Well known as a complication of primary inhalation anthrax, [1]meningitis may also complicate primary anthrax infections of the gastrointestinal tract, [8] skin, [7] and soft tissue [9] and it may occurr in patients with systemic anthrax with no recognized port of entry [10]. Newly revised anthrax treatment guidelines dictate more aggressive treatment protocols for patients with meningitis than for those without [11].
Radiographic and laboratory capacity may be insufficient to treat patients according to conventional standards in an anthrax mass causality incident. Crisis standards of care – intended to provide the greatest good to the greatest number in the shortest time – would likely be implemented; and patient triage would most likely be based on readily available data [12, 13]. Once healthcare personnel have triaged patients into suspected anthrax and non-anthrax groups, those with presumed anthrax will still need to be sorted into meningitis and non-meningitis subsets. Prior studies have proposed diagnostic criteria to differentiate inhalation anthrax from influenza-like illness [14] and community-acquired pneumonia [15]. However, previous reviews of anthrax meningitis have not proposed diagnostic criteria that could be used by clinicians during a mass casualty incident, in which diagnostic testing and antimicrobial availability may be severely limited. Therefore, we performed a systematic review of English-language published cases of systemic anthrax from 1880 through 2013 to identify the clinical presentation, patient factors, treatment, and outcomes of patients who developed anthrax meningitis. Our objectives were to: 1) improve the ability of clinicians to efficiently triage and monitor systemically ill patients for meningitis during an anthrax mass casualty incident, 2) identify patient and therapeutic factors associated with anthrax meningitis outcomes, and 3) inform public health policy regarding preparedness for and response to an anthrax mass casualty incident.
Guiding Questions for Literature Review
We asked what signs or symptoms could predict meningitis in patients with systemic anthrax, compared to definitive diagnostic tests such as lumbar puncture and culture. We also asked what clinical variables and therapies were associated with survival. We chose to use systemic (Appendix 1. Definitions, Systemic) rather than inhalation anthrax as our denominator because meningitis can complicate any type of anthrax [7] and notable inhalation anthrax outbreaks [2, 16] include cases of noninhalation anthrax.
Methods
Our case definitions are listed in Appendix 1.
Data Sources and Search String
We utilized systematic methods to identify published descriptions of cases with systemic anthrax. A search strategy was developed by anthrax subject matter experts (SMEs) and librarians at the Centers for Disease Control and Prevention (CDC) and Cornell Weill Medical College. Twelve databases were searched from inception through October 2013: Commonwealth Agricultural Bureau (1973 –), Cumulative Index to Nursing and Allied Health Literature (1981–), Defense Technical Information Center (1950 –), EconLit (1886 –), Embase (1988 –), Federal Research in Progress (1930 –), Global Health (1910 –), Medline (1946 –), National Technical Information Service (1964 –), Web of Science (1980 –), WorldCat (1967 –), and World Health Organization Library Database (1948 –).
Our search string terms captured English-language anthrax literature related to general triage of patients in an anthrax mass casualty incident, diagnostic testing for meningitis, and diagnostic test characteristics (Appendix 2: triage and meningitis search string syntax). A manual search of the bibliographies of review articles and SME-identified articles/government reports identified additional full-text articles/reports for review.
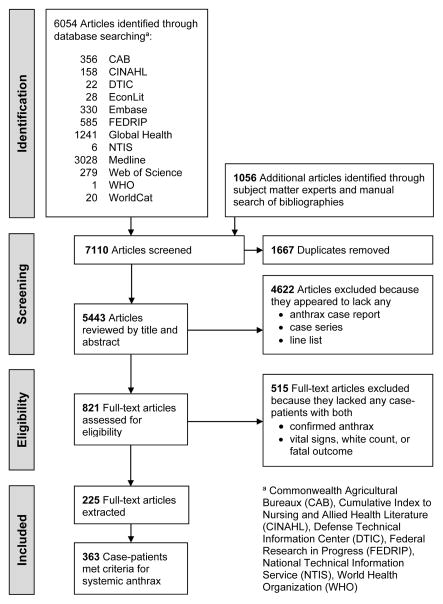
Study Selection
Following de-duplication, 4622 articles were excluded through title and abstract review because they appeared to lack a case report, case series, or line list describing any patients with anthrax (Appendix Figure 1: Flow Diagram of Study Selection Strategy). Following full text review, 515 of the remaining 821 articles were excluded because they lacked clinical information about individual patients with confirmed cases of anthrax whose reports included vital signs, white blood cell counts, or mention of death.
Figure 1.
Flow Diagram of Study Selection Strategy
Data Extraction and Cleaning
We abstracted the following data: author; title; demographic information; chief complaint; presence and timing of symptoms, signs, and laboratory and imaging findings; key laboratory results over the course of treatment; key complications and their timing; treatment(s); and outcomes including autopsy findings. Extraction of selected articles was completed independently and in duplicate; discrepancies were resolved through discussion of the original publication and by a third party arbiter as needed. Compiled case data were corrected for cases described in multiple publications.
Case Definitions and Completeness/Quality Assessment
Appendix 1 defines anthrax, systemic illness, meningitis, fulminant and prodromal status, geographic region, and publication type. We assessed case report completeness and quality by a 15-item checklist that was adapted from the CAse REport (CARE) checklist [17] and the toxicology case report checklist of Lavergne et al [18] and included demographic, medical, treatment, and outcome data (Appendix 1). If a case report contained extractable information for a given item it was scored 1; otherwise, 0.
Data Synthesis and Analysis
We report overall descriptive statistics including patient characteristics, primary route of transmission (if known), duration of illness prior to start of countermeasures (e.g., antimicrobials), symptoms and signs at presentation, initial laboratory results, treatment course, presence of meningitis, outcomes, and completeness score for patients of any age with systemic anthrax. Temporal and geographic trends are presented as supporting information. Since a number of authors [19–21] have suggested that the clinical presentation and prognosis of children may differ from those of adults, we stratified our results by age (0–17 years old, 18–50, >50).
We randomly divided adult cases with systemic anthrax into separate derivation (80%, n=246) and validation (20%, n=61) cohorts. Comparisons of sex, age, meningitis status, type of care, and time period showed the cohorts were comparable (data not shown). “Suspect” meningitis cases were excluded from the derivation (n=7) and validation cohorts (n=1) to improve the specificity of our findings. All pediatric cases (n=56) were included in a second validation cohort.
We used multivariable logistic regression to determine independent predictors of anthrax meningitis in the derivation cohort and tested differing combinations of predictors as a potential anthrax meningitis screening tool. We chose variables for this tool based on a combination of statistical significance on univariate analysis, accessibility (i.e., subjective and objective assessments that could be rapidly performed in the setting of a mass casualty incident), and historical clinical practice (e.g., elicitation of “meningeal signs”). Variables considered included age, sex, severe (e.g., “severe,” “throbbing,” and “worsening”) headache, altered mental status, meningeal signs (i.e., Kernig sign, Brudzinski sign, jolt accentuation test, nuchal rigidity, photophobia, and meningismus), other neurological signs (nonheadache, nonmeningeal signs including seizure, cranial nerve signs, limb weakness, and papilledema), fever/chills, and nausea/vomiting.
We selected the two best models based on calculated sensitivity, specificity, and positive and negative likelihood ratios (LR+, LR−) in the derivation cohort [22, 23]. These were then evaluated using data from the adult and pediatric validation cohorts. To assess possible reporting bias, a sensitivity analysis was conducted by applying the two models to cases with completeness scores in the top 50% and 75%.
Absence of reported data on symptoms, signs, laboratory results, and treatments was treated in the same manner as a negative response. We excluded cases with missing data from quantitative statistics as denoted in Tables 1 and 2. Symptoms and signs were considered to be “on presentation” if they occurred on or before the first day of hospitalization or autopsy or if the article mentioned a symptom or sign without specifying a timeframe. We considered a two-tailed p-value of <0.05 to be statistically significant and performed analyses using SAS software, version 9.3 (SAS Institute Inc., Cary, North Carolina).
Table 1A.
Patient Characteristics, Presenting Signs and Symptoms, and Outcome for Adultsa with Systemic Anthrax by Meningitis Status
| All Cases (n = 307) | Meningitis (n = 113) | No Meningitis (n = 194) | OR (95% CI)b | P-valuec | |
|---|---|---|---|---|---|
| Patient Characteristic | |||||
| Age (years), mean±STD (n) | 42.3±13.9 (293) | 43.1 ±12.8 (108) | 41.9±14.5 (185) | NA | 0.5 |
| Sexd | |||||
| Female, % (n) | 18 (54) | 14 (16) | 21 (38) | Reference | |
| Male, % (n) | 82 (238) | 86 (97) | 79 (141) | 1.6 (0.8–3.3) | 0.2 |
| Symptoms at Presentation | |||||
| Symptom onset to therapy (days), mean±STD (n) | 3.1±3.7 (148) | 3.2±4.1 (66) | 3.0±3.4 (82) | NA | 0.7 |
| Fever or chills, % (n) | 63 (193) | 77 (87) | 55 (106) | 2.8 (1.6–4.9) | 0.0001 |
| Fatigue or malaise, % (n) | 34 (105) | 34 (38) | 35 (67) | 1.0 (0.6–1.6) | >0.9 |
| Nausea or emesis, % (n) | 28 (87) | 44 (50) | 19 (37) | 3.4 (2.0–5.8) | <.0001 |
| Headache, % (n) | 28 (87) | 43 (49) | 20 (38) | 3.1 (1.8–5.4) | <.0001 |
| Severe headache, % (n) | 10 (30) | 19 (22) | 4 (8) | 5.6 (2.3–15.1) | <.0001 |
| Dyspnea, % (n) | 24 (75) | 20 (23) | 27 (52) | 0.7 (0.4–1.3) | 0.3 |
| Bleeding (e.g., hematemesis), % (n) | 12 (38) | 18 (20) | 9 (18) | 2.1 (1.0–4.4) | 0.0503 |
| Cough, % (n) | 18 (54) | 14 (16) | 20 (38) | 0.7 (0.3–1.3) | 0.3 |
| Chest Pain, % (n) | 13 (40) | 12 (13) | 14 (27) | 0.8 (0.4–1.7) | 0.7 |
| Clinical Signs at Presentationd | |||||
| Temperature (°C), mean±STD (n) | 38.4 ±1.3 (171) | 38.7±1.4 (58) | 38.3±1.2 (113) | NA | 0.03 |
| Fever (>38.0 °C), % (n) | 64 (133) | 68 (48) | 62 (85) | 1.3 (0.7–2.5) | 0.5 |
| Hypothermia (<36 °C), % (n) | 5 (10) | 6 (4) | 4 (6) | 1.3 (0.3–5.7) | 0.9 |
| Respiratory rate, mean±STD (n) | 26.8 ±8.5 (81) | 30.5±10.0 (26) | 25.0±7.1 (55) | NA | 0.007 |
| Tachypnea (≥30), % (n) | 26 (23) | 37 (11) | 21 (12) | 2.2 (0.7–6.4) | 0.2 |
| Heart rate, mean±STD (n) | 107.5 ±20.9 (121) | 111.0±25.2 (45) | 105.5±17.8 (76) | NA | 0.2 |
| Tachycardia (>90), % (n) | 81 (113) | 83 (43) | 80 (70) | 1.2 (0.4–3.2) | 0.9 |
| Systolic blood pressure, mean±STD (n) | 114.3 ±37.0 (61) | 120.7±35.7 (23) | 110.5±37.7 (38) | NA | 0.3 |
| Systolic hypotension (<90), % (n) | 27 (20) | 26 (8) | 29 (12) | 0.9 (0.3–2.8) | >0.9 |
| Fulminant phase, % (n) | 46 (142) | 63 (71) | 37 (71) | 2.9 (1.8–4.9) | <.0001 |
| Neurologic findings | |||||
| Altered mental status, % (n) | 37 (114) | 79 (89) | 13 (25) | 24.7 (13.0–48.7) | <.0001 |
| Loss of consciousness, % (n) | 15 (46) | 38 (43) | 2 (3) | 38.6 (11.8–200.8) | <.0001 |
| Meningeal signse, % (n) | 14 (42) | 40 (42) | 0 (0) | 178.2 (39.3-∞) | <.0001 |
| Other neurologic deficitse, % (n) | 16 (47) | 44 (47) | 0 (0) | 216.1 (47.7-∞) | <.0001 |
| Outcome | |||||
| Mortalityd, % (n) | 67 (202) | 95 (107) | 50 (95) | 17.7 (7.4–51.7) | <.0001 |
Adults: ≥18 years old.
Exact logistic regression used to calculate Odds Ratios (OR) and 95% Confidence Intervals (CI).
P-values reported for continuous variables were results of an independent two-sample t-test and were associated with the OR for categorical variables.
Adult systemic anthrax cases missing data include: sex (n=15), fever >38.0°C (n=98), hypothermia (n=98), tachypnea (n=220), tachycardia (n=168), systolic hypotension (n=234), and mortality (n=4).
Excludes suspected meningitis cases.
Table 2.
Patient Characteristics, Presenting Signs and Symptoms, and Treatments for Patients with Anthrax Meningitis by Fatality Status
| All Meningitis (n = 132) | Died (n = 122) | Lived (n = 10) | OR (95% CI)a | P-value b | |
|---|---|---|---|---|---|
| Patient Characteristic | |||||
| Age (years)c, mean±STD (n) | 38.4±16.4 (127) | 38.6±15.6 (117) | 36.1±24.7 (10) | NA | 0.6 |
| Age 0–12, % (n) | 7 (9) | 5 (6) | 30 (3) | Reference | |
| Age 13–17, % (n) | 8 (10) | 8 (9) | 10 (1) | 4.2 (0.3–261.9) | 0.5 |
| Age 18–50, % (n) | 61 (77) | 65 (76) | 10 (1) | 34.2 (2.4–2018.2) | 0.006 |
| Age >50, % (n) | 24 (31) | 22 (26) | 50 (5) | 2.5 (0.3–18.0) | 0.5 |
| Sex | |||||
| Female, % (n) | 17 (22) | 17 (21) | 10 (1) | Reference | |
| Male, % (n) | 83 (110) | 83 (101) | 90 (9) | 0.5 (0.0–4.3) | >0.9 |
| Symptoms at Presentation | |||||
| Symptom onset to therapy (days), mean±STD (n) | 3.1 ±3.9 (76) | 3.1±4.2 (66) | 2.7±1.3 (10) | NA | 0.8 |
| Fever or chills, % (n) | 78 (103) | 76 (93) | 100 (10) | 0.2 (0.0–1.2) | 0.2 |
| Nausea or emesis, % (n) | 46 (61) | 43 (53) | 80 (8) | 0.2 (0.0–1.0) | 0.06 |
| Fatigue or malaise, % (n) | 31 (41) | 31 (38) | 30 (3) | 1.1 (0.2–6.7) | >0.9 |
| Headache, % (n) | 44 (58) | 41 (50) | 80 (8) | 0.2 (0.0–0.9) | 0.04 |
| Severe headache, % (n) | 20 (26) | 17 (21) | 50 (5) | 0.2 (0.0–1.0) | 0.051 |
| Bleeding (e.g., hematemesis), % (n) | 16 (21) | 17 (21) | 0 (0) | 2.8 (0.6-∞) | 0.3 |
| Dyspnea, % (n) | 18 (24) | 18 (22) | 20 (2) | 0.9 (0.2–9.1) | >0.9 |
| Cough, % (n) | 13 (17) | 11 (14) | 30 (3) | 0.3 (0.1–2.0) | 0.2 |
| Chest Pain, % (n) | 10 (13) | 8 (10) | 30 (3) | 0.2 (0.0–1.5) | 0.1 |
| Clinical Signs at Presentation | |||||
| Arrhythmia, % (n) | 2 (2) | 1 (1) | 10 (1) | 0.1 (0.0–6.5) | 0.3 |
| Neurologic findings | |||||
| Altered mental status, % (n) | 77 (101) | 80 (97) | 40 (4) | 5.7 (1.2–29.8) | 0.02 |
| Loss of consciousness, % (n) | 40 (53) | 42 (51) | 20 (2) | 2.9 (0.5–28.7) | 0.3 |
| Meningeal signsd, % (n) | 40 (49) | 38 (44) | 71 (5) | 0.2 (0.0–1.6) | 0.2 |
| Other neurologic deficitsd, % (n) | 42 (52) | 42 (49) | 43 (3) | 1.0 (0.2–6.9) | >0.9 |
| First Laboratory Results | |||||
| WBCs (×109/L), mean±STD (n) | 19.2±13.0 (44) | 21.0±13.6 (36) | 10.9±4.9 (8) | NA | 0.047 |
| Serum sodium (mmol/L), mean±STD (n) | 134.8 ±10.4 (5) | 133.3±14.0 (3) | 137.0±4.2 (2) | NA | 0.8 |
| Cerebrospinal fluid | |||||
| Hemorrhagic, % (n) | 43 (57) | 46 (56) | 10 (1) | 7.5 (1.0–340.6) | 0.051 |
| Turbid, % (n) | 30 (40) | 30 (37) | 30 (3) | 1.0 (0.2–6.4) | >0.9 |
| RBCs (×109/L), mean±STD (n) | 5.7±8.1 (15) | 7.1±8.6 (12) | 0.4±0.6 (3) | NA | 0.2 |
| WBCs (×109/L), mean±STD (n) | 3.2±2.8 (36) | 3.6±2.8 (30) | 0.9±1.0 (6) | NA | 0.03 |
| Protein (g/L), mean±STD (n) | 6.3±6.3 (42) | 6.5±6.5 (37) | 4.6±5.1 (5) | NA | 0.5 |
| Glucose (mmol/L), mean±STD (n) | 3.1±2.4 (41) | 3.1±2.6 (35) | 2.8±1.7 (6) | NA | 0.8 |
| Subarachnoid hemorrhagee, % (n) | 46 (6) | 50 (6) | 0 (0) | 0.9 (0.0-∞) | >0.9 |
| Treatment | |||||
| Any therapy (antibiotics/antiserum), % (n) | 61 (81) | 58 (71) | 100 (10) | 0.1 (0.0–0.5) | 0.01 |
| Antimicrobial | |||||
| Monotherapy, % (n) | 29 (38) | 29 (35) | 30 (3) | 0.9 (0.2–5.9) | >0.9 |
| Multiple, % (n) | 29 (38) | 25 (31) | 70 (7) | 0.1 (0.0–0.7) | 0.01 |
| Bactericidal, % (n) | 56 (74) | 52 (64) | 100 (10) | 0.1 (0.0–0.4) | 0.005 |
| Penicillin, % (n) | 50 (66) | 48 (58) | 80 (8) | 0.2 (0.0–1.2) | 0.1 |
| Protein synthesis inhibitor, % (n) | 20 (26) | 18 (22) | 40 (4) | 0.3 (0.1–1.7) | 0.2 |
| Serum/Antitoxin therapy, % (n) | 6 (8) | 6 (7) | 10 (1) | 0.6 (0.1–27.4) | >0.9 |
| Steroids, % (n) | 11 (15) | 10 (12) | 30 (3) | 0.3 (0.1–1.7) | 0.2 |
Exact logistic regression used to calculate Odds Ratios (OR) and 95% Confidence Intervals (CI).
P-values reported for continuous variables were results of an independent two-sample t-test and were associated with the OR for categorical variables.
Five adult meningitis cases were missing age.
Excludes suspected meningitis cases.
Restricted to patients with imaging.
Findings
We identified 6,054 potentially relevant publications from our search and 1056 additional articles from SMEs and a manual search of review bibliographies. This strategy yielded 225 reports describing 363 systemic anthrax cases (Appendix Figure 1 and Appendix 3). Most were adult (84.6%), male (79.5%), and hospitalized (93.4%); 36.9% survived. A few (1.9%) received outpatient care and 3.0% died before presentation. Of the 132 (36.4%) with meningitis, 88.6% were confirmed; 5.3%, probable; and 6.1%, suspected. The primary routes of transmission for patients with systemic illness were: 51.8%, cutaneous; 22.9%, inhalation; 13.0%, ingestion; and 2.2%, injection. Thirty-two (8.8%) had no identifiable route of transmission. Date and location of report and temporal trends in treatment are described by Appendix Figures 2, 3, and 4. Case report quality on our 15-point scale ranged from five to 15, with a median score of 11 and 95% of reports scoring eight or higher.
Clinical Presentation and Diagnosis
Clinical signs and symptoms of all adults (≥18 years) and children (0 to 17 years) presenting with systemic anthrax are described in Tables 1A and 1B. We found no significant age or sex differences among adults with systemic anthrax with and without meningitis (Table 1A). Symptoms associated with ~ 3-fold increased risk of meningitis in univariate analysis included fever/chills, nausea/vomiting, and headache. Severe headache was uncommon, but was associated with a higher risk of meningitis. A number of neurological signs were much more common (>20-fold) in meningitis cases, including altered mental status, loss of consciousness, meningeal signs, and other neurological deficits (P<0.001 for each comparison). Adults with meningitis had higher respiratory rates than those without.
Table 1B.
Patient Characteristics, Presenting Signs and Symptoms, and Outcome for Childrena with Systemic Anthrax by Meningitis Status
| All Cases (n = 56) | Meningitis (n = 19) | No Meningitis (n = 37) | OR (95% CI)b | P-value c | |
|---|---|---|---|---|---|
| Patient Characteristic | |||||
| Age (years), mean±STD (n) | 10.7±6.0 (56) | 11.7±4.5 (19) | 10.1±6.7 (37) | NA | 0.4 |
| Age 0–1, % (n) | 12 (7) | 0 (0) | 19 (7) | Reference | |
| Age 2–5, % (n) | 14 (8) | 11 (2) | 16 (6) | 2.3 (0.3-∞) | 0.5 |
| Age 6–12, % (n) | 20 (11) | 37 (7) | 11 (4) | 12.4 (1.9-∞) | 0.02 |
| Age 13–17, % (n) | 54 (30) | 53 (10) | 54 (20) | 4.4 (0.8-∞) | 0.2 |
| Sexd | |||||
| Female, % (n) | 31 (17) | 32 (6) | 31 (11) | Reference | |
| Male, % (n) | 69 (37) | 68 (13) | 69 (24) | 1.0 (0.3–4.1) | >0.9 |
| Symptoms at Presentation | |||||
| Symptom onset to therapy (days), mean±STD (n) | 2.0 ±1.9 (23) | 2.0±2.0 (10) | 2.0±1.8 (13) | NA | >0.9 |
| Fever or chills, % (n) | 64 (36) | 84 (16) | 54 (20) | 4.4 (1.0–27.7) | 0.048 |
| Fatigue or malaise, % (n) | 11 (6) | 16 (3) | 8 (3) | 2.1 (0.3–17.4) | 0.7 |
| Nausea or emesis, % (n) | 30 (17) | 58 (11) | 16 (6) | 6.8 (1.7–30.5) | 0.004 |
| Headache, % (n) | 23 (13) | 47 (9) | 11 (4) | 7.1 (1.6–38.8) | 0.007 |
| Severe headache, % (n) | 7 (4) | 21 (4) | 0 (0) | 12.0 (1.9-∞) | 0.02 |
| Dyspnea, % (n) | 18 (10) | 5 (1) | 24 (9) | 0.2 (0.0–1.5) | 0.2 |
| Bleeding (e.g., hematemesis), % (n) | 16 (9) | 5 (1) | 22 (8) | 0.2 (0.0–1.8) | 0.2 |
| Cough, % (n) | 5 (3) | 5 (1) | 5 (2) | 1.0 (0.0–19.9) | >0.9 |
| Chest Pain, % (n) | 4 (2) | 0 (0) | 5 (2) | 0.8 (0.0–6.8) | 0.9 |
| Clinical Signs at Presentationd | |||||
| Temperature (°C), mean±STD (n) | 38.6±1.1 (34) | 38.9±1.2 (14) | 38.5±1.0 (20) | NA | 0.3 |
| Fever (>38.5 °C), % (n) | 60 (26) | 71 (10) | 55 (16) | 2.0 (0.4–10.8) | 0.5 |
| Hypothermia (<36 °C), % (n) | 0 (0) | 0 (0) | 0 (0) | ||
| Tachypnea, % (n) | 95 (19) | 88 (7) | 100 (12) | 0.7 (0.0–12.7) | 0.8 |
| Tachycardia, % (n) | 36 (10) | 25 (2) | 40 (8) | 0.5 (0.0–3.9) | 0.8 |
| Systolic hypotension, % (n) | 72 (13) | 100 (7) | 55 (6) | 6.3 (1.0-∞) | 0.1 |
| Fulminant phase, % (n) | 39 (22) | 58 (11) | 30 (11) | 3.2 (0.9–12.0) | 0.08 |
| Neurologic findings | |||||
| Altered mental status, % (n) | 29 (16) | 63 (12) | 11 (4) | 13.2 (3.0–74.6) | 0.0002 |
| Loss of consciousness, % (n) | 20 (11) | 53 (10) | 3 (1) | 36.7 (4.3–1781.6) | <.0001 |
| Meningeal signse, % (n) | 13 (7) | 39 (7) | 0 (0) | 28.8 (5.2-∞) | 0.0003 |
| Other neurologic deficitse, % (n) | 9 (5) | 28 (5) | 0 (0) | 17.4 (3.0-∞) | 0.005 |
| Outcome | |||||
| Mortalityd, % (n) | 44 (24) | 79 (15) | 25 (9) | 10.7 (2.6–56.2) | 0.0003 |
Children: 0–17 years old.
Exact logistic regression used to calculate Odds Ratios (OR) and 95% Confidence Intervals (CI).
P-values reported for continuous variables were results of an independent two-sample t-test and were associated with the OR for categorical variables.
Children systemic anthrax cases missing data include: sex (n=2), fever >38.5°C (n=13), hypothermia (n=13), tachypnea (n=36), tachycardia (n=28), systolic hypotension (n=38), and mortality (n=1).
Excludes suspected meningitis cases.
Six- to 12-year-olds were at increased risk of developing meningitis compared to 0- to 1-year-olds (p=0.0207) (Table 1B). While multiple signs and symptoms were strongly associated with meningitis in children (including nausea/emesis, severe headache, altered mental status, loss of consciousness, meningeal signs, and other neurologic deficits), age-defined tachypnea was present in most (95.0%) systemically ill children and showed no significant association with meningitis.
Cerebrospinal Fluid (CSF) Results in Anthrax Meningitis
The CSF was described as hemorrhagic in two-thirds of the 65 cases with meningitis who lacked evidence of subarachnoid hemorrhage (SAH) (by either imaging or autopsy) and underwent a lumbar puncture. Among those without SAH or frankly hemorrhagic CSF, the mean white blood cell (WBC) (n=13) and red blood cell (RBC) (n=7) counts were 3.4 × 109/L and 9.2 × 109/L and the mean protein (n=11) and glucose (n=11) levels were 7.3 g/L and 2.1 mmol/L, respectively.
Prognostic Indicators
Adults were more likely than children to die from systemic anthrax (P=0.002). The presence of meningitis increased mortality for systemic anthrax in both adults (Table 1A) and children (Table 1B). Adults and children with systemic anthrax with and without meningitis had similar durations of illness prior to onset of treatment (data not shown).
Mortality among cases who had meningitis was associated with multiple factors (Table 2). Compared to survivors, persons who died with meningitis were likelier to present with diminished mental status and higher peripheral white counts and CSF leukocytes; almost half of fatalities and one-tenth of survivors had hemorrhagic CSF (P=0.051). Cases aged 18–50 years appeared to be at increased risk of death compared to younger and older age-groups; although they accounted for a majority of the cases (61% versus 15% for younger and 24% for older), only one of ten survivors was in this age-group. Cases aged <18 years were more likely than those aged 18–50 to have a route of exposure other than inhalation or unknown (P=0.012) and to have received multiple antimicrobials (P=0.022). Cases >50 years old were more likely than those aged 18–50 to have cutaneous illness (P=0·036) and to have received a bactericidal antimicrobial (P<0·001) and steroids (P=0·018). On average, the younger and older age-groups were reported more recently (year 1972 and 1980, respectively) than the 18- to 50-year age-group (1960). After multivariable logistic analysis that adjusted for year (<1960 vs >1960), infection route (inhalation/unknown vs other), and treatment (bactericidal antimicrobials, multiple antimicrobials, or steroids vs other), children were still less likely to die than adults aged 18–50 (OR 0.06, 95% Confidence Interval (CI) 0.00–0.70), but both groups of adults had similar risk of death.
Survival among all anthrax meningitis cases was associated with presence of headache (P=0.039) and with nausea or vomiting (P=0.055) on admission. However, when the analysis was limited to either the 21 alert patients or the 56 non-comatose patients, these variables no longer predicted survivorship (P> 0.10 for each). All ten meningitis survivors received a bactericidal antimicrobial; seven survivors received multiple antimicrobials. Neither receipt of antitoxin/antiserum nor administration of systemic corticosteroids was associated with improved survival.
Variables associated with early (pre-hospital or first day of hospitalization) mortality included male sex, presentation with fever and/or chills, unconsciousness on presentation, and higher CSF RBCs.
Anthrax Meningitis Diagnostic Models
No single sign or symptom reliably identified anthrax meningitis in patients with systemic anthrax; for example, headache had a sensitivity of 35% and specificity of 82% in the derivation cohort (Table 3). In our derivation cohort, a four-item screening tool (severe headache, altered mental status, meningeal signs, and other neurological signs) that presumptively identified anthrax meningitis with either one (Model 4) or two (Model 5) positive responses, had better combined performance than three- or five-item screening tools. A negative response to all 4 questions (Model 4) had the lowest LR− (0.12) of the tested models in the derivation cohort; conversely, two or more positive responses to the same screening questions (Model 5) yielded a very high LR+ (26.5).
Table 3.
Assessment Toolsa for Meningitis in an Anthrax Mass Casualty Incident: Derivation, Validation, and Sensitivity Analyses
| Assessment Tools | TP b | FN | FP | TN | Sensitivity (95% CI), % | Specificity (95% CI), % | LR+ (95% CI) | LR− (95% CI) |
|---|---|---|---|---|---|---|---|---|
| Derivation Sample c | ||||||||
|
| ||||||||
| 1. Headache without: AMS, MS, ONSAMS, MS, ONS d | 6 | 11 | 24 | 109 | 35 (13–58) | 82 (75–88) | 2.0 (0.9–4.1) | 0.79 (0.55 – 1.13) |
| 2. One of the following: AMS, MS, ONS | 70 | 17 | 19 | 133 | 80 (72–89) | 88 (82–93) | 6.4 (4.2–9.9) | 0.22 (0.15 – 0.34) |
| 3. Two of the following: AMS, MS, ONS | 52 | 35 | 0 | 152 | 60 (49–70) | 100 (99–100)e | 182.6 (11.4–2921.1) e | 0.40 (0.31 – 0.52) |
| 4. One of the following: SHA, AMS, MS, ONS | 76 | 11 | 25 | 127 | 87 (80–94) | 84 (78–89) | 5.3 (3.7–7.7) | 0.15 (0.09 – 0.26) |
| 5. Two of the following: SHA, AMS, MS, ONS | 57 | 30 | 1 | 151 | 66 (56–76) | 99 (98–100) | 99.6 (14.0–706.6) | 0.35 (0.26 – 0.46) |
| 6. One of the following: SHA, AMS, MS, ONS, N/V | 77 | 10 | 50 | 102 | 89 (82–95) | 67 (60–75) | 2.7 (2.1–3.4) | 0.17 (0.09 – 0.31) |
| 7. Two of the following: SHA, AMS, MS, ONS, N/V | 65 | 22 | 7 | 145 | 75 (66–84) | 95 (92–99) | 16.2 (7.8–33.8) | 0.27 (0.18 – 0.38) |
|
| ||||||||
| Validation Samples c | ||||||||
|
| ||||||||
| 4. One of the following: SHA, AMS, MS, ONS | ||||||||
| Adult Sample (n = 61) | 17 | 2 | 6 | 36 | 89 (76–100) | 86 (75–96) | 6.3 (2.9–13.3) | 0.12 (0.03 – 0.46) |
| Pediatric Subset (n = 55) | 15 | 3 | 4 | 33 | 83 (66–100) | 89 (79–99) | 7.7 (3.0–19.9) | 0.19 (0.07 – 0.53) |
| 5. Two of the following: SHA, AMS, MS, ONS | ||||||||
| Adult Sample (n = 61) | 12 | 7 | 1 | 41 | 63 (41–85) | 98 (93–100) | 26.5 (3.7–189.6) | 0.38 (0.21 – 0.68) |
| Pediatric Subset (n = 55) | 7 | 11 | 0 | 37 | 39 (16–61) | 99 (95–100) e | 30.0 (1.8–497.9) e | 0.61 (0.42 – 0.88) |
|
| ||||||||
| Sensitivity Analyses c | ||||||||
|
| ||||||||
| 4. One of the following: SHA, AMS, MS, ONS | ||||||||
| Completeness Score >11 (n = 133) | 54 | 4 | 17 | 58 | 93 (87–100) | 77 (68–87) | 4.1 (2.7–6.3) | 0.09 (0.03 – 0.23) |
| Completeness Score >9 (n = 247) | 90 | 9 | 26 | 122 | 91 (85–97) | 82 (76–89) | 5.2 (3.6–7.4) | 0.11 (0.06 – 0.21) |
| 5. Two of the following: SHA, AMS, MS, ONS | ||||||||
| Completeness Score >11 (n = 133) | 40 | 18 | 0 | 75 | 69 (57–81) | 99 (98–100) e | 104.3 (6.6–1662.0) e | 0.31 (0.21 – 0.46) |
| Completeness Score >9 (n = 247) | 66 | 33 | 0 | 148 | 67 (57–76) | 100 (99–100) e | 198.2 (12.4–3164.7) e | 0.33 (0.25 – 0.44) |
… Suspected meningitis cases are excluded.
TP=true positive, FN=false negative, FP=false positive, TN=true negative, LR+=likelihood ratio positive, LR−=likelihood ratio negative.
Derivation sample based on an 80% random sample of adults (≥18 years) with systemic anthrax, adult validation sample is the remaining 20% of adults, pediatric subset (0–17 years old) is all children, and sensitivity analyses using completeness score are based on the full dataset of 363 case-patients.
AMS=altered mental status, MS=meningeal signs, ONS=other neurological signs, SHA=severe headache, N/V=nausea or vomiting.
Continuity correction factor of 0.5 added to TP, FN, FP, TN.
In the adult and pediatric validation cohorts, a single positive response (Model 4) had an LR+ of 6.26 and 7.71 and sensitivity of 89% and 83%, respectively. (See Table 4 for complete test characteristics.)
Sixteen (12%) cases known to have anthrax meningitis were not identified as such by our screening tool in either the derivation or validations cohorts (i.e. false negatives). However seven (44%) of these would have been identified after initial presentation had the screening tool been used for “monitoring” after day one.
Although reports on adults with meningitis did have more complete descriptions than those without (mean completeness score 11.3 [SD=2.0] vs 10.5 [2.1], P<0.001), restricting the analysis to cases with scores >11 (top 50%) or >9 (top 75%) had minimal impact on calculated test characteristics.
Interpretation
We found that a 4-item screening tool – assessing severe headache, altered mental status, meningeal signs, and other neurological signs – has good discriminatory power for two key tasks: (1) identifying patients with systemic anthrax who are likely to have anthrax meningitis and therefore should be started on specific therapies (e.g., triple antibiotic coverage including a bactericidal agent[11]) and (2) identifying patients with systemic anthrax who are unlikely to have anthrax meningitis and therefore do not require anthrax meningitis-specific countermeasures. Hence, patients with two or more positive responses may be categorized as presumptive anthrax meningitis, while patients with no positive responses may be categorized as non-meningitis systemic anthrax. Patients with only one positive response fall in the middle; in a crisis setting with overwhelming casualties, they may be managed by initiation of presumptive anti-anthrax meningitis treatment[24–27]. In a contingency setting, these patients may be prioritized for lumbar puncture and/or neuroimaging to individualize their treatment plans.
Four of our findings have important policy implications. First, our data, from the largest case series of systemic anthrax yet assembled, confirms that approximately one third of these cases are complicated by meningitis, and that these patients face double the mortality risk of non-meningitis cases (92.4% vs. 46.0%). Effective public health response to the intentional aerosol release of B. anthracis spores should, therefore, include rapid screening to identify those with anthrax meningitis to facilitate effective patient and resource management.
Second, both adults and children with anthrax meningitis either present with or develop a small number of signs and symptoms that can reliably distinguish them from patients with non-meningitic systemic anthrax. Our simple screening tool has excellent test characteristics, which we believe will be of use to public health agencies worldwide.
Third, we confirm increased survival of patients with meningitis who received bactericidal antimicrobials and/or multiple antimicrobials. Treatment of anthrax meningitis is hampered by the poor blood-brain barrier penetration of antitoxins [28, 29] and various antimicrobials. There may be a future role for neuroprotective agents in the management of anthrax meningitis, as some are beginning to show promise for the treatment of intracranial hemorrhage [30–32].
Fourth, we found that almost half of systemic anthrax patients who eventually developed meningitis (7 of 16) lacked suggestive signs or symptoms on presentation, underscoring the need for mandatory periodic reassessment of these patients.
Fatal anthrax meningitis cases in this series share a number of characteristics with patients who die from non-anthrax bacterial meningitis and SAH; these include presenting with altered mental status, increased peripheral and/or cerebrospinal fluid white count, and hemorrhagic CSF [33–35]. Due to the small number of reported observations, we could not assess the relation to outcome of comorbidities, arrhythmias, hypotension, hyponatremia, decreased platelets, or decreased hematocrit – known risk factors for poor outcome in non-anthrax meningitis and SAH [33–40].
Limitations
Publication bias (that is, preferential publication of the most dramatic or successfully treated cases) may have led us to conclusions that are not in fact representative of all anthrax meningitis cases. Data were extracted and assessed from English-language retrospective case reports and our conclusions rely on publications of varying origin, detail, and quality. Underlying medical comorbidity or pertinent negative clinical signs and symptoms may not have been reported in these cases. Finally, identified anthrax cases were published over a 100-year period, during which treatment options and supportive care techniques advanced greatly. Thus it is difficult to comment on the effectiveness of newer treatment modalities and guidelines.
Conclusions
We developed a simple four-item screening tool that accurately identifies systemic anthrax cases that are complicated by meningitis on the basis of clinical symptoms and signs alone. Efficient identification of these patients during an anthrax mass casualty incident may result in improved patient outcomes and more effective use of scarce resources. Our findings should be of interest and assistance to emergency response professionals and public health agencies worldwide.
Supplementary Material
Supplementary Figure 1. Temporal Trends in Patients Reported with Systemic Anthrax by Meningitis Status, 1880–2013
Supplementary Figure 2. Geographic Trends in Patients Reported with Systemic Anthrax, 1880–2013
Supplementary Figure 3. Temporal Trends in the Treatment of Systemic Anthrax, 1880–2013
Summary.
Systematic literature review includes 363 systemic anthrax cases; a third with meningitis. Presence of severe headache, meningeal signs, altered mental status, or other neurological symptoms on admission predicted anthrax meningitis. A screening tool is described based on these four items.
Acknowledgments
Funding: Office of Public Health Preparedness and Response, Centers for Disease Control and Prevention, Atlanta, GA.
We would like to thank Joanna Taliano MA, MLS, and Onnalee Gomez, MS, for conducting the searches for this systematic review; Paul J. Albert MA, MLS (Weill Medical College, Cornell University), for assistance with developing search strategies; Thomas Doker, DVM, MPH, and Sean Shadomy DVM, MPH, for insights in research design; Cheryl Isenhour, DVM, MPH; Tina Benoit, MPH; Leisha Nolen, MD, PHD; Kyung Park, MPH; Eileen Huang, MPH, MBA; Julie Guarnizo, BBA; Theresa Turski, MPH, CPH; and Jamechia Hoyle, DHSc, MPH, MS (Assistant Secretary for Preparedness and Response [ASPR]), for help in extracting data; and Patricia Yu, MPH, for programming expertise. Unless otherwise noted, the contributors listed above are affiliated with the Centers for Disease Control and Prevention.
Appendix 1. Definitions
Anthrax cases were confirmed in patients by microscopic identification (e.g., Gram stain after 1886), culture, paired sera, polymerase chain reaction, enzyme-linked immunosorbent assay, immunohistochemistry, animal inoculation, or by epidemiological linkage with a confirmed case or positive “environmental (e.g., shaving brush, hide)” culture.
Systemic illness was considered to be present in 1) adults 18 years of age and older who met any of the following criteria: temperature <36 or >38 degrees centigrade or described as “hypothermic,” pulse >90 per minute or described as “tachycardic,” respiratory rate >20 per minute or described as “tachypneic,” systolic blood pressure <90mm Hg or described as “hypotensive” or “in shock,” mean arterial pressure (MAP) <70mm Hg, or white blood cell count >12,000 cells/μL or <4,000 cells/μL; 2) children under 18 years of age who met any of the age-specific sepsis criteria described by Goldstein; and 3) any aged patient with a) any secondary organ involvement (e.g., gastrointestinal, pulmonary complications), b) evidence of B. anthracis from a normally sterile site (e.g., blood or CSF), c) evidence of ascites or pleural fluid, or d) death.
Anthrax meningitis was analyzed as a dichotomous variable but had confirmed, probable, and suspect subcategories dependent on the following criteria: Confirmed anthrax meningitis was defined as culture of B. anthracis from either the CSF or brain; microscopic evidence (e.g., Gram stain) from either the CSF or brain; or positive culture or microscopic evidence from a non-intracranial source accompanied by CSF findings consistent with meningitis (e.g., WBCs > .005 × 109/L). Probable anthrax meningitis was defined as CSF described as bloody, cloudy, or xanthochromic or CT, MRI, or autopsy evidence of a subarachnoid or intracranial hemorrhage or encephalitis. Suspect anthrax meningitis was defined as the presence of meningeal signs (i.e., Kernig sign, Brudzinski sign, nuchal rigidity, photophobia, meningismus) or nonheadache, nonmeningeal neurological signs (e.g., seizure, cranial nerve signs, limb weakness, papilledema).
Fulminant disease was considered to be present in patients who were described as being cyanotic or in shock; who, in the modern era, needed mechanical ventilation or vasopressors; or who died prior to or the day of arrival. Those who lacked these features were considered to have prodromal disease.
Country codes and geographic regions were assigned according to UN criteria.
To measure case report completeness and quality, we developed a previously described (See Case Definitions and Completeness/Quality Assessment) checklist from two sources. Our checklist comprised 15 items: age, sex, occupation or exposure, underlying medical condition, chief complaint, clinical findings, laboratory or imaging findings, timing of course of illness, treatments or interventions, countermeasure-specific treatment, timing of any treatments, fatal/nonfatal outcome, ICU admission, length of stay, morbidity description (or autopsy information).
Appendix 2. Triage and Meningitis Search String Syntax
Triage OR (acute ADJ care) OR EMS OR (emergenc* ADJ (medic* OR room* OR department*)) OR ER OR diagnos?s OR diagnostic* OR (symptom ADJ (assessment* OR evaluation*)) OR (prodromal ADJ2 (symptom* OR state* OR stage* OR sign* OR phase*)) OR (urgent ADJ care) OR (needs ADJ assessment*) OR symptom* OR (healthcare ADJ need*) OR (critical ADJ care) OR (constellation ADJ2 (sign* OR symptom*)) OR ((rapid OR quick) ADJ2 test*) OR (rapid ADJ assessment*) OR ((patient OR clinical) ADJ (assessment* OR evaluation*)) OR (disaster ADJ medicine) OR ICU OR (intensive ADJ care) OR (surge ADJ (capacity OR medical)) OR ((mass OR (large ADJ scale)) ADJ screening) OR (healthcare ADJ2 facilit*) OR (medical ADJ plan*) OR (medical ADJ priorit*) OR ((high ADJ risk) ADJ (screening OR patient*)) OR ((low ADJ risk) ADJ patient*) OR ((multiphasic OR medic*) ADJ screening) OR presentation* OR (decision ADJ making) OR prodom* OR fulmin* OR (gram ADJ stain) OR outpatient* OR discharge* OR hospitaliz* OR score OR sign OR toxemia OR effusion OR ascites OR shock OR h?emorrhag* OR CSF OR ( sensitivity ADJ specificity)
AND
Anthrax OR (Bacillus Anthracis) OR (B Anthracis) OR Charbon OR Charbonneuse OR Milzbrand OR Antraks OR Antrax OR Carbunclo OR Carbunco OR (woolsorter* OR (wool ADJ sorter)) OR ragpicker*
AND
Exp Humans/OR human.mp
Footnotes
Conflict of Interest
None of the authors have any conflicts of interest. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC.
Contributor Information
Stefan Katharios-Lanwermeyer, Division of High-consequence Pathogens and Pathology, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Jon-Erik Holty, Pulmonary, Critical Care and Sleep Medicine Section, VA Palo Alto Healthcare System Department of Medicine, Stanford University, Stanford, CA, USA.
Marissa Person, Division of High-consequence Pathogens and Pathology, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
James Sejvar, Division of High-consequence Pathogens and Pathology, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Dana Haberling, Division of High-consequence Pathogens and Pathology, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Heather Tubbs, Division of Preparedness and Emerging Infections, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Dana Meaney-Delman, National Center for Emerging Infections Diseases, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Satish K. Pillai, Division of Preparedness and Emerging Infections, Centers for Disease Control and Prevention, Atlanta, Georgia, USA
Nathaniel Hupert, Departments of Healthcare Policy and Research and of Medicine, Weill Medical College, Cornell University; New York Presbyterian Hospital, New York, NY, USA.
William A. Bower, Division of High-consequence Pathogens and Pathology, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Katherine Hendricks, Division of High-consequence Pathogens and Pathology, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
References
- 1.Holty JE, Bravata DM, Liu H, Olshen RA, McDonald KM, Owens DK. Systematic review: a century of inhalational anthrax cases from 1900 to 2005. Ann Intern Med. 2006;144(4):270–80. doi: 10.7326/0003-4819-144-4-200602210-00009. [DOI] [PubMed] [Google Scholar]
- 2.Jernigan DB, Raghunathan PL, Bell BP, et al. Investigation of bioterrorism-related anthrax, United States, 2001: epidemiologic findings. Emerg Infect Dis. 2002;8(10):1019–28. doi: 10.3201/eid0810.020353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barakat LA, Quentzel HL, Jernigan JA, et al. Fatal inhalational anthrax in a 94-year-old Connecticut woman. JAMA. 2002;287(7):863–8. doi: 10.1001/jama.287.7.863. [DOI] [PubMed] [Google Scholar]
- 4.Walsh J, Fraser G, Hunt E, et al. Inhalation anthrax associated with dried animal hides - Pennsylvania and New York City, 2006. MMWR Morb Mortal Wkly Rep. 2006;55(10):280–2. [PubMed] [Google Scholar]
- 5.Anaraki S, Addiman S, Nixon G, et al. Investigations and control measures following a case of inhalation anthrax in East London in a drum maker and drummer, October 2008. Euro Surveill. 2008;13(51) [PubMed] [Google Scholar]
- 6.Sprenkle MD, Griffith J, Marinelli W, et al. Lethal factor and anti-protective antigen IgG levels associated with inhalation anthrax, Minnesota, USA. Emerg Infect Dis. 2014;20(2):310–4. doi: 10.3201/eid2002.130245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lanska DJ. Anthrax meningoencephalitis. Neurology. 2002;59(3):327–34. doi: 10.1212/wnl.59.3.327. [DOI] [PubMed] [Google Scholar]
- 8.Tabatabaie P, Syadati A. Bacillus anthracis as a cause of bacterial meningitis. Pediatr Infect Dis J. 1993;12(12):1035–6. doi: 10.1097/00006454-199312000-00020. [DOI] [PubMed] [Google Scholar]
- 9.Berger T, Kassirer M, Aran AA. Injectional anthrax - new presentation of an old disease. Euro Surveill. 2014;19(32) doi: 10.2807/1560-7917.es2014.19.32.20877. [DOI] [PubMed] [Google Scholar]
- 10.Holty JE, Kim RY, Bravata DM. Anthrax: a systematic review of atypical presentations. Ann Emerg Med. 2006;48(2):200–11. doi: 10.1016/j.annemergmed.2005.11.035. [DOI] [PubMed] [Google Scholar]
- 11.Hendricks KA, Wright ME, Shadomy SV, et al. Centers for disease control and prevention expert panel meetings on prevention and treatment of anthrax in adults. Emerg Infect Dis. 2014;20(2) doi: 10.3201/eid2002.130687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iserson KV, Pesik N. Ethical resource distribution after biological, chemical, or radiological terrorism. Camb Q Healthc Ethics. 2003;12(04):455–65. doi: 10.1017/s0963180103124164. [DOI] [PubMed] [Google Scholar]
- 13.Burkle FM. Population-based Triage Management in Response to Surge-capacity Requirements during a Large-scale Bioevent Disaster. Acad Emerg Med. 2006;13(11):1118–29. doi: 10.1197/j.aem.2006.06.040. [DOI] [PubMed] [Google Scholar]
- 14.Fine AM, Wong JB, Fraser HS, Fleisher GR, Mandl KD. Is it influenza or anthrax? A decision analytic approach to the treatment of patients with influenza-like illnesses. Ann Emerg Med. 2004;43(3):318–28. doi: 10.1016/j.annemergmed.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 15.Kyriacou DN, Yarnold PR, Stein AC, et al. Discriminating inhalational anthrax from community-acquired pneumonia using chest radiograph findings and a clinical algorithm. Chest. 2007;131(2):489–96. doi: 10.1378/chest.06-1687. [DOI] [PubMed] [Google Scholar]
- 16.Meselson M, Guillemin J, Hugh-Jones M, et al. The Sverdlovsk anthrax outbreak of 1979. Science. 1994;266(5188):1202–8. doi: 10.1126/science.7973702. [DOI] [PubMed] [Google Scholar]
- 17.Gagnier JJ, Kienle G, Altman DG, Moher D, Sox H, Riley D. The CARE guidelines: consensus-based clinical case report guideline development. J Clin Epidemiol. 2014;67(1):46–51. doi: 10.1016/j.jclinepi.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 18.Lavergne V, Ouellet G, Bouchard J, et al. Guidelines for reporting case studies on extracorporeal treatments in poisonings: methodology. Semin Dial. 2014;27(4):407–14. doi: 10.1111/sdi.12251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bradley JS, Peacock G, Krug SE, et al. Pediatric anthrax clinical management: executive summary. Pediatrics. 2014;133(5):940–2. doi: 10.1542/peds.2014-0564. [DOI] [PubMed] [Google Scholar]
- 20.Bravata D, Holty J, Lewis R, Wang E, Wise P. Pediatric Anthrax: Implications for Bioterrorism Preparedness. Evidence Report/Technology Assessment Number 141. 2007 [PMC free article] [PubMed] [Google Scholar]
- 21.Hamele M, Poss WB, Sweney J. Disaster preparedness, pediatric considerations in primary blast injury, chemical, and biological terrorism. World J Crit Care Med. 2014;3(1):15–23. doi: 10.5492/wjccm.v3.i1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Simel DL, Samsa GP, Matchar DB. Likelihood ratios with confidence: sample size estimation for diagnostic test studies. J Clin Epidemiol. 1991;44(8):763–70. doi: 10.1016/0895-4356(91)90128-v. [DOI] [PubMed] [Google Scholar]
- 23.Worster A, Carpenter C. A brief note about likelihood ratios. CJEM. 2008;10(5):441–2. doi: 10.1017/s1481803500010538. [DOI] [PubMed] [Google Scholar]
- 24.World Health Organization. Health aspects of chemical and biological weapons: Report of a WHO group of consultants. 1970. [Google Scholar]
- 25.Bravata DM, Zaric GS, Holty J-EC, et al. Reducing mortality from anthrax bioterrorism: strategies for stockpiling and dispensing medical and pharmaceutical supplies. Biosecur Bioterror. 2006;4(3):244–62. doi: 10.1089/bsp.2006.4.244. [DOI] [PubMed] [Google Scholar]
- 26.Isukapalli S, Lioy P, Georgopoulos P. Mechanistic modeling of emergency events: Assessing the impact of hypothetical releases of anthrax. Risk Anal. 2008;28(3):723–40. doi: 10.1111/j.1539-6924.2008.01055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.US Congress. Technologies Underlying Weapons of Mass Destruction. Washington, DC: Office of Technology Assessment; 1993. [Google Scholar]
- 28.ANTHRASIL [package insert] U.S. Food and Drug Administration SS; MD: 2015. [Accessed April 15 2015]. Available at: http://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM439812.pdf. [Google Scholar]
- 29.RAXIBACUMAB [package insert] U.S. Food and Drug Administration SS; MD: 2015. [Accessed April 15, 2015]. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2012/125349s000lbl.pdf. [Google Scholar]
- 30.Fujimura M, Niizuma K, Inoue T, et al. Minocycline prevents focal neurological deterioration due to cerebral hyperperfusion after extracranial-intracranial bypass for moyamoya disease. Neurosurgery. 2014;74(2):163–70. doi: 10.1227/NEU.0000000000000238. [DOI] [PubMed] [Google Scholar]
- 31.Hwang BY, Appelboom G, Ayer A, et al. Advances in neuroprotective strategies: potential therapies for intracerebral hemorrhage. Cerebrovasc Dis. 2011;31(3):211–22. doi: 10.1159/000321870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Laskowitz DT, Kolls BJ. Neuroprotection in subarachnoid hemorrhage. Stroke. 2010;41(10 Suppl):S79–84. doi: 10.1161/STROKEAHA.110.595090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Claassen J, Carhuapoma JR, Kreiter KT, Du EY, Connolly ES, Mayer SA. Global cerebral edema after subarachnoid hemorrhage frequency, predictors, and impact on outcome. Stroke. 2002;33(5):1225–32. doi: 10.1161/01.str.0000015624.29071.1f. [DOI] [PubMed] [Google Scholar]
- 34.Dzupova O, Rozsypal H, Prochazka B, Benes J. Acute bacterial meningitis in adults: predictors of outcome. Scand J Infect Dis. 2009;41(5):348–54. doi: 10.1080/00365540902849391. [DOI] [PubMed] [Google Scholar]
- 35.Risselada R, Lingsma H, Bauer-Mehren A, et al. Prediction of 60 day case-fatality after aneurysmal subarachnoid haemorrhage: results from the International Subarachnoid Aneurysm Trial (ISAT) Eur J Epidemiol. 2010;25(4):261–6. doi: 10.1007/s10654-010-9432-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Frontera JA, Parra A, Shimbo D, et al. Cardiac arrhythmias after subarachnoid hemorrhage: risk factors and impact on outcome. Cerebrovasc Dis. 2008;26(1):71. doi: 10.1159/000135711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Neil-Dwyer G, Cruickshank J. The blood leucocyte count and its prognostic significance in subarachnoid haemorrhage. Brain. 1974;97(1):79–86. doi: 10.1093/brain/97.1.79. [DOI] [PubMed] [Google Scholar]
- 38.Sadamasa N, Yoshida K, Narumi O, Chin M, Yamagata S. Prediction of mortality by hematological parameters on admission in patients with subarachnoid hemorrhage. Neurol Med Chir. 2011;51(11):745–8. doi: 10.2176/nmc.51.745. [DOI] [PubMed] [Google Scholar]
- 39.Salekeen S, Mahmood K, Naqvi IH, Baig MY, Akhter ST, Abbasi A. Clinical course, complications and predictors of mortality in patients with tuberculous meningitis--an experience of fifty two cases at Civil Hospital Karachi, Pakistan. J Pak Med Assoc. 2013;63(5):563–7. [PubMed] [Google Scholar]
- 40.van de Beek D, de Gans J, Spanjaard L, Weisfelt M, Reitsma JB, Vermeulen M. Clinical features and prognostic factors in adults with bacterial meningitis. N Engl J Med. 2004;351(18):1849–59. doi: 10.1056/NEJMoa040845. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Temporal Trends in Patients Reported with Systemic Anthrax by Meningitis Status, 1880–2013
Supplementary Figure 2. Geographic Trends in Patients Reported with Systemic Anthrax, 1880–2013
Supplementary Figure 3. Temporal Trends in the Treatment of Systemic Anthrax, 1880–2013