Abstract
Novel aqueous biphasic systems (ABS) composed of phosphonium- or ammonium-based ionic liquids (ILs), combined with a buffered aqueous solution of potassium citrate/citric acid (pH=7.0), were investigated for the extraction of proteins. For that purpose, the phase diagrams, tie-lines and tie-line lengths were determined at 25ºC, and the performance of these ABS for the extraction of bovine serum albumin (BSA) was then evaluated. The obtained results reveal that, with the exception of the more hydrophobic ILs, most of the systems investigated allow the complete extraction of BSA for the IL-rich phase in a single-step. These remarkable extraction efficiencies are far superior to those afforded by more conventional extraction systems previously reported. The composition of the biphasic systems, i.e., the amount of phase-forming components, was also investigated aiming at reducing the overall costs of the process without losing efficiency on the protein extraction. It is shown that the extraction efficiencies of BSA are maintained at 100% up to high protein concentrations (at least up to 10 g.L-1). The recovery of the BSA from the IL-rich phase by dialysis is also shown in addition to the demonstration of the IL recyclability and reusability, at least for 3 times. In the sequential three-step extractions (BSA recovery/IL reusability), the extraction efficiencies of BSA for the IL-rich phase were maintained at 100%. For the improved ABS, the preservation of the protein native conformation was confirmed by Size Exclusion High-Performance Liquid Chromatography (used also as the quantification method) and by Fourier Transform Infra-Red spectroscopy. According to the results herein reported, ABS composed of phosphonium- or ammonium-based ILs and a biodegradable organic salt represent an alternative and remarkable platform for the extraction of BSA and may be extended to other proteins of interest.
Keywords: protein, aqueous biphasic system, ionic liquid, extraction, native conformation, bovine serum albumin (BSA)
1. Introduction
Proteins are biomolecules formed by a sequence of amino acids and which perform a wide array of functions within living organisms (Zhao, 2007). However, the proteins specific functions are dependent on the stability of their native structure (Roberts, 2007). Most proteins have low structural stability and may be denatured in presence of some chemical compounds, namely polymers, salts or organic solvents (Jiang and Schwendeman, 2000; Li et al., 2004; Raja et al., 2012; Wallqvist et al., 1998), high temperature and pressure conditions (Fang et al., 2014; Vasilchuk et al., 2014) or through pH changes (Dissanayake et al., 2013). Therefore, when foreseeing the extraction and/or purification of proteins, those parameters must be addressed aiming at maintaining the proteins structure - a major challenge in the biotechnology field.
Serum albumins are the major soluble protein constituents in body fluids and have many relevant physiological functions. Amongst several valuable characteristics, one of the most important relays on their function as carriers of various compounds within the circulatory system (Mandeville and Tajmir-Riahi, 2010). BSA (bovine serum albumin), a protein with some common features to other serum albumins, such as ESA (equine serum albumin), LSA (leporine serum albumin) and HSA (human serum albumin) (Bujacz, 2012), has been extensively studied as a model protein in a wide variety of fields. Some examples include studies on the interaction of serum albumins with chlorophylls (Gorza et al., 2014) and with ionic surfactants (Gelamo and Tabak, 2000) due to the particular similarity (in 76% of the amino acid sequence) between BSA and HAS (Gelamo et al., 2002; Huang et al., 2004). For these investigations, pure proteins are required. The extraction and purification of BSA was already investigated using various methodologies, such as reversed micelle approaches (Hebbar and Raghavarao, 2007; Hayes and Marchio, 1998; Lu et al., 2010), ultrafiltration (Corbatón-Báguena et al., 2014; Menon and Zydney, 2000) or tangential flow filtration (van Reis et al., 2000), precipitation (Lee et al., 2007; Ling et al., 2012) and by liquid-liquid approaches (Johansson et al., 1999; Puthirasigamany et al., 2014).
Amongst the several extraction and purification methods commonly applied to proteins, the use of aqueous biphasic systems (ABS) received a remarkable attention since they are mainly composed of water and thus are of high biocompatibility - favorable environment for the purification of biologically active biomolecules (Albertsson, 1958, Asenjo and Andrews, 2011). ABS fit within the liquid-liquid extraction/purification techniques and are environmentally-friendly since the use of volatile and hazardous organic solvents is avoided. ABS are typically composed of non-volatile pairs dissolved in aqueous media, such as two polymers, a polymer and a salt or two salts, which above given concentrations undergo liquid-liquid demixing. The selective partitioning of a target molecule, e.g. a protein, depends upon its affinity for each aqueous-rich phase, as well as on other parameters, such as pH, temperature and system composition. ABS have gained relevant attention since several early-stage processing steps, namely clarification, concentration and purification can be carried out in a single-stage. Moreover, the application of ABS at a large-scale has already been demonstrated (Asenjo and Andrews, 2011). Typical polymer-based ABS display, however, a high viscosity, which hinders the mass transfer and also lead to a slower phase separation. In the past decade, ionic-liquid-based ABS were proposed by Gutowski et al. (2003) as alternatives to polymer-based systems. Ionic liquids (ILs) belong to the molten salts group (with melting temperatures below 100°C) and are usually constituted by a large organic cation and an inorganic/organic anion. Due to their ionic character, most ILs present a negligible volatility at atmospheric conditions, non-flammability, high thermal and chemical stabilities, and a strong solvation capability for a large variety of compounds (Crowhurst et al., 2003; Seddon, 2003). Nevertheless, one of the most important features of ILs arises from their tailoring ability resulting from cation-anion combinations – a property that is further extended to IL-based ABS (Pereira et al., 2013). Due to the large amount of water and non-volatile nature of ILs, the respective ABS also appear as viable media for biocompatible extractions. IL-based ABS have been used in the separation of enzymes, proteins, amino acids and antibiotics (Dreyer and Kragl, 2008; Freire et al., 2012). Albeit these studies can be used as guidelines for the application of IL-based ABS in the extraction of proteins, in most of these works, imidazolium-based ILs and inorganic salts were used as phase-forming components (Freire et al., 2012). In particular, these IL-based ABS have been applied in the extraction of bovine serum albumin, lysozyme, trypsin, myoglobin, peroxidase, cytochrome c, globulins, hemoglobin and ovalbumin (Freire et al., 2012; Pei et al., 2009; Selvakumar et al., 2012; Yan et al., 2014; Zeng et al., 2013). Mainly ILs with anions with a strong alkaline or acidic character were investigated, and phosphate buffered solutions were employed to maintain the pH of the aqueous medium while avoiding the denaturation of proteins (Alvarez-Guerra and Irabien, 2012; Ito et al., 2013; Selvakumar et al., 2012). Nonetheless, phosphate ions can bind to metal ions, such as calcium, zinc or magnesium, which are essential to maintain the integrity of some proteins/enzymes (Good et al., 1966). These high-charge density phosphate-based salts can be adequately substituted by more biocompatible and biodegradable organic salts, such as citrate-based salts that combined with citric acid afford a wide buffered pH region. On the other hand, few studies have considered phosphonium- and ammonium-based ILs as phase-forming constituents of ABS for the extraction of proteins (Chen et al., 2014; Ito et al., 2013; Taha et al., 2014). Kragl and co-workers (Dreyer et al., 2009) studied ABS constituted by Ammoeng 110 to purify two alcohol dehydrogenases. The authors concluded that this IL stabilizes the enzymes and increases the solubility of hydrophobic species. More recently, Desai et al. (2014) studied ABS formed by an ammonium- or phosphonium-based IL and a phosphate-buffered solution to extract rubisco – a vegetable protein. These tetralkyl-based compounds are less expensive and thermally more stable than the equivalent imidazolium-based counterparts, are available on a multi-ton scale, and have already been used in industrial processes (Plechkova and Seddon, 2008). Still, to decrease the cost of IL-based processes, studies on the recovery and reusability of ILs are of outstanding importance when envisaging their scale-up and industrial implementation. For instance, imidazolium-based ILs employed in the extraction of gallic acid by ABS were recovered and reused by back-extraction approaches (Cláudio et al., 2014).
This work aims at investigating the extraction capacity of ABS formed by phosphonium- and ammonium-based ILs and a citrate-based salt for BSA, and to further evaluate the protein stability in the IL-rich phase, as well as the protein recovery and recyclability/reusability of ILs.
2. Materials and methods
2.1. Materials
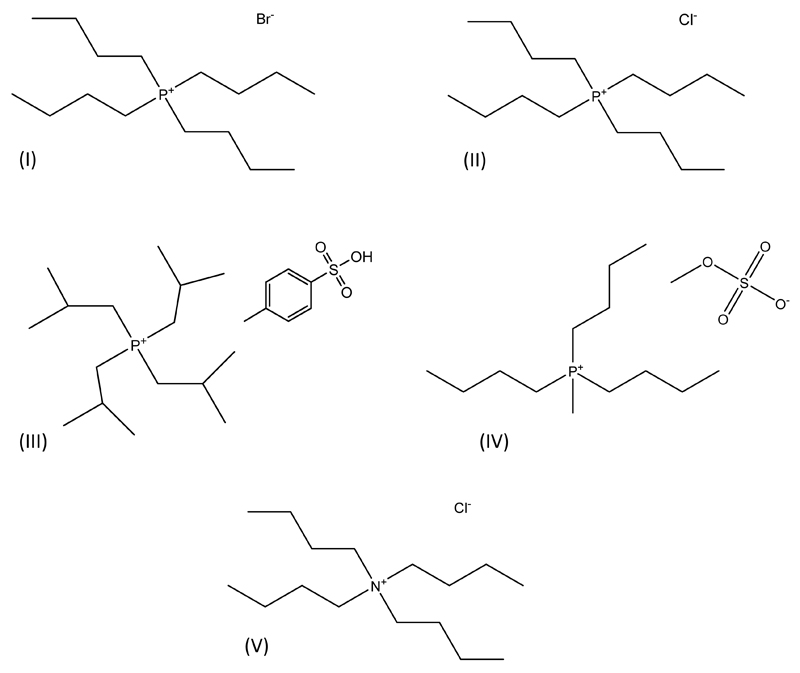
The ABS studied in this work are composed of a buffer aqueous solution constituted by potassium citrate, K3C6H5O7·H2O, ≥ 99 wt% pure from Sigma–Aldrich, and citric acid, C6H8O7·H2O, 100 wt% pure from Fisher Scientific. The ionic liquids studied were the following: tetrabutylammonium chloride ([N4444]Cl), > 97% pure, tetrabutylphosphonium bromide ([P4444]Br), > 96% pure, tetrabutylphosphonium chloride ([P4444]Cl), > 95% pure, tri(isobutyl)methylphosphonium tosylate ([Pi(444)1][Tos]), > 95% pure, and tri(butyl)methylphosphonium methylsulfate ([P4441][MeSO4]), > 99% pure. All phosphonium-based ILs were generously offered by Cytec Ind., while the ammonium-based compound was acquired from Sigma-Aldrich. The chemical structures of the ILs studied are depicted in Fig. 1. Bovine Serum Albumin (BSA), fraction V, pH 7.0, fat acid free, was purchased from Acros.
Fig. 1.
Chemical structure of the studied ILs: [P4444]Br (I); [P4444]Cl (II); [Pi(444)1][Tos] (III); [P4441][MeSO4] (IV); and [N4444]Cl (V).
2.2. Phase diagrams and tie-lines
Aqueous solutions of each IL ([P4444]Br, [P4444]Cl, [Pi(444)1][Tos], [P4441][MeSO4] and [N4444]Cl ) at circa 90 wt% and aqueous solutions of the mixture K3C6H5O7/C6H8O7 (as a buffer solution at pH = 7.0, mole ratio of ≈ 15:1) at ≈ 50 wt% were prepared and used for the determination of the binodal curves. The phase diagrams were determined through the cloud point titration method (Passos et al., 2012) at 25°C and atmospheric pressure. The system compositions were determined by the weight quantification of all components added within ± 10-4 g.
The tie-lines (TLs) were determined by a gravimetric method originally proposed by Merchuk et al. (1998). Different mixture points at the biphasic region were prepared in small glass ampoules (ca. 5 mL) especially designed for the purpose, vigorously stirred and allowed to reach equilibrium by the separation of the phases for at least 10 min at 25°C. After separation of the two phases, both the top and bottom phases were weighted. Each individual TL was determined by application of the lever-arm rule to the relationship between the top weight phase composition and the overall system composition. The experimental binodal curves were correlated using Eq. (1):
| (1) |
The determination of the TLs was accomplished through the solution of the following system of four equations (Eqs. (2) to (5)) aiming at determining four unknown values ([IL]IL, [IL]salt, [salt]IL and [salt]salt):
| (2) |
| (3) |
| (4) |
| (5) |
where the subscripts "IL", "salt" and "M" represent the top and the bottom phases and the mixture composition, respectively. The parameter α is the ratio between the weight of the top phase and the weight of the overall mixture. For the calculation of each tie-line length (TLL), Eq. (6) was employed:
| (6) |
2.3. Extraction of bovine serum albumin (BSA)
The ternary mixtures compositions used in the partitioning experiments were chosen based on the phase diagrams determined in this work for each IL-salt-water system. Different mixture compositions were studied to evaluate the effect of the concentration of the phase-forming components through the extraction of BSA, namely XXX. BSA at concentrations of circa 0.5, 1.0, 5.0 and 10.0 g.L-1 were also used as the aqueous solution. Each mixture was vigorously stirred, centrifuged for 10 min, and left to equilibrate for 10 min at 25°C to reach the BSA complete partitioning between the coexisting phases. After, a careful separation of the phases was performed and the amount of BSA in each phase was quantified by SE-HPLC (Size Exclusion High-Performance Liquid Chromatography). Each phase was diluted at a 1:10 (v:v) ratio in a phosphate buffer solution before injection. A Chromaster HPLC (VWR, Hitachi) coupled to an UV detector was used. SE-HPLC was performed with an analytical column (25 cm × 2 mm i.d., 25 μm), Lichrospher 100 RP-18, from Merck. A 100 mM phosphate buffer in MiliQ water (mobile phase) was run isocratically with a flow rate of 1 mL·min-1. The temperature of the column and auto-sampler was kept constant at 25°C. The injection volume was of 25 µL. The wavelength was set at 280 nm whereas the retention time of BSA was found to be circa 16 min within an analysis time of 40 min. The quantification of BSA in each phase was carried out by an external standard calibration method (R2=0.9997) - cf. the Supporting Information with the established calibration curve and associated standard deviations. The limit of detection of BSA was found to be 0.011 g.L-1. At least three independent ABS were prepared and 3 samples of each phase were quantified.
The percentage extraction efficiency of BSA, EEBSA%, is the percentage ratio between the amount of protein in the IL-rich aqueous phase to that in the total mixture, and is defined according to Eq. (7):
| (7) |
where and are the total weight of protein in the IL-rich and in the salt-rich aqueous phases, respectively. In all systems the top phase corresponds to the IL-rich phase whereas the bottom phase is mainly constituted by the citrate-based salt and water.
2.4. Recovery of bovine serum albumin (BSA) from the IL-rich phase and reusability of the ILs
In order to guarantee the recovery of BSA after the extraction for the IL-rich phase and the ILs recyclability, a three-step recovery/extraction process was here attempted. In this approach, the system composed of 30 wt% [Pi(444)1][Tos] + 30 wt% of K3C6H5O7/C6H8O7 + 40 wt% of an aqueous solution of BSA at 0.5 g.L-1 was chosen. After the initial extraction of BSA as described before, which occurs for the IL-rich phase, the protein was recovered by dialysis. The IL-rich phase containing BSA was dialyzed against 100 mL of ultra-pure water, for 12h at room temperature and under moderate agitation, using a dialysis tubing cellulose membrane (average flat width 10 mm (0.4 in.), molecular weight cut-off = 14,000) from Sigma. The dialysate solution containing BSA was recovered, and the aqueous phase containing the IL was dried under vacuum at 60ºC up to a constant weight, and then reused to form a new ABS with K3C6H5O7/C6H8O7 and an aqueous solution of BSA at 0.5 g.L-1 in order to perform a subsequent extraction of the protein. This step was repeated one more time to ascertain on the recyclability and reusability of the IL employed and its efficiency when employed as a phase-forming component of a given ABS for the extraction of proteins.
2.5. Fourier transform infrared spectroscopy (FT-IR)
FT-IR spectra were recorded using a Perkin Elmer Spectrum Bx spectrophotometer with a resolution of 4 cm−1. Several aqueous solutions containing BSA (1.0 g.L−1) and ILs at different concentrations were used to perform the FT-IR analysis. The spectra were obtained in the wavelength range from 1700 to 1500 cm−1.
3. Results
3.1. Phase diagrams and tie-lines
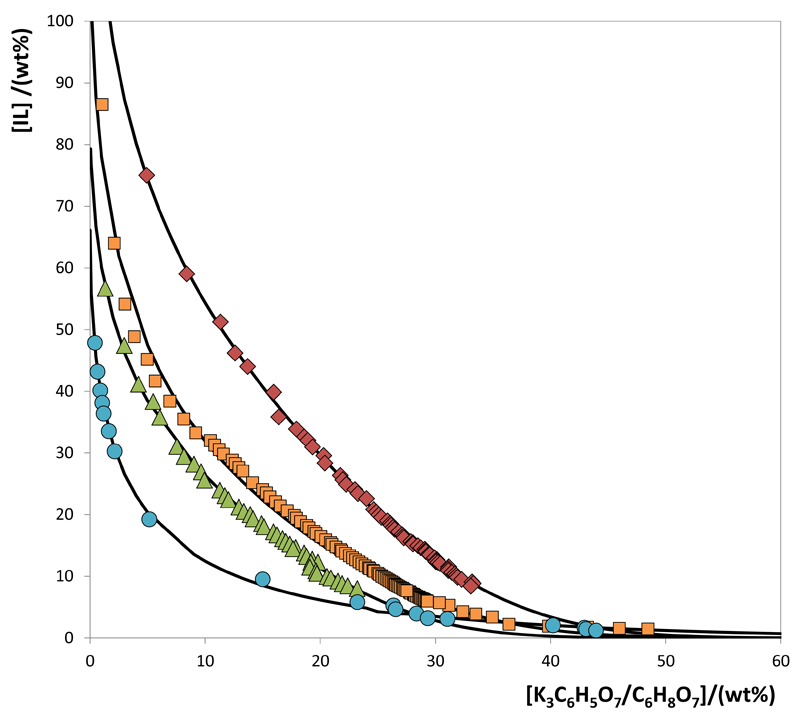
Novel phase diagrams for the various systems consisting of IL + water + K3C6H5O7/C6H8O7 buffer (pH = 7.0) at 25°C and atmospheric pressure were determined in this work. The respective phase diagrams are depicted in Figs. 2 and 3. The corresponding data are provided in the Supporting Information. All the calculations considering the weight fraction of the phase-forming components were carried out discounting the complexed water in the commercial citrate-based salt.
Fig. 2.
Phase diagrams for the systems composed of IL + K3C6H5O7/C6H8O7 + H2O at 25ºC and pH 7.0: [P4444]Br ( ); [P4441][MeSO4] (
); [P4441][MeSO4] ( ); [P4444]Cl (
); [P4444]Cl ( ); [Pi(444)1][Tos] (
); [Pi(444)1][Tos] ( ); and adjusted binodal data through Eq. (1) (–).
); and adjusted binodal data through Eq. (1) (–).
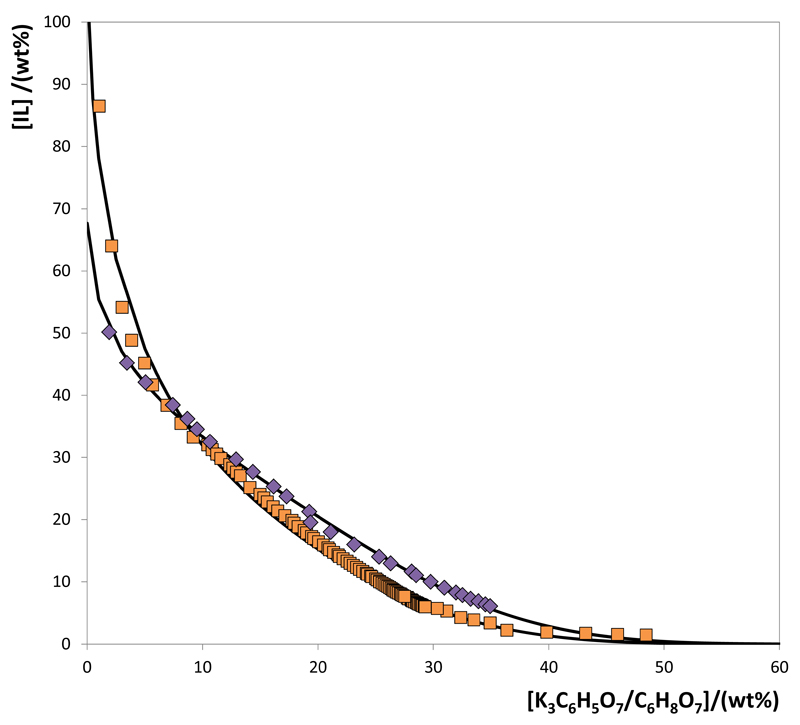
Fig. 3.
Phase diagrams for the systems composed of IL + K3C6H5O7/C6H8O7 + H2O at 25ºC and pH 7.0: [P4444]Cl ( ); [N4444]Cl (
); [N4444]Cl ( ); and adjusted binodal data through Eq. (1) (–).
); and adjusted binodal data through Eq. (1) (–).
Fig. 2 depicts the phase diagrams for the systems constituted by phosphonium-based ILs and allows the evaluation of the ILs ability to form ABS. In all phase diagrams, the two-phase region is positioned above the solubility curve while the monophasic region is localized below. Diagrams with the largest area above the binodal curve have therefore a higher ability to form two phases, i.e., the IL is more easily salted-out by the salt. Therefore, the capacity of phosphonium-based ILs to form ABS (or ILs more easily salted-out) follows the order: [P4444]Br > [P4441][MeSO4] > [P4444]Cl > [Pi(444)1][Tos]. Since not all the cations and anions are common there is a balanced effect of both ions to be hydrated and to form ABS. Nevertheless, the results obtained here are in close agreement with the trend previously described in the literature for ABS composed of phsophonium-based ILs + K3PO4 + water (Louros et al., 2010). In general, phosphonium-based ILs have a higher ability to udergo phase separation in ABS when compared with their imidazolium-based counterparts (Louros et al., 2010) – a major advantage when considering the amount of phase-forming compounds required to form liquid-liquid systems.
Since several ILs with different lengths at the cation alkyl chains were investigated, a direct comparison can only be made between [P4444]Br and [P4444]Cl, whereas the first is more able to undergo a phase separation. The Cl- anion presents a higher aptitude to be hydrated or to form hydration complexes, and thus more salt is required to induce the salting-out when compared with bromide-based ILs (Cláudio et al., 2014). Bromide has a weaker ability to hydrogen-bond with water or to form hydration complexes, and less salt is required for phase separation in ABS. In addition, when comparing the performance of [P4441][MeSO4] and [Pi(444)1][Tos], the [Tos]-based IL requires a higher amount of salt to undergo liquid-liquid demixing.
The phase diagrams for the systems composed of [N4444]Cl and [P4444]Cl are presented in Fig. 3. Although similar, the [P4444]-based IL presents a slightly higher ability to form ABS. The higher hydrophobicity of phosphonium-based ILs (when compared to the ammonium-based counterparts) was shown by Carvalho et al. (2014) and is reflected in the phase behavior of ternary systems composed of similar ILs, water and other salts (Passos et al., 2012). It is well-accepted that the water-IL miscibility derives essentially from hydrogen-bonding interactions mainly occurring with the anion (Freire et al., 2007). Nevertheless, the anion is similar in both cases, and the small deviations in the phase diagram are a result of the charge distribution at the central atom of the IL cation. Even so, both tetrabutyl-based cations are known for their high capacity to form ABS mainly due to the presence of heteroatoms surrounded by four alkyl chains and no aromatic character, and therefore with a low affinity for water.
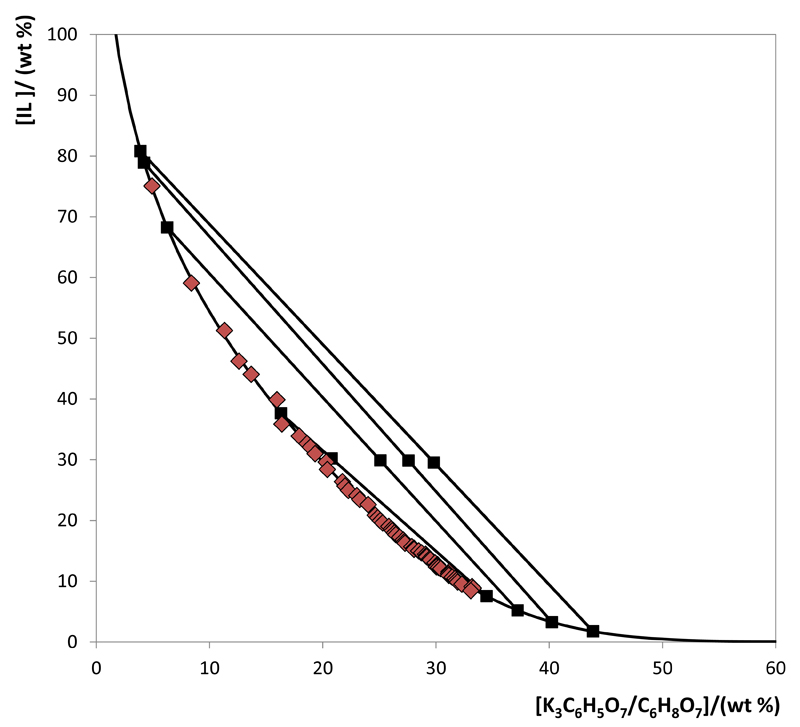
The experimental data corresponding to the binodal curves were fitted using Eq. (1) as shown in Figs. 2 and 3. The regression parameters estimated by least-squares regression, standard deviations (σ) and correlation coefficients (R2) are displayed in Table 1. Eq. (1) adequately describes the experimental binodal curves as confirmed by the good correlation coefficients obtained. The experimental TLs in each ABS, along with their respective length, and at the compositions for which the extraction studies of BSA were conducted are reported in Table 2. An example of the TLs obtained for the [Pi(444)1][Tos]-based system is depicted in Fig. 4. The graphical representation of the TLs for the remaining systems are presented in the Supporting Information.
Table 1.
Correlation parameters of Eq. (1) used to describe the experimental binodal data at 25ºC.
| IL | A ± σ | B ± σ | 105 (C ± σ) | R2 |
|---|---|---|---|---|
| IL + K3C6H5O7/C6H8O7 + water | ||||
| [P4444]Br | 66.13 ± 0.98 | -0.527 ± 0.013 | 0.21 ± 0.24 | 0.9988 |
| [P4444]Cl | 116.40 ± 2.65 | -0.399 ± 0.009 | 3.05 ± 0.20 | 0.9849 |
| [N4444]Cl | 69.16 ± 1.22 | -0.222 ± 0.007 | 2.78 ± 0.12 | 0.9978 |
| [Pi(444)1][Tos] | 149.90 ± 2.39 | -0.318 ± 0.006 | 2.83 ± 0.10 | 0.9953 |
| [P4441][MeSO4] | 85.89 ± 1.30 | -0.356 ± 0.007 | 5.40 ± 0.29 | 0.9971 |
Table 2.
Experimental TLs and TLLs of the ABS composed of ILs and C6H5K3O7/ C6H8O7 at 25ºC.
| Weight fraction composition / (wt %) | |||||||
|---|---|---|---|---|---|---|---|
| IL + K3C6H5O7/C6H8O7 + water | |||||||
| IL | [IL]IL | [salt]IL | [IL]M | [salt]M | [IL]salt | [salt]salt | TLL |
| [P4444]Br | 58.75 | 0.05 | 29.17 | 29.38 | 0.02 | 58.30 | 82.72 |
| 52.02 | 0.21 | 19.99 | 20.30 | 1.74 | 31.75 | 59.35 | |
| [P4444]Cl | 80.98 | 0.83 | 28.82 | 29.10 | 0.56 | 44.43 | 91.48 |
| 75.06 | 1.20 | 27.08 | 27.09 | 1.08 | 41.11 | 84.05 | |
| [N4444]Cl | 60.80 | 0.34 | 30.25 | 30.68 | 0.02 | 60.71 | 85.67 |
| 58.51 | 0.57 | 24.21 | 24.68 | 3.05 | 39.56 | 67.79 | |
| [Pi(444)1][Tos] | 80.78 | 3.90 | 29.51 | 29.83 | 1.74 | 43.87 | 85.57 |
| 78.90 | 4.21 | 29.86 | 27.57 | 3.28 | 40.23 | 83.76 | |
| 68.25 | 6.25 | 29.89 | 25.09 | 5.20 | 37.20 | 70.23 | |
| 37.62 | 16.32 | 30.19 | 20.80 | 7.52 | 34.48 | 35.15 | |
| [P4441][MeSO4] | 58.35 | 1.18 | 29.10 | 28.93 | 3.38E-04 | 56.55 | 80.44 |
| 41.87 | 4.03 | 19.61 | 19.88 | 1.72 | 32.62 | 49.29 | |
Fig. 4.
Phase diagram for the system composed of [Pi(444)1][Tos] + K3C6H5O7/C6H8O7 + H2O at 25ºC and pH 7.0: binodal curve data ( ); TL data (■); and adjusted binodal data through Eq. (1) (–).
); TL data (■); and adjusted binodal data through Eq. (1) (–).
3.2. Extraction of bovine serum albumin (BSA)
The extraction efficiencies of BSA in each IL-based ABS, and which allow the evaluation of the IL for such a purpose, were determined for a common mixture composition (30 wt% of IL, 30 wt% of K3C6H5O7/C6H8O7 and 40 wt% of an aqueous solution containing BSA at 0.5 g.·L-1). The results obtained are reported in Table 3. In all systems, the top phase corresponds to the IL-rich phase while the bottom phase is majorly composed of the citrate-based salt and water.
Table 3.
Extraction efficiency of BSA (EEBSA%) at 25ºC and pH 7.0 in the ABS composed of ILs and K3C6H5O7/C6H8O7.
| IL | Weight fraction composition / (wt %) |
EEBSA% | |
|---|---|---|---|
| IL | Salt | ||
| [P4444]Br | 29.98 ± 0.11 | 30.01 ± 0.93 | - |
| [P4444]Cl | 30.40 ± 0.80 | 30.48 ± 0.38 | 100 |
| [N4444]Cl | 31.30 ± 0.73 | 30.97 ± 1.30 | 100 |
| [Pi(444)1][Tos] | 29.92 ± 0.27 | 30.39 ± 0.32 | 100 |
| [P4441][MeSO4] | 31.30 ± 0.72 | 30.97 ± 1.30 | 100 |
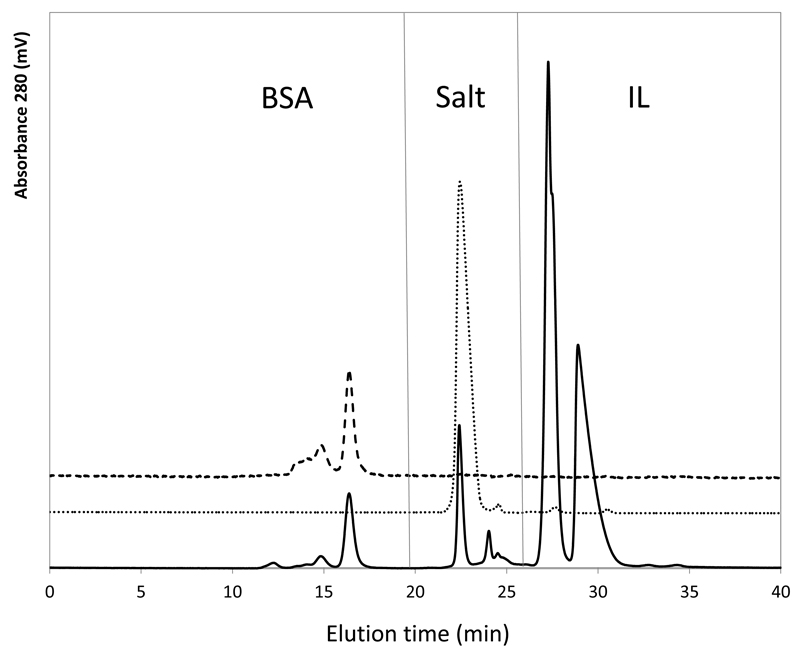
With the exception of the ABS composed of [P4444]Br, for which a complete precipitation of the protein was observed (no protein was detected at the coexisting phases as determined by SE-HPLC), all the other ABS are able to completely extract BSA for the IL-rich phase – with extraction efficiencies of 100% attained in a single-step. For these systems, the SE-HPLC chromatograms used for the BSA quantification confirm the presence of BSA only at the IL-rich phase, as well as the absence of BSA aggregates or the protein fragmentation – Fig. 5. Moreover, for these systems, no losses of protein by precipitation where observed.
Fig. 5.
Size exclusion chromatography results of a BSA standard solution (---); BSA in the salt rich-phase (⋅⋅⋅⋅); and BSA in the IL rich-phase (—–) corresponding to the ABS composed of [Pi(444)1][Tos] (20 wt%) + K3C6H5O7/ C6H8O7 (30 wt%).
[P4444]Br is the most hydrophobic IL investigated (from the ILs trend shown in Fig. 2) and leads to the complete precipitation and/or denaturation of BSA, being therefore not adequate for this type of extractions. If one compares the TL data presented in Table 2 for the similar mixture composition, it can be inferred that neither the IL content or water content at the IL-rich phase seem to be responsible for the protein precipitation. The composition of water in the [P4444]Br-rich phase is around 40 wt% and similar to that found in the [P4441][MeSO4]-rich phase. Therefore, these data indicate that the changes in the protein structure may result from specific interactions occurring between the protein and the IL. Hydrophobic interactions responsible for proteins precipitation have been reported with polymers (PEG and PPG), organic solvents (acetone, ethanol, methanol and dimethyl sulfoxide) and salts (Arakawa et al., 2007; Kumar et al., 2009; Tscheliessnig et al., 2014). For instance, the precipitation of rubisco was also observed with the molecular weight increase of the polymers used as phase-forming components of ABS, and that was related with a decrease in the free volume available as well as with the hydrophobicity increase in such a phase (Desai et al., 2014).
In general, and with the exception of the [P4444]Br-based ABS, BSA still preferentially partitions to the most hydrophobic phase, i.e., the IL-rich phase. This trend is similar to that observed in ABS formed by polyethylene glycol (PEG) and potassium citrate, where BSA preferentially partitions for the more hydrophobic PEG-rich phase (Lu et al., 2010). Lu et al. (2010) also reported that either an increase on the salt or polymer concentration, i.e. increase of the TLL, leads to a preferential partitioning of BSA for the polymer-rich phase. This behavior was explained based on the salting-out effect afforded by an increased concentration of salt in the system, leading thus to a preferential exclusion of the protein for the opposite phase (Lu et al., 2010). The isoelectric point of BSA is 4.7 (Yan et al., 2013). Hence, BSA is negatively charged at pH 7 and in all the studied ABS. Based on the overall results shown in Table III, the partitioning of BSA for the IL-rich phase seems to be driven by a combined effect of favorable dispersive interactions, salting-out effect exerted by the citrate-based salt, and electrostatic interactions between the positively charged IL cations and the negatively charged amino acid residues at the surface of the protein.
Overall, the extraction efficiencies achieved in this work are significantly higher than those observed in polymer-based ABS previously reported (Johansson et al., 2008; Porto et al., 2008; Saravanan et al., 2008). Saravanan et al. (2008) studied PEG4000-magnesium sulfate ABS to purify BSA with a maximum yield of 82.68%. In ABS composed of PEG and sodium citrate, Perumalsamy and Batcha (2011) reported extraction efficiencies lower than 50%. Thus, IL-based ABS seem to be more effective in the extraction of proteins from aqueous media than polymer-based systems. Lin et al. (2013) compared the effects of eight imidazolium-based ILs and identified the optimal conditions for the extraction of four proteins. Under the optimum conditions, the average recovery of BSA was 90.5%. Ding et al. (2014) also investigated the use of a series of functionalized alkylguanidinium ILs as phase-forming components of ABS, while applying single factor experiments to optimize the extraction performance, with a maximum extraction efficiency of BSA of 97%. Even in dye-affinity systems (containing the Reactive Red-120 dye) and after the optimization of the pH of the aqueous phase, temperature, and composition of the ABS, the maximum extraction efficiencies achieved of BSA for the IL-rich phase are below 100% (Sheikhian et al., 2013). Therefore, remarkable extraction efficiencies of BSA were obtained in this work with ABS composed of more stable and less expensive phosphonium- or ammonium-based ILs and a biodegradable organic salt. The ABS studied in this work seem to have an enhanced potential for the extraction of proteins and their application to the purification and concentration of added-value proteins is a main topic of further work at our laboratory.
3.3. Influence of the IL, salt and bovine serum albumin (BSA) concentrations
Aiming at reducing the amount of salt and IL required to form ABS, while keeping the complete extraction ability for BSA, a series of ABS containing variable concentrations of [Pi(444)1][Tos] (20-30 wt%) and salt (20-30 wt%) was prepared. [Pi(444)1][Tos] was selected since it is the only IL that is liquid at room temperature (from the set of ILs investigated) and therefore easier to handle and to prepare the target mixture compositions. The results obtained for the IL concentration effect are depicted in Table 4. In all systems, the complete extraction of BSA was attained. Therefore, the concentration of IL can be decreased down to 20 wt% without losing the ABS complete extraction performance.
Table 4.
Effect of the IL, salt and protein concentration in the extraction efficiencies of BSA (EEBSA%) for the system composed of [Pi(444)1][Tos] (from 20 to 30 wt%) + K3C6H5O7/C6H8O7 (from 20 to 30 wt%) at 25ºC and pH 7.0.
| [BSA] / (g.L-1) | Weight fraction composition / (wt %) |
EEBSA% | |
|---|---|---|---|
| IL | Salt | ||
| 0.5 | 30.22 ± 0.21 | 25.74 ± 0.04 | 100 |
| 31.56 ± 0.93 | 20.77 ± 0.03 | 100 | |
| 26.22 ± 0.36 | 30.48 ± 0.36 | 100 | |
| 20.41 ± 0.37 | 30.64 ± 0.09 | 100 | |
| 1.0 | 20.13 ± 0.07 | 30.41 ± 0.12 | 100 |
| 5.0 | 20.21 ± 0.06 | 30.32 ± 0.17 | 100 |
| 10.0 | 20.43 ± 0.15 | 30.47 ± 0.38 | 100 |
The effect of the salt concentration through the extraction efficiency of BSA is also shown in Table 4. All compositions allow the complete extraction of BSA, in a single-step, and without precipitation and/or denaturation of the protein. The variation of the salt amount has been reported to drive the migration of proteins to the top phase due to a decrease in the difference of the electrostatic potential between the coexisting phases in PEG-salt systems (Nascimento et al., 2010). Even so, in IL-salt-based ABS, the extraction efficiencies are kept at 100% within the salt concentration range evaluated. In fact, the mixture compositions (20 wt% of salt + 30 wt% of IL) and (30 wt% of salt + 20 wt% of IL) are very close to the binodal curve and correspond to minimum concentrations required to form ABS with [Pi(444)1][Tos].
The results presented in Table 5 reveal the extraction efficiencies of BSA, in a given ABS and composition, as a function of the protein concentration at the aqueous phase. It is here shown that the investigated [Pi(444)1][Tos]-based ABS can be used to completely extract BSA from aqueous solutions with a concentration up to 10 g·L-1 (without the saturation of the IL-rich phase and with no precipitation and/or denaturation effects observed).
3.4. Recovery of bovine serum albumin (BSA) from the IL-rich phase and reusability of the ILs
To guarantee the development of a sustainable and low-cost extraction procedure, we additionally evaluated the recovery of BSA from the IL-rich phase and further use of the IL to create a new ABS and to conduct subsequent extractions with new aqueous solutions containing BSA. In this step, the ABS composed of 30 wt% of [Pi(444)1][Tos], 30 wt% of K3C6H5O7/C6H8O7 and 40 wt% of an aqueous solution containing BSA at 0.5 g.·L-1 was investigated. After the first extraction, the top phase was separated, and BSA was recovered by dialysis. After a complete separation of BSA, the IL rich-phase was dried under vacuum at 60ºC. Then, the non-volatile components at the IL-rich phase, IL and salt, were used to create a new ABS by the addition of a fresh aqueous solution containing BSA at 0.5 g.·L-1, as well as more citrate-based salt to achieve the initial mixture composition. After the formation of the ABS and the conditions mentioned before for achieving the equilibrium, the phases were separated and the amount of BSA in each phase was again quantified. This overall procedure was repeated one more time. In all experiments of BSA recovery/IL reusability, the complete extraction of BSA (extraction efficiency of 100%) for the IL rich-phase was verified, meaning that ILs can be reused in ABS without a decrease on the complete extraction efficiency. However, it should be highlighted that unless cyclic, closed and more complex systems are used, inevitable losses of IL will occur by the simple transference of the fluid into different vials. Moreover, and from the TLs data shown in Table 2, there is also the loss of 1.74 wt% of [Pi(444)1][Tos] per extraction, which corresponds to the IL present in the salt-rich phase. In general, and for the three consecutive extractions investigated, a total loss of 9.96 wt% of [Pi(444)1][Tos] was found.
3.5. Bovine serum albumin (BSA) stability in ionic liquid solutions
Proteins are complex macromolecules which retain their structural and functional stability in their native environment; yet, small variations in this environment, such as temperature, pressure, pH and presence of some solutes can alter their native structure. Most works reported in literature focused on the extraction ability of IL-based ABS for proteins (Kohno et al., 2011; Shu et al., 2010; Tzeng et al., 2008), and few studies (Huang et al., 2013; Lin et al., 2013; Taha et al., 2014) have investigated the protein stability after being extracted into the IL-rich phase. However, if proteins are being extracted for a given phase, the protein stability is a prerequisite that cannot be discarded. The SE-HPLC chromatograms used for the BSA quantification, one example being displayed in Fig. 5, allow to endorse that there is no significant fragmentation or formation of aggregates of BSA under the studied conditions. In addition, the native structure of BSA at the IL-rich phase, in the systems investigated, was always confirmed by the size-exclusion chromatograms. The absence of BSA at the salt-rich phase also confirms the complete extraction of the protein for the IL-rich phase in the studied systems. Recently, Desai et al. (2014) demonstrated that other proteins, such as IgG and Rubisco, due to their higher molecular weight, tend to cluster into aggregates in presence of phosphonium-based ILs. Baker and Heller (2009) also reported the aggregation of HSA at an aqueous solution with 50 wt% of an imidazolium-based IL. However, BSA remained stable in all the IL-based ABS tested (with exception of the [P4444]Br-based system) supporting the viability of these systems to deal with the extraction/purification of proteins.
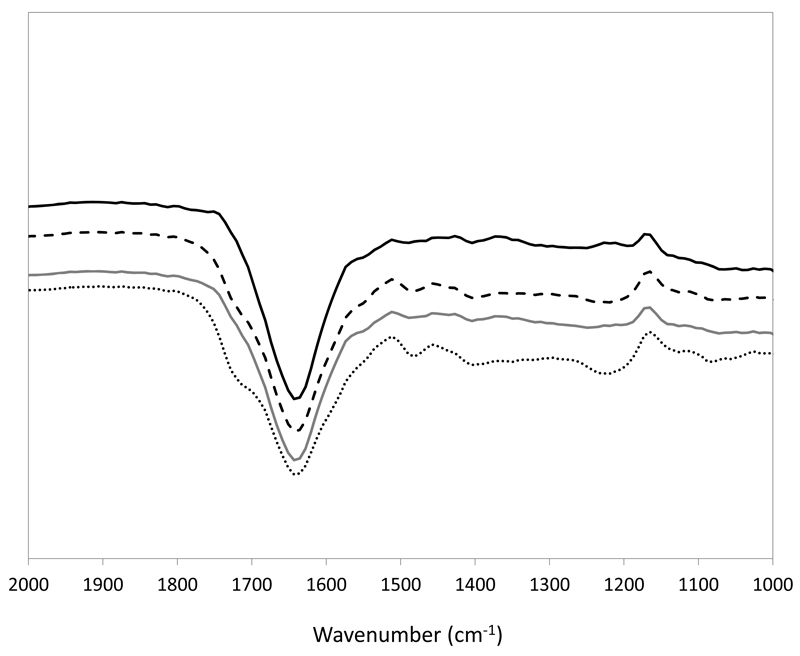
FT-IR spectra provide information for identifying the structure of proteins based on energy absorption bands of specific functional groups or chemical bonds. The most widely used absorption bands as structure probes for proteins are the amide I vibrations, which fall between 1690 and 1600 cm-1 (Barth, 2007). Fig. 6 depicts the FT-IR spectra of BSA in a buffered aqueous solution and BSA in an aqueous solution of [Pi(444)1][Tos] at different concentrations (up to 50 wt%). These results corroborate that there are no significant changes of the protein absorbance peaks suggesting that the protein may maintain its spatial structure in aqueous solutions of [Pi(444)1][Tos].
Fig. 6.
FT-IR spectra of a BSA standard solution (0.5 g.L-1) (—–); BSA in a 12.5 wt% [Pi(444)1][Tos] aqueous solution (-----); BSA in a 25 wt% [Pi(444)1][Tos] aqueous solution (—–); and BSA in a 50 wt% [Pi(444)1][Tos] aqueous solution (⋅⋅⋅⋅⋅⋅⋅).
4. Conclusions
In this work, the ability of a less studied class of IL-based ABS, composed of phosphonium- or ammonium-based ILs, was evaluated in what concerns their performance for the extraction of proteins, namely BSA. For that purpose, the respective phase diagrams, tie-lines and tie-line lengths were also ascertained. For almost all the studied systems, and at all the conditions analyzed, BSA preferentially migrates for the IL-rich phase, with the exception of the more hydrophobic IL investigated ([P4444]Br) that induced the precipitation of the protein. However, the remaining phosphonium- and ammonium-based ABS were able to completely extract BSA for the IL-rich phase. Remarkable extraction efficiencies of 100% were obtained in a single-step, without evidence of protein denaturation in concentrations up to 10 g.L-1 of BSA. Moreover, the recovery of the protein by dialysis and the IL reusability were also investigated in three-step consecutive extractions, and where it was demonstrated that IL-based ABS can be adequately reused without losses on their extraction performance. ABS composed of phosphonium- or ammonium-based ILs and a biodegradable organic salt are thus improved systems for the extraction of BSA when compared with the more traditional polymer-based or other IL-based ABS. Furthermore, tetralkylphosphonium- and tetralakylammonium-based ILs are less expensive and thermally and chemically more stable than their widely studied imidazolium-based counterparts. According to the reported results, these new ABS can be foreseen as alternative and cost-effective platforms for the separation and purification of other proteins of interest.
Supplementary Material
Acknowledgements
This work was financed by national funding from FCT - Fundação para a Ciência e a Tecnologia, through the project Pest-C/CTM/LA0011/2013. M. M. Pereira acknowledges the PhD grant (2740-13-3) and financial support from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Capes. M. G. Freire acknowledges the European Research Council (ERC) for the Starting Grant ECR-2013-StG-337753. The authors also acknowledge Cytec Industries Inc. for kindly supplying the phosphonium-based ionic liquids investigated in this work.
References
- Albertsson PA. Partition of cell particles and macromolecules: separation and purification of biomolecules, cell organelles, membranes, and cells in aqueous polymer two-phase systems and their use in biochemical analysis and biotechnology. Wiley; New York: 1986. [Google Scholar]
- Albertsson PA. Partition of proteins in liquid polymer-polymer two-phase systems. Nature. 1958;182:709–711. doi: 10.1038/182709a0. [DOI] [PubMed] [Google Scholar]
- Alvarez-Guerra E, Irabien A. Extraction of lactoferrin with hydrophobic ionic liquids. Sep Purif Technol. 2012;98:432–440. [Google Scholar]
- Arakawa T, Kita Y, Timasheff SN. Protein precipitation and denaturation by dimethyl sulfoxide. Biophys Chem. 2007;131:62–70. doi: 10.1016/j.bpc.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Asenjo JA, Andrews BA. Aqueous two-phase systems for protein separation: a perspective. J Chromatogr A. 2011;1218:8826–35. doi: 10.1016/j.chroma.2011.06.051. [DOI] [PubMed] [Google Scholar]
- Baker GA, Heller WT. Small-angle neutron scattering studies of model protein denaturation in aqueous solutions of the ionic liquid 1-butyl-3-methylimidazolium chloride. Chem Eng J. 2009;147:6–12. [Google Scholar]
- Barth A. Infrared spectroscopy of proteins. Biochim Biophys Acta. 2007;1767:1073–1101. doi: 10.1016/j.bbabio.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Carvalho PJ, Ventura SPM, Batista MLS, Schröder B, Gonçalves F, Esperança J, Mutelet F, Coutinho JAP. Understanding the impact of the central atom on the ionic liquid behavior: phosphonium vs ammonium cations. J Chem Phys. 2014;140 doi: 10.1063/1.4864182. 064505-1-0604505-11. [DOI] [PubMed] [Google Scholar]
- Chen J, Wang Y, Zeng Q, Ding X, Huang Y. Partition of proteins with extraction in aqueous two-phase system by hydroxyl ammonium-based ionic liquid. Anal Methods. 2014;6:4067–4076. [Google Scholar]
- Cláudio AFM, Swift L, Hallett JP, Welton T, Coutinho JAP, Freire MG. Extended scale for the hydrogen-bond basicity of ionic liquids. Phys Chem Chem Phys. 2014;16:6593–6601. doi: 10.1039/c3cp55285c. [DOI] [PubMed] [Google Scholar]
- Cláudio AFM, Marques CFC, Boal-Palheiros I, Freire MG, Coutinho JAP, Freire MG. Development of back-extraction and recyclability routes for ionic-liquid-based aqueous two-phase systems. Green Chem. 2014;16:259–268. [Google Scholar]
- Corbatón-Báguena M-J, Álvarez-Blanco S, Vincent-Vela M-C. Cleaning of ultrafiltration membranes fouled with BSA by means of saline solutions. Sep Purif Technol. 2014;125:1–10. [Google Scholar]
- Desai RK, Streefland M, Wijffels RH, Eppink MHM. Extraction and stability of selected proteins in ionic liquid based aqueous two phase systems. Green Chem. 2014;16:2670–2679. [Google Scholar]
- Ding X, Wang Y, Zeng Q, Chen J, Huang Y, Xu K. Design of functional guanidinium ionic liquid aqueous two-phase systems for the efficient purification of protein. Abal Chim Acta. 2014;815:22–32. doi: 10.1016/j.aca.2014.01.030. [DOI] [PubMed] [Google Scholar]
- Dissanayake M, Ramchandran L, Piyadasa C, Vasiljevic T. Influence of heat and pH on structure and conformation of whey proteins. Int Dairy J. 2013;28:56–61. [Google Scholar]
- Dreyer S, Kragl U. Ionic liquids for aqueous two-phase extraction and stabilization of enzymes. Biotechnol Bioeng. 2008;99:1416–1424. doi: 10.1002/bit.21720. [DOI] [PubMed] [Google Scholar]
- Dreyer S, Salim P, Kragl U. Driving forces of protein partitioning in an ionic liquid-based aqueous two-phase system. Biochem Eng J. 2009;46:176–185. [Google Scholar]
- Fang N-Y, Lee J, Yin S-J, Wang W, Wang Z-J, Yang J-M, Qian G-Y, Si Y-X, Park Y-D. Effects of osmolytes on arginine kinase from Euphausia superba: A study on thermal denaturation and aggregation. Process Biochem. 2014;49:936–947. [Google Scholar]
- Freire MG, Santos LMNBF, Fernandes AM, Coutinho JAP, Marrucho IM. An overview of the mutual solubilities of water-imidazolium-based ionic liquids systems. Fluid Phase Equilibr. 2007;261:449–454. [Google Scholar]
- Freire MG, Cláudio AFM, Araújo JMM, Coutinho JAP, Marrucho IM, Canongia Lopes JN, Rebelo LPN. Aqueous biphasic systems: a boost brought about by using ionic liquids. Chem Soc Rev. 2012;41:4966–4995. doi: 10.1039/c2cs35151j. [DOI] [PubMed] [Google Scholar]
- Gelamo EL, Silva CHTP, Imasato H, Tabak M. Interaction of bovine (BSA) and human (HSA) serum albumins with ionic surfactants: spectroscopy and modelling. Biochim Biophys Acta - Protein Struct Mol Enzymol. 2002;1594:84–99. doi: 10.1016/s0167-4838(01)00287-4. [DOI] [PubMed] [Google Scholar]
- Gelamo EL, Tabak M. Spectroscopic studies on the interaction of bovine (BSA) and human (HSA) serum albumins with ionic surfactants. Spectrochim Acta Part A Mol Biomol Spectrosc. 2000;56:2255–2271. doi: 10.1016/s1386-1425(00)00313-9. [DOI] [PubMed] [Google Scholar]
- Good NE, Winget GD, Winter W, Connolly TN, Izawa S, Singh RMM. Hydrogen ion buffers for biological research. Biochem. 1966;5:467–477. doi: 10.1021/bi00866a011. [DOI] [PubMed] [Google Scholar]
- Gorza FDS, Pedro GC, Trescher TF, da Silva RJ, Silva JR, de Souza NC. Morphological analysis and interaction of chlorophyll and BSA. Biomed Res Int. 2014;2014:872701. doi: 10.1155/2014/872701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutowski KE, Broker GA, Willauer HD, Huddleston JG, Swatloski RP, Holbrey JD, Rogers RD. Controlling the aqueous miscibility of ionic liquids: aqueous biphasic systems of water-miscible ionic liquids and water-structuring salts for recycle, metathesis, and separations. J Am Chem Soc. 2003;125:6632–6633. doi: 10.1021/ja0351802. [DOI] [PubMed] [Google Scholar]
- Hayes GG, Marchio C. Expulsion of proteins from water-in-oil microemulsions by treatment with cosurfactant. Biotechnol Bioeng. 1998;59:557–566. doi: 10.1002/(sici)1097-0290(19980905)59:5<557::aid-bit5>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Hebbar HU, Raghavarao KSMS. Extraction of bovine serum albumin using nanoparticulate reverse micelles. Process Biochem. 2007;42:1602–1608. [Google Scholar]
- Huang BX, Kim H-Y, Dass C. Probing three-dimensional structure of bovine serum albumin by chemical cross-linking and mass spectrometry. J Am Soc Mass Spectrom. 2004;15:1237–1247. doi: 10.1016/j.jasms.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Huang S, Wang Y, Zhou Y, Li L, Zeng Q, Ding X. Choline-like ionic liquid-based aqueous two-phase extraction of selected proteins. Anal Methods. 2013;5:3395–3402. [Google Scholar]
- Ito Y, Kohno Y, Nakamura N, Ohno H. Design of phosphonium-type zwitterion as an additive to improve saturated water content of phase-separated ionic liquid from aqueous phase toward reversible extraction of proteins. Int J Mol Sci. 2013;14:18350–61831. doi: 10.3390/ijms140918350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang W, Schwendeman SP. Formaldehyde-mediated aggregation of protein antigens: Comparison of untreated and formalinized model antigens. Biotechnol Bioeng. 2000;70:507–517. [PubMed] [Google Scholar]
- Johansson H-O, Persson J, Tjerneld F. Thermoseparating water/polymer system: A novel one-polymer aqueous two-phase system for protein purification. Biotechnol Bioeng. 1999;66:247–257. doi: 10.1002/(sici)1097-0290(1999)66:4<247::aid-bit6>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Johansson H-O, Ishii M, Minaguti M, Feitosa E, Penna TCV, Pessoa A. Separation and partitioning of Green Fluorescent Protein from Escherichia coli homogenate in poly(ethylene glycol)/sodium-poly(acrylate) aqueous two-phase systems. Sep Purif Technol. 2008;62:166–174. [Google Scholar]
- Kohno Y, Saita S, Murata K, Nakamura N, Ohno H. Extraction of proteins with temperature sensitive and reversible phase change of ionic liquid/water mixture. Polym Chem. 2011;2:862–867. [Google Scholar]
- Kumar V, Sharma VK, Kalonia DS. Effect of polyols on polyethylene glycol (PEG)-induced precipitation of proteins: Impact on solubility, stability and conformation. Int J Pharm. 2009;366:38–43. doi: 10.1016/j.ijpharm.2008.08.037. [DOI] [PubMed] [Google Scholar]
- Lee VE, Schulman JM, Stiefel EI, Lee CC. Reversible precipitation of bovine serum albumin by metal ions and synthesis, structure and reactivity of new tetrathiometallate chelating agents. J Inorg Biochem. 2007;101:1707–1718. doi: 10.1016/j.jinorgbio.2007.07.015. [DOI] [PubMed] [Google Scholar]
- Li S, Schöneich C, Borchard RT. Chemical instability of protein pharmaceuticals: Mechanisms of oxidation and strategies for stabilization. Biotechnol Bioeng. 2004;48:490–500. doi: 10.1002/bit.260480511. [DOI] [PubMed] [Google Scholar]
- Lin X, Wang Y, Zeng Q, Ding X, Chen J. Extraction and separation of proteins by ionic liquid aqueous two-phase system. Analyst. 2013;138:6445–6453. doi: 10.1039/c3an01301d. [DOI] [PubMed] [Google Scholar]
- Ling Y-Q, Nie H-L, Brandford-White C, Williams GR, Zhu L-M. Metal chelate affinity precipitation: purification of BSA using poly(N-vinylcaprolactam-co-methacrylic acid) copolymers. Colloids Surf B Biointerfaces. 2012;94:281–287. doi: 10.1016/j.colsurfb.2012.02.004. [DOI] [PubMed] [Google Scholar]
- Liu Y, Dong X, Sun Y. Protein separation by affinity extraction with reversed micelles of Span 85 modified with Cibacron Blue F3G-A. Sep Purif Technol. 2007;53:289–295. [Google Scholar]
- Louros CLS, Cláudio AFM, Neves CMSS, Freire MG, Marrucho IM, Jérôme P, Coutinho JAP. Extraction of biomolecules using phosphonium-based ionic liquids+ K3PO4 aqueous biphasic systems. Int J Mol Sci. 2010;11:1777–1791. doi: 10.3390/ijms11041777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y-M, Yang Y-Z, Zhao X-D, Xia C-B. Bovine serum albumin partitioning in polyethylene glycol (PEG)/potassium citrate aqueous two-phase systems. Food Bioprod Process. 2010;88:40–46. [Google Scholar]
- Mandeville JS, Tajmir-Riahi HA. Complexes of dendrimers with bovine serum albumin. Biomacromol. 2010;11:465–472. doi: 10.1021/bm9011979. [DOI] [PubMed] [Google Scholar]
- Merchuk JC, Andrews BA, Asenjo JA. Aqueous two-phase systems for protein separation. Studies on phase inversion. J Chromatogr B. 1998;711:285–293. doi: 10.1016/s0378-4347(97)00594-x. [DOI] [PubMed] [Google Scholar]
- Menon MK, Zydney AL. Effect of ion binding on protein transport through ultrafiltration membranes. Biotechnol Bioeng. 1999;63:298–307. [PubMed] [Google Scholar]
- Nascimento KS, Rosa PAJ, Nascimento KS, Cavada BS, Azevedo AM, Aires-Barros MR. Partitioning and recovery of Canavalia brasiliensis lectin by aqueous two-phase systems using design of experiments methodology. Sep Purif Technol. 2010;75:48–54. [Google Scholar]
- Passos H, Ferreira AR, Cláudio AFM, Coutinho JAP, Freire MG. Characterization of aqueous biphasic systems composed of ionic liquids and a citrate-based biodegradable salt. Biochem Eng J. 2012;67:68–76. [Google Scholar]
- Pereira JFB, Rebelo LPN, Rogers RD, Coutinho JAP, Freire MG. Combining ionic liquids and polyethylene glycols to boost the hydrophobic-hydrophilic range of aqueous biphasic systems. Phys Chem Chem Phys. 2013;15:19580–19583. doi: 10.1039/c3cp53701c. [DOI] [PubMed] [Google Scholar]
- Perumalsamy M, Batcha MI. Synergistic extraction of bovine serum albumin using polyethylene glycol based aqueous biphasic system. Process Biochem. 2011;46:494–497. [Google Scholar]
- Plechkova NV, Seddon KR. Applications of ionic liquids in the chemical industry. Chem Soc Rev. 2008;37:123–150. doi: 10.1039/b006677j. [DOI] [PubMed] [Google Scholar]
- Porto TS, Medeiros e Silva GM, Porto CS, Cavalcanti MTH, Neto BB, Lima-Filho JL, Converti A, Porto ALF, Pessoa A. Liquid–liquid extraction of proteases from fermented broth by PEG/citrate aqueous two-phase system. Chem Eng Process Process Intensif. 2008;47:716–721. [Google Scholar]
- Puthirasigamany M, Hamm I, van Winssen FA, Nikbin N, Kreis P, Gorak A, Zeiner T. Purification of biomolecules combining ATPS and membrane chromatography. Food Bioprod Process. 2014;92:152–160. [Google Scholar]
- Raja S, Murty VR, Thivaharan V, Rajasekar V, Ramesh V. Aqueous two phase systems for the recovery of biomolecules – a review. Sci Technol. 2012;1:7–16. [Google Scholar]
- Roberts CJ. Non-native protein aggregation kinetics. Biotechnol Bioeng. 2007;98:927–938. doi: 10.1002/bit.21627. [DOI] [PubMed] [Google Scholar]
- Saravanan S, Rao JR, Nair BU, Ramasami T. Aqueous two-phase poly(ethylene glycol)–poly(acrylic acid) system for protein partitioning: Influence of molecular weight, pH and temperature. Process Biochem. 2008;43:905–911. [Google Scholar]
- Seddon KR. Ionic liquids: A taste of the future. Nat Mater. 2003;2:363–365. doi: 10.1038/nmat907. [DOI] [PubMed] [Google Scholar]
- Selvakumar P, Ling TC, Walker S, Lyddiatt A. Partitioning of haemoglobin and bovine serum albumin from whole bovine blood using aqueous two-phase systems. Sep Purif Technol. 2012;90:182–188. [Google Scholar]
- Sheikhian L, Akhond M, Absalan G, Goltz DM. Dye-affinity partitioning of acidic, basic, and neutral proteins in ionic liquid-based aqueous biphasic systems. Sep Sci Technol. 2013;48:2372–2380. [Google Scholar]
- Shu Y, Chen X-W, Wang J-H. Ionic liquid-polyvinyl chloride ionomer for highly selective isolation of basic proteins. Talanta. 2010;81:637–642. doi: 10.1016/j.talanta.2009.12.059. [DOI] [PubMed] [Google Scholar]
- Taha M, Silva FA, Quental MV, Ventura SPM, Freire MG, Coutinho JAP. Good’s buffers as a basis for developing self-buffering and biocompatible ionic liquids for biological research. Green Chem. 2014;16:3149–3159. doi: 10.1039/C4GC00328D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tscheliessnig A, Satzer P, Hammerschmidt N, Schulz H, Helk B, Jungbauer A. Ethanol precipitation for purification of recombinant antibodies. J Biotechnol. 2014;188C:17–28. doi: 10.1016/j.jbiotec.2014.07.436. [DOI] [PubMed] [Google Scholar]
- Tzeng Y-P, Shen C-W, Yu T. Liquid-liquid extraction of lysozyme using a dye-modified ionic liquid. J Chromatogr A. 2008;1193:1–6. doi: 10.1016/j.chroma.2008.02.118. [DOI] [PubMed] [Google Scholar]
- van Reis R, Gadam S, Frautschy LN, Orlando S, Goodrich EM, Saksena S, Kuriyel R, Simpson CM, Pearl S, Zydney AL. High performance tangential flow filtration. Biotechnol Bioeng. 1997;56:71–82. doi: 10.1002/(SICI)1097-0290(19971005)56:1<71::AID-BIT8>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Vasilchuk D, Pandharipande PP, Suladze S, Sanchez-Ruiz JM, Makhatadze GI. Molecular determinants of expansivity of native globular proteins: a pressure perturbation calorimetry study. J Phys Chem B. 2014;118:6117–6122. doi: 10.1021/jp5028838. [DOI] [PubMed] [Google Scholar]
- Wallqvist A, Covell DG, Thirumalai D. Hydrophobic interactions in aqueous urea solutions with implications for the mechanism of protein denaturation. J Am Chem Soc. 1998;7863:427–428. [Google Scholar]
- Yan J, Du Y, Chen F, Yuan H, Hu F. Effect of proteins with different isoelectric points on the gene transfection efficiency mediated by stearic acid grafted chitosan oligosaccharide micelles. Mol Pharm. 2013;10:2568–2577. doi: 10.1021/mp300732d. [DOI] [PubMed] [Google Scholar]
- Yan J-K, Ma H-L, Pei J-J, Wang Z-B, Wu J-Y. Facile and effective separation of polysaccharides and proteins from Cordyceps sinensis mycelia by ionic liquid aqueous two-phase system. Sep Purif Technol. 2014;135:278–284. [Google Scholar]
- Zhao H. Directed evolution of novel protein functions. Biotechnol Bioeng. 2007;98:313–317. doi: 10.1002/bit.21455. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.