Abstract
The International Agency for Research on Cancer recently released an assessment classifying red and processed meat as “carcinogenic to humans” on the basis of the positive association between increased consumption and risk for colorectal cancer. Diet, however, can also decrease the risk for colorectal cancer and be used as a chemopreventive strategy. Bioactive dietary molecules, such as n-3 polyunsaturated fatty acids, curcumin, and fermentable fiber, have been proposed to exert chemoprotective effects, and their molecular mechanisms have been the focus of research in the dietary/chemoprevention field. Using these bioactives as examples, this review surveys the proposed mechanisms by which they exert their effects, from the nucleus to the cellular membrane. In addition, we discuss emerging technologies involving the culturing of colonic organoids to study the physiological effects of dietary bioactives. Finally, we address future challenges to the field regarding the identification of additional molecular mechanisms and other bioactive dietary molecules that can be utilized in our fight to reduce the incidence of colorectal cancer.
Keywords: n-3 polyunsaturated fatty acids, microRNAs, colonic organoids, membrane organization
INTRODUCTION
According to the National Cancer Institute, colorectal cancer accounted for 8% of all new cancer cases in 2015, making it the fourth most common cancer diagnosed in the United States (82). In addition, colorectal cancer accounted for 8.4% of all cancer deaths in 2015, the second leading cause of cancer-related death in the United States. Although the rate of new colorectal cancer cases has been dropping on average by 3.1% each year over the last 10 years, the death rates have not changed significantly over the same period (82). These statistics clearly highlight the importance of not only understanding the complex etiology of colorectal cancer, but also of developing effective prevention and treatment strategies for the disease.
The majority (95%) of colorectal cancers begin as noncancerous polyps of the intestinal epithelium on the inner lining of the colon or rectum that have accumulated oncogenic mutations over time (78, 203). Noncancerous polyps may become malignant and transform into adenomatous polyps if left undetected. Approximately 20% of colorectal cancer cases are attributed to patients with two or more first- or second-degree relatives with colorectal cancer (128, 184), suggesting that genetic factors play a minor role in the development of colon cancer. Various environmental factors contribute to the development of cancer. For example, of all cancer-related deaths, 25–30% can be attributed to tobacco, 30–35% are linked to diet, and 15–20% are due to infections (6). In particular for colon cancer, intestinal inflammation (e.g., Crohn's disease and ulcerative colitis) (53, 57) and obesity (19, 139) are additional risk factors that are associated with increased incidence of this form of cancer.
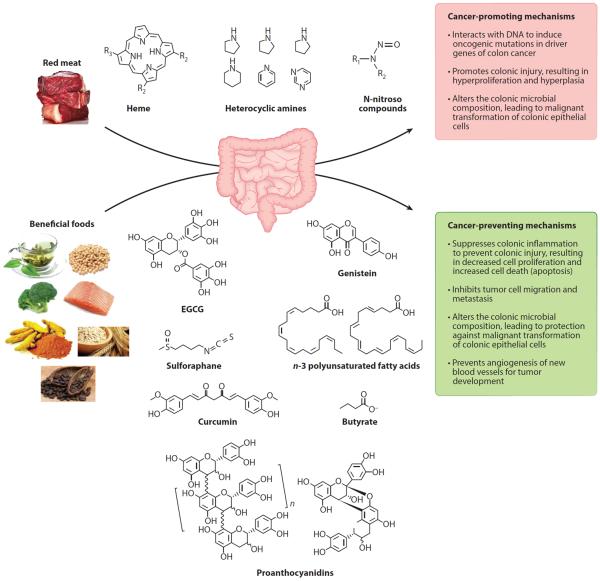
As mentioned above, epidemiological studies have established that diet can play a role in increasing the risk of developing colorectal cancer. In one study comparing US-born with foreign-born Japanese populations, the rate of colorectal cancer for US-born Japanese men was twice that of foreign-born Japanese men. Similarly, US-born Japanese women had a 40% higher incidence compared to foreign-born Japanese women (59). This trend of increased colon cancer risk in association with migration to the United States from another country has been confirmed in other migrating populations (80, 118, 161). Furthermore, studies have documented a correlation between increased colon cancer incidence and per capita consumption of meat, animal protein, and total fat (8, 173). Overwhelming evidence indicates that the consumption of red and processed meat is associated with an increased risk of colorectal cancer (14, 16, 27, 40, 116, 147, 175, 178). Various mechanisms have been proposed to link red and processed meat to colorectal cancer (see Figure 1). The high level of the iron-porphyrin pigment, heme, in red meat has been associated with colorectal cancer in epidemiological studies (11, 117). Heme is poorly absorbed by the small intestine; therefore, dietary heme can accumulate in the colon (225), where it induces colonic injury resulting in hyperproliferation and hyperplasia (86, 87, 188), which may lead to the development of colorectal cancer. With the recent focus on the gut microbiota, it is not surprising to find that heme can alter microbial composition and facilitate the malignant transformation of colonic epithelial cells (84, 85). The second potential compound found in red meat that may increase colorectal cancer risk is heterocyclic amines (40, 79, 148). These compounds are generated when red meat is cooked at high temperatures (41, 91, 201) and are thought to be genotoxic once they enter the cell and are metabolized to compounds that can interact with DNA to produce DNA adducts, inducing mutations in key oncogenic genes such as adenomatous polyposis coli (Apc), β-catenin, and K-Ras (201). A third class of compounds, N-nitroso compounds (NOCs), is also found in processed meat (121, 206). Although NOC can be endogenously synthesized from amines and amides with nitrosating agents derived from nitrites, the majority of exogenous exposure comes from the diet (83, 127). These compounds can interact with DNA to promote oncogenic mutations in driver genes (95, 119). Similar to the links of heme and heterocyclic amine with colorectal cancer, epidemiological studies have found a link between NOC and colorectal cancer (47, 125, 229).
Figure 1.
Effects of diet on promoting and preventing colorectal cancer. Compounds found in the diet have been linked to the development of colorectal cancer through the induction of DNA adducts, leading to mutations in oncogenic genes and promoting hyperproliferation and hyperplasia. Compounds found in many fruits, vegetables, and fish can counteract cancer-promoting compounds in a pleiotropic manner and therefore should be incorporated in a healthy human diet. Abbreviation: EGCG, epigallocatechin gallate.
The role of diet in promoting and preventing colorectal cancer can be context dependent, beneficial in one situation but detrimental in another context (101). One example of the dual modulatory role of diet in colorectal cancer is folate. This critical B vitamin is involved in the one-carbon transfer, where it is used as a substrate for the synthesis of nucleic acid purine bases and DNA methylation (61, 187). Thus folate plays an important role in regulating cell division, and folate deficiency is linked to many human health diseases, including congenital defects, adverse pregnancy outcomes, and cardiovascular diseases (198, 212). On the basis of these findings, an effort has been made to increase the dietary intake of folate/folic acid through supplementation in the food system (158), resulting in a significant reduction in congenital defects, such as neural tube defect, by as much as 50% postfortification (219). In cancer, however, one therapeutic strategy may be to interrupt DNA synthesis in rapidly replicating cells to suppress tumor growth. Indeed, folate deficiency has been shown to suppress DNA synthesis in neoplastic cells (102, 104). Epidemiological data on folate, however, have suggested that a high level of folate is associated with a reduction in risk of colorectal cancer (73, 97–99, 179). Some clinical studies have also demonstrated efficacy (93, 154, 221), although other clinical studies have shown no or even detrimental effects of folate supplementation on colorectal cancer (37, 58, 124, 214). These results highlight the duality of folate in cancer prevention and promotion. It has been suggested that folate deficiency may increase the risk of neoplastic transformation in normal colonic tissue, but as the disease progresses, folate deficiency may be beneficial in stopping the progression of the malignant transformation (reviewed in 103). In contrast, folate supplementation may be necessary for inhibiting normal colonic tissue from malignant transformation but may be detrimental once adenocarcinoma foci have developed in the colon (101). Folate may play a role in colorectal cancer in a context-dependent manner.
Another compound that has gained considerable attention in preventing colorectal cancer is aspirin, with the US Preventive Services Task Force releasing its systematic evidence review concluding that aspirin appears to reduce the risk of colorectal cancer incidence (35). It is thought that aspirin and other nonsteroidal anti-inflammatory drugs inhibit cyclooxygenase-2 (COX-2), which is often overexpressed in colorectal cancer tissue (26). Even though it is one of the most promising chemopreventive agents, potential serious side effects including gastrointestinal bleeding (25, 199) and cardiovascular events (142, 170) make it less than an ideal agent. Thus the search for innocuous, bioactive compounds for chemoprevention remains important.
Clearly, bioactive dietary compounds remain largely unexplored for their potential in providing a varied armamentarium for preventing the development of colorectal cancer (Figure 1). Long-chain n-3 polyunsaturated fatty acids (n-3 PUFAs), such as eicosapentaenoic acid (EPA, 20:5Δ5,8,11,14,17) and docosahexaenoic acid (DHA, 22:6Δ4,7,10,13,16,19), the most notable bioactive components found in fish oil, have been shown to prevent multiple forms of cancer. The increased consumption of fish, n-3 PUFA, EPA, or DHA has been associated with a significant risk reduction for colorectal cancer (69, 100, 105). Additional human studies have shown inverse relationships between intake of n-3 PUFA and risk of colon cancer (143, 180, 204). A meta-analysis of prospective cohort studies and a 22-year prospective study both showed reduced risk of colorectal cancer with increased consumption of fish and fatty acids from fish (68, 77). Additionally, individuals with high serum levels of n-3 PUFA have a decreased risk of developing colorectal cancer (76, 108, 165). Other dietary bioactives, such as curcumin (diferuloylmethane), a yellow color pigment of turmeric (Curcuma longa Linn) extracts, have also shown promise in suppressing colorectal cancer in experimental models and placebo-controlled clinical trials (145, 192). Utilizing n-3 PUFA, curcumin, and fermentable fiber as examples, this review highlights some of the putative mechanisms by which dietary bioactives may be used to prevent colorectal cancer, from the cell membrane to the regulation of transcription (Figure 2).
Figure 2.
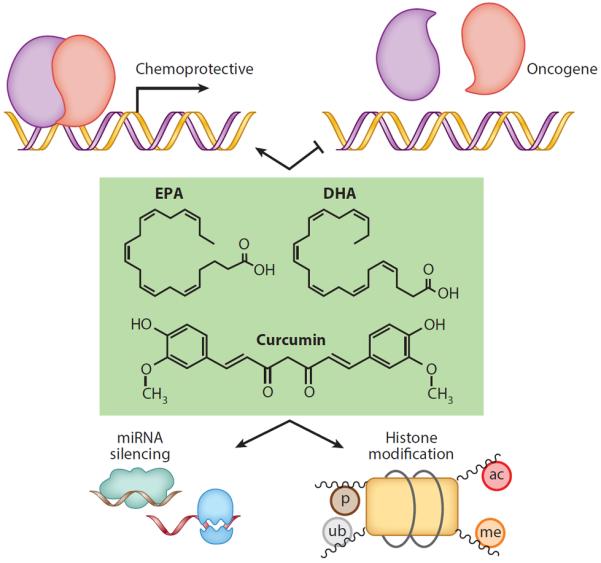
Potential mechanisms by which bioactive dietary molecules mediate chemoprotective effects in the nucleus. Bioactive dietary molecules such as EPA, DHA, and curcumin can activate transcription factors that regulate chemoprotective genes or inhibit transcription factors that drive oncogenesis. These molecules can also affect miRNA silencing as well as alter posttranslational modification of histones. Abbreviations: ac, histone acetylation; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; me, histone methylation; miRNA, microRNA; p, histone phosphorylation; ub, histone ubiquitination.
n-3 PUFA AND TRANSCRIPTIONAL MACHINERY
The bulk of dietary PUFAs is in the form of neutral fat or triglyceride and is well absorbed in the small intestine via emulsification, hydrolysis, and micelle formation (15). Following lipoprotein transport, PUFAs are incorporated into cell membrane glycerophospholipids by a remodeling pathway (Lands' cycle) to generate membrane asymmetry and diversity (194). This involves the concerted actions of phospholipases A2 and lysophospholipid acyltransferases.
Cell stimulation by a number of agonists triggers the formation of products of lipid hydrolysis, including DHA and EPA and their oxidative metabolites (63). These lipid mediators have been shown to interact directly with specific ligand-dependent nuclear receptors, including constitutive androstane receptor, hepatocyte nuclear factor 4 alpha (HNF4A), peroxisome proliferator-activated receptor gamma (PPARγ), pregnane X receptor (PXR), and retinoid X receptor alpha (RXRα) (157, 207). In this fashion, n-3 PUFA can regulate the function of nuclear receptors and impact transcriptional machinery. For example, DHA can bind and activate PPARγ, where it can transactivate genes associated with fatty acid transport and β-oxidation to control energy balance by regulating fatty acid homeostasis (163). Interestingly, impaired expression and function of PPARγ are associated with inflammatory bowel disease and colon cancer (52, 227). Therefore, one therapeutic application of n-3 PUFAs may be the activation of PPARγ and its associated fatty acid homeostasis pathways.
The liver X receptors (LXRs) are transcriptional regulators of cholesterol metabolism that control cholesterol uptake into the cell, its subsequent catabolism, and efflux out of the cell (176). This is noteworthy because cholesterol can control cell proliferation, and disruption in cholesterol metabolism has been associated with the development of colon cancer (164, 172, 228). LXRs also function by heterodimerizing with RXRα (176). The activation of LXRα by n-3 PUFA has been shown in preclinical studies to block proliferation of human colorectal cancer cells and to slow the growth of xenograft tumors in mice (123).
PXR (NR1I2) has been shown to regulate the expression of genes involved in the oxidation, conjugation, and transport of xenobiotics and to promote the metabolism, elimination, and detoxification of chemotherapeutic agents (167). It is noteworthy that the transcription of PXR increases in the presence of n-3 PUFA (114), and PXR can suppress the proliferation and tumorigenicity of colon cancer cells (151). The constitutive androstane receptor (NR1I3) is likewise transcriptionally increased by n-3 PUFAs in epithelial colorectal adenocarcinoma cells and similarly regulates genes involved in xenobiotic detoxification and energy homeostasis (114).
HNF4A maintains epithelial cell function and normal colon physiology via regulation of the balance between proliferation and differentiation, immune function, ion transport, epithelial barrier function, and oxidative stress (3, 22). P1-HNF4A, but not P2-HNF4A, expression is lost in colorectal carcinomas in humans, and it is predicted that treatments that increase nuclear P1-HNF4α protein levels, such as n-3 PUFA treatment, could help slow colon cancer progression (31, 150).
Since the original description of dietary fat as a regulator of gene expression more than a decade ago, many transcription factors have been identified as prospective indirect targets for n-3 PUFA regulation. For example, DHA can increase the activity of cyclic AMP response element-binding protein (CREB) binding protein, E1A-binding protein p300, and MYC and decrease the activity of nuclear factor-kappa B (NF-κB) (NFKB1) and signal transducer and activator of transcription 3 (STAT3) (157). However, DHA does not directly bind to this class of transcription factor. With respect to colon cancer, DHA exhibits a protective suppressive effect against hyperactivated STAT3 and may reestablish the equilibrium between STAT3 and PPARγ (42). The decrease in STAT3 activity may be associated with the ability of n-3 PUFA to trigger PPARγ-RXR heterodimers to localize at their cognate PPAR response elements and exchange corepressors for coactivators such as CREB binding protein and p300 (52).
The cytotoxic effects of DHA are also associated with signaling pathways involving lipid metabolism and endoplasmic reticulum (ER) stress. DHA can deplete free cholesterol in the ER, which leads to ER stress and growth arrest/apoptosis of metastatic tumor cells (92). It has been suggested that these alterations in the sterol content of the ER by DHA mediate growth partly by decreasing nuclear sterol regulatory element-binding protein, an important regulator of lipid homeostasis and cell growth regulation (185). Induction of ER stress mediators by DHA also promotes expression of protein kinase RNA-like endoplasmic reticulum kinase (PERK), which in turn increases translation of activating transcription factors 3, 4, and 6 (92). Furthermore, an elevation in PERK activity can increase levels of ER protein GADD34 (PPP1R15A) and the proapoptotic transcription factor DDIT3/CHOP along with its downstream target TRIB3 (197). The experimental details associated with differentially expressed target genes are described in a recent review (207).
Colon adenocarcinomas exhibit defective expression of the APC gene, which is a critical regulator of the Wnt signaling pathway. This and other developmental pathways play an important role in both genetic (familial) and sporadic epithelial cancers (71). From a chemoprevention perspective, in vivo studies demonstrate that fish-oil-derived n-3 PUFAs suppress the formation of intestinal tumors in mice and humans with a defective APC allele (36, 218). The downstream APC signaling oncogenic transcription factor MYC is an important regulator of cell proliferation, and the lack of MYC expression is associated with a reduced number of intestinal adenomas (156). Interestingly, patients with an amplified MYC gene and wild-type p53 have a greater response to anticancer therapies (7). In colon cancer cells, DHA increases the level of MYC, which is believed to induce a chemoprotective, proapoptotic phenotype (20).
DHA can inhibit NF-κB activity (217). This is relevant because NF-κB mediates signaling pathways that control the transcriptional activation of genes important for the regulation of many cellular processes and is aberrantly activated in many types of cancer (130, 138). n-3 PUFA treatment inhibits the expression and activity of NF-κB in many cell types; however, the exact mechanism is not fully understood (197). This has implications in chronic disease management because the DHA-mediated decrease in NF-κB activity has been shown to sensitize tumor cells to gamma-irradiation and promote the induction of apoptosis (227).
Curcumin also has been shown to inhibit cell proliferation, invasion, migration, angiogenesis, and inflammation and to induce cell cycle arrest and apoptosis in various cancers, such as breast, cervical, oral, gastric, melanoma, pancreatic, colon, and prostate (2, 74, 96). It exhibits its anticancer effects in part by regulating genes involved in cellular signaling pathways, including NF-κB, protein kinase B (Akt), mitogen-activated protein kinase, p53, and other pathways (152). Curcumin inhibits gene expression of epidermal growth factor receptor (EGFR) in human colon cancer cells (32) and also binds to the active sites of 5-lipoxygenase and COX-2 and inhibits their activity (169). It is noteworthy that curcumin modulates COX-2 and inducible nitric oxide synthase (163), similar to the effect induced by synthetic nonsteroidal anti-inflammatory drugs but without the side effects associated with these pharmaceutical agents (60).
EFFECTS OF n-3 PUFA, FIBER, AND CURCUMIN ON NONCODING MICRORNAs
High-throughput noncoding microRNA (miRNA) profiling studies have linked aberrant expression of miRNAs to the development of colon cancer (159, 196). For example, miR-21, a well-described “oncogenic” miRNA, is positively correlated with colorectal cancer metastasis (195). Elevated expression of miR-21 has been reported in breast (223), glioblastoma (67), pancreatic (140), and colon (9, 45, 216) cancers. The antiapoptotic properties of this miRNA target several tumor suppressors, including PTEN, PDCD4, BCL2, TIMP3, TGFβR2, SPRY3, and RECK (9, 120, 195, 216). Additionally, dysregulation of miRNA editing has been linked to aberrant EGFR signaling, which interacts with argonaute 2, thereby perturbing miRNA processing from precursor to mature miRNAs (48, 193). The fact that DHA antagonizes EGFR (209), which can modulate miRNA maturation through phosphorylation of argonaute 2 (48, 193), implicates a potential regulatory molecular mechanism involving fish oil and miRNAs. This epigenetic mechanism of action highlights a potential regulation of miRNAs by bioactive dietary components, and further study is needed to validate it.
The effects of n-3 PUFA on carcinogen-induced miRNA expression profiles during the early stages of colon tumorigenesis in rat colonic mucosa have been previously examined (44–46). The data indicate that translational alterations are far more extensive relative to transcriptional alterations in mediating malignant transformation. In contrast, changes in transcription are more extensive relative to translation in mediating the effects of diet. Therefore, during the early stage of colonic neoplasia, diet and carcinogen appear to predominantly regulate gene expression at multiple levels via unique mechanisms. Table 1 summarizes specific miRNAs modulated by a fish oil diet with respect to their validated mRNA target genes. miR-18a, miR-19b, miR-27b, miR-93, and miR-497 exhibited a coherent response at the total and polysomal mRNA levels by fish oil feeding. miR-132, miR-146b, miR-192, miR-206, and miR-218 were increased by fish oil feeding, with inverse relationships with their target genes. Specifically, azoxymethane treatment did not affect let-7d, miR-15b, miR-107, miR-191, and miR-324-5p expression in the fish-oil-fed animals. DHA in human colorectal adenocarcinoma cells modulated miR-141-3p, miR-221-3p, miR-192, miR-30c, miR-1283, let-7f, miR-181a, and miR-1 (72). Furthermore, DHA treatment of gastric cancer cells increased miR-15b and miR-16, resulting in a downregulation of Bcl-2 and the induction of apoptosis (202).
Table 1.
miRNAs differentially regulated by DHA, fiber, or curcumin in the colon
| Bioactive component | Differentially regulated genes | Validated mRNA targets | Cell line/organism | Target mRNA-involved pathway | Reference |
|---|---|---|---|---|---|
| n-3 PUFA | miR-18a | Runx1 | Rat colon | ERK-MAPK, Wnt/β-catenin, PTEN, apoptosis, EMT | 190 |
| miR-19b | Arid4b, Arpc3, Hipk3 | ||||
| miR-27b | Cxcl12, Runx1 | ||||
| miR-93 | Sqstm1, Stat3, Vegfa | ||||
| miR-497 | Bcl2, Bcl2ls, Eln | ||||
| miR-132 | Arhgap32, Btg2, Ep300, Foxo3, Kdm5a, Mecp2, Mmp9, Nr4a2, Paip2, Ptbp2 | ||||
| miR-146b | Nfkb1, Sirt1 | ||||
| miR-192 | Zeb2 | ||||
| miR-206 | Bdnf, Fstl1, Gja1, Hdac4, Hhip, Id1, Id2, Id3, Igsf5, Mmd, Notch3, Pax7, Pola1, Ptplad1, Spry1, Timp3, Utrn | ||||
| miR-218 | Onecut2 | ||||
| let-7d | Sept3 | 46 | |||
| miR-15b | Bcl2, Ccne1 | ||||
| miR-107 | Bace1, Serbp1 | ||||
| miR-191 | Mxi1, Riok3 | ||||
| miR-324 | Smo | ||||
| miR-1 | Calm1, Calm2, Gata4, Hdac4, Igf1, Mef2a | Human colorectal adenocarcinoma cells | EMT, cancer cell migration, invasion, tumor suppressor | 72 | |
| miR-30c | Ccne2, Celsr3, Egfr, HSPA4, Mdm2, Mtdh, Runx1, Smad1, Snai1, Timp3, Twf1, Vimentine | ||||
| miR-141 | Cdh11, Dlx5, Elavl4, Klf5, Mapk14, PTEN, Slc25a3, Stk3, Zeb1, Zeb2 | ||||
| miR-181a | Acvr2a, Bcl2, Cbx7, Cd69, Egr1, Gata6, Hoxa, Msx2, Rgs4, Runx1, Tbr1, Tcra, Tgfbi, Tox | ||||
| miR-192 | Zeb2 | ||||
| miR-221 | Arnt, Bnip3, Cdkn1b, Ddit4, Kit, Psmd9, Timp3 | ||||
| miR-1283 | |||||
| let-7f | |||||
| miR-15b | Arl2, Bcl2l2, Dedd | Human gastric cancer cells | 202 | ||
| miR-16 | App, Arl2, Bcl2, Bcl2l2, Cadm1, Ccnd1, Ccne1, Ccnt2, Cd40, Fgf2, Jag1, Jun, Mdm4, Slc6a4, Vegfa,Wnt3a | ||||
| n-3 PUFAs plus pectin | miR-16 | Arl2, App, Bcl2, Ccnd1, Jag1, Jun, Mdm4, Vegfa, Wnt3a | Rat colon | Adenocarcinoma, mTOR, PI3K/AKT, apoptosis, EMT | 190 |
| miR-21 | Eif4e3, Fasl, Peli1, Pdcd4, Pten, Reck, Smad7, Spry1, Spry2, Tgfbr3, Tnfaip8l2, YOD1, Yy1 | ||||
| miR-26b | Lef1, PDE4B | ||||
| miR-27b | Cxcl12, Mef2c, Pparg, Runx1, Smad3, Smad4, Smad5 | ||||
| miR-30b | Aicda, Snai1 | ||||
| miR-93 | Sqstm1, Stat3, Vegfa, VSIVgp2 | ||||
| miR-98 | Acvr1b, Il6, Mmp11 | ||||
| miR-130b | Meox2, Tgfb1 | ||||
| miR-182 | Adcy6, Clic5, Fbxw7, Foxo3, Tbx1 | ||||
| miR-200c | Bmi1, Flt1, Mapk14, Nog, Reln, Sox2, Vldlr, Zeb1, Zeb2, Zfpm2 | ||||
| miR-203 | Cav1, Ctnnb1, Rbm44, Snora62, Trp63, TCF4, Vcan, ZNF148 | ||||
| miR-206 | Adar, Bdnf, Clcn3, Eif4e3, Fn1, Fstl1, Fzd7, Gja1, Hdac4, Hhip, Hmgb3, Id1, Id2, Id3, Igsf5, Meox2, Mmd, Nfat5, Notch3, Pax7, Pdcd10, Pola1, Ptplad1, Rarb, Sh3bgrl, Spry1, Smarcb1, Smarcd2, Timp3, Utrn | ||||
| Curcumin | mir-21 | AP-1, Eif4e3, Fasl, Pdcd4, Peli1, Pten, Reck, Smad7, Spry1, Spry2, Tgfbr3, Tnfaip8l2, YOD1, Yy1 | Human colorectal adenocarcinoma cells | Apoptosis, proliferation, inflammation, Wnt, EGFR | 141 |
| mir-34a | Actb, Bcl2, Bcl6, Ccnd1, Dll1, Foxp1, Gas1, Notch-1, Pofut1, Ppp1r10, Sema4b, Sirt1, TGIF2, Trem2, Vcl, Vegfb | Human esophageal cancer cells | 200 | ||
| mir-17 | Specificity protein transcription factors, Bcl2l11, Mapk14, Rbl2, Sqstm1, Stat3, Tgfbr2 | Human colon carcinoma cell | 65 | ||
| mir-20a | App, Bmp4, Ep300, Fgf10, Hbp1, Mapk14, Osr1, Pten, Shox2, Sqstm1, Stat3, Tbx3, Tgfbr2, Trp53inp1, ULK1, Vegfa, Zbtb7a, Zfpm2 | ||||
| mir-27a | Alp, Bmp2, Bmpr1a, Creb1, Fbxw7, Il10, Odc1, Ppara, Pparg, Prdm16, Runx1, Runx2, Smad9, Spp1, Srm, Tcirg1 |
Abbreviations: EGFR, epidermal growth factor receptor; EMT, epithelial-mesenchymal transition; ERK, extracellular-signal-regulated kinase; MAPK, mitogen-activated protein kinase; PTEN, phosphatase and tensin homolog deleted on chromosome 10; PUFAs, polyunsaturated fatty acids.
A diverse population of microbiota colonizes the human gastrointestinal tract. The colon contains approximately 1011 cells per gram of contents, representing the densest population of microbes in the healthy adult (51). These predominantly anaerobic microbial populations are metabolically active, producing many metabolites that can exert both protective and pathogenic effects on the host. Some protective metabolites produced by the microbiota include short-chain fatty acids, such as acetate, butyrate, and propionate. A range of gut microbial structural components and metabolites directly interacts with host intestinal cells and stroma to influence nutrient uptake and epithelial resilience (75). For example, we and others have proposed that n-3 PUFA and butyrate, a four-carbon short-chain fatty acid produced during anaerobic fermentation of dietary fiber by endogenous bacteria in the colon, interact to profoundly suppress colon cancer (30, 110, 111). Dietary fish oil and the fermentable fiber pectin can act synergistically to protect against colon carcinogenesis primarily by enhancing apoptosis (110) (Figure 3). Table 2 summarizes animal and cell-line studies examining the synergism between n-3 PUFA and pectin or its fermentation product, butyrate.
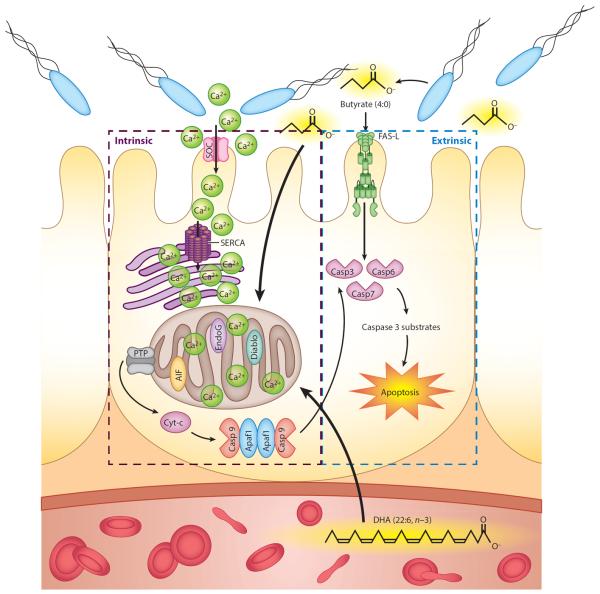
Figure 3.
Proposed mechanisms by which the interaction of dietary long-chain n-3 polyunsaturated fatty acids from fish oil and butyrate from bacterial fermentation of dietary fiber may reduce colon tumorigenesis. Butyrate induces colonocyte apoptosis via a nonmitochondrial, Fas-mediated, extrinsic pathway. Docosahexaenoic acid (DHA) and butyrate, in combination, synergistically perturb intracellular Ca2+, stimulating mitochondrial Ca2+ uptake. This directly or indirectly decreases cytosolic Ca2+ and promotes store-operated channel (SOC)-mediated entry via plasma membrane channels. Mitochondrial Ca2+ accumulation subsequently triggers the opening of the permeability transition pore (PTP) and release of proapoptotic molecules, such as cytochrome c, and other factors, such as apoptosis-inducing factor (AIF) and second mitochondrial activator of caspases (smac/Diablo). Together, these effects culminate in the induction of procaspases and downstream caspases (Casp) that execute cellular apoptosis. Abbreviations: Apaf1, apoptotic protease-activating factor 1; Cyt-c, cytochrome c; EndoG, endonuclease G; SERCA, sarcoendoplasmic reticulum Ca2+ ATPase.
Table 2.
n-3 Polyunsaturated fatty acids and butyrate act synergistically in the colon
| Cell-line experiments | |||
| Cell line | Treatment and duration | End point | Reference |
| YAMC | 0–200 mM DHA for 72 hours plus 0–10 mM butyrate for the final 6–24 hours | Mitochondrial Ca2+ and apoptosis (nucleosomal fragmentation assay) | 111 |
| HCT-116 | 50 mM DHA for 72 hours plus 5 mM butyrate for the final 12 hours | Apoptosis (TUNEL assay) | 110 |
| Animal studies | |||
| Animal | Diet component (% by weight) | AOM (mg/kg body weight) | End point |
| Rat | 11.5% fish oil plus 6% pectin | 15 mg/kg × 2 injections | miRNA quantification |
| Rat | 11.5% fish oil plus 6% pectin | 15 mg/kg × 2 injections | miRNA quantification |
| Mouse | 11.5% fish oil plus 6% pectin | 15 mg/kg × 4 injections | miRNA quantification, apoptosis, ACF |
Abbreviations: ACF, aberrant crypt foci; AOM, azoxymethane; DHA, docosahexaenoic acid; miRNA, microRNA.
The effects of dietary fish oil and pectin on miRNA expression during the early stages of colon tumorigenesis in preclinical models have been examined (46, 190). Table 1 summarizes specific miRNAs modulated by a combination fish oil and pectin diet with respect to their validated mRNA target genes. Surprisingly, miR-21 was decreased by the combination diet compared to the control diet (189, 190). This is noteworthy because, as previously noted, miR-21 is a well-known oncogenic miRNA, and its validated targets, PDCD4 and PTEN, are known tumor suppressor genes (9, 135, 229). Compared to the fish oil diet, miR-26b, miR-30b, miR-98, miR-130b, miR-182, miR-200c, and miR-203 were uniquely increased by fish oil and pectin combination feeding. Their validated targets in Table 1 are known to promote tumorigenesis.
In colon cancer, curcumin has been shown to modulate tumor suppressor genes and transcription factors. In RKO and HCT116 colon cancer cells, the expression of miR-21, which correlated with the inhibition of activator protein-1 binding to its promoter, was reduced following treatment with curcumin. Consequently, cell proliferation, tumor growth, invasion, and in vivo metastasis were suppressed while the expression of the tumor suppressor PDCD4, a target of miR-21, was upregulated (141). In another study, curcumin induced cell cycle arrest and apoptosis in TE-7 human esophageal cancer cells through downregulation of Notch-1-specific miR-21 and miR-34a and upregulation of tumor suppressor let-7a (200). Curcumin also inhibited the growth of RKO and SW480 colon cancer cells through induction of reactive oxygen species and repression of specificity protein (Sp) transcription factors through downregulation of miR-27a, miR-20a, and miR-17. These miRNAs regulate the Sp repressors zinc finger and BTB domain-containing proteins 4 and 10 (ZBTB4 and ZBTB10) (65). This regulation has important implications because Sp proteins are transcription factors that regulate genes involved in cell death and angiogenesis and are often overexpressed in tumors (1, 24). Moreover, curcumin is known to modulate DNA methylation in colorectal cancer cells (122), and recent advances in microarray and sequencing technologies have reported miRNA genes that are silenced by methylation in cancer (126). It has also been suggested that DHA enhances cell permissiveness to curcumin uptake (4, 137). Therefore, a diet containing both n-3 PUFA and curcumin may suppress colon cancer by acting on different molecular targets. An interesting future frontier will be the pursuit of epigenetic molecular complexes targeted by a combination of pleiotropic chemoprotective bioactive dietary compounds. This approach will likely avoid problems commonly associated with drug rejection.
Adult stem cells of the colon are of particular interest because they sustain self-renewal and are targets for cancer-initiating mutations (133, 220). Perturbations in adult stem cell dynamics are generally believed to represent an early step in colon tumorigenesis. Recently, several studies have demonstrated the role of miRNAs in the maintenance of colon cancer stem cells. For example, repression of the translation of select miRNAs in stem cells and differentiated daughter cells has been shown to be a means of regulating stem cell renewal and differentiation (56, 66, 94). Although evidence supports the beneficial effects of certain dietary components on suppression of colon cancer cells (46, 190), a comprehensive, comparative analysis of the effects of these dietary agents on colonic stem cells has not yet been conducted (189).
n-3 PUFA, DNA METHYLATION, AND HISTONE MODIFICATIONS
In addition to regulating transcriptional machinery and the expression of miRNAs, dietary bioactives can also exert chemoprotective effects through DNA modification. Genes are silenced by covalently adding a methyl group to cytosine, typically occurring in CpG dinucleotide islands and island shores (89). Typically, in the context of cancer, hypermethylation occurs at tumor suppressors, whereas hypomethylation occurs at tumor promoters. Another possibility of change in methylation is the presence of alternative RNA transcripts, as shown for the PIP5K1A locus in colon cancer (89). Several studies have shown that fish oil modulates the DNA methylation status of gene promoters. For example, in a high-fat-diet study of mice, fish oil supplementation abolished the decrease in Pparg2 promoter methylation in skeletal muscle, thereby suppressing the increase in Pparg2 expression (5). Another study has shown that EPA can decrease methylation at the CCAAT/enhancer-binding protein promoter for C/EBPβ and C/EBPδ to increase their expression in U937 leukemia cells (23). Interestingly, we have demonstrated that n-3 PUFA/DHA and pectin/butyrate reduce cancer risk in part via changes in the promoter methylation state of apoptosis-related genes, leading to induction of proapoptotic gene expression and increased apoptosis in colonocytes (33, 34). Similar to results in mouse models, in clinical studies n-3 PUFA has been shown to influence DNA methylation. In one study of the Yup'ik Alaska Natives, DNA methylation of 27 CpG sites was differentially regulated by n-3 PUFA (10). These findings highlight the effects of n-3 PUFA on regulating gene expression through DNA methylation in both preclinical and clinical studies, although more research is required to pinpoint the exact mechanisms by which n-3 PUFA alters the DNA methylation landscape.
Another epigenetic modification that can regulate gene expression is histone modification, which is composed of an array of posttranslational alterations of the histone tail that is associated with active and repressed gene expression. For example, histone H3 lysine 4 trimethylation is associated with active gene transcription, whereas H3 lysine 9 trimethylation is associated with repressed gene expression (18). We have demonstrated using rat colonic epithelial cells that there are differences in histone modification and proto-oncogene expression between proximal and distal colon (208). Studies are in progress to determine the effect of dietary fat and fiber composition on global histone posttranslational epigenetic programming in preclinical models of colon cancer.
The interaction of dietary fat and fiber-derived compounds in the colonic lumen can substantially impact the metabolism and kinetics of the colon epithelial cell population and suppress inflammation and neoplasia (29, 109, 210). For example, butyrate, formed via fiber fermentation, has pleiotropic effects in the colon (49, 81), acting as a principal energy source and a survival factor for normal colon cells while exerting antiproliferative, differentiation-inducing, and apoptosis-inducing effects in cancer cells (21). In addition to the regulation of basic cytokinetic processes, butyrate has also been shown to affect cell adhesion, morphology, invasiveness, metastasis, oxidative metabolism, and angiogenesis, as well as the activity of different enzymes and transcription factors. These effects are attributed in part to the function of butyrate as a histone deacetylase inhibitor, which mechanistically links it to gene expression (183).
ADDITIONAL MECHANISMS BY WHICH NUTRITIONAL BIOACTIVES EXERT CHEMOPROTECTIVE EFFECTS
The review thus far has focused on the binding of bioactive dietary compounds to transcription factors, or regulating transcription indirectly by regulating miRNA or DNA methylation. However, n-3 PUFA and its metabolites, as well as other amphiphilic dietary compounds such as curcumin, can modulate the function of membrane-associated receptors and exert additional effects on oncogenic cellular signaling.
The cellular plasma membrane is a heterogeneous composition of lipids and proteins. These lipids have many different head groups (glycerophosphocholine versus sphingomyelin), tail lengths (16:0 versus 18:0), and saturation indices (16:0 versus 16:1). Although membrane proteins have many different characteristics, such as transmembrane, lipid anchoring, and outer versus inner leaflets, recent studies have highlighted that these lipids and proteins are not just randomly distributed but rather are exquisitely organized. In fact, receptor activation can even be controlled by local spatial heterogeneity in ligand concentrations (136). Ultimately, most signals are propagated by the formation of signaling membrane domains composed of lipid-lipid, lipid-protein, and protein-protein interactions of differing size scales, such as micrometer (immunological synapse) and nanometer (Ras-Raf) platforms.
One view is that these membrane proteins exist in a balance of clustered and nonclustered states. The composition and organization of the membrane as well as the influence of cytoskeletal protein-protein interactions can stabilize these domains, favoring a shift of equilibrium to a clustered state. Interestingly, clustered proteins are not always efficient signaling domains because these clusters must be arranged in a particular conformation and be composed of the appropriate binding partners to propagate the signal (12, 132, 144). These membrane domains can be stabilized by transbilayer interactions between outer and inner leaflet lipids as well as the cytoskeleton (168).
Multidrug-resistance cancer cells have unique plasma membrane lipid composition and organization, specifically increased sphingomyelin and rigidity (213, 224). Increased rigidity is associated with increased lipid rafts, which typically facilitate efficient cellular signaling. It appears that these multidrug-resistance cells have remodeled their membrane to be in a state that is more receptive to activation. The plasma membrane, which is considered the outermost part of the cell, must receive and process extracellular signals. Because n-3 PUFAs such as DHA and EPA are physically incorporated into membrane phospholipids (Figure 4a), their main effect may originate at the membrane. Other compounds, such as curcuminoids, however, are not a physical part of the membrane. Instead, these compounds can intercalate into the membrane, where they can influence lipid-lipid and lipid-protein interactions (Figure 4b) (88). Effects of small-molecule agonists and antagonists can typically be predicted by structure-activity relationships, highlighting binding regions between proteins and said molecule. Unfortunately, it is very difficult to predict the effects of membrane-targeted dietary bioactives because the interaction involves modeling lipid and protein biophysics.
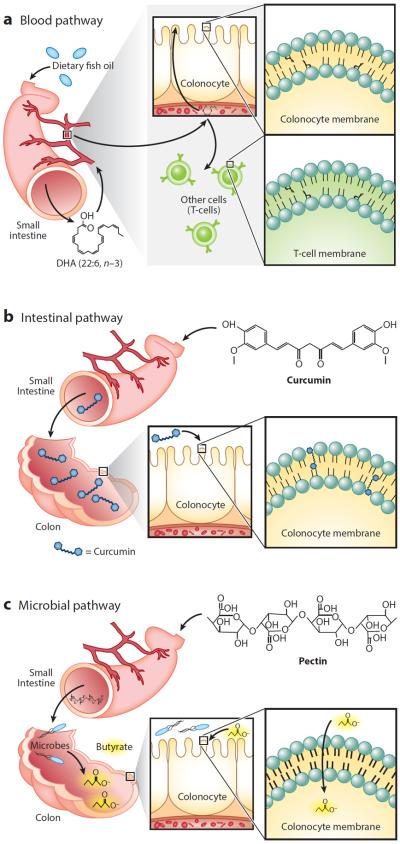
Figure 4.
Multiple pathways for the delivery of dietary bioactives to colonocytes. (a) Blood pathway. Polyunsaturated fatty acids are delivered to colonocytes and other cell types, such as T cells, through the bloodstream after digestion and absorption from the small intestine into the portal vein. In the colonocyte, the fatty acids are incorporated into phospholipids in the plasma membrane. Abbreviation: DHA, docosahexaenoic acid. (b) Intestinal pathway. Curcumin is poorly bioavailable and hence is transported, intact, to the colon, where it can intercalate between phospholipids in the plasma membrane of colonocytes. (c) Microbial pathway. Pectin is not digestible by human enzymes and therefore transits to the colon, where gut microbes ferment it to produce butyrate, which is rapidly taken up by colonocytes.
A criticism related to the physiological effects of dietary agents is that the bioavailability of most bioactive dietary compounds is very low and therefore membrane enrichment is low. However, from the standpoint of membrane biophysics, even small mole percentage increases of compounds can have drastic effects on biophysical properties of membranes (134). The magnitude and directionality of these effects depend on biophysical properties of the compounds themselves as well as those of the membrane. The colon is unique because many compounds that are not “bioavailable” and, therefore, not found in circulation are still bathing the colonic epithelium from the luminal side. Therefore, as opposed to the blood pathway shown in Figure 4a for PUFA effects on cell membranes, curcumin acts through an intestinal pathway, with the curcuminoid bioactive, for the most part, escaping absorption in the small intestine and being delivered to the colon intact (90), where it can become incorporated into membranes of the colonic epithelium and exert its physiological effects (Figure 4b). In comparison, fermentable fiber acts through a microbial pathway (Figure 4c), being converted to its bioactive form, butyrate, by the action of intestinal microbiota in the lumen of the colon.
NOVEL TECHNIQUES TO STUDY NUTRIENT-GENE INTERACTION IN COLON CANCER
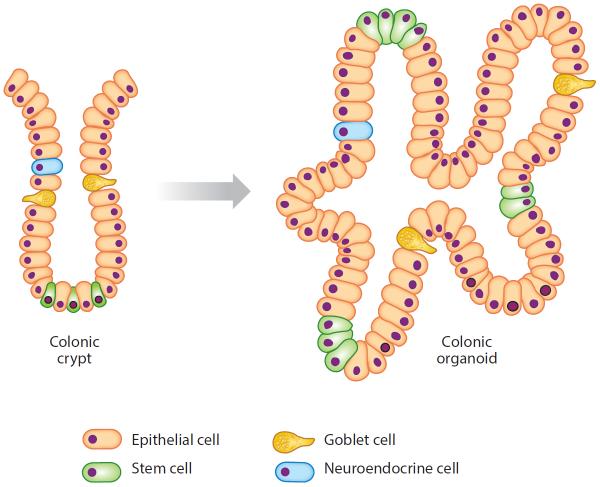
The intestinal epithelium is one of the most rapidly renewing tissues in vertebrate organisms. Self-renewal of the colonic epithelium is driven by the proliferation of stem cells and their progenitors located in crypts. In 2009, Sato et al. (182) established a long-term primary culture to generate epithelial organoids (enteroids) with crypt- and villus-like epithelial domains representing the complete census of progenitor and differentiated cells, mimicking the situation encountered by cells in the intestinal crypt niche (Figure 5). Later, they adapted the culture conditions to grow similar epithelial organoids from mouse colon and human small intestine and colon. The three-dimensional culture system of the native human colonic epithelium recapitulates the topological hierarchy of stem cell–driven tissue renewal, opening the methodological door for ex vivo studies designed to examine the effects of bioactive dietary compounds on colonic crypt metabolism (153).
Figure 5.
Culturing of colonic organoids. Colonic crypts, isolated from the colon by ethylenediamine tetra-acetic acid (EDTA) treatment, are cultured in Matrigel 3D matrix in media containing a mixture of recombinant growth factors. All cell lineages in the crypt are recapitulated in the developing organoid.
From the perspective of membrane-derived bioactives, it has been shown that prostaglandin E2 supports the growth of chicken embryo intestinal organoids in three-dimensional culture (160). Interestingly, we have provided evidence that exogenous prostaglandin E3, derived from n-3 PUFA, has diminished ability to support colonic stem cell expansion in mouse colonic organoids relative to prostaglandin E2, derived from n-6 PUFA, a known promoter of colon tumorigenesis (54, 191). The ability of bioactive compounds to alter colonic stem cell lineage and proliferation in this three-dimensional organoid culture system ex vivo strongly suggests that primary intestinal organoid cultures have widespread application for elucidating the molecular mechanisms of nutrient action on gut biology.
Evidence suggests that targeting cancer cell energy metabolism might be an effective therapeutic approach for selective ablation of malignancies. The Seahorse Extracellular Flux Analyzer is a novel platform designed to perform metabolic profiling of mitochondria, cells, or tissue. Using the Seahorse Analyzer, we have demonstrated that select bioactives known to affect gastrointestinal function and cancer risk can alter colonic mitochondrial function, both in vivo in crypts and in ex vivo organoid culture, by increasing respiration-induced proton leak, thereby inducing apoptosis, a marker of colon cancer risk (55).
The organoid culture system is applicable to basic and translational research. By using either CRISPR-Cas9 or lentivirus, Matano et al. (131) engineered diverse oncogenic mutations in organoids derived from normal colon, facilitated by selective culture conditions that encouraged the maintenance of the mutations. During tumorigenesis, niche factors often become dispensable, leading to a less stringent culture condition for cancer organoids as compared to wild-type organoids. Established cancer organoids can be xenotransplanted to recapitulate histopathology of the parental tumor from which they are derived. Cancer organoids reflect genetic lesions and gene expression patterns, opening up the possibility of in vitro drug testing for the prediction of clinical treatment response in patients (181).
An elegant model for examining changes in the colonic epithelium due to various intervention therapies is the noninvasive monitoring of gene expression in exfoliated colonocytes. Our group has developed methodology to extract host mRNA from stool samples for the purpose of RNA sequencing to examine gene expression profiles of the host organism (107). Indeed, we have demonstrated that the effects of chemotherapeutic diets on epithelial cell expression can be monitored noninvasively throughout the tumorigenic process (33). Because the sample is easily acquired, repeated sampling over time or after repeated treatments is possible, such as in a crossover intervention study. With this method, conflation of host transcriptome profiles with changes in microbiome can be achieved (50, 186).
FUTURE CHALLENGES
Although this review has focused on the pleiotropic mechanisms by which n-3 PUFA may be chemoprotective against colon cancer, two systematic reviews of n-3 PUFA on cancer risk qualitatively concluded that there is inadequate (129) or limited (70) evidence to suggest an association between long-chain n-3 PUFA intake and colorectal cancer risk. This discrepancy may be due to the source of n-3 PUFA, from fish oil, purified EPA, DHA, or a combination of the two n-3 PUFAs. This can result in variable ratios of EPA and DHA, and the administration of different doses. Consistent use of n-3 PUFA formulation, with particular attention to the ratio of EPA and DHA, as well as the standardization of the dose, may help resolve the discrepancy in the efficacy of n-3 PUFA in preventing colorectal cancer. Furthermore, work from our group suggests that the combined effects of n-3 PUFA in fish oils and highly fermentable fibers may act synergistically to enhance the chemopreventive potential, in part, by increasing apoptosis (Figure 3 and Table 2) (28, 33, 39, 109–111, 149, 177, 211). These results are in agreement with a recent prospective nested case-control study demonstrating significant colorectal cancer risk reduction among Seventh Day Adventist pescovegetarians, whose diets are high in both dietary fiber and n-3 PUFA-rich fish (HR: 0.57; 95% CI: 0.40, 0.82) (149). We postulate that the failure to address an interaction between dietary fat and fiber and their subtypes may explain why the interactive chemoprotective effects of n-3 PUFA and fermentable fiber have been obscured in prospective cohort studies.
Although this discussion has focused on n-3 PUFA and some of the nutrient-nutrient interactions, other diets may be chemoprotective against colorectal cancer as well. Indeed, the so-called Mediterranean diet, consisting of a high intake of vegetables, fruits, nuts, and olive oil, and a low consumption of red meat, may be beneficial in preventing colorectal cancer (174). It has been shown that Mediterranean countries have decreased colorectal cancer rates compared to Western countries (38, 205). Bioactive dietary molecules found in vegetables and fruits, such as epigallocatechin gallate in green tea (222), proanthocyanidins in apples and cocoa beans (146), genistein in soybeans (166), and sulforaphane in cruciferous vegetables such as broccoli (64), have also emerged as bioactive dietary compounds that may exert chemoprotective effects against colon cancer (Figure 1). More research on these and other emerging compounds is required to fully elucidate their chemoprotective efficacy.
Butyrate and propionate, produced in the colon by fermentation of dietary fiber by microbes in the human colon, are histone deacetylase inhibitors, regulating host transcriptome at the epigenetic level (21, 183). Not surprisingly, diet can change the microbial population in the colon (43, 215), and recent studies have begun to profile the changes to the microbiota associated with n-3 PUFA intake (155, 226). The continued study of how dietary bioactive molecules alter the microbiota as a chemoprotective strategy is needed for a full understanding of the dietary-host-microbiome nexus.
The overwhelming majority of colorectal cancers are initiated by activating mutations/deletions in the Wnt pathway (13, 106, 171). From a physiological standpoint, the Wnt signaling pathway is essential for the maintenance of the intestinal stem cell niche (112, 113, 115, 162). Therefore, environmental factors, which are capable of modulating Wnt signaling, will likely have a unique and central role in the physiology and pathology of the intestinal stem cell. A growing body of literature supports the hypothesis that dietary bioactive compounds (e.g., n-3 PUFA, folate, fermentable fiber) can modulate Wnt signaling by suppressing colonocyte nuclear β-catenin levels (17, 62). Nonetheless, our understanding of the mechanisms by which nutrient-gene interaction influences stem cells and colon cancer is still poorly developed. Therefore, certain issues in regard to colonic stem cells need to be addressed. For example, are the stem cell number and location fixed? Do these change during malignant transformation? Can a chemoprotective diet influence these events? These questions and others proposed in this review highlight that the interplay between diet and chemoprevention has not been fully explored and that more questions remain to be addressed in order to fully understand how diet can be used in the prevention of colon cancer.
ACKNOWLEDGMENTS
This work was supported by NIH grants R35CA197707, CA168312, and P30ES023512; the Cancer Prevention Research Institute of Texas (CPRIT); and the American Institute for Cancer Research (AICR). We thank Rachel Wright and Nadia Payne for creating graphics.
Glossary
- Apc
adenomatous polyposis coli gene
- NOCs
N-nitroso compounds
- COX-2
cyclooxygenase-2
- PUFAs
polyunsaturated fatty acids
- EPA
eicosapentaenoic acid
- DHA
docosahexaenoic acid
- PPARγ
proliferator-activated receptor gamma
- PXR
pregnane X receptor
- RXRα
retinoid X receptor alpha
- LXRs
liver X receptors
- NF-κB
nuclear factor-kappa B
- STAT3
signal transducer and activator of transcription 3
- ER
endoplasmic reticulum
- PERK
protein kinase RNA-like endoplasmic reticulum kinase
- EGFR
epidermal growth factor receptor
- miRNA
microRNA
- Sp
specificity protein
Footnotes
DISCLOSURE STATEMENT The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Abdelrahim M, Baker CH, Abbruzzese JL, Sheikh-Hamad D, Liu S, et al. Regulation of vascular endothelial growth factor receptor-1 expression by specificity proteins 1, 3, and 4 in pancreatic cancer cells. Cancer Res. 2007;67:3286–94. doi: 10.1158/0008-5472.CAN-06-3831. [DOI] [PubMed] [Google Scholar]
- 2.Aggarwal BB, Shishodia S. Molecular targets of dietary agents for prevention and therapy of cancer. Biochem. Pharmacol. 2006;71:1397–421. doi: 10.1016/j.bcp.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 3.Ahn SH, Shah YM, Inoue J, Morimura K, Kim I, et al. Hepatocyte nuclear factor 4α in the intestinal epithelial cells protects against inflammatory bowel disease. Inflamm. Bowel Dis. 2008;14:908–20. doi: 10.1002/ibd.20413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Altenburg JD, Bieberich AA, Terry C, Harvey KA, Vanhorn JF, et al. A synergistic antiproliferation effect of curcumin and docosahexaenoic acid in SK-BR-3 breast cancer cells: unique signaling not explained by the effects of either compound alone. BMC Cancer. 2011;11:149. doi: 10.1186/1471-2407-11-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amaral CL, Crisma AR, Masi LN, Martins AR, Hirabara SM, Curi R. DNA methylation changes induced by a high-fat diet and fish oil supplementation in the skeletal muscle of mice. J. Nutrigenet. Nutrigenom. 2014;7:314–26. doi: 10.1159/000381777. [DOI] [PubMed] [Google Scholar]
- 6.Anand P, Kunnumakkara AB, Sundaram C, Harikumar KB, Tharakan ST, et al. Cancer is a preventable disease that requires major lifestyle changes. Pharm. Res. 2008;25:2097–116. doi: 10.1007/s11095-008-9661-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arango D, Corner GA, Wadler S, Catalano PJ, Augenlicht LH. c-myc/p53 Interaction determines sensitivity of human colon carcinoma cells to 5-fluorouracil in vitro and in vivo. Cancer Res. 2001;61:4910–15. [PubMed] [Google Scholar]
- 8.Armstrong B, Doll R. Environmental factors and cancer incidence and mortality in different countries, with special reference to dietary practices. Int. J. Cancer. 1975;15:617–31. doi: 10.1002/ijc.2910150411. [DOI] [PubMed] [Google Scholar]
- 9.Asangani IA, Rasheed SA, Nikolova DA, Leupold JH, Colburn NH, et al. MicroRNA-21 (miR-21) post-transcriptionally downregulates tumor suppressor Pdcd4 and stimulates invasion, intravasation and metastasis in colorectal cancer. Oncogene. 2008;27:2128–36. doi: 10.1038/sj.onc.1210856. [DOI] [PubMed] [Google Scholar]
- 10.Aslibekyan S, Wiener HW, Havel PJ, Stanhope KL, O'Brien DM, et al. DNA methylation patterns are associated with n-3 fatty acid intake in Yup'ik people. J. Nutr. 2014;144:425–30. doi: 10.3945/jn.113.187203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Balder HF, Vogel J, Jansen MC, Weijenberg MP, van den Brandt PA, et al. Heme and chlorophyll intake and risk of colorectal cancer in The Netherlands Cohort Study. Cancer Epidemiol. Biomarkers Prev. 2006;15:717–25. doi: 10.1158/1055-9965.EPI-05-0772. [DOI] [PubMed] [Google Scholar]
- 12.Barcelo C, Paco N, Beckett AJ, Alvarez-Moya B, Garrido E, et al. Oncogenic K-ras segregates at spatially distinct plasma membrane signaling platforms according to its phosphorylation status. J. Cell Sci. 2013;126:4553–59. doi: 10.1242/jcs.123737. [DOI] [PubMed] [Google Scholar]
- 13.Barker N, Ridgway RA, van Es JH, van de Wetering M, Begthel H, et al. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature. 2009;457:608–11. doi: 10.1038/nature07602. [DOI] [PubMed] [Google Scholar]
- 14.Bastide NM, Chenni F, Audebert M, Santarelli RL, Tache S, et al. A central role for heme iron in colon carcinogenesis associated with red meat intake. Cancer Res. 2015;75:870–79. doi: 10.1158/0008-5472.CAN-14-2554. [DOI] [PubMed] [Google Scholar]
- 15.Beilstein F, Carriere V, Leturque A, Demignot S. Characteristics and functions of lipid droplets and associated proteins in enterocytes. Exp. Cell Res. 2016;340:172–79. doi: 10.1016/j.yexcr.2015.09.018. [DOI] [PubMed] [Google Scholar]
- 16.Bernstein AM, Song M, Zhang X, Pan A, Wang M, et al. Processed and unprocessed red meat and risk of colorectal cancer: analysis by tumor location and modification by time. PLOS ONE. 2015;10:e0135959. doi: 10.1371/journal.pone.0135959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bordonaro M, Lazarova DL, Sartorelli AC. Butyrate and Wnt signaling: a possible solution to the puzzle of dietary fiber and colon cancer risk? Cell Cycle. 2008;7:1178–83. doi: 10.4161/cc.7.9.5818. [DOI] [PubMed] [Google Scholar]
- 18.Brinkman AB, Roelofsen T, Pennings SW, Martens JH, Jenuwein T, Stunnenberg HG. Histone modification patterns associated with the human X chromosome. EMBO Rep. 2006;7:628–34. doi: 10.1038/sj.embor.7400686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat. Rev. Cancer. 2004;4:579–91. doi: 10.1038/nrc1408. [DOI] [PubMed] [Google Scholar]
- 20.Calviello G, Di Nicuolo F, Serini S, Piccioni E, Boninsegna A, et al. Docosahexaenoic acid enhances the susceptibility of human colorectal cancer cells to 5-fluorouracil. Cancer Chemother. Pharmacol. 2005;55:12–20. doi: 10.1007/s00280-004-0846-6. [DOI] [PubMed] [Google Scholar]
- 21.Canani RB, Costanzo MD, Leone L, Pedata M, Meli R, Calignano A. Potential beneficial effects of butyrate in intestinal and extraintestinal diseases. World J. Gastroenterol. 2011;17:1519–28. doi: 10.3748/wjg.v17.i12.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cattin AL, Le Beyec J, Barreau F, Saint-Just S, Houllier A, et al. Hepatocyte nuclear factor 4α, a key factor for homeostasis, cell architecture, and barrier function of the adult intestinal epithelium. Mol. Cell. Biol. 2009;29:6294–308. doi: 10.1128/MCB.00939-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ceccarelli V, Racanicchi S, Martelli MP, Nocentini G, Fettucciari K, et al. Eicosapentaenoic acid demethylates a single CpG that mediates expression of tumor suppressor CCAAT/enhancer-binding protein δ in U937 leukemia cells. J. Biol. Chem. 2011;286:27092–102. doi: 10.1074/jbc.M111.253609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chadalapaka G, Jutooru I, Chintharlapalli S, Papineni S, Smith R, 3rd, et al. Curcumin decreases specificity protein expression in bladder cancer cells. Cancer Res. 2008;68:5345–54. doi: 10.1158/0008-5472.CAN-07-6805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chan AT, Giovannucci EL, Meyerhardt JA, Schernhammer ES, Curhan GC, Fuchs CS. Long-term use of aspirin and nonsteroidal anti-inflammatory drugs and risk of colorectal cancer. JAMA. 2005;294:914–23. doi: 10.1001/jama.294.8.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chan AT, Ogino S, Fuchs CS. Aspirin and the risk of colorectal cancer in relation to the expression of COX-2. N. Engl. J. Med. 2007;356:2131–42. doi: 10.1056/NEJMoa067208. [DOI] [PubMed] [Google Scholar]
- 27.Chan DS, Lau R, Aune D, Vieira R, Greenwood DC, et al. Red and processed meat and colorectal cancer incidence: meta-analysis of prospective studies. PLOS ONE. 2011;6:e20456. doi: 10.1371/journal.pone.0020456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chang WC, Chapkin RS, Lupton JR. Predictive value of proliferation, differentiation and apoptosis as intermediate markers for colon tumorigenesis. Carcinogenesis. 1997;18:721–30. doi: 10.1093/carcin/18.4.721. [DOI] [PubMed] [Google Scholar]
- 29.Chapkin RS, Clark AE, Davidson LA, Schroeder F, Zoran DL, Lupton JR. Dietary fiber differentially alters cellular fatty acid-binding protein expression in exfoliated colonocytes during tumor development. Nutr. Cancer. 1998;32:107–12. doi: 10.1080/01635589809514727. [DOI] [PubMed] [Google Scholar]
- 30.Chapkin RS, McMurray DN, Lupton JR. Colon cancer, fatty acids and anti-inflammatory compounds. Curr. Opin. Gastroenterol. 2007;23:48–54. doi: 10.1097/MOG.0b013e32801145d7. [DOI] [PubMed] [Google Scholar]
- 31.Chellappa K, Robertson GR, Sladek FM. HNF4α: a new biomarker in colon cancer? Biomark. Med. 2012;6:297–300. doi: 10.2217/bmm.12.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen A, Xu J, Johnson AC. Curcumin inhibits human colon cancer cell growth by suppressing gene expression of epidermal growth factor receptor through reducing the activity of the transcription factor Egr-1. Oncogene. 2006;25:278–87. doi: 10.1038/sj.onc.1209019. [DOI] [PubMed] [Google Scholar]
- 33.Cho Y, Kim H, Turner ND, Mann JC, Wei J, et al. A chemoprotective fish oil- and pectin-containing diet temporally alters gene expression profiles in exfoliated rat colonocytes throughout oncogenesis. J. Nutr. 2011;141:1029–35. doi: 10.3945/jn.110.134973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cho Y, Turner ND, Davidson LA, Chapkin RS, Carroll RJ, Lupton JR. Colon cancer cell apoptosis is induced by combined exposure to the n-3 fatty acid docosahexaenoic acid and butyrate through promoter methylation. Exp. Biol. Med. (Maywood) 2014;239:302–10. doi: 10.1177/1535370213514927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chubak J, Kamineni A, Buist DSM, Anderson ML, Whitlock EP. Aspirin use for the prevention of colorectal cancer: an updated systematic evidence review for the U.S. Preventive Services Task Force. Agency Healthc. Res. Qual; Rockville, MD: 2015. Rep. No. 15-05228-EF-1. [PubMed] [Google Scholar]
- 36.Cockbain AJ, Toogood GJ, Hull MA. Omega-3 polyunsaturated fatty acids for the treatment and prevention of colorectal cancer. Gut. 2012;61:135–49. doi: 10.1136/gut.2010.233718. [DOI] [PubMed] [Google Scholar]
- 37.Cole BF, Baron JA, Sandler RS, Haile RW, Ahnen DJ, et al. Folic acid for the prevention of colorectal adenomas: a randomized clinical trial. JAMA. 2007;297:2351–59. doi: 10.1001/jama.297.21.2351. [DOI] [PubMed] [Google Scholar]
- 38.Cottet V, Bonithon-Kopp C, Kronborg O, Santos L, Andreatta R, et al. Dietary patterns and the risk of colorectal adenoma recurrence in a European intervention trial. Eur. J. Cancer Prev. 2005;14:21–29. doi: 10.1097/00008469-200502000-00004. [DOI] [PubMed] [Google Scholar]
- 39.Crim KC, Sanders LM, Hong MY, Taddeo SS, Turner ND, et al. Upregulation of p21Waf1/Cip1 expression in vivo by butyrate administration can be chemoprotective or chemopromotive depending on the lipid component of the diet. Carcinogenesis. 2008;29:1415–20. doi: 10.1093/carcin/bgn144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cross AJ, Ferrucci LM, Risch A, Graubard BI, Ward MH, et al. A large prospective study of meat consumption and colorectal cancer risk: an investigation of potential mechanisms underlying this association. Cancer Res. 2010;70:2406–14. doi: 10.1158/0008-5472.CAN-09-3929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cross AJ, Sinha R. Meat-related mutagens/carcinogens in the etiology of colorectal cancer. Environ. Mol. Mutagen. 2004;44:44–55. doi: 10.1002/em.20030. [DOI] [PubMed] [Google Scholar]
- 42.D'Archivio M, Scazzocchio B, Giammarioli S, Fiani ML, Vari R, et al. ω3-PUFAs exert anti-inflammatory activity in visceral adipocytes from colorectal cancer patients. PLOS ONE. 2013;8:e77432. doi: 10.1371/journal.pone.0077432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505:559–63. doi: 10.1038/nature12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Davidson LA, Nguyen DV, Hokanson RM, Callaway ES, Isett RB, et al. Chemopreventive n-3 polyunsaturated fatty acids reprogram genetic signatures during colon cancer initiation and progression in the rat. Cancer Res. 2004;64:6797–804. doi: 10.1158/0008-5472.CAN-04-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Davidson LA, Wang N, Ivanov I, Goldsby J, Lupton JR, Chapkin RS. Identification of actively translated mRNA transcripts in a rat model of early-stage colon carcinogenesis. Cancer Prev. Res. (Phila.) 2009;2:984–94. doi: 10.1158/1940-6207.CAPR-09-0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Davidson LA, Wang N, Shah MS, Lupton JR, Ivanov I, Chapkin RS. n-3 Polyunsaturated fatty acids modulate carcinogen-directed non-coding microRNA signatures in rat colon. Carcinogenesis. 2009;30:2077–84. doi: 10.1093/carcin/bgp245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dellavalle CT, Xiao Q, Yang G, Shu XO, Aschebrook-Kilfoy B, et al. Dietary nitrate and nitrite intake and risk of colorectal cancer in the Shanghai Women's Health Study. Int. J. Cancer. 2014;134:2917–26. doi: 10.1002/ijc.28612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Diederichs S, Haber DA. Dual role for argonautes in microRNA processing and posttranscriptional regulation of microRNA expression. Cell. 2007;131:1097–108. doi: 10.1016/j.cell.2007.10.032. [DOI] [PubMed] [Google Scholar]
- 49.Donohoe DR, Garge N, Zhang X, Sun W, O'Connell TM, et al. The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon. Cell Metab. 2011;13:517–26. doi: 10.1016/j.cmet.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Donovan SM, Wang M, Monaco MH, Martin CR, Davidson LA, et al. Noninvasive molecular fingerprinting of host-microbiome interactions in neonates. FEBS Lett. 2014;588:4112–19. doi: 10.1016/j.febslet.2014.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, et al. Diversity of the human intestinal microbial flora. Science. 2005;308:1635–38. doi: 10.1126/science.1110591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Edwards IJ, O'Flaherty JT. Omega-3 fatty acids and PPARγ in cancer. PPAR Res. 2008:358052. doi: 10.1155/2008/358052. 2008. doi: 10.1155/2008/358052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ekbom A, Helmick C, Zack M, Adami HO. Ulcerative colitis and colorectal cancer. A population-based study. N. Engl. J. Med. 1990;323:1228–33. doi: 10.1056/NEJM199011013231802. [DOI] [PubMed] [Google Scholar]
- 54.Fan YY, Davidson LA, Callaway ES, Goldsby JS, Chapkin RS. Differential effects of 2- and 3-series E-prostaglandins on in vitro expansion of Lgr5+ colonic stem cells. Carcinogenesis. 2014;35:606–12. doi: 10.1093/carcin/bgt412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fan YY, Davidson LA, Callaway ES, Wright GA, Safe S, Chapkin RS. A bioassay to measure energy metabolism in mouse colonic crypts, organoids, and sorted stem cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2015;309:G1–9. doi: 10.1152/ajpgi.00052.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fang Y, Xiang J, Chen Z, Gu X, Li Z, et al. miRNA expression profile of colon cancer stem cells compared to non-stem cells using the SW1116 cell line. Oncol. Rep. 2012;28:2115–24. doi: 10.3892/or.2012.2054. [DOI] [PubMed] [Google Scholar]
- 57.Feagins LA, Souza RF, Spechler SJ. Carcinogenesis in IBD: potential targets for the prevention of colorectal cancer. Nat. Rev. Gastroenterol. Hepatol. 2009;6:297–305. doi: 10.1038/nrgastro.2009.44. [DOI] [PubMed] [Google Scholar]
- 58.Figueiredo JC, Mott LA, Giovannucci E, Wu K, Cole B, et al. Folic acid and prevention of colorectal adenomas: a combined analysis of randomized clinical trials. Int. J. Cancer. 2011;129:192–203. doi: 10.1002/ijc.25872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Flood DM, Weiss NS, Cook LS, Emerson JC, Schwartz SM, Potter JD. Colorectal cancer incidence in Asian migrants to the United States and their descendants. Cancer Causes Control. 2000;11:403–11. doi: 10.1023/a:1008955722425. [DOI] [PubMed] [Google Scholar]
- 60.Flossmann E, Rothwell PM. British Doctors Aspirin Trial, UK-TIA Aspirin Trial. 2007. Effect of aspirin on long-term risk of colorectal cancer: consistent evidence from randomised and observational studies. Lancet. 369:1603–13. doi: 10.1016/S0140-6736(07)60747-8. [DOI] [PubMed] [Google Scholar]
- 61.Fox JT, Stover PJ. Folate-mediated one-carbon metabolism. Vitam. Horm. 2008;79:1–44. doi: 10.1016/S0083-6729(08)00401-9. [DOI] [PubMed] [Google Scholar]
- 62.Fujise T, Iwakiri R, Kakimoto T, Shiraishi R, Sakata Y, et al. Long-term feeding of various fat diets modulates azoxymethane-induced colon carcinogenesis through Wnt/β-catenin signaling in rats. Am. J. Physiol. Gastrointest. Liver Physiol. 2007;292:G1150–56. doi: 10.1152/ajpgi.00269.2006. [DOI] [PubMed] [Google Scholar]
- 63.Galli C, Marangoni F, Galella G. Modulation of lipid derived mediators by polyunsaturated fatty acids. Prostaglandins Leukot. Essent. Fatty Acids. 1993;48:51–55. doi: 10.1016/0952-3278(93)90009-l. [DOI] [PubMed] [Google Scholar]
- 64.Gamet-Payrastre L, Li P, Lumeau S, Cassar G, Dupont MA, et al. Sulforaphane, a naturally occurring isothiocyanate, induces cell cycle arrest and apoptosis in HT29 human colon cancer cells. Cancer Res. 2000;60:1426–33. [PubMed] [Google Scholar]
- 65.Gandhy SU, Kim K, Larsen L, Rosengren RJ, Safe S. Curcumin and synthetic analogs induce reactive oxygen species and decreases specificity protein (Sp) transcription factors by targeting microRNAs. BMC Cancer. 2012;12:564. doi: 10.1186/1471-2407-12-564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gangaraju VK, Lin H. MicroRNAs: key regulators of stem cells. Nat. Rev. Mol. Cell Biol. 2009;10:116–25. doi: 10.1038/nrm2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gaur AB, Holbeck SL, Colburn NH, Israel MA. Downregulation of Pdcd4 by mir-21 facilitates glioblastoma proliferation in vivo. Neuro Oncol. 2011;13:580–90. doi: 10.1093/neuonc/nor033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Geelen A, Schouten JM, Kamphuis C, Stam BE, Burema J, et al. Fish consumption, n-3 fatty acids, and colorectal cancer: a meta-analysis of prospective cohort studies. Am. J. Epidemiol. 2007;166:1116–25. doi: 10.1093/aje/kwm197. [DOI] [PubMed] [Google Scholar]
- 69.Gerber M. Background review paper on total fat, fatty acid intake and cancers. Ann. Nutr. Metab. 2009;55:140–61. doi: 10.1159/000229000. [DOI] [PubMed] [Google Scholar]
- 70.Gerber M. Omega-3 fatty acids and cancers: a systematic update review of epidemiological studies. Br. J. Nutr. 2012;107(Suppl. 2):S228–39. doi: 10.1017/S0007114512001614. [DOI] [PubMed] [Google Scholar]
- 71.Gerner EW, Ignatenko NA, Lance P, Hurley LH. A comprehensive strategy to combat colon cancer targeting the adenomatous polyposis coli tumor suppressor gene. Ann. N. Y. Acad. Sci. 2005;1059:97–105. doi: 10.1196/annals.1339.033. [DOI] [PubMed] [Google Scholar]
- 72.Gil-Zamorano J, Martin R, Daimiel L, Richardson K, Giordano E, et al. Docosahexaenoic acid modulates the enterocyte Caco-2 cell expression of microRNAs involved in lipid metabolism. J. Nutr. 2014;144:575–85. doi: 10.3945/jn.113.189050. [DOI] [PubMed] [Google Scholar]
- 73.Giovannucci E. Epidemiologic studies of folate and colorectal neoplasia: a review. J. Nutr. 2002;132:2350–55S. doi: 10.1093/jn/132.8.2350S. [DOI] [PubMed] [Google Scholar]
- 74.Gupta SC, Kim JH, Prasad S, Aggarwal BB. Regulation of survival, proliferation, invasion, angiogenesis, and metastasis of tumor cells through modulation of inflammatory pathways by nutraceuticals. Cancer Metastasis Rev. 2010;29:405–34. doi: 10.1007/s10555-010-9235-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ha CW, Lam YY, Holmes AJ. Mechanistic links between gut microbial community dynamics, microbial functions and metabolic health. World J. Gastroenterol. 2014;20:16498–517. doi: 10.3748/wjg.v20.i44.16498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hall MN, Campos H, Li H, Sesso HD, Stampfer MJ, et al. Blood levels of long-chain polyunsaturated fatty acids, aspirin, and the risk of colorectal cancer. Cancer Epidemiol. Biomarkers Prev. 2007;16:314–21. doi: 10.1158/1055-9965.EPI-06-0346. [DOI] [PubMed] [Google Scholar]
- 77.Hall MN, Chavarro JE, Lee IM, Willett WC, Ma J. A 22-year prospective study of fish, n-3 fatty acid intake, and colorectal cancer risk in men. Cancer Epidemiol. Biomarkers Prev. 2008;17:1136–43. doi: 10.1158/1055-9965.EPI-07-2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Heijstek MW, Kranenburg O, Borel Rinkes IH. Mouse models of colorectal cancer and liver metastases. Dig. Surg. 2005;22:16–25. doi: 10.1159/000085342. [DOI] [PubMed] [Google Scholar]
- 79.Helmus DS, Thompson CL, Zelenskiy S, Tucker TC, Li L. Red meat–derived heterocyclic amines increase risk of colon cancer: a population-based case-control study. Nutr. Cancer. 2013;65:1141–50. doi: 10.1080/01635581.2013.834945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ho GY, Figueroa-Valles NR, De La Torre-Feliciano T, Tucker KL, Tortolero-Luna G, et al. Cancer disparities between mainland and island Puerto Ricans. Rev. Panam. Salud Publica. 2009;25:394–400. doi: 10.1590/s1020-49892009000500003. [DOI] [PubMed] [Google Scholar]
- 81.Hofmanova J, Hyrslova Vaculova A, Kozubik A. Regulation of the metabolism of polyunsaturated fatty acids and butyrate in colon cancer cells. Curr. Pharm. Biotechnol. 2013;14:274–88. doi: 10.2174/1389201011314030004. [DOI] [PubMed] [Google Scholar]
- 82.Howlader N, Noone AM, Krapcho M, Garshell J, Miller D, et al. SEER Cancer Statistics Review, 1975–2012, National Cancer Institute. Natl. Cancer Inst.; Bethesda, MD: 2015. http://seer.cancer.gov/csr/1975_2012/ [Google Scholar]
- 83.Hughes R, Cross AJ, Pollock JR, Bingham S. Dose-dependent effect of dietary meat on endogenous colonic N-nitrosation. Carcinogenesis. 2001;22:199–202. doi: 10.1093/carcin/22.1.199. [DOI] [PubMed] [Google Scholar]
- 84.IJssennagger N, Belzer C, Hooiveld GJ, Dekker J, van Mil SW, et al. Gut microbiota facilitates dietary heme-induced epithelial hyperproliferation by opening the mucus barrier in colon. PNAS. 2015;112:10038–43. doi: 10.1073/pnas.1507645112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.IJssennagger N, Derrien M, van Doorn GM, Rijnierse A, van den Bogert B, et al. Dietary heme alters microbiota and mucosa of mouse colon without functional changes in host-microbe cross-talk. PLOS ONE. 2012;7:e49868. doi: 10.1371/journal.pone.0049868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.IJssennagger N, Rijnierse A, de Wit NJ, Boekschoten MV, Dekker J, et al. Dietary heme induces acute oxidative stress, but delayed cytotoxicity and compensatory hyperproliferation in mouse colon. Carcinogenesis. 2013;34:1628–35. doi: 10.1093/carcin/bgt084. [DOI] [PubMed] [Google Scholar]
- 87.IJssennagger N, Rijnierse A, de Wit N, Jonker-Termont D, Dekker J, et al. Dietary haem stimulates epithelial cell turnover by downregulating feedback inhibitors of proliferation in murine colon. Gut. 2012;61:1041–49. doi: 10.1136/gutjnl-2011-300239. [DOI] [PubMed] [Google Scholar]
- 88.Ingolfsson HI, Koeppe RE 2nd Andersen OS. Curcumin is a modulator of bilayer material properties. Biochemistry (Mosc.) 2007;46:10384–91. doi: 10.1021/bi701013n. [DOI] [PubMed] [Google Scholar]
- 89.Irizarry RA, Ladd-Acosta C, Wen B, Wu Z, Montano C, et al. The human colon cancer methylome shows similar hypo- and hypermethylation at conserved tissue-specific CpG island shores. Nat. Genet. 2009;41:178–86. doi: 10.1038/ng.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Irving GR, Howells LM, Sale S, Kralj-Hans I, Atkin WS, et al. Prolonged biologically active colonic tissue levels of curcumin achieved after oral administration—a clinical pilot study including assessment of patient acceptability. Cancer Prev. Res. (Phila.) 2013;6:119–28. doi: 10.1158/1940-6207.CAPR-12-0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jagerstad M, Skog K. Genotoxicity of heat-processed foods. Mutat. Res. 2005;574:156–72. doi: 10.1016/j.mrfmmm.2005.01.030. [DOI] [PubMed] [Google Scholar]
- 92.Jakobsen CH, Storvold GL, Bremseth H, Follestad T, Sand K, et al. DHA induces ER stress and growth arrest in human colon cancer cells: associations with cholesterol and calcium homeostasis. J. Lipid Res. 2008;49:2089–100. doi: 10.1194/jlr.M700389-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jaszewski R, Misra S, Tobi M, Ullah N, Naumoff JA, et al. Folic acid supplementation inhibits recurrence of colorectal adenomas: a randomized chemoprevention trial. World J. Gastroenterol. 2008;14:4492–98. doi: 10.3748/wjg.14.4492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ji Q, Karnak D, Hao P, Wang R, Xu L. No small matter: microRNAs—key regulators of cancer stem cells. Int. J. Clin. Exp. Med. 2010;3:84–87. [PMC free article] [PubMed] [Google Scholar]
- 95.Joosen AM, Kuhnle GG, Aspinall SM, Barrow TM, Lecommandeur E, et al. Effect of processed and red meat on endogenous nitrosation and DNA damage. Carcinogenesis. 2009;30:1402–7. doi: 10.1093/carcin/bgp130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Karunagaran D, Joseph J, Kumar TR. Cell growth regulation. Adv. Exp. Med. Biol. 2007;595:245–68. doi: 10.1007/978-0-387-46401-5_11. [DOI] [PubMed] [Google Scholar]
- 97.Kennedy DA, Stern SJ, Matok I, Moretti ME, Sarkar M, et al. Folate intake, MTHFR polymorphisms, and the risk of colorectal cancer: a systematic review and meta-analysis. J. Cancer Epidemiol. 2012;2012:952508. doi: 10.1155/2012/952508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kennedy DA, Stern SJ, Moretti M, Matok I, Sarkar M, et al. Folate intake and the risk of colorectal cancer: a systematic review and meta-analysis. Cancer Epidemiol. 2011;35:2–10. doi: 10.1016/j.canep.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 99.Kim DH, Smith-Warner SA, Spiegelman D, Yaun SS, Colditz GA, et al. Pooled analyses of 13 prospective cohort studies on folate intake and colon cancer. Cancer Causes Control. 2010;21:1919–30. doi: 10.1007/s10552-010-9620-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kim S, Sandler DP, Galanko J, Martin C, Sandler RS. Intake of polyunsaturated fatty acids and distal large bowel cancer risk in whites and African Americans. Am. J. Epidemiol. 2010;171:969–79. doi: 10.1093/aje/kwq032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kim Y-I. Current status of folic acid supplementation on colorectal cancer prevention. Curr. Pharmacol. Rep. 2016;2:21–33. [Google Scholar]
- 102.Kim YI. Folate and carcinogenesis: evidence, mechanisms, and implications. J. Nutr. Biochem. 1999;10:66–88. doi: 10.1016/s0955-2863(98)00074-6. [DOI] [PubMed] [Google Scholar]
- 103.Kim YI. Folate: a magic bullet or a double edged sword for colorectal cancer prevention? Gut. 2006;55:1387–89. doi: 10.1136/gut.2006.095463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kim YI. Folate and colorectal cancer: an evidence-based critical review. Mol. Nutr. Food Res. 2007;51:267–92. doi: 10.1002/mnfr.200600191. [DOI] [PubMed] [Google Scholar]
- 105.Kimura Y, Kono S, Toyomura K, Nagano J, Mizoue T, et al. Meat, fish and fat intake in relation to subsite-specific risk of colorectal cancer: the Fukuoka Colorectal Cancer Study. Cancer Sci. 2007;98:590–97. doi: 10.1111/j.1349-7006.2007.00425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Klaus A, Birchmeier W. Wnt signalling and its impact on development and cancer. Nat. Rev. Cancer. 2008;8:387–98. doi: 10.1038/nrc2389. [DOI] [PubMed] [Google Scholar]
- 107.Knight JM, Davidson LA, Herman D, Martin CR, Goldsby JS, et al. Non-invasive analysis of intestinal development in preterm and term infants using RNA-sequencing. Sci. Rep. 2014;4:5453. doi: 10.1038/srep05453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kojima M, Wakai K, Tokudome S, Suzuki K, Tamakoshi K, et al. Serum levels of polyunsaturated fatty acids and risk of colorectal cancer: a prospective study. Am. J. Epidemiol. 2005;161:462–71. doi: 10.1093/aje/kwi066. [DOI] [PubMed] [Google Scholar]
- 109.Kolar S, Barhoumi R, Jones CK, Wesley J, Lupton JR, et al. Interactive effects of fatty acid and butyrate-induced mitochondrial Ca2+ loading and apoptosis in colonocytes. Cancer. 2011;117:5294–303. doi: 10.1002/cncr.26205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kolar SS, Barhoumi R, Callaway ES, Fan YY, Wang N, et al. Synergy between docosahexaenoic acid and butyrate elicits p53-independent apoptosis via mitochondrial Ca2+ accumulation in colonocytes. Am. J. Physiol. Gastrointest. Liver Physiol. 2007;293:G935–43. doi: 10.1152/ajpgi.00312.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kolar SS, Barhoumi R, Lupton JR, Chapkin RS. Docosahexaenoic acid and butyrate synergistically induce colonocyte apoptosis by enhancing mitochondrial Ca2+ accumulation. Cancer Res. 2007;67:5561–68. doi: 10.1158/0008-5472.CAN-06-4716. [DOI] [PubMed] [Google Scholar]
- 112.Korinek V, Barker N, Moerer P, van Donselaar E, Huls G, et al. Depletion of epithelial stem-cell compartments in the small intestine of mice lacking Tcf-4. Nat. Genet. 1998;19:379–83. doi: 10.1038/1270. [DOI] [PubMed] [Google Scholar]
- 113.Korinek V, Barker N, Morin PJ, van Wichen D, de Weger R, et al. Constitutive transcriptional activation by a beta-catenin-Tcf complex in APC−/− colon carcinoma. Science. 1997;275:1784–87. doi: 10.1126/science.275.5307.1784. [DOI] [PubMed] [Google Scholar]
- 114.Kuan CY, Walker TH, Luo PG, Chen CF. Long-chain polyunsaturated fatty acids promote paclitaxel cytotoxicity via inhibition of the MDR1 gene in the human colon cancer Caco-2 cell line. J. Am. Coll. Nutr. 2011;30:265–73. doi: 10.1080/07315724.2011.10719969. [DOI] [PubMed] [Google Scholar]
- 115.Kuhnert F, Davis CR, Wang HT, Chu P, Lee M, et al. Essential requirement for Wnt signaling in proliferation of adult small intestine and colon revealed by adenoviral expression of Dickkopf-1. PNAS. 2004;101:266–71. doi: 10.1073/pnas.2536800100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Larsson SC, Wolk A. Meat consumption and risk of colorectal cancer: a meta-analysis of prospective studies. Int. J. Cancer. 2006;119:2657–64. doi: 10.1002/ijc.22170. [DOI] [PubMed] [Google Scholar]
- 117.Lee DH, Anderson KE, Harnack LJ, Folsom AR, Jacobs DR., Jr. Heme iron, zinc, alcohol consumption, and colon cancer: Iowa Women's Health Study. J. Natl. Cancer Inst. 2004;96:403–7. doi: 10.1093/jnci/djh047. [DOI] [PubMed] [Google Scholar]
- 118.Lee J, Demissie K, Lu SE, Rhoads GG. Cancer incidence among Korean-American immigrants in the United States and native Koreans in South Korea. Cancer Control. 2007;14:78–85. doi: 10.1177/107327480701400111. [DOI] [PubMed] [Google Scholar]
- 119.Lewin MH, Bailey N, Bandaletova T, Bowman R, Cross AJ, et al. Red meat enhances the colonic formation of the DNA adduct O6-carboxymethyl guanine: implications for colorectal cancer risk. Cancer Res. 2006;66:1859–65. doi: 10.1158/0008-5472.CAN-05-2237. [DOI] [PubMed] [Google Scholar]
- 120.Li X, Xin S, He Z, Che X, Wang J, et al. MicroRNA-21 (miR-21) post-transcriptionally downregulates tumor suppressor PDCD4 and promotes cell transformation, proliferation, and metastasis in renal cell carcinoma. Cell. Physiol. Biochem. 2014;33:1631–42. doi: 10.1159/000362946. [DOI] [PubMed] [Google Scholar]
- 121.Lijinsky W. Structure-activity relations in carcinogenesis by N-nitroso compounds. Cancer Metastasis Rev. 1987;6:301–56. doi: 10.1007/BF00144269. [DOI] [PubMed] [Google Scholar]
- 122.Link A, Balaguer F, Shen Y, Lozano JJ, Leung HC, et al. Curcumin modulates DNA methylation in colorectal cancer cells. PLOS ONE. 2013;8:e57709. doi: 10.1371/journal.pone.0057709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lo Sasso G, Bovenga F, Murzilli S, Salvatore L, Di Tullio G, et al. Liver X receptors inhibit proliferation of human colorectal cancer cells and growth of intestinal tumors in mice. Gastroenterology. 2013;144:1497–507.e13. doi: 10.1053/j.gastro.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 124.Logan RF, Grainge MJ, Shepherd VC, Armitage NC, Muir KR. Aspirin and folic acid for the prevention of recurrent colorectal adenomas. Gastroenterology. 2008;134:29–38. doi: 10.1053/j.gastro.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 125.Loh YH, Jakszyn P, Luben RN, Mulligan AA, Mitrou PN, Khaw KT. N-nitroso compounds and cancer incidence: the European Prospective Investigation into Cancer and Nutrition (EPIC)-Norfolk Study. Am. J. Clin. Nutr. 2011;93:1053–61. doi: 10.3945/ajcn.111.012377. [DOI] [PubMed] [Google Scholar]
- 126.Lopez-Serra P, Esteller M. DNA methylation-associated silencing of tumor-suppressor microRNAs in cancer. Oncogene. 2012;31:1609–22. doi: 10.1038/onc.2011.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lunn JC, Kuhnle G, Mai V, Frankenfeld C, Shuker DE, et al. The effect of haem in red and processed meat on the endogenous formation of N-nitroso compounds in the upper gastrointestinal tract. Carcinogenesis. 2007;28:685–90. doi: 10.1093/carcin/bgl192. [DOI] [PubMed] [Google Scholar]
- 128.Lynch HT, de la Chapelle A. Hereditary colorectal cancer. N. Engl. J. Med. 2003;348:919–32. doi: 10.1056/NEJMra012242. [DOI] [PubMed] [Google Scholar]
- 129.MacLean CH, Newberry SJ, Mojica WA, Khanna P, Issa AM, et al. Effects of omega-3 fatty acids on cancer risk: a systematic review. JAMA. 2006;295:403–15. doi: 10.1001/jama.295.4.403. [DOI] [PubMed] [Google Scholar]
- 130.Martinez-Augustin O, Lopez-Posadas R, Gonzalez R, Suarez MD, Zarzuelo A, Sanchez de Medina F. Genomic analysis of sulfasalazine effect in experimental colitis is consistent primarily with the modulation of NF-κB but not PPAR-γ signaling. Pharmacogenet. Genom. 2009;19:363–72. doi: 10.1097/FPC.0b013e3283299a73. [DOI] [PubMed] [Google Scholar]
- 131.Matano M, Date S, Shimokawa M, Takano A, Fujii M, et al. Modeling colorectal cancer using CRISPR-Cas9-mediated engineering of human intestinal organoids. Nat. Med. 2015;21:256–62. doi: 10.1038/nm.3802. [DOI] [PubMed] [Google Scholar]
- 132.Mazhab-Jafari MT, Marshall CB, Smith MJ, Gasmi-Seabrook GM, Stathopulos PB, et al. Oncogenic and RASopathy-associated K-RAS mutations relieve membrane-dependent occlusion of the effector-binding site. PNAS. 2015;112:6625–30. doi: 10.1073/pnas.1419895112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.McDonald SA, Preston SL, Lovell MJ, Wright NA, Jankowski JA. Mechanisms of disease: from stem cells to colorectal cancer. Nat. Clin. Pract. Gastroenterol. Hepatol. 2006;3:267–74. doi: 10.1038/ncpgasthep0473. [DOI] [PubMed] [Google Scholar]
- 134.Meerschaert RL, Kelly CV. Trace membrane additives affect lipid phases with distinct mechanisms: a modified Ising model. Eur. Biophys. J. 2015;44:227–33. doi: 10.1007/s00249-015-1017-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Meng F, Henson R, Wehbe-Janek H, Ghoshal K, Jacob ST, Patel T. MicroRNA-21 regulates expression of the PTEN tumor suppressor gene in human hepatocellular cancer. Gastroenterology. 2007;133:647–58. doi: 10.1053/j.gastro.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Meyer AS, Zweemer AJ, Lauffenburger DA. The AXL receptor is a sensor of ligand spatial heterogeneity. Cell Syst. 2015;1:25–36. doi: 10.1016/j.cels.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Midura-Kiela MT, Radhakrishnan VM, Larmonier CB, Laubitz D, Ghishan FK, Kiela PR. Curcumin inhibits interferon-gamma signaling in colonic epithelial cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2012;302:G85–96. doi: 10.1152/ajpgi.00275.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Mishra A, Chaudhary A, Sethi S. Oxidized omega-3 fatty acids inhibit NF-κB activation via a PPARα-dependent pathway. Arterioscler. Thromb. Vasc. Biol. 2004;24:1621–27. doi: 10.1161/01.ATV.0000137191.02577.86. [DOI] [PubMed] [Google Scholar]
- 139.Moghaddam AA, Woodward M, Huxley R. Obesity and risk of colorectal cancer: a meta-analysis of 31 studies with 70,000 events. Cancer Epidemiol. Biomarkers Prev. 2007;16:2533–47. doi: 10.1158/1055-9965.EPI-07-0708. [DOI] [PubMed] [Google Scholar]
- 140.Moriyama T, Ohuchida K, Mizumoto K, Yu J, Sato N, et al. MicroRNA-21 modulates biological functions of pancreatic cancer cells including their proliferation, invasion, and chemoresistance. Mol. Cancer Ther. 2009;8:1067–74. doi: 10.1158/1535-7163.MCT-08-0592. [DOI] [PubMed] [Google Scholar]
- 141.Mudduluru G, George-William JN, Muppala S, Asangani IA, Kumarswamy R, et al. Curcumin regulates miR-21 expression and inhibits invasion and metastasis in colorectal cancer. Biosci. Rep. 2011;31:185–97. doi: 10.1042/BSR20100065. [DOI] [PubMed] [Google Scholar]
- 142.Mukherjee D, Nissen SE, Topol EJ. Risk of cardiovascular events associated with selective COX-2 inhibitors. JAMA. 2001;286:954–59. doi: 10.1001/jama.286.8.954. [DOI] [PubMed] [Google Scholar]
- 143.Murff HJ, Shrubsole MJ, Cai Q, Smalley WE, Dai Q, et al. Dietary intake of PUFAs and colorectal polyp risk. Am. J. Clin. Nutr. 2012;95:703–12. doi: 10.3945/ajcn.111.024000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Nan X, Tamguney TM, Collisson EA, Lin LJ, Pitt C, et al. Ras-GTP dimers activate the mitogen-activated protein kinase (MAPK) pathway. PNAS. 2015;112:7996–8001. doi: 10.1073/pnas.1509123112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Nautiyal J, Banerjee S, Kanwar SS, Yu Y, Patel BB, et al. Curcumin enhances dasatinib-induced inhibition of growth and transformation of colon cancer cells. Int. J. Cancer. 2011;128:951–61. doi: 10.1002/ijc.25410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Nomoto H, Iigo M, Hamada H, Kojima S, Tsuda H. Chemoprevention of colorectal cancer by grape seed proanthocyanidin is accompanied by a decrease in proliferation and increase in apoptosis. Nutr. Cancer. 2004;49:81–88. doi: 10.1207/s15327914nc4901_11. [DOI] [PubMed] [Google Scholar]
- 147.Norat T, Lukanova A, Ferrari P, Riboli E. Meat consumption and colorectal cancer risk: dose-response meta-analysis of epidemiological studies. Int. J. Cancer. 2002;98:241–56. doi: 10.1002/ijc.10126. [DOI] [PubMed] [Google Scholar]
- 148.Ollberding NJ, Wilkens LR, Henderson BE, Kolonel LN, Le Marchand L. Meat consumption, heterocyclic amines and colorectal cancer risk: the Multiethnic Cohort Study. Int. J. Cancer. 2012;131:E1125–33. doi: 10.1002/ijc.27546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Orlich MJ, Singh PN, Sabate J, Fan J, Sveen L, et al. Vegetarian dietary patterns and the risk of colorectal cancers. JAMA Int. Med. 2015;175:767–76. doi: 10.1001/jamainternmed.2015.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Oshima T, Kawasaki T, Ohashi R, Hasegawa G, Jiang S, et al. Downregulated P1 promoter-driven hepatocyte nuclear factor-4αexpression in human colorectal carcinoma is a new prognostic factor against liver metastasis. Pathol. Int. 2007;57:82–90. doi: 10.1111/j.1440-1827.2006.02061.x. [DOI] [PubMed] [Google Scholar]
- 151.Ouyang N, Ke S, Eagleton N, Xie Y, Chen G, et al. Pregnane X receptor suppresses proliferation and tumourigenicity of colon cancer cells. Br. J. Cancer. 2010;102:1753–61. doi: 10.1038/sj.bjc.6605677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Park W, Amin AR, Chen ZG, Shin DM. New perspectives of curcumin in cancer prevention. Cancer Prev. Res. (Phila.) 2013;6:387–400. doi: 10.1158/1940-6207.CAPR-12-0410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Parris A, Williams MR. A human colonic crypt culture system to study regulation of stem cell-driven tissue renewal and physiological function. Methods Mol. Biol. 2015;1212:141–61. doi: 10.1007/7651_2015_197. [DOI] [PubMed] [Google Scholar]
- 154.Paspatis GA, Karamanolis DG. Folate supplementation and adenomatous colonic polyps. Dis. Colon Rectum. 1994;37:1340–41. doi: 10.1007/BF02257810. [DOI] [PubMed] [Google Scholar]
- 155.Patterson E, O'Doherty RM, Murphy EF, Wall R, O'Sullivan O, et al. Impact of dietary fatty acids on metabolic activity and host intestinal microbiota composition in C57BL/6J mice. Br. J. Nutr. 2014;111:1905–17. doi: 10.1017/S0007114514000117. [DOI] [PubMed] [Google Scholar]
- 156.Paulsen JE, Elvsaas IK, Steffensen IL, Alexander J. A fish oil derived concentrate enriched in eicosapentaenoic and docosahexaenoic acid as ethyl ester suppresses the formation and growth of intestinal polyps in the Min mouse. Carcinogenesis. 1997;18:1905–10. doi: 10.1093/carcin/18.10.1905. [DOI] [PubMed] [Google Scholar]
- 157.Pegorier JP, Le May C, Girard J. Control of gene expression by fatty acids. J. Nutr. 2004;134:2444–49S. doi: 10.1093/jn/134.9.2444S. [DOI] [PubMed] [Google Scholar]
- 158.Pfeiffer CM, Hughes JP, Lacher DA, Bailey RL, Berry RJ, et al. Estimation of trends in serum and RBC folate in the U.S. population from pre- to postfortification using assay-adjusted data from the NHANES 1988–2010. J. Nutr. 2012;142:886–93. doi: 10.3945/jn.111.156919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Piepoli A, Tavano F, Copetti M, Mazza T, Palumbo O, et al. miRNA expression profiles identify drivers in colorectal and pancreatic cancers. PLOS ONE. 2012;7:e33663. doi: 10.1371/journal.pone.0033663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Pierzchalska M, Grabacka M, Michalik M, Zyla K, Pierzchalski P. Prostaglandin E2 supports growth of chicken embryo intestinal organoids in Matrigel matrix. Biotechniques. 2012;52:307–15. doi: 10.2144/0000113851. [DOI] [PubMed] [Google Scholar]
- 161.Pinheiro PS, Sherman RL, Trapido EJ, Fleming LE, Huang Y, et al. Cancer incidence in first-generation U.S. Hispanics: Cubans, Mexicans, Puerto Ricans, and new Latinos. Cancer Epidemiol. Biomarkers Prev. 2009;18:2162–69. doi: 10.1158/1055-9965.EPI-09-0329. [DOI] [PubMed] [Google Scholar]
- 162.Pinto D, Gregorieff A, Begthel H, Clevers H. Canonical Wnt signals are essential for homeostasis of the intestinal epithelium. Genes Dev. 2003;17:1709–13. doi: 10.1101/gad.267103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Ponferrada A, Caso JR, Alou L, Colon A, Sevillano D, et al. The role of PPARγ on restoration of colonic homeostasis after experimental stress-induced inflammation and dysfunction. Gastroenterology. 2007;132:1791–803. doi: 10.1053/j.gastro.2007.02.032. [DOI] [PubMed] [Google Scholar]
- 164.Possidonio AC, Miranda M, Gregoracci GB, Thompson FL, Costa ML, Mermelstein C. Cholesterol depletion induces transcriptional changes during skeletal muscle differentiation. BMC Genomics. 2014;15:544. doi: 10.1186/1471-2164-15-544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Pot GK, Geelen A, van Heijningen EM, Siezen CL, van Kranen HJ, Kampman E. Opposing associations of serum n-3 and n-6 polyunsaturated fatty acids with colorectal adenoma risk: an endoscopy-based case-control study. Int. J. Cancer. 2008;123:1974–77. doi: 10.1002/ijc.23729. [DOI] [PubMed] [Google Scholar]
- 166.Qi W, Weber CR, Wasland K, Savkovic SD. Genistein inhibits proliferation of colon cancer cells by attenuating a negative effect of epidermal growth factor on tumor suppressor FOXO3 activity. BMC Cancer. 2011;11:219. doi: 10.1186/1471-2407-11-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Qiao EQ, Ji MH, Wu JZ, Ma R, Zhang XH, et al. Expression of the PXR gene in various types of cancer and drug resistance (review) Oncol. Lett. 2013;5:1093–100. doi: 10.3892/ol.2013.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Raghupathy R, Anilkumar AA, Polley A, Singh PP, Yadav M, et al. Transbilayer lipid interactions mediate nanoclustering of lipid-anchored proteins. Cell. 2015;161:581–94. doi: 10.1016/j.cell.2015.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Rao CV. Regulation of COX and LOX by curcumin. Adv. Exp. Med. Biol. 2007;595:213–26. doi: 10.1007/978-0-387-46401-5_9. [DOI] [PubMed] [Google Scholar]
- 170.Ray WA, Stein CM, Daugherty JR, Hall K, Arbogast PG, Griffin MR. COX-2 selective nonsteroidal anti-inflammatory drugs and risk of serious coronary heart disease. Lancet. 2002;360:1071–73. doi: 10.1016/S0140-6736(02)11131-7. [DOI] [PubMed] [Google Scholar]
- 171.Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434:843–50. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- 172.Robbins D, Chen T. Tissue-specific regulation of pregnane X receptor in cancer development and therapy. Cell Biosci. 2014;4:17. doi: 10.1186/2045-3701-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Rose DP, Boyar AP, Wynder EL. International comparisons of mortality rates for cancer of the breast, ovary, prostate, and colon, and per capita food consumption. Cancer. 1986;58:2363–71. doi: 10.1002/1097-0142(19861201)58:11<2363::aid-cncr2820581102>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 174.Rotelli MT, Bocale D, De Fazio M, Ancona P, Scalera I, et al. IN-VITRO evidence for the protective properties of the main components of the Mediterranean diet against colorectal cancer: a systematic review. Surg. Oncol. 2015;24:145–52. doi: 10.1016/j.suronc.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 175.Ruder EH, Thiebaut AC, Thompson FE, Potischman N, Subar AF, et al. Adolescent and mid-life diet: risk of colorectal cancer in the NIH-AARP Diet and Health Study. Am. J. Clin. Nutr. 2011;94:1607–19. doi: 10.3945/ajcn.111.020701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Sampath H, Ntambi JM. Polyunsaturated fatty acid regulation of genes of lipid metabolism. Annu. Rev. Nutr. 2005;25:317–40. doi: 10.1146/annurev.nutr.25.051804.101917. [DOI] [PubMed] [Google Scholar]
- 177.Sanders LM, Henderson CE, Hong MY, Barhoumi R, Burghardt RC, et al. An increase in reactive oxygen species by dietary fish oil coupled with the attenuation of antioxidant defenses by dietary pectin enhances rat colonocyte apoptosis. J. Nutr. 2004;134:3233–38. doi: 10.1093/jn/134.12.3233. [DOI] [PubMed] [Google Scholar]
- 178.Sandhu MS, White IR, McPherson K. Systematic review of the prospective cohort studies on meat consumption and colorectal cancer risk: a meta-analytical approach. Cancer Epidemiol. Biomarkers Prev. 2001;10:439–46. [PubMed] [Google Scholar]
- 179.Sanjoaquin MA, Allen N, Couto E, Roddam AW, Key TJ. Folate intake and colorectal cancer risk: a meta-analytical approach. Int. J. Cancer. 2005;113:825–28. doi: 10.1002/ijc.20648. [DOI] [PubMed] [Google Scholar]
- 180.Sasazuki S, Inoue M, Iwasaki M, Sawada N, Shimazu T, et al. Intake of n-3 and n-6 polyunsaturated fatty acids and development of colorectal cancer by subsite: Japan Public Health Center-based prospective study. Int. J. Cancer. 2011;129:1718–29. doi: 10.1002/ijc.25802. [DOI] [PubMed] [Google Scholar]
- 181.Sato T, Clevers H. SnapShot: growing organoids from stem cells. Cell. 2015;161:e1700–1. doi: 10.1016/j.cell.2015.06.028. [DOI] [PubMed] [Google Scholar]
- 182.Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262–65. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- 183.Scharlau D, Borowicki A, Habermann N, Hofmann T, Klenow S, et al. Mechanisms of primary cancer prevention by butyrate and other products formed during gut flora-mediated fermentation of dietary fibre. Mutat. Res. 2009;682:39–53. doi: 10.1016/j.mrrev.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 184.Schoen RE. Families at risk for colorectal cancer: risk assessment and genetic testing. J. Clin. Gastroenterol. 2000;31:114–20. doi: 10.1097/00004836-200009000-00005. [DOI] [PubMed] [Google Scholar]
- 185.Schonberg SA, Lundemo AG, Fladvad T, Holmgren K, Bremseth H, et al. Closely related colon cancer cell lines display different sensitivity to polyunsaturated fatty acids, accumulate different lipid classes and downregulate sterol regulatory element-binding protein 1. FEBS J. 2006;273:2749–65. doi: 10.1111/j.1742-4658.2006.05292.x. [DOI] [PubMed] [Google Scholar]
- 186.Schwartz S, Friedberg I, Ivanov IV, Davidson LA, Goldsby JS, et al. A metagenomic study of diet-dependent interaction between gut microbiota and host in infants reveals differences in immune response. Genome Biol. 2012;13:r32. doi: 10.1186/gb-2012-13-4-r32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Selhub J. Folate, vitamin B12 and vitamin B6 and one carbon metabolism. J. Nutr. Health Aging. 2002;6:39–42. [PubMed] [Google Scholar]
- 188.Sesink AL, Termont DS, Kleibeuker JH, Van der Meer R. Red meat and colon cancer: the cytotoxic and hyperproliferative effects of dietary heme. Cancer Res. 1999;59:5704–9. [PubMed] [Google Scholar]
- 189.Shah MS, Kim E, Davidson LA, Knight JM, Zoh RS, et al. Comparative effects of diet and carcinogen on microRNA expression in the stem cell niche of the mouse colonic crypt. Biochim. Biophys. Acta. 2016;1862:121–34. doi: 10.1016/j.bbadis.2015.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Shah MS, Schwartz SL, Zhao C, Davidson LA, Zhou B, et al. Integrated microRNA and mRNA expression profiling in a rat colon carcinogenesis model: effect of a chemo-protective diet. Physiol. Genom. 2011;43:640–54. doi: 10.1152/physiolgenomics.00213.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Shao J, Jung C, Liu C, Sheng H. Prostaglandin E2 stimulates the beta-catenin/T cell factor-dependent transcription in colon cancer. J. Biol. Chem. 2005;280:26565–72. doi: 10.1074/jbc.M413056200. [DOI] [PubMed] [Google Scholar]
- 192.Sharma RA, Euden SA, Platton SL, Cooke DN, Shafayat A, et al. Phase I clinical trial of oral curcumin: biomarkers of systemic activity and compliance. Clin. Cancer Res. 2004;10:6847–54. doi: 10.1158/1078-0432.CCR-04-0744. [DOI] [PubMed] [Google Scholar]
- 193.Shen J, Xia W, Khotskaya YB, Huo L, Nakanishi K, et al. EGFR modulates microRNA maturation in response to hypoxia through phosphorylation of AGO2. Nature. 2013;497:383–87. doi: 10.1038/nature12080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Shindou H, Hishikawa D, Harayama T, Eto M, Shimizu T. Generation of membrane diversity by lysophospholipid acyltransferases. J. Biochem. 2013;154:21–28. doi: 10.1093/jb/mvt048. [DOI] [PubMed] [Google Scholar]
- 195.Slaby O, Svoboda M, Fabian P, Smerdova T, Knoflickova D, et al. Altered expression of miR-21, miR-31, miR-143 and miR-145 is related to clinicopathologic features of colorectal cancer. Oncology. 2007;72:397–402. doi: 10.1159/000113489. [DOI] [PubMed] [Google Scholar]
- 196.Slaby O, Svoboda M, Michalek J, Vyzula R. MicroRNAs in colorectal cancer: translation of molecular biology into clinical application. Mol. Cancer. 2009;8:102. doi: 10.1186/1476-4598-8-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Slagsvold JE, Pettersen CH, Storvold GL, Follestad T, Krokan HE, Schonberg SA. DHA alters expression of target proteins of cancer therapy in chemotherapy resistant SW620 colon cancer cells. Nutr. Cancer. 2010;62:611–21. doi: 10.1080/01635580903532366. [DOI] [PubMed] [Google Scholar]
- 198.Sole-Navais P, Cavalle-Busquets P, Fernandez-Ballart JD, Murphy MM. Biochimie. 2016. Early pregnancy B vitamin status, one carbon metabolism, pregnancy outcome and child development. In press. doi: 10.1016/j.biochi.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 199.Sorensen HT, Mellemkjaer L, Blot WJ, Nielsen GL, Steffensen FH, et al. Risk of upper gastrointestinal bleeding associated with use of low-dose aspirin. Am. J. Gastroenterol. 2000;95:2218–24. doi: 10.1111/j.1572-0241.2000.02248.x. [DOI] [PubMed] [Google Scholar]
- 200.Subramaniam D, Ponnurangam S, Ramamoorthy P, Standing D, Battafarano RJ, et al. Curcumin induces cell death in esophageal cancer cells through modulating Notch signaling. PLOS ONE. 2012;7:e30590. doi: 10.1371/journal.pone.0030590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Sugimura T, Wakabayashi K, Nakagama H, Nagao M. Heterocyclic amines: mutagens/carcinogens produced during cooking of meat and fish. Cancer Sci. 2004;95:290–99. doi: 10.1111/j.1349-7006.2004.tb03205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Sun H, Meng X, Han J, Zhang Z, Wang B, et al. Anti-cancer activity of DHA on gastric cancer—an in vitro and in vivo study. Tumour Biol. 2013;34:3791–800. doi: 10.1007/s13277-013-0963-0. [DOI] [PubMed] [Google Scholar]
- 203.Terzic J, Grivennikov S, Karin E, Karin M. Inflammation and colon cancer. Gastroenterology. 2010;138:2101–14. e5. doi: 10.1053/j.gastro.2010.01.058. [DOI] [PubMed] [Google Scholar]
- 204.Theodoratou E, McNeill G, Cetnarskyj R, Farrington SM, Tenesa A, et al. Dietary fatty acids and colorectal cancer: a case-control study. Am. J. Epidemiol. 2007;166:181–95. doi: 10.1093/aje/kwm063. [DOI] [PubMed] [Google Scholar]
- 205.Trichopoulou A, Lagiou P, Kuper H, Trichopoulos D. Cancer and Mediterranean dietary traditions. Cancer Epidemiol. Biomarkers Prev. 2000;9:869–73. [PubMed] [Google Scholar]
- 206.Tricker AR. N-nitroso compounds and man: sources of exposure, endogenous formation and occurrence in body fluids. Eur. J. Cancer Prev. 1997;6:226–68. [PubMed] [Google Scholar]
- 207.Triff K, Kim E, Chapkin RS. Chemoprotective epigenetic mechanisms in a colorectal cancer model: modulation by n-3 PUFA in combination with fermentable fiber. Curr. Pharmacol. Rep. 2015;1:11–20. doi: 10.1007/s40495-014-0005-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Triff K, Konganti K, Gaddis S, Zhou B, Ivanov I, Chapkin RS. Genome-wide analysis of the rat colon reveals proximal-distal differences in histone modifications and proto-oncogene expression. Physiol. Genom. 2013;45:1229–43. doi: 10.1152/physiolgenomics.00136.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Turk HF, Barhoumi R, Chapkin RS. Alteration of EGFR spatiotemporal dynamics suppresses signal transduction. PLOS ONE. 2012;7:e39682. doi: 10.1371/journal.pone.0039682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Turk HF, Kolar SS, Fan YY, Cozby CA, Lupton JR, Chapkin RS. Linoleic acid and butyrate synergize to increase Bcl-2 levels in colonocytes. Int. J. Cancer. 2011;128:63–71. doi: 10.1002/ijc.25323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Vanamala J, Glagolenko A, Yang P, Carroll RJ, Murphy ME, et al. Dietary fish oil and pectin enhance colonocyte apoptosis in part through suppression of PPARδ/PGE2 and elevation of PGE3. Carcinogenesis. 2008;29:790–96. doi: 10.1093/carcin/bgm256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Verhaar MC, Stroes E, Rabelink TJ. Folates and cardiovascular disease. Arterioscler. Thromb. Vasc. Biol. 2002;22:6–13. doi: 10.1161/hq0102.102190. [DOI] [PubMed] [Google Scholar]
- 213.Vijayaraghavalu S, Peetla C, Lu S, Labhasetwar V. Epigenetic modulation of the biophysical properties of drug-resistant cell lipids to restore drug transport and endocytic functions. Mol. Pharm. 2012;9:2730–42. doi: 10.1021/mp300281t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Vollset SE, Clarke R, Lewington S, Ebbing M, Halsey J, et al. Effects of folic acid supplementation on overall and site-specific cancer incidence during the randomised trials: meta-analyses of data on 50,000 individuals. Lancet. 2013;381:1029–36. doi: 10.1016/S0140-6736(12)62001-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Walker AW, Ince J, Duncan SH, Webster LM, Holtrop G, et al. Dominant and diet-responsive groups of bacteria within the human colonic microbiota. ISME J. 2011;5:220–30. doi: 10.1038/ismej.2010.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Wang P, Zou F, Zhang X, Li H, Dulak A, et al. microRNA-21 negatively regulates Cdc25A and cell cycle progression in colon cancer cells. Cancer Res. 2009;69:8157–65. doi: 10.1158/0008-5472.CAN-09-1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Weber C, Erl W, Pietsch A, Danesch U, Weber PC. Docosahexaenoic acid selectively attenuates induction of vascular cell adhesion molecule-1 and subsequent monocytic cell adhesion to human endothelial cells stimulated by tumor necrosis factor-alpha. Arterioscler. Thromb. Vasc. Biol. 1995;15:622–28. doi: 10.1161/01.atv.15.5.622. [DOI] [PubMed] [Google Scholar]
- 218.West NJ, Clark SK, Phillips RK, Hutchinson JM, Leicester RJ, et al. Eicosapentaenoic acid reduces rectal polyp number and size in familial adenomatous polyposis. Gut. 2010;59:918–25. doi: 10.1136/gut.2009.200642. [DOI] [PubMed] [Google Scholar]
- 219.Williams J, Mai CT, Mulinare J, Isenburg J, Flood TJ, et al. Updated estimates of neural tube defects prevented by mandatory folic acid fortification—United States, 1995–2011. MMWR Morb. Mortal. Wkly. Rep. 2015;64:1–5. [PMC free article] [PubMed] [Google Scholar]
- 220.Willis ND, Przyborski SA, Hutchison CJ, Wilson RG. Colonic and colorectal cancer stem cells: progress in the search for putative biomarkers. J. Anat. 2008;213:59–65. doi: 10.1111/j.1469-7580.2008.00917.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Wu K, Platz EA, Willett WC, Fuchs CS, Selhub J, et al. A randomized trial on folic acid supplementation and risk of recurrent colorectal adenoma. Am. J. Clin. Nutr. 2009;90:1623–31. doi: 10.3945/ajcn.2009.28319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Yang CS, Lambert JD, Hou Z, Ju J, Lu G, Hao X. Molecular targets for the cancer preventive activity of tea polyphenols. Mol. Carcinog. 2006;45:431–35. doi: 10.1002/mc.20228. [DOI] [PubMed] [Google Scholar]
- 223.Yang Y, Chaerkady R, Beer MA, Mendell JT, Pandey A. Identification of miR-21 targets in breast cancer cells using a quantitative proteomic approach. Proteomics. 2009;9:1374–84. doi: 10.1002/pmic.200800551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Yi JS, Mun DG, Lee H, Park JS, Lee JW, et al. PTRF/cavin-1 is essential for multidrug resistance in cancer cells. J. Proteome Res. 2013;12:605–14. doi: 10.1021/pr300651m. [DOI] [PubMed] [Google Scholar]
- 225.Young GP, Rose IS, St John DJ. Haem in the gut. I. Fate of haemoproteins and the absorption of haem. J. Gastroenterol. Hepatol. 1989;4:537–45. doi: 10.1111/j.1440-1746.1989.tb00858.x. [DOI] [PubMed] [Google Scholar]
- 226.Yu HN, Zhu J, Pan WS, Shen SR, Shan WG, Das UN. Effects of fish oil with a high content of n-3 polyunsaturated fatty acids on mouse gut microbiota. Arch. Med. Res. 2014;45:195–202. doi: 10.1016/j.arcmed.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 227.Zand H, Rahimipour A, Salimi S, Shafiee SM. Docosahexaenoic acid sensitizes Ramos cells to Gamma-irradiation-induced apoptosis through involvement of PPAR-γ activation and NF-κB suppression. Mol. Cell. Biochem. 2008;317:113–20. doi: 10.1007/s11010-008-9838-x. [DOI] [PubMed] [Google Scholar]
- 228.Zhang X, Zhao XW, Liu DB, Han CZ, Du LL, et al. Lipid levels in serum and cancerous tissues of colorectal cancer patients. World J. Gastroenterol. 2014;20:8646–52. doi: 10.3748/wjg.v20.i26.8646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Zhu Y, Wang PP, Zhao J, Green R, Sun Z, et al. Dietary N-nitroso compounds and risk of colorectal cancer: a case-control study in Newfoundland and Labrador and Ontario, Canada. Br. J. Nutr. 2014;111:1109–17. doi: 10.1017/S0007114513003462. [DOI] [PMC free article] [PubMed] [Google Scholar]