The cerebellum and basal ganglia: a developing story
Martin Bareš 1,2 and Tomáš Kašpárek 1,3
1 Central European Institute of Technology, CEITEC MU, Behavioral and Social Neuroscience Research Group, Masaryk University, Brno, Czech Republic
2 First Department of Neurology, Faculty of Medicine, Masaryk University and St. Anne’s Teaching Hospital, Brno, Czech Republic
3 Department of Psychiatry, Faculty of Medicine, Masaryk University and St. Teaching Hospital, Brno, Czech Republic
Despite isolated studies disproving the role of the basal ganglia (BG) and the cerebellum in timekeeping [Harrington et al., 2004] recent research provides increasing evidence for the involvement of both of these structures in the processing of temporal information [Beudel et al., 2008]. While both the BG and the cerebellum were found to participate in time encoding, most experiments showed that they played different roles, such as encoding short versus long time intervals, dealing with explicit versus implicit timing or addressing timing versus temporal order [Ivry et al., 2004] [Dreher et al., 2002]. Many everyday skills, such as sports and the operation of motor vehicles or machinery require precise timing [Iacoboni, 2001]; neurological disorders that disrupt motor timing lead to dysmetric or inaccurate movements [Jahanshahi et al., 2010] [Bares et al., 2011]. Movements involve changes in muscle length over time, thus motor control and timing are inextricably related [Mauk et al., 2004].
The proceedings of the workshop entitled : „Cerebellum, Basal Ganglia And Cortical Connections Unmasked In Health And Disorder” held in Brno, Czech Republic on October 17th, 2013; synthesize the experimental, preclinical and clinical data suggesting that the cerebellum, BG and their connections play an important role in pathophysiology of various movement disorders (like Parkinson’s disease, atypical parkinsonian syndromes) or neurodevelopmental disorders (like autism and schizophrenia). The contributions from distinguished speakers cover the neuroanatomical research of complex networks, neuroimaging data showing that the cerebellum and BG are connected to a wide range of other central nervous system structures involved in movement control. Especially the cerebellum plays a more complex role in how the brain functions than previously thought [Vogel, 2005].
Cerebellar-basal ganglia communication: physiological evidence of a fast route for interaction
Michaela Loft, Stella Koutsikou, Nadia Cerminara, Richard Apps
School of Physiology and Pharmacology, University of Bristol, UK
The cerebellum is involved in a diverse array of functions, ranging from motor control (for review see [Manto et al., 2012]) to higher cognitive abilities e.g. language [Murdoch, 2010]. Given its uniform cytoarchitecture, it is generally thought that this functional diversity arises predominantly from regional differences in afferent and efferent connections. Inputs to the cerebellum have been described in considerable detail (e.g. [Cerminara et al., 2013]). In particular, climbing fibre afferents, which originate exclusively from the inferior olive, have been shown to be highly topographically organized, with specific olivary subregions providing climbing fibres that terminate in longitudinally arranged cortical zones of Purkinje cells with distinct phenotype [Cerminara et al., 2013]. Some alignment between mossy fibre and climbing fibre projections has also been found (e.g. [Pijpers et al., 2006]). In turn, Purkinje cells located within each zone provide a highly convergent projection to specific regions of the cerebellar and vestibular nuclei that also receive climbing fibre collaterals that terminate in the same zone [Voogd et al., 2013].
By comparison, much less is known about the organisation of cerebellar nuclear outputs to other parts of the CNS. However, a major target is the thalamus. From there, projections are sent to cerebral structures e.g. motor cortex. In turn, the cerebral cortex sends projections to pre-cerebellar nuclei, thereby forming multiple ‘cortico-cerebellar’ loops, reminiscent of cortico-basal ganglia loops (e.g. [Alexander et al., 1986]). Despite these similarities in organization, the cortico-basal ganglia and cortico-cerebellar loops are thought to operate largely independently. This is because basal ganglia and cerebellar outputs terminate in mainly separate thalamic territories [Percheron et al., 1996, Kuramoto et al., 2009, Groenewegen et al., 2004], which subsequently project to different layers of the cerebral cortex [Kuramoto et al., 2009]. However, recent studies have challenged this view, reporting an anatomical connection between the cerebellar dentate nucleus (DN) and the striatum via the centrolateral (CL) thalamus in both rodents and primates [Hoshi et al., 2005, Ichinohe et al., 2001, Ichinohe et al., 2000]. Presently, nothing is known about the functional significance of this connection.
As a first step towards addressing this question, we have investigated the physiology of the pathway, to determine whether it is a reliable route for communication; and whether the cerebellar projection influences all regions of the striatum or only discrete areas. Recordings were made in urethane (1.4mg/kg i.p) and ketamine/xylazine (25mg/kg and 2.5mg/kg i.p) anaesthetised rats (n=6) with the aim of mapping the location of neuronal responses in the striatum evoked by contralateral cerebellar DN stimulation. Evoked field potentials were recorded across the dorsal-ventral extent of the striatum at three medio-lateral co-ordinates relative to midline (2.5mm, 3.5mm and 4.0mm lateral to bregma). Stimulation of DN (single pulses, 0.2ms duration, inter pulse interval 3s, mean current strength 180 μA) evoked a localised field potential in the striatum with a mean onset latency of 4.1 ms and a peak latency of 7.4ms. Onset latencies did not vary with recording position (p= 0.84; Kruskal-Wallis with Dunn’s post-test).
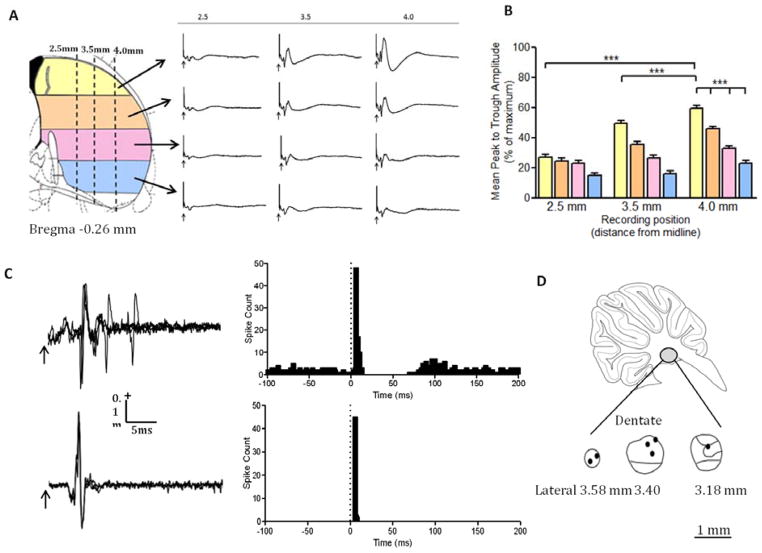
Amplitude of the evoked field potential varied as both a function of medio-lateral and dorsal-ventral recording position (Figure 1A). The evoked field potentials in the dorsolateral striatum were significantly larger than evoked fields recorded in any other striatal region (Figure 1B, p<0.001; Kruskal-Wallis with Dunn’s post-test).
Figure 1.
A) Topography of evoked striatal responses to contralateral dentate (DN) stimulation. Schematic diagram of a transverse section through the striatum (adapted from [Paxinos, 1998]). The striatum was subdivided into 4 regions dorso-ventrally. Evoked potentials recorded in the same subdivision were pooled for analysis. The three medio-lateral recording tracks are shown as dashed vertical lines. Waveform averages of evoked striatal field potentials in a single example case. Waveform averages were generated from 10 consecutive evoked field potentials. The evoked field potentials were largest in the most dorsolateral recording locations, and smallest in more ventromedial recording locations. For each trace an arrow indicates the stimulus onset. B) Changes in field potential amplitude as a function of medio-lateral and dorso-ventral recording position. Bar graph shows pooled mean peak to trough amplitude of evoked striatal fields in each track for all 4 striatal subdivisions (n=6 animals). Peak to trough amplitude was normalised to the largest response for each case. On average the largest response was found in the dorsolateral striatum (dorsal striatum, yellow bars, compared across medio-lateral recording sites i.e. 2.5mm vs. 4.0mm track and 3.5mm vs. 4.0mm track p<0.001; Kruskal-Wallis with Dunn’s post-test, n=6; field amplitudes recorded in the dorsal (yellow) region of the lateral 4.0mm track were compared to increasingly ventral recording positions shown as orange, pink and blue bars respectively p<0.001; Kruskal-Wallis with Dunn’s post-test, n=6). The error bars represent standard error of the mean. C) Single unit striatal responses evoked by contralateral DN stimulation. Left panel shows two examples of evoked single unit activity in the striatum. Each trace is an overlay of 3 consecutive sweeps. Arrow indicates stimulus onset. Right hand panel shows peri-stimulus time histograms (PSTHs) displaying the occurrence of single unit activity after DN stimulation. The PSTHs each represent 50 consecutive sweeps. The stimulus was given at time 0 (indicated by the vertical dotted line). D) Histological verification of the site of DN stimulation. Sagittal cerebellar section (adapted from [Paxinos, 1998]) showing three schematics of the DN at different medio-lateral co-ordinates. The black filled circles indicate the location of the stimulating electrode in the contralateral DN. In all 6 animals the electrode tip was found to be within the anatomical boundaries of the dentate nucleus.
Given the extensive interconnectivity between the cerebellum and the cerebral cortex, and the basal ganglia and the cerebral cortex, there is a possibility that the evoked field potentials were either a far field generated in the cerebral cortex, or genuinely localised in the striatum, but mediated via a dentate-thalamo-cerebral cortical-striatal pathway rather than a more direct dentate-thalamo-striatal projection. To address this issue, recordings were also made from the overlying cerebral cortex. In every experiment the evoked field potentials in the cerebral cortex were smaller in amplitude than those in the dorsal striatum. Moreover, the onset latency of evoked fields in the cerebral cortex was not significantly different from those recorded in the striatum. Taken together these results are therefore consistent with the recorded striatal responses being due to transmission via a subcortical route from cerebellum to striatum (presumably via the thalamus) rather than a product of cerebral cortical activity.
A small sample of single units (n=3) were also recorded in the striatum in response to single pulse stimulation of the contralateral DN. Each unit showed a brief, short-latency excitation in response to DN stimulation with a high probability of occurrence. The latencies of the responses ranged from 5.9–7.6 ms, with a mean latency of 6.7ms (Figure 1C).
In conclusion these data reveal that a powerful, short latency pathway connects the DN with the dorsolateral striatum in the rat. The dorsolateral striatum is known to be involved in habit formation [Yin et al., 2004], raising the possibility that the cerebellum plays an important role in modifying basal ganglia activity associated with habitual behaviour.
Acknowledgements
This work was supported by the MRC (grant G1100626 and a studentship to ML) and the BBSRC (Grant BB/G012717/1).
Diffusion Tensor Imaging: a Noninvasive Probe into Normal and Abnormal Brain White Matter
Zora Kikinis
Harvard Medical School, Boston, MA, U.S.A.
Magnetic Resonance Diffusion Tensor Imaging (DTI) is a method to visualize brain white matter in humans in a non-invasive way. DTI has been applied to detect structural changes of white matter in healthy subjects from childhood to adulthood [Lebel et al., 2011] and in patients with several diseases, including schizophrenia [White et al., 2008]. Due to the non-invasive feature of the method, changes in white matter might be followed over the course of the disease or in some instances even prior to symptom onset. This is of special interest to diseases such as schizophrenia, where changes in white matter prior to symptom onset have been reported, and suggested to reflect increased risk to develop psychosis.
To characterize structural abnormalities of white matter one has to reflect upon two features of those changes; namely their location, and their biological nature. The biological nature and location can be explored using DTI. The DTI method is sensitive to subtle changes in the diffusion properties of water molecules in tissue and brain white matter pathologies are revealed by changes in DTI measures such as Fractional Anisotropy (FA, which describes directionality of the diffusion), Mean Diffusivity (MD, average diffusion in all directions), axial diffusivity (AD, diffusion along the direction of the axon) and radial diffusion (RD, diffusion perpendicular to the axon). Examples of changes of biological nature are abnormalities in axonal myelination, inflammatory-like proceseses or abnormal development of white matter and were observed at different stages of schizophrenia. DTI studies comparing schizophrenia patients and control subjects typically report, in the chronic stage of disease, that the FA value decreases and RD increases, which is thought to reflect abnormalities in the microstructure of the axon and is interpreted as abnormalities of the myelin sheath [Seal et al., 2008, Song et al., 2002]. It has been recently speculated that at the time of first episode of psychosis, decreases in FA and increases in MD might point to a different scenario, to an acute and reversible inflammatory process [Pasternak et al., 2012]. Prior to the onset of psychosis, white matter changes have been also reported in subjects with increased clinical [Hohenberg et al., 2014], as well as genetic risk for schizophrenia. The genetic risk has been explored in siblings of schizophrenics, as well as in subjects with 22q11.2 deletion syndrome that have a 30% incidence of schizophrenia in adulthood. The later population has been characterized by reductions in FA and in AD, which presumably reflect changes of white matter, which might be interpreted as developmental abnormalities at the level of the axon [Kikinis et al., 2012, Radoeva et al., 2012]. The nature of these DTI abnormalities (lack of either RD or MD changes) suggests intact myelin and lack of neuroinflammation at this stage.
The location of the disease specific changes might be explored either by comparing whole brain white matter (using Voxel Based Morphometry, VBM, or Tract-Based Spatial Statistics, TBSS) or by performing tractography to reconstruct specific white matter tracts from DTI data. In schizophrenia the localization of the changes has not been established yet despite close to two-hundred of DTI studies being performed so far. Although a meta-analysis reports mostly changes to fronto-temporal connections [Ellison-Wright et al., 2009], other summaries of DTI studies favor the view that changes are scattered all over the brain [Melonakos et al., 2011, White et al., 2013]. The lack of the consensus of the localization is unexpected at the first glance, but might be explained by the heterogeneity of clinical presentations of patient group in each study. Schizophrenia is diagnosed by a number of symptoms, like hallucinations, delusions, disorganized speech or negative symptoms and may therefore comprise disorders with different trajectories. Thus, the data set of each study is as heterogeneous as the patients’ symptoms and the diverse areas impacted. Indeed, studies dividing schizophrenia patients into subgroups based either on symptoms, genetics, Research Domain Criteria (RDoc), hallucinations or movement deficits [Huttlova et al., 2014] are promising approaches to explore whether in a homogenous patient population the symptoms will be associated with changes localized to specific white matter tracts.
Is DTI a suitable method to explore white matter connections between the cerebellum, the basal ganglia and the cortex? Images acquired on scanners with magnetic fields of 1.5 and 3 Tesla allow the reconstruction of about 17 fiber tracts [Wakana et al., 2004], including three cerebellar tracts, such as the superior cerebellar peduncle (scp), the middle cerebellar peduncle (mcp) and the inferior cerebellar peduncle (icp) (Figure 2), but none of the specific tracts of basal ganglia. Connections to and from the basal ganglia are extensive and due to the small size of the subnuclei (few mm in diameter) the reconstruction of the individual tracts requires image resolution higher than 1mm × 1mm × 1mm, a better resolution than most of the DTI acquisitions using scanners of 3 Tesla can offer today. Sophisticated image post-processing methods such as multi-tensor tractography, as opposed to the most commonly used streamline tractography, were applied to explore corticostriatal connections on 3 Tesla data [Quan et al., 2013], but so far only few studies were successful.
Figure 2. Cerebellar tracts.
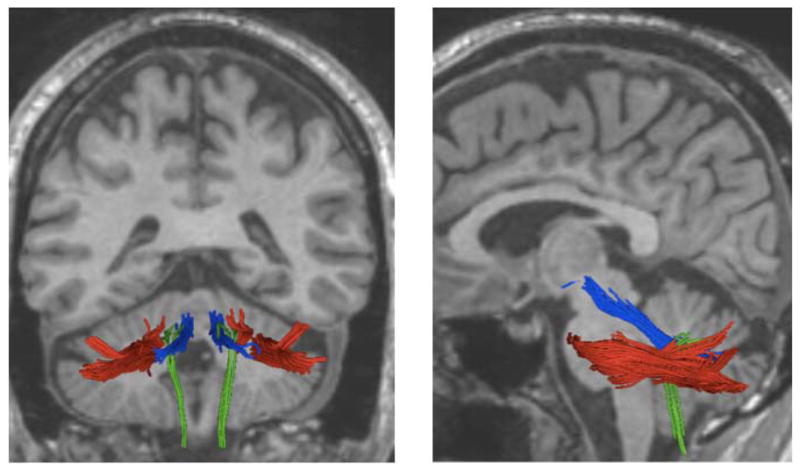
Middle cerebellar peduncle (mcp, colored in red), superior cerebellar peduncle (scp, in blue) and inferior cerebellar peduncle (icp, in green) were reconstructed from DTI images. Structural MRI image of the brain is in the background (black and white). Posterior view of the brain in the panel left, side view of the brain in the panel on the right.
Are connections between the cerebellum, basal ganglia and neocortex abnormal in schizophrenia? It is very likely that they are: abnormal corticostriatal connections [Quan et al., 2013] and abnormal connectivity in cerebellar tracts, structural as well as functional, were reported in schizophrenia patients [Huttlova et al., 2014, Kasparek et al., 2012, Liu et al., 2011]. So far, only few DTI studies have addressed these connections. One reason is that most of the schizophrenia studies have focused on cortical connections and not cerebellum. The second reason, as mentioned above, is that reconstruction of these specific connections in vivo poses technical challenges so far. Advances in imaging methods, either scanning at higher magnetics fields (7 Tesla) or improvement of acquisition techniques and image post-processing methods will allow the reconstruction of these tracts in the very near future.
Acknowledgements
The preparation of the manuscript was supported by Veterans Administration Merit Review and NIH/NIHM Subaward to 5R01MH64824
Imaging of the cerebellar nuclei in health and disease
Dagmar Timmann
Department of Neurology, University Clinic Essen, University of Duisburg-Essen, Hufelandstrasse 55, 45147 Essen, Germany
Deep cerebellar nuclei have rarely been assessed in human studies. Recent studies of our group focused on structural and functional magnetic resonance imaging (MRI) of the dentate nuclei in humans. Iron-content of the dentate nuclei is high. The paramagnetic effect of iron is used to visualize the dentate nuclei as hypointensities in susceptibility weighted images (SWI) [Diedrichsen et al., 2011]. One future application is to quantify the volume of cerebellar nuclei in patients with degenerative cerebellar disease. This is of particular interest in disorders, which have, based on histological data, atrophy of the cerebellar nuclei. The best known examples are spinocerebellar ataxia type 3 (SCA3) and Friedreich’s ataxia (FRDA) [Koeppen et al., 2013]. In our study on patients with FRDA, we were able to show atrophy of cerebellar nuclei using SWI images [Rabe et al., Submitted]. Because there is no or little degeneration of the cerebellar cortex, standard diagnostic MRI scans frequently show no cerebellar abnormality in FRDA.
Another application of SWI imaging is to determine the relationship between behavioral abnormalities and the location of lesions of the cerebellar nuclei. In patients with focal cerebellar lesions, for example due to stroke, SWI allows to perform lesion-symptom mapping on the level of the dentate nuclei. Here, lesions are outlined within the dentate nuclei [Maderwald et al., 2012]. The same region-of-interest-(ROI)-driven normalization technique is used to perform lesion-symptom-mapping, which had initially been developed by Diedrichsen et al. [Diedrichsen et al., 2009] for functional MRI of the dentate nuclei. In our initial study, we were able to show that more dorsal and rostral parts of the dentate nuclei were related to upper limb ataxia [Maderwald et al., 2012]. Findings are in good accordance with the dentate hand area shown in recent fMRI studies of our group and anatomical data in monkey discussed below. As yet, in most human cerebellar lesion studies high resolution T1-weighted MR images are available, but not SWI. Cerebellar nuclei are commonly not visible on T1-weighted images. However, some conclusions can still be drawn on the level of the cerebellar nuclei. Lesions are drawn on the T1-weighted images and include cerebellar cortex and nuclei to various extents. Normalized lesions are superimposed on cerebellar atlas templates, which include the cerebellar nuclei, for example the most recent version of the probabilistic atlas template of the cerebellum developed by Joern Diedrichsen [Diedrichsen et al., 2009, Diedrichsen et al., 2011]. Using this approach Winfried Ilg’s and our group were able to show that lesions of the posterolateral hemispheres and the dentate nuclei lead to abnormal treatmill tandem gait. Lesions of the medial cerebellum and interposed nuclei, on the other hand, are related to abnormal treatmill walking [Ilg et al., 2013].
On a structural level, dentate nuclei can be visualized using conventional (1.5T, 3T) and ultra-high-field (7T) MRI. Increasing field strength leads to better spatial resolution [Maderwald et al., 2012]. On a functional level, it is difficult to achieve robust activations of the dentate nuclei using conventional field strength. Ultra-high-field MRI together with optimized region-of-interest (ROI)-based normalization methods allow for reliable functional MRI studies at the level of the dentate nuclei [Diedrichsen et al., 2011]. We found evidence of a motor somatotopy within the human dentate nuclei [Küper et al., 2011]. In subsequent studies we were able to show that different areas within the dentate nuclei contribute to motor and cognitive tasks (verb generation and verbal working memory) [Thürling et al., 2012, Thürling et al., 2011]. Findings are consistent with Peter Strick’s anatomical data in the monkey. His group observed a motor domain within the dorso-rostral parts of the dentate nucleus and a non-motor domain within its ventro-caudal parts [Strick et al., 2009]. In an ongoing study we investigate different patterns of activations within the cerebellar cortex and nuclei in spinocerebellar ataxia type 6 (SCA6), which is thought to primarily the cerebellar cortex, and SCA3 and FRDA, which affect primarily the cerebellar nuclei [Koeppen et al., 2013].
In summary, SWI imaging allows to visualize dentate nuclei on individual scans. This has the diagnostic potential to reveal atrophy of the cerebellar nuclei in patients with cerebellar degeneration. Furthermore, lesion-symptom mapping is possible on the level of subdivisions of the dentate nuclei. Ultra-high-field MRI makes reliable fMRI studies of the dentate nuclei possible. In current studies we try to extend fMRI studies to the level of interposed nuclei using an eyeblink conditioning paradigm. Ultra-high field fMRI will be a helpful tool to understand the interactions between the cerebellar cortex and nuclei in future studies in healthy subjects and patients with cerebellar disease.
Acknowledgements
Studies were supported by the DFG (DFG TI 239/10-1, 10-2) and EU (Marie Curie Initial Training Network, ITN).
High Field Magnetic Resonance Spectroscopy in Movement Disorders: Biomarker and Surrogate Marker Potential
Gülin Öz
Center for Magnetic Resonance Research, Department of Radiology
University of Minnesota, Minneapolis, MN, U.S.A.
Proton magnetic resonance spectroscopy (1H MRS) enables the non-invasive quantification of a multitude of neurochemicals in selected brain regions at high and ultra-high fields [Emir, Auerbach, et al., 2012, Oz, 2013]. The neurochemicals that can be reliably quantified at 3 tesla (T) and higher fields include neurotransmitters, such as glutamate and γ-aminobutyric acid (GABA), antioxidants glutathione and ascorbate, and other important metabolites such as glutamine, in addition to those that are reliably quantified at 1.5 T, namely N-acetylaspartate (NAA), creatine, choline and, depending on acquisition parameters, myo-inositol and lactate. These “neurochemical profiles” are characteristic of brain regions [Emir, Auerbach, et al., 2012] and may provide markers of onset, progression and reversal of pathology in affected brain regions in many neurological diseases [Oz et al., 2014] including movement disorders. The need for such non-invasive biomarkers of cerebral and cerebellar disease is particularly urgent in the area of pre-clinical and clinical trials for neurodegenerative diseases because they are slowly progressive, show substantial phenotypic variability and therefore typically necessitate long clinical trials with large sample sizes. In addition, clinical outcome measures that are routinely used in such trials do not distinguish symptomatic from disease-modifying effects of drugs.
A number of recent studies focused on validating such in vivo markers of neurodegeneration in spinocerebellar ataxias (SCAs), hereditary movement disorders that cause atrophy and dysfunction of the cerebellum and in many cases also the brainstem. An ability to detect neurochemical alterations in SCA1, even in spectra from individual patients, was demonstrated first at 4T [Oz, Hutter, et al., 2010] and recently also at the widely available 3T platform [Emir, Hutter, et al., 2012]. In addition, significant correlations between the levels of select neurochemicals, namely the neuronal marker NAA, the putative gliosis marker myo-inositol and the neurotransmitter glutamate, and scores on the validated Scale for the Assessment and Rating of Ataxia (SARA) were observed [Oz, Hutter, et al., 2010], demonstrating that the neurochemical levels reflect clinical status in SCA1. Furthermore, MRS has shown potential to distinguish different SCA genotypes with similar clinical presentation [Oz, Iltis, et al., 2011].
Parallel studies with mouse models of SCA1 demonstrated that neurochemical levels measured by 1H MRS are sensitive to pre-symptomatic and progressive pathological changes in the cerebellum [Oz, Nelson, et al., 2010]. Interestingly, the same neurochemicals that reflected the clinical status in patients with SCA1 (NAA, myo-inositol, glutamate) were also those that showed the strongest correlations with semi-quantitative pathology measures in the mouse model, indicating these as robust markers of the neurodegenerative process [Oz, Nelson, et al., 2010]. Later, studies with a conditional transgenic SCA1 model demonstrated the sensitivity of 1H MRS measured neurochemical levels to disease reversal. Namely, upon suppression of transgene expression in this model, both the cerebellar pathology and abnormal neurochemical levels returned towards normal [Oz, Vollmers, et al., 2011]. Finally, studies with a knock-in SCA1 model that displays milder cerebellar pathology than the transgenic line used in prior studies showed that neurochemical alterations can be detected prior to overt pathology in SCA1 [Emir et al., 2013]. To summarize, 1) 1H MRS detects parallel neurochemical alterations in patients with SCA1 and SCA1 mouse models, 2) the same neurochemicals (NAA, myo-inositol, glutamate) reflect clinical status and pathological progression in SCA1, 3) neurochemical alterations are detectable prior to ataxia onset and overt pathology, and 4) reversal of neurochemical alterations with treatment is detectable by MRS.
The utility of MRS markers to detect neurochemical alterations and treatment response was also shown in Parkinson’s disease (PD), the most common movement disorder. Early involvement of caudal brainstem in PD, even prior to the characteristic degeneration of nigrostriatal dopaminergic neurons, was suggested about 10 years ago based on detailed pathological investigations [Braak et al., 2003] and has been gaining wide acceptance. Motivated by this work, neurochemical alterations in the brainstem and striatum in early-moderate stage PD were recently investigated at the ultra-high field of 7T. This study uncovered an elevation in the inhibitory neurotransmitter GABA in the pons [Emir, Tuite, et al., 2012], in addition to elevated striatal GABA levels previously shown in postmortem investigations [Kish et al., 1986]. A more pronounced GABA elevation in the pons than in the putamen was consistent with an earlier involvement of the lower brainstem in the disease pathology. Therefore, the altered GABAergic tone demonstrated by MRS in the lower brainstem and striatum in early-moderate PD may underlie disease pathogenesis and may provide a biomarker for disease staging. Another recent study focused on a potentially useful antioxidant therapy in PD. Reductions in the levels of the major antioxidant glutathione (GSH) were reported in PD [Sian et al., 1994], indicating the involvement of oxidative stress in the pathophysiology of the disease. In a pilot investigation, ultra-high field MRS was used to monitor increases in brain GSH levels in patients with PD and healthy controls in response to a single, intravenous administration of N-acetylcysteine, a known precursor to GSH [Holmay et al., 2013]. This study demonstrated the potential utility of MRS for monitoring treatment response by noninvasively measuring antioxidant levels in the brain.
The importance of spectral quality for the robustness of the above findings and reliable neurochemical quantification cannot be overemphasized. Thus, in all the above cited studies, in house developed, state-of-the art MRS methods were utilized. The critical need for standardization of MRS methodology for robust clinical applications was recently emphasized and the feasibility of implementing advanced MRS methods on clinical scanners was demonstrated in a multi-site setting in a recent consensus effort [Oz et al., 2014].
Acknowledgements
The preparation of this manuscript was supported by the National Institute of Neurological Disorders and Stroke (NINDS) grant R01 NS070815 (GÖ). The Center for Magnetic Resonance Research at the University of Minnesota is supported by National Center for Research Resources (NCRR) biotechnology research resource grant P41 RR008079, National Institute of Biomedical Imaging and Bioengineering (NIBIB) grant P41 EB015894 and the Institutional Center Cores for Advanced Neuroimaging award P30 NS076408.
Autism, Neural Timing, and the Cerebellum
James Ashe1,2,3 and Khalaf Bushara1,2,3
1 Department of Neuroscience, University of Minnesota, Minneapolis, MN, U.S.A.
2 Department of Neurology, University of Minnesota, Minneapolis, MN, U.S.A.
3 Neurology Service, VA Medical Center, Minneapolis, MN, U.S.A.
Autism is a severe developmental disorder characterized by abnormal social interaction, impaired language use and development, stereotypical repetitive movements, and resistance to change. It is remarkable that there is as yet no consensus on the neurological basis of this disorder first described in 1942 but now affecting more than 1 in every 100 children in the United States and in Europe. Although abnormalities have been documented in many different regions of the brain in those who died with this disorder, the most common abnormality has been a 30–40% reduction in the population of Purkinje cells in lobules VI and VII of the cerebellum. It is not immediately obvious based on the conventional understanding of cerebellar physiology how disrupting its function might lead to the types of behavioral disturbances in autism. Welsh and colleagues [Welsh et al., 2005] have provided a possible solution to this dilemma by proposing that disruption of a timing signal within the olivo-cerebellar circuit in infancy might lead to abnormal development of motor, cognitive and emotional processing [Schmahmann, 2004].
There is compelling evidence from experiments in vertebrate animals over a period of 40 years that cells in the inferior olive provide a rhythmic, synchronous output signal of 8–13 Hz that is conveyed via climbing fibres to the Purkinje cells to produce complex spikes. Despite these data there has been skepticism about the role and importance of a rhythmic signal generated in the inferior olive primarily because the few experiments that had addressed the issue in non-human primates have been either inconclusive or unsupportive depending on one’s perspective. We have recently demonstrated using functional neuroimaging in human subjects that the inferior olive is activated during the perception of the temporal [Xu et al., 2006] but not the non-temporal features [Liu et al., 2008] of changing visual stimuli. Furthermore, the sensitivity of the inferior olive to changes in stimulus timing is independent of awareness [Wu et al., 2011], which suggests that this structure may contribute to the temporal properties that underlie classical conditioning and implicit learning.
We believe that lesions of the olivo-cerebellum either prenatally or in early infancy may disrupt the neural processing of temporal information and lead to the abnormalities of motor, cognitive and emotional behavior in autism. Our next step in exploring this hypothesis is to attempt to reproduce in mice some of the behavioral abnormalities characteristic of autism by genetically engineering mice to produce focal ablations of populations of Purkinje cells in utero.
Acknowledgments
This workshop was supported by the project “CEITEC - Central European Institute of Technology” (CZ.1.05/1.1.00/02.0068) from European Regional Development Fund and by Ministry of Health of the Czech Republic / Ministry of Health’s Departmental Research and Development Program III (2010–2015) NT/13437.
References
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–81. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Bares M, Lungu OV, Liu T, Waechter T, Gomez CM, Ashe J. The neural substrate of predictive motor timing in spinocerebellar ataxia. Cerebellum. 2011;10(2):233–44. doi: 10.1007/s12311-010-0237-y. [DOI] [PubMed] [Google Scholar]
- Beudel M, Galama S, Leenders KL, de Jong BM. Time estimation in Parkinson’s disease and degenerative cerebellar disease. Neuroreport. 2008;19(10):1055–8. doi: 10.1097/WNR.0b013e328303b7b9. [DOI] [PubMed] [Google Scholar]
- Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24(2):197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- Cerminara NL, Aoki H, Loft M, Sugihara I, Apps R. Structural basis of cerebellar microcircuits in the rat. J Neurosci. 2013;33(42):16427–42. doi: 10.1523/JNEUROSCI.0861-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diedrichsen J, Maderwald S, Küper M, Thürling M, Rabe K, Gizewski ER, Ladd ME, Timmann D. Imaging the deep cerebellar nuclei: A probabilistic atlas and normalization procedure. NeuroImage. 2011;54(3):1786–1794. doi: 10.1016/j.neuroimage.2010.10.035. [DOI] [PubMed] [Google Scholar]
- Diedrichsen Jörn, Balsters Joshua H, Flavell Jonathan, Cussans Emma, Ramnani Narender. A probabilistic MR atlas of the human cerebellum. NeuroImage. 2009;46(1):39–46. doi: 10.1016/j.neuroimage.2009.01.045. [DOI] [PubMed] [Google Scholar]
- Dreher JC, Grafman J. The roles of the cerebellum and basal ganglia in timing and error prediction. Eur J Neurosci. 2002;16(8):1609–19. doi: 10.1046/j.1460-9568.2002.02212.x. [DOI] [PubMed] [Google Scholar]
- Ellison-Wright I, Bullmore E. Meta-analysis of diffusion tensor imaging studies in schizophrenia. Schizophr Res. 2009;108(1–3):3–10. doi: 10.1016/j.schres.2008.11.021. [DOI] [PubMed] [Google Scholar]
- Emir UE, Auerbach EJ, Moortele PF, Marjanska M, Ugurbil K, Terpstra M, Tkac I, Oz G. Regional neurochemical profiles in the human brain measured by 1H MRS at 7 T using local B1 shimming. NMR Biomed. 2012;25(1):152–60. doi: 10.1002/nbm.1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emir UE, Brent Clark H, Vollmers ML, Eberly LE, Oz G. Non-invasive detection of neurochemical changes prior to overt pathology in a mouse model of spinocerebellar ataxia type 1. J Neurochem. 2013;127(5):660–668. doi: 10.1111/jnc.12435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emir UE, Hutter D, Bushara KO, Gomez CM, Eberly LE, Oz G. MRS Biomarkers of Neurodegeneration in Spinocerebellar Ataxia type 1 (SCA1): Current and Future Potential. 20th Scientific Meeting of the ISMRM; Melbourne, Australia. 2012. [Google Scholar]
- Emir UE, Tuite PJ, Oz G. Elevated pontine and putamenal GABA levels in mild-moderate Parkinson disease detected by 7 tesla proton MRS. PLoS One. 2012;7(1):e30918. doi: 10.1371/journal.pone.0030918. PONE-D-11-17333 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenewegen HJ, Witter MP. Thalamus. In: Paxinos G, editor. The rat nervous system. San Diego (CA): Elsevier; 2004. pp. 407–453. [Google Scholar]
- Harrington DL, Lee RR, Boyd LA, Rapcsak SZ, Knight RT. Does the representation of time depend on the cerebellum? Effect of cerebellar stroke. Brain. 2004;127(Pt 3):561–74. doi: 10.1093/brain/awh065. [DOI] [PubMed] [Google Scholar]
- von Hohenberg Clemm, Pasternak O, Kubicki M, Ballinger T, Vu MA, Swisher T, Green K, Giwerc M, Dahlben B, Goldstein JM, Woo TU, Petryshen TL, Mesholam-Gately RI, Woodberry KA, Thermenos HW, Mulert C, McCarley RW, Seidman LJ, Shenton ME. White matter microstructure in individuals at clinical high risk of psychosis: a whole-brain diffusion tensor imaging study. Schizophr Bull. 2014;40(4):895–903. doi: 10.1093/schbul/sbt079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmay MJ, Terpstra M, Coles LD, Mishra U, Ahlskog M, Oz G, Cloyd JC, Tuite PJ. N-acetylcysteine Boosts Brain and Blood Glutathione in Gaucher and Parkinson Diseases. Clin Neuropharmacol. 2013;36(4):103–6. doi: 10.1097/WNF.0b013e31829ae713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshi E, Tremblay L, Feger J, Carras PL, Strick PL. The cerebellum communicates with the basal ganglia. Nat Neurosci. 2005;8(11):1491–3. doi: 10.1038/nn1544. [DOI] [PubMed] [Google Scholar]
- Huttlova J, Kikinis Z, Kerkovsky M, Bouix S, Vu MA, Makris N, Shenton M, Kasparek T. Abnormalities in myelination of the superior cerebellar peduncle in patients with schizophrenia and deficits in movement sequencing. Cerebellum. 2014;13(4):415–24. doi: 10.1007/s12311-014-0550-y. [DOI] [PubMed] [Google Scholar]
- Iacoboni M. Playing tennis with the cerebellum. Nat Neurosci. 2001;4(6):555–6. doi: 10.1038/88365. [DOI] [PubMed] [Google Scholar]
- Ichinohe N, Iwatsuki H, Shoumura K. Intrastriatal targets of projection fibers from the central lateral nucleus of the rat thalamus. Neurosci Lett. 2001;302(2–3):105–8. doi: 10.1016/s0304-3940(01)01666-4. [DOI] [PubMed] [Google Scholar]
- Ichinohe N, Mori F, Shoumura K. A di-synaptic projection from the lateral cerebellar nucleus to the laterodorsal part of the striatum via the central lateral nucleus of the thalamus in the rat. Brain Res. 2000;880(1–2):191–7. doi: 10.1016/s0006-8993(00)02744-x. [DOI] [PubMed] [Google Scholar]
- Ilg W, Christensen A, Mueller OM, Goericke SL, Giese MA, Timmann D. Effects of cerebellar lesions on working memory interacting with motor tasks of different complexities. Journal of Neurophysiology. 2013;110(10):2337–2349. doi: 10.1152/jn.00062.2013. [DOI] [PubMed] [Google Scholar]
- Ivry RB, Spencer RM. The neural representation of time. Curr Opin Neurobiol. 2004;14(2):225–32. doi: 10.1016/j.conb.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Jahanshahi M, Jones CR, Zijlmans J, Katzenschlager R, Lee L, Quinn N, Frith CD, Lees AJ. Dopaminergic modulation of striato-frontal connectivity during motor timing in Parkinson’s disease. Brain. 2010;133(Pt 3):727–45. doi: 10.1093/brain/awq012. [DOI] [PubMed] [Google Scholar]
- Kasparek T, Rehulova J, Kerkovsky M, Sprlakova A, Mechl M, Mikl M. Cortico-cerebellar functional connectivity and sequencing of movements in schizophrenia. BMC Psychiatry. 2012;12:17. doi: 10.1186/1471-244X-12-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikinis Z, Asami T, Bouix S, Finn CT, Ballinger T, Tworog-Dube E, Kucherlapati R, Kikinis R, Shenton ME, Kubicki M. Reduced fractional anisotropy and axial diffusivity in white matter in 22q11.2 deletion syndrome: a pilot study. Schizophr Res. 2012;141(1):35–9. doi: 10.1016/j.schres.2012.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kish SJ, Rajput A, Gilbert J, Rozdilsky B, Chang LJ, Shannak K, Hornykiewicz O. Elevated gamma-aminobutyric acid level in striatal but not extrastriatal brain regions in Parkinson’s disease: correlation with striatal dopamine loss. Ann Neurol. 1986;20(1):26–31. doi: 10.1002/ana.410200106. [DOI] [PubMed] [Google Scholar]
- Koeppen Arnulf H, Liane Ramirez R, Bjork Sarah T, Bauer Peter, Feustel Paul J. The Reciprocal Cerebellar Circuitry in Human Hereditary Ataxia. Cerebellum. 2013;12(4):493–503. doi: 10.1007/s12311-013-0456-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Küper Michael, Thürling Markus, Stefanescu Roxana, Maderwald Stefan, Roths Johannes, Elles Hans G, Ladd Mark E, Diedrichsen Jörn, Timmann Dagmar. Evidence for a motor somatotopy in the cerebellar dentate nucleus-An FMRI study in humans. Human Brain Mapping. 2011;33(11):2741–2749. doi: 10.1002/hbm.21400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuramoto Eriko, Furuta Takahiro, Nakamura Kouichi C, Unzai Tomo, Hioki Hiroyuki, Kaneko Takeshi. Two Types of Thalamocortical Projections from the Motor Thalamic Nuclei of the Rat: A Single Neuron-Tracing Study Using Viral Vectors. Cerebral Cortex. 2009;19(9):2065–2077. doi: 10.1093/cercor/bhn231. [DOI] [PubMed] [Google Scholar]
- Lebel C, Beaulieu C. Longitudinal development of human brain wiring continues from childhood into adulthood. J Neurosci. 2011;31(30):10937–47. doi: 10.1523/JNEUROSCI.5302-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Fan G, Xu K, Wang F. Changes in cerebellar functional connectivity and anatomical connectivity in schizophrenia: a combined resting-state functional MRI and diffusion tensor imaging study. J Magn Reson Imaging. 2011;34(6):1430–8. doi: 10.1002/jmri.22784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Xu D, Ashe J, Bushara K. Specificity of inferior olive response to stimulus timing. J Neurophysiol. 2008;100(3):1557–61. doi: 10.1152/jn.00961.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maderwald S, Thürling M, Küper M, Theysohn N, Müller O, Beck A, Aurich V, Ladd ME, Timmann D. Direct visualization of cerebellar nuclei in patients with focal cerebellar lesions and its application for lesion-symptom mapping. NeuroImage. 2012;63(3):1421–1431. doi: 10.1016/j.neuroimage.2012.07.063. [DOI] [PubMed] [Google Scholar]
- Manto Mario, Bower James M, Conforto AdrianaBastos, Delgado-García José M, Guarda SuzeteNascimentoFarias, Gerwig Marcus, Habas Christophe, Hagura Nobuhiro, Ivry RichardB, Mariën Peter, Molinari Marco, Naito Eiichi, Nowak Dennis A, Taib Nordeyn Oulad Ben, Pelisson Denis, Tesche Claudia D, Tilikete Caroline, Timmann Dagmar. Consensus Paper: Roles of the Cerebellum in Motor Control--The Diversity of Ideas on Cerebellar Involvement in Movement. The Cerebellum. 2012;11(2):457–487. doi: 10.1007/s12311-011-0331-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauk MD, Buonomano DV. The neural basis of temporal processing. Annu Rev Neurosci. 2004;27:307–40. doi: 10.1146/annurev.neuro.27.070203.144247. [DOI] [PubMed] [Google Scholar]
- Melonakos ED, Shenton ME, Rathi Y, Terry DP, Bouix S, Kubicki M. Voxel-based morphometry (VBM) studies in schizophrenia-can white matter changes be reliably detected with VBM? Psychiatry Res. 2011;193(2):65–70. doi: 10.1016/j.pscychresns.2011.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdoch Bruce E. The cerebellum and language: Historical perspective and review. Cortex. 2010;46(7):858–868. doi: 10.1016/j.cortex.2009.07.018. http://dx.doi.org/10.1016/j.cortex.2009.07.018. [DOI] [PubMed] [Google Scholar]
- Oz G. MR Spectroscopy in Health and Disease. In: Manto M, Gruol DL, Schmahmann JD, Koibuchi N, Rossi F, editors. Handbook of the Cerebellum and Cerebellar Disorders. Dordrecht: Springer; 2013. pp. 713–733. [Google Scholar]
- Oz G, Alger JR, Barker PB, Bartha R, Bizzi A, Boesch C, Bolan PJ, Brindle KM, Cudalbu C, Dincer A, Dydak U, Emir UE, Frahm J, Gonzalez RG, Gruber S, Gruetter R, Gupta RK, Heerschap A, Henning A, Hetherington HP, Howe FA, Huppi PS, Hurd RE, Kantarci K, Klomp DW, Kreis R, Kruiskamp MJ, Leach MO, Lin AP, Luijten PR, Marjanska M, Maudsley AA, Meyerhoff DJ, Mountford CE, Nelson SJ, Pamir MN, Pan JW, Peet AC, Poptani H, Posse S, Pouwels PJ, Ratai EM, Ross BD, Scheenen TW, Schuster C, Smith IC, Soher BJ, Tkac I, Vigneron DB, Kauppinen RA M. R. S. Consensus Group. Clinical proton MR spectroscopy in central nervous system disorders. Radiology. 2014;270(3):658–79. doi: 10.1148/radiol.13130531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oz G, Hutter D, Tkac I, Clark HB, Gross MD, Jiang H, Eberly LE, Bushara KO, Gomez CM. Neurochemical alterations in spinocerebellar ataxia type 1 and their correlations with clinical status. Mov Disord. 2010;25(9):1253–1261. doi: 10.1002/mds.23067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oz G, Iltis I, Hutter D, Thomas W, Bushara KO, Gomez CM. Distinct Neurochemical Profiles of Spinocerebellar Ataxias 1, 2, 6, and Cerebellar Multiple System Atrophy. Cerebellum. 2011;10(2):208–17. doi: 10.1007/s12311-010-0213-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oz G, Nelson CD, Koski DM, Henry PG, Marjanska M, Deelchand DK, Shanley R, Eberly LE, Orr HT, Clark HB. Noninvasive detection of presymptomatic and progressive neurodegeneration in a mouse model of spinocerebellar ataxia type 1. J Neurosci. 2010;30(10):3831–8. doi: 10.1523/JNEUROSCI.5612-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oz G, Vollmers ML, Nelson CD, Shanley R, Eberly LE, Orr HT, Clark HB. In vivo monitoring of recovery from neurodegeneration in conditional transgenic SCA1 mice. Exp Neurol. 2011;232(2):290–8. doi: 10.1016/j.expneurol.2011.09.021. S0014-4886(11)00329-3 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasternak O, Westin CF, Bouix S, Seidman LJ, Goldstein JM, Woo TU, Petryshen TL, Mesholam-Gately RI, McCarley RW, Kikinis R, Shenton ME, Kubicki M. Excessive extracellular volume reveals a neurodegenerative pattern in schizophrenia onset. J Neurosci. 2012;32(48):17365–72. doi: 10.1523/JNEUROSCI.2904-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G. The rat brain in stereotaxic co-ordinates. 4. Florida, USA: Academic Press; 1998. [Google Scholar]
- Percheron G, Francois C, Talbi B, Yelnik J, Fenelon G. The primate motor thalamus. Brain Research Reviews. 1996;22(2):93–181. [PubMed] [Google Scholar]
- Pijpers Angelique, Apps Richard, Pardoe Joanne, Voogd Jan, Ruigrok Tom JH. Precise Spatial Relationships between Mossy Fibers and Climbing Fibers in Rat Cerebellar Cortical Zones. J Neurosci. 2006;26(46):12067–12080. doi: 10.1523/jneurosci.2905-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan M, Lee SH, Kubicki M, Kikinis Z, Rathi Y, Seidman LJ, Mesholam-Gately RI, Goldstein JM, McCarley RW, Shenton ME, Levitt JJ. White matter tract abnormalities between rostral middle frontal gyrus, inferior frontal gyrus and striatum in first-episode schizophrenia. Schizophr Res. 2013;145(1–3):1–10. doi: 10.1016/j.schres.2012.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabe K, Kraff O, Minnerop M, Beck A, Schöls L, Ladd ME, Timmann D. Cerebellar pathology in Friedreich’s Ataxia: Atrophied nuclei with normal iron content. doi: 10.1016/j.nicl.2014.08.018. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radoeva PD, Coman IL, Antshel KM, Fremont W, McCarthy CS, Kotkar A, Wang D, Shprintzen RJ, Kates WR. Atlas-based white matter analysis in individuals with velo-cardio-facial syndrome (22q11.2 deletion syndrome) and unaffected siblings. Behav Brain Funct. 2012;8:38. doi: 10.1186/1744-9081-8-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmahmann JD. Disorders of the cerebellum: ataxia, dysmetria of thought, and the cerebellar cognitive affective syndrome. J Neuropsychiatry Clin Neurosci. 2004;16(3):367–78. doi: 10.1176/jnp.16.3.367. [DOI] [PubMed] [Google Scholar]
- Seal ML, Yucel M, Fornito A, Wood SJ, Harrison BJ, Walterfang M, Pell GS, Pantelis C. Abnormal white matter microstructure in schizophrenia: a voxelwise analysis of axial and radial diffusivity. Schizophr Res. 2008;101(1–3):106–10. doi: 10.1016/j.schres.2007.12.489. [DOI] [PubMed] [Google Scholar]
- Sian J, Dexter DT, Lees AJ, Daniel S, Agid Y, Javoy-Agid F, Jenner P, Marsden CD. Alterations in glutathione levels in Parkinson’s disease and other neurodegenerative disorders affecting basal ganglia. Ann Neurol. 1994;36(3):348–55. doi: 10.1002/ana.410360305. [DOI] [PubMed] [Google Scholar]
- Song SK, Sun SW, Ramsbottom MJ, Chang C, Russell J, Cross AH. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage. 2002;17(3):1429–36. doi: 10.1006/nimg.2002.1267. [DOI] [PubMed] [Google Scholar]
- Strick Peter L, Dum Richard P, Fiez Julie A. Cerebellum and Nonmotor Function. Annu Rev Neurosci. 2009;32(1):413–434. doi: 10.1146/annurev.neuro.31.060407.125606. [DOI] [PubMed] [Google Scholar]
- Thürling M, Hautzel H, Küper M, Stefanescu MR, Maderwald S, Ladd ME, Timmann D. Involvement of the cerebellar cortex and nuclei in verbal and visuospatial working memory: A 7T fMRI study. NeuroImage. 2012;62(3):1537–1550. doi: 10.1016/j.neuroimage.2012.05.037. [DOI] [PubMed] [Google Scholar]
- Thürling M, Küper M, Stefanescu R, Maderwald S, Gizewski ER, Ladd ME, Timmann D. Activation of the dentate nucleus in a verb generation task: A 7T MRI study. NeuroImage. 2011;57(3):1184–1191. doi: 10.1016/j.neuroimage.2011.05.045. [DOI] [PubMed] [Google Scholar]
- Vogel M. The cerebellum. Am J Psychiatry. 2005;162(7):1253. doi: 10.1176/appi.ajp.162.7.1253. [DOI] [PubMed] [Google Scholar]
- Voogd Jan, Shinoda Yoshikazu, Ruigrok Tom JH, Sugihara Izumi. Cerebellar Nuclei and the Inferior Olivary Nuclei: Organization and Connections. In: Manto Mario, Schmahmann Jeremy D, Rossi Ferdinando, Gruol Donna L, Koibuchi Noriyuki., editors. Handbook of the Cerebellum and Cerebellar Disorders. Dordrecht, Netherlands: Springer Science; 2013. pp. 377–436. [Google Scholar]
- Wakana S, Jiang H, Nagae-Poetscher LM, van Zijl PC, Mori S. Fiber tract-based atlas of human white matter anatomy. Radiology. 2004;230(1):77–87. doi: 10.1148/radiol.2301021640. [DOI] [PubMed] [Google Scholar]
- Welsh JP, Ahn ES, Placantonakis DG. Is autism due to brain desynchronization? Int J Dev Neurosci. 2005;23(2–3):253–63. doi: 10.1016/j.ijdevneu.2004.09.002. [DOI] [PubMed] [Google Scholar]
- White T, Ehrlich S, Ho BC, Manoach DS, Caprihan A, Schulz SC, Andreasen NC, Gollub RL, Calhoun VD, Magnotta VA. Spatial characteristics of white matter abnormalities in schizophrenia. Schizophr Bull. 2013;39(5):1077–86. doi: 10.1093/schbul/sbs106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White T, Nelson M, Lim KO. Diffusion tensor imaging in psychiatric disorders. Top Magn Reson Imaging. 2008;19(2):97–109. doi: 10.1097/RMR.0b013e3181809f1e. [DOI] [PubMed] [Google Scholar]
- Wu X, Ashe J, Bushara KO. Role of olivocerebellar system in timing without awareness. Proc Natl Acad Sci U S A. 2011;108(33):13818–22. doi: 10.1073/pnas.1104096108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D, Liu T, Ashe J, Bushara KO. Role of the olivo-cerebellar system in timing. J Neurosci. 2006;26(22):5990–5. doi: 10.1523/JNEUROSCI.0038-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Henry H, Knowlton Barbara J, Balleine Bernard W. Lesions of dorsolateral striatum preserve outcome expectancy but disrupt habit formation in instrumental learning. European Journal of Neuroscience. 2004;19(1):181–189. doi: 10.1111/j.1460-9568.2004.03095.x. [DOI] [PubMed] [Google Scholar]