Abstract
Background
Insulin action in the hypothalamus plays a critical role in the regulation of energy homeostasis, yet the intracellular signaling mechanisms mediating insulin action are incompletely understood. Although phosphodiesterase-3B (PDE3B) mediates insulin action in the adipose tissue and it is highly expressed in the hypothalamic areas implicated in energy homeostasis, its role, if any, in mediating insulin action in the hypothalamus is unknown. We tested the hypothesis that insulin action in the hypothalamus is mediated by PDE3B.
Methods
Using enzymatic assay, we examined the effects of peripheral or central administration of insulin on hypothalamic PDE3B activity in adult mice. Western blotting and immunohistochemistry also examined p-Akt and p-STAT3 levels in the hypothalamus. Effects of leptin on these parameters were also compared. We injected cilostamide, a PDE3 inhibitor, prior to central injection of insulin and examined 12–24 hr food intake and 24 hr body weight. Finally, we examined the effect of cilostamide on insulin-induced proopiomelanocortin (Pomc), neurotensin (Nt), neuropeptide Y (Npy) and agouti-related peptide (Agrp) gene expression in the hypothalamus by qPCR.
Results
peripheral or central injection of insulin significantly increased PDE3B activity in the hypothalamus in association with increased p-Akt levels but without any change in p-STAT3 levels. However, leptin–induced increase in PDE3B activity was associated with an increase in both p-Akt and p-STAT3 levels in the hypothalamus. Prior administration of cilostamide reversed the anorectic and body weight reducing effects as well as stimulatory effect of insulin on hypothalamic Pomc mRNA levels. Insulin did not alter Nt, Npy and Agrp mRNA levels.
Conclusion
Insulin induction of hypothalamic PDE3B activity, and the reversal of anorectic and body weight-reducing effects and stimulatory effect of insulin on hypothalamic Pomc gene expression by cilostamide suggest that activation of PDE3B is a novel mechanism of insulin signaling in the hypothalamus.
Keywords: Insulin, PDE3B, p-Akt, POMC, hypothalamus, feeding
Introduction
Insulin signaling in the hypothalamus plays a significant role in regulation of food intake and energy homeostasis. The circulating level of insulin is positively correlated with body weight and adiposity [1–4] and relays information on peripheral energy stores to the central nervous system (CNS) by acting on diverse neuronal populations. Along this line, chronic or acute intracerebroventricular (icv) administration of insulin decreases food intake and body weight in baboons, rats and mice [5–7]. Intranasal application of insulin reduces food intake and body weight in men [8, 9]. Reduction of insulin activity in the brain, including the hypothalamus, results in hyperphagia and weight gain [10–13]. Central insulin action is also essential for peripheral fat and glucose metabolism [14–17]. Furthermore, obese rats [18], sheep [19] and humans [20] have elevated cerebrospinal fluid insulin concentration, and in rats, imposition of a high-fat diet induces within 72 hours, a central insulin resistance independent of adiposity [21]. Thus, improving our understanding of the mechanisms of insulin signaling in the brain, particularly in the hypothalamus, is a vital step in analyzing the molecular and neural basis of food intake and body weight regulation and, ultimately, for the development of novel therapeutic approaches for the treatment of obesity and related disorders.
Although hypothalamic insulin action plays a critical role in the regulation of energy homeostasis, the intracellular signaling mechanisms mediating insulin action in the hypothalamus are incompletely understood. In this regard, (i) insulin is known to activate the phosphatidylinositol-3 kinase (PI3K)-Akt pathway in the hypothalamus [22], (ii) PI3K inhibitors reverse the anorectic and body weight reducing effects of insulin in the rat [22], and (iii) several signaling molecules downstream of Akt—including the mammalian target of rapamycin/S6 kinase [23, 24], and forkhead box O1 (FoxO1) [25]—have been implicated in insulin signaling in the hypothalamus and energy homeostasis [26–30]. In adipocytes, however, the anti-lipolytic effect of insulin [31], and insulin-induced glucose uptake and lipogenesis [32, 33] are mediated by activation of phosphodiesterase 3B (PDE3B) and regulation of the cAMP-signaling pathway. PDE3B, initially identified in adipose tissue and liver, was subsequently found in a variety of tissues including the hypothalamic arcuate nucleus (ARC), ventromedial nucleus (VMN), dorsomedial nucleus (DMN), lateral hypothalamic areas (LH), paraventricular nucleus (PVN), and perifornical hypothalamic areas that are implicated in food intake and body weight regulation [2, 34–40]. Furthermore, PDE3B is expressed in proopiomelanocortin- (Pomc) and neuropeptide Y (Npy)/agouti-related peptide (Agrp)-expressing neurons [34] that are the targets of both leptin and insulin signaling [1, 2, 40–43]. Thus, the question arises as to whether the PDE3B pathway mediates insulin signaling in the hypothalamus.
Whereas an appreciation of the role of the PDE3B pathway in mediating leptin signaling in the hypothalamus, particularly in the context of energy homeostasis, is gradually unfolding [44, 45], the role of this pathway in mediating hypothalamic insulin action is completely unknown. Therefore, the aim of the present study was to elucidate whether PDE3B pathway mediates hypothalamic action of insulin particularly in food intake and body weight regulation. To this end, we first tested whether insulin stimulates PDE3B activity in the hypothalamus and then examined the effects of cilostamide, a specific PDE3 inhibitor [31, 46–48], on the anorectic and body weight-reducing effects of central insulin.
Materials and Methods
Male C57BL/6J mice were available in our breeding colony, which was established by mating male and female C57BL/6J mice obtained from the Jackson Laboratory (Bar Harbor, Maine, USA). Mice were maintained in a light (lights on 0600 h to 1800 h) and temperature (22 °C)-controlled room with food (pelleted rodent chow) and water available ad libitum. Adult male mice of 12 to 16 weeks old were subjected to the following experiments, all of which were approved by the Institutional Animal Care and Use Committee of the University of Pittsburgh.
Experiment 1: Effects of peripheral insulin on PDE3B activity, p-Akt, and p-STAT3 levels in the hypothalamus of male mice: a comparison with the effects of leptin
Because insulin is known to increase PDE3B activity in the liver and adipose tissues [31, 49], we first examined whether insulin also increases PDE3B activity in the medial basal hypothalamus (MBH) of mice. Mice were subjected to a prolonged fast (20 hours) and then injected intraperitoneally (ip) with insulin (15 IU/kg) [22] or saline, without glucose, and sacrificed at 15 or 30 min post-injection. Because central leptin injection increases hypothalamic PDE3B activity in rats [44], we also compared the effect of insulin with that of leptin on PDE3B activation in the mouse hypothalamus. Thus, simultaneously, another group of mice was injected ip with leptin (3.5 mg/kg; A. F. Parlow, National Hormone & Peptide Program, Torrance, CA) and sacrificed 30 min post-injection. Brains were removed immediately and the MBH were dissected out, frozen in liquid nitrogen and kept at −80 C until processed for protein extraction. The MBH tissue was bounded rostrally by the posterior border of the optic chiasma, laterally by the lateral sulcus, and caudally by the mammillary bodies and cut to a depth of approximately 1.5 mm, as described previously by us [50]. Because recent evidence from our laboratory showed that Akt might not be an upstream regulator of the PDE3B pathway of leptin signaling in the rat hypothalamus [51], we also examined Akt phosphorylation (p-Aktser473) and STAT3 phosphorylation (p-STAT3Tyr705) in the MBH after insulin or leptin injection.
To examine whether p-Aktser473 and p-STAT3Tyr705 were altered in specific regions of the hypothalamus, some mice from each group at 30 min post-injection were anesthetized with pentobarbital and perfused intracardially with 0.9% saline, followed by ice-cold 4% paraformaldehyde in 50 mM phosphate buffer. The brains were dissected out and post-fixed in the same fixative for overnight and then kept in 25% sucrose solution at 4°C until they sank. Thereafter, the brains were frozen on dry ice, and coronal 25-μm free-floating sections were cut in five series through the hypothalamus on a freezing microtome (Leica Sliding Microtome; Leica Microsystems GmbH, Wetzlar, Germany), and stored in cryoprotectant at −20°C until use. One series was subjected to immunohistochemistry (IHC)
Measurement of PDE3B activity
PDE3B activity in the hypothalamus was measured according to a previously described protocol [52, 53], which has been used by us [44, 54]. The protocol used in the present study is essentially similar to that described recently by us [50]. MBH was homogenized in ice-cold extraction buffer (10 mM sodium phosphate, pH 7.2; 50 mM NaF, 150 mM NaCl; 3 mM benzamidine, 2 mM EDTA; 0.1% Triton X-100; 0.5% Igepal; containing cocktail of protease and phosphatase inhibitors from Roche Diagonostics, Indianapolis, IN, USA) and the homogenate was centrifuged at 38,000 g for 45 min at 4°C using an Optima TLX ultracentrifuge. The pellet was re-suspended in extraction buffer containing 1% NP-40 (Sigma Chemicals, St. Louis, MO, USA) and used for PDE3B assay, and supernatant was used to measure p-Akt and p-STAT3 levels by Western blotting. Protein was measured by Pierce BCA protein assay kit (Pierce Biotechnology, Inc., Rockford, IL, USA).
PDE3B assay was performed at 30°C in a total volume of 250 μl that included 12.5 μg of re-suspended MBH protein in a 50-μl sample volume and 200 μl reaction mixture [50 μl buffer A: 100 mM MOPS, pH 7.5, 4 mM EGTA, 1 mg/ml BSA; 50 μl buffer B: 100 mM MOPS, 75 mM Mg Acetate, ~100,000 cpm 3H-cAMP (Perkin-Elmer, Boston, MA, USA); 1 μM cAMP; 100 μl H2O] containing the specific PDE3 inhibitor cilostamide [31, 46–48] (100 mM) or no inhibitor. In this assay condition, cilostamide inhibited 30% of total PDE. PDE3B activity was calculated as pmol cAMP hydrolyzed per min/μg protein using an EXCEL Program (Microsoft Corp. Redmond, WA, USA). The values were then expressed in relation to the saline control.
Measurement of p-Akt and p-STAT3 levels in the hypothalamus by western blotting
Aliquots of supernatants from MBH protein extracts were resolved by SDS-PAGE (10% gel for p-Akt; 8% gel for p-STAT3) and transferred to polyscreen PVDF membranes. The membranes were blocked in 5% nonfat dry milk in Tris-buffered saline with 0.1% Tween-20, and incubated overnight with primary antibody for phospho-AktSer473 (Cell Signaling Technology, Inc., Danvers, MA; 1:2,000; catalog# 4051), or phospho-STAT3Tyr705 (p-STAT3, Santa Cruz Biotechnology, CA; 1:900; catalog# Sc-8059) at 4 °C followed by incubation for 60 min with HRP-conjugated secondary antibodies at room temperature. Immunoreactive bands were visualized by Western Lightning chemoluminescence Reagent Plus-ECL as described by the manufacturer (Perkin Elmer). Immunoreactive bands were scanned and analyzed by NIH Image Software. The membranes were stripped and then blotted for total Akt (Cell Signaling Technology, 1:1200; catalog# 9272) or STAT3 (Santa Cruz Biotechnology, 1:1000; catalog# Sc-483) as appropriate to measure Akt and STAT3 levels. The levels of p-Akt and p-STAT3 levels were first calculated as ratio to total Akt and STAT3, respectively, and then expressed as relative to the control saline group.
Immunohistochemistry (IHC) for p-Aktser473 and p-STAT3 localization in the hypothalamus
The IHC localization of p-Aktser473 immunoreactive protein in the hypothalamus was done according to the method described previously by us [50, 51]. Briefly, free-floating sections of fixed tissue were treated with 0.5% NaOH and 2% H2O2 in H2O for 20 min at room temperature followed by 2 h with blocking solution [10% normal goat serum in potassium PBS (KPBS), 1% BSA, 0.4% Triton X-100] at room temperature. Sections were then incubated with p-AktSer473 antibody (Cell Signaling Technology, 1:2000; Catalog# 4060) in antibody dilution buffer (5% normal goat serum in KPBS, 0.5% BSA, 0.4% Triton X-100) for approximately 38 h at 4 °C followed by washing in KPBS and incubation with secondary antibody (goat anti-rabbit, 1:1200; Vector Laboratories, Burlingame, CA, USA) in antibody dilution buffer for 90 min at room temperature. Sections were washed and incubated in Vectastain Elite ABC reagent (Vector Laboratories) in KPBS containing 0.4% Triton X-100 for 90 min at room temperature. Finally, sections were washed and the signal was developed by incubating in 0.04% 3,3′-diaminobenzidine solution containing 0.01% nickel chloride, giving a brown precipitate. For IHC localization of p-STAT3Tyr705 immunoreactive protein in the hypothalamus, we essentially used the method described previously by us [51, 55]. Briefly, free floating tissue sections were pretreated with 1% NaOH and 1% H2O2 in H2O for 20 min, 0.3% glycine for 10 min, and 0.03% sodium dodecyl sulfate for 10 min. Sections were then blocked for 1 h with blocking solution (5% normal goat serum in KPBS, 1% BSA, 0.4% Triton X-100), followed by incubation with p-STAT3Tyr705 antibody (Cell Signaling Technology, dilution 1:3000 in blocking solution; catalog# 9131) for overnight at 4°C. On the next day, the sections were washed, incubated with biotinylated secondary goat anti-rabbit antibody (Vector Laboratories, dilution1:4000 in blocking solution) for 90 min, and then treated with avidin-biotin-complex solution (Vector Laboratories) for 90 min. Finally, the signal was developed by diaminobenzidine solution containing 0.5% nickel sulfate, giving a dark-blue precipitate. The sections were then mounted on glass slide, dried, and processed through graded alcohol and clearing agent (Xylene), and then cover slipped with DPX.
For analysis of p-STAT3Tyr705-positive cells, the sections were photographed under a bright field using a QImaging camera (MicroPublisher 5.0 RTV, Surrey, BC, Canada) attached to a Leica DMBRE microscope (Leica Microsystems) and the matched-sections from different groups were compared. For analysis of p-Aktser473-positive cells, the sections were photographed as described and all cells immunoreactive for p-Aktser473 were counted in evenly-spaced, region matched sections (3 to 4) through the ARC of individual animals. Immunoreactive cells in the ARC, VMN and DMN areas were counted on both sides to determine the total number of p-Aktser473-positive cells per section.
Experiment 2: Effects of central insulin on PDE3B activity, p-Akt, and p-STAT3 levels in the hypothalamus of male mice: a comparison with the effects of leptin
Because in experiment 1 we observed peripheral insulin to increase hypothalamic PDE3B activity, which could be due to a direct or an indirect effect of insulin, we next examined whether central insulin administration increases PDE3B activity in the hypothalamus and also included a group receiving central leptin as a positive control [44]. For central injection, mice were anesthetized with pentobarbital (40 mg/kg) and then implanted stereotaxically with 28-gauge stainless steal cannula in the lateral cerebroventricle (LCV)(coordinates: AP: −0.5 mm, ML: 1 mm, DV: 2.5 mm) as described by Hill et al. [56]. After the recovery period of ten to 14 days, the icv location of the cannula was confirmed by drinking response after angiotensin II injection [57]. Mice with indwelling lateral cerebroventricular (LCV) cannula were fasted 22–24 hours and injected icv with insulin (4.4 mU/2μl) [58,59], leptin (4 μg/2μl) or saline (2μl). After 30 minutes, the mice were sacrificed and the MBH were dissected out, frozen in liquid nitrogen and kept at −80°Cuntil processed for protein extraction to measure PDE3B activity and p-AKT and p-STAT3 levels as described above. Trunk blood was collected to measure circulating insulin levels.
To examine whether p-Aktser473 and p-STAT3Tyr705 were altered in specific regions of the hypothalamus, some mice from each group were anesthetized with pentobarbital and perfused intracardially with 0.9% saline, followed by ice-cold 4% paraformaldehyde in 50 mM phosphate buffer. The brains were processed for IHC as described above.
Insulin determination
Plasma insulin levels were determined using Mercodia Mouse Insulin ELISA kit (Mercodia AB, Uppsala, Sweden). It is to be noted that this particular insulin ELISA kit detects both mouse and human insulin.
Experiment 3: Effects of cilostamide, a specific PDE3 inhibitor, on the anorectic and body weight-reducing effects of insulin
To learn whether the insulin-induced increase in hypothalamic PDE3B activity has any physiological significance, we investigated whether prior injection with cilostamide reversed the effects of central insulin on food intake and body weight in mice with indwelling LCV cannula. We used cilostamide because it is one of the well-established PDE3 inhibitors that are being used for PDE3 inhibition in many studies including ours (31, 44, 46–48, 60–62). After 10 to 14 days of recovery from the surgery and the confirmation of the icv location of the cannula by drinking response after angiotensin II injection [57], mice were injected icv with cilostamide (10 μg/μl) or DMSO (vehicle) five hours (i.e. at 13:00h) before the lights were turned off and food was withdrawn. Forty minutes later, the mice were injected icv with insulin (4.4 mU/2μl) or saline (2μl). Food (rodent chow) was given at 6:00 PM and food intake was measured at 12 and 24 hours; body weight was measured at 24 hours after the injection.
Experiment 4: Examine whether cilostamide reverses hypothalamic Pomc, Npy, Agrp and neurotensin (Nt) gene expression response to central insulin
Adult male mice with indwelling lateral cerebroventricular cannula, fasted for 24 hours, were injected icv with cilostamide (10 μg/μl) or DMSO, followed 40 minutes later with icv insulin (4.4 mU/2μl) or saline (2μl) injection. Two hours later, mice were sacrificed and the MBH were processed for Pomc, Nt, Npy and Agrp gene expression by qPCR, using a SYBR green master mix (Invitrogen) and run on the Applied Biosystems Prism 7900HT real-time PCR machine. Pomc, Nt, Npy and Agrp mRNA levels were expressed as relative to a house keeping gene, cyclophilin, in each sample.
RNA isolation and RT-qPCR
RNA isolation and RT-qPCR were done as described previously [63]. Briefly, total RNA from MBH was isolated by Trizol reagent according to manufacturer’s protocol (Life Technologies) and subjected to DNase treatment using RNase-Free DNase (Promega, Madison, WI) according to the manufacturer’s protocol and re-extracted with Trizol. cDNA was synthesized from 2 μg RNA using the High Capacity cDNA Reverse Transcription Kit (Life Technologies). The RT reaction consisted of 10 min incubation at 25 °C, 120 min incubation at 37 °C, followed by a 5 min 85 °C termination step, and the resulting cDNA was stored at −20 °C. qPCR reactions were performed in duplicate with 100 ng of cDNA using 2X Power SYBR Green PCR master mix (Life Technologies) containing 500 nm each primer and run on the Applied Biosystems Prism 7900HT real-time PCR machine at 95 °C for 10 min followed by 40 cycles of 95 °C (15 s) and 60 °C (60 s). The primer sequences are as follows: Pomc: sense-5′-atgccgagattctgctacagtcg-3′; anti-sense-5′-ttcatctccgttgcc-aggaaacac-3′; Nt: sense-5′-aatgtttgcagcctcataaataac-3′; anti-sense-5′-tgccaacaaggtcgtcatc-3′; Npy: sense-5′-cagaaaacgcccccagaa-3′; anti-sense-5′-aaaagtcgggagaacaagtttcatt-3′; Agrp: sense-5′-cggaggtgctagatccacaga-3′; anti-sense-5′-aggactcgtgcagccttacac-3′; cyclophilin: sense-5′-aaggtgaaagaaggcatgaac-3′; anti-sense-5′-agctgtccacagtcggaaatg-3′. The relative quantification of mRNA levels (fold change) normalized to cyclophilin was calculated using the ΔΔCT method from the Ct (threshold cycle) values obtained from ABI SDS software package.
Statistical analysis
All values are expressed as means ± standard error (SE). Statistical significance was determined using randomized one-way ANOVA with Fisher’s least significant difference (LSD) (protected t-tests) multiple-range tests to compare three or more groups or Student’s t test to compare two groups. Shapiro-Wilk test of normality was done for each data set before performing parametric analysis. All data had the probability > 0.05 and passed the normality test. All statistical analyses were done using GB-Stat software for the Macintosh (Dynamic Microsystems, Inc., Silver Spring, MD). P < 0.05 were considered to be significant.
Results
Experiment 1: Changes in PDE3B activity, p-Akt, and p-STAT3 levels in the hypothalamus of male mice following peripheral injection of insulin or leptin
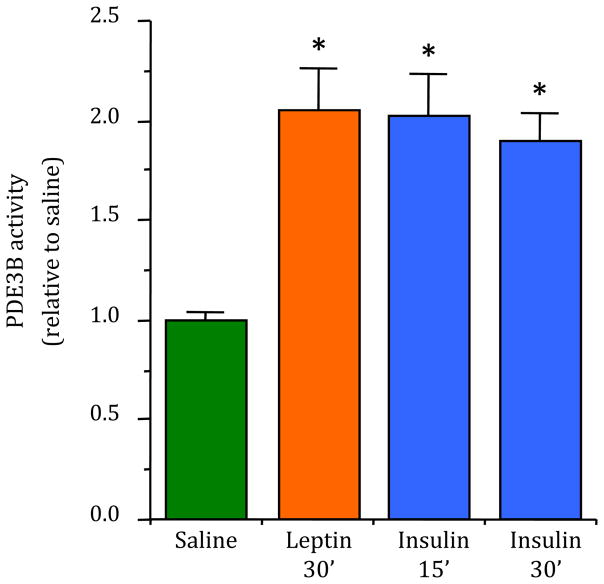
To examine if insulin utilizes PDE3B pathway in transducing its action in the hypothalamus in regulation of energy homeostasis, we first examined if peripheral injection of insulin increases PDE3B activity in the hypothalamus. Because we have previously demonstrated that leptin stimulates PDE3B activity in the hypothalamus [44, 50], we also examined the effect of peripheral leptin on PDE3B activity in the hypothalamus as a positive control. As expected, peripheral injection of leptin significantly increased PDE3B activity (p < 0.0001) in the MBH within 30 min post-injection (Fig. I). Most importantly, as shown in Figure 1, peripheral injection of insulin also significantly increased (p < 0.0001) PDE3B activity in the MBH at 15 or 30 min post-injection, which was associated with increased phosphorylation of Akt at serine473 (p-Aktser473) in the MBH (p < 0.01 and p < 0.05 at 15 and 30 min, respectively, Supplementary Figs. S1A and S1B). In addition, like insulin, leptin increased p-Aktser473 levels in the MBH (p < 0.05, Supplementary Figs. S1A and S1B). However, whereas leptin increased p-STAT3Tyr705 levels (p < 0.01) in the MBH, insulin failed to do so (Supplementary Figs. S1C and S1D). Notably, although glucose was not co-administered with insulin and hence the likelihood of provoking hypoglycemia and counter-regulatory responses exist, these responses are not known to trigger phosphorylation of Akt and STAT3, or to activate PDE3B. This also appears to be unlikely because we obtained essentially similar results following central administration of insulin (see below the results of Experiment 2).
Fig. 1.
Effects of peripheral injection of insulin or leptin on PDE3B activity in the hypothalamus. The values for PDE3B activity are expressed as relative to saline (control) group. PDE3B activity (pmol cAMP hydrolyzed per min/μg protein) in the control group was 1.10 ± 0.16. Values represent the mean ± SEM for 6–8 animals per group. * p < 0.0001 vs saline control group.
To address whether the changes in p-AktSer473 and p-STAT3Tyr705 levels seen in the MBH extract were confined to specific area(s) of the hypothalamus, we processed brain sections, at the level of the median eminence (ME)-arcuate region (ARC), for IHC with p-AktSer473 antibody or p-STAT3Tyr705 antibody to examine p-AktSer473-positive and p-STAT3Tyr705-positive cells, respectively. IHC confirmed the finding obtained by western blotting in that both insulin and leptin increased p-AktSer473-positive cells, but only leptin increased p-STAT3Tyr705-positive cells in the hypothalamus (Supplementary Fig. S2). In addition, whereas i.p. insulin injection increased the number of p-AktSer473-positive cells only in the ARC (p< 0.01), i.p. leptin injection increased the number of p-AktSer473-positive cells in the ARC (p <0.05), as well as in the VMN (p <0.05), and DMN (p <0.05) as compared to saline control (Supplementary Fig. S5A). Also the number of p-AktSer473-positive cells in the ARC was significantly increased (p<0.01) in the insulin treated group as compared to the leptin treated group (Supplementary Fig. S5A).
Experiment 2: Changes in PDE3B activity, p-Akt, and p-STAT3 levels in the hypothalamus of male mice following central injection of insulin or leptin
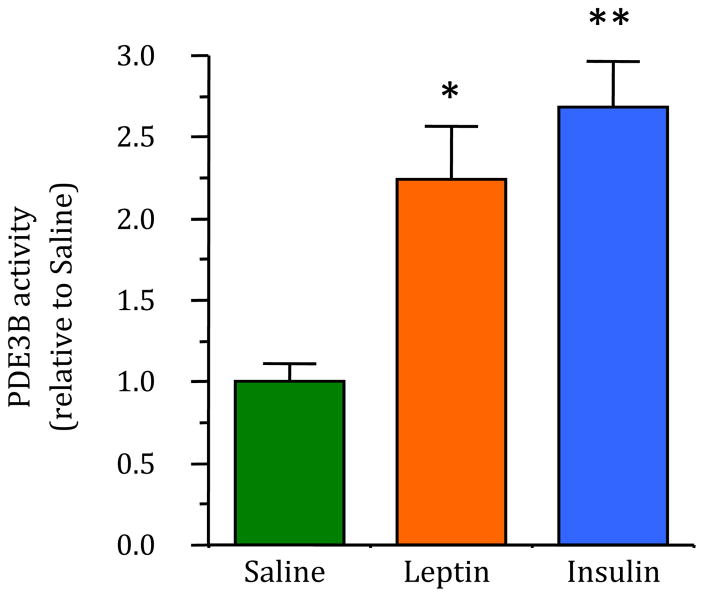
Because increased PDE3B activity and associated changes in p-Aktser473 and p-STAT3 levels in the MBH following peripheral insulin injection could be a direct or indirect effect of insulin, this study examined if the central injection of insulin increased PDE3B activity. As shown in Figure 2, intracerebroventricular injection of insulin increased PDE3B activity in the MBH by 2.5-fold (p < 0.001) as compared to that in the saline injected control. As a positive control, leptin increased PDE3B activity in the MBH as expected (p < 0.01, Fig. 2). Furthermore, as seen following peripheral injection, both insulin (p < 0.01) and leptin (p < 0.05) significantly increased p-Aktser473 levels, but only leptin increased p-STAT3Tyr705 levels (p < 0.01) in the hypothalamus (Supplementary Fig. S3). In addition, IHC demonstrated increased p-AktSer473-positive cells in the ARC, VMN and DMN following leptin or insulin injection (Supplementary Figs. S4B and S4C; Supplementary Fig. S5B) and only leptin, but not insulin, to increase p-STAT3Tyr705-positive cells in the hypothalamus (Supplementary Figs. S4E and S4F). Also, circulating insulin levels following icv injection did not differ among the groups (saline: 0.199 ± 0.025, leptin: 0.214 ± 0.057, insulin: 0.210 ± 0.034; ng/ml; mean ± SEM; n = 7/group), suggesting that the effects of insulin following central administration are direct, local actions.
Fig. 2.
Effects of central injection of insulin or leptin on PDE3B activity in the hypothalamus. The values for PDE3B are expressed as relative to saline (control) group. PDE3B activity (pmol cAMP hydrolyzed per min/μg protein) in the control group was 0.90 ± 0.10. Values represent the mean ± SEM for 6–8 animals per group. * p <0.01, ** p < 0.0001 vs saline control group.
Experiment 3: Cilostamide, a specific PDE3 inhibitor, reverses the anorectic and body weight-reducing effects of central insulin
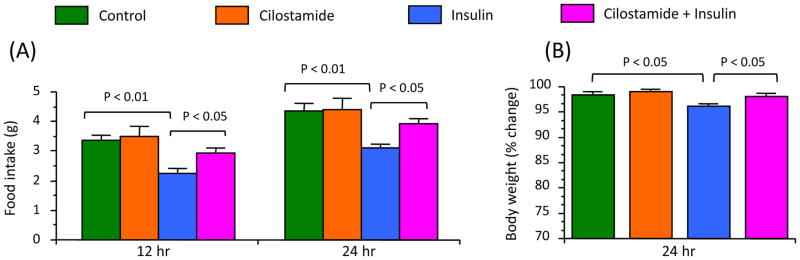
To address whether increased PBE3B activity has any physiological role in mediating insulin action on food intake and body weight regulation, this study examined whether PDE3B inhibition reverses the effect of insulin on these parameters. As shown in Figure 3A, central injection of insulin significantly decreased (p < 0.01) food intake at 12 or 24 hr post-injection, and this effect was blunted by prior injection of cilostamide. Similarly, central injection of insulin significantly decreased (p < 0.05) 24-hr body weight and prior cilostamide injection blunted this effect of insulin (Fig. 3B).
Fig. 3.
Effects of cilostamide, a specific PDE3 inhibitor, on anorectic and body weight reducing effects of central insulin. A, Food intake at 12 and 24 hr after insulin injection. B, Body weight at 24 hr after insulin injection. Cilostamide (10 μg/μl DMSO) or DMSO (vehicle control) was injected into the lateral cerebroventricle 4 hours before insulin (4.4 mU/2 μl) or saline injection. Values represent the mean ± SEM for 9–10 animals per group.
Experiment 4: Cilostamide reverses the stimulatory effect of insulin on Pomc gene expression in the hypothalamus
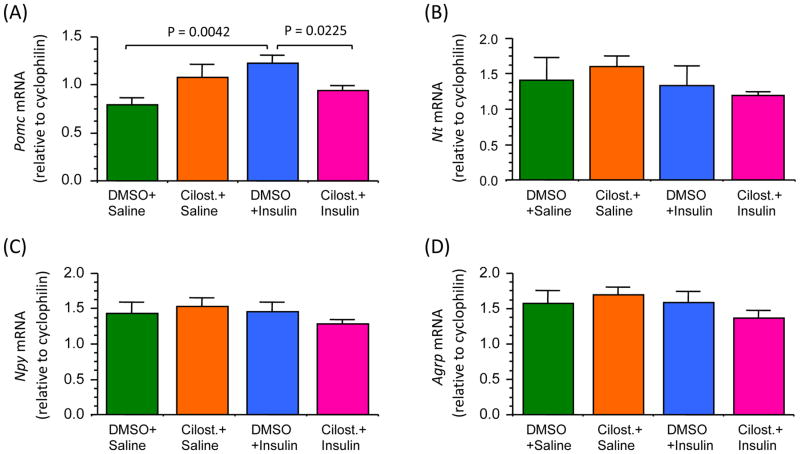
Because insulin signaling in POMC neurons plays an important role in energy homeostasis [64–66], we examined whether cilostamide reverses the insulin-induced increase in Pomc gene expression in the MBH. In addition, since NPY/AgRP neurons in the hypothalamus are also the known targets of insulin [67], and NT plays an important role in energy homeostasis [68–71], we also examined Npy, Agrp and Nt gene expression. As shown in Figure 4, central injection of insulin significantly increased (p < 0.01) Pomc mRNA levels in the MBH as compared to that following saline injection, and importantly, this effect of insulin was completely reversed by prior administration of cilostamide. Although there was a tendency towards an increase in Pomc mRNA levels following cilostamide alone, it was not significant and the underlying cause is currently unknown. However, in contrast to its stimulatory effect on Pomc gene expression, insulin did not have any effect on Nt, Npy or Agrp mRNA levels at this time point, and cilostamide had also no effect on these gene expressions (Fig. 4). Notably, previous studies have also reported central insulin having no effect on Npy and Agrp gene expression at 2 hour post-injection [7, 21].
Fig. 4.
Changes in hypothalamic mRNA levels for POMC (A), NT (B), NPY (C) and AgRP (D) following DMSO + insulin or cilostamide + insulin. Mice fasted for 24 hours were injected with cilostamide (10 μg/μl) or DMSO (control), followed 40 min later, with icv insulin (4.4 mU/2 μl) or saline. Two hours later the medial basal hypothalamus was harvested to measure Pomc, NTs, Npy and Agrp mRNA levels by qPCR. Values represent the mean ± SEM for 5 animals per group.
Discussion
The physiological role of the PDE3B pathway, if any, in mediating insulin action in the hypothalamus is completely unknown. In addition to providing first demonstration of the ability of peripherally or centrally administered insulin to stimulate PDE3B activity in the hypothalamus in mice, we demonstrate that a specific PDE3 inhibitor, cilostamide blunts the anorectic and body weight-reducing effects as well as the stimulatory effect of insulin on Pomc gene expression in the hypothalamus.
Cumulative evidence suggests that insulin action in the hypothalamus plays a significant role in energy homeostasis and in glucose and fat metabolism [1–4, 14–17]: yet, the intracellular signaling mechanisms mediating these actions of insulin are incompletely understood. Whereas the PI3K-Akt pathway has been established as the primary mechanism of insulin signaling in many tissues including the hypothalamus [22], there are several signaling molecules downstream of Akt –including mTOR/S6 kinase and Foxo1– have been implicated in insulin signaling in the hypothalamus and energy homeostasis [23–30]. Our laboratory is investigating the role of PDE3B in the hypothalamus in the regulation of energy homoeostasis and has demonstrated PDE3B signaling to play an important role in mediating leptin action in the hypothalamus [44,45]. Because PDE3B is localized in various hypothalamic sites which have been implicated in energy homeostasis and also reported to express insulin receptor (IR) [2, 34, 64–66], and because PDE3B pathway plays an important role in mediating insulin action in peripheral tissues, particularly in the adipose tissue and liver [31–33, 49,53], in the present study we sought to examine whether PDE3B plays any role in mediating insulin action in the hypothalamus in the regulation of energy homeostasis.
To establish the role of PDE3B in mediating insulin action, it is imperative to demonstrate if insulin stimulates PDE3B activity in the hypothalamus. Our demonstration that peripheral insulin administration increased PDE3B activity in the hypothalamus suggested for the first time the possibility of PDE3B to play a role in mediating hypothalamic action of insulin. We also confirmed the activation of PDE3B in the hypothalamus by peripheral leptin [50]. Furthermore, increased p-AktSer473 and p-STAT3 levels by insulin and leptin, respectively, confirmed the action of these peripherally administered hormones in the hypothalamus [22, 72].
Whereas increased PDE3B activity following peripheral insulin administration could be due to its direct and/or indirect effects in the hypothalamus, our finding that icv administration of insulin increased PDE3B activity in the hypothalamus clearly suggests that insulin directly acts in the hypothalamus to induce PDE3B activity. Increased PDE3B activity by central leptin not only confirms our previous finding in rats [44], it also validates the results with central insulin administration. Together the results obtained following peripheral and central administration of insulin clearly demonstrate that insulin induces PDE3B activation in the hypothalamus, an important first step toward establishing PDE3B as a target of insulin action in this part of the brain.
To establish the physiological significance of insulin-induced increase in hypothalamic PDE3B activity, we next examined if PDE3 inhibition could reverse the effects of central insulin on food intake and body weight. To this end, we used cilostamide that has been widely used as a specific PDE3 inhibitor [31, 46–48, 60–62]. Although there are some controversies whether central insulin reduces food intake or not [73, 74], our study shows that central injection of insulin significantly decreased food intake and body weight in our experimental system. This is in accord with the previous finding in mice [7]. Importantly, the finding that prior treatment with cilostamide blunted the anorectic and body weight reducing effects of insulin strongly suggests a role for the PDE3B pathway in mediating hypothalamic action of this hormone in the regulation of food intake and body weight.
To begin to examine the target neurons that mediate PDE3B pathway of insulin signaling in the hypothalamus in the regulation of energy homeostasis, in the present study we mainly focused on POMC, NT and NPY/AgRP neurons that have been implicated to play an obligatory role in energy homeostasis [41, 64, 67, 68–71]. Furthermore, POMC and NPY/AgRP neurons not only express both Lepr-b and IR [64, 66], they also express PDE3B [34]; and insulin is known to induce Pomc gene expression [21, 65] and inhibit Npy gene expression [67]. Whereas hypothalamic NT neurons are the targets of leptin signaling and have been implicated in energy homeostasis [68–71, 75, 76], and insulin increases Nt gene expression in a hypothalamic neuronal cell line [77], whether insulin also acts on these neurons in vivo is unknown. Therefore, we tested if cilostamide would modify the effect of insulin on Pomc, Nt and Npy/Agrp gene expression, if it does then it will provide the evidence in support of a role for the PDE3B pathway in mediating insulin action on these hypothalamic neurons. Our finding of cilostamide reversing the insulin-induced Pomc gene expression indeed suggests a role for PDE3B in mediating insulin action in POMC neurons, which is likely to mediate, at least partly, the hypothalamic action of insulin in regulating energy homeostasis. Notably, although almost all POMC neurons express PDE3B [34], it has been reported that the POMC neurons that respond to insulin are largely distinct from the ones that respond to leptin [78]. Thus, it is likely that the stimulatory effect of insulin on hypothalamic Pomc gene expression represent a direct action on insulin-sensitive POMC neurons. Whereas the mechanism(s) by which PDE3B mediates insulin action on POMC gene expression was not addressed in this study, insulin is known to increase POMC transcription by repressing FOXO1, which reduces POMC promoter activity [29]. In addition, insulin-stimulated PI3K—Akt activation is required for FOXO1 phosphorylation, which would translocate FOXO1 from the nucleus to cytoplasm resulting in removal of FOXO1 repression on POMC promoter activity and an increase in POMC gene expression [79, 80]. Presently, we do not know whether cilostamide inhibits insulin-stimulated phosphorylation of FOXO1 or whether activation of FOXO1 requires PDE3B activation. It is also unknown if the Pomc gene requires two signals, one from the phosphorylation and exclusion of FOXO1 from the nucleus and the other from activation of PDE3B. We speculate that FOXO1 and PDE3B are most likely two independent downstream signaling components of the PI3K-Akt pathway of insulin signaling in POMC neurons, which will be the subject of future investigation. Although central insulin administration had no effect on Npy, Agrp or Nt gene expression at 2 hour post-injection and it may require examining this response at a later time point [77] and therefore the effect of cilostamide could not be documented, because these neurons, particularly NPY/AgRP neurons, express PDE3B as well as insulin receptors [34, 66], involvement of these neurons in mediating insulin action through the PDE3B pathway can not be completely ruled out, which requires further investigation. Additionally, it is unknown if the effect of cilostamide is also mediated through a different class of neurons that may not be insulin-sensitive but indirectly modify the activity of insulin-responsive neurons. This possibility exists because, besides the ARC, PDEB is highly expressed in various other hypothalamic sites including the VMN, DMN, PVN and ventral premammillary nucleus regions that do not express POMC, NPY/AgRP or NT [34]. Moreover, whereas our previous study has shown PDE4 not to mediate leptin action in the hypothalamus [44], it remains to be determined if PDE4, particularly PDE4B that is highly expressed in the hypothalamus [81, 82], is involved in transducing insulin action in the hypothalamus.
Our finding that whereas insulin increased only p-Akt and not p-STAT3 levels, but leptin increased both p-Akt and p-STAT3 levels in the mouse hypothalamus deserves some additional comments. Particularly, based on the finding that PI3K inhibitor reversed the stimulatory effect of leptin on PDE3B activity and that leptin failed to increase p-Akt, we previously concluded that in rats, PI3K but not Akt is upstream of the PDE3B pathway of leptin signaling in the hypothalamus [51]. Although whether PI3K-Akt is upstream of the PDE3B pathway of insulin/leptin signaling was not specifically addressed in this study, the finding of increased p-Akt by insulin and leptin in association with increased PDE3B activity suggest the possibility that Akt may be upstream of the PDE3B pathway of both insulin and leptin signaling in the mouse hypothalamus. In addition, this study with biochemical and IHC approaches confirms previous reports of insulin not having any effect on p-STAT3 levels in the hypothalamus [51, 83]. It is noteworthy that p-Akt response to peripheral and central insulin was somewhat different in various hypothalamic regions. Specifically, peripheral insulin administration increased the number of p-AktSer473-positive cells mainly in the ARC but not in the VMN or DMN, whereas central insulin administration increased the number of p-AktSer473-positive cells in the ARC as well as in the VMN and DMN. Furthermore, the number of p-AktSer473-positive cells was significantly higher in the ARC following peripheral insulin as compared to central insulin administration. Although reason behind this discrepancy is not clear, a shorter duration of insulin treatment could be necessary to obtain optimal p-Akt activation in the hypothalamic neurons following central injection. However, both central and peripheral leptin treatment increased the number of p-AktSer473-positive cells in the ARC, VMN and DMN.
Whereas PI3K has been established to mediate both leptin and insulin signaling in the hypothalamus [22, 44, 84], whether PDE3B could be another signaling component in the hypothalamus that also mediates the action of both these hormones has not been addressed previously. Because both insulin and leptin resistance occur in the hypothalamus during the development of DIO, determining whether the PDE3B pathway integrates these two obligatory signals that regulate energy and glucose homeostasis by acting on hypothalamic neurons would be quite significant. It is particularly important because Pde3b-null mice show altered energy and glucose homeostasis [85] and DIO is associated with impaired PI3K-PDE3B pathway of leptin signaling in the hypothalamus [50, 55]. Thus, our present finding in mice of PDE3B activation in the hypothalamus by both insulin and leptin and reversal of anorectic and body weight reducing effect as well as stimulatory effect of insulin on POMC gene expression by PDE3B inhibitor cilostamide suggest the possibility that PDE3B could be a key signaling node mediating both leptin and insulin action in the hypothalamus in regulation of energy homeostasis. This position is also supported by our previous demonstration of cilostamide reversing anorectic and body weight reducing effects and stimulatory effect of leptin on POMC gene expression in the rat hypothalamus [44, 61].
Interestingly, despite an apparent role for PDE3B in the appetite-suppressing effects of both leptin [44] and insulin (present study), the Pde3b-null mice do not develop obesity and with less gonadal adipose tissue mass [85]. One of the reasons would be the development of compensations in the null mice. Also, because PDE3B is expressed in variety of tissues including the hypothalamus, liver and adipose tissues, etc., that play obligatory role in energy homeostasis, whole body knock-out may not produce the expected effect. It is also possible that reduced adipose tissue seen in the null mice, is most likely due to deletion of Pde3b in the adipocytes, presumably leading unopposed cAMP-dependent lipolysis. Nevertheless, the Pde3b-null mice exhibited all other metabolic phenotypes such as insulin resistance and altered glucose homeostasis seen during obesity [85], suggesting a critical role for PDE3B in energy homeostasis. In addition, it is unknown whether the altered metabolic phenotypes seen in the null mice were due to deletion of Pde3b in specific hypothalamic neurons. Answer to these critical questions would require studies in mice with tissue/neuron-specific knock-out of Pde3b as well as knock-down of PDE3B in adult hypothalamus, which would be the subject of future investigations.
In summary, we have demonstrated insulin induction of PDE3B activity in the mouse hypothalamus. This finding along with the reversal of anorectic and body weight-reducing effects as well as the stimulatory effect of insulin on Pomc mRNA levels by a specific PDE3 inhibitor have identified PDE3B pathway as a novel intracellular signaling mechanism of insulin action in the hypothalamus. Future studies are warranted towards understanding the physiological role of the PDE3B pathway in mediating insulin action in the hypothalamus in the regulation of energy homeostasis.
Supplementary Material
Acknowledgments
This work was supported by NIH RO1 Grant DK78068 to AS. Thanks are due to A. F. Parlow and the NIDDK National Hormone & Pituitary Program, Torrance, CA, for supplying the recombinant murine leptin.
Footnotes
Disclosure Statement
The authors have no conflicts of interest to disclose
References
- 1.Schwartz MW, Figlewicz DP, Baskin DG, Woods SC, Porte D., Jr Insulin in the brain: a hormonal regulator of energy balance. Endocr Rev. 1992;13:387–414. doi: 10.1210/edrv-13-3-387. [DOI] [PubMed] [Google Scholar]
- 2.Schwartz MW, Woods SC, Porte D, Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404:661–671. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- 3.Porte D, Jr, Baskin DG, Schwartz MW. Insulin signaling in the central nervous system: a critical role in metabolic homeostasis and disease from C. elegans to humans. Diabetes. 2005;54:1264–1276. doi: 10.2337/diabetes.54.5.1264. [DOI] [PubMed] [Google Scholar]
- 4.Plum L, Schubert M, Bruning JC. The role of insulin receptor signaling in the brain. Trends Endocrinol Metab. 2005;16:59–65. doi: 10.1016/j.tem.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 5.Woods SC, Lotter EC, McKay LD, Porte D., Jr Chronic intracerebroventricular infusion of insulin reduces food intake and body weight of baboons. Nature. 1979;282:503–505. doi: 10.1038/282503a0. [DOI] [PubMed] [Google Scholar]
- 6.Ikeda H, West DB, Pustek JJ, Figlewicz DP, Greenwood MR, Porte D, Jr, Woods SC. Intraventricular insulin reduces food intake and body weight of lean but not obese Zucker rats. Appetite. 1986;7:381–386. doi: 10.1016/s0195-6663(86)80006-x. [DOI] [PubMed] [Google Scholar]
- 7.Brown LM, Clegg DJ, Benoit SC, Woods SC. Intraventricular insulin and leptin reduce food intake and body weight in C57BL/6J mice. Physiol Behav. 2006;89:687–691. doi: 10.1016/j.physbeh.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 8.Hallschmid M, Benedict C, Schultes B, Fehm HL, Born J, Kern W. Intranasal insulin reduces body fat in men but not in women. Diabetes. 2004;53:3024–3029. doi: 10.2337/diabetes.53.11.3024. [DOI] [PubMed] [Google Scholar]
- 9.Hallschmid M, Higgs S, Thienel M, Ott V, Lehnert H. Postprandial administration of intranasal insulin intensifies satiety and reduces intake of palatable snacks in women. Diabetes. 2012;61:782–789. doi: 10.2337/db11-1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McGowan MK, Andrews KM, Grossman SP. Chronic intrahypothalamic infusions of insulin or insulin antibodies alter body weight and food intake in the rat. Physiol Behav. 1992;51:753–766. doi: 10.1016/0031-9384(92)90112-f. [DOI] [PubMed] [Google Scholar]
- 11.Strubbe JH, Mein CG. Increased feeding in response to bilateral injection of insulin antibodies in the VMH. Physiol Behav. 1977;19:309–313. doi: 10.1016/0031-9384(77)90343-2. [DOI] [PubMed] [Google Scholar]
- 12.Bruning JC, Gautam D, Burks DJ, Gillette J, Schubert M, Orban PC, Klein R, Krone W, Muller-Wieland D, Kahn CR. Role of brain insulin receptor in control of body weight and reproduction. Science. 2000;289:2122–2125. doi: 10.1126/science.289.5487.2122. [DOI] [PubMed] [Google Scholar]
- 13.Obici S, Feng Z, Karkanias G, Baskin DG, Rossetti L. Decreasing hypothalamic insulin receptors causes hyperphagia and insulin resistance in rats. Nat Neurosci. 2002;5:566–572. doi: 10.1038/nn0602-861. [DOI] [PubMed] [Google Scholar]
- 14.Plum L, Belgardt BF, Bruning JC. Central insulin action in energy and glucose homeostasis. J Clin Invest. 2006;116:1761–1766. doi: 10.1172/JCI29063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koch L, Wunderlich FT, Seibler J, Konner AC, Hampel B, Irlenbusch S, Brabant G, Kahn CR, Schwenk F, Bruning JC. Central insulin action regulates peripheral glucose and fat metabolism in mice. J Clin Invest. 2008;118:2132–2147. doi: 10.1172/JCI31073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Obici S, Zhang BB, Karkanias G, Rossetti L. Hypothalamic insulin signaling is required for inhibition of glucose production. Nat Med. 2002;8:1376–1382. doi: 10.1038/nm1202-798. [DOI] [PubMed] [Google Scholar]
- 17.Scherer T, O’Hare J, Diggs-Andrews K, Schweiger M, Cheng B, Lindtner C, Zielinski E, Vempati P, Su K, Dighe S, Milsom T, Puchowicz M, Scheja L, Zechner R, Fisher SJ, Previs SF, Buettner C. Brain insulin controls adipose tissue lipolysis and lipogenesis. Cell Metab. 2011;13:183–194. doi: 10.1016/j.cmet.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stein LJ, Dorsa DM, Baskin DG, Figlewicz DP, Ikeda H, Frankmann SP, Greenwood MR, Porte D, Jr, Woods SC. Immunoreactive insulin levels are elevated in the cerebrospinal fluid of genetically obese Zucker rats. Endocrinology. 1983;113:2299–2301. doi: 10.1210/endo-113-6-2299. [DOI] [PubMed] [Google Scholar]
- 19.Adam CL, Findlay PA, Aitken RP, Milne JS, Wallace JM. In vivo changes in central and peripheral insulin sensitivity in a large animal model of obesity. Endocrinology. 2012;153:3147–3157. doi: 10.1210/en.2012-1134. [DOI] [PubMed] [Google Scholar]
- 20.Owen OE, Reichard GA, Jr, Boden G, Shuman C. Comparative measurements of glucose, beta-hydroxybutyrate, acetoacetate, and insulin in blood and cerebrospinal fluid during starvation. Metabolism. 1974;23:7–14. doi: 10.1016/0026-0495(74)90098-5. [DOI] [PubMed] [Google Scholar]
- 21.Clegg DJ, Gotoh K, Kemp C, Wortman MD, Benoit SC, Brown LM, D’Alessio D, Tso P, Seeley RJ, Woods SC. Consumption of a high-fat diet induces central insulin resistance independent of adiposity. Physiol Behav. 2011;103:10–16. doi: 10.1016/j.physbeh.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Niswender KD, Morrison CD, Clegg DJ, Olson R, Baskin DG, Myers MG, Jr, Seeley RJ, Schwartz MW. Insulin activation of phosphatidylinositol 3-kinase in the hypothalamic arcuate nucleus: a key mediator of insulin-induced anorexia. Diabetes. 2003;52:227–231. doi: 10.2337/diabetes.52.2.227. [DOI] [PubMed] [Google Scholar]
- 23.Avruch J, Hara K, Lin Y, Liu M, Long X, Ortiz-Vega S, Yonezawa K. Insulin and amino-acid regulation of mTOR signaling and kinase activity through the Rheb GTPase. Oncogene. 2006;25:6361–6372. doi: 10.1038/sj.onc.1209882. [DOI] [PubMed] [Google Scholar]
- 24.Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Accili D, Arden KC. FoxOs at the crossroads of cellular metabolism, differentiation, and transformation. Cell. 2004;117:421–426. doi: 10.1016/s0092-8674(04)00452-0. [DOI] [PubMed] [Google Scholar]
- 26.Kim MS, Pak YK, Jang PG, Namkoong C, Choi YS, Won JC, Kim KS, Kim SW, Kim HS, Park JY, Kim YB, Lee KU. Role of hypothalamic Foxo1 in the regulation of food intake and energy homeostasis. Nat Neurosci. 2006;9:901–906. doi: 10.1038/nn1731. [DOI] [PubMed] [Google Scholar]
- 27.Ono H, Pocai A, Wang Y, Sakoda H, Asano T, Backer JM, Schwartz GJ, Rossetti L. Activation of hypothalamic S6 kinase mediates diet-induced hepatic insulin resistance in rats. J Clin Invest. 2008;118:2959–2968. doi: 10.1172/JCI34277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Woods SC, Seeley RJ, Cota D. Regulation of food intake through hypothalamic signaling networks involving mTOR. Annu Rev Nutr. 2008;28:295–311. doi: 10.1146/annurev.nutr.28.061807.155505. [DOI] [PubMed] [Google Scholar]
- 29.Kitamura T, Feng Y, Kitamura YI, Chua SC, Jr, Xu AW, Barsh GS, Rossetti L, Accili D. Forkhead protein FoxO1 mediates Agrp-dependent effects of leptin on food intake. Nat Med. 2006;12:534–540. doi: 10.1038/nm1392. [DOI] [PubMed] [Google Scholar]
- 30.Watterson KR, Bestow D, Gallagher J, Hamilton DL, Ashford FB, Meakin PJ, Ashford ML. Anorexigenic and orexigenic hormone modulation of Mammalian target of rapamycin complex 1 activity and the regulation of hypothalamic agouti-related protein mRNA expression. Neurosignals. 2013;21:28–41. doi: 10.1159/000334144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thompson PE, Manganiello V, Degerman E. Re-discovering PDE3 inhibitors--new opportunities for a long neglected target. Curr Top Med Chem. 2007;7:421–436. doi: 10.2174/156802607779941224. [DOI] [PubMed] [Google Scholar]
- 32.Zmuda-Trzebiatowska E, Oknianska A, Manganiello V, Degerman E. Role of PDE3B in insulin-induced glucose uptake, GLUT-4 translocation and lipogenesis in primary rat adipocytes. Cell Signal. 2006;18:382–390. doi: 10.1016/j.cellsig.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 33.Eriksson JW, Wesslau C, Smith U. The cGMP-inhibitable phosphodiesterase modulates glucose transport activation by insulin. Biochim Biophys Acta. 1994;1189:163–167. doi: 10.1016/0005-2736(94)90061-2. [DOI] [PubMed] [Google Scholar]
- 34.Sahu M, Litvin DG, Sahu A. Phosphodiesterase-3B is expressed in proopiomelanocortin and neuropeptide Y neurons in the mouse hypothalamus. Neurosci Lett. 2011;505:93–97. doi: 10.1016/j.neulet.2011.09.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reinhardt RR, Chin E, Zhou J, Taira M, Murata T, Manganiello VC, Bondy CA. Distinctive anatomical patterns of gene expression for cGMP-inhibited cyclic nucleotide phosphodiesterases. J Clin Invest. 1995;95:1528–1538. doi: 10.1172/JCI117825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao AZ, Zhao H, Teague J, Fujimoto W, Beavo JA. Attenuation of insulin secretion by insulin-like growth factor 1 is mediated through activation of phosphodiesterase 3B. Proc Natl Acad Sci USA. 1997;94:3223–3228. doi: 10.1073/pnas.94.7.3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nagaoka T, Shirakawa T, Kasuya J, Balon TW, Manganiello VC, Fujita-Yamaguchi Y. Cyclic nucleotide PDE-3. Quantitation of PDE-3A and -3B mRNAs in rat tissues by RNase protection assay. Cell Biochem Biophys. 1998;29:49–66. doi: 10.1007/BF02737828. [DOI] [PubMed] [Google Scholar]
- 38.Meacci E, Taira M, Moos M, Jr, Smith CJ, Movsesian MA, Degerman E, Belfrage P, Manganiello V. Molecular cloning and expression of human myocardial cGMP-inhibited cAMP phosphodiesterase. Proc Natl Acad Sci USA. 1992;89:3721–3725. doi: 10.1073/pnas.89.9.3721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Taira M, Hockman SC, Calvo JC, Belfrage P, Manganiello VC. Molecular cloning of the rat adipocytes hormone-sensitive cyclic GMP-inhibited cyclic nucleotide phosphodiesterase. J Biol Chem. 1993;268:18573–18579. [PubMed] [Google Scholar]
- 40.Kalra SP, Dube MG, Pu S, Xu B, Horvath TL, Kalra PS. Interacting appetite-regulating pathways in the hypothalamic regulation of body weight. Endocr Rev. 1999;20:68–100. doi: 10.1210/edrv.20.1.0357. [DOI] [PubMed] [Google Scholar]
- 41.Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. Central nervous system control of food intake and body weight. Nature. 2006;443:289–295. doi: 10.1038/nature05026. [DOI] [PubMed] [Google Scholar]
- 42.Sahu A. Leptin signaling in the hypothalamus: emphasis on energy homeostasis and leptin resistance. Front Neuroendocrinol. 2003;24:225–253. doi: 10.1016/j.yfrne.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 43.Sahu A. Minireview: A hypothalamic role in energy balance with special emphasis on leptin. Endocrinology. 2004;145:2613–2620. doi: 10.1210/en.2004-0032. [DOI] [PubMed] [Google Scholar]
- 44.Zhao AZ, Huan JN, Gupta S, Pal R, Sahu A. A phosphatidylinositol 3-kinase phosphodiesterase 3B-cyclic AMP pathway in hypothalamic action of leptin on feeding. Nat Neurosci. 2002;5:727–728. doi: 10.1038/nn885. [DOI] [PubMed] [Google Scholar]
- 45.Sahu A. Intracellular leptin-signaling pathways in hypothalamic neurons: the emerging role of phosphatidylinositol-3 kinase-phosphodiesterase-3B-cAMP pathway. Neuroendocrinology. 2011;93:201–210. doi: 10.1159/000326785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Beavo JA, Reifsnyder DH. Primary sequence of cyclic nucleotide phosphodiesterase isozymes and the design of selective inhibitors. Trends Pharmacol Sci. 1990;11:150–155. doi: 10.1016/0165-6147(90)90066-H. [DOI] [PubMed] [Google Scholar]
- 47.Sudo T, Tachibana K, Toga K, Tochizawa S, Inoue Y, Kimura Y, Hidaka H. Potent effects of novel anti-platelet aggregatory cilostamide analogues on recombinant cyclic nucleotide phosphodiesterase isozyme activity. Biochem Pharmacol. 2000;59:347–56. doi: 10.1016/s0006-2952(99)00346-9. [DOI] [PubMed] [Google Scholar]
- 48.Bender AT, Beavo JA. Cyclic nucleotide phosphodiesterases: molecular regulation to clinical use. Pharmacol Rev. 2006;58:488–520. doi: 10.1124/pr.58.3.5. [DOI] [PubMed] [Google Scholar]
- 49.Degerman E, Ahmad F, Chung YW, Guirguis E, Omar B, Stenson L, Manganiello V. From PDE3B to the regulation of energy homeostasis. Curr Opin Pharmacol. 2011;11:676–682. doi: 10.1016/j.coph.2011.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sahu M, Anamthathmakula P, Sahu A. Phosphodiesterase-3B-cAMP pathway of leptin signaling in the hypothalamus is impaired during the development of diet-induced obesity in FVB/N mice. J Neuroendocrinol. 2015;27:293–302. doi: 10.1111/jne.12266. [DOI] [PubMed] [Google Scholar]
- 51.Sahu A, Koshinaka K, Sahu M. Phosphatidylinositol 3-kinase is an upstream regulator of the phosphodiesterase 3B pathway of leptin signalling that may not involve activation of Akt in the rat hypothalamus. J Neuroendocrinol. 2013;25:168–179. doi: 10.1111/j.1365-2826.2012.02386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhao AZ, Bornfeldt KE, Beavo JA. Leptin inhibits insulin secretion by activation of phosphodiesterase 3B. J Clin Invest. 1998;102:869–873. doi: 10.1172/JCI3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhao AZ, Shinohara MM, Huang D, Shimizu M, Eldar-Finkelman H, Krebs EG, Beavo JA, Bornfeldt KE. Leptin induces insulin-like signaling that antagonizes cAMP elevation by glucagon in hepatocytes. J Biol Chem. 2000;275:11348–11354. doi: 10.1074/jbc.275.15.11348. [DOI] [PubMed] [Google Scholar]
- 54.Sahu A, Metlakunta AS. Hypothalamic phosphatidylinositol 3-kinase-phosphodiesterase 3B-cyclic AMP pathway of leptin signalling is impaired following chronic central leptin infusion. J Neuroendocrinol. 2005;17:720–726. doi: 10.1111/j.1365-2826.2005.01362.x. [DOI] [PubMed] [Google Scholar]
- 55.Metlakunta AS, Sahu M, Sahu A. Hypothalamic phosphatidylinositol 3-kinase pathway of leptin signaling is impaired during the development of diet-induced obesity in FVB/N mice. Endocrinology. 2008;149:1121–1128. doi: 10.1210/en.2007-1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hill JW, Williams KW, Ye C, Luo J, Balthasar N, Coppari R, Cowley MA, Cantley LC, Lowell BB, Elmquist JK. Acute effects of leptin require PI3K signaling in hypothalamic proopiomelanocortin neurons in mice. J Clin Invest. 2008;118:1796–1805. doi: 10.1172/JCI32964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Unger EK, Piper ML, Olofsson LE, Xu AW. Functional role of c-Jun-N-terminal kinase in feeding regulation. Endocrinology. 2010;151:671–682. doi: 10.1210/en.2009-0711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Caricilli AM, Penteado E, de Abreu LL, Quaresma PG, Santos AC, Guadagnini D, Razolli D, Mittestainer FC, Carvalheira JB, Velloso LA, Saad MJ, Prada PO. Topiramate treatment improves hypothalamic insulin and leptin signaling and action and reduces obesity in mice. Endocrinology. 2012;153:4401–4411. doi: 10.1210/en.2012-1272. [DOI] [PubMed] [Google Scholar]
- 59.Prada PO, Quaresma PG, Caricilli AM, Santos AC, Guadagnini D, Morari J, Weissmann L, Ropelle ER, Carvalheira JB, Velloso LA, Saad MJ. Tub has a key role in insulin and leptin signaling and action in vivo in hypothalamic nuclei. Diabetes. 2013;62:137–148. doi: 10.2337/db11-1388. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 60.Nikodemova M, Kasckow J, Liu H, Manganiello V, Aguilera G. Cyclic adenosine 3′,5′-monophosphate regulation of corticotropin-releasing hormone promoter activity in AtT-20 cells and in a transformed hypothalamic cell line. Endocrinology. 2003;144:1292–1300. doi: 10.1210/en.2002-220990. [DOI] [PubMed] [Google Scholar]
- 61.Sahu A. A role of phosphodiesterase-3B pathway in mediating leptin action on proopiomelanocortin and neurotensin neurons in the hypothalamus. Neuroscience Lett. 2010;479:18–21. doi: 10.1016/j.neulet.2010.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhai K, Chang Y, Wei B, Liu Q, Leblais V, Fischmeister R, Ji G. Phosphodiesterase types 3 and 4 regulate the phasic contraction of neonatal rat bladder smooth myocytes via distinct mechanisms. Cell Signal. 2014;26:1001–1010. doi: 10.1016/j.cellsig.2014.01.020. [DOI] [PubMed] [Google Scholar]
- 63.Anamthathmakula P, Sahu M, Sahu A. Evidence suggesting phosphodiesterase-3B regulation of NPY/AgRP gene expression in mHypoE-46 hypothalamic neurons. Neuroscience Lett. 2015;604:113–118. doi: 10.1016/j.neulet.2015.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Benoit SC, Air EL, Coolen LM, Strauss R, Jackman A, Clegg DJ, Seeley RJ, Woods SC. The catabolic action of insulin in the brain is mediated by melanocortins. J Neurosci. 2002;22:9048–9052. doi: 10.1523/JNEUROSCI.22-20-09048.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vogt MC, Bruning JC. CNS insulin signaling in the control of energy homeostasis and glucose metabolism - from embryo to old age. Trends Endocrinol Metab. 2013;24:76–84. doi: 10.1016/j.tem.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 66.Varela L, Horvath TL. Leptin and insulin pathways in POMC and AgRP neurons that modulate energy balance and glucose homeostasis. EMBO Rep. 2012;13:1079–1086. doi: 10.1038/embor.2012.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schwartz MW, Sipols AJ, Marks JL, Sanacora G, White JD, Scheurink A, Kahn SE, Baskin DG, Woods SC, Figlewicz DP, Porte D., Jr Inhibition of hypothalamic neuropeptide Y gene expression by insulin. Endocrinology. 1992;130:3608–3616. doi: 10.1210/endo.130.6.1597158. [DOI] [PubMed] [Google Scholar]
- 68.Luttinger D, King RA, Sheppard D, Strupp J, Nemeroff CB, Prange AJ., Jr The effect of neurotensin on food consumption in the rat. Eur J Pharmacol. 1982;81:499–503. doi: 10.1016/0014-2999(82)90116-9. [DOI] [PubMed] [Google Scholar]
- 69.Levine AS, Kneip J, Grace M, Morley JE. Effect of centrally administered neurotensin on multiple feeding paradigms. Pharmacol Biochem Behav. 1983;18:19–23. doi: 10.1016/0091-3057(83)90244-7. [DOI] [PubMed] [Google Scholar]
- 70.Stanley BG, Leibowitz SF, Eppel N, St-Pierre S, Hoebel BG. Suppression of norepinephrine-elicited feeding by neurotensin: evidence for behavioral, anatomical and pharmacological specificity. Brain Res. 1985;343:297–304. doi: 10.1016/0006-8993(85)90747-4. [DOI] [PubMed] [Google Scholar]
- 71.Leinninger GM, Opland DM, Jo YH, Faouzi M, Christensen L, Cappellucci LA, Rhodes CJ, Gnegy ME, Becker JB, Pothos EN, Seasholtz AF, Thompson RC, Myers MG., Jr Leptin action via neurotensin neurons controls orexin, the mesolimbic dopamine system and energy balance. Cell Metab. 2011;14:313–23. doi: 10.1016/j.cmet.2011.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vaisse C, Halaas JL, Horvath CM, Darnell JE, Jr, Stoffel M, Friedman JM. Leptin activation of Stat3 in the hypothalamus of wild-type and ob/ob mice but not db/db mice. Nat Genet. 1996;14:95–97. doi: 10.1038/ng0996-95. [DOI] [PubMed] [Google Scholar]
- 73.Mc Allister E, Pacheco-Lopez G, Woods SC, Langhans W. Inconsistencies in the hypophagic action of intracerebroventricular insulin in mice. Physiol Behav. 2015;151:623–628. doi: 10.1016/j.physbeh.2015.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Woods SC, Langhans W. Inconsistencies in the assessment of food intake. Am J Physiol Endocrinol Metab. 2012;303:E1408–E1418. doi: 10.1152/ajpendo.00415.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sahu A. Evidence suggesting that galanin (GAL), melanin-concentrating hormone (MCH), neurotensin (NT), proopiomelanocortin (POMC) and neuropeptide Y (NPY) are targets of leptin signaling in the hypothalamus. Endocrinology. 1998;139:795–798. doi: 10.1210/endo.139.2.5909. [DOI] [PubMed] [Google Scholar]
- 76.Sahu A, Caraway RE, Wang Y-P. Evidence that neurotensin mediates the central effect of leptin on food intake in rat. Brain Res. 2001;888:343–347. doi: 10.1016/s0006-8993(00)03107-3. [DOI] [PubMed] [Google Scholar]
- 77.Cui H, Cai F, Belsham DD. Anorexigenic hormones leptin, insulin, and alpha-melanocyte-stimulating hormone directly induce neurotensin (NT) gene expression in novel NT-expressing cell models. J Neurosci. 2005;25:9497–506. doi: 10.1523/JNEUROSCI.2269-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Williams KW, Margatho LO, Lee CE, Choi M, Lee S, Scott MM, Elias CF, Elmquist JK. Segregation of acute leptin and insulin effects in distinct populations of arcuate proopiomelanocortin neurons. J Neurosci. 2010;30:2472–2479. doi: 10.1523/JNEUROSCI.3118-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Belgardt BF, Husch A, Rother E, Ernst MB, Wunderlich FT, Hampel B, Klockener T, Alessi D, Kloppenburg P, Bruning JC. PDK1 deficiency in POMC-expressing cells reveals FOXO1-dependent and -independent pathways in control of energy homeostasis and stress response. Cell Metab. 2008;7:291–301. doi: 10.1016/j.cmet.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 80.Plum L, Lin HV, Dutia R, Tanaka J, Aizawa KS, Matsumoto M, Kim AJ, Cawley NX, Paik JH, Loh YP, DePinho RA, Wardlaw SL, Accili D. The obesity susceptibility gene Cpe links FoxO1 signaling in hypothalamic pro-opiomelanocortin neurons with regulation of food intake. Nat Med. 2009;15:1195–1201. doi: 10.1038/nm.2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhang R, Maratos-Flier E, Flier JS. Reduced adiposity and high-fat diet-induced adipose inflammation in mice deficient for phosphodiesterase 4B. Endocrinology. 2009;150:3076–3082. doi: 10.1210/en.2009-0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kelly MP, Adamowicz W, Bove S, Hartman AJ, Mariga A, Pathak G, Reinhart V, Romegialli A, Kleiman RJ. Select 3′,5′-cyclic nucleotide phosphodiesterases exhibit altered expression in the aged rodent brain. Cell Signal. 2014;26:383–397. doi: 10.1016/j.cellsig.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 83.Carvalheira JB, Siloto RM, Ignacchitti I, Brenelli SL, Carvalho CR, Leite A, Velloso LA, Gontijo JA, Saad MJ. Insulin modulates leptin induced STAT3 activation in rat hypothalamus. FEBS Lett. 2001;500:119–124. doi: 10.1016/s0014-5793(01)02591-1. [DOI] [PubMed] [Google Scholar]
- 84.Niswender KD, Morton GJ, Stearns WH, Rhodes CJ, Myers MG, Jr, Schwartz MW. Intracellular signaling. Key enzyme in leptin-induced anorexia. Nature. 2001;413:795–796. doi: 10.1038/35101657. [DOI] [PubMed] [Google Scholar]
- 85.Choi YH, Park S, Hockman S, Zmuda-Trzebiatowska E, Svennelid F, Haluzik M, Gavrilova O, Ahmad F, Pepin L, Napolitano M, Taira M, Sundler F, Stenson Holst L, Degerman E, Manganiello VC. Alterations in regulation of energy homeostasis in cyclic nucleotide phosphodiesterase 3B-null mice. J Clin Invest. 2006;116:3240–3251. doi: 10.1172/JCI24867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. New York, NY: Academic Press; 2004. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.