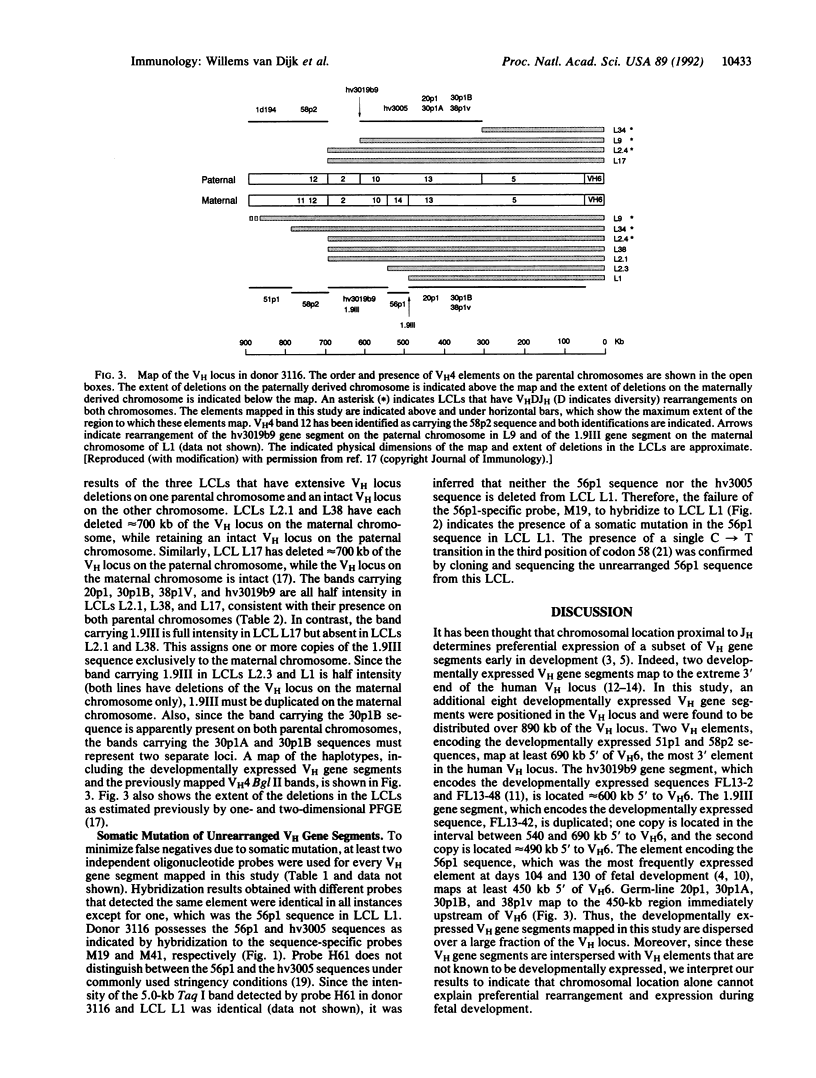
Abstract
The adult repertoire of antibody specificities is acquired in a developmentally programmed fashion that, in mouse and man, parallels the ordered rearrangement of a limited number of germ-line heavy chain variable region (VH) gene segments during development. It has been hypothesized that this developmental bias is a consequence of gene organization. In the mouse, rearrangement of VH gene segments proximal to the heavy chain joining region (JH) locus precedes rearrangement of genes located more distal to the JH locus. Similarly, in man, two VH elements located proximal to JH are expressed during fetal development. To test further this hypothesis in man, we have determined in a single individual the positions of an additional eight distinct VH elements known to comprise a significant fraction of the human developmental repertoire. These developmentally expressed VH elements were found to be dispersed over a region of 890 kilobases of the VH locus and were interspersed with other VH elements that are not known to be developmentally expressed. Thus, the ordered developmental expression of VH gene segments in man must involve mechanisms beyond physical proximity to the JH locus. Further, these results support the notion that fetal expression of VH gene segments is a regulated process and suggest that this regulation is important in the acquisition of immunocompetence.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alt F. W., Blackwell T. K., Yancopoulos G. D. Development of the primary antibody repertoire. Science. 1987 Nov 20;238(4830):1079–1087. doi: 10.1126/science.3317825. [DOI] [PubMed] [Google Scholar]
- Alt F. W., Yancopoulos G. D., Blackwell T. K., Wood C., Thomas E., Boss M., Coffman R., Rosenberg N., Tonegawa S., Baltimore D. Ordered rearrangement of immunoglobulin heavy chain variable region segments. EMBO J. 1984 Jun;3(6):1209–1219. doi: 10.1002/j.1460-2075.1984.tb01955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman J. E., Mellis S. J., Pollock R., Smith C. L., Suh H., Heinke B., Kowal C., Surti U., Chess L., Cantor C. R. Content and organization of the human Ig VH locus: definition of three new VH families and linkage to the Ig CH locus. EMBO J. 1988 Mar;7(3):727–738. doi: 10.1002/j.1460-2075.1988.tb02869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P. P., Liu M. F., Glass C. A., Sinha S., Kipps T. J., Carson D. A. Characterization of two immunoglobulin VH genes that are homologous to human rheumatoid factors. Arthritis Rheum. 1989 Jan;32(1):72–76. doi: 10.1002/anr.1780320112. [DOI] [PubMed] [Google Scholar]
- Chen P. P. Structural analyses of human developmentally regulated Vh3 genes. Scand J Immunol. 1990 Mar;31(3):257–267. doi: 10.1111/j.1365-3083.1990.tb02767.x. [DOI] [PubMed] [Google Scholar]
- Guillaume T., Rubinstein D. B., Young F., Tucker L., Logtenberg T., Schwartz R. S., Barrett K. J. Individual VH genes detected with oligonucleotide probes from the complementarity-determining regions. J Immunol. 1990 Sep 15;145(6):1934–1945. [PubMed] [Google Scholar]
- Jeong H. D., Teale J. M. VH gene family repertoire of resting B cells. Preferential use of D-proximal families early in development may be due to distinct B cell subsets. J Immunol. 1989 Oct 15;143(8):2752–2760. [PubMed] [Google Scholar]
- Johnston R. F., Pickett S. C., Barker D. L. Autoradiography using storage phosphor technology. Electrophoresis. 1990 May;11(5):355–360. doi: 10.1002/elps.1150110503. [DOI] [PubMed] [Google Scholar]
- Lichtman A. H., Tony H. P., Parker D. C., Abbas A. K. Antigen presentation by hapten-specific B lymphocytes. IV. Comparative ability of B cells to present specific antigen and anti-immunoglobulin antibody. J Immunol. 1987 May 1;138(9):2822–2825. [PubMed] [Google Scholar]
- Meek K., Eversole T., Capra J. D. Conservation of the most JH proximal Ig VH gene segment (VHVI) throughout primate evolution. J Immunol. 1991 Apr 1;146(7):2434–2438. [PubMed] [Google Scholar]
- Milner E. C., Lotshaw C. L., Willems van Dijk K., Charmley P., Concannon P., Schroeder H. W., Jr Isolation and mapping of a polymorphic DNA sequence pH30 on chromosome 4[HGM provisional no. D4S139]. Nucleic Acids Res. 1989 May 25;17(10):4002–4002. doi: 10.1093/nar/17.10.4002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickerson K. G., Berman J., Glickman E., Chess L., Alt F. W. Early human IgH gene assembly in Epstein-Barr virus-transformed fetal B cell lines. Preferential utilization of the most JH-proximal D segment (DQ52) and two unusual VH-related rearrangements. J Exp Med. 1989 Apr 1;169(4):1391–1403. doi: 10.1084/jem.169.4.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olee T., Yang P. M., Siminovitch K. A., Olsen N. J., Hillson J., Wu J., Kozin F., Carson D. A., Chen P. P. Molecular basis of an autoantibody-associated restriction fragment length polymorphism that confers susceptibility to autoimmune diseases. J Clin Invest. 1991 Jul;88(1):193–203. doi: 10.1172/JCI115277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlmutter R. M., Kearney J. F., Chang S. P., Hood L. E. Developmentally controlled expression of immunoglobulin VH genes. Science. 1985 Mar 29;227(4694):1597–1601. doi: 10.1126/science.3975629. [DOI] [PubMed] [Google Scholar]
- Raaphorst F. M., Timmers E., Kenter M. J., Van Tol M. J., Vossen J. M., Schuurman R. K. Restricted utilization of germ-line VH3 genes and short diverse third complementarity-determining regions (CDR3) in human fetal B lymphocyte immunoglobulin heavy chain rearrangements. Eur J Immunol. 1992 Jan;22(1):247–251. doi: 10.1002/eji.1830220136. [DOI] [PubMed] [Google Scholar]
- Sanz I., Kelly P., Williams C., Scholl S., Tucker P., Capra J. D. The smaller human VH gene families display remarkably little polymorphism. EMBO J. 1989 Dec 1;8(12):3741–3748. doi: 10.1002/j.1460-2075.1989.tb08550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasso E. H., Van Dijk K. W., Milner E. C. Prevalence and polymorphism of human VH3 genes. J Immunol. 1990 Oct 15;145(8):2751–2757. [PubMed] [Google Scholar]
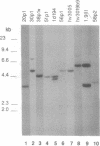
- Schroeder H. W., Jr, Hillson J. L., Perlmutter R. M. Early restriction of the human antibody repertoire. Science. 1987 Nov 6;238(4828):791–793. doi: 10.1126/science.3118465. [DOI] [PubMed] [Google Scholar]
- Schroeder H. W., Jr, Walter M. A., Hofker M. H., Ebens A., Willems van Dijk K., Liao L. C., Cox D. W., Milner E. C., Perlmutter R. M. Physical linkage of a human immunoglobulin heavy chain variable region gene segment to diversity and joining region elements. Proc Natl Acad Sci U S A. 1988 Nov;85(21):8196–8200. doi: 10.1073/pnas.85.21.8196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder H. W., Jr, Wang J. Y. Preferential utilization of conserved immunoglobulin heavy chain variable gene segments during human fetal life. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6146–6150. doi: 10.1073/pnas.87.16.6146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin E. K., Matsuda F., Nagaoka H., Fukita Y., Imai T., Yokoyama K., Soeda E., Honjo T. Physical map of the 3' region of the human immunoglobulin heavy chain locus: clustering of autoantibody-related variable segments in one haplotype. EMBO J. 1991 Dec;10(12):3641–3645. doi: 10.1002/j.1460-2075.1991.tb04930.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vakil M., Kearney J. F. Regulatory influences of neonatal multispecific antibodies on the developing B cell repertoire. Int Rev Immunol. 1988 Mar;3(1-2):117–131. doi: 10.3109/08830188809051185. [DOI] [PubMed] [Google Scholar]
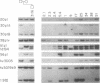
- Van Dijk K. W., Milner L. A., Milner E. C. Mapping of human H chain V region genes (VH4) using deletional analysis and pulsed field gel electrophoresis. J Immunol. 1992 May 1;148(9):2923–2931. [PubMed] [Google Scholar]
- Walter M. A., Dosch H. M., Cox D. W. A deletion map of the human immunoglobulin heavy chain variable region. J Exp Med. 1991 Aug 1;174(2):335–349. doi: 10.1084/jem.174.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter M. A., Surti U., Hofker M. H., Cox D. W. The physical organization of the human immunoglobulin heavy chain gene complex. EMBO J. 1990 Oct;9(10):3303–3313. doi: 10.1002/j.1460-2075.1990.tb07530.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willems van Dijk K., Schroeder H. W., Jr, Perlmutter R. M., Milner E. C. Heterogeneity in the human Ig VH locus. J Immunol. 1989 Apr 1;142(7):2547–2554. [PubMed] [Google Scholar]
- Yancopoulos G. D., Alt F. W. Regulation of the assembly and expression of variable-region genes. Annu Rev Immunol. 1986;4:339–368. doi: 10.1146/annurev.iy.04.040186.002011. [DOI] [PubMed] [Google Scholar]
- Yancopoulos G. D., Desiderio S. V., Paskind M., Kearney J. F., Baltimore D., Alt F. W. Preferential utilization of the most JH-proximal VH gene segments in pre-B-cell lines. Nature. 1984 Oct 25;311(5988):727–733. doi: 10.1038/311727a0. [DOI] [PubMed] [Google Scholar]
- van Dijk K. W., Sasso E. H., Milner E. C. Polymorphism of the human Ig VH4 gene family. J Immunol. 1991 May 15;146(10):3646–3651. [PubMed] [Google Scholar]