Abstract
One of the most consistent epidemiological associations between diet and human disease risk is the impact of red meat consumption (beef, pork, and lamb, particularly in processed forms). While risk estimates vary, associations are reported with all-cause mortality, colorectal and other carcinomas, atherosclerotic cardiovascular disease, type II diabetes, and possibly other inflammatory processes. There are many proposed explanations for these associations, some long discussed in the literature. Attempts to explain the effects of red meat consumption have invoked various red meat-associated agents, including saturated fat, high salt intake, Trimethylamine–N-oxide (TMAO) generation by microbiota, and environmental pollutants contaminating red meat, none of which are specific for red meat. Even the frequently mentioned polycyclic aromatic carcinogens arising from high temperature cooking methods are not red meat specific, as these are also generated by grilling poultry or fish, as well as by other forms of cooking. The traditional explanations that appear to be more red meat specific invoke the impact of N-nitroso compounds, heme iron, and the potential of heme to catalyze endogenous nitrosation. However, heme can be denatured by cooking, high levels of plasma hemopexin will block its tissue delivery, and much higher amounts of heme likely originate from red blood cell breakdown in vivo. Therefore, red meat-derived heme could only contribute to colorectal carcinoma risk, via direct local effects. Also, none of these mechanisms explain the apparent human propensity i.e., other carnivores have not been reported at high risk for all these diseases. A more recently proposed hypothesis involves infectious agents in beef from specific dairy cattle as agents of colorectal cancer. We have also described another mechanistic explanation for the human propensity for risk of red-meat associated diseases that is consistent with most observations: metabolic incorporation of a non-human sialic acid N-glycolylneuraminic acid (Neu5Gc) into the tissues of red meat consumers and the subsequent interaction with inflammation-provoking antibodies against this “xenoautoantigen”. Overall, we conclude that while multiple mechanisms are likely operative, many proposed theories to date are not specific for red meat, and that the viral and xenoautoantigen theories deserve further consideration. Importantly, there are potential non-toxic dietary antidotes, if the xenoautoantigen is indeed correct.
Keywords: Red meat, Disease Risk, Epidemiology, Inflammation, Carcinomas, Atherosclerosis, Diabetes
1.0. Introduction
1.1. Background Information and Scope of this Review
A recent World Health Organization-International Agency for Research on Cancer (WHO-IARC) monograph summary emphasized the carcinogenicity of consumption of red meat and processed red meat (Bouvard et al., 2015). By definition, red meat refers to all types of mammalian muscle meat, such as beef, veal, pork, lamb, mutton, horse, and goat. The term “processed meat” refers to meat that has been transformed through salting, curing, fermentation, smoking, or other processes to enhance flavor or improve preservation (Bouvard et al., 2015). Long-term consumption of red meat (and even more clearly processed meat) is associated with significant increase in all-cause mortality (Larsson and Orsini, 2014; Pan et al., 2012; Sinha et al., 2009a), likely contributing to the current epidemic of cardiovascular diseases (Micha et al., 2010; Micha et al., 2012), type 2 diabetes (Pan et al., 2011; Micha et al., 2010; Aune et al., 2009), and to increased risk of certain kinds of adenocarcinomas (cancers of mucosal epithelial origin), particularly colorectal cancer (Aune et al., 2013; Cross et al., 2010; Cross and Sinha, 2004). Aggravation of age-dependent macular degeneration (Ersoy et al., 2014; Chong et al., 2009) and of rheumatoid arthritis (Benito-Garcia et al., 2007; Choi, 2004; Oliver and Silman, 2006) have also been reported as being associated with red meat consumption. Corroborating with these facts, Seventh-Day Adventists consuming a vegetarian diet are at lower risks of cancer, diabetes mellitus, hypertension, and arthritis when compared to non-vegetarians from the same community (Fraser, 1999; Phillips et al., 1980). In fact, follow up studies show that a lifestyle pattern that includes a very low meat intake is associated with greater longevity (Singh et al., 2003; Singh et al., 2003).
There are many proposed mechanisms for the disease-promoting effects of red meat. These include DNA damage due to N-nitroso compounds (NOCs) and mutagens generation by high temperature grilling; high dietary intake of salt and saturated fat; pro-oxidant effects of heme and iron; and production of Trimethylamine–N-oxide (TMAO) by the gut microbiome. Here, we summarize and compare the major mechanistic hypotheses proposed to date. We will also outline a new theory regarding a virus present in beef, and then discuss a recent “xenoglycan” theory, which seems most consistent with available data, and is the one that could best explain the apparent human propensity of the risk.
1.2. Red Meat Consumption in Human Evolution and Reproductive Success
The evolution of the human species was much influenced by dietary changes, especially during the last two million years (Milton, 2003; Ye and Gu, 2011). With the improvement of stone tools, sustained running ability, scavenging and hunting, hominin ancestors in the genus Homo (Antón et al., 2014) began to access more animal-derived foods during the Pliocene period (Bramble and Lieberman, 2004; Domínguez-Rodrigo et al., 2005; Schoeninger, 2012). Diverging from other primates and earlier hominins whose diets mainly consisted of fruits and plants, the genus Homo appears to have transitioned to one rich in animal sources (particularly large game animals, i.e. “red meats”) which are energy dense and easily digestible foods that can provide all essential amino acids and micronutrients (Millward, 1999). Some writers have proposed that this dietary transition supported evolutionary selection for significant physiologic and anatomic changes in Homo, such as increase of the brain size and reduced gut volume (Aiello and Wheeler, 1995; Mann, 2000; Milton, 2003). In addition, emerging evidence indicates that human dietary habits contribute to microbiome diversity and its effects on human health (He et al., 2013b).
Although the introduction of animal products into ancestral human diet had many benefits, large-scale red meat consumption by modern society has contributed to epidemics of diseases such as zoonotic infections (Diaz-Sanchez et al., 2013), cardiovascular diseases and cancer, while also contributing to major environmental degradation, and even to global climate disruption (McMichael et al., 2007; Eshel et al., 2014; Springmann et al., 2016). Here, we focus on the impact of red meat consumption on current human diseases.
2. Existing Theories to Explain Increased Disease Risks of Red Meat Consumption
2.1. Theories That Are Not Specific to Red Meat
2.1.1. Carcinogenic compounds produced by cooking methods
Known mutagens found in red meat are heterocyclic amines (HCAs) and polycyclic aromatic hydrocarbons (PAHs), which are generated by cooking meat at high temperatures and for long durations (Skog et al., 1998; Knize et al., 1999; Turesky, 2007; Turesky and Le Marchand, 2011). In fact, PAHs are generated at various concentrations by a variety of cooking methods, including baking and grilling (Phillips, 1999; Turesky, 2007; Rose et al., 2015). Similar levels of PAHs are also generated in grilling chicken or fish (Sugimura et al., 2004; Yano et al., 1988), making dietary exposure of non-vegetarian humans to PAHs essentially unavoidable. In addition, PAHs are also generated during smoking of processed foods (Olatunji et al., 2014).
The formation of HCAs is induced by the endogenous reaction between creatine and amino acids or carbohydrates when muscle meat is cooked at high temperature (>150°C) (Jägerstad et al., 1991). Although the most common HCAs found in human diets have been shown to be carcinogenic in rodents (Shirai et al., 1995; Ito et al., 1997), epidemiologic studies have produced inconsistent data regarding associations of HCAs with human cancer (Augustsson et al., 1999; Joshi et al., 2015). In fact, while many authors have found a positive association between HCA’s and breast, prostate, lung, and renal cancer (Ferrucci et al., 2009; Cross et al., 2005; Tasevska et al., 2009; Daniel et al., 2012), a recent prospective analysis revealed that intake of these meat-derived mutagens was not significantly associated with colorectal cancer risk (Le et al., 2016). Although studies have shown carcinogenic effects of both HCA and PAH mutagens in animal models, these experiments typically used doses higher than usual human exposure (Ohgaki et al., 1986; Magee, 1989). Regardless, compounds derived from metabolism of dietary aromatic amines have been suggested as biomarkers, to monitor carcinogenic effects in humans (Guo et al., 2016; Turesky and Le Marchand, 2011).
While HCAs and PAHs are also present in processed or high temperature cooked fish, grilled or fried chicken actually contain higher levels of heterocyclic amines than does beef meat (Heddle et al., 2001). However, consumption of poultry or fish are generally not associated with cancer risk (Wiseman, 2008; Norat et al., 2002; Larsson and Wolk, 2006; Huxley et al., 2009; Cross et al., 2007; English et al., 2004). Based on all these facts, it seems reasonable to suggest that, while these mutagens may indeed be carcinogenic in humans, they do not provide a specific explanation for the carcinogenic effects of either processed or non-processed red meat per se.
2.1.2. Environmental Pollutants
Red meat also contains contaminating inorganic toxins, such as arsenic (As), cadmium (Cd), mercury (Hg), lead (Pb), pesticides among many others (Domingo and Nadal, 2016). These toxins can be derived from cooking processes, or from industrial sources during meat processing. These compounds are also found in comparable amounts in many dietary products, including fish, poultry, and vegetables, and may represent a general risk for human health. But again, they cannot explain the increased risk of disease specifically associated with red meat.
2.1.3. High Content of Saturated Fat
The fat content of red meat varies depending on animal species, age, sex, breed, feed, and the cut of the meat. Some have proposed that high intake of saturated fat (for which red meats can be a major source) contributes to obesity and general inflammation, insulin resistance, and intestinal dysbiosis (Calle and Kaaks, 2004; Schulz et al., 2014). In addition, oxidation of red meat derived fat leads to the formation of oxyesterols and aldehydes that may alter transforming growth factor beta (TGF-β)-mediated signal and cell proliferation (Biasi et al., 2008). Although all these factors together might contribute to the carcinogenic properties of red meat, they are not specific to red meat, as high content of saturated fats occur in other sources such as whole milk, cheeses, eggs and even certain vegetable sources e.g., coconut and palm oils. Moreover, recent epidemiological studies show inconsistent associations between saturated fat intake and the risk of prostate and breast carcinomas (Pelser et al., 2013; Xia et al., 2015).
2.1.4. High Salt Content of Processed Meats
Excessive salt intake can contribute to increased blood pressure (Weinberger, 1996; Weinberger, 2006) and thus secondarily to the development of cardiovascular and renal disease (Whelton et al., 2012; He et al., 2013a; Kotchen et al., 2013; Mozaffarian et al., 2014). High salt content would be particularly prominent in some kinds of processed meats. Highly salted foods have also been associated with some kinds of cancer risk (Tsugane et al., 2004). However, there are many alternative sources of salt in the diet, and there is no evidence that red meat represents a primary or even major culprit.
2.1.5. Production of Trimethylamine–N-oxide (TMAO) by the Gut Microbiome
Recent studies revealed the role of microbiota in generating compounds that affect the human host (Tremaroli and Bäckhed, 2012). Trimethylamine–N-oxide (TMAO) had been shown to arise from bacterial metabolism of choline or L-carnitine via an intermediate, trimethylamine (TMA), and subsequent hepatic oxidation to TMAO via flavin monooxygenase 3 (FMO3) (Wang et al., 2011; Koeth et al., 2013). Elevated levels of TMAO in the plasma have also been associated with increased risk of cardiovascular disease (CVD)(Wang et al., 2011; Koeth et al., 2013; Tang et al., 2013). The proposed mechanism involves inhibition of reverse cholesterol transport from the macrophages promoting foam cell formation in atherosclerosis lesion, as well as the alteration of bile acids pool size (Koeth et al., 2013). Recent studies also showed that TMAO has a direct effect on platelet function in vitro and in vivo – leading to enhanced thrombosis risk (Zhu et al., 2016) and that TMAO directly promotes enhanced arterial endothelial cell inflammatory gene expression changes in vivo (Seldin et al., 2016). Positive associations of plasma TMAO levels and colorectal cancer were recently reported as well (Bae et al., 2014; Xu et al., 2015). High plasma levels of carnitine were also reported to be significantly associated with incident risks for myocardial infarction, stroke, or death over a follow-up period of 3 years, but only in subjects with concurrently high TMAO levels (Koeth et al., 2013).
However, although the TMAO precursor L-carnitine is indeed found at higher levels in red meat (~100 mg in 100 g of beef) than in fish or chicken (~5 mg in 100 g codfish or chicken) (Traber et al., 1999), the much more abundant TMAO precursor choline is an essential nutrient present in most animal and some plant products; e.g., in egg yolk (250 mg in 100 g), meats and fish (~75 mg in 100 g), whole grains (~70 mg in 100 g), vegetable and fruits (~ 25mg in 100 g) (Patterson et al., 2008). Furthermore, some fish are significantly rich in TMAO (around 20 to 120 mg TMAO in 100 g) (Seibel and Walsh, 2002). Adults eating mixed diets that include red meat and other animal products ingest about 60–180 mg of carnitine per day (Rebouche, 2004), and about 300–400 mg of choline per day (Chiuve et al., 2007; Wallace and Fulgoni, 2016).
Meanwhile, supplementation with carnitine (approximately 3–6 g/day) has been reported to have potential benefits in some studies e.g., it has been claimed to improve mental dysfunction in older adults with early Alzheimer’s disease (Montgomery et al., 2003); to improve walking in patients with claudication (Brass et al., 2013); and to relieve nerve pain associated with diabetic neuropathy (Sima et al., 2005). Moreover, combinations of intravenous loading and oral ingestion of L-carnitine seems to have the potential to reduce short-term mortality following acute myocardial infarction (Tarantini et al., 2006). However, recent meta-analysis studies revealed conflicting effects for the secondary prevention of cardiovascular disease (CVD) by L-carnitine administration (DiNicolantonio et al., 2013; Shang et al., 2014).
Normal renal function maintains a narrow L-carnitine concentration in the circulation in the range of 40–60 μmol/L (Rebouche, 2004). Chronic kidney disease (CKD) patients who undergo hemodialysis are at risk for secondary carnitine deficiency because hemodialysis removes carnitine from the blood. While CVD is known as one of the major cause of death in CKD patients, the association between plasma TMAO level and CVD risk in CKD is debated (Kim et al., 2016; Stubbs et al., 2016; Tang et al., 2015). Overall, while TMAO derived from endogenous and exogenous sources of choline and carnitine may contribute to increased atherosclerotic vascular disease, red meat does not appear to be a major source of this compound, relative to other foods.
2.2. Theories That Appear More Specific to Red Meat
2.2.1. N-Nitroso-Compounds (NOCs) as Mutagens
One of the proposed mechanisms for the cancer-promoting effects of red meat is DNA damage due to the conversion of nitrates and nitrites in processed meat into NOCs, multi-site carcinogens that can proceed to form covalent adducts with DNA bases and potentially contribute to a wide range of malignancies (Mirvish, 1995; Bingham et al., 1996; Knekt et al., 1999; Parnaud et al., 2000; Santarelli et al., 2008; Kim et al., 2013; Dellavalle et al., 2014; Catsburg et al., 2014). In vitro exposure of human colorectal cells to NOCs can indeed induce DNA alkylation (Povey et al., 2002) and consequent mutation in genes involved in DNA damage control and in cell proliferation and differentiation such as K-RAS (Hebels et al., 2009; Hebels et al., 2010). In addition, endogenous formation of NOCs can be catalyzed by red meat-derived heme iron (Cross et al., 2002; Cross et al., 2003) and promote carcinogenesis in rats fed with low calcium diets. Thus, calcium present at physiologic concentrations is hypothesized to trap heme iron and thereby inhibit nitrosation as discussed below (Pierre et al., 2003).
In fact, human volunteers fed with diets rich in red meat have shown increased levels of fecal NOCs and NOC-specific DNA adducts in exfoliated colonic cells when compared to volunteers fed vegetarian diets (Lewin et al., 2006). Corroborating these data, a recent study has shown that high red meat intake is associated with increased levels of the O6-Methyl-2′-deoxyguanosine mutagenic adduct in rectal epithelial cells and that a concomitant intake of fiber-derived products, such as butyrylated high-amylose, prevented this red meat-induced adduct formation (Le Leu et al., 2015). Gastrointestinal diseases, such as inflammatory bowel disease (IBD), can also induce the production of NOCs and thus potentially increase the risk of cancer (de Kok et al., 2005).
While studies have shown a positive dose response in the levels of apparent total NOCs in the fecal samples of human volunteers given different quantities of red meat (Hughes et al., 2001; Bingham et al., 2002), many epidemiological studies found modest or no association between dietary NOCs and several types of cancer, including esophagus (Keszei et al., 2013; González et al., 2006), stomach (Keszei et al., 2013; Song et al., 2015), colorectal (Knekt et al., 1999; Dellavalle et al., 2014), and bladder cancer (Catsburg et al., 2014; Ferrucci et al., 2010; Zeegers et al., 2006). Of course, the absence of a biomarker for NOC long-term exposure as well as methods for distinguishing endogenous nitrosation from dietary intake of nitrate/nitrite limit our ability to validate the proposed association of NOC exposure with the risk of colorectal cancer. Thus, studies have also examined the role of nitrate and nitrite in relation to cancer incidence (Cross et al., 2010; Dubrow et al., 2010).
2.2.2. Oxidative and Chemical Transformative Properties of Heme Iron
The iron-carrying pigment heme is non-covalently associated with hemoglobin and myoglobin and gives red meat its distinctive color (Balla et al., 2007; Nagy et al., 2010; Livingston and Brown, 1981; Suman and Joseph, 2013). In light of the cytotoxic and potentially DNA-damaging oxidative properties of heme iron (Sesink et al., 2000), various epidemiological analyses have studied the association between intake of red meat-derived heme and development of carcinoma risk and found associations between these processes (Tasevska et al., 2009; Sinha et al., 2009b; Jakszyn et al., 2013), with the strongest association being found with colorectal cancer risk (Suman and Joseph, 2013; Bastide et al., 2011; Kim et al., 2013; Sesink et al., 1999). In fact, studies in rodents showed that a diet rich in heme increases proliferation and incidence of colon tumors, possibly due to increased levels of peroxyl radicals (Pierre et al., 2003). Yet another study showed a correlation between different levels of dietary heme and the promotion of colon carcinogenesis in rats (Pierre et al., 2004). More recently, additional in vivo studies showed that heme-induced oxidative stress and generation of free radicals can cause hyperproliferation and hyperplasia of mouse colon cells that eventually develop into colorectal cancer (IJssennagger et al., 2012b). Of note, heme is also bioavailable at high levels in the colon – more than 90% of heme reaches the colon since it is poorly absorbed in the small intestine (Young et al., 1989).
Nonetheless, it is very likely that any effects of dietary heme are limited to the gastrointestinal tract. This is because the vast stoichiometric excess of the high affinity heme scavenger protein hemopexin that is present in the plasma (Schaer et al., 2013) would quickly sequester any heme that enters the blood stream from the gut. In addition, heme derivatives released locally from damaged red blood cells within the abnormal neovasculature of cancers (Yin et al., 2015) and atheromas (Balla et al., 2007) seem much more likely to serve as the main sources of heme oxidant compounds in tissues throughout the body. Indeed, it is reported that the interior of advanced atheromatous lesions functions as a pro-oxidant environment in which erythrocytes breakdown and release both heme and iron to promote further lipid oxidation, thereby amplifying endothelial cell cytotoxicity (Balla et al., 1991; Balla et al., 1993; Belcher et al., 1993; Nagy et al., 2010). Likewise, neovascularization and hemorrhage are common features of malignant tumors and hemoglobin derived from extravasated RBC deposits heme iron within the tumor (Cermak et al., 1993). Overall, the relative contribution of dietary heme to disease risk via direct toxicity should be minimal, except by direct exposure in the gastrointestinal tract.
Dietary heme can also provoke changes in the gut microbiota that favor the hyperproliferation of colonic enterocytes and disturb the mucus barrier in the colon epithelia, amplifying the potential carcinogenic properties (IJssennagger et al., 2012b; IJssennagger et al., 2012a; Ijssennagger et al., 2015). Additional studies show that red meat-derived heme can promote fat peroxidation and generation of the carcinogenic compounds malondialdehyde, 4-hydroxyl-noneal and NOCs, providing plausible mechanisms underlying the role of heme iron in the promotion of colon cancer by processed red meat (Pierre et al., 2013; Bastide et al., 2015). The authors propose that this is a red meat-specific mechanism for the promotion of colorectal cancer since dietary intake of “white meat” as chicken did not induce the generation of the same carcinogenic compounds (Cross et al., 2003; Bastide et al., 2011).
One confounding issue is that exposure of heme-containing proteins to temperatures as high as 90°C leads to denaturation and drastic reduction of its pro-oxidative properties (Bou et al., 2008; Bou et al., 2010). An early study claimed that heating had no apparent effect on the heme content in fecal samples, but this was measured by a color detection test that is at best semi-quantitative (Schwartz and Ellefson, 1985). In fact, since many cuisines tend to cook their meat at high temperatures (turning the color of red meat to brown) much of the ingested heme may already be denatured prior to its consumption (Knöbel et al., 2007; Pierre et al., 2010).
Elemental iron released from heme is also considered toxic (Belcher et al., 2010). However, while subjects with hereditary hemochromatosis or acquired systemic iron overload are known to have a higher risk of developing colorectal (Shaheen et al., 2003) and liver cancer, the risk for the latter is specifically associated with chronic hepatocyte damage progressing to cirrhosis (Fonseca-Nunes et al., 2014). Such patients also do not show an obviously overall increased incidence of other tumors (Vinchi et al., 2014). Last but not least, red meat is also defined by its heme-derived red color, making heme intake already a direct proxy for overall red meat intake. Considering all these facts we can conclude that while dietary heme and iron from red meat may contribute to colorectal (and possibly esophageal) carcinoma risk, it plays a minor role if any in other cancers. Moreover, since patients with high iron burden do not exhibit an obviously increased incidence of atherosclerosis (Vinchi et al., 2014), it is also unlikely that red meat-derived iron aggravates the development of atherosclerosis.
2.3. Theories that Appear Specific to Red Meat, and Appear to be Human-specific
2.3.1. Species-specific Infectious Agent Found in Dairy Cattle Red Meat
The classic studies of zur Hausen (Boshart et al., 1984; Schwarz et al., 1985) eventually prevailed against the widespread skepticism of an etiologic role of viruses in human cancer development by showing that human cervical cancer is primarily caused by human papilloma virus infections, with the resulting introduction of effective vaccines and the awarding of the Nobel Prize in 2008 (zur Hausen, 2009). Zur Hausen has now suggested the possibility that consumption of red meat specifically derived from the species Bos taurus may best explain the global patterns of colorectal cancer risk (Zur Hausen, 2012). He has hypothesized that the intriguing epidemiological variance in the incidence of colorectal cancer between countries that consume high amounts of red meat can be explained by the fact that red meat-derived products from mammals of different species across the world are associated with greatly differing levels of contaminating cancer-causing viruses (zur Hausen and de Villiers, 2015). Thus, countries like Mongolia and Bolivia consume high levels of red meat derived from Yaks (Bos grunniens and Bos mutus) and several sub-species of Taurines (Bos taurus turano-mongolicus) (Maytsetseg, 2006) but nevertheless exhibit relatively low incidence rates of colorectal cancer. In contrast, the red meat and dairy products in regions with high risk for colorectal cancer are derived mostly from cattle of Bos taurus species (Zur Hausen, 2012).
Based on these epidemiological observations, the authors propose that Bos taurus could carry and transmit a factor involved in colon cancer etiology (Zur Hausen, 2012). Although not yet formally identified, they propose that single-stranded circular DNAs isolated from milk and serum of healthy cattle (Funk et al., 2014; Lamberto et al., 2014) might signal the presence of such factors. The authors postulate that these species-specific infectious agents could be potentially carcinogenic when transmitted to humans and act synergistically with compounds originated during processing or cooking of beef (zur Hausen and de Villiers, 2015). In fact, there is still no experimental evidence showing a direct correlation of these infectious agents with development of colorectal cancer. Nonetheless, this new hypothesis deserves more attention.
2.3.2. Human-Specific Mechanism Involving a Non-human glycan in Red Meat
2.3.2.1. Historical Background and Discovery
Besides being enriched in saturated fat and heme iron, red meats are also enriched (beef > pork & lamb) in glycans containing a particular variant of sialic acid called N-glycolylneuraminic acid (Neu5Gc) (Tangvoranuntakul et al., 2003; Samraj et al., 2014b) Samraj et al., 2014, #53932}. This Neu5Gc molecule is not naturally found in human tissues, due to a specific exon deletion mutation that occurred around 2–3 million years ago in the germ line of one of our hominin ancestors. The affected gene encodes the enzyme, cytidine monophospho-N-acetylneuraminic acid hydroxylase (CMAH), which is responsible for the generation of Neu5Gc from the precursor sialic acid N-acetylneuraminic acid (Neu5Ac) (Figure 1), (Chou et al., 1998; Chou et al., 2002; Hayakawa et al., 2006; Hayakawa et al., 2006), and the pseudogene state is now fixed in the genomes of all humans. Despite the inability of humans to produce Neu5Gc endogenously, this non-human isoform of sialic acid can be detected in small amounts in human epithelial and endothelial cells (Tangvoranuntakul et al., 2003) and also in human carcinomas (Malykh et al., 2001a; Samraj et al., 2014a) (Figure 1). Mice engineered to have a human-like mutation in the Cmah gene, which encodes the murine enzyme that generates Neu5Gc, show no evidence of any alternate pathway for Neu5Gc biosynthesis (Hedlund et al., 2007). Thus, metabolic incorporation via dietary consumption is the only possible source of the Neu5Gc that is found in human tissues.
Figure 1. Potential Disease Risks associated with Metabolic incorporation of the Red meat-derived Non-human Sialic Acid N-glycolylneuraminic acid (Neu5Gc).
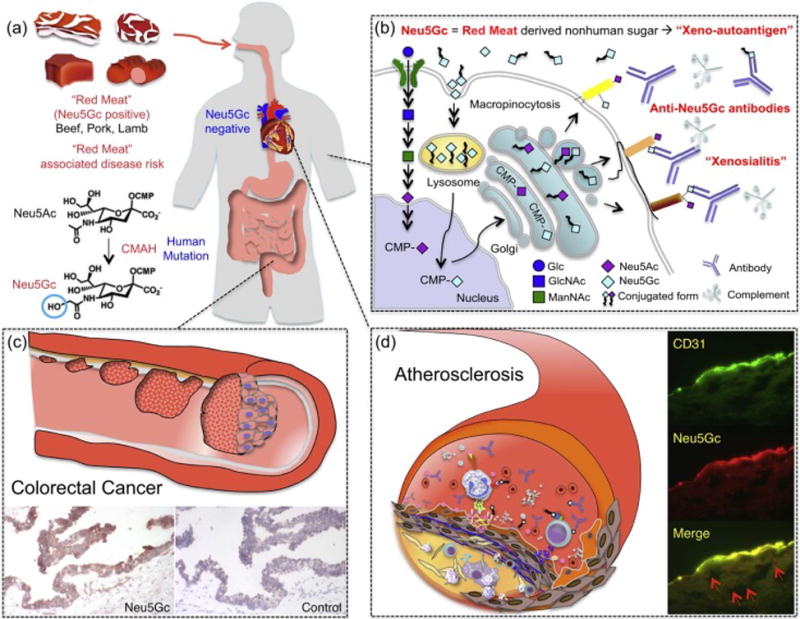
a) Red meat is a dietary source rich in (Neu5Gc) (beef, pork, lamb). Humans cannot synthesize Neu5Gc, due to a specific exon deletion in the gene encoding the enzyme cytidine monophospho-N-acetylneuraminic acid hydroxylase (CMAH), responsible for the generation of Neu5Gc from the precursor sialic acid N-acetylneuraminic acid (Neu5Ac). b) Neu5Gc can be incorporated into human cells through the same pathway used for Neu5Ac recycling. Endocytosed Neu5Gc is used as substrate for the synthesis of sialylated glycans in the Golgi. Cell surface glycans containing Neu5Gc may be targeted by circulating anti-Neu5Gc antibodies and complement, leading to a human specific inflammation, termed xenosialitis. c) Neu5Gc incorporation in human epithelia or endothelia and subsequent xenosialitis may be a risk factor for the promotion of colorectal tumor growth, atherosclerosis (d), and other inflammatory diseases associated with red meat consumption. Neu5Gc is detected in human colorectal cancer cells (c), in endothelial cells (CD31 positive) and subendothelial components (red arrow) in human atherosclerotic lesions (d). A part of (d) was originally published in Pham et al., 2009, Blood, 114: 5225–35. © by the American Society of Hematology.)
In keeping with this conclusion, human volunteer studies (Tangvoranuntakul et al., 2003) suggested that humans can metabolically incorporate and express Neu5Gc into cell surface glycoconjugates. Feeding of human epithelial cells with Neu5Gc leads to its incorporation (Bardor et al., 2005), with enrichment in mucins (Inoue et al., 2010). However, further studies are required to understand specific glycoconjugates on which Neu5Gc can be found in human tissues, and the nature of the associated underlying glycan structures. Regardless, taken together with the metabolic incorporation of Neu5Gc, Neu5Gc-containing glycans appear to act as “xenoautoantigens” that can be targeted by naturally circulating anti-Neu5Gc “xenoautoantibodies”, leading to an inflammatory process termed “xenosialitis” (Hedlund et al., 2008; Padler-Karavani et al., 2011; Pearce et al., 2014).
Xenosialitis can potentially affect both cancer initiation and progression. In fact, the tumor-promoting properties of auto-reactive antibodies are well described in other systems (Andreu et al., 2010; Wu et al., 2013). Indeed, such a process could be demonstrated in human-like Cmah null mice fed with Neu5Gc and infused with anti-Neu5Gc antibodies for other null mice that had been preimmunized (Samraj et al., 2014b). Moreover, when such Neu5Gc-fed mice were immunized to express anti-Neu5Gc antibodies and followed for over one year on a Neu5Gc-rich diet, they showed a markedly increased risk of adenoma-to-carcinoma progression in the liver, in association with Neu5Gc incorporation into the tumors (Samraj et al., 2014b) (see further discussion below). Taken together, these data indicate that the inflammatory xenosialitis triggered by red meat-derived Neu5Gc and anti-Neu5Gc antibodies represents a mechanism that is unique to humans and could be involved both in carcinogenesis and in promoting carcinoma progression. Overall, as argued below, we suggest that red meat-derived Neu5Gc-induced xenosialitis may be the only mechanism that provides an internally consistent explanation for human-specific aggravation of carcinoma risk associated with red meat consumption.
2.3.2.2. Complexities and Diversity of Human Anti-Neu5Gc Antibodies
Although their target was then unknown, anti-Neu5Gc antibodies were actually described almost a century ago, when Hanganutziu and Deicher observed that serum from patients that received animal antisera during therapy, could agglutinate animal red blood cells (Hanganutziu, 1924; Hanganutziu, 1924; Beer, 1936). Many years later, similar antibodies were also found in patients with autoimmune diseases, such as rheumatoid arthritis and Kawasaki disease and various types of cancer (Nishimaki et al., 1978a; Nishimaki et al., 1978b; Arita et al., 1982; Ikuta et al., 1982; Hokke et al., 1990; Gathuru et al., 1989; Higashihara et al., 1991). Subsequent studies then showed that these antibodies were directed against Neu5Gc and could target carcinoma antigens such as the monosialoganglioside (Neu5Gc)GM3 (Higashi et al., 1977; Asaoka et al., 1992; Malykh et al., 2001b). Because (Neu5Gc)GM3 was a well-known target for anti-Neu5Gc antibodies, it was used as capture antigen to detect anti-Neu5Gc antibodies in human individuals. ELISA assays using (Neu5Gc)GM3 revealed that less than (<1–2%) of total IgG were anti-Neu5Gc antibodies in healthy subjects (Merrick et al., 1978; Morito et al., 1982; Higashihara et al., 1991).
Sialic acids like Neu5Gc are terminal monosaccharides on both glycolipids and glycoproteins, generally attached to underlying glycans in an α2-3-linkage to Gal, an α2-6-linkage to Gal and GalNAc, or an α2-8 linkage to another sialic acid. Additionally, monosaccharides attached to Neu5Gc can also be part of the epitope recognized. Overall, there are hundreds of possible alternative Neu5Gc-containing epitopes. Thus, the use of (Neu5Gc)GM3 as the only target to detect anti-Neu5Gc led, for a long time, to the under-detection of the presence and diversity of these human antibodies. Using a more precise method that included glycans with α2-3 or α2-6-linkage to different underlying structures, we demonstrated that human anti-Neu5Gc antibodies are of broad and variable specificities (Padler-Karavani et al., 2008). In fact, while some individuals express high levels of anti-Neu5Gc IgGs that are reactive to most of the glycans structures tested, others showed no reactivity whatsoever. Comparisons of the presence of IgG, IgM or IgA subclasses of anti-Neu5Gc antibodies revealed a broad diversity between the individuals tested. In addition, this study showed that anti-Neu5Gc antibodies could be found in higher levels than some xenoreactive and natural blood group antibodies in some individuals (Padler-Karavani et al., 2008).
Precise serum analysis for anti-Neu5Gc antibodies requires the use of glycan arrays with multiple alternative molecular structures as capture antigens. However, not all possible structures are available for inclusion in such arrays, and there are only limited data available about the levels of specific anti-Neu5Gc antibodies in patients with inflammatory diseases related to red meat consumption. Also, there are no studies showing a correlation between the levels of anti-Neu5Gc antibodies and amounts of red meat consumed.
2.3.2.3. Role of Neu5Gc: Anti-Neu5Gc Antibody Interaction in Carcinoma Risk
Epidemiological studies have demonstrated that high consumption of red meat correlates with increased levels of a variety of inflammatory markers measured throughout the body (Azadbakht and Esmaillzadeh, 2009; van Woudenbergh et al., 2012; Ley et al., 2014; Montonen et al., 2013; Schulze et al., 2005). However, the precise mechanisms involved in the induction of this systemic response are not well understood, beyond the potential pro-inflammatory effects of saturated fat. We have found that red meat-derived Neu5Gc can be incorporated into the glycoconjugates present in various human tissues where they could encounter circulating anti-Neu5Gc antibodies. We have also demonstrated that Neu5Gc incorporation and interaction with anti-Neu5Gc antibodies contributes towards one of the hallmarks of cancer, tumor-promoting inflammation (Samraj et al., 2014b; Pearce et al., 2014).
Previous studies from our group demonstrated that congenic Cmah-wild-type murine tumors show accelerated growth in syngeneic Cmah−/− mice, after induction of anti-Neu5Gc antibodies (Hedlund et al., 2008). In addition, when Cmah−/− mice expressing anti-Neu5Gc antibodies were fed with a diet rich in Neu5Gc, high levels of plasma inflammatory markers were detected, which was associated with a marked increase in the incidence of hepatocellular carcinomas (Samraj et al., 2014b). These results suggest that anti-Neu5Gc antibodies have tumor-stimulating properties through induction of inflammatory processes. Importantly, we noted that the in vivo expression of diet-derived Neu5Gc in multiple epithelial cell types may serve to explain the well-documented association of red meat-associated increased risk of carcinomas in diverse epithelial tissues, such as the prostate (Amin et al., 2008; Bosetti et al., 2004; Hori et al., 2011; Kolonel, 2001), breast (Blackburn and Wang, 2007; Cho et al., 2006; Kabat et al., 2009; Linos et al., 2008; Pierce et al., 2007; Pierce, 2009; Taylor et al., 2007; Xia et al., 2015), pancreas (Larsson and Wolk, 2012; Rohrmann et al., 2013); Rohrmann et al., 2013, #82827}, esophagus (Jakszyn et al., 2013), and ovary (Kolahdooz et al., 2010; Wallin et al., 2011).
2.3.2.4. Potential Role of Neu5Gc/anti-Neu5Gc Antibodies in Aggravation of Atherosclerosis
Atherosclerotic CVD is the primary cause of myocardial infarction, ischemic heart failure, strokes, and peripheral vascular disease in humans (Grundy et al., 1999), but apparently not in other mammals (Varki et al., 2009). Indeed, while heart disease is common in great apes like chimpanzees, it results from processes that are distinct from those operating in humans (Varki et al., 2009). Chimpanzee “heart attacks” arise from arrhythmias due to diffuse interstitial myocardial fibrosis of unknown cause. In contrast, most human heart disease results from coronary artery atherosclerosis, which occludes blood supply. Accordingly, human-like myocardial infarction is very rare in the closely related great apes in captivity, despite the fact that apes have many of the major risk factors, including high LDL levels, sedentary conditions, stress, hypertension etc. (Varki et al., 2009).
Consumption of red meats and processed red meats is clearly associated with increased risk of CVD (Micha et al., 2012). As discussed earlier, current explanations include the impact of cholesterol and saturated fat (Swirski and Nahrendorf, 2013), conversion of choline and carnitine into proatherogenic TMAO (Wang et al., 2011; Wang et al., 2011; Koeth et al., 2013; Tang et al., 2013), and oxidant damage due to heme iron (Balla et al., 1991; Balla et al., 1993; Belcher et al., 1993; Nagy et al., 2010). Notably, some of these TMAO studies in mice used a dose of far higher L-carnitine dose – approximately 1700 mg/kg/day (Koeth et al., 2013) – than that typically encountered in a red meat-consuming human. Moreover, another study with doses of 87 or 352 mg/kg showed that TMAO actually had a protective effect against atherosclerosis (Collins et al., 2016). Regardless, as discussed earlier, most of these mechanisms are not specific to red meat, and dietary heme would be neutralized by hemopexin upon entering the circulation, and before it could reach the vasculature.
In contrast to these etiologic hypotheses, the process of xenosialitis can help both explain the association of red meat consumption with atherosclerosis and the human specificity of the risk. Thus, earlier studies from our group demonstrated that red meat-derived Neu5Gc can be detected in endothelium overlying atherosclerotic plaques as well as in subendothelial regions (Pham et al., 2009). In addition, incubation of human endothelial cells expressing Neu5Gc due to feeding, with human serum containing anti-Neu5Gc antibodies led to IgG and complement deposition. This in turn resulted in endothelial activation, selectin expression and increased cytokine secretion (Pham et al., 2009), the types of events common in early stages of atherogenesis. Importantly, these effects were blocked by Neu5Gc-alpha-methyl glycoside, a specific competitor of anti-Neu5Gc antibodies. Neu5Gc was also detected in the endothelium of Cmah−/− mice fed with a Neu5Gc-enriched diet (Banda et al., 2012). Overall, the data are consistent with the theory that Neu5Gc incorporation from red meat can induce xenosialitis in vascular endothelium, and may contribute to red meat-induced aggravation of atherosclerosis and CVD. Despite all this circumstantial evidence, further research is needed to confirm that this process is actually pro-atherogenic in vivo and thus a major causative factor in the development of CVD in humans.
2.3.2.6. Potential Role of Neu5Gc-induced Xenosialitis in Other Diseases Aggravated by Red Meat
While a strong epidemiological association between red meat intake and adult onset (type 2) diabetes (Pan et al., 2011; Micha et al., 2010; Aune et al., 2009) has been reported, the underlying mechanisms are less clear. However, it is notable that systemic inflammation aggravates type 2 diabetes via a variety of etiologic mechanisms (McNelis and Olefsky, 2014).
ELISA assays to detect anti-Neu5Gc antibodies were confirmed by sialoglycan microarrays (Padler-Karavani et al., 2013), and recently applied to kidney transplant recipients associated with rabbit-generated Neu5Gc-containing anti-thymocyte globulin therapy (Couvrat-Desvergnes et al., 2015). In addition, preliminary evidence suggests that Neu5Gc incorporation occurs at sites of tissue damage in muscular dystrophy (Chandrasekharan et al., 2010). Associations of red meat consumption with rheumatoid arthritis occurrence and progression have also been suggested (Grant, 2000; Grant, 2000; Choi, 2004; Oliver and Silman, 2006; Benito-Garcia et al., 2007). Although the association between xenosialitis and pathogenesis of these diseases is not well studied, it is reasonable to suggest that the inflammation induced by red meat-derived Neu5Gc and anti-Neu5Gc antibodies might aggravate some events during the progression of diseases. Studies have even shown that a diet restricted in red meat can reduce rates of recurrence in patients diagnosed with early stage colon cancer (Meyerhardt et al., 2007), perhaps by minimizing further inflammation that could stimulate growth of micrometastases (Weinberg, 2014).
The percentage of Neu5Gc versus Neu5Ac varies in red meats (beef > pork & lamb) (Samraj et al., 2014b), while being essentially absent in poultry and in fish steaks of various kinds. Neu5Gc is also present in significant amounts in goat and sheep milk (unlike cow milk), as well as in certain fish egg products such as caviar (Samraj et al., 2014b). However, since only small amounts of goat cheese and caviar are usually consumed in meals, it is unlikely that they represent important sources of dietary Neu5Gc. Also, what is called “cow’s milk” in some countries may be actually derived from other species such as buffalo, which have not been studied. Further studies of the distribution of Neu5Gc in various products of animal origin, as well as the bioavailability for metabolic incorporation are needed.
3. Potential Approaches to Addressing the Human-Specific Red Meat Risk
The most obvious approach to reduce the risk of diseases associated with red meat is to reduce or completely avoid ingestion of beef, pork, or lamb. However, especially for young healthy females of childbearing age, this would represent a loss of high quality protein as well as important micronutrients such as iron, zinc and vitamins, all of which are enriched in red meat (Mann, 2000). Also, despite increasing evidence and information regarding elevated disease risk resulting from red meat consumption, suggestions that everyone in every society should completely stop eating traditional red meat-based diets and specialty foods are highly unlikely to succeed. Instead, approaches to reduce the risks associated with this dietary habit are more reasonable. Such approaches that have been suggested to date that are designed to reduce the harmful effects of red meat include: A moderate increase in dietary intake of calcium, which could “trap” heme iron and potentially reduce endogenous fat peroxidation (Pierre et al., 2013; Allam et al., 2011; Santarelli et al., 2013) and a diet rich in fiber and/or fiber-derived products could reduce NOC-induced adducts in colonic cells (Le Leu et al., 2015). Although both approaches showed promising effects in reducing colorectal cancer incidence, little is known about the role of these dietary interventions for the other types of cancer or for other diseases associated with red meat intake.
If the xenosialitis hypothesis is validated, another possible approach would involve the use of genetically modified livestock as source of red meat. Pigs that do not endogenously express Neu5Gc (Cmah−/− pigs) are already available (Lutz et al., 2013; Burlak et al., 2013; Burlak et al., 2014; Wang et al., 2014) and have been used in studies related to xenotransplantation (Lutz et al., 2013). However, further studies are required for the public acceptance of meat derived from genetically modified animals, and there is also a theoretical possibility that such animals may be susceptible to new diseases, involving pathogens that recognize their altered sialic acid-containing receptors. Overall, this approach may be also impractical, at least in the near future.
Given all these considerations, the use of methods to eliminate pre-existing diet-derived Neu5Gc from human tissues seems worth investigating. In this regard, studies from our group showed that Neu5Gc could be eliminated from human cells in vitro by metabolic competition with Neu5Ac, the isoform of sialic acid predominantly expressed by human cells (Ghaderi et al., 2010). Thus, feeding Neu5Ac to the cells prevents Neu5Gc recycling in lysosomes and its reutilization during glycan biosynthesis. Also, adding excess Neu5Ac is thought to compete with any further incorporation of Neu5Gc derived from the culture medium (Bardor et al., 2005). The flushing out of preexisting Neu5Gc could thus be a useful approach to reduce inflammation induced by red meat and thus slow disease progression. At first glance, this approach would suggest the ingestion of a Neu5Gc competitor Neu5Ac along with red meat. In practice, it would be hard to arrange for such an antidote to be easily available as part of every meal in which red meat is consumed. However, it could be incorporated into processed meat products rich in Neu5Gc. An alternative would be to investigate the effects of periodic “flushing” with a bolus of oral Neu5Ac.
Genetically modified mice that spontaneously develop colorectal tumors that closely resemble human colon cancer have been developed (Hinoi et al., 2007). These can now be backcrossed into the human-like Cmah−/− background, as an important tool to evaluate not only the contribution of Neu5Gc/anti-Neu5Gc antibodies to colon cancer progression, but also the proposed protection by competition with Neu5Ac.
4. Conclusions and Future Perspectives
We have proposed that all traditional theories explaining disease risks associated with red meat consumption need to be further examined, with regard to their specificity for red meat consumption and human disease development. This does not negate the potential importance of any of these theories for general effects on disease, but it does indicate that new theories deserve attention. The beef-derived virus theory of Zur Hausen merits further research, as does the theory of Neu5Gc-induced xenosialitis. Based on the potential importance of Neu5Gc incorporation for the biology of human carcinomas, analytical methods for detection and quantification of Neu5Gc have been already been used (Samraj et al., 2014a; Wang et al., 2015). Although it is unquestionable that colon cancer is the type of cancer that has the strongest association with red meat consumption, quantitative analysis of the percentage of Neu5Gc in colorectal tumors still needs to be determined, to ascertain if this value correlates with cancer progression. Although not yet studied, it is also possible that meat processing improves the digestibility, absorption, and metabolic incorporation of Neu5Gc. Similarly, although not yet studied, we believe that xenosialitis may represent the missing link that connects red meat consumption to other inflammatory diseases such as atherosclerosis (Pham et al., 2009), type 2 diabetes (Pan et al., 2011), rheumatoid arthritis (Pan et al., 2011), macular degeneration (Ersoy et al., 2014; Chong et al., 2009) and possibly certain forms of infertility (Sroga et al., 2015). Interventional studies using animal models could be used to address these hypotheses further, eventually leading to potential solutions to this problem.
Acknowledgments
The authors deeply appreciate many helpful comments and critiques provided by Amanda Cross, Denis Corpet, Gregory Vercellotti, Harald Zur Hausen, John Pepper, Kana Wu, Karsten Zengler, Pascal Gagneux, Patricia Gaffney, Peter Ernst, Robert Turesky, Robert Weinberg, Shoib Siddiqui, Stanley Hazen and Walter Willett. Studies in the Varki laboratory have been supported by grants from the US National Institutes of Health. Frederico Alisson-Silva was supported by a fellowship from the Program Science Without Borders (Ciencias sem Fronteiras) – CAPES – Brazil.
Abbreviations
- CKD
chronic kidney disease
- CMAH
cytidine monophospho-N-acetylneuraminic acid hydroxylase
- CVD
cardiovascular disease
- FMO3
flavin monooxygenase 3
- GM3
monosialodihexosylganglioside
- HCAs
heterocyclic amines
- Neu5Ac
N-acetylneuraminic acid
- Neu5Gc
N-glycolylneuraminic acid
- NOCs
N-nitroso compounds
- PAHs
polycyclic aromatic hydrocarbons
- TGF-β
transforming growth factor beta
- TMA
trimethylamine
- TMAO
trimethylamine–N-oxide
- WHO-IARC
World Health Organization-International Agency for Research on Cancer
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aiello LC, Wheeler P. The Expensive-Tissue Hypothesis: The Brain and the Digestive System in Human and Primate Evolution. Curr Anthropol. 1995;36.2:199–221. [Google Scholar]
- Allam O, et al. Calcium carbonate suppresses haem toxicity markers without calcium phosphate side effects on colon carcinogenesis. Br J Nutr. 2011;105(3):384–92. doi: 10.1017/S0007114510003624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin M, et al. Dietary habits and prostate cancer detection: a case-control study. Can Urol Assoc J. 2008;2(5):510–15. [PMC free article] [PubMed] [Google Scholar]
- Andreu P, et al. FcRgamma activation regulates inflammation-associated squamous carcinogenesis. Cancer Cell. 2010;17(2):121–34. doi: 10.1016/j.ccr.2009.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antón SC, Potts R, Aiello LC. Human evolution. Evolution of early Homo: an integrated biological perspective. Science. 2014;345(6192):1236828. doi: 10.1126/science.1236828. [DOI] [PubMed] [Google Scholar]
- Arita K, et al. Heterophile Hanganutziu-Deicher antibodies in sera of patients with Kawasaki diseases. Biken J. 1982;25(4):157–62. [PubMed] [Google Scholar]
- Asaoka H, et al. Two chicken monoclonal antibodies specific for heterophil Hanganutziu-Deicher antigens. Immunol Lett. 1992;32:91–96. doi: 10.1016/0165-2478(92)90205-3. [DOI] [PubMed] [Google Scholar]
- Augustsson K, et al. Dietary heterocyclic amines and cancer of the colon, rectum, bladder, and kidney: a population-based study. Lancet. 1999;353(9154):703–7. doi: 10.1016/S0140-6736(98)06099-1. [DOI] [PubMed] [Google Scholar]
- Aune D, et al. Red and processed meat intake and risk of colorectal adenomas: a systematic review and meta-analysis of epidemiological studies. Cancer Causes Control. 2013;24(4):611–27. doi: 10.1007/s10552-012-0139-z. [DOI] [PubMed] [Google Scholar]
- Aune D, Ursin G, Veierød MB. Meat consumption and the risk of type 2 diabetes: a systematic review and meta-analysis of cohort studies. Diabetologia. 2009;52(11):2277–87. doi: 10.1007/s00125-009-1481-x. [DOI] [PubMed] [Google Scholar]
- Azadbakht L, Esmaillzadeh A. Red meat intake is associated with metabolic syndrome and the plasma C-reactive protein concentration in women. J Nutr. 2009;139(2):335–39. doi: 10.3945/jn.108.096297. [DOI] [PubMed] [Google Scholar]
- Bae S, et al. Plasma choline metabolites and colorectal cancer risk in the Women’s Health Initiative Observational Study. Cancer Res. 2014;74(24):7442–52. doi: 10.1158/0008-5472.CAN-14-1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balla G, et al. Hemin: a possible physiological mediator of low density lipoprotein oxidation and endothelial injury. Arterioscler Thromb. 1991;11(6):1700–11. doi: 10.1161/01.atv.11.6.1700. [DOI] [PubMed] [Google Scholar]
- Balla J, et al. Oxidized low-density lipoproteins and endothelium: oral vitamin E supplementation prevents oxidized low-density lipoprotein-mediated vascular injury. Trans Assoc Am Physicians. 1993;106:128–33. [PubMed] [Google Scholar]
- Balla J, et al. Heme, heme oxygenase, and ferritin: how the vascular endothelium survives (and dies) in an iron-rich environment. Antioxid Redox Signal. 2007;9(12):2119–37. doi: 10.1089/ars.2007.1787. [DOI] [PubMed] [Google Scholar]
- Banda K, et al. Metabolism of Vertebrate Amino Sugars with N-Glycolyl Groups:mechanisms underlying gastrointestinal incorporation of the non-human sialic acid xeno-autoantigen N-glycolylneuraminic acid. J Biol Chem. 2012;287(34):28852–64. doi: 10.1074/jbc.M112.364182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardor M, et al. Mechanism of uptake and incorporation of the non-human sialic acid N-glycolylneuraminic acid into human cells. J Biol Chem. 2005;280:4228–37. doi: 10.1074/jbc.M412040200. [DOI] [PubMed] [Google Scholar]
- Bastide NM, et al. A central role for heme iron in colon carcinogenesis associated with red meat intake. Cancer Res. 2015;75(5):870–79. doi: 10.1158/0008-5472.CAN-14-2554. [DOI] [PubMed] [Google Scholar]
- Bastide NM, Pierre FH, Corpet DE. Heme Iron from Meat and Risk of Colorectal Cancer: A Meta-analysis and a Review of the Mechanisms Involved. Cancer Prev Res (Phila) 2011;4(2):177–84. doi: 10.1158/1940-6207.CAPR-10-0113. [DOI] [PubMed] [Google Scholar]
- Beer P. The heterophile antibodies in infectious mononucleosis and after injection of serum. J Clin Invest. 1936;15(6):591–99. doi: 10.1172/JCI100811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belcher JD, et al. Vitamin E, LDL, and endothelium. Brief oral vitamin supplementation prevents oxidized LDL-mediated vascular injury in vitro. Arterioscler Thromb. 1993;13(12):1779–89. doi: 10.1161/01.atv.13.12.1779. [DOI] [PubMed] [Google Scholar]
- Belcher JD, et al. Heme degradation and vascular injury. Antioxid Redox Signal. 2010;12(2):233–48. doi: 10.1089/ars.2009.2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benito-Garcia E, et al. Protein, iron, and meat consumption and risk for rheumatoid arthritis: a prospective cohort study. Arthritis Res Ther. 2007;9(1):R16. doi: 10.1186/ar2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biasi F, Mascia C, Poli G. The contribution of animal fat oxidation products to colon carcinogenesis, through modulation of TGF-beta1 signaling. Carcinogenesis. 2008;29(5):890–94. doi: 10.1093/carcin/bgn106. [DOI] [PubMed] [Google Scholar]
- Bingham SA, Hughes R, Cross AJ. Effect of white versus red meat on endogenous N-nitrosation in the human colon and further evidence of a dose response. J Nutr. 2002;132(11 Suppl):3522S–5S. doi: 10.1093/jn/132.11.3522S. [DOI] [PubMed] [Google Scholar]
- Bingham SA, et al. Does increased endogenous formation of N-nitroso compounds in the human colon explain the association between red meat and colon cancer? Carcinogenesis. 1996;17(3):515–23. doi: 10.1093/carcin/17.3.515. [DOI] [PubMed] [Google Scholar]
- Blackburn GL, Wang KA. Dietary fat reduction and breast cancer outcome: results from the Women’s Intervention Nutrition Study (WINS) Am J Clin Nutr. 2007;86(3):s878–81. doi: 10.1093/ajcn/86.3.878S. [DOI] [PubMed] [Google Scholar]
- Bosetti C, et al. Food groups and risk of prostate cancer in Italy. Int J Cancer. 2004;110(3):424–28. doi: 10.1002/ijc.20142. [DOI] [PubMed] [Google Scholar]
- Boshart M, et al. A new type of papillomavirus DNA, its presence in genital cancer biopsies and in cell lines derived from cervical cancer. EMBO J. 1984;3(5):1151–57. doi: 10.1002/j.1460-2075.1984.tb01944.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bou R, et al. Effect of heating oxymyoglobin and metmyoglobin on the oxidation of muscle microsomes. J Agric Food Chem. 2008;56(20):9612–20. doi: 10.1021/jf8009848. [DOI] [PubMed] [Google Scholar]
- Bou R, et al. Effect of heating oxyhemoglobin and methemoglobin on microsomes oxidation. Meat Sci. 2010;85(1):47–53. doi: 10.1016/j.meatsci.2009.12.002. [DOI] [PubMed] [Google Scholar]
- Bouvard V, et al. Carcinogenicity of consumption of red and processed meat. Lancet Oncol. 2015;16:1599–600. doi: 10.1016/S1470-2045(15)00444-1. [DOI] [PubMed] [Google Scholar]
- Bramble DM, Lieberman DE. Endurance running and the evolution of Homo. Nature. 2004;432(7015):345–52. doi: 10.1038/nature03052. [DOI] [PubMed] [Google Scholar]
- Brass EP, et al. A systematic review and meta-analysis of propionyl-L-carnitine effects on exercise performance in patients with claudication. Vasc Med. 2013;18(1):3–12. doi: 10.1177/1358863X12467491. [DOI] [PubMed] [Google Scholar]
- Burlak C, et al. N-linked glycan profiling of GGTA1/CMAH knockout pigs identifies new potential carbohydrate xenoantigens. Xenotransplantation. 2013;20(5):277–91. doi: 10.1111/xen.12047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burlak C, et al. Reduced binding of human antibodies to cells from GGTA1/CMAH KO pigs. Am J Transplant. 2014;14(8):1895–900. doi: 10.1111/ajt.12744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer. 2004;4(8):579–91. doi: 10.1038/nrc1408. [DOI] [PubMed] [Google Scholar]
- Catsburg CE, et al. Dietary sources of N-nitroso compounds and bladder cancer risk: findings from the Los Angeles bladder cancer study. Int J Cancer. 2014;134(1):125–35. doi: 10.1002/ijc.28331. [DOI] [PubMed] [Google Scholar]
- Cermak J, et al. Tumor cell heme uptake induces ferritin synthesis resulting in altered oxidant sensitivity: possible role in chemotherapy efficacy. Cancer Res. 1993;53(21):5308–13. [PubMed] [Google Scholar]
- Chandrasekharan K, et al. A human-specific deletion in mouse Cmah increases disease severity in the mdx model of Duchenne muscular dystrophy. Sci Transl Med. 2010;2(42):42ra54. doi: 10.1126/scitranslmed.3000692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiuve SE, et al. The association between betaine and choline intakes and the plasma concentrations of homocysteine in women. Am J Clin Nutr. 2007;86(4):1073–81. doi: 10.1093/ajcn/86.4.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho E, et al. Red meat intake and risk of breast cancer among premenopausal women. Arch Intern Med. 2006;166(20):2253–59. doi: 10.1001/archinte.166.20.2253. [DOI] [PubMed] [Google Scholar]
- Choi HK. Diet and rheumatoid arthritis: red meat and beyond. Arthritis Rheum. 2004;50(12):3745–47. doi: 10.1002/art.20732. [DOI] [PubMed] [Google Scholar]
- Chong EW, et al. Red meat and chicken consumption and its association with age-related macular degeneration. Am J Epidemiol. 2009;169(7):867–76. doi: 10.1093/aje/kwn393. [DOI] [PubMed] [Google Scholar]
- Chou HH, et al. Inactivation of CMP-N-acetylneuraminic acid hydroxylase occurred prior to brain expansion during human evolution. Proc Natl Acad Sci U S A. 2002;99(18):11736–41. doi: 10.1073/pnas.182257399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou HH, et al. A mutation in human CMP-sialic acid hydroxylase occurred after the Homo-Pan divergence. Proc Natl Acad Sci USA. 1998;95:11751–56. doi: 10.1073/pnas.95.20.11751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins HL, et al. L-Carnitine intake and high trimethylamine N-oxide plasma levels correlate with low aortic lesions in ApoE(−)(/−) transgenic mice expressing CETP. Atherosclerosis. 2016;244:29–37. doi: 10.1016/j.atherosclerosis.2015.10.108. [DOI] [PubMed] [Google Scholar]
- Couvrat-Desvergnes G, et al. Rabbit antithymocyte globulin-induced serum sickness disease and human kidney graft survival. J Clin Invest. 2015;125(12):4655–65. doi: 10.1172/JCI82267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross AJ, et al. A large prospective study of meat consumption and colorectal cancer risk: an investigation of potential mechanisms underlying this association. Cancer Res. 2010;70(6):2406–14. doi: 10.1158/0008-5472.CAN-09-3929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross AJ, et al. A prospective study of red and processed meat intake in relation to cancer risk. PLoS Med. 2007;4(12):e325. doi: 10.1371/journal.pmed.0040325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross AJ, et al. A prospective study of meat and meat mutagens and prostate cancer risk. Cancer Res. 2005;65(24):11779–84. doi: 10.1158/0008-5472.CAN-05-2191. [DOI] [PubMed] [Google Scholar]
- Cross AJ, Pollock JR, Bingham SA. Red meat and colorectal cancer risk: the effect of dietary iron and haem on endogenous N-nitrosation. IARC Sci Publ. 2002;156:205–6. [PubMed] [Google Scholar]
- Cross AJ, Pollock JR, Bingham SA. Haem, not protein or inorganic iron, is responsible for endogenous intestinal N-nitrosation arising from red meat. Cancer Res. 2003;63(10):2358–60. [PubMed] [Google Scholar]
- Cross AJ, Sinha R. Meat-related mutagens/carcinogens in the etiology of colorectal cancer. Environ Mol Mutagen. 2004;44(1):44–55. doi: 10.1002/em.20030. [DOI] [PubMed] [Google Scholar]
- Daniel CR, et al. Large prospective investigation of meat intake, related mutagens, and risk of renal cell carcinoma. Am J Clin Nutr. 2012;95(1):155–62. doi: 10.3945/ajcn.111.019364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kok TM, et al. Inflammatory bowel disease stimulates formation of carcinogenic N-nitroso compounds. Gut. 2005;54(5):731. doi: 10.1136/gut.2004.057471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellavalle CT, et al. Dietary nitrate and nitrite intake and risk of colorectal cancer in the Shanghai Women’s Health Study. Int J Cancer. 2014;134(12):2917–26. doi: 10.1002/ijc.28612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Sanchez S, et al. Prevalence of Shiga toxin-producing Escherichia coli, Salmonella spp. and Campylobacter spp. in large game animals intended for consumption: relationship with management practices and livestock influence. Vet Microbiol. 2013;163(3–4):274–81. doi: 10.1016/j.vetmic.2012.12.026. [DOI] [PubMed] [Google Scholar]
- DiNicolantonio JJ, et al. L-carnitine in the secondary prevention of cardiovascular disease: systematic review and meta-analysis. Mayo Clin Proc. 2013;88(6):544–51. doi: 10.1016/j.mayocp.2013.02.007. [DOI] [PubMed] [Google Scholar]
- Domingo JL, Nadal M. Carcinogenicity of consumption of red and processed meat: What about environmental contaminants? Environ Res. 2016;145:109–15. doi: 10.1016/j.envres.2015.11.031. [DOI] [PubMed] [Google Scholar]
- Domínguez-Rodrigo M, et al. Cutmarked bones from Pliocene archaeological sites at Gona, Afar, Ethiopia: implications for the function of the world’s oldest stone tools. J Hum Evol. 2005;48(2):109–21. doi: 10.1016/j.jhevol.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Dubrow R, et al. Dietary components related to N-nitroso compound formation: a prospective study of adult glioma. Cancer Epidemiol Biomarkers Prev. 2010;19(7):1709–22. doi: 10.1158/1055-9965.EPI-10-0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- English DR, et al. Red meat, chicken, and fish consumption and risk of colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2004;13(9):1509–14. [PubMed] [Google Scholar]
- Ersoy L, et al. Nutritional risk factors for age-related macular degeneration. Biomed Res Int. 2014;2014:413150. doi: 10.1155/2014/413150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshel G, et al. Land, irrigation water, greenhouse gas, and reactive nitrogen burdens of meat, eggs, and dairy production in the United States. Proc Natl Acad Sci U S A. 2014;111(33):11996–2001. doi: 10.1073/pnas.1402183111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrucci LM, et al. Intake of meat, meat mutagens, and iron and the risk of breast cancer in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. Br J Cancer. 2009;101(1):178–84. doi: 10.1038/sj.bjc.6605118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrucci LM, et al. Meat and components of meat and the risk of bladder cancer in the NIH-AARP Diet and Health Study. Cancer. 2010;116(18):4345–53. doi: 10.1002/cncr.25463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca-Nunes A, Jakszyn P, Agudo A. Iron and Cancer Risk–A Systematic Review and Meta-analysis of the Epidemiological Evidence. Cancer Epidemiol Biomarkers Prev. 2014;23(1):12–31. doi: 10.1158/1055-9965.EPI-13-0733. [DOI] [PubMed] [Google Scholar]
- Fraser GE. Associations between diet and cancer, ischemic heart disease, and all-cause mortality in non-Hispanic white California Seventh-day Adventists. Am J Clin Nutr. 1999;70(3 Suppl):532S–8S. doi: 10.1093/ajcn/70.3.532s. [DOI] [PubMed] [Google Scholar]
- Funk M, et al. Isolation of protein-associated circular DNA from healthy cattle serum. Genome Announc. 2014;2(4) doi: 10.1128/genomeA.00846-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gathuru JK, et al. Use of biotinylated antibody for the assay of Hanganutziu-Deicher antibodies and antigens in fluids and tissues from cancer patients. Jpn J Vet Res. 1989;37:71–83. [PubMed] [Google Scholar]
- Ghaderi D, et al. Implications of the presence of N-glycolylneuraminic acid in recombinant therapeutic glycoproteins. Nat Biotechnol. 2010;28(8):863–67. doi: 10.1038/nbt.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González CA, et al. Meat intake and risk of stomach and esophageal adenocarcinoma within the European Prospective Investigation Into Cancer and Nutrition (EPIC) J Natl Cancer Inst. 2006;98(5):345–54. doi: 10.1093/jnci/djj071. [DOI] [PubMed] [Google Scholar]
- Grant WB. The role of meat in the expression of rheumatoid arthritis. Br J Nutr. 2000;84(5):589–95. doi: 10.1017/s0007114500001926. [DOI] [PubMed] [Google Scholar]
- Grundy SM, et al. Diabetes and cardiovascular disease: a statement for healthcare professionals from the American Heart Association. Circulation. 1999;100(10):1134–46. doi: 10.1161/01.cir.100.10.1134. [DOI] [PubMed] [Google Scholar]
- Guo J, et al. Multiclass Carcinogenic DNA Adduct Quantification in Formalin-Fixed Paraffin-Embedded Tissues by Ultraperformance Liquid Chromatography-Tandem Mass Spectrometry. Anal Chem. 2016;88(9):4780–87. doi: 10.1021/acs.analchem.6b00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanganutziu M. Hémagglutinines hétérogénétiques après injection de sérum de cheval. C.R Séances Soc Biol. 1924;91:1457–59. [Google Scholar]
- Hayakawa T, et al. Fixation of the Human-Specific CMP-N-Acetylneuraminic Acid Hydroxylase Pseudogene and Implications of Haplotype Diversity for Human Evolution. Genetics. 2006;172(2):1139–46. doi: 10.1534/genetics.105.046995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He FJ, Li J, Macgregor GA. Effect of longer term modest salt reduction on blood pressure: Cochrane systematic review and meta-analysis of randomised trials. BMJ. 2013a;346:f1325. doi: 10.1136/bmj.f1325. [DOI] [PubMed] [Google Scholar]
- He X, Marco ML, Slupsky CM. Emerging aspects of food and nutrition on gut microbiota. J Agric Food Chem. 2013b;61(40):9559–74. doi: 10.1021/jf4029046. [DOI] [PubMed] [Google Scholar]
- Hebels DG, et al. Radical mechanisms in nitrosamine- and nitrosamide-induced whole-genome gene expression modulations in Caco-2 cells. Toxicol Sci. 2010;116(1):194–205. doi: 10.1093/toxsci/kfq121. [DOI] [PubMed] [Google Scholar]
- Hebels DG, et al. Molecular signatures of N-nitroso compounds in Caco-2 cells: implications for colon carcinogenesis. Toxicol Sci. 2009;108(2):290–300. doi: 10.1093/toxsci/kfp035. [DOI] [PubMed] [Google Scholar]
- Heddle JA, et al. A test of the mutagenicity of cooked meats in vivo. Mutagenesis. 2001;16(2):103–7. doi: 10.1093/mutage/16.2.103. [DOI] [PubMed] [Google Scholar]
- Hedlund M, et al. Evidence for a human-specific mechanism for diet and antibody-mediated inflammation in carcinoma progression. Proc Natl Acad Sci U S A. 2008;105(48):18936–41. doi: 10.1073/pnas.0803943105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedlund M, et al. N-glycolylneuraminic acid deficiency in mice: implications for human biology and evolution. Mol Cell Biol. 2007;27(12):4340–46. doi: 10.1128/MCB.00379-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashi H, et al. Antigen of “serum sickness” type of heterophile antibodies in human sera: indentification as gangliosides with N-glycolylneuraminic acid. Biochem Biophys Res Commun. 1977;79:388–95. doi: 10.1016/0006-291x(77)90169-3. [DOI] [PubMed] [Google Scholar]
- Higashihara T, et al. Survey of Hanganutziu and Deicher antibodies in operated patients. Int Arch Allergy Appl Immunol. 1991;95:231–35. doi: 10.1159/000235434. [DOI] [PubMed] [Google Scholar]
- Hinoi T, et al. Mouse model of colonic adenoma-carcinoma progression based on somatic Apc inactivation. Cancer Res. 2007;67(20):9721–30. doi: 10.1158/0008-5472.CAN-07-2735. [DOI] [PubMed] [Google Scholar]
- Hokke CH, et al. Sialylated carbohydrate chains of recombinant human glycoproteins expressed in Chinese hamster ovary cells contain traces of N-glycolylneuraminic acid. FEBS Lett. 1990;275(1–2):9–14. doi: 10.1016/0014-5793(90)81427-p. [DOI] [PubMed] [Google Scholar]
- Hori S, Butler E, McLoughlin J. Prostate cancer and diet: food for thought? BJU Int. 2011;107(9):1348–59. doi: 10.1111/j.1464-410X.2010.09897.x. [DOI] [PubMed] [Google Scholar]
- Hughes R, et al. Dose-dependent effect of dietary meat on endogenous colonic N-nitrosation. Carcinogenesis. 2001;22(1):199–202. doi: 10.1093/carcin/22.1.199. [DOI] [PubMed] [Google Scholar]
- Huxley RR, et al. The impact of dietary and lifestyle risk factors on risk of colorectal cancer: a quantitative overview of the epidemiological evidence. Int J Cancer. 2009;125(1):171–80. doi: 10.1002/ijc.24343. [DOI] [PubMed] [Google Scholar]
- Ijssennagger N, et al. Gut microbiota facilitates dietary heme-induced epithelial hyperproliferation by opening the mucus barrier in colon. Proc Natl Acad Sci U S A. 2015;112(32):10038–43. doi: 10.1073/pnas.1507645112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IJssennagger N, et al. Dietary heme alters microbiota and mucosa of mouse colon without functional changes in host-microbe cross-talk. PLoS One. 2012a;7(12):e49868. doi: 10.1371/journal.pone.0049868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IJssennagger N, et al. Dietary haem stimulates epithelial cell turnover by downregulating feedback inhibitors of proliferation in murine colon. Gut. 2012b;61(7):1041–49. doi: 10.1136/gutjnl-2011-300239. [DOI] [PubMed] [Google Scholar]
- Ikuta K, et al. Hanganutziu-Deicher type-heterophile antigen-positive cells in human cancer tissues demonstrated by membrane immunofluorescence. Biken J. 1982;25:47–50. [PubMed] [Google Scholar]
- Inoue S, Sato C, Kitajima K. Extensive enrichment of N-glycolylneuraminic acid in extracellular sialoglycoproteins abundantly synthesized and secreted by human cancer cells. Glycobiology. 2010;20(6):752–62. doi: 10.1093/glycob/cwq030. [DOI] [PubMed] [Google Scholar]
- Ito N, et al. Carcinogenicity of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) in the rat. Mutat Res. 1997;376(1–2):107–14. doi: 10.1016/s0027-5107(97)00032-8. [DOI] [PubMed] [Google Scholar]
- Jägerstad M, et al. Formation of heterocyclic amines using model systems. Mutat Res. 1991;259(3–4):219–33. doi: 10.1016/0165-1218(91)90119-7. [DOI] [PubMed] [Google Scholar]
- Jakszyn P, et al. Meat and heme iron intake and esophageal adenocarcinoma in the European Prospective Investigation into Cancer and Nutrition study. Int J Cancer. 2013;133(11):2744–50. doi: 10.1002/ijc.28291. [DOI] [PubMed] [Google Scholar]
- Joshi AD, et al. Meat intake, cooking methods, dietary carcinogens, and colorectal cancer risk: findings from the Colorectal Cancer Family Registry. Cancer Med. 2015;4(6):936–52. doi: 10.1002/cam4.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabat GC, et al. Meat intake and meat preparation in relation to risk of postmenopausal breast cancer in the NIH-AARP diet and health study. Int J Cancer. 2009;124(10):2430–35. doi: 10.1002/ijc.24203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keszei AP, et al. Dietary N-nitroso compounds, endogenous nitrosation, and the risk of esophageal and gastric cancer subtypes in the Netherlands Cohort Study. Am J Clin Nutr. 2013;97(1):135–46. doi: 10.3945/ajcn.112.043885. [DOI] [PubMed] [Google Scholar]
- Kim E, Coelho D, Blachier F. Review of the association between meat consumption and risk of colorectal cancer. Nutr Res. 2013;33(12):983–94. doi: 10.1016/j.nutres.2013.07.018. [DOI] [PubMed] [Google Scholar]
- Kim RB, et al. Advanced chronic kidney disease populations have elevated trimethylamine N-oxide levels associated with increased cardiovascular events. Kidney Int. 2016;89(5):1144–52. doi: 10.1016/j.kint.2016.01.014. [DOI] [PubMed] [Google Scholar]
- Knekt P, et al. Risk of colorectal and other gastro-intestinal cancers after exposure to nitrate, nitrite and N-nitroso compounds: a follow-up study. Int J Cancer. 1999;80(6):852–56. doi: 10.1002/(sici)1097-0215(19990315)80:6<852::aid-ijc9>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Knize MG, et al. Food heating and the formation of heterocyclic aromatic amine and polycyclic aromatic hydrocarbon mutagens/carcinogens. Adv Exp Med Biol. 1999;459:179–93. doi: 10.1007/978-1-4615-4853-9_12. [DOI] [PubMed] [Google Scholar]
- Knöbel Y, et al. Ferric iron is genotoxic in non-transformed and preneoplastic human colon cells. Food Chem Toxicol. 2007;45(5):804–11. doi: 10.1016/j.fct.2006.10.028. [DOI] [PubMed] [Google Scholar]
- Koeth RA, et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. 2013;19(5):576–85. doi: 10.1038/nm.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolahdooz F, et al. Meat, fish, and ovarian cancer risk: Results from 2 Australian case-control studies, a systematic review, and meta-analysis. Am J Clin Nutr. 2010;91(6):1752–63. doi: 10.3945/ajcn.2009.28415. [DOI] [PubMed] [Google Scholar]
- Kolonel LN. Fat, meat, and prostate cancer. Epidemiol Rev. 2001;23:72–81. doi: 10.1093/oxfordjournals.epirev.a000798. [DOI] [PubMed] [Google Scholar]
- Kotchen TA, Cowley AW, Frohlich ED. Salt in health and disease–a delicate balance. N Engl J Med. 2013;368(13):1229–37. doi: 10.1056/NEJMra1212606. [DOI] [PubMed] [Google Scholar]
- Lamberto I, et al. Mycovirus-like DNA virus sequences from cattle serum and human brain and serum samples from multiple sclerosis patients. Genome Announc. 2014;2(4) doi: 10.1128/genomeA.00848-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson SC, Orsini N. Red meat and processed meat consumption and all-cause mortality: a meta-analysis. Am J Epidemiol. 2014;179(3):282–89. doi: 10.1093/aje/kwt261. [DOI] [PubMed] [Google Scholar]
- Larsson SC, Wolk A. Meat consumption and risk of colorectal cancer: a meta-analysis of prospective studies. Int J Cancer. 2006;119(11):2657–64. doi: 10.1002/ijc.22170. [DOI] [PubMed] [Google Scholar]
- Larsson SC, Wolk A. Red and processed meat consumption and risk of pancreatic cancer: meta-analysis of prospective studies. Br J Cancer. 2012;106(3):603–7. doi: 10.1038/bjc.2011.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Leu RK, et al. Butyrylated starch intake can prevent red meat-induced O6-methyl-2-deoxyguanosine adducts in human rectal tissue: a randomised clinical trial. Br J Nutr. 2015;114(2):220–30. doi: 10.1017/S0007114515001750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le NT, et al. A Prospective Analysis of Meat Mutagens and Colorectal Cancer in the Nurses’ Health Study and Health Professional Follow-up Study. Environ Health Perspect. 2016 doi: 10.1289/EHP238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewin MH, et al. Red Meat Enhances the Colonic Formation of the DNA Adduct O6-Carboxymethyl Guanine: Implications for Colorectal Cancer Risk. Cancer Res. 2006;66(3):1859–65. doi: 10.1158/0008-5472.CAN-05-2237. [DOI] [PubMed] [Google Scholar]
- Ley SH, et al. Associations between red meat intake and biomarkers of inflammation and glucose metabolism in women. Am J Clin Nutr. 2014;99(2):352–60. doi: 10.3945/ajcn.113.075663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linos E, et al. Red meat consumption during adolescence among premenopausal women and risk of breast cancer. Cancer Epidemiol Biomarkers Prev. 2008;17(8):2146–51. doi: 10.1158/1055-9965.EPI-08-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingston DJ, Brown WD. The chemistry of myoglobin and its reaction. Food Technol. 1981;35:244–52. [Google Scholar]
- Lutz AJ, et al. Double knockout pigs deficient in N-glycolylneuraminic acid and Galactose alpha-1,3-Galactose reduce the humoral barrier to xenotransplantation. Xenotransplantation. 2013;20(1):27–35. doi: 10.1111/xen.12019. [DOI] [PubMed] [Google Scholar]
- Magee PN. The experimental basis for the role of nitroso compounds in human cancer. Cancer Surv. 1989;8(2):207–39. [PubMed] [Google Scholar]
- Malykh YN, et al. Distribution and localization of CMP-N-acetylneuraminic acid hydroxylase and N-glycolylneuraminic acid-containing glycoconjugates in porcine lymph node and peripheral blood lymphocytes. Eur J Cell Biol. 2001a;80:48–58. doi: 10.1078/0171-9335-00139. [DOI] [PubMed] [Google Scholar]
- Malykh YN, Schauer R, Shaw L. N-Glycolylneuraminic acid in human tumours. Biochimie. 2001b;83(7):623–34. doi: 10.1016/s0300-9084(01)01303-7. [DOI] [PubMed] [Google Scholar]
- Mann N. Dietary lean red meat and human evolution. Eur J Nutr. 2000;39(2):71–79. doi: 10.1007/s003940050005. [DOI] [PubMed] [Google Scholar]
- Maytsetseg B. Changes and Actual State of Mongolian Meat Market and Distribution System. A Case Study of Ulaanbaatar City’s “Khuchit Shonhor Khuchit Shonhor Khuchit Shonhor” Food Market 2006 [Google Scholar]
- McMichael AJ, et al. Food, livestock production, energy, climate change, and health. Lancet. 2007;370(9594):1253–63. doi: 10.1016/S0140-6736(07)61256-2. [DOI] [PubMed] [Google Scholar]
- McNelis JC, Olefsky JM. Macrophages, immunity, and metabolic disease. Immunity. 2014;41(1):36–48. doi: 10.1016/j.immuni.2014.05.010. [DOI] [PubMed] [Google Scholar]
- Merrick JM, Zadarlik K, Milgrom F. Characterization of the Hanganutziu-Deicher (serum-sickness) antigen as gangliosides containing N-glycolylneuraminic acid. Int Arch Allergy Appl Immunol. 1978;57:477–80. doi: 10.1159/000232140. [DOI] [PubMed] [Google Scholar]
- Meyerhardt JA, et al. Association of dietary patterns with cancer recurrence and survival in patients with stage III colon cancer. JAMA. 2007;298(7):754–64. doi: 10.1001/jama.298.7.754. [DOI] [PubMed] [Google Scholar]
- Micha R, Michas G, Mozaffarian D. Unprocessed red and processed meats and risk of coronary artery disease and type 2 diabetes–an updated review of the evidence. Curr Atheroscler Rep. 2012;14(6):515–24. doi: 10.1007/s11883-012-0282-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micha R, Wallace SK, Mozaffarian D. Red and processed meat consumption and risk of incident coronary heart disease, stroke, and diabetes mellitus: a systematic review and meta-analysis. Circulation. 2010;121(21):2271–83. doi: 10.1161/CIRCULATIONAHA.109.924977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millward DJ. The nutritional value of plant-based diets in relation to human amino acid and protein requirements. Proc Nutr Soc. 1999;58(2):249–60. doi: 10.1017/s0029665199000348. [DOI] [PubMed] [Google Scholar]
- Milton K. The critical role played by animal source foods in human (Homo) evolution. J Nutr. 2003;133(11 Suppl 2):3886S–92S. doi: 10.1093/jn/133.11.3886S. [DOI] [PubMed] [Google Scholar]
- Mirvish SS. Role of N-nitroso compounds (NOC) and N-nitrosation in etiology of gastric, esophageal, nasopharyngeal and bladder cancer and contribution to cancer of known exposures to NOC. Cancer Lett. 1995;93(1):17–48. doi: 10.1016/0304-3835(95)03786-V. [DOI] [PubMed] [Google Scholar]
- Montgomery SA, Thal LJ, Amrein R. Meta-analysis of double blind randomized controlled clinical trials of acetyl-L-carnitine versus placebo in the treatment of mild cognitive impairment and mild Alzheimer’s disease. Int Clin Psychopharmacol. 2003;18(2):61–71. doi: 10.1097/00004850-200303000-00001. [DOI] [PubMed] [Google Scholar]
- Montonen J, et al. Consumption of red meat and whole-grain bread in relation to biomarkers of obesity, inflammation, glucose metabolism and oxidative stress. Eur J Nutr. 2013;52(1):337–45. doi: 10.1007/s00394-012-0340-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morito T, Kano K, Milgrom F. Hanganutziu-Deicher antibodies in infectious mononucleosis and other diseases. J Immunol. 1982;129(6):2524–28. [PubMed] [Google Scholar]
- Mozaffarian D, et al. Global sodium consumption and death from cardiovascular causes. N Engl J Med. 2014;371(7):624–34. doi: 10.1056/NEJMoa1304127. [DOI] [PubMed] [Google Scholar]
- Nagy E, et al. Red cells, hemoglobin, heme, iron, and atherogenesis. Arterioscler Thromb Vasc Biol. 2010;30(7):1347–53. doi: 10.1161/ATVBAHA.110.206433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimaki T, Kano K, Milgrom F. Studies on heterophile antibodies in rheumatoid arthritis. Arthritis Rheum. 1978a;21(6):634–38. doi: 10.1002/art.1780210604. [DOI] [PubMed] [Google Scholar]
- Nishimaki T, Kano K, Milgrom F. Studies on immune complexes in rheumatoid arthritis. Arthritis Rheum. 1978b;21(6):639–44. doi: 10.1002/art.1780210605. [DOI] [PubMed] [Google Scholar]
- Norat T, et al. Meat consumption and colorectal cancer risk: dose-response meta-analysis of epidemiological studies. Int J Cancer. 2002;98(2):241–56. doi: 10.1002/ijc.10126. [DOI] [PubMed] [Google Scholar]
- Ohgaki H, et al. Carcinogenicity in mice and rats of heterocyclic amines in cooked foods. Environ Health Perspect. 1986;67:129–34. doi: 10.1289/ehp.8667129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olatunji OS, et al. Determination of polycyclic aromatic hydrocarbons [PAHs] in processed meat products using gas chromatography – flame ionization detector. Food Chem. 2014;156:296–300. doi: 10.1016/j.foodchem.2014.01.120. [DOI] [PubMed] [Google Scholar]
- Oliver JE, Silman AJ. Risk factors for the development of rheumatoid arthritis. Scand J Rheumatol. 2006;35(3):169–74. doi: 10.1080/03009740600718080. [DOI] [PubMed] [Google Scholar]
- Padler-Karavani V, et al. Human xeno-autoantibodies against a non-human sialic acid serve as novel serum biomarkers and immunotherapeutics in cancer. Cancer Res. 2011;71(9):3352–63. doi: 10.1158/0008-5472.CAN-10-4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padler-Karavani V, et al. A Simple Method for Assessment of Human Anti-Neu5Gc Antibodies Applied to Kawasaki Disease. PLoS One. 2013;8(3):e58443. doi: 10.1371/journal.pone.0058443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padler-Karavani V, et al. Diversity in specificity, abundance, and composition of anti-Neu5Gc antibodies in normal humans: potential implications for disease. Glycobiology. 2008;18(10):818–30. doi: 10.1093/glycob/cwn072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan A, et al. Red meat consumption and mortality: results from 2 prospective cohort studies. Arch Intern Med. 2012;172(7):555–63. doi: 10.1001/archinternmed.2011.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan A, et al. Red meat consumption and risk of type 2 diabetes: 3 cohorts of US adults and an updated meta-analysis. Am J Clin Nutr. 2011;94(4):1088–96. doi: 10.3945/ajcn.111.018978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnaud G, et al. Endogenous N-nitroso compounds, and their precursors, present in bacon, do not initiate or promote aberrant crypt foci in the colon of rats. Nutr Cancer. 2000;38(1):74–80. doi: 10.1207/S15327914NC381_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson KY, et al. USDA Database for the Choline Content of Common Foods : Release Two. Agricultural Research Service, U.S Department of Agriculture; 2008. [Google Scholar]
- Pearce OM, et al. Inverse hormesis of cancer growth mediated by narrow ranges of tumor-directed antibodies. Proc Natl Acad Sci U S A. 2014;111(16):5998–6003. doi: 10.1073/pnas.1209067111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelser C, et al. Dietary fat, fatty acids, and risk of prostate cancer in the NIH-AARP diet and health study. Cancer Epidemiol Biomarkers Prev. 2013;22(4):697–707. doi: 10.1158/1055-9965.EPI-12-1196-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham T, et al. Evidence for a novel human-specific xeno-auto-antibody response against vascular endothelium. Blood. 2009;114(25):5225–35. doi: 10.1182/blood-2009-05-220400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips DH. Polycyclic aromatic hydrocarbons in the diet. Mutat Res. 1999;443(1–2):139–47. doi: 10.1016/s1383-5742(99)00016-2. [DOI] [PubMed] [Google Scholar]
- Phillips RL, et al. Mortality among California Seventh-Day Adventists for selected cancer sites. J Natl Cancer Inst. 1980;65(5):1097–107. [PubMed] [Google Scholar]
- Pierce JP. Diet and breast cancer prognosis: making sense of the Women’s Healthy Eating and Living and Women’s Intervention Nutrition Study trials. Curr Opin Obstet Gynecol. 2009;21(1):86–91. doi: 10.1097/gco.0b013e32831da7f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce JP, et al. Influence of a diet very high in vegetables, fruit, and fiber and low in fat on prognosis following treatment for breast cancer: the Women’s Healthy Eating and Living (WHEL) randomized trial. JAMA. 2007;298(3):289–98. doi: 10.1001/jama.298.3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierre F, et al. Beef meat and blood sausage promote the formation of azoxymethane-induced mucin-depleted foci and aberrant crypt foci in rat colons. J Nutr. 2004;134(10):2711–16. doi: 10.1093/jn/134.10.2711. [DOI] [PubMed] [Google Scholar]
- Pierre F, et al. Meat and cancer: haemoglobin and haemin in a low-calcium diet promote colorectal carcinogenesis at the aberrant crypt stage in rats. Carcinogenesis. 2003;24(10):1683–90. doi: 10.1093/carcin/bgg130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierre FH, et al. Calcium and α-tocopherol suppress cured-meat promotion of chemically induced colon carcinogenesis in rats and reduce associated biomarkers in human volunteers. Am J Clin Nutr. 2013;98(5):1255–62. doi: 10.3945/ajcn.113.061069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierre FH, et al. Freeze-dried ham promotes azoxymethane-induced mucin-depleted foci and aberrant crypt foci in rat colon. Nutr Cancer. 2010;62(5):567–73. doi: 10.1080/01635580903532408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Povey AC, et al. DNA alkylation and repair in the large bowel: animal and human studies. J Nutr. 2002;132(11 Suppl):3518S–21S. doi: 10.1093/jn/132.11.3518S. [DOI] [PubMed] [Google Scholar]
- Rebouche CJ. Kinetics, pharmacokinetics, and regulation of L-carnitine and acetyl-L-carnitine metabolism. Ann N Y Acad Sci. 2004;1033:30–41. doi: 10.1196/annals.1320.003. [DOI] [PubMed] [Google Scholar]
- Rohrmann S, et al. Meat and fish consumption and risk of pancreatic cancer: results from the European Prospective Investigation into Cancer and Nutrition. Int J Cancer. 2013;132(3):617–24. doi: 10.1002/ijc.27637. [DOI] [PubMed] [Google Scholar]
- Rose M, et al. Investigation into the formation of PAHs in foods prepared in the home to determine the effects of frying, grilling, barbecuing, toasting and roasting. Food Chem Toxicol. 2015;78:1–9. doi: 10.1016/j.fct.2014.12.018. [DOI] [PubMed] [Google Scholar]
- Samraj AN, et al. Involvement of a Non-Human Sialic Acid in Human Cancer. Front Oncol. 2014a;4:33. doi: 10.3389/fonc.2014.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samraj AN, et al. A red meat-derived glycan promotes inflammation and cancer progression. Proc Natl Acad Sci U S A. 2014b;112:542–47. doi: 10.1073/pnas.1417508112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarelli RL, et al. Calcium inhibits promotion by hot dog of 1,2-dimethylhydrazine-induced mucin-depleted foci in rat colon. Int J Cancer. 2013;133(11):2533–41. doi: 10.1002/ijc.28286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarelli RL, Pierre F, Corpet DE. Processed meat and colorectal cancer: a review of epidemiologic and experimental evidence. Nutr Cancer. 2008;60(2):131–44. doi: 10.1080/01635580701684872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaer DJ, et al. Hemolysis and free hemoglobin revisited: exploring hemoglobin and hemin scavengers as a novel class of therapeutic proteins. Blood. 2013;121(8):1276–84. doi: 10.1182/blood-2012-11-451229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoeninger MJ. Palaeoanthropology: the ancestral dinner table. Nature. 2012;487(7405):42–43. doi: 10.1038/487042a. [DOI] [PubMed] [Google Scholar]
- Schulz MD, et al. High-fat-diet-mediated dysbiosis promotes intestinal carcinogenesis independently of obesity. Nature. 2014;514(7523):508–12. doi: 10.1038/nature13398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze MB, et al. Dietary pattern, inflammation, and incidence of type 2 diabetes in women. Am J Clin Nutr. 2005;82(3):675–84. doi: 10.1093/ajcn.82.3.675. quiz 714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz S, Ellefson M. Quantitative fecal recovery of ingested hemoglobin-heme in blood: comparisons by HemoQuant assay with ingested meat and fish. Gastroenterology. 1985;89(1):19–26. doi: 10.1016/0016-5085(85)90740-1. [DOI] [PubMed] [Google Scholar]
- Schwarz E, et al. Structure and transcription of human papillomavirus sequences in cervical carcinoma cells. Nature. 1985;314(6006):111–14. doi: 10.1038/314111a0. [DOI] [PubMed] [Google Scholar]
- Seibel BA, Walsh PJ. Trimethylamine oxide accumulation in marine animals: relationship to acylglycerol storage. J Exp Biol. 2002;205(Pt 3):297–306. doi: 10.1242/jeb.205.3.297. [DOI] [PubMed] [Google Scholar]
- Seldin MM, et al. Trimethylamine N-Oxide Promotes Vascular Inflammation Through Signaling of Mitogen-Activated Protein Kinase and Nuclear Factor-κB. J Am Heart Assoc. 2016;5(2) doi: 10.1161/JAHA.115.002767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesink AL, et al. Red meat and colon cancer: the cytotoxic and hyperproliferative effects of dietary heme. Cancer Res. 1999;59(22):5704–9. [PubMed] [Google Scholar]
- Sesink AL, et al. Red meat and colon cancer: dietary haem, but not fat, has cytotoxic and hyperproliferative effects on rat colonic epithelium. Carcinogenesis. 2000;21(10):1909–15. doi: 10.1093/carcin/21.10.1909. [DOI] [PubMed] [Google Scholar]
- Shaheen NJ, et al. Association between hemochromatosis (HFE) gene mutation carrier status and the risk of colon cancer. J Natl Cancer Inst. 2003;95(2):154–59. doi: 10.1093/jnci/95.2.154. [DOI] [PubMed] [Google Scholar]
- Shang R, Sun Z, Li H. Effective dosing of L-carnitine in the secondary prevention of cardiovascular disease: a systematic review and meta-analysis. BMC Cardiovasc Disord. 2014;14:88. doi: 10.1186/1471-2261-14-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirai T, et al. Carcinogenicity of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) in rats: dose-response studies. Princess Takamatsu Symp. 1995;23:232–39. [PubMed] [Google Scholar]
- Sima AA, et al. Acetyl-L-carnitine improves pain, nerve regeneration, and vibratory perception in patients with chronic diabetic neuropathy: an analysis of two randomized placebo-controlled trials. Diabetes Care. 2005;28(1):89–94. doi: 10.2337/diacare.28.1.89. [DOI] [PubMed] [Google Scholar]
- Singh PN, Sabate J, Fraser GE. Does low meat consumption increase life expectancy in humans? Am J Clin Nutr. 2003;78(3 Suppl):526S–32S. doi: 10.1093/ajcn/78.3.526S. [DOI] [PubMed] [Google Scholar]
- Sinha R, et al. Meat intake and mortality: a prospective study of over half a million people. Arch Intern Med. 2009a;169(6):562–71. doi: 10.1001/archinternmed.2009.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, et al. Meat and meat-related compounds and risk of prostate cancer in a large prospective cohort study in the United States. Am J Epidemiol. 2009b;170(9):1165–77. doi: 10.1093/aje/kwp280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skog KI, Johansson MA, Jägerstad MI. Carcinogenic heterocyclic amines in model systems and cooked foods: a review on formation, occurrence and intake. Food Chem Toxicol. 1998;36(9–10):879–96. doi: 10.1016/s0278-6915(98)00061-1. [DOI] [PubMed] [Google Scholar]
- Song P, Wu L, Guan W. Dietary Nitrates, Nitrites, and Nitrosamines Intake and the Risk of Gastric Cancer: A Meta-Analysis. Nutrients. 2015;7(12):9872–95. doi: 10.3390/nu7125505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springmann M, et al. Global and regional health effects of future food production under climate change: a modelling study. Lancet. 2016;387(10031):1937–46. doi: 10.1016/S0140-6736(15)01156-3. [DOI] [PubMed] [Google Scholar]
- Sroga JM, et al. Detection of the dietary xenoglycan N-glycolylneuraminic acid (Neu5Gc) and anti-Neu5Gc antibodies within reproductive tracts of male and female infertility subjects. Clin Obstet Gynecol Reprod Med. 2015;1(3):72–78. [Google Scholar]
- Stubbs JR, et al. Serum Trimethylamine-N-Oxide is Elevated in CKD and Correlates with Coronary Atherosclerosis Burden. J Am Soc Nephrol. 2016;27(1):305–13. doi: 10.1681/ASN.2014111063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimura T, et al. Heterocyclic amines: Mutagens/carcinogens produced during cooking of meat and fish. Cancer Sci. 2004;95(4):290–99. doi: 10.1111/j.1349-7006.2004.tb03205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suman SP, Joseph P. Myoglobin chemistry and meat color. Annu Rev Food Sci Technol. 2013;4:79–99. doi: 10.1146/annurev-food-030212-182623. [DOI] [PubMed] [Google Scholar]
- Swirski FK, Nahrendorf M. Leukocyte behavior in atherosclerosis, myocardial infarction, and heart failure. Science. 2013;339(6116):161–66. doi: 10.1126/science.1230719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang WH, et al. Gut microbiota-dependent trimethylamine N-oxide (TMAO) pathway contributes to both development of renal insufficiency and mortality risk in chronic kidney disease. Circ Res. 2015;116(3):448–55. doi: 10.1161/CIRCRESAHA.116.305360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang WH, et al. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med. 2013;368(17):1575–84. doi: 10.1056/NEJMoa1109400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tangvoranuntakul P, et al. Human uptake and incorporation of an immunogenic nonhuman dietary sialic acid. Proc Natl Acad Sci U S A. 2003;100(21):12045–50. doi: 10.1073/pnas.2131556100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarantini G, et al. Metabolic treatment with L-carnitine in acute anterior ST segment elevation myocardial infarction. A randomized controlled trial. Cardiology. 2006;106(4):215–23. doi: 10.1159/000093131. [DOI] [PubMed] [Google Scholar]
- Tasevska N, et al. A prospective study of meat, cooking methods, meat mutagens, heme iron, and lung cancer risks. Am J Clin Nutr. 2009;89(6):1884–94. doi: 10.3945/ajcn.2008.27272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor EF, et al. Meat consumption and risk of breast cancer in the UK Women’s Cohort Study. Br J Cancer. 2007;96(7):1139–46. doi: 10.1038/sj.bjc.6603689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traber MG, et al. Modern nutrition in health and disease. Shils, ME. 1999:396–411. [Google Scholar]
- Tremaroli V, Bäckhed F. Functional interactions between the gut microbiota and host metabolism. Nature. 2012;489(7415):242–49. doi: 10.1038/nature11552. [DOI] [PubMed] [Google Scholar]
- Tsugane S, et al. Salt and salted food intake and subsequent risk of gastric cancer among middle-aged Japanese men and women. Br J Cancer. 2004;90(1):128–34. doi: 10.1038/sj.bjc.6601511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turesky RJ. Formation and biochemistry of carcinogenic heterocyclic aromatic amines in cooked meats. Toxicol Lett. 2007;168(3):219–27. doi: 10.1016/j.toxlet.2006.10.018. [DOI] [PubMed] [Google Scholar]
- Turesky RJ, Le Marchand L. Metabolism and biomarkers of heterocyclic aromatic amines in molecular epidemiology studies: lessons learned from aromatic amines. Chem Res Toxicol. 2011;24(8):1169–214. doi: 10.1021/tx200135s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Woudenbergh GJ, et al. Meat consumption and its association with C-reactive protein and incident type 2 diabetes: the Rotterdam Study. Diabetes Care. 2012;35(7):1499–505. doi: 10.2337/dc11-1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varki N, et al. Heart disease is common in humans and chimpanzees, but is caused by different pathological processes. Evolutionary Applications. 2009;2:101–12. doi: 10.1111/j.1752-4571.2008.00064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinchi F, et al. Atherogenesis and iron: from epidemiology to cellular level. Front Pharmacol. 2014;5:94. doi: 10.3389/fphar.2014.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace TC, Fulgoni VL., 3rd Assessment of Total Choline Intakes in the United States. J Am Coll Nutr. 2016;35(2):108–12. doi: 10.1080/07315724.2015.1080127. [DOI] [PubMed] [Google Scholar]
- Wallin A, Orsini N, Wolk A. Red and processed meat consumption and risk of ovarian cancer: a dose-response meta-analysis of prospective studies. Br J Cancer. 2011;104(7):1196–201. doi: 10.1038/bjc.2011.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, et al. LC-MS/MS glycomic analyses of free and conjugated forms of the sialic acids, Neu5Ac, Neu5Gc and KDN in human throat cancers. Glycobiology. 2015;25(12):1362–74. doi: 10.1093/glycob/cwv051. [DOI] [PubMed] [Google Scholar]
- Wang Z, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472(7341):57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZY, et al. Erythrocytes from GGTA1/CMAH knockout pigs: implications for xenotransfusion and testing in non-human primates. Xenotransplantation. 2014;21(4):376–84. doi: 10.1111/xen.12106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg Robert A. The Biology of Cancer. Second. New York: Garland Publishing; 2014. Supplement 11.7 How does diet affect colon cancer incidence? [Google Scholar]
- Weinberger MH. Salt sensitivity of blood pressure in humans. Hypertension. 1996;27(3 Pt 2):481–90. doi: 10.1161/01.hyp.27.3.481. [DOI] [PubMed] [Google Scholar]
- Weinberger MH. Pathogenesis of salt sensitivity of blood pressure. Curr Hypertens Rep. 2006;8(2):166–70. doi: 10.1007/s11906-006-0014-y. [DOI] [PubMed] [Google Scholar]
- Whelton PK, et al. Sodium, blood pressure, and cardiovascular disease: further evidence supporting the American Heart Association sodium reduction recommendations. Circulation. 2012;126(24):2880–89. doi: 10.1161/CIR.0b013e318279acbf. [DOI] [PubMed] [Google Scholar]
- Wiseman M. The Second World Cancer Research Fund/American Institute for Cancer Research expert report. Food, nutrition, physical activity, and the prevention of cancer: a global perspective. Proc Nutr Soc. 2008;67(3):253–56. doi: 10.1017/S002966510800712X. [DOI] [PubMed] [Google Scholar]
- Wu X, et al. Accelerated tumor growth mediated by sublytic levels of antibody-induced complement activation is associated with activation of the PI3K/AKT survival pathway. Clin Cancer Res. 2013;19(17):4728–39. doi: 10.1158/1078-0432.CCR-13-0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia H, et al. Meta-Analysis of Saturated Fatty Acid Intake and Breast Cancer Risk. Medicine (Baltimore) 2015;94(52):e2391. doi: 10.1097/MD.0000000000002391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu R, Wang Q, Li L. A genome-wide systems analysis reveals strong link between colorectal cancer and trimethylamine N-oxide (TMAO), a gut microbial metabolite of dietary meat and fat. BMC Genomics. 2015;16(Suppl 7):S4. doi: 10.1186/1471-2164-16-S7-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano M, et al. Presence of nitrosable mutagen precursors in cooked meat and fish. Mutat Res. 1988;202(1):119–23. doi: 10.1016/0027-5107(88)90172-8. [DOI] [PubMed] [Google Scholar]
- Ye K, Gu Z. Recent advances in understanding the role of nutrition in human genome evolution. Adv Nutr. 2011;2(6):486–96. doi: 10.3945/an.111.001024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin T, et al. Extravascular red blood cells and hemoglobin promote tumor growth and therapeutic resistance as endogenous danger signals. J Immunol. 2015;194(1):429–37. doi: 10.4049/jimmunol.1400643. [DOI] [PubMed] [Google Scholar]
- Young GP, Rose IS, St John DJ. Haem in the gut. I. Fate of haemoproteins and the absorption of haem. J Gastroenterol Hepatol. 1989;4(6):537–45. doi: 10.1111/j.1440-1746.1989.tb00858.x. [DOI] [PubMed] [Google Scholar]
- Zeegers MP, et al. Nitrate intake does not influence bladder cancer risk: the Netherlands cohort study. Environ Health Perspect. 2006;114(10):1527–31. doi: 10.1289/ehp.9098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W, et al. Gut Microbial Metabolite TMAO Enhances Platelet Hyperreactivity and Thrombosis Risk. Cell. 2016;165(1):111–24. doi: 10.1016/j.cell.2016.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- zur Hausen H. Papillomaviruses in the causation of human cancers – a brief historical account. Virology. 2009;384(2):260–65. doi: 10.1016/j.virol.2008.11.046. [DOI] [PubMed] [Google Scholar]
- zur Hausen H. Red meat consumption and cancer: reasons to suspect involvement of bovine infectious factors in colorectal cancer. Int J Cancer. 2012;130(11):2475–83. doi: 10.1002/ijc.27413. [DOI] [PubMed] [Google Scholar]
- zur Hausen H, de Villiers EM. Dairy cattle serum and milk factors contributing to the risk of colon and breast cancers. Int J Cancer. 2015;137(4):959–67. doi: 10.1002/ijc.29466. [DOI] [PubMed] [Google Scholar]


