Abstract
We have shown that zebrafish (Danio rerio) are an excellent model for evaluating the link between early life stage exposure to environmental chemicals and disease in adulthood and subsequent unexposed generations. Previously, we used this model to identify transgenerational effects of dioxin (2,3,7,8-tetrachlorodibenzo-p-dioxin [TCDD]) on skeletal development, sex ratio, and reproductive capacity. Transgenerational inheritance of TCDD toxicity, notably decreased reproductive capacity, appears to be mediated through the male germ line. Thus, we examine testicular tissue for structural and gene expression changes using histology, microarray, and quantitative reverse transcription polymerase chain reaction (qRT-PCR). Histological analysis revealed decreased spermatozoa with concurrent increase in spermatogonia, and decreased germinal epithelium thickness in TCDD-exposed males compared with controls. We also identified altered expression of genes associated with testis development, steroidogenesis, spermatogenesis, hormone metabolism, and xenobiotic response. Altered genes are in pathways involving lipid metabolism, molecular transport, small molecule biochemistry, cell morphology, and metabolism of vitamins and minerals. These data will inform future investigations to elucidate the mechanism of adult-onset and transgenerational infertility due to TCDD exposure in zebrafish.
Introduction
Toxicant exposure during development can affect health outcomes in the adult and subsequent generations. In humans, the developmental basis for adult-onset and transgenerational disease is difficult to study due to the time frame between cause and effect. Such studies in wild fish populations are complicated by the complexity of aquatic environments.1 Zebrafish are an excellent model system because they are vertebrates with short generation times, homogeneous genetic backgrounds, and modest housing needs that stabilize biotic and abiotic factors, thus facilitating single and multigenerational studies with minimal time, expense, and confounding variables.
Research using the zebrafish model has shown that exposure to low level dioxin (2,3,7,8-tetrachlorodibenzo-p-dioxin [TCDD]) causes toxic endpoints in multiple organs, including skeletal (spinal and craniofacial deformities), hydrostatic (uninflated swim bladders), respiratory/osmoregulatory (underdeveloped gills), cardiac (decreased output, ventricular hypertrophy, and pericardial edema), and reproductive (skewed sex ratios, body plan–gonadal mismatch, decreased egg release, decreased egg fertilization rates, and abnormal spawning behavior) systems.2,3 Exposures to low level TCDD at early life stages produce skeletal abnormalities and decreased reproductive capacity that persist for multiple, unexposed generations of zebrafish.4
TCDD is a potent, persistent environmental contaminant, and endocrine-disrupting chemical (EDC) that is primarily produced by industrial processes (waste incineration, fossil fuel combustion, manufacture of paper, pesticide, and metal products). EDCs are exogenous agents that interfere with the physiology of endogenous hormones, and often produce the most serious health consequences when exposure occurs early in development.5
In recent years, significant advances have occurred in linking EDCs with phenotypic endpoints and biomarkers of effect. For example, exposure to EDCs contributes to an array of reproductive disorders in both humans (e.g., reduced fertility, abnormal development and function of reproductive organs, poor sperm quality, and cancer)5–8 and fish (e.g., testicular oocytes or “intersex,” reduced recruitment, and elevated vitellogenin concentrations).1,6,9–11 Furthermore, we have shown that reproductive endpoints are observed in offspring of TCDD-lineage F2 male and control female spawnings,4 indicating that transgenerational inheritance of TCDD reproductive toxicity in zebrafish occurs through the male germ line.
Understanding the mechanism of toxin-induced reproductive disease is important because infertility, and particularly male-mediated infertility, is increasingly prevalent in people around the world,12 and associated with population-level consequences in wild fish.1 Research that uncovers critical pathways and biomarkers of effect can inform novel therapies, future research needs, and prevention and conservation strategies.
The purpose of this study is to identify structural and gene expression changes in testicular tissue from TCDD-exposed F0 generation zebrafish using histology, microarray, and quantitative reverse transcription polymerase chain reaction (qRT-PCR). It is widely known that TCDD acts as aryl hydrocarbon receptor (AhR) agonists that ultimately affect gene transcription, which mediates TCDD's toxic endpoints.13 When TCDD is translocated into the nucleus after AhR binding, AhR and the AhR nuclear translocator form a heterodimer that binds DNA promoter regions of a multitude of AhR-responsive genes. However, little is known about long-term changes associated with brief, low level TCDD exposure. Thus, identifying concurrent histological and transcriptomic changes, as well as molecular or cellular pathways affected, will inform future studies to investigate the mechanism of adult-onset and transgenerational reproductive disease due to TCDD exposure in zebrafish.
Materials and Methods
Fish husbandry
Zebrafish embryos (AB strain) were kept at 27°C–30°C in lightly buffered water (60 mg/L Instant Ocean Salts; Aquarium Systems, Mentor, OH) with a standard 14-h/10-h light/dark cycle as described by Westerfield.14 Fish were fed twice per day. When needed, fish were euthanized in tricaine methanesulfonate (1.67 mg/mL). The protocol for zebrafish use and maintenance was approved by the Research Animal Resources Center of the University of Wisconsin–Madison, which follows the National Institutes of Health Guide to the Care and Use of Laboratory Animals (Protocol No. M00489).
TCDD exposure
TCDD (>99% purity; Chemsyn) was used as a stock solution in dimethyl sulfoxide (0.1% DMSO). Zebrafish were exposed for 1 h to waterborne TCDD at 50 pg/mL or vehicle (DMSO) at both 3 and 7 weeks postfertilization (wpf; during sexual differentiation and gonad maturation) in small glass beakers with gentle rocking. This dose was determined based on previous dose response experiments and results.3 The number of fish per volume of dosing solution was 1 fish/mL at 3 wpf and 1 fish/2 mL at 7 wpf. This was changed due to growth between 3 and 7 wpf. TCDD exposures were accomplished in three blocks (replicates). Each replicate included eight vials of five fish, for a total n = 24. Mean percentage survival (±SEM) was recorded and the mean and standard error of the mean (SEM) were calculated. TCDD exposure produced survival rates similar to those found in control groups. All fish were raised using standard husbandry practices approved by the University of Wisconsin–Madison Institutional Animal Care and Use Committee.
Experimental design
These fish were part of a previously published study that showed a decrease in reproductive capacity in adult males and females after juvenile exposure to sublethal levels of TCDD.3 Adult male zebrafish were euthanized at 1 year of age. The testicular tissue was collected and analyzed using histopathology, microarray, and qRT-PCR.
Testis histology
Male fish were euthanized at 1 year of age with tricaine methanesulfonate (1.67 mg/mL). Whole fish were fixed in 10% Zn formalin, decalcified with Cal-ExII, and bisected along the sagittal plane. The specimens were dehydrated in a graded series of ethanol, cleared in xylene, embedded in paraffin, sectioned (5 μm), mounted onto slides, and stained with hematoxylin and eosin (H&E). Gonadal tissue was evaluated and nine seminiferous tubules from both testes were identified at 40× magnification and measured for each fish (six controls and six TCDD exposed). Sections were imaged using an Olympus DP72 digital camera on an Olympus S2X16 microscope.
The area (μm2) of each tubule and the four major testicular cell types were measured and recorded using ImageJ 1.49 software. The germinal epithelium thickness (μm) was also measured for each tubule. The areas of the following testicular cells types, spermatogonia (the least mature), spermatocyte, spermatid, and spermatozoa (most mature), were calculated and used to compare the TCDD-exposed male with the control (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/zeb). Student's t-test was used to identify differences between the two groups. A difference of p < 0.05 was considered significant.
RNA isolation
Both testes from five treated and five control zebrafish were extracted through macrodissection and frozen in liquid nitrogen and stored at −80°C. The testicular tissue of each fish served as an independent sample so that there were five independent biological replicates in each treatment group. Whole tissues were homogenized in QIAzol lysis reagent (Qiagen). RNA was isolated with the Qiagen RNEasy mini kit following the manufacturer's protocol. RNA was quantified and purity was assessed with Nanodrop-1000 (Thermo Fisher Scientific). RNA quality was assessed with the Agilent RNA 6000 Nano kit on an Agilent Bioanalyzer. RNA Integrity Number (RIN) values were between 5.7 and 8.0 (Supplementary Table S1). These numbers are low but are appropriate for gene expression studies through qRT-PCR.15
Microarray
Transcriptomic microarray analysis of testicular tissue was employed for discovery of expression changes across the whole genome in response to TCDD. The microarray processing, labeling, and hybridization were performed and analyzed following MIAME guidelines (Supplementary Table S2) by the University of Wisconsin Biotechnology Gene Expression Center.16 RNA from five treated and five control zebrafish was processed with the Ambion WT Expression Kit.
Each sample was assessed on an independent array. All samples were end-terminus labeled following the guidelines of the Affymetrix GeneChip WT terminal labeling and hybridization user manual target (P/N 702808 Rev. 7). After fragmentation, 10 μg of cRNA was hybridized for 16 h at 45°C on Zebrafish Gene 1.0 ST Genome Array. GeneChips were washed and stained in the Affymetrix Fluidics Station 450. GeneChips were scanned using the Affymetrix GeneArray Scanner GC3000 G7. The ZebGene 1.0 design was based on annotations associated with the Zv9 genome assembly.
The data were analyzed using Transcriptome Analysis Console (TAC; Affymetrix) and genes of interest were selected as having a p value ≤0.05 and an absolute fold change ≥1.5. The TAC software calculated gene intensity to perform normalization. For a particular gene, the gene intensity for each sample was calculated using the Tukey's Biweight average (log2 scale) for all the eligible exons (probe selection regions [PSRs]) intensities in that gene. Next, normalization of each PSR was performed using the gene intensity of that sample. Normalized intensities from Condition 1 (TCDD) were compared with normalized intensities from Condition 2 (control) using one-way between-subject ANOVA for the PSRs and junctions within a gene. Microarray data were uploaded to NCBI GEO database (GSE77335). The significantly altered genes (p value ≤0.05 and an absolute fold change ≥1.5) were uploaded into Ingenuity Pathway Analysis (IPA) for pathway analysis.
qRT-PCR
Genes of interest were selected based on the microarray results. RNA from the testes samples used in the microarray experiment was used to validate 23 genes using TaqMan Gene Expression Assays (Life Technologies) and analyzed on an Applied Biosystems 7900. Ten microliters of RNA, at 50 ng/μL, was reverse transcribed with random hexamers and Multiscribe MuLv from the High Capacity cDNA Reverse Transcription Kit (Thermo Fischer Scientific) per manufacturers protocol.
Ten microliters of the resulting cDNA was preamplified for genes selected through the microarray analysis or other genes of interest, using TaqMan Preamp Mastermix Kit (Thermo Fisher Scientific) for 40 cycles in a 50 μL reaction volume. Probes used were TaqMan gene expression assays (Thermo Fisher Scientific) for abcc2 (Dr03113584_m1), actb1 (Dr03432610_m1), apobb (Dr03122665_g1), asb16 (Dr03169566_s1), ca2 (Dr03433913_m1), cox6b1 (Dr03421412_m1), cyp1a (Dr03112441_m1), cyp2k22 (Dr03080917_mH), egr1 (Dr03074044_m1), hsp70 (Dr03205345_s1), id2a (Dr03113868_m1), igf2b (Dr03425647_m1), lyz (Dr03099438_g1), mtp (Dr03133295_g1), nr5a1b (Dr03088630_m1), nudt15 (Dr03425797_m1), si:dkey-208k22.3 (Dr03436005_g1), sox9a (Dr03112283_m1), sox9b (Dr03080049_m1), star (Dr03112289_m1), ucp1 (Dr03106289_m1), ugt5g1 (Dr03432374_m1), vasa (Dr03124942_g1), and vtg7 (Dr03442446_g1). Probes used were chosen for best coverage according to the Thermo Fisher database (Supplementary Table 3).
TaqMan gene expression assays are MIQE compliant and qRT-PCR was performed following MIQE guidelines (Supplementary Table S4).17,18 qRT-PCRs for the previously mentioned genes were performed with the TaqMan Universal Master Mix (Thermo Fisher Scientific) in a 20 μL reaction volume containing 2 μL of the preamplified cDNA. All experimental samples were analyzed for 10 genes at a time on 384-well plates. Thermal cycling parameters were carried out per manufacturer's protocol. Reactions were done in triplicate.
qRT-PCR analysis and calculations were performed in the Sequence Detection System v 2.4. All transcripts examined were normalized to β-actin (actb1) through the comparative Ct (cycle threshold; ΔΔCt) method using actb1 as a housekeeping gene. Actb1 was shown to be unaltered by TCDD in the microarray and qRT-PCR and thus was suitable to be used as the housekeeping gene. Student's t-test was used to identify differences between two groups. A difference of p < 0.05 was considered significant.
Results
Testis histology
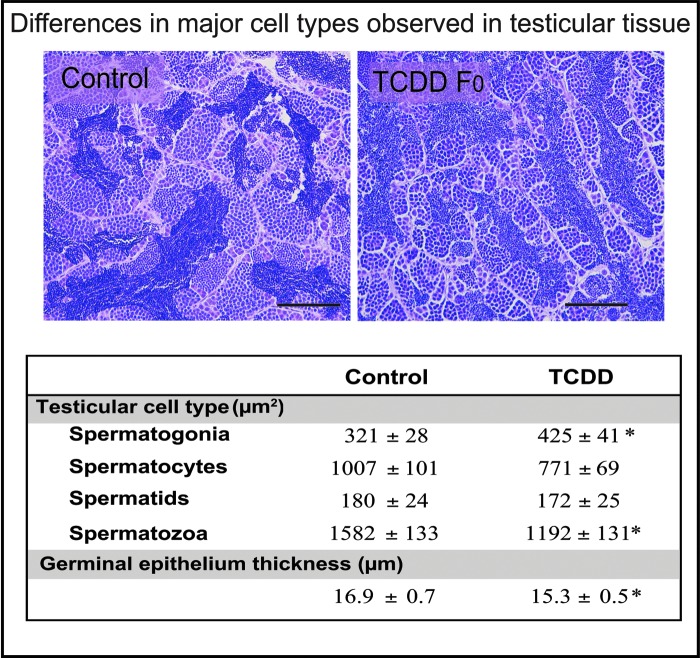
H&E staining and cell area quantification of testicular tissue revealed significant differences between the TCDD and control groups. There was no significant difference in the overall area of the tubules that were being compared between the two groups. Analysis of the four major cell types revealed that adult zebrafish exposed to TCDD during development have a higher area of immature cells (spermatogonia; p = 0.036) and a lower area of mature cells (spermatozoa; p = 0.038) than the controls. The intermediate cell types, spermatocytes (p = 0.055) and spermatids (p = 0.83), were not significantly different from the control. Germinal epithelium thickness was also measured for each tubule and was decreased in the fish exposed to TCDD (p = 0.048). Representative images of the control and TCDD group testicular tissue sections are shown in Figure 1. The average areas for each of the cell types, the germinal epithelium thickness, and the SEMs are also listed in Figure 1.
FIG. 1.
Histological analysis. H&E staining and cell type quantification (ImageJ) of testicular tissue revealed that zebrafish exposed to TCDD have a higher area of immature cells (spermatogonia), a lower area of mature cells (spermatozoa), and a decreased germinal epithelium thickness. Top panels show 40× sections from a control and TCDD-exposed fish. Numbers in the table indicate mean area of each cell type per tubule (μm2) or thickness (μm)+SEM. *Indicates significant difference (p < 0.05) from the control. H&E, hematoxylin and eosin; SEM, standard error of the mean; TCDD, 2,3,7,8-tetrachlorodibenzo-p-dioxin.
Microarray
We examined the effects of a low dose TCDD exposure to juvenile zebrafish on gene expression in the testis of the adult zebrafish by whole genome microarray analysis. Outliers were flagged and identified using Affymetrix Expression Console Software. One of the control samples was excluded from the microarray and qPCR analysis because the array Quality Control metrics flagged this sample as an outlier according to quality control All Probe Set RLE (relative log expression) Mean metric and the RLE signal graphs. According to Affymetrix,19 this metric measures “the signal of each probe set compared to the median signal value of this probe set in the study. The metric is the mean of these differences from all the probe sets. Unusually high values indicate that the signals on the array are different from others in the study, i.e., big values are bad.” The outlier had a value of 0.49 and the next highest sample was 0.29. The RLE and the rest of the QC metrics were similar between all other samples.
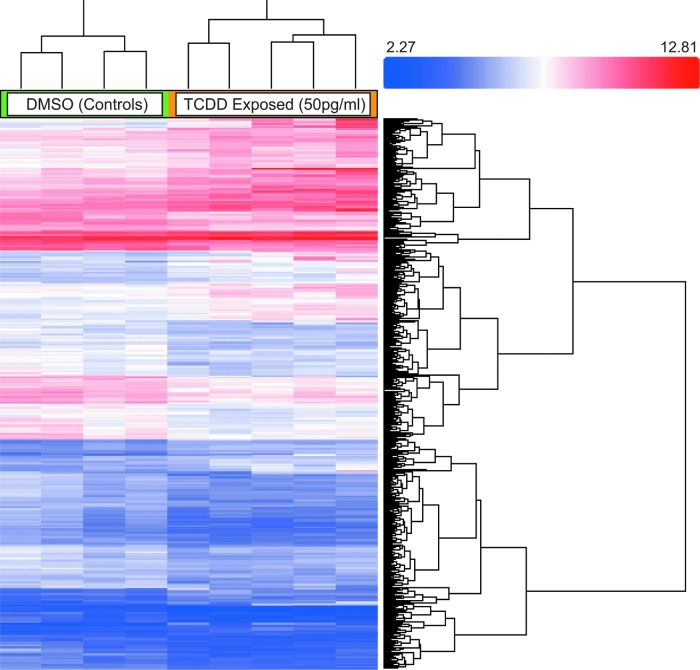
The data were analyzed using TAC (Affymetrix) and 722 genes were differentially regulated due to TCDD exposure, having a p value ≤0.05 and a fold change ≥1.5 when the normalized intensities were compared with one-way between-subject ANOVA. Of the genes, 42% were upregulated and 58% were downregulated. The most significant difference in an annotated gene was in apolipoprotein Bb with a fold change of 18.09 and a p value of 0.016. Microarray data were uploaded to NCBI GEO database (GSE77335) and the fold change and p values of the genes of interest are listed in Table 1. Using TAC software, hierarchal clustering and a heat map were generated to show correlation among samples (Fig. 2). Samples clustered into their respective treatment groups.
Table 1.
Gene Expression Changes
| Microarray | qRT-PCR | |||||
|---|---|---|---|---|---|---|
| Gene symbol | Gene description | FC | ANOVA p value | FC (2ΔΔct) | SEM | p |
| abcc2 | ATP-binding cassette, subfamily C, member 2 | 4.99 | 0.0370 | 63.12 | 56.7 | 0.0320 |
| apobb | Apolipoprotein Bb | 18.09 | 0.0170 | 37.10 | 12.7 | 0.0086 |
| asb16 | Ankyrin repeat and SOCS box containing 16 | −1.66 | 0.0011 | −2.17 | 0.43 | 0.0150 |
| ca2 | Carbonic anhydrase II | 1.69 | 0.0390 | 3.44 | 1.55 | 0.0360 |
| cox6b1 | Cytochrome c oxidase subunit 6b polypeptide 1 | 1.61 | 0.0005 | 1.91 | 0.35 | 0.0210 |
| cyp1a | Cytochrome P450, family 1, subfamily A, polypeptide 1 | 2.20 | 0.0610 | 20.23 | 11.5 | 0.0480 |
| cyp2k22 | Cytochrome P450, family 2, subfamily K, polypeptide 22 | 3.30 | 0.0560 | 19.86 | 10.6 | 0.0280 |
| egr1 | Early growth response 1 | 2.02 | 0.0016 | 3.13 | 0.79 | 0.0130 |
| hsp70 | Heat shock cognate 70-kd protein | −1.49 | 0.0220 | −3.20 | 0.51 | 0.0280 |
| id2a | Inhibitor of DNA binding 2, dominant negative helix-loop-helix protein, a | 1.34 | 0.0008 | 1.76 | 0.28 | 0.0190 |
| igf2b | Insulin-like growth factor 2b | 1.73 | 0.0260 | 2.09 | 0.33 | 0.0068 |
| lyz | Lysozyme | −2.43 | 0.0013 | −3.95 | 2.26 | 0.0290 |
| mtp | Microsomal triglyceride transfer protein | 1.62 | 0.0240 | 9.85 | 5.46 | 0.0460 |
| nr5a1b | Nuclear receptor subfamily 5, group A, member 1b (sf1) | 1.73 | 0.0034 | 1.68 | 0.26 | 0.0330 |
| nudt15 | Nudix (nucleoside diphosphate-linked moiety X)-type motif 15 | −3.03 | 0.0086 | −2.61 | 0.43 | 0.0005 |
| si:dkey-208k22.3 | Sanger Institute Chromosome 2: 23,495,059-23,498,694 forward strand | 2.17 | 0.0002 | 4.47 | 1.12 | 0.0038 |
| sox9a | SRY (sex determining region Y)-box 9a | 1.66 | 0.1080 | 2.03 | 0.46 | 0.0430 |
| sox9b | SRY (sex determining region Y)-box 9b | 1.36 | 0.0038 | 3.16 | 1.03 | 0.0300 |
| star | Steroidogenic acute regulatory protein | 1.42 | 0.0310 | 1.85 | 0.13 | 0.0010 |
| ucp1 | Uncoupling protein 1 | 6.58 | 0.0180 | 13.31 | 6.82 | 0.0190 |
| ugt5g1 | UDP glucuronosyltransferase 5 family, polypeptide A1 | 1.97 | 0.0180 | 2.25 | 0.36 | 0.0052 |
| vasa | Vasa homologue | −1.71 | 0.0120 | −1.69 | 0.23 | 0.0260 |
| vtg7 | Vitellogenin 7 | 1.44 | 0.0061 | 1.22 | 0.25 | 0.3780 |
Twenty-three genes were selected from the microarray data based on the criteria of the largest fold changes, most significant changes (lowest p value), classic TCDD-responsive genes, or genes involved in testicular development and spermatogenesis. These were validated with qPCR. The results for the microarray and qPCR with SEM are listed. FC indicates fold change and the p value was determined using ANOVA (microarray) and Student's t-test (qPCR).
qRT-PCR, quantitative reverse transcription polymerase chain reaction; SEM, standard error of the mean; TCDD, 2,3,7,8-tetrachlorodibenzo-p-dioxin.
FIG. 2.
Microarray results. Gene expression changes from ZebGene_1.0 ST Affyemtrix arrays. Transcriptome Analysis Console (Affymetrix) was used to analyze, hierarchically cluster the results, and generate the heat map. The heat map illustrates the level of expression of 722 transcripts that were significantly changed (p < 0.05; fold change >1.5) due to TCDD exposure. Samples are clustered into their respective treatment groups (TCDD and DMSO control). The scale displays the signal intensity range of the TCDD and control condition pair from minimum to maximum. DMSO, dimethyl sulfoxide.
The significantly altered genes (p value ≤0.05 and a fold change ≥1.5) were uploaded into IPA for pathway enrichment analysis. IPA converted the differentially expressed zebrafish genes into their known human homologues and these were analyzed for enrichment of known pathways. The most enriched molecular and cellular function pathways after TCDD treatment included lipid metabolism, molecular transport, small molecule biochemistry, cell morphology, and vitamin and mineral metabolism (Table 2).
Table 2.
Ingenuity Pathway Analysis Generated List of Molecular and Cellular Functions that are Enriched in the Testicular Tissue from 2,3,7,8-Tetrachlorodibenzo-p-Dioxin-Exposed Adult Males
| Molecular and cellular functions | p | No. of genes |
|---|---|---|
| Lipid metabolism | 1.35E-02–7.06E-07 | 105 |
| Concentration of triacylglycerol | 7.06E-07 | 26 |
| Concentration of acylglycerol | 7.36E-07 | 27 |
| Metabolism of terpenoid | 4.33E-06 | 24 |
| Concentration of lipid | 5.58E-06 | 53 |
| Steroid metabolism | 1.64E-05 | 21 |
| Oxidation of lipid | 4.11E-05 | 22 |
| Concentration of cholesterol | 5.10E-05 | 22 |
| Concentration of fatty acid | 6.34E-05 | 21 |
| Fatty acid metabolism | 6.91E-05 | 40 |
| Synthesis of fatty acid | 8.19E-05 | 22 |
| Molecular transport | 1.29E-02–7.06E-07 | 138 |
| Concentration of triacylglycerol | 7.06E-07 | 26 |
| Concentration of acylglycerol | 7.36E-07 | 27 |
| Concentration of lipid | 5.58E-06 | 53 |
| Concentration of cholesterol | 5.10E-05 | 22 |
| Concentration of fatty acid | 6.34E-05 | 21 |
| Uptake of d-glucose | 7.68E-05 | 20 |
| Concentration of d-glucose | 7.74E-05 | 24 |
| Quantity of steroid | 1.18E-04 | 33 |
| Secretion of lipid | 6.74E-04 | 16 |
| Abnormal quantity of lipid | 8.40E-04 | 11 |
| Small molecule biochemistry | 1.35E-02–7.06E-07 | 125 |
| Concentration of triacylglycerol | 7.06E-07 | 26 |
| Concentration of acylglycerol | 7.36E-07 | 27 |
| Metabolism of terpenoid | 4.33E-06 | 24 |
| Concentration of lipid | 5.58E-06 | 53 |
| Steroid metabolism | 1.64E-05 | 21 |
| Oxidation of lipid | 4.11E-05 | 22 |
| Metabolism of amino acids | 5.06E-05 | 16 |
| Concentration of cholesterol | 5.10E-05 | 22 |
| Concentration of fatty acid | 6.34E-05 | 21 |
| Fatty acid metabolism | 6.91E-05 | 40 |
| Cell morphology | 1.37E-02–3.87E-06 | 120 |
| Abnormal morphology of cells | 3.87E-06 | 76 |
| Morphology of cells | 2.59E-05 | 107 |
| Size of cells | 4.62E-03 | 27 |
| Morphology of epithelial cells | 7.12E-03 | 13 |
| Abnormal morphology of epithelial cells | 1.07E-02 | 11 |
| Morphology of endocrine cells | 1.26E-02 | 7 |
| Transmembrane potential of mitochondria | 1.37E-02 | 14 |
| Vitamin and mineral metabolism | 1.35E-02–4.33E-06 | 34 |
| Metabolism of terpenoid | 4.33E-06 | 24 |
| Steroid metabolism | 1.64E-05 | 21 |
| Metabolism of sterol | 4.96E-04 | 12 |
| Metabolism of cholesterol | 1.41E-03 | 10 |
| Homeostasis of cholesterol | 1.91E-03 | 9 |
| Synthesis of progesterone | 2.23E-03 | 7 |
| Metabolism of estrogen | 7.40E-03 | 3 |
| Synthesis of terpenoid | 1.06E-02 | 18 |
| Synthesis of steroid | 1.10E-02 | 17 |
| Abnormal quantity of vitamin D | 1.29E-02 | 2 |
| Quantity of fat-soluble vitamin | 1.35E-02 | 3 |
qRT-PCR
Twenty-three genes were selected from the microarray data with the criterion of the largest fold changes with the most lowest p value, classic TCDD-responsive genes, and genes involved in testicular development and spermatogenesis. These were examined through qRT-PCR. Expression of these genes was determined across all five TCDD-treated animals and all four control animals. Genes examined were all well within the limit of detection. Ct values for the housekeeping gene, actb1, were not significantly different between TCDD and control samples. The no template controls gave undetermined CTs. Triplicates showed little variation.
Although there are some quantitative differences between microarray and qRT-PCR, this does not raise concerns regarding the consistency of our findings as arrays and qRT-PCR are different measures. Arrays are hybridization and qRT-PCR is an amplification. We have commonly observed that the qRT-PCR tends to be more sensitive than arrays. Of the genes examined, 22 had significant expression changes and 1 was not validated (Table 1). The vtg7 gene was the only selected gene to have a false positive on microarray. Vtg7 showed significant upregulation according to the microarray but was not validated with qRT-PCR. The early-life germ cell marker vasa was significantly downregulated in TCDD-exposed males. Although increased expression of a particular splice variant of vasa was previously reported in male zebrafish exposed to TCDD, the microarray and qPCR probes used in our study target a region of the gene found in all splice variants of vasa, and the results reported here refer to overall vasa expression.20 The most significant differences in gene expression were abcc2, apobb, cyp1a, cyp2k22, and ucp1.
Discussion
To investigate TCDD-induced reproductive endpoints and concurrent changes in gene expression, we performed histological and transcriptomic analyses of testes in adult F0 generation zebrafish. Histological analysis showed no significant change in the area occupied by spermatocytes or spermatids within the seminiferous tubules, but the area occupied by spermatogonia was significantly increased, whereas area occupied by spermatozoa was significantly decreased in males exposed to low level TCDD during the developmental phase of sexual differentiation compared with that in control males. In addition, although no statistically significant change was found for spermatocytes (p = 0.055), the marked decrease may be biologically significant. Thus, TCDD-exposed males appear to have relatively immature testes culminating in hypospermatogenesis compared with controls.
Hypospermatogenesis was also found in similar studies using a rodent model, but a key difference in the rodent studies was that cells within the germinal epithelium were decreased at all stages of maturation.21,22 This key difference may be dose dependent because the rodent studies used considerably higher TCDD doses (0.5–50 μg/mL vs. 50 pg/mL) and more active routes of exposure (subcutaneous or intraperitoneal injection vs. immersion). Our results may be consistent with delayed spermiation (spermatid retention), in which spermatids are phagocytosed by Sertoli cells rather than released into the lumen of the seminiferous tubules to become spermatozoa.23
Delayed spermiation has been associated with toxicant exposure and hormone disruption, but spermiation is a highly complex process and the mechanism(s) involved in delayed spermiation remain unknown and are likely varied.23 Interestingly, oxidative stress can lead to junction and communication disruption between Sertoli cells and spermatids,12 and TCDD is known to induce oxidative stress in testis.24 If our delayed spermiation hypothesis is confirmed by future studies, to our knowledge, it would be the first finding of delayed spermiation due to TCDD, as well as the first in fish due to toxicant exposure.
Histology also revealed that overall germinal epithelium thickness was reduced in TCDD-treated fish compared with that in controls. Altered germinal epithelium was observed after TCDD exposure in rodent studies.21,25,26 According to these rodent studies, TCDD's adverse effect on germinal epithelium is dose dependent and concurrent with hypospermatogenesis. This is not surprising because changes to the localized hormonal environment of Sertoli cells, which includes both testosterone and estrogen, can alter cell adhesion within germinal epithelium, notably between Sertoli cells and spermatids.12 Testicular pathology in TCDD-treated zebrafish may be partially related to oxidative damage within the testis, but TCDD may also be indirectly affecting Leydig cell function and the Sertoli cell microenvironment through cross talk with estrogen and/or androgen receptors.27 In fact, this may lend support to our retained spermatid hypothesis because spermiation is particularly sensitive to hormonal fluctuations.12
In contrast, TCDD could cause direct damage to the germinal epithelium if the blood–testis barrier was compromised as a consequence of juvenile exposure. However, then we would expect that spermatogonia would decrease, or at least remain stable. It is possible that the apparent contradiction between reduced germinal epithelium and increased spermatogonia is due to relatively mild pathology after a low TCDD dose or long-term feedback/compensatory mechanism(s) in response to early disruptions in testes homeostasis.
The latter hypothesis highlights the value of assessing toxic endpoints over the course of multiple time points. Specifically, measuring endpoints at multiple time points or life stages after toxicant exposure has the potential to identify appropriate biomarkers for different types of exposure (acute, chronic, and pulsatile), stage of exposure, and the occurrence of phenotypic and genetic plasticity, as well as predict relevant toxic endpoints based on the life stage(s) exposed. To our knowledge, this is the first study to report changes in testicular germinal epithelium in fish after TCDD exposure.
The transcriptomic results showed significant changes in gene expression associated with lipid metabolism, molecular transport, small molecule biochemistry, cell morphology, and metabolism of vitamins and minerals. Genes of interest in these categories include ABCC2, APOA, EGR1, ID2, IGF2, MTTP, STAR, and UCP3. These genes are involved in lipid and steroid synthesis, lipid and cholesterol concentration, steroid, sterol, and cholesterol metabolism, lipid and steroid secretion, quantity of steroid hormones, cholesterol transport and translocation, or abnormal cell morphology.
In this study, expression of abcc2, apobb (and upstream promoter mtp), egr1, id2a, igf2b, star, and ucp1 was significantly increased. Interestingly, ucp1 is also part of the gene ontology pathway that is involved in cellular response to estrogen stimulus.28 Thus, ucp1 overexpression indicates the potential that testes of TCDD-treated zebrafish have altered estrogen level, which has been associated with reduced germinal epithelium,29,30 as was also seen in our histological results. From absence or underexpression studies, we know that apobb, egr1, id2a, igf2b, and star or their orthologs are important for testicular homeostasis,28,31–34 but little is known about the sequelae of overexpression of these genes. For example, expression of EGR1, a transcription factor that promotes Leydig cell steroidogenesis and differentiation of germinal epithelial cells, was increased in pulmonary epithelial cells after AhR activation.32,35,36 Due to tumorigenic properties, the authors concluded that EGR1 upregulation may contribute to the pathology associated with exposure to environmental toxicants.35
Similarly, expression of StAR, which is responsible for cholesterol transport that enables steroidogenesis in Leydig cells, was also induced by AhR activation, thus having implications for endocrine function.37 In contrast, increased transcription of ID2, a regulator of Sertoli cell function and meiotic cell divisions, has a stronger association with reproductive dysfunction characterized by maturation arrest of spermatogonia, leading to nonobstructive azoospermia in humans.34,38 Our pathway analysis highlights transcriptomes and molecular/cellular functions that are necessary for testicular and spermatogenic homeostasis, but further investigation will be required to understand their role in mediating the phenotypic endpoints described in this study and previously.3
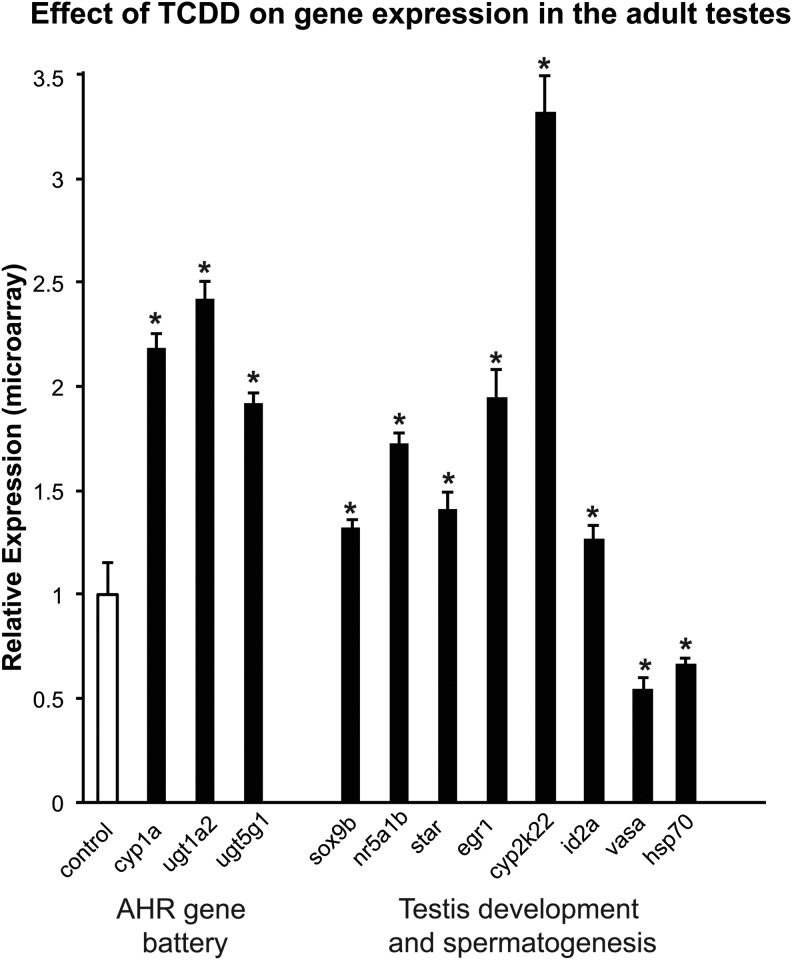
From microarray analysis and qRT-PCR validation, we found significant changes in other genes related to testes development, steroidogenesis, spermatogenesis, and hormone metabolism, namely ca2, cyp2k22, hsp70, lyz, nr5a1b, sox9b, and vasa (Figure 3).28,39–45 Notably, cyp2k22 had a greater than 19-fold increase in TCDD-treated zebrafish compared to controls. Induction of cyp2k22 is known to occur in response to androgens, possibly through cross talk with other nuclear receptors, and is a potential biomarker for the presence of androgens.41 This lends further evidence that the hormonal microenvironment within the testes of TCDD-treated zebrafish is altered.
FIG. 3.
Pathways and genes of interest. The bars represent the gene expression fold change from the microarray experiments for genes involved in the AhR gene battery, and testicular development and spermatogenesis. The scale is relative expression of the TCDD exposure compared with DMSO control, with the expression in the control (unfilled bar) set to 1. The error bars represent the SEM. *Indicates significant difference (p < 0.05) from the control. AhR, aryl hydrocarbon receptor.
Upregulation of sox9b was unexpected in this case because TCDD induces downregulation of sox9b within 72 h in the developing epicardium and skeleton, and is associated with heart and jaw malformations.46,47 Although it remains unknown whether sox9b downregulation occurs within testicular tissue acutely after TCDD exposure, and then has implications for testes development, sox9b upregulation may support our hypothesis that some of the transcriptomic changes may be due to long-term feedback/compensatory mechanism(s) in response to disruptions in testes homeostasis.
Also noteworthy, hsp70, a member of the ubiquitously conserved heat shock protein, was significantly downregulated. Certain hsp70 members are unique in that they are found exclusively in germ cells in mammals, become expressed early in development, and remain present at all stages of spermatogenesis.45,48 Mice lacking Hsp70-2 had increased frequency of apoptotic spermatocytes, resulting in failed production of postmeiotic spermatids and spermatozoa,48 and has been connected to cases of human male infertility.49,50 Although endpoints, such as sperm motility and acrosome reaction, were not evaluated in this study, alterations in lyz and ca2 may be involved in decreased egg fertilization that was reported previously in zebrafish,3,40,42 and thus may be further considered in future investigations.
Some of the genes highlighted by pathway and microarray analyses, namely abcc2, apobb, and ca2, also play a role in xenobiotic response and tissue defense.28,51 Markedly increased expression was observed for abcc2 (greater than 60-fold change) and apobb (greater than 35-fold change) in particular. Although the role of abcc2 is better described in liver, expression also occurs in the germinal epithelial cells of both humans and zebrafish.28,51,52 However, another xenobiotic-responsive gene was found to be downregulated; nudt15 protects against translational errors and oxygen radicals,53 so may be a factor in TCDD's overall pathology.
Not surprisingly, we also observed upregulation of cyp1a1, a ubiquitously expressed member of the AhR gene battery, as well as genes similar to the non-P450 genes in the AhR battery, specifically ugt1a1 and ugt5g (Fig. 3). The AhR gene battery was originally described in the murine model and consists of genes that are coordinately induced by AhR agonists, including TCDD.54 These genes play an important role in oxidative stress pathways and response, cell cycle regulation, and cellular fate.54 Since CYP1A1 is a canonical AhR response gene, it is not entirely unexpected that we observed a greater than 20-fold change in cyp1a1 expression. TCDD has a long half-life in tissues, and could thus cause prolonged AhR activation, which has been associated with toxic endpoints.55
Nonetheless, it is remarkable that these AhR-responsive genes are significantly upregulated 10 months after a brief, low level TCDD exposure. In fact, the long-term histological and transcriptomic changes in these TCDD-treated zebrafish testes highlight the developmental basis for adult-onset disease. Environmentally induced changes to the germ line during gonad development are a particularly sensitive time frame that could lead to permanent changes.56,57 In mammals, epigenetic reprogramming occurs during development,56 and thus could explain persistent disease in adulthood and transgenerationally. For example, changes in DNA methylation patterns in the germ line were responsible for transgenerational reproductive problems in rats.58 However, further investigation is necessary to determine whether epigenetic modifications are a factor in these zebrafish.
In summary, histological endpoints in these TCDD-exposed testes are concomitant with significant changes in the transcriptome and molecular/cellular functions that are associated with negative reproductive outcomes in adult zebrafish previously described.3 These findings bolster the value of zebrafish as a translational model for studying human male infertility, which accounts for ∼50% of human infertility cases.59
At the same time, the zebrafish model can provide insight into the effects of EDCs and environmental toxicants in wild fish. Toxicant exposure has been associated with declining and less sustainable wild fish populations,60–62 which are not only important indicators of aquatic ecosystem health but also an increasingly important protein source for human populations. Although the value of laboratory experiments to inform wild fish populations has been questioned due to plasticity and the dynamic, complex aquatic environment, a combination of controlled and field-based studies will likely continue to necessarily inform each other given the advantages/disadvantages of both approaches.
These results will inform future investigations to elucidate the mechanism of adult-onset and transgenerational infertility due to TCDD exposure in zebrafish. Understanding the mechanism(s) for disease and inheritance could help to improve treatment options in humans and identify wild fish populations that would benefit from environmental chemical mitigation/prevention strategies.
Supplementary Material
Acknowledgments
The authors thank the University of Wisconsin Biotechnology Center Gene Expression Center for providing Affymetrix GeneChip services. The authors also thank Dick Peterson and Christopher Bradfield for technical advice and Dorothy Nesbit for zebrafish maintenance and husbandry. Funding was provided by National Institute of Environmental Health Sciences from T32 ES007015 and National Center for Advancement of Translational Sciences support from K01 OD010462 Center for Urban Responses to Environmental Stressors (P30 ES020957) (to T.R.B.).
Disclosure Statement
No competing financial interests exist.
References
- 1.Hamilton PB, Cowx IG, Oleksiak MF, Griffiths AM, Grahn M, Stevens JR, et al. Population-level consequences for wild fish exposed to sublethal concentrations of chemicals—a critical review. Fish Fish 2015;17:1–22 [Google Scholar]
- 2.King Heiden TC, Spitsbergen J, Heideman W, Peterson RE. Persistent adverse effects on health and reproduction caused by exposure of zebrafish to 2,3,7,8-tetrachlorodibenzo-p-dioxin during early development and gonad differentiation. Toxicol Sci 2009;109:75–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baker TR, Peterson RE, Heideman W. Early dioxin exposure causes toxic effects in adult zebrafish. Toxicol Sci 2013;131:241–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baker TR, Peterson RE, Heideman W. Using zebrafish as a model system for studying the transgenerational effects of dioxin. Toxicol Sci 2014;138:403–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gore AC, Chappell VA, Fenton SE, Flaws JA, Nadal A, Prins GS, et al. Executive summary to EDC-2: the Endocrine Society's second scientific statement on endocrine-disrupting chemicals. Endocr Rev 2015;36:593–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hotchkiss AK, Rider CV, Blystone CR, Wilson VS, Hartig PC, Ankley GT, et al. Fifteen years after “Wingspread”—Endocrine disruptors and human and wildlife health: where we are today and where we need to go. Toxicol Sci 2008;105:235–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mocarelli P, Gerthoux PM, Needham LL, Patterson DG, Jr., Limonta G, Falbo R, et al. Perinatal exposure to low doses of dioxin can permanently impair human semen quality. Environ Health Perspect 2010;119:713–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Di Renzo GC, Conry JA, Blake J, DeFrancesco MS, DeNicola N, Martin JN Jr., et al. International Federation of Gynecology and Obstetrics opinion on reproductive health impacts of exposure to toxic environmental chemicals. Int J Gynaecol Obstet 2015;131:219–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Environmental Protection Agency (EPA): Special Report on Environmental Endocrine Disruption: an Effects Assessment and Analysis. EPA/630/R-96/012, 1997
- 10.Iwanowicz LR, Blazer VS, Pinkney AE, Guy CP, Major AM, Munney K, et al. Evidence of estrogenic endocrine disruption in smallmouth and largemouth bass inhabiting Northeast U.S. national wildlife refuge waters: a reconnaissance study. Ecotoxicol Environ Saf 2016;124:50–59 [DOI] [PubMed] [Google Scholar]
- 11.Purdom CE, Hardiman PA, Bye VJ, Eno NC, Tyler CR, Sumpter JP. Estrogenic effects of effluents from sewage treatment works. Chem Ecol 1994;8:275–285 [Google Scholar]
- 12.Wan HT, Mruk DD, Wong CK, Cheng CY. Targeting testis-specific proteins to inhibit spermatogenesis: lesson from endocrine disrupting chemicals. Expert Opin Ther Targets 2013;17:839–855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schmidt JV, Bradfield CA. Ah receptor signaling pathways. Ann Rev Cell Dev Biol 1996;12:55–89 [DOI] [PubMed] [Google Scholar]
- 14.Westerfield: The Zebrafish Book. A Guide for the Laboratory Use of Zebrafish (Danio rerio), 4th ed. University or Oregon Press, Eugene, OR, 2000 [Google Scholar]
- 15.Kap M, Oomen M, Arshad S, de Jong B, Riegman P. Fit for purpose frozen tissue collections by RNA integrity number-based quality control assurance at the Erasmus MC tissue bank. Biopreserv Biobank 2014;12:81–90 [DOI] [PubMed] [Google Scholar]
- 16.Brazma A, Hingamp P, Quackenbush J, Sherlock G, Spellman P, Stoeckert C, et al. Minimum information about a microarray experiment (MIAME)—toward standards for microarray data. Nat Genet 2001;29:365–371 [DOI] [PubMed] [Google Scholar]
- 17.ThermoFisher Scientific: Gene Expression Assay Performance Guaranteed With the TaqMan® Assays QPCR Guarantee Program [White paper]. 2010. https://tools.thermofisher.com/content/sfs/brochures/cms_088754.pdf (Accessed May10, 2016)
- 18.Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, et al. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem 2009;55:611–622 [DOI] [PubMed] [Google Scholar]
- 19.Affymetrix, Inc.: QC Metrics for Exon and Gene Design Expression Arrays; A Summary Based on the Affymetrix Quality Assessment of Exon and Gene Arrays White Paper. 2008. http://static1.1.sqspcdn.com/static/f/1438485/21486054/1359060361517/qc_metrics_exon_gene_qrc.pdf (Accessed May25, 2016)
- 20.Liu Q, Spitsbergen JM, Cariou R, Huang CY, Jiang N, Goetz G, et al. Histopathologic alterations associated with global gene expression due to chronic dietary TCDD exposure in juvenile zebrafish. PLoS One 2014;9:e100910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chahoud I, Hartmann J, Rune GM, Neubert D. Reproductive toxicity and toxicokinetics of 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin. Arch Toxicol 1992;66:567–572 [DOI] [PubMed] [Google Scholar]
- 22.Choi JS, Kim IW, Hwang SY, Shin BJ, Kim SK. Effect of 2, 3, 7, 8‐tetrachlorodibenzo‐p‐dioxin on testicular spermatogenesis‐related panels and serum sex hormone levels in rats. BJU Int 2008;101:250–255 [DOI] [PubMed] [Google Scholar]
- 23.O'Donnell L, Nicholls PK, O'Bryan MK, McLachlan RI, Stanton PG. Spermiation: the process of sperm release. Spermatogenesis 2011;1:14–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mohammadpour H, Murray WJ, Stohs SJ. 2, 3, 7, 8-Tetrachlorodibenzo-p-dioxin (TCDD)-induced lipid peroxidation in genetically responsive and non-responsive mice. Arch Environ Contam Toxicol 1988;17:645–650 [DOI] [PubMed] [Google Scholar]
- 25.Beytur A, Ciftci O, Aydin M, Cakir O, Timurkaan N, Yılmaz F. Protocatechuic acid prevents reproductive damage caused by 2, 3, 7, 8‐tetrachlorodibenzo‐p‐dioxin (TCDD) in male rats. Andrologia 2012;44:454–461 [DOI] [PubMed] [Google Scholar]
- 26.Kim W, Hwang S, Lee H, Song H, Kim S. Panax ginsengprotects the testis against 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin induced testicular damage in guinea pigs. BJU Int 1999;83:842–849 [DOI] [PubMed] [Google Scholar]
- 27.Ohtake F, Fujii-Kuriyama Y, Kawajiri K, Kato S. Cross-talk of dioxin and estrogen receptor signals through the ubiquitin system. J Steroid Biochem Mol Biol 2011;127:102–107 [DOI] [PubMed] [Google Scholar]
- 28.Sprague J, Bayraktaroglu L, Clements D, Conlin T, Fashena D, Frazer M, et al. The Zebrafish Information Network: the zebrafish model organism database. Nucl Acids Res 2006;34:D581–D585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gimeno S, Komen H, Jobling S, Sumpter J, Bowmer T. Demasculinisation of sexually mature male common carp, Cyprinus carpio, exposed to 4-tert-pentylphenol during spermatogenesis. Aquat Toxicol 1998;43:93–109 [Google Scholar]
- 30.Miller HD, Clark BW, Hinton DE, Whitehead A, Martin S, Kwok KW, et al. Anchoring ethinylestradiol induced gene expression changes with testicular morphology and reproductive function in the medaka. PLoS One 2012;7:e52479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Caron KM, Soo S, Wetsel WC, Stocco DM, Clark BJ, Parker KL. Targeted disruption of the mouse gene encoding steroidogenic acute regulatory protein provides insights into congenital lipoid adrenal hyperplasia. Proc Natl Acad Sci U S A 1997;94:11540–11545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lei N, Heckert LL. Sp1 and Egr1 regulate transcription of the Dmrt1 gene in Sertoli cells. Biol Reprod 2002;66:675–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang L, Voyiaziakis E, Chen HL, Rubin EM, Gordon JW. A novel functional role for apolipoprotein B in male infertility in heterozygous apolipoprotein B knockout mice. Proc Natl Acad Sci U S A 1996;93:10903–10907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sablitzky F, Moore A, Bromley M, Deed RW, Newton JS, Norton JD. Stage-and Subcellular-specific expression of Id proteins in male germ and Sertoli cells implicates distinctive regulatory roles for Id proteins during meiosis, spermatogenesis, and Sertoli cell function. Development 1998;29:30. [PubMed] [Google Scholar]
- 35.Martinez JM, Baek SJ, Mays DM, Tithof PK, Eling TE, Walker NJ. EGR1 is a novel target for AhR agonists in human lung epithelial cells. Toxicol Sci 2004;82:429–435 [DOI] [PubMed] [Google Scholar]
- 36.Tourtellotte WG, Nagarajan R, Bartke A, Milbrandt J. Functional compensation by Egr4 in Egr1-dependent luteinizing hormone regulation and Leydig cell steroidogenesis. Mol Cell Biol 2000;20:5261–5268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sugawara T, Nomura E, Sakuragi N, Fujimoto S. The effect of the arylhydrocarbon receptor on the human steroidogenic acute regulatory gene promoter activity. J Steroid Biochem Mol Biol 2001;78:253–260 [DOI] [PubMed] [Google Scholar]
- 38.Hai Y, Sun M, Niu M, Yuan Q, Guo Y, Li Z, et al. BMP4 promotes human Sertoli cell proliferation via Smad1/5 and ID2/3 pathway and its abnormality is associated with azoospermia. Discov Med 2015;19:311–325 [PubMed] [Google Scholar]
- 39.Castrillon DH, Quade BJ, Wang TY, Quigley C, Crum CP. The human VASA gene is specifically expressed in the germ cell lineage. Proc Natl Acad Sci U S A 2000;97:9585–9590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ekstedt E, Holm L, Ridderstråle Y. Carbonic anhydrase in mouse testis and epididymis; transfer of isozyme IV to spermatozoa during passage. J Mol Histol 2004;35:167–173 [DOI] [PubMed] [Google Scholar]
- 41.Fetter E, Smetanova S, Baldauf L, Lidzba A, Altenburger R, Schuttler A, et al. Identification and characterization of androgen-responsive genes in zebrafish embryos. Environ Sci Technol 2015;48:11789–11798 [DOI] [PubMed] [Google Scholar]
- 42.Irwin DM, Biegel JM, Stewart C. Evolution of the mammalian lysozyme gene family. BMC Evol Biol 2011;11:166–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lin L, Achermann JC. Steroidogenic factor-1 (SF-1, Ad4BP, NR5A1) and disorders of testis development. Sex Dev 2008;2:200–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tanaka SS, Toyooka Y, Akasu R, Katoh-Fukui Y, Nakahara Y, Suzuki R, et al. The mouse homolog of Drosophila Vasa is required for the development of male germ cells. Genes Dev 2000;14:841–853 [PMC free article] [PubMed] [Google Scholar]
- 45.Zakeri ZF, Wolgemuth DJ. Developmental-stage-specific expression of the hsp70 gene family during differentiation of the mammalian male germ line. Mol Cell Biol 1987;7:1791–1796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hofsteen P, Plavicki J, Johnson SD, Peterson RE, Heideman W. Sox9b is required for epicardium formation and plays a role in TCDD-induced heart malformation in zebrafish. Mol Pharmacol 2013;84:353–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xiong KM, Peterson RE, Heideman W. AHR-mediated downregulation of Sox9b causes jaw malformation in zebrafish embryos. Mol Pharmacol 2008;74:1544–1553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dix DJ, Allen JW, Collins BW, Morio C, Nakamura N, Poorman-Allen P, et al. Targeted gene disruption of Hsp70-2 results in failed meiosis, germ cell apoptosis, and male infertility. Proc Natl Acad Sci U S A 1996;93:3264–3268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Feng HL, Sandlow JI, Sparks AET. Decreased expression of the heat shock protein hsp70-2 is associated with the pathogenesis of male infertility. Feril Steril 2001;76:1136–1139 [DOI] [PubMed] [Google Scholar]
- 50.Lima S, Cedenho A, Cenedeze M, Bertolla R, Oehinger S, Srougi M. Male infertility and heat shock protein 70–72 (HSP70–2) expression. Feril Steril 2004;82:S181 [Google Scholar]
- 51.Leslie EM, Deeley RG, Cole SPC. Multidrug resistance proteins: role of P-glycoprotein, MRP1, MRP2, and BCRP (ABCG2) in tissue defense. Toxicol Appl Pharmacol 2005;204:216–237 [DOI] [PubMed] [Google Scholar]
- 52.Surowiak P, Materna V, Kaplenko I, Spaczynski M, Dolinska-Krajewska B, Gebarowska E, et al. ABCC2 (MRP2, cMOAT) can be localized in the nuclear membrane of ovarian carcinomas and correlates with resistance to cisplatin and clinical outcome. Clin Cancer Res 2006;12:7149–7158 [DOI] [PubMed] [Google Scholar]
- 53.Cai JP, Ishibashi T, Takagi Y, Hayakawa H, Sekiguchi M. Mouse MTH2 protein which prevents mutations caused by 8-oxoguanine nucleotides. Biochem Biophys Res Commun 2003;305:1073–1077 [DOI] [PubMed] [Google Scholar]
- 54.Nebert DW, Roe AL, Dieter MZ, Solis WA, Yang YI, Dalton TP. Role of the aromatic hydrocarbon receptor and [Ah] gene battery in the oxidative stress response, cell cycle control, and apoptosis. Biochem Pharmacol 2000;59:65–85 [DOI] [PubMed] [Google Scholar]
- 55.Lanham KA, Plavicki J, Peterson RE, Heideman W. Cardiac myocyte-specific AHR activation phenocopies TCDD-induced toxicity in zebrafish. Toxicol Sci 2014;141:141–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Skinner MK, Manikkam M, Guerrero-Bosagna C. Epigenetic transgenerational actions of environmental factors in disease etiology. Trends Endocrinol Metab 2010;21:214–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Manikkam M, Guerrero-Bosagna C, Tracey R, Haque MM, Skinner MK. Transgenerational actions of environmental compounds on reproductive disease and identification of epigenetic biomarkers of ancestral exposures. PLoS One 2012;7:e31901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Anway MD, Cupp AS, Uzumcu M, Skinner MK. Epigenetic transgenerational actions of endocrine disruptors and male fertility. Science 2005;308:1466–1469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.de Kretser DM. Male infertility. Lancet 1997;349:787–790 [DOI] [PubMed] [Google Scholar]
- 60.Kidd K, Blanchfield PJ, Mills KH, Palace VP, Evans RE, Lazorchak JM, et al. Collapse of a fish population after exposure to a synthetic estrogen. Proc Natl Acad Sci U S A 2006;104:8897–8901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Burdick GE, Harris EJ, Dea HJ, Walker TM, Skea J, Colby D. The accumulation of DDT in lake trout and the effect on reproduction. Trans Am Fish Soc 1964;93;127–136 [Google Scholar]
- 62.King-Heiden TC, Mehta V, Xiong KM, Lanham KA, Antkiewicz DS, Ganser A, et al. Reproductive and developmental toxicity of dioxin in fish. Mol Cell Endocrinol 2012;354:121–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.