Abstract
Relative to HIV-negative adults, HIV+ adults report elevated levels of early life stress (ELS). In non-HIV samples, high ELS has been linked to abnormalities in brain structure and function, as well as increased risk of neuropsychiatric symptoms. Yet, little is known about the neural effects of high ELS, and their relation to elevated neuropsychiatric symptoms, in HIV+ adults. Recent studies have revealed combined effects of HIV and high ELS on amygdala morphometry. Aberrant amygdala activity is prominently implicated in studies of neuropsychiatric symptomology in non-HIV samples. Hence, this preliminary study examined: 1) the combined effects of HIV and high ELS on amygdala activity, and 2) the relation between amygdala activity and neuropsychiatric symptoms in HIV+ adults. We included 28 HIV+ adults and 25 demographically-matched HIV-negative control (HC) adults. ELS exposure was quantified using a retrospective ELS questionnaire, which defined four groups: HIV+ Low-ELS (N=15); HIV+ High-ELS (N=13); HC Low-ELS (N=16); and HC High-ELS (N=9). Participants completed a battery of neuropsychiatric measures. BOLD fMRI assessed amygdala reactivity during explicit observation of fearful/angry faces. High-ELS participants demonstrated reduced levels of amygdala reactivity relative to Low-ELS participants. HIV+ High-ELS participants reported higher levels of neuropsychiatric symptoms than all other groups. In the HIV+ group, lower amygdala responses were associated with higher neuropsychiatric symptoms, particularly depression, anxiety, and alexithymia. Collectively, these results suggest that high ELS exposure is a significant risk factor for neuropsychiatric symptoms in HIV+ adults. Furthermore, our results implicate ELS-related abnormalities in amygdala activity in the etiology of neuropsychiatric symptoms in HIV+ adults.
Keywords: Amygdala, Adverse Childhood Experiences, Childhood Abuse, Childhood Trauma, Depression, Anxiety, Alexithymia
Introduction
Compared to HIV-negative adults, HIV-positive (HIV+) adults report higher rates of neuropsychiatric symptoms across a broad range of domains, with significant increases in depression, anxiety, apathy, alexithymia, and interpersonal problems (Benton 2008; Bing et al. 2001; Bogdanova et al. 2010; Ciesla and Roberts 2001; Clark et al. 2010a; Do et al. 2014; Morrison et al. 2002). There is growing evidence that levels of early life stress (ELS) exposure are also elevated in HIV+ cohorts relative to non-HIV samples (Machtinger et al. 2012; Paxton et al. 2004; Spies et al. 2012). Estimated rates of ELS exposure in HIV+ cohorts range from 32% to 76% (Simoni and Ng 2000; Spies et al. 2012; Villar-Loubet et al. 2014; Walton et al. 2011; Whetten et al. 2006), compared to 14% to 32% reported in non-HIV samples (Spies et al. 2012). The high prevalence of ELS events among HIV+ adults necessitates a greater understanding of the independent and combined effects of HIV infection and high ELS on mental health outcomes. High ELS exposure is associated with an increased risk of neuropsychiatric symptoms in non-HIV adult samples (Dube et al. 2003; Felitti et al. 1998; Kaufman and Charney 2001; McFarlane et al. 2005; Pechtel and Pizzagalli 2011; Pesonen and Raikkonen 2012; Repetti et al. 2002; Taylor 2010). In addition, neuroimaging studies indicate that high ELS exposure has lasting effects on brain regions implicated in affect regulation in non-HIV samples (Andersen et al. 2008; Baker et al. 2013; R. A. Cohen et al. 2006a; Dannlowski et al. 2012; Evans et al. 2015; Grant et al. 2011; Mehta et al. 2009; Taylor et al. 2006; Teicher et al. 2002; Tottenham et al. 2009a). Despite this evidence, research examining the neuropsychiatric sequelae of high ELS in the context of HIV infection is sparse. Moreover, we do not yet have a full appreciation of the degree to which neuropsychiatric symptoms in HIV+ cohorts stem from ELS-related or HIV-related factors.
Although the etiology of neuropsychiatric difficulties in HIV+ adults is still poorly understood, there is evidence that HIV-related neural abnormalities may play a contributory role. Results from neuroimaging studies point to both functional and structural abnormalities, such as abnormalities in serotonergic transmission (Hammoud et al. 2010), cortical and subcortical white matter (Smith et al. 2008; Stubbe-Drager et al. 2012), and grey matter volumes (Clark et al. 2015; Paul et al. 2005). In addition, some studies have observed significant associations between volumetric abnormalities in affective brain regions and markers of historical HIV-disease severity (e.g., nadir CD4 levels, the lowest ever T-cell count on record) (Clark et al. 2012; Clark et al. 2015). Taken together, these studies suggest that neuropsychiatric disorders in HIV+ adults may reflect a direct manifestation of the disease and its impact on neural systems involved in affect regulation.
Further elucidating the neural substrates of neuropsychiatric symptoms in HIV+ adults is of great importance, as the burden of neuropsychiatric disorders in this population is high. Moreover, neuropsychiatric disorders have a negative effect on critical HIV-disease outcomes (e.g., medication adherence) and have been linked to increased HIV-related mortality rates (Antelman et al. 2007; Catz et al. 2000; Cook et al. 2002; Horberg et al. 2008; Ickovics et al. 2001; Leserman 2008; Marshall et al. 2013; Mayne et al. 1996; Panos et al. 2014; Safren et al. 2001). Gaining a better understanding of the neural substrates of these highly prevalent disorders could stimulate improvements in diagnostic, preventative, and treatment strategies available to HIV+ adults, which in turn would improve both quality of life and health outcomes in this population. Furthermore, studies that begin to tease apart the effects of HIV and high ELS exposure on brain function and mental health outcomes could potentially enhance these pursuits by providing greater specificity regarding the demographic and neurobiological factors associated with elevated neuropsychiatric symptoms in HIV+ adults.
To our knowledge, only two studies have explored the combined effects of HIV infection and high ELS exposure on the brain (Clark et al. 2012; Spies et al. 2015). Findings from each of these studies suggest synergistic effects of HIV and high ELS on amygdala morphometry. The amygdala is important to a variety of neuropsychiatric and psychosocial functions (Bickart et al. 2014; Hilbert et al. 2014; Murray et al. 2011; Whalen and Phelps 2009). Moreover, converging data implicate amygdala abnormalities in the development and course of neuropsychiatric disorders in non-HIV samples (Blair et al. 2008; Etkin and Wager 2007; Groenewold et al. 2013; Li et al. 2014; Schienle et al. 2011). For example, abnormal levels of amygdala activation are commonly observed in adults with depression or anxiety (Damsa et al. 2009; Groenewold et al. 2013; Holzschneider and Mulert 2011), and there is evidence that amygdala responses normalize to some degree upon remission of these conditions (Arnone et al. 2012; Sheline et al. 2001). Overall, there has been minimal investigation of the amygdala in HIV+ cohorts, with most studies focusing on morphologic abnormalities (Ances et al. 2012; Behrman-Lay et al. 2015; Clark et al. 2012; Clark et al. 2015; Ortega et al. 2013; Spies et al. 2015). Hence, it remains unclear whether abnormalities in amygdala activation occur in HIV+ samples, and whether these abnormalities contribute to greater levels of neuropsychiatric symptoms in this patient population. Given prior observations of abnormal amygdala activity in non-HIV adult samples with high ELS (Dannlowski et al. 2012; Evans et al. 2015; Grant et al. 2011; Taylor et al. 2006), coupled with the high burden of ELS exposure in HIV+ cohorts, understanding the potential contribution of amygdala activity to neuropsychiatric symptoms in HIV+ adults appears particularly relevant. Yet, the combined effects of HIV and high ELS on amygdala activity, and the relation between amygdala activity and neuropsychiatric symptoms in HIV+ cohorts have not been investigated previously.
The current study was conducted to further elucidate the neural substrates of neuropsychiatric symptoms in HIV+ adults. We had two goals in conducting this preliminary study. Our first goal was to use functional magnetic resonance imaging (fMRI) to examine the independent and combined effects of HIV status and high ELS exposure on amygdala activity. Based on prior findings (Clark et al. 2012), we were particularly interested in assessing the effect of high ELS on amygdala activity in HIV+ adults. We hypothesized that HIV+ adults would exhibit abnormalities in amygdala reactivity relative to HIV-negative adults, and that HIV+ adults with high ELS would exhibit greater abnormalities than HIV+ adults with low ELS. The second goal was to examine whether abnormalities in amygdala reactivity, if present in HIV+ adults, were associated with elevated neuropsychiatric symptoms in our HIV+ sample. We assessed a number of symptoms commonly endorsed by HIV+ adults, including levels of depression, anxiety, apathy, alexithymia, and interpersonal problems (Benton 2008; Bing et al. 2001; Bogdanova et al. 2010; Ciesla and Roberts 2001; Clark et al. 2010a; Do et al. 2014; Fleishman et al. 2000; Hudson et al. 2001; Kamat et al. 2012; Morrison et al. 2002). Before assessing whether amygdala reactivity levels were associated with neuropsychiatric symptoms in HIV+ adults, we first determined whether HIV+ adults in our sample did indeed report experiencing greater neuropsychiatric symptoms. Here too, we sought to examine the effects of high ELS status on symptom levels in our HIV+ group. We hypothesized that HIV+ adults would report higher levels of neuropsychiatric symptoms relative to HIV-negative adults, and that this effect would be greatest in HIV+ adults with high ELS. We further hypothesized that degree of amygdala reactivity would covary with levels of neuropsychiatric symptoms in HIV+ adults, where greater abnormalities in amygdala reactivity would correlate with higher neuropsychiatric symptom levels.
Methods
Participants
This fMRI study included 28 HIV+ and 25 age-matched HIV-negative control (HC) adults. HIV+ participants were recruited from The Miriam Hospital in Providence, RI and from the Icahn School of Medicine at Mount Sinai in New York, NY. HC participants were recruited from the community at both sites (Table 1). One investigator (USC) oversaw all recruitment and testing procedures. Inclusion criteria were right-handedness, completion of 8 or more years of education, and being a native English speaker. All participants achieved a score of ≥ 25 points on the Mini-Mental Status Exam (MMSE) (Folstein et al. 1975). Exclusion criteria included reported history of uncorrected abnormal vision; developmental disability; learning disability; major psychiatric illness (e.g., schizophrenia, bipolar disorder, posttraumatic stress disorder); neurological illness affecting the central nervous system (e.g., stroke, progressive multifocal leukoencephalopathy, Parkinson’s disease); and traumatic head injury with loss of consciousness >10 minutes. Substance use exclusion criteria were reported current alcohol dependence; use of heroin/opiates or any intravenous drug within the past 6 months; use of cocaine within the past 3 months; and positive urine toxicology prior to MRI assessment (cocaine, opiates, methamphetamine, amphetamine, benzodiazepine, barbiturates, methadone, oxycodone). Individuals who tested positive for marijuana use (N=6) were not excluded. The Miriam Hospital’s Institutional Review Board and the Icahn School of Medicine at Mount Sinai’s Institutional Review Board approved this research. All participants gave their informed consent and were financially compensated for their time.
Table 1.
Demographic Characteristics of the Participant Groups
| Variable | HC Low-ELS (N=16) | HC High-ELS (N=9) | HIV+ Low-ELS (N=15) | HIV+ High-ELS (N=13) | F/t/χ2 | df | p | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||||
| Recruitment Site (% Providence, RI) | 68.8 | 55.6 | 53.3 | 61.5 | 0.88 | 3 | 0.832 | ||||
| % New York, NY | 31.3 | 44.4 | 46.7 | 38.5 | |||||||
| Age (years) | 46.8 | 13.1 | 48.6 | 12.3 | 46.3 | 8.9 | 49.0 | 10.2 | 0.18 | 3,49 | 0.907 |
| % Male | 56.3 | 77.8 | 60.0 | 53.8 | 1.49 | 3 | 0.686 | ||||
| Mini-Mental Status Exam (/30) | 28.8 | 1.3 | 28.8 | 1.0 | 29.1 | 0.9 | 28.0 | 1.7 | 1.94 | 3,49 | 0.136 |
| Ethnic composition (% Caucasian) | 43.8 | 77.8 | 20.0 | 38.5 | 7.82 | 3 | 0.050 | ||||
| % African American | 50.0 | 11.1 | 80.0 | 53.8 | |||||||
| % Native American | 7.7 | ||||||||||
| % Biracial or “Other” | 6.3 | 11.1 | |||||||||
| % Hepatitis C positive | 6.3 | 11.1 | 33.3 | 23.1 | 4.22 | 3 | 0.239 | ||||
| KMSK – Alcohol (/13) | 6.1 | 3.4 | 8.1 | 3.4 | 7.5 | 3.4 | 6.5 | 3.7 | 0.84 | 3,49 | 0.480 |
| KMSK – Cocaine (/16) | 3.1a | 5.6 | 1.3b,c | 3.6 | 7.5a,b | 6.7 | 7.4c | 6.3 | 3.32 | 3,49 | 0.027 |
| KMSK – Opiate (/13) | 0.6 | 1.8 | 0.2 | 0.7 | 2.7 | 4.6 | 1.0 | 2.3 | 2.03 | 3,49 | 0.121 |
| % With positive marijuana toxicology | 6.3 | 0.0 | 13.3 | 23.1 | 4.25 | 3 | 0.235 | ||||
| Nadir CD4 (cells/μl) | 244.5 | 258.8 | 290.7 | 262.0 | 0.46 | 25 | 0.651 | ||||
| Current CD4 (cells/μl) | 677.9 | 399.0 | 741.9 | 354.2 | 0.45 | 26 | 0.659 | ||||
| Current log10 viral load (copies/ml) | 2.4 | 1.3 | 2.2 | 1.1 | 0.48 | 26 | 0.634 | ||||
| Length of HIV infection (years) | 17.1 | 6.6 | 18.3 | 7.0 | 0.48 | 26 | 0.635 | ||||
| % On ARV medications | 100.0 | 92.3 | 0.464 | ||||||||
| Number of ELS events | 1.0a,b | 0.8 | 5.0a,c | 2.7 | 1.4c,d | 0.7 | 5.3b,d | 2.1 | 25.47 | 3,49 | <0.001 |
| Current stress – PSS (/70) | 12.4a | 7.3 | 20.1 | 8.0 | 13.7 | 6.5 | 22.5a | 10.2 | 5.13 | 3,49 | 0.004 |
| PTSD symptoms – PCLC (/85) | 20.4a | 3.9 | 23.3b | 11.4 | 24.1c | 10.4 | 38.6a,b,c | 16.7 | 7.11 | 3,49 | <0.001 |
| Anger & Fear facial emotion recognition (% correct) | 76.9a,b | 15.7 | 71.4 | 8.8 | 59.7a | 13.6 | 55.0b | 24.5 | 5.01 | 3,49 | 0.004 |
Note: HC = HIV-negative Control; KMSK = Kreek-McHugh-Schluger-Kellogg scale; ARV = antiretroviral; ELS = early life stress; PSS = Perceived Stress Scale; PTSD = Posttraumatic stress disorder; PCLC = Posttraumatic Checklist - Civilian. Across rows, means with the same superscript are significantly different at the p<.05 level.
Demographic Measures
Disease duration in the HIV+ group was estimated using self-report and verified against the medical record. History of antiretroviral (ARV) use, current CD4 levels, nadir CD4 levels (i.e., the lowest ever T-cell count on record), and viral loads were obtained from medical records. All but one HIV+ participant was prescribed ARV medications. Current CD4 levels ranged from 84 to 1509 cells/μl. Nadir CD4 levels ranged from 2 to 834 cells/μl. HIV viral load was log 10 transformed to normalize the distribution. Alcohol and drug use histories for all participants were quantified using the Kreek-McHugh-Schluger-Kellogg scale (KMSK) (Kellogg et al. 2003), which provided 3 subscales characterizing lifetime consumption of alcohol (KMSK-A), cocaine (KMSK-C), and opiates (KMSK-O). See Table 1 for group characteristics.
Early Life Stress Quantification
ELS exposure was quantified using the Early Life Stress Questionnaire (ELSQ) (R. A. Cohen et al. 2006b), which assessed the occurrence of 17 adverse childhood experiences (ACEs) (e.g., physical abuse, sexual abuse, neglect, family conflict, bullying) prior to age 18 years. HIV+ and HC participants were identified as having either high ELS or low ELS exposure based on their ELSQ responses. Although some studies examining ELS-related neural effects have compared individuals with no ELS exposure (i.e., zero ACEs) to individuals with high ELS (e.g., 3 or more ACEs) (R. A. Cohen et al. 2006a; Paul et al. 2008), this method has not been well suited to our HIV+ samples. We have found that less than 10% of our HIV+ participants report zero ACEs (Clark et al. 2012), consistent with rates observed in prior US studies (Mugavero et al. 2006). In the current sample only 2 HIV+ adults (7%) reported zero ACEs. Accordingly, low ELS was classified by endorsement of fewer than 3 ACEs, and high ELS was defined as an endorsement of 3 or more ACEs. Our use of this cutoff score is in line with prior studies examining ELS-related neural abnormalities in HIV+ cohorts and non-HIV samples (Clark et al. 2012; R. A. Cohen et al. 2006a; Paul et al. 2008; Seckfort et al. 2008). Using these criteria, four HIV-ELS groups were classified as follows: HIV+ Low-ELS (N=15); HIV+ High-ELS (N=13); HC Low-ELS (N=16); and HC High-ELS (N=9). Importantly, participants from all four groups were well represented at both testing sites (Table 1).
fMRI Task of Amygdala Reactivity
A block design paradigm known to elicit robust blood oxygen level dependent (BOLD) response in bilateral amygdala was employed during fMRI (Lieberman et al. 2007). This paradigm consisted of five discrete tasks during which participants viewed images of faces expressing negative emotion (anger or fear) (Tottenham et al. 2009b) or shapes. For the purposes of this study, we examined amygdala responses during one of the tasks, the Observe task, because it elicits the strongest degree of amygdala activation relative to the other four tasks in the paradigm (emotion-labeling, gender-labeling, emotion-matching, shapes-matching) (Lieberman et al. 2007). During the Observe task participants viewed a series of single emotional faces, which expressed anger or fear. Each task block began with a 3-second instruction screen (indicating the task type), followed by ten 5-second trials of that task (e.g., presentation of a single face). Trials within each block (5 fearful; 5 angry) were presented in random order using E-Prime software (http://www.pstnet.com). Each 50-second task block was followed by a 10-second fixation crosshair (rest). Participants completed two fMRI image acquisition runs of this paradigm, with one block of each of the five tasks occurring once per run in a counterbalanced order. Each run lasted 5′15″.
MRI Image Acquisition
Whole-brain echoplanar BOLD fMRI was conducted in Providence, RI and New York, NY using the same affective paradigm (described above). At both sites, whole-brain echoplanar BOLD images were acquired in the axial plane with AP phase encoding using a Siemens (Erlangen, Germany) 3 Tesla scanner and a 32-channel head coil. In Providence, RI images were acquired on a Siemens TIM TRIO 3T scanner (TE/TR=28/2500 ms, matrix=64×64 in 3 mm slices, FOV=192×192 mm). This procedure yielded 129 whole-brain volumes for each imaging run with a spatial resolution of 3 mm3 per voxel. In New York, NY images were acquired on a Siemens MAGNETOM Skyra 3T scanner (TE/TR=35/1000 ms, matrix=108×108 in 2.1 mm slices, FOV=228×228 mm). This procedure yielded 320 whole-brain volumes for each imaging run with a spatial resolution of 2.1 mm3 per voxel. At both sites, whole-brain high-resolution T1 images were acquired in the sagittal plane prior to the BOLD fMRI scans for anatomical reference (Providence, RI: TE/TR=2.98/1900 ms, 1 mm3, FOV=256×256 mm; New York, NY: TE/TR=2.07/2400 ms, 0.8 mm3, FOV=256×256 mm).
fMRI Data Analyses
All fMRI dataset processing and statistical analyses were performed with Analysis of Functional NeuroImages (AFNI) software (Cox 1996). For each run, the 3D+time datasets were slice-timing corrected, spatially aligned to the seventh volume (to minimize movement artifact), coregistered to high-resolution anatomical volumes, and transformed into standard stereotaxic space (Talairach and Tournoux 1988) before a three-dimensional 6-mm Gaussian kernel was applied. Data regarding participant movement were examined; none exhibited excess movement (i.e., movement greater than the spatial resolution of one voxel) during either run. General linear modeling (GLM) was utilized to quantify task specific activity for each brain voxel of individual datasets. The dependent variable in these single-subject analyses was BOLD signal over time; each of the five paradigm tasks (i.e., observe, emotion-labeling, etc.) was modeled as a fixed-response waveform convolved with the hemodynamic response (modeled as a gamma function); additional regressors included instruction screens, covariates (observed movement, linear drift), and resting baseline. For each participant, a general linear test (GLT) was used to compare brain responses during the Observe task to brain responses during rest. Brain regions demonstrating significant task-related effects during the Observe task (relative to rest) were identified using a whole-brain voxelwise one-sample t-test against a hypothetical mean of zero. This analysis was conducted across the entire sample in order to verify that our task elicited activation within expected regions (e.g., amygdala). Results were thresholded using a two-tailed p<.05, corrected for multiple comparisons using AFNI’s false discovery rate (FDR) procedure.
An a priori region of interest (ROI) analysis was conducted within the bilateral amygdala. ROIs were drawn as spheres with a 5 mm radius centered on Talairach coordinates for the amygdala (x= +/−23, y= 5, z= −15). For each participant, overlap between the bilateral ROI spheres and the right and left amygdala was verified, before calculating the mean BOLD response within the bilateral amygdala. Extracted data thus represented the mean BOLD response within the right and left amygdala during the Observe task relative to rest for each participant. These data served as the dependent variable in subsequent analyses conducted in SPSS (version 20, IBM Corporation).
Neuropsychiatric and Psychosocial Functions
A battery of self-report questionnaires was administered to assess several domains in which HIV+ adults are known to report increased difficulty. Neuropsychiatric symptoms, including current levels of depression, anxiety, and apathy were assessed using the Center for Epidemiological Studies-Depression Scale (CESD) (Radloff 1977), Beck Anxiety Inventory (BAI) (Beck et al. 1988), and the Apathy Evaluation Scale (AES) (Marin et al. 1991), respectively. Alexithymia, which is the inability to identify and describe internal emotions, was assessed with the Toronto Alexithymia Scale (TAS) (Bagby et al. 1994). Psychosocial functions were assessed using the Inventory of Interpersonal Problems (IIP) (Horowitz et al. 2000; Horowitz et al. 1988), which measured the degree of difficulty participants experienced in their interpersonal relationships. All measures were completed within two weeks of the MRI for all participants. On all measures, higher scores indicate greater symptomology.
Because the amygdalae are implicated in a wide diversity of neuropsychiatric and psychosocial functions (e.g., depression, anxiety, interpersonal interactions, social cognition, etc.) (Adolphs 2010; Bickart et al. 2014; Hilbert et al. 2014; Murray et al. 2011; Whalen and Phelps 2009), we sought to capture this diversity of function in a single index. We therefore created a composite score to estimate each participant’s global level of functioning across all neuropsychiatric and psychosocial domains assessed. For each participant, z-scores were calculated for each measure based on the mean of the HC group for that measure; the five z-scores were then averaged to create a composite index score for each participant. The index thus represents the overall degree of neuropsychiatric difficulty reported across all domains assessed, with the entire HC group serving as the normative sample. Higher scores on the composite index indicate greater global difficulty relative to HC.
Control Measures
Due to the known impact of current stress and posttraumatic stress disorder (PTSD) on amygdala response (Rosenberger et al. 2009; Shin et al. 2005; van Marle et al. 2009), we assessed the effects of current stress levels and PTSD-related symptomology on amygdala responses in our groups. Levels of current stress and PTSD-related symptoms were quantified using the Perceived Stress Scale (PSS-14) (S. Cohen et al. 1983) and Posttraumatic Checklist - Civilian (PCLC) (Weathers et al. 1993), respectively. We used these measures to assess whether the relation between high ELS and amygdala response was specific, and not confounded by recent stressful life events or subclinical PTSD-related abnormalities in amygdala response.
Prior studies suggest that individual differences in facial emotion recognition abilities are associated with differences in amygdala activation levels (H. Kim et al. 2003; H. Kim et al. 2004). HIV+ adults have been reported to exhibit reduced facial emotion recognition abilities relative to HC adults (Baldonero et al. 2013; Clark et al. 2010a; Clark et al. 2015; Heilman et al. 2013; Lane et al. 2012). Hence, we assessed the effects of facial emotion recognition abilities on amygdala responses in our sample. Facial emotion recognition abilities were assessed utilizing methods employed in prior studies (Circelli et al. 2013; Clark et al. 2010a; Clark et al. 2008, 2010b; Clark et al. 2015). Briefly, participants were presented with 70 Ekman and Friesen photographs (Ekman & Friesen, 1976) on a 15″ laptop computer. Ten images were displayed in random order from each of the six prototypic emotion categories (Anger, Disgust, Fear, Happy, Sad, Surprise) plus Neutral. For each image, participants indicated which of the seven emotion category labels best described the facial emotion expressed. We examined accuracy rates for angry and fearful facial stimuli only, to maintain correspondence with the affective content of facial stimuli presented in our fMRI paradigm (described above). Accordingly, for each participant, an average emotion recognition accuracy score was derived for angry and fearful faces (Table 1). This accuracy score was used as a covariate in analyses to control for differences in facial emotion recognition abilities. In this way we were able to assess whether HIV-related differences in amygdala response stemmed from HIV-related differences in emotion recognition.
Statistical Analyses
Group differences on demographic variables were assessed using one-way ANOVAs, independent t-tests, and chi-square tests. We verified that the fMRI task elicited significant activation within our bilateral amygdala ROI using a one-sample t-test (versus a hypothetical mean of zero).
The first goal of this study was to examine the independent and combined effects of HIV status and high ELS exposure on levels of amygdala activation. We thus examined the effects of HIV and high ELS on amygdala responses using a two-way ANCOVA with factors of HIV status (HIV, HC) and ELS status (High, Low). Covariates included MR acquisition site (Providence; New York), demographic variables on which the groups differed significantly (i.e., ethnic composition, cocaine use history; Table 1), and control measures described above (current stress [PSS], PTSD symptoms [PCLC], facial emotion recognition abilities). Partial eta-squared (ηp2) was used as an indicator of effect size, where values of .01, .06, and .14 indicate small, medium, and large effects, respectively (J. Cohen 1988). Next, we conducted a planned comparison to examine the effect of ELS status on amygdala reactivity in the HIV+ group.
Our second goal was to examine whether abnormal amygdala reactivity was associated with elevated neuropsychiatric symptoms in HIV+ adults. First, we determined whether HIV+ adults reported elevated neuropsychiatric symptoms by conducting a two-way ANCOVA with factors of HIV status (HIV, HC) and ELS status (High, Low), which allowed us to examine the effects of both HIV and high ELS on neuropsychiatric and psychosocial functions (composite index). Covariates included demographic variables on which the groups differed significantly (i.e., ethnic composition, cocaine use history; Table 1), as well as current stress levels (PSS), which were included to better control for potential confounding effects of group-related differences in current stress on reported neuropsychiatric symptoms. Next, we conducted a planned pair-wise comparison to examine the effects of ELS status on neuropsychiatric symptoms in the HIV+ group. Cohen’s d was calculated as a measure of effect size, where values of 0.2, 0.5, and 0.8 indicate small, medium, and large effects, respectively (J. Cohen 1988). Finally, a linear regression analysis, controlling for MR scanner (Providence; New York), was conducted in the HIV+ group to investigate the relation of amygdala reactivity to neuropsychiatric functions. Mean BOLD response within the bilateral amygdala was entered into the model as the dependent variable and neuropsychiatric composite index scores served as the independent variable; MR scanner was included in the model as a covariate.
Lastly, linear regression analyses were conducted to examine the relation between amygdala reactivity and key HIV-disease factors known to impact brain function, including nadir CD4 levels, current CD4 levels, current viral load, and length of HIV infection (Chang et al. 2002; Clark and Cohen 2010; R. A. Cohen et al. 2010; Cysique et al. 2006; Heaton et al. 2011). In each model, mean BOLD response within the bilateral amygdala was entered as the dependent variable and the HIV-disease factor of interest was entered as the independent variable; MR scanner was included in each model as a covariate. These analyses allowed us to address mechanistic questions related to our hypothesis that HIV infection is associated with amygdala abnormalities, by assessing whether specific markers of HIV-disease severity (e.g., viral load, current CD4, nadir CD4) were associated with amygdala reactivity levels in HIV+ adults.
Results
Participant Characteristics & Control Measures
Demographic data for the four HIV-ELS groups are reported in Table 1, including group means and statistics. With respect to demographic variables, groups differed significantly in the proportion of Caucasian to non-Caucasian participants and prior cocaine use history (KMSK-C). The HIV+ Low-ELS group reported higher rates of prior cocaine use than HC Low-ELS and HC High-ELS; rates were also higher in HIV+ High-ELS than in HC High-ELS (Table 1). With respect to the control measures, significant group differences were observed in reported levels of current stress, PTSD symptomology, and facial emotion recognition abilities (Table 1). Notably, mean scores on the PTSD symptom scale (PCLC) were below clinically significant levels (<44) for all groups. Demographic differences between the two HIV+ groups were also assessed with respect to HIV-disease variables. HIV+ Low-ELS and HIV+ High-ELS groups were well matched with regard to nadir and current CD4 levels, current viral load, and length of HIV infection (Table 1).
fMRI Task of Amygdala Reactivity
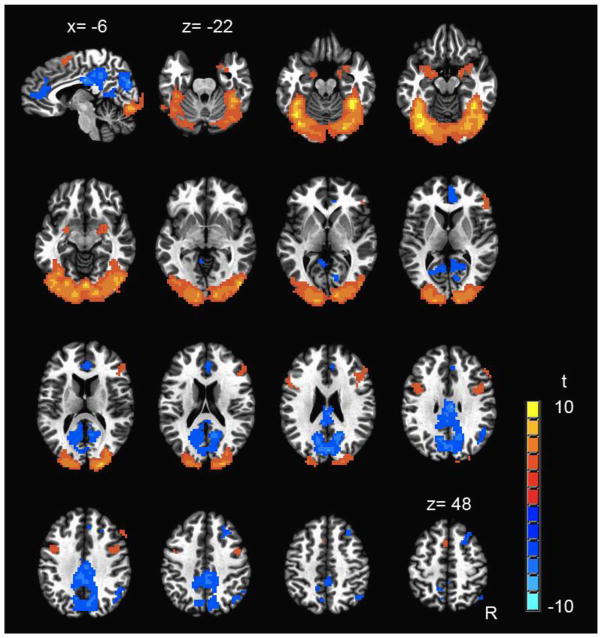
The Observe task elicited activation within several neural regions implicated in facial emotion recognition (Fusar-Poli et al. 2009), including the bilateral amygdala and ventral visual stream (Figure 1; Table 2). In addition, deactivation of several regions was also observed, including anterior cingulate cortex (ACC), posterior cingulate, and precuneus, bilaterally (Figure 1; Table 2) – a pattern that is consistent with active suppression of the default mode network (Raichle et al. 2001; Raichle and Snyder 2007).
Figure 1.
Regions of significant response during the Observe task (p<.05, FDR corrected) across the entire sample (N=53). One sagittal image (upper left) and 15 axial images are shown. For the sagittal image, x = −6. Axial images begin at z = −22 and increase in 5 mm increments. R = right.
Table 2.
Regions of Significant Response to the Observe Task Across the Entire Sample (N=53)
| Region | BA | Coordinates (x, y, z) | Cluster size (voxels) | t-score | p-value |
|---|---|---|---|---|---|
| L/R Lingual Gyrus/Fusiform Gyrus | 17, 18, 19, 37 | −1, 80, −4 | 3718 | 5.0512 | <0.0001 |
| L/R Posterior Cingulate/Precuneus | 7, 23, 31 | −1, 59, 27 | 1420 | −4.2261 | 0.0001 |
| R Inferior/Middle Frontal Gyrus | 6, 9, 45, 46 | −47, −21, 22 | 212 | 3.7373 | 0.0005 |
| R Amygdala and Parahippocampal Gyrus | 28, 34 | −26, 1, −17 | 137 | 3.8691 | 0.0003 |
| L/R Anterior Cingulate Cortex | 10, 24, 32 | −4, −40, 14 | 112 | −3.6712 | 0.0006 |
| R Superior/Middle Frontal Gyrus | 6, 8 | −27, −23, 46 | 92 | −3.5670 | 0.0008 |
| R Angular Gyrus | 39 | −45, 59, 37 | 81 | −3.5170 | 0.0009 |
| L Inferior/Middle Frontal Gyrus | 6, 9 | 43, −7, 28 | 78 | 3.4961 | 0.0010 |
| L Amygdala | 34 | 22, 3, −11 | 68 | 3.9859 | 0.0002 |
| L/R Medial Frontal Gyrus | 6 | 1, −5, 54 | 67 | 3.7423 | 0.0005 |
Note: Central voxel coordinates are listed using the Talairach and Tournoux atlas. BA = Brodmann area(s); L = left; R = right.
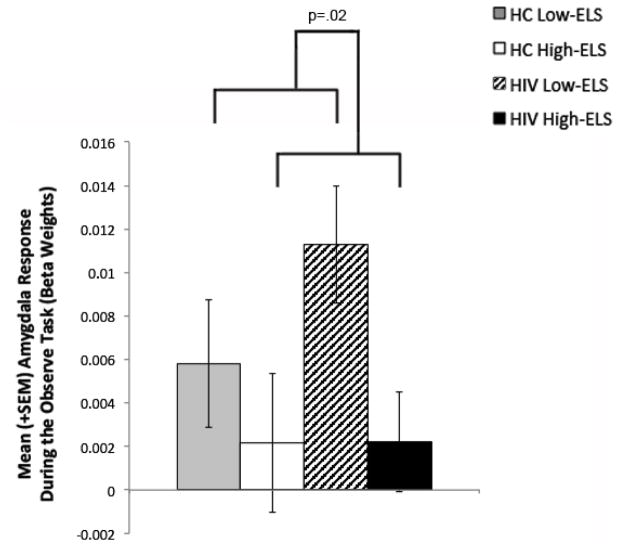
The Observe task elicited robust activation within the bilateral amygdala ROI across the entire sample, as expected (t[52]=4.01, p<.001). Results from the two-way ANCOVA (F[4,48]=3.60, p=.01) controlling for MR scanner revealed that High ELS participants (across both HIV+ and HC groups) demonstrated significantly lower amygdala activation levels than Low ELS participants (main effect of ELS: F[1,48]=5.70, p=.02, ηp2=.11) (Figure 2). The main effect of HIV and the HIV-ELS interaction were non-significant (F[1,48]=.79, p=.38, ηp2=.02; F[1,48]=.51, p=.48, ηp2=.01, respectively). Ethnicity, cocaine use history, marijuana use status (urine toxicology), current stress levels, PTSD symptomology, and facial emotion recognition abilities did not contribute significantly to the model when entered as covariates (p’s >.05) and were thus removed from the analysis. A planned comparison in the HIV+ group controlling for MR scanner indicated that the HIV+ High-ELS group exhibited lower amygdala activation levels than the HIV+ Low-ELS group (F[1,25]=6.86, p=.02, ηp2=.22). Notably, activation levels exhibited by HIV+ High-ELS and HC High-ELS did not differ significantly (F[1,19]=.01, p=.92, ηp2<.01; controlling for MR scanner), which underscores findings from the ANCOVA.
Figure 2.
High ELS status is associated with reduced BOLD response in the bilateral amygdala during observation of angry/fearful faces (versus rest). ELS = early life stress; BOLD = blood oxygen level dependent; SEM = standard error of the mean; HC = HIV-negative Control.
As indicated above, whole-brain voxelwise analyses conducted across the entire sample revealed significant task-related ACC deactivation during the Observe task (Figure 1; Table 2). Because the ACC shares extensive connections with the amygdala (Beckmann et al. 2009; Carmichael and Price 1995; Vogt and Pandya 1987), and is known to modulate amygdala activity in a top-down manner (Etkin et al. 2011), we conducted an explanatory ROI analysis within the ACC. Our goal was to explore whether group differences observed in amygdala activation would also be reflected in the ACC. We created an ROI sphere with a 5 mm radius centered on the Talairach coordinates of this empirically defined region (x= −4, y= −40, z= 14), and calculated the mean BOLD response within this ROI for each participant. Results from the two-way ANCOVA, controlling for MR scanner, were non-significant (F[4,48]=0.91, p=.47, ηp2=.07). While we did observe a tendency for the High ELS groups to exhibit greater ACC deactivation than Low ELS groups, which parallels prior observations of greater default mode network deactivation in adults with high ELS (Philip et al. 2013), this effect was non-significant (main effect of ELS: F=[1,48]=2.13, p=.15, ηp2=.04). Controlling for ethnicity, cocaine use history, current stress levels, PTSD symptomology, facial emotion recognition abilities, or marijuana use status (urine toxicology) did not significantly modify these results.
Neuropsychiatric and Psychosocial Functions
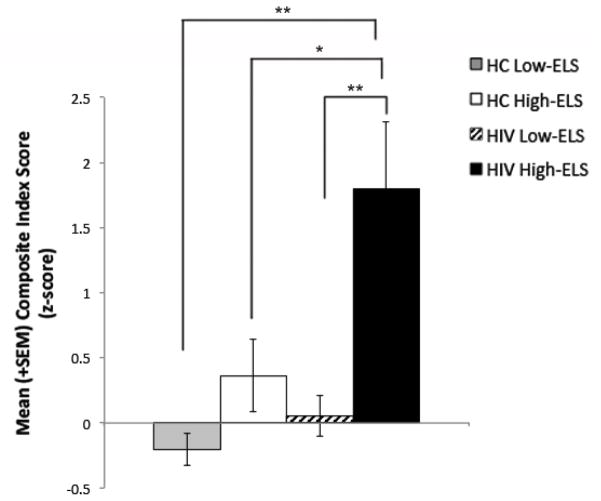
Group mean composite index scores are displayed in Figure 3. Statistical results are listed in Table 3. We conducted a two-way ANCOVA (p<.001) to examine the effects of HIV and ELS status on composite index scores, while controlling for the effect of current stress levels (PSS: p<.001). Ethnicity, cocaine use history, and marijuana use status (urine toxicology) did not contribute significantly to the model when entered as covariates (p’s >.05) and were thus removed from the analysis. Results from the ANCOVA revealed a significant main effect of HIV (p<.01), a main effect of ELS that trended toward significance (p=.07), and a significant HIV-ELS interaction (p=.02). These data indicate that the significant relative increase in the HIV+ groups’ neuropsychiatric symptoms was driven by the HIV+ High-ELS group. This is supported by results from the planned pair-wise comparison in the HIV+ group, which revealed that the HIV+ High-ELS group reported significantly higher symptom levels than HIV+ Low-ELS (t[14.3]=3.24, p<.01, Cohen’s d=1.30) (Figure 3). By contrast, the increase in mean symptom levels exhibited by HC High-ELS relative to HC Low-ELS was not nearly as large (Figure 3), although group comparisons did indicate a strong trend-level group difference in symptom levels (t[11.1]=1.86, p=.09, Cohen’s d=.90). Follow-up analyses also revealed that the HIV+ High-ELS group reported significantly higher symptom levels than HC High-ELS (t[20]=2.16, p=.04, Cohen’s d=.94) as well as HC Low-ELS (t[13.3]=3.79, p<.01, Cohen’s d=1.56) (Figure 3).
Figure 3.
HIV+ High-ELS participants report greater levels of neuropsychiatric/psychosocial difficulty (composite index score) than all other groups. SEM = standard error of the mean; HC = HIV-negative Control; ELS = early life stress. *p<.05, **p<.01.
Table 3.
Group Comparisons of Neuropsychiatric and Psychosocial Measures
| Omnibus | Main Effect of HIV | Main Effect of ELS | HIVxELS Interaction | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||||||||||
| Neuropsychiatric/Psychosocial Domain | F | df | p | ηp2 | F | df | p | ηp2 | F | df | p | ηp2 | F | df | p | ηp2 |
| Composite Index (z-score) | 18.86 | 4,48 | <.001 | .61 | 7.74 | 1,48 | <.01 | .14 | 3.57 | 1,48 | .07 | .07 | 5.70 | 1,48 | .02 | .11 |
| Depression (CESD) | 24.47 | 4,48 | <.001 | .67 | 11.11 | 1,48 | <.01 | .19 | 6.66 | 1,48 | .01 | .12 | 6.59 | 1,48 | .01 | .12 |
| Anxiety (BAI) | 7.29 | 4,48 | <.001 | .38 | 4.98 | 1,48 | .03 | .09 | 0.85 | 1,48 | .36 | .02 | 4.34 | 1,48 | .04 | .08 |
| Apathy (AES) | 5.41 | 4,47 | .001 | .31 | 3.15 | 1,47 | .08 | .06 | 0.79 | 1,47 | .38 | .02 | 0.07 | 1,47 | .80 | .00 |
| Alexithymia (TAS) | 7.45 | 4,48 | <.001 | .38 | 0.59 | 1,48 | .45 | .01 | 0.92 | 1,48 | .34 | .02 | 4.06 | 1,48 | .05 | .08 |
| Interpersonal Problems (IIP) | 2.26 | 4,47 | .076 | .16 | 0.00 | 1,47 | .96 | .00 | 0.44 | 1,47 | .51 | .01 | 0.50 | 1,47 | .48 | .01 |
Note: The Composite Index represents the overall degree of neuropsychiatric difficulty reported across all domains assessed, with the HC group serving as the normative sample. CESD = Center for Epidemiological Studies-Depression Scale; BAI = Beck Anxiety Inventory; AES = Apathy Evaluation Scale; TAS = Toronto Alexithymia Scale; IIP = Inventory of Interpersonal Problems. All analyses control for current stress levels, as reported on the Perceived Stress Scale.
We conducted explanatory analyses using ANCOVAs, controlling for current stress levels, to examine group differences on each individual neuropsychiatric/psychosocial measure (Table 3). These analyses suggested that the significant main effect of HIV reported above was most strongly driven by HIV+ participants’ elevated ratings on measures of depression (p<.01), anxiety (p=.03), and, to a lesser degree, apathy (p=.08, ηp2=.06) (Table 3). In addition, these explanatory analyses also indicated that the significant HIV-ELS interaction effect observed in the primary analysis was most strongly driven by measures of depression (p=.01), anxiety (p=.04), and alexithymia (p=.05) (Table 3). Follow-up analyses confirmed that, relative to HIV+ Low-ELS participants, HIV+ High ELS participants reported significantly higher levels of depression (t[16.8]=3.74, p<.01, Cohen’s d=1.48), anxiety (t[13.1]=2.27, p=.04, Cohen’s d=.92), apathy (t[25]=2.08, p=.05, Cohen’s d=.80), and alexithymia (t[26]=3.03, p<.01, Cohen’s d=1.15). For descriptive purposes, we report group mean scores for each of these measures in Table 4.
Table 4.
Group Mean Scores on Neuropsychiatric and Psychosocial Measures
| Neuropsychiatric/Psychosocial Domain | HC Low-ELS
|
HC High-ELS
|
HIV+ Low-ELS
|
HIV+ High-ELS
|
||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
|
|
|
|
|
|||||
| Composite Index (z-score) | −0.20 | 0.49 | 0.36 | 0.84 | 0.06 | 0.61 | 1.80 | 1.85 |
| Depression (CESD) | 3.00 | 3.52 | 8.56 | 7.04 | 5.67 | 6.34 | 20.54 | 13.07 |
| Anxiety (BAI) | 0.94 | 1.84 | 1.67 | 2.55 | 1.80 | 2.57 | 9.15 | 11.43 |
| Apathy (AES) | 23.56 | 4.76 | 26.78 | 7.65 | 26.21 | 4.46 | 30.46 | 6.08 |
| Alexithymia (TAS) | 37.94 | 8.81 | 40.33 | 11.02 | 35.80 | 9.82 | 49.54 | 14.06 |
| Interpersonal Problems (IIP) | 47.50 | 34.70 | 76.67 | 47.10 | 58.57 | 38.83 | 73.00 | 50.21 |
Note: The Composite Index represents the overall degree of neuropsychiatric difficulty reported across all domains assessed, with the HC group serving as the normative sample. The Composite Index is expressed as a z-score, whereas means for all other measures represent mean raw scores. Higher scores on all measures, including the Composite Index, indicate greater global difficulty. ELS = early life stress; HC = HIV-negative Control; CESD = Center for Epidemiological Studies-Depression Scale; BAI = Beck Anxiety Inventory; AES = Apathy Evaluation Scale; TAS = Toronto Alexithymia Scale; IIP = Inventory of Interpersonal Problems.
Relation of Amygdala Reactivity to Neuropsychiatric and Psychosocial Functions in HIV+ Adults
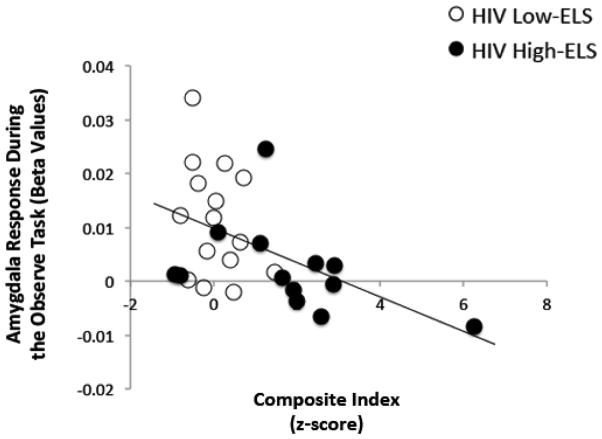
Results from the linear regression analysis are reported in Table 5. In the HIV+ group there was a significant association between amygdala activation and composite index scores, where lower activation was associated with higher reported neuropsychiatric symptoms (p=.01) (Figure 4). We conducted explanatory analyses to examine specific associations between amygdala reactivity and individual neuropsychiatric/psychosocial domains. Restricting these analyses to domains in which significant group differences were observed (depression, anxiety, apathy, alexithymia), we found that lower amygdala activation levels correlated significantly with higher levels of depression (p=.05), anxiety (p=.03), and alexithymia (p=.01) in HIV+ (Table 5).
Table 5.
Associations Between Amygdala BOLD Responses and Neuropsychiatric Measures in Each Group
| Neuropsychiatric/Psychosocial Domain | HC Group
|
HIV+ Group
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Model Statistics
|
Predictor
|
Model Statistics
|
Predictor
|
|||||||||||||
| R2 | F | df | p | β | t | p | R2 | F | df | p | β | t | p | |||
|
|
|
|
|
|||||||||||||
| Composite Index | .03 | .31 | 2,24 | .74 | −.06 | −0.29 | .78 | .44 | 9.70 | 2,27 | <.01 | −.44 | −2.90 | .01 | ||
| Depression (CESD) | .02 | .27 | 2,24 | .77 | −.01 | −0.03 | .98 | .35 | 6.84 | 2,27 | <.01 | −.33 | −2.03 | .05 | ||
| Anxiety (BAI) | .03 | .28 | 2,24 | .76 | −.03 | −0.14 | .89 | .38 | 7.71 | 2,27 | <.01 | −.37 | −2.33 | .03 | ||
| Apathy (AES) | .04 | .43 | 2,24 | .65 | −.12 | −0.57 | .58 | .29 | 4.79 | 2,26 | .02 | −.21 | −1.13 | .27 | ||
| Alexithymia (TAS) | .06 | .70 | 2,24 | .51 | .19 | 0.92 | .37 | .45 | 10.05 | 2,27 | <.01 | −.45 | −2.99 | .01 | ||
| Interpersonal Problems (IIP) | .08 | .96 | 2,24 | .39 | −.24 | −1.17 | .25 | .34 | 6.22 | 2,26 | .01 | −.31 | −1.85 | .08 | ||
Note: These data summarize results from linear regression analyses conducted in each group. Each model assesses the relation between bilateral amygdala response and a single neuropsychiatric domain (predictor) controlling for MR acquisition site. The Composite Index is a measure of the overall degree of neuropsychiatric difficulty reported across all domains assessed. BOLD = blood oxygen level dependent; HC = HIV-negative Control; CESD = Center for Epidemiological Studies-Depression Scale; BAI = Beck Anxiety Inventory; AES = Apathy Evaluation Scale; TAS = Toronto Alexithymia Scale; IIP = Inventory of Interpersonal Problems.
Figure 4.
Lower BOLD response in the bilateral amygdala is associated with greater neuropsychiatric and psychosocial difficulties (composite index) in HIV+ participants. BOLD = blood oxygen level dependent; ELS = early life stress.
Relation of Amygdala Reactivity to HIV-Disease Factors
The relation between HIV-disease factors (nadir CD4, current CD4 levels, current viral load, length of HIV infection) and amygdala activation in the HIV+ group were examined using linear regression. A significant association between nadir CD4 levels and amygdala activation was observed, where lower nadir CD4 counts (i.e., poorer historical immunological health) were associated with higher activation levels (R2=.48; F[2,26]=11.07, p<.01; β=−.46, [t=−2.88, p<.01]). No additional significant associations were observed.
Discussion
This study investigated whether HIV+ adults exhibit abnormal levels of amygdala activation, and assessed whether these abnormalities were associated with higher levels of neuropsychiatric symptoms. We examined the impact of HIV infection and high ELS on amygdala activation levels in our HIV+ sample based on the evidence that high ELS exposure is linked to abnormal amygdala activity in non-HIV cohorts (Dannlowski et al. 2012; Evans et al. 2015; Grant et al. 2011; Taylor et al. 2006) and abnormal amygdala morphometry in HIV+ adults (Clark et al. 2012). Briefly, we found that high ELS status was associated with reduced levels of amygdala activation. In addition, HIV+ adults with high ELS reported higher levels of neuropsychiatric symptoms relative to HIV+ adults with low ELS and healthy control adults. Lastly, we found that reduced levels of amygdala activation were associated with higher levels of neuropsychiatric symptoms in HIV+ adults. To our knowledge, this is the first evidence linking aberrant amygdala activity to elevated neuropsychiatric symptoms in HIV+ adults. Collectively, our results suggest that, for HIV+ adults, having a history of high ELS exposure is a significant risk factor for experiencing neuropsychiatric symptoms in adulthood. In addition, our findings implicate ELS-related abnormalities in amygdala activity in the etiology of neuropsychiatric symptoms in HIV+ adults.
ELS-related abnormalities in amygdala activity have been observed previously in non-HIV samples, though findings have varied. Studies have reported increased (Dannlowski et al. 2012; Evans et al. 2015; Grant et al. 2011), as well as decreased (Taylor et al. 2006) amygdala activation in non-HIV adult samples with high ELS. It is notable that ELS-related reductions in amygdala activity, as reported here and elsewhere (Taylor et al. 2006), are exclusive to studies that examine amygdala responses relative to a resting baseline (as opposed to an active baseline). There is some evidence that absolute blood flow in the amygdala at rest correlates positively with stress exposure (Canli et al. 2006). Hence, it is possible that reduced amygdala responses observed in our high ELS participants may reflect hyperactive baseline levels of amygdala activity, which create a ceiling effect, whereas participants with low ELS may be more able to generate increases in amygdala activation in response to threat stimuli. This suggestion is consistent with a number of studies documenting reduced amygdala responses to threat stimuli, and elevated baseline amygdala activity at rest in adults with high stress exposure, depression, or other related disorders (Canli et al. 2005; Drevets 1999; Drevets et al. 2002; Heinz et al. 2007; Lanius et al. 2010; Payer et al. 2012; Seeley et al. 2007; Sripada et al. 2012; Taylor et al. 2006). Thus, while reduced amygdala activation in high ELS adults may at first appear unexpected, the observed finding does in fact fit within the broader framework of the literature. Yet, it is also possible that reduced amygdala responses observed in our high ELS participants could reflect ELS-related abnormalities within regions known to modulate amygdala function (e.g., ACC, ventromedial prefrontal cortex). While our explanatory ROI analyses in the pregenual ACC did not reveal significant group-related effects, future investigations that focus on these regions will help to clarify this issue.
We found strong evidence that high ELS exposure was associated with elevated neuropsychiatric symptoms in HIV+ adults. Our data align with prior studies that have reported elevated depression and anxiety levels in HIV+ adults with a history of physical and/or sexual abuse (Mimiaga et al. 2009; Schafer et al. 2013; Simoni and Ng 2000; Spies et al. 2012; Welles et al. 2009). Notably, there has been little examination of the long-term mental health effects of other forms of childhood maltreatment, beyond physical and sexual abuse, in HIV+ adults (Spies et al. 2012). Investigation of a wider range of adverse childhood experiences (ACEs) is critical, as some ACEs, particularly poverty and neglect, persist at high rates in many societies, including the US (DeNavas-Walt and Proctor 2015; Gilbert et al. 2009). Our study thus adds to the prior literature by assessing exposure to a number of ACEs (e.g., physical abuse, sexual abuse, poverty, neglect), and by linking cumulative ACE exposure (i.e., high ELS) to a number of neuropsychiatric symptoms commonly endorsed by HIV+ adults (depression, anxiety, apathy, alexithymia). This result represents a novel finding in HIV+ cohorts, and parallels studies implicating high ELS as a significant risk factor for elevated neuropsychiatric symptoms in non-HIV samples (Dube et al. 2003; Felitti et al. 1998; Kaufman and Charney 2001; McFarlane et al. 2005; Pechtel and Pizzagalli 2011; Pesonen and Raikkonen 2012; Repetti et al. 2002; Taylor 2010).
We observed a strong association between elevated neuropsychiatric symptoms and aberrant amygdala responses in HIV+ patients. This effect was observed across a broad range of neuropsychiatric domains, and most notably for ratings of depression, anxiety, and alexithymia. Interestingly, we did not find a strong association between amygdala responses and apathy ratings, despite greater reports of apathy symptoms in the HIV+ group, which is in line with prior data indicating that the neural correlates of apathy and depression in HIV+ adults are distinct (Paul et al. 2005). The overall picture that emerges, however, is consistent with the larger literature documenting the importance of the amygdala to neuropsychiatric symptomology in non-HIV samples (Hilbert et al. 2014; Murray et al. 2011; Whalen and Phelps 2009). Additional studies would be needed to determine whether the association between ELS-related abnormalities in amygdala response and neuropsychiatric symptoms in HIV+ adults differs from that observed in HC adults presenting with a similar range of neuropsychiatric symptoms. Nevertheless, in the current study it is notable that while both the HIV+ High-ELS and HC High-ELS groups exhibited similar levels of amygdala responses, reported levels of neuropsychiatric symptoms were considerably higher in the HIV+ High-ELS group. This observation suggests that HIV-related abnormalities may be occurring in additional neural regions that may act in concert with ELS-related effects in the amygdala to elevate neuropsychiatric symptoms in HIV+ High-ELS adults. For example, in non-HIV cohorts, elevated neuropsychiatric symptoms have been associated with abnormalities within a number of regions, including the subgenual ACC (Damsa et al. 2009; Holzschneider and Mulert 2011; Mayberg 1997; Price and Drevets 2012), which is affected by HIV (Clark et al. 2015; Kuper et al. 2011). Future studies that examine potential additive effects of HIV-related and ELS-related abnormalities across a larger number of neural regions will provide a more comprehensive understanding of these networks and their relation to neuropsychiatric symptoms in HIV+ adults with high ELS.
Results from this study hint at the possibility that HIV infection and high ELS have independent effects on amygdala activity. Whereas high ELS status was associated with lower amygdala response, in contrast, greater historical immunological suppression in HIV+ patients (lower nadir CD4 levels) was associated with higher amygdala response. This finding suggests that severity of immunosuppression can exert a legacy effect on amygdala functions in HIV+ adults, consistent with several studies linking lower nadir CD4 levels to greater degree of neural abnormality in HIV+ adults (Clark et al. 2012; Clark et al. 2015; R. A. Cohen et al. 2010; Hua et al. 2013; Jernigan et al. 2011). HIV-related neural abnormalities include marked differences in frontal-lobe function (Castelo et al. 2006; Melrose et al. 2008). Accordingly, it is possible that the association between greater amygdala reactivity and lower nadir CD4 levels might be driven by HIV-related effects in frontal-lobe regions responsible for modulating amygdala response (M. J. Kim et al. 2011). Although we did not observe HIV-related differences in amygdala activation that were associated with neuropsychiatric symptoms, neuropsychiatric dysfunction in HIV+ adults is known to be multifactorial (Hammoud et al. 2010; Paul et al. 2005; Smith et al. 2008; Stubbe-Drager et al. 2012). It is likely that the neural mechanisms underlying neuropsychiatric dysfunction in HIV+ adults differ with respect to the different etiological factors involved (acute symptomatic illness, high ELS, etc.). Continued investigation of HIV-specific neural abnormalities is needed in order to advance individually-tailored prevention and intervention strategies for neuropsychiatric disorders in HIV+ adults.
Findings from this study have important clinical implications. First, our results suggest that assessing ELS histories in HIV+ patients could potentially enhance early identification of those at risk for developing neuropsychiatric symptoms. According to our data, HIV+ adults with high ELS exposure may be more likely to experience neuropsychiatric symptoms. These findings, coupled with those from similar studies (Mimiaga et al. 2009; Simoni and Ng 2000; Welles et al. 2009), have the potential to inform current conceptualizations of neuropsychiatric disorders in HIV+ patients (e.g., Rabkin 2008). Collectively, these studies suggest that high ELS exposure should be placed among other known risk factors of neuropsychiatric symptoms in HIV+ patients (e.g., acute symptomatic illness, stigma, substance abuse, social isolation). Second, the finding that the HIV+ High-ELS group exhibited significant elevations in neuropsychiatric symptoms relative to all other groups suggests that there might be a benefit in tailoring treatment approaches specifically to this group. At present, empirically supported interventions for HIV+ adults with high ELS exposure are scarce (Seedat 2012). Studies conducted in non-HIV samples indicate that psychotherapy is a critical component of successful symptom reduction for high ELS adults diagnosed with depression, even more so than pharmacotherapy alone (Blalock et al. 2013; Nemeroff et al. 2003; Zobel et al. 2011). Hence, treatment approaches that involve a psychotherapeutic component might be particularly effective for HIV+ High-ELS patients and thus warrant further examination. Third, our data suggest that ELS-related mechanisms (e.g., Carpenter et al. 2010a; Carpenter et al. 2010b; Irwin and Cole 2011; Johnson et al. 2013; Taylor 2010; Tyrka et al. 2013) might provide valuable targets for intervention when developing therapeutic strategies for neuropsychiatric disorders in HIV+ adults with high ELS. Accordingly, future studies are needed to determine whether biological mechanisms linked to high ELS exposure (e.g., increased cytokines) (Carpenter et al. 2010a; Tyrka et al. 2013) are associated with neural abnormalities in HIV+ adults with high ELS, as such studies may help to identify potential targets for intervention.
Some limitations of this preliminary study and considerations for future work include the following. First, our sample size was small, thereby limiting statistical power. However, we maximized power by using an ROI approach in our fMRI analysis and restricted our main analysis to one neural region (bilateral amygdala). Second, this study utilized retrospective reports of ELS history, which may be prone to report bias, revisionist recall error, and normal forgetting (Hardt and Rutter 2004; Maughan and Rutter 1997). It is notable that studies which utilize retrospective assessments of ELS and those that utilize objective documentation of ELS exposure (e.g., governmental records) find similar effects on neural, behavioral, and health outcomes (Clark et al. 2012; Felitti et al. 1998; Mehta et al. 2009; Pesonen and Raikkonen 2012; Tottenham et al. 2009a). Such findings support the notion that retrospective assays of ELS history provide data that have strong biological relevance. Third, in creating the neuropsychiatric composite index scores we utilized the entire HC group, rather than the HC Low-ELS group, as the normative sample. Using this approach, the resulting z-scores underestimate the relative level of symptomology reported by our High-ELS groups, thus dampening the magnitude of our ELS-related effects. Yet, using the entire HC group as the normative sample allows us to depict the degree to which our two HIV+ groups deviate from the larger random sample of demographically-matched HC adults. This approach thus increases the generalizability of our data. Additionally, the overall pattern of results reported herein remains the same whether the entire HC group or the HC Low-ELS group is used as the normative sample. Fourth, our groups differed significantly with respect to history of remote cocaine use. Although our analyses indicated that group differences in remote cocaine use did not contribute significantly to the observed effects, it remains possible that group differences in cocaine use history may have an indirect influence on our findings. Future studies should investigate the brain effects of HIV and ELS in samples in which remote substance use histories are more systematically controlled.
In summary, we observed evidence that high ELS exposure is a significant risk factor for elevated neuropsychiatric symptoms in HIV+ adults. This observation should guide future investigations of the neural etiology of neuropsychiatric disorders in HIV+ samples. Our data serve as an important first step in this direction, as they reveal ELS-related neural correlates of neuropsychiatric symptoms in HIV+ adults. More specifically, we found that in HIV+ adults, high ELS exposure is associated with altered amygdala response, which correlates with elevated neuropsychiatric symptoms. Future studies should be conducted to examine additional HIV-related and ELS-related brain effects that might play a role in the development of neuropsychiatric symptoms in HIV+ adults. In addition, future studies with larger sample sizes will help to confirm whether HIV and high ELS do indeed have independent effects as suggested here, or whether they might also have additive biological effects on amygdala function [e.g., through their impact on the immune system (Boyce et al. 1995; Glaser et al. 1986; Leserman 2003, 2008; Miller et al. 2002; Zachariae 2009)]. Collectively, such studies have the potential to improve diagnostic and treatment strategies for neuropsychiatric disorders in HIV+ adults by identifying potential biomarkers (e.g., neural signatures, cytokines) that could enhance early detection of HIV+ adults at risk of developing neuropsychiatric disorders, and by identifying specific targets for therapeutic intervention.
Acknowledgments
We thank all of the individuals who participated in this study. In addition, we gratefully acknowledge Karen T. Tashima, MD, Timothy P. Flanigan, MD, and the teams at The Miriam Hospital Immunology Center and the Manhattan HIV Brain Bank, who provided valuable aid in our recruitment efforts. Finally, we also thank Rachal Hegde for her assistance in preparing this manuscript.
Funding: This work was supported by grants from the National Institutes of Health (Grants K23 MH096628 [USC], R25 MH080663 [USC], R01 MH074368 [RAC], R25 MH083635 [USC], and P30 AI042853 [RAC]), the Brown University MRI Research Facility [USC], and the US Department of Veterans Affairs (Grant IK2 CX00724 [NSP]). The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the NIH or Department of Veterans Affairs.
Footnotes
Compliance with Ethical Standards
Conflict of Interest: The authors declare that they have no conflicts of interest.
Ethical Approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent: Informed consent was obtained from all individual participants included in the study.
References
- Adolphs R. What does the amygdala contribute to social cognition? Ann N Y Acad Sci. 2010;1191:42–61. doi: 10.1111/j.1749-6632.2010.05445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ances BM, Ortega M, Vaida F, Heaps J, Paul R. Independent effects of HIV, aging, and HAART on brain volumetric measures. J Acquir Immune Defic Syndr. 2012;59(5):469–477. doi: 10.1097/QAI.0b013e318249db17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen SL, Tomada A, Vincow ES, Valente E, Polcari A, Teicher MH. Preliminary evidence for sensitive periods in the effect of childhood sexual abuse on regional brain development. J Neuropsychiatry Clin Neurosci. 2008;20(3):292–301. doi: 10.1176/appi.neuropsych.20.3.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antelman G, Kaaya S, Wei R, Mbwambo J, Msamanga GI, Fawzi WW, et al. Depressive symptoms increase risk of HIV disease progression and mortality among women in Tanzania. J Acquir Immune Defic Syndr. 2007;44(4):470–477. doi: 10.1097/QAI.0b013e31802f1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnone D, McKie S, Elliott R, Thomas EJ, Downey D, Juhasz G, et al. Increased amygdala responses to sad but not fearful faces in major depression: relation to mood state and pharmacological treatment. Am J Psychiatry. 2012;169(8):841–850. doi: 10.1176/appi.ajp.2012.11121774. [DOI] [PubMed] [Google Scholar]
- Bagby RM, Parker JD, Taylor GJ. The twenty-item Toronto Alexithymia Scale--I. Item selection and cross-validation of the factor structure. J Psychosom Res. 1994;38(1):23–32. doi: 10.1016/0022-3999(94)90005-1. [DOI] [PubMed] [Google Scholar]
- Baker LM, Williams LM, Korgaonkar MS, Cohen RA, Heaps JM, Paul RH. Impact of early vs. late childhood early life stress on brain morphometrics. Brain Imaging Behav. 2013;7(2):196–203. doi: 10.1007/s11682-012-9215-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldonero E, Ciccarelli N, Fabbiani M, Colafigli M, Improta E, D’Avino A, et al. Evaluation of emotion processing in HIV-infected patients and correlation with cognitive performance. BMC Psychology. 2013;1(3):1–7. doi: 10.1186/2050-7283-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Epstein N, Brown GK, Steer RA. An inventory for measuring clinical anxiety: Psychometric properties. Journal of Consulting and Clinical Psychology. 1988;56(6):893–897. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- Beckmann M, Johansen-Berg H, Rushworth MF. Connectivity-based parcellation of human cingulate cortex and its relation to functional specialization. J Neurosci. 2009;29(4):1175–1190. doi: 10.1523/JNEUROSCI.3328-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrman-Lay AM, Paul RH, Heaps-Woodruff J, Baker LM, Usher C, Ances BM. Human immunodeficiency virus has similar effects on brain volumetrics and cognition in males and females. J Neurovirol. 2015 doi: 10.1007/s13365-015-0373-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton TD. Depression and HIV/AIDS. Curr Psychiatry Rep. 2008;10(3):280–285. doi: 10.1007/s11920-008-0045-y. [DOI] [PubMed] [Google Scholar]
- Bickart KC, Dickerson BC, Feldman Barrett L. The amygdala as a hub in brain networks that support social life. Neuropsychologia. 2014;63(0):235–248. doi: 10.1016/j.neuropsychologia.2014.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bing EG, Burnam MA, Longshore D, Fleishman JA, Sherbourne CD, London AS, et al. Psychiatric disorders and drug use among human immunodeficiency virus-infected adults in the United States. Arch Gen Psychiatry. 2001;58(8):721–728. doi: 10.1001/archpsyc.58.8.721. [DOI] [PubMed] [Google Scholar]
- Blair K, Shaywitz J, Smith BW, Rhodes R, Geraci M, Jones M, et al. Response to emotional expressions in generalized social phobia and generalized anxiety disorder: evidence for separate disorders. Am J Psychiatry. 2008;165(9):1193–1202. doi: 10.1176/appi.ajp.2008.07071060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blalock JA, Minnix JA, Mathew AR, Wetter DW, McCullough JP, Cinciripini PM. Relationship of childhood trauma to depression and smoking outcomes in pregnant smokers. J Consult Clin Psychol. 2013;81(5):821–830. doi: 10.1037/a0033381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdanova Y, Diaz-Santos M, Cronin-Golomb A. Neurocognitive correlates of alexithymia in asymptomatic individuals with HIV. Neuropsychologia. 2010;48(5):1295–1304. doi: 10.1016/j.neuropsychologia.2009.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce WT, Adams S, Tschann JM, Cohen F, Wara D, Gunnar MR. Adrenocortical and behavioral predictors of immune responses to starting school. Pediatr Res. 1995;38(6):1009–1017. doi: 10.1203/00006450-199512000-00030. [DOI] [PubMed] [Google Scholar]
- Canli T, Omura K, Haas BW, Fallgatter A, Constable RT, Lesch KP. Beyond affect: a role for genetic variation of the serotonin transporter in neural activation during a cognitive attention task. Proc Natl Acad Sci U S A. 2005;102(34):12224–12229. doi: 10.1073/pnas.0503880102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canli T, Qiu M, Omura K, Congdon E, Haas BW, Amin Z, et al. Neural correlates of epigenesis. Proc Natl Acad Sci U S A. 2006;103(43):16033–16038. doi: 10.1073/pnas.0601674103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael ST, Price JL. Limbic connections of the orbital and medial prefrontal cortex in macaque monkeys. J Comp Neurol. 1995;363(4):615–641. doi: 10.1002/cne.903630408. [DOI] [PubMed] [Google Scholar]
- Carpenter LL, Gawuga CE, Tyrka AR, Lee JK, Anderson GM, Price LH. Association between plasma IL-6 response to acute stress and early-life adversity in healthy adults. Neuropsychopharmacology. 2010a;35(13):2617–2623. doi: 10.1038/npp.2010.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter LL, Shattuck TT, Tyrka AR, Geracioti TD, Price LH. Effect of childhood physical abuse on cortisol stress response. Psychopharmacology (Berl) 2010b doi: 10.1007/s00213-010-2007-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelo JM, Sherman SJ, Courtney MG, Melrose RJ, Stern CE. Altered hippocampal-prefrontal activation in HIV patients during episodic memory encoding. Neurology. 2006;66(11):1688–1695. doi: 10.1212/01.wnl.0000218305.09183.70. [DOI] [PubMed] [Google Scholar]
- Catz SL, Kelly JA, Bogart LM, Benotsch EG, McAuliffe TL. Patterns, correlates, and barriers to medication adherence among persons prescribed new treatments for HIV disease. Health Psychol. 2000;19(2):124–133. [PubMed] [Google Scholar]
- Chang L, Ernst T, Witt MD, Ames N, Gaiefsky M, Miller E. Relationships among brain metabolites, cognitive function, and viral loads in antiretroviral-naive HIV patients. Neuroimage. 2002;17(3):1638–1648. doi: 10.1006/nimg.2002.1254. [DOI] [PubMed] [Google Scholar]
- Ciesla JA, Roberts JE. Meta-analysis of the relationship between HIV infection and risk for depressive disorders. Am J Psychiatry. 2001;158(5):725–730. doi: 10.1176/appi.ajp.158.5.725. [DOI] [PubMed] [Google Scholar]
- Circelli KS, Clark US, Cronin-Golomb A. Visual scanning patterns and executive function in relation to facial emotion recognition in aging. Aging, Neuropsychology and Cognition. 2013;20(2):148–173. doi: 10.1080/13825585.2012.675427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark US, Cohen RA. Brain dysfunction in the era of combination antiretroviral therapy: implications for the treatment of the aging population of HIV-infected individuals. Curr Opin Investig Drugs. 2010;11(8):884–900. [PMC free article] [PubMed] [Google Scholar]
- Clark US, Cohen RA, Sweet LH, Gongvatana A, Hana GN, Westbrook ML, et al. Effects of HIV and early life stress on amygdala morphometry and neurocognitive function. J Int Neuropsychol Soc. 2012;18(4):657–668. doi: 10.1017/S1355617712000434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark US, Cohen RA, Westbrook ML, Devlin KN, Tashima KT. Facial emotion recognition impairments in individuals with HIV. J Int Neuropsychol Soc. 2010a;16(6):1127–1137. doi: 10.1017/S1355617710001037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark US, Neargarder S, Cronin-Golomb A. Specific impairments in the recognition of emotional facial expressions in Parkinson’s disease. Neuropsychologia. 2008;46(9):2300–2309. doi: 10.1016/j.neuropsychologia.2008.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark US, Neargarder S, Cronin-Golomb A. Visual exploration of emotional facial expressions in Parkinson’s disease. Neuropsychologia. 2010b;48(7):1901–1913. doi: 10.1016/j.neuropsychologia.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark US, Walker KA, Cohen RA, Devlin KN, Folkers AM, Pina MJ, et al. Facial emotion recognition impairments are associated with brain volume abnormalities in individuals with HIV. Neuropsychologia. 2015;70:263–271. doi: 10.1016/j.neuropsychologia.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- Cohen RA, Grieve S, Hoth KF, Paul RH, Sweet L, Tate D, et al. Early life stress and morphometry of the adult anterior cingulate cortex and caudate nuclei. Biol Psychiatry. 2006a;59(10):975–982. doi: 10.1016/j.biopsych.2005.12.016. [DOI] [PubMed] [Google Scholar]
- Cohen RA, Harezlak J, Schifitto G, Hana G, Clark U, Gongvatana A, et al. Effects of nadir CD4 count and duration of human immunodeficiency virus infection on brain volumes in the highly active antiretroviral therapy era. J Neurovirol. 2010;16(1):25–32. doi: 10.3109/13550280903552420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen RA, Paul RH, Stroud L, Gunstad J, Hitsman BL, McCaffery J, et al. Early life stress and adult emotional experience: an international perspective. Int J Psychiatry Med. 2006b;36(1):35–52. doi: 10.2190/5R62-9PQY-0NEL-TLPA. [DOI] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24(4):385–396. [PubMed] [Google Scholar]
- Cook JA, Cohen MH, Burke J, Grey D, Anastos K, Kirstein L, et al. Effects of depressive symptoms and mental health quality of life on use of highly active antiretroviral therapy among HIV-seropositive women. J Acquir Immune Defic Syndr. 2002;30(4):401–409. doi: 10.1097/00042560-200208010-00005. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Cysique LA, Maruff P, Brew BJ. Variable benefit in neuropsychological function in HIV-infected HAART-treated patients. Neurology. 2006;66(9):1447–1450. doi: 10.1212/01.wnl.0000210477.63851.d3. [DOI] [PubMed] [Google Scholar]
- Damsa C, Kosel M, Moussally J. Current status of brain imaging in anxiety disorders. Curr Opin Psychiatry. 2009;22(1):96–110. doi: 10.1097/YCO.0b013e328319bd10. [DOI] [PubMed] [Google Scholar]
- Dannlowski U, Stuhrmann A, Beutelmann V, Zwanzger P, Lenzen T, Grotegerd D, et al. Limbic scars: long-term consequences of childhood maltreatment revealed by functional and structural magnetic resonance imaging. Biol Psychiatry. 2012;71(4):286–293. doi: 10.1016/j.biopsych.2011.10.021. [DOI] [PubMed] [Google Scholar]
- DeNavas-Walt C, Proctor BD. U. C. Bureau, editor. Current Population Reports, Income and Poverty in the United States: 2014. Washington, DC: 2015. [Google Scholar]
- Do AN, Rosenberg ES, Sullivan PS, Beer L, Strine TW, Schulden JD, et al. Excess burden of depression among HIV-infected persons receiving medical care in the united states: data from the medical monitoring project and the behavioral risk factor surveillance system. PLoS One. 2014;9(3):e92842. doi: 10.1371/journal.pone.0092842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC. Prefrontal cortical-amygdalar metabolism in major depression. Ann N Y Acad Sci. 1999;877:614–637. doi: 10.1111/j.1749-6632.1999.tb09292.x. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Bardgett ME, Reich T, Todd RD, Raichle ME. Glucose metabolism in the amygdala in depression: relationship to diagnostic subtype and plasma cortisol levels. Pharmacol Biochem Behav. 2002;71(3):431–447. doi: 10.1016/s0091-3057(01)00687-6. [DOI] [PubMed] [Google Scholar]
- Dube SR, Felitti VJ, Dong M, Giles WH, Anda RF. The impact of adverse childhood experiences on health problems: evidence from four birth cohorts dating back to 1900. Prev Med. 2003;37(3):268–277. doi: 10.1016/s0091-7435(03)00123-3. [DOI] [PubMed] [Google Scholar]
- Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn Sci. 2011;15(2):85–93. doi: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry. 2007;164(10):1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans GW, Swain JE, King AP, Wang X, Javanbakht A, Ho SS, et al. Childhood Cumulative Risk Exposure and Adult Amygdala Volume and Function. J Neurosci Res. 2015 doi: 10.1002/jnr.23681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, et al. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. Am J Prev Med. 1998;14(4):245–258. doi: 10.1016/s0749-3797(98)00017-8. [DOI] [PubMed] [Google Scholar]
- Fleishman JA, Sherbourne CD, Crystal S, Collins RL, Marshall GN, Kelly M, et al. Coping, conflictual social interactions, social support, and mood among HIV-infected persons. HCSUS Consortium. Am J Community Psychol. 2000;28(4):421–453. doi: 10.1023/a:1005132430171. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-mental state: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, Placentino A, Carletti F, Landi P, Allen P, Surguladze S, et al. Functional atlas of emotional faces processing: a voxel-based meta-analysis of 105 functional magnetic resonance imaging studies. J Psychiatry Neurosci. 2009;34(6):418–432. [PMC free article] [PubMed] [Google Scholar]
- Gilbert R, Widom CS, Browne K, Fergusson D, Webb E, Janson S. Burden and consequences of child maltreatment in high-income countries. Lancet. 2009;373(9657):68–81. doi: 10.1016/S0140-6736(08)61706-7. [DOI] [PubMed] [Google Scholar]
- Glaser R, Rice J, Speicher CE, Stout JC, Kiecolt-Glaser JK. Stress depresses interferon production by leukocytes concomitant with a decrease in natural killer cell activity. Behav Neurosci. 1986;100(5):675–678. doi: 10.1037//0735-7044.100.5.675. [DOI] [PubMed] [Google Scholar]
- Grant MM, Cannistraci C, Hollon SD, Gore J, Shelton R. Childhood trauma history differentiates amygdala response to sad faces within MDD. J Psychiatr Res. 2011;45(7):886–895. doi: 10.1016/j.jpsychires.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenewold NA, Opmeer EM, de Jonge P, Aleman A, Costafreda SG. Emotional valence modulates brain functional abnormalities in depression: evidence from a meta-analysis of fMRI studies. Neurosci Biobehav Rev. 2013;37(2):152–163. doi: 10.1016/j.neubiorev.2012.11.015. [DOI] [PubMed] [Google Scholar]
- Hammoud DA, Endres CJ, Hammond E, Uzuner O, Brown A, Nath A, et al. Imaging serotonergic transmission with [11C]DASB-PET in depressed and non-depressed patients infected with HIV. Neuroimage. 2010;49(3):2588–2595. doi: 10.1016/j.neuroimage.2009.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardt J, Rutter M. Validity of adult retrospective reports of adverse childhood experiences: review of the evidence. J Child Psychol Psychiatry. 2004;45(2):260–273. doi: 10.1111/j.1469-7610.2004.00218.x. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, Leblanc S, et al. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol. 2011;17(1):3–16. doi: 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilman KJ, Harden ER, Weber KM, Cohen M, Porges SW. Atypical autonomic regulation, auditory processing, and affect recognition in women with HIV. Biol Psychol. 2013;94(1):143–151. doi: 10.1016/j.biopsycho.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz A, Smolka MN, Braus DF, Wrase J, Beck A, Flor H, et al. Serotonin transporter genotype (5-HTTLPR): effects of neutral and undefined conditions on amygdala activation. Biol Psychiatry. 2007;61(8):1011–1014. doi: 10.1016/j.biopsych.2006.08.019. [DOI] [PubMed] [Google Scholar]
- Hilbert K, Lueken U, Beesdo-Baum K. Neural structures, functioning and connectivity in Generalized Anxiety Disorder and interaction with neuroendocrine systems: a systematic review. J Affect Disord. 2014;158:114–126. doi: 10.1016/j.jad.2014.01.022. [DOI] [PubMed] [Google Scholar]
- Holzschneider K, Mulert C. Neuroimaging in anxiety disorders. Dialogues Clin Neurosci. 2011;13(4):453–461. doi: 10.31887/DCNS.2011.13.4/kholzschneider. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horberg MA, Silverberg MJ, Hurley LB, Towner WJ, Klein DB, Bersoff-Matcha S, et al. Effects of depression and selective serotonin reuptake inhibitor use on adherence to highly active antiretroviral therapy and on clinical outcomes in HIV-infected patients. J Acquir Immune Defic Syndr. 2008;47(3):384–390. doi: 10.1097/QAI.0b013e318160d53e. [DOI] [PubMed] [Google Scholar]
- Horowitz LM, Alden LE, Wiggins JS, Pincus AL. IIP–64/IIP–32 professional manual. San Antonio, TX: Psychological Corporation; 2000. [Google Scholar]
- Horowitz LM, Rosenberg SE, Baer BA, Ureno G, Villasenor VS. Inventory of interpersonal problems: Psychometric properties and clinical applications. Journal of Consulting and Clinical Psychology. 1988;56(6):885–892. doi: 10.1037//0022-006x.56.6.885. [DOI] [PubMed] [Google Scholar]
- Hua X, Boyle CP, Harezlak J, Tate DF, Yiannoutsos CT, Cohen R, et al. Disrupted cerebral metabolite levels and lower nadir CD4 + counts are linked to brain volume deficits in 210 HIV-infected patients on stable treatment. Neuroimage Clin. 2013;3:132–142. doi: 10.1016/j.nicl.2013.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson AL, Lee KA, Miramontes H, Portillo CJ. Social interactions, perceived support, and level of distress in HIV-positive women. J Assoc Nurses AIDS Care. 2001;12(4):68–76. doi: 10.1016/S1055-3290(06)60218-5. [DOI] [PubMed] [Google Scholar]
- Ickovics JR, Hamburger ME, Vlahov D, Schoenbaum EE, Schuman P, Boland RJ, et al. Mortality, CD4 cell count decline, and depressive symptoms among HIV-seropositive women: longitudinal analysis from the HIV Epidemiology Research Study. Jama. 2001;285(11):1466–1474. doi: 10.1001/jama.285.11.1466. [DOI] [PubMed] [Google Scholar]
- Irwin MR, Cole SW. Reciprocal regulation of the neural and innate immune systems. Nat Rev Immunol. 2011;11(9):625–632. doi: 10.1038/nri3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jernigan TL, Archibald SL, Fennema-Notestine C, Taylor MJ, Theilmann RJ, Julaton MD, et al. Clinical factors related to brain structure in HIV: the CHARTER study. J Neurovirol. 2011;17(3):248–257. doi: 10.1007/s13365-011-0032-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SB, Riley AW, Granger DA, Riis J. The science of early life toxic stress for pediatric practice and advocacy. Pediatrics. 2013;131(2):319–327. doi: 10.1542/peds.2012-0469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamat R, Woods SP, Marcotte TD, Ellis RJ, Grant I, Group HIVNRP. Implications of apathy for everyday functioning outcomes in persons living with HIV infection. Arch Clin Neuropsychol. 2012;27(5):520–531. doi: 10.1093/arclin/acs055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Charney D. Effects of early stress on brain structure and function: implications for understanding the relationship between child maltreatment and depression. Dev Psychopathol. 2001;13(3):451–471. doi: 10.1017/s0954579401003030. [DOI] [PubMed] [Google Scholar]
- Kellogg SH, McHugh PF, Bell K, Schluger JH, Schluger RP, LaForge KS, et al. The Kreek-McHugh-Schluger-Kellogg scale: a new, rapid method for quantifying substance abuse and its possible applications. Drug Alcohol Depend. 2003;69(2):137–150. doi: 10.1016/s0376-8716(02)00308-3. [DOI] [PubMed] [Google Scholar]
- Kim H, Somerville LH, Johnstone T, Alexander AL, Whalen PJ. Inverse amygdala and medial prefrontal cortex responses to surprised faces. Neuroreport. 2003;14(18):2317–2322. doi: 10.1097/00001756-200312190-00006. [DOI] [PubMed] [Google Scholar]
- Kim H, Somerville LH, Johnstone T, Polis S, Alexander AL, Shin LM, et al. Contextual modulation of amygdala responsivity to surprised faces. J Cogn Neurosci. 2004;16(10):1730–1745. doi: 10.1162/0898929042947865. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Loucks RA, Palmer AL, Brown AC, Solomon KM, Marchante AN, et al. The structural and functional connectivity of the amygdala: from normal emotion to pathological anxiety. Behav Brain Res. 2011;223(2):403–410. doi: 10.1016/j.bbr.2011.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuper M, Rabe K, Esser S, Gizewski ER, Husstedt IW, Maschke M, et al. Structural gray and white matter changes in patients with HIV. Journal of Neurology. 2011;258(6):1066–1075. doi: 10.1007/s00415-010-5883-y. [DOI] [PubMed] [Google Scholar]
- Lane TA, Moore DM, Batchelor J, Brew BJ, Cysique LA. Facial emotional processing in HIV infection: relation to neurocognitive and neuropsychiatric status. Neuropsychology. 2012;26(6):713–722. doi: 10.1037/a0029964. [DOI] [PubMed] [Google Scholar]
- Lanius RA, Bluhm RL, Coupland NJ, Hegadoren KM, Rowe B, Theberge J, et al. Default mode network connectivity as a predictor of post-traumatic stress disorder symptom severity in acutely traumatized subjects. Acta Psychiatr Scand. 2010;121(1):33–40. doi: 10.1111/j.1600-0447.2009.01391.x. [DOI] [PubMed] [Google Scholar]
- Leserman J. HIV disease progression: depression, stress, and possible mechanisms. Biol Psychiatry. 2003;54(3):295–306. doi: 10.1016/s0006-3223(03)00323-8. [DOI] [PubMed] [Google Scholar]
- Leserman J. Role of depression, stress, and trauma in HIV disease progression. Psychosom Med. 2008;70(5):539–545. doi: 10.1097/PSY.0b013e3181777a5f. [DOI] [PubMed] [Google Scholar]
- Li Y, Yan J, Wang D, Sun M, Zhu Y, Zhu X, et al. Magnetic resonance study of the structure and function of the hippocampus and amygdala in patients with depression. Chin Med J (Engl) 2014;127(20):3610–3615. [PubMed] [Google Scholar]
- Lieberman MD, Eisenberger NI, Crockett MJ, Tom SM, Pfeifer JH, Way BM. Putting feelings into words: affect labeling disrupts amygdala activity in response to affective stimuli. Psychol Sci. 2007;18(5):421–428. doi: 10.1111/j.1467-9280.2007.01916.x. [DOI] [PubMed] [Google Scholar]
- Machtinger EL, Wilson TC, Haberer JE, Weiss DS. Psychological trauma and PTSD in HIV-positive women: a meta-analysis. AIDS Behav. 2012;16(8):2091–2100. doi: 10.1007/s10461-011-0127-4. [DOI] [PubMed] [Google Scholar]
- Marin RS, Biedrzycki RC, Firinciogullari S. Reliability and validity of the Apathy Evaluation Scale. Psychiatry Res. 1991;38(2):143–162. doi: 10.1016/0165-1781(91)90040-v. [DOI] [PubMed] [Google Scholar]
- Marshall R, Beach MC, Saha S, Mori T, Loveless MO, Hibbard JH, et al. Patient activation and improved outcomes in HIV-infected patients. J Gen Intern Med. 2013;28(5):668–674. doi: 10.1007/s11606-012-2307-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maughan B, Rutter M. Retrospective reporting of childhood adversity: issues in assessing long-term recall. J Pers Disord. 1997;11(1):19–33. doi: 10.1521/pedi.1997.11.1.19. [DOI] [PubMed] [Google Scholar]
- Mayberg HS. Limbic-cortical dysregulation: a proposed model of depression. J Neuropsychiatry Clin Neurosci. 1997;9(3):471–481. doi: 10.1176/jnp.9.3.471. [DOI] [PubMed] [Google Scholar]
- Mayne TJ, Vittinghoff E, Chesney MA, Barrett DC, Coates TJ. Depressive affect and survival among gay and bisexual men infected with HIV. Arch Intern Med. 1996;156(19):2233–2238. [PubMed] [Google Scholar]
- McFarlane A, Clark CR, Bryant RA, Williams LM, Niaura R, Paul RH, et al. The impact of early life stress on psychophysiological, personality and behavioral measures in 740 non-clinical subjects. J Integr Neurosci. 2005;4(1):27–40. doi: 10.1142/s0219635205000689. [DOI] [PubMed] [Google Scholar]
- Mehta MA, Golembo NI, Nosarti C, Colvert E, Mota A, Williams SC, et al. Amygdala, hippocampal and corpus callosum size following severe early institutional deprivation: the English and Romanian Adoptees study pilot. J Child Psychol Psychiatry. 2009;50(8):943–951. doi: 10.1111/j.1469-7610.2009.02084.x. [DOI] [PubMed] [Google Scholar]
- Melrose RJ, Tinaz S, Castelo JM, Courtney MG, Stern CE. Compromised fronto-striatal functioning in HIV: an fMRI investigation of semantic event sequencing. Behav Brain Res. 2008;188(2):337–347. doi: 10.1016/j.bbr.2007.11.021. [DOI] [PubMed] [Google Scholar]
- Miller GE, Cohen S, Ritchey AK. Chronic psychological stress and the regulation of pro-inflammatory cytokines: a glucocorticoid-resistance model. Health Psychol. 2002;21(6):531–541. doi: 10.1037//0278-6133.21.6.531. [DOI] [PubMed] [Google Scholar]
- Mimiaga MJ, Noonan E, Donnell D, Safren SA, Koenen KC, Gortmaker S, et al. Childhood sexual abuse is highly associated with HIV risk-taking behavior and infection among MSM in the EXPLORE Study. J Acquir Immune Defic Syndr. 2009;51(3):340–348. doi: 10.1097/QAI.0b013e3181a24b38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison MF, Petitto JM, Ten Have T, Gettes DR, Chiappini MS, Weber AL, et al. Depressive and anxiety disorders in women with HIV infection. Am J Psychiatry. 2002;159(5):789–796. doi: 10.1176/appi.ajp.159.5.789. [DOI] [PubMed] [Google Scholar]
- Mugavero M, Ostermann J, Whetten K, Leserman J, Swartz M, Stangl D, et al. Barriers to antiretroviral adherence: the importance of depression, abuse, and other traumatic events. AIDS Patient Care STDS. 2006;20(6):418–428. doi: 10.1089/apc.2006.20.418. [DOI] [PubMed] [Google Scholar]
- Murray EA, Wise SP, Drevets WC. Localization of dysfunction in major depressive disorder: prefrontal cortex and amygdala. Biol Psychiatry. 2011;69(12):e43–54. doi: 10.1016/j.biopsych.2010.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeroff CB, Heim CM, Thase ME, Klein DN, Rush AJ, Schatzberg AF, et al. Differential responses to psychotherapy versus pharmacotherapy in patients with chronic forms of major depression and childhood trauma. Proc Natl Acad Sci U S A. 2003;100(24):14293–14296. doi: 10.1073/pnas.2336126100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega M, Heaps JM, Joska J, Vaida F, Seedat S, Stein DJ, et al. HIV clades B and C are associated with reduced brain volumetrics. J Neurovirol. 2013;19(5):479–487. doi: 10.1007/s13365-013-0202-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panos SE, Del Re AC, Thames AD, Arentsen TJ, Patel SM, Castellon SA, et al. The impact of neurobehavioral features on medication adherence in HIV: evidence from longitudinal models. AIDS Care. 2014;26(1):79–86. doi: 10.1080/09540121.2013.802275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul RH, Brickman AM, Navia B, Hinkin C, Malloy PF, Jefferson AL, et al. Apathy is associated with volume of the nucleus accumbens in patients infected with HIV. J Neuropsychiatry Clin Neurosci. 2005;17(2):167–171. doi: 10.1176/appi.neuropsych.17.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul RH, Henry L, Grieve SM, Guilmette TJ, Niaura R, Bryant R, et al. The relationship between early life stress and microstructural integrity of the corpus callosum in a non-clinical population. Neuropsychiatr Dis Treat. 2008;4(1):193–201. doi: 10.2147/ndt.s1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxton KC, Myers HF, Hall NM, Javanbakht M. Ethnicity, serostatus, and psychosocial differences in sexual risk behavior among HIV-seropositive and HIV-seronegative women. AIDS Behav. 2004;8(4):405–415. doi: 10.1007/s10461-004-7325-2. [DOI] [PubMed] [Google Scholar]
- Payer DE, Nurmi EL, Wilson SA, McCracken JT, London ED. Effects of methamphetamine abuse and serotonin transporter gene variants on aggression and emotion-processing neurocircuitry. Transl Psychiatry. 2012;2:e80. doi: 10.1038/tp.2011.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pechtel P, Pizzagalli DA. Effects of early life stress on cognitive and affective function: an integrated review of human literature. Psychopharmacology (Berl) 2011;214(1):55–70. doi: 10.1007/s00213-010-2009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesonen AK, Raikkonen K. The lifespan consequences of early life stress. Physiol Behav. 2012;106(5):722–727. doi: 10.1016/j.physbeh.2011.10.030. [DOI] [PubMed] [Google Scholar]
- Philip NS, Sweet LH, Tyrka AR, Price LH, Carpenter LL, Kuras YI, et al. Early life stress is associated with greater default network deactivation during working memory in healthy controls: a preliminary report. Brain Imaging Behav. 2013;7(2):204–212. doi: 10.1007/s11682-012-9216-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JL, Drevets WC. Neural circuits underlying the pathophysiology of mood disorders. Trends Cogn Sci. 2012;16(1):61–71. doi: 10.1016/j.tics.2011.12.011. [DOI] [PubMed] [Google Scholar]
- Rabkin JG. HIV and depression: 2008 review and update. Curr HIV/AIDS Rep. 2008;5(4):163–171. doi: 10.1007/s11904-008-0025-1. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D Scale: A Self-Report Depression Scale for Research in the General Population. Applied Psychological Measurement. 1977;1(3):385–401. [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98(2):676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, Snyder AZ. A default mode of brain function: a brief history of an evolving idea. Neuroimage. 2007;37(4):1083–1090. doi: 10.1016/j.neuroimage.2007.02.041. discussion 1097–1089. [DOI] [PubMed] [Google Scholar]
- Repetti RL, Taylor SE, Seeman TE. Risky families: family social environments and the mental and physical health of offspring. Psychol Bull. 2002;128(2):330–366. [PubMed] [Google Scholar]
- Rosenberger C, Elsenbruch S, Scholle A, de Greiff A, Schedlowski M, Forsting M, et al. Effects of psychological stress on the cerebral processing of visceral stimuli in healthy women. Neurogastroenterol Motil. 2009;21(7):740–e745. doi: 10.1111/j.1365-2982.2009.01295.x. [DOI] [PubMed] [Google Scholar]
- Safren SA, Otto MW, Worth JL, Salomon E, Johnson W, Mayer K, et al. Two strategies to increase adherence to HIV antiretroviral medication: life-steps and medication monitoring. Behav Res Ther. 2001;39(10):1151–1162. doi: 10.1016/s0005-7967(00)00091-7. [DOI] [PubMed] [Google Scholar]
- Schafer KR, Gupta S, Dillingham R. HIV-infected men who have sex with men and histories of childhood sexual abuse: implications for health and prevention. J Assoc Nurses AIDS Care. 2013;24(4):288–298. doi: 10.1016/j.jana.2012.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schienle A, Ebner F, Schafer A. Localized gray matter volume abnormalities in generalized anxiety disorder. Eur Arch Psychiatry Clin Neurosci. 2011;261(4):303–307. doi: 10.1007/s00406-010-0147-5. [DOI] [PubMed] [Google Scholar]
- Seckfort DL, Paul R, Grieve SM, Vandenberg B, Bryant RA, Williams LM, et al. Early life stress on brain structure and function across the lifespan: A preliminary study. Brain Imaging and Behavior. 2008;2(1):49–58. [Google Scholar]
- Seedat S. Interventions to improve psychological functioning and health outcomes of HIV-infected individuals with a history of trauma or PTSD. Curr HIV/AIDS Rep. 2012;9(4):344–350. doi: 10.1007/s11904-012-0139-3. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27(9):2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Barch DM, Donnelly JM, Ollinger JM, Snyder AZ, Mintun MA. Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant treatment: an fMRI study. Biol Psychiatry. 2001;50(9):651–658. doi: 10.1016/s0006-3223(01)01263-x. [DOI] [PubMed] [Google Scholar]
- Shin LM, Wright CI, Cannistraro PA, Wedig MM, McMullin K, Martis B, et al. A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Arch Gen Psychiatry. 2005;62(3):273–281. doi: 10.1001/archpsyc.62.3.273. [DOI] [PubMed] [Google Scholar]
- Simoni JM, Ng MT. Trauma, coping, and depression among women with HIV/AIDS in New York City. AIDS Care. 2000;12(5):567–580. doi: 10.1080/095401200750003752. [DOI] [PubMed] [Google Scholar]
- Smith CA, Stebbins GT, Bartt RE, Kessler HA, Adeyemi OM, Martin E, et al. White matter anisotropy and depression symptoms in patients with HIV. J Neuropsychiatry Clin Neurosci. 2008;20(4):458–465. doi: 10.1176/jnp.2008.20.4.458. [DOI] [PubMed] [Google Scholar]
- Spies G, Afifi TO, Archibald SL, Fennema-Notestine C, Sareen J, Seedat S. Mental health outcomes in HIV and childhood maltreatment: a systematic review. Syst Rev. 2012;1:30. doi: 10.1186/2046-4053-1-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spies G, Ahmed-Leitao F, Fennema-Notestine C, Cherner M, Seedat S. Effects of HIV and childhood trauma on brain morphometry and neurocognitive function. J Neurovirol. 2015 doi: 10.1007/s13365-015-0379-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripada RK, King AP, Garfinkel SN, Wang X, Sripada CS, Welsh RC, et al. Altered resting-state amygdala functional connectivity in men with posttraumatic stress disorder. J Psychiatry Neurosci. 2012;37(4):241–249. doi: 10.1503/jpn.110069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubbe-Drager B, Deppe M, Mohammadi S, Keller SS, Kugel H, Gregor N, et al. Early microstructural white matter changes in patients with HIV: a diffusion tensor imaging study. BMC Neurol. 2012;12:23. doi: 10.1186/1471-2377-12-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain: 3-dimensional proportional system: an approach to cerebral imaging. Stuttgart; New York: Georg Thieme; 1988. [Google Scholar]
- Taylor SE. Mechanisms linking early life stress to adult health outcomes. Proc Natl Acad Sci U S A. 2010;107(19):8507–8512. doi: 10.1073/pnas.1003890107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SE, Eisenberger NI, Saxbe D, Lehman BJ, Lieberman MD. Neural responses to emotional stimuli are associated with childhood family stress. Biol Psychiatry. 2006;60(3):296–301. doi: 10.1016/j.biopsych.2005.09.027. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Andersen SL, Polcari A, Anderson CM, Navalta CP. Developmental neurobiology of childhood stress and trauma. Psychiatr Clin North Am. 2002;25(2):397–426. vii–viii. doi: 10.1016/s0193-953x(01)00003-x. [DOI] [PubMed] [Google Scholar]
- Tottenham N, Hare TA, Quinn BT, McCarry TW, Nurse M, Gilhooly T, et al. Prolonged institutional rearing is associated with atypically large amygdala volume and difficulties in emotion regulation. Developmental science. 2009a;13(1):46–61. doi: 10.1111/j.1467-7687.2009.00852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Tanaka JW, Leon AC, McCarry TW, Nurse M, Hare TA, et al. The NimStim set of facial expressions: Judgments from untrained research participants. Psychiatry Res. 2009b;168(3):242–249. doi: 10.1016/j.psychres.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyrka AR, Burgers DE, Philip NS, Price LH, Carpenter LL. The neurobiological correlates of childhood adversity and implications for treatment. Acta Psychiatr Scand. 2013;128(6):434–447. doi: 10.1111/acps.12143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Marle HJ, Hermans EJ, Qin S, Fernandez G. From specificity to sensitivity: how acute stress affects amygdala processing of biologically salient stimuli. Biol Psychiatry. 2009;66(7):649–655. doi: 10.1016/j.biopsych.2009.05.014. [DOI] [PubMed] [Google Scholar]
- Villar-Loubet OM, Illa L, Echenique M, Cook R, Messick B, Duthely LM, et al. Prenatal and mental health care among trauma-exposed, HIV-infected, pregnant women in the United States. J Assoc Nurses AIDS Care. 2014;25(1 Suppl):S50–61. doi: 10.1016/j.jana.2013.06.006. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Pandya DN. Cingulate cortex of the rhesus monkey: II. Cortical afferents. J Comp Neurol. 1987;262(2):271–289. doi: 10.1002/cne.902620208. [DOI] [PubMed] [Google Scholar]
- Walton G, Co SJ, Milloy MJ, Qi J, Kerr T, Wood E. High prevalence of childhood emotional, physical and sexual trauma among a Canadian cohort of HIV-seropositive illicit drug users. AIDS Care. 2011;23(6):714–721. doi: 10.1080/09540121.2010.525618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weathers F, Litz B, Herman D, Huska J, Keane T. The PTSD Checklist (PCL): Reliability, Validity, and Diagnostic Utility. Paper presented at the Annual Convention of the International Society for Traumatic Stress Studies; San Antonio, TX. October.1993. [Google Scholar]
- Welles SL, Baker AC, Miner MH, Brennan DJ, Jacoby S, Rosser BR. History of childhood sexual abuse and unsafe anal intercourse in a 6-city study of HIV-positive men who have sex with men. Am J Public Health. 2009;99(6):1079–1086. doi: 10.2105/AJPH.2007.133280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen PJ, Phelps EA, editors. The human amygdala. New York, NY: Guilford Press; 2009. [Google Scholar]
- Whetten K, Leserman J, Lowe K, Stangl D, Thielman N, Swartz M, et al. Prevalence of childhood sexual abuse and physical trauma in an HIV-positive sample from the deep south. Am J Public Health. 2006;96(6):1028–1030. doi: 10.2105/AJPH.2005.063263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachariae R. Psychoneuroimmunology: a bio-psycho-social approach to health and disease. Scand J Psychol. 2009;50(6):645–651. doi: 10.1111/j.1467-9450.2009.00779.x. [DOI] [PubMed] [Google Scholar]
- Zobel I, Kech S, van Calker D, Dykierek P, Berger M, Schneibel R, et al. Long-term effect of combined interpersonal psychotherapy and pharmacotherapy in a randomized trial of depressed patients. Acta Psychiatr Scand. 2011;123(4):276–282. doi: 10.1111/j.1600-0447.2010.01671.x. [DOI] [PubMed] [Google Scholar]