Abstract
Old age is the greatest risk factor for most neurodegenerative diseases. During recent decades there have been major advances in understanding the biology of aging, and the development of nutritional interventions that delay aging including calorie restriction (CR) and intermittent fasting (IF), and chemicals that influence pathways linking nutrition and aging processes. CR influences brain aging in many animal models and recent findings suggest that dietary interventions can influence brain health and dementia in older humans. The role of individual macronutrients in brain aging also has been studied, with conflicting results about the effects of dietary protein and carbohydrates. A new approach known as the Geometric Framework (GF) has been used to unravel the complex interactions between macronutrients (protein, fat, and carbohydrate) and total energy on outcomes such as aging. These studies have shown that low-protein, high-carbohydrate (LPHC) diets are optimal for lifespan in ad libitum fed animals, while total calories have minimal effect once macronutrients are taken into account. One of the primary purposes of this review is to explore the notion that macronutrients may have a more translational potential than CR and IF in humans, and therefore there is a pressing need to use GF to study the impact of diet on brain aging. Furthermore, given the growing recognition of the role of aging biology in dementia, such studies might provide a new approach for dietary interventions for optimizing brain health and preventing dementia in older people.
Keywords: ageing, cognition, geometric framework, macronutrients, intermittent fasting
Graphical abstract
Old age is the leading risk factor for neurodegenerative disease. Targeting nutrient sensing pathways through calorie restriction (CR) and intermittent fasting (IF) have proven to be effective treatments to delay ageing, increase healthspan, and postpone the onset of neurodegeneration. Anti-ageing compounds and low-protein, high-carbohydrate (LPHC) diet ratios have improved lifespan but the effects on delaying symptoms of neurodegeneration are still not well understood.
“Don’t dig your grave with your knife and fork.”
(English Proverb)s
1. Introduction
Old age is the major determinant of degenerative diseases of the brain that influence cognition including the various types of dementia (Mowszowski et al., 2010). There has been extensive research into these common diseases with a focus on establishing the etiology and developing therapies for their treatment and prevention (Partridge, 2014). This has generated profound insights into disease-specific cellular pathways, but the discovery of disease-modifying treatments has remained elusive. On the other hand, the relationships between aging biology of the brain and neurodegenerative diseases have been relatively understudied. In recent decades there have been major advances in the understanding of aging biology at the cellular and systemic levels, and the development of strategies that delay aging and the concomitant suite of age-related disorders and pathologies. In particular, there has been a concerted focus on the nutritional interventions that delay aging, especially calorie restriction (CR) and intermittent fasting (IF), and chemicals that act on the pathways that mediate the effects of nutrition on the aging process. More recent focus has been on altering dietary macronutrient ratios (Fat: F; Protein: P; Carbohydrate: C) and assessing the effects on lifespan and heathspan (Solon-Biet et al, 2014). Therefore, there is now the opportunity to consider whether those strategies that delay aging, and in particular macronutrient ratio modulation, might more generally impact brain aging and cognition, and potentially provide novel approaches to preventing age-related neurodegenerative disorders such as dementia.
2. The aging brain
There is a continuum between normal aging and disease in terms of pathological and biochemical changes in many tissues. There are ongoing efforts to differentiate disease from normal brain aging in both qualitative and quantitative terms. Even so, there is general agreement that old age in both animals and humans is associated with changes in brain volume, microscopic morphology, neurotransmitters and various phenotypic measures of cognition and behavior. While such changes may not be severe enough to interfere substantially with activities of daily living, they can reduce performance and increase brain vulnerability to neurodegenerative disorders such as Alzheimer’s disease (AD) and Parkinson’s disease (PD) (Anderton, 2002).
Those features that are usually considered to be associated with aging rather than disease are summarized in Figure 1. In a non-pathological brain, it appears as if the most robust changes that take place are in the medial temporal lobe and prefrontal cortex, with an overall relative decrease in brain volume. The hippocampus and prefrontal cortex experience the most volume loss with age (Anderton, 2002).
Figure 1.
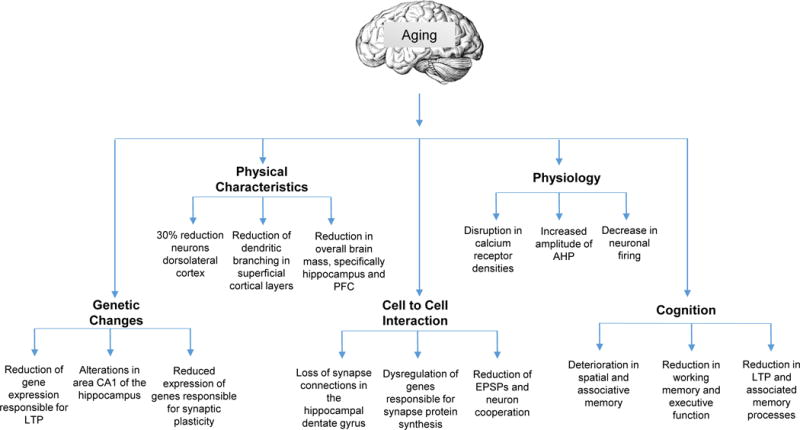
Changes in the brain that occur in aging. Genetic changes, physical characteristics, cell-to-cell interactions, physiological changes, and cognitive differences are included. It has been suggested that these underlying physiological underpinning contribute to the symptoms of cognitive decline. AHP, afterhyperpolarization; EPSPs, excitatory postsynaptic potentials; LTP, long-term potentiation; PFC, prefrontal cortex.
It was previously thought that rapid deterioration in the extent of dendritic branching in apical and basilar dendrites takes place in the hippocampus (Brody, 1955). Now it is known that there is hippocampal and frontal cortex dendritic and synaptic stability when comparing middle aged to old subjects (Hanks and Flood, 1991). However, more robust changes take place in the prefrontal cortex, with reduced dendritic branching in the superficial cortical layer (Grill and Riddle, 2002). Given the critical role of dendritic branching patterns in higher cognitive function processes, this might contribute to the reduction in cognitive function that occurs during the aging process (Cubelos and Nieto, 2010).
Aged neurons in the hippocampus and prefrontal cortex have disruptions in calcium homeostasis, which may be secondary to an increased density of calcium channels (Anderton, 2002; Verkhratsky and Toescu, 1998). After depolarization, the neurons utilize potassium channels to repolarize and there is a brief after-hyperpolarization potential (AHP). The AHP in the hippocampus and prefrontal cortex is increased in amplitude in aged organisms, taking the neuron longer to reset to resting potential (Matthews et al., 2009). This coincides with a decrease in levels of brain derived neurotrophic factor (BDNF) which correlates with age-related cognitive deficits (Navarro-Martinez et al., 2015).
With aging, neuron-to-neuron communication in the hippocampus deteriorates which may be due to a dysregulation of genes that are responsible for synaptic protein synthesis (Ryan et al., 2015). This may partially account for cognitive deficits associated with aging. Indeed, a progressive loss of synapses has been observed in some regions of the aging human brain, with this commencing at about twenty years of age (Terry and Katzman, 2001). This is associated with increased inflammation and oxidative stress during later years (Johnson and Johnson, 2015).
It should be noted that similar pathological features are evident in a normal aging brain when compared to a pathological brain (i.e., neurofibrillary tangles, senile plaques); however, these changes occur at a much faster rate in neurodegenerative conditions (Anderton, 1997). Transcriptome changes that take place in the aging human brain are similar to those that occur in the aging mouse brain, suggesting brain aging changes are conserved (Miller et al., 2010).
Age-related molecular and cellular changes in brain cells coincide with behavioral-cognitive changes, which have been observed in humans, monkeys, and rodents. The hippocampus and prefrontal cortex are heavily involved in spatial memory (Chersi and Burgess, 2015), and it is therefore not surprising that an increase in age coincides with a deterioration in spatial and associative memory (Weber et al., 2015). In addition to the hippocampus, the prefrontal cortex is crucially important for working memory, and is critical for high level cognitive and executive function (Stokes, 2015). It has been suggested that the deterioration of these two structures is largely responsible for the decline seen in cognitive function with age (West, 1996).
3. Unraveling the relationships among calorie restriction, lifespan, healthspan, and cognitive health
Given there are consistent age-related changes in the brain, the question arises whether strategies that delay aging have any impact on brain aging. To date, the main interventions that delay aging are nutritional. The effects of nutrition on aging, health and lifespan have been recognized since the beginning of recorded history. Ancient philosophers and physicians like Galen, Plato, and Hippocrates realized that particular diets are beneficial for health and can add quality years to life (Schafer, 2005). Moreover it was recognized that restricting food intake can be beneficial for both health span and lifespan. Philippus Paracelcus (1493 – 1541), one of the three fathers of Western medicine, claimed that ‘fasting is the greatest remedy’ while the Venetian, Luigi Conaro (1466–1566) wrote ‘renew yourselves and fast’. The first scientific demonstration of the effects of food restriction on aging was by Clive McCay and colleagues in 1935. In this landmark study, Fischer rats were given fewer calories than their ad libitum-fed counterparts in order to delay growth, and they subsequently experienced longer mean and maximum life spans (McCay et al., 1935). This type of nutritional intervention, now called calorie restriction (CR) has been reported to increase lifespan and/or health and healthspan in many species, ranging from yeast to humans and is generally considered to be the primary experimental approach to delay aging in animal models (Everitt, 2010; Fontana and Partridge, 2015; Mercken et al., 2012; Nakagawa et al., 2012; Speakman and Mitchell, 2011).
The mechanisms for the effects of caloric restriction on aging have been extensively studied. The main focus of research has been the nutrient sensing pathways (mTOR, sirtuins, AMPK, IGF1/insulin) which respond to changes in circulating nutrients (amino acids, glucose) or intracellular metabolites that reflect cellular energy supplies (NAD+, AMP). These master switches regulate most of the cellular processes linked with aging including mitochondrial function, autophagy, oxidative stress, gene expression, and intracellular metabolism with downstream tissue-level effects such as inflammation and vascular pathology (Koubova and Guarente, 2003).
There are caveats related to the effects of CR. It has been argued that laboratory diet and conditions allow rodents to become obese (Mattson, 2014), thus accounting for the apparent metabolic benefits of CR. Genetic factors may have an impact on the outcomes of CR. In one meta-analysis (Swindell, 2012) of studies of CR in mice and rats from 1934 to 2011, it was concluded that not only is the effect of CR on lifespan less pronounced in mice compared to rats, but it can also shorten the lifespan in particular mouse strains, thus indicating a strong genetic component. Recently, a CR regimen implemented in Rhesus macaques at the National Institute on Aging (NIA) improved healthspan but did not improve survival outcomes (Mattison et al., 2012). This contrasted the results of a similar study completed at the Wisconsin National Primate Center where lifespan was increased (Colman et al., 2009). Although these two studies led to controversy surrounding the effectiveness of CR, it is likely that their different dietary components contributed to the results. Importantly, the control group of monkeys in the Wisconsin study was fed ad libitum, whereas the control group in the NIA study was fed two portioned meals daily; therefore, the body weights and fat mass of the control Wisconsin monkeys was significantly greater compared to the control NIA monkeys (Mattison et al., 2012).
Due to the positive effects that CR has on healthspan and lifespan, scientists have recently begun to unravel potential mechanisms by which CR positively affects brain health. One of the fundamental theories of how CR contributes to heightened brain function is that the brain may have evolved to function optimally when there is a moderate lack of food and therefore the individual must outwit competitors in the search for nutrients (Mattson, 2015; Mattson, 2002). These challenges produce mild neuronal stress which engages signaling pathways that improve the ability of cells and organs to function optimally, and to resist age-related dysfunction and disease (Mattson, 2010, 2015).
Animal studies have shown that daily CR, intermittent fasting (IF), and alternate day fasting (ADF) not only modify the nutrient sensing pathways in the brain (Figure 2) but also increase synaptic plasticity, neurogenesis and neuroprotection (Fusco and Pani, 2013). CR and IF delay features of aging both on the microscopic and global levels (Pani, 2015). Some of the reported effects of CR and IF on the brain are summarized in Table 1.
Figure 2.
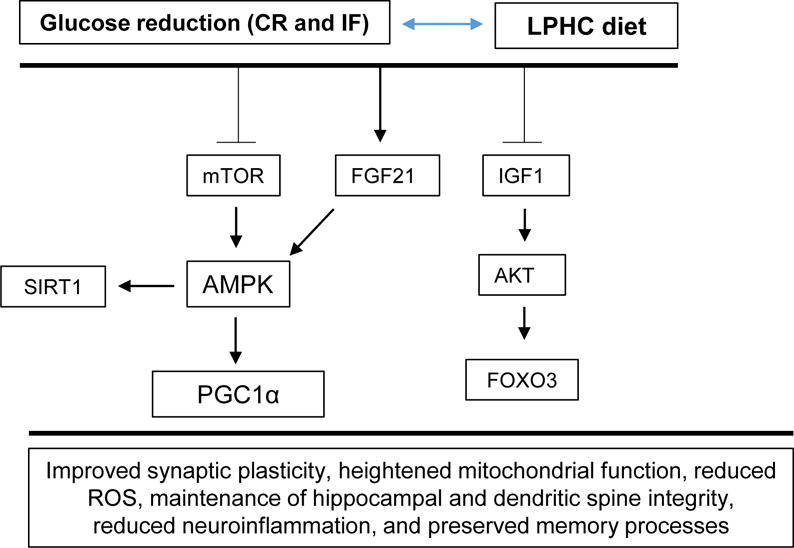
A LPHC diet and its effect on the associated mTOR and related nutrient-sensing pathways. A hypothesis as to how a LPHC diet may affect brain physiology and neuronal function is included. A LPHC diet reduces mTOR activation and increases FGF21 activation, which in turn drives AMPK levels, contributing to the production of beneficial proteins PGC1α and SIRT1. In parallel, reduced IGF-1 levels heighten the sensitivity of IGF-1 receptors to IGF-1 and contribute to the production of AKT, which drives FOXO3 production, leading to improved insulin sensitivity (Ruderman et al., 2010; Willette et al., 2012; Cheng et al., 2012; Solon-Biet et al., 2015a).
Table 1.
Calorie restriction is an effective treatment for many hallmarks of the aging brain, however the effects are still not known or controversial in higher order species.
| Aging brain pathology | Species | Calorie restriction effective treatment? | Reference(s) | Notes |
|---|---|---|---|---|
| Increased mitochondrial Reactive Oxygen Species (ROS) |
Rodents Non-human primates Humans |
✓ Unknown Unknown |
(Hyun et al., 2006) | Only several studies have been published in rodents. |
| Reduction in neurogenesis | Rodents Non-human primates Humans |
✓ Unknown Unknown |
(Kaptan et al., 2015) | Although heavily studies in rodents, still unknown in higher-order species perhaps due to a lack of feasible tissue for biochemical analysis. |
| Slowing of action potentials | Rodents Non-human primates Humans |
Unknown Unknown Unknown |
The one study in rodents was not cognitive and looked at lumbar motor neurons and corresponding hind-limb muscle activity. The authors concluded no CR effect (Kalmar et al., 2009). | |
| Tangles and plaques | Rodents Non-human primates Humans |
✓ Controversial/negative Unknown |
(Halagappa et al., 2007) (Sridharan et al., 2013) |
Although very few studies exist, there are some promising results in rodents. Presumably the lack of evidence in humans is due to the shortage of tissue for biochemical analysis. |
| Inflammation | Rodents Non-human primates Humans |
✓ ✓ ✓ |
(Vasconcelos et al., 2014) (Willette et al., 2013) (Johnson et al., 2007) |
Perhaps this is the most robust CR effect which has been heavily demonstrated in all three species. |
| Reduction in spatial, working, and associative memory |
Rodents Non-human primates Humans |
✓ Limited evidence Controversial |
(Brownlow et al., 2014; Kuhla et al., 2013) (Dal-Pan et al., 2011) (Cheatham et al., 2009) |
The results are controversial in humans mainly due to the differences in the memory tests and vastly different calorie restriction protocols. |
CR and IF may enhance synaptic plasticity through an increase in BDNF with downstream effects on neuronal bioenergetics and protein synthesis (Zaptan et al, 2015). BDNF also plays a crucial role in neural precursor cells (NPC) which reside in the dentate gyrus of the hippocampus where they are critical to the formation of new neurons that integrate into the hippocampal circuitry and play roles in spatial pattern separation, a fundamental domain of learning and memory (Marosi and Mattson, 2014; Vivar and van Praag, 2013). It has been shown that IF increases the survival of newly generated neurons in the dentate gyrus of rats (Mattson et al., 2001). Interestingly, it has also been shown that CR increases levels of the nutrient-sensing protein FOXO3 which increases the production of NPC (Renault et al., 2009). It is knows that CR decreases levels of circulating IGF-1, which is interesting considering IGF-1 plays important roles in neurogenesis and synaptic plasticity. The mechanisms. Whilst a beneficial effect of CR on neurogenesis has been demonstrated in rodents, the results are still uncertain in non-human primates and humans.
Neuroprotective effects are also seen through improved mitochondrial respiratory activity (Cerqueira et al., 2012). This is partly due to the up-regulation of PGC1α, which is a master regulator of mitochondrial respiration and contributes to mitochondrial biogenesis and detoxification (Liang and Ward, 2006). Furthermore, the upregulation of PGC1α modulates the expression of Nitric Oxide (NO) which has antioxidant and protective properties in the endothelium and may contribute to the preservation of brain microvasculature (Borniquel et al., 2006; Bernier et al. 2016). Additionally, PGC1α is critical for the maintenance of dendritic spines in the dentate gyrus of the hippocampus, highlighting its importance in memory functions (Cheng et al., 2012). Finally, it does appear that CR preserves hippocampal grey matter volume in Rhesus macaques (Willette et al., 2013) along with the preservation in grey matter volume of subcortical regions (Colman et al., 2009). There have not yet been studies on grey matter preservation with CR in humans.
Of particular interest has been the effect of CR on a family of proteins known as sirtuins that are implicated in healthspan and longevity (Braidy et al., 2015). CR has been shown to increase the activity of sirtuin 1 (SIRT1) thus resulting in lower inflammatory cytokine activity, and enhanced dendritic outgrowth and plasticity (Maalouf et al., 2009; Ng et al., 2015). SIRT1 is of particular interest because it is highly expressed in the hippocampus. Recently it was shown that SIRT1 knockout mice displayed worse cognitive abilities and spatial learning (Michan et al., 2010). SIRT1 also plays a key role in the reduction of brain inflammation. Activation of SIRT1 via resveratrol administration attenuated microglial inflammation in a mouse cell line, possibly through the modulation of several transcription factors including the pro-inflammatory cytokine NF-κB (Li et al., 2015).
Cognitive improvements have been demonstrated in rodents maintained on CR or IF diets. Numerous studies have shown that CR improves spatial, associative, working, and long-term memory in rodents. This has been demonstrated in a range of cognitive-behavioral tests including the Barnes maze, Morris water maze, and novel object recognition tests (Brownlow et al., 2014; Kuhla et al., 2013). CR enhances dendritic spine density in hippocampal dentate granule neurons which is associated with increased levels of BDNF in the hippocampus (Stranahan et al., 2009). Additionally, it was recently shown that 30% CR did improve some aspects of working memory in non-human primates but only on certain tasks (Dal-Pan et al., 2011). In humans the effects of CR on cognition and memory have thus far yielded mixed results. For example, Martin et al. suggested that a 6 month, 25% CR regiment produced no beneficial effect on verbal and visual memory in humans (Martin et al., 2007), whilst Witte et al. showed that a 3 month 30% CR program did improve measures of verbal memory in elderly patients (Witte et al., 2009).
ADF, the most commonly studied type of IF in rodents, has been reported to improve glucose metabolism and cardiovascular risk factors, and can protect cells against oxidative and metabolic stress (Castello et al., 2010; Longo and Mattson, 2014). For example, ADF reduces resting heart rate and blood pressure, increases heart rate variability, and improves cardiovascular stress adaptation in rats (Wan et al., 2003; Mager et al., 2006). In addition, the hearts of rats and mice maintained on an ADF diet exhibit resistance to ischemia-reperfusion injury in a model of myocardial infarction (Ahmet et al., 2005; Godar et al., 2014). IF also increases the resistance of neurons to dysfunction and degeneration in experimental models of a broad range of neurological disorders including: a focal ischemia-reperfusion model of stroke in rats and mice (Yu and Mattson, 1999; Arumugam et al., 2010); an excitotoxin model of epilepsy in rats (Bruce-Keller et al., 1999); mitochondrial toxin- and genetic mutation-based rat and mouse models of Huntington’s disease (Bruce-Keller et al., 1999; Duan et al., 2003); a mouse model of Alzheimer’s disease (Halagappa et al., 2007); a mitochondrial toxin- and genetic mutation-based mouse models of Parkinson’s disease (Duan and Mattson, 1999; Griffioen et al., 2010); and a rat model of bacterial sepsis-induced neuroinflammation (Vasconcelos et al., 2014). The cellular and molecular mechanisms by which ADF may counteract aging processes and protect against age-related disease are being elucidated and involve stimulation of the production of neurotrophic factors and antioxidant enzymes, enhancement of mitochondrial function and autophagy, and suppression of neuroinflammation (Mattson, 2012). Recent studies of ADF and two days/week fasting in human subjects suggest that IF also improves multiple health indicators in humans (Johnson et al., 2007; Varady and Hellerstein, 2007; Harvie et al., 2010).
4. Macronutrients, aging and the aging brain
There is minimal translational opportunity for daily CR for most humans who have essentially unlimited access to food and cannot sustain the rigors of voluntary CR. While some IF eating patterns are more readily incorporated into modern lifestyles (Mattson et al., 2015), most successful clinical trials have focused on obese or above-average body mass index (BMI) populations (reviewed in Seimon et al., 2015). It is also important to understand the effects of various dietary components on aging and disease risk (Sohal and Forster, 2014). Such studies have tended to focus on altering individual macronutrients in the diet (P; C; and F) although more recent studies have examined the effects of the interactions between macronutrients, i.e., addressing the question of what is the optimum balance of macronutrients for lifespan in ad libitum fed animals. It should be noted that without appropriate controls, which are seldom implemented, CR cannot differentiate between the effects of reduced calorie intake versus reduced intake of each of the macronutrients. Additionally, many CR studies are designed to give the animals less food, and therefore are potentially confounded by the effects of IF (Simpson et al., 2015). Intermittent fasting regimes seem to generate similar benefits as CR yet do not necessarily involve reduced calorie intake over longer periods of time because of compensatory periods of overeating. This suggests that IF and even periods of hunger might be important for the effects of CR on aging.
In addition to studies of CR and the aging brain, there has been a considerable amount of research on the effects of micronutrients and pharmaceutical agents on age-related changes cognitive function and dementia in an attempt to find a single therapeutic agent (Barnes et al., 2014; Granzotto and Zatta, 2014; Meramat et al., 2015). However, the role of macronutrients in brain health and aging has more recently been investigated. Many studies have examined the relationship between macronutrients and cognitive function (Table 2). Most of these studies in lower organisms have focused on chronic high-fat diets which exacerbate cognitive decline with aging (Beilharz et al., 2015). The roles that the macronutrients play in basic aspects of brain health and function have been relatively well established (Figure 3) (Jones et al., 2012; Solfrizzi et al., 2005; Solfrizzi et al., 2003a, b; Yon et al., 2013) and are described below. An understanding of the role of each of the macronutrients on brain function is important when designing a diet with a balance of macronutrients that optimizes brain health. It is not possible to alter the intake of a single macronutrient without influencing the intake of the other two macronutrients or the ratios between the macronutrients so it is likely that the optimum balance of macronutrients for healthy brain aging in an ad libitum fed diet will necessitate some trade offs between the beneficial and/or harmful effects of each macronutrient.
Table 2.
A selection of clinical trials which looked at individual macronutrient contributions to brain fitness and cognitive function.
| Reference and year | Year | Subjects and methods | Nutritional status or intervention | Diet results on cognitive function |
|---|---|---|---|---|
| (Gibson et al., 2013) | 2013 | Thirty-eight women, mean age 32.7±5.3 years; BMI 24.9±4.5kg/m2. Verbal recognition tasks, delayed matching to sample, and paired associate learning tasks were carried out |
Average daily macronutrient intake was assessed via a seven-day food diary. Mean protein (16.3±2.8%); carbohydrate (44.0±5.8%); fat (39.7±6.5%). | Highest fat intake was associated with poorer memory function in all three cognitive tests. |
| (Holloway et al., 2011) | 2011 | Sixteen men, mean age 22±1 years. Normal BMI; Double blind, cross over procedure after a 2-week washout period. The Cognitive Drug Research (CDR) computerized assessment battery was performed daily to measure attention, working memory, episodic memory, and self-reported mood and alertness were performed shortly after consumption. | Five days of either a high fat (70%), low carbohydrate (26%) diet or low fat (24%), high (50%) carbohydrate diet. | Cognitive testing showed impaired speed, attention, and mood after consumption of the high fat diet. |
| (Jones et al., 2012) | 2012 | Eighteen (5 males, 13 females), mean BMI 21.1kg/m2, mean age 19 years. Blind, placebo controlled, wash out, repeated measures design. Tests for spatial working memory, numeric working memory, spatial recall, and immediate word recall were performed shortly after consumption. | Either a 40 g protein in solution, 16 g fat emulsion, 40 g glucose solution, or an inert placebo over four days, with a 5 – 7 day wash out period. | In general, glucose and fat had had the fastest acting effects on memory, whereas protein ingestion had beneficial effects at later time-points. The authors suggested a complex interaction between macronutrients, with each affecting different memory systems. |
| (Holloway et al., 2011) | 2011 | Sixteen men, mean age 22±1 year. Normal BMI; Double blind, cross over procedure after a 2-week washout period. The Cognitive Drug Research (CDR) computerized assessment battery was performed daily to measure attention, working memory, episodic memory, and self-reported mood and alertness were performed shortly after consumption. | Five days of either a high fat (70%), low carbohydrate (26%) diet or low fat (24%), high (50%) carbohydrate diet. | Cognitive testing showed impaired speed, attention, and mood after consumption of the high fat diet. |
| (Jones et al., 2012) | 2012 | Eighteen (5 males, 13 females), mean BMI 21.1 kg/m2, mean age 19 years. Blind, placebo controlled, wash out, repeated measures design. Tests for spatial working memory, numeric working memory, spatial recall, and immediate word recall were performed shortly after consumption. | Either a 40 g protein in solution, 16 g fat emulsion, 40 g glucose solution, or an inert placebo over four days, with a 5 – 7 day wash out period. | In general, glucose and fat had had the fastest acting effects on memory, whereas protein ingestion had beneficial effects at later time-points. The authors suggested a complex interaction between macronutrients, with each affecting different memory systems. |
| (Roberts et al., 2012) | 2012 | 937 (51% male) participants. Mean BMI 27.7 kg/m2. Participants were instructed to record their dietary intake from the past 12 months in a food diary. Cognitive examination took place at 15 month intervals. | As a group, subjects consumed 232 g carbohydrate per day, 78 g protein/day, and 61g fat per day. | High daily carbohydrate percentages were associated with a higher risk of mild cognitive impairment, while higher fat and protein consumption was associated with lower risk of mild cognitive impairment. |
| (Krikorian et al., 2012) | 2012 | 23 participants (10 men, 13 women) with mild cognitive impairment as per Clinical Dementia Rating (CDR) assessment. Mean age 70.1±6.2 years. Subjects completed a 7-day diet diary prior to intervention. Trial Making Test Part B and Verbal Paired Associate Learning Test were used to assess executive ability, long term memory, and mood before and after dietary intervention. | High carbohydrate (50% of energy) and very low carbohydrate (5–10% of energy) diets with no restriction on total energy, protein or fat intake. | Very low percentage of carbohydrate consumption was associated with improved memory performance. |
| (D’Anci et al., 2009) | 2009 | 19 women, aged 22–55 years; repeated measures mixed-factor design. Profile of Mood States (POM) Questionnaire, five computer-based cognitive tasks assessing visuospatial memory, vigilance attention (CPT) with a concurrent secondary task, digit span (forward and backward), and both positive and negative consequences of food preoccupation were performed | Low-carbohydrate (0 g/day 1st week, 5 – 8 g/day 2nd week, 10–16 g/day 3rd week) vs. a low-calorie, macronutrient balanced diet (as per American Dietetic Association) | Low-carbohydrate was associated with less confusion (POMS) and faster response during an attention vigilance task (CPT) than low-calorie, macronutrient balanced dieters. |
| (Jakobsen et al., 2011) | 2011 | 23 healthy males aged 19–31 years. Randomised, single blinded, parallel intervention study. Addenbrooke Cognitive Examination, Tests for Attentional Performance. | Usual Protein (UP) diet (1.5 g protein/kg BW) for a 1-wk run-pre-intervention then UP or a High Protein (HP) diet (3.0 g protein/kg BW) for 3-wks with controlled intake of food and beverages. | High protein diet improved reaction time. |
Figure 3.
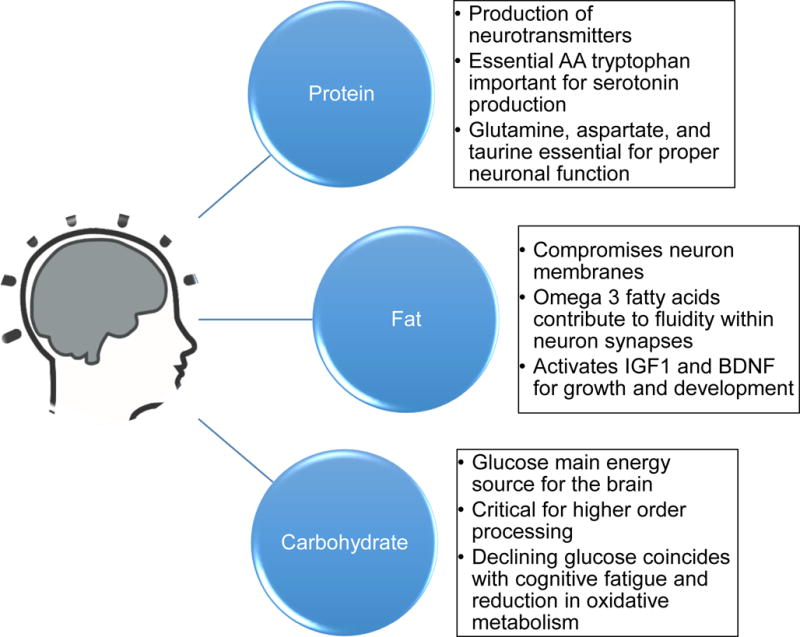
A brief description of the contribution of individual macronutrients to brain health and cognitive function. Whilst the individual contributions have been relatively well-established, what remains is elusive is their proper ratios in order to maintain maximal brain fitness in late life.
4.1 Protein and amino acids
One of the important roles of dietary protein is to provide a supply of amino acids for the production of neurotransmitters, such as catecholamines and serotonin (Bourre, 2006). Of particular interest is the essential amino acid tryptophan, which is a precursor to serotonin, a neurotransmitter involved in mood, information processing and cognitive function. For example, bioavailable tryptophan dietary supplements improved cognition in healthy middle-aged women (Mohajeri et al., 2015). Serotonin cannot cross the blood-brain barrier, however tryptophan crosses via specialized channels and is converted to 5- hydroxyl-tryptophan (5-HTP), which then undergoes further conversion to serotonin (Shabbir et al., 2013). Serotonin levels may decrease with age, possibly contributing to detrimental effects on cognitive function (Bourre, 2006; Melancon et al., 2014). In addition to serotonin other dietary-derived neurotransmitters such as glutamate, aspartate, and taurine are shuttled from surrounding astrocytes to neurons (Gundersen et al., 2015) and potentially could be influenced by dietary amino acids.
4.2 Fats
Dietary fat is an important macronutrient for the brain because it is necessary for the structure of neuronal membranes (Bourre, 2006). All neurons and organelles in the brain are rich in polyunsaturated omega 3 fatty acids (PUFA), which are of dietary origin. Of particular interest have been the omega-3 fatty acids, which make up about 30% of the membrane phospholipid composition in the brain. Whilst the majority of studies on rodents have found detrimental cognitive effects of a long-term high fat diet, a recent study reported that chronic high-fat diet treatment did not affect spatial memory in mice (Kesby et al., 2015). Nonetheless, the omega-3 fatty acid docosahexaenoic acid (DHA) has generated considerable interest due to its role in providing membrane fluidity and synaptic integrity, which in turn can affect cognitive function (Sidhu et al., 2016). It also activates neuronal cascades that affect BDNF and insulin-like growth factor 1 (IGF-1), which further activates signaling cascades at the pre- and post-synaptic levels, thereby affecting long-term potentiation (LTP) and associated memory processes (Gomez-Pinilla, 2008). These effects have been shown in clinical studies, where MRI has confirmed that omega-3 fatty acids can contribute to brain protection during aging (Denis et al., 2015).
4.3 Carbohydrates
Adequate carbohydrate consumption is essential for brain function, because glucose is the main energy source for the brain, and the brain requires about 25% of the total glucose energy consumed despite only compromising 2% of total body weight (Sokoloff, 1999). Measures of prefrontal cortex function increase transiently in response to glucose consumption (Kumar et al., 2015). Similarly, in aged rodent models complex cognitive behavioral tasks are rapidly improved post-glucose injection (Gold, 2005). On the other hand, it has been suggested that a chronic excess of glucose consumption can contribute to reduced synaptic plasticity and high levels of inflammation, which may contribute to cognitive deficits (Stefanidis and Watt, 2012). Interestingly, cognitive fatigue is common in older adults, which has been suggested to be partly due to a decline in neuronal glucose utilization with age (Galeffi et al., 2015), possibly as the result of reduced brain sensitivity to insulin (Ryu et al., 2014).
5. Anti-aging compounds, the aging brain and dementia
Recently it has been established that a number of compounds can activate or inhibit the nutrient sensing pathways that link diet with aging, and thereby potentially delay aging. Three examples are metformin, rapamycin, and resveratrol. Each of these drugs act via well-established nutrient sensing pathways (Maiese, 2015; Novelle et al., 2015; Pryor and Cabreiro, 2015). For example, the AMPK pathway has received attention as a target for delaying aging because of the availability of the AMPK agonist, metformin (Anisimov, 2013). Metformin has been shown to be a caloric restriction mimetic that prolongs life in various laboratory animals including mice (Martin-Montalvo et al., 2013). Metformin is also widely prescribed for the treatment of type II diabetes. A recent observational study found that diabetics who use metformin have lower mortality than age-matched non-diabetic controls suggesting that its anti-aging effects might also be apparent in humans (Bannister et al., 2014). Metformin has been promoted as a potential anti-dementia treatment based on its metabolic effects. Metformin has been reported to ameliorate cognitive impairment in some mouse models of AD (Li et al., 2012). Observational studies of metformin in diabetic humans, who have a very high risk of dementia as a result of their metabolic dysregulation, have yielded conflicting results with one study showing increased risk of cognitive impairment in diabetics taking metformin (Moore et al., 2013) while other studies have shown reduced risk (Ng et al., 2014). This might reflect different treatment doses, such that overtreatment might also increase the risk of dementia as the result of repeated episodes of hypoglycemia.
Rapamycin inhibits mTOR which plays a central role in signaling downstream pathways that control cell growth and proliferation and is heavily over-activated in certain cancers (Duzgun et al., 2015). Inhibition of mTOR activity with rapamycin is for the treatment of some cancers (Meng and Zheng, 2015) and as an immunosuppressant. Rapamycins increased lifespan in mice, including those mice fed standard diets and commenced on therapy in middle and late life (Harrison et al., 2009; Miller et al., 2011), and its role as a treatment of aging is being tested for feasibility and safety in older humans (Leslie, 2013). Intermittent dosing might be appropriate for long term aging therapy because this should reduce toxicity (Longo and Fontana, 2011). In AD patients, mTOR signaling is upregulated in the brain and correlates with disease severity (Sun et al., 2014) while down-regulation of mTOR or its inhibition with rapamycin can ameliorate pathology and cognitive impairment in mouse models of AD (Caccamo et al., 2014; Lin et al., 2013; Spilman et al., 2010).
Resveratrol, which is an allosteric activator of SIRT1, has the most beneficial effects under high fat diet conditions, where SIRT1 activity is greatly reduced (Baur et al., 2006; reviewed in Novelle et al. 2015). Some studies in humans have shown improvements in cardiometabolic function with resveratrol (Magyar et al., 2012; Poulsen et al., 2012; Timmers et al., 2012). It has also been shown that resveratrol reverses the phenotype, especially the metabolic changes, in transgenic progeria mice (Labbé et al., 2011) and frailty in old mice (Kane et al., 2016). The role of SIRT1 has also been studied in animal models of AD (Donmez, 2012); for example SIRT1 has recently been shown to mediate effects of metabolic stress on β-secretase (Wang et al., 2013). Overexpression of SIRT1 (Kim et al., 2007; Lalla and Donmez, 2013) and activation of SIRT1 with resveratrol (Porquet et al., 2013; Porquet et al., 2014) can prevent or ameliorate cognitive impairment in experimental models of AD and age-related dementia. Moreover, it was shown that two-year resveratrol supplementation ameliorated neuroinflammation and increased cerebral microvasculature density in Rhesus macaques on a high fat diet (Bernier et al. 2016).
Together these results suggest that rapamycin, resveratrol and metformin, which are agents that delay aging by modulating the nutrient sensing pathways, may also have promise as agents to delay brain aging and forestall the onset of dementia.
6. Linking dementia and aging
The observation that dietary and medical interventions that delay aging also delay brain aging and dementia suggests that there is evidence that dementia is part of the aging process, perhaps analogous to sarcopenia and immunosenescence. The prevalence of dementia under the age of 65 years is just 0.1% but this increases 300-fold beyond the age of 85 years, while the majority of people older than 90 years are diagnosed with dementia (Australian Institute of Health and Welfare, 2012; Yang et al., 2013). The powerful influence of aging on the risk of dementia in comparison to other risk factors is demonstrated by the ANU-ADRI scale. Simply living to the age of 85 years confers the same risk for AD as having every other known genetic (i.e., apolipoprotein ε4 allele) and environmental risk factor combined (Anstey et al., 2013). Although aging is a risk factor for many diseases (Sierra, 2016), such overwhelming age-dominance is unusual, perhaps shared only with the other as yet untreatable geriatric conditions of sarcopenia and frailty. Therefore, while dementia research has tended to focus on the role of genetic and modifiable risk factors for dementia, it has placed less emphasis on the role of the aging process in the pathogenesis of dementia and the possibility that the aging process is modifiable.
The influence of old age on dementia outweighs all other biomarkers, genes and risk factors (Brayne and Davis, 2012), and the heavily studied amyloid-based biomarkers become less accurate in older people (Mattsson et al., 2012). Numerous clinical trials targeting amyloid have not shown any effect on, and even worsened, cognitive outcomes (Schneider et al., 2014). This has led some people to question the validity and usefulness of the amyloid cascade hypothesis, which has been the major area of study during the past two decades (Drachman, 2014; Krstic and Knuesel, 2013).
From the clinical perspective it is of note that most chronic disorders that occur in older people – which may have a single etiopathogenesis in younger people – have multifactorial causes on a background of accruing cellular and tissue damage with aging. Thus it is not surprising that Alzheimer’s and other forms of dementia share similar trajectories to other disorders and diseases associated with old age in humans. The effects of age on mortality and AD are similar, with a doubling of mortality every 7.5 years from the age of 30, versus a doubling of dementia every 5 years (Raber et al., 2004). Dementia is also closely associated with multi-morbidity and frailty (Oosterveld et al., 2014), which are key clinical manifestations of old age.
Likewise, from the biological perspective it is clear that many of the major cellular and molecular changes of aging (such as characteristic changes in oxidative stress, mitochondrial function, DNA damage, telomere shortening, advanced glycation end products, autophagy) and major systemic changes (microvascular disease, inflammation have been documented in AD and dementia more broadly (de Cabo and Le Couteur, 2015) (Table 3). All of these processes have been linked individually and separately to dementia. In many cases it has been postulated that they contribute to disease causation.
Table 3.
Many of the common molecular hallmarks of aging are also present in dementia.
| Cellular process | Aging | Dementia reference |
|---|---|---|
| Increased oxidative stress | ✓(Finkel and Holbrook, 2000) | ✓(Wang et al., 2014) |
| Mitochondrial dysfunction | ✓(Balaban et al., 2005) | ✓(Ferrer, 2009) |
| mtDNA mutations | ✓(Piko et al., 1988) | ✓(Wang et al., 2014) |
| Shortened telomeres | ✓(Honig et al., 2012) | ✓(Honig et al., 2012) |
| Decreased autophagy | ✓(Tan et al., 2014) | ✓(Tan et al., 2014) |
| Microvascular abnormalities | ✓(Le Couteur and Lakatta, 2010) | ✓(Costanza et al., 2012) |
| Inflammation | ✓(Franceschi and Campisi, 2014) | ✓(Bettcher and Kramer, 2014) |
| Advanced glycation endproducts | ✓(Baynes, 2001) | ✓(Srikanth et al., 2013) |
Although the link between old age and dementia is overwhelming, there have been only a few studies about how the biological changes of aging might influence or even cause dementia. On the other hand, there has been a revolution in understanding the biology of aging in the last decade and this has led to the identification of pharmaceutical, genetic and nutritional interventions that delay aging and multiple age-associated pathologies in a range of species (Fontana et al., 2014). If dementias of all types are multifactorial manifestations of aging and not classical diseases with a discrete pathogenesis, then the therapeutic interventions being developed to act on the aging process could be harnessed and/or exploited for the treatment and prevention of dementia. This opens up a range of novel therapies and drug targets for dementia.
7. The Geometric Framework, diet and cognition
Whilst the contribution of individual macronutrients to cognitive fitness has been intensively studied, it is likely that the brain requires a finely tuned balance of macronutrients and micronutrients in order to function properly (Mallidou and Cartie, 2015). It has been shown that changes in diet, even in late life, can have a positive impact on cognition and possibly attenuate dementia (Hardman et al., 2015). An example of this is the Mediterranean dietary pattern. This dietary pattern is mainly followed by people living in the Mediterranean crescent and is largely composed of fresh fish and shellfish, red wine, breads, pasta, fruits, vegetables, and olive oil (Castro-Quezada, Román-Viñas, & Serra-Majem, 2014; Yannakoulia, Kontogianni, & Scarmeas, 2015). People who follow a Mediterranean dietary pattern have improved cognitive function during the transition to old age (Huhn, Kharabian Masouleh, Stumvoll, Villringer, & Witte, 2015; Lourida et al., 2013; Woodside, Gallagher, Neville, & McKinley, 2014). Another intervention that has been successful in delaying some symptoms of cognitive decline was the Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER) which combined exercise, cognitive training and diet with defined percentages of fat, carbohydrate and protein, and little sugar (<10%E) (Kivipelto et al., 2013). This multimodal approach was successful in reducing cognitive decline in a cohort of elderly patients (Ngandu et al., 2015). Although these two dietary approaches share some common features such as high intake of fruits, vegetables and wholegrain cereals with limited intake of added sugars, there are also some differences. For example, the FINGER diet is a well-defined diet based on the Finish Nutritional Recommendations and was developed based on a stringent set of specific nutritional guidelines (National Nutrition Council, 2005). On the other hand, the Mediterranean dietary pattern is not as strict in nutritional guidelines and allows for some leniency in food intake (Woodside et al., 2015). Another difference is the source of dietary lipids (rapeseed oil and vegetable margarine in the FINGER diet vs. olive oil and nuts in the Mediterranean diet). Followers of the Mediterranean dietary pattern consume high amounts of legumes which affects the ratio of animal to plant protein as well as the amounts of fibre and phytates in the diet. Contrary to the FINGER diet, moderate consumption of red wine – a moderate source of resveratrol – is recommended in the Mediterranean diet (Huhn et al., 2015).
When looking at the role of macronutrient consumption on aging, one of the main human populations studied in recent years has been the Okinawan people, who enjoy the longest lifespan in the world (Japan Ministry of Health, 2000). There is some evidence that their healthspan and cognitive function is also preserved later in life when compared to other similar populations (Willcox et al., 2014). Whilst there are varying factors involved in this lifespan extension including lifestyle choices and physical activity, perhaps one major factor is their diet, which is 9 percent protein, 85 percent carbohydrate, and very little fat (Reviewed in Le Couteur et al, 2016). Interestingly, these percentages are almost identical to the macronutrient ratios found to increase lifespan and healthspan in mice and insect models, even when compared to CR (Solon-Biet et al, 2014; Le Couteur et al, 2016). These results along with the Mediterranean dietary pattern and the FINGER diet study suggest that many components of the diet (and lifestyle) influence brain aging and that experimental approaches that target individual components of the diet maybe be overly simplistic. It should also be noted that these interventions seem to reduce brain aging as part of a broader outcome which could be termed ‘healthy aging’.
How can the complexity of diet be disentangled experimentally? One approach is the Geometric Framework (GF). GF provides a multidimensional approach that facilitates interpretation of how macronutrients and energy impact on outcomes such as lifespan, age-related phenotypes, and cognitive function (Simpson and Raubenheimer, 2007; Raubenheimer et al. 2016). Responses of individuals, such as cognitive behavior, are phenotypic features that can be superimposed on the experimental nutritional space by plotting these as response surfaces. In a recent study on aging of mice, the GF was used to analyze lifespan and age-related health in animals fed one of 25 diets varying in protein, carbohydrates, fat and total energy content (Solon-Biet et al., 2014). A major conclusion of the latter study is that LPHC diets optimize healthspan and lifespan. LPHC diets were found to inhibit hepatic mTOR, providing a mechanism linking this type of ad libitum diet to delayed aging. GF was utilized to unravel the complex effects of macronutrients on metabolic health, lifespan (Solon-Biet et al., 2014), immunity (Le Couteur et al., 2015), reproduction (Solon-Biet et al., 2015b) and microcirculation (Cogger et al., 2016). GF methodology is therefore likely to provide insight into how dietary energy and macronutrients influence brain aging and dementia.
8. Conclusion
Dietary factors, including energy intake and macronutrient composition, influence brain function, especially during the transition to later life. The study and understanding of aging biology has led to the identification of pharmaceutical and nutritional interventions that delay aging and multiple age-associated pathologies. Therefore, it is plausible that therapeutic interventions that delay the aging process could be used for the treatment and prevention of brain aging and dementia. Whilst the roles of individual macronutrients in the brain have been investigated, interactions among and between macronutrients and micronutrients, with regards to their effects on brain aging are unknown. Diet is complex and this complexity can be disentangled using novel approaches such as the GF, thereby informing future research and nutritional guidelines for the delay of brain aging and dementia.
Acknowledgments
Funding was provided from the Aging and Alzheimers Research Institute, National Health and Research Council of Australia (NHMRC) grants #571328 and #1084267 and our co-authors in studies cited in this review. RdC and MPM are funded by the Intramural Program of the National Institute on Aging, NIH. SMS-B is supported by the NHMRC Peter Doherty Early Career Fellowship (Grant 1110098).
References
- Ahmet I, Wan R, Mattson MP, Lakatta EG, Talan M. Cardioprotection by intermittent fasting in rats. Circulation. 2005;112:3115–3121. doi: 10.1161/CIRCULATIONAHA.105.563817. [DOI] [PubMed] [Google Scholar]
- Anderton BH. Changes in the ageing brain in health and disease. Phil T Roy Soc B. 1997;352:1781–1792. doi: 10.1098/rstb.1997.0162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderton BH. Ageing of the brain. Mech Ageing Dev. 2002;123:811–817. doi: 10.1016/s0047-6374(01)00426-2. [DOI] [PubMed] [Google Scholar]
- Anisimov VN. Metformin: do we finally have an anti-aging drug? Cell Cycle. 2013;12:3483–3489. doi: 10.4161/cc.26928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anstey KJ, Cherbuin N, Herath PM. Development of a new method for assessing global risk of Alzheimer’s disease for use in population health approaches to prevention. Prev Sci. 2013;14:411–421. doi: 10.1007/s11121-012-0313-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Australian Institute of Health and Welfare. Dementia in Australia Cat no AGE 70. Canberra: AIHW; 2012. [Google Scholar]
- Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120:483–495. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Bannister CA, Holden SE, Jenkins-Jones S, Morgan CL, Halcox JP, Schernthaner G, Mukherjee J, Currie CJ. Can people with type 2 diabetes live longer than those without? A comparison of mortality in people initiated with metformin or sulphonylurea monotherapy and matched, non-diabetic controls. Diabetes Obes Metab. 2014;16:1165–1173. doi: 10.1111/dom.12354. [DOI] [PubMed] [Google Scholar]
- Barnes JL, Tian M, Edens NK, Morris MC. Consideration of nutrient levels in studies of cognitive decline. Nutr Rev. 2014;72:707–719. doi: 10.1111/nure.12144. [DOI] [PubMed] [Google Scholar]
- Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Le Couteur D, Shaw RJ, Navas P, Puigserver P, Ingram DK, de Cabo R, Sinclair DA. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baynes JW. The role of AGEs in aging: causation or correlation. Exp Gerontol. 2001;36:1527–1537. doi: 10.1016/s0531-5565(01)00138-3. [DOI] [PubMed] [Google Scholar]
- Beilharz JE, Maniam J, Morris MJ. Diet-induced cognitive deficits: The role of fat and sugar, potential mechanisms and nutritional interventions. Nutrients. 2015;7:6719–6738. doi: 10.3390/nu7085307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernier M, Wahl D, Ali A, Allard J, Faulkner S, Wnorowski A, Sanghvi M, Moaddel R, Alfaras I, Mattison JA, Tarantini S, Tucsek A, Ungvari Z, Csiszar A, Pearson KJ, de Cabo R. Resveratrol supplementation confers neuroprotection in cortical brain tissue of nonhuman primates fed a high-fat/sucrose diet. Aging (Albany NY) 2016;8:899–916. doi: 10.18632/aging.100942. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettcher BM, Kramer JH. Longitudinal inflammation, cognitive decline, and Alzheimer’s disease: A mini-review. Clin Pharmacol Ther. 2014;96:464–469. doi: 10.1038/clpt.2014.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borniquel S, Valle I, Cadenas S, Lamas S, Monsalve M. Nitric oxide regulates mitochondrial oxidative stress protection via the transcriptional coactivator PGC-1 alpha. FASEB J. 2006;20:1889–1891. doi: 10.1096/fj.05-5189fje. [DOI] [PubMed] [Google Scholar]
- Bourre JM. Effects of nutrients (in food) on the structure and function of the nervous system: Update on dietary requirements for brain. Part 2: Macronutrients. J Nutr Health Aging. 2006;10:386–399. [PubMed] [Google Scholar]
- Braidy N, Poljak A, Grant R, Jayasena T, Mansour H, Chan-Ling T, Smythe G, Sachdev P, Guillemin GJ. Differential expression of sirtuins in the aging rat brain. Front Cell Neurosci. 2015;9:167. doi: 10.3389/fncel.2015.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brayne C, Davis D. Making Alzheimer’s and dementia research fit for populations. Lancet. 2012;380:1441–1443. doi: 10.1016/S0140-6736(12)61803-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody H. Organization of the Cerebral Cortex .3. A Study of Aging in the Human Cerebral Cortex. J Comp Neurol. 1955;102:511–556. doi: 10.1002/cne.901020206. [DOI] [PubMed] [Google Scholar]
- Brownlow ML, Joly-Amado A, Azam S, Elza M, Selenica ML, Pappas C, Small B, Engelman R, Gordon MN, Morgan D. Partial rescue of memory deficits induced by calorie restriction in a mouse model of tau deposition. Behav Brain Res. 2014;271:79–88. doi: 10.1016/j.bbr.2014.06.001. [DOI] [PubMed] [Google Scholar]
- Caccamo A, De Pinto V, Messina A, Branca C, Oddo S. Genetic reduction of mammalian target of rapamycin ameliorates Alzheimer’s disease-like cognitive and pathological deficits by restoring hippocampal gene expression signature. J Neurosci. 2014;34:7988–7998. doi: 10.1523/JNEUROSCI.0777-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castello L, Froio T, Maina M, Cavallini G, Biasi F, Leonarduzzi G, Donati A, Berhamini E, Poli G, Chiarpotto E. Alternate-day fasting protects the rat heart against age-induced inflammation and fibrosis by inhibiting oxidative damage and NF-kβ activation. Free Rad Biol Med. 2010;48:47–54. doi: 10.1016/j.freeradbiomed.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Castro-Quezada I, Román-Viñas B, Serra-Majem L. The mediterranean diet and nutritional adequacy: A review. Nutrients. 2014;6:231. doi: 10.3390/nu6010231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerqueira FM, Cunha FM, Laurindo FRM, Kowaltowski AJ. Calorie restriction increases cerebral mitochondrial respiratory capacity in a NO-mediated mechanism: Imact on neuronal survival. Free Rad Biol Med. 2012;52:1236–1241. doi: 10.1016/j.freeradbiomed.2012.01.011. [DOI] [PubMed] [Google Scholar]
- Cheatham RA, Roberts SB, Das SK, Gilhooly CH, Golden JK, Hyatt R, Lerner D, Saltzman E, Lieberman HR. Long-term effects of provided low and high glycemic load low energy diets on mood and cognition. Physiol Behav. 2009;98:374–379. doi: 10.1016/j.physbeh.2009.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng A, Wan R, Yang JL, Kamimura N, Son TG, Ouyang X, Luo Y, Okun E, Mattson MP. Involvement of PGC-1alpha in the formation and maintenance of neuronal dendritic spines. Nat Commun. 2012;3:1250. doi: 10.1038/ncomms2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chersi F, Burgess N. The Cognitive Architecture of Spatial Navigation: Hippocampal and Striatal Contributions. Neuron. 2015;88:64–77. doi: 10.1016/j.neuron.2015.09.021. [DOI] [PubMed] [Google Scholar]
- Cogger VC, Mohamad M, Solon-Biet SM, Senior AM, Warren A, O’Reilly JN, Tung BT, Svistounov D, McMahon AC, Fraser R, Raubenheimer D, Holmes AJ, Simpson SJ, Le Couteur DG. Dietary macronutrients and the aging liver sinusoidal endothelial cell. Am J Physiol Heart Circ Physiol. 2016;310:H1064–H1070. doi: 10.1152/ajpheart.00949.2015. [DOI] [PubMed] [Google Scholar]
- Colman RJ, Anderson RM, Johnson SC, Kastman EK, Kosmatka KJ, Beasley TM, Allison DB, Cruzen C, Simmons HA, Kemnitz JW, Weindruch R. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 2009;325:201–204. doi: 10.1126/science.1173635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costanza A, Xekardaki A, Kovari E, Gold G, Bouras C, Giannakopoulos P. Microvascular burden and Alzheimer-type lesions across the age spectrum. J Alzheimers Dis. 2012;32:643–652. doi: 10.3233/JAD-2012-120835. [DOI] [PubMed] [Google Scholar]
- Cubelos B, Nieto M. Intrinsic programs regulating dendrites and synapses in the upper layer neurons of the cortex. Commun Integr Biol. 2010;3:483–486. doi: 10.4161/cib.3.6.12755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dal-Pan A, Pifferi F, Marchal J, Picq JL, Aujard F. Cognitive performances are selectively enhanced during chronic caloric restriction or resveratrol supplementation in a primate. PLoS One. 2011;6:e16581. doi: 10.1371/journal.pone.0016581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Cabo R, Le Couteur DG. The Biology of Ageing. In: Longo DL, Fauci A, Kasper D, Hauser S, Jameson JL, Loscalzo J, editors. Harrisons Principles of Internal Medicine. 19th 2015. [Google Scholar]
- Denis I, Potier B, Heberden C, Vancassel S. Omega-3 polyunsaturated fatty acids and brain aging. Curr Opin Clin Nutr Metab Care. 2015;18:139–146. doi: 10.1097/MCO.0000000000000141. [DOI] [PubMed] [Google Scholar]
- D’Anci KE, Watts KL, Kanarek RB, Taylor HA. Low-carbohydrate weight-loss diets. Effects on cognition and mood. Appetite. 2009;52:96–103. doi: 10.1016/j.appet.2008.08.009. [DOI] [PubMed] [Google Scholar]
- Donmez G. The Effects of SIRT1 on Alzheimer’s Disease Models. Int J Alzheimers Dis. 2012;2012:509529. doi: 10.1155/2012/509529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drachman DA. The amyloid hypothesis, time to move on: Amyloid is the downstream result, not cause, of Alzheimer’s disease. Alzheimers Dement. 2014;10:372–380. doi: 10.1016/j.jalz.2013.11.003. [DOI] [PubMed] [Google Scholar]
- Duzgun Z, Eroglu Z, Biray Avci C. Role of mTOR in glioblastoma. Gene. 2015;575:187–190. doi: 10.1016/j.gene.2015.08.060. [DOI] [PubMed] [Google Scholar]
- Everitt A. Calorie restriction, ageing and longevity. Australas J Ageing. 2010;29:14–14. [Google Scholar]
- Ferrer I. Altered mitochondria, energy metabolism, voltage-dependent anion channel, and lipid rafts converge to exhaust neurons in Alzheimer’s disease. J Bioenerg Biomembr. 2009;41:425–431. doi: 10.1007/s10863-009-9243-5. [DOI] [PubMed] [Google Scholar]
- Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- Fontana L, Kennedy BK, Longo VD, Seals D, Melov S. Medical research: treat ageing. Nature. 2014;511:405–407. doi: 10.1038/511405a. [DOI] [PubMed] [Google Scholar]
- Fontana L, Partridge L. Promoting health and longevity through diet: From model organisms to humans. Cell. 2015;161:106–118. doi: 10.1016/j.cell.2015.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschi C, Campisi J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J Gerontol A Biol Sci Med Sci. 2014;69(Suppl 1):S4–9. doi: 10.1093/gerona/glu057. [DOI] [PubMed] [Google Scholar]
- Fusco S, Pani G. Brain response to calorie restriction. Cell Mol Life Sci. 2013;70:3157–3170. doi: 10.1007/s00018-012-1223-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galeffi F, Shetty PK, Sadgrove MP, Turner DA. Age-related metabolic fatigue during low glucose conditions in rat hippocampus. Neurobiol Aging. 2015;36:982–992. doi: 10.1016/j.neurobiolaging.2014.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson EL, Barr S, Jeanes YM. Habitual fat intake predicts memory function in younger women. Front Hum Neurosci. 2013;7:838. doi: 10.3389/fnhum.2013.00838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold PE. Glucose and age-related changes in memory. Neurobiol Aging. 2005;26(Suppl 1):60–64. doi: 10.1016/j.neurobiolaging.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Gomez-Pinilla F. Brain foods: the effects of nutrients on brain function. Nat Rev Neuro. 2008;9:568–578. doi: 10.1038/nrn2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granzotto A, Zatta P. Resveratrol and Alzheimer’s disease: message in a bottle on red wine and cognition. Front Aging Neurosci. 2014;6:95. doi: 10.3389/fnagi.2014.00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill JD, Riddle DR. Age-related and laminar-specific dendritic changes in the medial frontal cortex of the rat. Brain Res. 2002;937:8–21. doi: 10.1016/s0006-8993(02)02457-5. [DOI] [PubMed] [Google Scholar]
- Gundersen V, Storm-Mathisen J, Bergersen LH. Neuroglial transmission. Physiol Rev. 2015;95:695–726. doi: 10.1152/physrev.00024.2014. [DOI] [PubMed] [Google Scholar]
- Halagappa VK, Guo Z, Pearson M, Matsuoka Y, Cutler RG, Laferla FM, Mattson MP. Intermittent fasting and caloric restriction ameliorate age-related behavioral deficits in the triple-transgenic mouse model of Alzheimer’s disease. Neurobiol Dis. 2007;26:212–220. doi: 10.1016/j.nbd.2006.12.019. [DOI] [PubMed] [Google Scholar]
- Hanks SD, Flood DG. Region-specific stability of dendritic extent in normal human aging andregression in Alzheimers-disease .1. Ca1 of Hippocampus. Brain Res. 1991;540:63–82. doi: 10.1016/0006-8993(91)90493-f. [DOI] [PubMed] [Google Scholar]
- Hardman RJ, Kennedy G, Macpherson H, Scholey AB, Pipingas A. A randomised controlled trial investigating the effects of Mediterranean diet and aerobic exercise on cognition in cognitively healthy older people living independently within aged care facilities: the Lifestyle Intervention in Independent Living Aged Care (LIILAC) study protocol [ACTRN12614001133628] Nutr J. 2015;14:53. doi: 10.1186/s12937-015-0042-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter CS, Pahor M, Javors MA, Fernandez E, Miller RA. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–395. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloway CJ, Cochlin LE, Emmanuel Y, Murray A, Codreanu I, Edwards LM, Szmigielski C, Tyler DJ, Knight NS, Saxby BK, Lambert B, Thompson C, Neubauer S, Clarke K. A high-fat diet impairs cardiac high-energy phosphate metabolism and cognitive function in healthy human subjects. Am J Clin Nutr. 2011;93:748–755. doi: 10.3945/ajcn.110.002758. [DOI] [PubMed] [Google Scholar]
- Honig LS, Kang MS, Schupf N, Lee JH, Mayeux R. Association of shorter leukocyte telomere repeat length with dementia and mortality. Arch Neurol. 2012;69:1332–1339. doi: 10.1001/archneurol.2012.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huhn S, Kharabian Masouleh S, Stumvoll M, Villringer A, Witte AV. Components of a Mediterranean diet and their impact on cognitive functions in aging. Front Aging Neurosci. 2015;7:132. doi: 10.3389/fnagi.2015.00132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyun DH, Emerson SS, Jo DG, Mattson MP, de Cabo R. Calorie restriction up-regulates the plasma membrane redox system in brain cells and suppresses oxidative stress during aging. Proc Natl Acad Sci U S A. 2006;103:19908–19912. doi: 10.1073/pnas.0608008103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobsen LH, Kondrup J, Zellner M, Tetens I, Roth E. Effect of a high protein meat diet on muscle and cognitive functions: A randomised controlled dietary intervention trial in healthy men. Clin Nutr. 2011;30:303–311. doi: 10.1016/j.clnu.2010.12.010. [DOI] [PubMed] [Google Scholar]
- Japan Ministry of Health, Labor and Welfare Statistics and Information Division. Japan National Health Statistics for 2000. Tokyo, Japan: Japan Ministry of Health, Labor and Welfare; 2002. [Google Scholar]
- Johnson DA, Johnson JA. Nrf2-a therapeutic target for the treatment of neurodegenerative diseases. Free Radic Biol Med. 2015;88:253–267. doi: 10.1016/j.freeradbiomed.2015.07.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JB, Summer W, Cutler RG, Martin B, Hyun DH, Dixit VD, Pearson M, Nassar M, Telljohann R, Maudsley S, Carlson O, John S, Laub DR, Mattson MP. Alternate day calorie restriction improves clinical findings and reduces markers of oxidative stress and inflammation in overweight adults with moderate asthma. Free Radic Biol Med. 2007;42:665–674. doi: 10.1016/j.freeradbiomed.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones EK, Sunram-Lea SI, Wesnes KA. Acute ingestion of different macronutrients differentially enhances aspects of memory and attention in healthy young adults. Biol Psychol. 2012;89:477–486. doi: 10.1016/j.biopsycho.2011.12.017. [DOI] [PubMed] [Google Scholar]
- Kalmar JM, Button DC, Gardiner K, Cahill F, Gardiner PF. Caloric restriction does not offset age-associated changes in the biophysical properties of motoneurons. J Neurophysiol. 2009;101:548–557. doi: 10.1152/jn.90617.2008. [DOI] [PubMed] [Google Scholar]
- Kane AE, Hilmer SN, Boyer D, Gavin K, Nines D, Howlett SE, de Cabo R, Mitchell SJ. Impact of longevity interventions on a validated mouse clinical frailty index. J Gerontol A Biol Sci Med Sci. 2016;71:333–339. doi: 10.1093/gerona/glu315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaptan Z, Akgun-Dar K, Kapucu A, Dedeakayogullari H, Batu S, Uzum G. Long term consequences on spatial learning-memory of low-calorie diet during adolescence in female rats; hippocampal and prefrontal cortex BDNF level, expression of NeuN and cell proliferation in dentate gyrus. Brain Res. 2015;1618:194–204. doi: 10.1016/j.brainres.2015.05.041. [DOI] [PubMed] [Google Scholar]
- Kesby JP, Kim JJ, Scadeng M, Woods G, Kado DM, Olefsky JM, Jeste DV, Achim CL, Semenova S. Spatial cognition in adult and aged mice exposed to high-fat diet. PLoS One. 2015;10:e0140034. doi: 10.1371/journal.pone.0140034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Nguyen MD, Dobbin MM, Fischer A, Sananbenesi F, Rodgers JT, Delalle I, Baur JA, Sui G, Armour SM, Puigserver P, Sinclair DA, Tsai LH. SIRT1 deacetylase protects against neurodegeneration in models for Alzheimer’s disease and amyotrophic lateral sclerosis. EMBO J. 2007;26:3169–3179. doi: 10.1038/sj.emboj.7601758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kivipelto M, Solomon A, Ahtiluoto S, Ngandu T, Lehtisalo J, Antikainen R, Soininen H. The Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER): Study design and progress. Alzheimers Dement. 2013;9:657–665. doi: 10.1016/j.jalz.2012.09.012. [DOI] [PubMed] [Google Scholar]
- Koubova J, Guarente L. How does calorie restriction work? Genes Dev. 2003;17:313–321. doi: 10.1101/gad.1052903. [DOI] [PubMed] [Google Scholar]
- Krikorian R, Shidler MD, Dangelo K, Couch SC, Benoit SC, Clegg DJ. Dietary ketosis enhances memory in mild cognitive impairment. Neuro Aging. 2012;33:425.e419–425.e427. doi: 10.1016/j.neurobiolaging.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krstic D, Knuesel I. The airbag problem-a potential culprit for bench-to-bedside translational efforts: relevance for Alzheimer’s disease. Acta Neuropathol Commun. 2013;1:62. doi: 10.1186/2051-5960-1-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhla A, Lange S, Holzmann C, Maass F, Petersen J, Vollmar B, Wree A. Lifelong caloric restriction increases working memory in mice. PLoS One. 2013;8:e68778. doi: 10.1371/journal.pone.0068778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar N, Wheaton LA, Snow TK, Millard-Stafford M. Carbohydrate ingestion but not mouth rinse maintains sustained attention when fasted. Physiol Behav. 2015;153:33–39. doi: 10.1016/j.physbeh.2015.10.023. [DOI] [PubMed] [Google Scholar]
- Labbé A, Garand C, Cogger VC, Paquet ER, Desbiens M, Le Couteur DG, Lebel M. Resveratrol improves insulin resistance hyperglycemia and hepatosteatosis but not hypertriglyceridemia, inflammation, and life span in a mouse model for Werner syndrome. J Gerontol A Med Sci Biol Sci. 2011;66:264–278. doi: 10.1093/gerona/glq184. [DOI] [PubMed] [Google Scholar]
- Lalla R, Donmez G. The role of sirtuins in Alzheimer’s disease. Front Aging Neurosci. 2013;5:16. doi: 10.3389/fnagi.2013.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Couteur DG, Lakatta EG. A vascular theory of aging. J Gerontol A Biol Sci Med Sci. 2010;65:1025–1027. doi: 10.1093/gerona/glq135. [DOI] [PubMed] [Google Scholar]
- Le Couteur DG, Solon-Biet S, Cogger VC, Mitchell SJ, Senior A, de Cabo R, Raubenheimer D, Simpson SJ. The impact of low-protein high-carbohydrate diets on aging and lifespan. Cell Mol Life Sci. 2016;73:1237–1252. doi: 10.1007/s00018-015-2120-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Couteur DG, Solon-Biet S, Wahl D, Cogger VC, Willcox BJ, Willcox DC, Raubenheimer D, Simpson SJ. New horizons. Dietary protein, ageing and the Okinawan ratio. Age Ageing. 2016;0:1–5. doi: 10.1093/ageing/afw069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Couteur DG, Tay SS, Solon-Biet S, Bertolino P, McMahon AC, Cogger VC, Colakoglu F, Warren A, Holmes AJ, Pichaud N, Horan M, Correa C, Melvin RG, Turner N, Ballard JW, Ruohonen K, Raubenheimer D, Simpson SJ. The Influence of Macronutrients on Splanchnic and Hepatic Lymphocytes in Aging Mice. J Gerontol A Biol Sci Med Sci. 2015;70:1499–1507. doi: 10.1093/gerona/glu196. [DOI] [PubMed] [Google Scholar]
- Leslie M. Biomedicine. A putative antiaging drug takes a step from mice to men. Science. 2013;342:789. doi: 10.1126/science.342.6160.789. [DOI] [PubMed] [Google Scholar]
- Li J, Deng J, Sheng W, Zuo Z. Metformin attenuates Alzheimer’s disease-like neuropathology in obese, leptin-resistant mice. Pharmacol Biochem Behav. 2012;101:564–574. doi: 10.1016/j.pbb.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Sun Q, Li Y, Yang Y, Chang T, Man M, Zheng L. Overexpression of SIRT1 Induced by Resveratrol and Inhibitor of miR-204 Suppresses Activation and Proliferation of Microglia. J Mol Neurosci. 2015;56:858–867. doi: 10.1007/s12031-015-0526-5. [DOI] [PubMed] [Google Scholar]
- Liang HY, Ward WF. PGC-1 alpha: a key regulator of energy metabolism. Adv Physiol Educ. 2006;30:145–151. doi: 10.1152/advan.00052.2006. [DOI] [PubMed] [Google Scholar]
- Lin AL, Zheng W, Halloran JJ, Burbank RR, Hussong SA, Hart MJ, Javors M, Shih YY, Muir E, Solano Fonseca R, Strong R, Richardson AG, Lechleiter JD, Fox PT, Galvan V. Chronic rapamycin restores brain vascular integrity and function through NO synthase activation and improves memory in symptomatic mice modeling Alzheimer’s disease. J Cereb Blood Flow Metab. 2013;33:1412–1421. doi: 10.1038/jcbfm.2013.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo VD, Fontana L. Intermittent supplementation with rapamycin as a dietary restriction mimetic. Aging (Albany NY) 2011;3:1039–1040. doi: 10.18632/aging.100401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lourida I, Soni M, Thompson-Coon J, Purandare N, Lang IA, Ukoumunne OC, Llewellyn DJ. Mediterranean diet, cognitive function, and dementia: a systematic review. Epidemiology. 2013;24:479–489. doi: 10.1097/EDE.0b013e3182944410. [DOI] [PubMed] [Google Scholar]
- Maalouf M, Rho JM, Mattson MP. The neuroprotective properties of calorie restriction, the ketogenic diet, and ketone bodies. Brain Res Rev. 2009;59:293–315. doi: 10.1016/j.brainresrev.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magyar K, Halmosi R, Palfi A, Feher G, Czopf L, Fulop A, Battyany I, Sumegi B, Toth K, Szabados E. Cardioprotection by resveratrol: A human clinical trial in patients with stable coronary artery disease. Clin Hemorheol Microcirc. 2012;50:179–187. doi: 10.3233/CH-2011-1424. [DOI] [PubMed] [Google Scholar]
- Maiese K. Targeting molecules to medicine with mTOR, autophagy, and neurodegenerative disorders. Br J Clin Pharmacol. 2015 doi: 10.1111/bcp.12804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallidou A, Cartie M. Nutritional habits and cognitive performance of older adults. Nurs Manag (Harrow) 2015;22:27–34. doi: 10.7748/nm.22.3.27.e1331. [DOI] [PubMed] [Google Scholar]
- Marosi K, Mattson MP. BDNF mediates adaptive brain and body responses to energetic challenges. Trends Endocrinol Metab. 2014;25:89–98. doi: 10.1016/j.tem.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Montalvo A, Mercken EM, Mitchell SJ, Palacios HH, Mote PL, Scheibye-Knudsen M, Gomes AP, Ward TM, Minor RK, Blouin MJ, Schwab M, Pollak M, Zhang Y, Yu Y, Becker KG, Bohr VA, Ingram DK, Sinclair DA, Wolf NS, Spindler SR, Bernier M, de Cabo R. Metformin improves healthspan and lifespan in mice. Nat Commun. 2013;4:2192. doi: 10.1038/ncomms3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin CK, Anton SD, Han H, York-Crowe E, Redman LM, Ravussin E, Williamson DA. Examination of cognitive function during six months of calorie restriction: results of a randomized controlled trial. Rejuvenation Res. 2007;10:179–190. doi: 10.1089/rej.2006.0502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews EA, Linardakis JM, Disterhoft JF. The fast and slow afterhyperpolarizations are differentially modulated in hippocampal neurons by aging and learning. J Neurosci. 2009;29:4750–4755. doi: 10.1523/JNEUROSCI.0384-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattison JA, Roth GS, Beasley TM, Tilmont EM, Handy AM, Herbert RL, Longo DL, Allison DB, Young JE, Bryant M, Barnard D, Ward WF, Qi W, Ingram DK, de Cabo R. Impact of caloric restriction on health and survival in rhesus monkeys from the NIA study. Nature. 2012;489:318–321. doi: 10.1038/nature11432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP. Brain evolution and lifespan regulation: conservation of signal transduction pathways that regulate energy metabolism. Mech Ageing Dev. 2002;123:947–953. doi: 10.1016/s0047-6374(02)00032-5. [DOI] [PubMed] [Google Scholar]
- Mattson MP. The impact of dietary energy intake on cognitive aging. Front Aging Neurosci. 2010;2:5. doi: 10.3389/neuro.24.005.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP. Challenging oneself intermittently to improve health. Dose Response. 2014;12:600–618. doi: 10.2203/dose-response.14-028.Mattson. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP. Lifelong brain health is a lifelong challenge: from evolutionary principles to empirical evidence. Ageing Res Rev. 2015;20:37–45. doi: 10.1016/j.arr.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Duan W, Lee J, Guo Z. Suppression of brain aging and neurodegenerative disorders by dietary restriction and environmental enrichment: molecular mechanisms. Mech Ageing Dev. 2001;122:757–778. doi: 10.1016/s0047-6374(01)00226-3. [DOI] [PubMed] [Google Scholar]
- Mattsson N, Rosen E, Hansson O, Andreasen N, Parnetti L, Jonsson M, Herukka SK, van der Flier WM, Blankenstein MA, Ewers M, Rich K, Kaiser E, Verbeek MM, Rikkert MO, Tsolaki M, Mulugeta E, Aarsland D, Visser PJ, Schroder J, Marcusson J, de Leon M, Hampel H, Scheltens P, Wallin A, Eriksdotter-Jonhagen M, Minthon L, Winblad B, Blennow K, Zetterberg H. Age and diagnostic performance of Alzheimer disease CSF biomarkers. Neurology. 2012;78:468–476. doi: 10.1212/WNL.0b013e3182477eed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCay CM, Crowell MF, Maynard LA. The effect of retarded growth upon the length of life span and upon the ultimate body size. J Nutr. 1935;10:63–79. [PubMed] [Google Scholar]
- Melancon MO, Lorrain D, Dionne IJ. Exercise and sleep in aging: emphasis on serotonin. Pathol Biol (Paris) 2014;62:276–283. doi: 10.1016/j.patbio.2014.07.004. [DOI] [PubMed] [Google Scholar]
- Meng LH, Zheng XS. Toward rapamycin analog (rapalog)-based precision cancer therapy. Acta Pharmacol Sin. 2015;36:1163–1169. doi: 10.1038/aps.2015.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meramat A, Rajab NF, Shahar S, Sharif R. Cognitive Impairment, Genomic Instability and Trace Elements. Journal of Nutrition Health & Aging. 2015;19:48–57. doi: 10.1007/s12603-014-0489-1. [DOI] [PubMed] [Google Scholar]
- Mercken EM, Carboneau BA, Krzysik-Walker SM, de Cabo R. Of mice and men: The benefits of caloric restriction, exercise, and mimetics. Ageing Res Rev. 2012;11:390–398. doi: 10.1016/j.arr.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michan S, Li Y, Chou MM, Parrella E, Ge H, Long JM, Allard JS, Lewis K, Miller M, Xu W, Mervis RF, Chen J, Guerin KI, Smith LE, McBurney MW, Sinclair DA, Baudry M, de Cabo R, Longo VD. SIRT1 is essential for normal cognitive function and synaptic plasticity. J Neurosci. 2010;30:9695–9707. doi: 10.1523/JNEUROSCI.0027-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JA, Horvath S, Geschwind DH. Divergence of human and mouse brain transcriptome highlights Alzheimer disease pathways. Proc Natl Acad Sci USA. 2010;107:12698–12703. doi: 10.1073/pnas.0914257107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RA, Harrison DE, Astle CM, Baur JA, Boyd AR, de Cabo R, Fernandez E, Flurkey K, Javors MA, Nelson JF, Orihuela CJ, Pletcher S, Sharp ZD, Sinclair D, Starnes JW, Wilkinson JE, Nadon NL, Strong R. Rapamycin, but not resveratrol or simvastatin, extends life span of genetically heterogeneous mice. J Gerontol A Biol Sci Med Sci. 2011;66:191–201. doi: 10.1093/gerona/glq178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohajeri MH, Wittwer J, Vargas K, Hogan E, Holmes A, Rogers PJ, Goralczyk R, Gibson EL. Chronic treatment with a tryptophan-rich protein hydrolysate improves emotional processing, mental energy levels and reaction time in middle-aged women. Br J Nutr. 2015:1–16. doi: 10.1017/S0007114514003754. [DOI] [PubMed] [Google Scholar]
- Moore EM, Mander AG, Ames D, Kotowicz MA, Carne RP, Brodaty H, Woodward M, Boundy K, Ellis KA, Bush AI, Faux NG, Martins R, Szoeke C, Rowe C, Watters DA, Investigators, A Increased risk of cognitive impairment in patients with diabetes is associated with metformin. Diabetes Care. 2013;36:2981–2987. doi: 10.2337/dc13-0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mowszowski L, Batchelor J, Naismith SL. Early intervention for cognitive decline: can cognitive training be used as a selective prevention technique? Int Psychogeriatr. 2010;22:537–548. doi: 10.1017/S1041610209991748. [DOI] [PubMed] [Google Scholar]
- Nakagawa S, Lagisz M, Hector KL, Spencer HG. Comparative and meta-analytic insights into life extension via dietary restriction. Aging Cell. 2012;11:401–409. doi: 10.1111/j.1474-9726.2012.00798.x. [DOI] [PubMed] [Google Scholar]
- National Nutrition Council. Finnish nutrition recommendations—Diet and physical activity in balance. Oy, Finland: Edita; 2005. p. 57. [Google Scholar]
- Navarro-Martinez R, Fernandez-Garrido J, Buigues C, Torralba-Martinez E, Martinez-Martinez M, Verdejo Y, Mascaros MC, Cauli O. Brain-derived neurotrophic factor correlates with functional and cognitive impairment in non-disabled older individuals. Exp Gerontol. 2015;72:129–137. doi: 10.1016/j.exger.2015.10.001. [DOI] [PubMed] [Google Scholar]
- Ng F, Wijaya L, Tang BL. SIRT1 in the brain-connections with aging-associated disorders and lifespan. Front Cell Neurosci. 2015;9:64. doi: 10.3389/fncel.2015.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng TP, Feng L, Yap KB, Lee TS, Tan CH, Winblad B. Long-term metformin usage and cognitive function among older adults with diabetes. J Alzheimers Dis. 2014;41:61–68. doi: 10.3233/JAD-131901. [DOI] [PubMed] [Google Scholar]
- Ngandu T, Lehtisalo J, Solomon A, Levälahti E, Ahtiluoto S, Antikainen R, Bäckman L, Hänninen T, Jula A, Laatikainen T, Lindström J, Mangialasche F, Paajanen T, Pajala S, Peltonen M, Rauramaa R, Stigsdotter-Neely A, Strandberg T, Tuomilehto J, Soininen H, Kivipelto M. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. The Lancet. 2015;385:2255–2263. doi: 10.1016/S0140-6736(15)60461-5. [DOI] [PubMed] [Google Scholar]
- Novelle MG, Wahl D, Dieguez C, Bernier M, de Cabo R. Resveratrol supplementation: Where are we now and where should we go? Ageing Res Rev. 2015;21:1–15. doi: 10.1016/j.arr.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojo JO, Rezaie P, Gabbott PL, Stewart MG. Impact of age-related neuroglial cell responses on hippocampal deterioration. Front Aging Neurosci. 2015;7:57. doi: 10.3389/fnagi.2015.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oosterveld SM, Kessels RP, Hamel R, Ramakers IH, Aalten P, Verhey FR, Sistermans N, Smits LL, Pijnenburg YA, van der Flier WM, Olde Rikkert MG, Melis RJ. The Influence of Co-Morbidity and Frailty on the Clinical Manifestation of Patients with Alzheimer’s Disease. J Alzheimers Dis. 2014;42:501–509. doi: 10.3233/JAD-140138. [DOI] [PubMed] [Google Scholar]
- Pani G. Neuroprotective effects of dietary restriction: Evidence and mechanisms. Semin Cell Dev Biol. 2015;40:106–114. doi: 10.1016/j.semcdb.2015.03.004. [DOI] [PubMed] [Google Scholar]
- Partridge L. Intervening in ageing to prevent the diseases of ageing. Trends Endocrinol Metab. 2014;25:555–557. doi: 10.1016/j.tem.2014.08.003. [DOI] [PubMed] [Google Scholar]
- Piko L, Hougham AJ, Bulpitt KJ. Studies of sequence heterogeneity of mitochondrial DNA from rat and mouse tissues: evidence for an increased frequency of deletions/additions with aging. Mech Ageing Dev. 1988;43:279–293. doi: 10.1016/0047-6374(88)90037-1. [DOI] [PubMed] [Google Scholar]
- Porquet D, Casadesus G, Bayod S, Vicente A, Canudas AM, Vilaplana J, Pelegri C, Sanfeliu C, Camins A, Pallas M, del Valle J. Dietary resveratrol prevents Alzheimer’s markers and increases life span in SAMP8. Age (Dordr) 2013;35:1851–1865. doi: 10.1007/s11357-012-9489-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porquet D, Grinan-Ferre C, Ferrer I, Camins A, Sanfeliu C, Del Valle J, Pallas M. Neuroprotective role of trans-resveratrol in a murine model of familial Alzheimer’sdisease. J Alzheimers Dis. 2014;42:1209–1220. doi: 10.3233/JAD-140444. [DOI] [PubMed] [Google Scholar]
- Poulsen MM, Vestergaard PF, Clasen BF, Radko Y, Christensen LP, Stodkilde-Jorgensen H, Moller N, Jessen N, Pedersen SB, Jorgensen JO. High-dose resveratrol supplementation in obese men: An investigator-initiated, randomized, placebocontrolled clinicaltrial of substrate metabolism, insulin sensitivity, and body composition. Diabetes. 2012;62:1186–1195. doi: 10.2337/db12-0975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryor R, Cabreiro F. Repurposing metformin: an old drug with new tricks in its binding pockets. Biochem J. 2015;471:307–322. doi: 10.1042/BJ20150497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raber J, Huang Y, Ashford JW. ApoE genotype accounts for the vast majority of AD risk and AD pathology. Neurobiol Aging. 2004;25:641–650. doi: 10.1016/j.neurobiolaging.2003.12.023. [DOI] [PubMed] [Google Scholar]
- Raubenheimer D, Simpson SJ, Le Couteur DG, Solon-Biet SM, Coogan SCP. Nutritional ecology and the evolution of aging. Exp Geront. 2016 doi: 10.1016/j.exger.2016.04.007. in press. [DOI] [PubMed] [Google Scholar]
- Renault VM, Rafalski VA, Morgan AA, Salih DA, Brett JO, Webb AE, Villeda SA, Thekkat PU, Guillerey C, Denko NC, Palmer TD, Butte AJ, Brunet A. FoxO3 regulates neural stem cell homeostasis. Cell Stem Cell. 2009;5:527–539. doi: 10.1016/j.stem.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts RO, Roberts LA, Geda YE, Cha RH, Pankratz VS, O’Connor HM, Knopman DS, Petersen RC. Relative Intake of Macronutrients Impacts Risk of Mild Cognitive Impairment or Dementia. J Alzheimers Dis. 2012;32:329–339. doi: 10.3233/JAD-2012-120862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruderman NB, Xu XJ, Nelson L, Cacicedo JM, Saha AK, Lan F, Ido Y. AMPK and SIRT1: a long-standing partnership? Am J Physiol Endocrinol Metab. 2010;298:E751–E760. doi: 10.1152/ajpendo.00745.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan MM, Guevremont D, Luxmanan C, Abraham WC, Williams JM. Aging alters long-term potentiation–related gene networks and impairs synaptic protein synthesis in the rat hippocampus. Neurobiol Aging. 2015;36:1868–1880. doi: 10.1016/j.neurobiolaging.2015.01.012. [DOI] [PubMed] [Google Scholar]
- Ryu SY, Coutu JP, Rosas HD, Salat DH. Effects of insulin resistance on white matter microscructure in middle-aged and older adults. Neurology. 2014;82:1862–1870. doi: 10.1212/WNL.0000000000000452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer D. Aging, longevity, and diet: historical remarks on calorie intake reduction. Gerontology. 2005;51:126–130. doi: 10.1159/000082198. [DOI] [PubMed] [Google Scholar]
- Schneider LS, Mangialasche F, Andreasen N, Feldman H, Giacobini E, Jones R, Mantua V, Mecocci P, Pani L, Winblad B, Kivipelto M. Clinical trials and late-stage drug development for Alzheimer’s disease: an appraisal from 1984 to 2014. J Intern Med. 2014;275:251–283. doi: 10.1111/joim.12191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seimon RV, Roekenes JA, Zibellini J, Zhu B, Gibson AA, Hills AP, Wood RE, King NA, Byrne NM, Sainsbury A. Do intermittent diets provide physiological benefits over continuous diets for weight loss? A systematic review of clinical trials. Mol Cell Endocrinol. 2015;418:153–172. doi: 10.1016/j.mce.2015.09.014. [DOI] [PubMed] [Google Scholar]
- Shabbir F, Patel A, Mattison C, Bose S, Krishnamohan R, Sweeney E, Sandhu S, Nel W, Rais A, Sandhu R, Ngu N, Sharma S. Effect of diet on serotonergic neurotransmission in depression. Neurochem Int. 2013;62:324–329. doi: 10.1016/j.neuint.2012.12.014. [DOI] [PubMed] [Google Scholar]
- Sierra F. The emergence ofgeroscience as an interdisciplinary approach to the enhancement of health span and life span. Cold Spring Harb Perspect Med. 2016 doi: 10.1101/cshperspect.a025163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidhu VK, Huang BX, Desai A, Kevala K, Kim HY. Role of DHA in aging-related changes in the mouse brain synaptic plasma membrane proteome. Neurobiol Age. 2016;41:73–85. doi: 10.1016/j.neurobiolaging.2016.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson SJ, Le Couteur DG, Raubenheimer D. Putting the Balance Back in Diet. Cell. 2015;161:18–23. doi: 10.1016/j.cell.2015.02.033. [DOI] [PubMed] [Google Scholar]
- Simpson SJ, Raubenheimer D. Caloric restriction and aging revisited: the need for a geometric analysis of the nutritional bases of aging. J Gerontol A Biol Sci Med Sci. 2007;62:707–713. doi: 10.1093/gerona/62.7.707. [DOI] [PubMed] [Google Scholar]
- Sohal RS, Forster MJ. Caloric restriction and the aging process: a critique. Free Radic Biol Med. 2014;73:366–382. doi: 10.1016/j.freeradbiomed.2014.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokoloff L. Energetics of functional activation in neural tissues. Neurochemical Research. 1999;24:321–329. doi: 10.1023/a:1022534709672. [DOI] [PubMed] [Google Scholar]
- Solfrizzi V, D’Introno A, Colacicco AM, Capurso C, Del Parigi A, Capurso S, Gadaleta A, Capurso A, Panza F. Dietary fatty acids intake: possible role in cognitive decline and dementia. Exp Gerontol. 2005;40:257–270. doi: 10.1016/j.exger.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Solfrizzi V, Panza F, Capurso A. The role of diet in cognitive decline. J Neural Transm (Vienna) 2003a;110:95–110. doi: 10.1007/s00702-002-0766-8. [DOI] [PubMed] [Google Scholar]
- Solfrizzi V, Panza F, Capurso A. The role of diet in cognitive decline. J Neural Transm. 2003b;110:95–110. doi: 10.1007/s00702-002-0766-8. [DOI] [PubMed] [Google Scholar]
- Solon-Biet SM, McMahon AC, Ballard JW, Ruohonen K, Wu LE, Cogger VC, Warren A, Huang X, Pichaud N, Melvin RG, Gokarn R, Khalil M, Turner N, Cooney GJ, Sinclair DA, Raubenheimer D, Le Couteur DG, Simpson SJ. The ratio of macronutrients, not caloric intake, dictates cardiometabolic health, aging, and longevity in ad libitum-fed mice. Cell Metab. 2014;19:418–430. doi: 10.1016/j.cmet.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solon-Biet SM, Mitchell SJ, de Cabo R, Raubenheimer D, Le Couteur DG, Simpson SJ. Macronutrients and caloric intake in health and longevity. J Endocrinol. 2015a;226:R17–28. doi: 10.1530/JOE-15-0173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solon-Biet SM, Walters KA, Simanainen UK, McMahon AC, Ruohonen K, Ballard JW, Raubenheimer D, Handelsman DJ, Le Couteur DG, Simpson SJ. Macronutrient balance, reproductive function, and lifespan in aging mice. Proc Natl Acad Sci U S A. 2015b;112:3481–3486. doi: 10.1073/pnas.1422041112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speakman JR, Mitchell SE. Caloric restriction. Mol Aspects Med. 2011;32:159–221. doi: 10.1016/j.mam.2011.07.001. [DOI] [PubMed] [Google Scholar]
- Spilman P, Podlutskaya N, Hart MJ, Debnath J, Gorostiza O, Bredesen D, Richardson A, Strong R, Galvan V. Inhibition of mTOR by rapamycin abolishes cognitive deficits and reduces amyloid-beta levels in a mouse model of Alzheimer’s disease. PLoS One. 2010;5:e9979. doi: 10.1371/journal.pone.0009979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridharan A, Pehar M, Salamat MS, Pugh TD, Bendlin BB, Willette AA, Anderson RM, Kemnitz JW, Colman RJ, Weindruch RH, Puglielli L, Johnson SC. Calorie restriction attenuates astrogliosis but not amyloid plaque load in aged rhesus macaques: a preliminary quantitative imaging study. Brain Res. 2013;1508:1–8. doi: 10.1016/j.brainres.2013.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srikanth V, Westcott B, Forbes J, Phan TG, Beare R, Venn A, Pearson S, Greenaway T, Parameswaran V, Munch G. Methylglyoxal, cognitive function and cerebral atrophy in older people. J Gerontol A Biol Sci Med Sci. 2013;68:68–73. doi: 10.1093/gerona/gls100. [DOI] [PubMed] [Google Scholar]
- Stefanidis A, Watt MJ. Does too much sugar make for lost memories? J Physiol. 2012;590:3633–3634. doi: 10.1113/jphysiol.2012.235028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes MG. ‘Activity-silent’ working memory in prefrontal cortex: a dynamic coding framework. Trends Cogn Sci. 2015;19:394–405. doi: 10.1016/j.tics.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun YX, Ji X, Mao X, Xie L, Jia J, Galvan V, Greenberg DA, Jin K. Differential activation of mTOR complex 1 signaling in human brain with mild to severe Alzheimer’s disease. J Alzheimers Dis. 2014;38:437–444. doi: 10.3233/JAD-131124. [DOI] [PubMed] [Google Scholar]
- Swindell WR. Dietary restriction in rats and mice: a meta-analysis and review of the evidence for genotype-dependent effects on lifespan. Ageing Res Rev. 2012;11:254–270. doi: 10.1016/j.arr.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan CC, Yu JT, Tan MS, Jiang T, Zhu XC, Tan L. Autophagy in aging and neurodegenerative diseases: implications for pathogenesis and therapy. Neurobiol Aging. 2014;35:941–957. doi: 10.1016/j.neurobiolaging.2013.11.019. [DOI] [PubMed] [Google Scholar]
- Terry RD, Katzman R. Life span and synapses: will there be a primary senile dementia? Neurobiol Aging. 2001;22:347–348. doi: 10.1016/s0197-4580(00)00250-5. [DOI] [PubMed] [Google Scholar]
- Timmers S, Auwerx J, Schrauwen P. The journey of resveratrol from yeast to human. Aging (Albany NY) 2012;4:146–158. doi: 10.18632/aging.100445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasconcelos AR, Yshii LM, Viel TA, Buck HS, Mattson MP, Scavone C, Kawamoto EM. Intermittent fasting attenuates lipopolysaccharide-induced neuroinflammation and memory impairment. J Neuroinflammation. 2014;11 doi: 10.1186/1742-2094-11-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkhratsky A, Toescu EC. Calcium and neuronal ageing. Trends Neurosci. 1998;21:2–7. doi: 10.1016/s0166-2236(97)01156-9. [DOI] [PubMed] [Google Scholar]
- Vivar C, van Praag H. Functional circuits of new neurons in the dentate gyrus. Front Neural Circuits. 2013;7:15. doi: 10.3389/fncir.2013.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Li JJ, Diao S, Kwak YD, Liu L, Zhi L, Bueler H, Bhat NR, Williams RW, Park EA, Liao FF. Metabolic stress modulates Alzheimer’s beta-secretase gene transcription via SIRT1-PPARgamma-PGC-1 in neurons. Cell Metab. 2013;17:685–694. doi: 10.1016/j.cmet.2013.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Wang W, Li L, Perry G, Lee HG, Zhu X. Oxidative stress and mitochondrial dysfunction in Alzheimer’s disease. Biochim Biophys Acta. 2014;1842:1240–1247. doi: 10.1016/j.bbadis.2013.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber M, Wu T, Hanson JE, Alam NM, Solanoy H, Ngu H, Lauffer BE, Lin HH, Dominguez SL, Reeder J, Tom J, Steiner P, Foreman O, Prusky GT, Scearce-Levie K. Cognitive deficits, changes in synaptic function, and brain pathology in a mouse model of normal aging(1,2,3) eNeuro. 2015;2 doi: 10.1523/ENEURO.0047-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West RL. An application of prefrontal cortex function theory to cognitive aging. Psychol Bull. 1996;120:272–292. doi: 10.1037/0033-2909.120.2.272. [DOI] [PubMed] [Google Scholar]
- Willcox DC, Scapagnini G, Willcox BJ. Healthy aging diets other than the mediterranean: A focus on the Okinawan diet. Mech Age Devel. 2014:136–137. doi: 10.1016/j.mad.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willette AA, Bendlin BB, Colman RJ, Kastman EK, Field AS, Alexander AL, Sridharan A, Allison DB, Anderson R, Voytko ML, Kemnitz JW, Weindruch RH, Johnson SC. Calorie restriction reduces the influence of glucoregulatory dysfunction on regional brain volume in aged rhesus monkeys. Diabetes. 2012;61:1036–1042. doi: 10.2337/db11-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willette AA, Coe CL, Birdsill AC, Bendlin BB, Colman RJ, Alexander AL, Allison DB, Weindruch RH, Johnson SC. Interleukin-8 and interleukin-10, brain volume and microstructure, and the influence of calorie restriction in old rhesus macaques. Age (Dordr) 2013;35:2215–2227. doi: 10.1007/s11357-013-9518-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte AV, Fobker M, Gellner R, Knecht S, Floel A. Caloric restriction improves memory in elderly humans (vol 106, pg 1255, 2009) Proc Natl Acad Sci USA. 2009;106:6023–6023. doi: 10.1073/pnas.0808587106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodside JV, Gallagher NE, Neville CE, McKinley MC. Mediterranean diet interventions to prevent cognitive decline–opportunities and challenges. Eur J Clin Nutr. 2014;68:1241–1244. doi: 10.1038/ejcn.2014.178. [DOI] [PubMed] [Google Scholar]
- Yang ZX, Slavin MJ, Sachdev PS. Dementia in the oldest old. Nat Rev Neurol. 2013;9:382–393. doi: 10.1038/nrneurol.2013.105. [DOI] [PubMed] [Google Scholar]
- Yannakoulia M, Kontogianni M, Scarmeas N. Cognitive health and Mediterranean diet: just diet or lifestyle pattern? Ageing Res Rev. 2015;20:74–78. doi: 10.1016/j.arr.2014.10.003. [DOI] [PubMed] [Google Scholar]
- Yon MA, Mauger SL, Pickavance LC. Relationships between dietary macronutrients and adult neurogenesis in the regulation of energy metabolism. Br J Nutr. 2013;109:1573–1. doi: 10.1017/S000711451200579X. [DOI] [PubMed] [Google Scholar]
- Zaptan A, Akgün-Dar, Kapucu A, Dedeakayoğullari H, Batu Ş, Üzüm G. Long term consequences on spatial learning-memory of low-calorie diet durung adoloscence in female rats; hippocampal and prefrontal cortex BDNF level, expression of NeuN and cell proliferation in the dentate gyrus. Brain Res. 2015;1618:194–204. doi: 10.1016/j.brainres.2015.05.041. [DOI] [PubMed] [Google Scholar]


