Abstract
Differentiating erythroblasts are exposed to an oxidative environment. The dynamics of oxidative status during terminal erythropoiesis and how it affects cell differentiation in response to erythropoietin are unclear. Here we show that erythropoietin induces reactive oxygen species (ROS) production in the early stages of terminal erythropoiesis. The levels of ROS correlate with CD71 surface expression and the uptake of iron and transferrin. ROS decreases in the late stage of terminal erythropoiesis when the cells are preparing for enucleation. Consistently, treatment of erythroblasts with a low dose (5 mM) of N-acetyl-cysteine (NAC), an ROS scavenger, promotes enucleation. However, high dose (20 mM) NAC leads to significant cell death. Our study reveals an important function of erythropoietin in regulating the dynamics of oxidative status and enucleation.
Keywords: Erythropoietin, erythropoiesis, enucleation, reactive oxygen species, transferrin, iron
Introduction
Erythropoietin (Epo) plays a pivotal role in regulating the differentiation, proliferation, and survival of erythroid progenitors (1). Epo binds to the cell surface Epo receptor (EpoR) on early-stage erythroid progenitors to activate many signaling transduction pathways that induce a spectrum of erythroid-specific genes including transferrin receptor CD71 and globins (2). CD71 is responsible for the binding and uptake of transferrin required for hemoglobin synthesis. In a well-established two-day mouse fatal liver erythroblast in vitro culture system, CD71 is gradually upregulated on the cell surface of purified erythroid progenitors on day one in culture in the presence of Epo (3, 4). CD71 gradually decreases on day two while the cells undergo terminal maturation, enrichment of hemoglobin and iron, overexpression of surface Ter119 antigen, and enucleation.
Presumably, synthesis and accumulation of hemoglobin with growing amount of iron in the maturing erythroblasts would lead to the increased production of reactive oxygen species (ROS). Unchecked accumulation of ROS often results in a shortened red blood cell lifespan and hemolysis, as evidenced by the fact that deficiencies in several ROS scavengers cause anemia (5, 6). However, recent work from others and ours revealed that ROS decreases steadily in the late stages of terminal erythropoiesis (7,8). Although this could be explained by the autophagic clearance of mitochondria where ROS is generated (9), the dynamic changes of oxidative status throughout terminal erythropoiesis, how Epo is involved in this process, and whether ROS affects differentiation and enucleation, are unclear.
In this study, we discovered that Epo and transferrin are required for ROS generation, which positively correlates with CD71 expression. Suppression of ROS with low dose ROS scavenger increased enucleation, but high dose ROS scavenger led to cell death. These results reveal an unexpected effect of Epo induced oxidative stress on enucleation.
Materials and Methods
Purification and culture of fetal liver cells
Purification and culture of mouse fetal liver erythroblasts from E13.5 C57BL/6 embryos (Jackson Laboratories) were performed as previously reported (7,10). Briefly, embryonic day 13.5 mouse fetal livers were harvested. Ter119 negative erythroblasts were purified by negative selection using magnetic beads. The purified Ter119 negative erythroid progenitor cells were cultured in different media as detailed in the main text. SCF medium contains stem cell factor (SCF), IL-6, Flt-3 ligand plus fetal bovine serum. Epo medium contains Epo, fetal bovine serum, insulin, holo-transferrin, and BSA. Protocols for all animal experiments were approved by the Institutional Animal Care and Use Committee of Northwestern University.
Flow cytometric analysis
Flow cytometric analysis of differentiation status of cultured mouse fetal erythroblasts was described previously (7). Briefly, cells were immunostained with allophycocyanin (APC)–conjugated anti-Ter119 (1:200) (eBioscience) and fluorescein isothiocyanate (FITC)–conjugated anti-CD71 (1:200) (eBioscience) antibodies for 20 min at room temperature. Propidium iodide was added to exclude dead cells from analysis. For enucleation analysis, cells were stained with 10 μg/ml Hoechst 33342 (DNA staining) in addition to stains mentioned above. Apoptosis was analyzed using an Annexin V Apoptosis Detection Kit (eBioscience) according to the manufacturer's instructions. All stained cells were analyzed on a FACSCalibur (Becton Dickinson) flow cytometer.
Quantitative analysis of intracellular ROS
Freshly isolated fetal liver cells were washed and resuspended in pre-warmed PBS with 10 μM 5-(and 6-)-chloromethyl-2’, 7’-dichlorodihydrofluorescein diacetate (CM-H2DCFDA; Invitrogen), together with allophycocyanin (APC)–conjugated anti-Ter119 (1:200) (eBioscience) and phycoerythrin (PE)–conjugated anti-CD71 (1:200) (eBioscience) antibodies. After incubation for 30 min at 37°C, 5% CO2, the cells were washed and resuspended in PBS. Propidium iodide was added to exclude dead cells from analysis. The stained cells were immediately analyzed by flow cytometric assay.
Quantification of hemoglobin concentration
Hemoglobin was quantified by lysing 3×106 cells in 200 ml Drabkin's reagent according to the manufacture's instruction (Sigma Aldrich). Spectrophotometric reading was then performed at 540 nm.
Cytospin preparations and histologic stainings
2 × 104 cultured cells were centrifuged onto slides for 3 min at 800 rpm (Cytospin 3; Thermo Shandon, Pittsburgh, PA) and air-dried. Cells were fixed in −20°C methanol for 2 minutes and stained with 3,3’-diaminobenzidine and Giemsa stains according to the manufacturer's recommendations (Sigma).
Results
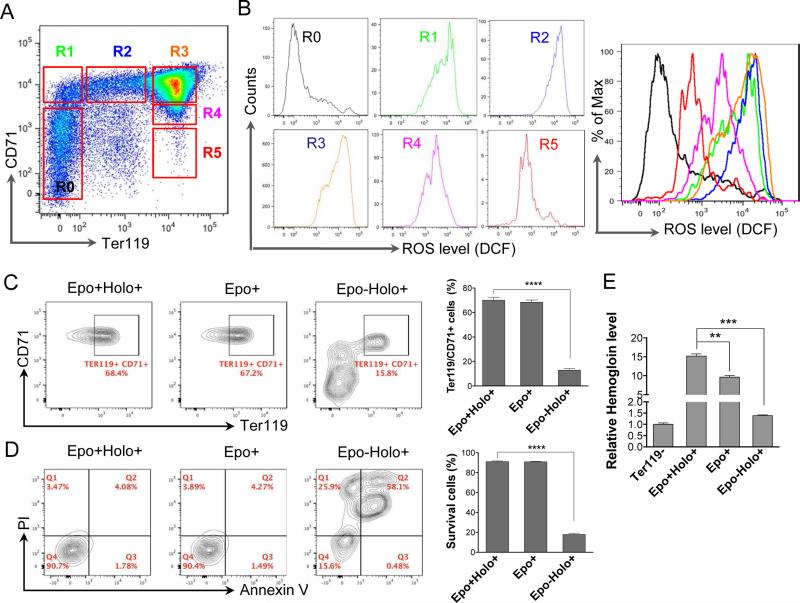
To determine the dynamic changes of oxidative status during terminal erythropoiesis, we first analyzed ROS levels in different stages of mouse fetal liver erythropoiesis. To this end, total fetal liver cells were purified from embryonic day 13.5 (E13.5) pregnant mouse when most of the cells in the fetal liver are erythroid. The cells were gated into six groups based on their cell surface expression of CD71 (transferrin receptor) and Ter119 (a mature red cell surface marker) by flow cytometry. R0 population represents the least differentiated erythroid progenitors whereas R5 cells are mostly late stage orthochromatic erythroblasts and enucleated reticulocytes (11) (Fig. 1A). When analyzed in parallel, we found that ROS level significantly increased when the cells differentiated from R0 to R1. The ROS level maintained at a high level through R3 and underwent a rapid decrease at R4 and R5 stages, which correlated with the changes of the cell surface CD71 levels (Fig. 1A, 1B).
Fig. 1.
Erythropoietin and transferrin regulate the generation of reactive oxygen species during mouse fetal liver terminal erythropoiesis. (A) Flow cytometric analysis of E13.5 mouse fetal liver cells stained with CD71 and Ter119. R0 to R5 populations were gated based on the cells’ relative expression levels of CD71 and Ter119. (B) Flow cytometric analysis of the levels of reactive oxygen species (ROS) based on the intensity of CM-H2DCFDA in R0-R5. The colors of the curves (right) match the gated populations (left). (C-D) Flow cytometric analysis of differentiation (C) and apoptosis (D) of cultured mouse fetal liver erythroblasts based on the Ter119 and CD71 level, and annexin V and propidium iodine (PI), respectively. Ter119 negative fetal liver erythroblasts were purified and cultured in SCF medium for 8 h followed by 24 h culture in indicated medium. Statistical analysis with student's t-test is on the right. Data were obtained from three independent experiments and presented as mean ± SD. **** p <0.0001. Epo: Erythropoietin; Holo: holo-transferrin. (E) Relative hemoglobin levels from cells in C. Equal number of the cells were collected. Total hemoglobin was quantified by measuring light absorbance at 540 nm using Drabkin's reagent. ** p <0.01, *** p <0.001.
While the reduction of ROS from R3 to R4-R5 could be due to mitochondrial autophagy (9), the increased ROS level in the early stage of terminal erythropoiesis may be related to Epo-induced CD71 cell surface expression and accumulation of transferrin and iron. To test this hypothesis, we first cultured the purified E13.5 mouse fetal liver Ter119 negative erythroblasts in Epo free medium but containing stem cell factor (SCF), IL-3, and Flt-3 ligand (we call it SCF medium) to maintain the progenitor status of the purified cells and eliminate the effect of residual endogenous Epo (10). After 4 h of SCF medium culture, the cells were changed into Epo containing medium with holo-transferrin, Epo containing medium without holo-transferrin, or Epo free medium with holo-transferrin, and cultured for additional 24 h. As expected, flow cytometric analysis demonstrated compromised differentiation status and increased apoptosis of cells cultured in Epo free medium compared to those in Epo medium with holo-transferrin (Fig. 1C, 1D). Cells cultured in the presence of Epo, but an absence of holo-transferrin showed intact Ter119 and CD71 expression and no increased apoptosis (Fig. 1C, 1D). However, hemoglobin synthesis was significantly downregulated (Fig 1E). This indicates that absence of holotransferrin does not affect Epo-induced cell surface protein expression or anti-apoptotic effects.
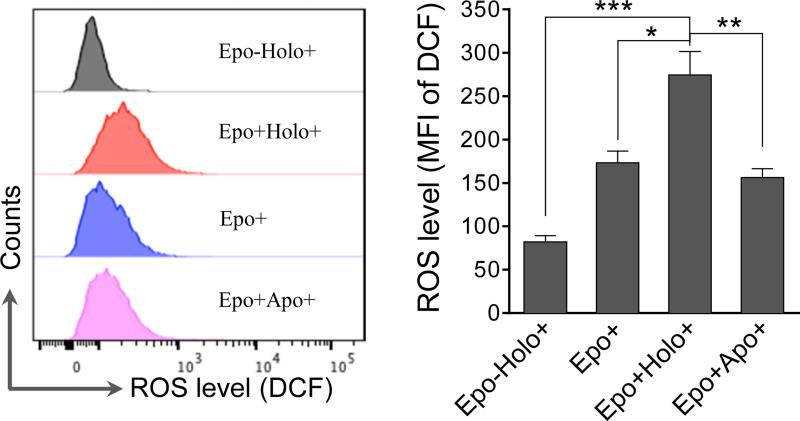
We next tested the ROS levels in these cells to confirm the role of Epo, iron, and transferrin in the generation of ROS. In this experiment, we also examined cells cultured with Epo and apotransferrin to definitively determine the role of iron in ROS production. As expected, robust ROS upregulation was only observed in cells cultured with Epo and holo-transferrin (Fig. 2). Together, these results establish a positive relationship between Epo-induced transferrin and iron uptake and cellular oxidative status.
Fig. 2.
Effect of erythropoietin and transferrin on ROS generation in cultured mouse fetal liver erythroblasts. Ter119 negative fetal liver erythroblasts were purified and cultured in SCF medium for 8 h followed by 24 h culture in indicated medium. Epo: Erythropoietin; Holo: holo-transferrin; Apo: apo-transferrin. Intracellular ROS levels were analyzed and presented as the mean fluorescence intensity (MFI) of DCF, an oxidation product of CM-H2DCFHDA. Results are shown as the means ± SD of three independent experiments. * p <0.05; ** p <0.01; *** p <0.001.
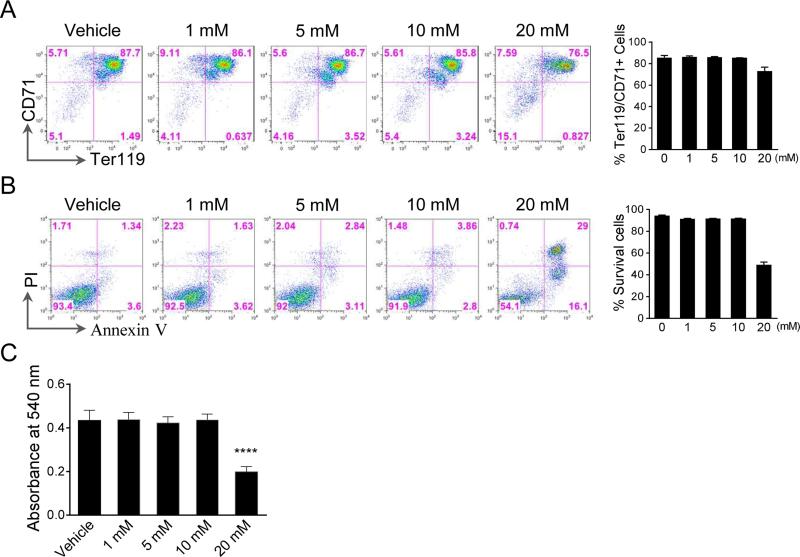
Our recent study indicated that Epo negatively affects enucleation in a dose dependent manner in vitro (7). To determine whether this is related to the cellular ROS level, we treated the Ter119 negative erythroblasts with different concentration of ROS scavenger N-acetyl-cysteine (NAC) in the presence of high Epo level (2 units/ml). NAC did not affect cell differentiation or apoptosis at low doses (1-10 mM) (Fig. 3A, 3B). Hemoglobin concentration of cells treated with low doses (1-10 mM) of NAC remained unchanged compared to the control cells, which further confirms the intact differentiation (Fig. 3C). However, a higher concentration of NAC (20 mM) induced significant differentiation block and apoptosis (Fig. 3A-C), which indicates that low level of ROS is necessary for mouse terminal erythropoiesis.
Fig. 3.
Inhibition of ROS with low dose of ROS scavenger does not affect differentiation. (A-B) Flow cytometric analysis of differentiation (A) and apoptosis (B) of Ter119 negative mouse fetal liver erythroblasts cultured in Epo (2 units/ml) containing medium in the presence of different concentration of NAC for 48 h. Statistical analysis with student's t-test is on the right. (C) Hemoglobin concentration of the indicated cells in panel (A). An equal number of the cells were collected. Total hemoglobin was quantified by measuring light absorbance at 540 nm using Drabkin's reagent. All data were obtained from three independent experiments and presented as mean±SD. *** p <0.0001.
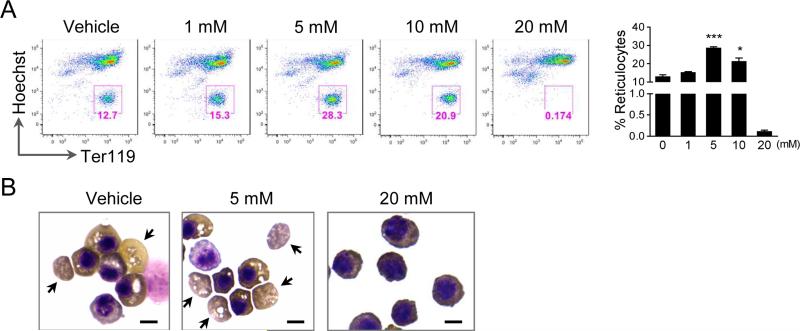
Since increased concentration of Epo negatively affects enucleation, we next determined whether inhibition of Epo-induced increase of ROS could contribute to this effect. To this end, the Ter119 negative erythroblasts were treated with different concentration of NAC at the beginning of culture and analyzed by flow cytometry after 2 d of culture using Ter119 and Hoechst 33342 (DNA staining) staining. The percentage of reticulocytes (defined as HoechstlowTer119high cells) represents enucleation rate. Indeed, low doses of NAC (5 and 10 mM) significantly increased the enucleation efficiency (Fig. 4A). Benzidine-Giemsa stains of the cells treated with NAC also showed an increased enucleation rate compared to the controls (Fig. 4B). However, there were no detectable enucleated reticulocytes in the cells with a high dose of NAC (20 mM). In addition, their cytoplasms were less hemoglobinized and the nuclei were bigger than those of the control cells (Fig. 4B), indicating differentiation block which indirectly affected enucleation.
Fig. 4.
Inhibition of ROS promotes enucleation. (A) Flow cytometric analysis of enucleation of Ter119 negative mouse fetal liver erythroblasts cultured in Epo (2 units/ml) containing medium in the presence of different concentration of NAC for 48 h. Hoechst was used for the DNA staining. Statistical analysis with student's t-test is on the right. Data were obtained from three independent experiments and presented as mean ± SD. * p <0.05; *** p <0.001. (B) Benzidine–Giemsa staining demonstrating cell morphology of the in vitro cultured erythroblasts in the present of NAC for 48 h. Arrows indicate incipient reticulocytes. Scale bar: 5 μm.
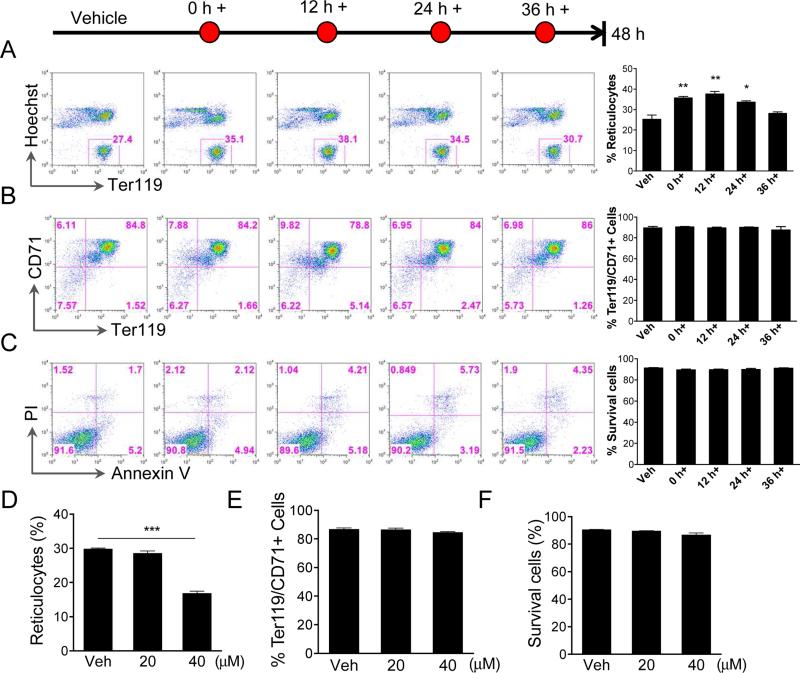
To further determine the role of ROS in enucleation, we added a low concentration of NAC (5 mM) at different time points in culture (Fig. 5A). Flow cytometric analysis of enucleation at 48 h in culture demonstrated that introduction of low concentration of NAC in the early stages of terminal erythropoiesis when the ROS level is high, significantly increased the enucleation efficiency (Fig 5A). Enucleation rate slightly increased when Ter119-negative mouse fetal erythroid progenitors were treated after 36 h in culture when enucleation was initiating. NAC treatment with this low dose in these experiments did not affect cell differentiation or apoptosis (Fig. 5B, 5C). These data demonstrate that the high level of ROS in the early stage of terminal erythropoiesis negatively affects enucleation. To further confirm this, we treated the cells with hydrogen peroxide, a physiological ROS precursor, in the early stage of terminal erythropoiesis. As we expected, cells treated with hydrogen peroxide showed dramatically decreased enucleation rate compared to control cells (Fig. 5D), and differentiation block and apoptosis were not observed under the same condition (Fig 5E, 5F).
Fig. 5.
Inhibition of ROS at different stages of fetal liver erythropoiesis affects enucleation. (A-C) Flow cytometric analysis of enucleation (A), differentiation (B), and apoptosis (C) of Ter119 negative mouse fetal liver erythroblasts cultured in Epo (2 units/ml) containing medium for 48 h. NAC (5 μM) was added at indicated time points. Statistical analysis with Student's t-test is on the right. (D-F) Treatment of mouse fetal liver erythroblasts with hydrogen peroxide inhibited enucleation. The mouse fetal liver Ter119 negative erythroblasts were purified and cultured in Epo medium. Hydrogen peroxide was added at 30 h in culture. Enucleation (D), differentiation (E), and apoptosis (F) were analyzed at 48 h in culture. All data were obtained from three independent experiments and presented as mean ± SD. * p <0.05; ** p <0.01. *** p <0.001.
Discussion
Our study illustrates that erythroid progenitors in the CFU-E stage present with lower ROS level than erythroblasts in later stages. This is consistent with a previous report that the low metabolic activity in the early progenitor cells can prevent them from the damage of metabolic products such as ROS (12). Binding of Epo to EpoR triggers the process of terminal erythropoiesis that ultimately leads to the generation of enucleated reticulocytes. In the early stage of terminal erythropoiesis, Epo activated JAK2-STAT5 and GATA1 signaling, induces CD71 and hemoglobin expression that causes the accumulation of transferrin and ROS. In this process, our experiments demonstrate that transferrin is also required for ROS generation since erythroblasts cultured in medium with transferrin exhibit higher ROS level than the cells in the transferrin-free medium.
High ROS level is detrimental to early-stage erythroblasts so that the cells establish many self-protective mechanisms to spare the damage of the oxidative stress. For example, Epo-JAK2-STAT5 pathway drives the expression of Bcl-xL to prevent apoptosis induced by cytochrome c release (13,14). Pleckstrin-2 upregulated by Epo plays a similar function in the early stage of terminal erythropoiesis by preventing the mitochondrial localization of cofilin that can cause apoptosis (7). On the other hand, erythroid cells contain a strong arsenal of antioxidant enzymes that protect the cells against oxygen radicals (15). The FoxO family of transcription factors was recently shown to have essential functions in the regulation of oxidative stress in erythroid cells. FoxO3 is the first identified EpoR-regulated transcription factor that is critical for regulating oxidative stress in vivo during erythropoiesis, which is at least partly through the upregulated transcription of the ROS-scavenging enzymes (16,17).
It is unexpected that Epo induced ROS negatively affects enucleation. This suggests that ROS decreases in the late stage of terminal erythropoiesis, mainly through autophagy of mitochondria or ROS-scavenging enzymes, to prepare the final stages of terminal erythropoiesis. However, high dose of ROS inhibitor also affects normal erythropoiesis, indicating that low level of ROS could also play certain roles in terminal erythropoiesis. In this respect, it was reported that in human erythroid cells, ROS induces the activation of caspases (18), which is well known to be required for early stage terminal erythropoiesis (19, 20, 21).
Taken together, these results revealed a close relationship of Epo and cellular ROS levels during mouse terminal erythropoiesis. Future in vivo studies could be valuable to determine whether the anti-oxidative agents, besides their demonstrated anti-apoptotic role in erythroid cells (17), could synergistically enhance the efficiency of Epo in the treatment of anemia, through the improvement of enucleation.
Highlights.
➢ Erythropoietin induces reactive oxygen species (ROS) production in early-stage erythroblasts.
➢ The oxidative status correlates with CD71 expression and the uptake of iron and transferrin.
➢ ROS level decreases in the late stage of terminal erythropoiesis.
➢ ROS scavenger promotes enucleation.
Acknowledgement
We thank the flow cytometry core facility of the Robert H. Lurie Comprehensive Cancer Center of Northwestern University for the help with the flow cytometric analysis. We thank Ganesan Keerthivasan for helpful discussion of the manuscript. The authors report no potential conflicts of interests. This study was supported by NIH R00 HL102154, NIH R01 DK102718, and an American Society of Hematology Scholar award to P.J.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
BZ and PJ designed the research. BZ, YM, and JY performed the experiments. BZ and PJ analyzed the data. BZ and PJ wrote the paper. All authors have reviewed and approved the final manuscript.
Competing interests
The authors declare no competing financial interests.
References
- 1.Bunn HF. Erythropoietin. Cold Spring Harbor perspectives in medicine. 2013;3:a011619. doi: 10.1101/cshperspect.a011619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Richmond TD, Chohan M, Barber DL. Turning cells red: signal transduction mediated by erythropoietin. Trends in cell biology. 2005;15(3):146–55. doi: 10.1016/j.tcb.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 3.Zhang J, Socolovsky M, Gross AW, Lodish HF. Role of Ras signalling in erythroid differentiation of mouse foetal liver cells: functional analysis by a flow cytometer-based novel culture system. Blood. 2003;102(12):3938–46. doi: 10.1182/blood-2003-05-1479. [DOI] [PubMed] [Google Scholar]
- 4.Ji P, Jayapal SR, Lodish HF. Enucleation of cultured mouse foetal erythroblasts requires Rac GTPases and mDia2. Nature cell biology. 2008;10(3):314–21. doi: 10.1038/ncb1693. [DOI] [PubMed] [Google Scholar]
- 5.Friedman JS, Rebel VI, Derby R, Bell K, Huang TT, Kuypers FA, Epstein CJ, Burakoff SJ. Absence of mitochondrial superoxide dismutase results in a murine hemolytic anaemia responsive to therapy with a catalytic antioxidant. The Journal of experimental medicine. 2001;193(8):925–34. doi: 10.1084/jem.193.8.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kong Y, Zhou S, Kihm AJ, Katein AM, Yu X, Gell DA, Mackay JP, Adachi K, Foster-Brown L, Louden CS, Gow AJ, Weiss MJ. Loss of alpha-haemoglobin-stabilizing protein impairs erythropoiesis and exacerbates beta-thalassemia. The Journal of clinical investigation. 2004;114(10):1457–66. doi: 10.1172/JCI21982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao B, Keerthivasan G, Mei Y, Yang J, McElherne J, Wong P, Doench JG, Feng G, Root DE, Ji P. Targeted shRNA screening identified critical roles of pleckstrin-2 in erythropoiesis. Haematologica. 2014;99(7):1157–67. doi: 10.3324/haematol.2014.105809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu Y, Swartz KL, Siu KT, Bhattacharyya M, Minella AC. Fbw7-dependent cyclin E regulation ensures terminal maturation of bone marrow erythroid cells by restraining oxidative metabolism. Oncogene. 2014;33(24):3161–71. doi: 10.1038/onc.2013.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sandoval H, Thiagarajan P, Dasgupta SK, Schumacher A, Prchal JT, Chen M, Wang J. Essential role for Nix in autophagic maturation of erythroid cells. Nature. 2008;454(7201):232–5. doi: 10.1038/nature07006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao B, Mei Y, Yang J, Ji P. Mouse foetal liver culture system to dissect target gene functions at the early and late stages of terminal erythropoiesis. Journal of Visualized Experiments. 2014;91:e51894. doi: 10.3791/51894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shearstone JR, Pop R, Bock C, Boyle P, Meissner A, Socolovsky M. Global DNA demethylation during mouse erythropoiesis in vivo. Science. 2011;334(6057):799–802. doi: 10.1126/science.1207306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ghaffari S. Oxidative stress in the regulation of normal and neoplastic hematopoiesis. Antioxidants & redox signalling. 2008;10(11):1923–40. doi: 10.1089/ars.2008.2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Socolovsky M, Fallon AE, Wang S, Brugnara C, Lodish HF. Fetal anaemia and apoptosis of red cell progenitors in Stat5a−/−5b−/− mice: a direct role for Stat5 in Bcl-X(L) induction. Cell. 1999;98(2):181–91. doi: 10.1016/s0092-8674(00)81013-2. [DOI] [PubMed] [Google Scholar]
- 14.Socolovsky M, Nam H, Fleming MD, Haase VH, Brugnara C, Lodish HF. Ineffective erythropoiesis in Stat5a(−/−)5b(−/−) mice due to decreased survival of early erythroblasts. Blood. 2001;98(12):3261–73. doi: 10.1182/blood.v98.12.3261. [DOI] [PubMed] [Google Scholar]
- 15.Johnson RM, Goyette G, Jr., Ravindranath Y, Ho YS. Haemoglobin autoxidation and regulation of endogenous H2O2 levels in erythrocytes. Free radical biology & medicine. 2005;39(11):1407–17. doi: 10.1016/j.freeradbiomed.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 16.Wang S, Dale GL, Song P, Viollet B, Zou MH. AMPKalpha1 deletion shortens erythrocyte life span in mice: role of oxidative stress. The Journal of biological chemistry. 2010;285(26):19976–85. doi: 10.1074/jbc.M110.102467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marinkovic D, Zhang X, Yalcin S, Luciano JP, Brugnara C, Huber T, Ghaffari S. Foxo3 is required for the regulation of oxidative stress in erythropoiesis. The Journal of clinical investigation. 2007;117(8):2133–44. doi: 10.1172/JCI31807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dallalio G, Means RT., Jr Effects of oxidative stress on human erythroid colony formation: Modulation by gamma-interferon. Journal of laboratory and clinical medicine. 2003;141(6):395–400. doi: 10.1016/S0022-2143(03)00041-6. [DOI] [PubMed] [Google Scholar]
- 19.Carlile GW, Smith DH, Wiedmann M. Caspase-3 has a nonapoptotic function in erythroid maturation. Blood. 2004;103(11):4310–6. doi: 10.1182/blood-2003-09-3362. [DOI] [PubMed] [Google Scholar]
- 20.Zermati Y, Garrido C, Amsellem S, Fishelson S, Bouscary D, Valensi F, Varet B, Solary E, Hermine O. Caspase activation is required for terminal erythroid differentiation. The Journal of experimental medicine. 2001;193(2):247–54. doi: 10.1084/jem.193.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao B, Mei Y, Schipma M, Roth E, Bleher R, Rappoport J, Wickrema A, Yang J, Ji P. Nuclear condensation during mouse erythropoiesis requires caspase-3-mediated nuclear opening. Developmental cell. 2016;36:498–10. doi: 10.1016/j.devcel.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]