Abstract
Epidemiological studies link fructose consumption with metabolic disease, an association attributable in part to fructose mediated lipogenesis. The mechanisms governing fructose-induced lipogenesis and disease remain debated. Acutely, fructose increases de novo lipogenesis through the efficient and uninhibited action of Ketohexokinase and Aldolase B, which yields substrates for fatty-acid synthesis. Chronic fructose consumption further enhances the capacity for hepatic fructose metabolism via activation of several key transcription factors (i.e. SREBP1c and ChREBP), which augment expression of lipogenic enzymes, increasing lipogenesis, further compounding hypertriglyceridemia, and hepatic steatosis. Hepatic insulin resistance develops from diacylglycerol-PKCε mediated impairment of insulin signaling and possibly additional mechanisms. Initiatives that decrease fructose consumption and therapies that block fructose mediated lipogenesis are needed to avert future metabolic pandemics.
Introduction
We find ourselves in the midst of a health epidemic that is assuredly linked to our modern lifestyle. Yet we are unable to pinpoint the precise causes and avoid the ominous consequences. Over two thirds of American adults are considered overweight or obese (and 1 in 20 are considered to be extremely obese) [1]. Alarmingly, nearly a third of American children are also overweight or obese [1]. The outlook is even darker when considering the rising prevalence of obesity-associated conditions, such as type 2 diabetes, nonalcoholic fatty liver disease and atherosclerosis. Though this epidemic is multifactorial, sugar consumption is often posited as a key contributor to the obesity epidemic and associated cardiometabolic disease. Certainly, ecological data show temporal associations between increased total sugar consumption, increased use of high-fructose corn syrup (HFCS), and the obesity epidemic [2]. The fructose component of sugar sweeteners (nearly equivalent in sucrose and HFCS) is considered a singularly harmful macronutrient. In particular, increased consumption of fructose, a more lipogenic sugar, has been suggested to lead to obesity, hyperlipidemeia and insulin resistance, key risk factors for common conditions including type 2 diabetes, nonalcoholic fatty liver disease and cardiovascular disease [3]. Sugar-sweetened beverages (SSBs) are the highest single contributor to dietary fructose intake in the US diet and are a reasonable proxy for sugar and fructose consumption in epidemiological studies [4]. Observational studies performed in the Framingham cohort have found that daily consumption of SSBs are linked to increased visceral adiposity, hypertriglyceridemia, non-alcoholic fatty liver disease, and insulin resistance [5–7]. This review will focus on the biology of fructose. Specifically, we will consider the effects of fructose on metabolic health, how fructose is metabolized distinctly from glucose, and the impact of fructose metabolism on cellular signaling pathways, nutrient storage, and insulin action.
High fructose consumption rapidly impacts metabolism
Short-term studies lasting several weeks and using high-doses of fructose have supported the causal relationship between high-fructose consumption, obesity and cardiometabolic disease suggested by ecological and epidemiological data. Stanhope et al. performed a comprehensive set of studies in overweight and obese individuals receiving 25% of their daily caloric intake as either a glucose sweetened beverage or fructose sweetened beverage [8]. Though both groups experienced similar weight gain over the 10-weeks of feeding, the metabolic impact was markedly different. First, the fructose supplemented group had a greater increase in intra-abdominal adipose mass. Second, consuming fructose, but not glucose containing beverages, increased post-prandial plasma triglyceride concentrations (in as early as two weeks). This occurred in association with increased de novo lipogenesis. Moreover, fructose consumption increased both fasting glucose and insulin concentrations and worsened glucose tolerance.
The rapidity with which excessive dietary fructose consumption exerts adverse metabolic effects is striking. Schwarz et al. demonstrated that de novo lipogenesis was ~60% higher in healthy men fed an isocaloric, high-fructose diet (25% calories) for nine days relative to when the subjects ate a complex carbohydrate diet [9]. Le et al. compared normoglycemic, lean, insulin sensitive individuals with normoglycemic and lean, but insulin resistant offspring of patients with T2DM before and after 7 days of a hypercaloric diet in which an additional 35% of their calories were derived from fructose [10]. Both groups of patients had similar increases in VLDL triglyceride, intrahepatic and intramyocellular lipid content associated with a reduction in hepatic insulin sensitivity. The increase in VLDL was markedly higher in the offspring of patients with T2DM, suggesting that insulin resistance enhances the pathogenicity of fructose. Even within 24 hours, Teff et al., observed that fructose sweetened beverages increased plasma triglycerides relative to glucose sweetened beverages in obese men and women [11]. In this cohort, the presence of pre-existing insulin resistance further exacerbated fructose-induced hypertriglyceridemia. Thus, short term exposure to large doses of fructose can acutely and robustly impact glucose and lipid metabolism, and insulin resistance may potentiate sugar-induced hypertriglyceridemia.
Metabolic effects of typical dietary sugar consumption
Some question the public health relevance of these short-term, mechanistic studies, as the fructose doses greatly exceed typical quantities consumed by most people. For instance, Stanhope et al. studied subjects receiving 25% calories from glucose, fructose or HFCS [12], whereas in the US, fructose consumption on average accounts for ~10% of consumed calories or 50–60 grams / day [4]. Recent studies have sought to determine whether “typical” doses of sugar or fructose might regulate traits associated with cardiometabolic disease. Young, non-obese subjects were prospectively assigned to isocaloric diets in which they would receive 0, 10, 17.5 or 25% calories from high-fructose corn syrup (~ 4.5, 7.9 and 11% from fructose) for ~ 2 weeks. There were dose dependent increases in weight gain and serum lipid concentrations, suggesting that even low doses of fructose (akin to what many consume) have detrimental health consequences [13]. Additional studies have been conducted to determine whether commonly ingested amounts of fructose or sucrose can acutely regulate pathophysiological processes implicated in the development of metabolic disease and cardiac risk factors. Theytaz and colleagues gave non-obese, young human subjects a single meal that contained ~30gm of fructose (or ~7.5% of a daily caloric requirement) and demonstrated increases in both chylomicron and VLDL triglyceride concentrations several hours after the test meal [14]. Thus, fructose exposures that better mirror typical consumption still promote lipogenesis and hyperlipidemia. Longer-term prospective studies are required to establish the full health impact of “modestly” increased sugar or fructose consumption.
Biochemistry of fructose metabolism
Fructose is transported into cells via GLUT5, a specific fructose transporter highly expressed along the brush border of in the small intestine, and GLUT2 [15], a transporter for both glucose and fructose expressed in the liver, small intestine (along the basolateral membrane) and pancreas. The majority of ingested fructose passes via the portal circulation to the liver where it is rapidly cleared. Very little of this escapes the liver first-pass, limiting peak peripheral fructose levels to high micromolar or low millimolar concentrations [11, 16]. Intracellularly, fructose is rapidly phosphorylated by Ketohexokinase (KHK) into fructose 1-phosphate (F1P) (Figure 1). F1P is subsequently cleaved by Aldolase B (Aldob) into glyceraldehyde and dihydoxyacetone phosphate (DHAP). Glyceraldehyde requires an additional phosphorylation by a triose kinase likely dihydroxyacetone kinase 2 (DAK), a mammalian homologue of a yeast enzyme with triose kinase activity ([17]). The triose phosphates resulting from “fructolysis” become substrates for gluconeogenesis, lipogenesis, or cellular respiration. Increased use of fructose-derived substrate for lipogenesis (both fatty-acid synthesis and fatty-acid esterification into triglyceride) may largely account for the increases in plasma triglyceride concentrations after short-term, high fructose exposure. However, chronic fructose intake may trigger signaling events to further enhance lipogenesis as will be discussed in detail later.
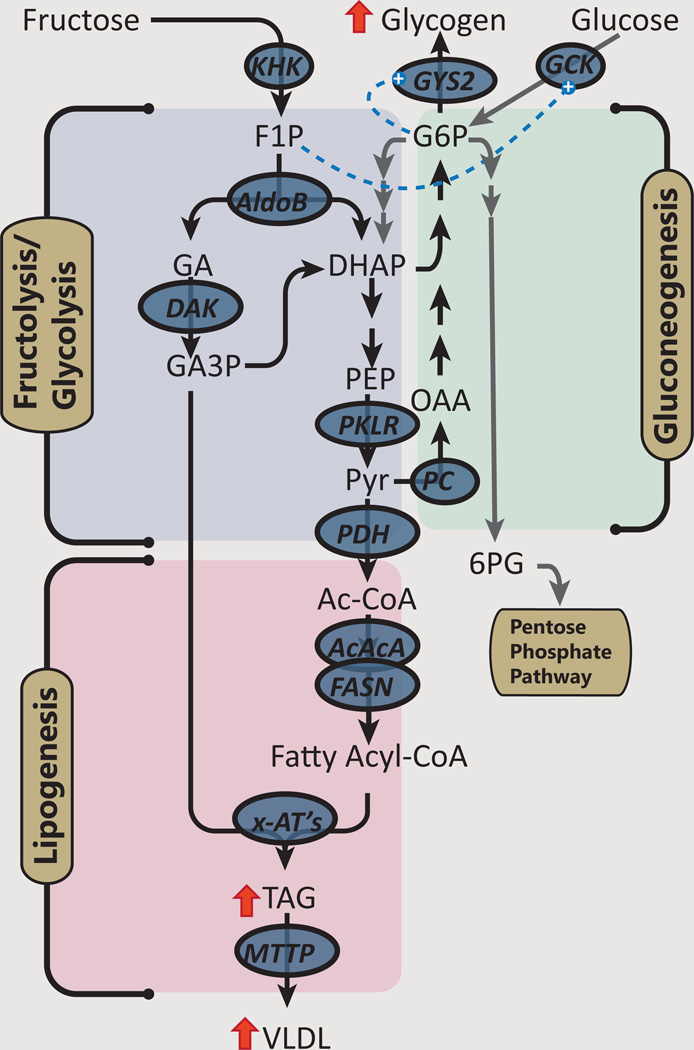
Figure 1. Fructose provides substrate for lipogenesis and glycogenesis.
After acute consumption, fructose is efficiently metabolized by ketohexokinase (KHK) into fructose 1-phosphatase. Fructose 1-phosphate has only one pathy: cleavage by aldolase B (AldoB) into dihydroxyacetone phosphate (DHAP) and glyceraldhyde. The latter is phosphorylated by dihydroxyacetone kinase-2 (DAK) to provide glyceraldhyde 3-phosphate. In contrast, glucose 6-phosphatae can be used for glycogen synthesis, glycolysis or enter the pentose phosphate pathway. The triose-phosphates formed from fructolysis (or glycolysis) can resynthesized into hexoses for glycogen synthesis or further metabolized into acetyl CoA, which can be oxidized or used for lipogenesis. Fructose metabolism augments glycogen synthesis from glucose via activating glucokinase (GCK). Additionally, glucose 6-phosphate (G6P) produced from either direct phosphorylation of glucose, or indirect resynthesis from triose phosphates can allosterically activate glycogen synthase (GYS2). Other abbreviations: PKLR: Pyruvate Kinase-Liver/Red Blood Cell; PC: pyruvate carboxylase; PDH: pyruvate dehydrogenase complex; ACACA: acetyl-CoA carboxylase alpha; FASN: fatty acid synthase; x-AT connotes various acetyl transferases (e.g MGAT, DGAT, etc.); F1P: Fructose 1-phosphate; PEP: phosphenolpyruvate; OAA: oxaloacetate; Ac-CoA: acetyl CoA;
Fructose-derived carbons are also stored in hepatic glycogen. A recent study assessing the pathways of glycogen synthesis in sucrose fed rats compared to regular chow fed rats found ~ 4-fold increase in the contribution of triose phosphates to glycogen synthesis which reflects the contribution of fructose [18]. Fructose, or specifically F1P, activates glucokinase (Gck), by promoting the release of Gck from Glucokinase Regulatory Protein (Gckr) in the nucleus [19, 20]. Thus, F1P can enhance G6P production from glucose which may further activate glycogen synthase, inhibit glycogen phosphorylase and further enhance glycogen deposition [21].
Activation of Gck occurs with “catalytic” amounts of fructose: replacement of ~10% of a glucose load with fructose increased hepatic glycogen synthesis [22], improved glucose tolerance, and reduced insulin secretion in normal subjects and patients with type 2 diabetes [23].
KHK as a gatekeeper for fructose metabolism
Fructose per se is likely not a bioactive molecule; both the substrate-level and signaling effects of fructose require fructose metabolism. KHK catalyzes the first step in intracellular fructose metabolism (Figure 1). This enzyme exists in two splice isoforms, the more widely expressed, but less active KHK-A, and a selectively expressed (liver, kidney, small intestine) but more biologically active KHK-C. Whereas ATP generated via glycolysis inhibits phosphofructokinase to limit further glycolytic flux [24], KHK activity is not inhibited by ATP [25]. Additionally, fructose-derived triose phosphates enter the glycolytic pathway distal to phosphofructokinase. Thus, fructolysis will continue unabated, even when glycolysis is inhibited by positive cellular energy balance.
While KHK is essential for fructose metabolism, it is not essential for life. Homozygous loss-of-function mutations in KHK cause the benign human condition Essential Fructosuria, characterized by large and persistent increases in blood and urine fructose after sucrose or fructose ingestion [26]. Though data on these individuals is sparse, they appear to have no evidence of metabolic disease [26].
To explore the roles of KHK isoforms in fructose metabolism and metabolic disease, Diggle and colleagues created two separate mouse strains lacking KHK-A alone (KHK-A−/−) or both KHK isoforms (KHK-A/C−/−) [27]. Preliminary phenotypic assessments did not find marked differences between KHK-A−/− or KHK-A/C−/− compared to wild-type mice, other than for a marked increase in plasma fructose concentrations in both knockout models. Body weight, plasma glucose, insulin and lipid concentrations were unchanged. However, significant differences emerged when these mice were challenged with a high-fructose diet (30% fructose water) [28]. The KHK-A/C−/− mice demonstrated fructosuria and gained less weight than wild type mice on a high-fructose diet. Fructose-fed KHK-A/C−/− mice had decreased adiposity, hepatic lipid content and serum cholesterol and insulin concentrations compared to fructose-fed control mice. In contrast, selectively knocking out the KHK-A isoform exacerbated the adverse metabolic phenotype associated with fructose feeding - increasing adiposity, hepatic lipid content and plasma insulin concentration [28]. KHK-A/C−/− mice were also protected from steatohepatitis from chronic high fat (36% calories)/high-fructose (30% calories) feeding [29]. These results suggest that fructose metabolism in tissues expressing KHK-C such as liver are required for fructose-induced metabolic disease, and ablating KHK-A may shunt fructose to tissues expressing KHK-C further exacerbating disease-associated processes like hepatic lipogenesis.
KHK may also regulate specific aspects of enterocyte fructose metabolism. Deletion of KHK diminished the increase in enterocyte GLUT5 expression in response to high-fructose feeding [30]. The mechanistic links between enterocyte fructose metabolism and regulation of GLUT5 and other genes remains obscure, though changes in cyclic AMP [31], phosphatidylinositol 3-kinase [32], and histone deacetylation of specific regions of enterocyte chromatin [33, 34], have all been implicated. How KHK expression is itself regulated is also unclear, though some data suggest that chronic fructose feeding increases hepatic KHK expression, possibly due to the activity of the carbohydrate-sensing transcription factor, Carbohydrate-Responsive Element-Binding Protein (ChREBP) [35, 36], or possibly cellular uric acid [37].
Whereas glucose-derived, G6P has multiple potential metabolic fates including incorporation into glycogen or flux through glycolysis or the pentose phosphate pathway, F1P has a single fate – cleavage by Aldob to yield DHAP and glyceraldehyde (Figure 1). Like KHK, Aldob is induced by feeding and chronic fructose exposure [38], and also appears to be regulated by ChREBP [35, 39]. Deficiencies in Aldob result in Hereditary Fructose Intolerance characterized by energetic crises following fructose ingestion, with marked depletion of ATP and increases in uric acid, due to the action of purine salvage pathways [40, 41]. Aldob knockout mice fail to thrive on diets containing even small amounts of fructose and demonstrate marked toxicity when challenged with a high-fructose diet, with 100% mortality and marked liver damage [42]. Presumably, this toxicity is related to accumulation of F1P or some other fructose-derived metabolite. Interestingly, even on a fructose free diet, Aldob−/− mice develop some steatosis, potentially due to persistent toxic metabolic effects of aberrant metabolism of endogenously synthesized fructose [43].
Chronic Fructose consumption activates a program of lipogenesis
To review the prior section, acute fructose ingestion contributes to lipid synthesis via the flux of fructose carbons into lipogenic precursors. However, we’ve also known for several decades that chronic fructose feeding can further enhance the capacity to metabolize fructose into lipid. Some of this adaptation may occur in the small intestine. As previously mentioned, chronic fructose exposure may increase enterocytes’ capacity to absorb fructose by upregulating GLUT5, and may also enhance enterocyte lipid synthesis [44, 45]. However, the bulk of lipid synthesis from fructose likely occurs in the liver, where the activities of key enzymes regulating lipid synthesis, such as malic enzyme [46], pyruvate dehydrogenase (PDH) [47] and fatty acid synthase (FASN) [48], are clearly increased by chronic fructose exposure. The following sections will review some of the signaling mechanisms whereby chronic fructose consumption may promote hepatic lipid synthesis (Figure 2).
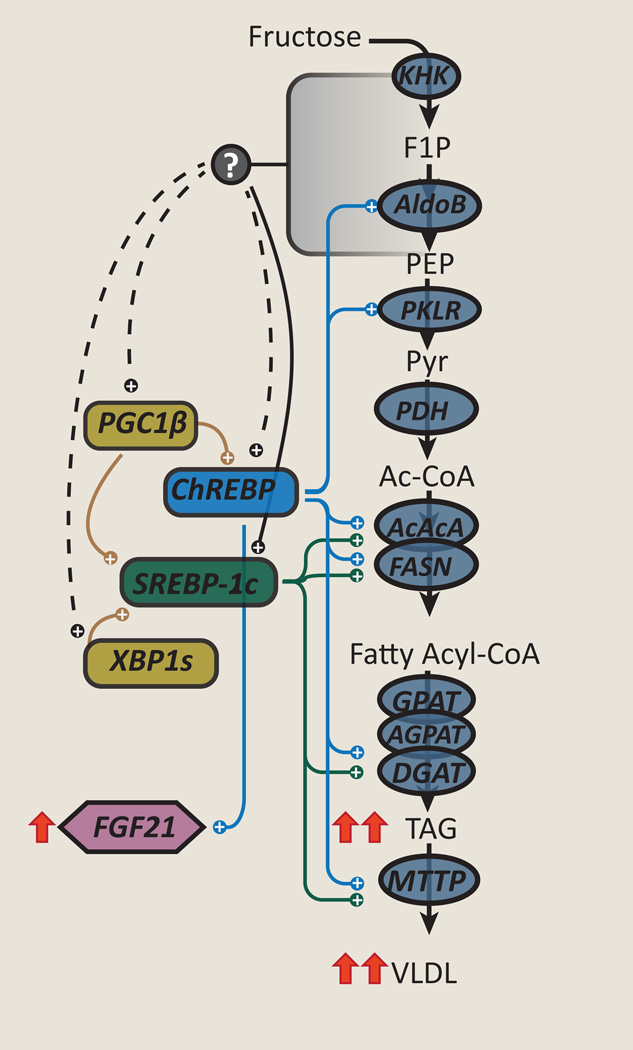
Figure 2. Chronic fructose exposure activates coordinate programs of lipogenesis.
Fructose exposure can increase activity of sterol response element binding protein 1c (SREBP1c) and carbohydrate-responsive element-binding protein (ChREBP), which can increase expression of several enzymes involved in lipogenesis from fructose. This lipogenic program is further enhanced by various other factors, such as peroxisome proliferator-activated receptor gamma coactivator 1-beta (PGC1β) and X-box binding protein 1s (XBP1s). The increased expression of these enzymes can further enhance de novo lipogenesis and worsen hypertriglyceridemia. In addition, fructose can increase FGF21 in a ChREBP dependent fashion. The metabolic signal(s) from fructose metabolism that activates these pathways are not known.
Sterol regulatory element-binding protein 1c (SREBP1c)
SREBP1c, a master regulator of lipid synthesis, is itself regulated by nutrients and hormones at the transcriptional and post-translational level. Additionally, via a feed-forward loop, SREBP1c increases its own transcription further amplifying a lipogenic stimulus. Chronic high-fructose feeding increases hepatic SREBP1c expression in rodents [49, 50] (Figure 2). Insulin is considered to be a potent activator of SREBP1c, both by increasing mRNA expression and proteolytic processing of the SREBP1c precursor protein into a mature, nuclear transcription factor [51]. Insulin signaling also increases the activity of mechanistic target of rapamycin complex 1 (mTORC1) which may also regulate SREBP1c mRNA expression [52] and processing [53]. Thus, hyperinsulinemia induced by chronic fructose or sucrose feeding may be one factor that enhances SREBP1c activity and lipogenesis. But, fructose-induced activation of SREBP1c may be partly insulin-independent. Liver-specific insulin receptor knockout (LIRKO) mice still exhibit prandial activation of a lipogenic program including SREBP1c, when placed on a 60% high-fructose diet for 1-week, though the increases in lipogenic gene expression were attenuated in comparison to wild-type mice [54]. In contrast to wild-type mice, liver triglyceride content did not increase in fructose-fed LIRKO mice, suggesting that insulin action may be required for the development of fructose-induced hepatic steatosis. Thus, chronic fructose feeding may activate SREBP1c-mediated lipogenesis via insulin-signaling dependent and independent pathways.
ChREBP
ChREBP, also known as Mlx interacting protein-like (Mlxipl), like SREBP1c, is another master transcriptional regulator of lipogenic gene expression programs, and also regulates other metabolic programs including glycolysis [55]. ChREBP is highly expressed in key metabolic tissues including liver, adipose tissue, small intestine, pancreatic islets, and kidney and, as its name suggests, is activated by products of carbohydrate metabolism in an insulin-independent manner [36, 54, 55]. A specific role for ChREBP in regulating fructose consumption was suggested by the observation that ChREBP knockout mice were intolerant to diets containing fructose, but tolerant of diets containing dextrose [36]. Additionally, binding of ChREBP to its transcriptional targets as well as ChREBP-target gene expression was markedly higher in rats fed high-fructose compared to high-glucose diets [56] (Figure 2). High-carbohydrate diets have also been reported to increase hepatic expression of the novel, potent ChREBP-β isoform in mice, but a specific or selective effect of fructose has not yet been reported [57, 58]. The mechanisms by which carbohydrates and specifically fructose activate ChREBP remain controversial and may include allosteric activation by carbohydrate metabolites or post-translational modifications including phosphorylation, acetylation, and O-GlcNAcylation (reviewed in [59]).
A role for ChREBP in human cardiometabolic disease is suggested from genome-wide association studies which show that single nucleotide polymorphisms in the ChREBP locus associate with multiple features of the metabolic syndrome, including serum triglyceride and HDL-cholesterol levels [60, 61], elevated serum liver enzymes - a marker of fatty liver disease [62], and hyperuricemia [63]. Erion and colleagues demonstrated that knocking down hepatic ChREBP using ChREBP targeted antisense oligonucleotides (ASOs) in high-fructose fed rats lowers serum triglycerides and increases peripheral insulin sensitivity [35]. Intriguingly, though ChREBP ASO decreased the expression of lipogenic enzymes and de novo lipogenesis in fructose-fed rats, hepatic steatosis and hepatic insulin sensitivity did not improve. This discordance was attributed in part to a ChREBP ASO mediated reduction in the expression of microsomal triglyceride transfer protein (MTTP), a key enzyme in VLDL packaging and secretion. Notably, ChREBP ASO had minimal effects in high-fat fed rats [35]. These studies further substantiate ChREBP’s role as a central regulator of hepatic lipid metabolism in response to carbohydrates.
A Sugar-Responsive ChREBP-FGF21 Signaling Axis
Fibroblast growth factor 21 (FGF21) is a recently discovered hormone produced primarily by key metabolic tissues including the liver and adipose tissue [64–66] which has numerous actions on glucose and lipid homeostasis [67]. Although originally identified as a circulating factor that mediates an adaptive response to fasting or starvation in rodents [64, 68, 69], recent evidence indicates that FGF21 is regulated by ChREBP [39, 70] and also responds to fructose or sucrose consumption [71]. Dushay and colleagues recently demonstrated that fructose ingestion (75g) acutely and robustly increased circulating levels of FGF21 in humans, achieving a 4-fold increase within 2 hours [72]. This hormone-like FGF21 response to fructose is suggestive of a ChREBP-FGF21 signaling axis that may mediate an adaptive response to sucrose or fructose ingestion. Indeed, FGF21 may have a role in sugar preferences: exogenous FGF21 suppressed sweetened beverage consumption in mice and primates and FGF21 KO mice prefer sweet beverages [73, 74]. However, in humans, polymorphisms in the FGF21 locus that increase circulating FGF21 levels associate with increased, rather than decreased, carbohydrate consumption [75, 76]. Altogether these data support an emerging role for a ChREBP-FGF21 signaling axis as a regulator of sugar consumption, although more information is needed to complete our understanding.
Peroxisome proliferator-activated receptor gamma coactivator 1-beta (PGC1β)
SREBP1c and CHREBP regulated lipogenesis may be enhanced by other co-factors. PGC1β is a homolog of PGC1α [77] which, like PGC1α, increases the activities of many key transcription factors, such as peroxisome proliferator-activated receptor gamma (PPARγ), PPARα, estrogen related receptors (ERR), and liver X receptor (LXR) [78]. PGC1β is distinguished from PGC1α by its ability to potently bind to and transactivate SREBP1c [79] and ChREBP [80]. Nagai et al. used ASOs to decrease PGC1β expression in normal rats that were fed either a control chow or high-fructose diet, to assess the role of PGC1β in fructose induced lipogenesis [81]. While fructose feeding dramatically induced SREBP1c expression in comparison to regular chow, this was prevented by PGC1β ASO. PGC1 β ASO also prevented increases in adiposity, glycemia, and plasma insulin and triglycerides in fructose-fed rats. The decrease in SREBP1c expression attenuated induction of lipogenic enzymes such as FASN, which in turn accounted for the decreased accumulation of di- and triacylglycerol within the livers of fructose-fed rats. Recently, Chambers et al. showed that PGC1β, but not PGC1α, also interacts directly with ChREBP, and that PGC1β is required for carbohydrate-mediated upregulation of ChREBP targets [80]. Thus, the effects of PGC1β on lipogenic gene expression may be mediated through coordinate regulation of both SREBP1c and ChREBP, and potentially other binding partners yet to be identified.
Other putative mechanisms have also been described to explain the activation of lipogenesis with chronic fructose exposure. For example, some have suggested that fructose exposure may activate the unfolded protein response (UPR) or ER stress pathway [82, 83]. Recent studies suggest that fructose feeding activates mTOR which may suppress autophagy, a cellular process that helps maintain cellular homeostasis in part by removing misfolded proteins [83]. This occurred quickly, 6–12h after mice were fed a 35% fructose diet. Chronic activation of the UPR may increase expression of lipogenic enzymes. For example, inositol-requiring transmembrane kinase/endonuclease 1 (IRE1)-X-box binding protein 1 (XBP1) axis of the UPR regulates lipogenesis, possibly to support ER membrane expansion [84]. IRE1 is responsible for atypical splicing of the XBP1 mRNA into XBP1s, which encodes a transcription factor. Increases in XBP1s protein expression is evident in mice fed a 60% fructose diet [82] and conditional deletion of XBP1 (XBP1Δ) decreased expression of s everal key lipogenic enzymes, reduced hepatic lipid accumulation and protected mice from hepatic insulin resistance [82, 85].
Fructose and Insulin Resistance
High fructose feeding leads to insulin resistance. Stanhope et al. found that nine weeks of high-fructose feeding resulted in glucose intolerance and insulin resistance in humans [8]. Aeberli et al. fed a group of young volunteers diets supplemented with SSB’s providing either an extra 40g (~9% total energy consumption) or 80g of fructose (~14% of total energy consumption), in comparison to 80g of glucose or sucrose, for three weeks [86]. Hepatic insulin resistance was only evident in the higher fructose fed group. Schwarz et al. fed eight healthy men an isocaloric weight maintaining diet for nine days in which they received either complex carbohydrate or fructose (25% of energy requirements) [9]. High fructose feeding increased lipogenesis and impaired insulin-mediated suppression of hepatic glucose production. Thus, when fructose intake is sufficient to stimulate lipogenesis, hepatic insulin resistance also develops.
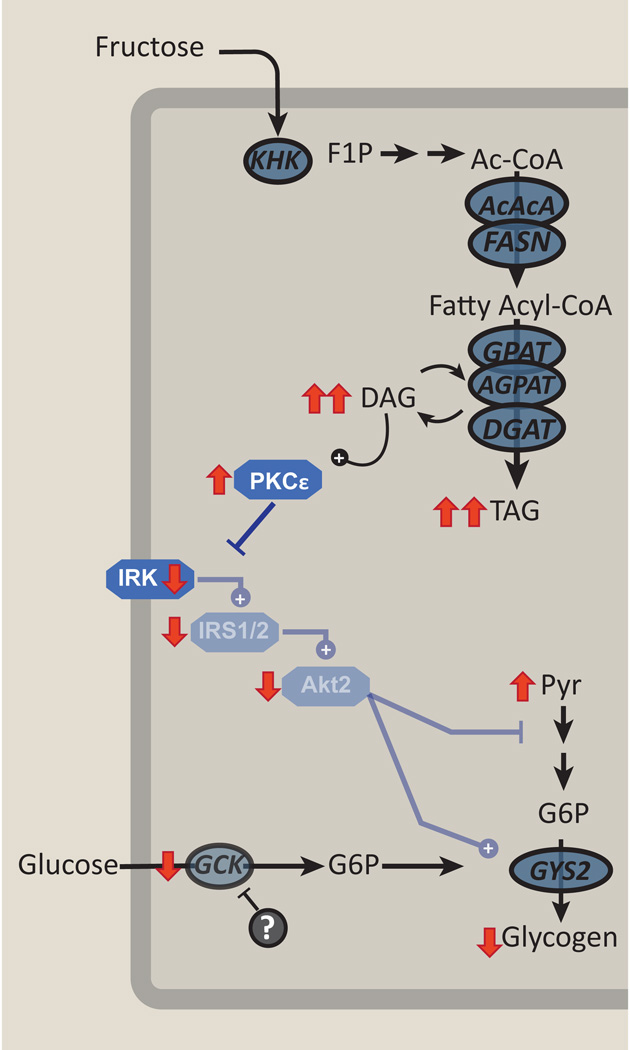
The mechanisms linking hepatic steatosis and hepatic insulin resistance have been more thoroughly investigated in high-fat feeding paradigms. Short-term, high-fat feeding in rodents is associated with steatosis and marked hepatic insulin resistance without peripheral resistance [87, 88]. This is associated with increased hepatic fatty acyl CoA and diacylglycerol content without any change in muscle lipid. Studies examining the mechanisms of fat-induced muscle insulin resistance in rodents and humans suggested that diacylglycerol-mediated activation of a novel protein kinase C (PKC) isoform, PKC-θ, could interfere with insulin signaling [89–91]. PKCε is a novel PKC that is highly expressed in liver. PKCε is activated with hepatic steatosis and associated with hepatic insulin resistance in numerous rodent models and in humans [87, 92–94]. With diacylglycerol accumulation, PKCε activation impairs insulin receptor kinase (IRK) activation. While PKCε ASO treatment did not prevent hepatic steatosis in fat-fed rats, it did preserve hepatic insulin signaling [95]. Fructose feeding itself has been associated with diacylglycerol accumulation, PKC activation and impaired insulin mediated Akt2 activation [81, 85] (Figure 3). However, fructose feeding may lead to insulin resistance through additional mechanisms. Coate and colleagues demonstrated that dogs fed a high-fat diet or high-fructose (17% of calories from fructose) diet for four weeks developed a similar degree of insulin resistance [96]. Both diets produced similar increases in body weight and plasma and liver triglycerides. High-fat feeding impaired insulin-stimulated Akt phosphorylation but the high-fructose diet did not. Instead, chronic high-fructose feeding markedly impaired Gck protein expression, Gck activity and glycogen synthesis. Though similar changes in Gck activity were also present in high-fat fed dogs, the extent was greater with high-fructose feeding (Figure 3). Thus, mechanisms linking fructose metabolism to the development of insulin resistance may be distinct from diacylglycerol and PKCε mediated changes in insulin signaling.
Figure 3. Chronic fructose exposure will cause hepatic insulin resistance.
Persistent fructose mediated lipogenesis will increase hepatic diacyglycerol (DAG) content, leading to activation of protein kinase c-epsilon (PKCε). This impairs insulin receptor kinase activation (IRK), decreasing the activation of downstream kinases, such as insulin receptor substrate-1/2 (IRS1/2) and Akt2, ultimately limiting the ability of insulin to suppress gluconeogenesis and activate glycogen synthesis. Chronic fructose exposure may act through other mechanisms to further impair activation of glucokinase.
Concluding remarks and future perspectives
Sugar has built empires and industries but now, sugar consumption threatens global health. Though controversy exists about the contribution of current fructose consumption to the rising prevalence of metabolic disease, several aspects are clear. Fructose is a lipogenic dietary sugar. The pathways governing its acute metabolism favor its rapid metabolism into the precursors used for lipid synthesis. Chronic high fructose consumption elicits a series of coordinated changes that further enhance the capacity for lipid synthesis from fructose. And, increases in hepatic steatosis and plasma lipid concentrations may contribute to insulin resistance and promote cardiovascular disease. The various mechanisms that underpin these adaptations may provide novel opportunities for therapeutic interventions.
Of course, behavioral changes could also help. Aerobic exercise may prevent some of the adverse metabolic consequences of a high-fructose diet. In a small study, subjects who exercised for four days while consuming a high fructose (30% total energy) diet were protected from fructose induced triglyceride synthesis. Reducing fructose intake is also critical. The American Academy of Pediatrics provides clear guidelines to limit juice and sweetened beverage intake in children [97], and advocates limiting sales of sugar sweetened beverages (SSB) in schools [98]. Decreasing SSB has been shown to lead to weight loss in overweight children [99]. Mexico enacted a 1 peso/liter excise tax of SSB in 2013 and recent data has shown that this maneuver has decreased purchases of SSB [100]. However, similar legislation has failed to gain traction in the United States. Further research into the role of dietary fructose in the development of metabolic diseases and the mechanisms by which chronic fructose consumption leads to adaptive changes to further enhance lipogenesis are required, to better inform clinical investigators and policy makers. An informed, coordinated and comprehensive approach will provide the needed path out of our current epidemic and back to a healthy society.
Trends Box.
Fructose comprises ~ 50% of the dietary sugars sucrose and high-fructose corn syrup, and when consumed in excess exacerbates cardiometabolic risk factors including dyslipidemia, fatty liver disease and insulin resistance.
Ketohexokinase (KHK) may be a therapeutic target. Complete knockout of all KHK isoforms prevents fructose-induced disease. In contrast, selective knockout of the ubiquitous, low-activity KHK-A isoform exacerbates fructose-induced disease, possibly by increasing flux through the KHK-C isoform expressed in key metabolic tissues, like liver.
Fructose contributes to lipogenesis and associated pathologies like steatosis, dyslipidemia, and hepatic insulin resistance both by providing substrate and coordinating expression of lipogenic enzymes via Srebp1c and ChREBP.
Limiting fructose intake and regulating fructose metabolism may represent a promising therapeutic strategy to reduce cardiometabolic risk factors.
Outstanding Questions Box.
Consumption of large doses of fructose by humans and animals can rapidly increase cardiometabolic risk factors but whether fructose consumed in more typical quantities is a major contributor to epidemic of obesity and its associated comorbidities remains uncertain.
Metabolism of fructose in tissues expressing KHK-C like liver, kidney, and small intestine appear to be important for fructose-induced metabolic disease but what are the relative contributions of these tissues? For instance, fructose consumption regulates intestinal gene expression. How does this occur and and what contribution does this make to systemic metabolism?
Fructose enhances de novo lipogenesis (DNL) by providing substrate and by activating lipogenic gene expression programs. What are the metabolic signals from fructose metabolism that converge on the activation of transcription factors such as Srebp1c and ChREBP?
Emerging evidence suggests that fructose-mediated activation of ChREBP regulates circulating FGF21 levels and that FGF21 regulates sugar consumption and sweet taste preferences. Does FGF21 contribute to an adaptive metabolic response to sugar consumption in other ways?
High fructose feeding leads to hepatic insulin resistance and this may be in part mediated by fructose-induced accumulation of diacylglycerol which activates PKC and impairs insulin signaling. However, recent data suggests that fructose may induce hepatic insulin resistance by additional means. What are additional mechanisms by which fructose regulates hepatic glucose metabolism and hepatic insulin signaling?
Acknowledgments
MAH is supported by NIH R01 DK100425.
VTS is supported by VA I01 BX000901
References
- 1.Ogden CL, et al. Prevalence of Obesity Among Adults and Youth: United States, 2011–2014. NCHS Data Brief. 2015:1–8. [PubMed] [Google Scholar]
- 2.Bray GA, et al. Consumption of high-fructose corn syrup in beverages may play a role in the epidemic of obesity. Am J Clin Nutr. 2004;79:537–543. doi: 10.1093/ajcn/79.4.537. [DOI] [PubMed] [Google Scholar]
- 3.Lim JS, et al. The role of fructose in the pathogenesis of NAFLD and the metabolic syndrome. Nat Rev Gastroenterol Hepatol. 7:251–264. doi: 10.1038/nrgastro.2010.41. [DOI] [PubMed] [Google Scholar]
- 4.Marriott BP, et al. National Estimates of Dietary Fructose Intake Increased from 1977 to 2004 in the United States. The Journal of Nutrition. 2009;139:1228S–1235S. doi: 10.3945/jn.108.098277. [DOI] [PubMed] [Google Scholar]
- 5.Green AK, et al. Sugar-sweetened beverages and prevalence of the metabolically abnormal phenotype in the Framingham Heart Study. Obesity (Silver Spring) 2014;22:E157–E163. doi: 10.1002/oby.20724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ma J, et al. Sugar-sweetened beverage consumption is associated with abdominal fat partitioning in healthy adults. J Nutr. 2014;144:1283–1290. doi: 10.3945/jn.113.188599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ma JFC, Speliotes EK, Hoffmann U, Smith CE, Saltzman E, Jacques PF, McKeown NM. Sugar-sweetened beverage intake is associated with fatty liver in the Framingham Offspring Study. The FASEB Journal. 2014;28 267.263. [Google Scholar]
- 8.Stanhope KL, et al. Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J Clin Invest. 2009;119:1322–1334. doi: 10.1172/JCI37385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schwarz JM, et al. Effect of a High-Fructose Weight-Maintaining Diet on Lipogenesis and Liver Fat. J Clin Endocrinol Metab. 2015;100:2434–2442. doi: 10.1210/jc.2014-3678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Le KA, et al. Fructose overconsumption causes dyslipidemia and ectopic lipid deposition in healthy subjects with and without a family history of type 2 diabetes. Am J Clin Nutr. 2009;89:1760–1765. doi: 10.3945/ajcn.2008.27336. [DOI] [PubMed] [Google Scholar]
- 11.Teff KL, et al. Endocrine and Metabolic Effects of Consuming Fructose- and Glucose-Sweetened Beverages with Meals in Obese Men and Women: Influence of Insulin Resistance on Plasma Triglyceride Responses. J Clin Endocrinol Metab. 2009;94:1562–1569. doi: 10.1210/jc.2008-2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stanhope KL, et al. Consumption of Fructose and High Fructose Corn Syrup Increase Postprandial Triglycerides, LDL-Cholesterol, and Apolipoprotein-B in Young Men and Women. The Journal of Clinical Endocrinology and Metabolism. 2011;96:E1596–E1605. doi: 10.1210/jc.2011-1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stanhope KL, et al. A dose-response study of consuming high-fructose corn syrup-sweetened beverages on lipid/lipoprotein risk factors for cardiovascular disease in young adults. Am J Clin Nutr. 2015;101:1144–1154. doi: 10.3945/ajcn.114.100461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Theytaz F, et al. Metabolic fate of fructose ingested with and without glucose in a mixed meal. Nutrients. 2014;6:2632–2649. doi: 10.3390/nu6072632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Colville CA, et al. Kinetic analysis of the liver-type (GLUT2) and brain-type (GLUT3) glucose transporters in Xenopus oocytes: substrate specificities and effects of transport inhibitors. Biochemical Journal. 1993;290:701–706. doi: 10.1042/bj2900701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prieto PG, et al. Plasma D-glucose, D-fructose and insulin responses after oral administration of D-glucose, D-fructose and sucrose to normal rats. J Am Coll Nutr. 2004;23:414–419. doi: 10.1080/07315724.2004.10719386. [DOI] [PubMed] [Google Scholar]
- 17.Cabezas A, et al. Identification of human and rat FAD-AMP lyase (cyclic FMN forming) as ATP-dependent dihydroxyacetone kinases. Biochem Biophys Res Commun. 2005;338:1682–1689. doi: 10.1016/j.bbrc.2005.10.142. [DOI] [PubMed] [Google Scholar]
- 18.Delgado TC, et al. 2H enrichment distribution of hepatic glycogen from 2H2O reveals the contribution of dietary fructose to glycogen synthesis. American Journal of Physiology - Endocrinology and Metabolism. 2013;304:E384–E391. doi: 10.1152/ajpendo.00185.2012. [DOI] [PubMed] [Google Scholar]
- 19.Brown KS, et al. Glucokinase Regulatory Protein May Interact With Glucokinase in the Hepatocyte Nucleus. Diabetes. 1997;46:179–186. doi: 10.2337/diab.46.2.179. [DOI] [PubMed] [Google Scholar]
- 20.Niculescu L, et al. Investigation on the mechanism by which fructose, hexitols and other compounds regulate the translocation of glucokinase in rat hepatocytes. Biochem J. 1997;321(Pt 1):239–246. doi: 10.1042/bj3210239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Agius L. Glucokinase and molecular aspects of liver glycogen metabolism. Biochem J. 2008;414:1–18. doi: 10.1042/BJ20080595. [DOI] [PubMed] [Google Scholar]
- 22.Petersen KF, et al. Stimulating effects of low-dose fructose on insulin-stimulated hepatic glycogen synthesis in humans. Diabetes. 2001;50:1263–1268. doi: 10.2337/diabetes.50.6.1263. [DOI] [PubMed] [Google Scholar]
- 23.Moore MC, et al. Acute fructose administration decreases the glycemic response to an oral glucose tolerance test in normal adults. J Clin Endocrinol Metab. 2000;85:4515–4519. doi: 10.1210/jcem.85.12.7053. [DOI] [PubMed] [Google Scholar]
- 24.Kagimoto T, Uyeda K. Hormone-stimulated phosphorylation of liver phosphofructokinase in vivo. Journal of Biological Chemistry. 1979;254:5584–5587. [PubMed] [Google Scholar]
- 25.Adelman RC, et al. Purification and Properties of Rat Liver Fructokinase. Journal of Biological Chemistry. 1967;242:3360–3365. [PubMed] [Google Scholar]
- 26.Steinmann B, et al. Disorders of Fructose Metabolism. In: Valle D, et al., editors. Scriver's Online Metabolic and Molecular Bases of Inherited Disease. 2006. [Google Scholar]
- 27.Diggle CP, et al. Both isoforms of ketohexokinase are dispensable for normal growth and development. Physiological Genomics. 2010;42A:235–243. doi: 10.1152/physiolgenomics.00128.2010. [DOI] [PubMed] [Google Scholar]
- 28.Ishimoto T, et al. Opposing effects of fructokinase C and A isoforms on fructose-induced metabolic syndrome in mice. Proceedings of the National Academy of Sciences. 2012;109:4320–4325. doi: 10.1073/pnas.1119908109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ishimoto T, et al. High-fat and high-sucrose (western) diet induces steatohepatitis that is dependent on fructokinase. Hepatology. 2013;58:1632–1643. doi: 10.1002/hep.26594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patel C, et al. Fructose-induced increases in expression of intestinal fructolytic and gluconeogenic genes are regulated by GLUT5 and KHK. Am J Physiol Regul Integr Comp Physiol. 2015;309:R499–R509. doi: 10.1152/ajpregu.00128.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mahraoui L, et al. Regulation of expression of the human fructose transporter (GLUT5) by cyclic AMP. Biochem J. 1994;301(Pt 1):169–175. doi: 10.1042/bj3010169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cui XL, et al. Fructose-induced increases in neonatal rat intestinal fructose transport involve the PI3-kinase/Akt signaling pathway. Am J Physiol Gastrointest Liver Physiol. 2005;288:G1310–G1320. doi: 10.1152/ajpgi.00550.2004. [DOI] [PubMed] [Google Scholar]
- 33.Honma K, et al. Induction by fructose force-feeding of histone H3 and H4 acetylation at their lysine residues around the Slc2a5 gene and its expression in mice. Biosci Biotechnol Biochem. 2013;77:2188–2191. doi: 10.1271/bbb.130300. [DOI] [PubMed] [Google Scholar]
- 34.Suzuki T, et al. Diet-induced epigenetic regulation in vivo of the intestinal fructose transporter Glut5 during development of rat small intestine. Biochem J. 2011;435:43–53. doi: 10.1042/BJ20101987. [DOI] [PubMed] [Google Scholar]
- 35.Erion DM, et al. The role of the carbohydrate response element-binding protein in male fructose-fed rats. Endocrinology. 2013;154:36–44. doi: 10.1210/en.2012-1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Iizuka K, et al. Deficiency of carbohydrate response element-binding protein (ChREBP) reduces lipogenesis as well as glycolysis. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:7281–7286. doi: 10.1073/pnas.0401516101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lanaspa MA, et al. Uric Acid Stimulates Fructokinase and Accelerates Fructose Metabolism in the Development of Fatty Liver. PLoS ONE. 2012;7:e47948. doi: 10.1371/journal.pone.0047948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koo H-YY, et al. Dietary fructose induces a wide range of genes with distinct shift in carbohydrate and lipid metabolism in fed and fasted rat liver. Biochimica et biophysica acta. 2008;1782:341–348. doi: 10.1016/j.bbadis.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 39.Ma L, et al. ChREBP•Mlx Is the Principal Mediator of Glucose-induced Gene Expression in the Liver. Journal of Biological Chemistry. 2006;281:28721–28730. doi: 10.1074/jbc.M601576200. [DOI] [PubMed] [Google Scholar]
- 40.Oberhaensli RD, et al. Study of hereditary fructose intolerance by use of 31P magnetic resonance spectroscopy. Lancet. 1987;2:931–934. doi: 10.1016/s0140-6736(87)91419-x. [DOI] [PubMed] [Google Scholar]
- 41.Boesiger P, et al. Changes of liver metabolite c oncentrations in adults with disorders of fructose metabolism after intravenous fructose by 31P magnetic resonance spectroscopy. Pediatr Res. 1994;36:436–440. doi: 10.1203/00006450-199410000-00004. [DOI] [PubMed] [Google Scholar]
- 42.Oppelt SA, et al. Aldolase-B knockout in mice phenocopies hereditary fructose intolerance in humans. Mol Genet Metab. 2015;114:445–450. doi: 10.1016/j.ymgme.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 43.Lanaspa MA, et al. Endogenous fructose production and metabolism in the liver contributes to the development of metabolic syndrome. Nat Commun. 2013;4:2434. doi: 10.1038/ncomms3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hayashi AA, et al. Intestinal SR-BI is upregulated in insulin-resistant states and is associated with overproduction of intestinal apoB48-containing lipoproteins. Am J Physiol Gastrointest Liver Physiol. 2011;301:G326–G337. doi: 10.1152/ajpgi.00425.2010. [DOI] [PubMed] [Google Scholar]
- 45.Haidari M, et al. Fasting and postprandial overproduction of intestinally derived lipoproteins in an animal model of insulin resistance. Evidence that chronic fructose feeding in the hamster is accompanied by enhanced intestinal de novo lipogenesis and ApoB48-containing lipoprotein overproduction. J Biol Chem. 2002;277:31646–31655. doi: 10.1074/jbc.M200544200. [DOI] [PubMed] [Google Scholar]
- 46.Sugawa-Katayama Y, Morita N. Effects of a high fructose diet on lipogenic enzyme activities in some organs of rats fed ad libitum. J Nutr. 1975;105:1377–1383. doi: 10.1093/jn/105.11.1377. [DOI] [PubMed] [Google Scholar]
- 47.Park OJ, et al. Mechanisms of fructose-induced hypertriglyceridaemia in the rat. Activation of hepatic pyruvate dehydrogenase through inhibition of pyruvate dehydrogenase kinase. Biochem J. 1992;282(Pt 3):753–757. doi: 10.1042/bj2820753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Katsurada A, et al. Effects of nutrients and hormones on transcriptional and post-transcriptional regulation of fatty acid synthase in rat liver. European Journal of Biochemistry. 1990;190:427–433. doi: 10.1111/j.1432-1033.1990.tb15592.x. [DOI] [PubMed] [Google Scholar]
- 49.Miyazaki M, et al. Stearoyl-CoA desaturase 1 gene expression is necessary for fructose-mediated induction of lipogenic gene expression by sterol regulatory element-binding protein-1c-dependent and -independent mechanisms. J Biol Chem. 2004;279:25164–25171. doi: 10.1074/jbc.M402781200. [DOI] [PubMed] [Google Scholar]
- 50.Nagai Y, et al. Amelioration of high fructose-induced metabolic derangements by activation of PPARalpha. Am J Physiol Endocrinol Metab. 2002;282:E1180–E1190. doi: 10.1152/ajpendo.00471.2001. [DOI] [PubMed] [Google Scholar]
- 51.Brown MS, Goldstein JL. Selective versus total insulin resistance: a pathogenic paradox. Cell Metab. 2008;7:95–96. doi: 10.1016/j.cmet.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 52.Li S, et al. Bifurcation of insulin signaling pathway in rat liver: mTORC1 required for stimulation of lipogenesis, but not inhibition of gluconeogenesis. Proceedings of the National Academy of Sciences. 2010;107:3441–3446. doi: 10.1073/pnas.0914798107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Peterson Timothy R, et al. mTOR Complex 1 Regulates Lipin 1 Localization to Control the SREBP Pathway. Cell. 2011;146:408–420. doi: 10.1016/j.cell.2011.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Haas JT, et al. Hepatic insulin signaling is required for obesity-dependent expression of SREBP-1c mRNA but not for feeding-dependent expression. Cell Metabolism. 2012;15:873–884. doi: 10.1016/j.cmet.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Uyeda K, Repa JJ. Carbohydrate response element binding protein, ChREBP, a transcription factor coupling hepatic glucose utilization and lipid synthesis. Cell Metabolism. 2006;4:107–110. doi: 10.1016/j.cmet.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 56.Koo HY, et al. Replacing dietary glucose with fructose increases ChREBP activity and SREBP-1 protein in rat liver nucleus. Biochem Biophys Res Commun. 2009;390:285–289. doi: 10.1016/j.bbrc.2009.09.109. [DOI] [PubMed] [Google Scholar]
- 57.Herman MA, et al. A novel ChREBP isoform in adipose tissue regulates systemic glucose metabolism. Nature. 2012;484:333–338. doi: 10.1038/nature10986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stamatikos AD, et al. Tissue Specific Effects of Dietary Carbohydrates and Obesity on ChREBPalpha and ChREBPbeta Expression. Lipids. 2016;51:95–104. doi: 10.1007/s11745-015-4090-0. [DOI] [PubMed] [Google Scholar]
- 59.Filhoulaud G, et al. Novel insights into ChREBP regulation and function. Trends in endocrinology and metabolism: TEM. 2013;24:257–268. doi: 10.1016/j.tem.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 60.Kooner JS, et al. Genome-wide scan identifies variation in MLXIPL associated with plasma triglycerides. Nature genetics. 2008;40:149–151. doi: 10.1038/ng.2007.61. [DOI] [PubMed] [Google Scholar]
- 61.Kathiresan S, et al. Six new loci associated with blood low-density lipoprotein cholesterol, high-density lipoprotein cholesterol or triglycerides in humans. Nature genetics. 2008;40:189–197. doi: 10.1038/ng.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chambers JC, et al. Genome-wide association study identifies loci influencing concentrations of liver enzymes in plasma. Nature genetics. 2011;43:1131–1138. doi: 10.1038/ng.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kottgen A, et al. Genome-wide association analyses identify 18 new loci associated with serum urate concentrations. Nature genetics. 2013;45:145–154. doi: 10.1038/ng.2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Badman MK, et al. Hepatic fibroblast growth factor 21 is regulated by PPARalpha and is a key mediator of hepatic lipid metabolism in ketotic states. Cell Metab. 2007;5:426–437. doi: 10.1016/j.cmet.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 65.Kharitonenkov A, et al. FGF-21 as a novel metabolic regulator. J Clin Invest. 2005;115:1627–1635. doi: 10.1172/JCI23606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dushay J, et al. Increased fibroblast growth factor 21 in obesity and nonalcoholic fatty liver disease. Gastroenterology. 2010;139:456–463. doi: 10.1053/j.gastro.2010.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gimeno RE, Moller DE. FGF21-based pharmacotherapy--potential utility for metabolic disorders. Trends in endocrinology and metabolism: TEM. 2014;25:303–311. doi: 10.1016/j.tem.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 68.Potthoff MJ, et al. FGF21 induces PGC-1alpha and regulates carbohydrate and fatty acid metabolism during the adaptive starvation response. Proc Natl Acad Sci U S A. 2009;106:10853–10858. doi: 10.1073/pnas.0904187106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Inagaki T, et al. Endocrine regulation of the fasting response by PPARalpha-mediated induction of fibroblast growth factor 21. Cell Metab. 2007;5:415–425. doi: 10.1016/j.cmet.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 70.Iizuka K, et al. Glucose induces FGF21 mRNA expression through ChREBP activation in rat hepatocytes. FEBS letters. 2009;583:2882–2886. doi: 10.1016/j.febslet.2009.07.053. [DOI] [PubMed] [Google Scholar]
- 71.Sanchez J, et al. Response to carbohydrate and fat refeeding in the expression of genes involved in nutrient partitioning and metabolism: striking effects on fibroblast growth factor-21 induction. Endocrinology. 2009;150:5341–5350. doi: 10.1210/en.2009-0466. [DOI] [PubMed] [Google Scholar]
- 72.Dushay JR, et al. Fructose ingestion acutely stimulates circulating FGF21 levels in humans. Mol Metab. 2015;4:51–57. doi: 10.1016/j.molmet.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Talukdar S, et al. FGF21 Regulates Sweet and Alcohol Preference. Cell Metab. 2016;23:344–349. doi: 10.1016/j.cmet.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.von Holstein-Rathlou S, et al. FGF21 Mediates Endocrine Control of Simple Sugar Intake and Sweet Taste Preference by the Liver. Cell Metab. 2016;23:335–343. doi: 10.1016/j.cmet.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chu AY, et al. Novel locus including FGF21 is associated with dietary macronutrient intake. Human molecular genetics. 2013;22:1895–1902. doi: 10.1093/hmg/ddt032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tanaka T, et al. Genome-wide meta-analysis of observational studies shows common genetic variants associated with macronutrient intake. Am J Clin Nutr. 2013;97:1395–1402. doi: 10.3945/ajcn.112.052183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lin J, et al. Peroxisome Proliferator-activated Receptor Î3 Coactivator 1Î2 (PGC-1Î2), A Novel PGC-1-related Transcription Coactivator Associated with Host Cell Factor. Journal of Biological Chemistry. 2002;277:1645–1648. doi: 10.1074/jbc.C100631200. [DOI] [PubMed] [Google Scholar]
- 78.Lin J, et al. Metabolic control through the PGC-1 family of transcription coactivators. Cell Metabolism. 2005;1:361–370. doi: 10.1016/j.cmet.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 79.Lin J, et al. Hyperlipidemic effects of dietary saturated fats mediated through PGC-1β coactivation of SREBP. Cell. 2005;120:261–273. doi: 10.1016/j.cell.2004.11.043. [DOI] [PubMed] [Google Scholar]
- 80.Chambers KT, et al. PGC-1beta and ChREBP partner to cooperatively regulate hepatic lipogenesis in a glucose concentration-dependent manner. Mol Metab. 2013;2:194–204. doi: 10.1016/j.molmet.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nagai Y, et al. The role of peroxisome proliferator-activated receptor gamma coactivator-1 beta in the pathogenesis of fructose-induced insulin resistance. Cell Metab. 2009;9:252–264. doi: 10.1016/j.cmet.2009.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lee AH, et al. Regulation of hepatic lipogenesis by the transcription factor XBP1. Science. 2008;320:1492–1496. doi: 10.1126/science.1158042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang H, et al. Restoration of autophagy alleviates hepatic ER stress and impaired insulin signalling transduction in high fructose-fed male mice. Endocrinology. 2015;156:169–181. doi: 10.1210/en.2014-1454. [DOI] [PubMed] [Google Scholar]
- 84.Glimcher LH. XBP1: the last two decades. Annals of the Rheumatic Diseases. 2010;69:i67–i71. doi: 10.1136/ard.2009.119388. [DOI] [PubMed] [Google Scholar]
- 85.Jurczak MJ, et al. Dissociation of inositol-requiring enzyme (IRE1alpha)-mediated c-Jun N-terminal kinase activation from hepatic insulin resistance in conditional X-box-binding protein-1 (XBP1) knock-out mice. J Biol Chem. 2012;287:2558–2567. doi: 10.1074/jbc.M111.316760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Aeberli I, et al. Moderate amounts of fructose consumption impair insulin sensitivity in healthy young men: a randomized controlled trial. Diabetes Care. 2013;36:150–156. doi: 10.2337/dc12-0540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Samuel VT, et al. Mechanism of hepatic insulin resistance in non-alcoholic fatty liver disease. J Biol Chem. 2004;279:32345–32353. doi: 10.1074/jbc.M313478200. [DOI] [PubMed] [Google Scholar]
- 88.Kraegen EW, et al. Development of muscle insulin resistance after liver insulin resistance in high-fat-fed rats. Diabetes. 1991;40:1397–1403. doi: 10.2337/diab.40.11.1397. [DOI] [PubMed] [Google Scholar]
- 89.Griffin ME, et al. Free fatty acid-induced insulin resistance is associated with activation of protein kinase C theta and alterations in the insulin signaling cascade. Diabetes. 1999;48:1270–1274. doi: 10.2337/diabetes.48.6.1270. [DOI] [PubMed] [Google Scholar]
- 90.Kim JK, et al. PKC-theta knockout mice are protected from fat-induced insulin resistance. J Clin Invest. 2004;114:823–827. doi: 10.1172/JCI22230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Itani SI, et al. Increased protein kinase C theta in skeletal muscle of diabetic patients. Metabolism. 2001;50:553–557. doi: 10.1053/meta.2001.22512. [DOI] [PubMed] [Google Scholar]
- 92.Savage DB, et al. Reversal of diet-induced hepatic steatosis and hepatic insulin resistance by antisense oligonucleotide inhibitors of acetyl-CoA carboxylases 1 and 2. J Clin Invest. 2006;116:817–824. doi: 10.1172/JCI27300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Varela GM, et al. Inhibition of ADRP prevents diet-induced insulin resistance. Am J Physiol Gastrointest Liver Physiol. 2008;295:G621–G628. doi: 10.1152/ajpgi.90204.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kumashiro N, et al. Cellular mechanism of insulin resistance in nonalcoholic fatty liver disease. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:16381–16385. doi: 10.1073/pnas.1113359108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Samuel VT, et al. Inhibition of protein kinase Cepsilon prevents hepatic insulin resistance in nonalcoholic fatty liver disease. J Clin Invest. 2007;117:739–745. doi: 10.1172/JCI30400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Coate KC, et al. Hepatic glucose uptake and disposition during short-term high-fat vs. high-fructose feeding. 2014 doi: 10.1152/ajpendo.00083.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Committee on, N. The Use and Misuse of Fruit Juice in Pediatrics. Pediatrics. 2001;107:1210–1213. doi: 10.1542/peds.107.5.1210. [DOI] [PubMed] [Google Scholar]
- 98.Murray R, et al. Snacks, Sweetened Beverages, Added Sugars, and Schools. Pediatrics. 2015;135:575–583. doi: 10.1542/peds.2014-3902. [DOI] [PubMed] [Google Scholar]
- 99.Ebbeling CB, et al. Effects of Decreasing Sugar-Sweetened Beverage Consumption on Body Weight in Adolescents: A Randomized, Controlled Pilot Study. Pediatrics. 2006;117:673–680. doi: 10.1542/peds.2005-0983. [DOI] [PubMed] [Google Scholar]
- 100.Colchero MA, et al. Beverage purchases from stores in Mexico under the excise tax on sugar sweetened beverages: observational study. BMJ. 2016;352 doi: 10.1136/bmj.h6704. [DOI] [PMC free article] [PubMed] [Google Scholar]