Abstract
This report defines criteria and reviews the epidemiology, pathophysiology, and management of the following common anorectal disorders: fecal incontinence (FI), functional anorectal pain, and functional defecation disorders. FI is defined as the recurrent uncontrolled passage of fecal material for at least 3 months. The clinical features of FI are useful for guiding diagnostic testing and therapy. Anorectal manometry and imaging are useful for evaluating anal and pelvic floor structure and function. Education, antidiarrheals, and biofeedback therapy are the mainstay of management; surgery may be useful in refractory cases. Functional anorectal pain syndromes are defined by clinical features and categorized into 3 subtypes. In proctalgia fugax, the pain is typically fleeting and lasts for seconds to minutes. In levator ani syndrome and unspecified anorectal pain, the pain lasts more than 30 minutes, but in levator ani syndrome there is puborectalis tenderness. Functional defecation disorders are defined by ≥2 symptoms of chronic constipation or irritable bowel syndrome with constipation, and with ≥2 features of impaired evacuation, that is, abnormal evacuation pattern on manometry, abnormal balloon expulsion test, or impaired rectal evacuation by imaging. It includes 2 subtypes: dyssynergic defecation and inadequate defecatory propulsion. Pelvic floor biofeedback therapy is effective for treating levator ani syndrome and defecatory disorders.
Keywords: Anorectal Disorders, Fecal Incontinence, Constipation, Dyssynergic Defecation, Levator Ani Syndrome, Anorectal Pain, Biofeedback Therapy
Anorectal disorders are defined by specific symptoms and, in the case of functional disorders of defecation, also with abnormal diagnostic tests. Our understanding of these disorders continues to evolve with the availability of newer techniques to characterize anorectal structure and function.1–3 Consequently, the distinction between “organic” and “functional” anorectal disorders may be difficult in individual patient. 1–3
Anorectal disorders, such as fecal incontinence, are usually defined by specific symptoms, but functional disorders of defecation require symptoms and anorectal physiological testing.4 While bowel symptoms recorded by questionnaires and bowel diaries are correlated,5 some patients may not accurately recall bowel symptoms6; hence, symptom diaries may be more reliable.
In this report, we examine the prevalence and pathophysiology of anorectal disorders, listed in Table 1, and provide recommendations for diagnostic evaluation and management. These supplement practice guidelines recommended by the American Gastroenterological Association7 and American College of Gastroenterology.8 We will not address anorectal symptoms secondary to a neurologic or systemic disorder. The revised diagnostic criteria include a minimum duration of symptoms that were selected arbitrarily to avoid the inclusion of self-limited conditions.
Table 1.
Functional Anorectal Disorders
| F. Functional anorectal disorders |
| F1. Fecal incontinence |
| F2. Functional anorectal pain |
| F2a. Levator ani syndrome |
| F2b. Unspecifed functional anorectal pain |
| F2c. Proctalgia fugax |
| F3. Functional defecation disorders |
| F3a. Dyssynergic defecation |
| F3b. Inadequate defecatory propulsion |
F1. Fecal Incontinence
Definition
Fecal incontinence (FI) is defined as the recurrent uncontrolled passage of fecal material for at least 3 months. We recognize that fecal staining of underwear may reflect poor hygiene, prolapsing hemorrhoids, or rectal prolapse rather than true FI, but for practical purposes it is included in the definition of FI. Clear mucus secretion must be excluded by careful questioning. Flatus incontinence is often included in the definition of anal incontinence but not in the current diagnosis of FI because it is difficult to define when isolated passage of flatus is abnormal. FI is often multifactorial and occurs in conditions that cause diarrhea, impair colorectal storage capacity, and/or weaken the pelvic floor (Table 2). FI is considered abnormal after toilet training has been achieved, generally around 4 years of age.9
Table 2.
Common Causes of Fecal Incontinence
| Anal sphincter weakness |
| Traumatic: obstetric, surgical (eg, hemorrhoidectomy, internal sphincterotomy, fistulectomy) |
| Nontraumatic: scleroderma, idiopathic internal sphincter degeneration |
| Neuropathy |
| Peripheral (eg, pudendal) or generalized (eg, diabetes mellitus) |
| Pelvic floor disorders |
| Rectal prolapse, descending perineum syndrome |
| Disorders affecting rectal capacity and/or sensationa |
| Inflammatory conditions: radiation proctitis, Crohn’s disease, ulcerative colitis |
| Anorectal surgery (pouch, anterior resection) |
| Rectal hyposensitivity |
| Rectal hypersensitivity |
| Central nervous system disorders |
| Dementia, stroke, brain tumors, multiple sclerosis, spinal cord lesions |
| Psychiatric diseases, behavioral disorders |
| Bowel disturbances |
| Irritable bowel syndrome, post-cholecystectomy diarrhea |
| Constipation and fecal retention with overflow |
These conditions may also be associated with diarrhea.
Epidemiology
Prevalence
Several large community-based studies10–17 have suggested that FI is common, with a prevalence ranging from 7% to 15% in community-dwelling women, 18% to 33% in hospitals, and 50% to 70% in nursing homes.18,19 The prevalence is either comparable16,20 or lower in men than women.21,22 Some11,13,17,23 but not all16,24 studies reported a lower prevalence in African-American than white women, but similar prevalence across races in men.24 Interestingly, the majority of patients seen in clinical practice are women.
Variations in the prevalence of FI among studies may reflect differences in survey methods, screening questions, reference time frame10,16,25 (1 year or past month), and definition of incontinence. Two studies evaluated the incidence of FI.23,26 In a community study (65 years and older), the incidence of FI at 4 years was 17%, with 6% having FI at least monthly.23 In a follow-up community study (50 years and older), the incidence of FI was 7.0%.26
Impact on quality of life and psychosocial factors
Persons with FI report that poor bowel control restricts their social life; other issues pertain to toilet location, hygiene/odor issues, coping strategies, fear, physical activities, embarrassment, and unpredictability of bowel habits.27 Co-existent psychological problems may include anxiety and depression, 28,29 poor self-esteem, and problems with sexual relationships.30 Quality of life issues can be evaluated by generic or disease-specific instruments, such as the Rockwood Fecal Incontinence Quality of Life Scale, modified Manchester Health Questionnaire, Fecal Incontinence and Constipation Assessment Quality of Life scale. FI symptoms can also be assessed by Pelvic Organ Prolapse/Incontinence Sexual Questionnaire–IUGA (International Urogynecology Association).31–34 There is a significant correlation between symptom severity and QOL in FI.31,35 FI was associated with increased mortality in some, but not all studies.36–38 but whether it is due to FI per se or conditions associated with FI (age and comorbidity) is unknown.16
Etiology and risk factors for fecal incontinence
The etiology of incontinence is often multifactorial. Therefore, it is more appropriate to focus on associated conditions, especially when they precede the onset of FI, and on risk factors for FI. In community surveys, bowel disturbances, especially diarrhea and rectal urgency, and the burden of chronic illness were more important and independent risk factors for FI than obstetric-related pelvic floor injury (eg, forceps use, complicated episiotomy). 2,16,26,39–41 In a community-based cohort of 176 randomly selected women with FI and 176 without FI, the independent risk factors for FI were diarrhea (odds ratio [OR] = 53; 95% confidence interval [CI]: 6.1–471), cholecystectomy (OR = 4.2l 95% CI: 1.2–15), current smokers (OR = 4.7; 95% CI: 1.4–15), rectocele (OR = 4.9; 95% CI: 1.3–19), stress urinary incontinence (OR = 3.1; 95% CI: 1.4–6.5), and body mass index (per unit, OR = 1.1; 95% CI: 1.004–1.1).41 Smoking, external sphincter atrophy, and obesity are also risk factors for FI.2,11,13,17,41 Other conditions associated with FI include advanced age, disease burden (comorbidity count, diabetes), anal sphincter trauma (obstetrical injury, prior surgery), and decreased physical activity.11,16,17,42,43 Several diseases that affect anorectal sensorimotor dysfunctions and/or alter bowel habits are also associated with FI in clinical practice (Table 2). Some of these conditions do not emerge as risk factors in community studies, possibly because their prevalence is relatively low. Consistent with the findings of community-based studies, the vast majority of women with FI who consult a physician might not have a neurologic or inflammatory disorder, but rather have bowel disturbances, typically diarrhea, often associated with a history of obstetric risk factors. However, neurologic deficit can only be identified with neurophysiological tests, and these are not widely available.
The incidence of FI after vaginal delivery was 8% in a recent series.44 This may reflect improvements in obstetrical practices, including decreased use of instrumented vaginal delivery (eg, forceps), less frequent and more selective use of episiotomy, and increased use of cesarean sections, although a Cochrane review showed no demonstrable difference between cesarean sections and vaginal deliveries.45 Third-degree (ie, involving the external anal sphincter) and fourth-degree lacerations (ie, extending through the external and internal anal sphincters) are strong risk factors for anal and fecal incontinence.46 A prospective National Institutes of Health trial identified a nearly 2-fold increased OR of FI for women with sphincter injury during childbirth compared with a control group.47 The risk is highest for instrument-assisted deliveries, with increased odds of 1.5 for anal incontinence and a higher risk with forceps than vacuum extraction.48 Among women in the community, the median age of onset of FI is in the 7th decade, that is, many decades after vaginal delivery11 and, therefore, how obstetric injury predisposes to FI is unclear.
Anorectal surgery for fistula, fissures, or hemorrhoidectomy and anorectal carcinoma can damage the sphincters.49 Impaired rectal compliance, as can occur with proctitis or after creation of a pouch, and fecal impaction with overflow diarrhea, can all cause FI.50–52
F1. Diagnostic Criteria a for Fecal Incontinence.
Recurrent uncontrolled passage of fecal material in an individual with a developmental age of at least 4 years
aCriteria fulfilled for the last 3 months. For research studies, consider onset of symptoms for at least 6 months previously with 2–4 episodes of FI over 4 weeks.
Justification for Changes in Diagnostic Criteria
The earlier definition of functional fecal incontinence was cumbersome, did not facilitate management, and was seldom used in clinical practice or research studies. Therefore, we recommend the generic term fecal incontinence.
We recognize that newer sensitive diagnostic tools (eg, anal ultrasonography, pelvic magnetic resonance imaging [MRI], and high resolution/3-dimenstional high-definition anorectal pressure topography) often reveal disturbances of anorectal structure and/or function in a majority of patients with FI, but their relationship to symptoms is unclear, especially as some have more dysfunction(s) than others. Therefore, it can be challenging to attribute symptoms with confidence to an organic or functional cause and more studies are needed.
Pathophysiology
Physiological factors
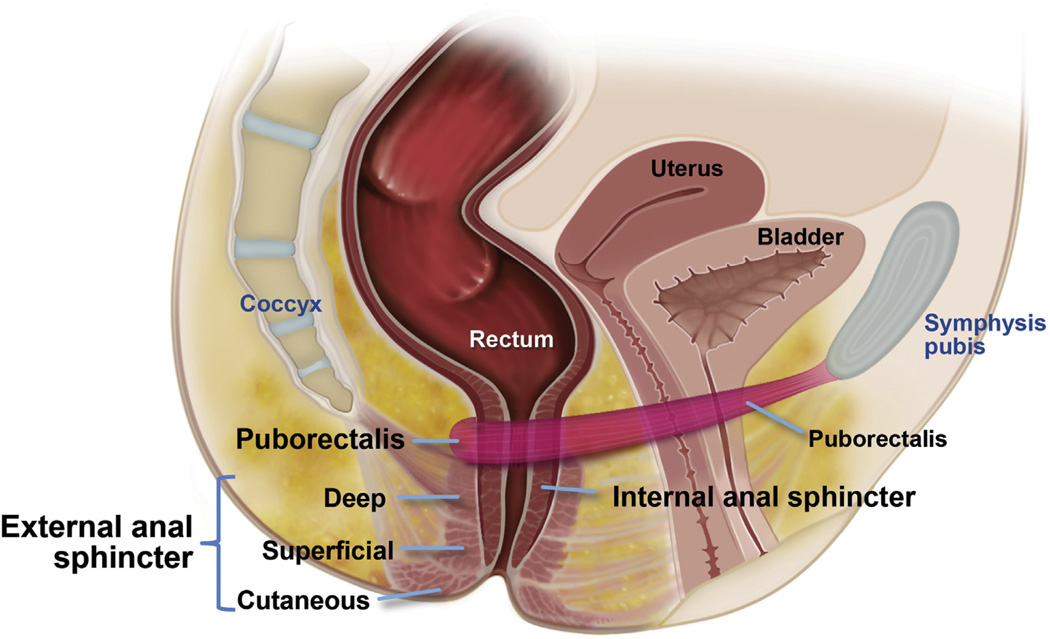
Continence is maintained by several mechanisms, including anatomical factors (endovascular cushions, integrity of anal sphincter, and puborectalis muscle), rectoanal sensation, rectal compliance, neuronal innervation, stool consistency, mobility, and psychological factors (Figure 1).53
Figure 1.
Anatomy of the anal canal and rectum, which displays the key physiologic mechanisms for continence and defecation.
Anorectal and pelvic floor musculature
Anal sphincter weakness is the most frequently identified abnormality in FI. Among older women, approximately 40% had reduced anal resting pressure and 80% reduced squeeze pressure.54 Internal anal sphincter dysfunction is characterized by exaggerated spontaneous relaxation of the internal anal sphincter (sampling reflex)55 or decreased resting pressure.54,55 The latter is associated with structural disturbances, that is, defects (after obstetric injury) and/or thinning (scleroderma, advanced age). This is best visualized by ultrasonography. Among postpartum women, the severity of FI was greater in women with internal anal sphincter defects.56
External anal sphincter weakness can result from one or more of the following factors: sphincter damage, neuropathy, myopathy, or reduced corticospinal input. In addition to the anal sphincters, the levator ani muscles also contribute to the pelvic barrier.57 One study suggested that the reduced inward traction exerted by the puborectalis in patients with FI correlated more closely with symptoms than did squeeze pressures, and improved after biofeedback therapy.58 Whereas the anal sphincters and endovascular cushions seal the anal canal, the levator ani and puborectalis maintain continence of solid stool by a flap-valve action.59–62 Patients with excessive perineal descent have a more obtuse anorectal angle, suggesting that the flap valve that normally maintains continence when intra-abdominal pressure increases is impaired.57
FI in men who generally have fecal soiling or leakage rather than gross incontinence may be associated with normal sphincteric function63–67; iatrogenic anal injury (eg, after perianal procedures); or dyssynergic defecation,68 wherein high anal resting pressure entraps feces during defecation and subsequently expels them69 ; radiation therapy70; or isolated weakness of the internal anal sphincter.
Rectal compliance and rectoanal sensation
Stool is often transferred into the rectum by colonic high-amplitude propagated contractions, which tend to occur after awakening or meals.71 Rectal distention by stool is associated with several processes that serve to preserve continence or, if appropriate, proceed to defecation. Rectal distention induces reflex relaxation of the internal anal sphincter and is perceived as a sensation of rectal fullness, as if the rectum were uncomfortably full of flatus or feces. If defecation is inconvenient, the desire to defecate prompts voluntary contraction of the external sphincter and puborectalis muscle72 ; this sensation wanes, together with the sense of urgency, as the rectum accommodates to hold more stool.
The sphincter pressures alone do not always distinguish continent from incontinent subjects. Reduced rectal sensation allows stool to enter the anal canal and perhaps leak before the external sphincter contracts.55,72,73 Decreased rectal sensitivity (rectal hyposensitivity) and increased rectal compliance can also contribute to fecal retention by decreasing the frequency and intensity of the urge (and hence the motivation) to defecate. Conversely, fecal retention may reduce rectal sensation, perhaps by altering rectal tone and viscoelastic properties, or by affecting afferent nerve pathways.74
Rectal hypersensitivity, perhaps a marker of concomitant irritable bowel syndrome (IBS),55,75 may be associated with reduced rectal compliance and repetitive rectal contractions during rectal distention. 54,76 Rectal capacity is also reduced in women with FI and associated with the symptom of urgency54,77 In addition, rectal hypersensitivity cannot be entirely explained by disturbances in rectal compliance. Anal sphincter relaxation may occur during, or independent of, rectal distention, or along with colonic high-amplitude propagated contractions, which enables the anal lining to periodically “sample” rectal contents and ascertain whether rectal contents are gas, liquid, or stool. 74,78 Sampling occurred less frequently in incontinent patients, perhaps depriving them of sensory information.74 In addition to anorectal dysfunctions, continence can also be affected by disturbances of stool consistency and/or delivery, impaired mental faculties, and mobility. These observations confirm that FI is a heterogeneous disorder and that patients often exhibit more than one deficit (Table 2).
Clinical Evaluation
History
It is essential to develop a rapport with FI patients and, with tact and skill, evaluate its severity, awareness for stooling, and conditions that predispose, including the type (solid, liquid, and/or gas), quantity, and frequency. Staining, soiling, and seepage reflect the nature and severity of FI.20 Soiling indicates leakage that is more extensive than staining of underwear and can be specified further (ie, soiling of underwear or furnishing/bedding). Seepage refers to leakage of small amounts of stool.
Characterization of bowel habit is important and the Bristol Stool Form Scale and bowel diaries can be useful.79 Constipation with fecal impaction is a significant risk in nursing homes.36,80 Factors that cause or exacerbate incontinence via loose stools (eg, laxatives, artificial sweeteners) and anorectal surgical procedures (eg, lateral sphincterotomy) or other mechanisms (eg, smoking, obesity) should be considered. Conversely, agents that cause constipation may predispose to fecal retention and overflow. Recognizing the timing of incontinence (eg, whether predominantly during or after events such as meals, bowel movements, exercise, or at night) can provide clues to etiology and management.40 History taking should also include consideration of conditions that are a secondary cause of FI, such as multiple sclerosis, diabetic neuropathy, or scleroderma.
Urge vs passive FI can provide clues to the pathophysiology. Incontinence for solid stool suggests more severe sphincter weakness than liquid stool alone.81 Patients with urge incontinence have a sensation of the desire to defecate before leakage, but cannot reach the toilet on time. Conversely, patients with passive incontinence have diminished or no awareness of the desire to defecate before the incontinent episode. Patients with urge incontinence often have reduced squeeze pressures82 and/or squeeze duration,83 reduced rectal capacity, and increased perception of rectal balloon distention,54,84 whereas patients with passive incontinence often have lower resting pressures.82,83
Several FI instruments–Wexner (Cleveland Clinic), Vaizey (St Marks), Rockwood, Fecal Incontinence and Constipation Assessment, and the bowel version of the International Consultation of Incontinence questionnaire–are currently used in clinical studies to rate the severity of FI 10,31,35,85–87 and are validated instruments. Currently, success in therapeutic trials is typically defined as a 50% reduction in the number of episodes of FI or days per week,88 although a patient’s perspective may differ and more meaningful outcome measures are required. 80,90
Physical examination including digital rectal evaluation
A multisystem and abdominal examination and focused neurologic examination is often necessary in FI patients with neurologic symptoms.
A digital rectal examination (DRE) should be conducted in the left lateral position and before enemas or laxatives are given. Inspection may reveal scars from previous surgery or obstetric injury or a patulous sphincter or perianal fecal soiling or dermatitis. An absent anocutaneous reflex in response to gentle stroking of the perianal region suggests nerve impairment. After inspection, anorectal digital palpation should be conducted. This may reveal external anal sphincter and/or puborectalis weakness or defects,91 stool impaction, and presence of dyssynergia during simulated defecation. A meticulous DRE performed by an experienced examiner had a positive predictive value of 67% and 81% for identifying low resting and squeeze pressures, respectively.92
Diagnostic testing
Testing should be tailored to the patient’s clinical problem, severity, possible etiology, impact on quality of life, and response to medical management.
Endoscopy
Endoscopic assessment of the rectosigmoid mucosa or full colonoscopy with biopsies may be considered in patients with diarrhea or recent change in bowel habit.
Manometry evaluation
Anorectal manometry (ARM) assesses continence and defecatory mechanisms by determining:
resting anal pressure, which is predominantly (ie, approximately 70%) attributable to internal anal sphincter function;
squeeze pressure: the strength and duration of voluntary external anal sphincter contraction and puborectalis contraction;
presence of an internal anal sphincter inhibitory reflex;
threshold volume of rectal distention required to elicit the first sensation of distention, a sustained feeling of urgency to defecate, and the maximum tolerable volume;
whether attempted defecation is accompanied by increased intra-abdominal pressure and relaxation of the pelvic floor muscles (normal), or by paradoxical contraction of the pelvic floor muscles, which may be relevant to symptoms; and
rectal compliance can be evaluated by assessing the pressure–volume relationship during stepwise distention of a latex balloon, but it is preferable to do so with an infinitely compliant polyethylene balloon and a barostat.
The methods used for ARM, including solid-state probe, high-resolution ARM, and 3-dimensional high-definition ARM systems, and its measurements and interpretation are detailed elsewhere.93–95
Anal endosonography
Anal endosonography identifies anal sphincter thinning and/or defects that are often clinically unrecognized and may be amenable to surgical repair. 96,97 Endosonography reliably identifies anatomic defects or thinning of the internal sphincter, whereas interpretation of external sphincter images may pose technical challenges. In contrast, 3-dimensional endosonography can measure the length and volume of the external anal sphincter and atrophy.98 Endoanal MRI, 99,100 and vaginal ultrasound can provide additional information.101
Defecography
Defecography is useful only for selected patients with FI, particularly before surgery, to identify or confirm structural alterations of the pelvic floor.
Pelvic magnetic resonance imaging
MRI is the only imaging modality that can visualize both anal sphincter anatomy and global pelvic floor motion (ie, anterior, middle, and posterior compartments) in real time without radiation exposure.102 Endosonography is the first choice for anal sphincter imaging in FI because it is widely available and the internal sphincter is visualized more clearly. MRI is more useful for identifying external sphincter atrophy and a patulous anal canal, which is a marker of not only anal sphincter injury, but disturbances beyond sphincter injury, such as damage to the anal cushions or anal denervation.54,103
Neurophysiologic tests
Neurophysiological tests can characterize disturbances in the motor and sensory innervation of the anorectum and pelvic floor muscles. These tests include pudendal nerve terminal motor latencies, electromyography (EMG), rectoanal sensory tests, and motor evoked potentials. There are several methodological limitations to pudendal nerve terminal motor latencies, and the utility of this measurement has been questioned.7 Needle EMG can identify normal, neurogenic, or muscle injury. 57,104 Recently, prolonged rectal and anal motor evoked potentials have been shown in a majority of FI patients, suggesting that neurophysiologic dysfunction plays an important role.105
Treatment
Management of FI must be tailored toward correction of clinical manifestations.
Bowel habit modification with dietary or pharmacological interventions
Loose stools are a major risk factor for FI.2,40 Correction of reversible factors like laxatives or other medications can help. Dietary trials (eg, low lactose or low fructose) in selected patients can normalize stool form. Among fiber supplements, only psyllium but not gum arabic or carboxymethylcellulose, improved FI compared with placebo.106 Loperamide given at an adequate dose (ie, 2–4 mg, 30 minutes before meals) can improve stool consistency and increase internal sphincter tone, thereby reducing incontinence.107 Diphenoxylate, combined with atropine, is an alternative to loperamide, but there may be anticholinergic side effects.108 In an open-label study of 18 patients, amitriptyline (20 mg daily), which has anticholinergic effects, improved FI in most patients.109
Patients with constipation, fecal impaction, and overflow incontinence often benefit from a program to increase emptying of the colorectum by various means. For example, a regimen consisting of a daily osmotic laxative (lactulose 10 mL twice daily) plus a weekly enema was useful in the majority of elderly patients with FI, including those with dementia.110 However, loosening the stool may aggravate FI. Other measures aimed at improving rectal emptying, such as the use of suppositories or enemas, fiber supplementation, oral laxatives, and correction of any abnormal toileting behavior, or positioning and biofeedback may be helpful.111
Rectal cleansing and anal plug devices
In patients who fail bowel modification and biofeedback therapy, periodic rectal cleansing is a practical solution. It should be considered particularly in patients with neurogenic bowel dysfunction.112–115 Plug devices may also be useful in some patients with seepage.115
Biofeedback therapy
Biofeedback is based on the principle of operant conditioning or instrumental learning.116 One randomized controlled trial showed that biofeedback therapy is superior to Kegel exercises.117
Surgical approaches
Anal sphincter repair, although well established, does not appear to be effective in the long-term.118 Sacral nerve stimulation and anal submucosal injection of dextranomer in stabilized hyaluronic acid [NASHA Dx]), a bulking agent, are both approved by the US Food and Drug Administration for the treatment of FI. In the pivotal US multicenter study of sacral nerve stimulation, at 5-year follow-up, 76 of 120 (63%) patients were available, of whom, 36% reported complete continence and 89% were deemed a therapeutic success.119 However, this and nearly all other studies with sacral nerve stimulation have been uncontrolled. In a crossover study of 34 patients, the number of episodes of FI declined by 90% during stimulation vs 76% without stimulation.120
In the pivotal trial of NASHA Dx (206 patients), the proportion of patients achieving a 50% FI episode reduction was higher for NASHA Dx (52%) than sham injections (31%), this response was sustained up to 3 years in some patients.121,122
F2. Functional Anorectal Pain
Three types of functional anorectal pain disorders have been described: proctalgia fugax, levator ani syndrome, and unspecified. They are primarily distinguished on the basis of the duration of pain and the presence or absence of anorectal tenderness. Despite some differences, there is significant overlap among these conditions.123
F2a. Levator Ani Syndrome
Definition
In levator ani syndrome, the pain is often described as a vague, dull ache or pressure sensation high in the rectum that is often worse with sitting than with standing or lying down. Physical examination may reveal spasm of levator ani muscles and tenderness on palpation, more often on the left than right side, or of the pelvic floor or vagina.124
Epidemiology
In one survey, the prevalence of anorectal pain due to all causes and symptoms of levator ani syndrome elicited by questionnaire were 11.6% and 6.6%, respectively.125
F2a. Diagnostic criteriaa for Levator Ani Syndrome.
Must include all of the following:
Chronic or recurrent rectal pain or aching
Episodes last 30 minutes or longer
Tenderness during traction on the puborectalis
Exclusion of other causes of rectal pain, such as inflammatory bowel disease, intramuscular abscess and fissure, thrombosed hemorrhoids, prostatitis, coccygodynia, and major structural alterations of the pelvic floor.
aCriteria fulfilled for the last 3 months with symptom onset at least 6 months before diagnosis.
F2b. Diagnostic Criteria for Unspecified Functional Anorectal Pain
Symptom criteria for chronic levator ani syndrome but no tenderness during posterior traction on the puborectalis muscle
Justification for Changes in Diagnostic Criteria
The previous classification included chronic proctalgia that was subcategorized into levator ani syndrome, unspecified anorectal pain, and proctalgia fugax. Because chronic proctalgia includes many other conditions, it has been deleted, but the 3 subentities are retained. There are very limited published data on the duration of pain, but we believed the revised duration may facilitate better distinction between these entities. Reflecting the limited spatial discrimination of visceral pain in humans, the location of pain in proctalgia fugax has been revised to “rectum” instead of “anal canal or lower rectum.”
Pathophysiology
Physiological factors
Levator ani syndrome is hypothesized to result from spasm of pelvic floor muscles and elevated anal resting pressures.123 However, a recent randomized controlled study found features of dyssynergic defecation and a majority (85%) had levator muscle tenderness. The dyssynergia reversed after successful biofeedback, suggesting that rectoanal incoordination may be a pathophysiological explanation for levator anisyndrome.126
Clinical evaluation
Diagnosis is based primarily on the presence of characteristic symptoms and physical examination findings (see definition). Evaluation often includes sigmoidoscopy, ultrasonography, and pelvic imaging to exclude alternative diseases.
Treatment
Treatments include electrogalvanic stimulation; biofeedback training; muscle relaxants, such as methocarbamol, diazepam, and cyclobenzaprine; digital massage of the levator ani muscles; and sitz baths. However, only 2 randomized controlled trials have been reported. In one, 157 patients with chronic proctalgia received either electrical stimulation or digital massage of the levator ani and warm sitz baths or pelvic floor biofeedback plus psychological counseling.126 Among patients who reported tenderness on palpation, the intent-to-treat analysis showed that 87% reported adequate relief of rectal pain after biofeedback, compared with 45% for electrical stimulation and 22% for massage. This improvement was maintained 12 months later. In another randomized controlled trial, 12 patients were randomized to anal sphincter injections of either botulinum A toxin or placebo administered at an interval of 3 months; botulinum toxin injections were similar to placebo injections.127
F2c. Proctalgia Fugax
Definition
Proctalgia fugax is defined as sudden, severe pain in the rectal area, lasting for a few seconds to several minutes (rarely up to 30 minutes), and then disappearing completely.128,129 Pain is localized to the rectum in 90% of cases.130 Attacks are infrequent, typically occurring fewer than 5 times per year in 51% of patients.130 The pain has been described as cramping, gnawing, aching, or stabbing and may range from uncomfortable to unbearable.129 Almost 50% of patients had to interrupt their normal activities during an attack.131 The symptoms may awaken the patient from sleep.
Epidemiology
The prevalence of proctalgia fugax has ranged from 8% to 18% with no difference between the sexes.125,128 Symptoms rarely begin before puberty, but there have been cases reported in 7-year old children.128,129
F2c. Diagnostic Criteriaa for Proctalgia Fugax.
Must include all of the following:
Recurrent episodes of pain localized to the rectum and unrelated to defecation
Episodes last from seconds to minutes, with a maximum duration of 30 minutes
There is no anorectal pain between episodes.
Exclusion of other causes of rectal pain, such as inflammatory bowel disease, intramuscular abscess and fissure, thrombosed hemorrhoids, prostatitis, coccygodynia, and major structural alterations of the pelvic floor.
aFor research purposes, criteria must be fulfilled for 3 months with symptom onset at least 6 months before diagnosis.
Pathophysiology
Physiological factors
The short duration and sporadic, infrequent nature of this disorder have made the identification of physiological mechanisms difficult, but abnormal smooth muscle contractions may be responsible for the pain.132–134 Two studies cited families in which a hereditary form of proctalgia fugax was found to be associated with hypertrophy of the internal anal sphincter and comorbid constipation.135,136 Attacks of proctalgia fugax are often precipitated by stressful life events or anxiety.137 In an uncontrolled unblinded study, a majority of patients were perfectionistic, anxious, and/or hypochondriacal.138
Clinical evaluation
Diagnosis is based on the presence of characteristic symptoms as described and exclusion of anorectal and pelvic pathophysiology.
Treatment
For most patients, the episodes are so brief that remedial treatment is impractical and prevention is not feasible, and because it is harmless, treatment will normally consist of reassurance and explanation. However, patients with frequent symptoms will require treatment. A randomized controlled trial showed that inhalation of salbutamol was more effective than placebo for shortening the duration of episodes of proctalgia for patients in whom episodes lasted 20 minutes or longer.139
F3. Functional Defecation Disorders
Definition
Chronic constipation is commonly classified as either slow colonic transit or outlet dysfunction, although some patients may have neither and others fulfill criteria for both. A large subset of outlet dysfunction has a functional defecation disorder (FDD), which is characterized by paradoxical contraction or inadequate relaxation of the pelvic floor muscles during attempted defecation and/or inadequate propulsive forces during attempted defecation. These disorders are frequently associated with symptoms such as excessive straining, feeling of incomplete evacuation, and digital facilitation of bowel movements.140 However, symptoms (eg, digital disimpaction, anal pain) do not consistently distinguish patients with FDDs from those without.141–143 Thus, the criteria for FDDs must rely on both symptoms and physiological testing.
Several investigators have described the association of paradoxical anal contraction with constipation and have described dyssynergia patterns.144,145 Likewise, studies have shown inadequate propulsive forces as identified by decreased or absent intrarectal pressure during attempted defecation.141,142,145–148 These patients are clinically indistinguishable from patients with dyssynergic defecation. Recently, a large controlled study showed that dyssynergic defecation, inadequate propulsive forces, and a hybrid of both disturbances were uncorrelated, suggesting that the pathophysiology of dyssynergic defecation and inadequate propulsive forces are distinct.142 Also, these patterns are observed in asymptomatic controls,94,141,149,150 and by themselves they have limited utility for discriminating between health and defecatory disorders. Hence, FDDs are best identified by a combination of dyssynergic patterns during attempted defecation and other findings (see diagnostic criteria).
Epidemiology
The prevalence of FDDs in the general population is unknown because the diagnosis requires laboratory testing. At tertiary referral centers, the prevalence of dyssynergic defecation among patients with chronic constipation has ranged widely, from 20% to 81%.151–155 However, the prevalence of dyssynergia may have been overestimated due to the high false-positive rates seen in some studies.156,157 In one tertiary care center, the prevalence of dyssynergia was 3 times higher in women than men, but was similar in younger and older individuals.140
F3. Diagnostic Criteriaa for Functional Defecation Disorders.
The patient must satisfy diagnostic criteria for functional constipation and/or irritable bowel syndrome with constipation
-
During repeated attempts to defecate, there must be features of impaired evacuation, as demonstrated by 2 of the following 3 tests:
Abnormal balloon expulsion test
Abnormal anorectal evacuation pattern with manometry or anal surface EMG
Impaired rectal evacuation by imaging
Subcategories F3a and F3b apply to patients who satisfy criteria for FDD
F3a. Diagnostic Criteria for Inadequate Defecatory Propulsion
Inadequate propulsive forces as measured with manometry with or without inappropriate contraction of the anal sphincter and/or pelvic floor musclesb
F3b. Diagnostic Criteria for Dyssynergic Defecation
Inappropriate contraction of the pelvic floor as measured with anal surface EMG or manometry with adequate propulsive forces during attempted defecation b
aCriteria fulfilled for the last 3 months with symptom onset at least 6 months before diagnosis.
bThese criteria are defined by age- and sex-appropriate normal values for the technique.
Justification for Changes in Diagnostic Criteria
In the previous classification, only patients with functional constipation, but not constipation-predominant IBS, were eligible to be diagnosed with a defecatory disorder. Since then, an association between IBS and pelvic floor dysfunction has been recognized,158,159 and patients with dyssynergic defecation can be effectively treated with biofeedback therapy irrespective of coexistent IBS.160 Hence, the Rome IV criteria have been revised to include patients with constipation-predominant or mixed IBS. The criteria for “inadequate propulsive forces” and “inappropriate contraction of the anal sphincter and/or pelvic floor muscles” are no longer specified because they vary among different techniques.141,142,161
Clinical Evaluation
A detailed assessment of bowel symptoms (eg, prolonged or excessive straining, feeling of incomplete evacuation after defecation, digital facilitation of defecation) and a meticulous DRE often raise suspicion for an FDD. Bowel diaries avoid the limitation of recall bias inherent to questionnaires and an interview. In a single study, the DRE has a sensitivity of 75% and specificity of 87% for detecting dyssynergia,162 which is associated with contraction or failure to relax the puborectalis and/or anal sphincter muscle and reduced perineal descent when patients try to expel the examining finger.
Physiologic studies should be considered if there is insufficient response to conservative treatment, for example, education regarding normal bowel habits, increased dietary fiber and liquids, and elimination of medications with constipating side effects whenever possible. These studies should include balloon expulsion test, anorectal manometry, and if necessary, defecography-imaging to aid in diagnosis of FDD. There is no single “gold standard” diagnostic test to diagnose FDD and limited agreement among various tests.
Balloon expulsion test
Rectal expulsion can be evaluated by asking patients to expel balloons filled with water or air from the rectum. 143,146,163 The time required to expel the balloon depends on the method used and ranges from 1 minute to expel a 50-mL balloon filled with water 145,164,165 to 2 minutes.148 It is recommended that the patient sit on a commode chair behind a privacy screen.148 The balloon expulsion test is a useful screening test for FDD, but it does not define the mechanism of disordered defecation. Because the balloon may not mimic the patients’ stool, a normal balloon expulsion study does not always exclude a defecation disorder.141
Manometric assessment
Traditionally, ARM has been considered essential for diagnosis of FDDs. This assessment includes measurement of intrarectal pressures during attempted defecation, and measurement of anal pressures and/or EMG activity during attempted defecation. However, given the overlap of findings in asymptomatic people and patients with FDD, the precise criteria and utility of manometry for diagnosing defecatory disorders is in evolution. Also, body position and manometry systems may influence findings.
A normal pattern is characterized by increased intrarectal pressure associated with anal relaxation. A study of 100 patients with a 6-sensor, solid-state manometry system identified 4 patterns of FDD.141 Two patterns, types I and III, describe dyssynergic defecation. Type I pattern is characterized by increased intrarectal pressure (≥45 mm Hg) and increased anal pressure reflecting contraction of the anal sphincter. Type III pattern is characterized by increased intrarectal pressure (≥45 mm Hg) with absent or insufficient (<20%) relaxation of the anal sphincter. Inadequate propulsion (intrarectal pressure <45 mm Hg) may be associated with paradoxical contraction (type II pattern) or insufficient relaxation (<20%) of anal sphincter (type IV pattern). During testing 1 month later, the abnormal patterns were reproducible in 51 of 53 patients.141 Levels of inter-observer agreement for identifying these patterns was substantial for types I and IV dyssynergia, moderate for normal defecation pattern, and fair for types II and III dyssynergia.161 A study using high-resolution manometry in 62 healthy women and 295 women with chronic constipation identified 3 phenotypes (high anal, low rectal, and hybrid) that discriminated among patients with normal and abnormal balloon expulsion time with 75% sensitivity and 75% specificity.165
Defecography
Defecography is a radiologic technique used to evaluate the rectum and pelvic floor during attempted defecation.3,166 This test can detect structural abnormalities (rectocele, enterocele, intussusception, rectal prolapse, and megarectum) and assess functional parameters (anorectal angle at rest and during straining, perineal descent, anal diameter, indentation of the puborectalis, degree of rectal emptying).
The diagnostic value of defecography is unclear,7 but is still employed when ARM and balloon expulsion test are equivocal, or for patients who are unable to evacuate a balloon, but who relax the pelvic floor normally during simulated defecation. In several European countries, defecography is the primary modality for identifying FDD.
Magnetic resonance defecography images anorectal motion and rectal evacuation in real time. Advantages include better resolution of soft tissue surrounding the rectum, improved ability to visualize anal sphincter and levator ani muscles, and lack of radiation. MRI is particularly useful in patients with normal balloon expulsion to identify structural lesions and disordered defecation, and to guide surgical therapy, for example for rectoceles and cystoceles.102,167
Radio-opaque marker test of whole gut transit time
By itself, slow colonic transit is not diagnostic of a primary colonic motility disorder because slow transit constipation exists independent of, or co-exists with, FDDs and up to two-thirds of patients with a defecation disorder also have delayed colonic transit.141,168,169 In one study, colonic transit improved after biofeedback therapy for outlet dysfunction, which suggests that outlet dysfunction was responsible for delayed colonic transit.168 Colonic transit time can be measured by obtaining abdominal radiographs after patients ingest radio-opaque markers,170 a wireless motility capsule,171–173 or by scintigraphy. The wireless motility capsule and scintigraphy can also measure gastric emptying and small intestinal transit, which may also be delayed in constipated patients.174
Utility of anorectal testing for functional defecation disorders
The role of diagnostic testing was evaluated by assessing anorectal manometry, balloon expulsion test, defecography, and colonic transit in 100 consecutive patients with symptoms of difficult defecation.141 In this group, anal manometry and balloon expulsion were normal in 30%. Among 70 patients with abnormal manometry, balloon expulsion was abnormal in 42 patients (60%) indicative of FDD. Among 28 patients with abnormal manometry and normal balloon expulsion, defecography showed features of dyssynergic defecation in 7 patients (25%). Because a considerable proportion of healthy people exhibit dyssynergia when tested with high-resolution manometry, the utility of high-resolution manometry for identifying DD is unclear.142,161 Based on these results, abnormal findings with 2 of 3 tests (ie, anorectal manometry, balloon expulsion test, and defecography) are required to confirm the diagnosis of FDD.
Pathophysiology
FDDs are probably acquired but subliminal behavioral disorders, particularly in patients who learn to relax the external anal sphincter and puborectalis muscles appropriately when provided with biofeedback training.
Anxiety and/or psychological stress may also contribute to the development of dyssynergic defecation by increasing skeletal muscle tension,175 and one study found that patients with dyssynergic defecation had higher scores for anxiety, depression, paranoid ideation, hostility, and obsessive compulsiveness than those patients with slow transit constipation.176 Psychological distress seem to have a negative impact on the outcome of biofeedback therapy.177 Uncontrolled studies have reported sexual abuse in 22% of women with FDD, and 40% of women with functional lower gut disorders, including FDD.140,178
Treatment
Historically, 2 types of pelvic floor training involving behavioral modification have been advocated: biofeedback training in which pressure sensors or EMG placed inside the anus and rectum provide feedback to the patient on muscle activity179 and simulated defecation in which the patient practices evacuating an artificial stool surrogate.179,180 Simulated defecation has been combined with diaphragmatic muscle training by some investigators.179,181 Recent randomized controlled trials have used multicomponent biofeedback treatment,182 which includes the following four steps (Table 3):
Patient education: Explain to patients that they inadvertently squeeze or fail to relax their anus when they are straining.
Enhance push effort: Teach the patients to effectively push, when straining, by appropriately increasing the intra-abdominal pressure; use feedback from rectal sensor regarding abdominal and diaphragmatic push effort to expel stool.
Train to relax pelvic floor muscles: Teach patients to relax their pelvic floor muscles when straining. This skill can be taught by providing visual feedback regarding anal canal pressure or EMG activity (Figure 2).
Practice simulated defecation: Educate patient to practice defecation and expulsion of a lubricated, inflated balloon while the therapist assists by gently pulling on the catheter.
Table 3.
Summary of Randomized Controlled Trials of Biofeedback Therapy for functional defecation disorder
| Variable | Chiarioni et al184 | Rao et al183 | Chiarioni et al168 | Heymen et al186 | Rao et al185 |
|---|---|---|---|---|---|
| Trial design | EMG Biofeedback vs PEG 14.6 g |
Biofeedback (manometry pressure) vs standard treatment vs sham biofeedback |
EMG biofeedback for slow transit vs dyssynergia |
EMG biofeedback vs diazepam 5 mg vs placebo |
Biofeedback (manometry pressure) vs standard therapy |
| Subjects and randomization and intervention(s) |
109 (104 women) 54 biofeedback 55 polyethylene glycol |
77 (69 women) 1:1:1 distribution Standard: diet, exercise, laxatives Sham: progressive muscle relaxation with anorectal probe |
52 (49 women) 34 dyssynergia 12 slow transit 6 mixed |
84 (71 women) 30 biofeedback 30 diazepam 24 placebo |
52 = short-term therapy 26 = long-term study 12 = biofeedback 13 = standard therapy Standard: diet, exercise, laxatives (titrated) |
| Duration and no. of biofeedback sessions |
6 mo, 1 y, 5 weekly, 30-min training sessions performed by physician investigator |
3 mo, every other week, 1 h, maximum of 6 sessions over 3 mo, performed by biofeedback nurse therapist |
1-6-12-24 mo 5 weekly 30-min training sessions, performed by physician investigator |
6 every other week, 1-h sessions |
1y 6 active therapy sessions and 3 reinforcement sessions at 3-mo intervals |
| Primary outcomes | Global improvement of symptoms Worse = 0 No improvement =1 Mild = 2 Fair = 3 Major improvement = 4 |
Presence of dyssynergia Balloon expulsion time No. of CSBMs Global satisfaction |
Symptom improvement None = 1 Mild = 2 Fair = 3 Major = 4 |
Global symptom relief |
No. of CSBMs Secondary outcome; Presence of dyssynergia Balloon expulsion time Global satisfaction |
| Dyssynergia corrected or symptoms improved |
79.6% reported major improvement at 6 and 12 mo 81.5% reported persistent major improvement at 24 mo |
Dyssynergia corrected at 3 months in 79% with biofeedback vs 4% sham and 6% in standard group; CSBM = biofeedback group vs sham or Standard; P < .05 |
71% with dyssynergia and 8% with slow transit alone reported fair improvement in symptoms both at short and long-term follow-up intervals |
70% improved with biofeedback compared to 38% with placebo and 30% with diazepam; P < .01 |
No. of CSBMs/wk increased significantly in biofeedback; P < .001 Dyssynergia pattern normalized; P < .0010 Balloon expulsion improved; P < .001 Colonic transit normalized; P < .01 |
CSBM, complete spontaneous bowel movement; PEG, polyethylene glycol.
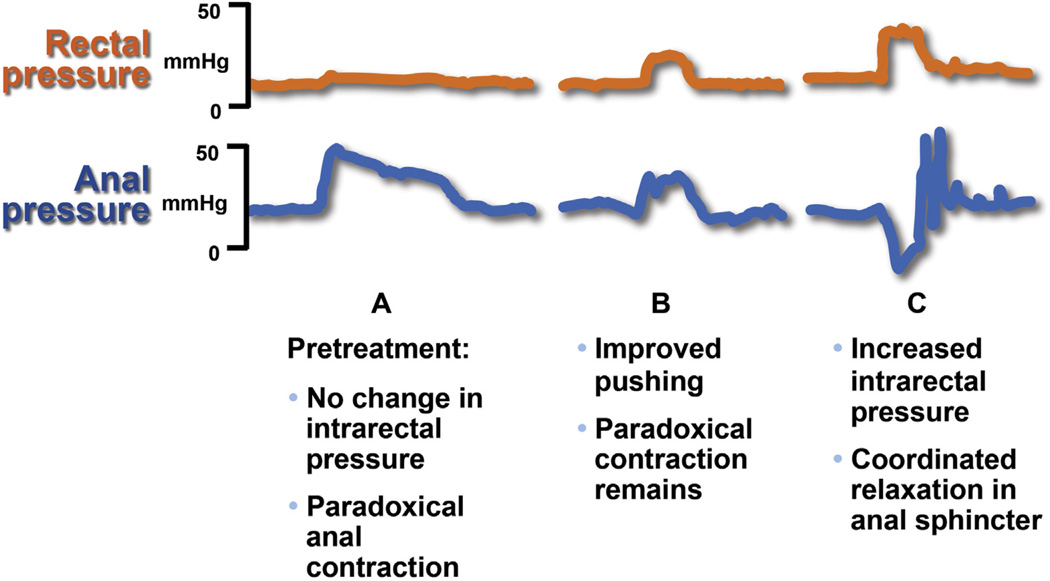
Figure 2.
Effect of biofeedback therapy on dyssynergia in 1 patient before and after treatment. Panel A shows baseline intrarectal and anal sphincter pressures. There is inadequate propulsion and paradoxical anal contraction. Panel B shows that after learning diaphragmatic breathing technique, the pushing effort has improved but patient still shows paradoxical contraction. Panel C shows coordinated relaxation, with an increase in intrarectal pressure and relaxation of the anal sphincter. Adapted from Rao, with permission.187
Several randomized controlled trials have demonstrated that biofeedback is safe and effective treatment for dyssynergic defecation (Table 3). Biofeedback therapy was more effective than sham feedback, pelvic floor exercises, laxatives, and muscle relaxant drugs, both on a short- and long-term basis without side effects.116–117,183–185 Biofeedback therapy is to be regarded as first-choice treatment for FDD whenever dedicated expertise is available. Biofeedback therapy is not effective for constipated patients without FDD.116 Whether biofeedback is as effective for altered defecatory propulsion as it is for dyssynergic defecation is not known.
Recommendations for Future Research
Multicenter studies of normal physiology of defecation and fecal continence using newer diagnostic modalities in large groups of subjects stratified for age, sex, and parity.
Define the role of rectal contraction and sensation in disordered defecation, especially to understand dyssynergic defecation vs inadequate propulsion.
Evaluate interaction(s) between stool consistency, sphincter weakness, sphincter defects, rectal sensation and compliance, and neurogenic sphincter injury in FI.
Randomized, blinded controlled study of biofeedback treatment for dyssynergic defecation, especially to examine its generalizability for FDD.
Examine the natural history, duration, and phenotype of anorectal pain syndromes and perform randomized studies of drugs, biofeedback, and other treatments for levator ani syndrome.
Randomized controlled trials of bowel management, biofeedback, sacral nerve stimulation, anal bulking agents and sphincteroplasty in FI, including mechanistic understanding and well-designed outcome measures.
Acknowledgments
Funding
SSCR is supported in part by grant National Institutes of Health, 5 R21DK104127-02. AEB was supported in part by grant R01 DK78924 from the National Institutes of Health, US Department of Health and Human Services.
Abbreviations used in this paper
- ARM
anorectal manometry
- CI
confidence interval
- DRE
digital rectal examination
- EMG
electromyography
- FDD
functional defecation disorder
- FI
fecal incontinence
- IBS
irritable bowel syndrome
- MRI
magnetic resonance imaging
- OR
odds ratio
Footnotes
Supplementary Material
Note: The first 50 references associated with this article are available below in print. The remaining references accompanying this article are available online only with the electronic version of the article. Visit the online version of Gastroenterology at www.gastrojournal.org, and at http://dx.doi.org/10.1053/j.gastro.2016.02.009.
Conflicts of interest
The authors disclose the following: AEB is an inventor of the portable anorectal manometry catheter that has been licensed to Medspira Inc; AEB and Mayo Clinic have contractual rights to receive royalties from the licensing of this technology. GC is an advisory board member and speaker for Shire Italia and Takeda Italia. The remaining authors disclose no conflicts.
References
- 1.Bharucha AE, Rao SSC. An update on anorectal disorders for gastroenterologists. Gastroenterology. 2014;146:37–45. doi: 10.1053/j.gastro.2013.10.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bharucha AE, Fletcher JG, Melton LJ, 3rd, et al. Obstetric trauma, pelvic floor injury and fecal incontinence: a population-based case-control study. Am J Gastroenterol. 2012;107:902–911. doi: 10.1038/ajg.2012.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shorvon PJ, McHugh S, Diamant NE, et al. Defecography in normal volunteers: results and implications. Gut. 1989;30:1737–1749. doi: 10.1136/gut.30.12.1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bharucha AE, Wald A, Enck P, Rao S. Functional anorectal disorders. Gastroenterology. 2006;130:1510–1518. doi: 10.1053/j.gastro.2005.11.064. [DOI] [PubMed] [Google Scholar]
- 5.Bharucha AE, Seide BM, Zinsmeister AR, et al. Insights into normal and disordered bowel habits from bowel diaries. Am J Gastroenterol. 2008;103:692–698. doi: 10.1111/j.1572-0241.2007.01631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ashraf W, Park F, Lof J, Quigley EM. An examination of the reliability of reported stool frequency in the diagnosis of idiopathic constipation. Am J Gastroenterol. 1996;91:26–32. [PubMed] [Google Scholar]
- 7.Diamant NE, Kamm MA, Wald A, et al. AGA technical review on anorectal testing techniques. Gastroenterology. 1999;116:735–760. doi: 10.1016/s0016-5085(99)70195-2. [DOI] [PubMed] [Google Scholar]
- 8.Wald A, Bharucha AE, Cosman BC, et al. ACG Clinical guidelines: management of benign anorectal disorders. Am J Gastroenterol. 2014;109:1141–1157. doi: 10.1038/ajg.2014.190. [DOI] [PubMed] [Google Scholar]
- 9.Bongers MEJ, Tabbers MM, Benninga MA. Functional nonretentive fecal incontinence in children. J Ped Gastroenterol Nutr. 2007;44:5–13. doi: 10.1097/01.mpg.0000252187.12793.0a. [DOI] [PubMed] [Google Scholar]
- 10.Bharucha AE, Zinsmeister AR, Locke GR, et al. Prevalence and burden of fecal incontinence: a population based study in women. Gastroenterology. 2005;129:42–49. doi: 10.1053/j.gastro.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 11.Melville JL, Fan MY, Newton K, et al. Fecal incontinence in US women: a population-based study. Am J Obstet Gynecol. 2005;193:2071–2076. doi: 10.1016/j.ajog.2005.07.018. [DOI] [PubMed] [Google Scholar]
- 12.Quander CR, Morris MC, Melson J, et al. Prevalence of and factors associated with fecal incontinence in a large community study of older individuals. Am J Gastroenterol. 2005;100:905–909. doi: 10.1111/j.1572-0241.2005.30511.x. [DOI] [PubMed] [Google Scholar]
- 13.Varma MG, Brown JS, Creasman JM, et al. Reproductive Risks for Incontinence Study at Kaiser Research Group. Fecal incontinence in females older than aged 40 years: who is at risk? Dis Colon Rectum. 2006;49:841–851. doi: 10.1007/s10350-006-0535-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Siproudhis L, Pigot F, Godeberge P, et al. Defecation disorders: a French population survey. Dis Colon Rectum. 2006;49:219–227. doi: 10.1007/s10350-005-0249-8. [DOI] [PubMed] [Google Scholar]
- 15.Nygaard I, Barber MD, Burgio KL, et al. Pelvic Floor Disorders Network. Prevalence of symptomatic pelvic floor disorders in US women. JAMA. 2008;300:1311–1316. doi: 10.1001/jama.300.11.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Whitehead WE, Borrud L, Goode PS, et al. Pelvic Floors Disorders Network. Fecal incontinence in US adults: epidemiology and risk factors. Gastroenterology. 2009;137:512–517. doi: 10.1053/j.gastro.2009.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matthews CA, Whitehead WE, Townsend MK, et al. Risk factors for urinary, fecal, or dual incontinence in the nurses’ health study. Obstet Gynecol. 2013;122:539–545. doi: 10.1097/AOG.0b013e31829efbff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nelson R, Furner S, Jesudason V. Fecal incontinence in Wisconsin nursing homes: prevalence and associations. Dis Colon Rectum. 1998;41:1226–1229. doi: 10.1007/BF02258218. [DOI] [PubMed] [Google Scholar]
- 19.Bliss DZ, Harms S, Garrard JM, et al. Prevalence of incontinence by race and ethnicity of older people admitted to nursing homes. J Am Med Dir Assoc. 2013;14:451, e1–e7. doi: 10.1016/j.jamda.2013.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perry S, Shaw C, McGrother C, et al. Prevalence of faecal incontinence in adults aged 40 years or more living in the community. Gut. 2002;50:480–484. doi: 10.1136/gut.50.4.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Landefeld CS, Bowers BJ, Feld AD, et al. National Institutes of Health state-of-the-science conference statement: prevention of fecal and urinary incontinence in adults. Ann Intern Med. 2008;148:449–458. doi: 10.7326/0003-4819-148-6-200803180-00210. [DOI] [PubMed] [Google Scholar]
- 22.Ditah I, Devaki P, Luma HN, et al. Prevalence, trends, and risk factors for fecal incontinence in United States adults, 2005–2010. Clin Gastroenterol Hepatol. 2014;12:636–643. e1–e2. doi: 10.1016/j.cgh.2013.07.020. [DOI] [PubMed] [Google Scholar]
- 23.Markland AD, Goode PS, Burgio KL, et al. Incidence and risk factors for fecal incontinence in black and white older adults: a population-based study. J Am Geriatr Soc. 2010;58:1341–1346. doi: 10.1111/j.1532-5415.2010.02908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goode PS, Burgio KL, Halli AD, et al. Prevalence and correlates of fecal incontinence in community-dwelling older adults. J Am Geriatr Soc. 2005;53:629–635. doi: 10.1111/j.1532-5415.2005.53211.x. [DOI] [PubMed] [Google Scholar]
- 25.Bharucha AE. Outcome measures for fecal incontinence: anorectal structure and function. Gastroenterology. 2004;126(Suppl 1):S90–S98. doi: 10.1053/j.gastro.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 26.Rey E, Choung RS, Schleck CD, et al. Onset and risk factors for fecal incontinence in a US community. Am J Gastroenterol. 2010;105:412–419. doi: 10.1038/ajg.2009.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cotterill N, Norton C, Avery KNL, et al. A patient-centered approach to developing a comprehensive symptom and quality of life assessment of anal incontinence. Dis Colon Rectum. 2008;51:82–87. doi: 10.1007/s10350-007-9069-3. [DOI] [PubMed] [Google Scholar]
- 28.Maeda Y, Vaizey CJ, Hollington P, et al. Physiological, psychological and behavioural characteristics of men and women with faecal incontinence. Colorectal Dis. 2009;11:927–932. doi: 10.1111/j.1463-1318.2008.01717.x. [DOI] [PubMed] [Google Scholar]
- 29.Koloski NA, Jones M, Kalantar J, et al. Psychological impact and risk factors associated with new onset fecal incontinence. J Psychosom Res. 2012;73:464–468. doi: 10.1016/j.jpsychores.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 30.Visscher AP, Lam TJ, Hart N, et al. Fecal incontinence, sexual complaints, and anorectal function after third-degree obstetric anal sphincter injury (OASI): 5-year follow-up. Int Urogynecol J. 2014;25:607–613. doi: 10.1007/s00192-013-2238-0. [DOI] [PubMed] [Google Scholar]
- 31.Bharucha AE, Zinsmeister AR, Locke GR, et al. Symptoms and quality of life in community women with fecal incontinence. Clin Gastroenterol Hepatol. 2006;4:1004–1009. doi: 10.1016/j.cgh.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 32.Rockwood TH, Church JM, Fleshman JW, et al. Fecal incontinence quality of life scale: quality of life instrument for patients with fecal incontinence. Dis Colon Rectum. 2000;43:9–16. doi: 10.1007/BF02237236. discussion 16–17. [DOI] [PubMed] [Google Scholar]
- 33.Kwon S, Visco AG, Fitzgerald MP, et al. Pelvic Floor Disorders Network. Validity and reliability of the Modified Manchester Health Questionnaire in assessing patients with fecal incontinence. Dis Colon Rectum. 2005;48:323–331. doi: 10.1007/s10350-004-0899-y. discussion 331 –334. [DOI] [PubMed] [Google Scholar]
- 34.Rogers RG, Rockwood TH, Constantine ML, et al. A new measure of sexual function in women with pelvic floor disorders (PFD): the Pelvic Organ Prolapse/Incontinence Sexual Questionnaire, IUGA-Revised (PISQ-IR) Intern Urogyn J. 2013;24:1091–1103. doi: 10.1007/s00192-012-2020-8. [DOI] [PubMed] [Google Scholar]
- 35.Rockwood TH, Church JM, Fleshman JW, et al. Patient and surgeon ranking of the severity of symptoms associated with fecal incontinence: the fecal incontinence severity index. Dis Colon Rectum. 1999;42:1525–1532. doi: 10.1007/BF02236199. [DOI] [PubMed] [Google Scholar]
- 36.Chassagne P, Landrin I, Neveu C, et al. Fecal incontinence in the institutionalized elderly: incidence, risk factors, and prognosis. Am J Med. 1999;106:185–190. doi: 10.1016/s0002-9343(98)00407-0. [DOI] [PubMed] [Google Scholar]
- 37.Nakanishi N, Tatara K, Shinsho F, et al. Mortality in relation to urinary and faecal incontinence in elderly people living at home. Age Ageing. 1999;28:301–306. doi: 10.1093/ageing/28.3.301. [DOI] [PubMed] [Google Scholar]
- 38.Alameel T, Basheikh M, Andrew MK. Digestive symptoms in older adults: prevalence and associations with institutionalization and mortality. Can J Gastroenterol. 2012;26:881–884. doi: 10.1155/2012/324602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bharucha AE, Zinsmeister AR, Locke GR, et al. Risk factors for fecal incontinence: a population based study in women. Am J Gastroenterol. 2006;101:1305–1312. doi: 10.1111/j.1572-0241.2006.00553.x. [DOI] [PubMed] [Google Scholar]
- 40.Bharucha AE, Seide B, Zinsmeister AR, et al. Relation of bowel habits to fecal incontinence in women. Am J Gastroenterol. 2008;103:1470–1475. doi: 10.1111/j.1572-0241.2008.01792.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bharucha AE, Zinsmeister AR, Schleck CD, et al. Bowel disturbances are the most important risk factors for late onset fecal incontinence: a population-based case-control study in women. Gastroenterology. 2010;139:1559–1566. doi: 10.1053/j.gastro.2010.07.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nelson R, Norton N, Cautley E, et al. Community-based prevalence of anal incontinence. JAMA. 1995;274:559–561. [PubMed] [Google Scholar]
- 43.Rommen K, Schei B, Rydning A, et al. Prevalence of anal incontinence among Norwegian women: a cross-sectional study. BMJ Open. 2012;2(4) doi: 10.1136/bmjopen-2012-001257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rogers RG, Leeman L, Borders A, et al. Does cesarean delivery protect against pelvic floor dysfunction at 6 months postpartum? Female Pelvic Med Reconstruct Surg. 2012;18:S73. [Google Scholar]
- 45.Nelson RL, Furner SE, Westercamp M, et al. Cesarean delivery for the prevention of anal incontinence. Cochrane Database Syst Rev. 2010 Feb;17(2):CD006756. doi: 10.1002/14651858.CD006756.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bols EMJ, Hendriks EJM, Berghmans BCM, et al. A systematic review of etiological factors for postpartum fecal incontinence. Acta Obstet Gynecol Scand. 2010;89:302–314. doi: 10.3109/00016340903576004. [DOI] [PubMed] [Google Scholar]
- 47.Borello-France D, Burgio KL, et al. Pelvic Floor Disorders Network. Fecal and urinary incontinence in primiparous women. Obstet Gynecol. 2006;108:863–872. doi: 10.1097/01.AOG.0000232504.32589.3b. [DOI] [PubMed] [Google Scholar]
- 48.Pretlove SJ, Thompson PJ, Toozs-Hobson PM, et al. Does the mode of delivery predispose women to anal incontinence in the first year postpartum? A comparative systematic review. BJOG. 2008;115:421–434. doi: 10.1111/j.1471-0528.2007.01553.x. [DOI] [PubMed] [Google Scholar]
- 49.Nyam DC, Pemberton JH. Long-term results of lateral internal sphincterotomy for chronic anal fissure with particular reference to incidence of fecal incontinence. Dis Colon Rectum. 1999;42:1306–1310. doi: 10.1007/BF02234220. [DOI] [PubMed] [Google Scholar]
- 50.Buchmann P, Mogg GA, Alexander-Williams J, et al. Relationship of proctitis and rectal capacity in Crohn’s disease. Gut. 1980;21:137–140. doi: 10.1136/gut.21.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
Supplementary References
- 51.Read NW, Abouzekry L, Read MG, et al. Anorectal function in elderly patients with fecal impaction. Gastroenterology. 1985;89:959–966. doi: 10.1016/0016-5085(85)90194-5. [DOI] [PubMed] [Google Scholar]
- 52.Varma JS, Smith AN, Busuttil A. Correlation of clinical and manometric abnormalities of rectal function following chronic radiation injury. Br J Surg. 1985;72:875–878. doi: 10.1002/bjs.1800721107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rao SS. Pathophysiology of adult fecal incontinence. Gastroenterology. 2004;126(Suppl 1):S14–S22. doi: 10.1053/j.gastro.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 54.Bharucha AE, Fletcher JG, Harper CM, et al. Relationship between symptoms and disordered continence mechanisms in women with idiopathic fecal incontinence. Gut. 2005;54:546–555. doi: 10.1136/gut.2004.047696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sun WM, Donnelly TC, Read NW. Utility of a combined test of anorectal manometry, electromyography, and sensation in determining the mechanism of ‘idiopathic’ faecal incontinence. Gut. 1992;33:807–813. doi: 10.1136/gut.33.6.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Richter HE, Fielding JR, Bradley CS, et al. Pelvic Floor Disorders Network. Endoanal ultrasound findings and fecal incontinence symptoms in women with and without recognized anal sphincter tears. Obstet Gynecol. 2006;108:1394–1401. doi: 10.1097/01.AOG.0000246799.53458.bc. [DOI] [PubMed] [Google Scholar]
- 57.Bartolo DC, Jarratt JA, Read MG, et al. The role of partial denervation of the puborectalis in idiopathic faecal incontinence. Br J Surg. 1983;70:664–667. doi: 10.1002/bjs.1800701108. [DOI] [PubMed] [Google Scholar]
- 58.Fernandez-Fraga X, Azpiroz F, Malagelada JR. Significance of pelvic floor muscles in anal incontinence. Gastroenterology. 2002;123:1441–1450. doi: 10.1053/gast.2002.36586. [DOI] [PubMed] [Google Scholar]
- 59.Phillips SF, Edwards DA. Some aspects of anal continence and defaecation. Gut. 1965;6:396–406. doi: 10.1136/gut.6.4.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu J, Guaderrama N, Nager CW, et al. Functional correlates of anal canal anatomy: puborectalis muscle and anal canal pressure. Am J Gastroenterol. 2006;101:1092–1097. doi: 10.1111/j.1572-0241.2006.00596.x. [DOI] [PubMed] [Google Scholar]
- 61.Jung S-A, Pretorius DH, Weinstein M, et al. Closure mechanism of the anal canal in women: assessed by three-dimensional ultrasound imaging. Dis Colon Rectum. 2008;51:932–939. doi: 10.1007/s10350-008-9221-8. [DOI] [PubMed] [Google Scholar]
- 62.Raizada V, Bhargava V, Karsten A, Mittal RK. Functional morphology of anal sphincter complex unveiled by high definition anal manometery and three dimensional ultrasound imaging. Neurogastroenterol Motil. 2011;23:1013–1019. doi: 10.1111/j.1365-2982.2011.01782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chun AB, Rose S, Mitrani C, Silvestre AJ, Wald A. Anal sphincter structure and function in homosexual males engaging in anoreceptive intercourse. Am J Gastroenterol. 1997;92:465–468. [PubMed] [Google Scholar]
- 64.Mitrani C, Chun A, Desautels S, Wald A. Anorectal manometric characteristics in men and women with idiopathic fecal incontinence. J Clin Gastroenterol. 1998;26:175–178. doi: 10.1097/00004836-199804000-00005. [DOI] [PubMed] [Google Scholar]
- 65.Chen H, Humphreys MS, Kettlewell MG, et al. Anal ultrasound predicts the response to nonoperative treatment of fecal incontinence in men. Ann Surg. 1999;229:739–743. doi: 10.1097/00000658-199905000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Burgell RE, Bhan C, Lunniss PJ, et al. Fecal incontinence in men: coexistent constipation and impact of rectal hyposensitivity. Dis Colon Rectum. 2012;55:18–25. doi: 10.1097/DCR.0b013e318237f37d. [DOI] [PubMed] [Google Scholar]
- 67.Paramor KA, Ibrahim QI, Sadowski DC. Clinical parameters and symptom severity in males with fecal leakage and incontinence. Neurogastroenterol Motil. 2014;26:361–367. doi: 10.1111/nmo.12270. [DOI] [PubMed] [Google Scholar]
- 68.Rao SS, Ozturk R, Stessman M. Investigation of the pathophysiology of fecal seepage. Am J Gastroenterol. 2004;99:2204–2209. doi: 10.1111/j.1572-0241.2004.40387.x. [DOI] [PubMed] [Google Scholar]
- 69.Parellada CM, Miller AS, Williamson ME, Johnston D. Paradoxical high anal resting pressures in men with idiopathic fecal seepage. Dis Colon Rectum. 1998;41:593–597. doi: 10.1007/BF02235265. [DOI] [PubMed] [Google Scholar]
- 70.Krol R, Smeenk RJ, van Lin EN, et al. Systematic review: anal and rectal changes after radiotherapy for prostate cancer. Int J Colorectal Dis. 2014;29:273–283. doi: 10.1007/s00384-013-1784-8. [DOI] [PubMed] [Google Scholar]
- 71.Bharucha AE. High amplitude propagated contractions. Neurogastroenterol Motil. 2012;24:977–982. doi: 10.1111/nmo.12019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sun WM, Read NW, Miner PB. Relation between rectal sensation and anal function in normal subjects and patients with faecal incontinence. Gut. 1990;31:1056–1061. doi: 10.1136/gut.31.9.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Buser WD, Miner PB. Delayed rectal sensation with fecal incontinence. Successful treatment using anorectal manometry. Gastroenterology. 1986;91:1186–1191. doi: 10.1016/s0016-5085(86)80015-4. [DOI] [PubMed] [Google Scholar]
- 74.Miller R, Bartolo DC, Cervero F, et al. Anorectal sampling: a comparison of normal and incontinent patients. Br J Surg. 1988;75:44–47. doi: 10.1002/bjs.1800750116. [DOI] [PubMed] [Google Scholar]
- 75.Whitehead WE, Palsson OS. Is rectal pain sensitivity a biological marker for irritable bowel syndrome: psychological influences on pain perception. Gastroenterology. 1998;115:1263–1271. doi: 10.1016/s0016-5085(98)70099-x. [DOI] [PubMed] [Google Scholar]
- 76.Andrews C, Bharucha AE, Camilleri M, et al. Rectal sensorimotor dysfunction in women with fecal incontinence. Am J Physiol. 2007;292:G282–G289. doi: 10.1152/ajpgi.00176.2006. [DOI] [PubMed] [Google Scholar]
- 77.Felt-Bersma RJ, Sloots CE, Poen AC, et al. Rectal compliance as a routine measurement: extreme volumes have direct clinical impact and normal volumes exclude rectum as a problem. Dis Colon Rectum. 2000;43:1732–1738. doi: 10.1007/BF02236859. [DOI] [PubMed] [Google Scholar]
- 78.Malcolm A, Camilleri M. Coloanal motor coordination in association with high-amplitude colonic contractions after pharmacological stimulation. Am J Gastroenterol. 2000;95:715–719. doi: 10.1111/j.1572-0241.2000.01840.x. [DOI] [PubMed] [Google Scholar]
- 79.Heaton KW, Parker D, Cripps H. Bowel function and irritable bowel symptoms after hysterectomy and cholecystectomy–a population based study. Gut. 1993;34:1108–1111. doi: 10.1136/gut.34.8.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schnelle JF, Simmons SF, Beuscher L, et al. Prevalence of constipation symptoms in fecally incontinent nursing home residents. J Am Geriatr Soc. 2009;57:647–652. doi: 10.1111/j.1532-5415.2009.02215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Read NW, Bartolo DC, Read MG. Differences in anal function in patients with incontinence to solids and in patients with incontinence to liquids. Br J Surg. 1984;71:39–42. doi: 10.1002/bjs.1800710112. [DOI] [PubMed] [Google Scholar]
- 82.Engel AF, Kamm MA, Bartram CI, et al. Relationship of symptoms in faecal incontinence to specific sphincter abnormalities. Int J Colorectal Dis. 1995;10:152–155. doi: 10.1007/BF00298538. [DOI] [PubMed] [Google Scholar]
- 83.Chiarioni G, Scattolini C, Bonfante F, et al. Liquid stool incontinence with severe urgency: anorectal function and effective biofeedback treatment. Gut. 1993;34:1576–1580. doi: 10.1136/gut.34.11.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Siproudhis L, El Abkari M, El Alaoui M, et al. Low rectal volumes in patients suffering from fecal incontinence: what does it mean? Alimen Pharmacol Therap. 2005;22:989–996. doi: 10.1111/j.1365-2036.2005.02675.x. [DOI] [PubMed] [Google Scholar]
- 85.Jorge JM, Wexner SD. Etiology and management of fecal incontinence. Dis Colon Rectum. 1993;36:77–97. doi: 10.1007/BF02050307. [DOI] [PubMed] [Google Scholar]
- 86.Vaizey CJ, Carapeti E, Cahill JA, et al. Prospective comparison of faecal incontinence grading systems. Gut. 1999;44:77–80. doi: 10.1136/gut.44.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bharucha AE, Locke GR, Seide B, et al. A New Questionnaire for Constipation and Fecal Incontinence. Aliment Pharmacol Therap. 2004;20:355–364. doi: 10.1111/j.1365-2036.2004.02028.x. [DOI] [PubMed] [Google Scholar]
- 88.Wexner SD, Coller JA, Devroede G, Hull T, et al. Sacral nerve stimulation for fecal incontinence: results of a 120-patient prospective multicenter study. Ann Surg. 2010;251:441–449. doi: 10.1097/SLA.0b013e3181cf8ed0. [DOI] [PubMed] [Google Scholar]
- 89.Wald A. Clonidine and botulinum toxin: a tale of two treatments. Clin Gastroenterol Hepatol. 2014;12:852–853. doi: 10.1016/j.cgh.2013.08.022. [DOI] [PubMed] [Google Scholar]
- 90.Wald A. New treatments for fecal incontinence: update for the gastroenterologist. Clin Gastroenterol Hepatol. 2014;12:1783–1788. doi: 10.1016/j.cgh.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 91.Sultan AH, Kamm MA, Hudson CN, et al. Anal-sphincter disruption during vaginal delivery. N Engl J Med. 1993;329:1905–1911. doi: 10.1056/NEJM199312233292601. [DOI] [PubMed] [Google Scholar]
- 92.Hill J, Corson RJ, Brandon H, et al. History and examination in the assessment of patients with idiopathic fecal incontinence. Dis Colon Rectum. 1994;37:473–477. doi: 10.1007/BF02076194. [DOI] [PubMed] [Google Scholar]
- 93.Rao SSC, Azpiroz F, Diamant N, et al. Minimum standards of anorectal manometry. Neurogastroenterol motil. 2002;14:553–559. doi: 10.1046/j.1365-2982.2002.00352.x. [DOI] [PubMed] [Google Scholar]
- 94.Noelting J, Ratuapli SK, Bharucha AE, et al. Normal values for high-resolution anorectal manometry in healthy women: effects of age and significance of rectoanal gradient. Am J Gastroenterol. 2012;107:1530–1536. doi: 10.1038/ajg.2012.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Coss-Adame E, Rao SS, Valestin J, et al. Accuracy and reproducibility of high-definition anorectal manometry and pressure topography analyses in healthy subjects. Clin Gastroenterol Hepatol. 2015;13:1143–1150. doi: 10.1016/j.cgh.2014.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Felt-Bersma RJ. Endoanal ultrasound in benign anorectal disorders: clinical relevance and possibilities. Exp Rev Gastroenterol Hepatol. 2008;2:587–606. doi: 10.1586/17474124.2.4.587. [DOI] [PubMed] [Google Scholar]
- 97.Abdool Z, Sultan AH, Thakar R. Ultrasound imaging of the anal sphincter complex: a review. Br J Radiol. 2012;85:865–875. doi: 10.1259/bjr/27314678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Williams AB, Bartram CI, Halligan S, et al. Alteration of anal sphincter morphology following vaginal delivery revealed by multiplanar anal endosonography. BJOG. 2002;109:942–946. doi: 10.1111/j.1471-0528.2002.00251.x. [DOI] [PubMed] [Google Scholar]
- 99.Dobben AC, Terra MP, Slors JFM, et al. External anal sphincter defects in patients with fecal incontinence: comparison of endoanal MR imaging and endoanal US. Radiology. 2007;242:463–471. doi: 10.1148/radiol.2422051575. [DOI] [PubMed] [Google Scholar]
- 100.West RL, Dwarkasing S, Briel JW, et al. Can three-dimensional endoanal ultrasonography detect external anal sphincter atrophy? A comparison with endoanal magnetic resonance imaging. Inl J Colorectal Dis. 2005;20:328–333. doi: 10.1007/s00384-004-0693-2. [DOI] [PubMed] [Google Scholar]
- 101.Roos AM, Abdool Z, Sultan AH, et al. The diagnostic accuracy of endovaginal and transperineal ultrasound for detecting anal sphincter defects: the PREDICT study. Clin Radiol. 2011;66:597–604. doi: 10.1016/j.crad.2010.11.017. [DOI] [PubMed] [Google Scholar]
- 102.Bharucha AE, Fletcher JG, Seide B, et al. Phenotypic variation in functional disorders of defecation. Gastroenterology. 2005;128:1199–1210. doi: 10.1053/j.gastro.2005.03.021. [DOI] [PubMed] [Google Scholar]
- 103.Prichard D, Harvey DM, Fletcher JG, et al. Relationship among anal sphincter injury, patulous anal canal, and anal pressures in patients with anorectal disorders. Clin Gastroenterol Hepatol. 2015;13:1793–1800. doi: 10.1016/j.cgh.2015.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bharucha AE, Daube J, Litchy W, et al. Anal sphincteric neurogenic injury in asymptomatic nulliparous women and fecal incontinence. Am J Physiol. 2012;303:G256–G262. doi: 10.1152/ajpgi.00099.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tantiphlachiva K, Attaluri A, Valestin J, et al. Translumbar and transsacral motor-evoked potentials: a novel test for spino-anorectal neuropathy in spinal cord injury. Am J Gastroenterol. 2011;106:907–914. doi: 10.1038/ajg.2010.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bliss DZ, Savik K, Jung HJ, et al. Dietary fiber supplementation for fecal incontinence: a randomized clinical trial. Res Nursing Health. 2014;37:367–378. doi: 10.1002/nur.21616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Read M, Read NW, Barber DC, et al. Effects of loperamide on anal sphincter function in patients complaining of chronic diarrhea with fecal incontinence and urgency. Dig Dis Sci. 1982;27:807–814. doi: 10.1007/BF01391374. [DOI] [PubMed] [Google Scholar]
- 108.Omar IM, Alexander EC. Drug treatment for faecal incontinence in adults. Cochrane Database Syst Rev. 2013 Jun 11;6:CD002116. doi: 10.1002/14651858.CD002116.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Santoro GA, Eitan BZ, Pryde A, et al. Open study of low-dose amitryptalinee in the treatment of patients with idiopathic fecal incontinence. Am J Gastroenterol. 2000;43:1676–1681. doi: 10.1007/BF02236848. [DOI] [PubMed] [Google Scholar]
- 110.Tobin GW, Brocklehurst JC. Faecal incontinence in residential homes for the elderly: prevalence, aetiology and management. Age Ageing. 1986;15:41–46. doi: 10.1093/ageing/15.1.41. [DOI] [PubMed] [Google Scholar]
- 111.Bharucha AE, Pemberton JH, Locke GR., 3rd American gastroenterological association technical review on constipation. Gastroenterology. 2013;144:218–238. doi: 10.1053/j.gastro.2012.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cazemier M, Felt-Bersma RJ, Mulder CJ. Anal plugs and retrograde colonic irrigation are helpful in fecal incontinence or constipation. World J Gastroenterol. 2007;13:3101–3105. doi: 10.3748/wjg.v13.i22.3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Christensen P, Krogh K, Buntzen S, et al. Long-term outcome and safety of transanal irrigation for constipation and fecal incontinence. Dis Colon Rectum. 2009;52:286–292. doi: 10.1007/DCR.0b013e3181979341. [DOI] [PubMed] [Google Scholar]
- 114.Preziosi G, Gosling J, Raeburn A, et al. Transanal irrigation for bowel symptoms in patients with multiple sclerosis. Dis Colon Rectum. 2012;55:1066–1073. doi: 10.1097/DCR.0b013e3182653bd1. [DOI] [PubMed] [Google Scholar]
- 115.Collins E, Hibberts F, Lyons M, et al. Outcomes in non-surgical management for bowel dysfunction. Br J Nursing. 2014;23:776–780. doi: 10.12968/bjon.2014.23.14.776. [DOI] [PubMed] [Google Scholar]
- 116.Rao SSC, Beninga MA, Bharucha AE, et al. ANMS-ESNM position paper and consensus guidelines on biofeedback therapy for anorectal disorder. Neurogastroenterol Motil. 2015;27:594–609. doi: 10.1111/nmo.12520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Heymen S, Scarlett Y, Jones K, et al. Randomized controlled trial shows biofeedback to be superior to alternative treatments for fecal incontinence. Dis Colon Rectum. 2009;52:1730–1737. doi: 10.1007/DCR.0b013e3181b55455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Malouf AJ, Norton CS, Engel AF, et al. Long-term results of overlapping anterior anal-sphincter repair for obstetric trauma. Lancet. 2000;355:260–265. doi: 10.1016/S0140-6736(99)05218-6. [DOI] [PubMed] [Google Scholar]
- 119.Hull T, Giese C, Wexner SD, et al. SNS Study Group. Long-term durability of sacral nerve stimulation therapy for chronic fecal incontinence. Dis Colon Rectum. 2013;56:234–245. doi: 10.1097/DCR.0b013e318276b24c. [DOI] [PubMed] [Google Scholar]
- 120.Leroi AM, Parc Y, Lehur PA, et al. Study Group. Efficacy of sacral nerve stimulation for fecal incontinence: results of a multicenter double-blind crossover study. Ann Surg. 2005;242:662–669. doi: 10.1097/01.sla.0000186281.09475.db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Graf W, Mellgren A, Matzel KE, et al. Efficacy of dextranomer in stabilised hyaluronic acid for treatment of faecal incontinence: a randomised, sham-controlled trial. Lancet. 2011;377:997–1003. doi: 10.1016/S0140-6736(10)62297-0. [DOI] [PubMed] [Google Scholar]
- 122.Mellgren A, Matzel KE, Pollack J, et al. Long-term efficacy of NASHA Dx injection therapy for treatment of fecal incontinence. Neurogastroenterol Motil. 2014;26:1087–1094. doi: 10.1111/nmo.12360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Grimaud JC, Bouvier M, Naudy B, et al. Manometric and radiologic investigations and biofeedback treatment of chronic idiopathic anal pain. Dis Colon Rectum. 1991;34:690–695. doi: 10.1007/BF02050352. [DOI] [PubMed] [Google Scholar]
- 124.Grant SR, Salvati EP, Rubin RJ. Levator syndrome: an analysis of 316 cases. Dis Colon Rectum. 1975;18:161–163. doi: 10.1007/BF02587168. [DOI] [PubMed] [Google Scholar]
- 125.Drossman DA, Li Z, Andruzzi E, et al. US householder survey of functional gastrointestinal disorders Prevalence, sociodemography, and health impact. Dig Dis Sci. 1993;38:1569–1580. doi: 10.1007/BF01303162. [DOI] [PubMed] [Google Scholar]
- 126.Chiarioni G, Nardo A, Vantini I, et al. Biofeedback is superior to electrogalvanic stimulation and massage for treatment of levator ani syndrome. Gastroenterology. 2010;138:1321–1329. doi: 10.1053/j.gastro.2009.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Rao SS, Paulson J, Mata M, et al. Clinical trial: effects of botulinum toxin on Levator ani syndrome-a double blind, placebo-controlled study. Alimen Pharmacol Therap. 2009;29:985–991. doi: 10.1111/j.1365-2036.2009.03964.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Thompson WG, Heaton KW. Proctalgia fugax. J R Coll Phys London. 1980;14:247–248. [PMC free article] [PubMed] [Google Scholar]
- 129.de Parades V, Etienney I, Bauer P, et al. Proctalgia fugax: demographic and clinical characteristics What every doctor should know from a prospective study of 54 patients. Dis Colon Rectum. 2007;50:893–898. doi: 10.1007/s10350-006-0754-4. [DOI] [PubMed] [Google Scholar]
- 130.Thompson WG. Proctalgia fugax in patients with the irritable bowel, peptic ulcer, or inflammatory bowel disease. Am J Gastroenterol. 1984;79:450–452. [PubMed] [Google Scholar]
- 131.Thompson WG. The irritable bowel. Gut. 1984;25:305–320. doi: 10.1136/gut.25.3.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Eckardt VF, Dodt O, Kanzler G. Anorectal function and morphology in patients with sporadic proctalgia fugax. Dis Colon Rectum. 1996;39:755–762. doi: 10.1007/BF02054440. [DOI] [PubMed] [Google Scholar]
- 133.Harvey RF. Colonic motility in proctalgia fugax. Lancet. 1979;2:713–714. doi: 10.1016/s0140-6736(79)90642-1. [DOI] [PubMed] [Google Scholar]
- 134.Rao SS, Hatfield RA. Paroxysmal anal hyperkinesis: a characteristic feature of proctalgia fugax. Gut. 1996;39:609–612. doi: 10.1136/gut.39.4.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Celik AF, Katsinelos P, Read NW, et al. Hereditary proctalgia fugax and constipation: report of a second family. Gut. 1995;36:581–584. doi: 10.1136/gut.36.4.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Guy RJ, Kamm MA, Martin JE. Internal anal sphincter myopathy causing proctalgia fugax and constipation: further clinical and radiological characterization in a patient. Eur J Gastroenterol Hepatol. 1997;9:221–224. doi: 10.1097/00042737-199702000-00023. [DOI] [PubMed] [Google Scholar]
- 137.Karras JD, Angelo G. Proctalgia fugax Clinical observations and a new diagnostic aid. Dis Colon Rectum. 1963;6:130–134. doi: 10.1007/BF02633466. [DOI] [PubMed] [Google Scholar]
- 138.Pilling LF, Swenson WM, Hill JR. The psychologic aspects of proctalgia fugax. Dis Colon Rectum. 1965;8:372–376. doi: 10.1007/BF02627263. [DOI] [PubMed] [Google Scholar]
- 139.Eckardt VF, Dodt O, Kanzler G, et al. Treatment of proctalgia fugax with salbutamol inhalation. Am J Gastroenterol. 1996;91:686–689. [PubMed] [Google Scholar]
- 140.Rao SSC, Tuteja AK, Vellema T, et al. Dyssynergic defecation: demographics, symptoms, stool patterns, and quality of life. J Clin Gastroenterol. 2004;38:680–685. doi: 10.1097/01.mcg.0000135929.78074.8c. [DOI] [PubMed] [Google Scholar]
- 141.Rao SS, Mudipalli RS, Stessman M, et al. Investigation of the utility of colorectal function tests and Rome II criteria in dyssynergic defecation (Anismus) Neurogastroenterol Motil. 2004;16:589–596. doi: 10.1111/j.1365-2982.2004.00526.x. [DOI] [PubMed] [Google Scholar]
- 142.Ratuapli S, Bharucha AE, Noelting J, et al. phenotypic identification and classification of functional defecatory disorders using high resolution anorectal manometry. Gastroenterology. 2013;144:314–322. doi: 10.1053/j.gastro.2012.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Minguez M, Herreros B, Sanchiz V, et al. Predictive value of the balloon expulsion test for excluding the diagnosis of pelvic floor dyssynergia in constipation. Gastroenterology. 2004;126:57–62. doi: 10.1053/j.gastro.2003.10.044. [DOI] [PubMed] [Google Scholar]
- 144.Roberts JP, Womack NR, Hallan RI, et al. Evidence from dynamic integrated proctography to redefine anismus. Br J Surg. 1992;79:1213–1215. doi: 10.1002/bjs.1800791140. [DOI] [PubMed] [Google Scholar]
- 145.Rao SS. Dyssynergic defecation. Gastroenterol Clin N Am. 2001;30:97–114. doi: 10.1016/s0889-8553(05)70169-2. [DOI] [PubMed] [Google Scholar]
- 146.Rao SS, Welcher KD, Leistikow JS. Obstructive defecation: a failure of rectoanal coordination. Am J Gastroenterol. 1998;93:1042–1050. doi: 10.1111/j.1572-0241.1998.00326.x. [DOI] [PubMed] [Google Scholar]
- 147.Halligan S, Bartram CI, Park HJ, et al. Proctographic features of anismus. Radiology. 1995;197:679–682. doi: 10.1148/radiology.197.3.7480738. [DOI] [PubMed] [Google Scholar]
- 148.Chiarioni G, Kim SM, Vantini I, et al. Validation of the balloon evacuation test: reproducibility and agreement with findings from anorectal manometry and electromyography. Clin Gastroenterol Hepatol. 2014;12:2049–2054. doi: 10.1016/j.cgh.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 149.Rao SS, Hatfield R, Soffer E, et al. Manometric tests of anorectal function in healthy adults. Am J Gastroenterol. 1999;94:773–783. doi: 10.1111/j.1572-0241.1999.00950.x. [DOI] [PubMed] [Google Scholar]
- 150.Voderholzer WA, Neuhaus DA, Klauser AG, et al. Paradoxical sphincter contraction is rarely indicative of anismus. Gut. 1997;41:258–262. doi: 10.1136/gut.41.2.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kuijpers HC. Application of the colorectal laboratory in diagnosis and treatment of functional constipation. Dis Colon Rectum. 1990;33:35–39. doi: 10.1007/BF02053199. [DOI] [PubMed] [Google Scholar]
- 152.Wald A, Caruana BJ, Freimanis MG, et al. Contributions of evacuation proctography and anorectal manometry to evaluation of adults with constipation and defecatory difficulty. Dig Dis Sci. 1990;35:481–487. doi: 10.1007/BF01536923. [DOI] [PubMed] [Google Scholar]
- 153.Surrenti E, Rath DM, Pemberton JH, et al. Audit of constipation in a tertiary referral gastroenterology practice. Am J Gastroenterol. 1995;90:1471–1475. [PubMed] [Google Scholar]
- 154.Rao S, Patel RS. How useful are manometric tests of anorectal function in the management of defecation disorders? Am J Gastroenterol. 1997;92:469–475. [PubMed] [Google Scholar]
- 155.Nullens S, Nelsen T, Camilleri M, et al. Regional colon transit in patients with dyssynergic defaecation or slow transit in patients with constipation. Gut. 2012;61:1132–1139. doi: 10.1136/gutjnl-2011-301181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Duthie GS, Bartolo DC. Anismus: the cause of constipation? Results of investigation and treatment. World J Surg. 1992;16:831–835. doi: 10.1007/BF02066978. [DOI] [PubMed] [Google Scholar]
- 157.Schouten WR. Anismus: Fact or fiction. Dis Colon Rectum. 1997;40:1033–1041. doi: 10.1007/BF02050925. [DOI] [PubMed] [Google Scholar]
- 158.Prott G, Shim L, Hansen R, et al. Relationships between pelvic floor symptoms and function in irritable bowel syndrome. Neurogastroenterol Motil. 2010;22:764–769. doi: 10.1111/j.1365-2982.2010.01503.x. [DOI] [PubMed] [Google Scholar]
- 159.Suttor VP, Prott GM, Hansen RD, et al. Evidence for pelvic floor dyssynergia in patients with irritable bowel syndrome. Dis Colon Rectum. 2010;53:156–160. doi: 10.1007/DCR.0b013e3181c188e8. [DOI] [PubMed] [Google Scholar]
- 160.Patcharatrakul T, Gonlachanvit S. Outcome of biofeedback therapy in dyssynergic defecation patients with and without irritable bowel syndrome. J Clin Gastroenterol. 2011;45:593–598. doi: 10.1097/MCG.0b013e31820c6001. [DOI] [PubMed] [Google Scholar]
- 161.Grossi U, Carrington EV, Bharucha AE, et al. Diagnostic accuracy study of anorectal manometry for diagnosis of dyssynergic defecation. Gut. 2016;65:447–455. doi: 10.1136/gutjnl-2014-308835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Tantiphlachiva K, Rao P, Attaluri A, et al. Digital rectal examination is a useful tool for identifying patients with dyssynergia. Clin Gastroenterol Hepatol. 2010;8:955–960. doi: 10.1016/j.cgh.2010.06.031. [DOI] [PubMed] [Google Scholar]
- 163.Pezim ME, Pemberton JH, Levin KE, et al. Parameters of anorectal and colonic motility in health and in severe constipation. Dis Colon Rectum. 1993;36:484–491. doi: 10.1007/BF02050015. [DOI] [PubMed] [Google Scholar]
- 164.Rao SSC, Kavlock R, Rao S. Influence of body position and stool characteristics on defecation in humans. Am J Gastroenterol. 2006;101:2790–2796. doi: 10.1111/j.1572-0241.2006.00827.x. [DOI] [PubMed] [Google Scholar]
- 165.Ratuapli S, Bharucha AE, Harvey D, et al. Comparison of rectal balloon expulsion test in seated and left lateral positions. Neurogastroenterol Motil. 2013;25:e813–e820. doi: 10.1111/nmo.12208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ekberg O, Mahiew PHG, Bartram CI, et al. Defecography: dynamic radiological imaging and proctology. Gastroenterol Int. 1990;3:93–99. [Google Scholar]
- 167.Kaufman HS, Buller JL, Thompson JR, et al. Dynamic pelvic magnetic resonance imaging and cystocolpoproctography alter surgical management of pelvic floor disorders. Dis Colon Rectum. 2001;44:1575–1583. doi: 10.1007/BF02234374. [DOI] [PubMed] [Google Scholar]
- 168.Chiarioni G, Salandini L, Whitehead WE. Biofeedback benefits only patients with outlet dysfunction, not patients with isolated slow transit constipation. Gastroenterology. 2005;129:86–97. doi: 10.1053/j.gastro.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 169.Ravi K, Bharucha AE, Camilleri M, et al. Phenotypic variation of colonic motor functions in chronic constipation. Gastroenterology. 2009;138:89–97. doi: 10.1053/j.gastro.2009.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Metcalf AM, Phillips SF, Zinsmeister AR, et al. Simplified assessment of segmental colonic transit. Gastroenterology. 1987;92:40–47. doi: 10.1016/0016-5085(87)90837-7. [DOI] [PubMed] [Google Scholar]
- 171.Rao SS, Kuo B, McCallum RW, et al. Investigation of colonic and whole gut transit with wireless motility capsule and radioopaque markers in constipation. Clin Gastroenterol Hepatol. 2009;7:537–544. doi: 10.1016/j.cgh.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 172.Camilleri M, Thorne NK, Ringel R, et al. Wireless pH-motility capsule for colonic transit: prospective comparison with radiopaque markers in chronic constipation. Neurogastroenterol Motil. 2010;22:874–e233. doi: 10.1111/j.1365-2982.2010.01517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Rao SSC, Coss-Adame E, Valestin J, et al. Evaluation of constipation in older adults: radioopaque markers (ROMs) versus wireless motility capsule (WMC) Arch Gerontol Geriatr. 2012;55:289–294. doi: 10.1016/j.archger.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 174.Deiteren A, Camilleri M, Bharucha AE, et al. Performance characteristics of scintigraphic colon transit measurement in health and irritable bowel syndrome and relationship to bowel functions. Neurogastroenterol Motil. 2010;22:415–423. doi: 10.1111/j.1365-2982.2009.01441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Heymen S, Wexner SD, Gulledge AD. MMPI assessment of patients with functional bowel disorders. Dis Colon Rectum. 1993;36:593–596. doi: 10.1007/BF02049867. [DOI] [PubMed] [Google Scholar]
- 176.Rao SSC, Seaton K, Miller MJ, et al. Psychological profiles and quality of life differ between patients with dyssynergia and those with slow transit constipation. J Psychosom Res. 2007;63:441–449. doi: 10.1016/j.jpsychores.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 177.Nehra V, Bruce BK, Rath-Harvey DM, et al. Psychological disorders in patients with evacuation disorders and constipation in a tertiary practice. Am J Gastroenterol. 2000;95:1755–1758. doi: 10.1111/j.1572-0241.2000.02184.x. [DOI] [PubMed] [Google Scholar]
- 178.Leroi AM, Berkelmans I, Denis P, et al. Anismus as a marker of sexual abuse Consequences of abuse on anorectal motility. Dig Dis Sci. 1995;40:1411–1416. doi: 10.1007/BF02285184. [DOI] [PubMed] [Google Scholar]
- 179.Rao SS, Welcher KD, Pelsang RE. Effects of biofeedback therapy on anorectal function in obstructive defecation. Dig Dis Sci. 1997;42:2197–2205. doi: 10.1023/a:1018846113210. [DOI] [PubMed] [Google Scholar]
- 180.Bleijenberg G, Kuijpers HC. Treatment of the spastic pelvic floor syndrome with biofeedback. Dis Colon Rectum. 1987;30:108–111. doi: 10.1007/BF02554946. [DOI] [PubMed] [Google Scholar]
- 181.Koutsomanis D, Lennard-Jones JE, Roy AJ, et al. Controlled randomised trial of visual biofeedback versus muscle training without a visual display for intractable constipation. Gut. 1995;37:95–99. doi: 10.1136/gut.37.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Chiarioni G, Whitehead WE. Biofeedback therapy for constipation. In: Parkman HP, McCallum RW, Rao SSC, editors. GI Motility Testing: A Laboratory and Office Handbook. Vol. 1. Thorofare, NJ: Slack Inc; 2011. pp. 79–187. [Google Scholar]
- 183.Rao SS, Seaton K, Miller M, et al. Randomized controlled trial of biofeedback, sham feedback, and standard therapy for dyssynergic defecation. Clin Gastroenterol Hepatol. 2007;5:331–338. doi: 10.1016/j.cgh.2006.12.023. [DOI] [PubMed] [Google Scholar]
- 184.Chiarioni G, Whitehead WE, Pezza V, et al. Biofeedback is superior to laxatives for normal transit constipation due to pelvic floor dyssynergia. Gastroenterology. 2006;130:657–664. doi: 10.1053/j.gastro.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 185.Rao SS, Valestin J, Brown CK, et al. Long-term efficacy of biofeedback therapy for dyssynergic defecation: randomized controlled trial. Am J Gastroenterol. 2010;105:890–896. doi: 10.1038/ajg.2010.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Heymen S, Scarlett Y, Jones K, et al. Randomized, controlled trial shows biofeedback to be superior to alternative treatments for patients with pelvic floor dyssynergia-type constipation. Dis Colon Rectum. 2007;50:428–441. doi: 10.1007/s10350-006-0814-9. [DOI] [PubMed] [Google Scholar]
- 187.Rao SS. Constipation: evaluation and treatment. Gastroenterol Clin North Am. 2003;32:659–683. doi: 10.1016/s0889-8553(03)00026-8. [DOI] [PubMed] [Google Scholar]