Abstract
Cell-based gene therapy holds a great promise for the treatment of human malignancy. Among different cells, mesenchymal stem cells (MSCs) are emerging as valuable anti-cancer agents that have the potential to be used to treat a number of different cancer types. They have inherent migratory properties, which allow them to serve as vehicles for delivering effective therapy to isolated tumors and metastases. MSCs have been engineered to express anti-proliferative, pro-apoptotic, and anti-angiogenic agents that specifically target different cancers. Another field of interest is to modify MSCs with the cytokines that activate pro-tumorigenic immunity or to use them as carriers for the traditional chemical compounds that possess the properties of anti-cancer drugs. Although there is still controversy about the exact function of MSCs in the tumor settings, the encouraging results from the preclinical studies of MSC-based gene therapy for a large number of tumors support the initiation of clinical trials.
Background
The poor prognosis of patients with different cancers due to insufficient treatment efficacy indicates the necessity to explore more effective anti-tumor therapy. The broad application of agents that control cell proliferation is limited by their short biological half-life or excessive toxicity. Recently, cell-based therapy has been considered a promising approach to enhance anti-cancer effect. Among different cell types, mesenchymal stem cells (MSCs) have attracted increased attention, because they exhibit unique biological properties in vivo. Accumulating evidence indicates that MSCs transplanted in different pathological conditions are home to the sites of tissue injury and induce the recruitment of endogenous cells, tissue remodeling, and anti-inflammatory activities [1–3].
It has been recently shown that MSCs also have a natural ability to migrate toward tumors, being attracted by the plethora of chemo-attractants facilitating cell homing to active cancer sites with posterior transdifferentiation due to the local microenvironmental cues [4]. The population of cancer-attracted MSCs actually support the tumor growth and progression in different cancer types [5,6]. However, anti-tumor properties of MSCs have also been reported, rendering them very attractive to researchers and clinicians [7,8]. To circumvent the problem with the duality of MSC influence on the tumor cells, a delivery of exogenous, engineered MSCs could present some solution for converting them into the unequivocal therapeutic tools.
The engineering strategies of MSCs equip them for targeted delivery of different factors using more focused biological approaches. MSCs can be modified to become the carriers of suicide genes, which, in turn, would produce toxic products that would inhibit tumor expansion, whereas the surrounding healthy tissues remain intact [9–11]. MSCs may also be employed as the carriers of anti-angiogenesis factors that contribute to the inhibition of tumor growth and to prevent metastasis [12,13]. Yet another approach is the induction of cytokine gene expression in MSCs, which, in turn, will attract and modulate processes, making the tumor cells more exposed to the host immune system response [14–16]. Besides this, anti-mitotic factors could be a rational target for the MSC-based anti-cancer engineering [17].
Ultimately, growing interest is focused on the use of exosomes as biological delivery vehicles for miRNA transfer, as exosomes do not elicit acute immune rejection and risk of tumor formation [18].
In this article, we will focus on some recent advances in cell-based cancer therapies using genetically engineered MSCs as well as on the potential side effects of MSC delivery strategies.
Heterogeneity of MSCs
In the 1970s, Friedenstein and his coworkers identified within the bone marrow a subpopulation of nonhematopoietic cells with a fibroblast-like morphology designated as colony-forming unit fibroblasts [19]. Afterward, the term “MSCs” was adopted by the Caplan group to define a population of stem cells with a three-lineage differentiation potential [20].
In 2006, the International Society for Cell Therapy (ISCT) proposed the minimal criteria for MSCs: adherence to plastic when cultured in vitro; possession of a trilineage mesodermal differentiation capacity toward chondrocytes, osteocytes, and adipocytes. Additional requirements for MSCs include the expression of the cell surface molecules such as CD73 (ecto 5′ nucleotidase), CD90 (Thy-1), and CD105 (endoglin) as well as the absence of hematopoietic markers, including CD45, CD34, CD14 or CD11b, CD79α, and the MHC II class cellular receptor HLA-DR [21].
However, these criteria have been proved to be inadequate. The expression of this broad set of markers was also found on fibroblasts and on the surface of the other cell types [22]. In fact, the isolation of MSCs according to ISCT criteria produces heterogeneous, nonclonal cultures of stromal cells containing stem cells with different multipotential properties, committed progenitors, and differentiated cells [23]. In addition, it was recently postulated that only a minor subpopulation of pluripotent stem cells among MSCs, called multilineage-differentiating stress-enduring (Muse) cells, are responsible for the broad spectrum of differentiation abilities previously attributed to the whole MSC population [24].
Bone marrow remains one of the major sources of MSCs for clinical use; however, these cells can be successfully isolated from other tissues such as adipose, umbilical cord blood, and Wharton's jelly. MSCs from various sources share some common features but exhibit many differences, including the variable potential for differentiation and functional abilities. Moreover, a study by Lv et al. demonstrated that only a small part of cells among the MSC population are stem cells, with trilineage differentiation potential, whereas others represent a mixture of different cell types with support functions. Researchers proposed novel specific markers that associate with the stemness of MSCs, including Stro-1, SSEA-4, and CD146. They revisited the antigens expressed on the surface of MSCs from different sources, aiming in their potential as MSC markers, and suggested to define the relevant panel for future investigation [25].
Some recent papers demonstrated significant differences between MSCs obtained from neonatal and adult sources, favorable neonatal tissues as less differentiated with higher proliferation rate and clonality [26]. Recently, specific markers regarding the source of MSCs have also been identified. CD271 has been described as the best marker for characterization of the bone marrow MSC population [27].
Moreover, despite the similar pattern of surface antigen expression, global expression patterns vary significantly in MSC population isolated from bone marrow, adipose tissue, and umbilical cord blood. Numerous publications indicate that MSCs have multiple developmental origins and belong to pericytes, fibroblasts, or neural crest cells but issues regarding ontogeny of MSCs are still very controversial [28,29]. Clonal assays demonstrated that in an MSC population, multiple types of cells with different developmental potential exist [30].
Due to this heterogeneous nature of MSCs, their precise characterization in the absence of known accurately defining biomarkers poses a challenge for their further use in cell therapy. However, the problems with detailed identification should not interfere with future investigation of their therapeutic properties, even if the results among studies somewhat vary.
Inherent Anti-Cancer Properties of MSCs
Several studies postulated that naïve, nonengineered MSCs may exert anti-tumor activities [31–33]. However, it should be noted that this alluring from the therapeutic point-of-view feature of MSCs is still under debate due to contradictory data reported on MSC influence on tumor cells [34]. For instance, MSCs derived from different tissues may stimulate or suppress glioblastoma cell proliferation as reported by Akimoto et al. that adipose tissue–derived MSCs (AT-MSCs) induced and umbilical cord blood–derived MSCs (UCB-MSCs) inhibited the progression of the glioblastoma cells [35].
Even more remarkably, MSCs of the same origin, cultured in the same conditions in vitro, promote or restrain tumor progression depending on the protocol applied during the experiment, that is, human fallopian tube MSCs (hftMSCs) used in a murine breast adenocarcinoma study [36]. In this study, hftMSCs participated in tumor progression when coinjected subcutaneously with tumor cells, but they exerted antitumor effects when administrated intraperitoneally to the animals already bearing tumor cells.
In addition, attention must be paid to senescent MSCs with therapeutic purposes, due to the contradictory data obtained, as on the one hand, that type of cells promotes cancer cell migration and proliferation, that is, by the galectin-3 secretion in the case of AT-MSCs [37], or by the secretion of IL-6 in the case of umbilical cord–derived MSCs (UC-MSCs) [38]; however, when UC-MSCs with tumor progression-fomenting properties are pretreated with IL-6, they exert anti-tumor properties [39]. On the other hand, senescent AT-MSCs were proved to inhibit tumor growth, but when these cells were primed by tumor cells, their anti-tumor effect was abolished [40].
Furthermore, senescent bone marrow–derived MSCs (BM-MSCs) triggered senescent phenotype in proliferating MSCs, confirming the notion that secreted factors from MSCs might promote senescence in neighboring cells that, in turn, might have an application in the anti-cancer cell-based approaches [41]. Thus, taking into consideration several aspects of the MSC biology, including altered molecular characteristics caused by cellular senescence [42], in spite of the anti-tumor properties, naïve MSCs ought to be used with caution [43].
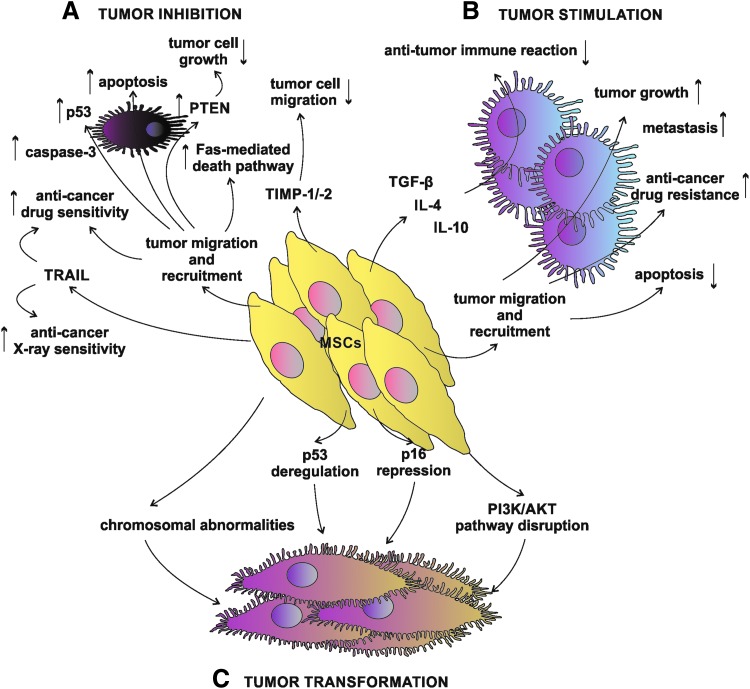
Nonetheless, to limit the story to the productive side of MSCs, there are several examples of beneficial effects elicited by the unmodified MSC administration in various cancer types, suggesting that MSCs do possess intrinsic anti-tumor properties that are worthy of interest and further investigation (Fig. 1A). There are several prominent examples from anti-glioblastoma experiments [44–47], followed by other anti-cancer MSC application such as in breast cancer [48], liver cancer [49], pancreatic cancer [50], prostate cancer [7], colon cancer [51], myeloma [52], and sarcoma [53], up to the case of the human BM-MSCs use in an anti-lymphoma [54]. Interestingly, neither positive nor negative effects were attributed to human Wharton's jelly-derived MSC secretome on lung cancer cells in vitro [55].
FIG. 1.
Complex interaction between MSCs and cancer cells. (A) Naïve MSCs may act through various pathways to induce tumor inhibition. PTEN–phosphatase and tensin homolog deleted on chromosome 10 protein, TIMP-1/-2, TRAIL. (B) MSCs possess natural ability to migrate toward the tumor and recruit to the tumor cell mass, contributing to the tumor progression by the decrease of apoptosis rate and anti-tumor immune response; besides, MSCs elicit anti-cancer drug resistance and stimulate metastasis. (C) Spontaneous MSC tumor transformation is also one of the possible ways of MSC biological activities. MSCs, mesenchymal stem cells; TIMP-1/-2, tissue inhibitor of metalloproteinase-1 and -2; TRAIL, tumor necrosis factor-related apoptosis-inducing ligand.
Due to their inherent ability to migrate toward lesion sites, MSCs seem to be very attractive in future anti-tumor therapies. For instance, UC-MSCs contributed to the increased overall anti-tumor effects when they were administrated in vivo into tumor-bearing mice followed by the therapeutic irradiation exposure. In this case, human tumor cells had been implanted to the dorsal skin folds to generate bilateral xenotumors followed by the intraperitoneal MSC administration. In the next step, only one tumor-containing site was irradiated, leaving the opposite tumor site for the study of “bystander effect” [56]. Due to their positive impact on radiotherapy effects, UC-MSCs might find their application in the tumor radio-sensitization approaches [56].
After BM-MSC administration into mice bearing hepatocellular carcinoma (HCC), upregulation of p53 and caspase-3 genes was reported in liver tissue, which eventually led to the apoptosis induction [32]. In addition, tumor cells were found to be in their majority in the G0/G1 phase, with the concomitant S-phase decline.
Furthermore, MSCs could limit the growth of liver cancer cells by releasing Dickkopf-1 (Dkk-1) factor, which was reported to be an inhibitor of Wnt/β-catenin signaling pathway [57], which, in turn, is frequently elevated in various tumors and contributes to their expansion [58]. Dkk-1-secreting MSCs caused a decline in the expression of Wnt/β-catenin signaling pathway-related factors, that is, bcl-2 and survivin in human liver carcinoma cell line in vitro [59]; likewise, Dkk-1 released from UC-MSCs elicited the suppression of the Wnt/β-catenin signaling pathway in human breast adenocarcinoma cells in vitro [60].
Time-dependent anti-tumor activity was noticed in an in vivo study with MSC intravenous administration [61]. In this study, implanted BM-MSCs contributed to the elevated HCC cell apoptosis; however, changes in tumor apoptotic and anti-apoptotic genes were not conclusive, and reported effects were reduced with time. In another in vivo study with BM-MSC tail vein injection in artificial pulmonary metastatic mouse and ascitogenous hepatoma model, BM-MSC treatment brought more encouraging results in the form of reported substantial improvement of the lifetime of mice and inhibition of the development of both tumor types [62].
In vivo, UCB-MSCs triggered PTEN stimulation not only in glioblastoma cells that were in direct contact but also in those in the vicinity [44]. PTEN upregulation influenced Akt expression pattern, causing PI3K/Akt pathway disruption and leading to the inhibition of growth and migration of cancer cells [44].
Glioblastoma tumor progression needs new blood vessel formation. Ho et al. indicated that BM-MSCs could exert anti-tumor effects in this regard [45]. It was shown that glioma tumor angiogenesis might be unsettled by BM-MSC paracrine action on endothelial progenitor cell recruitment, with concomitant downregulation of proangiogenic factors [45]. Further, BM-MSC secreted factors that inhibited endothelial cell tube formation in vitro and participated in the decline of microvessel density in vivo in the subcutaneous glioma tumor mouse model.
In an in vitro study, glioblastoma multiforme stem-like cells entered senescence, but not apoptosis, with a hallmark of cyclin D1 increased level on account of exposure to BM-MSC conditioned media [47]. An increased sensitivity to the anti-cancer drugs, that is, Temozolomide and 5-Fluoro-Uracil, was also noticed [47]. Similar results were observed in the case of human breast cancer cells that were co-cultured with AT-MSCs [63].
Another in vitro study brings data on apoptosis and differentiation induction in the U251 human glioma cell line after stimulation with MSC conditioned media from different MSC origins: AT-MSCs and UC-MSCs. In this study, after the MSC conditioned media exposure, elevated levels of mRNAs for caspase-3 and -9 were detected with the simultaneous decline in mRNA levels of survivin and X-linked inhibitor of apoptosis protein (XIAP) in U251 cells. Additionally, G0/G1 growth cell arrest had been reported in these cells. Besides, MSC conditioned media called out U251 cell differentiation toward normal phenotype glial cells that were manifested with GFAP presence and cell migration decline [46]. On the other hand, there are some data claiming that MSC origin is crucial, because different tissue-derived MSCs act in completely different ways [35].
UC-MSCs exert their inhibitory effect by initiating cell cycle arrest on breast cancer cells both in vitro and in vivo [48], and BM-MSCs were proved to reduce metastasis in vivo [64]. Yoon et al. performed experiments on BM-MSC activation to arouse their anti-cancer properties [65]. BM-MSCs were stimulated with TNF-α, and DNA/RNA was released from apoptotic cancer cells.
Culture medium from osteo-induced AT-MSCs contributed to the in vitro proliferation inhibition and apoptosis induction in breast cancer cells [66]. Such a positive influence might be dependent not only on the differentiation state of AT-MSCs but also on the origin of the therapeutic MSCs [67]. These data are reinforced by observation of the immortalized MSC line that produced and secreted tissue inhibitor of metalloproteinase-1 and -2 (TIMP-1, TIMP-2). The elevated presence of TIMP-1 and TIMP-2 in the extracellular matrix, undoubtedly, interferes with the tumor cell migration processes that are crucial for tumor progression through metastasis [68].
Suicide Gene Approaches
The herpes simplex virus-thymidine kinase (HSV-TK) became one of the most used enzymes employed in suicide gene anti-cancer approaches [69]. In the case of MSCs, the advantage of this approach is that HSV-TK modified MSCs could be effectively delivered to the area of interest and ganciclovir, a substrate for HSV-TK, could be safely administrated systemically. The toxic product is exclusively produced from ganciclovir by the HSV-TK-bearing MSCs, which, in practice, would target the region occupied by tumor cells (Fig. 2A). As a consequence, only tumor cells will be affected, whereas surrounding tissues would remain intact [70].
FIG. 2.
Suicide gene delivery system mediated by MSCs. (A) The hsv-tk gene is one of the suicide genes employed in MSC-based anti-tumor studies. Nontoxic pro-drug GCV is converted into toxic compound GCV-triphosphate (GCV-ppp) due to HSV-TK activity in HSV-TK-producing MSCs. GCV-ppp is then delivered to the target tumor cells through the gap-junctional system, and it is subsequently incorporated into cell metabolic pathways, causing eventual cell death. (B) CD also found its application in MSC-based studies. MSCs modified do produce a CD carry-out enzymatic reaction of nontoxic pro-drug 5-FC to produce the toxic product 5-FU, which is secreted and reaches target tumor cells, eliciting tumor cell death. (C) iCas-9 system. Inducible caspase-9 system is based on the pro-apoptotic activity of the caspase-9 delivered to the target tumor cell by adenoviral transfection. First, MSCs are genetically engineered to produce an invasive form of adenoviral construct containing the icas-9 gene. After target tumor cell delivery, iCas-9 protein is effectively produced followed by the small-molecule CID stimulation, which triggers iCas-9 dimerization and apoptosis pathway induction. 5-FC, 5-fluorocytosine; 5-fluorouracil; CD, cytosine deaminase; CID, chemical inducer of dimerization; GCV, Ganciclovir; HSV-TK, herpes simplex virus-thymidine kinase.
HSV-TK engineered MSCs act mainly through the “bystander effect,” which consists of an action of the toxic compound not only in the cells where it had been produced but also after its delivery to the surrounding target cells [71,72]. Additionally, this mechanism is reported to be very effective for anti-tumor acting BM-MSCs [73]. In the case of HSV-TK-based anti-cancer cell therapy, the gap-junctional intercellular communication system between therapeutic and target cells is crucial due to its involvement in the toxic compound transmission [74]; thus in practice, this phenomenon may lead to the insufficient toxic compound penetration throughout the tumor site, leading to incomplete treatment. For this reason, advanced monitoring methods are desired to exert control over ongoing processes in vivo [75,76].
On the other hand, the HSV-TK bystander effect might be enhanced by the simultaneous connexin 43 overexpression in targeted glioma cells, leading to the increase in the number of gap-junctional connections between those cells and therapeutic BM-MSCs [77], or through chemical stimulation such as by histone deacetylase inhibitor 4-phenylbutyrate (4-PB) [78], or valproic acid [79].
In spite of the reported drawbacks, this kind of MSC modification poses an attractive solution for anti-cancer treatment, since no adverse effects were reported for tumor neighboring normal cells [80] and no considerable negative side effects were found for HSV-TK engineered BM-MSCs themselves [81]. The HSV-TK engineered MSCs were applied in a variety of studies on different tumor types such as pancreatic carcinoma [82,83], HCC [84], prostate tumor [9], pulmonary melanoma metastasis [85], and a cluster of experiments with the aim of battling gliomas [86–91]. Besides, HSV-TK BM-MSCs have been already employed in I/II clinical trials on gastrointestinal tumors [92].
In many cases, HSV-TK engineering might be enforced by additional modifications aiming at the increase of therapeutic effects of modified cells, that is, HSV-TK overexpression was accompanied with cytosine deaminase (CD) expression with an example of the lung metastases treated with engineered AT-MSCs [93] and ovarian carcinoma studies utilizing UCB-MSCs [94]. Furthermore, the HSV-TK engineering of BM-MSCs could be improved by the co-transfection of the tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) gene [95,96]. To improve the efficiency of gene therapy, some investigators have combined HSV-TK suicide gene therapy with classical radiotherapy or Temozolomide, both of which are commonly used to treat glioblastoma patients. It was shown that this combination was more efficient than HSV-TK alone [97].
CD was proposed to be a good candidate for the pro-drug anti-cancer strategies, even claimed to be the most effective pro-drug system compared with others [98], and considered harmless for basal biologic properties of CD-producing AT-MSCs [99] and BM-MSCs [100] (Fig. 2B). CD converts a low-toxic substrate 5-fluorocytosine into a 5-fluorouracil, which is known as a potent anti-cancer agent, but the systemic administration is associated with the adverse side effects such as severe myelin degeneration [101]; hence, the MSC cell-based therapy is an interesting proposal to address this question. However, CD-producing cells induce immune responses, leading to the rapid clearance of the engineered cells and causing limited duration of their anti-cancer action [102].
Nonetheless, CD overexpressing MSCs were successfully used in glioma [103–106], glioblastoma [10], osteosarcoma [107], gastric cancer [108], medullary thyroid carcinoma [109], and melanoma experiments [110], showing the evident anti-cancer activity of the therapeutic cells with good prognosis for future clinical trials and with an example of the ongoing clinical trial NCT01156584 on malignant glioma. Concordantly, a significant inhibition of tumor growth was observed in the colon cancer and prostate cancer mice models after the CD-engineered AT-MSC administration [111,112].
Finally, the inducible Caspase-9 (iCas-9) suicide gene system might find its application in the MSC-based anti-tumor therapies (Fig. 2C). The iCas-9 system includes activation of the Caspase-9 by the small-molecule chemical inducer of dimerization (CID), which is designed to interact with the iCasp9 [113]. For instance, iCas-9-producing BM-MSCs treated with CID initiated apoptotic pathways, causing their relatively fast clearance in vivo [114]. This feature was utilized in the anti-lung cancer BM-MSC-based experiment. In this case, the Bortezomid stimulation of the iCas-9-producing BM-MSCs led to the increase of pro-apoptotic activity via the Caspase-3 stabilization, confirming feasibility of the iCas-9 system usage in the field of anti-tumor activities [115].
Anti-Angiogenesis Approaches
The tumor progression is not possible without the participation of new blood vessels. For this reason, the induction of angiogenesis inhibition is one of the worth considering issues in the field of anti-cancer treatment (Fig. 3A). The vascular endothelial growth factor (VEGF), a core element involved in the angiogenesis [116], was found plausible as a potential target in the anti-angiogenic cancer therapy [117].
FIG. 3.
Diversity of anti-tumor MSC-based approaches. (A) Several anti-tumor angiogenesis approaches could be appreciated. Some of them target the VEGF–VEGF–R axis, with the examples of soluble vascular endothelial growth factor receptor-1 (sFlt-1) or PEDF. Others recruit Thrombospondin 1 (TSP-1) or interferon-inducible protein-10 (IP-10), both of which are stated anti-angiogenic factors. (B) Various cytokines found their application in anti-tumor studies: interferons (IFNs), tumor necrosis factor super family proteins (TNFs), and interleukins (ILs). TRAIL, LIGHT, homologous to Lymphotoxin, exhibits inducible expression and competes with HSV Glycoprotein D for binding to Herpesvirus entry mediator, a receptor expressed on T lymphocytes. (C) A plethora of miRNAs found their application in MSC-based studies on tumor cell progression. After target tumor cell delivery, miRNAs might contribute to the decrease in tumor angiogenesis, viability and growth, invasion and migration. Furthermore, miRNAs might cause chemosenzitization of tumor cells for anti-tumor drugs. Additionally, synthetic small interfering miRNAs (siRNA) might be also employed as in the case of IL-6 production silencing. (D) MSCs could be used as vehicles for regular chemical anti-cancer acting compounds, that is, PTX, DOX, or GCB. DOX, Doxorubicin; GCB, Gemcitabine; PTX, Paclitaxel; PEDF, pigment epithelium-derived factor; VEGF, vascular endothelial growth factor; VEGF-R, VEGF–Receptor.
Apart from this, the application of a soluble VEGF receptor-1 (sFlt-1) in the treatment of solid human ovarian carcinoma in the mice model was found to be beneficial, contributing to the reduction of tumor growth and vascularity [118]. In the case of BM-MSC-based studies, the sFlt-1 modified BM-MSCs were administrated to the metastasis mice model of Lewis lung cancer and colon cancer carcinoma. sFlt-1 was secreted by the engineered cells in the tumor loci, participating in the anti-angiogenic and pro-apoptotic processes within tumor tissue [119].
The physiological function of endostatin is the inhibition of endothelial cell proliferation, angiogenesis, and tumor growth [120]. Besides, endostatin was described as an inducer of endothelial cell apoptosis [121]. The anti-tumor effects of endostatin were investigated in the colorectal peritoneal carcinomatosis mouse model, where the endostatin overexpressing placenta-derived MSCs (plMSCs) were intraperitoneally administered. In in vivo studies, a significant reduction in the tumor nodules and the prolongation of survival after the transgenic cell administration were observed [122].
It is believed that Kringle 1–5 protein (K1-5) is a stronger inhibitor of angiogenesis than endostatin and angiostatin [13]. Human plMSCs expressing K1-5 showed effective inhibition of angiogenesis both in vitro and in vivo. Furthermore, this result suggested that the fiber-modified viral vector transfection method could be effectively adapted in an anti-angiogenic strategy in cancer therapy.
Based on the data that the high-mobility group box1 proteins (HMGB1) are involved in the tumor angiogenesis as strong modulators [123,124], these proteins were selected as yet another target for the anti-angiogenic therapies. It was established that another DNA-binding domain of HMGB1, the A box, competed with the HMGB1 for the same binding sites. For this reason, the A box protein was utilized in the anti-HMGB1 trials [125].
The A box encoding gene was chosen for the BM-MSC transfection to produce the A box protein secreting cells, which were found to efficiently migrate toward colon cancer and to block the activity of HMGB1, cooperating with significant tumor growth [17].
Another important factor is thrombospondin-1 (TSP-1), a matricellular protein that is widely considered to be involved in the inhibition of angiogenesis and tumorigenesis [126,127]. A recent study demonstrated that BM-MSCs expressing a novel variant of TSP-1 significantly inhibited tumor progression and had an anti-angiogenic effect on glioblastoma cells [128].
The pigment epithelium-derived factor (PEDF) is a potent antagonist of VEGF in the regulation of angiogenesis [129], thus another anti-angiogenic strategy was proposed [130]. BM-MSCs were engineered to overexpress the PEDF and used in studies on the HCC in nude mice. The exogenous cells properly migrated to the sites of tumor growth with the posterior inhibition of tumor progression and participated in the decline of pulmonary metastases development.
In a different study, the PEDF overexpressing AT-MSCs were reported to reduce the tumor growth and endothelial cell tube formation in a prostate cancer model [131].
In both cases, the engineered MSCs had strong anti-angiogenic activity [130,131]. Based on a recent report, BM-MSCs overexpressing PEDF could inhibit tumor angiogenesis and increase apoptosis of gliomas [132]. The study conducted in mice reported that intravenously administrated PEDF-modified MSCs migrated and delivered PEDF to target glioma cells, prolonging survival of glioma-bearing mice. It is believed that this strategy might be a novel and promising therapeutic approach for refractory brain tumors.
Recent reports confirmed the effectiveness of IL-12 as a powerful strategy in MSC-mediated tumor therapy [133,134]. IL-12 is considered the most efficient cytokine; it exhibits anti-tumor effects, has antiangiogenic effects, and activates natural killers and T lymphocytes [133]. The anti-tumor effect of IL-12 was shown in the mouse orthotopic glioblastoma model, where IL-12 overexpressing BM-MSCs were intracranially administrated. In this study, the significant retardation of tumor growth and an increase of mouse survival were observed [133]. In another report, the anti-tumor effect of MSCs that produced IL-12 was also confirmed in kidney cancer, cervical cancer, and Ewing sarcoma models [135–137].
Another protein with potential future application in MSC-based therapy trials is the interferon-inducible protein-10 (IP-10), which was previously found to be a strong anti-angiogenic chemokine [138]. AT-MSCs were successfully transfected with the IP-10 encoding gene and initially characterized in terms of changes in the expression levels of other genes that are potentially important in anti-cancer therapies such as VEGF, CXCR4, and SDF-1 [139].
MSCs Modified to Express Anti-Tumor Cytokines
Another field of interest in the anti-cancer attempts employing MSCs as therapeutic cells deals with the MSCs overexpressing the various cytokines (Fig. 3B). It is well known that inflammation is one of the consistent features of the tumor microenvironment. Immune cell infiltration of tumors can have a dual role, leading to either an anti-tumor response or inhibition of an immune reaction, promoting growth and spread of malignant disease. Thus, in noninflammatory “silent” conditions, therapeutic strategy to activate pro-tumorigenic immunity, based on the local production of cytokines by MSCs transduced to express specific genes of interest, is intensively explored [140]. It was shown that MSCs overexpressing different cytokines have suppressed tumor growth by the selective induction of apoptosis of cancer cells or activation of immune response [141].
According to the type of cancer, a series of anti-cancer genes, including interferons (IFNs), tumor necrosis factor (TNF) super family proteins, or interleukins (ILs) genes, have been transfected into MSCs. Interferons released by engineered MSCs demonstrated anti-proliferative activities on tumor cells [142].
IFN-β is the most therapeutically attractive member of the IFN family. It was proved that IFN-β gene modified BM-MSCs (BM-MSCs/IFN-β) effectively inhibited the proliferation of HCC or prostate cancer (PC-3) cell lines in vitro [143]. Co-culture of BM-MSCs/IFN-β with tumor cells accompanied with high levels of IFN-β secretion decreased the percentage of hepatoma cells with incorporated BrdU [143]. Systemic transplantation of BM-MSCs/IFN-β caused a significant reduction of tumor burden, suppression of metastasis, and prolonged animal survival in HCC xenograft-bearing mice [143].
A co-injection of MSCs and cancer cells was also described. The authors used transfected human MSCs with IFN-β to treat melanoma xenografts in mice [144]. An injection of MSCs/IFN-β into the peripheral circulation resulted in reduced tumor growth and an increased animal lifespan. These results are in concordance with other findings, in which MSCs/IFN-β potentially inhibited breast carcinoma [145], glioma [146], melanoma [147], pancreatic cancer [50] and gastrointestinal carcinoma [148]. In each of these tumor experimental models, the treatment showed efficacy in the inhibition of local tumor growth.
The mechanism by which IFN-β caused cancer cell growth attenuation still remains an open question. Dedoni et al. have shown that IFN-β treatment downregulates the PI3K/Akt pathway in neuroblastoma cells [149]. These data provide a line of evidence for the regulatory effect of MSCs/IFN-β on Akt signaling cascade inhibition in HCC cells [143].
The extent of the CD8+ T-cell infiltrate positively correlates with good prognosis in many cancers. Tumor-specific IFN-γ-producing CD8+ T-cell immune response is important to the outcomes of cancer-bearing animals [150]. It supports the notion that to inhibit tumor growth, delivery of immune-stimulatory genes by MSCs is valuable [151]. ILs that regulate immune response are known to have an anti-tumor effect via positive modulation of the endogenous immune system [152]. The delivery of ILs via MSCs has been advantageous to improve the anti-cancer immune surveillance by activating cytotoxic lymphocytes and natural killer cells [152]. The value of IL-12, IL-15, IL-18, and IL-21 engineered MSCs for immunotherapy of different cancers has been confirmed [15,136,137,153,154].
It is well known that IL-12 is a key cytokine inducing a pro-inflammatory pattern of immune response [155]. BM-MSCs engineered to express IL-12 have been shown to slow tumor progression in mice bearing renal cell carcinoma [136] or head-and-neck tumors [137]. In vivo administration of MSCs/IL-12 prevented metastases into the lymph nodes and the other organs, as well as increased tumor cell apoptosis in different tumor models of melanoma or hepatoma tumors [156]. Similarly, murine BM-MSCs retrovirally engineered to secrete IL-12 significantly reduced breast cancer growth when delivered subcutaneously in syngeneic and allogeneic hosts [157]. This treatment resulted in high density of intratumoral T-cell infiltration, suggesting that the therapeutic effect was dependent on a T-cell immune response.
MSCs have also been engineered with other immune stimulatory ILs, such as IL-15, which is associated with long-lasting T-cell anti-tumor immunity and cancer cell apoptosis in pancreatic tumor-bearing mice [153]. It was observed that in addition to NK and CD8+ T cells, γδ lymphocytes were also expanded in the tumor site after MSCs/IL-15 treatment. Furthermore, tumor-specific memory T cells were induced by IL-15 gene modified MSCs, giving the possibility to reject subsequent re-challenges with identical tumor cells by mice cured of previous tumors in such a setting [153].
In another study, human MSCs engineered to express IL-18 were tested in mice bearing noninvasive and invasive gliomas [154]. In these settings, transplantation of IL-18 secreting MSCs was associated with enhanced T-cell infiltration and long-term anti-tumor immunity in the hosts.
More recent studies have revealed the anti-tumor effects of MSCs expressing IL-21. Previously, IL-21 has been shown to activate NK and T cells and to induce a strong cell-mediated immune response in the tumor vaccine approaches [158]. In experimental settings, human UC-MSCs (hUC-MSCs) transduced by the IL-21 gene induced tumoricidal activities in ovarian tumor-bearing mice [15]. This was accompanied with enhanced NK cytotoxicity and elevated serum levels of IFN-γ and TNF-α, which were probably related to the secretion of IL-21 from transplanted hUC-MSCs/IL21. Both cytokines have an important function in anti-tumor immunity by increasing cytotoxic potential of NK cells.
Moreover, hUC-MSCs/IL-21 could inhibit tumor growth through the negative regulation of the Wnt signaling pathway being involved in metabolism, proliferation, and cell cycle. Hence, transplantation of hUC-MSCs/IL-21 downregulated the expression of β-catenin and cyclin D1 in ovarian cancers [15]. This alteration may refer to the inhibition of tumor growth confirmed histopathologically.
TNF-α is another major mediator of tumor growth. This cytokine was originally named owing to its anti-tumor properties; however, TNF-α has been shown to have a paradoxical effect on cancers by both inhibiting and promoting tumor growth depending on the tumor type and the level of TNF-α protein [159]. At the cellular point, the positive anti-cancer role of TNF-α has been shown to induce apoptosis and necrosis in the malignant cells in vitro.
In vivo, TNF-α exerts a stronger anti-tumor activity, because it acts toward other cells in the tumor microenvironment, especially toward endothelial cells, inducing their apoptosis and leading to vessel destruction [160]. Moreover, TNF-α can enhance anti-tumor immunity via stimulating T-cell infiltration into the tumor tissue [161]. TNF-α is also capable of regulating other cytokines, showing synergism with their cytotoxic effects [162].
AT-MSCs transduced to express TNF-α gene induced apoptosis of tumor cell lines of different origins in vitro, and when injected subcutaneously with melanoma cells into nude mice resulted in tumor growth inhibition [16]. This was in concordance with the previous studies of Al-Zoubi et al., in which delivery of TNF-α by engineered MSCs completely prevented breast cancer tumor formation in mice [163]. Significant inhibition of tumor mass described in vivo may be probably also a result of MSCs/TNF-α stimulation to secrete other inhibitory factors and cytokines by the host [163].
In addition to TNF-α presence, the upregulation of gene transcripts associated with inflammation and senescence, that is, of IL-1β, IL-6, IL-8, and MCP-1, were observed in vivo [163]. We can even speculate that an insufficient level of TNF-α could be a factor facilitating tumorigenesis in people.
Among the other factors could be TRAIL, which is a molecule that selectively kills transformed and cancer cells but not the normal cells, making it an attractive target for anti-cancer therapy [164,165]. A number of studies have shown the therapeutic efficacy of MSCs engineered to express TRAIL in either cell lines or different experimental models of cancers both in vitro and in vivo.
Loebinger et al. have shown that human BM-MSCs transduced with the TRAIL gene caused lung, breast, squamous, and cervical cancer cell apoptosis and death in co-culture experiments [140]. Subcutaneously xenotransplanted TRAIL-expressing MSCs contributed to the tumor mass reduction and inhibited metastases of breast cancer cells in NOD/SCID mice. Similarly, human MSCs genetically engineered to express TRAIL induced apoptosis in glioma cell lines, had profound anti-tumor effects in orthotopic glioma tumors in mice, and resulted in a significant increase in animal survival [166–168].
Human pancreas–derived MSCs (pan-MSCs), modified to express a soluble form of recombinant TRAIL, have also been explored for anti-tumor effects in gastrointestinal carcinomas [169]. TRAIL has been shown to induce cytotoxic effects on pancreatic cancer cells [169]. Similarly, in a human pancreatic carcinoma mouse xenograft model, the engineered MSCs expressing TRAIL slowed tumor growth and reduced the incidence of metastases [170]. Similarly, MSCs/TRAIL induced apoptosis of human colorectal cancer cell lines, including DLD1 in vitro; however, they failed to affect colorectal adenocarcinoma xenograft growth secondary to pulmonary engraftment in mice after systemic MSCs/TRAIL transplantation [171]. This observation highlights not only the strong potential of TRAIL-mediated anti-tumor effect but also some limitations of therapies using genetically engineered MSCs.
LIGHT, another member of the TNF super-family [172], was used in the anti-tumor studies, exposing its role as a strong inducer of anti-tumor immunity. Systemic administration of UCB-MSCs transfected by the LIGHT gene into gastric cancer-bearing nude mice suppressed tumor growth [173]. The average volume of tumors in UCB-MSCs/LIGHT was smaller than in the control group with a larger tumor necrosis area. LIGHT, homologous to lymphotoxin, can enhance the extravasation and homing of naïve T cells [174].
Further data demonstrated that forced expression of LIGHT in the tumor induces a massive infiltration of T lymphocytes into tumor tissue, leading to its rejection [175]. It was shown that LIGHT sustains effector T-cell functions at the site of various tumors without defining tumor antigens [176]. Delivering LIGHT protein in genetically engineered MSCs into breast cancer-bearing mice resulted in tumor growth repression [177]. The stable proliferation rate of cancer cells indicated that tumor growth arrest was not involved in the therapeutic efficacy of MSCs/LIGHT. However, the tumors showed CD4+ and CD8+ T-cell infiltration, which may contribute to maintenance of tumor control.
Moreover, MSCs-/LIGHT-treated mice induce pro-inflammatory cytokines, that is, IFN-γ, IL-6 in local tumor tissue [177]. In addition, IFN-γ-producing T-cell level also increased in lymphoid tissues of tumor-bearing hosts after MSCs-/LIGHT-mediated tumor control caused by the primed antitumor immunity. This phenomenon was not due to the direct contact inhibition of tumor cells with LIGHT-producing MSCs. Yu et al. have shown that LIGHT gene delivery inside fibrosarcoma cells inoculated in mice recruited naive T lymphocytes into the tumor microenvironment [174].
High expression of LIGHT inside the tumor promoted cytokine changes that may contribute to the activation, expansion, and conversion of CD8+ T cells into fully functional effectors. Moreover, targeting primary tumors with the LIGHT gene elicited tumor-specific cytotoxic T lymphocytes that migrate into the distal tumors and eradicated them as observed in mammary carcinoma-bearing mice [175]. It seems that LIGHT is capable of generating comprehensive immune responses against established tumors and spontaneous metastases [176].
Another example of the apoptosis induced in tumor cells is the case of the tumor-suppressor phosphatase with tensin homology (PTEN) overexpressing pan-MSCs [178]. PTEN acts as an inhibitor of the anti-apoptotic pathway, so its overexpression drives the induction of the apoptotic signaling.
Janus Face of MSC-Derived miRNA in Oncology
There is constantly growing evidence of miRNA involvement in the process of oncogenesis [179]. In that context, it has been shown that tumor-recruited MSCs enhance tumorigenic properties of breast cancers through an increase of miR-199a [180]. The miR-221 paracrine secretion from gastric cancer-derived MSCs supports growth and migration of the tumor HGC-27 cell line [181]. In turn, let-7 family miRNA has been downregulated in prostate cancer–derived MSCs [182]. In both cases mentioned earlier, the genetic intervention to normalize miRNA expression/secretion abolished the tumor-promoting properties of cancer-derived MSCs. Moreover, the exogenous miRNA can have anti-tumor activity [183,184]. The exosomes derived from naïve MSCs carry the miR-16, which downregulates VEGF, and through that mechanism inhibits angiogenesis and limits the growth of tumors [185] (Fig. 3C).
Furthermore, the MSCs can be engineered to express specific and desired anti-tumor miRNA, and such prepared MSCs through active recruitment by a cancer tissue could also serve as a Trojan horse. The one strategy for tumor targeting is finding miRNAs downregulated in cancer cells and supplying them with missing miRNA. Particularly, the low expression of miR-145 and miR-124 was found in glioma cells [186]. The genetically engineered MSCs were capable of efficient delivery of these miRNAs through gap junctions and exosomes to the glioma cells, which resulted in the migratory inhibition properties of the latter cells [187]. Instead of genetic engineering, the MSCs can be equipped with synthetic miRNA, which then will be enveloped in exosomes and released. It was shown that effective supplementation of miR-143, downregulated in osteosarcoma cells, suppressed their migratory properties [188].
It has been previously shown that miR-122 can also determine resistance to chemotherapy of HCC cells [189]. In fact, the MSC-derived exosomes bearing miR-122 revealed chemosensitizing properties [189]. Such a strategy of supplementation of tumor cells with lacking miRNA for therapeutic purposes could be considered a personalized medicine approach. The contribution of EGFR amplification to the glioma invasiveness is a well-known phenomenon [190], and the delivery of EGFR-mRNA-silencing miR-146b through MSC-derived exosomes reduced its invasion, migration, and viability, thus abolishing a negative consequence of EGFR amplification [191]. The production of miR-21 by MSCs also tunes the supportive/inhibitory influence of MSC-derived exosomes on tumor growth [192].
Although the miRNA-centered approaches mentioned earlier were focused on the supplementation of tumor cells with missing miRNA to facilitate their homeostasis and reduce malignancy, the continuous search in using miRNA as therapeutics revealed that miRNA is capable of having a direct cytotoxic effect on the glioblastoma cells, whereas not on normal astrocytes [193]. It is likely that this miRNA could be delivered to the tumor through MSC-derived exosomes. Although the suppression of tumor cell invasion is highly desirable, it has also a dark side—it can produce a dormant state of tumor cells in metastatic niches, not approachable to the current chemotherapeutic regimes.
In particular, the exosomal transfer of miR-23b from BM-MSCs promoted the long-lasting local presence of breast cancer cells, which might be responsible for delayed recurrence on the unknown as yet signaling mechanism [194]. Therefore, the miRNA may also determine the chronicity of cancer disease and may require therapeutic targeting. The power of miRNA can be best presented in the case of miR-145. Its loss in glioma cells upregulates a palette of cell proliferation and migration-related proteins: cyclinD1, c-myc, N-myc, N-cadherin, and E-cadherin, which emphasizes the central role of miRNA in the regulation of cellular fate through simultaneous action on many genes [195].
Although the multigene regulatory characteristic of miRNA predisposes it as an anti-cancer weapon, there are also positive effects with the use of more specific small RNA molecules such as siRNA, and myeloma-stimulating naïve MSCs have been converted into anti-cancer acting cells by IL-6 siRNA transfection to silence myeloma arousal effects of IL-6 production [196].
MSC-Assisted Nanodrug Delivery to Tumor Sites
Additionally, MSCs can be used as carriers for the traditional chemical compounds that possess the properties of anti-cancer drugs, with the example of the paclitaxel (PTX)-loaded nanoparticles delivered to MSCs (Fig. 3D). MSCs loaded with nanoparticles containing paclitaxel, a cytotoxic drug, showed active tumor-tropism so that the release of paclitaxel took place locally [197]. This strategy characterized by high efficiency and low systematic toxicity has the potential to be developed as a cancer targeting tumor therapy in humans.
Recently, Pessina et al. demonstrated that paclitaxel-releasing MSCs had a potent therapeutic effect and suppressed the growth of a murine B16 melanoma in vitro. They also confirmed that paclitaxel-loaded MSC treatment inhibited lung metastasis [198]. Another study showed that PTX-loaded MSCs, in a mouse orthotopic glioblastoma model, migrated to the tumor microenvironment and exerted an anti-tumor effect [199]. A significant number of recent approaches demonstrated new methods of injecting nanoparticles containing classical anti-cancer drugs to MSCs [200,201]. However, up to now, no effective therapeutic treatment, anchoring drug-loaded nanoparticles to MSCs, has been discovered.
Similarly, the study of Li et al. has proved that the silica nanorattle system may be a promising vehicle for drug delivery to MSCs. Silica nanorattle-doxorubicin (SN-DOX) anchored MSCs are capable of accumulating in the vicinity of tumor tissues to slowly release the stored drug. The study also reported that increased and prolonged doxorubicin levels secreted by MSCs significantly enhanced apoptosis in human glioma cell xenografts compared with the effect elicited by the drug alone [202]. An SN-DOX drug delivery system was efficiently conjugated to MSCs by specific monoclonal antibodies that bind to the membrane proteins CD73 and CD90 [202]. Uploading with up to 1,500 nanoparticles did not affect the viability of MSCs [202]. The intracellular retention time of the silica nanorattle was sufficient, as cell-directed tumor tropism amounted to no less than 48 h [202].
In vivo experiments proved that the loaded MSCs could trace the glioma tumor cells and deliver doxorubicin with wider distribution and longer retention lifetime in tumor tissues, compared with free DOX and silica nanorattle encapsulated DOX [202]. The increased and prolonged DOX distribution into the tumor site further contributed to the enhanced tumor cell apoptosis. This strategy has potential to be developed as a high, efficient, and meaningful method for tumor targeting therapy in humans.
Malignant gliomas are aggressive and difficult-to-treat tumors. Therefore, the effective drug delivery methods that are also characterized with the capability of infiltration of the tumor site are highly desirable. One of the biggest limitations is low targeting efficiency for nanoparticle drug delivery system-based cancer therapy. Thus, in brain tumor therapy, alternative strategies are applied to enhance the delivery and homing activity. Recently, researchers have demonstrated that MSCs in an organometallic complex Ferrociphenol lipid nanocapsule delivery system can be used as promising treatment. In that study, a subpopulation of human BM-MSCs isolated adult multilineage inducible cells (MIAMI), combined with nanoparticles, migrated to the tumor microenvironment and exerted a cytotoxic effect on glioma cells [203]. In addition, this strategy could be effectively combined with classical radiotherapy.
Another alternative anti-cancer therapy is to use MSCs as the targeting vehicles to uptake and release another important drug, gemcitabine (GCB). GCB-loaded MSCs integrated into the tumor mass, therefore, were capable of delivering much higher amounts of the drug in situ than could be achieved by intravenous injection [204].
Inherent Potential of MSCs for Oncogenic Transformation
The achievement of therapeutic effect in clinical use of MSCs requires transplantation of large amounts of cells. However, the limiting factor in terms of single-dose injection is the number of isolated MSCs. Therefore, in vitro propagation is often required to reach a sufficient number of cells. During this process, MSCs may undergo genetic alterations that, subsequently, increase the probability of spontaneous malignant transformation. For that reason, there is a presumable risk for patients receiving in vitro expanded MSCs.
Issues regarding MSC transformation are very controversial, and both spontaneous and induced transformation have been reported [205,206]. On the one hand, there are some reports demonstrating that MSC expansion in long-term in vitro culture is safe and deprived of acquired chromosomal aberrations [207–210].
One of the first results confirming stability of human MSCs in long-term in vitro culture was published by Bernardo et al.; researchers studied genetic changes in human MSCs at different stages with the use of karyotyping and comparative genomic hybridization methods [207]. MSCs obtained from bone marrow of 10 healthy donors were propagated in vitro until reaching senescence or till passage 25 and did not exhibit any sings of malignant transformation. Alternatively, other studies that investigated both osteosarcoma patient and healthy donor showed no evidence of neoplastic changes in human MSCs during long-term in vitro culture [210].
One of the greatest advantages of the potential MSC therapy is that these cells are safe and maintain chromosomal stability after transplantation; moreover, there are no results demonstrating tumor formation after their transplantation in humans [211]. However, there are some reports demonstrating malignant transformation of administrated MSCs in rodent models [212]. These studies confirm that genetically unmodified mice MSCs can undergo chromosomal abnormalities even at early passages in vitro and form malignant tumors when transplanted in vivo [213] (Fig. 1C).
Most surveys on the transformation process have been conducted in mice, but there have been few reports on spontaneous human MSC transformation that occurred during in vitro culture. Results published by Rubio et al. showed the primary molecular mechanism of transformation of human AT-MSCs after senescence caused by regulating c-myc and repressing p16 [214].
Many other papers confirmed deregulation of p53, Retinoblastoma, PI3K-AKT, and MAPK pathways involved in tumor transformation of MSCs [215,216]. Recently, researchers described the epigenetic mechanism implicated in spontaneous transformation of MSCs in long-term in vitro culture [217]. Particularly, the epigenetic modification of the p16 gene, including histone H3 lysine methylation (H3K27/9me) and DNA methylation, was investigated in in vitro cultured adult rat BM-MSCs at different stages during the transformation process [218]. These findings are in agreement with observations made by a number of other groups. These results suggest that MSCs used for transplantation should be carefully monitored in terms of chromosomal status during their expansion in vitro [219].
To confirm the real identity of transformed cells among the MSC population, other tests such as fingerprinting and short tandem repeat analysis should be employed [220,221]. According to the recent data, the main mechanism for protecting human MSCs against spontaneous transformation includes telomere shortening and lacks both telomerase reverse transcriptase expression and telomerase activity [222]. Therefore, the reports that support the existence of the human MSC transformation process are based on telomerase-immortalized human MSCs that give rise to a tumor formation. Thus, it appears that using telomerase to help production of large numbers of cells is effective, but on the other hand, it has an impact on neoplastic transformation [223].
Tumor Propagation Mediated by MSCs
Some features of MSCs such as immunomodulation or paracrine effects, which may be beneficial in regenerative medicine approaches, could be also destructive in cell therapy against cancer. Some studies have shown MSC contribution in tumor growth, metastasis, and development of anti-cancer drug resistance.
Results from co-culture of MSCs, breast cancer cells, and peripheral blood mononuclear cells (PBMCs) showed that MSCs negatively affected proliferation and migration of PBMCs and favored the shift from Th1 toward Th2 response [224]. An increase in the number of regulatory T cells (Tregs) was also observed, which could be at least partially due to MSC-derived anti-inflammatory cytokine, TGF-β [224] (Fig. 1B). In vivo studies on mammary carcinoma-bearing mice showed that intravenously administered human peripheral blood–derived MSCs (human PB-MSCs) might support tumor growth through stimulating the Tregs and secretion of immunosuppressive factors, that is, TGF-β, IL-4, and IL-10 with a simultaneous decrease in the cytotoxic capacity of CD8 T lymphocytes and NK cells [225].
Furthermore, MSCs infiltrate tumors and support their progression [6]. It was observed that the majority of the mouse glioma cell line (GL261) consisted of cells with mesenchymal features (Lin-Sca-1+CD9+CD44+CD166+ phenotype; trilineage differentiation capacity) [6]. However, most of the MSC-like cells in a tumor mass were of host origin, which indicated that endogenous MSCs were recruited to the tumor site. Moreover, brain tumor–derived MSCs co-injected with GL261 cells significantly reduced mice survival rate, compared with GL261 cell injections alone [6]. Similarly, other studies on different tumors such as head and neck cancer [226], colorectal cancer [227,228], or breast cancer [229] also confirmed the positive effect of MSCs on tumor growth.
Additionally, MSCs could contribute to promotion of metastasis formation [230]. Only few microscopic metastases were found in lungs of mice grafted with breast cancer cells (BCC) alone, whereas injections of a mixture of BCC and BM-MSCs increased the number of metastases in distant organs [230].
There are also a number of studies reporting MSC participation in the development of anti-cancer drug resistance. Mammary adenocarcinoma and Lewis lung carcinoma cells increased their resistance to paclitaxel or doxorubicin when exposed to BM-MSC conditioned medium [231]. Greater viability was due to decreased caspase-3 activity and Annexin-V expression.
Similarly, doxorubicin resistance was observed among triple-negative breast cancer cells after AT-MSC conditioned medium treatment [232]. Further studies revealed that the medium containing IL-8 caused an increase in BCRP protein expression (breast cancer resistance protein, one of the ABC transporters that are ATP-binding cassette transmembrane proteins engaged in the transport of a wide range of molecules across cell membranes, frequently against their concentration gradient, leading to the multidrug resistance in case of cancer cells [233]).
Other results showed that MSC-derived exosomes could restrain apoptosis of gastric cancer cells and enhanced the expression of multi-drug resistance-associated proteins after exposure to 5-fluorouracil [234]. In turn, Vianello et al. reported that co-culturing chronic myeloid leukemia cells with BM-MSCs prevented them from imatinib-induced apoptosis via the SDF-1/CXCR4 axis. Inhibition of CXCR4 abolished this resistance [235].
Because of the heterogeneity of the MSC population, Waterman et al. proposed a distinction between those cells into two phenotypes [236]. According to this hypothesis, MSCs can be polarized, depending on which Toll-like receptor (TLR) was previously activated on the cell surface, into pro-inflammatory MSC1 (TLR-4-primed) or immunosuppressive MSC2 (TLR-3-primed). Both in vitro and in vivo studies showed that MSC1 hampered tumor cell growth and invasion, whereas MSC2 supported these processes [237].
Conclusions
Genetically modified MSCs seem to be a promising strategy to improve cell-based therapy, enabling delivery of a plethora of factors that effectively repress tumor growth. A review of currently published studies has shown that the effects of MSCs engineered to express different genes or to serve as a vehicle for therapeutic agents for cancer therapy are multiple and may depend on the state of tumor and interactions with other cell types in the tumor microenvironment. These results are sometimes contradictory, and the factors released from modified MSCs have been implicated in both pro- and anti-tumor growth and/or metastases. Hence, the selective control of therapeutic gene expression by MSCs within the defined tumor settings provides new options to use modified MSCs for cancer therapy in patients.
Acknowledgments
This study was supported by grants from the Polish National Centre for Research and Development STRATEGMED1/235773/19/NCBR/2016 “EXPLORE ME” and NIH R01 NS091100-01A1.
Author Disclosure Statement
All authors declare that no competing financial interests exist.
References
- 1.Nowakowski A, Walczak P, Janowski M. and Lukomska B. (2015). Genetic engineering of mesenchymal stem cells for regenerative medicine. Stem Cells Dev 24:2219–2242 [DOI] [PubMed] [Google Scholar]
- 2.Nowakowski A, Walczak P, Lukomska B. and Janowski M. (2016). Genetic engineering of mesenchymal stem cells to induce their migration and survival. Stem Cell Int 2016:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nowakowski A, Andrzejewska A, Janowski M, Walczak P. and Lukomska B. (2013). Genetic engineering of stem cells for enhanced therapy. Acta Neurobiol Exp (Wars) 73:1–18 [DOI] [PubMed] [Google Scholar]
- 4.Barcellos-de-Souza P, Gori V, Bambi F. and Chiarugi P. (2013). Tumor microenvironment: bone marrow-mesenchymal stem cells as key players. Biochim Biophys Acta 1836:321–335 [DOI] [PubMed] [Google Scholar]
- 5.Houthuijzen JM, Daenen LG, Roodhart JM. and Voest EE. (2012). The role of mesenchymal stem cells in anti-cancer drug resistance and tumour progression. Br J Cancer 106:1901–1906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Behnan J, Isakson P, Joel M, Cilio C, Langmoen IA, Vik‐Mo EO. and Badn W. (2014). Recruited brain tumor‐derived mesenchymal stem cells contribute to brain tumor progression. Stem Cells 32:1110–1123 [DOI] [PubMed] [Google Scholar]
- 7.Han I, Yun M, Kim EO, Kim B, Jung MH. and Kim SH. (2014). Umbilical cord tissue-derived mesenchymal stem cells induce apoptosis in PC-3 prostate cancer cells through activation of JNK and downregulation of PI3K/AKT signalling. Stem Cell Res Ther 5:54. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 8.Kucerova L, Zmajkovic J, Toro L, Skolekova S, Demkova L. and Matuskova M. (2014). Tumor-driven molecular changes in human mesenchymal stromal cells. Cancer Microenviron 8:1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cavarretta IT, Altanerova V, Matuskova M, Kucerova L, Culig Z. and Altaner C. (2010). Adipose tissue-derived mesenchymal stem cells expressing prodrug-converting enzyme inhibit human prostate tumor growth. Mol Ther 18:223–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Altaner C, Altanerova V, Cihova M, Ondicova K, Rychly B, Baciak L. and Mravec B. (2014). Complete regression of glioblastoma by mesenchymal stem cells mediated prodrug gene therapy simulating clinical therapeutic scenario. Int J Cancer 134:1458–1465 [DOI] [PubMed] [Google Scholar]
- 11.Amara I, Touati W, Beaune P. and de Waziers I. (2014). Mesenchymal stem cells as cellular vehicles for prodrug gene therapy against tumors. Biochimie 105:4–11 [DOI] [PubMed] [Google Scholar]
- 12.Keung EZ, Nelson PJ. and Conrad C. (2013). Concise review: genetically engineered stem cell therapy targeting angiogenesis and tumor stroma in gastrointestinal malignancy. Stem Cells 31:227–235 [DOI] [PubMed] [Google Scholar]
- 13.Chu Y, Liu H, Lou G, Zhang Q. and Wu C. (2014). Human placenta mesenchymal stem cells expressing exogenous kringle1-5 protein by fiber-modified adenovirus suppress angiogenesis. Cancer Gene Ther 21:200–208 [DOI] [PubMed] [Google Scholar]
- 14.Shahrokhi S, Daneshmandi S. and Menaa F. (2014). Tumor necrosis factor-α/CD40 ligand-engineered mesenchymal stem cells greatly enhanced the antitumor immune response and lifespan in mice. Hum Gene Ther 25:240–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Y, Wang J, Ren M, Li M, Chen D, Chen J, Shi F, Wang X. and Dou J. (2014). Gene therapy of ovarian cancer using IL-21-secreting human umbilical cord mesenchymal stem cells in nude mice. J Ovarian Res 7:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tyciakova S, Matuskova M, Bohovic R, Polakova K, Toro L, Skolekova S. and Kucerova L. (2015). Genetically engineered mesenchymal stromal cells producing TNFα have tumour suppressing effect on human melanoma xenograft. J Gene Med 17:54–67 [DOI] [PubMed] [Google Scholar]
- 17.Kikuchi H, Yagi H, Hasegawa H, Ishii Y, Okabayashi K, Tsuruta M, Hoshino G, Takayanagi A. and Kitagawa Y. (2014). Therapeutic potential of transgenic mesenchymal stem cells engineered to mediate anti-high mobility group box 1 activity: targeting of colon cancer. J Surg Res 190:134–143 [DOI] [PubMed] [Google Scholar]
- 18.Hu G, Drescher KM. and Chen XM. (2012). Exosomal miRNAs: biological properties and therapeutic potential. Front Genet 3:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Friedenstein A, Gorskaja JF. and Kulagina NN. (1976). Fibroblast precursors in normal and irradiated mouse hematopoietic organs. Exp Hematol 4:267–274 [PubMed] [Google Scholar]
- 20.Caplan AI. and Dennis JE. (2006). Mesenchymal stem cells as trophic mediators. J Cell Biochem 98:1076–1084 [DOI] [PubMed] [Google Scholar]
- 21.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj. and Horwitz E. (2006). Minimal criteria for defining multipotent mesenchymal stromal cells. The international society for cellular therapy position statement. Cytotherapy 8:315–317 [DOI] [PubMed] [Google Scholar]
- 22.Bianco P, Robey PG. and Simmons PJ. (2008). Mesenchymal stem cells: revisiting history, concepts, and assays. Cell Stem Cell 2:313–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Squillaro T, Peluso G. and Galderisi U. (2016). Clinical trials with mesenchymal stem cells: an update. Cell Transplant 25:829–848 [DOI] [PubMed] [Google Scholar]
- 24.Kuroda Y. and Dezawa M. (2014). Mesenchymal stem cells and their subpopulation, pluripotent muse cells, in basic research and regenerative medicine. Anat Rec (Hoboken) 297:98–110 [DOI] [PubMed] [Google Scholar]
- 25.Lv FJ, Tuan RS, Cheung KM. and Leung VY. (2014). Concise review: the surface markers and identity of human mesenchymal stem cells. Stem Cells 32:1408–1419 [DOI] [PubMed] [Google Scholar]
- 26.Hass R, Kasper C, Böhm S. and Jacobs R. (2011). Different populations and sources of human mesenchymal stem cells (MSC): a comparison of adult and neonatal tissue-derived MSC. Cell Commun Signal 9:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Álvarez-Viejo M, Menéndez-Menéndez Y. and Otero-Hernández J. (2015). CD271 as a marker to identify mesenchymal stem cells from diverse sources before culture. World J Stem Cells 7:470–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feng J, Mantesso A, De Bari C, Nishiyama A. and Sharpe PT. (2011). Dual origin of mesenchymal stem cells contributing to organ growth and repair. Proc Natl Acad Sci U S A 108:6503–6508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Isern J, García-García A, Martín AM, Arranz L, Martín-Pérez D, Torroja C, Sánchez-Cabo F. and Méndez-Ferrer S. (2014). The neural crest is a source of mesenchymal stem cells with specialized hematopoietic stem cell niche function. Elife 3:e03696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li Z, Zhang C, Weiner LP, Zhang Y. and Zhong JF. (2013). Molecular characterization of heterogeneous mesenchymal stem cells with single-cell transcriptomes. Biotechnol Adv 31:312–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chanda D, Isayeva T, Kumar S, Hensel JA, Sawant A, Ramaswamy G, Siegal GP, Beatty MS. and Ponnazhagan S. (2009). Therapeutic potential of adult bone marrow-derived mesenchymal stem cells in prostate cancer bone metastasis. Clin Cancer Res 15:7175–7185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abd-Allah SH, Shalaby SM, El-Shal AS, Elkader EA, Hussein S, Emam E, Mazen NF, El Kateb M. and Atfy M. (2014). Effect of bone marrow-derived mesenchymal stromal cells on hepatoma. Cytotherapy 16:1197–1206 [DOI] [PubMed] [Google Scholar]
- 33.Nasuno M, Arimura Y, Nagaishi K, Isshiki H, Onodera K, Nakagaki S, Watanabe S, Idogawa M, Yamashita K, et al. (2014). Mesenchymal stem cells cancel azoxymethane-induced tumor initiation. Stem Cells 32:913–925 [DOI] [PubMed] [Google Scholar]
- 34.Lazennec G. and Jorgensen C. (2008). Concise review: adult multipotent stromal cells and cancer: risk or benefit? Stem Cells 26:1387–1394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Akimoto K, Kimura K, Nagano M, Takano S, To'a Salazar G, Yamashita T. and Ohneda O. (2013). Umbilical cord blood-derived mesenchymal stem cells inhibit, but adipose tissue-derived mesenchymal stem cells promote, glioblastoma multiforme proliferation. Stem Cells Dev 22:1370–1386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jazedje T, Ribeiro AL, Pellati M, de Siqueira Bueno HM, Nagata G, Trierveiler M, Rodrigues EG. and Zatz M. (2015). Human mesenchymal stromal cells transplantation may enhance or inhibit 4T1 murine breast adenocarcinoma through different approaches. Stem Cells Int 2015:796215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li Y, Xu X, Wang L, Liu G, Li Y, Wu X, Jing Y, Li H. and Wang G. (2015). Senescent mesenchymal stem cells promote colorectal cancer cells growth via galectin-3 expression. Cell Biosci 5:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Di GH, Liu Y, Lu Y, Liu J, Wu C. and Duan HF. (2014). IL-6 secreted from senescent mesenchymal stem cells promotes proliferation and migration of breast cancer cells. PLoS One 9:e113572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang M, Cai J, Huang F, Zhu M, Zhang Q, Yang T, Zhang X, Qian H. and Xu W. (2015). Pre-treatment of human umbilical cord-derived mesenchymal stem cells with interleukin-6 abolishes their growth-promoting effect on gastric cancer cells. Int J Mol Med 35:367–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Özcan S1, Alessio N, Acar MB, Toprak G, Gönen ZB, Peluso G. and Galderisi U. (2015). Myeloma cells can corrupt senescent mesenchymal stromal cells and impair their anti-tumor activity. Oncotarget 6:39482–39492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Severino V, Alessio N, Farina A, Sandomenico A, Cipollaro M, Peluso G, Galderisi U. and Chambery A. (2013). Insulin-like growth factor binding proteins 4 and 7 released by senescent cells promote premature senescence in mesenchymal stem cells. Cell Death Dis 4:e911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kundrotas G, Gasperskaja E, Slapsyte G, Gudleviciene Z, Krasko J, Stumbryte A. and Liudkeviciene R. (2016). Identity, proliferation capacity, genomic stability and novel senescence markers of mesenchymal stem cells isolated from low volume of human bone marrow. Oncotarget 7:10788–10802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wong RS. (2011). Mesenchymal stem cells: angels or demons? J Biomed Biotechnol 2011:459510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dasari VR, Kaur K, Velpula KK, Gujrati M, Fassett D, Klopfenstein JD, Dinh DH. and Rao JS. (2010). Upregulation of PTEN in glioma cells by cord blood mesenchymal stem cells inhibits migration via downregulation of the PI3K/Akt pathway. PLoS One 5:e10350. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 45.Ho IA, Toh HC, Ng WH, Teo YL, Guo CM, Hui KM. and Lam PY. (2013). Human bone marrow-derived mesenchymal stem cells suppress human glioma growth through inhibition of angiogenesis. Stem Cells 31:146–155 [DOI] [PubMed] [Google Scholar]
- 46.Yang C, Lei D, Ouyang W, Ren J, Li H, Hu J. and Huang S. (2014). Conditioned media from human adipose tissue-derived mesenchymal stem cells and umbilical cord-derived mesenchymal stem cells efficiently induced the apoptosis and differentiation in human glioma cell lines in vitro. Biomed Res Int 2014:109389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kološa K, Motaln H, Herold-Mende C, Koršič M. and Lah TT. (2015). Paracrine effects of mesenchymal stem cells induce senescence and differentiation of glioblastoma stem-like cells. Cell Transplant 24:631–644 [DOI] [PubMed] [Google Scholar]
- 48.Ma Y, Hao X, Zhang S. and Zhang J. (2012). The in vitro and in vivo effects of human umbilical cord mesenchymal stem cells on the growth of breast cancer cells. Breast Cancer Res Treat 133:473–485 [DOI] [PubMed] [Google Scholar]
- 49.Qiao L, Xu Z, Zhao T, Zhao Z, Shi M, Zhao RC, Ye L. and Zhang X. (2008). Suppression of tumorigenesis by human mesenchymal stem cells in a hepatoma model. Cell Res 18:500–507 [DOI] [PubMed] [Google Scholar]
- 50.Kidd S, Caldwell L, Dietrich M, Samudio I, Spaeth EL, Watson K, Shi Y, Abbruzzese J, Konopleva M, Andreeff M. and Marini FC. (2010). Mesenchymal stromal cells alone or expressing interferon-beta suppress pancreatic tumors in vivo, an effect countered by anti-inflammatory treatment. Cytotherapy 12:615–625 [DOI] [PubMed] [Google Scholar]
- 51.Ohlsson LB, Varas L, Kjellman C, Edvardsen K. and Lindvall M. (2003). Mesenchymal progenitor cell-mediated inhibition of tumor growth in vivo and in vitro in gelatin matrix. Exp Mol Pathol 75:248–255 [DOI] [PubMed] [Google Scholar]
- 52.Ciavarella S, Caselli A, Tamma AV, Savonarola A, Loverro G, Paganelli R, Tucci M. and Silvestris F. (2015). A peculiar molecular profile of umbilical cord-mesenchymal stromal cells drives their inhibitory effects on multiple myeloma cell growth and tumor progression. Stem Cells Dev 24:1457–1470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Khakoo AY, Pati S, Anderson SA, Reid W, Elshal MF, Rovira II, Nguyen AT, Malide D, Combs CA, et al. (2006). Human mesenchymal stem cells exert potent antitumorigenic effects in a model of Kaposi's sarcoma. J Exp Med 203:1235–1247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Secchiero P, Zorzet S, Tripodo C, Corallini F, Melloni E, Caruso L, Bosco R, Ingrao S, Zavan B. and Zauli G. (2010). Human bone marrow mesenchymal stem cells display anti-cancer activity in SCID mice bearing disseminated non-Hodgkin's lymphoma xenografts. PLoS One 5:e11140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hendijani F, Javanmard ShH, Rafiee L. and Sadeghi-Aliabadi H. (2015). Effect of human Wharton's jelly mesenchymal stem cell secretome on proliferation, apoptosis and drug resistance of lung cancer cells. Res Pharm Sci 10:134–142 [PMC free article] [PubMed] [Google Scholar]
- 56.de Araújo Farias V, O'Valle F, Lerma BA, Ruiz de Almodóvar C, López-Peñalver JJ, Nieto A, Santos A, Fernández BI, Guerra-Librero A, et al. (2015). Human mesenchymal stem cells enhance the systemic effects of radiotherapy. Oncotarget 6:31164–31180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rachner TD, Göbel A, Browne A, Hötzel J, Rauner M. and Hofbauer LC. (2015). P38 regulates the Wnt inhibitor Dickkopf-1 in breast cancer. Biochem Biophys Res Commun 466:728–732 [DOI] [PubMed] [Google Scholar]
- 58.de Sousa E. Melo F. and Vermeulen L. (2016). Wnt signaling in cancer stem cell biology. Cancers (Basel) 8:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hou L, Wang X, Zhou Y, Ma H, Wang Z, He J, Hu H, Guan W. and Ma Y. (2014). Inhibitory effect and mechanism of mesenchymal stem cells on liver cancer cells. Tumour Biol 35:1239–1250 [DOI] [PubMed] [Google Scholar]
- 60.Sun B, Yu KR, Bhandari DR, Jung JW, Kang SK. and Kang KS. (2010). Human umbilical cord blood mesenchymal stem cell-derived extracellular matrix prohibits metastatic cancer cell MDA-MB-231 proliferation. Cancer Lett 296:178–185 [DOI] [PubMed] [Google Scholar]
- 61.Li T, Song B, Du X, Wei Z. and Huo T. (2013). Effect of bone-marrow-derived mesenchymal stem cells on high-potential hepatocellular carcinoma in mouse models: an intervention study. Eur J Med Res 18:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang L, Su XS, Ye JS, Wang YY, Guan Z. and Yin YF. (2015). Bone marrow mesenchymal stem cells suppress metastatic tumor development in mouse by modulating immune system. Stem Cell Res Ther 6:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kucerova L, Skolekova S, Matuskova M, Bohac M. and Kozovska Z. (2013). Altered features and increased chemosensitivity of human breast cancer cells mediated by adipose tissue-derived mesenchymal stromal cells. BMC Cancer 13:535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Meleshina AV, Cherkasova EI, Shirmanova MV, Klementieva NV, Kiseleva EV, Snopova LB, Prodanets NN. and Zagaynova EV. (2015). Influence of mesenchymal stem cells on metastasis development in mice in vivo. Stem Cell Res Ther 6:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yoon N, Park MS, Peltier GC. and Lee RH. (2015). Pre-activated human mesenchymal stromal cells in combination with doxorubicin synergistically enhance tumor-suppressive activity in mice. Cytotherapy 17:1332–1341 [DOI] [PubMed] [Google Scholar]
- 66.Librizzi M, Tobiasch E. and Luparello C. (2016). The conditioned medium from osteo-differentiating human mesenchymal stem cells affects the viability of triple negative MDA-MB231 breast cancer cells. Cell Biochem Funct 34:7–15 [DOI] [PubMed] [Google Scholar]
- 67.Eiró N, Sendon-Lago J, Seoane S, Bermúdez MA, Lamelas ML, Garcia-Caballero T, Schneider J, Perez-Fernandez R. and Vizoso FJ. (2014). Potential therapeutic effect of the secretome from human uterine cervical stem cells against both cancer and stromal cells compared with adipose tissue stem cells. Oncotarget 5:10692–10708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Clarke MR, Imhoff FM. and Baird SK. (2015). Mesenchymal stem cells inhibit breast cancer cell migration and invasion through secretion of tissue inhibitor of metalloproteinase-1 and -2. Mol Carcinog 54:1214–1219 [DOI] [PubMed] [Google Scholar]
- 69.Pulkkanen KJ. and Yla-Herttuala S. (2005). Gene therapy for malignant glioma: current clinical status. Mol Ther 12:585–598 [DOI] [PubMed] [Google Scholar]
- 70.Uchibori R, Okada T, Ito T, Urabe M, Mizukami H, Kume A. and Ozawa K. (2009). Retroviral vector-producing mesenchymal stem cells for targeted suicide cancer gene therapy. J Gene Med 11:373–381 [DOI] [PubMed] [Google Scholar]
- 71.Mori K, Iwata J, Miyazaki M, Osada H, Tange Y, Yamamoto T, Aiko Y, Tamura M. and Shiroishi T. (2010). Bystander killing effect of tymidine kinase gene-transduced adult bone marrow stromal cells with ganciclovir on malignant glioma cells. Neurol Med Chir (Tokyo) 50:545–553 [DOI] [PubMed] [Google Scholar]
- 72.Matuskova M, Baranovicova L, Kozovska Z, Durinikova E, Pastorakova A, Hunakova L, Waczulikova I, Nencka R. and Kucerova L. (2012). Intrinsic properties of tumour cells have a key impact on the bystander effect mediated by genetically engineered mesenchymal stromal cells. J Gene Med 14:776–787 [DOI] [PubMed] [Google Scholar]
- 73.Li S, Gu C, Gao Y, Amano S, Koizumi S, Tokuyama T. and Namba H. (2012). Bystander effect in glioma suicide gene therapy using bone marrow stromal cells. Stem Cell Res 9:270–276 [DOI] [PubMed] [Google Scholar]
- 74.Mesnil M. and Yamasaki H. (2000). Bystander effect in herpes simplex virus-thymidine kinase/ganciclovir cancer gene therapy: role of gap-junctional intercellular communication. Cancer Res 60:3989–3999 [PubMed] [Google Scholar]
- 75.Alieva M, Bagó JR, Aguilar E, Soler-Botija C, Vila OF, Molet J, Gambhir SS, Rubio N. and Blanco J. (2012). Glioblastoma therapy with cytotoxic mesenchymal stromal cells optimized by bioluminescence imaging of tumor and therapeutic cell response. PLoS One 7:e35148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Leten C, Trekker J, Struys T, Roobrouck VD, Dresselaers T, Vande Velde G, Lambrichts I, Verfaillie CM. and Himmelreich U. (2016). Monitoring the bystander killing effect of human multipotent stem cells for treatment of malignant brain tumors. Stem Cells Int 2016:4095072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Huang Q, Liu XZ, Kang CS, Wang GX, Zhong Y. and Pu PY. (2010). The anti-glioma effect of suicide gene therapy using BMSC expressing HSV/TK combined with overexpression of Cx43 in glioma cells. Cancer Gene Ther 17:192–202 [DOI] [PubMed] [Google Scholar]
- 78.Ammerpohl O, Thormeyer D, Khan Z, Appelskog IB, Gojkovic Z, Almqvist PM. and Ekström TJ. (2004). HDACi phenylbutyrate increases bystander killing of HSV-tk transfected glioma cells. Biochem Biophys Res Commun 324:8–14 [DOI] [PubMed] [Google Scholar]
- 79.Ryu CH, Park KY, Kim SM, Jeong CH, Woo JS, Hou Y. and Jeun SS. (2012). Valproic acid enhances anti-tumor effect of mesenchymal stem cell mediated HSV-TK gene therapy in intracranial glioma. Biochem Biophys Res Commun 421:585–590 [DOI] [PubMed] [Google Scholar]
- 80.Amano S, Gu C, Koizumi S, Tokuyama T. and Namba H. (2011). Tumoricidal bystander effect in the suicide gene therapy using mesenchymal stem cells does not injure normal brain tissues. Cancer Lett 306:99–105 [DOI] [PubMed] [Google Scholar]
- 81.Lee WY, Zhang T, Lau CP, Wang CC, Chan KM. and Li G. (2013). Immortalized human fetal bone marrow-derived mesenchymal stromal cell expressing suicide gene for anti-tumor therapy in vitro and in vivo. Cytotherapy 15:1484–1497 [DOI] [PubMed] [Google Scholar]
- 82.Zischek C, Niess H, Ischenko I, Conrad C, Huss R, Jauch KW, Nelson PJ. and Bruns C. (2009). Targeting tumor stroma using engineered mesenchymal stem cells reduces the growth of pancreatic carcinoma. Ann Surg 250:747–753 [DOI] [PubMed] [Google Scholar]
- 83.Conrad C, Hüsemann Y, Niess H, von Luettichau I, Huss R, Bauer C, Jauch KW, Klein CA, Bruns C. and Nelson PJ. (2011). Linking transgene expression of engineered mesenchymal stem cells and angiopoietin-1-induced differentiation to target cancer angiogenesis. Ann Surg 253:566–571 [DOI] [PubMed] [Google Scholar]
- 84.Niess H, Bao Q, Conrad C, Zischek C, Notohamiprodjo M, Schwab F, Schwarz B, Huss R, Jauch KW, Nelson PJ. and Bruns CJ. (2011). Selective targeting of genetically engineered mesenchymal stem cells to tumor stroma microenvironments using tissue-specific suicide gene expression suppresses growth of hepatocellular carcinoma. Ann Surg 254:767–774; discussion 774–775 [DOI] [PubMed] [Google Scholar]
- 85.Zhang TY, Huang B, Yuan ZY, Hu YL, Tabata Y. and Gao JQ. (2014). Gene recombinant bone marrow mesenchymal stem cells as a tumor-targeted suicide gene delivery vehicle in pulmonary metastasis therapy using non-viral transfection. Nanomedicine 10:257–267 [DOI] [PubMed] [Google Scholar]
- 86.Amano S, Li S, Gu C, Gao Y, Koizumi S, Yamamoto S, Terakawa S. and Namba H. (2009). Use of genetically engineered bone marrow-derived mesenchymal stem cells for glioma gene therapy. Int J Oncol 35:1265–1270 [DOI] [PubMed] [Google Scholar]
- 87.Gu C, Li S, Tokuyama T, Yokota N. and Namba H. (2010). Therapeutic effect of genetically engineered mesenchymal stem cells in rat experimental leptomeningeal glioma model. Cancer Lett 291:256–262 [DOI] [PubMed] [Google Scholar]
- 88.Kinoshita Y, Kamitani H, Mamun MH, Wasita B, Kazuki Y, Hiratsuka M, Oshimura M. and Watanabe T. (2010). A gene delivery system with a human artificial chromosome vector based on migration of mesenchymal stem cells towards human glioblastoma HTB14 cells. Neurol Res 32:429–437 [DOI] [PubMed] [Google Scholar]
- 89.Matuskova M, Hlubinova K, Pastorakova A, Hunakova L, Altanerova V, Altaner C. and Kucerova L. (2010). HSV-tk expressing mesenchymal stem cells exert bystander effect on human glioblastoma cells. Cancer Lett 290:58–67 [DOI] [PubMed] [Google Scholar]
- 90.Amano S, Gu C, Koizumi S, Tokuyama T. and Namba H. (2011). Timing of ganciclovir administration in glioma gene therapy using HSVtk gene-transduced mesenchymal stem cells. Cancer Genomics Proteomics 8:245–250 [PubMed] [Google Scholar]
- 91.de Melo SM, Bittencourt S, Ferrazoli EG, da Silva CS, da Cunha FF, da Silva FH, Stilhano RS, Denapoli PM, Zanetti BF, et al. (2015). The anti-tumor effects of adipose tissue mesenchymal stem cell transduced with HSV-Tk Gene on U-87-driven brain tumor. PLoS One 10:e0128922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Niess H, von Einem JC, Thomas MN, Michl M, Angele MK, Huss R, Günther C, Nelson PJ, Bruns CJ. and Heinemann V. (2015). Treatment of advanced gastrointestinal tumors with genetically modified autologous mesenchymal stromal cells (TREAT-ME1): study protocol of a phase I/II clinical trial. BMC Cancer 15:237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Matuskova M, Kozovska Z, Toro L, Durinikova E, Tyciakova S, Cierna Z, Bohovic R. and Kucerova L. (2015). Combined enzyme/prodrug treatment by genetically engineered AT-MSC exerts synergy and inhibits growth of MDA-MB-231 induced lung metastases. J Exp Clin Cancer Res 34:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jiang J, Wei D, Sun L, Wang Y, Wu X, Li Y, Fang Z, Shang H. and Wei Z. (2014). A preliminary study on the construction of double suicide gene delivery vectors by mesenchymal stem cells and the in vitro inhibitory effects on SKOV3 cells. Oncol Rep 31:781–787 [DOI] [PubMed] [Google Scholar]
- 95.Kim SW, Kim SJ, Park SH, Yang HG, Kang MC, Choi YW, Kim SM, Jeun SS. and Sung YC. (2013). Complete regression of metastatic renal cell carcinoma by multiple injections of engineered mesenchymal stem cells expressing dodecameric TRAIL and HSV-TK. Clin Cancer Res 19:415–427 [DOI] [PubMed] [Google Scholar]
- 96.Martinez-Quintanilla J, Bhere D, Heidari P, He D, Mahmood U. and Shah K. (2013). Therapeutic efficacy and fate of bimodal engineered stem cells in malignant brain tumors. Stem Cells 31:1706–1714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rainov NG, Fels C, Droege JW, Schäfer C, Kramm CM. and Chou TC. (2001). Temozolomide enhances herpes simplex virus thymidine kinase/ganciclovir therapy of malignant glioma. Cancer Gene Ther 8:662–668 [DOI] [PubMed] [Google Scholar]
- 98.Nouri FS, Wang X. and Hatefi A. (2015). Genetically engineered theranostic mesenchymal stem cells for the evaluation of the anticancer efficacy of enzyme/prodrug systems. J Control Release 200:179–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kucerova L, Poturnajova M, Tyciakova S. and Matuskova M. (2012). Increased proliferation and chemosensitivity of human mesenchymal stromal cells expressing fusion yeast cytosine deaminase. Stem Cell Res 8:247–258 [DOI] [PubMed] [Google Scholar]
- 100.Park JS, Chang DY, Kim JH, Jung JH, Park J, Kim SH, Lee YD, Kim SS. and Suh-Kim H. (2013). Retrovirus-mediated transduction of a cytosine deaminase gene preserves the stemness of mesenchymal stem cells. Exp Mol Med 45:e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Weng Q, Tan B, Wang J, Wang J, Zhou H, Shi J, He Q. and Yang B. (2014). 5-Fluorouracil causes severe CNS demyelination by disruption of TCF7L2/HDAC1/HDAC2 complex in adolescent mice. Toxicology 325C:144–150 [DOI] [PubMed] [Google Scholar]
- 102.Haack K, Linnebacher M, Eisold S, Zöller M, von Knebel Doeberitz M. and Gebert J. (2000). Induction of protective immunity against syngeneic rat cancer cells by expression of the cytosine deaminase suicide gene. Cancer Gene Ther 7:1357–1364 [DOI] [PubMed] [Google Scholar]
- 103.Chang DY, Yoo SW, Hong Y, Kim S, Kim SJ, Yoon SH, Cho KG, Paek SH, Lee YD, Kim SS. and Suh-Kim H. (2010). The growth of brain tumors can be suppressed by multiple transplantation of mesenchymal stem cells expressing cytosine deaminase. Int J Cancer 127:1975–1983 [DOI] [PubMed] [Google Scholar]
- 104.Fei S, Qi X, Kedong S, Guangchun J, Jian L. and Wei Q. (2012). The antitumor effect of mesenchymal stem cells transduced with a lentiviral vector expressing cytosine deaminase in a rat glioma model. J Cancer Res Clin Oncol 138:347–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kosaka H, Ichikawa T, Kurozumi K, Kambara H, Inoue S, Maruo T, Nakamura K, Hamada H. and Date I. (2012). Therapeutic effect of suicide gene-transferred mesenchymal stem cells in a rat model of glioma. Cancer Gene Ther 19:572–578 [DOI] [PubMed] [Google Scholar]
- 106.Jung JH, Kim AA, Chang DY, Park YR, Suh-Kim H. and Kim SS. (2015). Three-dimensional assessment of bystander effects of mesenchymal stem cells carrying a cytosine deaminase gene on glioma cells. Am J Cancer Res 5:2686–2696 [PMC free article] [PubMed] [Google Scholar]
- 107.NguyenThai QA, Sharma N, Luong do H, Sodhi SS, Kim JH, Kim N, Oh SJ. and Jeong DK. (2015). Targeted inhibition of osteosarcoma tumor growth by bone marrow-derived mesenchymal stem cells expressing cytosine deaminase/5-fluorocytosine in tumor-bearing mice. J Gene Med 17:87–99 [DOI] [PubMed] [Google Scholar]
- 108.You MH, Kim WJ, Shim W, Lee SR, Lee G, Choi S, Kim DY, Kim YM, Kim H. and Han SU. (2009). Cytosine deaminase-producing human mesenchymal stem cells mediate an antitumor effect in a mouse xenograft model. J Gastroenterol Hepatol 24:1393–1400 [DOI] [PubMed] [Google Scholar]
- 109.Kucerova L, Matuskova M, Hlubinova K, Bohovic R, Feketeova L, Janega P, Babal P. and Poturnajova M. (2011). Bystander cytotoxicity in human medullary thyroid carcinoma cells mediated by fusion yeast cytosine deaminase and 5-fluorocytosine. Cancer Lett 311:101–112 [DOI] [PubMed] [Google Scholar]
- 110.Kucerova L, Matuskova M, Pastorakova A, Tyciakova S, Jakubikova J, Bohovic R, Altanerova V. and Altaner C. (2008). Cytosine deaminase expressing human mesenchymal stem cells mediated tumour regression in melanoma bearing mice. J Gene Med 10:1071–1082 [DOI] [PubMed] [Google Scholar]
- 111.Kucerova L, Altanerova V, Matuskova M, Tyciakova S. and Altaner C. (2007). Adipose tissue-derived human mesenchymal stem cells mediated prodrug cancer gene therapy. Cancer Res 67:6304–6313 [DOI] [PubMed] [Google Scholar]
- 112.Abrate A, Buono R, Canu T, Esposito A, Del Maschio A, Lucianò R, Bettiga A, Colciago G, Guazzoni G, et al. (2014). Mesenchymal stem cells expressing therapeutic genes induce autochthonous prostate tumour regression. Eur J Cancer 50:2478–2488 [DOI] [PubMed] [Google Scholar]
- 113.Straathof KC, Pulè MA, Yotnda P, Dotti G, Vanin EF, Brenner MK, Heslop HE, Spencer DM. and Rooney CM. (2005). An inducible caspase 9 safety switch for T-cell therapy. Blood 105:4247–4254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ramos CA, Asgari Z, Liu E, Yvon E, Heslop HE, Rooney CM, Brenner MK. and Dotti G. (2010). An inducible caspase 9 suicide gene to improve the safety of mesenchymal stromal cell therapies. Stem Cells 28:1107–1115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ando M, Hoyos V, Yagyu S, Tao W, Ramos CA, Dotti G, Brenner MK. and Bouchier-Hayes L. (2014). Bortezomib sensitizes non-small cell lung cancer to mesenchymal stromal cell-delivered inducible caspase-9-mediated cytotoxicity. Cancer Gene Ther 21:472–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Crafts TD, Jensen AR, Blocher-Smith EC. and Markel TA. (2014). Vascular endothelial growth factor: therapeutic possibilities and challenges for the treatment of ischemia. Cytokine 71:385–393 [DOI] [PubMed] [Google Scholar]
- 117.Bertolini F, Marighetti P, Martin-Padura I, Mancuso P, Hu-Lowe DD, Shaked Y. and D'Onofrio A. (2011). Anti-VEGF and beyond: shaping a new generation of anti-angiogenic therapies for cancer. Drug Discov Today 16:1052–1060 [DOI] [PubMed] [Google Scholar]
- 118.Sallinen H, Anttila M, Narvainen J, Koponen J, Hamalainen K, Kholova I, Heikura T, Toivanen P, Kosma VM, et al. (2009). Antiangiogenic gene therapy with soluble VEGFR-1, -2, and -3 reduces the growth of solid human ovarian carcinoma in mice. Mol Ther 17:278–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hu M, Yang JL, Teng H, Jia YQ, Wang R, Zhang XW, Wu Y, Luo Y, Chen XC, et al. (2008). Anti-angiogenesis therapy based on the bone marrow-derived stromal cells genetically engineered to express sFlt-1 in mouse tumor model. BMC Cancer 8:306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.O'Reilly MS, Boehm T, Shing Y, Fukai N, Vasios G, Lane WS, Flynn E, Birkhead JR, Olsen BR. and Folkman J. (1997). Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell 88:277–285 [DOI] [PubMed] [Google Scholar]
- 121.Dhanabal M, Ramchandran R, Waterman MJ, Lu H, Knebelmann B, Segal M. and Sukhatme VP. (1999). Endostatin induces endothelial cell apoptosis. J Biol Chem 274:11721–11726 [DOI] [PubMed] [Google Scholar]
- 122.Zhang D, Zheng L, Shi H, Chen X, Wan Y, Zhang H, Li M, Lu L, Luo S, et al. (2014). Suppression of peritoneal tumorigenesis by placenta-derived mesenchymal stem cells expressing endostatin on colorectal cancer. Int J Med Sci 11:870–879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.van Beijnum JR, Nowak-Sliwinska P, van den Boezem E, Hautvast P, Buurman WA. and Griffioen AW. (2013). Tumor angiogenesis is enforced by autocrine regulation of high-mobility group box 1. Oncogene 32:363–374 [DOI] [PubMed] [Google Scholar]
- 124.Wang W, Jiang H, Zhu H, Zhang H, Gong J, Zhang L. and Ding Q. (2013). Overexpression of high mobility group box 1 and 2 is associated with the progression and angiogenesis of human bladder carcinoma. Oncol Lett 5:884–888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yang H, Ochani M, Li J, Qiang X, Tanovic M, Harris HE, Susarla SM, Ulloa L, Wang H, et al. (2004). Reversing established sepsis with antagonists of endogenous high-mobility group box 1. Proc Natl Acad Sci U S A 101:296–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lawler PR. and Lawler J. (2012). Molecular basis for the regulation of angiogenesis by thrombospondin-1 and -2. Cold Spring Harb Perspect Med 2:a006627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Jeanne A, Schneider C, Martiny L. and Dedieu S. (2015). Original insights on thrombospondin-1-related antireceptor strategies in cancer. Front Pharmacol 6:252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Choi SH, Tamura K, Khajuria RK, Bhere D, Nesterenko I, Lawler J. and Shah K. (2015). Antiangiogenic variant of TSP-1 targets tumor cells in glioblastomas. Mol Ther 23:235–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Tong JP. and Yao YF. (2006). Contribution of VEGF and PEDF to choroidal angiogenesis: a need for balanced expressions. Clin Biochem 39:267–276 [DOI] [PubMed] [Google Scholar]
- 130.Gao Y, Yao A, Zhang W, Lu S, Yu Y, Deng L, Yin A, Xia Y, Sun B. and Wang X. (2010). Human mesenchymal stem cells overexpressing pigment epithelium-derived factor inhibit hepatocellular carcinoma in nude mice. Oncogene 29:2784–2794 [DOI] [PubMed] [Google Scholar]
- 131.Zolochevska O, Yu G, Gimble JM. and Figueiredo ML. (2012). Pigment epithelial-derived factor and melanoma differentiation associated gene-7 cytokine gene therapies delivered by adipose-derived stromal/mesenchymal stem cells are effective in reducing prostate cancer cell growth. Stem Cells Dev 21:1112–1123 [DOI] [PubMed] [Google Scholar]
- 132.Wang Q, Zhang Z, Ding T, Chen Z. and Zhang T. (2013). Mesenchymal stem cells overexpressing PEDF decrease the angiogenesis of gliomas. Biosci Rep 33:e00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hong X, Miller C, Savant-Bhonsale S. and Kalkanis SN. (2009). Antitumor treatment using interleukin-12-secreting marrow stromal cells in an invasive glioma model. Neurosurgery 64:1139–1146; discussion 1146–1147 [DOI] [PubMed] [Google Scholar]
- 134.Ryu CH, Park SH, Park SA, Kim SM, Lim JY, Jeong CH, Yoon WS, Oh WI, Sung YC. and Jeun SS. (2011). Gene therapy of intracranial glioma using interleukin 12-secreting human umbilical cord blood-derived mesenchymal stem cells. Hum Gene Ther 22:733–743 [DOI] [PubMed] [Google Scholar]
- 135.Duan X, Guan H, Cao Y. and Kleinerman ES. (2009). Murine bone marrow-derived mesenchymal stem cells as vehicles for interleukin-12 gene delivery into Ewing sarcoma tumors. Cancer 115:13–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Gao P, Ding Q, Wu Z, Jiang H. and Fang Z. (2010). Therapeutic potential of human mesenchymal stem cells producing IL-12 in a mouse xenograft model of renal cell carcinoma. Cancer Lett 290:157–166 [DOI] [PubMed] [Google Scholar]
- 137.Seo SH, Kim KS, Park SH, Suh YS, Kim SJ, Jeun SS. and Sung YC. (2011). The effects of mesenchymal stem cells injected via different routes on modified IL-12-mediated antitumor activity. Gene Ther 18:488–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Strieter RM, Kunkel SL, Arenberg DA, Burdick MD. and Polverini PJ. (1995). Interferon gamma-inducible protein 10 (IP-10), a member of the C-X-C chemokine family, is an inhibitor of angiogenesis. Biochem Biophys Res Commun 210:51–57 [DOI] [PubMed] [Google Scholar]
- 139.Razmkhah M, Jaberipour M. and Ghaderi A. (2014). Downregulation of MMP2 and Bcl-2 in adipose derived stem cells (ASCs) following transfection with IP-10 gene. Avicenna J Med Biotechnol 6:27–37 [PMC free article] [PubMed] [Google Scholar]
- 140.Loebinger MR, Eddaoudi A, Davies D. and Janes SM. (2009). Mesenchymal stem cell delivery of TRAIL can eliminate metastatic cancer. Cancer Res 69:4134–4142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Rodríguez R, García-Castro J, Trigueros C, García Arranz M. and Menéndez P. (2012). Multipotent mesenchymal stromal cells: clinical applications and cancer modeling. Adv Exp Med Biol 741:187–205 [DOI] [PubMed] [Google Scholar]
- 142.Wang GX, Zhan YA, Hu HL, Wang Y. and Fu B. (2012). Mesenchymal stem cells modified to express interferon-β inhibit the growth of prostate cancer in a mouse model. J Int Med Res 40:317–327 [DOI] [PubMed] [Google Scholar]
- 143.Xie C, Xie DY, Lin BL, Zhang GL, Wang PP, Peng L. and Gao ZL. (2013). Interferon-β gene-modified human bone marrow mesenchymal stem cells attenuate hepatocellular carcinoma through inhibiting AKT/FOXO3a pathway. Br J Cancer 109:1198–1205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Studeny M, Marini FC, Champlin RE, Zompetta C, Fidler IJ. and Andreeff M. (2002). Bone marrow-derived mesenchymal stem cells as vehicles for interferon-beta delivery into tumors. Cancer Res 62:3603–3608 [PubMed] [Google Scholar]
- 145.Studeny M, Marini FC, Dembinski JL, Zompetta C, Cabreira-Hansen M, Bekele BN, Champlin RE. and Andreeff M. (2004). Mesenchymal stem cells: potential precursors for tumor stroma and targeted-delivery vehicles for anticancer agents. J Natl Cancer Inst 96:1593–1603 [DOI] [PubMed] [Google Scholar]
- 146.Nakamizo A, Marini F, Amano T, Khan A, Studeny M, Gumin J, Chen J, Hentschel S, Vecil G, et al. (2005). Human bone marrow-derived mesenchymal stem cells in the treatment of gliomas. Cancer Res 65:3307–3318 [DOI] [PubMed] [Google Scholar]
- 147.Seo KW, Lee HW, Oh YI, Ahn JO, Koh YR, Oh SH, Kang SK. and Youn HY. (2011). Anti-tumor effects of canine adipose tissue-derived mesenchymal stromal cell-based interferon-β gene therapy and cisplatin in a mouse melanoma model. Cytotherapy 13:944–955 [DOI] [PubMed] [Google Scholar]
- 148.Bao Q, Zhao Y, Niess H, Conrad C, Schwarz B, Jauch KW, Huss R, Nelson PJ. and Bruns CJ. (2012). Mesenchymal stem cell-based tumor-targeted gene therapy in gastrointestinal cancer. Stem Cells Dev 21:2355–2363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Dedoni S, Olianas MC. and Onali P. (2010). Interferon-β induces apoptosis in human SH-SY5Y neuroblastoma cells through activation of JAK-STAT signalling and down-regulation of PI3K/Akt pathway. J Neurochem 115:1421–1433 [DOI] [PubMed] [Google Scholar]
- 150.Zitvogel L, Kepp O. and Kroemer G. (2010). Decoding cell death signals in inflammation and immunity. Cell 140:798–804 [DOI] [PubMed] [Google Scholar]
- 151.Yang X, Du J, Xu X, Xu C. and Song W. (2014). IFN-γ-secreting-mesenchymal stem cells exert an antitumor effect in vivo via the TRAIL pathway. J Immunol Res 31:8098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Shah K. (2012). Mesenchymal stem cells engineered for cancer therapy. Adv Drug Deliv Rev 64:739–748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Jing W, Chen Y, Lu L, Hu X, Shao C, Zhang Y, Zhou X, Zhou Y, Wu L, et al. (2014). Human umbilical cord blood-derived mesenchymal stem cells producing IL15 eradicate established pancreatic tumor in syngeneic mice. Mol Cancer Ther 13:2127–2137 [DOI] [PubMed] [Google Scholar]
- 154.Xu X, Yang G, Zhang H. and Prestwich GD. (2009). Evaluating dual activity LPA receptor pan-antagonist/autotaxin inhibitors as anti-cancer agents in vivo using engineered human tumors. Prostaglandins Other Lipid Mediat 89:140–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zundler S. and Neurath MF. (2015). Interleukin-12: functional activities and implications for disease. Cytokine Growth Factor Rev 26:559–568 [DOI] [PubMed] [Google Scholar]
- 156.Chen X, Lin X, Zhao J, Shi W, Zhang H, Wang Y, Kan B, Du L, Wang B, et al. (2008). A tumor-selective biotherapy with prolonged impact on established metastases based on cytokine gene-engineered MSCs. Mol Ther 16:749–756 [DOI] [PubMed] [Google Scholar]
- 157.Eliopoulos N, Francois M, Boivin MN, Martineau D. and Galipeau J. (2008). Neo-organoid of marrow mesenchymal stromal cells secreting interleukin-12 for breast cancer therapy. Cancer Res 68:4810–4818 [DOI] [PubMed] [Google Scholar]
- 158.Dou J, Chu L, Zhao F, Tang Q, Zhang A, Zhang L, Wang Y, Li Y, Cao M. and Gu N. (2007). Study of immunotherapy of murine myeloma by an IL-21-based tumor vaccine in BALB/C mice. Cancer Biol Ther 6:1871–1879 [DOI] [PubMed] [Google Scholar]
- 159.Balkwill F. (2009). Tumour necrosis factor and cancer. Nat Rev Cancer 9:361–371 [DOI] [PubMed] [Google Scholar]
- 160.Johansson A, Hamzah J, Payne CJ. and Ganss R. (2012). Tumor-targeted TNFα stabilizes tumor vessels and enhances active immunotherapy. Proc Natl Acad Sci U S A 109:7841–7846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Eggermont A, Robert C, Soria JC. and Zitvogel L. (2014). Harnessing the immune system to provide long-term survival in patients with melanoma and other solid tumors. Oncoimmunology 3:e27560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Enderlin M, Kleinmann EV, Struyf S, Buracchi C, Vecchi A, Kinscherf R, Kiessling F, Paschek S, Sozzani S, et al. (2009). TNF-alpha and the IFN-gamma-inducible protein 10 (IP-10/CXCL-10) delivered by parvoviral vectors act in synergy to induce antitumor effects in mouse glioblastoma. Cancer Gene Ther 16:149–160 [DOI] [PubMed] [Google Scholar]
- 163.Al-Zoubi M, Salem AF, Martinez-Outschoorn UE, Whitaker-Menezes D, Lamb R, Hulit J, Howell A, Gandara R, Sartini M, et al. (2013). Creating a tumor-resistant microenvironment: cell-mediated delivery of TNFα completely prevents breast cancer tumor formation in vivo. Cell Cycle 12:480–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Perlstein B, Finniss SA, Miller C, Okhrimenko H, Kazimirsky G, Cazacu S, Lee HK, Lemke N, Brodie S, et al. (2013). TRAIL conjugated to nanoparticles exhibits increased anti-tumor activities in glioma cells and glioma stem cells in vitro and in vivo. Neuro Oncol 15:29–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Wu GS. (2009). TRAIL as a target in anti-cancer therapy. Cancer Lett 285:1–5 [DOI] [PubMed] [Google Scholar]
- 166.Menon LG, Kelly K, Yang HW, Kim SK, Black PM. and Carroll RS. (2009). Human bone marrow-derived mesenchymal stromal cells expressing S-TRAIL as a cellular delivery vehicle for human glioma therapy. Stem Cells 27:2320–2330 [DOI] [PubMed] [Google Scholar]
- 167.Sasportas LS, Kasmieh R, Wakimoto H, Hingtgen S, van de Water JA, Mohapatra G, Figueiredo JL, Martuza RL, Weissleder R. and Shah K. (2009). Assessment of therapeutic efficacy and fate of engineered human mesenchymal stem cells for cancer therapy. Proc Natl Acad Sci U S A 106:4822–4827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Yang B, Wu X, Mao Y, Bao W, Gao L, Zhou P, Xie R, Zhou L. and Zhu J. (2009). Dual-targeted antitumor effects against brainstem glioma by intravenous delivery of tumor necrosis factor-related, apoptosis-inducing, ligand-engineered human mesenchymal stem cells. Neurosurgery 65:610–624 [DOI] [PubMed] [Google Scholar]
- 169.Moniri MR, Sun XY, Rayat J, Dai D, Ao Z, He Z, Verchere CB, Dai LJ. and Warnock GL. (2012). TRAIL-engineered pancreas-derived mesenchymal stem cells: characterization and cytotoxic effects on pancreatic cancer cells. Cancer Gene Ther 19:652–658 [DOI] [PubMed] [Google Scholar]
- 170.Mohr A, Albarenque SM, Deedigan L, Yu R, Reidy M, Fulda S. and Zwacka RM. (2010). Targeting of XIAP combined with systemic mesenchymal stem cell-mediated delivery of sTRAIL ligand inhibits metastatic growth of pancreatic carcinoma cells. Stem Cells 28:2109–2120 [DOI] [PubMed] [Google Scholar]
- 171.Luetzkendorf J, Mueller LP, Mueller T, Caysa H, Nerger K. and Schmoll HJ. (2010). Growth inhibition of colorectal carcinoma by lentiviral TRAIL-transgenic human mesenchymal stem cells requires their substantial intratumoral presence. J Cell Mol Med 14:2292–2304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Mauri DN, Ebner R, Montgomery RI, Kochel KD, Cheung TC, Yu GL, Ruben S, Murphy M, Eisenberg RJ, et al. (1998). LIGHT, a new member of the TNF superfamily, and lymphotoxin alpha are ligands for herpesvirus entry mediator. Immunity 8:21–30 [DOI] [PubMed] [Google Scholar]
- 173.Zhu X, Su D, Xuan S, Ma G, Dai Z, Liu T, Tang D, Mao W. and Dong C. (2013). Gene therapy of gastric cancer using LIGHT-secreting human umbilical cord blood-derived mesenchymal stem cells. Gastric Cancer 16:155–166 [DOI] [PubMed] [Google Scholar]
- 174.Yu P, Lee Y, Liu W, Chin RK, Wang J, Wang Y, Schietinger A, Philip M, Schreiber H. and Fu YX. (2004). Priming of naive T cells inside tumors leads to eradication of established tumors. Nat Immunol 5:141–149 [DOI] [PubMed] [Google Scholar]
- 175.Yu P, Lee Y, Wang Y, Liu X, Auh S, Gajewski TF, Schreiber H, You Z, Kaynor C, Wang X. and Fu YX. (2007). Targeting the primary tumor to generate CTL for the effective eradication of spontaneous metastases. J Immunol 179:1960–1968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Yu P. and Fu YX. (2008). Targeting tumors with LIGHT to generate metastasis-clearing immunity. Cytokine Growth Factor Rev 19:285–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Zou W, Zheng H, He TC, Chang J, Fu YX. and Fan W. (2012). LIGHT delivery to tumors by mesenchymal stem cells mobilizes an effective antitumor immune response. Cancer Res 72:2980–2989 [DOI] [PubMed] [Google Scholar]
- 178.Yang ZS, Tang XJ, Guo XR, Zou DD, Sun XY, Feng JB, Luo J, Dai LJ. and Warnock GL. (2014). Cancer cell-oriented migration of mesenchymal stem cells engineered with an anticancer gene (PTEN): an imaging demonstration. Onco Targets Ther 7:441–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Shimono Y, Mukohyama J, Nakamura S. and Minami H. (2016). MicroRNA regulation of human breast cancer stem cells. J Clin Med 5:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Cuiffo BG, Campagne A, Bell GW, Lembo A, Orso F, Lien EC, Bhasin MK, Raimo M, Hanson SE, et al. (2014). MSC-regulated microRNAs converge on the transcription factor FOXP2 and promote breast cancer metastasis. Cell Stem Cell 15:762–774 [DOI] [PubMed] [Google Scholar]
- 181.Wang M, Zhao C, Shi H, Zhang B, Zhang L, Zhang X, Wang S, Wu X, Yang T, et al. (2014). Deregulated microRNAs in gastric cancer tissue-derived mesenchymal stem cells: novel biomarkers and a mechanism for gastric cancer. Br J Cancer 110:1199–1210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Sung SY, Liao CH, Wu HP, Hsiao WC, Wu IH, Jinpu , Yu , Lin SH. and Hsieh CL. (2013). Loss of let-7 microRNA upregulates IL-6 in bone marrow-derived mesenchymal stem cells triggering a reactive stromal response to prostate cancer. PLoS One 8:e71637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Chen Y, Gao DY. and Huang L. (2014). In vivo delivery of miRNAs for cancer therapy: challenges and strategies. Adv Drug Deliv Rev 81C:128–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Gandhi NS, Tekade RK. and Chougule MB. (2014). Nanocarrier mediated delivery of siRNA/miRNA in combination with chemotherapeutic agents for cancer therapy: current progress and advances. J Control Release 194C:238–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Lee JK, Park SR, Jung BK, Jeon YK, Lee YS, Kim MK, Kim YG, Jang JY. and Kim CW. (2013). Exosomes derived from mesenchymal stem cells suppress angiogenesis by down-regulating VEGF expression in breast cancer cells. PLoS One 8:e84256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Kim TM, Huang W, Park R, Park PJ. and Johnson MD. (2011). A developmental taxonomy of glioblastoma defined and maintained by MicroRNAs. Cancer Res 71:3387–3399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Lee HK, Finniss S, Cazacu S, Bucris E, Ziv-Av A, Xiang C, Bobbitt K, Rempel SA, Hasselbach L, et al. (2013). Mesenchymal stem cells deliver synthetic microRNA mimics to glioma cells and glioma stem cells and inhibit their cell migration and self-renewal. Oncotarget 4:346–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Shimbo K, Miyaki S, Ishitobi H, Kato Y, Kubo T, Shimose S. and Ochi M. (2014). Exosome-formed synthetic microRNA-143 is transferred to osteosarcoma cells and inhibits their migration. Biochem Biophys Res Commun 445:381–387 [DOI] [PubMed] [Google Scholar]
- 189.Xu Y, Xia F, Ma L, Shan J, Shen J, Yang Z, Liu J, Cui Y, Bian X, Bie P. and Qian C. (2011). MicroRNA-122 sensitizes HCC cancer cells to adriamycin and vincristine through modulating expression of MDR and inducing cell cycle arrest. Cancer Lett 310:160–169 [DOI] [PubMed] [Google Scholar]
- 190.Katakowski M, Zheng X, Jiang F, Rogers T, Szalad A. and Chopp M. (2010). MiR-146b-5p suppresses EGFR expression and reduces in vitro migration and invasion of glioma. Cancer Invest 28:1024–1030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Katakowski M, Buller B, Zheng X, Lu Y, Rogers T, Osobamiro O, Shu W, Jiang F. and Chopp M. (2013). Exosomes from marrow stromal cells expressing miR-146b inhibit glioma growth. Cancer Lett 335:201–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Shin KK, Lee AL, Kim JY, Lee SY, Bae YC. and Jung JS. (2012). miR-21 modulates tumor outgrowth induced by human adipose tissue-derived mesenchymal stem cells in vivo. Biochem Biophys Res Commun 422:633–638 [DOI] [PubMed] [Google Scholar]
- 193.Kefas B, Floyd DH, Comeau L, Frisbee A, Dominguez C, Dipierro CG, Guessous F, Abounader R. and Purow B. (2013). A miR-297/hypoxia/DGK-α axis regulating glioblastoma survival. Neuro Oncol 15:1652–1663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Ono M, Kosaka N, Tominaga N, Yoshioka Y, Takeshita F, Takahashi RU, Yoshida M, Tsuda H, Tamura K. and Ochiya T. (2014). Exosomes from bone marrow mesenchymal stem cells contain a microRNA that promotes dormancy in metastatic breast cancer cells. Sci Signal 7:ra63. [DOI] [PubMed] [Google Scholar]
- 195.Rani SB, Rathod SS, Karthik S, Kaur N, Muzumdar D. and Shiras AS. (2013). MiR-145 functions as a tumor-suppressive RNA by targeting Sox9 and adducin 3 in human glioma cells. Neuro Oncol 15:1302–1316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Teoh HK, Chong PP, Abdullah M, Sekawi Z, Tan GC, Leong CF. and Cheong SK. (2016). Small interfering RNA silencing of interleukin-6 in mesenchymal stromal cells inhibits multiple myeloma cell growth. Leuk Res 40:44–53 [DOI] [PubMed] [Google Scholar]
- 197.Dai T, Yang E, Sun Y, Zhang L, Zhang L, Shen N, Li S, Liu L, Xie Y, Wu S. and Gao Z. (2013). Preparation and drug release mechanism of CTS-TAX-NP-MSCs drug delivery system. Int J Pharm 456:186–194 [DOI] [PubMed] [Google Scholar]
- 198.Pessina A, Leonetti C, Artuso S, Benetti A, Dessy E, Pascucci L, Passeri D, Orlandi A, Berenzi A, et al. (2015). Drug-releasing mesenchymal cells strongly suppress B16 lung metastasis in a syngeneic murine model. J Exp Clin Cancer Res 34:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Pacioni S, D'Alessandris QG, Giannetti S, Morgante L, De Pascalis I, Coccè V, Bonomi A, Pascucci L, Alessandri G, et al. (2015). Mesenchymal stromal cells loaded with paclitaxel induce cytotoxic damage in glioblastoma brain xenografts. Stem Cell Res Ther 6:194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Porada CD. and Almeida-Porada G. (2010). Mesenchymal stem cells as therapeutics and vehicles for gene and drug delivery. Adv Drug Deliv Rev 62:1156–1166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Roger M, Clavreul A, Venier-Julienne MC, Passirani C, Sindji L, Schiller P, Montero-Menei C. and Menei P. (2010). Mesenchymal stem cells as cellular vehicles for delivery of nanoparticles to brain tumors. Biomaterials 31:8393–83401 [DOI] [PubMed] [Google Scholar]
- 202.Li L, Guan Y, Liu H, Hao N, Liu T, Meng X, Fu C, Li Y, Qu Q, et al. (2011). Silica nanorattle-doxorubicin-anchored mesenchymal stem cells for tumor-tropic therapy. ACS Nano 5:7462–7470 [DOI] [PubMed] [Google Scholar]
- 203.Roger M, Clavreul A, Huynh NT, Passirani C, Schiller P, Vessières A, Montero-Menei C. and Menei P. (2012). Ferrociphenol lipid nanocapsule delivery by mesenchymal stromal cells in brain tumor therapy. Int J Pharm 423:63–68 [DOI] [PubMed] [Google Scholar]
- 204.Bonomi A, Sordi V, Dugnani E, Ceserani V, Dossena M, Coccè V, Cavicchini L, Ciusani E, Bondiolotti G, et al. (2015). Gemcitabine-releasing mesenchymal stromal cells inhibit in vitro proliferation of human pancreatic carcinoma cells. Cytotherapy 17:1687–1695 [DOI] [PubMed] [Google Scholar]
- 205.Tang Q, Chen Q, Lai X, Liu S, Chen Y, Zheng Z, Xie Q, Maldonado M, Cai Z, et al. (2013). Malignant transformation potentials of human umbilical cord mesenchymal stem cells both spontaneously and via 3-methycholanthrene induction. PLoS One 8:e81844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Wang Y, Zhang Z, Chi Y, Zhang Q, Xu F, Yang Z, Meng L, Yang S, Yan S, et al. (2013). Long-term cultured mesenchymal stem cells frequently develop genomic mutations but do not undergo malignant transformation. Cell Death Dis 4:e950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Bernardo ME, Zaffaroni N, Novara F, Cometa AM, Avanzini MA, Moretta A, Montagna D, Maccario R, Villa R, et al. (2007). Human bone marrow derived mesenchymal stem cells do not undergo transformation after long-term in vitro culture and do not exhibit telomere maintenance mechanisms. Cancer Res 67:9142–9149 [DOI] [PubMed] [Google Scholar]
- 208.Meza-Zepeda LA, Noer A, Dahl JA, Micci F, Myklebost O. and Collas P. (2008). High-resolution analysis of genetic stability of human adipose tissue stem cells cultured to senescence. J Cell Mol Med 12:553–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Chen G, Yue A, Ruan Z, Yin Y, Wang R, Ren Y. and Zhu L. (2014). Human umbilical cord-derived mesenchymal stem cells do not undergo malignant transformation during long-term culturing in serum-free medium. PLoS One 9:e98565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Buddingh EP, Ruslan SE, Reijnders CM, Szuhai K, Kuijjer ML, Roelofs H, Hogendoorn PC, Egeler RM, Cleton-Jansen AM. and Lankester AC. (2015). Mesenchymal stromal cells of osteosarcoma patients do not show evidence of neoplastic changes during long-term culture. Clin Sarcoma Res 5:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.von Bahr L, Batsis I, Moll G, Hägg M, Szakos A, Sundberg B, Uzunel M, Ringden O. and Le Blanc K. (2012). Analysis of tissues following mesenchymal stromal cell therapy in humans indicates limited long-term engraftment and no ectopic tissue formation. Stem Cells 30:1575–1578 [DOI] [PubMed] [Google Scholar]
- 212.Jeong JO, Han JW, Kim JM, Cho HJ, Park C, Lee N, Kim DW. and Yoon YS. (2011). Malignant tumor formation after transplantation of short-term cultured bone marrow mesenchymal stem cells in experimental myocardial infarction and diabetic neuropathy. Circ Res 108:1340–1347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Rosland GV, Svendsen A, Torsvik A, Sobala E, McCormack E, Immervoll H, Mysliwietz J, Tonn JC, Goldbrunner R, et al. (2009). Long-term cultures of bone marrow-derived human mesenchymal stem cells frequently undergo spontaneous malignant transformation. Cancer Res 69:5331–5339 [DOI] [PubMed] [Google Scholar]
- 214.Rubio D, Garcia S, Paz MF, De la Cueva T, Lopez-Fernandez LA, Lloyd AC, Garcia-Castro J. and Bernad A. (2008). Molecular characterization of spontaneous mesenchymal stem cell transformation. PLoS One 3:e1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Rubio R, Abarrategi A, Garcia-Castro J, Martinez-Cruzado L, Suarez C, Tornin J, Santos L, Astudillo A, Colmenero I, et al. (2014). Bone environment is essential for osteosarcoma development from transformed mesenchymal stem cells. Stem Cells 32:1136–1148 [DOI] [PubMed] [Google Scholar]
- 216.Velletri T, Xie N, Wang Y, Huang Y, Yang Q, Chen X, Chen Q, Shou P, Gan Y, et al. (2016). P53 functional abnormality in mesenchymal stem cells promotes osteosarcoma development. Cell Death Dis 7:e2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Wagner W. (2012). Implications of long-term culture for mesenchymal stem cells: genetic defects or epigenetic regulation? Stem Cell Res Ther 3:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Zheng Y, He L, Wan Y. and Song J. (2013). H3K9me-enhanced DNA hypermethylation of the p16INK4a gene: an epigenetic signature for spontaneous transformation of rat mesenchymal stem cells. Stem Cells Dev 22:256–267 [DOI] [PubMed] [Google Scholar]
- 219.Borghesi A, Avanzini MA, Novara F, Mantelli M, Lenta E, Achille V, Cerbo RM, Tzialla C, Longo S, et al. (2013). Genomic alterations in human umbilical cord-derived mesenchymal stromal cells call for stringent quality control before any possible therapeutic approach. Cytotherapy 15:1362–1373 [DOI] [PubMed] [Google Scholar]
- 220.Garcia S, Bernad A, Martín MC, Cigudosa JC, Garcia-Castro J. and de la Fuente R. (2010). Pitfalls in spontaneous in vitro transformation of human mesenchymal stem cells. Exp Cell Res 316:1648–1650 [DOI] [PubMed] [Google Scholar]
- 221.Torsvik A, Rosland GV. and Bjerkvig R. (2012). Spontaneous transformation of stem cells in vitro and the issue of cross-contamination. Int J Biol Sci 8:1051–1052; discussion 1053–1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.He L, Zheng Y, Wan Y. and Song J. (2014). A shorter telomere is the key factor in preventing cultured human mesenchymal stem cells from senescence escape. Histochem Cell Biol 142:257–267 [DOI] [PubMed] [Google Scholar]
- 223.Kono K, Takada N, Yasuda S, Sawada R, Niimi S, Matsuyama A. and Sato Y. (2015). Characterization of the cell growth analysis for detection of immortal cellular impurities in human mesenchymal stem cells. Biologicals 43:146–149 [DOI] [PubMed] [Google Scholar]
- 224.Patel SA, Meyer JR, Greco SJ, Corcoran KE, Bryan M. and Rameshwar P. (2010). Mesenchymal stem cells protect breast cancer cells through regulatory T cells: role of mesenchymal stem cell-derived TGF-β. J Immunol 184:5885–5894 [DOI] [PubMed] [Google Scholar]
- 225.Ljujic B, Milovanovic M, Volarevic V, Murray B, Bugarski D, Przyborski S, Arsenijevic N, Lukic ML. and Stojkovic M. (2013). Human mesenchymal stem cells creating an immunosuppressive environment and promote breast cancer in mice. SciRep 3:2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Kansy BA, Dißmann PA, Hemeda H, Bruderek K, Westerkamp AM, Jagalski V, Schuler P, Kansy K, Lang S, Dumitru CA. and Brandau S. (2014). The bidirectional tumor-mesenchymal stromal cell interaction promotes the progression of head and neck cancer. Stem Cell Res Ther 5:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.de Boeck A, Pauwels P, Hensen K, Rummens JL, Westbroek W, Hendrix A, Maynard D, Denys H, Lambein K, Braems G. and Gespach C. (2013). Bone marrow-derived mesenchymal stem cells promote colorectal cancer progression through paracrine neuregulin 1/HER3 signalling. Gut 62:550–560 [DOI] [PubMed] [Google Scholar]
- 228.Huang WH, Chang MC, Tsai KS, Hung MC, Chen HL. and Hung SC. (2013). Mesenchymal stem cells promote growth and angiogenesis of tumors in mice. Oncogene 32:4343–4354 [DOI] [PubMed] [Google Scholar]
- 229.Mandel K, Yang Y, Schambach A, Glage S, Otte A. and Hass R. (2013). Mesenchymal stem cells directly interact with breast cancer cells and promote tumor cell growth in vitro and in vivo. Stem Cells Dev 22:3114–3127 [DOI] [PubMed] [Google Scholar]
- 230.Karnoub AE, Dash AB, Vo AP, Sullivan A, Brooks MW, Bell GW, Richardson AL, Polyak K, Tubo R. and Weinberg RA. (2007). Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature 449:557–563 [DOI] [PubMed] [Google Scholar]
- 231.Bergfeld SA, Blavier L. and DeClerck YA. (2014). Bone marrow–derived mesenchymal stromal cells promote survival and drug resistance in tumor cells. Mol Cancer Ther 13:962–975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Chen DR, Lu DY, Lin HY. and Yeh WL. (2014). Mesenchymal stem cell-induced doxorubicin resistance in triple negative breast cancer. Biomed Res Int 2014:532161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Fletcher JI, Williams RT, Henderson MJ, Norris MD. and Haber M. (2016). ABC transporters as mediators of drug resistance and contributors to cancer cell biology. Drug Resist Updat 26:1–9 [DOI] [PubMed] [Google Scholar]
- 234.Ji R, Zhang B, Zhang X, Xue J, Yuan X, Yan Y, Wang M, Zhu W, Qian H. and Xu W. (2015). Exosomes derived from human mesenchymal stem cells confer drug resistance in gastric cancer. Cell Cycle 14:2473–2483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Vianello F, Villanova F, Tisato V, Lymperi S, Ho KK, Gomes AR, Marin D, Bonnet D, Apperley J, Lam EW. and Dazzi F. (2010). Bone marrow mesenchymal stromal cells non-selectively protect chronic myeloid leukemia cells from imatinib-induced apoptosis via the CXCR4/CXCL12 axis. Haematologica 95:1081–1089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Waterman RS, Tomchuck SL, Henkle SL. and Betancourt AM. (2010). A new mesenchymal stem cell (MSC) paradigm: polarization into a pro-inflammatory MSC1 or an Immunosuppressive MSC2 phenotype. PLoS One 5:e10088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Waterman RS, Henkle SL. and Betancourt AM. (2012). Mesenchymal stem cell 1 (MSC1)-based therapy attenuates tumor growth whereas MSC2-treatment promotes tumor growth and metastasis. PLoS One 7:e45590. [DOI] [PMC free article] [PubMed] [Google Scholar]