Abstract
Background
Irritable bowel syndrome (IBS) costs the National Health Service almost £12 million per annum. Despite national guidelines advising primary care management, these have failed to stem secondary care referrals of patients with likely IBS for unnecessary and costly assessment and investigation without necessarily achieving resolution of their symptoms.
Methods
In 2011, an integrated team from primary and secondary care developed a business case using baseline data to create a Somerset-wide IBS pathway using Clinical Commissioning Group funding. This provided face-to-face general practitioners (GP) education, developed a diagnostic pathway and funded faecal calprotectin (FC) testing to exclude inflammatory pathology for patients aged 16–45 years with likely IBS and no alarm symptoms. For those with FC≤50 μg/g, we provided a management algorithm and community-based dietetic treatment. Audit results measured usage and outcomes from FC testing, changes in patterns and costs of new patients reviewed in gastroenterology outpatients and dietetic IBS treatment outcomes.
Results
The proportion of new patient slots used reduced from 14.3% to 8.7% over 10 months while overall costs reduced by 25% for patients with no alarm symptoms and likely IBS aged 16–45 years. FC results confirmed research findings with no inflammatory pathology, if FC≤50 μg/g over 2 years. 63% of patients had satisfactory control of their IBS after specialist dietetic input with 74% reporting improved quality of life.
Conclusions
The combination of GP education, providing diagnosis and management pathways, using FC to exclude inflammatory pathology and providing an effective treatment for patients with likely IBS appeared successful in our pilot. This proved cost-effective, reduced secondary care involvement and improved patient care.
Keywords: DIET, FUNCTIONAL BOWEL DISORDER, IRRITABLE BOWEL SYNDROME, NUTRITION, QUALITY OF LIFE
Introduction
Irritable bowel syndrome (IBS) is a chronic and debilitating condition, which places a significant burden on the National Health Service (NHS), both in terms of financial cost and strain on primary and secondary care.1–3 The total attributable cost of IBS in the UK was almost £12 million per annum in 2012–2013. Despite the National Institute for Health and Care Excellence (NICE) and British Society of Gastroenterology guidelines recommending that IBS management should take place within primary care,4 5 a significant proportion of patients aged 16–45 years with likely IBS are still referred to secondary care despite a low probability of pathology. Research suggests that general practitioners (GP) still see IBS as a diagnosis of exclusion often due to uncertainty about diagnosis and in the belief that negative diagnostic tests are useful.5–7 Overall demands for inpatient and outpatient diagnostic endoscopies are increasing steadily.1 Hence, better GP education around diagnosis of IBS within primary care, along with an effective management pathway, should lead to direct NHS savings, which would allow secondary care gastroenterology services to target their resources more effectively.
Methods
In 2011, an integrated Flexible Healthcare Gastroenterology Clinical Team was created in Somerset with the aim of breaking down barriers between organisations to improve patient care. This team contained clinicians and managers from secondary care gastroenterology (Taunton and Somerset NHS Foundation Trust, Yeovil District NHS Foundation Trust) community dietetics (Somerset Partnership NHS Foundation Trust), GPs and Somerset Clinical Commissioning Group (SCCG).
To obtain a snapshot of the care available within gastroenterology, new outpatient slots in secondary care in Taunton were audited in May 2011 to determine number of patients aged 16–45 years referred from primary care with likely IBS and no alarm symptoms. IBS was diagnosed using Rome III criteria.8 See online supplementary files S1 and S2.
flgastro-2016-100727supp_files.pdf (264.6KB, pdf)
Using these audit results and extrapolating the expenditure over a 24-month period to include all patients covered by Yeovil and Taunton Hospital catchment areas, this group of low-risk patients would have cost the NHS in excess of £161,000. See online supplementary file S3.
In order to investigate the ‘revolving door’ effect of ongoing symptoms leading to repeated referral and investigation, which is well-described in IBS,9 an audit of six consecutive months (from 1 October 2011 to 31 March 2012) of new patient referrals to gastroenterology secondary care outpatient clinics in Taunton and Somerset NHS Foundation Trust for those aged 16–45 years with no alarm symptoms was undertaken. This aimed to establish the percentage that had been seen in the previous 5 years, for either gastroenterology outpatient review, radiological or endoscopic investigations for the same symptoms.
In 2011, a community dietitian based in primary care in South Somerset undertook a pilot project with patients with IBS referred directly from local GPs. This pilot study looked at outcomes using dietary intervention including the low fermentable oligosaccharides, disaccharides, monosaccharides and polyols (FODMAP) diet.10–13 For 55 patients, informal feedback data on postintervention quality of life were recorded by asking each patient, ‘Have the results of dietary intervention improved your quality of life?’, with 69% (38/55) responding positively.
After collecting baseline data, the focus of the future project was to empower GPs to make a positive diagnosis of IBS in order to avoid secondary care referrals. This was done by providing GPs with the following:
‘Diagnosis of IBS’ algorithm supplied as an innovative desktop app (see online supplementary appendix 1).
‘Management of IBS’ algorithm supplied as an innovative desktop app (see online supplementary appendix 2).
County-wide GP teaching sessions led by a specialist dietitian and/or consultant gastroenterologist.
Provide faecal calprotectin (FC) testing to exclude inflammatory pathologies in patients aged 16–45 years with likely IBS and thus avoiding secondary care referrals in those with FC ≤50 μg/g.
Provide an innovative community-based dietetic-led gastroenterology service using dietary interventions such as the low FODMAP diet for patients with IBS with no alarm symptoms, normal blood tests and FC results and intractable symptoms.
flgastro-2016-100727supp_appendices.pdf (943.2KB, pdf)
Overall, the project aimed to identify those patients who despite symptoms were at low risk of pathology, and gear the focus of their management towards symptom control using specialist dietetic input to avoid expensive and unnecessary secondary care referral and investigations in a time of limited resources. The money saved by avoiding secondary care referrals and investigations would be used to fund FC testing in primary care and specialist dietetic time. The figures from within our business case are shown in table 1.
Table 1.
Cost of new pathway with associated savings comparisons using secondary care costs based on secondary care audit data collected in May 2011
| Costs of FC testing at £31 per test on a cost per case basis based on funding for up to 350 tests | £10 850 |
| Cost of dietetics service with 15% on costs, band six specialist dietitian and 0.2 WTE administrator support | £48 003 |
| Total cost of new pathway | £58 853 |
| Present annual secondary care costs for patients with likely IBS aged 16–45 years with no alarm symptoms when seen in secondary care | £161 198 |
| Savings comparison | £102 345 |
FC, faecal calprotectin; IBS, irritable bowel syndrome; WTE, whole time equivalent.
The electronic FC request form included a compulsory pop-up audit, allowing review of the indications for the test. Use of a single laboratory for FC testing rather than point-of-care testing allowed consistency of reporting and assay (Barnsley and Rotherham Joint Pathology Services using the Immundiagnostik ELISA test). The result was returned directly to the GP as a paper copy with basic advice around levels obtained. Using the laboratory reference range and based on information available in 2011–2012, a level ≤50 µg/g was chosen to avoid referral to secondary care.14 15 Our approach was later endorsed by NICE.20
First-line dietetic advice was supplied either by Somerset Partnership NHS Trust community dietitians based within each GP practice in the county or directly by GPs. If symptoms proved intractable, then onward referral to the specialist dietitian-led gastroenterology community clinic could be made by community dietitians, GPs and other community-based healthcare professionals. Secondary care teams were not given access to this clinic as the business case revolved around avoiding referral to secondary care, reinforcing to GPs that IBS should be managed in primary care. Having specialist dietitians in a primary care setting was an important safety step as they were able to reassess patients for alarm symptoms and return the patient to their GP if they felt that further investigation in secondary care gastroenterology was needed.
These new specialist dietetic clinics provided the capacity to see 30 new referrals per month with each patient requiring two appointments with an 8-week gap between them. To assess the outcomes, patients filled in symptom evaluation forms at the start and end of treatment. The evaluation form was based on a data collection tool for assessing patient primary outcomes, using the internationally validated Gastrointestinal Symptom Rating Scale-IBS scoring system.7 16 An additional validated global symptom satisfaction question used as the current standard in IBS trials was included in the evaluation form,17 along with informal feedback data on quality of life.
SCCG agreed funding in June 2012 with an initial audit planned at 1 year. Outcome data were collected for the new dietetic service from 1 May 2013 to 30 April 2016, and analysis of FC testing ran from 1 November 2013 to 31 October 2015. The audit of outpatient data was repeated 10 months into the FC testing project (August 2014) to inform the end of first year analysis for SCCG.
Results
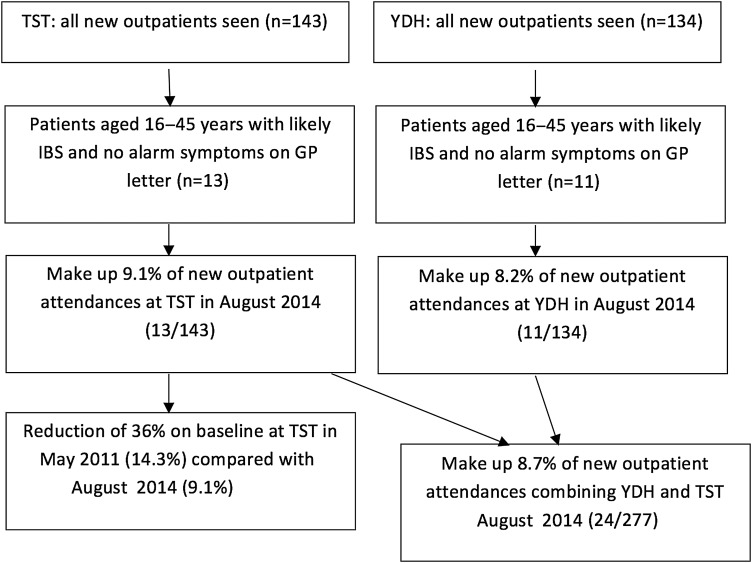
The secondary care outpatient data analysis originally carried out in Taunton in May 2011 (figure 1) was repeated in August 2014 for Taunton and Yeovil to see if the pilot study using FC testing and dietetic treatment had made any difference to the use of new patient slots in gastroenterology clinics. This analysis determined the number of relevant patients and established investigations undertaken, overall outcome and costs.
Figure 1.
Audit data for new outpatient attendances in August 2014 following GP referral at Taunton and Somerset NHS Foundation Trust (TST) and Yeovil District NHS Foundation Trust (YDH) aged 16–45 years with no alarm symptoms and likely irritable bowel syndrome (IBS).
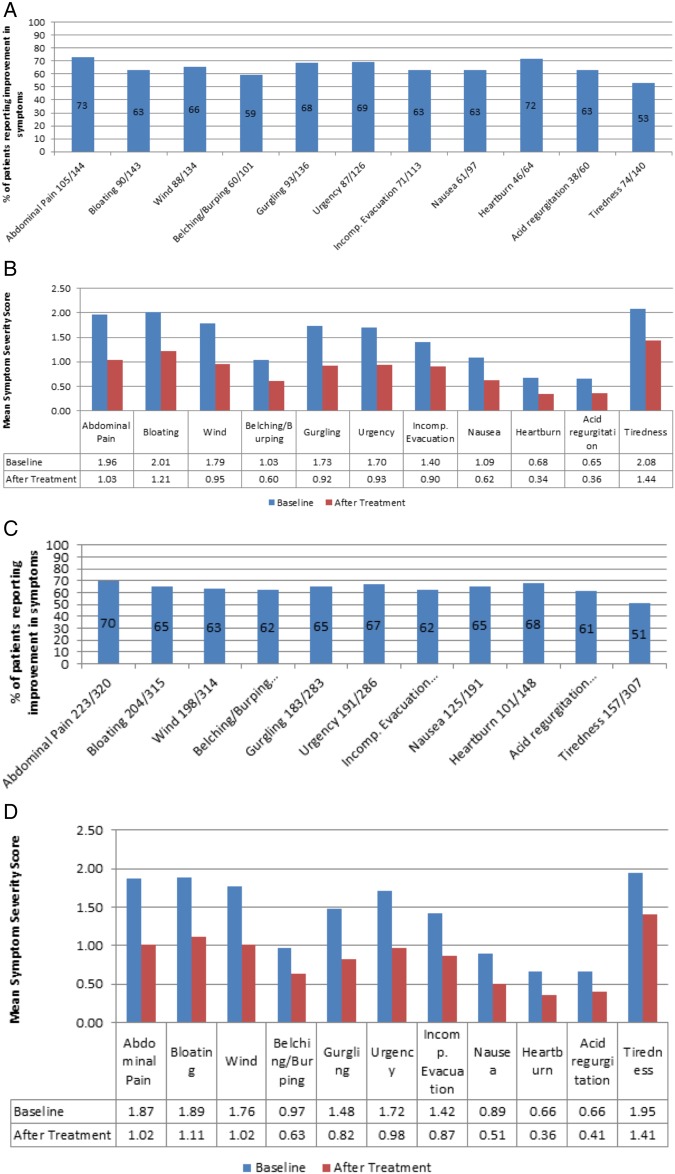
Figure 2.
A, B, C, D show outcome results for individual symptoms using GSRS-IBS scoring system and mean symptom severity scores before and after IBS dietetic management who completed treatment between 1 May 2013 and 30 April 2016. A and B (n=146) patients aged 16-45 years. C and D (n=335) adult patients of any age.
When figures from both hospitals were extrapolated over 12 months, the total costs from these patients were approximately £120 000, including their outpatient slot, endoscopy, radiology and serological investigations. This was a 25% saving over baseline compared with costs from the 2011 Taunton audit data of approximately £161 000. The overall reduction in referrals of patients aged 16–45 years with likely IBS from GPs to secondary care using Taunton data was 36%.
The potential revolving door data collected using the audit of 6 months of new patient slots for patients aged 16–45 years with likely IBS and no alarm symptoms showed that of the 117 patients fitting these criteria, 38 had been seen previously within the last 5 years for endoscopy, radiology or gastroenterology review (32.5%).
Over 2 years, 308 FC tests were requested by primary care as part of this project. Analysis showed that pathology was highly unlikely unless FC >150 µg/g, with no gastrointestinal pathology found at levels ≤50 µg/g. These data should reinforce the confident use of this pathway in the future (table 2). For those patients with FC >50 µg/g where referral did not take place, contact was made either by telephone or letter with their GP to determine their current gastrointestinal health. This helped to educate GPs and encourage them to seek a secondary care opinion if patients had FC ≥50 µg/g and their symptoms remained troublesome.
Table 2.
Audit results for GP requests for faecal calprotectin levels across Somerset from 1 November 2013 to 31 October 2015 (n=308 patients)
| Referral to secondary care | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| FC result | Number of tests | Referral made | Referral not made | Number of patients with GI pathology identified if referred and investigated | ||||||
| Total | 308 | Yes | Referred but DNA appointment(s) | No | Patient refused referral/investigation | Moved away/lost to follow-up | Infection present in microbiology stool sample | UC/Crohn's disease | Other GI pathology | Normal |
| ≤50 μg/g | 210 | 43 | 6 | 167 | – | – | 0 | 0 | 0 | 37 |
| 51–150 μg/g | 63 | 38 | 5 | 19 (one awaiting repeat FC) | – | 3 | 3 | 1 (tiny localised aphthoid ulcers in rectosigmoid histology non-diagnostic) | One panniculitis one bile acid malabsorption | 30 |
| ≥151 μg/g | 35 | 31 | 1 | 4 | 2 | 0 | 2 | 11 | Two bile acid malabsorption | 17 |
FC, faecal calprotectin; GI, gastrointestinal; UC, ulcerative colitis.
In the case of 12 out of 13 patients with FC 50–150 µg/g who were not referred to secondary care, on checking with their GPs, there had been no other relevant consultations and the GP added a file note to their records in each case. One patient awaits a repeat FC. For those cases where coexistent infection was discovered, symptoms settled after antibiotics.
Our specialist community gastroenterology dietitians received their referrals from around Somerset, providing clinics in four separate locations throughout the county to allow for increased patient access. Only those aged 16–45 years, who had completed their dietary intervention period and who were referred specifically for management of IBS, have been included in the data (n=146). The mean age was 32 years with 83% female predominance.
Using the validated global primary outcome question, ‘Do you have satisfactory relief from your IBS symptoms?’, of those who noted ‘no’ at the initial appointment (n=139), 63% were then able to respond with ‘yes’ following dietary intervention.
Graphs A–D show outcome results and mean symptom severity scores for dietetic intervention for patients referred for IBS management who had completed dietary treatment. Graphs A and B: n=146 aged 16–45 years; graphs C and D: n=335 all patients.
Graph A shows that over 70% of patients reported improvement in abdominal pain and heartburn. Urgency also responded well to dietary intervention with 69% of patients reporting improvement. Using the Wilcoxon signed-rank test, graph B shows that all symptoms showed a statistically significant reduction following dietetic intervention with all p values <0.001.
When considering the informal feedback on the improvement in quality of life, 74% (108/146) noted that dietary intervention had improved their quality of life with comments such as, ‘This diet has turned my life around’, ‘I am so grateful that something has finally worked to help me’.
In total, the specialist dietetic clinic saw 335 patients of all ages over the data collection period and graphs C and D show results for all patients indicating similar trends to those in the age group of 16–45 years. The mean age overall was 48.1 with 84% female predominance. The similarity of these data imply that IBS dietary management is effective for all age groups irrespective of the referral source, although for older patients, pathological causes of their symptoms would need ruling out by endoscopic or radiological means.
Discussion
Despite the 2008 IBS NICE guidance, research shows that referrals of patients with IBS to secondary care are steadily increasing at a national level.1 5 However, by using cost-effective patient-focused care, this pathway has managed to reverse that trend with a reduction in combined referrals and costs in two district general hospitals.
While many centres have focused on using FC to triage secondary care referrals after outpatient review, we understand that this is the first pathway which has piloted the funding of GP education, aided diagnosis by using FC to exclude inflammatory pathology, provided an effective treatment plan and used the cost savings which have been realised across an integrated healthcare community to fund specialist dietetic services based in the community.
While the 2008 NICE report highlighted that IBS should be managed within primary care, they also noted that healthcare professionals would need to be appropriately trained in IBS diagnostic criteria, if the guidance was to be successfully implemented.5 Hence, the focus of the Somerset project was to educate GPs and to give them the necessary tools to help their patients with IBS.
As this project has taken several years of negotiation and a business case to reach the project stage from its initial inception in 2011, more UK data are now available regarding the use of FC to reduce unnecessary referral and investigation where the result is likely to be negative.18 19 What is reassuring is that subsequent NICE advice (DG11) has validated our initial chosen cut-off level for FC to rule out inflammatory disease (<50 µg/g).20 While use of FC reduces unnecessary endoscopic investigations, for those patients who are referred with FC levels of 50–150 µg/g, there is still a low likelihood of pathology and in these cases, an outpatient review can determine the right course of action. An alternative approach which is supported by recent evidence, is to repeat the FC for equivocal cases in 6–8 weeks and refer only if the result remains elevated.21
Other areas have found that with time GPs tend to extend their use of the FC test to include patients where they would otherwise have been happy to make a clinical diagnosis, potentially negating any financial benefits.18 To counteract this in Somerset, we have insisted that FC should be used only if the patient is aged between 16 and 45 years, with no alarm symptoms and would otherwise be referred to secondary care. All referrals require completion of a pop-up GP audit form in order to obtain authorisation and ongoing GP education is essential.
Dietetic outcomes were encouraging and our data have contributed to Kings College London research in long-term outcomes, where it has been shown that 70% of patients continue to have satisfactory relief 6–18 months postdischarge.22 Nevertheless, there will be a significant number of patients who do not respond to dietary and lifestyle measures for a variety of reasons. We have made basic suggestions about using medication in our ‘management of IBS’ pathway (see online supplementary appendix 2). However, we should consider hypnotherapy and cognitive behavioural therapy within this pathway as a future development as these have been endorsed by the NICE Clinical Guideline 61 published in 2015.12 These were not included in our initial pathway as their availability was limited in Somerset and would have required additional funding and appointment of new staff.
Since 2012, we have received over 35 enquiries from across the UK from other primary and secondary care teams to find out how this project was set up and funded both for the specialist dietetic clinic and the overall pathway. In August 2014, a survey (n=31) of those making enquiries revealed that despite using our data to make a strong business case, only 12 were offering a dedicated IBS dietetic service with just 6 of these being established as a specialist dietetic-led community gastroenterology clinic. Interestingly, 46% of the present or planned dietetic services were to be placed within secondary care which may negate any cost-efficiency benefit as secondary care referral may raise expectations around investigation and medical review in outpatients. One of the strengths of our project was to avoid referral into secondary care to prevent this happening. For those centres without a service, 92% cited ‘lack of funding’ as the main obstacle, while 29% cited lack of secondary care support amid concerns about missing underlying pathology such as cancer and losing money from secondary care services to provide funding.23
The pathway used in Somerset was established in the knowledge that there is a general community dietitian in every GP practice in the county. Hence, to gatekeep referrals into the specialist gastroenterology dietetic service, first-line advice was given by these general community dietitians or GPs themselves. However, it is acknowledged that the situation may differ in other areas of the UK and hence the pathway may need to be altered according to local needs. Indeed, first-line advice could be given in dietitian-led group sessions, or GPs can use the British Dietetic Association IBS first-line information sheet, which is endorsed by NICE and is freely available on the internet.24
Overall, the suspicion that in 2011 our secondary care outpatient clinics were not effectively managing symptoms with little reassurance or lasting benefit from normal test results was confirmed by an appreciable revolving door effect which necessitated a new approach. This IBS pathway was set up as a pragmatic way to manage increasing demand for secondary care services, by providing an alternative cost-effective patient-centred solution.
Conclusion
Our model highlights that there is no single solution to IBS and that it is essential to have the multidisciplinary approaches of effective dietetic intervention, reliable faecal biomarkers and GP education in order to encourage long-term and effective patient self-management. With this combination it is possible to have a successful and cost-effective pathway, which delivers significant NHS savings and also high levels of patient satisfaction in a community setting while allowing secondary care gastroenterology services to target their resources more effectively.
Key messages.
What is already known on this topic?
In 2008, the National Institute for Health and Care Excellence Clinical Guideline 61 advised that irritable bowel syndrome (IBS) diagnosis and management should take place in primary care; however, referrals to secondary care have continued to rise for patients with likely IBS despite a low probability of finding pathology if investigations are carried out.
The low FODMAP diet has gained increasing credibility for managing IBS symptoms, when provided by specifically trained dietitians.
What this study adds?
Providing general practitioners (GP) education along with diagnosis and management pathways can lead to a reduction in secondary care referrals.
Dietary intervention works to significantly reduce all IBS symptoms in patients of all ages when advice is given by appropriately trained dietitians.
How might it impact on clinical practice in the foreseeable future?
Supply GP with an alternative pathway for patients with IBS to help reduce secondary care referrals.
Encourage the use of audited faecal calprotectin testing as a reliable tool within primary care when ruling out inflammatory bowel disease in patients aged 16–45 years with likely IBS and no alarm symptoms.
Encourage the use of primary care-based specialist dietetic intervention for patients with IBS.
Encourage Clinical Commissioning Group to set up IBS management within primary care to prevent unnecessary secondary care referrals and investigation and thus reduce National Health Service costs.
Footnotes
Acknowledgements: Richard Blackwell, Information Analysis Manager, South West Academic Health Science Network, UK. Dr David James, Consultant Chemical Pathologist, Taunton and Somerset NHS Foundation Trust, UK. Dr Tom Johnston, Specialty Registrar in Gastroenterology, Severn Deanery, UK. Dumiso Ncube, Directorate Manager, Yeovil District NHS Foundation Trust, UK.
Contributors: All authors were responsible for design of study and critical revisions to the manuscript. MW, LS, EG, SG: data collection. LS, EG, SG: data analysis. EG, MW: authorship and original idea.
Competing interests: EG: non-financial support to cover travel to, accommodation for and attendance of European Crohn’s and Colitis Organisation meeting funded by Ferring 20–22 February 2014 and 16–18 February 2012. EG: Financial payment for attendance of Shire GI Advisory Board 2008. EG: Financial payment for attendance of Ferring Inflammatory Bowel Disease Advisory Board 2007.
Provenance and peer review: Not commissioned; internally peer reviewed.
References
- 1.Soubieres A, Wilson P, Poullis A, et al. . Burden of irritable bowel syndrome in an increasingly cost-aware National Health Service. Frontline Gastroenterol 2015;6:229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maxion-Bergemann S, Thielecke F, Abel F, et al. . Costs of irritable bowel syndrome in the UK and US. Pharmacoeconomics 2006;24:21–37. doi:10.2165/00019053-200624010-00002 [DOI] [PubMed] [Google Scholar]
- 3.Thompson WG, Heaton KW, Smyth GT, et al. . Irritable bowel syndrome in general practice: prevalence, characteristics, and referral. Gut 2000;46:78–82. doi:10.1136/gut.46.1.78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.British Society of Gastroenterology. IBS/Functional Symptoms: Commissioning Report: Clinical 2014. http://www.bsg.org.uk/clinical/commissioning-report/ibs/functional-symptoms.html
- 5.National Institute for Health and Care Excellence. Irritable Bowel Syndrome: Costing report implementing NICE guidance. CG61 Manchester: NICE, 2008. https://www.nice.org.uk/guidance/cg61/resources/costing-report-196660189 [Google Scholar]
- 6.Bellini M, Tosetti C, Costa F, et al. . The general practitioners approach to irritable bowel syndrome: from intention to practice. Dig Liver Dis 2005;37:934–9. doi:10.1016/j.dld.2005.06.011 [DOI] [PubMed] [Google Scholar]
- 7.Spiegel BM, Farid M, Esrailian E, et al. . Is irritable bowel syndrome a diagnosis of exclusion? A survey of primary care providers, gastroenterologists and IBS experts. Am J Gastroenterol 2010;105:848–58. doi:10.1038/ajg.2010.47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drossman DA, Dumitrascu DL. Rome III: new standard for functional gastrointestinal disorders. J Gastrointestin Liver Dis 2006;15:237–41. [PubMed] [Google Scholar]
- 9.Hungin APS, Whorwell PJ, Tack J, et al. . The Prevalence, patterns and impact of irritable bowel syndrome: an international survey of 40,000 subjects. Aliment Pharmacol Ther 2003;17:643–50. doi:10.1046/j.1365-2036.2003.01456.x [DOI] [PubMed] [Google Scholar]
- 10.Staudacher HM, Whelan K, Irving PM, et al. . Comparison of symptom response following advice for a diet low in fermentable carbohydrates (FODMAPs) versus standard dietary advice in patients with irritable bowel syndrome. J Hum Nutr Diet 2011;24:487–95. doi:10.1111/j.1365-277X.2011.01162.x [DOI] [PubMed] [Google Scholar]
- 11.Gibson PR, Shepherd SJ. Evidence-based dietary management of functional gastrointestinal symptoms: The FODMAP approach. J Gastroenterol Hepatol 2010;25:252–8. doi:10.1111/j.1440-1746.2009.06149.x [DOI] [PubMed] [Google Scholar]
- 12.National Institute for Health and Care Excellence. Irritable bowel syndrome in adults: diagnosis and management of irritable bowel syndrome in primary care. CG61. Manchester: NICE, 2015. [Google Scholar]
- 13.McKenzie YA, Alder A, Anderson W, et al. . British Dietetic Association evidence-based guidelines for the dietary management of irritable bowel syndrome in adults. J Hum Nutr Diet 2012;25:260–74. doi:10.1111/j.1365-277X.2012.01242.x [DOI] [PubMed] [Google Scholar]
- 14.Map of Medicine. Irritable bowel syndrome (IBS). International View. London: Map of Medicine, 2010. [Google Scholar]
- 15.NHS Purchasing and Supply Agency. Centre for Evidence-based Purchasing. Economic report: value of calprotectin in screening out irritable bowel syndrome. London, 2010. [Google Scholar]
- 16.Svedlund J, Sjödin I, Ottosson JO,et al. . Controlled study of psychotherapy in irritable bowel syndrome. Lancet 1983;589–92. doi:10.1016/S0140-6736(83)90678-5 [DOI] [PubMed] [Google Scholar]
- 17.Wiklund IK, Fullerton S, Hawkey CJ, et al. . An irritable bowel syndrome-specific symptom questionnaire: development and validation. Scand J Gastroenterol 2003;38:947–54. doi:10.1080/00365520310004209 [DOI] [PubMed] [Google Scholar]
- 18.Pavlidis P, Chedgy FJ, Tibble JA. Diagnostic accuracy and clinical application of faecal calprotectin in adult patients presenting with gastrointestinal symptoms in primary care. Scand J Gastroenterol 2013;48:1048–54. doi:10.3109/00365521.2013.816771 [DOI] [PubMed] [Google Scholar]
- 19.Tavabie OD, Hughes SA, Loganayagam A. The role of faecal calprotectin in the differentiation of organic from functional bowel disorders. Br J Gen Pract 2014;64:595–6. doi:10.3399/bjgp14X682525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Institute for Health and Care Excellence. Faecal calprotectin diagnostic tests for inflammatory diseases of the b owel. DG11. Manchester: NICE, 2013. https://www.nice.org.uk/guidance/dg11 [Google Scholar]
- 21.Seenan JP, Thomson F, Rankin K, et al. . Are we exposing patients with a mildly elevated faecal calprotectin to unnecessary investigations? Frontline Gastroenteroly 2015;6:156–60. doi:10.1136/flgastro-2014-100467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martin LvVC, Seamark L, Williams M, et al. . Low FODMAP advice for patients with irritable bowel syndrome: long-term outcomes for symptoms and dietary intake. Gut 2015;OC-104(Supp 1):A51. [Google Scholar]
- 23.Williams M. Issues in establishing a dietetic-led gastroenterology clinic. Personal Audit Data, 2014. [Google Scholar]
- 24.McKenzie Y, Reeves L, Williams M. Irritable Bowel Syndrome and Diet—Food Fact Sheet. British Dietetic Association, 2016. https://www.bda.uk.com/foodfacts/IBSfoodfacts.pdf [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
flgastro-2016-100727supp_files.pdf (264.6KB, pdf)
flgastro-2016-100727supp_appendices.pdf (943.2KB, pdf)