Abstract
Objective
Significant regional variation in surgical rates has been identified following multiple surgical procedures. However, limited data have examined the regional variability in patient selection and treatment of abdominal aortic aneurysms (AAA). This study aims to evaluate regional variation in patient selection, perioperative management and operative approach to AAA.
Methods
All patients undergoing open or endovascular repair (EVAR) of an AAA in the Vascular Quality Initiative from 2009 to 2014 were identified. All regional groups were de-identified, and those with less than 100 open repairs were combined into a single region.
Results
17,269 elective repairs (EVAR: 13,759, Open: 3,510) and 1,462 ruptured AAAs (EVAR:749, Open:713) were identified. There was significant regional variation in the use of EVAR for elective (Range: 66–88% P < .01) and ruptured AAA (40–80% P < .01). The median diameter for elective repair was similar among regions (EVAR: 5.4 cm, Open: 5.7 cm). There was wide variation in the treatment of small aneurysms in male patients (<5.5 cm) for EVAR (34–49% P < .01) and open repair (17–38% P < .01) and variation in the treatment of small aneurysms in female patients (<5 cm) for EVAR (14–32% P < .01), but not significant for open repair (6–24%). For elective cases, pre-operative aspirin (EVAR: 50–75% P <.01; Open 49–78% P < .01) and statin use (EVAR: 61–75% P < .01, Open: 56–80% P < .01) varied widely. Among elective cardiac patients preoperative management varied significantly including beta-blocker use (EVAR: 66–78% P < .01, Open: 69–88%, P = 0.01) and the frequency of stress-tests (EVAR: 33–64% P < .01, Open: 31–73% P < .01). Among open repairs for aneurysms extending at or beyond the juxta-renal segment, there was wide variation in the use of retroperitoneal exposures (7–70%, P < .01) and adjunctive renal protective measures (cold renal perfusion: 2–43% P < .01, mannitol: 47–92%, P < .01).
Conclusion
Significant regional variation exists in patient selection, perioperative management, and operative approach for the repair of AAA. Definitive evidence is lacking in many aspects of operative care including the use of the retroperitoneal approach and renal protective strategies. However, this variation emphasizes the importance of research to determine best practice in the areas of greatest variation. Furthermore, where current clinical process measures exist and data is clear, such as the use of statin and antiplatelet agents, the high degree of variation should serve as an impetus for regional quality improvement projects.
Introduction
Regional variation in the rates of surgical procedures have been documented both in the United States and abroad, and despite increasing evidence evaluating the impact of regional variation, it does not appear to be decreasing.1–4 Research by Birkmeyer et al, and the Dartmouth Atlas of Health Care suggest that illness burden and diagnostic practices explain only a small degree of the variation seen, and the majority can be better correlated to physician belief about surgical indications and patient preferences.1, 4
Variation can be divided into two categories: acceptable and unwarranted. Variation that is acceptable includes patient demographics and operative techniques where best practice is undefined. Unwarranted variation occurs when evidence-based medicine has created clear benchmarks for care and variation in practice patterns still exists.1, 4 This type of variation is more concerning and can result in unnecessary adverse outcomes for patients. Reducing unwarranted variation represents a significant opportunity for improvement in morbidity and mortality as well as reductions in health care expenditures.5
In an effort to improve the quality of vascular care, the Society for Vascular Surgery released consensus statements for the care of patients with vascular disease aimed at guiding physician decision making by clearly defining best practices where strong evidence exists.6 Process measures specific to abdominal aortic aneurysms (AAA) include: pre-operative cardiac evaluation and noninvasive stress-tests for those with known cardiac disease or multiple risk factors, elective repair for aneurysms greater than 5.5 centimeters (cm) and consideration of repair for aneurysms 5–5.4 cm for women and young healthy patients. Additionally, recommendations for technique include an endovascular first approach for ruptured aneurysms when anatomically feasible, the use of a retroperitoneal approach for aneurysmal disease extending to the juxta-renal or visceral segment, and high resolution preoperative CT imaging to define proximal clamp location.6 However, despite the widespread publication of these guidelines, limited data have documented how well these benchmarks are being met.
In an effort to better understand regional variation in the surgical care of AAA, this study aims to evaluate the regional variation in patient selection, pre-operative management, and operative technique for the repair of AAAs. In addition to evaluating the impact of current process measures and the identification of quality improvement projects where compliance with best practice guidelines is variable, this study will also identify the areas of widest variation where future research is needed to define best practice.
Methods
Dataset
The Vascular Quality Initiative (VQI) was utilized to identify all patients undergoing endovascular (EVAR) or open repair for AAA from 2009 to 2014. Details about this registry can be found at http://www.vascularqualityinitiative.org/. At the time of this study, the VQI consisted of 16 regional quality groups across the United States. These geographic regions were organized according to regional affiliations, and do not reflect any known variation in patient characteristics. Each region was de-identified, and those regions performing less than 100 open aneurysms repairs were grouped into a single combined region. As a result of this grouping, this analysis has 14 regions. Intact and ruptured aneurysm repairs were evaluated separately. The Beth Israel Deaconess Medical Center Institutional Review Board approved this study, and patient consent was waived due to the de-identified nature of this dataset.
Variables
Patient demographics, comorbid conditions, pre-operative medical and cardiac management, and operative technique data were collected from all patients. All variables analyzed had less than 5% missing data, with the majority with less than 1% missing data. Small aneurysms were defined as less than 5.5 cm for men and less than 5 cm for women. Small aneurysms with concurrent iliac aneurysms greater than 3 cm were excluded from analysis of small aneurysms. Prior documented cardiac disease was defined as previous coronary artery bypass, percutaneous cardiac intervention, congestive heart failure, or coronary artery disease. Chlorhexidine skin preparation use was documented when it was used as a single agent or as part of a multiple antimicrobial surgical scrub.
Statistical Analysis
Statistical analysis was completed using the SPSS software package (version 21.0) and figures were produced using GraphPad (version 6.0). Chi-square was utilized for binary variables. Continuous variables were evaluated using ANOVA, Mann-Whitney or Kruskal-Wallace tests as appropriate. Forrest plots were utilized to represent the range of variables of interest by region including a symbol for each of the 14 regions studied. A vertical line was used to represent the median within the VQI. Pearson correlation testing was utilized to assess the relationship between the use of EVAR in the treatment of elective and ruptured AAA. Statistical significance was defined as P < 0.05.
Results
We identified 17,269 elective AAA repairs (13,759 EVAR and 3,510 open) and 1,462 ruptured AAAs (749 EVARs and 713 open). A total of 14 regions were analyzed including a single combined group that was composed of 3 regions performing fewer than 100 open AAA repairs each. The number of open AAA repairs ranged from 119 to 1,357 per region (median: 205). The range of endovascular repairs ranged from 375 to 3,491 (median: 907).
Patient Selection and Demographics
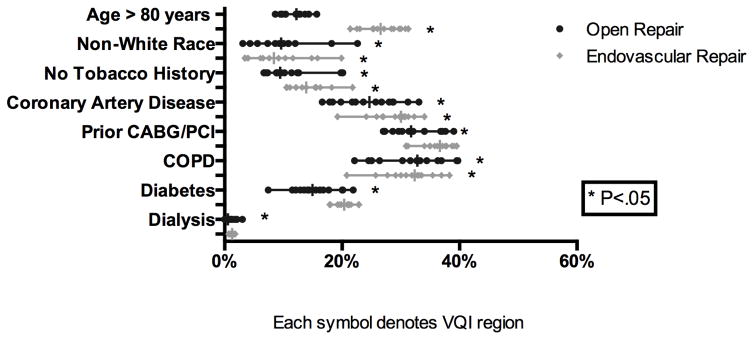
Among patients undergoing elective repair, male patients underwent the majority of EVAR (80%) and open repairs (73%) across all regions with no significant variation noted. The mean age of patients also did not vary significantly among regions (EVAR: 72–75 years, Open: 68–71 years). There was wide variation in the proportion of non-white patients treated with EVAR (3–20%, P < .01) and open repair (3–22%, P <. 01). Significant variation in patient comorbidities was also seen. The frequency of AAA repair among patients with coronary artery disease (EVAR: 19–34% P < .01, Open: 17–33% P < .01), prior cardiac intervention (EVAR: 31–40% P < .01, Open: 27–39% P < .01), and chronic obstructive pulmonary disease (EVAR: 21–38%, P < .01, Open: 22–40% P < .01) varied significantly for both EVAR and open repair respectively. Significant variation in the proportion with diabetes (7–22% P = 0.01) and patients on hemodialysis (0–3%, P < .01) was seen in open repair but not EVAR (Figure 1).
Figure 1.
Variation in Patient Selection (Presentation) for Elective Aortic Aneurysm Repair
Among patients treated for ruptured AAA there was no regional variation in gender with men representing 78% of patients undergoing EVAR and 77% of patients undergoing open repair. The mean age of patients treated with EVAR was 73 years (66–80, P = 0.03), and for open repair was 72 years (67–74, P = 0.08). Similar to elective repair, non-white race was widely variable across regions for both EVAR and open repair (EVAR: 0–29% P = 0.02, Open: 0–29%, P < .01). Among ruptured AAAs, regional variation was seen only in the proportion of hemodialysis noted pre-operatively (EVAR: 0–9% P = 0.04, Open: 0–5%, P = 0.04). Variation in the rates of coronary artery disease was significant for EVAR only (11–39%, P = 0.04). There was no significant regional variation in other demographic or comorbid conditions among patients treated with open repair (Supplement Figure 1).
Pre-operative Management
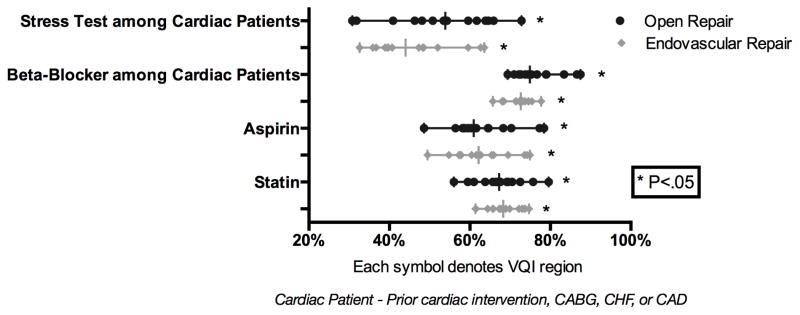
Prior to elective AAA repair there was wide variation across regions in treatment and work-up of patients. The preoperative use of aspirin and statins among patients undergoing elective AAA varied widely. Aspirin use ranged from 50–75% (P < .01) for EVAR and 49–78% (P < .01) for open repair. Statin use ranged from 56–80% prior to EVAR (P < .01) and 61–75% prior to open repair (P < .01). Despite SVS guidelines for the preoperative management of patients with known cardiac disease, there was also wide variation in the frequency of pre-operative stress testing (EVAR: 33–64% P < .01, Open: 31–73% P < .01) and the use of beta-blockers (EVAR: 66–78%, P < .01, Open: 69–88%, P = 0.01) (Figure 2). Given the emergent nature of ruptured AAA pre-operative medical management was not evaluated.
Figure 2.
Preoperative Management for Elective Aortic Aneurysm Repair
Operative Approach
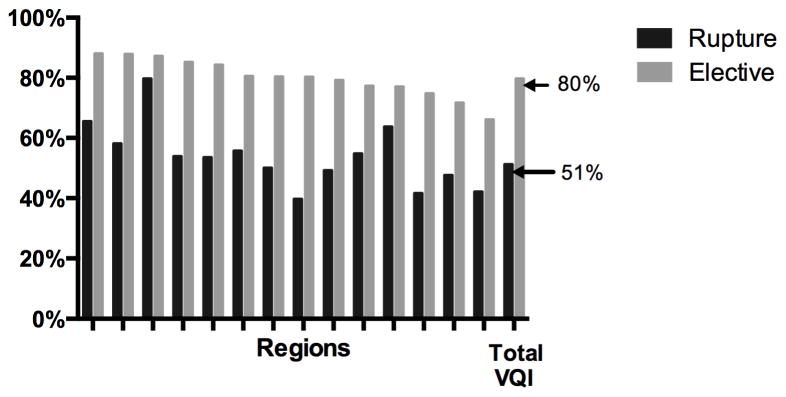
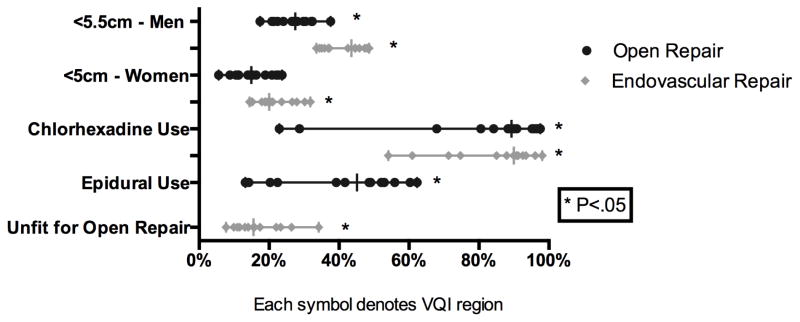
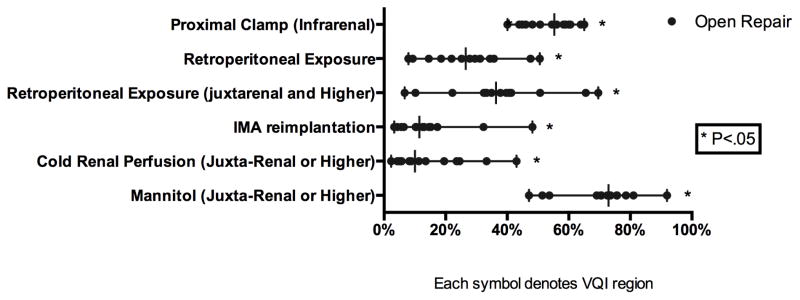
For elective repair there was significant variation in the use of EVAR (66–88% P < 0.01) (Figure 3). Additionally, the frequency of open repair for elective infra-renal aneurysms (40–65% P < .01) was also widely variable. Furthermore, there was significant variation in the percentage of AAA repairs performed for rupture (5–11%, P < .01). The use of EVAR for elective and ruptured cases was moderately correlated (R=0.6). The median aneurysm diameter for those undergoing elective repair was 5.4 cm (P < .01) for EVAR and 5.7 cm (P < .01) for open repair. Additionally, the treatment of small aneurysms, less than 5.5 cm, among male patients varied widely for both EVAR (34–49%, P < .01) and open repair (17–38%, P< .01). Among female patients, the treatment of small aneurysms, less than 5 cm, varied for EVAR (14–32% P < .01), but did not achieve significance among open repair (6–24% P = 0.22), likely due to smaller numbers (Figure 4). There was significant variation in the proportion of small aneurysms treated that were symptomatic among both men (2–12% P < .01) and women (3–33%, P = 0.01) (Supplement Figure 2). Chlorhexidine use also varied widely (EVAR: 54–98% P < .01, Open: 23–98% P < .01). Despite recommendations for the use of an epidural analgesia for open repair, epidural use was widely variable (13–62%, P < .01) (Figure 4). Among patients undergoing open repair the frequency of inferior mesenteric artery (IMA) re-implantation (3–32% P < .01) also varied significantly. Additionally, despite recommendations for retroperitoneal exposure for aneurysms that require clamping of the juxta-renal or visceral aortic segment, retroperitoneal exposure for such aneurysms (7–70%, P < .01) had significant regional variation. Finally, among patients requiring clamping of the juxta-renal or visceral aortic segment, there was extensive variation in the use of cold renal perfusion (2–43% P < .01) and mannitol (47–92%, P < .01) (Figure 5).
Figure 3.
Proportion of Repairs Completed with EVAR
Figure 4.
Operative Approach for Elective Aortic Aneurysm Repair
Figure 5.
Operative Technique for Elective Open Aortic Aneurysm Repair
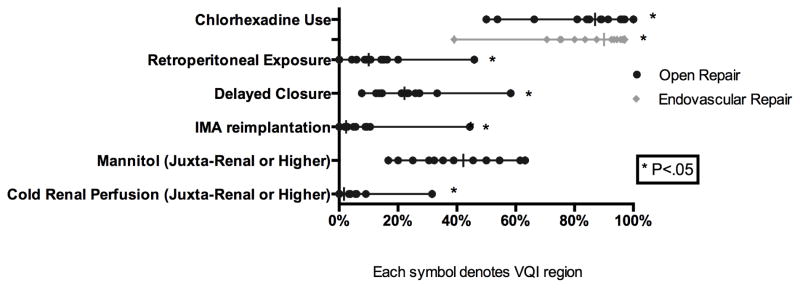
Among ruptured AAAs, the use of EVAR varied widely (40–80% P < .01) (Figure 3). The median aneurysm diameter did not vary for EVAR (7.1, P = 0.36) or open repair (7.9, P = 0.16). Similar to elective repairs, wide variation was seen the use of Chlorhexidine skin preparation (EVAR: 39–97% P < .01, Open: 50–100% P < .01). Among ruptured AAAs treated with open repair, delayed closure (8–58%, P = 0.03), retroperitoneal exposure (0–46%, P < .01), and IMA re-implantation (0–44% P < .01) were significantly variable. When aneurysms required a juxta-renal or visceral aortic clamp, the use of cold renal perfusion (0–32%, P < .01) was widely variable, while mannitol use (17–63%, P = 0.06) also varied, but did not achieve significance (Figure 6).
Figure 6.
Operative Technique for Ruptured Aortic Aneurysm Repair
Discussion
This study found that significant variation exists in patient selection, pre-operative management, and operative approach to AAA among arbitrarily selected geographic groups across the U.S. as represented in VQI. Some of the variation identified in this study is unwarranted and represents variation in clinical practice despite clearly defined clinical process measures that are meant to serve as benchmarks for quality of care. However, other variables represent acceptable variation and serve to identify important targets for future research where optimal treatment remains undefined.
Current SVS guidelines for the care of patients with abdominal aortic aneurysms recommend elective surgical repair of an AAA greater than 5.5 cm for men or 5 cm among women with surveillance for smaller aneurysms.6 This recommendation is supported by both the UK Small Aneurysm Trial and the ADAM trial which found no long-term survival benefit in the treatment of small aneurysms compared with surveillance.7, 8 However, despite these guidelines, significant regional variation exists in the elective treatment of small aneurysms among male and female patients. While symptomatic AAA may contribute to the rates of repair found in this study, the indication for repair is unclear for the majority of these small AAA. It is difficult to determine from the VQI the indication for elective repair, and circumstances such as recent enlargement, strong family history of rupture, saccular versus fusiform aneurysm, or patient preference may have also played roles in treating small aneurysms.
In addition to indication for surgery, pre-operative management of patients with cardiac disease is also defined by SVS guidelines, which suggest consideration of a non-invasive stress test for patients with known cardiac disease or multiple risk factors.6 While open repair patients with known cardiac disease were more likely to undergo a noninvasive stress test, there was wide variation in use among cardiac patients. It is possible that some of this variation may be attributed to utilization of the American Heart Association (AHA) guidelines, which also consider functional capacity in the determination for noninvasive stress tests. However, the AHA also suggests that exercise stress testing may be considered for patients undergoing elevated risk procedures.9
In addition to indications for treatment and pre-operative care, current literature recommends several important operative techniques to improve morbidity and mortality. Specifically, the SVS recommends an EVAR first approach for ruptured AAA in those with feasible anatomy.6 Despite extensive evidence supporting improved outcomes among patients treated with an EVAR first approach, the frequency of its use varied from 40–80%.10–12 Issues related to transfer eligibility, surgeon experience, and institutional resources may contribute to the ability to perform EVAR for ruptured aneurysms.13 Evidence also exists regarding the superiority of Chlorhexidine over povidone-iodine or alcohol-based iodine paint for the prevention of surgical site infections.14–16 However the utilization of Chlorhexidine remains below 50% in multiple regions regardless of operative approach, and no formal recommendation regarding Chlorhexidine use exists for the treatment of AAA.
Open surgical approach for aneurysms involving the visceral or renal arteries is also widely variable despite a SVS recommendation for retroperitoneal exposure in this setting.6 It should be noted that this recommendation is based on a low quality of evidence, and while several studies have identified increased return of bowel function, fewer incisional hernias, and improved visualization with this approach the results are still mixed.6, 17–19 This may explain the on-going variation and represents an important area for future research.
While pre-operative management may be clearly defined in the surgical literature, it is important to note that some of the variation seen in this study can be classified as acceptable. Specifically, the variation in patient demographics and co-morbidities likely reflect diverse and variable patient populations seen in different geographic areas of the United States, as well as a variable prevalence of specific disease processes which reflect the diversity among regions. Additionally, there are many areas of practice where best practice is currently unknown. These include the use of renal protective strategies, as well as the previously noted controversy over the widespread use of the retroperitoneal approach for open aneurysms.
This study found a wide range of both acceptable and unwarranted variation in patient selection and the treatment of AAA within the VQI. Much variation exists in clinical areas where strong evidence to guide best practice is lacking, and this highlights a clear need for targeted research efforts moving forward. Unwarranted variation; however is more challenging to address due to its likely multifactorial causes. Given that physician belief and preference is the most common cause of variation, efforts to improve dissemination and education are paramount.1, 5 Previous studies have shown that distribution of updated systemic reviews have been successful in achieving moderate improvement (9–14%) in practice performance.5 An additional proven mechanism is performance feedback using clinical process measures and peer performance, which has been successful in improving physician practice in both the VQI and cardiac registries.20, 21 Finally, system-level interventions including funding restriction and regulatory changes can effect physician practice; however this can lead to significant and unintended adverse consequences including reduction in overall quality and access to care for both necessary and unwarranted surgery.5
This study has several notable limitations. First, this is a retrospective analysis of all hospitals participating in the VQI, and is not population based. Limitations associated with this database include a potential for coding error, missing data, and self-reporting at each site. However, in an effort to minimize these factors, the VQI completes an annual audit and review of billing data to confirm reported records. Additionally, the VQI may not include all advanced technology used in the assessment and measurement of aneurysm size, therefore it is possible that aneurysm size may not be estimated differently based on the practice of each center. An additional limitation of this study is a lack of uniformity in the regions evaluated. Specifically, the number and size of hospitals in each region are variable suggesting smaller regions may be impacted by large centers. Finally, this analysis does not adjust for geographic variation in patient comorbidities. However, this decision was made to allow data to be compared to SVS guidelines that do not adjust for variation in epidemiologic trends among regions.
Conclusion
Despite well-defined evidence-based guidelines, significant variation exists across regions in the United States in the pre-operative management AAA patients with cardiac risk factors, the use of EVAR for ruptured AAA, and operative approach for the repair of AAA. This variation suggests that the publication of guidelines alone are not sufficient to enact significant change in practice patterns, and further efforts to improve dissemination and physician feedback are necessary. Additionally, evidence is lacking to guide many aspects of operative practice including adjunctive therapy for supra-renal clamping and exposure technique. These topics of high regional variation serve as important targets for future research to determine best practice.
Supplementary Material
Footnotes
Presented at 41st Annual Meeting of the New England Society for Vascular Surgery, Boston, MA, September 11-14th 2014.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Birkmeyer JD, Reames BN, McCulloch P, Carr AJ, Campbell WB, Wennberg JE. Understanding of regional variation in the use of surgery. Lancet. 2013;382(9898):1121–9. doi: 10.1016/S0140-6736(13)61215-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hanstede MM, Burger MJ, Timmermans A, Burger MP. Regional and temporal variation in hysterectomy rates and surgical routes for benign diseases in the Netherlands. Acta Obstet Gynecol Scand. 2012;91(2):220–5. doi: 10.1111/j.1600-0412.2011.01309.x. [DOI] [PubMed] [Google Scholar]
- 3.Matlock DD, Groeneveld PW, Sidney S, Shetterly S, Goodrich G, Glenn K, et al. Geographic variation in cardiovascular procedure use among Medicare fee-for-service vs Medicare Advantage beneficiaries. JAMA. 2013;310(2):155–62. doi: 10.1001/jama.2013.7837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goodney PR, Dzebisashvili N, Goodman DC, Bronner KK. Variation in the Care of Surgical Conditions. The Dartmouth Institute for Health Policy and Clinical Practice; 2015. [PubMed] [Google Scholar]
- 5.McCulloch P, Nagendran M, Campbell WB, Price A, Jani A, Birkmeyer JD, et al. Strategies to reduce variation in the use of surgery. Lancet. 2013;382(9898):1130–9. doi: 10.1016/S0140-6736(13)61216-7. [DOI] [PubMed] [Google Scholar]
- 6.Chaikof EL, Brewster DC, Dalman RL, Makaroun MS, Illig KA, Sicard GA, et al. The care of patients with an abdominal aortic aneurysm: The Society for Vascular Surgery practice guidelines. J Vasc Surg. 2009;50(4):S2–S49. doi: 10.1016/j.jvs.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 7.Participants UKSAT. Long-term outcomes of immediate repair compared with surveillance of small abdominal aortic aneurysms. N Engl J Med. 2002;346(19):1445–52. doi: 10.1056/NEJMoa013527. [DOI] [PubMed] [Google Scholar]
- 8.Lederle FA, Wilson SE, Johnson GR, Reinke DB, Littooy FN, Acher CW, et al. Immediate repair compared with surveillance of small abdominal aortic aneurysms. N Engl J Med. 2002;346(19):1437–44. doi: 10.1056/NEJMoa012573. [DOI] [PubMed] [Google Scholar]
- 9.Fleisher LA, Fleischmann KE, Auerbach AD, Barnason SA, Beckman JA, Bozkurt B, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;130(24):2215–45. doi: 10.1161/CIR.0000000000000105. [DOI] [PubMed] [Google Scholar]
- 10.Moore R, Nutley M, Cina CS, Motamedi M, Faris P, Abuznadah W. Improved survival after introduction of an emergency endovascular therapy protocol for ruptured abdominal aortic aneurysms. J Vasc Surg. 2007;45(3):443–50. doi: 10.1016/j.jvs.2006.11.047. [DOI] [PubMed] [Google Scholar]
- 11.Starnes BW, Quiroga E, Hutter C, Tran NT, Hatsukami T, Meissner M, et al. Management of ruptured abdominal aortic aneurysm in the endovascular era. J Vasc Surg. 2010;51(1):9–17. doi: 10.1016/j.jvs.2009.08.038. discussion-8. [DOI] [PubMed] [Google Scholar]
- 12.Mehta M, Byrne J, Darling RC, III, Paty PSK, Roddy SP, Kreienberg PB, et al. Endovascular repair of ruptured infrarenal abdominal aortic aneurysm is associated with lower 30-day mortality and better 5-year survival rates than open surgical repair. J Vasc Surg. 2013;57(2):368–75. doi: 10.1016/j.jvs.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 13.Mell MW, Schneider PA, Starnes BW. Variability in transfer criteria for patients with ruptured abdominal aortic aneurysm in the western United States. J Vasc Surg. 2015;62(2):326–30. doi: 10.1016/j.jvs.2015.03.032. [DOI] [PubMed] [Google Scholar]
- 14.Dumville JC, McFarlane E, Edwards P, Lipp A, Holmes A, Liu Z. Preoperative skin antiseptics for preventing surgical wound infections after clean surgery. Cochrane Database Syst Rev. 2015;4:Cd003949. doi: 10.1002/14651858.CD003949.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Darouiche RO, Wall MJ, Jr, Itani KM, Otterson MF, Webb AL, Carrick MM, et al. Chlorhexidine-Alcohol versus Povidone-Iodine for Surgical-Site Antisepsis. N Engl J Med. 2010;362(1):18–26. doi: 10.1056/NEJMoa0810988. [DOI] [PubMed] [Google Scholar]
- 16.Kalish JA, Farber A, Homa K, Trinidad M, Beck A, Davies MG, et al. Factors associated with surgical site infection after lower extremity bypass in the Society for Vascular Surgery (SVS) Vascular Quality Initiative (VQI) J Vasc Surg. 2014;60(5):1238–46. doi: 10.1016/j.jvs.2014.05.012. [DOI] [PubMed] [Google Scholar]
- 17.Borkon MJ, Zaydfudim V, Carey CD, Brophy CM, Guzman RJ, Dattilo JB. Retroperitoneal repair of abdominal aortic aneurysms offers postoperative benefits to male patients in the Veterans Affairs Health System. Ann Vasc Surg. 2010;24(6):728–32. doi: 10.1016/j.avsg.2010.02.026. [DOI] [PubMed] [Google Scholar]
- 18.Twine CP, Lane IF, Williams IM. The retroperitoneal approach to the abdominal aorta in the endovascular era. J Vasc Surg. 2012;56(3):834–8. doi: 10.1016/j.jvs.2012.04.021. [DOI] [PubMed] [Google Scholar]
- 19.Cambria RP, Brewster DC, Abbott WM, Freehan M, Megerman J, LaMuraglia G, et al. Transperitoneal versus retroperitoneal approach for aortic reconstruction: a randomized prospective study. J Vasc Surg. 1990;11(2):314–24. doi: 10.1067/mva.1990.17353. discussion 24–5. [DOI] [PubMed] [Google Scholar]
- 20.De Martino RR, Hoel AW, Beck AW, Eldrup-Jorgensen J, Hallett JW, Upchurch GR, et al. Participation in the Vascular Quality Initiative is associated with improved perioperative medication use, which is associated with longer patient survival. J Vasc Surg. 2015;61(4):1010–9. doi: 10.1016/j.jvs.2014.11.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grover FL, Shroyer ALW, Hammermeister K, Edwards FH, Ferguson TB, Dziuban SW, et al. A Decade’s Experience With Quality Improvement in Cardiac Surgery Using the Veterans Affairs and Society of Thoracic Surgeons National Databases. Ann Surg. 2001;234(4):464–74. doi: 10.1097/00000658-200110000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.