Abstract
Objective
To examine the effects of antenatal steroids on severe intraventricular hemorrhage (IVH) in infants born during the IVH vulnerable period (<28 weeks gestational age) and to evaluate rates of IVH correlated with the time interval between treatment or retreatment and birth.
Study design
429 infants (< 28 weeks gestation), who delivered ≥24 hours after the first BMZ course (two doses), were divided into groups based on the interval between the first course of BMZ and delivery: <10 days or ≥10 days. The primary outcome was severe IVH. Multiple regression analyses were performed to adjust for potential confounders.
Results
392 infants delivered after a single BMZ course (312 delivered <10 days; 80 ≥10 days). The incidence of severe IVH was 17% for infants delivered ≥10 days and 7% for those delivered <10 days after a single BMZ course (aOR 4.16; 95% CI 1.59–10.87, p=0.004). 37 infants (born ≥10 days from the first BMZ course) received a second/rescue BMZ course. The incidence of severe IVH among infants receiving a second/rescue course was 8%, which was similar to the incidence among infants born <10 days (aOR 1.7; 95% CI 0.41–6.6, p=0.48).
Conclusion
In infants born before 28 weeks gestation, delivery ≥10 days from the first BMZ course is associated with a higher incidence of severe IVH; a second/rescue course may reverse this effect.
Keywords: intracranial hemorrhage, newborn, corticosteroids, premature birth
Although randomized controlled trials (RCTs) demonstrate that infants exposed to antenatal corticosteroids have a decreased incidence of death, respiratory distress syndrome (RDS) and severe grades (grades 3 and 4) (1) of intraventricular hemorrhage (IVH) (2), there are unanswered questions about the duration of the beneficial effects and whether retreatment is required. In vitro (3) and in vivo (4) studies suggest that BMZ’s beneficial effects on the preterm lung are reversible and begin to wane after the first week. In addition, a meta-analysis of the RCTs that examined the use of repeat doses of corticosteroids in women still at risk of preterm birth 1–2 weeks after an initial course found that retreatment decreases the incidence of RDS (5).
Whether the beneficial effects of antenatal corticosteroids on other morbidities (e.g., severe IVH) also wane with time is much less clear. None of the corticosteroid retreatment RCTs (5) found a difference in the incidence of severe IVH between infants who received a single course and those who received repeat dosing (5–11). Although these findings suggest that retreatment may not affect the incidence of severe IVH, and that the beneficial effects of corticosteroids on severe IVH may not wane with time, there are important considerations that need to be examined before one can accept this conclusion. Severe IVH occurs almost exclusively in infants born before 28 weeks gestation; the incidence is 27% among infants born below 26 weeks, but less than 2% among those born at 28 weeks or older (12). Unfortunately, the infants that delivered before 28 weeks gestation in the “retreatment” RCTs made up only 7% (range 0–16%) of the study population and the overall incidence of severe IVH was only 2.6% (range 0–5%) (6–11). Therefore, we designed an observational study to examine the effects of antenatal steroids on severe IVH in infants born during the IVH vulnerable period (<28 weeks gestation). We hypothesized that the beneficial effects of antenatal steroids on severe IVH are time-limited and wane with time, in neonates born before 28 weeks gestation, and that retreatment with a second course of antenatal BMZ can restore its beneficial effects.
METHODS
This project was approved by the University of California San Francisco’s Institutional Review Board. A single neonatologist prospectively evaluated and recorded the perinatal and neonatal risk factors and outcome measures from all infants born at ≤27–6/7 weeks’ gestation and admitted, within the first 24 hours of birth, to the William H. Tooley Intensive Care Nursery at the University of California San Francisco between January 1998 and December 2015. Infants with major congenital malformations were excluded. Six hundred sixty-seven patients were eligible for the study. Perinatal characteristics of the study population are listed in Table I (available at www.jpeds.com).
Table 1; online.
Characteristics of infants who received no betamethasone or betamethasone ≤6 hours prior to delivery and those who received a two-dose course of betamethasone ≥24 hours prior to delivery
| Characteristic | Time to delivery after 1st dose of betamethasone | ||
|---|---|---|---|
|
| |||
| None or ≤ 6 hours N= 183 |
≥24 hours N= 429 |
P value | |
|
| |||
| Birth year epoch (%) | 0.03 | ||
| 1998 – 2003 | 46 | 37 | |
| 2004 – 2009 | 36 | 35 | |
| 2010 – 2015 | 19 | 28 | |
|
| |||
| Preeclampsia (%) | 4 | 23 | <0.0001 |
|
| |||
| Gestational Diabetes (%) | 3 | 8 | 0.03 |
|
| |||
| Clinical Chorioamnionitis (%) | 14 | 26 | 0.02 |
|
| |||
| Cesarean Delivery (%) | 59 | 69 | 0.02 |
|
| |||
| Presentation at delivery (%) | 0.94 | ||
| Vertex | 59 | 60 | |
| Breech | 35 | 34 | |
| Transverse | 6 | 6 | |
|
| |||
| Multiple Birth (%) | 28 | 35 | 0.11 |
|
| |||
| Caucasian (%) | 35 | 48 | 0.02 |
|
| |||
| Outborn (%) | 80 | 9 | <0.0001 |
|
| |||
| Gestational age <26 weeks (%) | 58 | 39 | <0.0001 |
|
| |||
| Gestational age (mean ± SD) | 25.6 ± 1.2 | 26.1 ± 1.1 | <0.0001 |
|
| |||
| Small for gestational age (%) | 6 | 20 | <0.0001 |
|
| |||
| Birth weight (mean ± SD) | 830 ± 188 | 803 ± 197 | 0.12 |
|
| |||
| Male | 59 | 49 | 0.04 |
|
| |||
| 5 minute Apgar score ≥6 (%) | 55 | 71 | <0.0001 |
|
| |||
| Prophylactic indomethacin (%) | 72 | 70 | 0.77 |
|
| |||
| Early Onset Sepsis (%) | 4 | 5 | 0.64 |
Criteria used to evaluate specific neonatal and perinatal risk factors that may affect the incidence of severe IVH have been previously described (13, 14). Gestational age was determined by the date of last menstrual period and early ultrasounds (before 24 weeks gestation). If there were discrepancies, the early ultrasound dating was used. Intrauterine growth restriction was defined as birthweight less than the tenth-percentile for gestational age using the growth curves from Olsen et al (15).
Detailed descriptions of our approach to respiratory and hemodynamic support have been published elsewhere (13, 16, 17). Oxygen Saturation target limits for this population were 88–94% throughout the study period.
All infants were examined with serial bedside cranial ultrasounds that included a study on postnatal day 3 or 4, followed by weekly or biweekly studies for the first 4 weeks. A single neonatologist (RIC) prospectively reviewed all of the cranial ultrasound examinations with an ultrasonographer. IVH was classified using the four-level grading system (2). Grades 3 and 4 IVH were considered “severe IVH” (2).
Bronchopulmonary dysplasia (BPD) was defined by a modified physiologic room-air challenge test performed between 36 and 37 weeks postmenstrual age (18).
Necrotizing enterocolitis (NEC) was defined as Bell’s classification II or greater (this included NEC that was treated medically or surgically, and “spontaneous perforations” that occur before 7 days) (19).
The criteria for diagnosis, follow up and treatment of ROP have been previously described (14).
Infants were divided into groups depending on the interval between the first dose of the first course of antenatal BMZ and delivery. A complete course of antenatal BMZ consisted of two 12-mg doses that were administered 24 hours apart. Group A consisted of infants who were either never treated or delivered within 6 hours of the initiation of antenatal BMZ. Group B consisted of infants who delivered between 7 and 23 hours of the initiation of antenatal BMZ. Group C consisted of infants who delivered between 24 hours and 9 days (group C-1, between 24 and 47 hours; group C-2, between 48 hours and 7 days; and group C-3 between 8 and 9 days) after the initiation of antenatal BMZ. Group D consisted of infants who delivered 10 days or more after the initiation of antenatal BMZ.
Statistical analyses
Statistical analyses were performed using STATA (StataCorp. 2015. Stata Statistical Software: Release 14. College Station, TX: StataCorp LP). A Chi Square test was used to compare categorical baseline characteristics between infants who delivered at different times after the initiation of antenatal BMZ treatment. The Student t-test was used to compare continuous parametric variables (gestational age, birth weight).
Because our study period spanned 17 years, we were concerned that unmeasured changes in practice or risk factors may have occurred that could have affected the rates of our primary study outcome (severe IVH). Therefore, we created a categorical variable (“birth year epoch”) that divided the study period into the 3 epochs (1998–2003, 2004–2009, 2010–2015). We included the “birth year epoch” variable in all of our multivariate regression analyses to adjust for any unmeasured practice changes that may have occurred over time (see below).
We first examined the relationship between the time interval from the first dose of BMZ to delivery and neonatal morbidity by conducting a multivariate regression analysis. Logistic regression was used for categorical outcomes and linear regression was used for continuous outcome variables. Regression covariates were selected a priori. For these analyses the following covariates were included in the model: gestational age, birth year epoch, pregnancy complications (small for gestational age, multiple gestation, gestational diabetes, chorioamnionitis, preeclampsia), delivery mode, out born status and race. For all regression models, sensitivity analyses were conducted for other baseline characteristics (including male sex, prophylactic indomethacin and 5 minute Apgar score) that were not included in the primary models. Addition of these variables to the model did not change the point estimates.
We next investigated the waning effects of a complete (two-dose) course of BMZ on neonatal morbidity by examining a subset of our population who either delivered between 24 hours and 9 days (Group C), or delivered 10 days or more (Group D) after the initiation of antenatal BMZ. Multivariate regression model covariates were selected a prior and included gestational age, birth year epoch, small for gestation, preeclampsia, multiple gestation and gestational diabetes. Variation inflation factors were used to check for collinearity between variables in our models. If the distribution of an outcome variable was skewed, bootstrapping (with 100 repetitions) was employed to overcome the normality assumption of the linear regression model and bias corrected confidence intervals were calculated and reported.
For all statistical tests, a p-value of 0.05 was considered significant.
RESULTS
Among the 667 infants in the study, 28% delivered prior to or within 6 hours of the first dose of BMZ, 8% delivered between 7 and 23 hours of the first dose, and 64% delivered after completing the full two-dose course of BMZ (≥24 hours after the first dose). We first examined the relationship between neonatal morbidity and the interval from the first dose of BMZ to delivery to see how our population compared with previous study populations that were used to examine the effects of BMZ on neonatal morbidity. Infants who delivered after completing the full two-dose course of BMZ differed from those who delivered prior to or within 6 hours of receiving the first dose of BMZ in several of the perinatal baseline characteristics (Table I). These differences are similar to what have been observed in prior observational studies that have examined this issue (20–23). We used multivariate regression analyses to adjust for these confounders and found that delivery after completing the full two-dose BMZ course was associated with a significant decrease in the incidence of severe IVH, a decrease in the need for higher levels of respiratory support at 24 hours of life (both intubated-mechanical ventilation and Respiratory Severity Score), and a decrease in the incidence of death compared with infants who were inadequately treated with BMZ (Table II). There was no association between completing a two-dose course of BMZ and the incidence of BPD, NEC, or severe ROP (Table II). These findings are similar to those of previous studies that have examined the effects of BMZ in infants born before 28 weeks gestation (20–23).
Table 2.
Relationship between the time to delivery after the first dose of betamethasone and Neonatal Morbidities
| Neonatal Morbidity | Time to delivery after 1st dose of betamethasone | Unadjusted Odds ratio (or Coefficient) for Time to delivery ≥ 24 hours (95% CI) | Adjusted* Odds ratio (or Coefficient) for Time to delivery ≥24 hours (95% CI) | P value† | ||
|---|---|---|---|---|---|---|
| ≤6 hrs N= 183 |
7–23 hrs N = 55 |
≥24 hrs N = 429 |
||||
| Severe IVH‡ (%) | 35 | 19 | 9 | 0.18 (0.12, 0.29) | 0.13 (0.07, 0.27) | <0.0001 |
| Intubated-Mechanical Ventilation at 24h (%) | 88 | 67 | 76 | 0.45 (0.27, 0.73) | 0.47 (0.23, 0.98) | 0.043 |
| Respiratory Severity Score at 24h (mean ± SD)§ | 2.7±2.3 | 1.6 ± 0.6 | 2.1 ± 1.4 | −0.57 (−0.98, −0.26) | −0.42 (−0.72, − 0.01) | 0.014 |
| Death (%) | 35 | 25 | 17 | 0.39 (0.32, 1.25) | 0.25 (0.13, 0.47) | <0.0001 |
| BPD** (%) | 33 | 29 | 32 | 0.96 (0.62, 1.48) | 0.82 (0.43, 1.56) | 0.541 |
| BPD or death (%) | 55 | 47 | 42 | 0.58 (0.41, 0.82) | 0.46 (0.26, 0.79) | 0.005 |
| NEC†† (%) | 21 | 21 | 14 | 0.63 (0.38, 1.03) | 0.62 (0.30, 1.28) | 0.200 |
| NEC or death (%) | 43 | 31 | 26 | 0.45 (0.32, 0.65) | 0.34 (0.25, 0.65) | 0.001 |
| ROP‡‡ requiring treatment (%) | 16 | 19 | 12 | 0.76 (0.42, 1.36) | 1.30 (0.51, 3.12) | 0.615 |
Betamethasone ≤6 hours referent. Adjusted for gestational age, small for gestation, gestational diabetes, race, preeclampsia, multiple birth, outborn, chorioamnionitis, delivery mode and birth year epoch.
P value for adjusted odds ratio or coefficient.
Severe IVH, grade 3 or 4 among infants who survived at least 4 days. N= 639 (some infants died prior to the time the morbidity was able to be determined)
Respiratory Severity Score (RSS) = mean airway pressure x fraction of inspired oxygen. By convention, mean airway pressure = liter flow rate when infants received nasal cannula flows of ≤3 liter/min; measurements made at 24 hours after birth. N= 654 (some infants died prior to the 24-hour time point). Linear regression with bootstrapping coefficient and bias corrected confidence interval reported.
Bronchopulmonary Dysplasia (BPD), physiologic definition by room air challenge at 36 weeks post-menstrual age. N= 528 (some infants died prior to 36 weeks post- menstrual age)
Necrotizing enterocolitis (NEC), Bell’s classification ≥II (treated medically or surgically) or “spontaneous perforations”. N= 564 (some infants died prior to the time the morbidity was able to be determined, i.e., prior to 21 days postnatal age or prior to receiving enteral feedings greater than 80 ml/kg/day)
Retinopathy of Prematurity (ROP), requiring treatment. N= 524 (some infants died prior to the time the morbidity was able to be determined at 38 weeks post-menstrual age)
Our primary goal was to determine if the IVH lowering effects of BMZ might be time-limited, because this would increase the risk that a severe IVH might occur if an infant delivered more than a week (≥10 days) after BMZ treatment and still within the IVH vulnerable period (<28 weeks gestation). Four hundred twenty-nine infants were exposed to antenatal BMZ for ≥24 hours. The Figure shows the incidence of severe IVH stratified by the time to delivery after the first dose of BMZ.
Figure.
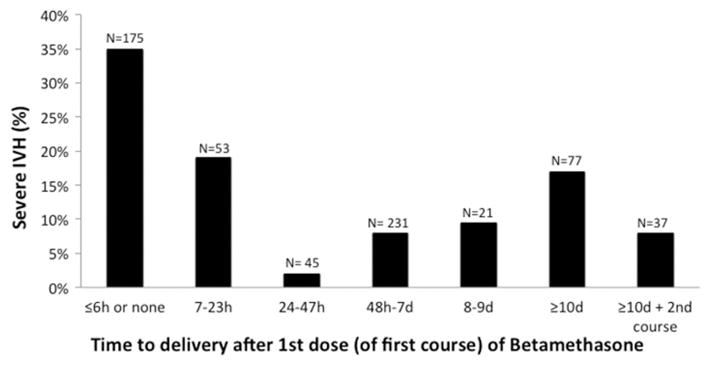
Relationship between the time to delivery after the first course of betamethasone and the incidence of severe (grades 3 and 4) intraventricular hemorrhage. Among the 667 infants in the study population, 28 died before 4 days and before the highest grade of intraventricular hemorrhage could be determined: n=8, in the group that delivered ≤6 hours from the initiation of BMZ; n=2, in the group that delivered between 7 and 23 hours; n=3, in the group that delivered between 24 and 47 hours; n=12, in the group that delivered between 48 hours and 7 days; n=0, in the group that delivered between 8 and 9 days; n=3, in the group that delivered 10 days or more after a single course of BMZ; and, n=0, in the group that delivered after a second/rescue course of BMZ.
Three hundred ninety-two infants received only a single (two-dose) course of BMZ: 312 delivered less than 10 days and 80 delivered ≥10 days after the first dose of a single course of BMZ (Figure and Table III). Several perinatal variables (birth year epoch, preeclampsia, gestational diabetes, multiple gestation, gestational age and small for gestational age) differed significantly between infants born <10 days and those born ≥10 days after a single BMZ course (Table III). After adjusting for these potential confounders we found that infants who delivered ≥10days after the first dose of BMZ had an increased risk of developing a severe IVH (Table IV). The incidence of severe IVH was 17% in infants born ≥10 days and 7% in those born <10 days after a single completed course of BMZ.
Table 3.
Characteristics of infants who received a single course (two doses) of betamethasone: comparison of those who delivered less than 10 days after the first betamethasone dose with those who delivered greater than or equal to 10 days after the first betamethasone dose
| Characteristic | Time to delivery after 1st dose of betamethasone | P value | |
|---|---|---|---|
|
| |||
| <10 days (N = 312) |
≥10 days (N = 80) |
||
|
| |||
| Birth year epoch (%) | 0.012 | ||
| 1998 – 2003 | 39 | 21 | |
| 2004 – 2009 | 33 | 43 | |
| 2010 – 2015 | 28 | 36 | |
|
| |||
| Preeclampsia (%) | 26 | 13 | 0.013 |
|
| |||
| Gestational Diabetes (%) | 6 | 14 | 0.030 |
|
| |||
| Clinical Chorioamnionitis (%) | 25 | 29 | 0.494 |
|
| |||
| Cesarean Delivery (%) | 69 | 71 | 0.726 |
|
| |||
| Presentation at delivery (%) | 0.483 | ||
| Vertex | 60 | 66 | |
| Breech | 34 | 28 | |
| Transverse | 6 | 6 | |
|
| |||
| Multiple Gestation (%) | 29 | 48 | 0.001 |
|
| |||
| Caucasian (%) | 46 | 55 | 0.158 |
|
| |||
| Outborn (%) | 10 | 10 | 0.917 |
|
| |||
| Gestational age <26 weeks (%) | 48 | 11 | <0.0001 |
|
| |||
| Gestational age (mean ± SD) | 25.8 ± 1.1 | 26.7 ± 0.7 | <0.0001 |
|
| |||
| Small for gestational age (%) | 26 | 5 | <0.0001 |
|
| |||
| Birth weight-gm (mean ± SD) | 766 ±193 | 919 ± 183 | <0.0001 |
|
| |||
| Male (%) | 50 | 43 | 0.231 |
|
| |||
| 5 minute Apgar score ≥6 (%) | 69 | 73 | 0.561 |
|
| |||
| Prophylactic indomethacin (%) | 69 | 71 | 0.726 |
|
| |||
| Early Onset Sepsis (%) | 4 | 8 | 0.216 |
|
| |||
| Time to delivery *- days (mean ± SD) | 3.9 ± 2.1 | 14.9 ± 4.0 | <0.0001 |
Time to delivery after 1st dose of betamethasone; all infants delivered at least 24 hours after the 1st dose of betamethasone
Table 4.
Waning effects of a single course of antenatal betamethasone on neonatal morbidly
| Neonatal Morbidity | Unadjusted Odds ratio (or Coefficient) for Time to delivery ≥ 10 days (95% CI) | Adjusted* Odds ratio (or Coefficient) for Time to delivery ≥ 10 days (95% CI) | P value† |
|---|---|---|---|
| Severe IVH‡ (%) | 2.67 (1.26–5.61) | 4.16 (1.59–10.87) | 0.004 |
| Intubated-Mechanical Ventilation at 24h (%) | 1.05 (0.58–1.89) | 3.23(1.59–6.57) | 0.001 |
| Respiratory Severity Score at 24h (mean ± SD)§ | 0.19 (CI −0.22, 0.59) | 0.39 (CI 0.10–0.84) | 0.029 |
| Death (%) | 0.40 (0.09–1.80) | 1.37 (0.58–3.20) | 0.468 |
| BPD** or death (%) | 1.50 (0.64–3.60) | 1.26(0.68–2.4) | 0.460 |
| NEC†† or death (%) | 1.00 (0.39–2.60) | 1.30 (0.65–2.6) | 0.468 |
Note: all infants completed a two dose course of antenatal betamethasone
Adjusted for gestational age at birth, small for gestational age, preeclampsia, multiple birth, gestational diabetes and birth year epoch.
P value for adjusted odds ratio or coefficient
Grade 3 or 4
Respiratory Severity Score (RSS) = mean airway pressure x fraction of inspired oxygen. By convention, mean airway pressure = liter flow rate when infants received nasal cannula flows of ≤3 liter/min; measurements made at 24 hours after birth; linear regression with bootstrapping coefficient and bias corrected confidence interval reported
Bronchopulmonary Dysplasia (BPD), physiologic definition by room air challenge at 36 weeks post menstrual age
Necrotizing enterocolitis, Bell’s classification ≥II (treated medically or surgically) and “spontaneous perforations”
Thirty-seven infants received a second, or “rescue”, course of antenatal BMZ when their mothers remained pregnant for more than 9 days after their first BMZ course. All 37 infants delivered within 8 days of the second/rescue course of BMZ (gestational age = 26.5±0.9 weeks; time to delivery after the 1st course of BMZ = 17.9±5.3 days; time to delivery after the 2nd course of BMZ = 3.05±2.3 days). Despite the prolonged interval between the first course of BMZ and delivery, the incidence of severe IVH in infants, whose mothers received a second/rescue course of BMZ, was only 8% (Figure). This is similar to the rate of severe IVH (7%) among infants born <10 days after the first completed BMZ course. After adjusting for the potential confounders listed in Table IV, we found no difference in the incidence of severe IVH when infants who received a second/rescue course of BMZ were compared with infants who delivered within 9 days of the first completed BMZ course (adjusted OR 1.7; 95% CI 0.41–6.6, p=0.48).
We also examined whether the risks of other morbidities, that are modified by antenatal BMZ treatment (eg, levels of respiratory support at 24 hours after birth and death)(2) (Table II), waned with time in our population (Table IV). We found that the association between BMZ and the need for initial respiratory support (both mechanical ventilation and Respiratory Severity Score) appeared to wane with time (Table IV), and that a repeat or rescue course of BMZ was associated with persistence of BMZ’s beneficial effects. There was no difference in the need for mechanical ventilation (adjusted OR 0.98; 95% CI 0.38–2.54, p=0.97) or in the Respiratory Severity Score (adjusted Coefficient = 0.20; 95% CI −0.28 to 0.6.8, p=0.42)) when infants who received a second/rescue course of BMZ were compared with infants who delivered within 9 days of the first BMZ course. On the other hand, the effects of BMZ on neonatal mortality did not appear to be time-limited (Table IV). Our findings about the need for respiratory support and death after BMZ treatment are similar to those of previous studies (20–23).
DISCUSSION
Although the benefits of antenatal corticosteroids in infants born before 28 weeks gestation have not been proven in RCTs, evidence from observational studies suggests their benefits. As in prior observational studies (21–26), we also found that in infants delivering before 28 weeks gestation exposure to a two-dose course of antenatal BMZ was associated with a decreased incidence of severe IVH, need for increased levels of respiratory support at 24 hours after birth, and death (but not BPD, NEC, or severe ROP) (Table II).
Among infants delivered before 28 weeks gestation, the most consistent and largest change associated with BMZ administration was a decreased incidence of severe IVH (21–23) (Table II). Therefore, we felt that it was important to determine whether the IVH sparing effects associated with antenatal BMZ were persistent or waned with time. We found that the risk of severe IVH increased in infants who delivered ≥10 days after the first dose of antenatal BMZ (Table IV). We also found that a repeat or rescue course of BMZ was associated with persistence of BMZ’s IVH sparing effects in infants who delivered remote from their first course of BMZ but still within the period of IVH vulnerability (before 28 weeks). Our findings differ from most of the prior studies that have examined the waning effects of antenatal BMZ on severe IVH. We suggest that this difference may be due to the fact that most of the prior studies examined populations with an insufficient number of infants that actually delivered during the IVH vulnerable period. Even in these studies a relationship can be seen between the incidence of severe IVH and the BMZ-to-delivery time interval, if infants delivering at the youngest gestational ages are examined separately (4, 24, 27–29).
The causes of IVH are multifactorial. They depend on the fragility of the immature germinal matrix microvasculature, fluctuations in cerebral blood flow, and coagulation disorders (30–34). In addition to altering the severity of an infant’s lung disease, antenatal corticosteroid administration stabilizes the germinal matrix vasculature by downregulating vascular endothelial growth factor, suppressing angiogenesis, increasing coverage of nascent endothelial cells with pericytes and astrocyte foot processes, increasing basal lamina fibronectin levels, inhibiting neurovascular proteases, and increasing vascular tone and blood pressure stability (35–37). In the future, knowing which of these effects are reversible with time may identify crucial pathways for future manipulation.
There are several limitations to our study. Our study uses a prospectively collected, single center, observational data set that spans 17 years. Thus, our findings may not be generalizable to other centers. Our study was performed over a prolonged time interval. Even though we adjusted our analyses for the epoch in which the infants were born, there may have been unmeasured changes in practice that may have occurred during the study period that could have affected the rates of severe IVH. As an observational study, the reason for the non-administration of antenatal BMZ or for the timing of delivery after the BMZ course could not be controlled. Although we controlled for important confounders that differed between study groups, there is still the possibility of unmeasured residual confounding. The confidence intervals for our adjusted OR estimate of 4.16 are wide, (1.59–10.87) (Table IV). Therefore, the OR of severe IVH in these infants could be as low as 1.59 or as high as 10.87. However, most of the 95% confidence interval is above our point estimate suggesting the “true” effect may be larger than what we have observed. Certain obstetrical practices were introduced during the later third of the study period (magnesium for brain protection and delayed cord clamping). We were unable to reliably collect data about them during the early phases of their implementation and therefore have not included them in our data set. However, as of this time, neither antenatal Magnesium (38, 39) nor delayed cord clamping (40) has been shown to alter the incidence of severe IVH in preterm infants. It is possible that our study was underpowered to detect small differences in neonatal outcomes; the number of infants that delivered ≥10 days after the first dose of BMZ was small and the cohort that received a second course of steroids was even smaller.
On the other hand, there are also strengths to our study. The single center aspect of the study meant that the same consensus-driven, standardized approaches to respiratory, hemodynamic, fluid, nutrition and PDA management were consistent among the infants in each of the study eras. The same neonatologist reviewed all of the infants’ cranial ultrasounds in addition to prospectively following the clinical course of all of the study infants and recording all of the study data.
Currently the American Congress of Obstetricians and Gynecologists recommends that a “single rescue course of antenatal corticosteroids may be considered if the antecedent treatment was given more than 2 weeks prior, the gestational age is less than 32 6/7 weeks, and the women are judged by the clinician to be likely to give birth within the next week” (41). As corticosteroid treatment is initiated earlier in gestation, in an attempt to resuscitate periviable infants, the optimal timing of a repeat course has become a clinical dilemma. Unfortunately, obstetricians are limited in their ability to predict when preterm delivery will occur and are likely to both overestimate the risks of delivery, and administer the corticosteroids too early (42, 43), when the beneficial effects might dissipate, as well as to underestimate the risks (9), and administer them too late, when imminent delivery might preclude completion of the planned treatment. Recent studies suggest that long-term neurodevelopmental morbidity after preterm birth is most highly correlated with IVH, BPD and ROP (44) but not RDS. Depending on the goals for treatment, knowing that rescue BMZ does not decrease the risks of BPD or ROP, and might only be beneficial in decreasing the incidence of IVH (if infants deliver before 28 weeks) and RDS (if infants deliver before 34 weeks), makes the question of when to use a rescue dose even more of a conundrum. Replication of this study in other observational data sets is warranted and if our results are confirmed could help guide recommendations on the timing of rescue courses of betamethasone.
Acknowledgments
Supported by the National Heart, Lung, and Blood Institute (HL109199) and the Jamie and Bobby Gates Foundation.
Abbreviations
- RCT
randomized controlled trial
- PDA
patent ductus arteriosus
- NEC
necrotizing enterocolitis
- ROP
retinopathy of prematurity
- IVH
intraventricular/intracranial hemorrhage
- RDS
respiratory distress syndrome
- BPD
bronchopulmonary dysplasia
- BMZ
betamethasone
- OR
odds ratio
Footnotes
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weight < 1500 grams. J Pediatr. 1978;92:529–34. doi: 10.1016/s0022-3476(78)80282-0. [DOI] [PubMed] [Google Scholar]
- 2.Roberts D, Dalziel S. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. 2006:CD004454. doi: 10.1002/14651858.CD004454.pub2. [DOI] [PubMed] [Google Scholar]
- 3.Vidaeff AC, Ramin SM, Gilstrap LC, 3rd, Alcorn JL. Characterization of corticosteroid redosing in an in vitro cell line model. Am J Obstet Gynecol. 2004;191:1403–8. doi: 10.1016/j.ajog.2004.06.049. [DOI] [PubMed] [Google Scholar]
- 4.Ring AM, Garland JS, Stafeil BR, Carr MH, Peckman GS, Pircon RA. The effect of a prolonged time interval between antenatal corticosteroid administration and delivery on outcomes in preterm neonates: a cohort study. Am J Obstet Gynecol. 2007;196:457, e1–6. doi: 10.1016/j.ajog.2006.12.018. [DOI] [PubMed] [Google Scholar]
- 5.Crowther CA, McKinlay CJ, Middleton P, Harding JE. Repeat doses of prenatal corticosteroids for women at risk of preterm birth for improving neonatal health outcomes. Cochrane Database Syst Rev. 2015;7:CD003935. doi: 10.1002/14651858.CD003935.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guinn DA, Atkinson MW, Sullivan L, Lee M, MacGregor S, Parilla BV, et al. Single vs weekly courses of antenatal corticosteroids for women at risk of preterm delivery: A randomized controlled trial. JAMA. 2001;286:1581–7. doi: 10.1001/jama.286.13.1581. [DOI] [PubMed] [Google Scholar]
- 7.Wapner RJ, Sorokin Y, Thom EA, Johnson F, Dudley DJ, Spong CY, et al. Single versus weekly courses of antenatal corticosteroids: evaluation of safety and efficacy. Am J Obstet Gynecol. 2006;195:633–42. doi: 10.1016/j.ajog.2006.03.087. [DOI] [PubMed] [Google Scholar]
- 8.Crowther CA, Haslam RR, Hiller JE, Doyle LW, Robinson JS. Neonatal respiratory distress syndrome after repeat exposure to antenatal corticosteroids: a randomised controlled trial. Lancet. 2006;367:1913–9. doi: 10.1016/S0140-6736(06)68846-6. [DOI] [PubMed] [Google Scholar]
- 9.Peltoniemi OM, Kari MA, Tammela O, Lehtonen L, Marttila R, Halmesmaki E, et al. Randomized trial of a single repeat dose of prenatal betamethasone treatment in imminent preterm birth. Pediatrics. 2007;119:290–8. doi: 10.1542/peds.2006-1549. [DOI] [PubMed] [Google Scholar]
- 10.Murphy KE, Hannah ME, Willan AR, Hewson SA, Ohlsson A, Kelly EN, et al. Multiple courses of antenatal corticosteroids for preterm birth (MACS): a randomised controlled trial. Lancet. 2008;372:2143–51. doi: 10.1016/S0140-6736(08)61929-7. [DOI] [PubMed] [Google Scholar]
- 11.Garite TJ, Kurtzman J, Maurel K, Clark R. Impact of a ‘rescue course’ of antenatal corticosteroids: a multicenter randomized placebo-controlled trial. Am J Obstet Gynecol. 2009;200:248, e1–9. doi: 10.1016/j.ajog.2009.01.021. [DOI] [PubMed] [Google Scholar]
- 12.Stoll BJ, Hansen NI, Bell EF, Walsh MC, Carlo WA, Shankaran S, et al. Trends in Care Practices, Morbidity, and Mortality of Extremely Preterm Neonates, 1993–2012. JAMA. 2015;314:1039–51. doi: 10.1001/jama.2015.10244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jhaveri N, Moon-Grady A, Clyman RI. Early surgical ligation versus a conservative approach for management of patent ductus arteriosus that fails to close after indomethacin treatment. J Pediatr. 2010;157:381–7. 7 e1. doi: 10.1016/j.jpeds.2010.02.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Catenacci M, Miyagi S, Wickremasinghe AC, Lucas SS, de Alba Campomanes AG, Good WV, et al. Dopamine-resistant hypotension and severe retinopathy of prematurity. J Pediatr. 2013;163:400–5. doi: 10.1016/j.jpeds.2013.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Olsen IE, Groveman SA, Lawson ML, Clark RH, Zemel BS. New intrauterine growth curves based on United States data. Pediatrics. 2010;125:e214–24. doi: 10.1542/peds.2009-0913. [DOI] [PubMed] [Google Scholar]
- 16.Liebowitz MC, Clyman RI. Predicting the Need for Home Oxygen Therapy in Preterm Infants Born Before 28 Weeks’ Gestation. Am J Perinatol. 2016;33:34–9. doi: 10.1055/s-0035-1555122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clyman RI, Wickremasinghe A, Merritt TA, Solomon T, McNamara P, Jain A, et al. Hypotension following patent ductus arteriosus ligation: the role of adrenal hormones. J Pediatr. 2014;164:1449–55. doi: 10.1016/j.jpeds.2014.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walsh MC, Yao Q, Gettner P, Hale E, Collins M, Hensman A, et al. Impact of a physiologic definition on bronchopulmonary dysplasia rates. Pediatrics. 2004;114:1305–11. doi: 10.1542/peds.2004-0204. [DOI] [PubMed] [Google Scholar]
- 19.Bell MJ, Ternberg JL, Feigin RD, Keating JP, Marshall R, Barton L, et al. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann Surg. 1978;187:1–7. doi: 10.1097/00000658-197801000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garite TJ, Rumney PJ, Briggs GG, Harding JA, Nageotte MP, Towers CV, et al. A randomized, placebo-controlled trial of betamethasone for the prevention of respiratory distress syndrome at 24 to 28 weeks’ gestation. Am J Obstet Gynecol. 1992;166:646–51. doi: 10.1016/0002-9378(92)91691-3. [DOI] [PubMed] [Google Scholar]
- 21.Carlo WA, McDonald SA, Fanaroff AA, Vohr BR, Stoll BJ, Ehrenkranz RA, et al. Association of antenatal corticosteroids with mortality and neurodevelopmental outcomes among infants born at 22 to 25 weeks’ gestation. JAMA. 2011;306:2348–58. doi: 10.1001/jama.2011.1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mori R, Kusuda S, Fujimura M Neonatal Research Network J. Antenatal corticosteroids promote survival of extremely preterm infants born at 22 to 23 weeks of gestation. J Pediatr. 2011;159:110–4. e1. doi: 10.1016/j.jpeds.2010.12.039. [DOI] [PubMed] [Google Scholar]
- 23.Costeloe K, Hennessy E, Gibson AT, Marlow N, Wilkinson AR. The EPICure study: outcomes to discharge from hospital for infants born at the threshold of viability. Pediatrics. 2000;106:659–71. doi: 10.1542/peds.106.4.659. [DOI] [PubMed] [Google Scholar]
- 24.Melamed N, Shah J, Soraisham A, Yoon EW, Lee SK, Shah PS, et al. Association Between Antenatal Corticosteroid Administration-to-Birth Interval and Outcomes of Preterm Neonates. Obstet Gynecol. 2015;125:1377–84. doi: 10.1097/AOG.0000000000000840. [DOI] [PubMed] [Google Scholar]
- 25.Abbasi S, Oxford C, Gerdes J, Sehdev H, Ludmir J. Antenatal corticosteroids prior to 24 weeks’ gestation and neonatal outcome of extremely low birth weight infants. Am J Perinatol. 2010;27:61–6. doi: 10.1055/s-0029-1223269. [DOI] [PubMed] [Google Scholar]
- 26.Wei JC, Catalano R, Profit J, Gould JB, Lee HC. Impact of antenatal steroids on intraventricular hemorrhage in very-low-birth weight infants. J Perinatol. 2016;36:352–6. doi: 10.1038/jp.2016.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sehdev HM, Abbasi S, Robertson P, Fisher L, Marchiano DA, Gerdes JS, et al. The effects of the time interval from antenatal corticosteroid exposure to delivery on neonatal outcome of very low birth weight infants. Am J Obstet Gynecol. 2004;191:1409–13. doi: 10.1016/j.ajog.2004.06.055. [DOI] [PubMed] [Google Scholar]
- 28.Vermillion ST, Bland ML, Soper DE. Effectiveness of a rescue dose of antenatal betamethasone after an initial single course. Am J Obstet Gynecol. 2001;185:1086–9. doi: 10.1067/mob.2001.117633. [DOI] [PubMed] [Google Scholar]
- 29.Chawla S, Natarajan G, Rane S, Thomas R, Cortez J, Lua J. Outcomes of extremely low birth weight infants with varying doses and intervals of antenatal steroid exposure. J Perinat Med. 2010;38:419–23. doi: 10.1515/jpm.2010.060. [DOI] [PubMed] [Google Scholar]
- 30.Ballabh P. Pathogenesis and prevention of intraventricular hemorrhage. Clin Perinatol. 2014;41:47–67. doi: 10.1016/j.clp.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duppre P, Sauer H, Giannopoulou EZ, Gortner L, Nunold H, Wagenpfeil S, et al. Cellular and humoral coagulation profiles and occurrence of IVH in VLBW and ELWB infants. Early Hum Dev. 2015;91:695–700. doi: 10.1016/j.earlhumdev.2015.09.008. [DOI] [PubMed] [Google Scholar]
- 32.Van Bel F, Van de Bor M, Stijnen T, Baan J, Ruys JH. Aetiological role of cerebral blood-flow alterations in development and extension of peri-intraventricular haemorrhage. Dev Med Child Neurol. 1987;29:601–14. doi: 10.1111/j.1469-8749.1987.tb08502.x. [DOI] [PubMed] [Google Scholar]
- 33.Vinukonda G, Dummula K, Malik S, Hu F, Thompson CI, Csiszar A, et al. Effect of prenatal glucocorticoids on cerebral vasculature of the developing brain. Stroke. 2010;41:1766–73. doi: 10.1161/STROKEAHA.110.588400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ballabh P, Xu H, Hu F, Braun A, Smith K, Rivera A, et al. Angiogenic inhibition reduces germinal matrix hemorrhage. Nat Med. 2007;13:477–85. doi: 10.1038/nm1558. [DOI] [PubMed] [Google Scholar]
- 35.Liu J, Feng ZC, Yin XJ, Chen H, Lu J, Qiao X. The role of antenatal corticosteroids for improving the maturation of choroid plexus capillaries in fetal mice. Eur J Pediatr. 2008;167:1209–12. doi: 10.1007/s00431-007-0649-y. [DOI] [PubMed] [Google Scholar]
- 36.Yang D, Baumann JM, Sun YY, Tang M, Dunn RS, Akeson AL, et al. Overexpression of vascular endothelial growth factor in the germinal matrix induces neurovascular proteases and intraventricular hemorrhage. Sci Transl Med. 2013;5:193ra90. doi: 10.1126/scitranslmed.3005794. [DOI] [PubMed] [Google Scholar]
- 37.Moise AA, Wearden ME, Kozinetz CA, Gest AL, Welty SE, Hansen TN. Antenatal steroids are associated with less need for blood pressure support in extremely premature infants. Pediatrics. 1995;95:845–50. [PubMed] [Google Scholar]
- 38.Doyle LW, Crowther CA, Middleton P, Marret S, Rouse D. Magnesium sulphate for women at risk of preterm birth for neuroprotection of the fetus. Cochrane Database Syst Rev. 2009:CD004661. doi: 10.1002/14651858.CD004661.pub3. [DOI] [PubMed] [Google Scholar]
- 39.Hirtz DG, Weiner SJ, Bulas D, DiPietro M, Seibert J, Rouse DJ, et al. Antenatal Magnesium and Cerebral Palsy in Preterm Infants. J Pediatr. 2015;167:834–9. e3. doi: 10.1016/j.jpeds.2015.06.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rabe H, Diaz-Rossello JL, Duley L, Dowswell T. Effect of timing of umbilical cord clamping and other strategies to influence placental transfusion at preterm birth on maternal and infant outcomes. Cochrane Database Syst Rev. 2012;8:CD003248. doi: 10.1002/14651858.CD003248.pub3. [DOI] [PubMed] [Google Scholar]
- 41.Practice ACoO. ACOG Committee Opinion No. 475: antenatal corticosteroid therapy for fetal maturation. Obstet Gynecol. 2011;117:422–4. doi: 10.1097/AOG.0b013e31820eee00. [DOI] [PubMed] [Google Scholar]
- 42.McLaughlin KJ, Crowther CA, Vigneswaran P, Hancock E, Willson K. Who remains undelivered more than seven days after a single course of prenatal corticosteroids and gives birth at less than 34 weeks? Aust N Z J Obstet Gynaecol. 2002;42:353–7. doi: 10.1111/j.0004-8666.2002.00353.x. [DOI] [PubMed] [Google Scholar]
- 43.Modi N, Lewis H, Al-Naqeeb N, Ajayi-Obe M, Dore CJ, Rutherford M. The effects of repeated antenatal glucocorticoid therapy on the developing brain. Pediatric Research. 2001;50:581–5. doi: 10.1203/00006450-200111000-00008. [DOI] [PubMed] [Google Scholar]
- 44.Schmidt B, Asztalos EV, Roberts RS, Robertson CM, Sauve RS, Whitfield MF. Impact of bronchopulmonary dysplasia, brain injury, and severe retinopathy on the outcome of extremely low-birth-weight infants at 18 months: results from the trial of indomethacin prophylaxis in preterms. JAMA. 2003;289:1124–9. doi: 10.1001/jama.289.9.1124. [DOI] [PubMed] [Google Scholar]


