Abstract
We have recently discovered a unique CD34loCD133lo cell population in the human fetal liver (FL) that gives rise to cells in the hepatic lineage. In this study, we further characterized the biological functions of FL CD34loCD133lo cells. Our findings show that these CD34loCD133lo cells express markers of both endodermal and mesodermal lineages and have the capability to differentiate into hepatocyte and mesenchymal lineage cells by ex vivo differentiation assays. Furthermore, we show that CD34loCD133lo cells express growth factors that are important for human hematopoietic stem cell (HSC) expansion: stem cell factor (SCF), insulin-like growth factor 2 (IGF2), C-X-C motif chemokine 12 (CXCL12), and factors in the angiopoietin-like protein family. Co-culture of autologous FL HSCs and allogenic HSCs derived from cord blood with CD34loCD133lo cells supports and expands both types of HSCs.These findings are not only essential for extending our understanding of the HSC niche during the development of embryonic and fetal hematopoiesis but will also potentially benefit adult stem cell transplantations in clinics because expanded HSCs demonstrate the same capacity as primary cells to reconstitute the human immune system and mediate long-term hematopoiesis in vivo. Together, CD34loCD133lo cells not only serve as stem/progenitor cells for liver development but are also an essential component of the HSC niche in the human FL.
Keywords: epithelial–mesenchymal transition, fetal liver niche, hematopoietic cell expansion, hematopoietic cell transplantation
INTRODUCTION
Human adult hematopoietic stem cells (HSCs) are normally quiescent in the bone marrow (BM) and undergo limited self-renewal. By contrast, within the human fetal liver (FL), there is a higher frequency of cycling HSCs undergoing self-renewal, which suggests that the FL provides a microenvironment that is more conducive in supporting HSCs. Thus, the FL is an ideal place to study hematopoiesis and characterize the specialized HSC niches created by the surrounding stromal cells. Although the exact cellular niches that support HSC self-renewal in the FL have not been defined, endothelial cells, stromal cells, and perhaps developing hepatocytes likely provide cues to the hematopoietic microenvironment.1 In rodents, Zhang et al. showed that liver-derived cell lines express proteins such as IGF2 and angiopoietin-like factors that are associated with supporting HSCs.2 Recently, it has been reported that hepatic progenitors in mouse FLs are stromal cells that support HSCs.3 However, little is known about the human FL niche due to ethical constraints and limited access to human samples.
For decades, various human stem cells, such as hematopoietic, mesenchymal and other stem cells within fetal tissue transplants, have been utilized clinically to treat a spectrum of hematological malignancies, cancers, and genetic immune disorders.4,5,6,7,8 Different stem cell sources, such as CB, peripheral blood, and BM are used for transplantation.4,5,6,7,8 However, due to a scarcity of donors and a limited number of cells from these organs, stem cell use has been restricted to low body-weight recipients.9 In recent years, a great deal of research has been focused on expanding and maintaining the number of HSCs in culture. These experiments range from supplementing the culture with undefined factors such as serum to the addition of feeder cells.2,10,11,12 However, there remains a need for more efficient protocols to expand HSCs while maintaining their potency and stemness. Increasing our depth of understanding of stem cell environments will not only increase the number of patients benefiting from allogenic transplantation but also reduce the overall mortality rate.13
In a previous study, we discovered a novel human hepatic stem/progenitor cell population in the FL that has a phenotype of CD34loCD133lo14. Despite its ability to differentiate into human hepatocytes, little is known about this population. Therefore, further characterization of this CD34loCD133lo cell population is required to explore more of its biological functions. Here, we report that CD34loCD133lo cells are an important component of the FL niche because they are capable of developing into various endodermal and mesodermal cell types. In this sense, CD34loCD133lo cells are not just restricted to the hepatic lineages. Furthermore, CD34loCD133lo cells express principal growth factors that are important for HSC expansion and are capable of supporting the ex vivo expansion of HSCs.
MATERIALS AND METHODS
Mice
NOD-SCIDIL2Rγ −/− (NSG) mice were obtained from the Jackson Laboratory and bred in the animal facility at A*STAR, Biological Resource Centre. Neonate mice were sublethally irradiated (100 rads) within 24 hours of birth and infused with cells via intra-hepatic injection. The International Animal Care and Use Committee (IACUC) at A*STAR specifically approved this study with the protocol number BRC #120716. All animal experimental procedures were performed in accordance with this protocol.
FL processing and cell isolation
Human FL samples from male and female donors 16–23 weeks of age were obtained from Kandang Kerbau Women's and Children's Hospital (KKH) with written consent from donors. SingHealth and National Health Care Group Research Ethics Committees Singapore specifically approved this study (CIRB Ref: 2012/064/B). FL processing was performed by dissociating the liver and digesting it with collagenase VI (2 mg/ml in Dulbecco's modified Eagle's medium (DMEM)) for 15 minutes at 37 °C with constant rotation. A single-cell suspension was prepared by passing the digested tissue through a 100 µm mesh. Human cord blood (CB) was obtained from Singapore Cord Blood Bank. CD34+ cells were isolated via a CD34-positive selection kit (Stem Cell Technologies, Vancouver, BC), as previously described.14
In vitro differentiation
Endothelial differentiation: To induce endothelial differentiation, CD34loCD133lo cells were cultured with endothelial medium for 2 weeks with medium changes twice weekly. Endothelial differentiation medium consists of Iscove's modified Dulbecco's medium (IMDM) (Sigma) supplemented with 20% FBS (Thermo Scientific), 20 ng/mL stem cell factor (SCF) (R&D), 20 ng/mL vascular endothelial growth factor (VEGF) (Peprotech), 10 ng/mL fibroblast growth factor 2 (FGF2) (Peprotech), 20 ng/mL transforming growth factor beta 3 (TGFβ3) (R&D), 20 ng/mL epidermal growth factor (EGF) (Peprotech), 10−6 mol/L Hydrocortisol (Sigma), 50 µMβ-mercaptoethanol (Gibco), and 100 units penicillin/streptomycin (Gibco). Endothelial differentiation was assessed by Von Willebrand factor (vWF) staining on a Fluoview inverted confocal (multi-photon) microscope (Olympus). Osteogenic differentiation: To induce osteogenic differentiation, CD34loCD133lo cells were cultured with StemMACSOsteoDiff Medium (MiltenyiBiotec) for 2 weeks with medium changes twice weekly. Osteogenesis was assessed by alizarin red staining. Adipogenic differentiation: To induce adipogenic differentiation, CD34loCD133lo cells were treated with StemMACSAdipoDiff Medium (MiltenyiBiotec) for two weeks with medium changes twice weekly. Adipogenesis was assessed by Oil Red O Staining. Hepatogenic differentiation: CD34loCD133lo cells were trans-differentiated over 3 weeks in a two-step protocol. Step 1 differentiation medium consisted of IMDM (Sigma), 100 ng/mL ActivinA (R&D), 10 ng/mL FGF2 (Peprotech), 200 µL insulin-transferrin-sodium selenite media supplement (ITS) (Sigma), 50 µMβ-mercaptoethanol (Gibco), and 100 units penicillin/streptomycin (Gibco). Step 2 medium contained 20 ng/mL hepatocyte growth factor (HGF) (R&D), 10 ng/mL FGF2 (Peprotech), 0.61 mg/mL Nicotinamide (Sigma), 50 ng/mL ITS (Sigma), 50 µMβ-mercaptoethanol (Gibco), and 100 units penicillin/streptomycin (Gibco). Medium changes were performed twice weekly, and hepatogenesis was assessed with a Fluoview inverted confocal (multi-photon) microscope (Olympus) to check for albumin (ALB) production.
Immunostaining
For immunofluorescence staining, deparaffinized sections or cells were blocked with 3% BSA and 0.2% Triton-X in PBS. Then, they were stained with the following antibodies: mouse anti-human CD34 (Abcam), rabbit anti-human ALB (Abcam), rabbit anti-human SCF (Abcam), mouse anti-human Delta-like 1 homolog (DLK1) (Abcam), or rabbit anti-human CXCL12 (Abcam). An Alexa Fluor 488 goat anti-mouse IgG antibody (Invitrogen) and an Alexa Fluor 546 goat anti-rabbit IgG antibody (Invitrogen) served as the secondary antibodies. Slides were cover-slipped with Prolong Gold anti-fade reagent containing DAPI (Invitrogen), and examined with a Fluoview inverted confocal (multi-photon) microscope (Olympus).
HSC expansion and transwell culture
Using a FACS Aria II cell sorter (BD, Franklin Lakes, NJ), CD34+ FL cells were separated into CD34hiCD133hi HSCs and CD34loCD133lo cells. Three culture conditions were set up: HSCs alone, CD34loCD133lo cells alone and a co-culture at a ratio of 1:7. Prior to this experiment, a range of co-culture conditions were tested, including 1:3, 1:5, 1:7, 1:10, and 1:20 (data not shown). The ratio of 1:7 was chosen as it was found to be the condition showing the highest viability, expansion, and stemness of HSCs. Cells were cultured for 7 days in serum-free StemSpan medium (Stem Cell Technologies) in the presence of defined factors: 20 ng/mL SCF (R&D), 10 ng/mL thrombopoietin (TPO) (R&D), 10 ng/mL fibroblast growth factor 1 (FGF1) (Gibco), 100 ng/mL insulin-like growth factor binding protein 2 (IGFBP2) (R&D), 10 ng/mL Heparin (Sigma), 50 µMβ-mercaptoethanol (Gibco), and 100 units penicillin/streptomycin (Gibco). At different time points, cells were resuspended, counted, and stained on ice for 30 minutes with anti-human CD34 and CD133 antibodies and analyzed by flow cytometry. An experiment using the same conditions with the addition of transwells (Millipore) was designed to test if cell–cell contact is essential for HSC expansion.
Flow cytometry
Conjugated antibodies specific for human CD45 (HI30), CD34 (561), CD14 (M5E2), CD56 (HCD56), CD3 (HIT3a), CD19 (HIB19), CD303 (201A), ILT7 (17G10.2), and mouse CD45.1 (A20) were obtained from BioLegend; antibodies against CD133 (EMK08) were purchased from Miltenyi. Cells were stained with appropriate antibodies in 100 μl PBS containing 0.2% BSA and 0.05% sodium azide for 30 minutes on ice. Flow cytometry was performed on a LSRII flow cytometer with FACSDiva software (BD, Franklin Lakes, NJ); 5000 events were collected per sample, and the data were analyzed on FlowJo software 8.8.6 (Treestar, Ashland, OR, USA). Cell sorting was performed on an Aria cell sorter (Beckton Dickinson); the purity of sorted cells was between 90% and 99%.
Secondary transplantation and limiting dilution assay
HSCs and CD34loCD133lo cells were mixed at a ratio of 1:7 and cultured for 7 days. CD34hiCD133hi HSCs were re-purified from the co-culture by cell sorting. CD34hiCD133hi HSCs freshly isolated from the same FL donor were used as control. Cells were counted, and 400, 1000, 5000, or 100 000 HSCs were injected into each sublethally irradiated neonate NSG mouse. Eight weeks after injection, BM was harvested and to determine the amount of human cell reconstitution. Single-cell suspensions were prepared from the BM by flushing it with a 27 gauge needle and syringe. BM cells were stained with anti-mouse CD45.1, anti-human CD45, CD34, and CD133 and analyzed by flow cytometry. Chimerism was calculated by the following equation: [%hCD45+/(%hCD45+ + %mCD45+)].
RNA isolation and quantitative RT-PCR (qPCR)
RNA isolation was performed with RNeasy Mini and Micro kits (Qiagen). Reverse transcription was performed using the iScript cDNA Synthesis Kit (BIO-RAD) according to manufacturer's specifications. All values were normalized with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as an endogenous control. Data were analyzed with the comparative CT method in which gene expression is calculated as 2−ΔΔCT, where Delta Ct = (Ct gene of interest – Ct GADPH internal control). The primer sequences used to detect endoderm, general endoderm, and mesoderm genes are listed in Supplementary Table 1.
Statistical analysis
Data are represented by mean ± standard error of the mean (SEM).
RESULTS
CD34loCD133lo fetal liver cells are able to develop into liver and mesodermal cell lineages
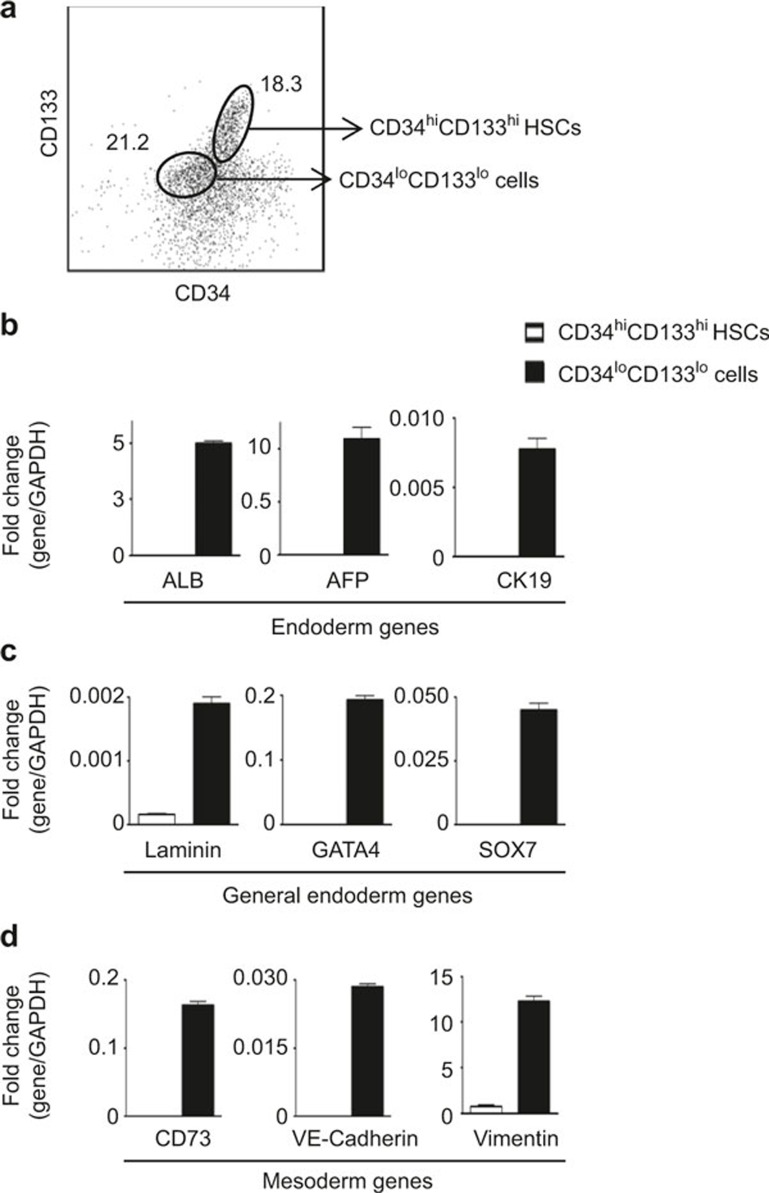
We have recently reported that of FL CD34+ cells, CD34hiCD133hi cells are HSCs andCD34loCD133lo cells give rise to hepatocyte-like cells.14 CD34hiCD133hi and CD34hiCD133neg are hematopoietic stem/progenitor cells which do not express ALB, whereas CD34loCD133lo cells expresses ALB.14 Therefore, ALB expression can be used to distinguish between CD34+ hematopoietic cells and CD34loCD133lo cells. To locate CD34+ hematopoietic cells and CD34loCD133lo cells in situ, we stained FL paraffin sections by immunofluorescence. As shown in Supplementary Figure 1, we were able to identify a number of CD34+ cells within the FL, and ∼10% of these CD34+ cells stained positive for ALB. Therefore, these CD34+ALB+ cells detected in the liver represent CD34loCD133lo cells in situ. In this study, we have further characterized the phenotypes of CD34hiCD133hi and CD34loCD133lo cells. CD34hiCD133hi HSCs and CD34loCD133lo cells were purified (Figure 1A) and analyzed for a panel of endodermal- and mesodermal-associated genes. The results showed that CD34loCD133lo cells expressed the liver-associated endodermal markers ALB, alpha-fetoprotein (AFP), cytokeratin 19 (CK19) (Figure 1B), other general endodermal markers such as laminin, GATA4, and SOX7 (Figure 1C) and mesodermal markers including CD73, VE-cadherin, and vimentin (Figure 1D), whereas HSCs from the same donors did not express these genes.
Figure 1.
Phenotyping of CD34+ cells purified from human FLs. (A) Purified CD34+ cells from human FLs (n = 8) were stained for CD34 and CD133 and analyzed by flow cytometry. Representative staining profile for CD34 versus CD133 is shown. The numbers shown are the frequencies of the gated cells. (B–D) CD34hiCD133hi HSCs and CD34loCD133lo cells were purified by cell sorting. Total RNA was isolated from purified cells, assayed by qPCR and normalized to GADPH. The data shown are obtained from three different FL samples. Comparison of the relative transcript levels of liver-associated endoderm- (B), general endoderm- (C), and mesoderm (D)-specific genes in CD34hiCD133hi HSCs and CD34loCD133lo cells. Data are represented as the mean ± SEM.
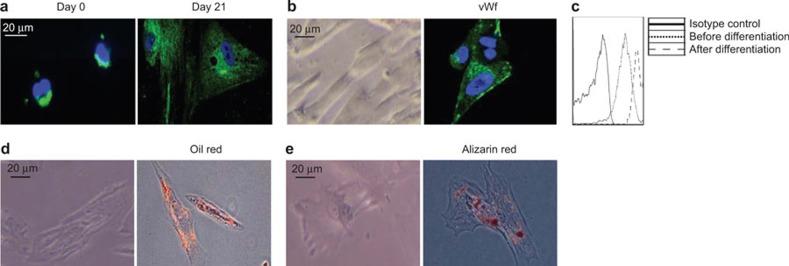
In light of the expression of both endodermal and mesodermal markers by CD34loCD133lo cells, we investigated whether these cells have the capacity to develop into liver and mesodermal cell lineages. We first purified CD34loCD133lo cells by cell sorting and cultured them in differentiation medium to allow for specific lineage development. In hepatocyte differentiation medium, CD34loCD133lo cells demonstrated morphological changes, eventually resembling ALB+ hepatocyte-like cells after 21 days in culture (Figure 2A). In endothelium induction medium, CD34loCD133lo cells developed into spindle-shaped cells, 90% of which expressed the endothelial marker vWF (Figure 2B). CD31 is another marker for endothelial cells. Although CD34loCD133lo cells endogenously expressed CD31, the expression of CD31 was further upregulated in the differentiated endothelial cells (Figure 2C). In adipocyte induction media, the cells increased in size, and 40% stained positive for fat (using Oil Red O) (Figure 2D). When cultured in osteocyte differentiation medium, the cells became elongated and 30% stained positive for calcium deposits (using Alizarin Red S) (Figure 2E). These data clearly demonstrate that CD34loCD133lo cells contain progenitor cells that can give rise to hepatocyte-like and mesodermal-like cell lineages.
Figure 2.
Ex vivo differentiation of CD34loCD133lo cells. CD34loCD133lo cells were purified and cultured in various differentiation media. (A) CD34loCD133lo cells were cultured in hepatocyte differentiation medium. Representative images of cultured CD34loCD133lo cells immunofluorescently stained on day 0 and 21 post-culture for human ALB (green) and DAPI (blue). (B) Representative phase-contrast micrographs (left) and immunofluorescence stains for human Von Willebrand Factor (vWf) (green) and DAPI (blue) (right) are shown for endothelial cell differentiation cultures after 7 days in vitro. (C) Cells from endothelial cell differentiation cultures were stained with a CD31 antibody and analyzed by flow cytometry. The histograms of the CD31 stains of CD34loCD133lo cells before and after differentiation are shown. (D) Representative phase-contrast micrographs (left) and stains for Oil Red O (red) (right) are shown for adipocyte differentiation cultures after 7 days in vitro. (E) Representative phase-contrast micrographs (left) and stains for Alizarin Red S (red) (right) are shown for osteocyte differentiation cultures after14 days in vitro. The scale bar applies to all panels.
Co-culture of CD34loCD133lo cells enhances the expansion of HSCs
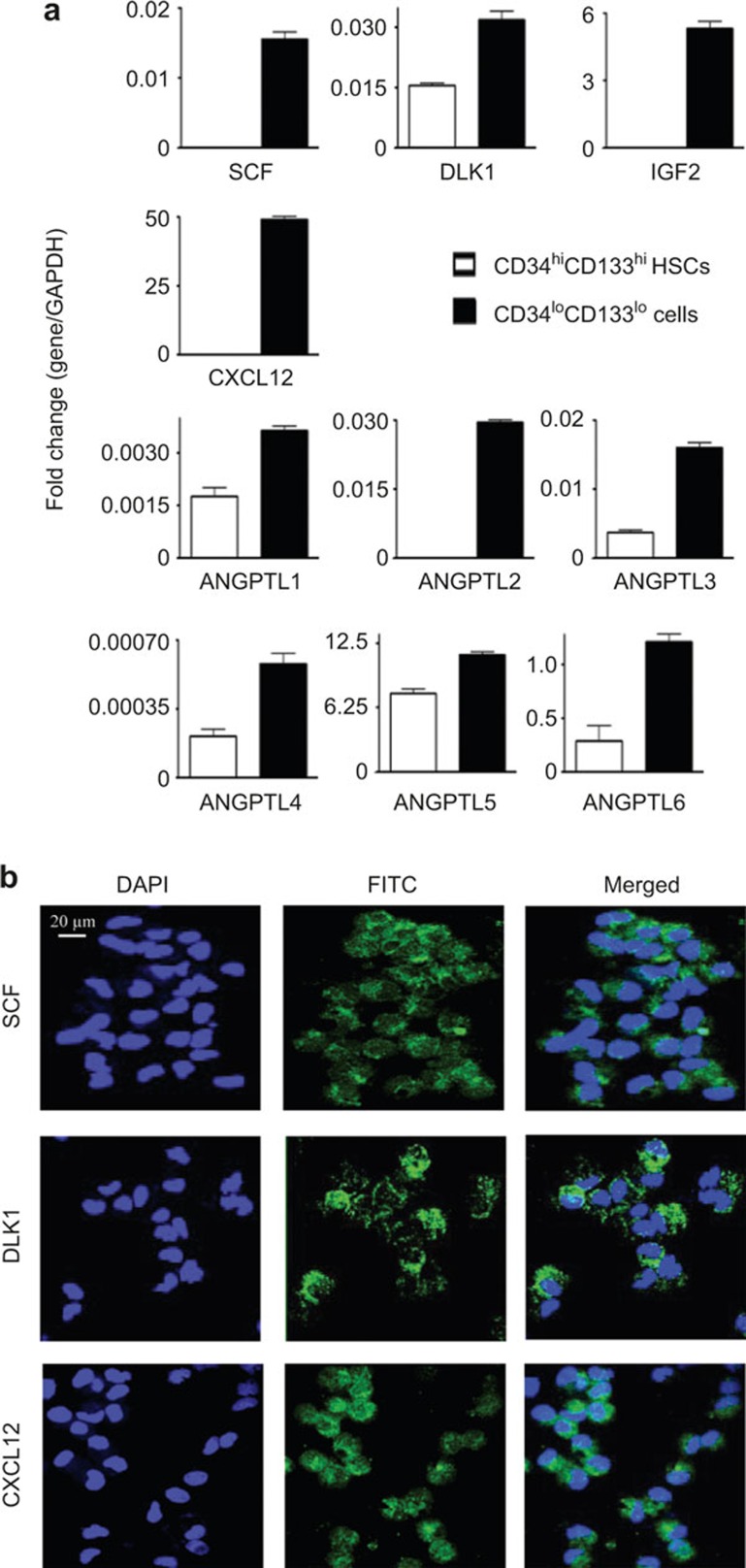
To investigate whether CD34loCD133lo cells could serve as supportive stromal cells for HSCs in the FL niche, CD34loCD133lo cells were purified and analyzed for the expression of known important HSC growth factors at both the RNA and protein levels. mRNA transcripts of SCF, DLK1, and IGF2, which are key factors in HSC expansion3, were found to be highly enriched in CD34loCD133lo cells, but not in CD34hiCD133hi HSCs (Figure 3A). Importantly, CXCL12, which is an HSC trafficking regulator secreted by stromal cells,15 was also found to be highly expressed by CD34loCD133lo cells (Figure 3A). Members of the angiopoietin-like protein (ANGPTL) family are known to play an important role in maintaining HSC homeostasis.2 We were able to detect ANGPTLs (ANGPTL1 through ANGPTL6) mRNA enrichment in CD34loCD133lo cells (Figure 3A). ANGPTL 2, 3, 5, and 6 were particularly highly expressed. Immunofluorescence stains confirmed the protein expression of SCF, DLK1, and CXCL12 in CD34loCD133lo cells (Figure 3B).
Figure 3.
CD34loCD133lo cells express growth factors that support HSC expansion. (A) Comparison of the relative transcript levels of human growth factor genes in CD34hiCD133hi HSCs and CD34loCD133lo cells. Total RNA was isolated from purified cells, assayed by qPCR</emph> and normalized to GAPDH. The data shown were obtained from two different FL samples. Data are represented as the mean ± SEM. (B) Cytospins were prepared with purified CD34loCD133lo cells</emph> and stained with antibodies specific for human SCF, DLK1, and CXCL12. Blue: DAPI. Green: SCF, DLK1, and CXCL12. Representative images from two different experiments are shown. The scale bar applies to all panels.
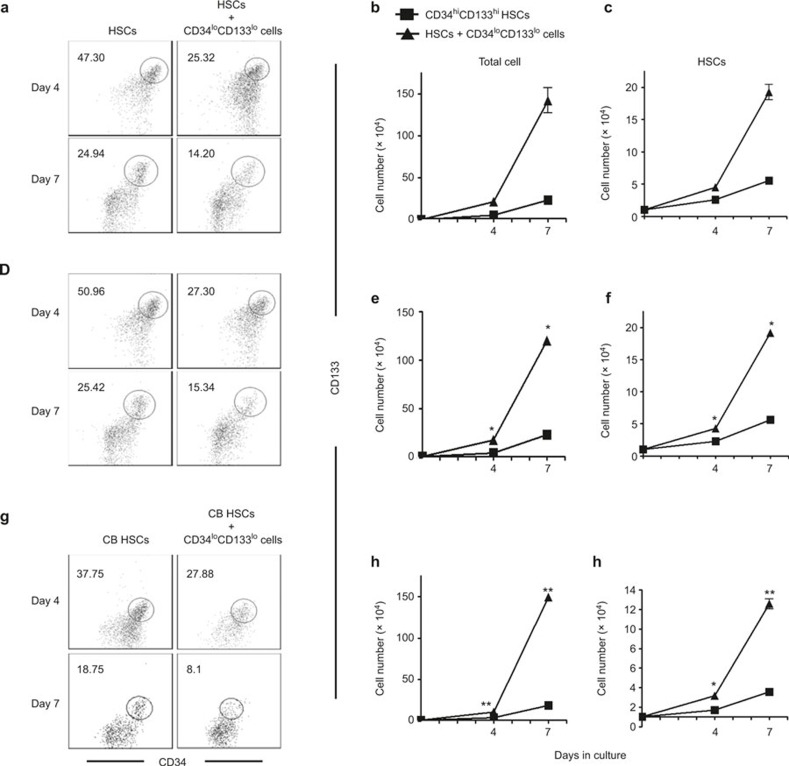
To determine whether CD34loCD133lo cells support the ex vivo expansion of HSCs, CD34hiCD133hi HSCs and CD34loCD133lo cells were purified by cell sorting and seeded at a ratio of 1:7, respectively. Cultures of HSCs alone were used as a control. At the beginning of the co-culture, >95% of the initial HSCs were CD34hiCD133hi. After culture for 4 days, the percentage of CD34hiCD133hi cells decreased to ∼25% in the co-cultures and ∼45% in the control cultures (Figure 4A). The percentages continued to decrease, and by day 7 CD34hiCD133hi cells accounted for ∼14% of cells in the co-cultures and ∼25% in the control cultures. When CD34loCD133lo cells were cultured alone in the same HSC expansion medium, they attached to the bottom of wells and did not grow; hence, we counted the cells grown in suspension as expanded hematopoietic cells. Despite the decrease in the percentages of CD34hiCD133hi cells in the cultures because the total cell numbers increased dramatically (∼140-fold in co-culture versus ∼16-fold in control on day 7) (Figure 4B), the actual numbers of CD34hiCD133hi HSCs were increased ∼18-fold in co-cultures, and ∼3-fold in control cultures at day 7 (Figure 4C).
Figure 4.
Ex vivo expansion of HSCs by co-culture with CD34loCD133lo cells. CD34hiCD133hi HSCs and CD34loCD133lo cells were purified from FL CD34+ cells by sorting. CD34hiCD133hi CB HSCs were purified from CB. Co-cultures were prepared by mixing HSCs and CD34loCD133lo cells at a ratio of 1:7. HSCs alone were used as a control culture. Non-adherent cells in the CD34loCD133lo cell co-cultures and control cultures were harvested at 4 and 7 days, counted, and analyzed for CD34 and CD133 expression. All the experiments were repeated twice. (A–C) FL CD34hiCD133hi HSCs and CD34loCD133lo cells were used for culture. (A) Representative CD34 versus CD133 staining profiles of cultured cells at day 4 and day 7 are shown. The number indicates the percentage of CD34hiCD133hi HSCs in the gated region. (B) The amount of total non-adherent cells, and (C) CD34hiCD133hi HSCs in culture. (D–F) FL CD34hiCD133hi HSCs and CD34loCD133lo cells were used for culture. Transwells were used to block cell–cell contact between HSCs and CD34loCD133lo cells. (D) Representative CD34 versus CD133 staining profiles of cultured cells at day 4 and day 7 are shown. The number indicates the percentage of CD34hiCD133hi HSCs in the gated region. (E) Numbers of total non-adherent cells and (F) CD34hiCD133hi HSCs in culture. (G–I) CB CD34hiCD133hi HSCs and CD34loCD133lo cells were used for culture. (G) Representative CD34 versus CD133 staining profiles of cultured cells at day 4 and day 7 are shown. The number indicates the percentage of CD34hiCD133hi HSCs in the gated region. (H) Numbers of total non-adherent cells and (I) CD34hiCD133hi HSCs in culture. Data are shown as the mean ± SEM of 2 independent experiments. *p <0.05.
To investigate whether direct cell–cell contact is required for the HSC-CD34loCD133lo co-culture cell expansion, the contact between HSCs and CD34loCD133lo cells was blocked by a transwell. Compared to co-culture without the transwell, the percentage of CD34hiCD133hi cells (Figure 4D), total and CD34hiCD133hi cell numbers (Figure 4E and F) were not significantly different. This result suggests that the HSC expansion is supported by soluble growth factors rather than contact-dependent signals produced by CD34loCD133lo cells.
The use of CB transplants has increased for both children and adults to cure blood diseases. Given that the HSC expansion supported by CD34loCD133lo cells is independent of autologous cell–cell contact, it is interesting to test whether our co-culture system could also support the expansion of allogeneic CB HSCs. Purified CD34+CD133+ CB HSCs were co-cultured with CD34loCD133lo cells at a ratio of 1:7. Similar to FL HSCs, CB HSCs expanded well in co-culture with CD34loCD133lo cells (Figure 4G). The results of this experiment demonstrated that the numbers of total cells (Figure 4H) and CD34+CD133+ CB HSCs (Figure 4I) dramatically increased by 150-fold and 12-fold, respectively, in co-culture. These results show that the growth factors produced by CD34loCD133lo cells also support the expansion of allogeneic HSCs.
Expanded HSCs maintain long-term repopulating activity in vivo
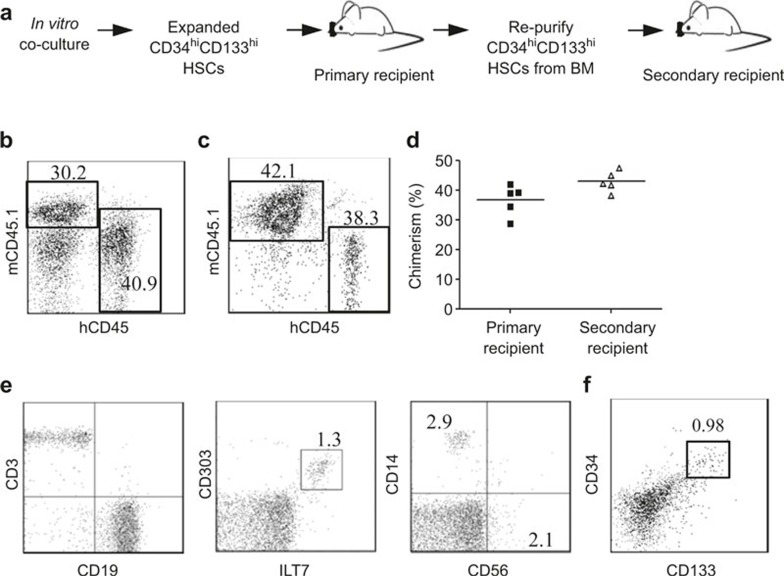
To investigate whether the HSCs expanded in our co-culture system maintained their stemness and long-term self-renewing capacities, we assessed the repopulating activity of expanded cells by in vivo secondary transplantation and serial limiting dilution in immunodeficient NSG mice. For secondary transplantation (Figure 5A), CD34hiCD133hi HSCs were purified from CD34loCD133lo cells from the co-cultures and injected into sublethally irradiated neonates at 100 000 cells per mouse. Eight weeks post-transplantation, the human chimerism in the mouse peripheral blood and BM was analyzed by the staining of human CD45 and mouse CD45.1. Among total peripheral blood mononuclear cells (PBMCs), human CD45+ leukocyte reconstitution levels ranged from 10% to 60% (Figure 5B). In BM, the chimera levels ranged from 15% to 45% (Figure 5C). Additionally, CD34+ cells were purified from BM mononuclear cells (MNCs) and transplanted into sublethally irradiated secondary recipient neonates at 100 000 CD34hiCD133hi HSCs per mouse. Eight weeks post transplantation, PBMCs and BM MNCs were analyzed by staining human CD45 and mouse CD45.1. The human CD45+ cell chimerism in primary and secondary recipient mice was similar (Figure 5D). All the major immune cell types, including human CD56+ NK cells, CD14+ monocytes/macrophages, CD3+ T cells, CD19+ B cells, and CD303+ILT7+ plasmacytoid dendritic cells, were found in the secondary recipient mice (Figure 5E). Moreover, CD34hiCD133hi HSCs were still detectable, with a frequency of ∼1% in the BM of secondary recipient mice (Figure 5F). These results indicate that despite ∼15-fold expansion, HSCs from our co-culture system maintain their long-term repopulating capability.
Figure 5.
Long-term repopulating capacity of expanded HSCs. CD34hiCD133hi HSCs and CD34loCD133lo cells were purified from FL CD34+ cells by cell sorting. Co-cultures were set up by mixing HSCs and CD34loCD133lo cells at a ratio of 1:7 to expand HSCs. CD34hiCD133hi HSCs were purified from the day 7 co-culture and injected into sublethally irradiated NSG pups at 100 000 cells/mouse (primary recipient mice, n = 5). Eight weeks later, PBMCs and BM MNCs were prepared from primary recipient mice and analyzed by flow cytometry. Subsequently, CD34+ cells were purified from the BM of primary recipient mice and injected into sublethally irradiated NSG pups at 100 000 CD34hiCD133hi HSCs/mouse (secondary recipient mice, n = 5). Eight weeks later, PBMCs and BM MNCs were prepared from secondary recipient mice for flow analysis. (A) Experimental flow of secondary transplantation. Representative human CD45 (hCD45) versus mouse CD45.1 (mCD45.1) staining profile of PMBCs (B) BM MNCs (C) from primary recipients is shown. The number indicates the percentage of cells in the gated region. (D) Plots indicating the chimerism levels in the BM of individual primary and secondary mice. Chimerism was calculated using the formula [%hCD45+/(%hCD45+ + %mCD45+)]. Each symbol represents one mouse, and the horizontal line indicates the mean value. (E) CD3 versus CD19, CD303 versus ILT7, and CD56 versus CD14 staining profiles gated on human CD45+ cells in PBMCs from secondary mice. Representative plots are shown. The numbers indicate the percentage of cells in the gated regions. (F) A representative CD133 versus CD133 staining profile is shown human CD45+ cells from BM MNCs from secondary mice. The number indicates the percentage of cells in the gated region.
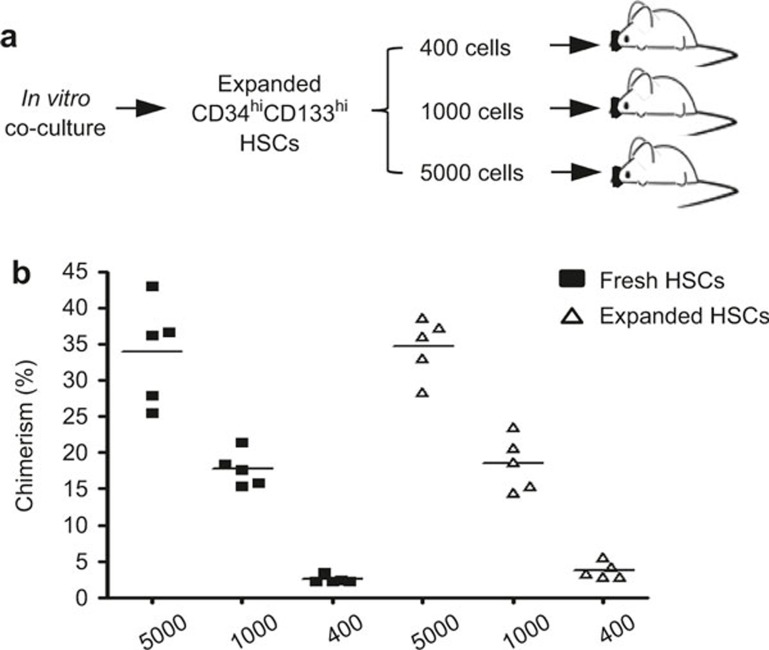
For the serial limiting dilution assay, CD34hiCD133hi HSCs were purified from CD34loCD133lo cells from the co-cultures. As a comparison, CD34hiCD133hi HSCs freshly isolated from the same FL donor were used. Both the fresh and expanded CD34hiCD133hi HSCs were diluted, and 400, 1000, or 5000 cells were injected into each sublethally irradiated neonate (Figure 6A). Even with these limited number of HSCs engrafted, considerable levels of human chimerism were achieved in the BM of recipient mice. Furthermore, both the expanded and fresh HSCs gave rise to similar levels of chimerism (Figure 6B). These results show that despite dramatic expansion, the cultured HSCs maintained the same self-renewing and repopulating capacities found in fresh HSCs.
Figure 6.
In vivo transplantation of expanded HSCs with limiting dilutions. CD34hiCD133hi HSCs were purified from the day 7 co-cultures and injected into sublethally irradiated NSG pups at 400, 1000, or 5000 cells/mouse (n = 5 of each group). Freshly isolated CD34hiCD133hi HSCs from the same donor were used as a control. Eight weeks later, BM MNCs were prepared and stained for mouse CD45.1 and human CD45. (A) Experimental flow of the limiting dilution assay. (B) Plots indicating the level of chimerism in the BM of individual mice injected with indicated number of HSCs. Each symbol represents one mouse, and the horizontal line indicates the mean value.
DISCUSSION
Our discovery of CD34loCD133lo cells may provide new insights into the early embryonic liver development. CD34loCD133lo cells are different from the previously reported hepatic progenitor cells because they express liver-associated endodermal genes (ALB, AFP, and CK19), general endodermal genes (laminin, GATA4, and SOX7) and mesodermal genes (CD73, VE-Cadherin, and vimentin). CD34loCD133lo cells uniformly express ALB, AFP, SCF, DLK1, and CXCL12 on the protein level, and therefore they are likely not a mixture of mesodermal and endodermal lineage cells but are likely a unique population. CD34loCD133lo cells have multipotent properties that enable them to differentiate into many types of endodermal and mesodermal cell lineages, such as hepatocytes, adipocytes, osteocytes, and endothelial cells, suggesting that they may be mesenchymal–epithelial transitional cells. Many groups have shown that mesenchymal stem cells (MSCs)16 or mesodermal multipotent adult progenitor cells17 in both mice and humans can differentiate into hepatocytes. Recently, Dan et al. reported the isolation of multipotent progenitor cells from human FL (hFLMPCs) might have a mesendodermalorigin.18 These hFLMPCs can differentiate into hepatocytic and mesodermal lineage cells but do not express hepatic markers such as ALB and AFP. CD34loCD133lo cells are clearly different from MSCs, multipotent adult progenitor cells, and hFLMPCs because CD34loCD133lo cells express endodermal markers. It has been hypothesized that the mesoderm and endoderm arise from a bipotential germ layer called the mesendoderm.19 CD34loCD133lo cells carry characteristics of both mesoderm and endoderm; however, whether they originated from mesendodermremains unknown.
From previous studies, it is known that the fate of HSCs is highly dependent on their in vivo niche.20 There have been studies attempting to characterize HSC niches in major hematopoietic organs such as CB, adult BM, and fetal tissues by using stromal cells such as MSCs from these organs to mimic the in vivo environment in ex vivo co-culture systems to support HSCs expansion.12,21,22,23,24,25,26 Depending on the co-culture conditions, 80- to 800-fold increases in total cell numbers and 4- to 100-fold increases in CD34+ cells were reported. As shown in Figure 1A, CD34loCD133lo cells occupy ∼20% of CD34+ FL cells, and CD34hiCD133hi HSCs have a similar frequency. From fresh FLs collected at 16–23 gestation weeks, we found that the frequency of CD34+ cells is approximately 3–15% (unpublished data). Thus, CD34loCD133lo cells constitute approximately 0.6∼3% of cells in the FL. It has been observed that HSCs exponentially increase in number within the liver during early embryonic development.27 Given their relative abundance, we believe that CD34loCD133lo cells are crucial in creating a microenvironment for the expansion of HSCs in the FL. In our previous study, we demonstrated that CD34hiCD133hi FL cells are long-term HSCs with high in vivo repopulating and ex vivo colony-forming capacities, whereas CD34hiCD133− cells are hematopoietic progenitor cells that are more differentiated and do not repopulate NSG mice in vivo.14 Therefore, in this study, we used CD34 and CD133 co-expression in cultured cells as markers for long-term HSC activity. We verified that CD34loCD133lo cells have a high expression of growth factors important for HSC homeostasis and expansion, including SCF, DLK1, IGF2, CXCL12, and ANGPTLs. Furthermore, we showed that CD34loCD133lo cells were able to support an extensive expansion of both FL and CB HSCs ex vivo, achieving a ∼130-fold increase in the total cell number and a ∼18-foldexpansion of CD34hiCD133hi HSCs within 7 days. Although some studies have suggested that cell contact between HSCs and feeder cells are essential to expand the HSCs,12,28,29 we determined that CD34loCD133lo cell-mediated HSC expansion is largely independent of direct cell contact.
A variety of assays have been developed to determine the stemness or stem cell activity of expanded HSCs. The key assays demonstrate that stem cells are functional: they must exhibit self-renewal and the ability to generate appropriate tissues. The best assays available to study this property in putative human HSCs involve xeno-transplantation into immune-deficient mice. Our in vivo assessment showed that even after extensive expansion, the HSCs in CD34loCD133lo cell co-cultures were able to maintain their phenotype and stemness, as demonstrated by in vivo transplantation and serial limiting dilutions. Most importantly, the co-culture system also successfully applies to the expansion of allogenic HSCs, i.e., CB HSCs and potentially other adult HSCs, which broadens the application of this system within cell therapy areas in clinics.
In summary, our findings indicate that CD34+ FL cells can be classified into CD34hi hematopoietic lineages (CD34hiCD133hi HSCs and CD34hiCD133− hematopoietic progenitors) and CD34loCD133lo cells. Phenotyping and functional assays of CD34loCD133lo cells indicate that this unique cell population is an important component in organ development and the stem cell microenvironment. It would be of great interest to identify the critical growth factors secreted by CD34loCD133lo cells and use these to optimize a feeder-cell-free protocol for HSC expansion, which would greatly benefit the treatment of blood diseases. Given the high demand for stem cell therapy and a cure for cancer, this population of cells has a potential clinical significance, and discovering its underlying mechanisms can open doors for many unanswered questions.
AUTHORS' CONTRIBUTIONS
Kylie Su Mei Yong designed and performed experiments, analyzed and interpreted data, and prepared the manuscript; Choong Tat Keng performed experiments; Shu Qi Tan, Eva Loh, Kenneth TE Chang, and ThiamChye Tan provided the research tools and reagents; and Qingfeng Chen and Wanjin Hong conceived the study, supervised the project and prepared the manuscript.
Acknowledgments
We thank Sue Yee Tan and Jessica Jie Ying Ong for providing excellent technical support. This study was supported by the Institute of Molecular and Cell Biology, the Agency for Science, Technology and Research (A*STAR), Singapore and A*STAR Joint Council Office Development Programme 1334k00082.
The authors have no financial conflict of interest.
Footnotes
Supplementary information accompanies the manuscript on Cellular & Molecular Immunology's website (http://www.nature.com/cmi/)
Supplementary Information accompanies the paper on Cellular & Molecular Immunology website.
Supplementary Information
References
- Martinez-Agosto JA, Mikkola HKA, Hartenstein V, Banerjee U. The hematopoietic stem cell and its niche: a comparative view. Genes Dev 2007; 21: 3044–3060. [DOI] [PubMed] [Google Scholar]
- Zhang CC, Kaba M, Ge G, Xie K, Tong W, Hug C et al. Angiopoietin-like proteins stimulate ex vivo expansion of hematopoietic stem cells. Nat Med 2006; 12: 240–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou S, Lodish HF. Fetal liver hepatic progenitors are supportive stromal cells for hematopoietic stem cells. PNAS 2010; 107: 7799–7804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartier N, Hacein-Bey-Abina S, Bartholomae CC, Veres G, Schmidt M, Kutschera I et al. Hematopoietic stem cell gene therapy with a lentiviral vector in X-linked adrenoleukodystrophy. Science 2009; 326: 818–823. [DOI] [PubMed] [Google Scholar]
- Le Blanc K, Ringdén O. Mesenchymal stem cells: properties and role in clinical bone marrow transplantation. Curr Opin Immunol 2006; 18:586–591. [DOI] [PubMed] [Google Scholar]
- Ishii T, Eto K. Fetal stem cell transplantation: past, present, and future. World J Stem Cells 2014; 6: 404–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehman K, Iqbal MJ, Zahra N, Akash MSH. Liver stem cells: from preface to advancements. Curr Stem Cell Res Ther 2014; 9: 10–21. [DOI] [PubMed] [Google Scholar]
- Herrmann RP, Sturm MJ. Adult human mesenchymal stromal cells and the treatment of graft versus host disease. Stem Cells and Cloning 2014: 7: 45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner JE, Barker JN, DeFor TE, Baker KS, Blazar BR, Eide C et al. Transplantation of unrelated donor umbilical cord blood in 102 patients with malignant and nonmalignant diseases: influence of CD34 cell dose and HLA disparity on treatment-related mortality and survival. Blood 2002; 100: 1611–1618. [DOI] [PubMed] [Google Scholar]
- Colter DC, Class R, DiGirolamo CM, Prockop DJ. Rapid expansion of recycling stem cells in cultures of plastic-adherent cells from human bone marrow. PNAS 2000; 97: 3213–3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake AC, Khoury M, Leskov I, Iliopoulou BP, Fragoso M, Lodish H et al. Human CD34+ CD133+ hematopoietic stem cells cultured with growth factors including Angptl5 efficiently engraft adult NOD-SCID Il2rc2/2 (NSG) mice. PLoS One 2011; 6: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoury M, Drake A, Chen Q, Dong D, Leskov I, Fragoso MF et al. Mesenchymal stem cells secreting angiopoietin-like-5 support efficient expansion of human hematopoietic stem cells without compromising their repopulating potential. Stem Cells Dev 2011; 20: 1371–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walasek MA, Os Rv, Haan Gd. Hematopoietic stem cell expansion: challenges and opportunities. Ann New York Acad Sci 2012; 1266: 138–150. [DOI] [PubMed] [Google Scholar]
- Chen Q, Khoury M, Limmon G, Choolani M, Chan JKY, Chen J. Human fetal hepatic progenitor cells are distinct from, but closely related to, hematopoetic stem/progenitor cells. Stem Cells 2013; 31: 1160–1169. [DOI] [PubMed] [Google Scholar]
- Sugiyama T, Kohara H, Noda M, Nagasawa T. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity 2006; 25: 977–988. [DOI] [PubMed] [Google Scholar]
- Christ B, Stock P. Mesenchymal stem cell-derived hepatocytes for functional liver replacement. Front Immunol 2012; 3: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz RE, Reyes M, Koodie L, Jiang Y, Blackstad M, Lund T et al. Multipotent adult progenitor cells from bone marrow differentiate into functional hepatocyte-like cells. J Clin Investig 2002; 109: 1291–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dan YY, Riehle KJ, Lazaro C, Teoh N, Haque J, Campbell JS et al. Isolation of multipotent progenitor cells from human fetal liver capable of differentiating into liver and mesenchymal lineages. PNAS 2006; 103: 9912–9917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodaway A, Patient R. Mesendoderm: an ancient germ layer? Cell 2001; 105: 169–172. [DOI] [PubMed] [Google Scholar]
- Noll JE, Williams SA, Purton LE, Zannettino ACW. Tug of war in the haematopoietic stem cell niche: do myeloma plasma cells compete for the HSC niche? Blood Cancer J 2012; 2: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walenda T, Bork S, Horn P, Wein F, Saffrich R, Diehlmann A et al. Mesenchymal stem cells in the Wharton's jelly of the human umbilical cord. J Cell Mol Med 2010; 14: 337–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H-S, Hung S-C, Peng S-T, Huang C-C, Wei H-M, Guo Y-J et al. Mesenchymal stem cells in the Wharton's jelly of the human umbilical cord. Stem Cells 2004; 22: 1330–1337. [DOI] [PubMed] [Google Scholar]
- Bakhshi T, Zabriskie RC, Bodie S, Kidd S, Ramin S, Paganessi LA et al. Mesenchymal stem cells from the Wharton's jelly of umbilical cord segments provide stromal support for the maintenance of cord blood hematopoietic stem cells during long-term ex vivo culture. Transfusion 2008; 48: 2638–2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee OK, Kuo TK, Chen W-M, Lee K-D, Hsieh S-L, Chen T-H. Isolation of multipotent mesenchymal stem cells from umbilical cord blood. Blood 2004; 103: 1669–1675. [DOI] [PubMed] [Google Scholar]
- Huang G-P, Pan Z-J, Jia B-B, Zheng Q, Xie C-G, Gu J-H et al. Ex vivo expansion and transplantation of hematopoietic stem/progenitor cells supported by mesenchymal stem cells from human umbilical cord blood. Cell Transplant 2007; 16: 579–585. [DOI] [PubMed] [Google Scholar]
- Campagnoli C, Roberts IAG, Kumar S, Bennett PR, Bellantuono I, Fisk NM. Identification of mesenchymal stem/progenitor cells in human first-trimester fetal blood, liver, and bone marrow. Blood 2001; 98: 2396–2402. [DOI] [PubMed] [Google Scholar]
- Ivanovs A, Rybtsov S, Welch L, Anderson RA, Turner ML, Medvinsky A. Highly potent human hematopoietic stem cells first emerge in the intraembryonic aorta-gonad-mesonephros region. J Exp Med 2011; 208: 2417–2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isern J, Martín-Antonio B, Ghazanfari R, Martín AM, López JA, Toro Rd et al. Self-renewing human bone marrow mesenspheres promote hematopoietic stem cell expansion. Cell 2013; 3: 1714–1724. [DOI] [PubMed] [Google Scholar]
- Dzierzak EA, Harvey KN. Cell-cell contact and anatomical compatibility in stromal cell-mediated HSC support during development. Stem Cells 2004; 22: 253–258. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.