Anaesthesia and evidence-based medicine (EBM) are considered as two of the 15 most important medical milestones.[1] The birth of anaesthesia on 16th October 1846 and the subsequent publication of this discovery as a case study 33 days later in the high-impact Boston Medical and Surgical Journal (the current New England Journal of Medicine) resulted in its widespread use that dramatically changed surgical practice.[2] Likewise, EBM, born at McMaster University in the early 90s, has had a considerable impact on the modern day health-care practice.[3]
WHAT IS EVIDENCE-BASED MEDICINE?
The term EBM first appeared in the published literature in 1991;[3] 5 years later, the most-cited EBM landmark article described EBM as the conscientious, explicit and judicious use of the current best evidence in making decisions about the care of individual patients.[4] Another characterisation, appearing a few years later, described EBM as a systematic approach to clinical problem-solving that allows integration of the best available research evidence with clinical expertise and patient values.[5] A final characterisation highlights three key principles underlying optimal clinical practice: systematic summaries of the best evidence, a schema for deciding what constitutes the best evidence and the prominent consideration of individual patient values and preferences.[6]
To understand the importance of EBM, one can begin with an appreciation of how medicine was practiced before EBM.
Period before evidence-based medicine
Before EBM thinking began to impact on its structure, clinical practice relied on expert advice – often driven by physiological reasoning and individual clinicians' experience. This emphasis resulted in a significant gap between the available evidence and the actual clinical practice. Two examples will demonstrate why this was a problem.
In 1992, Antman et al. published findings of their study comparing recommendations by experts with results of meta-analyses of randomised controlled trials (RCTs) (the best available evidence at the time recommendations were made) for treating myocardial infarction (MI).[7] For thrombolytic therapy for MI, after publication of 30 trials with >6000 patients, a 25% reduction in odds of death was observed. Despite this, additional trials recruiting another 40,000 patients, half of whom did not receive the proven benefits of thrombolytic therapy, were conducted. Disagreement between the experts providing recommendations of this therapy for the treatment of MI necessitated producing essentially redundant evidence, long after the answer was in. Only a decade after benefits were securely established, when evidence became completely overwhelming, did experts finally achieve a consensus regarding the use of thrombolytic therapy in ST-elevation MI.
In another example, despite accumulating RCT evidence demonstrating no benefit and a suggestion of possible increased mortality from prophylactic lignocaine as antiarrhythmic agent for MI, for two decades, experts continued to recommend its use. Similar examples are available in our specialty. Despite evidence of lack of benefit regarding the effectiveness of cricoid pressure during rapid sequence induction for intubation during general anaesthesia,[8] this technique continues to be practiced and recommended by experts.[9]
The extreme examples of inconsistency between evidence and expert recommendations are due to non-availability of systematic summaries to make rational clinical decisions, resulting in reliance on preconceptions and low-quality evidence from individual studies.
How to practice evidence-based medicine for improving patient care? The evidence-based medicine process
When clinicians encounter patient care decisions, optimal practice demands knowledge and application of the relevant evidence. This can be achieved by following the 5A technique: ask a structured question, acquire relevant evidence, appraise the evidence, evaluate applicability of the findings to the patient care (generalisability and significance to the patient) and finally, act, involving the patient in the decision-making process [Figure 1].[10]
Figure 1.
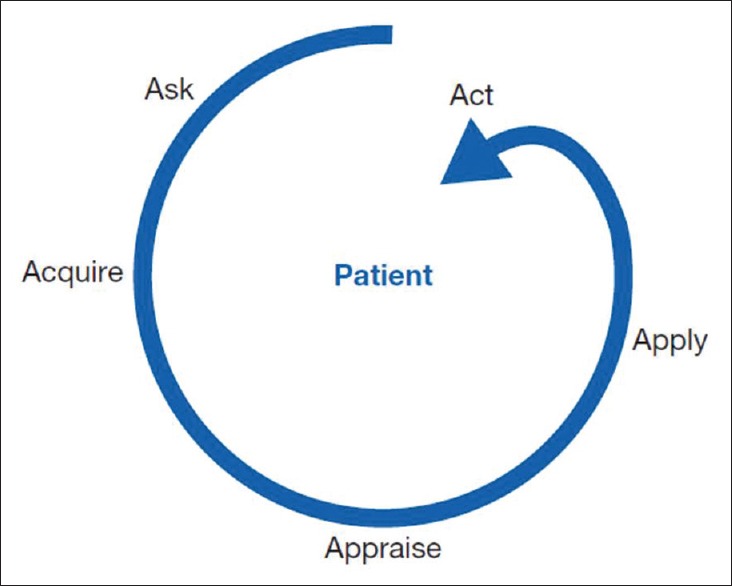
The 5 A approach to using the medical literature to provide optimal patient care. (Reproduced with permission from Guyatt G, Rennie D, Meade MO, Cook DJ. Users' Guides to the Medical Literature: A Manual for Evidence-Based Clinical Practice, 3rd ed. New York, NY: McGraw-Hill; 2015. http://jamaevidence.com. Copyright 2015 American Medical Association.)
A 6th A, ‘Assess’ is also used to evaluate the patients before the beginning of the EBM cycle and also to assess performance of the patients at the end of this process.
Appraising the evidence involves the recognition that some evidence is more trustworthy than other evidence, and an understanding of how one can distinguish between the more and the less trustworthy. Appraisal skills allow patients and physicians to make clinical decisions based on the best available evidence; allow health-care policy makers to frame and implement wise decisions and guide researchers in designing, implementing and disseminating higher quality studies.
In the older hierarchy of evidence, RCTs were placed at the highest level and case series were placed at the lowest level based on the probability of bias and risk of systematic errors.[11] However, evidence from RCTs is not always of the highest quality, and not all research questions can be answered through RCTs for either practical (eg., incidence of post-operative cognitive dysfunction) or ethical reasons (eg., cardiopulmonary resuscitation).
Over several years, it was recognised that the quality of evidence depends not only on study design but also on a number of other factors, including the extent of risk of bias in study implementation, imprecision, inconsistency, indirectness (inapplicability to the patient at hand), the possibility of publication bias,[12] the magnitude of treatment effect and the presence of a dose-response gradient. One can apply the new hierarchy of evidence to the questions of diagnosis, therapy and prognosis, and ultimately classify evidence on a spectrum from high to low quality.[13]
Finding the current best evidence
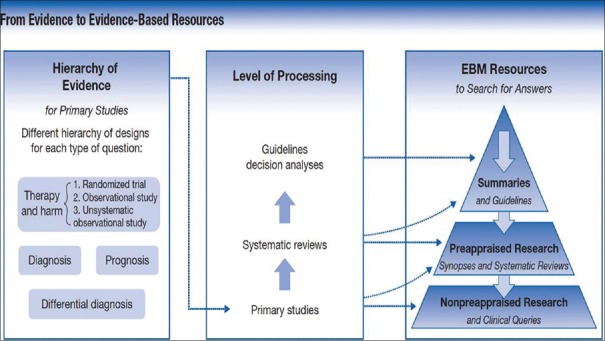
About 2000 articles are indexed in PubMed every day,[14] and a search, for example, on post-operative nausea and vomiting on PubMed provides approximately 8000 citations. This makes it extremely difficult to identify the current best evidence from a mix of RCTs, reviews, case reports and editorials for relevance and application in clinical practice. Pre-appraised EBM resources provide rapid and efficient path for searching answers for clinical questions. Finding the best evidence involves an understanding of hierarchies of evidence that we have described, the level of processing of the information and the desirability of beginning one's search for information with the maximum level of processing (such as trustworthy clinical practice guidelines) [Figure 2].[15]
Figure 2.
From evidence to evidence-based resources: the new hierarchy. (Reproduced with permission from Guyatt G, Rennie D, Meade MO, Cook DJ. Users' Guides to the Medical Literature: A Manual for Evidence-Based Clinical Practice, 3rd ed. New York, NY: McGraw-Hill; 2015. http://jamaevidence.com. Copyright 2015 American Medical Association.)
Although it is possible that the highest quality evidence may not exist for a particular clinical problem, using this approach increases the efficiency of finding the best current evidence for application into clinical practice and reduces the burden of decision-making.
Importance of high-quality recommendations which have good evidence summaries using grading of recommendation, assessment, development and evaluation
The new hierarchy of evidence is best expressed in the grading of recommendation, assessment, development and evaluation (GRADE) framework, which provides guidance on evaluating and rating the quality of body of evidence in healthcare.[16] This system is endorsed by >100 organisations and scientific societies such as the World Health Organization, the Cochrane Collaboration, the Agency for Healthcare Research and Quality and the National Institute for Health and Care Excellence. The system provides a transparent method to judge the quality of evidence for individual outcomes summarised in systematic reviews (ideally summarised in a meta-analysis), though in a pinch when a systematic review is not available, one can apply it to less systematic summaries. This approach includes higher rating for evidence from RCTs and then possibly rating down based on five key factors mentioned previously: inconsistency, indirectness, imprecision, risk of bias and publication bias. Non-RCTs begin as low-quality evidence but can be rated up if the magnitude of effect is large, and if there is evidence to suggest a dose-response effect. The GRADE (Grading of Recommendations Assessment, Development and Evaluation) framework also provides rationale for judgements regarding the strength of recommendations. This approach has been utilised in anaesthesia literature such as to summarise the best evidence and develop recommendations on central venous access device placements,[17] for anaesthesia and perioperative management of patients with neuromuscular disorders[18] and Scandinavian Society of Anaesthesiology and Intensive Care Medicine clinical practice guideline on pre-hospital airway management.[19]
In summary, EBM addresses the deficiencies in clinical care that rely on expert opinion based on physiological reasoning and unstructured use of evidence and provides in its place a coherent framework for assessing and applying the best available evidence to clinical care decisions.
REFERENCES
- 1.What is the most important medical advance since 1840? [Last cited on 2016 Jul 19];BMJ. 2007 334(Suppl 1) Available from: http://www.bmj.com/content/suppl/2007/01/18/334.suppl_1.DC3 . [Google Scholar]
- 2.Bigelow HJ. Insensibility during surgical operations produced by inhalation. [Last cited on 2016 Jul 19];Boston Med Surg J. 1846 35:309–17. Available from: http://www.nejm.org/doi/abs/10.1056/NEJM184611180351601 . [Google Scholar]
- 3.Guyatt GH. Evidence-based medicine. [Last accessed on 2015 May 14];ACP J Club. 1991 114:A16. Available from: http://www.acpjc.org/Content/114/2/issue/ACPJC-1991-114-2-A16.htm . [Google Scholar]
- 4.Sackett DL, Rosenberg WM, Gray JA, Haynes RB, Richardson WS. Evidence based medicine: What it is and what it isn't. Br Med J. 1996;312:71–2. doi: 10.1136/bmj.312.7023.71. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2349778/pdf/bmj00524-0009.pdf . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sackett DL, Strauss SE, Richardson WS, Rosenberg W, Haynes RB. Evidence-Based Medicine: How to Practice and Teach EBM. 2nd ed. Edinburgh: Churchill Livingstone; 2000. [Google Scholar]
- 6.Guyatt G, Jaeschke R, Wilson MC, Montori V, Richardson WS. What is evidence-based medicine? In: Guyatt GH, Rennie D, Meade MO, Cook DJ, editors. Users' Guide to the Medical Literature: A Manual for Evidence-Based Clinical Practice. 3rd ed. New York: McGraw-Hill; 2015. pp. 7–14. [Google Scholar]
- 7.Antman EM, Lau J, Kupelnick B, Mosteller F, Chalmers TC. A comparison of results of meta-analyses of randomized control trials and recommendations of clinical experts. Treatments for myocardial infarction. JAMA. 1992;268:240–8. [PubMed] [Google Scholar]
- 8.Algie CM, Mahar RK, Tan HB, Wilson G, Mahar PD, Wasiak J. Effectiveness and risks of cricoid pressure during rapid sequence induction for endotracheal intubation. In: Wasiak J, editor. Cochrane Database of Systematic Reviews. Chichester, UK: John Wiley and Sons, Ltd; 2015. [Last cited on 2016 Jul 22]. Available from: http://www.doi.wiley.com/10.1002/14651858.CD011656.pub2 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Difficult Airway Society. Intubation Guidelines – Rapid Sequence Induction. [Last cited on 2016 Jul 20]. Available from: https://www.das.uk.com/guidelines/rsi.html .
- 10.Guyatt G, Meade MO. How to use the medical literature -and this book -to improve your patient care. In: Guyatt G, Rennie D, Meade MO, Cook DJ, editors. Users' Guide to the Medical Literature: A Manual of Evidence Based Clinical Practice. 3rd ed. New York: McGraw-Hill; 2015. pp. 3–6. [Google Scholar]
- 11.Sackett DL. Rules of evidence and clinical recommendations on the use of antithrombotic agents. Chest. 1989;95(2 Suppl):2S–4S. [PubMed] [Google Scholar]
- 12.Guyatt GH, Oxman AD, Montori V, Vist G, Kunz R, Brozek J, et al. GRADE guidelines: 5.Rating the quality of evidence - publication bias. J Clin Epidemiol. 2011;64:1277–82. doi: 10.1016/j.jclinepi.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 13.Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–6. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Glasziou P, Burls A, Gilbert R. Evidence based medicine and the medical curriculum. BMJ. 2008;337:a1253. doi: 10.1136/bmj.a1253. [DOI] [PubMed] [Google Scholar]
- 15.Agoritsas T, Vandvik PO, Neumann I, Rochwerg B, Jaeschke R, Hayward R, et al. Finding current best evidence. In: Guyatt G, Rennie D, Meade MO, Cook DJ, editors. Users' Guides to the Medical Literature: A Manual of Evidence-Based Clinical Practice. 3rd ed. New York: McGraw-Hill; 2015. pp. 29–50. [Google Scholar]
- 16.Guyatt GH, Oxman AD, Schünemann HJ, Tugwell P, Knottnerus A. GRADE guidelines: A new series of articles in the Journal of Clinical Epidemiology. J Clin Epidemiol. 2011;64:380–2. doi: 10.1016/j.jclinepi.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 17.Moureau N, Lamperti M, Kelly LJ, Dawson R, Elbarbary M, van Boxtel AJ, et al. Evidence-based consensus on the insertion of central venous access devices: Definition of minimal requirements for training. Br J Anaesth. 2013;110:347–56. doi: 10.1093/bja/aes499. [DOI] [PubMed] [Google Scholar]
- 18.Racca F, Mongini T, Wolfler A, Vianello A, Cutrera R, Del Sorbo L, et al. Recommendations for anesthesia and perioperative management of patients with neuromuscular disorders. Minerva Anestesiol. 2013;79:419–33. [PubMed] [Google Scholar]
- 19.Rehn M, Hyldmo PK, Magnusson V, Kurola J, Kongstad P, Rognås L, et al. Scandinavian SSAI clinical practice guideline on pre-hospital airway management. Acta Anaesthesiol Scand. 2016;60:852–64. doi: 10.1111/aas.12746. [DOI] [PMC free article] [PubMed] [Google Scholar]