Abstract
The CPS1 gene was identified as a virulence factor in the maize pathogen Cochliobolus heterostrophus. Hypothesizing that the homologous gene in Coccidioides posadasii could be important for virulence, we created a Δcps1 deletion mutant which was unable to cause disease in three strains of mice (C57BL/6, BALB/c, or the severely immunodeficient NOD-scid,γcnull [NSG]). Only a single colony was recovered from 1 of 60 C57BL/6 mice following intranasal infections of up to 4,400 spores. Following administration of very high doses (10,000 to 2.5 × 107 spores) to NSG and BALB/c mice, spherules were observed in lung sections at time points from day 3 to day 10 postinfection, but nearly all appeared degraded with infrequent endosporulation. Although the role of CPS1 in virulence is not understood, phenotypic alterations and transcription differences of at least 33 genes in the Δcps1 strain versus C. posadasii is consistent with both metabolic and regulatory functions for the gene. The in vitro phenotype of the Δcps1 strain showed slower growth of mycelia with delayed and lower spore production than C. posadasii, and in vitro spherules were smaller. Vaccination of C57BL/6 or BALB/c mice with live Δcps1 spores either intranasally, intraperitoneally, or subcutaneously resulted in over 95% survival with mean residual lung fungal burdens of <1,000 CFU from an otherwise lethal C. posadasii intranasal infection. Considering its apparently complete attenuation of virulence and the high degree of resistance to C. posadasii infection when used as a vaccine, the Δcps1 strain is a promising vaccine candidate for preventing coccidioidomycosis in humans or other animals.
INTRODUCTION
Coccidioides species (C. immitis and C. posadasii) are the causative agents of coccidioidomycosis (valley fever), an important emerging disease endemic to the southwestern United States and elsewhere in the Western Hemisphere (1–3). Inhalation of a 2- to 4-μm arthroconidium (spore) initiates a respiratory infection and grows as a unique parasitic phase structure, the spherule, to 80 to 100 μm in diameter (4). During spherule maturation, which in mice takes approximately 4 days, internal cell division and septation results in hundreds of endospores that, if released, can reinitiate spherule growth in the infected tissue. Although many infections resolve without medical intervention, about 40% of infections cause respiratory illnesses that often last weeks to many months (5). In a small percentage of patients, infection disseminates from the lungs hematogenously to produce progressive, protracted, and even fatal complications. With or without clinical illness, most infections produce lifelong immunity to a second coccidioidal infection, and it is this observation that suggests a preventative vaccine could be developed (6).
Interest in CPS1 came first from a search for general virulence factors in the maize pathogen Cochliobolus heterostrophus. In this and other ascomycete cereal grain pathogens, deletion of the CPS1 gene results in reduced virulence on host plants with production of smaller lesions (7). We hypothesized that disruption of the homolog of CPS1 in Coccidioides might also alter its pathogenicity. In this report, we show that deletion of CPS1 by gene replacement (Δcps1 mutant) results in a complete attenuation of the pathogenicity exhibited by wild-type C. posadasii for mice. Moreover, mice vaccinated with the live Δcps1 mutant become highly resistant to a subsequent respiratory infection with wild-type C. posadasii that otherwise would be lethal. The striking safety and efficacy demonstrated in these studies support the Δcps1 strain as a promising vaccine candidate to prevent coccidioidomycosis in humans and other mammals. In addition, since we found that the capacity of the Δcps1 strain to grow into arthroconidia (spores) is only slightly reduced, this mutant may readily lend itself to manufacturing processes and formulations that would be needed for a live spore-based vaccine to become clinically useful.
MATERIALS AND METHODS
Strains, media, and growth conditions.
All manipulations of viable cultures were performed at biosafety level 3 (BSL3). Wild-type C. posadasii (strain Silveira, ATCC 28868) was cultured on 2× GYE medium (2% glucose, 1% yeast extract, and 1.5% agar) at room temperature (approximately 24°C). Mutant strains were selected and maintained on 2× GYE media supplemented with 50 μg/ml of hygromycin, also at room temperature. Arthroconidia (spores) of C. posadasii and Δcps1 strains were harvested from 4- and 8-week-old cultures, respectively, using sterile water by the mini-stir bar method described previously (8) and stored in sterile water at 4°C. Spore numbers were determined with a hemocytometer and viable counts by serial dilution and plating.
Spherules were grown in vitro in modified Converse medium at 37°C, 20% CO2 with shaking at 180 rpm as described previously (9).
Mice.
Six- to 8-week-old female C57BL/6NHsd (B6) and BALB/cAnNHsd (BALB/c) mice were purchased from Harlan Sprague Dawley (renamed Envigo, Indianapolis, IN). They were housed and utilized according to National Institutes of Health guidelines. NOD.Cg-Prkdcscid IL2rgtm1Wjl/SzJ (NSG) mice were originally obtained from Leonard Schultz, Jackson Laboratory, and bred onsite under specific-pathogen free (SPF) conditions. NSG mice lack mature T cells, B cells, and functional NK cells and are also deficient in cytokine signaling (10); because of their severe immunodeficiency, they were housed under SPF conditions until transfer into the animal BSL3 laboratory for infection with the Δcps1 mutant. All studies were conducted with the approval of the Institutional Animal Care and Use Committee at the University of Arizona.
Creation of a CPS1 gene deletion mutant in C. posadasii.
The CPS1 gene of Coccidioides (GenBank accession number XM_001243861.2; Broad Institute) was identified using the C. heterostrophus sequence (GenBank accession number AF332878) (7). A CPS1 gene deletion cassette for C. posadasii was constructed in multiple steps using primers listed in Table 1. DNA from C. posadasii was used as the template to generate PCR fragments representing 1.1 kb of the 5′ flanking region and 1.2 kb of the 3′ flanking region of the CPS1 gene using primers OAM 1190 and OAM 1192 as well as OAM 1193 and OAM 1194, respectively. Primers OAM 1192 and OAM 1193 contain sequences complementary to the ends of the hygromycin resistance gene cassette (hphB) of plasmid pCB1004 (11). The hygromycin (hphB) gene was amplified from plasmid pCB1004 using primers OAM 597 and OAM 598 (12). The PCR products of the CPS1 5′ and 3′ flanking sequences were mixed with that of the hphB gene and amplified with nested primers OAM 1191 and 1195, containing EcoRI sites, using double-joint PCR (13). The resulting PCR product was then ligated into pGEM-T Easy (Promega, Madison, WI). The construct, designated pAM1567, was verified by restriction analysis and PCR, and the hphB insert gene of the plasmid was sequenced to determine that no mutations had been introduced. The gene replacement construct from pAM1567, containing CPS1 5′-untranslated region (UTR)-hphB-CPS1 3′UTR, was cloned into the binary vector pAM1145 as an EcoRI fragment, producing plasmid pAM1594. Plasmid pAM1594 was transformed into Agrobacterium tumefaciens strain EHA105 (14) by electroporation, and the resulting strain was named A1594.
TABLE 1.
Primers used in this study
| Primer | Orientation | Sequence (5′-3′) |
|---|---|---|
| 5′ UTR | ||
| OAM1190 | Sense | GTGGGTATCAGTTGTTTGTAGGAAG |
| OAM1192 | Antisense | GCTCCTTCAATATCAGTTAACGTCGAGTTAAACGCCAATCAGTATCGTCGTTTCG |
| 3′ UTR | ||
| OAM1193 | Sense | AGATGCCGACCGGGAACCAGTTAACATAGACATGAGGATTGCTCGGCTTTGTC |
| OAM1194 | Antisense | TCACGATGTCGTACGGGCCAGTTTG |
| Nested | ||
| OAM1191 | Sense | GGGAATTCGAATTCGCGTGGTCTGGTAGTCGCGTTGAGAGCC |
| OAM1195 | Antisense | GAGCCGGAATTCCCTAAATGCATAGCCATTCCACAAATAC |
| CPS1 internal | ||
| OAM1288 | Sense | CAACCGCAGGTCAGTGTATG |
| OAM1289 | Antisense | TCCCGCTATTATTGGAATCG |
C. posadasii was transformed using A. tumefaciens strain A1594 as described previously (12). Briefly, 1 × 107 spore germlings and 1 × 109 induced A. tumefaciens cells were mixed and dispersed onto six 0.45-μm, 82-mm-diameter nitrocellulose filters (Millipore Corporation, Bedford, MA, USA) on plates containing AB induction media. Following cocultivation at room temperature for 48 h, the nitrocellulose filters were transferred to selection plates supplemented with 50 μg/ml hygromycin (selection for transformed strains) and 100 μg/ml kanamycin (counterselection to prevent growth of A. tumefaciens). Transformants were isolated after incubation at room temperature for 1 to 2 weeks and grown on selection plates for sporulation. Monoconidial isolates were selected via two conidial passages as described previously (12).
Twenty-seven hygromycin-resistant transformants were analyzed by PCR to test for the presence of the hphB gene, and 20 of these were subjected to Southern hybridization using the hph gene, a CPS1 gene internal probe, and 5′ and 3′ flanking sequences as probes. Probes for hybridization included the hphB gene, generated by PCR amplification from plasmid pCB1004 using primers OAM 597 and OAM 598, an internal fragment of CPS1 generated by amplification from C. posadasii genomic DNA using primers OAM 1288 and OAM 1289, and a 5′ flanking region fragment generated using C. posadasii DNA as the template with primers OAM 1190 and OAM 1192 (Table 1). When hybridized with the hphB gene probe, seven strains (11, 19, 28, 30, 47, 52, and 53) contained the predicted 10.2-kb EcoRI fragment, indicative of a homologous gene replacement event defined by the EcoRI sites that are present in the 5′ and 3′ flanking regions. The transformant DNA blots were hybridized with an internal probe of the CPS1 gene, and as predicted, the putative gene replacement strains (11, 19, 28, 30, 47, 52, and 53) lacked the CPS1 gene, while others contained the 5.1-kb EcoRI fragment that was present in C. posadasii.
Ten transformants, including six putative gene replacement strains (11, 19, 28, 30, 52, and 53) and four putative ectopic strains (6, 13, 45, and 48), were analyzed further. A 5′ flanking region probe was used to confirm replacement of the CPS1 gene with hphB in strains 11, 19, 28, 30, 52, and 53. In all of these strains, the 5′ probe hybridized to the same 10.2-kb EcoRI fragment as the hphB probe, as predicted. The putative ectopic strains 6, 13, 45, and 48 contained two bands that hybridized to the 5′ probe: a 5.1-kb hybridizing band, as seen with the CPS1 internal probe, identical to the band in C. posadasii, as well as an additional, variable-sized band identifying the ectopically introduced construct. These results indicate that strains 11, 19, 28, 30, 52, and 53 each arose from a single homologous integration event of the CPS1 deletion cassette while strains 6, 13, 45, and 48 are ectopic transformants. Strains 19, 52, and 53 were selected for in vivo virulence studies. Following these studies, strain 19 was used for further studies and is referred to here as the Δcps1 strain.
Measurements of in vitro growth.
Radial growth of Δcps1 strain mycelia was compared to that of mycelia of C. posadasii by measuring colonial diameters following inoculation of plates with 6-mm-diameter agar plugs of young cultures. Triplicate plates of C. posadasii and Δcps1 strains in 2× GYE were grown at room temperature and 37°C. Colony diameters were recorded daily for days 3 to 7 and day 14 postinoculation. Conidiation of the Δcps1 strain was compared to that of C. posadasii by plating ∼1 × 106 spores and allowing cultures to mature for 4 to 8 weeks at room temperature. Twelve to 20 plates were harvested and combined, and the spores were enumerated using hemocytometer counts and serial dilution plating.
In vitro spherule sizes of Δcps1 and C. posadasii strains were compared by harvesting spherules at 24-h intervals up to 120 h, fixing in 10% formaldehyde, and staining them with cotton blue. At least 50 spherules were measured with an ocular micrometer at each time point.
Effect of oxidative stress on C. posadasii and Δcps1 strains was assessed on medium containing H2O2 (15). Hydrogen peroxide from 2 mM to 20 mM was added to GYE media, and strains were grown at either room temperature or 37°C. Radial growth was measured as described above on days 3, 5, and 7.
Fungal metabolite analysis.
For extraction of secreted metabolites, C. posadasii and Δcps1 strains were grown on 2× GYE agar media. Four plates per strain were inoculated with mycelial plugs from the periphery of actively growing cultures and incubated at room temperature for 1 month. To extract metabolites, 10 ml of methanol was added to each plate and incubated for 5 min, and then the agar was cut into 0.5-cm cubes and transferred with the methanol to a 500-ml flask. An additional 60 ml of methanol was added to each flask and incubated with agitation for 4 h. The methanol extract was filtered through Whatman no. 1 paper to remove agar and then sterilized using a 0.22-μm filter. Crude extracts were analyzed on thin-layer chromatography (TLC) plates using either hexane-ethyl acetate (50:50) or 100% ethyl acetate as the solvent, or they were subjected to further purification. Extracts were purified by methanol evaporation followed by resuspension and partitioning between distilled water and ethyl acetate. Ethyl acetate extracts were dried, resuspended in methanol, and subjected to TLC analysis on silica gel 60 RP-18 F254 plates. Extracts were separated using methanol or methanol-water mixtures (90:10, 80:20, and 60:40) as solvent.
RNA-seq.
To perform genome-wide transcriptome expression profiling (RNA-seq), spherules from C. posadasii and Δcps1 strains were prepared and RNA isolated (16). Strains were grown in duplicate by inoculating 100 ml of modified Converse liquid medium with 3 × 108 C. posadasii or Δcps1 strain freshly harvested spores and growing them at 38°C and 20% CO2, with shaking at 180 rpm for 48 h. Spherule RNAs were resuspended in diethyl pyrocarbonate-treated H2O prior to assessment of their quality and concentration with an Agilent Bioanalyzer (Agilent Technologies, Palo Alto, CA) and with the Quant-iT RiboGreen RNA assay kit (Life Technologies, Grand Island, NY).
RNA-seq was performed, in duplicate, on the C. posadasii and Δcps1 strain RNAs by 100-bp paired-end reads on an Illumina HiSeq2000 at the University of Arizona Genetics Core facility. Sequencing produced between 2.05 × 107 and 2.37 × 107 paired-end reads per library. Differential gene expression analysis was performed utilizing the Tuxedo 2.0 suite of programs hosted on the iPlant Cyberinfrastructure at the University of Arizona (17). Gene identification was performed using the C. immitis RS annotated sequence provided by the Broad Institute (http://www.broadinstitute.org/scientific-community/science/projects/fungal-genome-initiative/coccidioides-genomes). These sequences are no longer available at the Broad site but are accessible via GenBank BioProject PRJNA46299.
Murine virulence studies.
Pathogenicity of the Δcps1 strain was assessed in a series of studies using B6, BALB/c, and NSG mice. Initially, 8-week-old B6 mice were infected intranasally (i.n.) under ketamine (80 mg/kg of body weight)-xylazine (8 mg/kg) anesthesia as described previously (18). Twelve mice per group were infected by intranasal insufflation of 30 μl of spores suspended in 0.9% saline with doses ranging from 50 to 5,000 spores of three independent CPS1 deletion strains (19, 52, and 53). As controls, B6 mice were given a lethal dose (50 spores) of C. posadasii. Two mice from each group were sacrificed at 14 days postinfection (p.i.) for histopathology, and the other 10 were maintained for 28 days or until they appeared moribund.
In a second experiment, six NSG mice received 1,030 spores of the Δcps1 strain. Two mice were sacrificed on day 6, with lungs fixed and processed for histopathology, and the other four were sacrificed on day 14 p.i. Two mice sacrificed on day 14 were processed for histopathology, and the entire lungs of the other two were cultured to assess fungal burden (19).
BALB/c and NSG mice were further evaluated in histopathology studies to observe the fate of the Δcps1 strain. Two NSG and two BALB/c mice received 10,000 spores of the Δcps1 strain, and one mouse of each strain was euthanized on day 1 or day 3 p.i. and the lungs fixed for histopathology. In a final experiment, eight BALB/c mice were given 2.5 × 107 spores i.n. and sacrificed on days 1 (n = 2), 3 (n = 1), 4 (n = 1), 5 (n = 2), 7 (n = 1), and 10 (n = 1) p.i., and the lungs were fixed for histopathology (19).
Protection of mice against C. posadasii infection by Δcps1 mutant vaccination.
Mice were primed, boosted 2 weeks later, and challenged intranasally 4 weeks following the boost. Control immunogens consisted of rAg2/PRA1–106-CSA with MPL-SE (25 μg)/CpG (10 μg) adjuvant as previously described for the protection control and adjuvant only as the negative control (20). Groups of eight B6 mice were vaccinated with 50,000 viable spores of the Δcps1 strain, either subcutaneously (s.c.) or intraperitoneally (i.p.), or with control vaccines and then infected with 90 C. posadasii spores. Mice were sacrificed 2 weeks later and lung fungal burden quantitated; spleens were cultured whole to determine dissemination (20). Groups of 10 BALB/c mice were vaccinated i.n. or s.c. with 10,000 viable spores of the Δcps1 strain or with control reagents and infected with 46 spores of C. posadasii. Mice were observed for survival for 28 days. Surviving mice were sacrificed and lungs and spleens cultured.
Histopathology.
Lungs were fixed in 10% buffered formalin for a minimum of 24 h. For the B6 mouse virulence studies (2 mice per group), one slide (5-μm section) from each mouse was stained with hematoxylin and eosin (H&E) for review. The NSG mice, given 1,030 spores, were sacrificed on days 6 and 14, and 5-μm sections were cut through the entire lungs with every fifth section affixed to slides and stained with H&E. For the studies of BALB/c and NSG mice infected with 10,000 or 25 million spores, five pairs of sections, also separated by five serial sections, were prepared on slides. One slide from each pair was stained with H&E, and the other was immunohistochemically stained with a polyclonal goat anti-Ag2/PRA antibody that is specific for Coccidioides as previously described (21). Historical immunohistochemically stained slides of early development of C. posadasii in mouse lungs were used for comparison (22).
Statistical analysis.
Differences in mycelial growth, spherule size, and murine survival following vaccinations were analyzed using the Kruskal-Wallis test. The effect of hydrogen peroxide oxidative stress was analyzed by analysis of variance (ANOVA). Lung fungal burdens were log transformed and compared by ANOVA. Results were considered significant at P ≤ 0.05.
Nucleotide sequence accession number.
The RNA-seq data has been deposited in GEO under accession number GSE85364.
RESULTS
Deletion of the Coccidioides CPS1 gene.
The C. immitis strain RS CPS1 gene (CIMG_03303.3; GenBank accession no. XM_001243861) was identified via tblastn analysis of the Coccidioides genome database using the Cochliobolus heterostrophus Cps1 protein (GenBank accession no. AF332878) (7). The CPS1 gene was deleted, via homologous recombination, in Coccidioides posadasii strain Silveira by Agrobacterium-mediated transformation (23). Seven CPS1 deletion strains were identified out of 27 hygromycin-resistant transformed strains analyzed by PCR and DNA hybridization (data not shown). These strains contained a single insertion of the hphB gene in place of the coding sequences of the CPS1 locus, resulting in a 6.0-kb deletion of the full coding region of the CPS1 gene. CPS1 deletion strains 19, 52, and 53 were used for further analyses.
Growth characteristics in vitro of Δcps1 strain.
The radial growth rate of Δcps1 and C. posadasii strains was the same for the first 7 days. Subsequently, at room temperature but not at 37°C, the Δcps1 strain showed a slight but significant slowing in radial growth compared to C. posadasii. At room temperature on day 14, the mean ± standard deviation (SD) diameter was 4.1 ± 0.1 cm for Δcps1 colonies compared to 4.7 ± 0.1 cm for C. posadasii (P = 0.014). C. posadasii cultures harvested after 4 weeks of growth yielded an average of 2.3 × 108 CFU per plate (100 mm), while harvests of Δcps1 spores after 4 weeks yielded 10% that amount, 2 × 107 CFU/plate. By 45 days, Δcps1 spores yielded 1.4 × 108 CFU per plate, still less than C. posadasii. Despite these differences in growth rates and spore yields, Δcps1 spores were morphologically similar to those of wild-type C. posadasii.
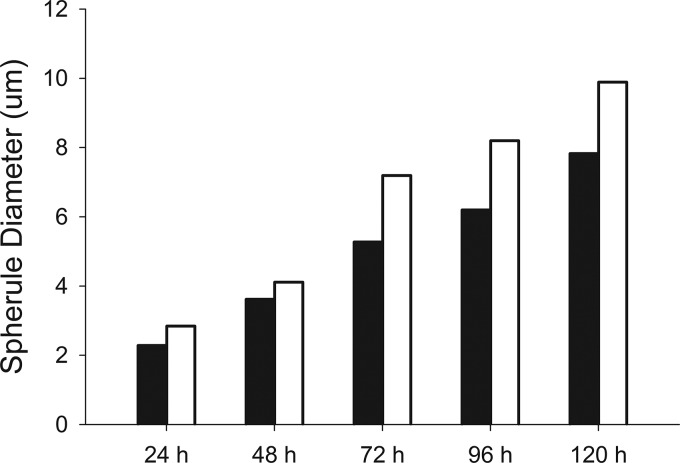
In the host, inhaled spores undergo a dimorphic shift and round up into spherules that range from 10 to 100 μm in diameter. In the laboratory, we can induce spores to undergo spherulation by growing in Converse medium under 20% CO2 at 37°C. We determined that spores of the Δcps1 strain initiated spherulation similar to C. posadasii, but Δcps1 spherules were significantly smaller than those of C. posadasii on each day measured (P < 0.001), with an increasing size disparity over time (Fig. 1).
FIG 1.
Mean in vitro spherule size of Δcps1 strain (black bars) and C. posadasii (open bars) from 24 h to 120 h following initiation of spherulation at 37°C, 20% CO2, in Converse medium. Δcps1 spherules are significantly smaller than those of C. posadasii at all time points (P < 0.001).
When the Δcps1 strain was grown on plates at room temperature, a dark green pigment was observed in the medium around growing colonies that was absent with C. posadasii or with transformed strains where the CPS1 deletion construct integrated ectopically. It is unclear whether the pigment observed is due to accumulation of a metabolic intermediate, as occurs in Aspergillus nidulans when the sterigmatocystin pathway is blocked (24), or a response to stress. Sensitivity to oxidative stress was tested by exposure to H2O2, and as shown in Fig. 2, the Δcps1 strain was actually less rather than more susceptible to oxidative stress than C. posadasii (P < 0.001). Irrespective of the temperature, C. posadasii was unable to grow in the presence of >2 mM H2O2, whereas Δcps1 colony growth diminished as the concentration increased; growth was eliminated beyond 6 mM H2O2 at room temperature and 8 mM at 37°C. TLC analysis indicated the differential presence of small molecules, with the Δcps1 strain producing novel compounds of low polarity relative to C. posadasii (data not shown). The definition of the pigment in future studies should clarify whether it is an intermediate in a CPS1 metabolic pathway.
FIG 2.
Mean colony diameters of Δcps1 (triangles) and C. posadasii (circles) strains on 2× GYE with 0 to 10 mM hydrogen peroxide at room temperature (gray) and 37°C (black) on days 3 and 7 after inoculation of plates with a 6-mm plug. At both temperatures, C. posadasii fails to grow with concentrations >2 mM, while Δcps1 mutant growth diminished at higher hydrogen peroxide levels (P < 0.001).
Gene expression is modified during spherule development of the Δcps1 mutant.
RNA-seq demonstrated changes in the expression level of many transcripts in the absence of Cps1. Figure 3 shows a volcano plot of the differentially regulated genes, with 167 genes reaching potential significance based on an uncorrected P value of 0.0005 or lower. In order to avoid spurious observations, we omitted genes with inconsistencies between biological replicates and focused on those gene changes with a q value (the Benjamini-Hochberg false discovery rate-adjusted P value) of ≤0.05. In addition, gene changes with an induction or repression of less than 2-fold were excluded.
FIG 3.
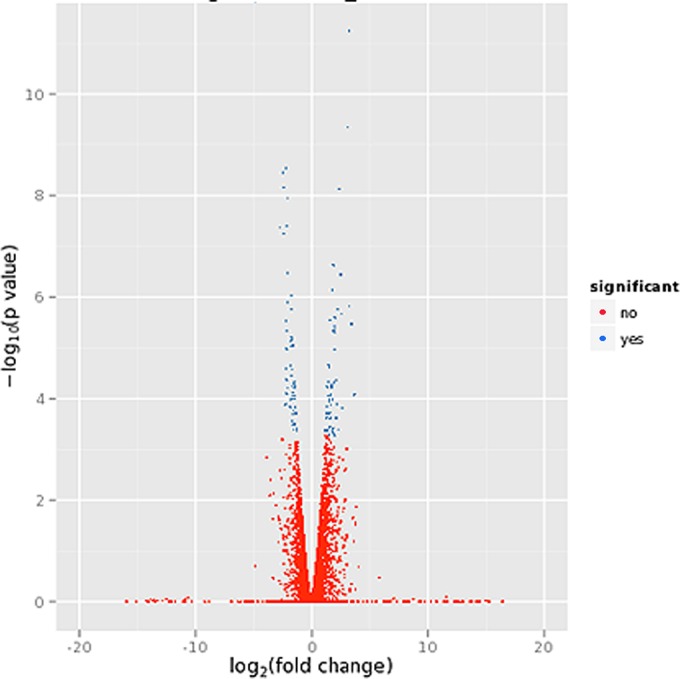
Volcano plot representing differences in transcription in C. posadasii and Δcps1 strains. RNA-seq data from two biological replicates of RNA transcripts for 48-h spherules are shown. Deviations to the left and right are increased and decreased transcripts, respectively, relative to C. posadasii. Red dots represent transcripts that are not significantly different between C. posadasii and Δcps1 strains, while blue dots indicate significant difference. There were 167 genes reaching potential significance based on an uncorrected P value of 0.0005 or lower. In order to avoid spurious observations, we omitted genes with inconsistencies between biological replicates and focused on 33 differentially expressed genes with a q value (the Benjamini-Hochberg false discovery rate-adjusted P value) of <0.05 or lower.
By these criteria, 33 genes were identified, of which 17 were downregulated during spherulation of the Δcps1 strain and 16 were upregulated (Table 2). Also shown in Table 2 are genes nominally identified as similar because of a total similarity of at least 50 and a query overlap of at least 50% at the amino acid level. Data are given for Saccharomyces cerevisiae because of its extensive annotation and separately for other fungal gene products deposited in GenBank. Downregulated genes include conserved fungal proteins with similarity to genes involved in metabolism (coenzyme A synthesis and pyruvate dehydrogenase), cellular respiration, gene regulation (a Ser/Thr protein kinase and a Ser/Thr protein phosphatase), cell adhesion, and conidiation. Three downregulated genes encode proteins that, based on GenBank sequences, are unique to Coccidioides alone or Coccidioides and its close relative, Uncinocarpus reesii. Genes with increased expression in the Δcps1 strain versus C. posadasii during spherulation include the secreted saprobic-phase member of the cerato-platanin family known as the Coccidioides heat-stable antigen (25), three major facilitator superfamily (MFS) transporters, and two heat shock proteins. This analysis indicates that CPS1 deletion impacts multiple pathways, both regulatory and metabolic, suggesting that the gene has pleiotropic functions.
TABLE 2.
Gene expression differences between wild-type Coccidioides posadasii and the CPS1 gene replacement mutant
| Gene ID |
Fold change in expression | P value | Similar gene products in other fungi and their putative functions |
||
|---|---|---|---|---|---|
| GenBank accession no. | Broad assignment | S. cerevisiaea | BLAST results | ||
| Δcps1 strain expression less than wild-type C. posadasii (n = 17) | |||||
| XM_001247405 | CIMG_01177 | 3.16 | 0.001023 | None | Phosphopantothenoylcysteine decarboxylase (coenzyme A synthesis) |
| XM_001247797 | CIMG_01569 | 4.25 | 3.35E−05 | Gor1 (glyoxylate reductase, glyoxylate catabolic process) | Glyoxylate reductase, D-isomer-specific 2-hydroxyacid dehydrogenase |
| XM_001243619 | CIMG_03061 | 6.87 | 2.27E−10 | None | Hypothetical protein, Coccidioides-Uncinocarpus conserved |
| XM_001243862 | CIMG_03304 | 3.21 | 0.000695 | YHR202Wp-like protein (unknown function) | Ser/Thr protein phosphatase family (regulatory protein) |
| XM_001243900 | CIMG_03342 | 4.73 | 9.42E−07 | None | Hypothetical cysteine-rich PLAC8 domain-containing protein |
| XM_001244172 | CIMG_03614 | 3.19 | 0.000695 | Msc1p (protein of unknown function; mutant is defective in directing meiotic recombination events to homologous chromatids) | Nuclear envelope stress response protein Ish1, meiotic sister chromatid recombination protein Ish1/Msc1 (plays a role in maintaining cell viability during stationary phase) |
| XM_001245109 | CIMG_04551 | 5.38 | 0.002733 | None | Hypothetical protein, related to A. nidulans ConF conidiation protein (important for conidial germination and stress response) |
| XM_001246018 | CIMG_05460 | 6.80 | 0.00012 | None | Pyruvate dehydrogenase dihydrolipoamide acetyltransferase component (metabolic enzyme producing acetyl-coenzyme A for the citric acid cycle) |
| XM_001246157 | CIMG_05599 | 5.05 | 7.11E−06 | None | Hypothetical protein, Coccidioides-Uncinocarpus specific |
| XM_001241955 | CIMG_05852 | 3.17 | 0.001904 | None | Fasciclin domain containing protein (involved in cell adhesion) |
| XM_001240537 | CIMG_07701 | 2.97 | 0.002474 | None | Conserved fungal hypothetical protein (putative BTB domain transcription factor with possible role in conidial maturation) |
| XM_001240127 | CIMG_09749 | 4.03 | 0.000556 | None | Conserved hypothetical protein |
| XM_001238874 | CIMG_09897 | 3.08 | 0.013808 | None | Conserved hypothetical protein |
| XM_001238997 | CIMG_10020 | 4.37 | 3.41E−06 | Gad1p (response to oxidative stress) | Glutamate decarboxylase |
| XM_012358897 | CIMG_10785 | 3.28 | 0.002138 | None | Coccidioides-specific protein |
| XM_001245313 | CIMG_11390 | 3.37 | 0.005166 | Ifa38 (monocarboxylic acid metabolic process) | Short-chain dehydrogenase/reductase, estradiol 17-beta-dehydrogenase |
| XM_001243347 | CIMG_13127 | 7.22 | 5.72E−06 | None | Serine/threonine protein kinase (regulatory proteins) |
| Δcps1 strain expression greater than wild-type C. posadasii (n = 16) | |||||
| XM_001247153 | CIMG_00925 | 9.34 | 0.000131 | None | Putative clock-controlled protein, Ccg-6; also like Metarhizium Mmc protein (important for microcycle conidiation) |
| XM_001247409 | CIMG_01181 | 12.47 | 7.78E−10 | None | Coccidioides heat-stable antigen (CSA), related to allergen Aspf15 in the cerato-platanin family |
| XM_001247652 | CIMG_01424 | 2.96 | 0.003585 | None | Ankryin repeat protein (mediates protein-protein interactions), Coccidioides-Uncinocarpus conserved protein |
| XM_001248576 | CIMG_02348 | 5.34 | 5.71E−05 | None | FAD binding domain protein |
| XM_001248747 | CIMG_02519 | 4.47 | 0.007859 | Pcf11p (DNA-templated transcription, termination) | mRNA cleavage factor complex component Pcf11 (3′ mRNA processing) |
| XM_001249051 | CIMG_02823 | 4.26 | 1.78E−05 | Ccp1p (response to oxidative stress) | Cytochrome c peroxidase |
| XM_001244274 | CIMG_03716 | 5.42 | 1.52E−06 | None | 30-kDa heat shock protein, HSP30-like |
| XM_001244778 | CIMG_04220 | 6.48 | 3.39E−05 | Tpo1p (membrane, transmembrane transport) | MFS multidrug transporter |
| XM_001244884 | CIMG_04326 | 5.91 | 0.000191 | Lot6p (response to oxidative stress) | NADPH-dependent FMN reductase |
| XM_001244971 | CIMG_04413 | 4.15 | 0.001529 | None | GMC (glucose-methanol-choline) oxidoreductase |
| XM_001245445 | CIMG_04887 | 3.74 | 5.13E−05 | Ydj1p (protein folding, protein targeting) | DnaJ/Hsp40 chaperone; mitochondrial import protein (stress response to unfolded proteins) |
| XM_001245812 | CIMG_05254 | 5.01 | 0.0007 | None | SUN domain-containing beta-glucosidase protein (cell wall synthesis) |
| XM_001242081 | CIMG_05978 | 3.21 | 0.000976 | Tna1p (nicotinamide mononucleotide transport) | MFS transporter, Tna1 (nicotinic acid transporter) |
| XM_001242139 | CIMG_06036 | 3.74 | 0.002738 | None | DNA repair and transcription factor Ada; methylated DNA-protein-cysteine-methyltransferase |
| XM_001242511 | CIMG_06408 | 4.65 | 0.000125 | Rgt2p (membrane, transmembrane transporter activity) | MFS monosaccharide transporter (transmembrane glucose/monosaccharide sensor/transporter) |
| XM_001242605 | CIMG_06502 | 9.34 | 4.78E−07 | None | Conserved hypothetical protein |
S. cerevisiae putative functions are descriptions of those proteins, based on mutants, from http://www.yeastgenome.org/.
CPS1 deletion mutants are avirulent in mice.
Naive B6 mice challenged with C. posadasii spores died by 19 days p.i., while all animals inoculated with 50 to 4,400 spores of the CPS1 deletion strain 52, 53, or 19 (the Δcps1 strain) remained healthy until scheduled sacrifice. From quantitative cultures of lungs of the 40 mice inoculated with strain 19, only a single colony was recovered from one mouse inoculated with 810 spores. All lungs from mice infected with strains 52 and 53 were sterile. No fungus grew from the spleens of any of these mice. PCR analysis of the single colony recovered from a mouse given the Δcps1 strain verified that it was the mutant strain (data not shown). Supporting the lack of growth from nearly all mice, histopathology of two mice from each group infected with any of the CPS1 deletion strains revealed no spherules or inflammation in lung sections, while innumerable spherules invaded 50 to 80% of lung tissue from C. posadasii-infected mice or mice infected with strain 45, the one with the ectopic insertion of the CPS1 deletion construct.
To further assess the avirulence noted in B6 mice, we inoculated six immunocompromised NSG mice intranasally with 1,030 Δcps1 spores. An equivalent dose of C. posadasii spores would be lethal to immunocompetent mice within 10 days of infection. NSG mice appeared healthy until sacrifice, including the four animals kept for 2 weeks. Histology of step sections through the entire lungs of 2 mice each on days 6 and 14 p.i. revealed no spherules. However, while lung cultures of one mouse at day 14 p.i. had no fungal growth, the other grew 8,200 CFU, demonstrating the capacity of the mutant strain to persist for at least 14 days while not causing illness. PCR of selected colonies verified the gene deletion.
Because we were unable to observe the mutant spherules histologically with a 1,000-spore inoculation yet the culture-positive mouse supported at least transient growth of the strain, we inoculated two NSG and two BALB/c mice with 10,000 Δcps1 spores and sacrificed one each on days 1 and 3 p.i. to look earlier and with a 10-fold higher inoculum. With this infectious dose, no abnormalities were seen on day 1 p.i., and only a few spherules were visualized in both strains on day 3 p.i. (a maximum of 11 spherules per 200× field in the NSG mouse and 4 in the BALB/c mouse), ranging from 0 to approximately two dozen per slide. Organisms were unevenly distributed in the lungs, and most fields were devoid of spherules in both strains. The Δcps1 spherules generally appeared empty with thin, irregular, and broken walls. They were often degraded and surrounded by or filled with neutrophils (Fig. 4A). This was in contrast to 3-day C. posadasii spherules in historical controls, which are characterized by thick walls, uniform roundness, and internal content (endospores) which reacts strongly with the Coccidioides-specific stain. The C. posadasii spherules have a mild macrophage infiltrate surrounding them but few or no neutrophils (Fig. 4B). Following identification of spherules by Coccidioides-specific staining, evaluation of the H&E slides from the NSG and BALB/c mice at higher power (600×) better demonstrated the neutrophilic inflammation in and around the broken, empty Δcps1 spherules (Fig. 4C).
FIG 4.
Spherules of Δcps1 strain compared to wild-type strain 3 days postinfection following 10,000-spore inoculation. (A) Δcps1 spherule in lung of NSG mouse with thin, degenerating wall and neutrophils inside (arrowhead) (Coccidioides-specific stain); (B) wild-type spherule in historical control lungs with thick, round wall, dark red-staining contents (developing endospores), and no neutrophilic infiltrate (Coccidioides-specific stain); (C) high-power image of degenerating Δcps1 spherule in NSG mouse showing wall remnants (arrows) and neutrophils (arrowhead) both within and surrounding the spherule (H&E stain). Bars: 25 μm (A and B) and 20 μm (C).
Because of the paucity of spherules seen following the 10,000-spore infection of BALB/c and NSG mice and the degraded appearance of the spherules at day three, BALB/c mice were infected with a very high dose of Δcps1 spores (2.5 × 107) and their lungs were studied starting 1 day after infection. H&E-stained sections from day one p.i. revealed suppurative bronchopneumonia with edema, but no organisms were seen. The huge inflammatory response was presumed to be a reaction to the large number of foreign particles instilled in the lungs. By day three, we observed extensive suppurative bronchial and alveolar infiltrates with large numbers of Δcps1 spherules, most of which appeared degenerated with neutrophilic inflammation within and surrounding them. Endospores were infrequently observed in the Δcps1 spherules on days four and five, and among these we observed a failure of the Δcps1 endospores to disperse and a dense accumulation of neutrophils surrounding them (Fig. 5A). In contrast, in wild-type infections, endospores were dispersing both individually and in clusters with a mixed inflammatory response more loosely aggregated between them (Fig. 5B).
FIG 5.
Ruptured Δcps1 strain and wild-type spherules on day 5 p.i. after administration of 25 million spores. (A) Δcps1 strain in a BALB/c mouse showing a thick mantle of neutrophils (N) surrounding thin-walled endospores (arrowheads) which are failing to disperse despite the degeneration of the spherule wall. (B) Wild-type endospores (dark red) in a historical control are dispersing from the ruptured spherule in the midst of a mixed inflammatory infiltrate of macrophages and neutrophils. Coccidioides-specific stain was used. Bars in panels A and B are 25 μm.
Lungs from the two high-dose Δcps1 mutant-infected mice sacrificed on days 7 and 10 had a >90% visual decrease in spherules compared to the day five lungs (data not shown). Small, sporadic lesions were characterized as mature granulomas with a fibrogranulomatous mantle and a necrotic center of debris, degenerate and nondegenerate neutrophils, and occasional small, empty spherules. In summary, these highly susceptible BALB/c mice had almost completely resolved this extreme dose (approximately 5 logs higher than the lethal dose of the C. posadasii strain) within 7 to 10 days p.i.
Immunization with the Δcps1 strain provides protection against infection by wild-type C. posadasii.
Following vaccination with the Δcps1 strain or controls, B6 mice were infected with the lethal dose of 90 C. posadasii spores. After 14 days, they were clinically well, and cultures revealed that the Δcps1 mutant viable spore vaccination provided a highly significant reduction in lung fungal burden, 3 logs less than mice receiving the chimeric antigen (P < 0.001) and almost 5 logs less than those receiving the adjuvant alone (P < 0.001) (Fig. 6). Mice in the control group had a mean lung fungal burden of 5.3 × 107 CFU (range, 8.0 × 102 to 11.9 × 107 CFU), while the mean fungal burden of all mice vaccinated with the Δcps1 strain was 2.1 × 102 CFU (range, 1 to 1.7 × 103 CFU).
FIG 6.
Protection of C57BL/6 mice by vaccination with viable Δcps1 strain. Total lung CFU 14 days following challenge with 90 spores of C. posadasii were significantly reduced in mice vaccinated with the Δcps1 strain (d-cps1 IP or d-cps1 SC) compared to the positive-control chimeric antigen (Chim Ag) (P = 0.001) or adjuvant alone (Adj Only) (P = 0.001).
Vaccination with the Δcps1 strain of the more susceptible BALB/c mice, either i.n. or s.c., and infection with 46 C. posadasii spores resulted in 19 of 20 mice surviving until day 28 p.i., while control mice that received the chimeric antigen vaccine or adjuvant only all died by day 15 p.i. (P < 0.001) (Fig. 7A). As shown in Fig. 7B, the lung fungal burdens of surviving Δcps1 strain-vaccinated BALB/c mice at 28 days p.i. was less than 1,000 CFU per lung for 18 of 19 mice, with 7 producing no growth from their lungs. Fungal burden was not measured in the mouse that died before day 28. Although fungal burdens were lower in the i.n. than in the s.c. Δcps1 strain-vaccinated groups, differences were not significant (P = 0.241). One animal from the Δcps1 strain s.c. vaccinated group lost weight during the last week and was found to have 4.6 × 104 CFU in the lungs, and spleen cultures were positive when the animal was euthanized. With this one exception, spleen cultures were negative in Δcps1 strain-vaccinated mice, indicating the vaccine also prevented fungal dissemination. Spleens from the recombinant vaccine and adjuvant groups that died were universally positive for fungal growth. Thus, Δcps1 strain vaccination is highly effective in preventing disease or death from respiratory coccidioidal infection in both B6 and BALB/c mice.
FIG 7.
(A) Survival results for BALB/c mice vaccinated i.n. (circles) or s.c. (triangles) with the Δcps1 strain, recombinant chimeric antigen (squares), or adjuvant alone (diamonds) and challenged with 46 spores of C. posadasii i.n. Survival of mice vaccinated with the Δcps1 strain is significantly better (P < 0.001) than that of mice vaccinated with chimeric antigen or adjuvant only. (B) Twenty-eight-day residual lung fungal burden of mice vaccinated i.n. or s.c. is not different (P = 0.242). Parenthetical numbers are mice with 0 (n = 3 s.c., n = 4 i.n.) or 1 (n = 1 i.n.) CFU at sacrifice.
DISCUSSION
In this report, we used data about a virulence factor in the phytopathogen C. heterostrophus (7) as the basis for our discovery that the CPS1 gene in C. posadasii is essential to its pathogenicity in mice. While in C. heterostrophus CPS1 mutations result in only a partial reduction in lesion size on maize (7), deletion of CPS1 in Coccidioides virtually eliminated its pathogenicity in mice, even at high intranasal inocula or in immunodeficient mice. This is a dramatic example of using virulence gene discovery in a fungal phytopathogen as the point of discovery for a critical virulence factor in a medically important fungus.
CPS1 is highly conserved among the Ascomycota with genes of high similarity also present in lower fungi, although they apparently are absent from the Basidiomycota (data not shown).
Experimental evidence of the function of CPS1 genes in ascomycetes is lacking. By motif analysis, CPS1 in C. heterostrophus was shown to belong to the AMP-binding superfamily and phylogenetic analysis initially suggested it could be part of a nonribosomal peptide synthase (NRPS) (7), but further evidence suggests it groups outside most NRPSs (26). CPS1 is part of the adenylate forming domain superfamily of proteins that includes the disco-interacting proteins (Dip2), such as DIP2 of Drosophila and Dip2A in mice and humans (27–29). Dip2A is a membrane receptor protein that binds secreted follistatin-like proteins which appear to regulate downstream gene expression. Like the Dip2 proteins, Cps1 contains a DMAP1 binding domain at its N terminus, with DMAP being a transcriptional corepressor. In silico analysis of Cps1 suggests it is also a transmembrane protein, indicating it functions by interacting with an extracellular signal, resulting in a cellular response. In C. posadasii, our RNA-seq analysis is also consistent with a regulatory role for Cps1, since multiple genes and pathways are impacted by CPS1 deletion. The differential appearance of products between WT and Δcps1 strains during saprobic growth using TLC analysis indicates modified metabolism upon deletion of CPS1. Detailed analysis of these products could shed light on the biochemical consequences of the CPS1 deletion.
What little is known or hypothesized about the function of Cps1 at this time does not explain the level of avirulence observed in mice infected with the Δcps1 strain. Although deletion of CPS1 resulted in a modest reduction of both saprobic and first-generation spherule growth in vitro, those assays showed that it is competent to make spores and to transform into spherules. The mutant (i) has a mycelial radial growth rate similar to that of C. posadasii initially but shows retarded growth after 2 weeks and (ii) sporulates at a reduced rate, producing 10% of the spores of C. posadasii after 30 days, and still a reduced number of spores compared to C. posadasii at 45 days. Finally, in vitro spherules are reduced in size by about 25% at 72, 96, and 120 h. Because initial studies in mice showed complete avirulence and failed to identify inflammation or residual organisms in 39/40 mice, it was unknown whether the Δcps1 strain was unable to make the dimorphic switch in the host or if it was very susceptible to the host immune system. Histopathological observations at early time points and in NSG mice lacking adaptive components of their immune system demonstrated that (i) the Δcps1 strain does undergo spherulation in the host; (ii) the vast majority of the organisms appear to degrade with or without the presence of the host immune system; and (iii) near complete clearance of even large inocula from the lungs occurs within 10 days in susceptible but immunocompetent mice. These observations strongly indicate that the attenuation of the Δcps1 strain does not depend upon an unusual susceptibility to host immunologic defenses but rather that the Δcps1 strain is simply unfit for propagation within mammalian tissue. If corroborated by further studies, such as clearance of high inocula from the lungs of immunodeficient mice, this characteristic has profound implications for the possible safety of the Δcps1 strain as a vaccine candidate.
When used as a vaccine, the Δcps1 strain produced exceptional protection against subsequent C. posadasii infection, measured both by dramatically enhanced survival and reduction in fungal burden, regardless of parenteral i.p. and s.c. or i.n. routes of administration. In BALB/c mice, 95% or greater survival was observed following either subcutaneous or intranasal immunization. Notably, a recombinant vaccine, which in past studies was found to be protective in C75BL/6 mice (20), showed no prolongation in survival under these conditions. In going forward with a vaccine, these model studies have demonstrated that a subcutaneous route of administration, practical for humans and dogs, is just as effective as other routes.
The Δcps1 strain is not the first attenuated Coccidioides mutant to protect mice when used as a vaccine. Subcutaneous but not intranasal vaccination with temperature-sensitive mutants protected against intranasal infection (30). Protection also was produced by another live attenuated mutant created by the deletion of two of the eight C. posadasii chitinases (CTS2 and CTS3) and disruption of a third gene (ARD1) contiguous to CTS3 (31). Of note, both the Δcps1 strain and the previously published live attenuated vaccines initiated spherule development before growth was arrested. In contrast, vaccination of mice with an RYP1 knockout strain of C. posadasii, which does not undergo transition to the spherule phase in C. posadasii (32), did not induce protection to a subsequent coccidioidal infection (M. A. Mandel, M. J. Orbach, and L. F. Shubitz, unpublished data). In earlier studies, spherule vaccines were found to be protective against respiratory murine coccidioidal infections whereas mycelial vaccines were not (33). This pattern is consistent with the possibility that spherule initiation as occurs in the Δcps1 strain is critical for a live vaccine to stimulate protective immunity against a respiratory infection.
The search for a clinically useful vaccine to prevent coccidioidomycosis has been ongoing for over half a century (34). Whole killed spherule vaccines provided excellent protection in mice when given repetitively in milligram quantities, but injection site reactions were severe, including caseous granulomas that sometimes produced skin breakdown. Protection from this vaccine was sufficiently impressive in mice to merit human trials, but at a dose that was protective in mice, adverse injection site reactions in humans were severe (35) and no differences were seen in incidence of Valley Fever in vaccinated and control populations (36). Because of this, lower doses of whole-cell preparations or specific subcellular components seemed necessary for a tenable vaccine. The chimeric peptide recombinant vaccine used in this study for comparisons to the Δcps1 strain was a potential subcellular candidate. It was proposed by a large collaborative program as a vaccine candidate for use in clinical trials because of its protection in B6 mice (20) despite its limitations in BALB/c mice, as shown here. Those trials were never conducted, in large part because the cost of manufacturing and formulation were prohibitive for a small-market vaccine such as one to prevent coccidioidomycosis. Such practical considerations make the Δcps1 strain particularly attractive as a vaccine candidate for clinical development. Spores, as used in our murine studies, are, in essence, the vaccine formulation. It would still be necessary to develop methods to harvest spores and dispense them into stable and storable units. However, identifying and applying industrial technologies for managing fungal spores would seem to be a much less difficult requirement and more feasible than the formulation required for a polypeptide vaccine to allow clinical trials to proceed. Although our ultimate objective is to use the Δcps1 strain as a vaccine to prevent coccidioidomycosis in humans, there is a similar need for a canine vaccine (37), and we hope to achieve that goal as well.
ACKNOWLEDGMENTS
We thank Leslie Gunatilaka and Kithsiri Wijeratne for their help with extraction and TLC analysis of secreted metabolites. We thank Gillian Turgeon for helpful discussions. We thank Sharon Dial for assistance with photomicrographs and the Arizona Veterinary Diagnostic Laboratory (Tucson, AZ) for preparation of immunohistochemical slides.
REFERENCES
- 1.Pappagianis D. 1988. Epidemiology of coccidioidomycosis. Curr Top Med Mycol 2:199–238. doi: 10.1007/978-1-4612-3730-3_6. [DOI] [PubMed] [Google Scholar]
- 2.Fisher MC, Koenig GL, White TJ, San-Blas G, Negroni R, Alvarez IG, Wanke B, Taylor JW. 2001. Biogeographic range expansion into South America by Coccidioides immitis mirrors New World patterns of human migration. Proc Natl Acad Sci U S A 98:4558–4562. doi: 10.1073/pnas.071406098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nguyen C, Barker BM, Hoover S, Nix DE, Ampel NM, Frelinger JA, Orbach MJ, Galgiani JN. 2013. Recent advances in our understanding of the environmental, epidemiological, immunological, and clinical dimensions of coccidioidomycosis. Clin Microbiol Rev 26:505–525. doi: 10.1128/CMR.00005-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cole GT, Sun SH. 1985. Arthroconidium-spherule-endospore transformation in Coccidioides immitis, p 281–333. In Szaniszlo PJ, Harris JL (ed), Fungal dimorphism–with emphasis on fungi pathogenic for humans. Plenum Press, New York, NY. [Google Scholar]
- 5.Tsang CA, Anderson SM, Imholte SB, Erhart LM, Chen S, Park BJ, Christ C, Komatsu KK, Chiller T, Sunenshine RH. 2010. Enhanced surveillance of coccidioidomycosis, Arizona, USA, 2007-2008. Emerg Infect Dis 16:1738–1744. doi: 10.3201/eid1611.100475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cole GT, Xue JM, Okeke CN, Tarcha EJ, Basrur V, Schaller RA, Herr RA, Yu JJ, Hung CY. 2004. A vaccine against coccidioidomycosis is justified and attainable. Med Mycol 42:189–216. doi: 10.1080/13693780410001687349. [DOI] [PubMed] [Google Scholar]
- 7.Lu SW, Kroken S, Lee BN, Robbertse B, Churchill ACL, Yoder OC, Turgeon BG. 2003. A novel class of gene controlling virulence in plant pathogenic ascomycete fungi. Proc Natl Acad Sci U S A 100:5980–5985. doi: 10.1073/pnas.0931375100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huppert M, Sun S, Gross A. 1972. Evaluation of an experimental animal model for testing antifungal substances. Antimicrob Agents Chemother 1:367–372. doi: 10.1128/AAC.1.5.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Converse JL, Besemer AR. 1959. Nutrition of the parasitic phase of Coccidioides immitis in a chemically defined liquid medium. J Bacteriol 78:231–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shultz LD, Brehm MA, Garcia-Martinez JV, Greiner DL. 2012. Humanized mice for immune system investigation: progress, promise and challenges. Nat Rev Immunol 12:786–798. doi: 10.1038/nri3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carroll A, Sweigard J, Valent B. 1994. Improved vectors for selecting resistance to hygromycin. Fung Genet Newsl 41:22. [Google Scholar]
- 12.Kellner EM, Orsborn KI, Siegel EM, Mandel MA, Orbach MJ, Galgiani JN. 2005. Coccidioides posadasii contains a single 1,3-beta-glucan synthase gene that appears to be essential for growth. Eukaryot Cell 4:111–120. doi: 10.1128/EC.4.1.111-120.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu JH, Hamari Z, Han KH, Seo JA, Reyes-Dominguez Y, Scazzocchio C. 2004. Double-joint PCR: a PCR-based molecular tool for gene manipulations in filamentous fungi. Fung Genet Biol 41:973–981. doi: 10.1016/j.fgb.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 14.Hood EE, Gelvin SB, Melchers LS, Hoekema A. 1993. New Agrobacterium helper plasmids for gene-transfer to plants. Transgenic Res 2:208–218. doi: 10.1007/BF01977351. [DOI] [Google Scholar]
- 15.Thon M, Al-Abdallah Q, Hortschansky P, Brakhage AA. 2007. The thioredoxin system of the filamentous fungus Aspergillus nidulans–impact on development and oxidative stress response. J Biol Chem 282:27259–27269. doi: 10.1074/jbc.M704298200. [DOI] [PubMed] [Google Scholar]
- 16.Mandel MA, Galgiani JN, Kroken S, Orbach MJ. 2006. Coccidioides posadasii contains single chitin synthase genes corresponding to classes I to VII. Fung Genet Biol 43:775–788. doi: 10.1016/j.fgb.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 17.Trapnell C, Roberts A, Goff L, Pertea G, Kim D, Kelley DR, Pimentel H, Salzberg SL, Rinn JL, Pachter L. 2012. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat Protoc 7:562–578. doi: 10.1038/nprot.2012.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shubitz L, Peng T, Perrill R, Simons J, Orsborn K, Galgiani JN. 2002. Protection of mice against Coccidioides immitis intranasal infection by vaccination with recombinant antigen 2/PRA. Infect Immun 70:3287–3289. doi: 10.1128/IAI.70.6.3287-3289.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shubitz LF, Trinh HT, Perrill RH, Thompson CM, Hanan NJ, Galgiani JN, Nix DE. 2014. Modeling nikkomycin Z dosing and pharmacology in murine pulmonary coccidioidomycosis preparatory to phase 2 clinical trials. J Infect Dis 209:1949–1954. doi: 10.1093/infdis/jiu029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shubitz LF, Yu JJ, Hung CY, Kirkland TN, Peng T, Perrill R, Simons J, Xue J, Herr RA, Cole GT, Galgiani JN. 2006. Improved protection of mice against lethal respiratory infection with Coccidioides posadasii using two recombinant antigens expressed as a single protein. Vaccine 24:5904–5911. doi: 10.1016/j.vaccine.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 21.Shubitz LF, Dial SM, Perrill R, Casement R, Galgiani JN. 2008. Vaccine-induced cellular immune responses differ from innate responses in susceptible and resistant strains of mice infected with Coccidioides posadasii. Infect Immun 76:5553–5564. doi: 10.1128/IAI.00885-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shubitz LF, Perrill R, Lewis ML, Dial SM, Galgiani JN. 2011. Early post-infection detection of Coccidioides in intranasally infected mice, p 40 In Catanzaro A, Blair JE, Ampel N, Clemmons K (ed), Proceedings of the 55th Annual Coccidioidomycosis Study Group. University of California–Davis, Davis, CA. [Google Scholar]
- 23.Abuodeh RO, Orbach MJ, Mandel MA, Das A, Galgiani JN. 2000. Genetic transformation of Coccidioides immitis facilitated by Agrobacterium tumefaciens. J Infect Dis 181:2106–2110. doi: 10.1086/315525. [DOI] [PubMed] [Google Scholar]
- 24.Shaaban MI, Bok JW, Lauer C, Keller NP. 2010. Suppressor mutagenesis identifies a velvet complex remediator of Aspergillus nidulans secondary metabolism. Eukaryot Cell 9:1816–1824. doi: 10.1128/EC.00189-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pan S, Cole GT. 1995. Molecular and biochemical characterization of a Coccidioides immitis-specific antigen. Infect Immun 63:3994–4002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bushley KE, Turgeon BG. 2010. Phylogenomics reveals subfamilies of fungal nonribosomal peptide synthetases and their evolutionary relationships. BMC Evol Biol 10:26. doi: 10.1186/1471-2148-10-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mukhopadhyay M, Pelka P, DeSousa D, Kablar B, Schindler A, Rudnicki MA, Campos AR. 2002. Cloning, genomic organization and expression pattern of a novel Drosophila gene, the disco-interacting protein 2 (dip2), and its murine homolog. Gene 293:59–65. doi: 10.1016/S0378-1119(02)00694-7. [DOI] [PubMed] [Google Scholar]
- 28.Ouchi N, Asaumi Y, Ohashi K, Higuchi A, Sono-Romanelli S, Oshima Y, Walsh K. 2010. DIP2A functions as a FSTL1 receptor. J Biol Chem 285:7127–7134. doi: 10.1074/jbc.M109.069468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tanaka M, Murakami K, Ozaki S, Imura Y, Tong XP, Watanabe T, Sawaki T, Kawanami T, Kawabata D, Fujii T, Usui T, Masaki Y, Fukushima T, Jin ZX, Umehara H, Mimori T. 2010. DIP2 disco-interacting protein 2 homolog A (Drosophila) is a candidate receptor for follistatin-related protein/follistatin-like 1-analysis of their binding with TGF-beta superfamily proteins. FEBS J 277:4278–4289. doi: 10.1111/j.1742-4658.2010.07816.x. [DOI] [PubMed] [Google Scholar]
- 30.Walch HA, Kalvoda A. 1971. Immunization of mice with induced mutants of Coccidioides immitis. 1. Characterization of mutants and preliminary studies of their use as viable vaccines. Sabouraudia 9:173–184. [PubMed] [Google Scholar]
- 31.Xue JM, Chen X, Selby D, Hung CY, Yu JJ, Cole GT. 2009. A genetically engineered live attenuated vaccine of Coccidioides posadasii protects BALB/c mice against coccidioidomycosis. Infect Immun 77:3196–3208. doi: 10.1128/IAI.00459-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wickramage AS, Mandel MA, Orbach MJ. 2011. RIG1 and RYP1–homologous genes essential for fungal pathogenicity in plant and animal hosts. Abstr 26th Fung Genet Conf. [Google Scholar]
- 33.Levine HB, Cobb JM, Smith CE. 1960. Immunity to coccidioidomycosis induced in mice by purified spherule, arthrospore, and mycelial vaccines. Trans NY Acad Sci 22:436–447. doi: 10.1111/j.2164-0947.1960.tb00711.x. [DOI] [PubMed] [Google Scholar]
- 34.Galgiani JN. 2008. Vaccines to prevent systemic mycoses: holy grails meet translational realities. J Infect Dis 197:938–940. doi: 10.1086/529205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Williams PL, Sable DL, Sorgen SP, Pappagianis D, Levine HB, Brodine SK, Brown BW, Grumet FC, Stevens DA. 1984. Immunological responsiveness and safety associated with the Coccidioides immitis spherule vaccine in volunteers of white, black and Filipino ancestry. Am J Epidemiol 119:591–602. [DOI] [PubMed] [Google Scholar]
- 36.Pappagianis D, Brown BW, Cunningham R, Einstein H, Ellsworth R, Galgiani J, Hampson CR, Holeman CW, Johnson RH, Larwood TR, Levine HB, Massa DA, Nichols J, Stevens DA, Tidball JS, Whitfield R, Williams PL. 1993. Evaluation of the protective efficacy of the killed Coccidioides immitis spherule vaccine in humans. Am Rev Respir Dis 148:656–660. doi: 10.1164/ajrccm/148.3.656. [DOI] [PubMed] [Google Scholar]
- 37.Graupmann-Kuzma A, Valentine BA, Shubitz LF, Dial SM, Watrous B, Tornquist SJ. 2008. Coccidioidomycosis in dogs and cats: a review. J Am Anim Hosp Assoc 44:226–235. doi: 10.5326/0440226. [DOI] [PubMed] [Google Scholar]