Abstract
Here, we characterized the first OXA-72-producing Acinetobacter baumannii isolate (designated MAL) recovered from a urine sample from a Serbian patient. Antimicrobial susceptibility testing, plasmid analysis, and whole-genome sequencing (WGS) were performed to fully characterize the resistome of the A. baumannii MAL clinical isolate. The isolate was multidrug resistant and remained susceptible only to colistin and tigecycline. PCR analysis revealed the presence of the carbapenemase OXA-72, an OXA-40 variant. Extraction by the Kieser method revealed the presence of two plasmids, and one of these, a ca. 10-kb plasmid, harbored the blaOXA-72 gene. WGS revealed 206 contigs corresponding to a genome of 3.9 Mbp in size with a G+C content of 38.8%. The isolate belonged to sequence type 492 and to worldwide clone II (WWCII). Naturally occurring β-lactamase-encoding genes (blaADC-25 and blaOXA-66) were also identified. Aminoglycoside resistance genes encoding one aminoglycoside adenyltransferase (aadA2), three aminoglycoside phosphatases (strA, strB, aphA6), and one 16S RNA methylase (armA) conferring resistance to all aminoglycosides were identified. Resistance to fluoroquinolones was likely due to mutations in gyrA, parC, and parE. Of note, the resistome matched perfectly with the antibiotic susceptibility testing results.
INTRODUCTION
The circumstances that allow Acinetobacter to assume a pathogenic role are not well understood. Accordingly, most Acinetobacter species are considered opportunistic pathogens responsible for nosocomial infections, mostly in debilitated patients and/or external device carriers (1, 2). Acinetobacter baumannii was frequently involved in nosocomial outbreaks that occurred mostly in intensive care units (2). The A. baumannii-related infections ranged from septicemia to pneumonia and urinary tract infections, often associated with catheters (2). During the last 3 decades, multidrug resistance has increasingly been reported in A. baumannii, resulting in carbapenems being considered the last-resort antibiotic for the treatment of infections with this pathogen (3).
Unfortunately, the overuse of carbapenems has rapidly resulted in the worldwide dissemination of carbapenem-resistant A. baumannii strains, raising serious concerns about antimicrobial treatment options. In Acinetobacter spp., the most prevalent mechanism responsible for carbapenem resistance is the production of a carbapenem-hydrolyzing Ambler class D β-lactamase (CHDL) (3). These CHDLs include both (i) acquired carbapenemases of the OXA-23, OXA-24/40, OXA-58, OXA-143, and OXA-235 types (4) and (ii) enzymes with the ISAba1-enhanced expression of the intrinsic chromosomal blaOXA-51-like gene (3). Except for their detection in a few isolates of OXA-23-producing Proteus mirabilis (5, 6), CHDLs are currently restricted to Acinetobacter species.
Aminoglycosides are also currently used in the treatment of severe infections. In A. baumannii, aminoglycoside resistance might rely on (i) decreased outer membrane permeability, (ii) active efflux, (iii) modifications in ribosomal proteins, and (iv) most often, enzymatic modification of the drug. Recently, methylation of 16S rRNA has emerged in Gram-negative organisms, leading to methylation of the 16S rRNA target, which subsequently confers a high level of resistance to all clinically useful aminoglycosides (i.e., amikacin, gentamicin, and tobramycin) (7). Among the currently described 16S rRNA methylases, the ArmA enzyme is predominant in A. baumannii. In addition, it is often associated with the carbapenemase OXA-23 (3).
Here, using a whole-genome sequencing (WGS) approach, we have characterized an OXA-72-producing A. baumannii isolate recovered from a urine sample from a Serbian patient.
MATERIALS AND METHODS
Antimicrobial susceptibility testing.
Antimicrobial susceptibility was determined by the disk diffusion method and interpreted according to EUCAST guidelines, as updated in 2015 (www.eucast.org). The MICs were determined using Etest (bioMérieux, La Balme-les-Grottes, France) on Mueller-Hinton agar at 37°C.
Plasmid identification and transformation.
Plasmid DNA of the A. baumannii MAL strain was extracted using the Kieser method, as previously described (8). Transfer of the β-lactam resistance markers was attempted by electroporation of the plasmid DNA suspension into electrocompetent A. baumannii CIP 70.10 cells. Transformants were selected on Trypticase soy agar supplemented with ticarcillin at 100 μg/ml. Plasmids of ca. 154, 66, 48, and 7 kb from Escherichia coli NCTC 50192 were used as plasmid size markers. Plasmid DNAs of A. baumannii MAL and its transformants were analyzed by agarose gel electrophoresis. In addition, the blaOXA-72-harboring plasmid, named pAB-MAL-1, was extracted from the transformant using a QIAprep spin miniprep kit (Qiagen, Courtaboeuf, France) and sequenced separately, as described below.
Detection of carbapenemase activity.
The carbapenemase activity was searched for using the CarbAcineto NP test as previously described (9). The CarbAcineto NP test, which detects imipenemase activity, was performed using a bacterial inoculum taken directly from the antibiogram on Mueller-Hinton medium.
WGS and sequence analysis.
Briefly, total DNA was isolated using an UltraClean microbial DNA isolation kit (Mo Bio Laboratories) from overnight cultures on blood agar (Bio-Rad, Marnes-la-Coquette, France). Genomic DNA quantifications were performed using a Qubit fluorometer (Life Technologies, Carlsbad, CA), and DNA quantities were adjusted to 0.2 ng/μl. Library preparation was performed using a Nextera XT DNA sample preparation kit (Illumina, San Diego, CA). Sequencing was performed on an Illumina MiSeq 2000 sequencer with v3 chemistry using two 75-bp paired-end reads at a raw cluster density of ∼1,300,000 clusters/mm2.
The contigs were fully annotated using the RAST server (http://rast.nmpdr.org/), which predicted 3,710 coding sequences in the genome.
Total raw data sequences of the A. baumannii MAL were subjected to analysis with the ResFinder (v2.1) server (http://www.genomicepidemiology.org/), which is dedicated to the identification of acquired antimicrobial resistance genes (10). Genes conferring resistance to aminoglycosides, β-lactams, fluoroquinolones, fosfomycin, macrolides, lincosamide, streptogramin B, phenicol, rifampin, sulfonamide, tetracycline, and trimethoprim were sought using a 98% threshold for nucleotide sequence identity and 60% for the minimum length coverage.
Multilocus sequence typing (MLST) was performed using the MLST (v1.8) server (http://www.genomicepidemiology.org/) as previously described (11). The two different MLST schemes (the Oxford and Pasteur schemes) available for A. baumannii were tested (http://pubmlst.org/abaumannii/) (12, 13).
Accession number(s).
The A. baumannii MAL genome sequence corresponds to GenBank accession number LKIB00000000 (14). GenBank accession numbers for plasmids pAB-MAL-1 and pAB-MAL-2 are KX230793 and KX230794, respectively.
RESULTS
Clinical history and characteristics of the A. baumannii MAL clinical isolate.
A man in his 70s living in Serbia was admitted to a Serbian hospital for acute urinary retention. An indwelling long-term urethral catheter was inserted, and the patient was discharged. One month later, he was diagnosed with a urinary tract infection and treated with cefixime for 2 weeks; the urethral catheter was not removed. Two weeks after the end of the antimicrobial treatment, the patient was admitted to the emergency unit of a hospital in the suburbs of Paris, France, because of high fever, perineal pain, and dysuria. The urethral catheter was removed, and ceftriaxone and gentamicin were administered. Culture of the urine grew an Enterococcus faecalis isolate (105 CFU/ml) and an A. baumannii isolate named MAL (106 CFU/ml). Antimicrobial susceptibility testing revealed that the E. faecalis isolate possessed a wild-type phenotype and that A. baumannii MAL was resistant to all tested antimicrobial molecules except colistin and tigecycline (Table 1). The diagnosis of E. faecalis prostatitis and catheter-associated asymptomatic bacteriuria with A. baumannii MAL was made. Amoxicillin and gentamicin treatment was initiated, and this was followed by sulfamethoxazole-trimethoprim treatment. The fever rapidly disappeared, and the urinary flow was completely restored.
TABLE 1.
MICs of β-lactams for A. baumannii MAL, A. baumannii CIP 70.10 transformed with the natural plasmid carrying blaOXA-72 (pAB-MAL-1), and the A. baumannii CIP 70.10 reference strain
| β-Lactam | MIC (mg/liter) |
||
|---|---|---|---|
| A. baumannii MAL | A. baumannii CIP 70.10(pAB-MAL-1) | A. baumannii CIP 70.10 | |
| Ticarcillin | >256 | >256 | 8 |
| Ticarcillin + CLAa | >256 | >256 | 8 |
| Piperacillin | >256 | >256 | 4 |
| Piperacillin + TZBb | >256 | 128 | 4 |
| Cefotaxime | >64 | 32 | 32 |
| Ceftazidime | >64 | 4 | 4 |
| Cefepime | 64 | 16 | 16 |
| Imipenem | >32 | >32 | 0.25 |
| Meropenem | >32 | >32 | 0.5 |
| Doripenem | >32 | >32 | 0.25 |
CLA, clavulanic acid (4 mg/liter).
TZB, tazobactam (4 mg/liter).
In order to characterize the underlying mechanism responsible for carbapenem resistance in A. baumannii MAL and rapidly implement infection control measures (including placement of the patient in an individual room and the use of dedicated medical staff), the CarbAcineto NP test was performed directly on the antibiogram as previously described (9). This test confirmed in less than 2 h the presence of a carbapenem-hydrolyzing enzyme; thus, proper infection control measures could be implemented rapidly, preventing the further spread of this highly drug-resistant bacterium.
Genomic features of A. baumannii MAL.
WGS of the A. baumannii MAL isolate gave a total of 2,611,036 reads with an average length of 75.17 bp (14). Two hundred six contigs were obtained using the CLC genomic workbench (v8.5), corresponding to a total of 3,914,647 bp, including the chromosome and plasmids, with a G+C content of 38.8%, which is in accordance with that for A. baumannii species.
The global analysis of A. baumannii MAL sequences identified the blaOXA-72 gene encoding the OXA-72 carbapenemase, which is responsible for carbapenem hydrolysis and which was detected with the CarbAcineto NP test (Table 2). The resistance to all β-lactams was completed by the overexpression of the intrinsic chromosomally encoded ADC-25 cephalosporinase due to the insertion of ISAba1 upstream of the blaADC-25 gene. No insertion sequence was identified upstream of the intrinsic chromosomally encoded OXA-66 oxacillinase, suggesting a likely basal expression. Five genes encoding aminoglycoside resistance determinants were identified: one aminoglycoside adenylase (aadA2), three aminoglycoside phosphatases (strA, strB, aphA6), and one 16S RNA methylase (armA) responsible for resistance to all aminoglycosides (Table 2). Although resistance to macrolides was mediated by the impermeability of Gram-negative rods, two macrolide resistance genes were identified, namely, mph(E) and msr(E). The products of the sul1, sul2, and dfrA12 genes caused resistance to sulfamethoxazole-trimethoprim, and tetracycline resistance was mediated by tet(B). The localization of all acquired resistance genes is shown in Table 2. No acquired genes encoding resistance to quinolones were found in A. baumannii MAL. However, after comparison of the sequence of A. baumannii MAL with that of the A. baumannii ATCC 19606 reference strain, we identified substitutions in GyrA (Ser-81-Leu), ParC (Ser-84-Leu, Ser-467-Gly), and ParE (V-237-Ala). These mutations in GyrA and ParC have previously been shown to be responsible for resistance to fluoroquinolones in Acinetobacter species (15–17).
TABLE 2.
WGS-detected resistance genes and in silico-deduced antimicrobial resistance phenotype
| Antibiotic family | Resistance gene | % identity | Localization | Enzyme |
GenBank accession no. | Antimicrobial resistancec |
||
|---|---|---|---|---|---|---|---|---|
| Name | Function | In silico deduced | Observed | |||||
| Aminoglycosides | aadA2 | 100 | Chromosome | AAD | Aminoglycoside adenylase | JQ364967 | GEN, TOB | GEN, TOB, NET, AMK |
| strA | 100 | Chromosome | APH(3″)-1b | Aminoglycoside phosphatase | M96392 | TOB, AMK | GEN, TOB, NET, AMK | |
| strB | 100 | Chromosome | APH(6)-1d | Aminoglycoside phosphatase | M96392 | STR | GEN, TOB, NET, AMK | |
| aphA6 | 100 | Chromosome | APH(3′)-VIa | Aminoglycoside phosphatase | X07753 | AMK | GEN, TOB, NET, AMK | |
| armA | 100 | Chromosome | ArmA | 16S RNA methylase | AY220558 | GEN, TOB, NET, AMK | GEN, TOB, NET, AMK | |
| β-Lactams | blaADC-25 | 99.91a | Chromosome | ADC-25 | Cephalosporinase | EF016355 | AMX, AMC, cephalosporins | All β-lactams |
| blaOXA-66 | 100 | Chromosome | OXA-66 | CHDLb | FJ360530 | Noned | All β-lactams | |
| blaOXA-72 | 100 | Plasmid | OXA-72 | CHDL | GU199039 | All β-lactams except cephalosporins | All β-lactams | |
| Macrolides, lincosamide, and streptogramin | mphE | 100 | Chromosome | MphE | Macrolide phosphatase | EU294228 | ERY | ERY |
| msrE | 100 | Chromosome | MsrE | Macrolide efflux protein | EU294228 | ERY | ERY | |
| Sulfonamide | sul1 | 100 | Chromosome | Sul1 | Alternate dihydropteroate synthetase | CP002151 | SUL | SUL |
| sul2 | 100 | Chromosome | Sul2 | Alternate dihydropteroate synthetase | GQ421466 | SUL | SUL | |
| Trimethoprim | dfrA12 | 100 | Chromosome | DfrA12 | Alternate dihydropteroate reductase | AB571791 | TMP | TMP |
| Tetracycline | tetB | 100 | Chromosome | TetB | Efflux pump | AP000342 | TET | TET |
This 99.91% nucleotide identity with blaADC-25 corresponds to 100% amino acid similarity with ADC-25.
CHDL, carbapenem-hydrolyzing Ambler class D β-lactamase.
GEN, gentamicin; TOB, tobramycin; AMK, amikacin; NET, netilmicin; STR, streptomycin; AMX, amoxicillin; AMC, amoxicillin-clavulanic acid; ERY, erythromycin; SUL, sulfonamide; TMP, trimethoprim; TET, tetracycline.
OXA-66 is a chromosomally encoded CHDL which is normally not expressed. If blaOXA-66 is associated with an upstream insertion sequence (IS), it leads to the expression of the CHDL, resulting in decreased susceptibility to carbapenems.
Using the MLST (v1.8) server, we found that A. baumannii MAL belongs to sequence type (ST) 492 (ST492), according to the Pasteur scheme, and to a novel ST, according to the Oxford database.
The genome of A. baumannii MAL was further analyzed, especially in respect to the sequences of different porins, which were compared to the sequences of porins encoded by the A. baumannii ATCC 19606 reference genome. The sequence of the carO gene exhibited a low percent identity with the sequence of carO from A. baumannii ATCC 19606 (73% amino acid identity), with 57 different substitutions and a deletion of 3 amino acids in the protein. Three substitutions (Ala-137-Glu Cys-163-Arg, and Asp-172-Glu) were identified in OmpA, and two substitutions (Leu-47-Iso and Thr-48-Ser) were identified in OprD. A last porin, namely, the 33- to 36-kDa Omp, was analyzed and showed a perfect identity with that of A. baumannii ATCC 19606.
The regulatory efflux pumps AdeR and AdeS of A. baumannii MAL showed 99% (3 substitutions) and 97% (10 substitutions) amino acid sequence identities, respectively, with those of A. baumannii ATCC 19606, whereas AdeN and AdeB of A. baumannii MAL showed 99% amino acid sequence identities with those of A. baumannii ATCC 19606.
A. baumannii MAL possesses an AbaR element within the comM gene but an intact uspA gene, giving rise to the minimal form of the AbaR variant, which might be involved in the acquisition of several antibiotic resistance genes identified by WGS.
Plasmid characterization.
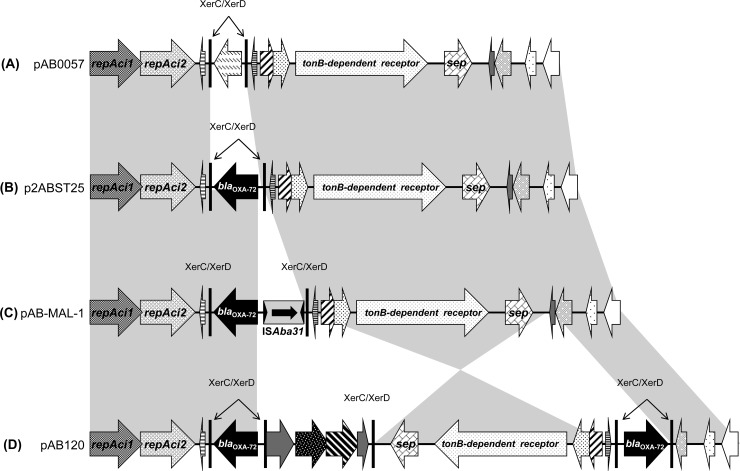
Using the Kieser extraction method (8), two distinct plasmids of ca. ∼10 kb and ∼67 kb were identified (data not shown). Transformation of the Kieser extracts into A. baumannii CIP 70.10 revealed that the blaOXA-72 gene encoding the carbapenemase was located on the ca. ∼10-kb plasmid, named pAB-MAL-1 (Table 1). WGS revealed that plasmid pAB-MAL-1 was 9,810 bp in size and belonged to the GR2 plasmid family. This plasmid was 99% identical to the recently published pA105-2 plasmid (GenBank accession number KR535993.1) recovered from an A. baumannii strain of ST636 (18). It showed strong similarity with plasmid p2ABST25 (GenBank accession number AEPA01000396.1) (19), differing only by the insertion of ISAba31 upstream of the blaOXA-72 gene (Fig. 1). In addition, these two plasmids were highly similar to plasmid pAB0057 (GenBank accession number NC_011585.1) (20). The major difference with plasmid pAB0057 consisted of the replacement of the XerC/XerD flanking sequence by a nucleotide sequence containing the blaOXA-72 gene and ISAba31 (Fig. 1). Another similar plasmid is pAB120 (GenBank accession number NC_019359.1), recovered from a carbapenem-resistant A. baumannii isolate recovered in Lithuanian hospitals (Fig. 1) (21). The main differences between pAB-MAL-1 and pAB120 reside in the number of blaOXA-72 gene copies (two in pAB120) and the inversion of a ca. ∼4.4-kb-long sequence containing the septocolysin (sep) and the TonB-dependent receptor genes (Fig. 1).
FIG 1.
Schematic representation of pAB-MAL-1, the blaOXA-72 gene-harboring plasmid of A. baumannii MAL, and of its related plasmids. Common features are highlighted with gray shading. (A) Plasmid pAB0057 from A. baumannii AB0057 (GenBank accession number NC_011585.1) (20); (B) plasmid p2ABST25 (GenBank accession number AEPA01000396.1) (19); (C) plasmid pAB-MAL-1; (D) plasmid pAB120 from a carbapenem-resistant A. baumannii isolate from Lithuania (GenBank accession number NC_019359.1) (21). Gene names are as follows: repAci, replicase gene; sep, septicolysin-encoding gene; ISAba31, insertion sequence of A. baumannii 31; XerC/XerD, specific site of the tyrosine recombinases XerC and XerD.
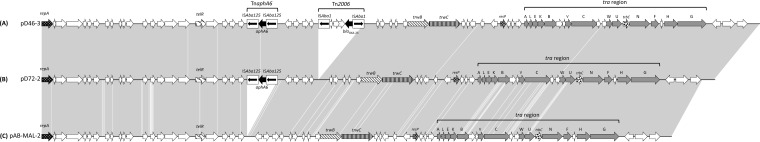
The second plasmid, named pAB-MAL-2 (67,025 bp), was related to two previously described repAci6 plasmids named pD72-2 and pD46-3 (GenBank accession number KM977710) (Fig. 2) (22). Since no antibiotic resistance genes are located on this plasmid, no transformant could be obtained. As shown in Fig. 2, pAB-MAL-2 and pD72-2 differed by only 61 single nucleotide polymorphisms (SNPs) and a composite transposon TnaphA6 containing the aphA6 gene flanked by two copies of ISAba125 inserted at nucleotide position 21,406 bp. Plasmids pD46-3 and pD72-2 shared 99.99% sequence identity with only five SNPs in their backbone and differed only by the insertion of Tn2006, which includes the blaOXA-23 gene.
FIG 2.
Schematic representation of pAB-MAL-2 of A. baumannii MAL and of its closely related plasmids. Common features are highlighted with gray shading. (A) Plasmid pD46-3 plasmid from A. baumannii D46 (GenBank accession number KM977710) (22); (B) plasmid pD72-2 from A. baumannii D72 (GenBank accession number NC_025111) (22); (C) plasmid pAB-MAL-2. Gene names are as follows; repA, replicase gene; telR, tellurite resistance gene; IS, insertion sequence; Tn, composite transposon; aphA6, aminoglycoside phosphotransferase A6; trwB, gene encoding an inner membrane nucleoside triphosphate-binding protein involved in plasmid transfer; trwC, gene encoding a helicase/relaxase involved in plasmid transfer; resP, gene encoding a putative resolvase; tra, plasmid transfer genes; trbC, gene involved in mating pair formation; white arrows, putative open reading frames with an unknown function.
DISCUSSION
To the best of our knowledge, this is the first description of an OXA-72-producing A. baumannii isolate from Serbia. OXA-72 is a single-amino-acid variant of OXA-24/40 (G224D). In contrast to OXA-23, which is the most common acquired carbapenemase in A. baumannii worldwide, OXA-72-producing isolates have been reported in only a few countries, including Brazil, Colombia, Taiwan, France, Croatia, Poland, Italy, Lithuania, and Sweden (19, 21, 23–30). The clonal spread of OXA-72-producing A. baumannii isolates was previously reported in the Balkan region, in a Croatian university hospital (24). Although this outbreak was related to a WWCII of A. baumannii, the plasmidic location of the blaOXA-72 was not evidenced. In addition, we found that the A. baumannii MAL belonged to a novel ST, ST492. This ST is a single-locus variant (SLV) of ST2 (with a single mutation in the fusA allele), indicating that the A. baumannii MAL isolate belongs to worldwide clone II (or global clone GC-2) (31). This clone is widely distributed and responsible for the dissemination of carbapenemases, including OXA-23, and OXA-24/40-like enzymes, including OXA-72 (31). Interestingly, blaOXA-72 was mostly identified in GC-2 isolates and was rarely reported from non-GC-2 A. baumannii isolates (32, 33). Our findings suggest that A. baumannii ST492 may possess all the features necessary for the efficient dissemination of OXA-72.
In A. baumannii, permeability defects, such as porin modification of efflux system overexpression, might be involved in antimicrobial resistance. As an example, the CarO porin has been advocated to play a role in carbapenem resistance (34). Since the CarO porin of A. baumannii MAL shares only 73% amino acid sequence identity with wild-type CarO, we could not exclude the possibility that it might also add to carbapenem resistance. In addition, although no direct evidence of the role of the porins OmpA and OprD in antimicrobial resistance has been demonstrated, the observed changes may act on their functionality and, thus, on membrane permeability to several antimicrobial compounds. Similarly, we have analyzed the sequence of efflux pump regulatory genes, which are known to be involved in antibiotic resistance, namely, the AdeR/S two-component system and AdeN and AdeB, which regulate the AdeIJK and AdeAB efflux pumps, respectively (35–37). Although only a few substitutions were found in efflux pump regulatory genes, further investigations using reverse transcription-PCR will be necessary to correlate these substitutions with overexpression of these efflux pumps.
The AbaR variant element is a putative class II transposon involved in the acquisition of resistance genes in A. baumannii, as exemplified by the discovery that AbaR1 possesses more than 14 resistance genes inserted within the comM gene (38). More than 20 different AbaR variants have been characterized to date (39). Two different AbaR variants were described: either the AbaR3-like variant, a large genetic element carrying numerous resistance genes, or its minimal form (40). These two forms differ by the acquisition of a large composite transposon within the uspA gene (40). A. baumannii MAL possesses the minimal form of AbaR, which might be involved in the acquisition of antibiotic resistance genes.
Globally, the in silico antimicrobial susceptibility profile, predicted using WGS, perfectly correlates with the observed phenotype of the A. baumannii MAL isolate.
Two plasmids were identified in our isolate. The first one carried the blaOXA-72 gene and shared similarity with the pAB120 plasmid, which was recovered from a carbapenem-resistant A. baumannii isolate recovered in Lithuanian hospitals and which carried two copies of the blaOXA-72 gene (21). Our study suggests that our plasmid carrying blaOXA-72 (pAB-MAL-1) might be derived from the p2ABST25 plasmid (only with an insertion of ISAba31 upstream of the blaOXA-72 gene), which itself might be considered an intermediate between pAB0057, which does not carry blaOXA-72 but with which p2ABST25 shares a common backbone, and pAB120, which carries two copies of the blaOXA-72 gene with an inversion in the common backbone (Fig. 1). The second plasmid (pAB-MAL-2) was found to share high similarity and a common backbone with the recently described pAD46-3 and pA105-2 plasmids, recovered from an A. baumannii isolate collected in Australia in 2010 (Fig. 2) (22) and Sweden in 2013 (18), respectively. As shown in Fig. 2, the pAB-MAL-2 plasmid might be considered the progenitor of pD72-2, in which a composite transposon (TnaphA6) has been inserted. Plasmid pD72-2 might itself be considered the progenitor of plasmid pD46-3, in which Tn2006 containing the blaOXA-23 gene has been inserted (Fig. 2) (22). Sixty-one SNPs were identified between pAB-MAL-2 and pD72-2, suggesting an ancient acquisition of TnaphA6 by pAB-MAL-2. In comparison, only five SNPs were found in the plasmid backbones of pD46-3 and pD72-2, suggesting a very recent acquisition of Tn2006 by pD72-2 (Fig. 2). The pAD46-3 plasmid has been reported to be a conjugative plasmid, which highlights the possible role of such repAci6 plasmids in the spread of antimicrobial resistance determinants worldwide. Hence, further studies are needed to evaluate the exact role of these types of plasmids in the global spread of antibiotic resistance genes in A. baumannii.
ACKNOWLEDGMENTS
We have no conflicts to declare.
This work was partially funded by grants from the University Paris-Sud, France, from the European Community (MAGIC-BULLET, FP7/HEALTH-F3-2001-27823), and from the Joint Program Initiative on Antimicrobial Resistance (ANR-14-JAMR-0002). L.D., S.B., D.G., N.F., and T.N. are members of the Laboratory of Excellence LERMIT, supported by a grant from ANR (ANR-10-LABX-33).
Funding Statement
This work was partially funded by grants from the University Paris-Sud, France, from the European Community (MAGIC-BULLET, FP7/HEALTH-F3-2001-27823), and from the Joint Program Initiative on Antimicrobial Resistance (ANR-14-JAMR-0002). L.D., S.B., D.G., N.F., and T.N. are members of the Laboratory of Excellence LERMIT, supported by a grant from ANR (ANR-10-LABX-33).
REFERENCES
- 1.Joly-Guillou ML. 2005. Clinical impact and pathogenicity of Acinetobacter. Clin Microbiol Infect 11:868–873. doi: 10.1111/j.1469-0691.2005.01227.x. [DOI] [PubMed] [Google Scholar]
- 2.Peleg AY, Seifert H, Paterson DL. 2008. Acinetobacter baumannii: emergence of a successful pathogen. Clin Microbiol Rev 21:538–582. doi: 10.1128/CMR.00058-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Potron A, Poirel L, Nordmann P. 2015. Emerging broad-spectrum resistance in Pseudomonas aeruginosa and Acinetobacter baumannii: mechanisms and epidemiology. Int J Antimicrob Agents 45:568–585. doi: 10.1016/j.ijantimicag.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 4.Higgins PG, Perez-Llarena FJ, Zander E, Fernandez A, Bou G, Seifert H. 2013. OXA-235, a novel class D β-lactamase involved in resistance to carbapenems in Acinetobacter baumannii. Antimicrob Agents Chemother 57:2121–2126. doi: 10.1128/AAC.02413-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonnet R, Marchandin H, Chanal C, Sirot D, Labia R, De Champs C, Jumas-Bilak E, Sirot J. 2002. Chromosome-encoded class D β-lactamase OXA-23 in Proteus mirabilis. Antimicrob Agents Chemother 46:2004–2006. doi: 10.1128/AAC.46.6.2004-2006.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Osterblad M, Karah N, Halkilahti J, Sarkkinen H, Uhlin BE, Jalava J. 2016. Rare detection of the Acinetobacter class D carbapenemase blaOXA-23 gene in Proteus mirabilis. Antimicrob Agents Chemother 60:3243–3245. doi: 10.1128/AAC.03119-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doi Y, Arakawa Y. 2007. 16S ribosomal RNA methylation: emerging resistance mechanism against aminoglycosides. Clin Infect Dis 45:88–94. doi: 10.1086/518605. [DOI] [PubMed] [Google Scholar]
- 8.Kieser T. 1984. Factors affecting the isolation of CCC DNA from Streptomyces lividans and Escherichia coli. Plasmid 12:19–36. doi: 10.1016/0147-619X(84)90063-5. [DOI] [PubMed] [Google Scholar]
- 9.Dortet L, Poirel L, Errera C, Nordmann P. 2014. CarbAcineto NP test for rapid detection of carbapenemase-producing Acinetobacter spp. J Clin Microbiol 52:2359–2364. doi: 10.1128/JCM.00594-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zankari E, Hasman H, Kaas RS, Seyfarth AM, Agerso Y, Lund O, Larsen MV, Aarestrup FM. 2013. Genotyping using whole-genome sequencing is a realistic alternative to surveillance based on phenotypic antimicrobial susceptibility testing. J Antimicrob Chemother 68:771–777. doi: 10.1093/jac/dks496. [DOI] [PubMed] [Google Scholar]
- 11.Larsen MV, Cosentino S, Rasmussen S, Friis C, Hasman H, Marvig RL, Jelsbak L, Sicheritz-Ponten T, Ussery DW, Aarestrup FM, Lund O. 2012. Multilocus sequence typing of total-genome-sequenced bacteria. J Clin Microbiol 50:1355–1361. doi: 10.1128/JCM.06094-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bartual SG, Seifert H, Hippler C, Luzon MA, Wisplinghoff H, Rodriguez-Valera F. 2005. Development of a multilocus sequence typing scheme for characterization of clinical isolates of Acinetobacter baumannii. J Clin Microbiol 43:4382–4390. doi: 10.1128/JCM.43.9.4382-4390.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diancourt L, Passet V, Nemec A, Dijkshoorn L, Brisse S. 2010. The population structure of Acinetobacter baumannii: expanding multiresistant clones from an ancestral susceptible genetic pool. PLoS One 5:e10034. doi: 10.1371/journal.pone.0010034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dortet L, Bonnin RA, Girlich D, Imanci D, Bernabeu S, Fortineau N, Naas T. 2015. Whole genome sequence of a European clone II and OXA-72-producing Acinetobacter baumannii from Serbia. Genome Announc 3(6):e01390-15. doi: 10.1128/genomeA.01390-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gu DX, Hu YJ, Zhou HW, Zhang R, Chen GX. 2015. Substitutions of Ser83Leu in GyrA and Ser80Leu in ParC associated with quinolone resistance in Acinetobacter pittii. Microb Drug Resist 21:345–351. doi: 10.1089/mdr.2014.0057. [DOI] [PubMed] [Google Scholar]
- 16.Vila J, Ruiz J, Goni P, Jimenez de Anta T. 1997. Quinolone-resistance mutations in the topoisomerase IV parC gene of Acinetobacter baumannii. J Antimicrob Chemother 39:757–762. doi: 10.1093/jac/39.6.757. [DOI] [PubMed] [Google Scholar]
- 17.Vila J, Ruiz J, Goni P, Marcos A, Jimenez de Anta T. 1995. Mutation in the gyrA gene of quinolone-resistant clinical isolates of Acinetobacter baumannii. Antimicrob Agents Chemother 39:1201–1203. doi: 10.1128/AAC.39.5.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karah N, Dwibedi CK, Sjostrom K, Edquist P, Johansson A, Wai SN, Uhlin BE. 2016. Novel aminoglycoside resistance transposons and transposon-derived circular forms detected in carbapenem-resistant Acinetobacter baumannii clinical isolates. Antimicrob Agents Chemother 60:1801–1818. doi: 10.1128/AAC.02143-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Di Nocera PP, Rocco F, Giannouli M, Triassi M, Zarrilli R. 2011. Genome organization of epidemic Acinetobacter baumannii strains. BMC Microbiol 11:224. doi: 10.1186/1471-2180-11-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adams MD, Goglin K, Molyneaux N, Hujer KM, Lavender H, Jamison JJ, MacDonald IJ, Martin KM, Russo T, Campagnari AA, Hujer AM, Bonomo RA, Gill SR. 2008. Comparative genome sequence analysis of multidrug-resistant Acinetobacter baumannii. J Bacteriol 190:8053–8064. doi: 10.1128/JB.00834-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Povilonis J, Seputiene V, Krasauskas R, Juskaite R, Miskinyte M, Suziedelis K, Suziedeliene E. 2013. Spread of carbapenem-resistant Acinetobacter baumannii carrying a plasmid with two genes encoding OXA-72 carbapenemase in Lithuanian hospitals. J Antimicrob Chemother 68:1000–1006. doi: 10.1093/jac/dks499. [DOI] [PubMed] [Google Scholar]
- 22.Nigro SJ, Holt KE, Pickard D, Hall RM. 2015. Carbapenem and amikacin resistance on a large conjugative Acinetobacter baumannii plasmid. J Antimicrob Chemother 70:1259–1261. doi: 10.1093/jac/dku486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barnaud G, Zihoune N, Ricard JD, Hippeaux MC, Eveillard M, Dreyfuss D, Branger C. 2010. Two sequential outbreaks caused by multidrug-resistant Acinetobacter baumannii isolates producing OXA-58 or OXA-72 oxacillinase in an intensive care unit in France. J Hosp Infect 76:358–360. doi: 10.1016/j.jhin.2010.05.026. [DOI] [PubMed] [Google Scholar]
- 24.Franolic-Kukina I, Bedenic B, Budimir A, Herljevic Z, Vranes J, Higgins PG. 2011. Clonal spread of carbapenem-resistant OXA-72-positive Acinetobacter baumannii in a Croatian university hospital. Int J Infect Dis 15:e706–e709. doi: 10.1016/j.ijid.2011.05.016. [DOI] [PubMed] [Google Scholar]
- 25.Kuo SC, Yang SP, Lee YT, Chuang HC, Chen CP, Chang CL, Chen TL, Lu PL, Hsueh PR, Fung CP. 2013. Dissemination of imipenem-resistant Acinetobacter baumannii with new plasmid-borne blaOXA-72 in Taiwan. BMC Infect Dis 13:319. doi: 10.1186/1471-2334-13-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lu PL, Doumith M, Livermore DM, Chen TP, Woodford N. 2009. Diversity of carbapenem resistance mechanisms in Acinetobacter baumannii from a Taiwan hospital: spread of plasmid-borne OXA-72 carbapenemase. J Antimicrob Chemother 63:641–647. doi: 10.1093/jac/dkn553. [DOI] [PubMed] [Google Scholar]
- 27.Majewski P, Wieczorek P, Ojdana D, Sacha PT, Wieczorek A, Tryniszewska EA. 2014. In vitro activity of rifampicin alone and in combination with imipenem against multidrug-resistant Acinetobacter baumannii harboring the blaOXA-72 resistance gene. Scand J Infect Dis 46:260–264. doi: 10.3109/00365548.2013.865141. [DOI] [PubMed] [Google Scholar]
- 28.Saavedra SY, Cayo R, Gales AC, Leal AL, Saavedra CH. 2014. Early dissemination of OXA-72-producing Acinetobacter baumannii strain in Colombia: a case report. Braz J Infect Dis 18:678–680. doi: 10.1016/j.bjid.2014.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vasconcelos AT, Barth AL, Zavascki AP, Gales AC, Levin AS, Lucarevschi BR, Cabral BG, Brasiliense DM, Rossi F, Furtado GH, Carneiro IC, da Silva JO, Ribeiro J, Lima KV, Correa L, Britto MH, Silva MT, da Conceicao ML, Moreira M, Martino MD, de Freitas MR, Oliveira MS, Dalben MF, Guzman RD, Cayo R, Morais R, Santos SA, Martins WM. 2015. The changing epidemiology of Acinetobacter spp. producing OXA carbapenemases causing bloodstream infections in Brazil: a BrasNet report. Diagn Microbiol Infect Dis 83:382–385. doi: 10.1016/j.diagmicrobio.2015.08.006. [DOI] [PubMed] [Google Scholar]
- 30.Zarrilli R, Giannouli M, Rocco F, Loman NJ, Haines AS, Constantinidou C, Pallen MJ, Triassi M, Di Nocera PP. 2011. Genome sequences of three Acinetobacter baumannii strains assigned to the multilocus sequence typing genotypes ST2, ST25, and ST78. J Bacteriol 193:2359–2360. doi: 10.1128/JB.00245-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zarrilli R, Pournaras S, Giannouli M, Tsakris A. 2013. Global evolution of multidrug-resistant Acinetobacter baumannii clonal lineages. Int J Antimicrob Agents 41:11–19. doi: 10.1016/j.ijantimicag.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 32.Tian GB, Adams-Haduch JM, Bogdanovich T, Pasculle AW, Quinn JP, Wang HN, Doi Y. 2011. Identification of diverse OXA-40 group carbapenemases, including a novel variant, OXA-160, from Acinetobacter baumannii in Pennsylvania. Antimicrob Agents Chemother 55:429–432. doi: 10.1128/AAC.01155-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wright MS, Haft DH, Harkins DM, Perez F, Hujer KM, Bajaksouzian S, Benard MF, Jacobs MR, Bonomo RA, Adams MD. 2014. New insights into dissemination and variation of the health care-associated pathogen Acinetobacter baumannii from genomic analysis. mBio 5:e00963-13. doi: 10.1128/mBio.00963-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mussi MA, Limansky AS, Viale AM. 2005. Acquisition of resistance to carbapenems in multidrug-resistant clinical strains of Acinetobacter baumannii: natural insertional inactivation of a gene encoding a member of a novel family of β-barrel outer membrane proteins. Antimicrob Agents Chemother 49:1432–1440. doi: 10.1128/AAC.49.4.1432-1440.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rosenfeld N, Bouchier C, Courvalin P, Perichon B. 2012. Expression of the resistance-nodulation-cell division pump AdeIJK in Acinetobacter baumannii is regulated by AdeN, a TetR-type regulator. Antimicrob Agents Chemother 56:2504–2510. doi: 10.1128/AAC.06422-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sun JR, Chan MC, Chang TY, Wang WY, Chiueh TS. 2010. Overexpression of the adeB gene in clinical isolates of tigecycline-nonsusceptible Acinetobacter baumannii without insertion mutations in adeRS. Antimicrob Agents Chemother 54:4934–4938. doi: 10.1128/AAC.00414-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yoon EJ, Courvalin P, Grillot-Courvalin C. 2013. RND-type efflux pumps in multidrug-resistant clinical isolates of Acinetobacter baumannii: major role for AdeABC overexpression and AdeRS mutations. Antimicrob Agents Chemother 57:2989–2995. doi: 10.1128/AAC.02556-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fournier PE, Vallenet D, Barbe V, Audic S, Ogata H, Poirel L, Richet H, Robert C, Mangenot S, Abergel C, Nordmann P, Weissenbach J, Raoult D, Claverie JM. 2006. Comparative genomics of multidrug resistance in Acinetobacter baumannii. PLoS Genet 2:e7. doi: 10.1371/journal.pgen.0020007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roca I, Espinal P, Vila-Farres X, Vila J. 2012. The Acinetobacter baumannii oxymoron: commensal hospital dweller turned pan-drug-resistant menace. Front Microbiol 3:148. doi: 10.3389/fmicb.2012.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bonnin RA, Poirel L, Nordmann P. 2012. AbaR-type transposon structures in Acinetobacter baumannii. J Antimicrob Chemother 67:234–236. doi: 10.1093/jac/dkr413. [DOI] [PubMed] [Google Scholar]