Abstract
We evaluated the diagnostic and clinical usefulness of blood specimens to detect Middle East respiratory syndrome coronavirus infection in 21 patients from the 2015 outbreak in South Korea. Viral RNA was detected in blood from 33% of patients at initial diagnosis, and the detection preceded a worse clinical course.
Keywords: Middle East respiratory syndrome, coronavirus, blood, prognosis, real-time PCR, viruses, MERS-CoV, zoonoses
Middle East respiratory syndrome coronavirus (MERS-CoV) is a zoonotic, betacoronavirus lineage C RNA virus that was first identified in Saudi Arabia in 2012 (1). MERS-CoV causes respiratory and renal illness in humans, and infection often progresses to severe pneumonia, acute respiratory distress syndrome, renal failure, or death in a subset of patients (2). Risk factors, including patient age, preexisting health conditions, and high viral load in upper respiratory specimens, have been suggested to be related to disease severity and death (3,4). However, pathogenesis and clinical characteristics promoting recovery from infection or progression to serious organ failure have not been well elucidated.
Respiratory specimens are preferred for viral RNA detection and confirmatory diagnosis of MERS-CoV infection in humans (5). MERS-CoV has broad tissue tropism, including the kidney, intestinal tract, liver, histiocytes, macrophages, and T lymphocytes, but viral RNA has been found inconsistently in blood, urine, and fecal specimens (6–10). Reports have described small numbers of cases with extrapulmonary virus; therefore, it remains unclear whether extrapulmonary specimens have any diagnostic usefulness in determining infection or whether extrapulmonary viral detection has clinical implications in disease management.
A large MERS-CoV outbreak occurred in 2015 in South Korea. This outbreak comprised the first imported case and subsequent infection of 185 patients (11). Our study aimed to evaluate the diagnostic utility of blood specimens for MERS-CoV infection by using large numbers of patients with a single viral origin and to determine the relationship between blood viral detection and clinical characteristics.
The Study
We collected 21 pairs of EDTA whole blood and serum specimens from 21 patients with MERS-CoV after admission to the National Medical Center in Seoul, South Korea. MERS-CoV infection initially was diagnosed by the Korea Centers for Disease Control and Prevention using respiratory specimens (11). After admission, each patient was reassessed for epidemiologic information and clinically managed with monitoring.
Specimens were stored at −80°C before analyses. Viral RNA was extracted and eluted with a MagNA Pure LC 2.0 automated nucleic acid extractor and MagNA Pure LC total nucleic acid isolation kit (both from Roche Diagnostics, Mannheim, Germany) according to the manufacturer’s instructions. Specimen volumes were 100 μL EDTA whole blood and 200 μL serum, and elution volumes were 100 μL for EDTA whole blood and 50 μL for serum. One-step, real-time reverse transcription PCR (rRT-PCR) was performed for the 3 MERS-CoV gene regions (upstream envelope [upE], open reading frame [ORF] 1a, and nucleocapsid) with an AgPath-ID One-step RT-PCR kit (Applied Biosystems, Foster City, CA, USA) and an ABI7500 real-time PCR system (Applied Biosystems). Human ribonuclease (RNase) P was amplified in parallel for sample quality control (5,12,13). In each test, the viral RNA was considered detected when amplification before cutoff was observed from at least 2 different targets in MERS-CoV with pass of sample quality control. The robustness of rRT-PCR was demonstrated for qualitative concordance of positivity or negativity by using different types of specimens (Technical Appendix Table 1). We calculated the viral copy concentration in the blood using standard curves constructed from the cycle threshold (Ct) of serially diluted 105 copies/μL of upE RNA (provided by the University of Bonn Medical Center, Bonn, Germany).
The results of the blood viral RNA analyses did not affect clinical management. We assessed the relationships among clinical and molecular diagnostic factors with the IBM SPSS Statistics program 22.0 (SPSS Inc., Chicago, IL, USA). In each test, p<0.05 was considered statistically significant. The institutional review board of the National Medical Center approved this study (H-1508-057-002).
We assessed patient demographics, their clinical features, and disease outcomes (Tables 1, 2; Technical Appendix Table 2). The time difference was an average of 1.5 days (median 1, range 0–5 days) between when the initial diagnostic respiratory specimens and blood specimens were obtained. At admission, viral RNA was detected in 6 (29%) of 21 EDTA whole blood and 6 (29%) of 21 serum samples from infected patients. Two patients showed viral positivity in either specimen subtype of EDTA whole blood or serum; therefore, the overall detection rate for MERS-CoV was 33% (7/21) in blood. The concordance rate of viral assay was 90% (19/21) between EDTA whole blood and serum specimens. Blood virus concentration was 3,130 copies/mL EDTA whole blood (range 2,080–17,400 copies/mL), equivalent to a median upE Ct of 37.55, and 1,300 copies/mL serum (range 490–10,200 copies/mL, median upE Ct 36.88).
Table 1. Demographic characteristics of 21 Middle East respiratory syndrome coronavirus–infected patients, South Korea, 2015.
| Characteristic | Value |
|---|---|
| No. |
21 |
| Median age, y (range) |
64 (23–86) |
| Sex, no. | |
| M | 9 |
| F |
12 |
| Median hospitalization, d (range) |
17 (2–138) |
| Death rate, % |
23.8 |
| Median no. days exposed to virus (range)* |
3 (1–20) |
| Median duration between symptom onset and initial diagnosis, d (range) | 2 (0–12)† |
*Based on contact history. †Two asymptomatic patients were excluded from analysis.
Table 2. Differences in Middle East respiratory syndrome coronavirus detection by rRT-PCR among specimen types, South Korea, 2015*.
| Characteristic |
Respiratory specimen type |
Blood specimen type | |
|---|---|---|---|
| EDTA whole blood |
Serum |
||
| No. specimens |
20 sputum; 1 endotracheal aspirate |
21 |
21 |
| Days from initial confirmatory diagnosis using respiratory specimens to blood sampling |
Set as day 0 |
1.4 (median 1,
range 0–5) |
1.5 (median 1,
range 0–5) |
| Viral gene region rRT-PCR | |||
| Upstream of E | |||
| Total positive results/total tests (%) | 21/21 (100) | 6/21 (29) | 6/21 (29) |
| Median Ct (range) | 28.48 (19.70–33.46)† | 37.55 (35.34–38.07) | 36.88 (34.24–38.14) |
| Open reading frame 1a | |||
| Total positive results/total tests (%) | 21/21 (100) | 0/21 (0) | 0/21 (0) |
| Median Ct (range) | 29.28 (21.00–34.21)† | Not detected | Not detected |
| Nucleocapsid | |||
| Total positive results/total tests (%) | Not performed | 7/21 (33) | 7/21 (33) |
| Median Ct (range) | Not performed | 36.37 (35.62–38.04) | 34.62 (32.93–38.84) |
*Ct, cycle threshold; rRT-PCR, real-time reverse transcription PCR. †One patient whose Ct was not reported was excluded.
Blood viral RNA positivity at admission was associated with fever >37.5°C on the sampling date (p = 0.007), requirement for mechanical ventilation during the following clinical course (p = 0.003) and extracorporeal membrane oxygenation (p = 0.025), and patient death (p = 0.025, all by 2-tailed Fisher exact test; Figure). Blood viral RNA positivity was not associated with viral Ct in the initial diagnostic lower respiratory specimens, or requirement of oxygen supplementation during the following clinical course. Between the blood viral RNA-positive and -negative groups, we found no differences in age, duration from symptom onset to diagnosis of MERS-CoV infection, or an invasive procedure before the specimens were obtained (Technical Appendix Table 3).
Viral loads in the lower respiratory specimens at the initial confirmatory diagnosis showed no effect on patient survival (Figure). Patient death was not associated with length of time from symptom onset to diagnosis of MERS-CoV infection (Technical Appendix Table 3).
Figure.
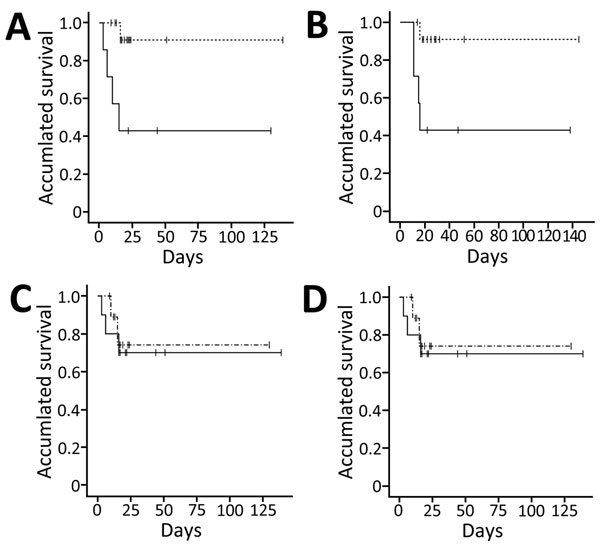
Differences in survival among Middle East respiratory syndrome coronavirus–infected patients, South Korea, 2015. A, B) Survival difference between the blood viral RNA-positive (solid line) and -negative (broken line) groups. Survival was defined as the time from initial confirmatory diagnosis to death before hospital discharge (A) (Kaplan-Meier survival analysis, log rank p = 0.009; Breslow p = 0.006) and as the time from symptom onset to death (B) (Kaplan-Meier survival analysis, log rank p = 0.017; Breslow p = 0.015). C, D) Survival difference between the high respiratory viral load (solid line) and low respiratory viral load (broken line) groups. Viral loads were classified into 2 groups: patients who harbored viral loads above the median load of patients and patients who harbored below. Survival was defined as time from initial confirmatory diagnosis to death. Cycle threshold (Ct) values were calculated for real-time reverse transcription PCRs targeting the upstream of envelope region (C) and open reading frame 1a region (D) (Kaplan-Meier survival analysis, log rank p = 0.739; Breslow p = 0.630). Tick marks along data lines indicate data-censored time points.
Our results showed that the detection rate of blood viral RNA was low in the early phase of infection in patients with a confirmed diagnosis, similar to results from a previous study (14). These findings contrasted with those of severe acute respiratory syndrome coronavirus infection (15). Therefore, in the case of MERS-CoV infection, blood does not have the highest diagnostic yield for the initial confirmatory diagnosis. The viral load in blood was low, even in detected cases. A proportion of MERS-CoV isolates in the 2015 Korea outbreak harbored a C→T substitution in the third nucleotide of the ORF1a primer binding site (GenBank accession nos. KT374052–374055). This mismatch may partially contribute to the insensitivity of ORF1a assay observed in this study. An alternative sensitive target replacing ORF1a might be useful in studies using blood specimens.
Blood viral RNA has been detected in a few case reports of MERS-CoV fatalities (8–10). Our data of 42 specimens from cross-sectional time points focusing on early viremia showed that blood viral RNA was present in a subpopulation of patients and that these patients had significantly poorer prognoses, as demonstrated by the need for more frequent mechanical ventilation and the increased risk for death. Further large studies using serial daily specimens that are collected throughout the admission period–both upper and lower respiratory specimens and paired measurement of viral RNA and antibody in blood–might help overcome the limitation of the current study, which included relatively small numbers of deceased patients (24% [5/21]).
Conclusions
Our data showed a detection rate of 33% for viral RNA in blood at initial diagnosis, which was insufficient for initial confirmatory diagnosis. Blood viral RNA at the early phase was related to a worse clinical course in infected patients and might be a good prognostic indicator of severe outcome. Measuring blood viral RNA at hospital admission might be useful.
Sensitivity, reproducibility, and inhibitory effect among different specimen types for the Middle Ease respiratory syndrome coronavirus (MERS-CoV) real-time reverse transcription PCR; viral RNA analyses of MERS-CoV–infected patients; and statistical relationships among clinical and molecular diagnostic factors.
Acknowledgments
We thank Christian Drosten for providing the in vitro transcribed RNAs of MERS-CoV genes.
This research was supported by a research program funded by the National Medical Center Research Institute, grant number NMC-2015-MS-02.
Biography
Dr. Kim is a medical doctor at the Department of Laboratory Medicine of National Medical Center in South Korea. Her research interests include molecular diagnostics and infectious diseases.
Footnotes
Suggested citation for this article: Kim SY, Park SJ, Cho SY, Cha RH, Jee HG, Kim G, et al. Viral RNA in blood as indicator of severe outcome in Middle East respiratory syndrome coronavirus infection. Emerg Infect Dis. 2016 Oct [date cited]. http://dx.doi.org/10.3201/eid2210.160218
References
- 1.Zaki AM, van Boheemen S, Bestebroer TM, Osterhaus AD, Fouchier RA. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367:1814–20 http://. 10.1056/NEJMoa1211721 [DOI] [PubMed] [Google Scholar]
- 2.Zumla A, Hui DS, Perlman S. Middle East respiratory syndrome. Lancet. 2015;386:995–1007. 10.1016/S0140-6736(15)60454-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Majumder MS, Kluberg SA, Mekaru SR, Brownstein JS. Mortality risk factors for Middle East respiratory syndrome outbreak, South Korea, 2015. Emerg Infect Dis. 2015;21:2088–90. 10.3201/eid2111.151231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feikin DR, Alraddadi B, Qutub M, Shabouni O, Curns A, Oboho IK, et al. Association of higher MERS-CoV virus load with severe disease and death, Saudi Arabia, 2014. Emerg Infect Dis. 2015;21:2029–35. 10.3201/eid2111.150764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization. Laboratory testing for Middle East respiratory syndrome coronavirus. Interim guidance (revised) [cited 2015 Oct 15]. http://apps.who.int/iris/bitstream/10665/176982/1/WHO_MERS_LAB_15.1_eng.pdf
- 6.Chan JF, Chan KH, Choi GK, To KK, Tse H, Cai JP, et al. Differential cell line susceptibility to the emerging novel human betacoronavirus 2c EMC/2012: implications for disease pathogenesis and clinical manifestation. J Infect Dis. 2013;207:1743–52. 10.1093/infdis/jit123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chu H, Zhou J, Wong BH, Li C, Chan JF, Cheng ZS, et al. Middle East respiratory syndrome coronavirus efficiently infects human primary T lymphocytes and activates the extrinsic and intrinsic apoptosis pathways. J Infect Dis. 2016;213:904–14. 10.1093/infdis/jiv380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guery B, Poissy J, el Mansouf L, Séjourné C, Ettahar N, Lemaire X, et al. ; MERS-CoV study group. Clinical features and viral diagnosis of two cases of infection with Middle East respiratory syndrome coronavirus: a report of nosocomial transmission. Lancet. 2013;381:2265–72. 10.1016/S0140-6736(13)60982-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Drosten C, Seilmaier M, Corman VM, Hartmann W, Scheible G, Sack S, et al. Clinical features and virological analysis of a case of Middle East respiratory syndrome coronavirus infection. Lancet Infect Dis. 2013;13:745–51. 10.1016/S1473-3099(13)70154-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Poissy J, Goffard A, Parmentier-Decrucq E, Favory R, Kauv M, Kipnis E, et al. ; MERS-CoV Biology Group. Kinetics and pattern of viral excretion in biological specimens of two MERS-CoV cases. [Erratum in: J Clin Virol. 2015,63:94.. ]. J Clin Virol. 2014;61:275–8. 10.1016/j.jcv.2014.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Korea Centers for Disease Control and Prevention. Middle East respiratory syndrome coronavirus outbreak in the Republic of Korea, 2015. [Erratum in: Osong Public Health Res Perspect. 2016. ]. Osong Public Health Res Perspect. 2015;6:269–78. 10.1016/j.phrp.2015.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corman VM, Eckerle I, Bleicker T, Zaki A, Landt O, Eschbach-Bludau M, et al. Detection of a novel human coronavirus by real-time reverse-transcription polymerase chain reaction. Euro Surveill. 2012;17:20285. [DOI] [PubMed] [Google Scholar]
- 13.Lu X, Whitaker B, Sakthivel SK, Kamili S, Rose LE, Lowe L, et al. Real-time reverse transcription–PCR assay panel for Middle East respiratory syndrome coronavirus. J Clin Microbiol. 2014;52:67–75. 10.1128/JCM.02533-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Corman VM, Albarrak AM, Omrani AS, Albarrak MM, Farah ME, Almasri M, et al. Viral shedding and antibody response in 37 patients with Middle East respiratory syndrome coronavirus infection. Clin Infect Dis. 2016;62:477–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ng EK, Hui DS, Chan KC, Hung EC, Chiu RW, Lee N, et al. Quantitative analysis and prognostic implication of SARS coronavirus RNA in the plasma and serum of patients with severe acute respiratory syndrome. Clin Chem. 2003;49:1976–80. 10.1373/clinchem.2003.024125 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sensitivity, reproducibility, and inhibitory effect among different specimen types for the Middle Ease respiratory syndrome coronavirus (MERS-CoV) real-time reverse transcription PCR; viral RNA analyses of MERS-CoV–infected patients; and statistical relationships among clinical and molecular diagnostic factors.


