Abstract
Objective
To examine whether changes in diet quality predict changes in central adiposity among post-menopausal women.
Methods
At baseline and 3-year follow-up, Women’s Health Initiative Observational Study participants completed food frequency questionnaires and waist circumference was measured (WC, n=67,175). In a subset, trunk fat was measured via Dual Energy X-ray Absorptiometry (DXA, n=4,254). Using multivariable linear regression, we examined 3-year changes in dietary patterns (Healthy Eating Index-2010, Alternate Healthy Eating Index-2010, Alternate Mediterranean Diet [aMED], and Dietary Approaches to Stop Hypertension) as predictors of concurrent changes in WC and, secondarily, DXA.
Results
Mean (SD) age, and 3-year changes in weight and WC, were 63 (7) years, 0.52 (4.26) kilograms and 0.94 (6.65) cm. A 10% increase in any dietary pattern score, representing improved diet quality, was associated with 0.07–0.43 cm smaller increase in WC over 3 years (all p<0.05). After adjusting for weight change, associations attenuated to 0.02–0.10 cm, but remained statistically significant for all patterns except aMED. Results were similar for DXA trunk fat.
Conclusions
3-year improvements in diet quality are modestly protective against gain in WC and partially explained by less weight gain. Achieving and maintaining a healthful diet after menopause may protect against gains in central adiposity.
Introduction
While U.S. obesity prevalence has plateaued, waist circumference (WC) continues to increase: from 1999–2012, WC increased by 4 centimeters (cm) among women, with abdominal obesity prevalence (WC>88cm) reaching 65%.1 Most epidemiologic studies utilize body mass index (BMI); however, WC provides additional information. For example, elevated WC is associated with higher cardiovascular mortality even among normal-weight women and predicts insulin resistance and chronic disease better than BMI.2 Additionally, since fat and lean mass are lost at different rates during aging, change in WC may be a more sensitive indicator of fat gain than change in BMI or weight among middle-aged and older populations.3
Limited evidence suggests that adherence to a healthful dietary pattern may influence body fat distribution,4 raising the possibility that dietary modification could mitigate the increasing abdominal obesity prevalence and associated chronic diseases.5 In contrast to the study of single foods or nutrients, dietary patterns incorporate the combinations and quantities in which foods and nutrients are consumed and their synergistic and cumulative effects.6 Thus, public health messages may be more easily adopted by consumers when described by this composite measure of diet quality. Additionally, while most previous research assesses diet quality and WC at single time-points,7, 8 examining changes over time better addresses whether achieving or maintaining a healthful diet prevents increases in WC.
This study of ethnically diverse post-menopausal women examines whether changes in four healthful dietary patterns predict changes in WC over 3 years. Secondarily, we examine whether age or race/ethnicity modify associations, and whether changes in dietary patterns predict changes in dual energy X-ray absorptiometry (DXA) trunk fat mass.
Methods
Study population
The methods of the Women’s Health Initiative (WHI) are described elsewhere.9 From 1993–1998, postmenopausal women 50–79 years old were recruited into overlapping clinical trials or an observational study (OS). Of the n= 93,676 women enrolled in the WHI-OS, the present analysis includes those with complete food frequency questionnaires (FFQs), plausible energy intakes (>600 kcals/day-<5,000 kcals/day) and weight and WC measurements at both baseline and 3-year follow-up (n=67,378). To achieve normality and to limit the potential for residual confounding due to unmeasured changes in health status influencing both weight and diet, we excluded women with 3-year weight changes>20 kilograms (kg), resulting in an analytic sample of 67,175 women. In sensitivity analyses, we retained women with large weight changes. Institutional review boards at participating institutions approved procedures and protocols. All participants provided written informed consent.
Anthropometric measurements
At baseline and 3-year follow-up, trained staff used a standardized protocol to measure weight to the nearest 0.1 kg using a balance beam scale, height to the nearest 0.1 cm using a wall-mounted stadiometer and WC to the nearest 0.1 cm during expiration at the narrowest section of the torso. BMI was calculated as weight in kg divided by height in meters squared (kg/m2). With use of standardized protocols, whole-body dual X-ray absorptiometry (DXA) scans were obtained for women in three pre-designated WHI centers (Birmingham, Tucson/Phoenix, and Pittsburgh) using Hologic QDR scanners (QDR 2000, 2000+, or 4500W; Hologic, Inc., Waltham, MA). Scanner performance was monitored longitudinally using spine and whole-body phantom scans. Quality control procedures were implemented at the University of California, San Francisco Coordinating Center. In secondary analyses, we examined change in trunk fat mass among n=4,254 WHI-OS women with DXA at baseline and 3-year follow-up. This subset of women was comparable to our full sample with respect to baseline age (63 years), waist circumference (84 cm), diet quality (e.g., DASH score 23 versus 24 points), and race/ethnicity (81% versus 86% non-Hispanic white).
Diet assessment
Diet was measured at baseline and 3-year follow-up using a self-administered food frequency questionnaire (FFQ) developed and validated for WHI.10 The FFQ captured foods relevant for multiethnic and geographically diverse populations, and produced reliable estimates of foods and nutrients comparable to those from four 24-hour dietary recalls and 4-day food records.10 We used a nutrient database from the Nutrition Data Systems for Research (NDSR), v.2005 (Nutrition Coordinating Center, University of Minnesota).11 MyPyramid equivalents were computed using the FFQ data with a customized link between NDSR and the MyPyramid Equivalents Database, v.2.0 (USDA).12 MyPyramid equivalents translate foods, as eaten, into standardized quantities: e.g., an equivalent is an amount considered nutritionally equal to 1 cup in the vegetable, fruit, and dairy components or 1 ounce (28.35 g) in grains or protein foods.
We calculated four a priori dietary patterns previously associated with lower morbidity and mortality in WHI.13–15 These indices were : 1) the alternate Mediterranean Diet (aMED), reflecting a Mediterranean-style dietary pattern characterized by high consumption of minimally processed plant-based foods; olive oil as the principal source of fat; low-to-moderate consumption of dairy products, fish, and poultry; low consumption of red meat; and low-to-moderate consumption of wine;16, 17 2) the Healthy Eating Index-2010 (HEI-2010), created by the USDA and the National Cancer Institute to align with the 2010 Dietary Guidelines for Americans;18–20 3) the Alternate Healthy Eating Index-2010 (AHEI-2010), which adapted the Dietary Guidelines to incorporate food components predictive of chronic disease, including greater intakes of vegetables and fruits, whole grains, nuts and legumes, long-chain omega-3 fatty acids, and polyunsaturated fatty acids; lower intake of sugar-sweetened beverages and fruit juice, red/processed meat, trans-fat, sodium; and moderate alcohol consumption;5 and 4) Dietary Approaches to Stop Hypertension (DASH) index, based on controlled-feeding studies21, 22 that administered diets rich in vegetables, fruits, and low-fat dairy products, inclusive of whole grains, poultry, fish, and nuts, low in saturated fat, red meat, sweets, and sugary beverages and reduced in sodium.23, 24 Further details on each score’s components and index/population-specific cut-points are shown in Supplemental Table 1. For all dietary patterns, higher scores reflect higher quality diets.
Covariates
At baseline, women reported demographics, health behaviors, and medical histories. We categorized as follows: race/ethnicity (non-Hispanic white, non-Hispanic black, Hispanic, other race/ethnicity, or missing [0.26%]); education (<GED, some college, college, postgraduate, or missing [0.74%]); and smoking (never, past, current, or missing [1.21%]). Women reported physical activity using the WHI brief physical activity inventory, which is reliable (weighted κ ranging from 0.67 to 0.71) and valid compared with accelerometers (r=0.73).25 We calculated metabolic equivalent (MET)-hours per week of recreational activity categorized into quintiles (0-<2, 2-<7, 7–13, >13–23, >23 MET-hours/week, or missing [0.97%]). Women reported use of hormone therapies (HT, unopposed estrogen and/or estrogen plus progesterone) via pills/patches, which we classified as never, past, current use or missing (1.84%).
Statistical analysis
We treated baseline dietary pattern scores categorically, in quartiles. To compare across scores, we examined 10% increments based on each score’s theoretical range: 3.2-points on DASH, 1-point in aMED, 11-points in AHEI-2010, and 10-points in HEI-2010. We calculated changes by subtracting values at enrollment from those at 3-year follow-up for WC in cm, weight in kg, and each dietary score. We treated 3-year changes in scores continuously, per 10% increment, and comparing women who increased or decreased by 10% in a given score to women who maintained diet quality within 10% of baseline scores. We categorized women’s diet quality as 1) consistently low, scoring below the baseline median for a dietary pattern at both baseline and 3-year follow-up; 2) consistently high, scoring above the baseline median at both visits; 3) decreasing, moving from above to below the median; or 4) increasing, moving from below to above the median baseline score.
We calculated Pearson correlations between scores, and means, standard deviations (SD), and frequencies of demographic/lifestyle characteristics by quartiles of baseline scores. Using multivariable linear regression (covariates selected a priori), we examined associations of our main outcome (change in WC) with, first, baseline diet scores, and second, 3-year changes in diet scores. Models adjusted sequentially for baseline WC and age in years, then education, race/ethnicity, smoking, diabetes family history, postmenopausal hormone therapy, daily energy intake, and physical activity, and, finally, for 3-year change in weight.
We hypothesized associations of dietary patterns with WC change would differ by age and race/ethnicity: younger WHI women gained the most weight,26 clinical studies suggest differences by age/ethnicity with respect to location of body fat depots,27 and there are differing patterns of consumption in the foods making up the dietary indices by race/ethnicity.28 We tested interaction of our main exposure (10% changes in dietary scores) with race/ethnicity using likelihood ratio tests and age (>65/<65 years) using Wald χ2 tests.
We repeated change analyses with change in DXA trunk fat mass as the outcome among the subset of women with DXA, adjusting for covariates listed above and baseline trunk fat.
All analyses were conducted using SAS, version 9.3 (SAS Institute, Inc., Cary, North Carolina). All tests were 2-sided with statistical significance set at P<0.05.
Results
At baseline, the mean (SD) age was 63 (7) years, WC was 83 (13) cm and BMI was 27 (5) kg/m2. The median (range) for each dietary pattern score was 51 (16–93) for aHEI-2010; 24 (8–38) for DASH; 68 (20–95) for HEI-2010; and 4 (0–9) for aMED (Table 1). From baseline to 3-year follow-up, changes in weight, WC and trunk fat were small at mean (SD) of 0.52 (4) kilograms, 0.94 (7) cm, and 308 (2,177) grams, respectively. Univariate correlations between the four diet quality indices were statistically significant (P <0.001), ranging from ρ=0.54 (HEI-2010 and aMED) to 0.72 (HEI-2010 and DASH). Correlations among changes in diet scores (range ρ=0.37–0.55, P <0.001) followed a similar pattern.
Table 1.
Sample Characteristics by Quartile of Baseline Diet Scores among Post-Menopausal Women in the WHI Observational Study (N=67,175)
| AHEI-2010 | DASH | HEI-2010 | aMED | |||||
|---|---|---|---|---|---|---|---|---|
| Median score (possible range) | 51 (0–110) | 24 (8–40) | 68 (0–100) | 4 (0–9) | ||||
| Quartile 1 | Quartile 4 | Quartile 1 | Quartile 4 | Quartile 1 | Quartile 4 | Quartile 1 | Quartile 4 | |
| Median score (observed range) | 38 (16 – 43) | 64 (59 – 93) | 18 (8 – 20) | 30 (28 – 38) | 54 (20 – 61) | 79 (75 – 95) | 2 (0 – 2) | 6 (6 – 9) |
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | |||||
| DASH | 20 (4) | 28 (3) | 18 (2) | 30 (2) | 20 (4) | 28 (3) | 19 (3) | 28 (3) |
| HEI-2010 | 59 (10) | 75 (7) | 56 (9) | 76 (7) | 53 (6) | 80 (4) | 58 (11) | 74 (8) |
| aHEI-2010 | 37 (5) | 65 (5) | 41 (8) | 61 (9) | 42 (9) | 59 (9) | 41 (8) | 60 (9) |
| aMED | 3 (1) | 6 (1) | 3 (1) | 6 (1) | 3 (2) | 5 (2) | 2 (1) | 7 (1) |
| Energy in Kcal | 1658 (643) | 1535 (602) | 1535 (666) | 1649 (595) | 1791 (864) | 1435 (470) | 1310 (552) | 1816 (689) |
| MET hours/week | 10 (12) | 19 (16) | 9 (12) | 19 (16) | 10 (12) | 18 (15) | 10 (13) | 18 (15) |
| Age, years | 63 (7) | 64 (7) | 63 (7) | 64 (7) | 63 (7) | 65 (7) | 63 (7) | 64 (7) |
| BMI, kg/m2 | 28 (6) | 26 (5) | 28 (6) | 26 (5) | 29 (6) | 26 (5) | 29 (6) | 26 (5) |
| Weight, kg | 74 (16) | 67 (13) | 74 (16) | 67 (13) | 75 (17) | 67 (13) | 73 (16) | 68 (14) |
| Waist circumference, cm | 88 (14) | 81 (12) | 89 (14) | 81(12) | 88 (14) | 81 (12) | 86 (14) | 82 (12) |
| DXA trunk fat, kg | 15 (6) | 12 (5) | 16 (7) | 12 (5) | 16 (6) | 12 (5) | 15 (6) | 13 (6) |
| % | % | % | % | |||||
| ≥College education | 10 | 13 | 10 | 14 | 10 | 14 | 10 | 13 |
| Race/ethnicity | ||||||||
| White | 83 | 89 | 78 | 91 | 81 | 89 | 83 | 88 |
| Black | 10 | 4 | 12 | 4 | 10 | 5 | 8 | 5 |
| Asian | 2 | 4 | 3 | 2 | 3 | 3 | 2 | 4 |
| Latino | 4 | 2 | 5 | 2 | 5 | 2 | 5 | 2 |
| Other | 1 | 1 | 2 | 1 | 2 | 1 | 2 | 1 |
| Current smoker | 8 | 3 | 10 | 2 | 10 | 3 | 9 | 3 |
| Family history of diabetes | 33 | 29 | 33 | 29 | 33 | 29 | 32 | 29 |
| Current hormone therapy | 45 | 53 | 45 | 52 | 44 | 51 | 45 | 52 |
WHI = Women’s Health Initiative; AHEI = Alternate Healthy Eating Index; DASH = Dietary Approaches to Stopping Hypertension; HEI = Healthy Eating Index; aMED = Alternate Mediterranean Diet; SD = Standard Deviation; MET = Metabolic Equivalent Task; BMI = Body Mass Index; DXA = Dual Energy X-Ray Absorptiometry
Comparing women with the lowest quality baseline diets (first quartile of dietary pattern scores), women with higher quality diets (fourth quartile) were more likely to be physically active, current hormone therapy users and non-Hispanic white, less likely to be smokers, and were slightly older, with lower BMI and WC (Table 1).
Diet quality and 3-year changes in WC
Across indices, higher baseline diet quality had a protective association with subsequent increases in WC: each 10% higher score was associated with a 0.10–0.20 cm smaller increase in WC over 3-years (all p<0.05, Table 2). Associations of 3-year changes in diet scores were smaller in magnitude: each 10% increase from baseline to 3-year follow-up was associated with a 0.07–0.43 cm smaller increase in WC before adjusting for weight change (all p<0.05, Table 3). Compared to women who maintained dietary scores within 10%, women who increased by >10% had between 0.12cm (aMED) and 0.58cm (aHEI-2010) smaller gains WC, while women whose scores decreased >10% experienced between 0.10 cm (aMED) and 0.71 cm (aHEI-2010) greater gains in WC. After adjusting for weight change, these associations were attenuated: only the association of decreases in diet quality and greater gains in WC was independent of weight change.
Table 2.
Baseline Diet Quality Scores and Change in Waist Circumference Over Three Years: Results from the Women’s Health Initiative Observational Study, n=67,175
| Mean Difference in Change in Waist Circumference, Centimeters (95% Confidence Interval) | |||||
|---|---|---|---|---|---|
| Baseline diet quality score | Median score (Min – Max) |
Age and baseline waist-adjusted1 | Lifestyle-adjusted2 | Weight change-adjusted2 | |
| AHEI-2010 | Quartile 1 | 38 (16 – 43) | 0.60 (0.45, 0.74) | 0.26 (0.11, 0.41) | 0.45 (0.32, 0.58) |
| 2 | 47 (43 – 51) | 0.42 (0.29, 0.56) | 0.23 (0.09, 0.38) | 0.29 (0.17, 0.42) | |
| 3 | 55 (51 – 59) | 0.18 (0.04, 0.32) | 0.09 (−0.05, 0.23) | 0.11 (−0.01, 0.24) | |
| 4 (higher quality) | 64 (59 – 93) | REF. | REF. | REF. | |
| Per 10% higher baseline score | −0.25 (−0.30, −0.20) | −0.13 (−0.18, −0.07) | −0.20 (−0.24, −0.15) | ||
| DASH | Quartile 1 | 18 (8 – 20) | 0.58 (0.43, 0.72) | 0.24 (0.09, 0.39) | 0.48 (0.34, 0.62) |
| 2 | 23 (21 – 24) | 0.34 (0.20, 0.48) | 0.19 (0.05, 0.33) | 0.26 (0.14, 0.38) | |
| 3 | 26 (25 – 27) | 0.15 (0.01, 0.30) | 0.08 (−0.06, 0.23) | 0.10 (−0.03, 0.23) | |
| 4 (higher quality) | 30 (28 – 38) | REF. | REF. | REF. | |
| Per 10% higher baseline score | −0.17 (−0.20, −0.14) | −0.08 (−0.12, −0.05) | −0.14 (−0.18, −0.11) | ||
| HEI-2010 | Quartile 1 | 54 (20 – 61) | 0.56 (0.42, 0.70) | 0.20 (0.05, 0.35) | 0.48 (0.34, 0.61) |
| 2 | 65 (61 – 68) | 0.33 (0.19, 0.46) | 0.18 (0.04, 0.32) | 0.27 (0.15, 0.40) | |
| 3 | 71 (68 – 75) | 0.14 (0.00, 0.27) | 0.08 (−0.06, 0.21) | 0.15 (0.02, 0.27) | |
| 4 (higher quality) | 79 (75 – 95) | REF. | REF. | REF. | |
| Per 10% higher baseline score | −0.21 (−0.26, −0.17) | −0.08 (−0.13, −0.03) | −0.18 (−0.22, −0.13) | ||
| aMED | Quartile 1 | 2 (0 – 2) | 0.49 (0.34, 0.64) | 0.31 (0.15, 0.47) | 0.48 (0.34, 0.62) |
| 2 | 4 (3 – 4) | 0.40 (0.27, 0.52) | 0.30 (0.16, 0.43) | 0.33 (0.21, 0.44) | |
| 3 | 5 (5 – 5) | 0.20 (0.05, 0.35) | 0.16 (0.01, 0.31) | 0.20 (0.07, 0.34) | |
| 4 (higher quality) | 6 (6 – 9) | REF. | REF. | REF. | |
| Per 10% higher baseline score | −0.11 (−0.13, −0.08) | −0.07 (−0.10, −0.04) | −0.10 (−0.12, −0.07) | ||
Models adjust for age and waist circumference at enrollment.
Models additionally adjust for education, race/ethnicity, smoking status, family history of diabetes, HRT status, total energy intake, and physical activity and 3-year change in weight.
AHEI = Alternate Healthy Eating Index; DASH = Dietary Approaches to Stopping Hypertension; HEI = Healthy Eating Index; aMED = Alternate Mediterranean Diet; REF = Reference Value
Table 3.
Change in Diet Quality Scores and Concurrent Change in Waist Circumference Over Three Years: Results from the Women’s Health Initiative Observational Study, n=67,175
| Mean Difference in Change in Waist Circumference, Centimeters (95% Confidence Interval) | |||||
|---|---|---|---|---|---|
| 3-year change in diet quality | n | Median change (Min, Max) |
Age and baseline waist-adjusted1 | Lifestyle-adjusted2 | Weight change-adjusted3 |
| AHEI-2010 | |||||
| 10% increase | 8,063 | 15 (11, 50) | −0.56 (−0.72, −0.41) | −0.58 (−0.73, −0.43) | −0.02 (−0.15, 0.12) |
| 10% decrease | 5,882 | −14 (−51, −11) | 0.75 (0.57, 0.92) | 0.71 (0.54, 0.89) | 0.31 (0.16, 0.47) |
| <10% change | 53,230 | 0 (−11, 11) | REF. | REF. | REF. |
| Per continuous 10% increase | 67,175 | 1 (−51, 50) | −0.43 (−0.49, −0.37) | −0.43 (−0.49, −0.37) | −0.10 (−0.15, −0.04) |
| DASH | |||||
| 10% increase | 10,870 | 5 (4, 22) | −0.35 (−0.49, −0.22) | −0.39 (−0.53, −0.26) | 0.01 (−0.11, 0.13) |
| 10% decrease | 10,261 | −5 (−19, −4) | 0.58 (0.44, 0.72) | 0.57 (0.43, 0.71) | 0.28 (0.16, 0.41) |
| <10% change | 46,044 | 0 (−3, 3) | REF. | REF. | REF. |
| Per continuous 10% increase | 67,175 | 0 (−19, 22) | −0.27 (−0.32, −0.23) | −0.28 (−0.32, −0.24) | −0.07 (−0.11, −0.03) |
| HEI-2010 | |||||
| 10% increase | 9,215 | 14 (10, 54) | −0.36 (−0.51, −0.22) | −0.46 (−0.61, −0.32) | 0.02 (−0.11, 0.15) |
| 10% decrease | 6,691 | −14 (−51, −10) | 0.81 (0.64, 0.97) | 0.77 (0.60, 0.93) | 0.37 (0.22, 0.52) |
| <10% change | 51,269 | 0 (−10, 10) | REF. | REF. | REF. |
| Per continuous 10% increase | 67,175 | 1 (−51, 54) | −0.37 (−0.43, −0.32) | −0.40 (−0.46, −0.35) | −0.11 (−0.16, −0.06) |
| aMED | |||||
| 10% increase | 24,656 | 1 (1, 7) | −0.13 (−0.26, 0.00) | −0.12 (−0.25, 0.01) | −0.06 (−0.17, 0.05) |
| 10% decrease | 26,205 | −1 (−8, −1) | 0.11 (−0.01, 0.24) | 0.10 (−0.03, 0.22) | 0.01 (−0.10, 0.12) |
| <10% change | 16,314 | 0 (0, 0) | REF. | REF. | REF. |
| Per continuous 10% increase | 67,175 | 0 (−8, 7) | −0.07 (−0.10, −0.05) | −0.07 (−0.10, −0.04) | −0.02 (−0.04, 0.01) |
Models adjust for age and waist circumference at enrollment.
Models additionally adjust for education, race/ethnicity, smoking status, family history of diabetes, HRT status, total energy intake, and physical activity.
Models additionally adjust for 3-year change in weight.
AHEI = Alternate Healthy Eating Index; DASH = Dietary Approaches to Stopping Hypertension; HEI = Healthy Eating Index; aMED = Alternate Mediterranean Diet; REF = Reference Value
Figure 1 shows associations of diet quality changes from above to below the median score on each dietary pattern with concurrent changes in WC, with and without adjustment for weight change. Consistently higher diet quality (scoring above the median score on any dietary pattern at both enrollment and 3-year follow-up) was associated with a 0.45–0.54 cm smaller gain in WC compared to consistently lower diet quality (all p<0.05), independent of weight change and regardless of the score examined. Before adjustment for weight change, improving diet quality (from below to above the median scores) was also associated with smaller increases in WC (0.26–0.35 cm) independent of weight change. Before adjustment for weight change, decreases in diet quality (from above to below the median) as measured by DASH and HEI-2010 were associated with greater increases in WC, while decreases in aMED and AHEI-2010 were not.
Figure 1.
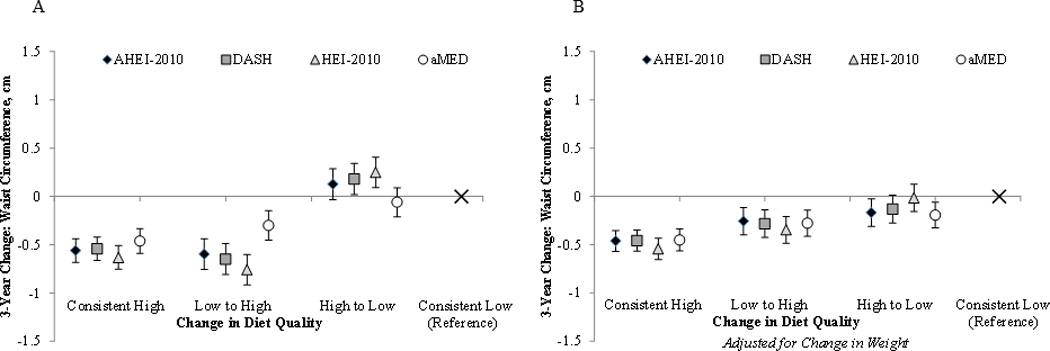
Three Year Changes in Diet Quality Scores and Changes in Waist Circumference: With and Without Adjustment for Changes in Weight1, 2
1 All models adjust for age at enrollment, baseline waist circumference, education, race/ethnicity, smoking status, family history of diabetes, HRT status, total energy intake, and physical activity; where indicated (Figure 1B) models adjust for 3-year change in weight.
2 “Consistent high” indicates always being in the top two quartiles for diet quality, whereas “consistent low” indicates always being in the bottom two. “Low to high” is moving from one of the lower quartiles to one of the higher quartiles, whereas “high to low” is moving from one of the upper quartiles to one of the lower quartiles.
The median baseline value for each dietary pattern scores were as follows: AHEI-2010 (51); DASH (24); HEI-2010 (68); and aMED (4). The “n; median change” for the “consistent high” category was n=25,040; 1 point for AHEI-2010; n=23,013; 0 points for DASH; n=24,493; 1 point for HEI-2010 n=19,444; 0 points for aMED. These values for the “low to high” category were n=8,548; 11 points for AHEI-2010; n=23,013; 0 points for DASH; n=9,095; 11 points for HEI-2010 n=9,382; 2 points for aMED. For the “high to low” category the values were n=8,548; −10 points for AHEI-2010; n=8,196; −4 points for DASH; n=9,095; −9 points for HEI-2010 n=10,158; −2 points for aMED, and for the “consistent low” category the n=25039; 1 point for AHEI-2010; n=27,682; 0 points for DASH; n=24,492; 1 points for HEI-2010 n=28,191; 0 points for aMED.
Predictors of change in DXA fat mass
Table 4 shows results for change in DXA trunk fat mass. Similar to WC, each 10% increase in diet scores from baseline to 3-year follow-up was associated with a 49–284 g smaller increase in trunk fat before adjusting for weight change, which attenuated after adjusting for weight change (Table 4). In categorical analyses, compared to maintaining dietary pattern scores within 10% of baseline values, women who increased by >10% had between 41g (aMED) and 607g (aHEI-2010) smaller gains trunk fat mass, while women who decreased >10% experienced between 145g (aMED) and 443g (aHEI-2010) greater gains in trunk fat mass. After adjusting for weight change, these associations attenuated.
Table 4.
Change in Diet Quality Scores and Concurrent Change in DXA Trunk Fat Over Three Years: Results from the Women’s Health Initiative Observational Study, n=4,254
| Mean Difference in Change in DXA Trunk Fat, Grams (95% Confidence Interval) | |||||
|---|---|---|---|---|---|
| 3-year change in diet quality | n | Median change (Min, Max) |
Age and baseline waist-adjusted1 | Lifestyle-adjusted2 | Weight change-adjusted3 |
| AHEI-2010 | |||||
| 10% increase | 506 | 15 (11, 37) | −608 (−804, −412) | −607 (−803, −412) | −195 (−327, −62) |
| 10% decrease | 386 | −14 (−38, −11) | 234 (13, 455) | 228 (8, 449) | −14 (−163, 135) |
| <10% change | 3362 | 0 (−11, 11) | REF. | REF. | REF. |
| Per continuous 10% increase | 4254 | 1 (−38, 37) | −284 (−362, −207) | −284 (−362, −207) | −73 (−126, −21) |
| DASH | |||||
| 10% increase | 658 | 5 (4, 16) | −322 (−500, −144) | −336 (−514, −159) | −130 (−249, −10) |
| 10% decrease | 670 | −5 (−15, −4) | 211 (34, 388) | 228 (51, 404) | 77 (−42, 195) |
| <10% change | 2926 | 0 (−3, 3) | REF. | REF. | REF. |
| Per continuous 10% increase | 4254 | 0 (−15, 16) | −150 (−206, −95) | −160 (−215, −104) | −45 (−83, −7) |
| HEI-2010 | |||||
| 10% increase | 606 | 14 (10, 38) | −371 (−554, −188) | −341 (−525, −157) | −66 (−190, 58) |
| 10% decrease | 450 | −14 (−36, −10) | 457 (250, 664) | 443 (235, 651) | 93 (−48, 233) |
| <10% change | 3198 | 0 (−10, 10) | REF. | REF. | REF. |
| Per continuous 10% increase | 4254 | 1 (−36, 38) | −275 (−345, −205) | −266 (−337, −195) | −63 (−111, −15) |
| aMED | |||||
| 10% increase | 1551 | 1 (1, 7) | −15 (−181, 150) | −41 (−206, 124) | 3 (−108, 114) |
| 10% decrease | 1660 | −1 (−6, −1) | 117 (−46, 280) | 125 (−38, 288) | 43 (−66, 152) |
| <10% change | 1043 | 0 (0, 0) | REF. | REF. | REF. |
| Per continuous 10% increase | 4254 | 0 (−6, 7) | −39 (−76, −1) | −49 (−87, −12) | −7 (−32, 18) |
Models adjust for age and waist circumference at enrollment.
Models additionally adjust for education, race/ethnicity, smoking status, family history of diabetes, HRT status, total energy intake, and physical activity.
Models additionally adjust for 3-year change in weight.
DXA = Dual Energy X-Ray Absorptiometry ; AHEI = Alternate Healthy Eating Index; DASH = Dietary Approaches to Stopping Hypertension; HEI = Healthy Eating Index; aMED = Alternate Mediterranean Diet; REF = Reference Value
Age differences
The association of change in diet quality with change in WC varied significantly by age at baseline; thus, in Table 5 we separate younger <65 years (mean [SD] age: 58 [4] years, n=36,300) and older post-menopausal women >65 years (mean [SD] age: 70 [4] years, n=30,875). While results were in the same direction but stronger among younger women, whose baseline scores were slightly lower (e.g., median baseline HEI-2010 score of 67 versus 69) and whose changes in diet quality, weight and waist over the 3-year follow-up were slightly greater (e.g., median changes in HEI-2010 of 1.14 versus 0.54 points, in waist of 1.00 versus 0.50 cm, and in weight of 1.00 versus 0.10 kg), data not shown.
Table 5.
Change in Diet Quality Scores and Concurrent Change in Waist Circumference Over Three Years by Age at Baseline Results from the Women’s Health Initiative Observational Study
| Mean Difference in Change in Waist Circumference, Centimeters (95% Confidence Interval) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Median change (Min, Max) |
<65 years old at baseline n=36,300 |
n | Median change (Min, Max) |
>65 years old at baseline n=30,875 |
P for interaction 3 |
|||
| 3-year change in diet quality |
Lifestyle-adjusted1 | Weight change- adjusted2 |
Lifestyle-adjusted1 | Weight change- adjusted2 |
|||||
| AHEI-2010 | |||||||||
| 10% increase | 4,634 | 15 (11, 50) | −0.78 (−0.98, − 0.58) | −0.07 (−0.25, 0.10) | 3,429 | 14 (11, 50) | −0.30 (−0.53, −0.07) | 0.07 (−0.14, 0.28) | |
| 10% decrease | 3,043 | −14 (−39, −11) | 1.08 (0.84, 1.32) | 0.46 (0.24, 0.67) | 2,839 | −14 (−51, −11) | 0.32 (0.07, 0.57) | 0.16 (−0.07, 0.38) | |
| <10% change | 28,623 | 1 (−11, 11) | REF. | REF. | 24,607 | 0 (−11, 11) | REF. | REF. | |
| Per continuous 10% increase |
36,300 | 1 (−39, 50) | −0.58 (−0.66, −0.5) | −0.13 (−0.2, −0.06) | 30,875 | 0 (−51, 50) | −0.24 (−0.33, – 0.15) | −0.06 (−0.14, 0.02) | <.0001 |
| DASH | |||||||||
| 10% increase | 6,005 | 5 (4, 22) | −0.49 (−0.67, −0.3) | 0.04 (−0.12, 0.2) | 4,865 | 5 (4, 16) | −0.28 (−0.48, − 0.08) | −0.02 (−0.20, 0.16) | |
| 10% decrease | 5,594 | −5 (−18, −4) | 0.76 (0.57, 0.95) | 0.32 (0.16, 0.49) | 4,667 | −5 (−19, −4) | 0.35 (0.15, 0.56) | 0.23 (0.05, 0.42) | |
| <10% change | 24,701 | 0 (−3, 3) | REF. | REF. | 21,343 | 0 (−3, 3) | REF. | REF. | |
| Per continuous 10% increase |
36,300 | 0 (−18, 22) | −0.37 (−0.43, − 0.31) | −0.08 (−0.14, − 0.03) | 30,875 | 0 (−19, 16) | −0.17 (−0.23, −0.1) | −0.05 (−0.11, 0.01) | <.0001 |
| HEI-2010 | |||||||||
| 10% increase | 5,450 | 14 (10, 49) | −0.53 (−0.72, − 0.34) | 0.06 (−0.11, 0.22) | 3,765 | 14 (10, 54) | −0.36 (−0.58, -0.13) | −0.03 (−0.23, 0.17) | |
| 10% decrease | 3,548 | −14 (−48, −10) | 1.17 (0.94, 1.39) | 0.51 (0.31, 0.70) | 3,143 | −13 (−51, − 10) | 0.32 (0.08, 0.56) | 0.21 (−0.01, 0.42) | |
| <10% change | 27,302 | 1 (−10, 10) | REF. | REF. | 23,967 | 0 (−10, 10) | REF. | REF. | |
| Per continuous 10% increase |
36,300 | 1 (−48, 49) | −0.51 (−0.58, − 0.43) | −0.12 (−0.19, − 0.05) | 30,875 | 1 (−51, 54) | −0.26 (−0.35, −0.18) | −0.10 (−0.17, −0.02) | 0.0002 |
| aMED | |||||||||
| 10% increase | 13,486 | 1 (1, 7) | −0.18 (−0.36, − 0.01) | −0.04 (−0.19, 0.12) | 11,170 | 1 (1, 7) | −0.05 (−0.23, 0.14) | −0.09 (−0.26, 0.08) | |
| 10% decrease | 14,020 | −1 (−8, −1) | 0.11 (−0.06, 0.28) | 0.01 (−0.14, 0.16) | 12,185 | −1 (−8, −1) | 0.09 (−0.09, 0.28) | 0.01 (−0.15, 0.18) | |
| <10% change | 8,794 | 0 (0, 0) | REF. | REF. | 7,520 | 0 (0, 0) | REF. | REF. | |
| Per continuous 10% increase |
36,300 | 0 (−8, 7) | −0.10 (−0.13, − 0.06) | −0.01 (−0.05, 0.02) | 30,875 | 0 (−8, 7) | −0.04 (−0.08, 0.00) | −0.02 (−0.06, 0.02) | 0.03 |
Models adjust for continuous age at baseline within age categories, waist circumference at enrollment, education, race/ethnicity, smoking status, family history of diabetes, HRT status, total energy intake, and physical activity.
Models additionally adjust for 3-year change in weight
P for interaction calculated via Wald test of interaction term for the product term of continuous 10% change in dietary score * age (<65 years versus >65 years).
AHEI = Alternate Healthy Eating Index; DASH = Dietary Approaches to Stopping Hypertension; HEI = Healthy Eating Index; aMED = Alternate Mediterranean Diet; REF = Reference Value
Sensitivity analyses including women with large changes in weight (>20kg over three years) yielded virtually identical results.
Discussion
Our results suggest that achieving and adhering to a healthful dietary pattern is one strategy to attenuate increases in overall and abdominal adiposity. In this study of 67,175 post-menopausal women, higher diet quality and incremental improvements in diet quality were associated with smaller gains in WC and trunk fat even over a brief 3-year period, largely explained by smaller gains in weight. Results were consistent across racial/ethnic subgroups, but stronger among younger women.
Few studies are directly comparable, but our results are consistent with findings from the Nurses’ Health Study where improvements in aHEI-2010, DASH, or aMED scores were associated with less weight gain over 4 years, with stronger results among younger women.29 In our study, while incremental increases in diet quality were associated with smaller gains in WC, results were stronger before weight change adjustment and among younger women. One explanation is that after menopause and during aging, body fat depots shift from peripheral to abdominal stores, total abdominal fat increases, and the ratio of subcutaneous to visceral fat declines.27, 30 Much of the weight gained among post-menopausal women is likely at abdominal;31 thus, it is expected that much of the relationship of diet to waist change would be explained by weight change. Results were independent of weight change among younger but not among older women. In older women WC might not adequately capture the shifting ratio of subcutaneous to visceral fat (changes in WC and weight were smaller in older women). Another reason for weaker associations among older versus younger women could be higher baseline diet quality and smaller magnitude of change over 3 years.
While no prior study examined changes in diet quality and changes in central adiposity, the magnitude and direction of associations in our study were similar to those in prior studies using single measures of diet to predict later WC or WC change.32–34 With respect to DXA, small interventions find improvements in DASH scores are associated with decreases in fat mass independent of energy intake.35 In absolute terms, changes in WC in WHI-OS were small; yet, they may be clinically meaningful over longer follow-up given the strong, adverse associations of WC with risk of chronic disease. For example, in the Cardiovascular Health Study 3-year gains in WC>10 cm were associated with 70% higher diabetes risk.36 Though only 5% of WHI-OS women gained >10cm over 3 years, a majority (56%) gained, and the average gain was 5 cm. This suggests improvements in diet quality might mitigate waist gain and thereby diabetes risk in a majority of post-menopausal women. Consistent with overall and central adiposity partially mediating the relationship of diet quality to diabetes, in a prior study we found adjustment for BMI and/or WC attenuated but did not eliminate the inverse associations of healthful dietary patterns with diabetes.13
Multiple healthful dietary patterns were associated with smaller gains in central adiposity. Prior literature focused on Mediterranean-style diets, which consistently predict smaller WC and smaller WC change.37 For example, the Prevención con dieta mediterránea trial showed a Mediterranean-style diet supplemented with olive oil or nuts reduced WC compared to a low fat diet.38 Few studies measure adherence to national guidelines, but in the Multi-Ethnic Study of Atherosclerosis, higher HEI-2005 scores predicted lower WC 18-months later.39 Despite differences between these patterns, there is remarkable consistency in the characteristics of healthful diets. Noting this, the scientific report of the 2015 Dietary Guidelines for Americans Advisory Committee concluded that a “healthy dietary pattern is higher in vegetables, fruits, whole grains, low- or non-fat dairy, seafood, legumes, and nuts; moderate in alcohol (among adults); lower in red and processed meat; and low in sugar-sweetened foods and drinks and refined grains.”40 Additionally, as there are multiple ways to achieve high scores on each index, strategies to improve diet quality may be tailored to individual tastes, preferences, or cultures. A 10-point increase in the AHEI-2010 can be achieved by eliminating sugar-sweetened beverages or reducing intake of red/processed meat to <2.5 ounces/day. This level of improvement is both realistic and beneficial: in our study, women who improved diet quality by moving from above to below the median AHEI-2010 score increased their scores on average by 10 points. This degree of improvement was associated with smaller increases in WC.
Of note, we found that weaker associations of aMED with WC change than with other dietary patterns. This runs counter to evidence linking adherence to a Mediterranean-style diet to lower WC. One possibility is that the benefits of plant-source mono-unsaturated fat intake (e.g., olive oil, a signature component of Mediterranean diets) may be confounded by meat intake in this U.S. population. Thus, aMED represents a diet that includes both healthy and unhealthy habits. Alternatively, the narrower score range (0–9 versus 0–110 for AHEI-2010) may not distinguish diet quality as precisely or detect small improvements.
Strengths and Limitations
By examining changes in dietary indices and changes in WC and trunk fat independent of changes in weight, the current study provides evidence that central adiposity is an important mechanism through which dietary patterns reduce chronic disease risk. Most studies examine single measures of diet and adiposity, do not have DXA data, or examine Mediterranean-style diets only. Further, the size and ethnic diversity of WHI enhance precision and generalizability.
Among limitations, neither WC nor the available DXA data distinguished visceral fat. Future visceral fat assessment will be possible if new software is applied to existing DXA scans. An additional limitation is that random error in the assessment of diet or WC may be magnified when examining changes, potentially attenuating associations._ENREF_56 In this observational study, residual confounding is possible. However, whether women with extreme changes in weight were included or excluded results were identical, suggesting that unmeasured changes in health status or behavior related to both diet and body size/shape are not a major concern. Further, examining changes in diet quality and WC may mitigate confounding by personal characteristics that are constant over a 3-year period, e.g., health consciousness. Finally, the magnitude of change in WC and diet quality over a 3-year period is small, resulting in a narrow range of exposure and modest associations. Despite the small magnitude of change and the influence of measurement error, we still detected associations of changes in dietary indices with changes in WC over the short follow-up time examined.
Conclusion
Our findings suggest that achieving and maintaining high diet quality contributes to mitigating abdominal fat gain in post-menopausal women.
Supplementary Material
Study importance.
Even as overall obesity prevalence plateaus in the United States, abdominal obesity prevalence continues to increase.
Healthful dietary patterns are associated with reduced risk of chronic disease, possibly through reductions in overall and central adiposity.
Most prior research assesses diet at a single time-point; whether achieving a healthful diet can protect against the increases in central adiposity that accompany aging is unknown.
Among a large, ethnically diverse group of postmenopausal women, our study finds that improved adherence to healthful dietary patterns predicts smaller gains in waist circumference; among younger women, this is independent of changes in weight.
Acknowledgments
FUNDING: The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts HHSN268201100046C, HHSN268201100001C, HHSN268201100002C, HHSN268201100003C, HHSN268201100004C, and HHSN271201100004C.
Support for Dr. Waring provided by NIH grant KL2TR000160.
Footnotes
DISCLOSURE: The authors have no conflicts of interest to disclose.
Dr. K. Asao became an employee of Eli Lilly Japan, K.K. as of March 1, 2016. The work presented in this manuscript was conducted while Dr. K. Asao was employed at the University of Tennessee Health Science Center. Eli Lilly Japan, K.K. has no role in this work.
References
- 1.Ford ES, Maynard LM, Li C. Trends in mean waist circumference and abdominal obesity among US adults, 1999–2012. Jama. 2014;312:1151–1153. doi: 10.1001/jama.2014.8362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang C, Rexrode KM, van Dam RM, Li TY, Hu FB. Abdominal obesity and the risk of all-cause, cardiovascular, and cancer mortality: sixteen years of follow-up in US women. Circulation. 2008;117:1658–1667. doi: 10.1161/CIRCULATIONAHA.107.739714. [DOI] [PubMed] [Google Scholar]
- 3.Bigaard J, Tjonneland A, Thomsen BL, Overvad K, Heitmann BL, Sorensen TI. Waist circumference, BMI, smoking, and mortality in middle-aged men and women. Obesity research. 2003;11:895–903. doi: 10.1038/oby.2003.123. [DOI] [PubMed] [Google Scholar]
- 4.Newby PK, Muller D, Hallfrisch J, Qiao N, Andres R, Tucker KL. Dietary patterns and changes in body mass index and waist circumference in adults. The American journal of clinical nutrition. 2003;77:1417–1425. doi: 10.1093/ajcn/77.6.1417. [DOI] [PubMed] [Google Scholar]
- 5.Chiuve SE, Fung TT, Rimm EB, et al. Alternative dietary indices both strongly predict risk of chronic disease. The Journal of nutrition. 2012;142:1009–1018. doi: 10.3945/jn.111.157222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hu FB. Dietary pattern analysis: a new direction in nutritional epidemiology. Current opinion in lipidology. 2002;13:3–9. doi: 10.1097/00041433-200202000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Romaguera D, Angquist L, Du H, et al. Dietary determinants of changes in waist circumference adjusted for body mass index - a proxy measure of visceral adiposity. PloS one. 2010;5:e11588. doi: 10.1371/journal.pone.0011588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feldeisen SE, Tucker KL. Nutritional strategies in the prevention and treatment of metabolic syndrome. Applied physiology, nutrition, and metabolism = Physiologie appliquee, nutrition et metabolisme. 2007;32:46–60. doi: 10.1139/h06-101. [DOI] [PubMed] [Google Scholar]
- 9.Women's Health Initiative Study Group. Design of the Women's Health Initiative clinical trial and observational study. The Women's Health Initiative Study Group. Controlled clinical trials. 1998;19:61–109. doi: 10.1016/s0197-2456(97)00078-0. [DOI] [PubMed] [Google Scholar]
- 10.Patterson RE, Kristal AR, Tinker LF, Carter RA, Bolton MP, Agurs-Collins T. Measurement characteristics of the Women's Health Initiative food frequency questionnaire. Annals of epidemiology. 1999;9:178–187. doi: 10.1016/s1047-2797(98)00055-6. [DOI] [PubMed] [Google Scholar]
- 11.Schakel SF, Sievert YA, Buzzard IM. Sources of data for developing and maintaining a nutrient database. Journal of the American Dietetic Association. 1988;88:1268–1271. [PubMed] [Google Scholar]
- 12. [Accessed November 5 2014];MyPyramid Equivalents Database, 2.0 for USDA Survey Foods, 2003–2004. 2008 [Online] http://www.ars.usda.gov/SP2UserFiles/Place/80400530/pdf/mped/mped2_doc.pdf.
- 13.Cespedes E, Hu F, Tinker L, et al. Multiple Healthful Dietary Patterns and Type 2 Diabetes in the Women’s Health Initiative. American journal of epidemiology. 2015 doi: 10.1093/aje/kwv241. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qiao Y, Tinker L, Olendzki BC, et al. Racial/ethnic disparities in association between dietary quality and incident diabetes in postmenopausal women in the United States: the Women's Health Initiative 1993–2005. Ethnicity & health. 2014;19:328–347. doi: 10.1080/13557858.2013.797322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.George SM, Ballard-Barbash R, Manson JE, et al. Comparing indices of diet quality with chronic disease mortality risk in postmenopausal women in the Women's Health Initiative Observational Study: evidence to inform national dietary guidance. American journal of epidemiology. 2014;180:616–625. doi: 10.1093/aje/kwu173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trichopoulou A, Costacou T, Bamia C, Trichopoulos D. Adherence to a Mediterranean diet and survival in a Greek population. The New England journal of medicine. 2003;348:2599–2608. doi: 10.1056/NEJMoa025039. [DOI] [PubMed] [Google Scholar]
- 17.Fung TT, McCullough ML, Newby PK, et al. Diet-quality scores and plasma concentrations of markers of inflammation and endothelial dysfunction. The American journal of clinical nutrition. 2005;82:163–173. doi: 10.1093/ajcn.82.1.163. [DOI] [PubMed] [Google Scholar]
- 18.Guenther PM, Reedy J, Krebs-Smith SM. Development of the Healthy Eating Index-2005. Journal of the American Dietetic Association. 2008;108:1896–1901. doi: 10.1016/j.jada.2008.08.016. [DOI] [PubMed] [Google Scholar]
- 19.Guenther PM, Casavale KO, Reedy J, et al. Update of the Healthy Eating Index: HEI-2010. Journal of the Academy of Nutrition and Dietetics. 2013;113:569–580. doi: 10.1016/j.jand.2012.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. [Accessed November 3 2014];Dietary guidelines for Americans. 2010 www.cnpp.usda.gov/dietaryguidelines.htm.
- 21.Appel LJ, Moore TJ, Obarzanek E, et al. A clinical trial of the effects of dietary patterns on blood pressure DASH Collaborative Research Group. The New England journal of medicine. 1997;336:1117–1124. doi: 10.1056/NEJM199704173361601. [DOI] [PubMed] [Google Scholar]
- 22.Sacks FM, Obarzanek E, Windhauser MM, et al. Rationale and design of the Dietary Approaches to Stop Hypertension trial (DASH). A multicenter controlled-feeding study of dietary patterns to lower blood pressure. Annals of epidemiology. 1995;5:108–118. doi: 10.1016/1047-2797(94)00055-x. [DOI] [PubMed] [Google Scholar]
- 23.Fung TT, Chiuve SE, McCullough ML, Rexrode KM, Logroscino G, Hu FB. Adherence to a DASH-style diet and risk of coronary heart disease and stroke in women. Archives of internal medicine. 2008;168:713–720. doi: 10.1001/archinte.168.7.713. [DOI] [PubMed] [Google Scholar]
- 24. [Accessed November 3 2014];Lowering Your Blood Pressure With DASH. 2009 http://www.nhlbi.nih.gov/files/docs/public/heart/dash_atglance.pdf.
- 25.Langer RD, White E, Lewis CE, Kotchen JM, Hendrix SL, Trevisan M. The Women's Health Initiative Observational Study: baseline characteristics of participants and reliability of baseline measures. Annals of epidemiology. 2003;13:S107–S121. doi: 10.1016/s1047-2797(03)00047-4. [DOI] [PubMed] [Google Scholar]
- 26.Caan B, Neuhouser M, Aragaki A, et al. Calcium plus vitamin D supplementation and the risk of postmenopausal weight gain. Archives of internal medicine. 2007;167:893–902. doi: 10.1001/archinte.167.9.893. [DOI] [PubMed] [Google Scholar]
- 27.Enzi G, Gasparo M, Biondetti PR, Fiore D, Semisa M, Zurlo F. Subcutaneous and visceral fat distribution according to sex, age, and overweight, evaluated by computed tomography. The American journal of clinical nutrition. 1986;44:739–746. doi: 10.1093/ajcn/44.6.739. [DOI] [PubMed] [Google Scholar]
- 28.Gao SK, Fitzpatrick AL, Psaty B, et al. Suboptimal nutritional intake for hypertension control in 4 ethnic groups. Archives of internal medicine. 2009;169:702–707. doi: 10.1001/archinternmed.2009.17. [DOI] [PubMed] [Google Scholar]
- 29.Fung TT, Pan A, Hou T, et al. Long-Term Change in Diet Quality Is Associated with Body Weight Change in Men and Women. The Journal of nutrition. 2015;145:1850–1856. doi: 10.3945/jn.114.208785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ley CJ, Lees B, Stevenson JC. Sex- and menopause-associated changes in body-fat distribution. The American journal of clinical nutrition. 1992;55:950–954. doi: 10.1093/ajcn/55.5.950. [DOI] [PubMed] [Google Scholar]
- 31.Toth M, Tchernof A, Sites C, Poehlman E. Effect of menopausal status on body composition and abdominal fat distribution. International journal of obesity. 2000;24:226–231. doi: 10.1038/sj.ijo.0801118. [DOI] [PubMed] [Google Scholar]
- 32.Funtikova AN, Benitez-Arciniega AA, Gomez SF, Fito M, Elosua R, Schroder H. Mediterranean diet impact on changes in abdominal fat and 10-year incidence of abdominal obesity in a Spanish population. The British journal of nutrition. 2014;111:1481–1487. doi: 10.1017/S0007114513003966. [DOI] [PubMed] [Google Scholar]
- 33.Rumawas ME, Meigs JB, Dwyer JT, McKeown NM, Jacques PF. Mediterranean-style dietary pattern, reduced risk of metabolic syndrome traits, and incidence in the Framingham Offspring Cohort. The American journal of clinical nutrition. 2009;90:1608–1614. doi: 10.3945/ajcn.2009.27908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tande DL, Magel R, Strand BN. Healthy Eating Index and abdominal obesity. Public health nutrition. 2010;13:208–214. doi: 10.1017/S1368980009990723. [DOI] [PubMed] [Google Scholar]
- 35.Nazare J-A, Smith J, Borel A-L, et al. Changes in both global diet quality and physical activity level synergistically reduce visceral adiposity in men with features of metabolic syndrome. The Journal of nutrition. 2013;143:1074–1083. doi: 10.3945/jn.113.175273. [DOI] [PubMed] [Google Scholar]
- 36.Biggs ML, Mukamal KJ, Luchsinger JA, et al. Association between adiposity in midlife and older age and risk of diabetes in older adults. Jama. 2010;303:2504–2512. doi: 10.1001/jama.2010.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kastorini CM, Milionis HJ, Esposito K, Giugliano D, Goudevenos JA, Panagiotakos DB. The effect of Mediterranean diet on metabolic syndrome and its components: a meta-analysis of 50 studies and 534,906 individuals. Journal of the American College of Cardiology. 2011;57:1299–1313. doi: 10.1016/j.jacc.2010.09.073. [DOI] [PubMed] [Google Scholar]
- 38.Estruch R, Ros E, Salas-Salvado J, et al. Primary prevention of cardiovascular disease with a Mediterranean diet. The New England journal of medicine. 2013;368:1279–1290. doi: 10.1056/NEJMoa1200303. [DOI] [PubMed] [Google Scholar]
- 39.Gao SK, Beresford SA, Frank LL, Schreiner PJ, Burke GL, Fitzpatrick AL. Modifications to the Healthy Eating Index and its ability to predict obesity: the Multi-Ethnic Study of Atherosclerosis. The American journal of clinical nutrition. 2008;88:64–69. doi: 10.1093/ajcn/88.1.64. [DOI] [PubMed] [Google Scholar]
- 40.Scientific Report of the Dietary Guidelines Advisory Committee: Advisory to the Secretary of Health and Human Services and the Secretary of. 2015 Agriculture http://www.health.gov/dietaryguidelines/2015-scientific-report/PDFs/Scientific-Report-of-the-2015-Dietary-Guidelines-Advisory-Committee.pdf.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


