Abstract
This review discusses two distinct, yet related, mechanisms of sodium-glucose cotransporter 2 (SGLT2) inhibition: Calorie restriction mimicry (CRM) and pro-ketogenic effect, which may explain their cardiovascular benefits. We term these adaptive CRM and pro-ketogenic effects of SGLT2 inhibition, the Robin Hood hypothesis. In English history, Robin Hood was a “good person,” who stole from the rich and helped the poor. He supported redistribution of resources as he deemed fit for the common good. In a similar fashion, SGLT2 inhibition provides respite to the overloaded glucose metabolism while utilizing lipid stores for energy production.
Keywords: Calorie restriction mimicry canagliflozin, cardiovascular outcomes, dapagliflozin, diabetes, EMPA-REG outcome, empagliflozin, ketogenesis, ketogenic diet, liraglutide
INTRODUCTION
Recent publication of the EMPA-REG outcome trial has highlighted the beneficial effects of empagliflozin and perhaps other sodium-glucose cotransporter 2 (SGLT2) inhibitors not only on cardiovascular outcomes but also on all-cause mortality as well.[1] Although much discussion has focused on the possible pharmaco-physiologic factors which mediate this benefit, ambiguity still persists.[2,3,4,5] Importantly, it has been difficult to correlate the magnitude of the observed effect, to any of the well-known cardiometabolic mechanisms.[6,7]
The benefits reported with empagliflozin are in stark contrast to reports of adverse events, such as euglycemic ketosis with the same class.[8,9] The SGLT2 inhibitors have a ketogenic effect, which has been reported in both animal and human studies.[10,11,12,13,14,15,16] As long as the body has adequate insulin to meet basic demands of insulin-dependent tissues and avoid ketoacidosis, this remains an orderly, regulated process and works as an adaptive mechanism.
SGLT2 inhibitors act by enhancing glucose (and calorie) excretion through urine, thus acting as “calorie restriction mimetics (CRMs).”[17] In this regard, they are similar to interventions such as calorie restriction per se, metformin, and glucagon-like peptide-1 receptor agonists (GLP1RA), all of which are known to improve cardiovascular outcomes and/or longevity.[18,19,20,21] Here, too, as long as calorie restriction does not precipitate malnutrition, it works as an adaptive mechanism which promotes good health. While GLP1RA do act as CRMs, however, they tend to suppress ketogenesis.
This review discusses these distinct, yet related, mechanisms of SGLT2 inhibition: CRM and pro-ketogenic effect, which may explain their cardiovascular benefits. We term these adaptive CRM and pro-ketogenic effects of SGLT2 inhibition, the Robin Hood hypothesis. In English history, Robin Hood was a “good person,” who stole from the rich and helped the poor. He supported redistribution of resources as he deemed fit for the common good.
CALORIE RESTRICTION MIMICRY
The CRM effect mediated via modulation of the adenosine monophosphate (AMP)-activated protein kinase pathway[21] may be a potential explanation for the cardiovascular benefit seen in the EMPA-REG outcome study. This pathway is a well-studied, scientifically robust explanation of health benefits noted with various nonpharmacologic and pharmacologic interventions[21] and should be studied further in the context of SGLT2 inhibition.
An increased availability of fatty acids has been observed to stimulate AMP-kinase activity and in turn, enhance fatty acid oxidation in skeletal muscles.[22] Following the initial step of calorie loss in the urine, SGLT2 inhibitors mediate a metabolic switch from glucose to lipid utilization as the predominant substrate.[23] This metabolic switch is the principle mechanism for weight loss mediated by SGLT2 inhibitors. Regarding the effect of SGLT2 inhibition on plasma adiponectin or leptin levels, current evidence is sparse. Nevertheless, like Robin Hood, SGLT2 inhibitors apparently hit the foundational pathophysiology of type 2 diabetes (T2D), by taxing the long-held unutilized wealth of lipids and sparing the already harassed glucose metabolism. The resultant improvement in peripheral insulin sensitivity leads to reduction in the hyperinsulinemic stress of type-2 diabetes, evident by a small reduction in the inherent insulin secretion, and in the requirement for external insulin.[23]
PRO-KETOGENIC EFFECT
A separate, but closely linked, explanation for the cardiovascular benefit noted with empagliflozin has been termed the “thrifty substrate” hypothesis.[24] This is based on the ketogenic potential of SGLT2 inhibitors which increase the production of ketone bodies: 3-hydroxybutyrate, acetoacetate, and acetone in the liver, by increasing glucagon levels and by reducing the insulin: glucagon ratio.[23,24,25,26] These ketone bodies offer an alternative, and more efficient, fuel for organ cells facing privation in the setting of CRM.
This hypothesis finds support from recently published evidence, which compares and contrasts calorie restriction with a ketogenic diet.[27] Gumbiner and colleagues observed that even when diabetic patients are administered low-calorie diets, the dietary composition may significantly influence ketogenesis. In the study, 2 groups of obese diabetic patients were administered low caloric diets for 3 weeks; their dietary compositions differing in the carbohydrate content (≈24 g/d and ≈94g/d respectively)." As expected, the lower carbohydrate diet resulted in significantly greater levels of circulating ketones, which was strongly associated with a lower hepatic glucose output. Interestingly, there was a strong inverse correlation between circulating ketones and hepatic glucose output, suggesting that higher levels of ketones are associated with more favorable effects on glycemic control in diabetics.[27]
SODIUM-GLUCOSE COTRANSPORTER 2 INHIBITORS: THE ROBIN HOOD OF DIABETES PHARMACOTHERAPY
SGLT2 inhibition provides the twin benefits of CRM and physiological, regulated ketogenesis. SGLT2 inhibitors enhance calorie loss from the body, thus offloading unnecessary calories from a maladaptive, overburdened body. This is similar to what Robin Hood did when he redistributed resources from the rich to the poor. The drugs promote mild ketogenesis in an orderly, self-regulated fashion and create an efficient, alternative fuel which can supply the requirements of critical organs such as brain and liver. A 100 g of acetoacetate is able to generate 9.4 kg adenosine triphosphate (ATP) and 100 g of 3-hydroxybutyrate yields 10.5 kg ATP while 100 g glucose produces only 8.7 kg ATP.[28] This allows the body to maintain efficient fuel production in the face of calorie loss.
SGLT2 inhibition encourages burning of fat (lipolysis) while sparing carbohydrates from having to be metabolized by cells. This is similar to effects noted with the ketogenic diet (a high fat and low carbohydrate diet) which encourages metabolism of fat (lipolysis) to fatty acid and fatty acid beta-oxidation to acetoacetate, 3β-hydroxybutyrate, and acetone. It must be mentioned here that human nutrition begins with a ketogenic diet: Colostrum is a ketogenic diet which serves the needs of the newborn neonate completely.[29]
THE CARDIOVASCULAR SYSTEM
The cardiac muscle is an omnivore, which uses diverse substrates as sources of fuel, preferring free fatty acids, followed by glucose, ketone bodies, lactate, pyruvate, glycogen, and amino acids.[30,31] The diabetic heart is marred by lipotoxicity due to a chronic accumulation of free fatty acids in the heart. While the metabolic switch caused by CRM ensures efficient lipid oxidation in the peripheral tissues, free fatty acids prove to be relatively inefficient substrates for the heart as they require more oxygen to release an equivalent amount of energy as compared to glucose or ketone bodies. Moreover, free fatty acids get converted to triglycerides and ceramide and generate reactive oxygen species which cause further myocardial damage and apoptosis.[30,31,32]
KETONE BODIES: THE ROBIN HOOD OF METABOLISM
Ketone bodies offer possible beneficial effects on the myocardial metabolism, for the failing diabetic heart.[30,31,32] Veech has designated ketone bodies as “superfuel,” for their superiority over fatty acids or glucose, in ensuring efficient cardiac metabolism.[32] In a nonclinical model of heart failure, ketone bodies increased the cardiac hydraulic efficiency by 28%. This effect was comparable to the well-known effect of insulin on the failing heart that facilitates myocardial uptake of glucose for preferential utilization as a substrate. Moreover, ketone bodies inhibit the uptake and oxidation of free fatty acids in the lipid-loaded diabetic myocardium, thus attenuating free radical-induced injury. The energy reserves of the heart improve as the acetyl-CoA content of the myocardium increases.[30,31,32] Apart from these mechanisms, empagliflozin increases the hematocrit by nearly 5% and reduces blood pressure without an associated increase in heart rate.[4] These effects improve the metabolic efficiency of the heart, providing a thrifty substrate and enhanced oxygen delivery to the myocardium, without an increase in the cardiac workload.
THE NERVOUS SYSTEM
SGLT 2 inhibition, with its mild, physiological ketosis, without acidosis, may have a protective effect on other cells of the body, such as neurons. This mimics the beneficial effects reported with ketogenic diet. Such a diet may make neurons more resilient to seizures by changing the method of ATP production, altering brain pH, inhibiting ion channel transport, or favoring synthesis of gamma-aminobutyric acid. It may also help brain cells receive more energy by offering an additional energy substrate directly while simultaneously upregulating ketone transporter and glucose transporter type 1 activity, as well as capillary density. Ketogenic diet is now considered an established part of an integrative approach, along with drug therapy, in major epilepsy centers worldwide.[33]
EXAPTATION OF SODIUM-GLUCOSE COTRANSPORTER 2 INHIBITION
Examples abound of the exaptation of endocrine pharmacologic discoveries to fit nonendocrine uses.[34] Similar uses may be proposed for SGLT2 inhibitors in future [Table 1]. Such diseases can be classified as disease of substrate insufficiency or insulin resistance, diseases resulting from free radical damage, and disease resulting from hypoxia. It is possible that SGLT2 inhibition may be studied in neurological diseases such as epilepsy, Alzheimer disease, and Parkinson's disease, where ketogenic diet has been shown to enhance mitochondrial function and improve clinical outcomes.[35] They may also help in certain cancers where delivery of ketones is facilitated while that of glucose is attenuated, forcing a shift in substrate use, and thus preventing growth of tumor cells. A similar mechanism may work in cardiac ischemia, where mitochondrial energetics are made more efficient with use of alternative fuel. Other potential uses for SGLT2 inhibitors include acne and polycystic ovary syndrome, where ketogenic diet is postulated to have beneficial effects, albeit with limited evidence.
Table 1.
Potential exaptation of sodium glucose transporter 2 inhibition

SUMMARY
This communication seeks to highlight two discrete yet overlapping mechanisms which may explain the beneficial cardiovascular effects seen with empagliflozin. We hope that the Robin Hood hypothesis will stimulate more research into these aspects of SGLT2 inhibitor biology.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We acknowledge the help of Arnav Kalra, All India Institute of Medical Sciences, Rishikesh, India, in crafting Figure 1.
Figure 1.
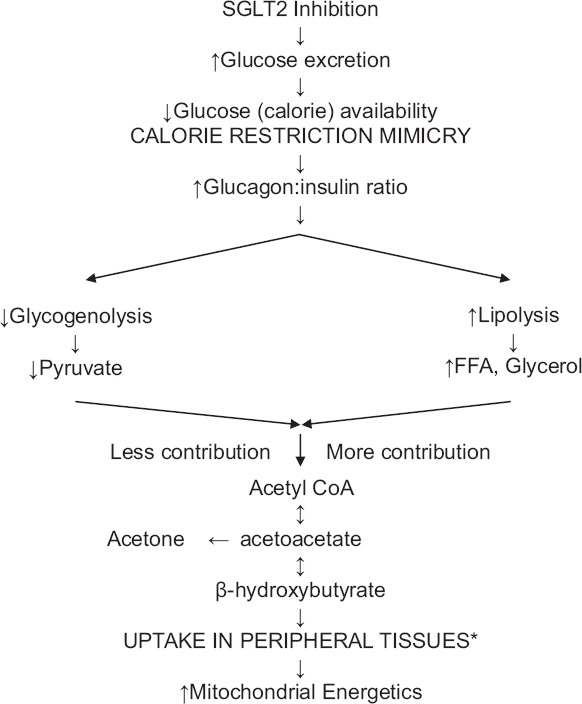
Sodium-glucose cotransporter 2 inhibition: Robin Hood effect (*heart, muscle, and renal cortex. Brain utilizes ketones only in prolonged starvation. Erythrocytes do not utilize ketones as they do not have mitochondria. Liver does not utilize ketones as it does not have the enzyme thiophorase)
REFERENCES
- 1.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med. 2015;373:2117–28. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 2.Abdul-Ghani M, Del Prato S, Chilton R, DeFronzo RA. SGLT2 inhibitors and cardiovascular risk: Lessons learned from the EMPA-REG OUTCOME study. Diabetes Care. 2016;39:717–25. doi: 10.2337/dc16-0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scheen AJ. Reduction in cardiovascular and all-cause mortality in the EMPA-REG OUTCOME trial: A critical analysis. Diabetes Metab. 2016;42:71–6. doi: 10.1016/j.diabet.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 4.Sattar N, McLaren J, Kristensen SL, Preiss D, McMurray JJ. SGLT2 Inhibition and cardiovascular events: Why did EMPA-REG Outcomes surprise and what were the likely mechanisms? Diabetologia. 2016 doi: 10.1007/s00125-016-3956-x. DOI: 10.1007/s00125-016-3956-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kalra S. One small step for empagliflozin, one giant leap for diabetology. Diabetes Ther. 2015;6:405–9. doi: 10.1007/s13300-015-0144-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.John M. Empagliflozin: Not just a glorified diuretic. Indian J Endocrinol Metab. 2016;20:154–6. doi: 10.4103/2230-8210.176361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marx N, McGuire DK. Sodium-glucose cotransporter-2 inhibition for the reduction of cardiovascular events in high-risk patients with diabetes mellitus. Eur Heart J. 2016 doi: 10.1093/eurheartj/ehw110. pii: Ehw110. [DOI] [PubMed] [Google Scholar]
- 8.Dutta D, Khandelwal D. Sodium glucose transporter 2 inhibition, euglycemic ketosis and bone mineral loss: Refining clinical practices. Indian J Endocrinol Metab. 2015;19:854–5. doi: 10.4103/2230-8210.167558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ogawa W, Sakaguchi K. Euglycemic diabetic ketoacidosis induced by SGLT2 inhibitors: Possible mechanism and contributing factors. J Diabetes Investig. 2016;7:135–8. doi: 10.1111/jdi.12401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Devenny JJ, Godonis HE, Harvey SJ, Rooney S, Cullen MJ, Pelleymounter MA. Weight loss induced by chronic dapagliflozin treatment is attenuated by compensatory hyperphagia in diet-induced obese (DIO) rats. Obesity (Silver Spring) 2012;20:1645–52. doi: 10.1038/oby.2012.59. [DOI] [PubMed] [Google Scholar]
- 11.Yokono M, Takasu T, Hayashizaki Y, Mitsuoka K, Kihara R, Muramatsu Y, et al. SGLT2 selective inhibitor ipragliflozin reduces body fat mass by increasing fatty acid oxidation in high-fat diet-induced obese rats. Eur J Pharmacol. 2014;727:66–74. doi: 10.1016/j.ejphar.2014.01.040. [DOI] [PubMed] [Google Scholar]
- 12.Suzuki M, Takeda M, Kito A, Fukazawa M, Yata T, Yamamoto M, et al. Tofogliflozin, a sodium/glucose cotransporter 2 inhibitor, attenuates body weight gain and fat accumulation in diabetic and obese animal models. Nutr Diabetes. 2014;4:e125. doi: 10.1038/nutd.2014.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferrannini E, Baldi S, Frascerra S, Astiarraga B, Heise T, Bizzotto R, et al. Shift to fatty substrate utilization in response to sodium-glucose cotransporter 2 inhibition in subjects without diabetes and patients with type 2 diabetes. Diabetes. 2016;65:1190–5. doi: 10.2337/db15-1356. [DOI] [PubMed] [Google Scholar]
- 14.Nishimura R, Tanaka Y, Koiwai K, Inoue K, Hach T, Salsali A, et al. Effect of empagliflozin monotherapy on postprandial glucose and 24-hour glucose variability in Japanese patients with type 2 diabetes mellitus: A randomized, double-blind, placebo-controlled, 4-week study. Cardiovasc Diabetol. 2015;14:11. doi: 10.1186/s12933-014-0169-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nishimura R, Osonoi T, Kanada S, Jinnouchi H, Sugio K, Omiya H, et al. Effects of luseogliflozin, a sodium-glucose co-transporter 2 inhibitor, on 24-h glucose variability assessed by continuous glucose monitoring in Japanese patients with type 2 diabetes mellitus: A randomized, double-blind, placebo-controlled, crossover study. Diabetes Obes Metab. 2015;17:800–4. doi: 10.1111/dom.12481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Inagaki N, Kondo K, Yoshinari T, Takahashi N, Susuta Y, Kuki H. Efficacy and safety of canagliflozin monotherapy in Japanese patients with type 2 diabetes inadequately controlled with diet and exercise: A 24-week, randomized, double-blind, placebo-controlled, phase III study. Expert Opin Pharmacother. 2014;15:1501–15. doi: 10.1517/14656566.2014.935764. [DOI] [PubMed] [Google Scholar]
- 17.Kalra S, Jacob JJ, Gupta Y. Newer antidiabetic drugs and calorie restriction mimicry. Indian J Endocrinol Metab. 2016;20:142–6. doi: 10.4103/2230-8210.172242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Colman RJ, Anderson RM, Johnson SC, Kastman EK, Kosmatka KJ, Beasley TM, et al. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 2009;325:201–4. doi: 10.1126/science.1173635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eurich DT, Majumdar SR, McAlister FA, Tsuyuki RT, Johnson JA. Improved clinical outcomes associated with metformin in patients with diabetes and heart failure. Diabetes Care. 2005;28:2345–51. doi: 10.2337/diacare.28.10.2345. [DOI] [PubMed] [Google Scholar]
- 20.Beiroa D, Imbernon M, Gallego R, Senra A, Herranz D, Villarroya F, et al. GLP-1 agonism stimulates brown adipose tissue thermogenesis and browning through hypothalamic AMPK. Diabetes. 2014;63:3346–58. doi: 10.2337/db14-0302. [DOI] [PubMed] [Google Scholar]
- 21.Coughlan KA, Valentine RJ, Ruderman NB, Saha AK. AMPK activation: A therapeutic target for type 2 diabetes? Diabetes Metab Syndr Obes. 2014;7:241–53. doi: 10.2147/DMSO.S43731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Watt MJ, Steinberg GR, Chen ZP, Kemp BE, Febbraio MA. Fatty acids stimulate AMP-activated protein kinase and enhance fatty acid oxidation in L6 myotubes. J Physiol. 2006;574(Pt 1):139–47. doi: 10.1113/jphysiol.2006.107318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferrannini E, Muscelli E, Frascerra S, Baldi S, Mari A, Heise T, et al. Metabolic response to sodium-glucose cotransporter 2 inhibition in type 2 diabetic patients. J Clin Invest. 2014;124:499–508. doi: 10.1172/JCI72227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferrannini E, Mark M, Mayoux E. CV protection in the EMPA-REG OUTCOME trial: A ‘thrifty substrate’ hypothesis. Diabetes Care. 2016 doi: 10.2337/dc16-0330. [In press] [DOI] [PubMed] [Google Scholar]
- 25.Kalra S, Sahay R, Gupta Y. Sodium glucose transporter 2 (SGLT2) inhibition and ketogenesis. Indian J Endocrinol Metab. 2015;19:524–8. doi: 10.4103/2230-8210.157859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kalra S, Gupta Y, Patil S. Sodium-glucose cotransporter-2 inhibition and the insulin: Glucagon ratio: Unexplored dimensions. Indian J Endocrinol Metab. 2015;19:426–9. doi: 10.4103/2230-8210.152793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gumbiner B, Wendel JA, McDermott MP. Effects of diet composition and ketosis on glycemia during very-low-energy-diet therapy in obese patients with non-insulin-dependent diabetes mellitus. Am J Clin Nutr. 1996;63:110–5. doi: 10.1093/ajcn/63.1.110. [DOI] [PubMed] [Google Scholar]
- 28.Manninen AH. Metabolic effects of the very-low-carbohydrate diets: Misunderstood “villains” of human metabolism. J Int Soc Sports Nutr. 2004;1:7–11. doi: 10.1186/1550-2783-1-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Rooy L, Hawdon J. Nutritional factors that affect the postnatal metabolic adaptation of full-term small- and large-for-gestational-age infants. Pediatrics. 2002;109:E42. doi: 10.1542/peds.109.3.e42. [DOI] [PubMed] [Google Scholar]
- 30.Kolwicz SC, Jr, Airhart S, Tian R. Ketones step to the plate: A game changer for metabolic remodeling in heart failure? Circulation. 2016;133:689–91. doi: 10.1161/CIRCULATIONAHA.116.021230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schugar RC, Moll AR, André d’Avignon D, Weinheimer CJ, Kovacs A, Crawford PA. Cardiomyocyte-specific deficiency of ketone body metabolism promotes accelerated pathological remodeling. Mol Metab. 2014;3:754–69. doi: 10.1016/j.molmet.2014.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Veech RL. The therapeutic implications of ketone bodies: The effects of ketone bodies in pathological conditions: Ketosis, ketogenic diet, redox states, insulin resistance, and mitochondrial metabolism. Prostaglandins Leukot Essent Fatty Acids. 2004;70:309–19. doi: 10.1016/j.plefa.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 33.Medina JM, Tabernero A. Lactate utilization by brain cells and its role in CNS development. J Neurosci Res. 2005;79:2–10. doi: 10.1002/jnr.20336. [DOI] [PubMed] [Google Scholar]
- 34.Kalra S, Bantwal G, John M. The exaptation of endocrine pharmacology. Indian J Endocrinol Metab. 2012;16:329–30. doi: 10.4103/2230-8210.95658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kossoff E. The fat is in the fire: Ketogenic diet for refractory status epilepticus. Epilepsy Curr. 2011;11:88–9. doi: 10.5698/1535-7511-11.3.88. [DOI] [PMC free article] [PubMed] [Google Scholar]


