Skeletal muscle from aged (17–25 yr) compared with young (2 yr) American Quarter Horses was characterized by a change in fiber-type composition and a decline in mitochondrial density and cytochrome-c oxidase activity, without impairment of 3-hydroxyacyl-CoA dehydrogenase (3-HADH) and mitochondrial respiration. Our findings suggest that aged American Quarter Horses were either at a transition point of aging before overt decline in mitochondrial function, or, alternatively, display skeletal muscle aging different from traditional animal models and humans.
Keywords: high-resolution respirometry, myosin heavy chain isoform, cytochrome-c oxidase, citrate synthase, 3-hydroxyacyl-CoA dehydrogenase
Abstract
Skeletal muscle function, aerobic capacity, and mitochondrial (Mt) function have been found to decline with age in humans and rodents. However, not much is known about age-related changes in Mt function in equine skeletal muscle. Here, we compared fiber-type composition and Mt function in gluteus medius and triceps brachii muscle between young (age 1.8 ± 0.1 yr, n = 24) and aged (age 17-25 yr, n = 10) American Quarter Horses. The percentage of myosin heavy chain (MHC) IIX was lower in aged compared with young muscles (gluteus, P = 0.092; triceps, P = 0.012), while the percentages of MHC I (gluteus; P < 0.001) and MHC IIA (triceps; P = 0.023) were increased. Mass-specific Mt density, indicated by citrate synthase activity, was unaffected by age in gluteus, but decreased in aged triceps (P = 0.023). Cytochrome-c oxidase (COX) activity per milligram tissue and per Mt unit decreased with age in gluteus (P < 0.001 for both) and triceps (P < 0.001 and P = 0.003, respectively). Activity of 3-hydroxyacyl-CoA dehydrogenase per milligram tissue was unaffected by age, but increased per Mt unit in aged gluteus and triceps (P = 0.023 and P < 0.001, respectively). Mt respiration of permeabilized muscle fibers per milligram tissue was unaffected by age in both muscles. Main effects of age appeared when respiration was normalized to Mt content, with increases in LEAK, oxidative phosphorylation capacity, and electron transport system capacity (P = 0.038, P = 0.045, and P = 0.007, respectively), independent of muscle. In conclusion, equine skeletal muscle aging was accompanied by a shift in fiber-type composition, decrease in Mt density and COX activity, but preserved Mt respiratory function.
NEW & NOTEWORTHY
Skeletal muscle from aged (17–25 yr) compared with young (2 yr) American Quarter Horses was characterized by a change in fiber-type composition and a decline in mitochondrial density and cytochrome-c oxidase activity, without impairment of 3-hydroxyacyl-CoA dehydrogenase (3-HADH) and mitochondrial respiration. Our findings suggest that aged American Quarter Horses were either at a transition point of aging before overt decline in mitochondrial function, or, alternatively, display skeletal muscle aging different from traditional animal models and humans.
aging is a process during which tissue functions progressively decline (4, 16). Age-associated impairment of structure and function of skeletal muscle, one of the body's most metabolically active tissues, has substantial consequences for the well-being of the aging individual. In humans, loss of muscle mass and function, namely sarcopenia, starts to become apparent in the fourth decade of life, progressively worsening in individuals over the age of 70 yr (10). These age-related changes lead to reduced mobility and, consequentially, to an impairment of independent living (4). While research on skeletal muscle aging in humans, primates, and rodent models has led to numerous publications, equine skeletal muscle aging is less explored. The horse is among the most athletic animals (89) and used as a companion animal in work and competitive and leisure activities. In recent decades, the number of horses over the age of 15 yr, which is considered aged, has steadily increased in the US (58), in part due to more specialized care and veterinary advances. Several recent surveys have pointed out that horses can still actively work and perform athletically well into their 20's (8, 9), an age physiologically analogous to a 65-yr-old human. The growing population of aged and geriatric horses in the US and their continued use for work and athletic activities raises the question of whether age-associated changes in skeletal muscle that have been observed in aged humans and laboratory animals also occur in the horse. Moreover, with the willingness of owners to use and work with this older horse population, understanding the physiology of equine aging is warranted. There are some studies that have documented skeletal muscle functional capacity in horses (reviewed by Ref. 54), and most of these studies used horses younger than 12 yr of age (5, 20, 55, 76, 77), therefore not addressing the problems and phenomena associated with aging into the geriatric stage of life. In the present study, we recruited sedentary horses between the ages of 17 and 25 yr to investigate the impact of physiological aging on skeletal muscle function compared with a group of young horses.
Skeletal muscle is highly malleable and able to adapt to altered functional demands and stimuli (21). A common adaptation is a change in muscle fiber metabolism through modifying fiber-type composition. Skeletal muscle myosin heavy chain (MHC), an essential component of the contractile apparatus, exists in different isoforms, which are associated with mode of energy production and muscle fiber function. In large animals, based on the MHC isoform present, skeletal muscle fibers are classified as type I, associated with predominantly oxidative energy metabolism; type IIX (or historically IIB), associated with predominantly glycolytic energy metabolism; or type IIA, which is an intermediate fiber type, exploiting predominantly oxidative energy production with functional features characteristic of glycolytic fibers. Importantly, hybrid fibers exist, which express more than one isoform, and are more prevalent in muscles undergoing transition, such as aging, or exercise adaptation (reviewed by Ref. 63). Furthermore, muscle groups very rarely express only one fiber type: they are composed of a combination of the three fiber types. Fiber-type distribution in skeletal muscle has been investigated in untrained horses to reflect alterations in muscle function with advancing age. However, the data are contradictory, with some studies reporting an increase in oxidative fibers (74), and others suggesting an age-related shift from oxidative to glycolytic fibers (43, 53). In addition, whether an age-related alteration in fiber-type composition is associated with changes in muscle oxidative capacity has not been well investigated in the horse (43, 74).
Skeletal muscle oxidative metabolism occurs in the mitochondria. Mitochondria are prominent in skeletal muscle and provide ATP for muscle function through the process of oxidative phosphorylation (OXPHOS), carried out by the complexes of the mitochondrial electron transport system (ETS). Increasing evidence shows that aging is associated with compromised capacity for OXPHOS in human muscle (15, 42), most likely due to a decline in mitochondrial density and/or function (83). Numerous mitochondrial components have been tested during the past two decades as biomarkers of muscle oxidative capacity in human (6, 59, 73), as well as in horses, such as citrate synthase (CS), 3-HADH (43, 70), cytochrome-c oxidase (COX; complex IV of the mitochondrial ETS) (100), and succinate dehydrogenase (82). Among them, activities of CS, COX, and 3-HADH have been used as biomarkers of mitochondrial content and oxidative capacity (13, 49, 72). Moreover, OXPHOS is a complicated process, which requires the coordinated interaction of multiple enzyme complexes. The mere assessment of enzymatic function of individual components of this system may be insufficient to reveal how well the system functions as a whole, and whether and where age-related defects occur (44, 80, 94). High-resolution respirometry (HRR) with saponin-permeabilized muscle fibers allows the study of OXPHOS in skeletal muscle mitochondria in situ. Oxygen consumption of mitochondria still situated within the muscle fiber can be sensitively monitored and evaluated by titrating energy substrates, ADP, complex inhibitors, and uncouplers to the permeabilized fibers. To date, only a few groups have applied this technology in horse studies to investigate the effect of training on muscle function (95, 96, 100). We are unaware of any studies using HRR with permeabilized muscle fibers to study the impact of aging on integrated mitochondrial respiratory function in horse skeletal muscle.
The objective of this study was to examine the age-associated changes in skeletal muscle fiber-type composition and mitochondrial function in the American Quarter Horse. We sampled the gluteus medius and triceps brachii, muscles of the extremities with distinct locomotor functions attributed to and associated with different muscle fiber-type composition (92). To address the question of whether alterations in fiber-type composition, determined electrophoretically, are associated with changes in muscle oxidative capacity, both the classical spectrophotometric method of enzyme activity assays and the more comprehensive HRR were used to assess mitochondrial OXPHOS capacity. Furthermore, the comparison of mitochondrial function between gluteus medius and triceps brachii muscle allowed us to evaluate muscle-specific responses to aging.
MATERIALS AND METHODS
Animals
All procedures performed in this study were approved by the University of Florida Institute of Food and Agricultural Sciences Animal Research Committee. A total of 34 American Quarter Horses, including 24 young (1.8 ± 0.1 yr old, 14 fillies and 10 geldings) and 10 aged subjects (17–25 yr old, 9 mares and 1 gelding) were utilized in this study. Horse body condition score (BCS) was assessed using the Henneke scoring system, with a scale from 1 to 9 (1 = emaciated and 9 = extremely obese) (30). Compared with the young horses, which had a BCS of 5.00, the aged horses had a significantly higher average BCS (5.80 ± 0.25; P < 0.001). All horses used in this study were owned by the University of Florida. None of the young horses had undergone any type of conditioning; and none of the aged horses had been athletes in the past, nor had been used in competitions, and had been exposed to only low-intensity exercise/training in the past, if any. None of the horses used in this study had received forced exercise for 6 mo before the study.
Skeletal Muscle Sampling
Skeletal muscle samples were taken from the right or left gluteus medius (n = 34: 24 young + 10 aged) and from the triceps brachii of a subset of young horses (n = 12) and of all aged horses (n = 10), under local anesthesia, using a 14-gauge SuperCore biopsy needle (Angiotech, Gainesville, FL). The gluteus medius was located on the croup by tracing approximately one-third down a line from the tuber coxae to the tailhead; and the long head of the triceps brachii was located at the intersection of a line traced between the scapulohumeral and radiohumeral joint and a vertical line extending down from the tricipital crest of the scapula. Briefly, the sample collection site (3 × 3 cm) was shaved, and the skin was cleaned with 7.5% povidone-iodine and rinsed with 70% ethanol. Horses were sedated with ∼0.3 ml of detomidine hydrochloride (Dormosedan, Pfizer Animal Health, Exton, PA) administered intravenously. The sampling site was then anesthetized by subcutaneous injection of 0.2–0.3 ml of 2% mepivacaine hydrochloride (Carbocaine-V, Pfizer Animal Health). The muscle sample (∼30–40 mg tissue) was taken at a standardized depth of 5 cm within the respective muscle through an initial skin puncture created with a 14-gauge needle and subsequent insertion of the biopsy needle. To collect a sufficient quantity of tissue sample, the biopsy needle was reinserted two to three times into the same initial puncture at different angles (same depth). Samples were immediately transferred into 1 ml ice-cold biopsy preservation solution [BIOPS; 2.77 mM CaK2EGTA, 7.23 mM K2EGTA, 5.77 mM Na2ATP, 6.56 mM MgCl2·6H2O, 20 mM taurine, 15 mM Na2phosphocreatine, 20 mM imidazole, 0.5 mM DTT, and 50 mM MES; pH 7.1 (80)] for subsequent respiration measurement on fresh tissue sample. The remaining tissue sample was snap-frozen in liquid nitrogen and stored in a dry shipper (MVE SC4/2V, CHART, Ball Ground, GA) for transport to the laboratory and later stored at −80°C.
Preparation of Permeabilized Muscle Fibers
The preparation of permeabilized muscle fibers was described previously (47). Briefly, muscle samples were immersed in ice-cold BIOPS solution during transportation to the laboratory. Before further preparation of fibers, fat and connective tissue were removed, and muscle fibers gently separated using two pairs of forceps. Myofiber bundles were transferred to 1 ml fresh BIOPS solution containing 50 μg/ml saponin, and the cell membrane was permeabilized for 30 min at 4°C on a rotator. After the permeabilization, the myofibers were rinsed in ice-cold mitochondrial respiration medium (MiR05; 110 mM sucrose, 60 mM potassium lactobionate, 0.5 mM EGTA, 3 mM MgCl2·6H2O, 20 mM taurine, 10 mM KH2PO4, 20 mM HEPES, and 1 g/l BSA; pH 7.1) on a rotator for 10 min at 4°C. Mitochondrial respiration of these permeabilized myofibers was immediately assessed using HRR.
High-Resolution Respirometry
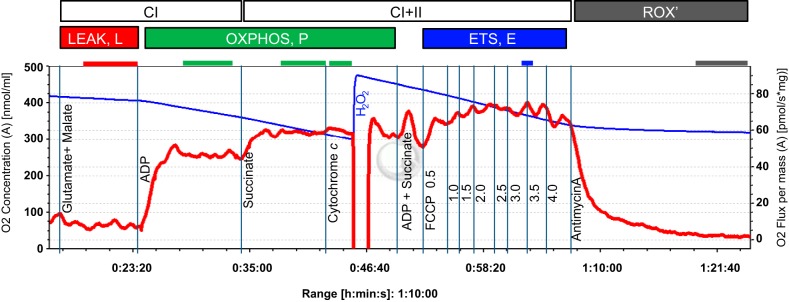
Mitochondrial respiration (O2 flux) was determined in duplicate using the high-resolution respirometer Oroboros O2k (OROBOROS Instruments, Innsbruck, Austria) at 37°C and in hyperoxic conditions (220–450 μM O2), according to methods previously described (47). Unless stated otherwise, the concentrations of the following reagents used are final concentrations in the respirometer chamber. Permeabilized fibers (2–3 mg wet weight) were added to the respirometer chamber containing 2 ml of MiR05. The respiration medium was supplemented with 20 mM creatine to saturate mitochondrial creatine kinase, which facilitates mitochondrial ADP transport (79, 98). Oxygen flux was determined with the following titration protocol (Fig. 1): electron flow through complex I (CI) of the ETS was supported by the NADH-linked substrates glutamate (10 mM) and malate (2 mM) (LEAK respiration, L), followed by addition of adenosine diphosphate (ADP; 2.5 mM) to stimulate respiration (OXPHOSCI, PCI); the addition of succinate (10 mM) supported convergent electron flow through CI and complex II (CII) of the ETS (PCI+II); additional ADP (1.25 mM) and succinate (5 mM) were then titrated to evaluate whether OXPHOS capacity could be increased any further. No further increase was observed, indicating that OXPHOS capacity was measured at a saturating ADP concentration. Subsequent addition of cytochrome c (cyt c; 10 μM) tested the integrity of the mitochondrial outer membrane. Only samples with response to cyt c below a 15% increase in respiration were included in the analysis. Titration of the uncoupler carbonyl cyanide 4-(trifluoromethoxy) phenylhydrazone (0.5-μl steps of a 0.1 mM stock solution, to a final concentration between 1.5 and 2.0 μM) measured maximum noncoupled respiration (ETS capacity; E). The coupling control ratio L/P was calculated as the ratio of LEAK to OXPHOS capacity (L/PCI+II), and the PCI+II-to-E coupling control ratio (P/E) was calculated as the ratio of OXPHOS capacity (PCI+II) to ETS capacity.
Fig. 1.
Respirometric protocol with permeabilized fibers from American Quarter Horse gluteus medius muscle. Shown is a typical trace of oxygen consumption after permeabilized fiber preparation with glutamate/malate and succinate substrate combinations to support electron flow through complex I (CI) and complex II (CII), respectively, of the mitochondrial electron transport system (ETS), and its activation by ADP. Cytochrome c was added as a quality control (see text for details), FCCP to induce uncoupling and evaluate ETS capacity, and antimycin A (inhibitor of complex III of the ETS) to evaluate residual oxygen consumption (ROX). The blue line represents the oxygen concentration (nmol/ml), the red line the muscle mass-specific O2 flux (pmol O2·s−1·mg wet wt−1; negative slope of the blue line normalized to tissue weight). Marked sections correspond to steady-state fluxes at different coupling states (L, P, and E; see text for explanations).
Sample Preparation and SDS-PAGE for MHC Analysis
Frozen muscle was powdered in liquid nitrogen using a BioPulverizer (BioSpec Products, Bartlesville, OK). Tissue powder was subsequently homogenized with 30 volumes of extraction buffer (50 mM Tris·HCl, pH 8.3, 10 mM EDTA) using a glass tissue grinder (Kontes Dual, size 20, Kimble Chase, Vineland, NJ). The muscle homogenate was transferred to a microcentrifuge tube and diluted with equal volume of protein denaturation buffer (4% SDS, 20% glycerol, and 125 mM Tris·HCl, pH 6.8). The sample was then heated at 50°C for 20 min and centrifuged at 14,000 g for 20 min (101). Protein content was determined using the bicinchoninic acid protein assay kit, according to the manufacturer's instructions (Thermo Scientific, Rockford, IL). Samples were stored in equal volume of glycerol at −20°C until further processing.
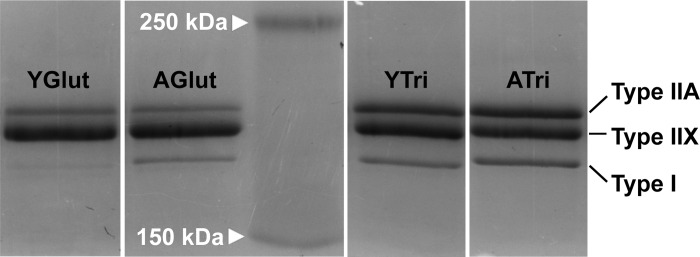
MHC isoforms in muscle homogenates were analyzed using a slightly modified protocol described previously (88). Briefly, the protocol was modified as follows: 1) samples were heated at 95°C for 3 min; 2) electrophoresis (SDS-PAGE) proceeded at 120 V for 18 h; 3) protein load per lane was 2.5 μg. Following electrophoretic protein separation, the gel was stained with Coomassie brilliant blue R250 (Bio-Rad Laboratories, Hercules, CA) for 2 h, and the proteins were visualized using a cooled charge-coupled device camera and acquisition software (G-BOX imaging system, Syngene, Frederick, MD). To verify the nature of the separated bands, a separate gel electrophoresis with a representative subset of muscle sample homogenates was performed. Instead of Coomassie staining, the proteins were transferred to a PVDF membrane using a semidry blotter at 20 V for 60 min (Trans-Blot Bio-Rad Laboratories, Hercules, CA). The nature of the protein bands was confirmed as MHC IIA, MHC IIX, and MHC I isoforms by immunoblotting using primary MHC antibodies SC-71 (MHC IIA, 1:150), 6H1 (MHC IIX, 1:100), and BA-D5 (MHC I, 1:100) (all from Developmental Studies Hybridoma Bank), and horseradish peroxidase-conjugated goat anti-mouse IgG secondary antibody (1:80,000, Sigma, St. Louis, MO). Bands were visualized using a horseradish peroxidase substrate (DuoLuX, Vector Laboratories, Burlingame, CA), and the chemiluminescence was captured with the G-Box imaging system. The intensity of the Coomassie blue stained protein bands (Fig. 2), as well as the chemiluminescence intensity was analyzed and quantified by densitometry using the manufacturer's analysis software (GeneTools, Syngene). For each sample, percentages of each MHC isoforms were calculated as a percentage of the total (100%) MHC isoforms in each lane.
Fig. 2.
Representative SDS polyacrylamide gel stained with Coomassie blue following electrophoretic separation. Bands shown are MHC isoforms type I, type IIA, and type IIX, in gluteus muscle from young (YGlut) and aged (AGlut), and triceps muscle from young (YTri) and aged (ATri) American Quarter Horses. The middle lane shows the molecular mass markers at 250 and 150 kDa.
Spectrophotometric Determination of Enzyme Activities
Enzymatic activities of CS, COX, and 3-HADH in muscle homogenates were measured as previously described (22, 85) using a microplate reader (Synergy HT, BioTek Instruments, Winooski, VT). Briefly, CS activity was assessed at 412 nm by measuring the initial rate of reaction of free CoA-SH with DTNB; COX activity was determined by measuring the maximal, linear rate of oxidation of fully reduced cyt c at 550 nm; and 3-HADH was assayed by measuring the oxidation of NADH at 340 nm using acetoacetyl-CoA as substrate.
Statistical Analyses
Data are reported as means ± SE, with the number of samples per group noted in the figure legends. Statistical analyses were performed using SigmaPlot version 12.0 (Systat Software, San Jose, CA). All data were compared between different age groups and across skeletal muscles using two-way ANOVA, followed by Student-Newman-Keuls multiple-comparison tests. For data that did not follow a normal distribution (COX activity/CS activity; mass-specific 3-HADH activity), a log-transformation was performed before statistical analysis, but the original data were presented in the graph. In all comparisons, differences with P ≤ 0.05 were considered statistically significant, and trends were reported if 0.05 < P < 0.1.
RESULTS
Aging and Muscle Fiber-Type Composition
The composition of MHC isoforms was significantly altered in muscles from aged compared with young horses, with increase of MHC I and IIA, and decrease of MHC IIX, independent of muscle type (Table 1; main effect of age: MHC I, P = 0.002; MHC IIA, P = 0.032; MHC IIX, P = 0.004). More specifically, in gluteus muscle, the percentage of MHC I was significantly higher in aged compared with young horses (P < 0.001). In addition, there was a trend for a decreased percentage of MHC IIX in aged gluteus muscle (P = 0.092), whereas the proportion of MHC IIA was unaltered (P = 0.520). In triceps muscle, no difference in percent MHC I was detected between the two age groups (P = 0.224). However, aged horses exhibited a higher percentage of MHC IIA (P = 0.023) and a lower percentage of MHC IIX (P = 0.012) relative to young animals. When gluteus and triceps muscles were compared, the MHC composition significantly differed between muscles, and this difference was maintained in both age groups (main effect of muscle type, P < 0.001 for all fiber types). More specifically, gluteus muscle had a lower percentage of MHC I and MHC IIA, and a higher percentage of MHC IIX compared with triceps.
Table 1.
Fiber-type composition of gluteus medius and triceps brachii from young and aged American Quarter Horses
| Gluteus Medius |
Triceps Brachii |
||||
|---|---|---|---|---|---|
| MHC Isoforms | Young | Aged | Young | Aged | Main Effect of Muscle Type, P Value |
| n | 24 | 9 | 11 | 9 | |
| MHC-I | 3.39 ± 0.35 | 6.68 ± 0.87‡ | 10.57 ± 0.99 | 11.85 ± 0.71 | <0.001 |
| MHC-IIA | 21.35 ± 0.85 | 23.18 ± 2.65 | 33.79 ± 1.22 | 41.42 ± 4.53* | <0.001 |
| MHC-IIX | 75.26 ± 0.92 | 70.14 ± 2.82† | 55.64 ± 1.59 | 46.73 ± 4.92* | <0.001 |
Values are means ± SE in %. †P < 0.1, ‡P < 0.001 (young vs. aged within gluteus),
P < 0.05 (young vs. aged within triceps).
Effect of Age on Mitochondrial Density and Enzyme Activity
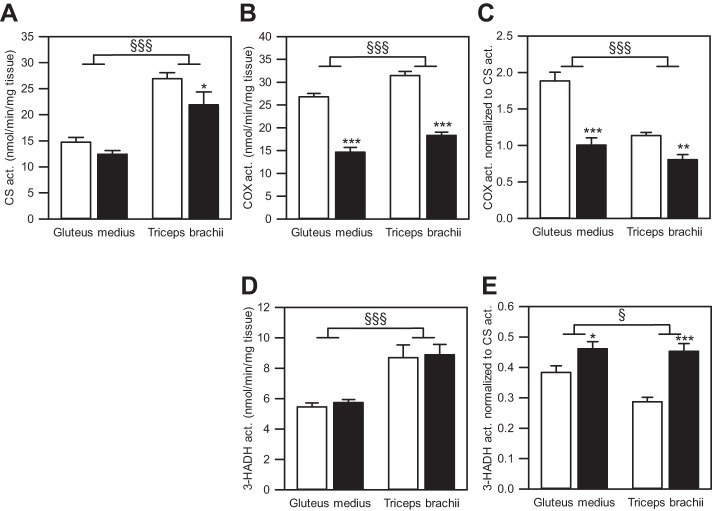
We found a main effect of age on CS activity per milligram tissue (P = 0.014; Fig. 3A). Specifically, CS activity did not differ between age groups in gluteus muscle (P = 0.230), but was lower in triceps muscle from aged horses (P = 0.023). When the two muscles were compared, CS activity in the triceps was approximately twofold greater compared with the gluteus (main effect of muscle type: P < 0.001), independent of age, which is consistent with the observed almost twofold difference in percentage of MHC I and IIA, traditionally associated with mitochondria-rich muscle fibers.
Fig. 3.
Enzyme activities in muscle tissue homogenates from gluteus medius and triceps brachii from young and aged American Quarter Horses. A: activity of citrate synthase (CS) per milligram tissue of gluteus medius and triceps brachii from young (n = 24 for gluteus, n = 11 for triceps) and aged (n = 9 for gluteus, n = 10 for triceps) horses. B–E: activity of cytochrome-c oxidase (COX; B and C) and 3-hydroxyacyl-CoA dehydrogenase (3-HADH; D and E) normalized to milligram tissue (B and D) and to CS activity (C and E) in gluteus medius and triceps brachii from young (n = 23–24 for gluteus, n = 11–12 for triceps) and aged (n = 9–10 for gluteus, n = 9–10 for triceps) horses. Values are means ± SE. Open bars represent young horses; solid bars, aged horses. Young vs. aged: *P < 0.05, **P < 0.01, ***P < 0.001. Gluteus vs. triceps: §P < 0.05, §§§P < 0.001.
There was a main effect of age on COX activity per milligram muscle tissue (P < 0.001; Fig. 3B). Specifically, COX activity per milligram tissue decreased significantly with age in both muscles (P < 0.001). Furthermore, COX activity per milligram tissue was significantly lower in gluteus compared with triceps muscle (main effect of muscle type: P < 0.001). When COX activity was normalized to CS activity to reflect the oxidative capacity on the mitochondrial level (Fig. 3C), there was a significant effect of age (main effect of age: P < 0.001). Specifically, COX activity per Mt unit was 47% lower in aged gluteus (P < 0.001), and 30% lower in aged triceps muscle (P = 0.003). When muscles were compared, COX activity per Mt unit was lower in triceps compared with gluteus muscle (main effect of muscle type: P < 0.001).
Activity of 3-HADH per milligram muscle tissue was not affected by age in either gluteus or triceps muscle (main effect of age: P = 0.125; Fig. 3D). Compared with the gluteus, the triceps muscle exhibited higher 3-HADH activity per milligram muscle tissue (main effect of muscle type: P < 0.001). When 3-HADH activity was evaluated per Mt unit by normalizing its activity to CS activity (Fig. 3E), 3-HADH activity was significantly elevated with age in both muscles (main effect of age: P < 0.001; gluteus: P = 0.023; triceps: P < 0.001). When muscles were compared, 3-HADH activity per mitochondrial unit was lower in triceps compared with gluteus muscle (main effect of muscle type: P = 0.044).
Effects of Age on Mitochondrial Respiration
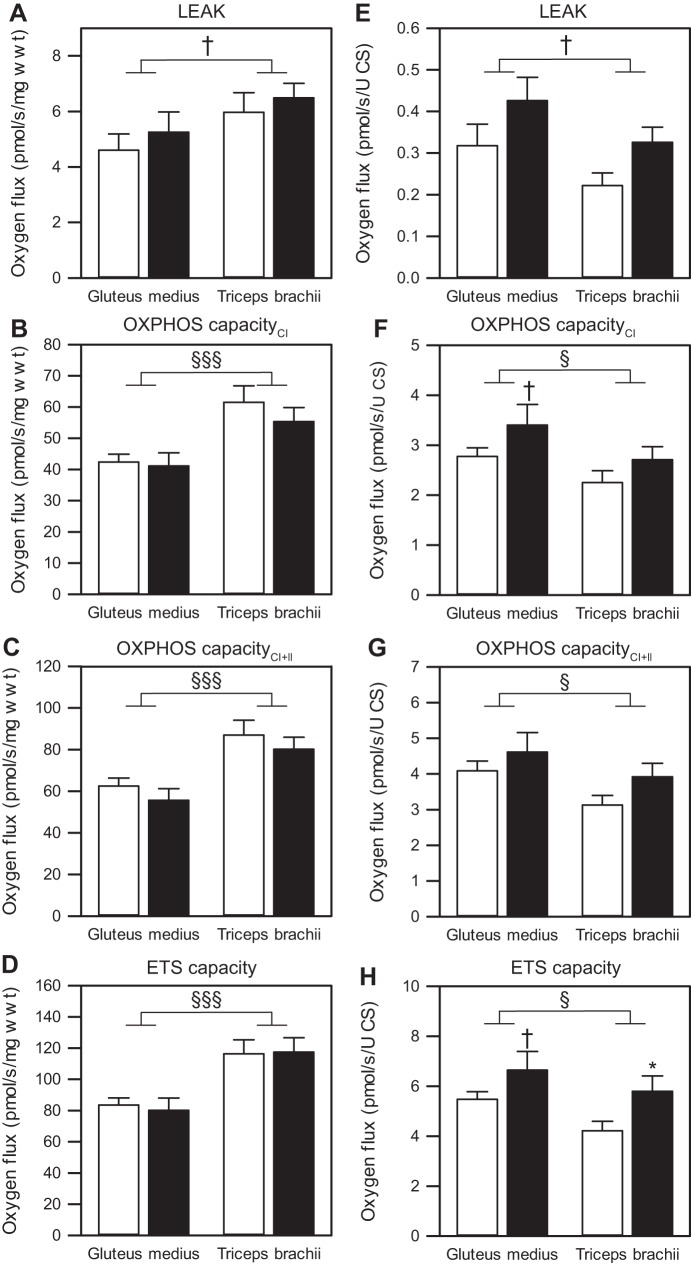
There were no apparent age-associated changes in mass-specific Mt respiration (O2 flux; pmol O2·s−1·mg wet wt−1) in either gluteus or triceps muscle for any of the assessed respiratory states (LEAK: gluteus, P = 0.487; triceps, P = 0.606; PCI: gluteus, P = 0.820; triceps, P = 0.318; PCI+II: gluteus, P = 0.386; triceps, P = 0.422; ETS capacity: gluteus, P = 0.738; triceps, P = 0.918; Fig. 4, A–D). When muscles were compared, significant differences were detected between gluteus and triceps muscle (Fig. 4, A–D). Specifically, the triceps exhibited higher OXPHOS capacity (PCI and PCI+II) and ETS capacity compared with the gluteus muscle (main effect of muscle type: P < 0.001 for all).
Fig. 4.
Mitochondrial respiration of permeabilized skeletal muscle fibers from gluteus medius and triceps brachii. Mass-specific O2 flux (pmol O2·s−1·mg wet wt−1; A–D) and O2 flux normalized to CS activity (pmol·s−1·unit CS−1; E–H), respectively, with LEAK respiration (A and E), OXPHOS capacityCI (B and F), OXPHOS capacityCI+II (C and G), and maximal ETS capacity (D and H) are shown. Values are means ± SE; n = 17–18 (young-gluteus), 12 (young-triceps), 9 (aged-gluteus), and 9 (aged-triceps). Open bars represent young horses; solid bars, aged horses. Young vs. aged: †P < 0.1, *P < 0.05. Gluteus vs. triceps: †P < 0.1, §P < 0.05, §§§P < 0.001.
When O2 flux was normalized to CS activity (Fig. 4, E–H), we found a significant effect of age for all but OXPHOS capacity supported by glutamate, malate, and succinate (PCI+II), independent of muscle type (main effect of age: LEAK, P = 0.038; PCI, P = 0.045; PCI+II, P = 0.073; ETS capacity, P = 0.007). In particular, PCI and ETS capacity tended to be higher in aged gluteus (P = 0.086 and P = 0.085, respectively; Fig. 4, F and H), and ETS capacity was significantly higher in aged triceps (P = 0.033; Fig. 4H) compared with the young counterparts. When the two muscles were compared, PCI, PCI+II, and ETS capacity were significantly higher in gluteus compared with triceps (main effect of muscle type: P = 0.025, P = 0.027, and P = 0.038, respectively; Fig. 4, E–H). These findings are consistent with COX activity per mitochondrial unit and suggest that the higher oxidative capacity of triceps muscle results from a higher mitochondrial density rather than from oxidative capacity of individual mitochondria.
Effect of Aging on Coupling Control Ratios
We used the O2 flux in different respiratory states to calculate coupling control ratios. The average L/P coupling control ratio (LEAK/PCI+II), an indicator for coupling of oxygen consumption and phosphorylation, was between 0.06 and 0.07 for young triceps and gluteus muscles, and between 0.08 and 0.1 for aged triceps and gluteus muscles, suggesting a tight coupling of mitochondria in permeabilized fibers (Fig. 5A). There were no main effects of aging or differences between muscle types on the L/P coupling control ratio (main effect of age: P = 0.110; main effect of muscle type: P = 0.153; effect of age in triceps: P = 0.671), but a tendency for elevation of L/P in gluteus muscle from aged compared with young horses (P = 0.055).
Fig. 5.
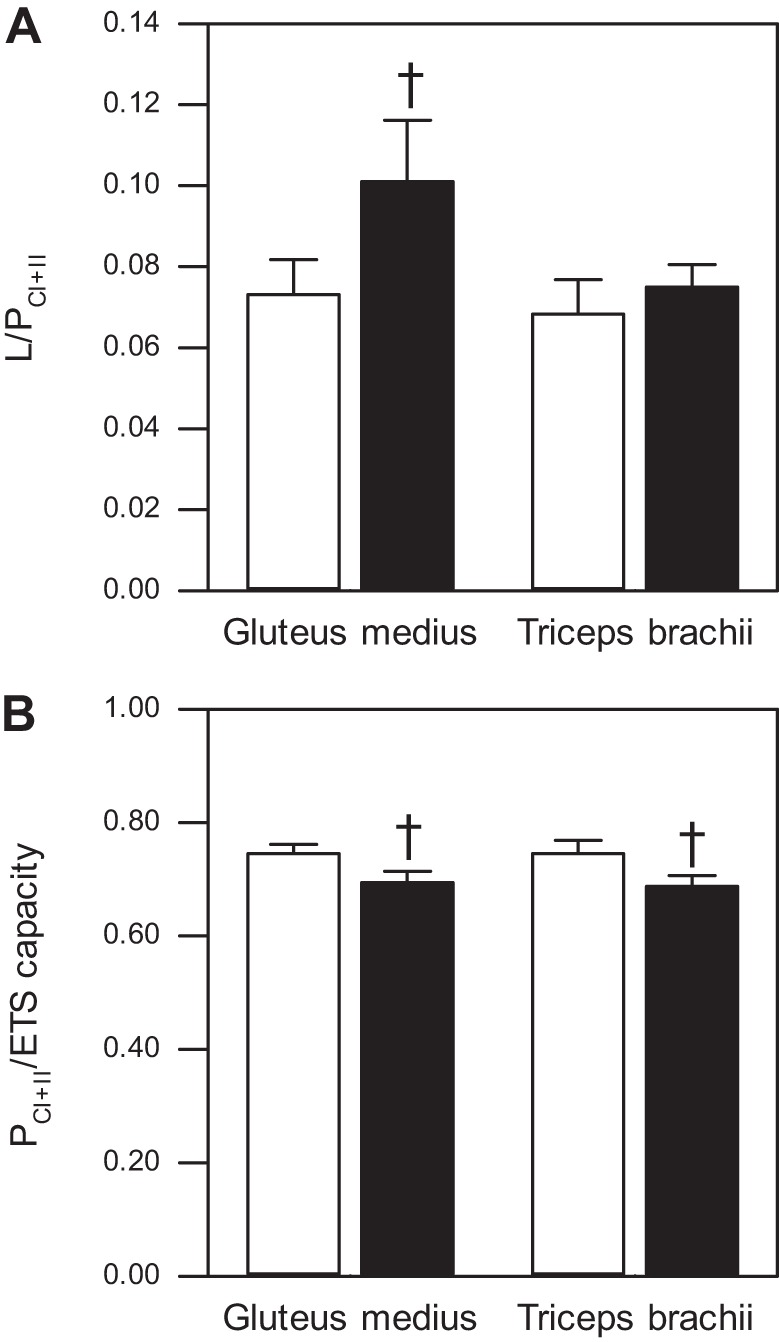
Mitochondrial coupling control ratios of permeabilized skeletal muscle fibers from gluteus medius and triceps brachii. A: L/P coupling control ratio (LEAK/PCI+II) of gluteus medius and triceps brachii from young (n = 18 for gluteus, n = 12 for triceps) and aged (n = 9 for gluteus, n = 8 for triceps) American Quarter Horses. B: P/E coupling control ratio (PCI+II/ETS capacity) of gluteus medius and triceps brachii from young (n = 18 for gluteus, n = 12 for triceps) and aged (n = 9 for gluteus, n = 9 for triceps) American Quarter Horses. Values are means ± SE. Open bars represent young horses; solid bars, aged horses. Young vs. aged: †P < 0.1.
To examine the extent to which OXPHOS exploits the full capacity of the ETS, we calculated the P/E coupling control ratio (PCI+II/fully noncoupled ETS capacity; Fig. 5B). We found that P/E was significantly affected by age (main effect of age: P = 0.011), but, when the effect in the individual muscles was evaluated, there was only a tendency for P/E to decrease with age (gluteus: P = 0.076; triceps: P = 0.057). No difference in P/E was detected between the two muscles (main effect of muscle type: P = 0.854).
DISCUSSION
In the present study, we compared skeletal muscle metabolic phenotype by analyzing muscle fiber-type composition and mitochondrial OXPHOS capacity in American Quarter Horses of two different age groups. For this comparison, we chose the gluteus medius and the triceps brachii muscle based on their functions. The gluteus muscle is located in the hindlimb and used for explosive propulsive movement, while the triceps muscle is a postural muscle located in the forelimb, where it supports the body weight during long periods of standing. These different functional demands dictate a different muscle fiber-type composition and energy metabolism. Based on differential susceptibility of muscle fiber types to age-related changes that has been reported in humans and rodent models (34, 42, 64), we expected to see different responses to age in fiber-type composition and mitochondrial function in these two types of equine muscles.
Fiber-Type Composition in Aged Horse Skeletal Muscle
MHC isoform has been used for muscle fiber-type classification and skeletal muscle fiber-type composition in numerous studies (2, 42, 75). Our findings in the American Quarter Horse are in agreement with previous findings in horses (92). Both gluteus and triceps muscles had a relatively high content of MHC II isoforms (88–96%), which might partially explain the great capacity for explosive speed of this horse breed. However, gluteus muscle had relatively more MHC IIX fibers than the triceps, while the triceps contained a greater percentage of MHC I fibers. This is consistent with observations in other horse breeds at the same biopsy sampling depth (92) and is in accordance with the muscles' different functional demands for explosive power for propulsion (gluteus) or for postural support (triceps), respectively.
In our study, aging was associated with a shift in skeletal muscle fiber-type composition toward a MHC I or IIA phenotype, but with a differential response in the two muscles investigated. Aging has been shown to affect fiber-type distribution in various horse breeds (20, 43, 53, 74) (Table 2). For example, Kim and coworkers (43) found a decline in percentage of type I fibers with age, but no correlation of age and percentage of type IIA or IIX fibers in the semitendinosus muscle from a group of horses of different breeds, with ages ranging from 2 to over 30 yr. Likewise, Lehnhard et al. (53) reported a decrease in MHC I and IIA, and an increase in MHC IIX content in gluteus medius from old (20+ yr old) compared with young (4–8 yr old) Standardbred mares. On the contrary, our results suggest an age-associated increase in MHC I-containing fibers in the gluteus and MHC IIA-containing fibers in the triceps muscle, concomitant with a relative decrease in MHC IIX-containing fibers in both muscles. This is in agreement with findings in humans (99), rodents (61, 86, 87), and two reports in horses (20, 74) (Table 2). A shift to a more oxidative phenotype was reported by Lanza et al. (48) in aging humans. Using phosphorous magnetic resonance spectroscopy of contracting skeletal muscle in vivo, the authors demonstrated that older men relied more on OXPHOS and less on glycolysis for ATP production compared with young men. However, our collective data do not support a strong association of MHC isoform and metabolic phenotype, an observation that warrants caution when interpreting MHC expression.
Table 2.
Effect of age on fiber-type composition in equine skeletal muscle
| Horse Breed | Age | Muscle | Methodology | Effect of Age | Ref. No. |
|---|---|---|---|---|---|
| SB | 2 mo to 28 yr | Gm, Tb | Histochemistry | Gm: type I, IIA ↑, type IIB ↓; Tb: type I ↔, type IIA ↑, type IIB ↓ | 20 |
| AL, A | 2–3 yr vs. 10–24 yr | Gm | Histochemistry | %type I, IIA ↑; %type IIB ↓ | 74 |
| SB | 4–8 yr vs. 20+ yr | Gm | SDS-PAGE & Coomassie blue staining | %type I, IIA ↓, %type IIX ↑ | 53 |
| TB, SB, QH, CB | 2 yr to 30 yr | S | SDS-PAGE & Coomassie blue staining | %type I ↓, type IIA, IIB ↔ | 43 |
Horse breeds are as follows: TB, Thoroughbred; SB, Standardbred; QH, American Quarter Horse; CB, crossbred; AL, Andalusian; A, Arabian. Muscles investigated are as follows: Gm, gluteus medius; Tb, triceps brachii; S, semimembranosus. Arrows indicate increased, decreased, or unchanged percentage of fiber type.
The discrepancies between our results and some previously published findings in equine muscle could have resulted from the choice of breed and the age-range investigated. Compared with other breeds, the skeletal muscle of the American Quarter Horse is rich in type IIX fibers (91), which could have accentuated the shift in fiber-type distribution compared with breeds with a more balanced distribution. In addition, the age of the young horses investigated in this study was ∼2 yr, an age at which the skeletal muscle is still developing (71), which could have affected the outcome of our measurement compared with other studies.
There are several potential mechanisms that could underlie the age-associated change in relative fiber-type composition. A selective age-related susceptibility to atrophy of a specific fiber type could lead to relative increase in the other fiber types (12). Selective atrophy of mainly type II(X) fibers and predominantly fast-twitch muscles has been widely accepted and described to occur in rodents (56, 90) and humans (42). For example, in elderly humans, the elevated relative increase of type I fibers was mainly caused by a selective atrophy of type II fibers (52). Muscle fiber atrophy observed with age can, at least in part, be driven by several mechanisms, such as a decrease in protein synthesis (reviewed by Ref. 102), or mitochondria-dependent and -independent apoptosis (57). To the best of our knowledge, apoptosis or protein degradative mechanisms in skeletal muscle have not yet been investigated in the aged horse. Wagner et al. (97) reported that whole body protein synthesis was unaffected by age in mixed-breed horses, but that there seems to be a disturbance of skeletal muscle-specific protein synthesis (namely in the mammalian target of rapamycin signaling pathway). However, muscle structure, muscle fiber features, and other effectors of the mammalian target of rapamycin pathway, such as atrophy and autophagy proteins, were not assessed in this study.
An alternative explanation for the altered fiber-type distribution is an age-induced fast-to-slow fiber-type transition within a given fiber (reviewed by Ref. 50). This transition could be caused by denervation of type II fibers followed by either atrophy of the denervated fiber or by subsequent reconstitution of larger motor units with slow motoneurons, inducing a fiber-type switch (63). Studies in rodents undergoing fast-to-slow conversion indicated that the transition in MHC isoforms follows a sequential order from MHC IIB to MHC IIX to MHC IIA to MHC I (40). Based on our data, we speculate that, if there was an age-related conversion of fiber types in the muscle of American Quarter Horses, it occurred to a differential degree or in a differential time line in the two muscles. With MHC IIX decreasing in both muscles, the gluteus displayed an increase in MHC I and the triceps in MHC IIA portion, which suggest that gluteus could have been affected at an earlier age or to a more severe degree compared with triceps. Similar muscle-specific changes associated with age were suggested by Essen and coworkers (20) in a study on racing horses, in which the authors compared age-related responses in fiber-type distribution in the gluteus with that in other muscles (including triceps). In this study, the gluteus was the only muscle that displayed an increased type I-to-type II ratio with age. In rodents, fiber-type distribution of different muscles was differentially affected by aging. For example, in rats, age-related alterations were only observed in slow-twitch soleus muscle but not in fast-twitch tibialis anterior muscle of the same individual (51). Further studies need to be conducted to investigate a differential time line or severity of changes in fiber-type distribution between different muscles. In addition, to distinguish between loss of fibers and fiber-type conversion, total fiber number, cross-sectional area of fibers, and muscle weight would be ideal parameters to determine. However, the lack of practicality prohibits some of those measures in the study of live animals in which only small muscle biopsies can be acquired.
In summary, our results are in agreement with extensive literature on humans and rodents. However, at this point, we cannot distinguish between selective fiber atrophy or fiber-type conversion, or the development of hybrid fibers. In addition, it needs to be emphasized that the literature is all but consistent in the observation of age-related changes in fiber-type composition. Purves-Smith et al. (69) recently critically reviewed and evaluated the methodology leading to the various and often contradictory findings. The authors concluded that the presence of hybrid fibers, which is rarely assessed, may draw a different picture of age-related changes in fiber-type contribution altogether, and that the lack of identification of hybrid fibers could account for the contradictory results on the effect of age on fiber-type composition.
Mitochondrial Oxidative Function
Our next aim was to compare the observed shift in MHC isoform composition with the metabolic phenotype on a subcellular level. Specifically, we asked whether the age-induced increase in MHC I and MHC IIA was concomitant with altered function and OXPHOS capacity of skeletal muscle mitochondria, and whether muscle oxidative capacity was altered through modifying mitochondrial content, mitochondrial respiratory capacity, or both.
Mitochondrial density.
CS activity was used as a biomarker of mitochondrial content and total cristae area (49). When we compared the two muscles, we found that CS activity was twofold higher in triceps compared with gluteus muscle, which corresponds with the twofold higher proportion of MHC IIA + MHC I in triceps compared with gluteus muscle. We furthermore found that gluteus and triceps muscles were affected differently by age. More specifically, CS activity decreased with age in the triceps muscle, but not in the gluteus muscle. A decline in CS activity has not often been documented in old sedentary horses (43). But findings similar to ours were reported in human studies that revealed an age-associated decline in CS activity in the oxidative portion of the gastrocnemius muscle (34), but not in the relatively more glycolytic vastus lateralis (25, 34). The explanation for this divergent change in CS activity between different muscles with aging is not clearly evident, but it may be partially explained by MHC distribution. In the American Quarter Horse, CS activity was unaffected by age in gluteus muscle. However, this “preservation” of mitochondrial density did not align with the proportional increase in presumably mitochondria-rich type I fibers (deduced from MHC I content), suggesting that the increased proportion of type I fibers did not cause a proportional increase in mitochondrial density. What is more, CS activity diminished with age in the triceps muscle, despite the observed relative increase in MHC IIA content. In contrast to our findings, Kim et al. (43) reported that CS activity in equine semimembranosus, a predominantly glycolytic muscle, was negatively correlated with age, and concomitant with a decreased MHC I proportion. However, we cannot deduce from Kim's data whether the decrease in CS activity, and deduced from this mitochondrial density, was proportional to the decline in oxidative fibers. The underlying cause for the age-associated decline in CS activity (29) and mitochondrial density described for human muscle (14) might be a decreased rate of mitochondrial protein synthesis (28), or alterations in mitochondrial biogenesis, which is associated with mitochondrial protein synthesis (42). Alternatively, an age-associated imbalance between autophagic removal of mitochondria, namely mitophagy, and mitochondrial biogenesis would diminish mitochondrial density. However, impaired rather than increased mitophagy has been widely observed in aged muscle (reviewed in Ref. 31). To the best of our knowledge, neither mitochondrial biogenesis nor mitophagy have been assessed in aging equine skeletal muscle, and they are the current subject of our laboratory's investigations.
Mitochondrial function.
Next we addressed the question of whether the age-related decrease in mitochondrial density (deduced from CS activity) correlated with decreased oxidative capacity. We assessed muscle mitochondrial function, namely OXPHOS capacity, by both spectrophotometric measurement of COX activity in muscle homogenates, and HRR of skeletal muscle mitochondria in situ. In addition to OXPHOS capacity, we also assessed activity of 3-HADH, an enzyme of the β-oxidation pathway, to evaluate the capacity to metabolize fatty acids for energy production. There is some debate on whether differences in muscle tissue OXPHOS are due to mitochondrial content and/or composition of muscle fiber types, or function of the individual mitochondria, or both. Initially, it was suggested that differences in muscle respiration may be attributed solely to the differences in mitochondrial quantity (33, 81). Recently, a greater number of studies have revealed that differences in muscle oxidative capacity depended on the function of the mitochondria themselves (1, 36). Here, we present COX and 3-HADH activities and O2 flux relative to muscle weight, as well as per unit mitochondria. The normalization of mitochondrial functional markers to a mitochondrial marker such as CS activity has been recommended by Pesta and Gnaiger (62) to separate the effects of mitochondrial quality (function) from mitochondrial quantity.
3-HADH activity.
In agreement with studies in humans (72) and horses (43), our data on 3-HADH activity suggest that fatty acid oxidation capacity was not impaired on a muscle level and was even elevated on a mitochondria level in muscles from our aged American Quarter Horses. However, others reported an age-related decline of 3-HADH activity in humans (13) and a decreased ability to utilize fatty acids for mitochondrial respiration in mice (41). The preserved ability to metabolize fatty acids suggested by our data in aged horses seems to be concurrent with the structural shift toward a higher percentage of type I and IIA muscle fibers. Gueugneau et al. (27) reported a higher lipid content in type I and IIA compared with type IIX fibers in human vastus lateralis muscle, and an increase in intramyocellular lipid content with age, although this was not accompanied with a fiber-type shift.
COX activity.
COX activity has been validated as a marker of mitochondrial function, specifically of OXPHOS capacity (49). Our data showed that COX activity declined with age in both the gluteus and the triceps muscle, whether normalized to wet weight or to mitochondrial unit, suggesting an impairment of OXPHOS capacity in older horses, not only through decline in mitochondrial number, but also through impaired function of the mitochondria themselves. Joseph et al. (42) found that COX activity (per unit muscle weight) in elderly, low-functioning humans was lower than that in young individuals, but the authors did not measure any indicator of mitochondrial density. We observed an over 40% reduction in COX activity with age in both muscles. This decline was more severe in the gluteus muscle, especially when the COX activity was normalized to mitochondrial unit. Given that gluteus mitochondrial density was not severely affected by age, it appears that the oxidative capacity is primarily impaired on the individual mitochondrial level. On the other hand, in the triceps muscle, which displayed a significant decrease in mitochondrial density, the impairment of COX activity on the mitochondrial level seemed less severe. In the present investigation, we did not measure production of reactive oxygen species (ROS) or markers of (oxidative) damage to mitochondrial components, which could potentially underlie the pronounced reduction in COX activity in gluteus muscle. Compared with oxidative muscles, muscles of a more glycolytic phenotype have been shown to produce more ROS, to have lower free-radical scavenging capacity, and to consequently display higher oxidative damage, such as lipid peroxidation (3, 65, 66). Comparing the two muscles, independent of age, an interesting finding was that triceps muscle exhibited higher COX activity than gluteus muscle when normalized to muscle weight, but lower COX activity per mitochondrial unit, which suggests that, in triceps muscle, the higher COX activity on the tissue level was achieved by a higher mitochondrial content. To further explore the muscle-specific impairment of mitochondrial function with aging, we measured mitochondrial respiratory function in situ using HRR.
Mitochondrial respiration.
Although our results demonstrated that COX activity in skeletal muscle homogenates was dependent on age and muscle type, we cannot deduce whether this alteration reflects a change of muscle mitochondrial OXPHOS. Saponin-permeabilized muscle fibers allow examination of the integrative mitochondrial function in a relatively preserved cytoarchitecture (46, 80). Our mass-specific respirometry data demonstrated no age-related difference in muscle respiratory capacity across all respiratory states, indicating that the capacity for muscle OXPHOS remained high in both muscles from our aged American Quarter Horses. When expressed relative to CS activity, LEAK, OXPHOS (PCI), and ETS capacity were elevated with age (significant main effect of age), independent of muscle type, although only aged triceps muscle displayed a significant increase in ETS capacity. Products of oxidative stress are known to accumulate with age and are reported to activate mitochondrial uncoupling proteins and the adenine nucleotide transporter (18, 19). Both could have caused an increased proton LEAK and, consequently, increased LEAK respiration. Moreover, the “uncoupling to survive” hypothesis of aging suggests that mild uncoupling, particularly in aged tissue, protects against mitochondrial ROS production and subsequent oxidative damage to mitochondria and other cellular components (7, 24, 84). The elevated LEAK respiration in aged muscle explains only a minor part of the concurrently increased ETS capacity. It is possible that this increase reflects on a functional level the increasing reliance on oxidative energy production in aged fibers. Jacobs et al. (38) recently showed in mice that mitochondria-specific ETS capacity was elevated with age in the primarily glycolytic gastrocnemius muscle, but not in the primarily oxidative soleus muscle.
Both mass-specific and mitochondria-specific activated respiration supported by CI- and CII-linked substrates (PCI+II) were not affected by age, which is contrary to the age-related decline in COX activity that we determined in muscle homogenates. A similar case is reported by Chabi et al. (11), who assessed state 3 respiration (OXPHOS in the presence of glutamate, malate, and ADP) of isolated mitochondria and COX activity in muscle homogenates. The control of mitochondrial respiration is shared between all of the complexes and electron carriers in the respiratory ETS (78), suggesting no rate-limiting step per se. In addition, it was evident in human mitochondria that maximal complex IV activity is in excess of what is required for OXPHOS (23, 94). This is supported by the observation that inhibition of complex IV activity had to exceed a critical value (40–60%) to cause a detectable decrease in mitochondrial respiration in both isolated mitochondria (17, 93) and permeabilized muscle fibers (45). These suggestions could explain why we did not detect a decrease in OXPHOS with aging in either muscle as a whole when we assessed mitochondrial respiration with substrates supporting CI and CII. We did not assess mitochondrial respiration supported by fatty acid oxidation. In rodents, fatty acid-supported respiration was impaired with age (32, 41), while fat oxidation capacity was similar in muscle from young and old sedentary men (72). The unaltered 3-HADH activity on a whole muscle level observed here suggests that the capacity to oxidize fatty acids was preserved in skeletal muscle form aged horses. Future studies will have to determine whether capacity for fatty acid β-oxidation is correlated with utilization of this energy by the Mt electron transport chain.
The difference between mass- and mitochondria-specific respiration when comparing gluteus and triceps muscles is consistent with our results for COX activity. Triceps muscle had higher mass-specific respiratory rates across all states (except LEAK), which could be due to its considerably higher mitochondrial density. However, mitochondria-specific respiration in triceps was lower (with the exception of LEAK). This difference in mass- and mitochondria-specific respiration between different equine muscles is similar to observations in humans and laboratory animals (1, 36). For example, in old mice, mass-specific activated respiration was higher and mitochondria-specific activated respiration lower in the oxidative soleus compared with the glycolytic gastrocnemius muscle (38), which suggests a mitochondrial specialization with muscle and/or muscle fiber type. Moreover, the finding that mitochondria in glycolytic muscles possessed a higher oxidative capacity than those in oxidative muscles is consistent with observations in other mammals (36, 37, 65). Specific mitochondrial phenotypes have been postulated to exist across skeletal muscle types, with different composition and morphology. For example, others have reported that mitochondrial composition can vary by differential expression of respiratory complex subunit isoforms to sustain the tissue-specific energy demands (26, 35, 44), and Jacobs et al. (38) determined differential protein expression of all ETS complexes in mouse skeletal muscle homogenates of three different muscles. We thus speculate that mitochondria from gluteus muscle might express more COX protein, reflected in the higher mitochondria-specific COX activity and respiratory capacity compared with triceps mitochondria. At this point, we cannot substantiate our speculation, and further analyses will have to quantify mitochondrial components in the different muscles. In addition, mitochondria vary morphometrically between slow and fast fibers. Fast-twitch muscle mitochondria are arranged in a thinner and longer reticular network, whereas mitochondria in slow-twitch muscle possess a thicker and more truncated network (60), which could be related to the lower mitochondria-specific respiratory capacity in triceps muscle.
We found no difference in L/P or P/E between the two muscles or with age. A study with elite athletes pointed out that differences in mitochondrial OXPHOS capacity and mitochondrial coupling control were apparent only when substrates linked with both CI and CII are provided (39). We hence calculated L/P and P/E using OXPHOS capacity with a physiological substrate combination that supports electron flow through CI and CII (PCI+II). In the young horses, both muscles exhibited low L/P, indicating a good coupling between respiration and phosphorylation, and the L/P for young horse in our study are similar to those reported by Votion et al. (96) for mature horses with the same respiratory substrates [note: these authors determined the respiratory control ratio (RCR); and 1/RCR = L/P]. In contrast, age affected the P/E phosphorylation control ratio (main effect of age), independent of muscle type. In young horses, P/E was around 0.75 in both muscles, consistent with a report on healthy, untrained mature horses with a P/E of 0.8 (96). The age-associated reduction in P/E together with the elevation of ETS capacity indicate a limitation of OXPHOS capacity by the phosphorylation system and suggest that, even though aged horses displayed elevated maximal ETS capacity, constraints of the phosphorylation system may underlie a lower relative activated respiration, thereby possibly limiting the energy supply in the aging muscle. However, a limited mitochondrial ATP generation might be necessary to reduce the production of ROS (7). ATP production relies on a high proton gradient, which may, in turn, be associated with ROS generation. In this situation, an elevated proton leak, suggested by the higher LEAK respiration in the older muscles, might help to limit oxidative stress and damage.
The data on mitochondrial respiration on the tissue, as well as mitochondrial level, suggest that, despite significant decline in mitochondrial content (CS activity) and COX activity, as well as shift in MHC-isoform composition, integrated mitochondrial function and intrinsic mitochondrial functionality were not negatively impacted in skeletal muscle from American Quarter Horses ∼20 yr of age. Several interpretations come to mind. 1) In this study, we assessed integrated mitochondrial function in situ in permeabilized muscle fibers, a technique that helps to attenuate or even prevent disruptions due to preparative technique (67). But, similar to assessment of isolated mitochondria, the mitochondria in situ were deprived of their intra- and extracellular milieu, which could have affected mitochondrial function in vivo (reviewed in Ref. 31). 2) It is possible that the American Quarter Horse, which can remain active well beyond the age assessed in this study, does not responds to aging with impaired oxidative capacity like it has been described for rodents and humans. And lastly, 3) the horses investigated in our study might not have reached the age at which integrated and/or intrinsic mitochondrial function becomes overtly impaired and were instead at a transition age, displaying cellular adaptions rather than impairments, similar to observations made in aging rhesus monkeys (68). Taken together, future studies are needed to test those possibilities, for example, by using in vivo, noninvasive technologies to assess oxidative capacity within the feasibility of application in large animals, and by expanding the age range of subjects.
Conclusions
Based on our collective data, we conclude that, despite some age-associated changes, there was no overt mitochondrial dysfunction in skeletal muscle from aged American Quarter Horses. If a transition age exists in the horse, defining it might provide insights about a beneficial time point to apply interventions aiming to delay the onset of overt muscle oxidative dysfunction and decline in physical performance. On the other hand, if the horse ages differently from traditional animal models and humans, it will be of interest to characterize the underlying differences.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
C.L., L.K.W., and S.E.W. conception and design of research; C.L., S.H.W., and S.E.W. performed experiments; C.L. and S.E.W. analyzed data; C.L., S.H.W., L.K.W., and S.E.W. interpreted results of experiments; C.L. and S.E.W. prepared figures; C.L. and S.E.W. drafted manuscript; C.L., S.H.W., L.K.W., and S.E.W. edited and revised manuscript; C.L., S.H.W., L.K.W., and S.E.W. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the staff and students at the University of Florida's Equine Sciences Center and Horse Teaching Unit, and Kailey Mansour for technical assistance.
Present address of S. H. White: College of Health Sciences, University of Kentucky, Lexington, KY.
REFERENCES
- 1.Amara CE, Shankland EG, Jubrias SA, Marcinek DJ, Kushmerick MJ, Conley KE. Mild mitochondrial uncoupling impacts cellular aging in human muscles in vivo. Proc Natl Acad Sci U S A 104: 1057–1062, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andersen JL. Muscle fibre type adaptation in the elderly human muscle. Scand J Med Sci Sports 13: 40–47, 2003. [DOI] [PubMed] [Google Scholar]
- 3.Anderson EJ, Neufer PD. Type II skeletal myofibers possess unique properties that potentiate mitochondrial H2O2 generation. Am J Physiol Cell Physiol 290: C844–C851, 2006. [DOI] [PubMed] [Google Scholar]
- 4.Anton SD, Woods AJ, Ashizawa T, Barb D, Buford TW, Carter CS, Clark DJ, Cohen RA, Corbett DB, Cruz-Almeida Y, Dotson V, Ebner N, Efron PA, Fillingim RB, Foster TC, Gundermann DM, Joseph AM, Karabetian C, Leeuwenburgh C, Manini TM, Marsiske M, Mankowski RT, Mutchie HL, Perri MG, Ranka S, Rashidi P, Sandesara B, Scarpace PJ, Sibille KT, Solberg LM, Someya S, Uphold C, Wohlgemuth S, Wu SS, Pahor M. Successful aging: advancing the science of physical independence in older adults. Ageing Res Rev 24: 304–327, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barrey E, Valette JP, Jouglin M, Blouin C, Langlois B. Heritability of percentage of fast myosin heavy chains in skeletal muscles and relationship with performance. Equine Vet J Suppl 30: 289–292, 1999. [DOI] [PubMed] [Google Scholar]
- 6.Boushel R, Gnaiger E, Schjerling P, Skovbro M, Kraunsoe R, Dela F. Patients with type 2 diabetes have normal mitochondrial function in skeletal muscle. Diabetologia 50: 790–796, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brand MD. Uncoupling to survive? The role of mitochondrial inefficiency in ageing. Exp Gerontol 35: 811–820, 2000. [DOI] [PubMed] [Google Scholar]
- 8.Brosnahan MM, Paradis MR. Assessment of clinical characteristics, management practices, and activities of geriatric horses. J Am Vet Med Assoc 223: 99–103, 2003. [DOI] [PubMed] [Google Scholar]
- 9.Brosnahan MM, Paradis MR. Demographic and clinical characteristics of geriatric horses: 467 cases (1989–1999). J Am Vet Med Assoc 223: 93–98, 2003. [DOI] [PubMed] [Google Scholar]
- 10.Buford TW, Anton SD, Judge AR, Marzetti E, Wohlgemuth SE, Carter CS, Leeuwenburgh C, Pahor M, Manini TM. Models of accelerated sarcopenia: critical pieces for solving the puzzle of age-related muscle atrophy. Ageing Res Rev 9: 369–383, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chabi B, Ljubicic V, Menzies KJ, Huang JH, Saleem A, Hood DA. Mitochondrial function and apoptotic susceptibility in aging skeletal muscle. Aging Cell 7: 2–12, 2008. [DOI] [PubMed] [Google Scholar]
- 12.Ciciliot S, Rossi AC, Dyar KA, Blaauw B, Schiaffino S. Muscle type and fiber type specificity in muscle wasting. Int J Biochem Cell Biol 45: 2191–2199, 2013. [DOI] [PubMed] [Google Scholar]
- 13.Coggan AR, Spina RJ, King DS, Rogers MA, Brown M, Nemeth PM, Holloszy JO. Histochemical and enzymatic comparison of the gastrocnemius muscle of young and elderly men and women. J Gerontol 47: B71–B76, 1992. [DOI] [PubMed] [Google Scholar]
- 14.Conley KE, Jubrias SA, Esselman PC. Oxidative capacity and ageing in human muscle. J Physiol 526: 203–210, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cooper JM, Mann VM, Schapira AH. Analyses of mitochondrial respiratory chain function and mitochondrial DNA deletion in human skeletal muscle: effect of ageing. J Neurol Sci 113: 91–98, 1992. [DOI] [PubMed] [Google Scholar]
- 16.Crescenzo R, Bianco F, Mazzoli A, Giacco A, Liverini G, Iossa S. Skeletal muscle mitochondrial energetic efficiency and aging. Int J Mol Sci 16: 10674–10685, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.D'Aurelio M, Pallotti F, Barrientos A, Gajewski CD, Kwong JQ, Bruno C, Beal MF, Manfredi G. In vivo regulation of oxidative phosphorylation in cells harboring a stop-codon mutation in mitochondrial DNA-encoded cytochrome c oxidase subunit I. J Biol Chem 276: 46925–46932, 2001. [DOI] [PubMed] [Google Scholar]
- 18.Echtay KS, Pakay JL, Esteves TC, Brand MD. Hydroxynonenal and uncoupling proteins: a model for protection against oxidative damage. Biofactors 24: 119–130, 2005. [DOI] [PubMed] [Google Scholar]
- 19.Echtay KS, Roussel D, St-Pierre J, Jekabsons MB, Cadenas S, Stuart JA, Harper JA, Roebuck SJ, Morrison A, Pickering S, Clapham JC, Brand MD. Superoxide activates mitochondrial uncoupling proteins. Nature 415: 96–99, 2002. [DOI] [PubMed] [Google Scholar]
- 20.Essen B, Lindholm A, Thornton J. Histochemical properties of muscle fibres types and enzyme activities in skeletal muscles of Standardbred trotters of different ages. Equine Vet J 12: 175–180, 1980. [DOI] [PubMed] [Google Scholar]
- 21.Fluck M. Functional, structural and molecular plasticity of mammalian skeletal muscle in response to exercise stimuli. J Exp Biol 209: 2239–2248, 2006. [DOI] [PubMed] [Google Scholar]
- 22.Fong JC, Schulz H. On the rate-determining step of fatty acid oxidation in heart. Inhibition of fatty acid oxidation by 4-pentenoic acid. J Biol Chem 253: 6917–6922, 1978. [PubMed] [Google Scholar]
- 23.Gnaiger E, Lassnig B, Kuznetsov A, Rieger G, Margreiter R. Mitochondrial oxygen affinity, respiratory flux control and excess capacity of cytochrome c oxidase. J Exp Biol 201: 1129–1139, 1998. [DOI] [PubMed] [Google Scholar]
- 24.Greco M, Villani G, Mazzucchelli F, Bresolin N, Papa S, Attardi G. Marked aging-related decline in efficiency of oxidative phosphorylation in human skin fibroblasts. FASEB J 17: 1706–1708, 2003. [DOI] [PubMed] [Google Scholar]
- 25.Grimby G, Danneskiold-Samsoe B, Hvid K, Saltin B. Morphology and enzymatic capacity in arm and leg muscles in 78–81 year old men and women. Acta Physiol Scand 115: 125–134, 1982. [DOI] [PubMed] [Google Scholar]
- 26.Grossman LI, Lomax MI. Nuclear genes for cytochrome c oxidase. Biochim Biophys Acta 1352: 174–192, 1997. [DOI] [PubMed] [Google Scholar]
- 27.Gueugneau M, Coudy-Gandilhon C, Theron L, Meunier B, Barboiron C, Combaret L, Taillandier D, Polge C, Attaix D, Picard B, Verney J, Roche F, Feasson L, Barthelemy JC, Bechet D. Skeletal muscle lipid content and oxidative activity in relation to muscle fiber type in aging and metabolic syndrome. J Gerontol A Biol Sci Med Sci 70: 566–576, 2015. [DOI] [PubMed] [Google Scholar]
- 28.Guillet C, Prod'homme M, Balage M, Gachon P, Giraudet C, Morin L, Grizard J, Boirie Y. Impaired anabolic response of muscle protein synthesis is associated with S6K1 dysregulation in elderly humans. FASEB J 18: 1586–1587, 2004. [DOI] [PubMed] [Google Scholar]
- 29.Hebert SL, Marquet-de Rougé P, Lanza IR, McCrady-Spitzer SK, Levine JA, Middha S, Carter RE, Klaus KA, Therneau TM, Highsmith EW, Nair KS. Mitochondrial aging and physical decline: insights from three generations of women. J Gerontol A Biol Sci Med Sci 70: 1409–1417, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Henneke DR, Potter GD, Kreider JL, Yeates BF. Relationship between condition score, physical measurements and body fat percentage in mares. Equine Vet J 15: 371–372, 1983. [DOI] [PubMed] [Google Scholar]
- 31.Hepple RT. Mitochondrial involvement and impact in aging skeletal muscle. Front Aging Neurosci 6: 211, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hey-Mogensen M, Jeppesen J, Madsen K, Kiens B, Franch J. Obesity augments the age-induced increase in mitochondrial capacity for H2O2 release in Zucker fatty rats. Acta Physiol (Oxf) 204: 354–361, 2012. [DOI] [PubMed] [Google Scholar]
- 33.Hoppeler H, Hudlicka O, Uhlmann E. Relationship between mitochondria and oxygen consumption in isolated cat muscles. J Physiol 385: 661–675, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Houmard JA, Weidner ML, Gavigan KE, Tyndall GL, Hickey MS, Alshami A. Fiber type and citrate synthase activity in the human gastrocnemius and vastus lateralis with aging. J Appl Physiol (1985) 85: 1337–1341, 1998. [DOI] [PubMed] [Google Scholar]
- 35.Huttemann M, Kadenbach B, Grossman LI. Mammalian subunit IV isoforms of cytochrome c oxidase. Gene 267: 111–123, 2001. [DOI] [PubMed] [Google Scholar]
- 36.Jackman MR, Willis WT. Characteristics of mitochondria isolated from type I and type IIb skeletal muscle. Am J Physiol Cell Physiol 270: C673–C678, 1996. [DOI] [PubMed] [Google Scholar]
- 37.Jacobs RA, Diaz V, Meinild AK, Gassmann M, Lundby C. The C57Bl/6 mouse serves as a suitable model of human skeletal muscle mitochondrial function. Exp Physiol 98: 908–921, 2013. [DOI] [PubMed] [Google Scholar]
- 38.Jacobs RA, Diaz V, Soldini L, Haider T, Thomassen M, Nordsborg NB, Gassmann M, Lundby C. Fast-twitch glycolytic skeletal muscle is predisposed to age-induced impairments in mitochondrial function. J Gerontol A Biol Sci Med Sci 68: 1010–1022, 2013. [DOI] [PubMed] [Google Scholar]
- 39.Jacobs RA, Lundby C. Mitochondria express enhanced quality as well as quantity in association with aerobic fitness across recreationally active individuals up to elite athletes. J Appl Physiol (1985) 114: 344–350, 2013. [DOI] [PubMed] [Google Scholar]
- 40.Jaschinski F, Schuler M, Peuker H, Pette D. Changes in myosin heavy chain mRNA and protein isoforms of rat muscle during forced contractile activity. Am J Physiol Cell Physiol 274: C365–C370, 1998. [DOI] [PubMed] [Google Scholar]
- 41.Johnson ML, Lalia AZ, Dasari S, Pallauf M, Fitch M, Hellerstein MK, Lanza IR. Eicosapentaenoic acid but not docosahexaenoic acid restores skeletal muscle mitochondrial oxidative capacity in old mice. Aging Cell 14: 734–743, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Joseph AM, Adhihetty PJ, Buford TW, Wohlgemuth SE, Lees HA, Nguyen LM, Aranda JM, Sandesara BD, Pahor M, Manini TM, Marzetti E, Leeuwenburgh C. The impact of aging on mitochondrial function and biogenesis pathways in skeletal muscle of sedentary high- and low-functioning elderly individuals. Aging Cell 11: 801–809, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim JS, Hinchcliff KW, Yamaguchi M, Beard LA, Markert CD, Devor ST. Age-related changes in metabolic properties of equine skeletal muscle associated with muscle plasticity. Vet J 169: 397–403, 2005. [DOI] [PubMed] [Google Scholar]
- 44.Kunz WS. Different metabolic properties of mitochondrial oxidative phosphorylation in different cell types–important implications for mitochondrial cytopathies. Exp Physiol 88: 149–154, 2003. [DOI] [PubMed] [Google Scholar]
- 45.Kunz WS, Kudin A, Vielhaber S, Elger CE, Attardi G, Villani G. Flux control of cytochrome c oxidase in human skeletal muscle. J Biol Chem 275: 27741–27745, 2000. [DOI] [PubMed] [Google Scholar]
- 46.Kuznetsov AV, Mayboroda O, Kunz D, Winkler K, Schubert W, Kunz WS. Functional imaging of mitochondria in saponin-permeabilized mice muscle fibers. J Cell Biol 140: 1091–1099, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kuznetsov AV, Veksler V, Gellerich FN, Saks V, Margreiter R, Kunz WS. Analysis of mitochondrial function in situ in permeabilized muscle fibers, tissues and cells. Nat Protoc 3: 965–976, 2008. [DOI] [PubMed] [Google Scholar]
- 48.Lanza IR, Befroy DE, Kent-Braun JA. Age-related changes in ATP-producing pathways in human skeletal muscle in vivo. J Appl Physiol (1985) 99: 1736–1744, 2005. [DOI] [PubMed] [Google Scholar]
- 49.Larsen S, Nielsen J, Hansen CN, Nielsen LB, Wibrand F, Stride N, Schroder HD, Boushel R, Helge JW, Dela F, Hey-Mogensen M. Biomarkers of mitochondrial content in skeletal muscle of healthy young human subjects. J Physiol 590: 3349–3360, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Larsson L, Ansved T. Effects of ageing on the motor unit. Prog Neurobiol 45: 397–458, 1995. [DOI] [PubMed] [Google Scholar]
- 51.Larsson L, Edstrom L. Effects of age on enzyme-histochemical fibre spectra and contractile properties of fast- and slow-twitch skeletal muscles in the rat. J Neurol Sci 76: 69–89, 1986. [DOI] [PubMed] [Google Scholar]
- 52.Larsson L, Sjodin B, Karlsson J. Histochemical and biochemical changes in human skeletal muscle with age in sedentary males, age 22–65 years. Acta Physiol Scand 103: 31–39, 1978. [DOI] [PubMed] [Google Scholar]
- 53.Lehnhard RA, McKeever KH, Kearns CF, Beekley MD. Myosin heavy chain profiles and body composition are different in old versus young Standardbred mares. Vet J 167: 59–66, 2004. [DOI] [PubMed] [Google Scholar]
- 54.Leisson K, Jaakma U, Seene T. Adaptation of equine locomotor muscle fiber types to endurance and intensive high speed training. J Equine Vet Sci 28: 395–401, 2008. [Google Scholar]
- 55.Lopez-Rivero JL, Morales-Lopez JL, Galisteo AM, Aguera E. Muscle fibre type composition in untrained and endurance-trained Andalusian and Arab horses. Equine Vet J 23: 91–93, 1991. [DOI] [PubMed] [Google Scholar]
- 56.Lowe DA, Surek JT, Thomas DD, Thompson LV. Electron paramagnetic resonance reveals age-related myosin structural changes in rat skeletal muscle fibers. Am J Physiol Cell Physiol 280: C540–C547, 2001. [DOI] [PubMed] [Google Scholar]
- 57.Marzetti E, Hwang JC, Lees HA, Wohlgemuth SE, Dupont-Versteegden EE, Carter CS, Bernabei R, Leeuwenburgh C. Mitochondrial death effectors: Relevance to sarcopenia and disuse muscle atrophy. Biochim Biophys Acta 1800: 235–244, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McKeever KH. Exercise physiology of the older horse. Vet Clin North Am Equine Pract 18: 469–490, 2002. [DOI] [PubMed] [Google Scholar]
- 59.Mogensen M, Bagger M, Pedersen PK, Fernstrom M, Sahlin K. Cycling efficiency in humans is related to low UCP3 content and to type I fibres but not to mitochondrial efficiency. J Physiol 571: 669–681, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ogata T, Yamasaki Y. Ultra-high-resolution scanning electron microscopy of mitochondria and sarcoplasmic reticulum arrangement in human red, white, and intermediate muscle fibers. Anat Rec 248: 214–223, 1997. [DOI] [PubMed] [Google Scholar]
- 61.Pehme A, Alev K, Kaasik P, Seene T. Age-related changes in skeletal-muscle myosin heavy-chain composition: effect of mechanical loading. J Aging Phys Act 12: 29–44, 2004. [DOI] [PubMed] [Google Scholar]
- 62.Pesta D, Gnaiger E. High-resolution respirometry: OXPHOS protocols for human cells and permeabilized fibers from small biopsies of human muscle. Methods Mol Biol 810: 25–58, 2012. [DOI] [PubMed] [Google Scholar]
- 63.Pette D, Staron RS. Myosin isoforms, muscle fiber types, and transitions. Microsc Res Tech 50: 500–509, 2000. [DOI] [PubMed] [Google Scholar]
- 64.Phillips T, Leeuwenburgh C. Muscle fiber specific apoptosis and TNF-alpha signaling in sarcopenia are attenuated by life-long calorie restriction. FASEB J 19: 668–670, 2005. [DOI] [PubMed] [Google Scholar]
- 65.Picard M, Csukly K, Robillard ME, Godin R, Ascah A, Bourcier-Lucas C, Burelle Y. Resistance to Ca2+-induced opening of the permeability transition pore differs in mitochondria from glycolytic and oxidative muscles. Am J Physiol Regul Integr Comp Physiol 295: R659–R668, 2008. [DOI] [PubMed] [Google Scholar]
- 66.Picard M, Ritchie D, Thomas MM, Wright KJ, Hepple RT. Alterations in intrinsic mitochondrial function with aging are fiber type-specific and do not explain differential atrophy between muscles. Aging Cell 10: 1047–1055, 2011. [DOI] [PubMed] [Google Scholar]
- 67.Picard M, Taivassalo T, Ritchie D, Wright KJ, Thomas MM, Romestaing C, Hepple RT. Mitochondrial structure and function are disrupted by standard isolation methods. PLoS One 6: e18317, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pugh TD, Conklin MW, Evans TD, Polewski MA, Barbian HJ, Pass R, Anderson BD, Colman RJ, Eliceiri KW, Keely PJ, Weindruch R, Beasley TM, Anderson RM. A shift in energy metabolism anticipates the onset of sarcopenia in rhesus monkeys. Aging Cell 12: 672–681, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Purves-Smith FM, Sgarioto N, Hepple RT. Fiber typing in aging muscle. Exerc Sport Sci Rev 42: 45–52, 2014. [DOI] [PubMed] [Google Scholar]
- 70.Revold T, Mykkanen AK, Karlstrom K, Ihler CF, Poso AR, Essen-Gustavsson B. Effects of training on equine muscle fibres and monocarboxylate transporters in young Coldblooded Trotters. Equine Vet J Suppl 38: 289–295, 2010. [DOI] [PubMed] [Google Scholar]
- 71.Rietbroek NJ, Dingboom EG, Joosten BJ, Eizema K, Everts ME. Effect of show jumping training on the development of locomotory muscle in young horses. Am J Vet Res 68: 1232–1238, 2007. [DOI] [PubMed] [Google Scholar]
- 72.Rimbert V, Boirie Y, Bedu M, Hocquette JF, Ritz P, Morio B. Muscle fat oxidative capacity is not impaired by age but by physical inactivity: association with insulin sensitivity. FASEB J 18: 737–739, 2004. [DOI] [PubMed] [Google Scholar]
- 73.Ritov VB, Menshikova EV, Kelley DE. Analysis of cardiolipin in human muscle biopsy. J Chromatogr B Analyt Technol Biomed Life Sci 831: 63–71, 2006. [DOI] [PubMed] [Google Scholar]
- 74.Rivero JL, Galisteo AM, Aguera E, Miro F. Skeletal muscle histochemistry in male and female Andalusian and Arabian horses of different ages. Res Vet Sci 54: 160–169, 1993. [DOI] [PubMed] [Google Scholar]
- 75.Rivero JL, Talmadge RJ, Edgerton VR. A sensitive electrophoretic method for the quantification of myosin heavy chain isoforms in horse skeletal muscle: histochemical and immunocytochemical verifications. Electrophoresis 18: 1967–1972, 1997. [DOI] [PubMed] [Google Scholar]
- 76.Roneus M. Muscle characteristics in standardbreds of different ages and sexes. Equine Vet J 25: 143–146, 1993. [DOI] [PubMed] [Google Scholar]
- 77.Roneus M, Lindholm A, Asheim A. Muscle characteristics in Thoroughbreds of different ages and sexes. Equine Vet J 23: 207–210, 1991. [DOI] [PubMed] [Google Scholar]
- 78.Rossignol R, Letellier T, Malgat M, Rocher C, Mazat JP. Tissue variation in the control of oxidative phosphorylation: implication for mitochondrial diseases. Biochem J 347: 45–53, 2000. [PMC free article] [PubMed] [Google Scholar]
- 79.Saks VA, Belikova YO, Kuznetsov AV, Khuchua ZA, Branishte TH, Semenovsky ML, Naumov VG. Phosphocreatine pathway for energy transport: ADP diffusion and cardiomyopathy. Am J Physiol Heart Circ Physiol 261: H30–H38, 1991. [DOI] [PubMed] [Google Scholar]
- 80.Saks VA, Veksler VI, Kuznetsov AV, Kay L, Sikk P, Tiivel T, Tranqui L, Olivares J, Winkler K, Wiedemann F, Kunz WS. Permeabilized cell and skinned fiber techniques in studies of mitochondrial function in vivo. Mol Cell Biochem 184: 81–100, 1998. [PubMed] [Google Scholar]
- 81.Schwerzmann K, Hoppeler H, Kayar SR, Weibel ER. Oxidative capacity of muscle and mitochondria: correlation of physiological, biochemical, and morphometric characteristics. Proc Natl Acad Sci U S A 86: 1583–1587, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Serrano AL, Quiroz-Rothe E, Rivero JL. Early and long-term changes of equine skeletal muscle in response to endurance training and detraining. Pflügers Arch 441: 263–274, 2000. [DOI] [PubMed] [Google Scholar]
- 83.Short KR, Bigelow ML, Kahl J, Singh R, Coenen-Schimke J, Raghavakaimal S, Nair KS. Decline in skeletal muscle mitochondrial function with aging in humans. Proc Natl Acad Sci U S A 102: 5618–5623, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Speakman JR, Talbot DA, Selman C, Snart S, McLaren JS, Redman P, Krol E, Jackson DM, Johnson MS, Brand MD. Uncoupled and surviving: individual mice with high metabolism have greater mitochondrial uncoupling and live longer. Aging Cell 3: 87–95, 2004. [DOI] [PubMed] [Google Scholar]
- 85.Spinazzi M, Casarin A, Pertegato V, Salviati L, Angelini C. Assessment of mitochondrial respiratory chain enzymatic activities on tissues and cultured cells. Nat Protoc 7: 1235–1246, 2012. [DOI] [PubMed] [Google Scholar]
- 86.Sugiura T, Matoba H, Miyata H, Kawai Y, Murakami N. Myosin heavy chain isoform transition in ageing fast and slow muscles of the rat. Acta Physiol Scand 144: 419–423, 1992. [DOI] [PubMed] [Google Scholar]
- 87.Sullivan VK, Powers SK, Criswell DS, Tumer N, Larochelle JS, Lowenthal D. Myosin heavy chain composition in young and old rat skeletal muscle: effects of endurance exercise. J Appl Physiol (1985) 78: 2115–2120, 1995. [DOI] [PubMed] [Google Scholar]
- 88.Talmadge RJ, Roy RR. Electrophoretic separation of rat skeletal muscle myosin heavy-chain isoforms. J Appl Physiol (1985) 75: 2337–2340, 1993. [DOI] [PubMed] [Google Scholar]
- 89.Taylor CR, Maloiy GM, Weibel ER, Langman VA, Kamau JM, Seeherman HJ, Heglund NC. Design of the mammalian respiratory system. III. Scaling maximum aerobic capacity to body mass: wild and domestic mammals. Respir Physiol 44: 25–37, 1981. [DOI] [PubMed] [Google Scholar]
- 90.Thompson LV, Brown M. Age-related changes in contractile properties of single skeletal fibers from the soleus muscle. J Appl Physiol (1985) 86: 881–886, 1999. [DOI] [PubMed] [Google Scholar]
- 91.Valberg S. Muscle anatomy, physiology and adaptations to exercise and training. In: The Athletic Horse: Principles and Practice of Equine Sports Medicine, edited by Hodgson DR, McGowan CM, and McKeever KH. St. Louis, MO: Elsevier/Saunders, 2013, p. 174–201. [Google Scholar]
- 92.van den Hoven R, Wensing T, Breukink HJ, Meijer AE, Kruip TA. Variation of fiber types in the triceps brachii, longissimus dorsi, gluteus medius, and biceps femoris of horses. Am J Vet Res 46: 939–941, 1985. [PubMed] [Google Scholar]
- 93.Villani G, Attardi G. In vivo control of respiration by cytochrome c oxidase in wild-type and mitochondrial DNA mutation-carrying human cells. Proc Natl Acad Sci U S A 94: 1166–1171, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Villani G, Greco M, Papa S, Attardi G. Low reserve of cytochrome c oxidase capacity in vivo in the respiratory chain of a variety of human cell types. J Biol Chem 273: 31829–31836, 1998. [DOI] [PubMed] [Google Scholar]
- 95.Votion DM, Fraipont A, Goachet AG, Robert C, van Erck E, Amory H, Ceusters J, de la Rebiere de Pouyade G, Franck T, Mouithys-Mickalad A, Niesten A, Serteyn D. Alterations in mitochondrial respiratory function in response to endurance training and endurance racing. Equine Vet J Suppl 38: 268–274, 2010. [DOI] [PubMed] [Google Scholar]
- 96.Votion DM, Gnaiger E, Lemieux H, Mouithys-Mickalad A, Serteyn D. Physical fitness and mitochondrial respiratory capacity in horse skeletal muscle. PLoS One 7: e34890, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wagner AL, Urschel KL, Betancourt A, Adams AA, Horohov DW. Effects of advanced age on whole-body protein synthesis and skeletal muscle mechanistic target of rapamycin signaling in horses. Am J Vet Res 74: 1433–1442, 2013. [DOI] [PubMed] [Google Scholar]
- 98.Walsh B, Tonkonogi M, Soderlund K, Hultman E, Saks V, Sahlin K. The role of phosphorylcreatine and creatine in the regulation of mitochondrial respiration in human skeletal muscle. J Physiol 537: 971–978, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Welle S, Bhatt K, Thornton CA. High-abundance mRNAs in human muscle: comparison between young and old. J Appl Physiol (1985) 89: 297–304, 2000. [DOI] [PubMed] [Google Scholar]
- 100.White SH, Warren LK, Li C, Wohlgemuth S. Mitochondrial adaptations to submaximal exercise training in the gluteus medius and triceps brachii of young equine athletes (Abstract). J Equine Vet Sci 35: 395, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wojtysiak D, Poltowicz K. Carcass quality, physico-chemical parameters, muscle fibre traits and myosin heavy chain composition of m. longissimus lumborum from Pulawska and Polish Large White pigs. Meat Sci 97: 395–403, 2014. [DOI] [PubMed] [Google Scholar]
- 102.Yarasheski KE. Exercise, aging, and muscle protein metabolism. J Gerontol A Biol Sci Med Sci 58: M918–M922, 2003. [DOI] [PubMed] [Google Scholar]