Abstract
Objective
To estimate the risk of stroke associated with new antidepressant use among older adults with traumatic brain injury (TBI).
Participants
64,214 Medicare beneficiaries aged ≥65 years meeting inclusion criteria and hospitalized with a TBI during 2006 to 2010.
Design
New user design. Generalized estimating equations were used to estimate the relative risks (RR) of stroke.
Main Measures
Primary exposure was new antidepressant use following TBI identified through Medicare Part D claims. The primary outcome was stroke following TBI. Ischemic and hemorrhagic stroke were secondary outcomes.
Results
20,859 (32%) beneficiaries used an antidepressant at least once following TBI. Selective serotonin reuptake inhibitors (SSRIs) accounted for the majority of antidepressant use. SSRI use was associated with an increased risk of hemorrhagic stroke (relative risk (RR)= 1.26; 95% confidence interval (CI)= 1.06, 1.50), but not ischemic stroke (RR=1.04; 95% CI= 0.94, 1.15). The SSRIs escitalopram (RR 1.33, 95% CI: 1.02, 1.74) and sertraline (RR=1.46; 95% CI= 1.10, 1.94) were associated with an increase in the risk of hemorrhagic stroke.
Conclusion
Findings from this study will aid prescribers in choosing appropriate antidepressants to treat depression in older adults with TBI.
Keywords: Older adults, Medicare, Traumatic Brain Injury, SSRI, Stroke
Traumatic brain injury (TBI) is a major health problem among older adults.1,2 In the United States, more than 50% of TBIs occur in adults aged 50 years and older.3 Moreover, the rates of TBI hospitalization and mortality among adults over 65 years old are greater than that of the general population.4–6
TBI is associated with an increased risk of stroke.7–9 A large study conducted in the US reported a significant increase in the hazard of stroke among patients with TBI aged 50 and older compared to trauma patients without TBI.7 A study of Medicare beneficiaries aged 65 and older compared the rates of ischemic and hemorrhagic stroke prior to and following TBI and reported hazard rates for hemorrhagic and ischemic stroke of 6.3 and 1.3, respectively.9
Among older adults, depression also is common following TBI.10–11 Selective serotonin reuptake inhibitors (SSRIs) are the most commonly prescribed pharmacological treatment for depression in patients with a TBI.12–13 However, SSRIs decrease platelet aggregation, which can increase the risk of hemorrhagic stroke.14,15 SSRIs also may increase the risk of ischemic stroke through vasoconstriction caused by serotoninergic activation.16,17 Consequently, SSRI use may amplify the risk of stroke in individuals with TBI. Thus, the objective of this study was to estimate the risk of stroke among older Medicare beneficiaries with TBI who use SSRIs. We hypothesized that SSRI use would be associated with an increased risk of stroke following TBI among older Medicare beneficiaries. A secondary objective was to assess the risk of stroke associated with other antidepressant use.
Methods
Data and Sample
This study used Medicare administrative claims data from the Centers for Medicare and Medicaid Services (CMS) Chronic Condition Data Warehouse (CCW) that included 100% of Medicare beneficiaries aged 65 years and older with an inpatient TBI claim from January 1, 2006 through December 31, 2010. TBI was defined using the International Classification of Disease, 9th Revision, Clinical Modification (ICD-9-CM) codes 800.xx, 801.xx, 803.xx, 804.xx, 850.xx–854.1x, 950.1–950.3, and 959.01 in any position. The Centers for Disease Control and Prevention uses these codes to define TBI and they have been reported to have high sensitivity and positive predictive value to detect moderate to severe TBI when compared to medical records.18,19 Any TBI occurring within 14 days of the previous TBI was collapsed as a single TBI event.
We used a nested-cohort study with a new user design to assess the risk of stroke associated with new use of antidepressants among Medicare beneficiaries hospitalized with TBI. In a nested cohort study, all study participants have an initial exposure, in this case TBI, and then a second exposure is examined, in this case new antidepressant use. The new user study design created a wash-out period during which there could be no antidepressant use. This ensured that all antidepressant users were ‘new’ and helped in reducing prevalent user bias. Prevalent user bias occurs when inclusion of prevalent users biases study results by underestimating early adverse events related to the study drug(s).20 New use of antidepressants was defined as any use after a six month washout period.
We required at least six months of Medicare Parts A, B, and D coverage prior to TBI. We also continuous Medicare parts A, B, and D coverage following TBI. Beneficiaries with any antidepressant use six months prior to their TBI were excluded. Beneficiaries also were excluded if they did not survive the TBI hospital stay and if they had Medicare Part C (Medicare Advantage) coverage because their administrative claims aren’t consistently available.
Exposure
The exposure of interest for this study was SSRI use following TBI. SSRI use was ascertained per 30-day period before and after the TBI hospitalization by searching Medicare Part D claims. Prescriptions filled and proportion of days covered (PDC) information was used to determine SSRI use per period. PDC was calculated as the number of days a drug was available divided by the number of days in a period. Beneficiaries who had an SSRI prescription filled during a 30-day period or had a PDC greater than 0 were classified as SSRI users for that period. Both prescriptions filled and PDC information were used to determine SSRI use. This was because some prescriptions may be written for multiple months; therefore, while there may not have been a prescription filled every month, there could be a PDC > 0 for the month. Medicare beneficiaries with a skilled nursing facility (SNF) stay following TBI discharge had missing information for SSRI use up to the first 100 days of a SNF stay as medication information could not be ascertained.
Similarly, we assessed use of other antidepressants, including serotonin norepinephrine reuptake inhibitors (SNRIs), tricyclic antidepressants (TCAs), tetracyclic antidepressants (TetCAs), phenylpiperazine antidepressants (PPAs), and monoamine oxidase inhibitors (MAOIs) (Table 1). A small proportion (ranging from 2–5% over the course of study follow-up) of beneficiaries who had concomitant use of two or more different antidepressant classes were excluded from analysis because the class effects of antidepressants could not be distinguished.
Table 1.
List of antidepressants by class
| Class | Antidepressant |
|---|---|
| SSRI | Citalopram |
| Escitalopram | |
| Fluvoxamine | |
| Fluoxetine | |
| Paroxetine | |
| Sertraline | |
| SNRI | Duloxetine |
| Desvenlafaxine | |
| Venlafaxine | |
| TCA | Amitriptyline |
| Amoxapine | |
| Clomipramine | |
| Desipramine | |
| Doxepin | |
| Imipramine | |
| Nortriptyline | |
| Protiptyline | |
| Trimipramine | |
| TetCA | Maprotiline |
| Mirtazapine | |
| PPA | Nefazadone |
| Trazadone | |
| MAOI | Isocarboxazid |
| Pargyline | |
| Phenelzine | |
| Selegiline |
Abbreviations: SSRI, selective serotonin reuptake inhibitor; SNRI, serotonin norepinephrine reuptake inhibitor; TCA, tricyclic antidepressant; TetCA, tetracyclic antidepressant; PPA, phenylpiperazine antidepressants; MAOI, monoamine oxidase inhibitor
Outcome
The outcome of interest for the study was stroke. Stroke was defined by inpatient claims using ICD-9-CM codes 430.xx–432.xx (hemorrhagic stroke) and 433.xx, 434.xx, 435.xx, 437.0x, 437.1x (ischemic stroke) in any position. These codes have been used in prior Medicare claims analysis and have shown to accurately detect stroke events.21,22 We also examined the risk of hemorrhagic and ischemic stroke separately.
Covariates
Demographic characteristics included age, sex, and race. The CCW identifies chronic conditions through Medicare claims using ICD-9-CM based algorithms.23 CCW data identifies the date of first diagnosis of a condition going back to 1999 and contains annual flags indicating that the algorithm was met for the condition.24 Beneficiaries were categorized as having a chronic condition at baseline if the date of first diagnosis of the condition was prior to TBI. Other conditions that may be associated with stroke were identified by searching inpatient claims data for the following ICD-9-CM codes: 286.x, 287.x (coagulation defect); 291.x, 303.x, 305.0x, 571.0–571.3 (alcohol abuse/dependence); 394.x–397.x, 398.9x, 398.9x, V42.2, V43.3 (valvular heart disease (VHD)), 571.2x, 571.5x, 571.6x, 070.0x, 070.2x, 070.4x, 070.6x, 070.71, 348.3x, 456.0x–456.2x, 572.2x–572.4x, 782.4x, 789.59, 155.x, V42.7, 50.5, 471.35, 471.36 (chronic liver disease); and 332.x, 340.x, 342–344, 436.xx (neurological disease). Prior hemorrhagic and ischemic stroke, identified by inpatient claims, were included as covariates. Warfarin use in the period was included as anticoagulants are associated with stroke and warfarin is the most commonly prescribed anticoagulant in the US.21,25,26 Length of hospital stay and discharge to a SNF were included as covariates as they may be indicative of TBI severity.21
Depression is an important confounder because it has been independently linked to an increased risk of stroke and is highly correlated with antidepressant use.27–29 We used the CCW date of first diagnosis of depression in conjunction with annual flags to create two depression variables. We created an indicator variable for history of depression prior to TBI using the first ever diagnosis of depression flag and a second variable indicating recently diagnosed depression following TBI using the annual depression flags.
Statistical Analysis
Associations between antidepressant use and demographic variables were tested using Chi-square and Student’s t-tests. Trends in antidepressant use throughout the study period were assessed using the Cochran trend test.
Discrete time analysis was used to quantify the risk of stroke associated with antidepressant use. Discrete time analysis is a type of survival analysis that can be used with longitudinal data to assess the impact of time-varying exposures and covariates.30 The risk of all stroke, ischemic stroke, and hemorrhagic stroke per period following TBI as a function of SSRI use were modelled using generalized estimating equations (GEE) with a binomial distribution and complimentary log-log link. GEE models the correlation between multiple observations per Medicare beneficiary and can be used with longitudinal data.31 SSRI use was lagged by one period to ensure antidepressant exposure preceded the outcome. Thus, the risk of stroke was modelled based on exposure to SSRIs in the preceding period. Beneficiaries were censored after experiencing the event, at death, or the end of the study period. Potential covariates and confounders identified in literature and bivariate analysis were included in the adjusted models. We also modelled the risk of stroke as a function of other antidepressant class use. Relative risks (RR) and 95% confidence intervals (CI) were reported.
To better understand stroke risk associated with SSRI use, we examined the risk of ischemic and hemorrhagic stroke associated with individual SSRIs using discrete time analysis and lagging use by one period.
Sensitivity analysis was conducted by incorporating inverse probability treatment weights (IPTWs). IPTWs were used to control for selection bias due to the non-random assignment of treatment.32 Separate IPTWs were constructed for ischemic stroke, hemorrhagic stroke and any stroke by first modeling antidepressant use as a function of risk factors associated with ischemic stroke, hemorrhagic stroke and any stroke, respectively, and then calculating the inverse probability of antidepressant treatment. The weights were normalized prior to their incorporation in the models.
All analyses were performed with SAS version 9.2 (Cary, NC). This study was approved by the University of Maryland Baltimore’s IRB. Statistical significance was set at p < 0.001.
Results
A total of 105,315 Medicare beneficiaries meeting inclusion criteria were hospitalized for a TBI during 2006 to 2010. The study sample consisted of 64,214 beneficiaries with no antidepressant use during the six months prior to TBI. Among these, 43,355 (68%) did not use antidepressants following TBI (unexposed) and 20,859 (32%) used an antidepressant (exposed) at least once following TBI (Table 2).
Table 2.
Characteristics of older Medicare beneficiaries hospitalized for traumatic brain injury by new antidepressant use status post-injury (n=64,214)
| Antidepressant Used | ||||
|---|---|---|---|---|
| Characteristics | Total Sample (n=64,214) |
No Use (n=43,355) |
Any Use (n=20,859) |
P-valuee |
| Mean age (SD) | 82.8 (8.0) | 82.8 (8.1) | 82.8 (7.9) | 0.480 |
| Sex, n (%) | <0.001 | |||
| Male | 25,881 (40.2) | 18,291 (42.2) | 7,520 (36.1) | |
| Female | 38,403 (59.8) | 25,064 (58.8) | 13,339 (63.9) | |
| Race, n (%) | <0.001 | |||
| White | 54,228 (84.5) | 36,242 (83.6) | 17,986 (86.2) | |
| Black | 4,572 (7.1) | 3,315 (7.6) | 1,257 (6.0) | |
| Hispanic | 2,146 (3.3) | 1,600 (3.7) | 546 (2.6) | |
| Asian | 1,885 (2.9) | 1,191 (2.8) | 694 (3.3) | |
| Othera | 1,383 (2.2) | 1,007 (2.3) | 376 (1.8) | |
| Warfarin use month of TBI, n (%) | 15,934 (24.8) | 10,354 (23.9) | 5,580 (26.8) | <0.001 |
| Chronic conditions, n (%)b | ||||
| Depressionc | 15,874 (24.7) | 7,468 (17.2) | 8,406 (40.3) | <0.001 |
| CHF | 29,956 (46.7) | 19,600 (45.2) | 10,356 (49.7) | <0.001 |
| Hypertension | 56,826 (88.5) | 37,949 (87.6) | 18,877 (90.6) | <0.001 |
| History of AMI | 5,194 (8.1) | 3,468 (8.0) | 1,726 (8.3) | 0.230 |
| Diabetes | 25,731 (40.1) | 16,875 (38.9) | 8,856 (42.5) | <0.001 |
| Atrial fibrillation | 17,486 (27.2) | 11,697 (27.0) | 5,789 (27.8) | 0.039 |
| Coagulation defect | 5,528 (8.6) | 3,718 (8.6) | 1,810 (8.7) | 0.667 |
| Alcohol abuse/dependence | 1,609 (2.5) | 966 (2.2) | 643 (3.1) | <0.001 |
| Valvular heart disease | 5,344 (8.3) | 3,509 (8.1) | 1,835 (8.8) | 0.003 |
| Liver disease | 3,051 (4.8) | 1,962 (4.5) | 1,089 (5.2) | <0.001 |
| Chronic liver disease | 3,051 (4.8) | 1,962 (4.5) | 1,089 (5.2) | <0.001 |
| ADRD | 19,332 (30.1) | 11,817 (27.3) | 7,515 (36.0) | <0.001 |
| Length of hospital stay | <0.001 | |||
| 0–2 days | 16,239 (25.3) | 11,667 (26.9) | 4,572 (21.9) | |
| 3–5 days | 22,680 (35.3) | 15,260 (35.2) | 7,420 (35.6) | |
| 6–8 days | 9,602 (15.0) | 6,375 (14.7) | 3,227 (15.5) | |
| ≥ 9 days | 15, 693 (24.4) | 10,053 (23.2) | 5,640 (27.0) | |
| Mean days follow up post TBI (SD) | 653.3 (418.6) | 621.5 (426.5) | 719.5 (393.7) | <0.001 |
| Discharged to SNF, n (%) | 23,290 (36.3) | 14,183 (32.7) | 9,107 (43.7) | <0.001 |
| Previous hemorrhagic event, n (%) | 759 (1.2) | 493 (1.1) | 266 (1.3) | 0.285 |
| Previous ischemic event, n (%) | 5,496 (8.6) | 3,391 (7.8) | 2,105 (10.1) | <0.001 |
Other races include Native American, other and unknown race
Chronic conditions present at time of traumatic brain injury
History of depression prior to injury
Beneficiaries were categorized as non-users if they did not use antidepressants at any time during follow-up, while beneficiaries were categorized as users if they had any antidepressant use at any time during follow-up
P-value from chi-square for categorical variables and Student’s t-test for mean age and reflects comparison between antidepressant users and non-users.
Abbreviations: TBI, traumatic brain injury; SD, standard deviation; SNF, skilled nursing facility; CHF, congestive heart failure; AMI, acute myocardial infarction; ADRD, Alzheimer’s disease and related dementias
The mean age of the study population was 83 years (standard deviation 8.0) and the sample was predominantly white (85%) and female (60%) (Table 2). Compared to non-users, antidepressant users were significantly more likely to have hypertension and atrial fibrillation, conditions that are associated with stroke.33,34 Antidepressant users also were more likely to have a history of depression at the time of TBI than non-users (40% vs 17% of non-users, p<0.001). Additionally, antidepressant users were more likely to have had a prior ischemic stroke (10% vs. 8% of non-users, p<0.001). Antidepressant users were more likely have a longer length of TBI hospital stay (27% of antidepressant users had a stay longer than nine days versus to 23% of non-users, p<0.001). Antidepressant users also were more likely to be discharged to a SNF (44% vs. 33%, p<0.001) (Table 2).
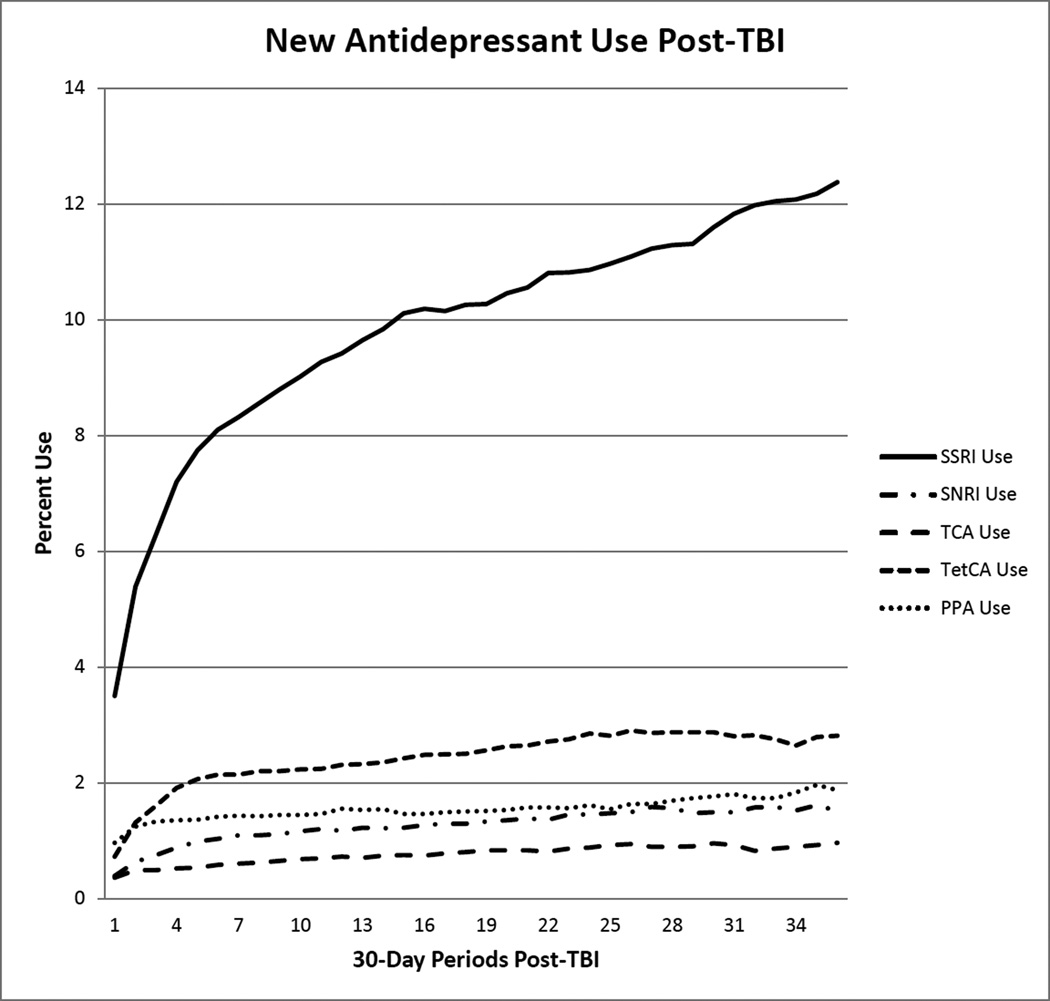
Figure 1 displays the trends in antidepressant use among all beneficiaries during the follow-up period. Throughout the study period, SSRIs were the most commonly used antidepressant, while TCAs were the least commonly used. SSRI use steadily increased during the follow up (p<0.001). SNRI, TCA, TetCA, and PPA use also increased throughout the study period (p<0.001). We did not the measure time trends or assess the risk of stroke associated with use of monoamine oxidase inhibitors (MAOIs) due to small numbers of users.
Figure 1.
Antidepressant use per month following TBI among older Medicare beneficiaries, n= 64,214
Abbreviations: TBI, traumatic brain injury; SSRI, selective serotonin reuptake inhibitor; SNRI, serotonin norepinephrine reuptake inhibitor; TCA, tricyclic antidepressant; TetCA, tetracyclic antidepressant; PPA, phenylpiperazine antidepressants
Among antidepressant users, 949 (4.6%) had a hemorrhagic stroke and 2,413 (11.7%) had an ischemic stroke. Among those who did not use antidepressants, 1,448 (3.4%) had a hemorrhagic stroke and 3,121 (7.5%) had an ischemic stroke.
Multivariable Models
All adjusted generalized estimating equation models included age, sex, race, discharge to SNF, length of hospital stay, warfarin use in period, depression (history of and recently diagnosed), atrial fibrillation, Alzheimer’s disease and related dementias (ADRD), and hypertension. The ischemic stroke model also included hyperlipidemia, diabetes, ischemic heart disease, congestive heart failure, and prior ischemic stroke; while the hemorrhagic stroke model also included liver disease, chronic kidney disease, neurological disease, alcohol abuse, anemia, coagulation defect, valvular heart disease and prior hemorrhagic stroke. The composite stroke model included all variables from both ischemic and hemorrhagic stroke models.
In our adjusted models, new use of SNRIs (RR 1.37; 95% CI 1.10, 1.72) and PPAs (RR 1.33; 95% CI 1.07, 1.66) following TBI was associated with increased risk of our composite stroke variable (Table 3). No other antidepressants were significantly associated with increased risk of stroke.
Table 3.
Relative Risk (95% CI) of all stroke, ischemic stroke, and hemorrhagic stroke among older Medicarebeneficiaries with TBI
| All Stroke | ||
| Antidepressant Usea | Unadjusted | Adjustedb |
| No Use | Reference | Reference |
| SSRI Use (n=13,827) | 1.27 (1.16, 1.39) | 1.07 (0.98, 1.18) |
| SNRI Use (n=1,690) | 1.62 (1.30, 2.02) | 1.37 (1.10, 1.72) |
| TCA Use (n=1,460) | 1.15 (0.82, 1.61) | 1.13 (0.81, 1.59) |
| TetCA Use (n=3,649) | 1.16 (0.96, 1.41) | 0.96 (0.79, 1.17) |
| PPA Use (n=2,738) | 1.48 (1.18, 1.84) | 1.33 (1.07, 1.66) |
| Ischemic Stroke | ||
| Antidepressant Usea | Unadjusted | Adjustedb |
| No Use | Reference | Reference |
| SSRI Use (n=13,827) | 1.29 (1.17, 1.42) | 1.04 (0.94, 1.15) |
| SNRI Use (n=1,690) | 1.70 (1.34, 2.16) | 1.36 (1.06, 1.73) |
| TCA Use (n=1,460) | 1.14 (0.78, 1.67) | 1.11 (0.76, 1.63) |
| TetCA Use (n=3,649) | 1.23 (0.99, 1.52) | 0.95 (0.77, 1.18) |
| PPA Use (n=2,738) | 1.48 (1.15, 1.90) | 1.31 (1.02, 1.68) |
| Hemorrhagic Stroke | ||
| Antidepressant Usea | Unadjusted | Adjustedb |
| No Use | Reference | Reference |
| SSRI Use (n=13,827) | 1.33 (1.13, 1.57) | 1.26 (1.06, 1.50) |
| SNRI Use (n=1,690) | 1.20 (0.73, 1.96) | 1.19 (0.72, 1.95) |
| TCA Use (n=1,460) | 1.09 (0.57, 2.10) | 1.11 (0.58, 2.14) |
| TetCA Use (n=3,649) | 0.91 (0.60, 1.38) | 0.84 (0.55, 1.27) |
| PPA Use (n=2,738) | 1.44 (0.96, 2.18) | 1.30 (0.86, 1.97) |
Numbers associated with specific drug classes reflect that total number of beneficiaries that had a fill for the specific drug class at any time following TBI
All models adjusted for: age, race, sex, warfarin use in period, length of hospital stay, discharge to a skilled nursing facility, history of depression, incident depression, atrial fibrillation, Alzheimer’s disease and related dementias, hypertension. Ischemic stroke model also included hyperlipidemia, ischemic heart disease, congestive heart failure, prior ischemic stroke, and diabetes. Hemorrhagic stroke model also included liver disease, chronic kidney disease, alcohol abuse, anemia, coagulation defect, valvular heart disease, neurological disease, prior hem stroke. All stroke model adjusted for all variables in the ischemic and hemorrhagic stroke models
Abbreviations: TBI, traumatic brain injury; CI, confidence interval; SSRI, selective serotonin reuptake inhibitor; SNRI, serotonin norepinephrine reuptake inhibitor; TCA, tricyclic antidepressant; TetCA, tetracyclic antidepressant; PPA, phenylpiperazine antidepressants
Similarly, new use of SNRIs (RR 1.36; 95% CI 1.06, 1.73) and PPAs (RR 1.31; 95% CI 1.02, 1.68) following TBI was associated with increased risk of ischemic stroke (Table 3). No other antidepressants were significantly associated with increased risk of ischemic stroke.
New use of SSRIs (RR 1.26; 95% CI 1.06, 1.50) following TBI was associated with an increase in the risk of hemorrhagic stroke (Table 3). No other antidepressant drug class was significantly associated with an increased risk of hemorrhagic stroke. Among individual SSRIs, use of escitalopram (RR=1.33; 95% CI= 1.02, 1.74) and sertraline (RR= 1.46; 95% CI= 1.10, 1.94) following TBI was associated with increased risk of hemorrhagic stroke (Table 4).
Table 4.
Relative Risk (95% CI) of ischemic stroke by SSRI use and hemorrhagic stroke by SSRI among older Medicare beneficiaries with TBI
| Ischemic Stroke | ||
| SSRI Usea | Unadjusted | Adjustedb |
| No Use | Reference | Reference |
| Citalopram (n=4,047) | 1.21 (1.02, 1.43) | 0.95 (0.79, 1.13) |
| Escitalopram (n=3,612) | 1.38 (1.17, 1.64) | 1.07 (0.91, 1.28) |
| Fluvoxamine (n=18) | N/Ac | N/Ac |
| Fluoxetine (n=918) | 1.51 (1.08, 2.11) | 1.27 (0.91, 1.77) |
| Paroxetine (n=1,077) | 1.01 (0.70, 1.45) | 0.87 (0.61, 1.26) |
| Sertraline (n=3,239) | 1.25 (1.04, 1.51) | 1.02 (0.84, 1.23) |
| Hemorrhagic Stroke | ||
| SSRI Usea | Unadjusted | Adjustedb |
| No Use | Reference | Reference |
| Citalopram (n=4,047) | 1.33 (1.02, 1.75) | 1.23 (0.93, 1.63) |
| Escitalopram (n=3,612) | 1.47 (1.13, 1.92) | 1.33 (1.02, 1.74) |
| Fluvoxamine (n=18) | N/Ac | N/Ac |
| Fluoxetine (n=918) | 1.42 (0.81, 2.51) | 1.40 (0.79, 2.48) |
| Paroxetine (n=1,077) | 1.45 (0.87, 2.41) | 1.47 (0.88, 2.44) |
| Sertraline (n=3,239) | 1.54 (1.17, 2.03) | 1.46 (1.10, 1.94) |
Numbers associated with specific drugs reflect that total number of beneficiaries that had a fill for the specific drug at any time following TBI.
All models adjusted for: age, race, sex, warfarin use in period, length of hospital stay, discharge to a skilled nursing facility, history of depression, incident depression, atrial fibrillation, Alzheimer’s disease and related dementias, hypertension. Ischemic stroke model also included hyperlipidemia, ischemic heart disease, congestive heart failure, prior ischemic stroke, and diabetes. Hemorrhagic stroke model also included liver disease, chronic kidney disease, alcohol abuse, anemia, coagulation defect, valvular heart disease, neurological disease, prior hem stroke
N/A—estimate is too small and unreliable.
Abbreviations: TBI, traumatic brain injury; CI, confidence interval; SSRI, selective serotonin reuptake inhibitor
In sensitivity analysis incorporating IPTW, PPA use increased the risk of hemorrhagic stroke (RR= 1.40; 95% CI= 1.02, 1.93). All other associations remained unchanged.
Discussion
In this national sample of older Medicare beneficiaries, one-third started new use of antidepressants following hospitalization for TBI, highlighting the need for information on the safety of antidepressants in this population. SSRI use was associated with an increased risk of hemorrhagic, but not ischemic, stroke. Specifically, the SSRIs escitalopram and sertraline were associated with increased the risk of hemorrhagic stroke. One possible explanation for their heightened risk for stroke is that both escitalopram and sertraline decrease platelet aggregation and coagulation, increasing the risk of hemorrhage.14
Similar to Sertraline, use of both fluoxetine and paroxetine was associated with a 40% increase in risk of hemorrhagic stroke; however, these associations were not statistically significant. Throughout the study follow-up, 918 beneficiaries received fluoxetine and 1,077 beneficiaries received paroxetine. Because hemorrhagic stroke is a rare outcome, our study may have been underpowered to test the associations of these SSRIs with increased risk of hemorrhagic stroke.
Prior studies assessing the risk of stroke associated with SSRIs in non-TBI populations have reported contradictory results.35–38 Two case-control studies did not find that SSRI use was associated with hemorrhagic stroke; however, those studies were not limited to the older adults.35,36 Two cohort studies among older adults reported that SSRI use was associated with stroke.37,38 No study has assessed the risk of stroke among older TBI patients who use SSRIs.
SNRI and PPA use were associated with increased risk of ischemic stroke. This may be related to their low affinity towards serotonin transporters.16,39,40 Antidepressants with low affinity towards serotonin transporters may increase platelet aggregation, which can potentially increase the risk of ischemic stroke.40 Additional analysis examining individual SNRIs and PPAs was not possible due to the small number of beneficiaries taking these antidepressants. Less than two percent of the study population was prescribed SNRIs and PPAs, suggesting that while SNRIs and PPAs may increase the risk of stroke, they are rarely prescribed in older adults with TBI.
SSRI use was not associated with an increased risk of ischemic stroke. This is important because the absolute risk of ischemic stroke is three and a half times that of hemorrhagic stroke among older adults with TBI.9 In this study, there were twice as many ischemic stroke events than hemorrhagic stroke events, making SSRIs a safer option for the treatment of depression for older patients with TBI compared to other antidepressant classes.
This study was limited by a lack of TBI severity information. Beneficiaries with severe TBI may be at a higher risk of stroke than those with less severe TBI. We controlled for length of hospital stay and discharge to SNF, possible proxy measures for TBI severity, in our regression models to address this limitation. This approach has been previously used in Medicare claims data.21
Strengths of this study include a large national sample of older Medicare beneficiaries that permitted examination of the impact of individual SSRIs on the rare outcome of hemorrhagic stroke. Moreover, the nested-cohort, new user study design helped reduce bias. The nested cohort design decreases selection bias as all exposure groups suffered a TBI, while the new user aspect creates a wash-out period for antidepressant users and reduces prevalent user bias.41 Including prevalent users in a pharmacoepidemiology study may bias the results by underestimating early adverse events related to the study drug.20
In conclusion, the SSRIs escitalopram and sertraline were associated with an increased risk of hemorrhagic stroke among older Medicare beneficiaries with TBI. Citalopram, fluoxetine and paroxetine were not associated with increased risk of stroke and may present a safer alternative among SSRIs. Findings from this study will aid prescribers in choosing appropriate antidepressants for the treatment of depression among older adults with TBI. These findings suggest that clinicians should consider multiple factors, such as risk of stroke, when prescribing certain antidepressants to older adults with TBI.
Acknowledgments
Source of Funding: Mr. Khokhar was supported by National Institutes of Health grant T32AG000262-14 (Magaziner, PI). Dr. Albrecht was supported by National Institutes of Health grant K12HD43489-13. (Tracey, PI)
Footnotes
Conflicts of Interest:
The authors declare no conflicts of interest.
References
- 1.Coronado VG, McGuire LC, Sarmiento K, et al. Trends in traumatic brain injury in the US and the public health response: 1995–2009. J Safety Res. 2012;43(4):299–307. doi: 10.1016/j.jsr.2012.08.011. [DOI] [PubMed] [Google Scholar]
- 2.Thompson HJ, Dikmen S, Temkin N. Prevalence of comorbidity and its association with traumatic brain injury and outcomes in older adults. Res Gerontol Nurs. 2012;5(1):17. doi: 10.3928/19404921-20111206-02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cuthbert JP, Harrison-Felix C, Corrigan JD, et al. Epidemiology of adults receiving acute inpatient rehabilitation for a primary diagnosis of traumatic brain injury in the United States. J Head Trauma Rehabil. 2015;30(2):122–135. doi: 10.1097/HTR.0000000000000012. [DOI] [PubMed] [Google Scholar]
- 4.Thompson HJ, McCormick WC, Kagan SH. Traumatic brain injury in older adults: epidemiology, outcomes, and future implications. J Am Geriatr Soc. 2006;54(10):1590–1595. doi: 10.1111/j.1532-5415.2006.00894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coronado VG, Xu L, Basavaraju SV, et al. Surveillance for traumatic brain injury-related deaths: United States, 1997–2007. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention; 2011. [Google Scholar]
- 6.Coronado VG, Thomas KE, Sattin RW, Johnson RL. The CDC traumatic brain injury surveillance system: characteristics of persons aged 65 years and older hospitalized with a TBI. J Head Trauma Rehabil. 2005;20(3):215–228. doi: 10.1097/00001199-200505000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Burke JF, Stulc JL, Skolarus LE, Sears ED, Zahuranec DB, Morgenstern LB. Traumatic brain injury may be an independent risk factor for stroke. Neurology. 2013;81(1):33–39. doi: 10.1212/WNL.0b013e318297eecf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen Y-H, Kang J-H, Lin H-C. Patients With Traumatic Brain Injury Population-Based Study Suggests Increased Risk of Stroke. Stroke. 2011;42(10):2733–2739. doi: 10.1161/STROKEAHA.111.620112. [DOI] [PubMed] [Google Scholar]
- 9.Albrecht JS, Liu X, Smith GS, et al. Stroke Incidence Following Traumatic Brain Injury in Older Adults. J Head Trauma Rehabil. 2015;30(2):E62–E67. doi: 10.1097/HTR.0000000000000035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Menzel JC. Depression in the elderly after traumatic brain injury: a systematic review. Brain Inj. 2008;22(5):375–380. doi: 10.1080/02699050802001492. [DOI] [PubMed] [Google Scholar]
- 11.Albrecht JS, Kiptanui Z, Tsang Y, et al. Patterns of Depression Treatment in Medicare Beneficiaries with Depression after Traumatic Brain Injury. J Neurotrauma. 2015;32(16):1223–1229. doi: 10.1089/neu.2014.3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fann JR, Hart T, Schomer KG. Treatment for depression after traumatic brain injury: a systematic review. J Neurotrauma. 2009;26(12):2383–2402. doi: 10.1089/neu.2009.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Warden DL, Gordon B, McAllister TW, et al. Guidelines for the pharmacologic treatment of neurobehavioral sequelae of traumatic brain injury. J Neurotrauma. 2006;23(10):1468–1501. doi: 10.1089/neu.2006.23.1468. [DOI] [PubMed] [Google Scholar]
- 14.de Abajo FJ. Effects of Selective Serotonin Reuptake Inhibitors on Platelet Function. Drugs Aging. 2011;28(5):345–367. doi: 10.2165/11589340-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 15.Zagaria M. Bleeding risk with SSRIs: surgery and seniors. US Pharm. 2013;38(11):20–22. [Google Scholar]
- 16.Trifirò G, Dieleman J, Sen EF, Gambassi G, Sturkenboom MC. Risk of ischemic stroke associated with antidepressant drug use in elderly persons. J Clin Psychopharmacol. 2010;30(3):252–258. doi: 10.1097/JCP.0b013e3181dca10a. [DOI] [PubMed] [Google Scholar]
- 17.Gartlehner G, Hansen R, Reichenpfader U, Kaminski A. Drug Class Review: Second-Generation Antidepressants: Final Update 5 Report. Portland (OR): Oregon Health & Science University; 2011. ( http://www.ncbi.nlm.nih.gov/books/NBK54355/) [PubMed] [Google Scholar]
- 18.Carroll CP, Cochran JA, Guse CE, Wang MC. Are we underestimating the burden of traumatic brain injury? Surveillance of severe traumatic brain injury using centers for disease control International classification of disease, ninth revision, clinical modification, traumatic brain injury codes. Neurosurgery. 2012;71(6):1064–1070. doi: 10.1227/NEU.0b013e31826f7c16. [DOI] [PubMed] [Google Scholar]
- 19.Marr AL, Coronado VG. Central nervous system injury surveillance data submission standards—2002. Atlanta, GA: Centers for Disease Control and Prevention, National Center for Injury Prevention and Control; 2004. [Google Scholar]
- 20.Ray WA. Evaluating medication effects outside of clinical trials: new-user designs. Am J Epidemiol. 2003;158(9):915–920. doi: 10.1093/aje/kwg231. [DOI] [PubMed] [Google Scholar]
- 21.Albrecht JS, Liu X, Baumgarten M, et al. Benefits and risks of anticoagulation resumption following traumatic brain injury. JAMA Intern Med. 2014;174(8):1244–1251. doi: 10.1001/jamainternmed.2014.2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arnason T, Wells P, Van Walraven C, Forster A. Accuracy of coding for possible warfarin complications in hospital discharge abstracts. Thromb Res. 2006;118(2):253–262. doi: 10.1016/j.thromres.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 23.Centers For Medicare and Medicaid Services Chronic Condition Data Warehouse. Medicare Administrative Data User Guide. 2014 https://www.ccwdata.org/web/guest/user-documentation.
- 24.Centers For Medicare and Medicaid Services Chronic Condition Data Warehouse. Condition Categories. [Accessed June 15, 2014]; http://www.ccwdata.org/web/guest/condition-categories.
- 25.Teles JS, Fukuda EY, Feder D. Warfarin: pharmacological profile and drug interactions with antidepressants. Einstein (Sao Paulo) 2012;10(1):110–115. doi: 10.1590/s1679-45082012000100024. [DOI] [PubMed] [Google Scholar]
- 26.Sayal K, Duncan-McConnell D, McConnell H, Taylor D. Psychotropic interactions with warfarin. Acta Psychiatr Scand. 2000;102(4):250–255. doi: 10.1034/j.1600-0447.2000.102004250.x. [DOI] [PubMed] [Google Scholar]
- 27.Henderson KM, Clark CJ, Lewis TT, et al. Psychosocial distress and stroke risk in older adults. Stroke. 2013;44(2):367–372. doi: 10.1161/STROKEAHA.112.679159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pan A, Sun Q, Okereke OI, Rexrode KM, Hu FB. Depression and risk of stroke morbidity and mortality: a meta-analysis and systematic review. JAMA. 2011;306(11):1241–1249. doi: 10.1001/jama.2011.1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Whooley MA. Depression and cardiovascular disease: healing the broken-hearted. JAMA. 2006;295(24):2874–2881. doi: 10.1001/jama.295.24.2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Allison PD. Discrete-time methods for the analysis of event histories. Sociol Methodol. 1982;13(1):61–98. [Google Scholar]
- 31.Zeger SL, Liang K-Y, Albert PS. Models for longitudinal data: a generalized estimating equation approach. Biometrics. 1988:1049–1060. [PubMed] [Google Scholar]
- 32.Robins JM, Hernan MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000;11(5):550–560. doi: 10.1097/00001648-200009000-00011. [DOI] [PubMed] [Google Scholar]
- 33.Greenlund KJ, Croft JB, Mensah GA. Prevalence of heart disease and stroke risk factors in persons with prehypertension in the United States, 1999–2000. Arch Intern Med. 2004;164(19):2113–2118. doi: 10.1001/archinte.164.19.2113. [DOI] [PubMed] [Google Scholar]
- 34.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22(8):983–988. doi: 10.1161/01.str.22.8.983. [DOI] [PubMed] [Google Scholar]
- 35.Bak S, Tsiropoulos I, Kjærsgaard JO, et al. Selective Serotonin Reuptake Inhibitors and the Risk of Stroke A Population-Based Case-Control Study. Stroke. 2002;33(6):1465–1473. doi: 10.1161/01.str.0000018589.56991.ba. [DOI] [PubMed] [Google Scholar]
- 36.Kharofa J, Sekar P, Haverbusch M, et al. Selective serotonin reuptake inhibitors and risk of hemorrhagic stroke. Stroke. 2007;38(11):3049–3051. doi: 10.1161/STROKEAHA.107.491472. [DOI] [PubMed] [Google Scholar]
- 37.Coupland C, Dhiman P, Morriss R, Arthur A, Barton G, Hippisley-Cox J. Antidepressant use and risk of adverse outcomes in older people: population based cohort study. BMJ. 2011;343 doi: 10.1136/bmj.d4551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hung C-C, Lin C-H, Lan T-H, Chan C-H. The association of selective serotonin reuptake inhibitors use and stroke in geriatric population. Am J Geriatr Psychiatry. 2013;21(8):811–815. doi: 10.1016/j.jagp.2013.01.018. [DOI] [PubMed] [Google Scholar]
- 39.Wu C-S, Wang S-C, Cheng Y-C, Gau SS-F. Association of cerebrovascular events with antidepressant use: a case-crossover study. Am J Psychiatry. 2011;168(5):511–521. doi: 10.1176/appi.ajp.2010.10071064. [DOI] [PubMed] [Google Scholar]
- 40.Sauer WH, Berlin JA, Kimmel SE. Effect of antidepressants and their relative affinity for the serotonin transporter on the risk of myocardial infarction. Circulation. 2003;108(1):32–36. doi: 10.1161/01.CIR.0000079172.43229.CD. [DOI] [PubMed] [Google Scholar]
- 41.Schneeweiss S, Patrick AR, Stürmer T, et al. Increasing levels of restriction in pharmacoepidemiologic database studies of elderly and comparison with randomized trial results. Med Care. 2007;45(10 SUPL):S131. doi: 10.1097/MLR.0b013e318070c08e. [DOI] [PMC free article] [PubMed] [Google Scholar]