Abstract
Neutrophils play a major role in inflammation by releasing large amounts of reactive oxygen species (ROS) produced by NADPH oxidase (NOX) and myeloperoxidase (MPO). This ROS overproduction is mediated by phosphorylation of the NOX subunits with an uncontrolled manner. Therefore, targeting neutrophil subunits would represent a promising strategy to moderate NOX activity, lower ROS, and other inflammatory agents, such as cytokines and leukotrienes, produced by neutrophils. For this purpose, we investigated the effects of protectin DX (PDX) - a docosahexaenoic acid (DHA) di-hydroxylated product which inhibits blood platelet aggregation - on neutrophil activation in vitro. We found that PDX decreases ROS production, inhibits NOX activation and MPO release from neutrophils. We also confirm, that PDX is an anti-aggregatory and anti-inflammatory agent by inhibiting both cyclooxygenase-1 and -2 (COX-1 and COX-2, E.C. 1.14.99.1) as well as COX-2 in lipopolysaccharides (LPS)-treated human neutrophils. However, PDX has no effect on the 5-lipoxygenase pathway that produces the chemotactic agent leukotriene B4 (LTB4). Taken together, our results suggest that PDX could be a protective agent against neutrophil invasion in chronic inflammatory diseases.
Keywords: Cyclooxygenase 1; Cyclooxygenase 2; N-Formylmethionine Leucyl-Phenylalanine; NADPH Oxidase; Neutrophils; Peroxidase; Phosphorylation; Prostaglandins; Reactive Oxygen Species; Serine; Superoxides; Tetradecanoylphorbol Acetate; Cyclooxygenase Inhibitors; Time Factors; Docosahexaenoic Acids; Dose-Response Relationship, Drug; Humans; Immunoblotting; Isomerism; Lipopolysaccharides; Lipoxygenase
Introduction
Chronic inflammation is involved in various diseases including atherosclerosis [1], bowel disease [2, 3], cancer [4], cardiovascular diseases [5], the metabolic syndrome [6], and rheumatoid arthritis [7]. They all have in common [8] excessive production of pro-inflammatory mediators such as interleukin (IL)-1, IL-6, IL-8 [9], granulocyte macrophage-colony stimulating factor (GM-CSF) [10], leukotriene B4 (LTB4), platelet-activating factor (PAF), and tumor necrosis factor-α (TNF-α) [8]. Highly activated inflammatory cells such as neutrophils, monocytes and macrophages [11], and production of reactive oxygen species (ROS) [12–15] are common features. A major source of ROS at inflammation sites is the reduction of an oxygen molecule to superoxide anion (O2−·) by neutrophil enzyme complex NADPH oxidase (NOX) [16], and hypochloric acid (HOCl) produced by myeloperoxidase (MPO) from hydrogen peroxide [13]. In physiological situations, NOX activation in neutrophils is tightly regulated to avoid tissue damage. However, a dysregulation occurs in chronic inflammatory states where NOX subunits are strongly phosphorylated and translocated to cell plasma membrane, resulting in highly active oxidase overproducing ROS [17]. Targeting NOX subunits to decrease ROS overproduction would then represent a strategy to develop new anti-inflammatory approaches. Dietary manipulation is a possible way to prevent inflammatory diseases that involve polymorphonuclear neutrophil (PMN) activation and oxidative stress. As a matter of fact, dietary long-chain n-3 polyunsaturated fatty acids (PUFA), eicosapentaenoic (EPA) and docosahexaenoic (DHA) acids, present in marine food, have been widely used. As an example, they have been shown to attenuate inflammation and neutrophil infiltration in an experimental model of colitis [18]. N-3 PUFA may be converted into active oxygenated derivatives named protectins and resolvins which may fight inflammation [19]. Moreover, conjugated double bond-containing PUFA, like conjugated linoleic (CLA) and linolenic (ClnA) acids have been reported to decrease the incidence of tumors in mice, to inhibit cell proliferation and invasion of cancer cells by promoting apoptosis [20–22]. We have previously found that punicic acid, a conjugated triene PUFA found in high percentage (70%) in pomegranate seed oil, exerts a potent anti-inflammatory effect through inhibition of TNFα-induced priming of neutrophil NOX, by targeting the p38MAPK/Ser345-p47phox axis, and by decreasing neutrophil degranulation and MPO release [23]. On the other hand, oral administration of punicic acid or pomegranate seed oil to rats inhibits trinitrobenzene sulfonic acid (TNBS)-induced colitis and decreases ROS-induced tissue damage through a down regulation of neutrophil activation [23].
Recently, our group identified new di-oxygenated derivatives from PUFA, called poxytrins, which inhibit blood platelet aggregation [24]. Among them, protectin DX (PDX) deriving from DHA [25] shares some structure similarity with other conjugated triene fatty acids, like punicic acid and oleostearic acid. PDX is an isomer of protectin/neuroprotectin D1, which is known to have anti-inflammatory properties [19, 26]. More recently, PDX (called PD1 isomer, even PD1 through the paper) has been shown to inhibit the influenza virus replication [27], which makes it a relevant molecule to fight this infectious disease. The aim of the present study was to test whether PDX may have anti-inflammatory effect by modulating neutrophil activation. A second goal was to precise the anti-inflammatory potential of PDX through cyclooxygenase inhibition. We found that PDX decreases ROS produced by neutrophils stimulated with formyl-methionyl-leucyl-phenylalanine (fMLF), and phorbol myristate acetate (PMA) in vitro, and partly inhibited neutrophil degranulation and MPO release by fMLF-triggered neutrophils. Also, PDX is an inhibitor of COX-1, as well as COX-2 which the activity is enhanced during inflammation, but fails to inhibit the 5-lipoxygenase pathway. Altogether these effects provide evidence that PDX may be an anti-inflammatory agent acting through original mechanisms.
Materials and Methods
Reagents and antibodies
PMA, fMLF, protease and phosphatase inhibitors, DHA, arachidonic acid (ARA), soybean lipoxygenase (sLOX) (E.C. 1.13.11.12, Type 1-B), COX-1 from sheep, and human recombinant COX-2 (E.C. 1.14.99.1), N,O-Bis(trimethylsilyl)-trifluoroacetamide (BSTFA) were from Sigma-Aldrich (St Quentin Fallavier, France). Endotoxin-free buffers and salt solutions were from Invitrogen/Life Technologies SAS (St Aubin, France). Anti phospho-Ser345-p47phox and anti-p47phox were from Cell Signaling/Ozyme (St Quentin Yvelines, France). Anti MPO antibody was from Abcam. Organic solvents were from Carlo-Erba. All chemicals used were reagent grade or with the highest quality available. [3H]-ARA (6.6 × 109 Bq/mM) was from Perkin Elmer, USA.
PDX synthesis
PDX was prepared as previously described [25]. Briefly, 200 μM DHA was incubated with soybean lipoxygenase in sodium-borate buffer (pH 9.0) on ice for 30 min, synthesized hydroperoxides were then reduced by NaBH4 for 15 min, and the reduction step was stopped by adding acetic acid to pH 3.0. PDX was extracted by using a C18-Sep Pak cartridge, collected by reverse phase high performance liquid chromatography (RP-HPLC), and measured according to its UV absorbance.
Preparation of human neutrophils and measurement of ROS production
Human neutrophils were obtained from healthy donors, in using LPS-free conditions by onestep purification on polymorphprep dextran sedimentation as previously described [28]. Briefly, freshly drawn blood was added to cold dextran solution (0.1 mM dextran T500, 40 mM EDTA, 120 mM NaCl, pH 7.4) in a screw-cap polypropylene tubes, and mixed gently. After at least 1 hour on melting ice, the upper solution was collected and mixed with phosphate-buffered saline (PBS) pH 7.4. Cold Ficoll (density 1.077) was added carefully to the bottom of the tubes and were further centrifuged at 500 g for 20 min at 4°C. After centrifugation, lymphocytes were removed and the pellets were re-suspended in cold PBS. Red cells were lysed by hypotonic shock. Neutrophils were then washed twice with PBS and kept on ice until analysis.
ROS production was measured with a luminol-amplified chemiluminescence method: human neutrophils were suspended in Hank’s balanced salt solution (HBSS) containing 10 μM luminol, and pre-heated at 37°C in the thermostated chamber of the luminometer (Berthold-Biolumat LB937) prior to treatment with PDX. Neutrophils were then stimulated with PMA (0.162 mM) or 0.1 μM fMLF, for chemiluminescence recording.
Measurement of released MPO from neutrophils
The release of MPO was performed by measuring its activity in using the H2O2-dependent tetramethyl benzidine (TMB)-oxidation assay at 650 nm. Human neutrophils were suspended in HBSS and pre-heated at 37 °C. The cells were treated with PDX, and then stimulated or not with 0.1 μM fMLF. Neutrophils were then centrifuged and MPO activity was measured in supernatants. Total MPO activity has been expressed in mU/min relative to a standard curve established with known amounts of triton X-100-treated neutrophils.
Immunoblotting of MPO released by fMLF stimulated neutrophils was done with primary rabbit antibodies against MPO (dilution: 1/10,000), and with a horseradish peroxidase (HRP)-labeled-goat anti-rabbit antibody (dilution: 1/10,000). The reaction was measured by using enhanced chemiluminescence (ECL) reagents.
Detection of Ser345 phosphorylation in neutrophils
Neutrophils were incubated with different concentrations of PDX for 20 min, then treated with 0.1 μM fMLF for 20 min. Cell proteins were denatured in Laemmli buffer [29]. The samples were analyzed by 10% SDS-polyacrylamide gel electrophoresis (PAGE), and immunoblotting was done with primary rabbit antibodies against phospho-Ser345 (dilution: 1/10,000) or against p47phox (dilution: 1/5,000), then with HRP-labeled-goat anti-rabbit antibody (dilution: 1/10,000). The reaction was measured by using ECL reagents.
Measurement of 5-lipoxygenase activity in neutrophils
Human neutrophils, suspended in a Tyrode-HEPES buffer containing 2 mM of Ca2+, were pre-incubated with 1μM PDX for 3 min at 37°C, and were then triggered with 1 μM of ionophore A23187 plus 10 μM of ARA for 10 min. The reaction was terminated by acidifying the media to pH 3.0 with acetic acid. Oxygenated metabolites (5-hydroxy-6E,8Z,11Z,14Z-eicosatetraenoic acid (5-HETE), LTB4 and its isomers) were then extracted by solid phase extraction on Sep-Pak C18-cartridges. They were analyzed by RP-HPLC on a Waters XBridge C18 column (4.6×250 mm, 3.5 μm) by using a linear solvent gradient (1 mL/min): solvent A was acetonitrile/water (10:90, v/v) pH 3.0, and solvent B was acetonitrile. Mono-hydroxylated conjugated diene and di-hydroxylated conjugated triene fatty acids were detected with a diode array detector at 235 nm and 270 nm, respectively.
GC-MS measurement of COX-1 and COX-2 activities
Commercial COX-1 and COX-2 were pre-incubated for 15 min in a 0.1 M Tris HCl buffer pH 8.0 with or without 0.5 or 5 μM of PDX, and incubated with 10 μM ARA as a substrate for 10 min. Albumin (45 g/L) was added to enhance the formation of prostaglandin (PG) D2 and E2 from PGH2 at the end of incubation [30]. Reaction was stopped by acidification to pH 3.0 with acetic acid. PGD2 and E2 were then extracted by 10 volumes of diethyl ether. Deuterated PGD2 and E2 were used as internal standards for their quantification. Prostaglandins were derivatized into methoxime, pentafluorobenzyl ester and trimethysilyl ether, and further analyzed by NICI GC-MS. Selected ions corresponding to [M-181]− (loss of the PFB group): m/z: 524 for both derivatized PGD2 and E2 and m/z: 528 for their corresponding deuterated internal standards, were used for their measurement.
Measurement of LPS-induced COX activities in human neutrophils
Human neutrophils were treated by LPS (100 ng/mL) in order to overexpress COX-2. After 4 hours, cells were pre-incubated in presence or absence of 10 μM PDX for 3 min at 37°C, and 10 μM radiolabelled ARA (333 Bq/nmol) was added as a substrate for 10 min. After acidification to pH 3.0 with acetic acid, lipids were extracted by 10 volumes of diethyl ether and separated by thin-layer chromatography (TLC). Radioactivity of extracted gel spots corresponding to PGD2 and E2 were measured by scintillation counting.
PDX incorporation into neutrophils
10 μM PDX was incubated with isolated neutrophils for 3 min at 37°C. Cells were washed twice with buffer containing 45 g/L fatty acid free albumin. After centrifugation at 500 g for 20 min at 4°C, pellets were extracted using methanol/acidified water and PDX was measured by LC-MS/MS (AB Sciex, Qtrap 4500), using the double 15-lipoxygenase product of arachidonic acid (8,15-diHETE) as an internal standard. Quantification was done using multiple reaction monitoring mode (MRM). Parent ions were m/z: 359 and m/z: 335 and daughter ions m/z 153 and m/z 235 for PDX and 8,15-diHETE respectively.
Statistical analysis
All results are expressed as means ± standard error of the mean (SEM) or means ± standard deviation (SD). One away ANOVA followed by Fisher’s protected least significant difference (PLSD) post hoc test was used to assess differences between groups. P-value of 0.05 was assumed to represent the level of significance. Statistical significance was determined by using 2-tailed Student’s t tests in case of comparison between two groups of values.
Results
PDX inhibits fMLF and PMA-induced superoxide anion from neutrophils
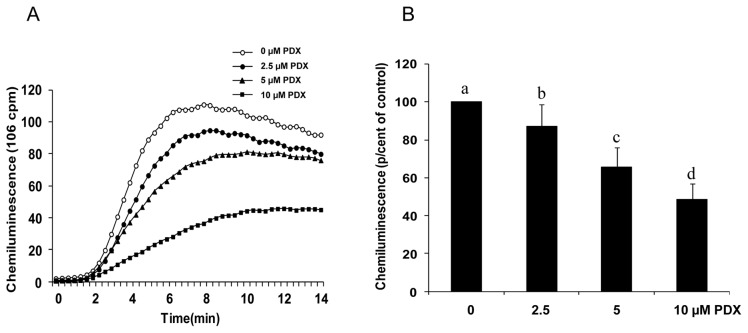
PDX was tested on ROS production in response to PMA. In presence of PDX for 20 min, PMA-induced neutophil production of ROS was decreased. Our results (Fig. 1) show that in absence of PDX, PMA induced a marked ROS production compared to baseline (Fig. 1A), and incubation of neutrophils with PDX prior to the addition of PMA, induced a significant decrease in ROS production (Fig. 1B), indicating that PDX inhibited NOX activity, and/or scavenged the produced ROS. The effect of PDX was dose-dependent from 2.5 μM to 10 μM. At these concentrations, PDX did not affect PMN viability as determined by trypan blue exclusion test (not shown).
Figure 1. Effect of PDX on PMA-induced superoxide anion production by human neutrophils.
Human neutrophils (2 × 106 cells/mL HBSS) were incubated in the absence or presence of different concentrations of PDX for 20 min. PMA (0.162 mM) was added, and O2° production was measured by chemiluminescence technique in the presence of 10 μM luminol. One curve is representative of four experiments (A). Total chemiluminescence in each condition was quantified and presented as mean ± SEM of 4 experiments (B). Different letters indicate significant differences between the groups at p< 0.001 level (one way ANOVA followed by Fisher’s PLSD test).
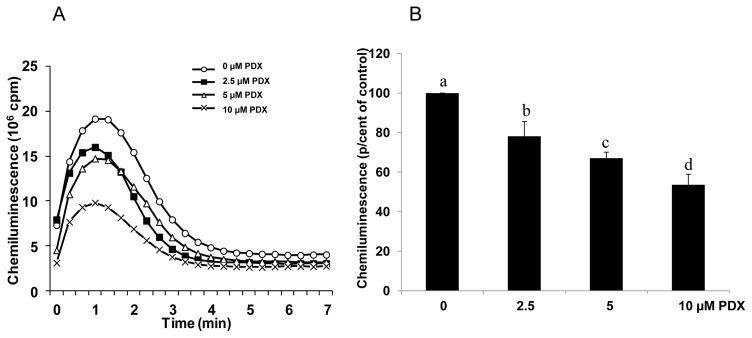
We then analyzed the effect of PDX on the neutrophil respiratory burst induced by 0.1 μM fMLF. Figure 2A shows that, similarly to the PMA experiments, the luminescence of neutrophils stimulated with fMLF is increased and lowered by PDX, in a dose-dependent manner, also from 2.5 μM to 10 μM (Fig. 2B). In contrast, PDX had no effect on superoxide production by the in vitro xanthine/xanthine oxidase system, and had no effect on H2O2 availability as measured by the peroxidase-catalyzed reaction (data not shown). These data suggest that PDX did neither scavenge superoxide anion, nor H2O2.
Figure 2. Effect of PDX on fMLF-induced superoxide anion production by human neutrophils.
Human neutrophils (2 × 106 cells/mL HBSS) were incubated in the absence or presence of different concentrations of PDX for 20 min. fMLF (0.1 μM) was added, and O2° production was measured by chemiluminescence technique in the presence of 10 μM luminol. One curve is representative of four experiments (A). Total chemiluminescence in each condition was quantified and presented as mean ± SEM of 4 experiments (B). Different letters indicate significant differences between the groups at p< 0.005 level (one way ANOVA followed by Fisher’s PLSD test).
PDX inhibits fMLF-induced MPO release from neutrophils
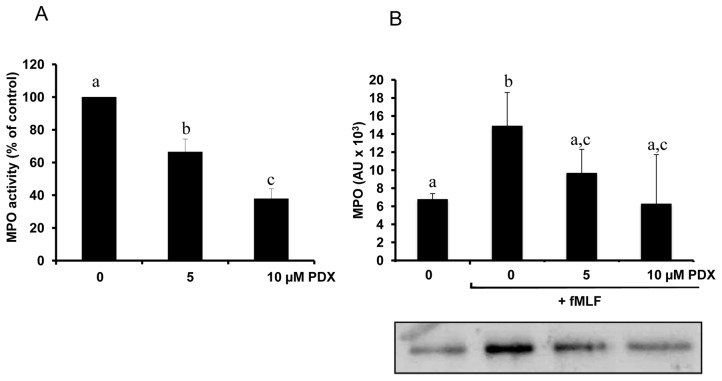
Neutrophil NOX produces superoxide anion (O2−) that is the source of other ROS. O2− is spontaneously dismutated into H2O2 at acidic pH, within the phagosome or the extracellular medium. The release of MPO catalyzes the conversion of H2O2 into the toxic molecule HOCl in presence of chloride ions [31–33]. Also, reaction between H2O2 and OCl− produces singlet oxygen, another reactive oxygen species. Therefore, the activation of NOX synergizes with MPO for inflammatory processes. The effect of PDX was then investigated on neutrophil release of MPO. Human neutrophils were incubated in HBSS medium in presence or absence of PDX for 20 min at 37°C. After washing and centrifugation, PMN were stimulated with 0.1 μM fMLF, and MPO release was assessed by measuring its activity in supernatant of all samples. Figure 3A shows that MPO activity from neutrophils pre-treated with PDX and stimulated with 0.1 μM fMLF, was significantly decreased in a dose-dependent manner as compared to control (neutrophils treated without PDX, but stimulated with fMLF). To test whether this inhibitory effect may reflect decreased neutrophil degranulation, or is related to decreased enzymatic activity, we performed a western blot analysis of the supernatant from fMLF-stimulated samples, using an antibody directed against human MPO. Results (Fig. 3B) show that the amount of MPO was lower in 5 μM and 10 μM PDX-treated neutrophils than in control (neutrophils treated without PDX, but stimulated with fMLF), whereas we did not find any effect of PDX on the enzymatic activity determined in vitro on recombinant MPO activity (data not shown). Taken together our results suggest that PDX exerts an inhibitory effect on stimulated neutrophil oxidative burst and degranulation.
Figure 3. Inhibitory effect of PDX on neutrophil release of MPO.
Human neutrophils (2 × 106 cells/mL HBSS) were pretreated or not (control) with 5 or 10 μM PDX for 20 min, then stimulated with fMLF (0.1 μM) for 3 min. Cells were centrifuged and MPO activity (A) was determined in the supernatants using H2O2 and TMB as described in Materials and Methods. Different letters indicate significant difference between groups at p< 0.001 level. MPO protein (B) was determined by immunoblotting with anti MPO antibody. Data are presented as mean ± SEM of 4 experiments. Different letters indicate significant differences between the groups at p< 0.05 level (one way ANOVA followed by Fisher’s PLSD test).
PDX inhibits fMLF-induced p47phox phosphorylation on Ser345
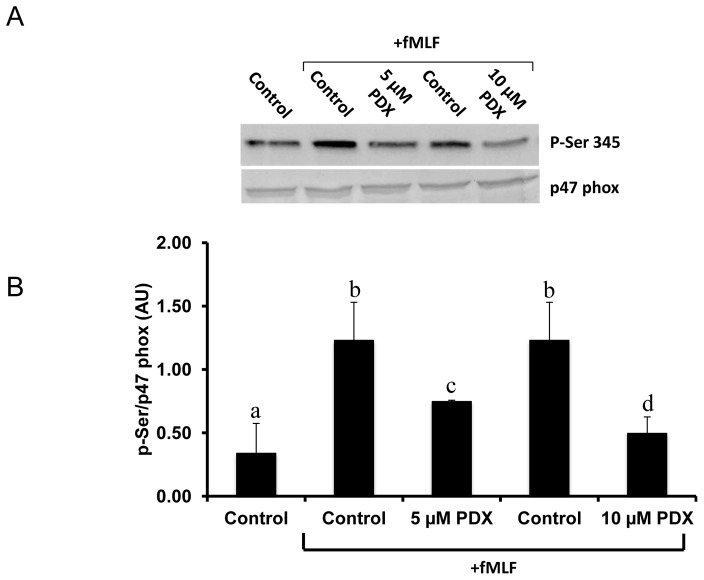
We have previously shown that production of ROS by NOX results from the phosphorylation of its cytosolic subunits, particularly p47phox on Ser345. The effect of PDX on this process is shown in Fig. 4. The level of phosphorylated 345P-Ser on p47phox has been corrected from the amount of total p47phox, which shows that PDX significantly lowers the phosphorylation of this protein when induced by fMLF treatment of neutrophils (Fig. 4A and B).
Figure 4. Effects of PDX on fMLF-induced p47phox phosphorylation on Ser345.
Human neutrophils (2 × 106 cells/mL HBSS) were pretreated with 5 or 10 μM PDX for 20 minutes, then incubated with or without (control) fMLF (0.1 μM) for 20 minutes. Cells were then lysed and proteins were analyzed with SDS-PAGE and immunoblotting with anti-phospho-ser345 antibody (pSer345) or anti-p47phox antibody (p47phox) (A). Western blots from different experiments were scanned, phosphorylated and total p47phox were quantified by densitometry, and the intensity of phosphorylated p47phox was corrected for the amount of p47phox (B). Data are representative of 3 independent experiments using cells from different healthy donors. Results are expressed as mean ± SD (n=3). Different letters indicate significant differences between the groups at p< 0.05 level. One away ANOVA followed by Fisher’s PLSD test.
Effect of PDX on COX-1 and COX-2 activities
The inhibition of purified cyclooxygenases (COX-1 and COX-2) by 0.5 μM and 5 μM of PDX were assessed by GC-MS measurement of both PGD2 and E2 which represent the global prostaglandin synthesis after albumin addition and acidification that convert PGH2 into PGD2 and E2 (see methods). Results reported in Table 1 show that 0.5 μM and 5 μM PDX significantly decreased prostaglandin production through COX-1 by around 55% and 75%, respectively. Interestingly, the inhibition of COX-2 by PDX seems even more efficient as the decreased prostaglandin production through COX-2 attained 70% and 80% in response to 0.5 μM and 5 μM PDX, respectively. This means that the decreased production of pro-inflammatory prostaglandins by PDX may substantially contribute in its anti-inflammatory effects.
Table 1.
Effect of PDX on COX activities
| Control | PDX 0.5 μM | PDX 5 μM | |
|---|---|---|---|
| COX-1 | 3.4 ± 0.4 | 1.5 ± 0.04* | 0.9±0.02* |
| COX-2 | 1.6 ± 0.7 | 0.5 ± 0.02* | 0.3±0.01* |
Each result is expressed in nanomoles of PGD2 + E2, representatives of total prostaglandin production (see methods). The results represent the mean ± SD of 3 determinations,
p< 0.05 as compared to control.
Effect of PDX on prostaglandin production in human neutrophils treated by LPS
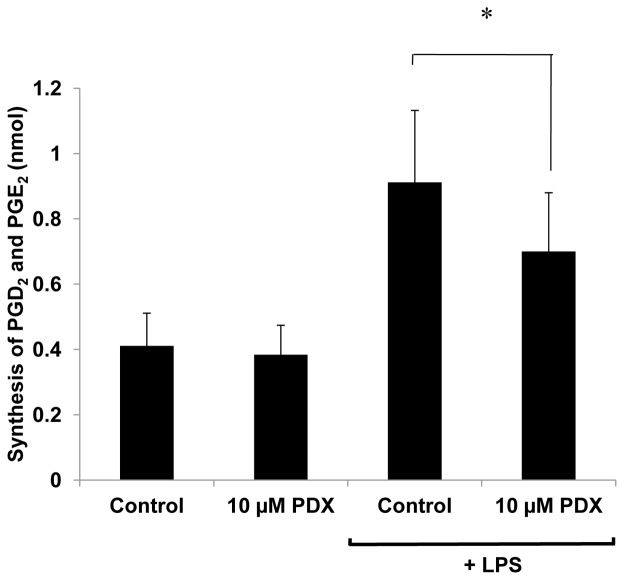
Neutrophils were treated by LPS in order to overexpress COX-2. Radiolabelled ARA was then used as a substrate for the neutrophil cyclooxygenase in the presence or absence of 10 μM PDX, in order to exclude the contribution of released endogenous ARA in response to LPS. COX activity was assessed by measuring the radioactive PGD2 and E2 as representatives of prostaglandin production. Results (Fig. 5) show that such a production was enhanced by 121% following the LPS treatment, compared to non-stimulated cells, reflecting the expected COX-2 overexpression. 10 μM PDX significantly decreased prostaglandin production by around 25% in LPS-treated neutrophils. The inhibition by PDX was not visible in non-treated neutrophils compared to control (not shown).
Figure 5. Effect of PDX on the production of PGD2 and E2 in human neutrophils treated ornot by LPS.
Human neutrophils (2 × 106 cells/mL HBSS) were treated in the presence or absence of 100 ng/mL LPS for 4 hours. Cells were then pre-incubated with and without 10 μM PDX for 3 min at 37°C and incubated with 10 μM radiolabelled ARA for 10 min. Lipids were extractedafter acidification, and PGD2 and E2, representative of prostaglandin production, were separated by TLC. Their corresponding spots were detected by dichlorofluorescein, scrapped off and the radioactivity was counted. Results are expressed in nanomoles of PGD2 and E2 synthesized and represent the mean ± SEM of 4 determinations. * p< 0.05 as compared to control.
Effect of PDX on 5-lipoxygenase activity in activated human isolated PMN
The 5-lipoxygenase pathway is a relevant one to promote inflammation through the ARA metabolite LTB4. The 5-LOX end-products are LTB4 and its geometrical and stereochemical isomers (LTB4 isomers), as well as 5-HETE. Measuring all those end-products provides a global view of the 5-lipoxygenase pathway acting upon ARA. Results summarized in Table 2 show that even at 10 μM, PDX did not inhibit the 5-lipoxygenase pathway, meaning that the inhibition of the cyclooxygenase reported above is rather specific.
Table 2.
Effect of PDX on the 5-lipoxygenase in activated human neutrophils
| Control | PDX 1 μM | PDX 10 μM | |
|---|---|---|---|
| LTB4s | 9.0±1.6 | 9.0±3.0 | 9.1±2.4 |
| 5-HETE | 0.8±0.3 | 0.6±0.3 | 0.6±0.4 |
Each result is expressed in nanomoles of the sum of the different 5-lipoxygenase metabolites: LTB4s (LTB4 + LTB4 isomers) and 5-HETE and represents the mean ± SD of 3 determinations.
PDX incorporation into neutrophils
The IC50 for the inhibition of isolated COX enzymes by PDX being much lower (less than 0.5 μM) than in neutrophils (more than 10μM), studies on the uptake of PDX has been done. Incubation of neutrophils with 10 μM PDX led to low incorporation (8.5 +/− 0.7 %) as measured by LC-MS/MS. No degradation products were detected, and the remaining PDX (not incorporated) was measured in the supernatant fraction.
Discussion
The present study shows that PDX inhibits both ROS production and MPO release by human neutrophils. Also, the inhibition of fMLF-induced activation of NOX results from the inhibition of the phosphorylation of p47phox on Ser345.
Several cytokines, including TNF-α, are involved in many inflammatory states. As a matter of fact, anti-TNF-α antibodies are used to treat some severe forms of inflammatory bowel disease [34]. This cytokine primes the neutrophil ROS production that is a key process in inflammation [35]. Primed neutrophils have been reported in diverse inflammatory states [36, 37]. One molecular mechanism involved in this priming process is the phosphorylation of p47phox on Ser345. Anti-inflammatory interventions could use nutritional approaches to decrease such a phosphorylation and subsequently ROS overproduction, without altering that one which is required for innate immunity and host defence. The present study shows that PDX, a double lipoxygenase product of DHA, is able to slow-down NOX activation and subsequent ROS-derived oxidative stress. MPO activity was also decreased by PDX to the same extent. As MPO and ROS (especially H2O2) generate toxic molecules at the inflammatory sites, the inhibition by PDX supports the potential action of this DHA metabolite as an anti-inflammatory agent. However, this protective effect needs to be confirmed in vivo.
Other fatty acids may affect ROS production in neutrophils. Linoleic acid (LA), the main consumed fatty acid in western countries [38], may act synergistically with TNF-α to affect ROS production [39, 40]. The LA product ARA can directly activate neutrophils [41] through enzymatic conversion into pro-inflammatory oxygenated metabolites such as LTB4 and PGE2 [42–44]. By contrast, n-3 PUFA such as EPA and DHA, provided by marine lipids, are oxygenated into less inflammatory and even anti-inflammatory derivatives counteracting the effects of ARA-derived eicosanoids [45]. Indeed, Hardy et al. have shown that pre-treatment with ARA, enhances fMLF- or PMA-stimulated neutrophils, then inducing more superoxide anion production than in non ARA-enriched cells [46]. In contrast, a recent study reported that EPA and DHA could increase the response of neutrophils to TNF-α [47]. None of these in vitro and in vivo experiments have addressed the molecular mechanism that might explain the increased ROS production.
Nutritional doses of EPA and DHA have been used in humans to prevent inflammatory states, and to lower the level of circulating inflammation markers [45]. The inhibitory effect of PDX on prostaglandin synthesis by either sheep COX-1 or human recombinant COX-2 is quite relevant in this respect, especially at 0.5 μM. This was also observed to a lesser extent in neutrophils treated by LPS. However, this inhibition is in the same order if we consider that only 8% of PDX was incorporated into the neutrophils after 10 μM incubation. This means that PDX would be more relevant when produced inside neutrophils through their own 15-LOX.
In addition to the COX-1 inhibition, already observed in relation with the inhibition of platelet aggregation [24], PDX appears to be an even more potent inhibitor of COX-2 which is the inducible enzyme, although detectable at low level in most normal tissues. It becomes abundant in activated macrophages and other cells at sites of inflammation. The anti-inflammatory potential of PDX is quite specific since it fails to inhibit the 5-lipoxygenase pathway. Therefore, PDX appears to be an original anti-inflammatory agent to play a protective role in chronic inflammatory diseases.
We conclude that PDX inhibits fMLF-induced ROS production by targeting the neutrophil p47phox/NOX axis and MPO release as well. Besides its inhibitory effects on platelet aggregation through inhibition of COX-1, that is confirmed against purified COX-1, PDX inhibits COX-2 and then could protect against inflammation. It might be used for the prevention and treatment of various inflammatory diseases. However, further in vivo studies are needed to confirm those in vitro findings.
Acknowledgments
This study was supported by Inserm and the French Ministry of Education and Research. Miao Liu was granted from China Scholarship Council.
Abbreviations
- ARA
arachidonic acid
- BSTFA
N,O-Bis(trimethylsilyl)-trifluoroacetamide
- CLA
conjugated linoleic acid
- ClnA
conjugated linolenic acid
- COX
cyclooxygenase
- DHA
docosahexaenoic acid
- ECL
enhanced chemiluminescence
- EPA
eicosapentaenoic acid
- 5-HETE
5-hydroxy-6E,8Z,11Z,14Z-eicosatetraenoic acid
- fMLF
formyl-methionyl-leucyl-phenylalanine
- GM-CSF
granulocyte macrophage-colony stimulating factor
- HBSS
Hank’s balanced salt solution
- HOCl
hypochloric acid
- HRP
horseradish peroxidase
- IBD
inflammatory bowel disease
- IL
interleukin
- LA
linoleic acid
- LPS
lipopolysaccharides
- LTB4
leukotriene B4
- MPO
myeloperoxidase
- NOX
NADPH oxidase
- PAF
platelet-activating factor
- PAGE
polyacrylamide gel electrophoresis
- PBS
phosphate-buffered saline
- PDX
protectin DX
- PG
prostaglandin
- phox
phagocyte oxidase
- PMA
phorbol myristate acetate
- PMN
polymorphonuclear neutrophil
- PUFA
polyunsaturated fatty acids
- ROS
reactive oxygen species
- RP-HPLC
reverse phase high performance liquid chromatography
- sLOX
soybean lipoxygenase
- TLC
thin-layer chromatography
- TMB
tetramethyl benzidine
- TNBS
trinitrobenzene sulfonic acid
- TNF-α
tumor necrosis factor-α
References
- 1.Mullenix PS, Andersen CA, Starnes BW. Atherosclerosis as Inflammation. Ann Vasc Surg. 2005;19:130–138. doi: 10.1007/s10016-004-0153-z. [DOI] [PubMed] [Google Scholar]
- 2.Fiocchi C. Inflammatory bowel disease: etiology and pathogenesis. Gastroenterology. 1998;115:182–205. doi: 10.1016/s0016-5085(98)70381-6. [DOI] [PubMed] [Google Scholar]
- 3.Podolsky DK. Inflammatory bowel disease. N Engl J Med. 2002;347:417–429. doi: 10.1056/NEJMra020831. [DOI] [PubMed] [Google Scholar]
- 4.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 5.Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, 3rd, Criqui M, Fadl YY, Fortmann SP, Hong Y, Myers GL, Rifai N, Smith SC, Jr, Taubert K, Tracy RP, Vinicor F. Markers of Inflammation and Cardiovascular Disease: application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 6.Esposito K, Giugliano D. The metabolic syndrome and inflammation: association or causation? Nutr Metab Cardiovasc Dis. 2004;14:228–232. doi: 10.1016/s0939-4753(04)80048-6. [DOI] [PubMed] [Google Scholar]
- 7.Choy EH, Panayi GS. Cytokine Pathways and Joint Inflammation in Rheumatoid Arthritis. N Engl J Med. 2001;344:907–916. doi: 10.1056/NEJM200103223441207. [DOI] [PubMed] [Google Scholar]
- 8.Nathan C. Points of control in inflammation. Nature. 2002;420:846–852. doi: 10.1038/nature01320. [DOI] [PubMed] [Google Scholar]
- 9.Santana Reyes C, García-Muñoz F, Reyes D, González G, Dominguez C, Domenech E. Role of cytokines (interleukin-1beta, 6, 8, tumour necrosis factor-alpha, and soluble receptor of interleukin-2) and C-reactive protein in the diagnosis of neonatal sepsis. Acta Paediatr. 2003;92:221–227. doi: 10.1111/j.1651-2227.2003.tb00530.x. [DOI] [PubMed] [Google Scholar]
- 10.Bayne LJ, Beatty GL, Jhala N, Clark CE, Rhim AD, Stanger BZ, Vonderheide RH. Tumor-derived granulocyte-macrophage colony-stimulating factor regulates myeloid inflammation and T cell immunity in pancreatic cancer. Cancer Cell. 2012;21:822–835. doi: 10.1016/j.ccr.2012.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ortega-Gómez A, Perretti M, Soehnlein O. Resolution of inflammation: an integrated view. EMBO Mol Med. 2013;5:661–674. doi: 10.1002/emmm.201202382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Menshchikova E, Zenkov N, Tkachev V, Potapova O, Cherdantseva L, Shkurupiy V. Oxidative Stress and Free-Radical Oxidation in BCG Granulomatosis Development. Oxid Med Cell Longev. 2013;2013:452546. doi: 10.1155/2013/452546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klebanoff SJ. Myeloperoxidase: friend and foe. J Leukoc Biol. 2005;77:598–625. doi: 10.1189/jlb.1204697. [DOI] [PubMed] [Google Scholar]
- 14.Roos D, van Bruggen R, Meischl C. Oxidative killing of microbes by neutrophils. Microbes Infect. 2003;5:1307–1315. doi: 10.1016/j.micinf.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 15.Babior BM. Oxidants from phagocytes: agents of defense and destruction. Blood. 1984;64:959–966. [PubMed] [Google Scholar]
- 16.Babior BM, Lambeth JD, Nauseef W. The neutrophil NADPH oxidase. Arch Biochem Biophys. 2002;397:342–344. doi: 10.1006/abbi.2001.2642. [DOI] [PubMed] [Google Scholar]
- 17.Sheppard FR, Kelher MR, Moore EE, McLaughlin NJ, Banerjee A, Silliman CC. Structural organization of the neutrophil NADPH oxidase: phosphorylation and translocation during priming and activation. J Leukoc Biol. 2005;78:1025–1042. doi: 10.1189/jlb.0804442. [DOI] [PubMed] [Google Scholar]
- 18.Whiting CV, Bland PW, Tarlton JF. Dietary n-3 polyunsaturated fatty acids reduce disease and colonic proinflammatory cytokines in a mouse model of colitis. Inflamm Bowel Dis. 2005;11:340–349. doi: 10.1097/01.mib.0000164016.98913.7c. [DOI] [PubMed] [Google Scholar]
- 19.Serhan CN. Resolvins and protectins: novel lipid mediators in anti-inflammation and resolution. Scand J Food Nutr. 2006;50:68–78. [Google Scholar]
- 20.Kohli P, Levy BD. Resolvins and protectins: mediating solutions to inflammation. Br J Pharmacol. 2009;158:960–971. doi: 10.1111/j.1476-5381.2009.00290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Song HJ, Sneddon AA, Barker PA, Bestwick C, Choe SN, McClinton S, Grant I, Rotondo D, Heys SD, Wahle KW. Conjugated linoleic acid inhibits proliferation and modulates protein kinase C isoforms in human prostate cancer cells. Nutr Cancer. 2004;49:100–108. doi: 10.1207/s15327914nc4901_14. [DOI] [PubMed] [Google Scholar]
- 22.Flowers M, Thompson PA. t10c12 conjugated linoleic acid suppresses HER2 protein and enhances apoptosis in SKBr3 breast cancer cells: possible role of COX2. PLoS One. 2009;4:e5342. doi: 10.1371/journalpone0005342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boussetta T, Raad H, Lettéron P, Gougerot-Pocidalo MA, Marie JC, Driss F, El-Benna J. Punicic acid a conjugated linolenic acid inhibits TNFα-induced neutrophil hyperactivation and protects from experimental colon inflammation in rats. PLoS One. 2009;4:e6458. doi: 10.1371/journalpone0006458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen P, Véricel E, Lagarde M, Guichardant M. Poxytrins, a class of oxygenated products from polyunsaturated fatty acids, potently inhibit blood platelet aggregation. FASEB J. 2011;25:382–388. doi: 10.1096/fj.10-161836. [DOI] [PubMed] [Google Scholar]
- 25.Chen P, Fenet B, Michaud S, Tomczyk N, Véricel E, Lagarde M, Guichardant M. Full characterization of PDX, a neuroprotectin/protectin D1 isomer, which inhibits blood platelet aggregation. FEBS Lett. 2009;583:3478–3484. doi: 10.1016/j.febslet.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 26.Serhan CN, Gotlinger K, Hong S, Lu Y, Siegelman J, Baer T, Yang R, Colgan SP, Petasis NA. Anti-inflammatory actions of neuroprotectin D1/protectin D1 and its natural stereoisomers: assignments of dihydroxy-containing docosatrienes. J Immunol. 2006;176:1848–1859. doi: 10.4049/jimmunol.176.3.1848. [DOI] [PubMed] [Google Scholar]
- 27.Morita M, Kuba K, Ichikawa A, Nakayama M, Katahira J, Iwamoto R, Watanebe T, Sakabe S, Daidoji T, Nakamura S, Kadowaki A, Ohto T, Nakanishi H, Taguchi R, Nakaya T, Murakami M, Yoneda Y, Arai H, Kawaoka Y, Penninger JM, Arita M, Imai Y. The lipid mediator protectin D1 inhibits influenza virus replication and improves severe influenza. Cell. 2013;153:112–125. doi: 10.1016/j.cell.2013.02.027. [DOI] [PubMed] [Google Scholar]
- 28.Dang PM, Stensballe A, Boussetta T, Raad H, Dewas C, Kroviarski Y, Hayem G, Jensen ON, Gougerot-Pocidalo MA, El-Benna J. A specific p47phox -serine phosphorylated by convergent MAPKs mediates neutrophil NADPH oxidase priming at inflammatory sites. J Clin Invest. 2006;116:2033–2043. doi: 10.1172/JCI27544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 30.Watanabe T, Narumiya S, Shimizu T, Hayaishi O. Characterization of the biosynthetic pathway of prostaglandin D2 in human platelet-rich plasma. J Biol Chem. 1982;257:14847–14853. [PubMed] [Google Scholar]
- 31.Klebanoff SJ. Myeloperoxidase. Proc Assoc Am Physicians. 1999;111:383–389. doi: 10.1111/paa.1999.111.5.383. [DOI] [PubMed] [Google Scholar]
- 32.Winterbourn CC, Kettle AJ. Biomarkers of myeloperoxidase-derived hypochlorous acid. Free Radic Biol Med. 2000;29:403–409. doi: 10.1016/s0891-5849(00)00204-5. [DOI] [PubMed] [Google Scholar]
- 33.Stamp LK, Khalilova I, Tarr JM, Senthilmohan R, Turner R, Haigh RC, Winyard PG, Kettle AJ. Myeloperoxidase and oxidative stress in rheumatoid arthritis. Rheumatology (Oxford) 2012;51:1796–1803. doi: 10.1093/rheumatology/kes193. [DOI] [PubMed] [Google Scholar]
- 34.Papadakis KA, Targan SR. Tumor necrosis factor: biology and therapeutic inhibitors. Gastroenterology. 2000;119:1148–1157. doi: 10.1053/gast.2000.18160. [DOI] [PubMed] [Google Scholar]
- 35.Dewas C, Dang PM, Gougerot-Pocidalo MA, El-Benna J. TNF-alpha induces phosphorylation of p47 (phox) in human neutrophils: partial phosphorylation of p47phox is a common event of priming of human neutrophils by TNF-alpha and granulocyte-macrophage colony-stimulating factor. J Immunol. 2003;171:4392–4398. doi: 10.4049/jimmunol.171.8.4392. [DOI] [PubMed] [Google Scholar]
- 36.El-Benna J, Dang PM, Gougerot-Pocidalo MA. Priming of the neutrophil NADPH oxidase activation: role of p47phox phosphorylation and NOX2 mobilization to the plasma membrane. Semin Immunopathol. 2008;30:279–289. doi: 10.1007/s00281-008-0118-3. [DOI] [PubMed] [Google Scholar]
- 37.Larmonier CB, Midura-Kiela MT, Ramalingam R, Laubitz D, Janikashvili N, Larmonier N, Ghishan FK, Kiela PR. Modulation of neutrophil motility by curcumin: implications for inflammatory bowel disease. Inflamm Bowel Dis. 2011;17:503–515. doi: 10.1002/ibd.21391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zock PL, Katan MB. Linoleic acid intake and cancer risk: a review and meta-analysis. Am J Clin Nutr. 1998;68:142–153. doi: 10.1093/ajcn/68.1.142. [DOI] [PubMed] [Google Scholar]
- 39.Hatanaka E, Levada-Pires AC, Pithon-Curi TC, Curi R. Systematic study on ROS production induced by oleic, linoleic, and gamma-linolenic acids in human and rat neutrophils. Free Radic Biol Med. 2006;41:1124–113239. doi: 10.1016/j.freeradbiomed.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 40.Martins de Lima-Salgado T, Coccuzzo Sampaio S, Cury-Boaventura MF, Curi R. Modulatory effect of fatty acids on fungicidal activity, respiratory burst and TNF-α and IL-6 production in J774 murine macrophages. Br J Nutr. 2011;105:1173–1179. doi: 10.1017/S0007114510004873. [DOI] [PubMed] [Google Scholar]
- 41.Wanten GJ, Janssen FP, Naber AH. Saturated triglycerides and fatty acids activate neutrophils depending on carbon chain-length. Eur J Clin Invest. 2002;32:285–289. doi: 10.1046/j.1365-2362.2002.00959.x. [DOI] [PubMed] [Google Scholar]
- 42.Lewis RA, Austen KF, Soberman RJ. Leukotrienes and other products of the 5-lipoxygenase pathway. Biochemistry and relation to pathobiology in human diseases. N Engl J Med. 1990;323:645–655. doi: 10.1056/NEJM199009063231006. [DOI] [PubMed] [Google Scholar]
- 43.Sakai M, Kakutani S, Horikawa C, Tokuda H, Kawashima H, Shibata H, Okubo H, Sasaki S. Arachidonic acid and cancer risk: a systematic review of observational studies. BMC Cancer. 2012;12:606. doi: 10.1186/1471-2407-12-606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harbige LS. Fatty acids, the immune response, and autoimmunity: a question of n-6 essentiality and the balance between n-6 and n-3. Lipids. 2003;38:323–341. doi: 10.1007/s11745-003-1067-z. [DOI] [PubMed] [Google Scholar]
- 45.Siriwardhana N, Kalupahana NS, Moustaid-Moussa N. Health benefits of n-3 polyunsaturated fatty acids: eicosapentaenoic acid and docosahexaenoic acid. Adv Food Nutr Res. 2012;65:211–222. doi: 10.1016/B978-0-12-416003-3.00013-5. [DOI] [PubMed] [Google Scholar]
- 46.Hardy SJ, Robinson BS, Poulos A, Harvey DP, Ferrante A, Murray AW. The neutrophil respiratory burst. Responses to fatty acids, N-formylmethionylleucylphenylalanine and phorbol ester suggest divergent signalling mechanisms. Eur J Biochem. 1994;198:801–806. doi: 10.1111/j.1432-1033.1991.tb16084.x. [DOI] [PubMed] [Google Scholar]
- 47.Paschoal VA, Vinolo MA, Crisma AR, Magdalon J, Curi R. Eicosapentaenoic (EPA) and docosahexaenoic (DHA) acid differentially modulate rat neutrophil function in vitro. Lipids. 2013;48:93–103. doi: 10.1007/s11745-012-3726-6. [DOI] [PubMed] [Google Scholar]